Abstract
This quality improvement study uses tumor size to assess which patients are at low risk for papillary thyroid cancer and may be candidates for active surveillance as a treatment strategy.
In 2015, the American Thyroid Association (ATA) guidelines for the first time endorsed active surveillance (AS) as an alternative to immediate surgery in select patients with low-risk papillary thyroid cancer (PTC).1 To date, very few patients in the US are being treated with AS. The numbers of newly diagnosed patients with PTC who may be AS candidates in the coming years are not known.
Methods
The study was deemed exempt from review by the Memorial Sloan Kettering Cancer Center institutional review board. Using the Surveillance, Epidemiology, and End Results (SEER) Database (SEER Database 18) of the National Cancer Institute (NCI) and SEER*Stat statistical software (version 8.3.4, NCI), we pulled the age-adjusted incidence and raw numbers of PTC from 2004 to 2016, and then converted to US population numbers with 2010 Census Bureau statistics. Predicted rates beyond 2016 were calculated using joinpoint regression analysis statistical software (version 4.5.0.1, NCI) to identify the most recent inflection point(s) in the annual percent change (APC) in the incidence of PTC.
Available 2016 data show the possible beginning of a plateau in rates; we therefore modeled 2 future predicted rates with 1 and 2 inflection points to describe a range of possibilities and raw numbers for future trends. The first APC model with 1 inflection point (APC, 1.41%) reflects a maximum number of patients expected to be diagnosed and treated for PTC starting in 2017; this is based on the overall rising incidence in the several years prior. The second APC model with 2 inflection points (APC, 2.09%) describes the possible leveling off of increased incidence seen from 2014 to 2016, and therefore suggests there may be lower numbers and expected trends.
We report a range of tumor sizes: based on Japanese data,2 the 2015 ATA guidelines used papillary microcarcinoma (<1 cm) as an example of very low-risk tumors for which AS can be considered, whereas current available literature from the Memorial Sloan Kettering Cancer Center cohort suggests safety of AS for tumors up to 1.5 cm,3 and the most recent literature examining tumor size and prognosis describes no survival differences based on size in any T1 tumors (≤2 cm).4 We therefore defined a group of patients with low-risk PTC (LR-PTC) as those with papillary histology staged clinically T1, clinically node-negative neck (N0), no distant metastases, and subgroups of patients with tumors 1.5 cm or smaller, and those with tumors 1.0 cm or smaller.
A risk-stratification system proposed by Brito et al5 characterizes patients with LR-PTC as inappropriate candidates for AS if their tumors show extracapsular extension, are in a subcapsular location adjacent to the recurrent laryngeal nerve (RLN), or invade the RLN. Similar risk stratification systems in Japanese cohorts describe 3% to 14% of tumors as being inappropriate for AS.2 Given the range from 3% to 14%, for the present analysis, we conservatively used an estimate of inappropriate candidates of 14%.
Results
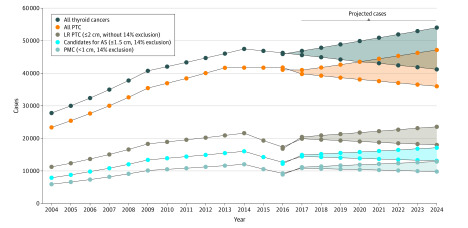
Between 2004 to 2016, 87% of patients with thyroid cancer had histologically confirmed PTC, 50% of which were LR-PTC (tumor ≤2.0 cm or TI). Among patients with T1 tumors, 84.5% had tumors of 1.5 cm or smaller, and 63.5% had tumors of 1.0 cm or smaller. Age-adjusted incidence rates, converted to US population statistics are seen in the Figure, with a range in future years after last available data in 2016 projected using the 2 different joinpoint models. The estimated total number of patients with T1 tumors in the next 5 years (2020-2024) will be 92 635 to 113 395. Using a size cutoff of 1.5 cm or smaller and 14% exclusion of inappropriate for AS, the estimated total number of patients eligible for AS in the next 5 years will be 67 344 to 82 405. Using a further size cutoff of less than 1 cm and 14% exclusion of inappropriate for AS, the estimated total number of patients eligible for AS in the next 5 years will be 50 578 to 61 925.
Figure. Projected Estimates of Thyroid Cancer Cases Eligible for Active Surveillance, 2020 to 2024.
AS indicates active surveillance; LR PTC, low-risk papillary thyroid cancer; PMC, papillary thyroid microcarcinomas. Beyond the available data to 2016, the upper and lower lines represent the 2 joinpoint-projected estimates, and the shaded area represents the range between the estimates. Incidence rates are age adjusted to 2000 US standard population and converted to US population statistics. For the subgroup of estimated patients eligible for active surveillance (≤1.5 cm) and those with papillary microcarcinoma (≤1 cm), the figure reflects an additional 14% exclusion of patients assumed to be inappropriate for surveillance.
Discussion
Our projections suggest that there will be 212 215 to 259 785 new thyroid cancer diagnoses with at least 67 344 patients having tumors of 1.5 cm or smaller, and at least 50 578 having tumors smaller than 1 cm over the next 5 years who will be potential candidates for active surveillance as a treatment strategy. Thus, there will be a pressing need for further development of high-quality data on treatment options (including active surveillance and local ablative therapies), guidelines, and support systems at the organizational and patient level geared specifically for this challenge.
References
- 1.Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133. doi: 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: a review of active surveillance trials. Eur J Surg Oncol. 2018;44(3):307-315. doi: 10.1016/j.ejso.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 3.Tuttle RM, Fagin JA, Minkowitz G, et al. Natural history and tumor volume kinetics of papillary thyroid cancers during active surveillance. JAMA Otolaryngol Head Neck Surg. 2017;143(10):1015-1020. doi: 10.1001/jamaoto.2017.1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen XV, Roy Choudhury K, Tessler FN, Hoang JK. Effect of tumor size on risk of metastatic disease and survival for thyroid cancer: implications for biopsy guidelines. Thyroid. 2018;28(3):295-300. doi: 10.1089/thy.2017.0526 [DOI] [PubMed] [Google Scholar]
- 5.Brito JP, Ito Y, Miyauchi A, Tuttle RM. A clinical framework to facilitate risk stratification when considering an active surveillance alternative to immediate biopsy and surgery in papillary microcarcinoma. Thyroid. 2016;26(1):144-149. doi: 10.1089/thy.2015.0178 [DOI] [PMC free article] [PubMed] [Google Scholar]