This case-control study assesses the association between macular abnormalities over time and the use of 5α-reductase inhibitors for treatment of benign prostatic hypertrophy and/or alopecia.
Key Points
Question
Is there an association between the male sex hormone antagonist 5α-reductase inhibitor and abnormalities in the eye?
Findings
In this case-control study of 31 male participants, medication that inhibits androgenic hormones for the treatment of benign prostatic hypertrophy and androgenic alopecia in male patients was associated with macular optical coherence tomography imaging abnormalities including cystoid abnormalities, foveal cavitation, or hypolucent outer foveal defects.
Meaning
The findings of this investigation suggest that macular abnormalities may be associated with a male sex hormone antagonist.
Abstract
Importance
The neuroprotective action of sex hormones has been described. Data on the association between 5α-reductase inhibitor (5-ARI), a male sex hormone antagonist, and macular abnormalities are lacking to date.
Objective
To assess the association between the use of 5-ARI for treatment of benign prostate hypertrophy and/or androgenic alopecia in men and macular abnormalities on optical coherence tomography imaging.
Design, Setting, and Participants
This retrospective case-control, cross-sectional study included electronic health record data from 31 male patients who showed foveal cavitation on spectral-domain optical coherence tomography imaging from January 1, 2016, to June 30, 2019.
Exposures
Receipt of 5-ARI for at least 2 years as treatment of benign prostate hypertrophy and/or androgenic alopecia.
Main Outcomes and Measures
Clinical data and multimodal imaging findings and the proportion of 5-ARI users.
Results
Among 31 male patients with foveal cavitation, 5-ARI was used for 10 of 14 patients (71.4%) with macular abnormalities of unknown origin and for 2 of 17 patients (11.8%) with macular abnormalities of well-known specific origin (P = .001). The mean age of these 14 patients was 74.7 years (range, 60.1-88.0 years). In the 15 eyes of 10 patients who had received 5-ARI for macular abnormalities of unknown origin, mean (SD) age was 72.8 (7.5) years, mean (SD) length of time receiving 5-ARI was 72.3 (39.2) months, and mean (SD) logMAR visual acuity was 0.08 (0.10) (Snellen equivalents, 20/24 [20/25]). Optical coherence tomography imaging showed a disease spectrum ranging from tiny foveal cavitation to an impending macular hole. Of the total male patients, 80.0% (8 of 10) had no symptoms.
Conclusions and Relevance
The findings suggest that macular abnormalities associated with 5-ARI are characterized by cystoid abnormalities and foveal cavitation in male patients, which may progress to outer foveal defect and macular hole. These macular abnormalities associated with a male sex hormone antagonist suggested by this investigation warrant further corroboration.
Introduction
There have been reports of various retinal diseases associated with the use of sex hormones.1,2,3,4 The high incidence of retinal disease noted after menopause or associated with patient sex may indicate the role of sex hormones. However, the mechanism associated with this end point is not known precisely yet. Menopause is associated with the development of idiopathic macular hole and macular telangiectasia (MacTel) type 2.1,5,6 Reports of sex hormone–associated retinal diseases have mostly concentrated on female patients, and few reports are published that involve men.7
Tamoxifen, a female sex hormone antagonist, is well known to be a selective estrogen receptor modulator and is associated with retinopathy.8,9 Recent studies8,10 of patients who received tamoxifen therapy for breast cancer reported retinal alterations similar to those observed in patients with MacTel type 2. Both tamoxifen-associated retinopathy and MacTel type 2 have a neurodegenerative pathogenesis in which retinal Müller cells may play a role and manifest retinal findings, such as bilateral refractile crystals, a loss of retinal transparency in the parafovea, and foveal cystoid spaces on optical coherence tomography (OCT).8,10 Recently, studies11,12 in male patients who received androgen deprivation therapy have reported the development of MacTel and age-related macular degeneration, but the exact role is not clear yet. In this study, we report a series of patients who showed abnormalities similar to tamoxifen-associated retinopathy and MacTel type 2 after receiving 5α-reductase inhibitor (5-ARI), a male sex hormone antagonist. 5-ARI is widely used for the management of benign prostatic hyperplasia (BPH) and alopecia. The frequency of 5-ARI use among Korean individuals after adjustment for age and estimated indirectly from the prevalence of BPH in 2016 was approximately 21.5%.13,14 As life expectancy increases, the number of patients with these diseases will increase, and the use of 5-ARI is expected to increase accordingly.13,15 However, to our knowledge, there has been no report to date addressing the association of 5-ARI with abnormalities in the eye. The purpose of the current study was to assess the clinical spectrum of macular findings associated with the use of 5-ARI in male patients.
Methods
This retrospective, case-control, cross-sectional study was approved by the institutional review board of Samsung Medical Center, Seoul, Korea, and conformed to the tenets of the Declaration of Helsinki.16 Electronic medical records of all male patients who visited Samsung Medical Center from January 1, 2016, to June 30, 2019, were screened. The institutional review board waived the requirement for a signed consent because the research presented no more than minimal risk of harm to persons and involves no procedures.
The electronic medical records system of the hospital provides a search engine (the seventh stage, the highest level of Healthcare Information Management and Systems Society) in which clinic notes of individual patients can be searched. The tomographic keywords foveal cavitation and inner retinal cyst were the preferentially used descriptive terms in the search to identify patients in the electronic medical records system. The following medical and demographic data were collected and analyzed: age, sex, chief complaint or reason for visit, medical history, medication history, and best-corrected visual acuity. Specifically, the history of receiving 5-ARI, which are also known as dihydrotestosterone (DHT) blockers (eg, finasteride, dutasteride), was documented. In addition, fluorescein angiography (Spectralis HRA + OCT; Heidelberg Engineering Inc), OCT (Spectralis HRA + OCT; Heidelberg Engineering Inc), and OCT angiography (DRI-OCT Triton; Topcon Company) were performed.
National Health Insurance Service data17 were used to determine the frequency of 5-ARI users in the entire Korean population. The number of patients who met the criteria of the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) diagnosis code for BPH (N40) and received a prescription for 5-ARI (finasteride, dutasteride) during 2016 were investigated in the study.
Statistical Analysis
Statistical analysis was performed using SAS, version 9.4 (SAS Institute Inc) and R 3.5.1 (The R Foundation). We used the comparison of proportions test (z test, Fisher exact test). P < .05 was considered statistically significant.
Results
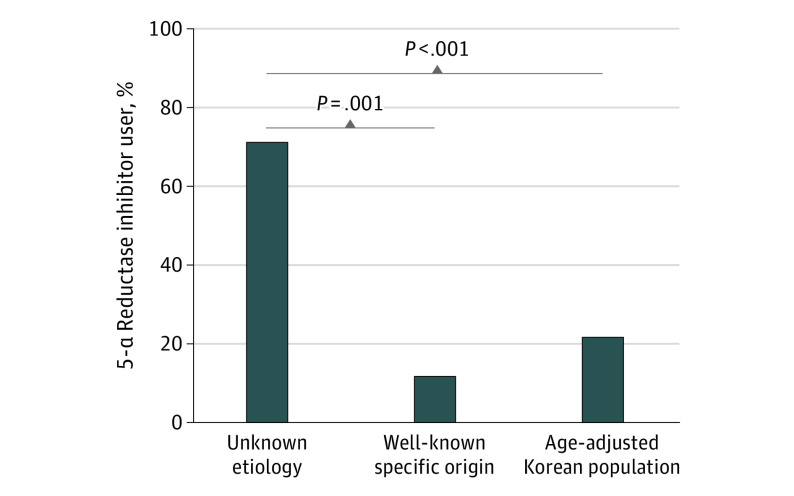
Of 31 male patients identified, 17 were excluded from this study because they had well-known specific causes of cystic change in the fovea; specifically, 9 patients had MacTel types 1 or 2, 2 patients had retinal vein occlusion, 2 had vitreous or membrane traction, and 1 patient each had diabetic macular edema, radiation retinopathy, myopic choroidal neovascularization, and retinoschisis. The remaining 14 patients had macular abnormalities of unknown cause. No vascular changes were seen on fluorescein angiography and OCT angiography in 14 patients, thus excluding the possibility of cystic changes because of systemic diseases, such as diabetes or hypertension. In addition, 12 eyes showed complete posterior vitreous attachment, and 2 eyes showed complete posterior vitreous detachment. If vitreomacular traction was present, patients were classified as having macular abnormalities with a known origin. The mean age of these 14 patients was 74.7 years (range, 60.1-88.0 years). Of the 14 patients with macular abnormalities of unknown origin, 10 (71.4%) had received 5-ARIs and 4 (28.6%) had not received this treatment. Of 17 patients with macular abnormalities of well-known specific origin, 2 (11.8%) had received 5-ARIs for BPH and 15 (88.2%) had not received this treatment. Of these 2 patients, OCT showed a foveal cystic lesion below focal vitreous traction for one and epiretinal membrane with multiple cystoid lesions for the other. A Fisher exact test revealed a significant difference in the proportion of patients receiving 5-ARI between the macular abnormalities of unknown origin group and the macular abnormalities of well-known specific origin group (71.4% vs 11.7%; P = .001) (Figure 1). During 2016, of 931 015 patients received a diagnosis of BPH, 382 042 patients received 5-ARI. The estimated frequency of 5-ARI use among the general population was also significantly lower than the proportion of individuals who received 5-ARI within the macular abnormalities of unknown origin group (21.5% vs 71.4%; P < .001, z test) (Figure 1).
Figure 1. Comparison of Male Patients With Foveal Cavitation on Spectral-Domain Optical Coherence Tomography.
Among the 10 patients with macular abnormalities of unknown origin who received 5-ARI, 5 had bilateral and unilateral presentations. The demographic and clinical data of these patients are summarized in the Table. The mean (SD) age of these 10 patients was 72.8 (7.5) years (range, 60.1-84.2 years). Mean (SD) length of 5-ARI treatment was 72.3 (39.2) months (range, 24.0-122.4 months). Mean (SD) refractive error of 15 eyes was 0.63 (1.87) D. Mean (SD) logMAR visual acuity was 0.08 (0.10) (Snellen equivalents, 20/24 [20/25]). Most of the included patients were asymptomatic; however, 1 patient presented with decreased vision due to cataract and another with distorted vision. Of these patients, 7 were receiving 5-ARI treatment for BPH, 2 were receiving 5-ARIs for alopecia, and 1 was receiving 5-ARIs for both BPH and alopecia. With regard to the 5-ARIs used, 5 patients were receiving dutasteride, 4 patients were receiving finasteride, and 1 patient was receiving both medications. The duration of dutasteride treatment ranged from 24 to 116 months. The cumulative dose ranged from 365.0 mg to 1746.0 mg. The duration of finasteride treatment was from 36 to 122 months. The cumulative dose was from 5490.0 mg to 21 900.0 mg.
Table. Characteristics of Patients Treated With 5α-Reductase Inhibitors.
| Case | Diagnosis | Other diseases | 5α-Reductase inhibitor, treatment duration (cumulative dose) | Reason for visit | Eye involved | Best-corrected visual acuity, Snellen | Refractive error | OCT finding | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Right eye | Left eye | Right eye | Left eye | |||||||
| 1 | BPH | Diabetes | Finasteride, 31 mo (4690 mg) and dutasteride, 23 mo (360.5 mg) | Diabetic retinopathy follow-up | Left | 20/20 | 20/25 | +0.25 | +0.50 | Foveal cavitation |
| 2 | BPH | Diabetes, hypertension, colon cancer | Finasteride, 36 mo (5490.0 mg) | Diabetic retinopathy follow-up | Both | 20/40 | 20/20 | +1.75 | +0.50 | Foveal cavitation |
| 3 | BPH | Hypertension | Dutasteride, 41 mo (620.5 mg) | Medical evaluation | Left | 20/20 | 20/20 | +1.00 | +2.00 | Foveal cavitation |
| 4 | BPH | Parkinson disease | Finasteride, 102 mo (15 490.0 mg) | Cataract, preoperative evaluation | Both | 20/32 | 20/25 | +2.00 | +2.00 | Lamellar hole |
| 5 | BPH | Hypertension, arrhythmia, COPD | Dutasteride, 116 mo (1746.0 mg) | RVO on other eye | Left | 20/20 | 20/20 | –0.25 | +0.75 | Foveal cavitation |
| 6 | BPH | Hypertension | Dutasteride, 36 mo (547.5 mg) | Cataract, preoperative evaluation | Both | 20/25 | 20/20 | +0.50 | +2.50 | Foveal cavitation |
| 7 | BPH | Hypertension | Dutasteride, 24 mo (365.0 mg) | RVO on other eye | Right | 20/25 | 20/50 | +2.50 | +1.25 | Foveal cavitation |
| 8 | BPH, alopecia | Diabetes, hypertension | Finasteride, 120 mo (21 900.0 mg) | Medical evaluation | Left | 20/20 | 20/20 | +1.50 | +2.00 | Foveal cavitation |
| 9 | Alopecia | Hypertension | Dutasteride, 72 mo (1095.0 mg) | Diabetic retinopathy follow-up | Both | 20/20 | 20/20 | –0.50 | –0.75 | Foveal cavitation |
| 10 | Alopecia | None | Finasteride, 122 mo (4637.5mg) | Complaint of metamorphosis | Both | 20/32 | 20/25 | –4.00 | –2.25 | Impending macular hole |
Abbreviations: BPH, benign prostate hypertrophy; COPD, chronic obstructive pulmonary disease; OCT, optical coherence tomography; RVO, retinal vein occlusion.
Color fundus photographs showed a pseudohole appearance or loss of the foveal reflex. On OCT, all patients had a foveal lesion either unilaterally or bilaterally. With progression, OCT showed enlarged foveal lesions resembling lamellar holes and outer foveal defects resembling impending macular holes for 2 patients. Fluorescein angiography did not reveal any leakage of dye in any eyes. On OCT angiography, no vascular abnormality was detected in either the superficial or deep retinal capillary slab in any eyes.
Case 1
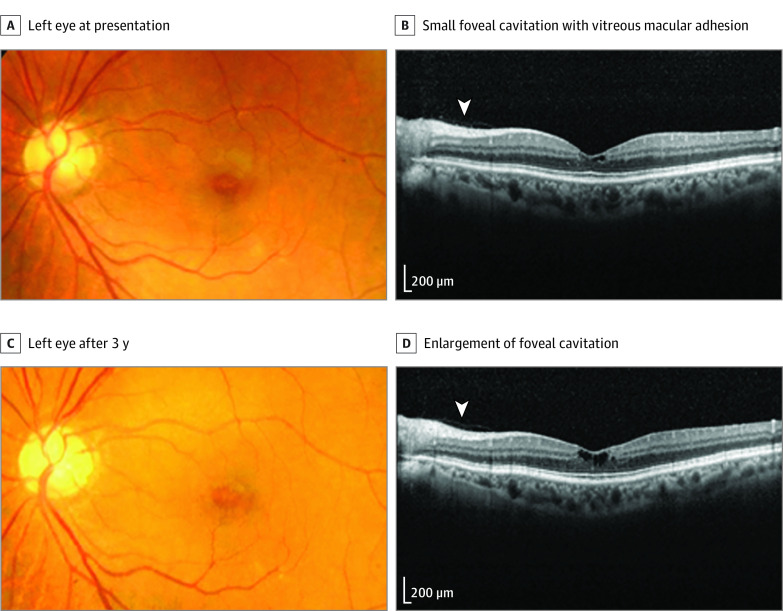
A patient with diabetes was referred to our clinic for a macular abnormality, which was noted during a routine fundus examination. He had not noticed any vision problem. However, his best-corrected visual acuity was 20/20 OD and 20/25 OS. His medical history included diabetes and BPH treated with a 5-ARI. He had received finasteride, 5 mg, daily for 31 months (cumulative dose of 4690.0 mg) and dutasteride, 0.5 mg, daily for 23 months (cumulative dose of 360.5 mg) for a total of 54 months. The anterior segment and intraocular pressure were within the normal limits in both eyes. Fundus examination revealed no diabetic retinopathy in either eye; however, an irregular fovea light reflex with a yellow spot was observed in the left eye. Fluorescein angiography showed no leakage in the context of any lesion. Optical coherence tomography revealed foveal cavitation but no tractional components, such as vitreomacular traction or epiretinal membrane. Although further deterioration of the vision in the patient’s left eye was not noted after an additional 21 months, a progressive enlargement of the cavitary space was detected during that same period (Figure 2).
Figure 2. Findings for a Patient Treated With 5α-Reductase Inhibitors Finasteride and Dutasteride for Benign Prostate Hypertrophy (Case 1).
A, At presentation, a color fundus photograph of the left eye shows the loss of the foveal reflex. B, Spectral-domain optical coherence tomography reveals small foveal cavitation, with persistent vitreous adhesion in the macula (arrowhead indicates the margin of the vitreous cortex). C, Fundus photograph of the left eye 5 years later shows no interval change. D, Spectral-domain optical coherence tomography reveals further enlargement of the foveal cavitation with persistent vitreous adhesion (arrowhead).
Case 9
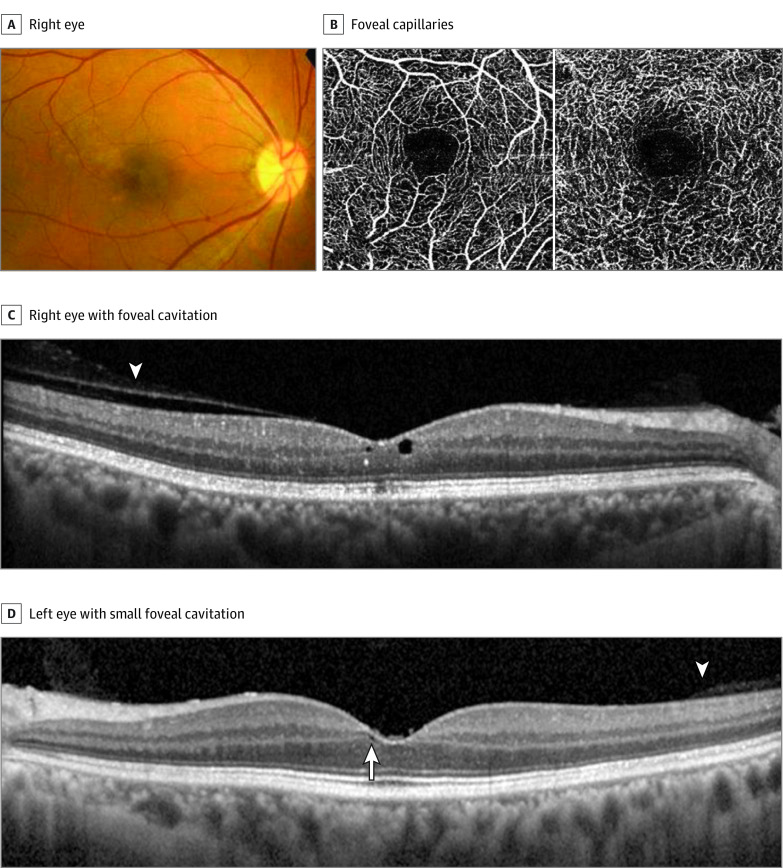
A patient was referred to our clinic with a suspicion of foveal disease. He had no visual complaint. However, during his annual medical examination, fundus photographs revealed pigmentary lesions and the loss of the foveal reflex in both eyes. The patient had no history of ocular disease. His medical history included hypertension and alopecia treated with a 5-ARI. He had received dutasteride, 0.5 mg, daily for 72 months (cumulative dose of 1095.0 mg). His visual acuity was 20/20 OU. Slitlamp examination findings were within the normal limits. However, fundoscopy revealed a loss of the foveal reflex and a depigmentary lesion in the right eye. Optical coherence tomography of the right eye showed foveal cavitation with attached posterior vitreous cortex and disrupted ellipsoid zone and interdigitation zone; OCT of the left eye showed relatively tiny foveal cavitation. Optical coherence tomography angiography showed no remarkable foveal vascular abnormalities (Figure 3).
Figure 3. Findings for a Patient Treated With 5α-Reductase Inhibitor Dutasteride for Alopecia (Case 9).
A, Color fundus photograph of the right eye shows mild hypopigmentation in the temporal side of the macula. B, Spectral-domain optical coherence tomographic angiography shows the normality of the foveal capillaries. C, Spectral-domain optical coherence tomography shows foveal cavitation and disrupted ellipsoid zone and interdigitation zone with persistent vitreous adhesion in the macula (arrowhead). D, Left eye with a small foveal cavitation (arrow) with persistent vitreous adhesion in the macula (arrowhead).
Case 10
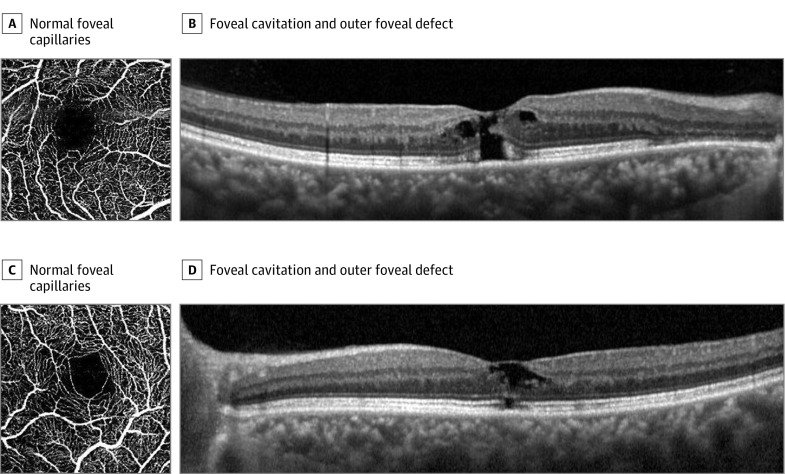
A patient was referred to our clinic for the diagnosis of a foveal lesion. He had complained of decreased and distorted vision. Alopecia was being treated with a 5-ARI. He had received finasteride, 1.25 mg, daily for 122 months (cumulative dose of 4637.5 mg). His visual acuity was 20/32 OD and 20/25 OS. Fundoscopy revealed a loss of the foveal reflex with drusen and retinal pigmentary changes outside the fovea. Fluorescein angiography showed multiple hyperfluorescent spots due to window defects, but vascular leakage or aneurysmal changes were not detected. Optical coherence tomography showed a focal defect of the outer retinal layer and foveal cavitation, simulating an impending macular hole in both eyes. However, the posterior vitreous cortex remained attached, and there was no evidence of vitreofoveal traction (Figure 4).
Figure 4. Patient Treated With 5α-Reductase Inhibitor Finasteride for Alopecia (Case 10).
A-D, Spectral-domain optical coherence tomographic angiography shows the normality of the capillaries of the fovea (A, C) and foveal cavitation and outer foveal defect, simulating an impending macular hole with persistent vitreous adhesion in the macula (B, D).
Discussion
Because of the antiandrogen effect of 5-ARI agents, such as finasteride and dutasteride, these treatments have been used widely for BPH, urinary retention, and androgenic alopecia.18,19,20 5-ARI treatment is used for transgender women as a component of gender-affirming hormone therapy.21 Although 5-ARI has recently been used in transgender women and women with hair loss, the US Food and Drug Administration has approved its use only in men to treat BPH or androgenic alopecia. Several adverse events of 5-ARIs have been reported to date, including those affecting sexual function, the cardiovascular system, bone metabolism, and neuropsychiatric status.22,23 However, to our knowledge, adverse events of 5-ARIs in the eye have not been previously reported.
The androgen DHT is formed from the conversion of circulating testosterone to DHT by 5α-reductase.24 Dihydrotestosterone is a more potent androgen than testosterone, in that it binds more strongly to androgen receptors; the dissociation rate of DHT is slower than that of testosterone.24,25 Dihydrotestosterone plays an essential role in androgen-dependent conditions, such as hirsutism and hair loss, and prostate diseases, such as BPH and prostate cancer.26 The 5-ARIs that prevent DHT synthesis are thus effective in the treatment of these conditions. The use of 5-ARIs decreases levels of DHT and their metabolites. A metabolite of DHT, 3β-androstanediol, is a potent and selective agonist of the estrogen receptor (ER)β and also binds to ERα with a low affinity.27 Thus, 3β-androstanediol acts as an estrogen and 5-ARIs may also have antiestrogenic effects.
Estrogen receptor is divided into ERα and ERβ.28 The ERα is predominantly expressed in reproductive tissues (eg, uterus, breast, and ovaries), the liver, and the central nervous system, whereas ERβ is expressed in other tissues, including bone, endothelium, the lungs, the urogenital tract, the ovaries, the central nervous system, and the prostate.29 In a previous study by Manaut et al,30 ERα was found to be present in human retina and ERβ was observed in the ganglion cell layer and choroid. In a rat model developed by Mäkelä et al,31 ERβ was upregulated in the vascular wall after injury and ERα remained unchanged. Another report32 suggested that not only estrogen itself but also ER, including ERβ, may play a neuroprotective role in the retina through the inhibition of glutamate secretion. Therefore, 5-ARIs may be associated with neurotoxic effects due to unexpected changes in metabolites.
Tamoxifen is the primary treatment option for ERα-positive tumors.33 However, 5% to 10% of patients with ERα-negative tumors have shown responsiveness to tamoxifen.28,33 Therefore, interest in ERβ has increased. However, although several studies27,28,34 on this subject have been conducted, further research is still needed. Tamoxifen inhibits the glutamate-aspartate transporters that may result in Müller cell dysfunction and neuronal apoptosis,35 which leads to the formation of cavitary spaces on OCT. In MacTel type 2, histopathologic studies have shown a loss of Müller cell markers in the clinically altered area, suggesting Müller cell death.10,36 This degeneration of Müller cells has been shown to manifest as a loss of macular pigment and hyperreflective cavities on OCT.36 In this study, male patients who received 5-ARI treatment also showed foveal cavitation on OCT. We speculated that the antiestrogenic effect of 5-ARIs was associated with foveal atrophy or cavitation. In addition, the frequency of 5-ARI used among individuals with macular abnormalities of unknown origin was significantly higher than that in the age-adjusted Korean population and among those with known specific origin of macular abnormalities. These statistical differences, as well as pathologic mechanisms, suggest that there may be an association between 5-ARI medication and macular changes.
The incidence of MacTel type 2 is associated with some systemic factors, such as hypertension, diabetes, coronary artery disease, and smoking.36 In this study, 7 of 10 patients had hypertension, 2 had both hypertension and diabetes, and 1 had diabetes. Similar to MacTel type 2, these findings suggest that the presence of systemic vascular disease may be a risk factor for 5-ARI associated with macular abnormalities. If a macular abnormality develops after cumulative drug toxic effects, bilateral involvement would be expected. However, 5 of 10 patients showed only unilateral involvement. Previous research on tamoxifen-associated retinopathy had also suggested a high rate of unilateral involvement (7 of 18 patients).9 Most studies8,9,37 have reported an asymmetric level of involvement in bilateral cases. As such, neurodegenerative findings associated with tamoxifen macular abnormalities are not necessarily found equally in both eyes. Similarly, in the present study, asymmetric involvement was also noted in bilateral cases. Furthermore, the retinal lesions were observed to be larger in bilateral cases than in unilateral cases, and this could be speculated to represent the existence of further progressed lesions in bilateral cases.
In this study, 2 patients had lamellar holes and impending macular holes. In case 4, the patient had an enlarged foveal cavitation that looked similar to lamellar holes. He also received a diagnosis of Parkinson disease. Although the use of 5-ARIs was associated with an increased risk of incident dementia, excessive secretion of glutamate in the brain is thought to be the developmental mechanism of Parkinson disease.38,39 This pathophysiology may synergistically work with 5-ARI intake. In case 10, the patient had no medical history other than hair loss, and the only medication received was finasteride. Furthermore, the cumulative dose was not substantially higher than that for the other patients. However, the patient also had an enlarged foveal cavitation and progressive outer foveal defect that resembled an impending macular hole. Thus, the extent of morphologic changes in the retina may be different individually.
Tamoxifen-associated macular abnormalities and MacTel type 2 are rare and usually asymptomatic.36,37,40 Drug-polar lipid complexes that form owing to the amphiphilic properties of tamoxifen may be involved in crystal formation.41 Recently, the screening of patients receiving tamoxifen has become necessary for foveal cavitation even in the absence of crystals or visual complaints.8 Similarly, many patients in this study had no significant symptoms or crystalline retinopathic findings. Because 5-ARIs have hydrophobic properties, the pharmacologic effect of these medications would appear slowly.42 Thus, the cases were identified incidentally by OCT. However, as shown in some cases, the progression of disease severity may result in decreased vision. For this reason, we believe that patients receiving 5-ARI medications should also undergo ophthalmic examinations for the development of foveal changes.
Three types of isoenzymes were reported for 5α-reductase. Of the 3 isoforms of 5α-reductase, dutasteride inhibits all types (I, II, and III), whereas finasteride inhibits type II and III.43 In addition, dutasteride is 3 times more potent than finasteride in inhibiting type II and 100 times more potent in inhibiting type I.24 These results suggest that dutasteride is also more likely to have adverse effects. In this study, 6 of 10 patients received dutasteride. The minimum duration of dutasteride treatment was 2.0 years, and the cumulative dose was 365.0 mg. The minimum duration of finasteride treatment was 2.5 years. and the cumulative dose was 5490.0 mg. However, the dose-response relationship could not be described in this small sample size study. Further investigation should be completed to determine the minimum duration and cumulative dose associated with the development of retinopathy.
Limitations
There are several limitations in this study. First, the study design was retrospective and cross-sectional. Because this study involved a retrospective medical record review, we could not ask patients to stop taking 5-ARIs. Additional longitudinal studies are necessary to elucidate the course of macular change with or without suspending 5-ARI treatment. Second, the study population was small for both groups (ie, unknown origin and well-known specific origin). To confirm a cause and effect relationship, a randomized clinical trial will be needed. Third, our study reported the association between macular abnormalities and 5-ARIs. However, we could not identify a dose-response relationship. Additional study is required to define a toxic ocular dose as well as causation.
Conclusions
In this study, a spectrum of foveal abnormalities was associated with use of 5-ARIs for BPH and alopecia. We speculate that MacTel type 2, tamoxifen-associated retinopathic findings, and macular abnormalities associated with 5-ARIs may share the same developmental mechanism. If foveal cavitation and/or macular holes are observed in male patients, recording of history for 5-ARI medication use is warranted. Although the macular abnormalities associated with a male sex hormone inhibitor suggested by this investigation warrant further corroboration, we believe that ophthalmologists should consider retinal evaluation in male patients receiving 5-ARI therapy.
References
- 1.Feskanich D, Cho E, Schaumberg DA, Colditz GA, Hankinson SE. Menopausal and reproductive factors and risk of age-related macular degeneration. Arch Ophthalmol. 2008;126(4):519-524. doi: 10.1001/archopht.126.4.519 [DOI] [PubMed] [Google Scholar]
- 2.Nudleman E, Witmer MT, Kiss S, Williams GA, Wolfe JD. Central serous chorioretinopathy in patients receiving exogenous testosterone therapy. Retina. 2014;34(10):2128-2132. doi: 10.1097/IAE.0000000000000198 [DOI] [PubMed] [Google Scholar]
- 3.Kwon HJ, Lee SM, Pak KY, Park SW, Lee JE, Byon IS. Gender differences in the relationship between sex hormone deficiency and soft drusen. Curr Eye Res. 2017;42(11):1527-1536. doi: 10.1080/02713683.2017.1337155 [DOI] [PubMed] [Google Scholar]
- 4.Nuzzi R, Scalabrin S, Becco A, Panzica G. Gonadal hormones and retinal disorders: a review. Front Endocrinol (Lausanne). 2018;9:66. doi: 10.3389/fendo.2018.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans JR, Schwartz SD, McHugh JD, et al. Systemic risk factors for idiopathic macular holes: a case-control study. Eye (Lond). 1998;12(pt 2):256-259. doi: 10.1038/eye.1998.60 [DOI] [PubMed] [Google Scholar]
- 6.Heeren TFC, Holz FG, Charbel Issa P. First symptoms and their age of onset in macular telangiectasia type 2. Retina. 2014;34(5):916-919. doi: 10.1097/IAE.0000000000000082 [DOI] [PubMed] [Google Scholar]
- 7.Yanyali AC, Freund KB, Sorenson JA, Slakter JS, Wheatley HM. Tamoxifen retinopathy in a male patient. Am J Ophthalmol. 2001;131(3):386-387. doi: 10.1016/S0002-9394(00)00781-9 [DOI] [PubMed] [Google Scholar]
- 8.Doshi RR, Fortun JA, Kim BT, Dubovy SR, Rosenfeld PJ. Pseudocystic foveal cavitation in tamoxifen retinopathy. Am J Ophthalmol. 2014;157(6):1291-1298.e3. doi: 10.1016/j.ajo.2014.02.046 [DOI] [PubMed] [Google Scholar]
- 9.Gorin MB, Day R, Costantino JP, et al. Long-term tamoxifen citrate use and potential ocular toxicity [published correction appears in Am J Ophthalmol. 1998;126(2):338]. Am J Ophthalmol. 1998;125(4):493-501. doi: 10.1016/S0002-9394(99)80190-1 [DOI] [PubMed] [Google Scholar]
- 10.Powner MB, Gillies MC, Tretiach M, et al. Perifoveal müller cell depletion in a case of macular telangiectasia type 2. Ophthalmology. 2010;117(12):2407-2416. doi: 10.1016/j.ophtha.2010.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller S, Allam JP, Bunzek CG, Clemons TE, Holz FG, Charbel Issa P. Sex steroids and macular telangiectasia type 2. Retina. 2018;38(suppl 1):S61-S66. doi: 10.1097/iae.0000000000001789 [DOI] [PubMed] [Google Scholar]
- 12.Lin S-Y, Lin C-L, Chang C-H, Wu H-C, Lin C-H, Kao C-H. Risk of age-related macular degeneration in patients with prostate cancer: a nationwide, population-based cohort study. Ann Oncol. 2017;28(10):2575-2580. doi: 10.1093/annonc/mdx402 [DOI] [PubMed] [Google Scholar]
- 13.Jang WS, Son IP, Yeo IK, et al. The annual changes of clinical manifestation of androgenetic alopecia clinic in Korean males and females: a outpatient-based study. Ann Dermatol. 2013;25(2):181-188. doi: 10.5021/ad.2013.25.2.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go T-H, Kim HS, Kang DR, et al. The benign prostatic hyperplasia incidence rate in Korea: using National Health Insurance Service data. J Health Info Stat. 2018;43(3):217-222. doi: 10.21032/jhis.2018.43.3.217 [DOI] [Google Scholar]
- 15.Kang JY, Min GE, Son H, Kim HT, Lee H-L. National-wide data on the treatment of BPH in Korea. Prostate Cancer Prostatic Dis. 2011;14(3):243-247. doi: 10.1038/pcan.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Lee JS, Park S-H, Shin SA, Kim K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46(2):e15. [DOI] [PubMed] [Google Scholar]
- 18.Finn DA, Beadles-Bohling AS, Beckley EH, et al. A new look at the 5alpha-reductase inhibitor finasteride. CNS Drug Rev. 2006;12(1):53-76. doi: 10.1111/j.1527-3458.2006.00053.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adil A, Godwin M. The effectiveness of treatments for androgenetic alopecia: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77(1):136-141.e5. doi: 10.1016/j.jaad.2017.02.054 [DOI] [PubMed] [Google Scholar]
- 20.Dahm P, Brasure M, MacDonald R, et al. Comparative effectiveness of newer medications for lower urinary tract symptoms attributed to benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol. 2017;71(4):570-581. doi: 10.1016/j.eururo.2016.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radix A. Hormone therapy for transgender adults. Urol Clin North Am. 2019;46(4):467-473. doi: 10.1016/j.ucl.2019.07.001 [DOI] [PubMed] [Google Scholar]
- 22.Traish AM, Melcangi RC, Bortolato M, Garcia-Segura LM, Zitzmann M. Adverse effects of 5α-reductase inhibitors: what do we know, don’t know, and need to know? Rev Endocr Metab Disord. 2015;16(3):177-198. doi: 10.1007/s11154-015-9319-y [DOI] [PubMed] [Google Scholar]
- 23.Traish AM, Hassani J, Guay AT, Zitzmann M, Hansen ML. Adverse side effects of 5α-reductase inhibitors therapy: persistent diminished libido and erectile dysfunction and depression in a subset of patients. J Sex Med. 2011;8(3):872-884. doi: 10.1111/j.1743-6109.2010.02157.x [DOI] [PubMed] [Google Scholar]
- 24.Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S. Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5α-reductase inhibitor. J Clin Endocrinol Metab. 2004;89(5):2179-2184. doi: 10.1210/jc.2003-030330 [DOI] [PubMed] [Google Scholar]
- 25.Askew EB, Gampe RT Jr, Stanley TB, Faggart JL, Wilson EM. Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J Biol Chem. 2007;282(35):25801-25816. doi: 10.1074/jbc.M703268200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Vignera S, Condorelli RA, Russo GI, Morgia G, Calogero AE. Endocrine control of benign prostatic hyperplasia. Andrology. 2016;4(3):404-411. doi: 10.1111/andr.12186 [DOI] [PubMed] [Google Scholar]
- 27.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5α-androstane-3β,17β-diol, in modulating oestrogen receptor β-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21(4):351-358. doi: 10.1111/j.1365-2826.2009.01840.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618-629. doi: 10.1056/NEJMra022219 [DOI] [PubMed] [Google Scholar]
- 29.Paterni I, Granchi C, Katzenellenbogen JA, Minutolo F. Estrogen receptors alpha (ERα) and beta (ERβ): subtype-selective ligands and clinical potential. Steroids. 2014;90:13-29. doi: 10.1016/j.steroids.2014.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munaut C, Lambert V, Noël A, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol. 2001;85(7):877-882. doi: 10.1136/bjo.85.7.877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mäkelä S, Savolainen H, Aavik E, et al. Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors alpha and beta. Proc Natl Acad Sci U S A. 1999;96(12):7077-7082. doi: 10.1073/pnas.96.12.7077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryant DN, Dorsa DM. Roles of estrogen receptors alpha and beta in sexually dimorphic neuroprotection against glutamate toxicity. Neuroscience. 2010;170(4):1261-1269. doi: 10.1016/j.neuroscience.2010.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Droog M, Nevedomskaya E, Dackus GM, et al. Estrogen receptor α wields treatment-specific enhancers between morphologically similar endometrial tumors [correction published in Proc Natl Acad Sci USA. 2018;115(5):E1075]. Proc Natl Acad Sci USA. 2017;114(8):E1316-E1325. doi: 10.1073/pnas.1615233114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonsson P, Katchy A, Williams C. Support of a bi-faceted role of estrogen receptor β (ERβ) in ERα-positive breast cancer cells. Endocr Relat Cancer. 2014;21(2):143-160. doi: 10.1530/ERC-13-0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mäenpää H, Mannerström M, Toimela T, Salminen L, Saransaari P, Tähti H. Glutamate uptake is inhibited by tamoxifen and toremifene in cultured retinal pigment epithelial cells. Pharmacol Toxicol. 2002;91(3):116-122. doi: 10.1034/j.1600-0773.2002.910305.x [DOI] [PubMed] [Google Scholar]
- 36.Charbel Issa P, Gillies MC, Chew EY, et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013;34:49-77. doi: 10.1016/j.preteyeres.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Noureddin BN, Seoud M, Bashshur Z, Salem Z, Shamseddin A, Khalil A. Ocular toxicity in low-dose tamoxifen: a prospective study. Eye (Lond). 1999;13(pt 6):729-733. doi: 10.1038/eye.1999.217 [DOI] [PubMed] [Google Scholar]
- 38.Obeso JA, Stamelou M, Goetz CG, et al. Past, present, and future of Parkinson’s disease: a special essay on the 200th anniversary of the shaking palsy. Mov Disord. 2017;32(9):1264-1310. doi: 10.1002/mds.27115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welk B, McArthur E, Ordon M, Morrow SA, Hayward J, Dixon S. The risk of dementia with the use of 5 alpha reductase inhibitors. J Neurol Sci. 2017;379:109-111. doi: 10.1016/j.jns.2017.05.064 [DOI] [PubMed] [Google Scholar]
- 40.Heier JS, Dragoo RA, Enzenauer RW, Waterhouse WJ. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol. 1994;117(6):772-775. doi: 10.1016/S0002-9394(14)70321-6 [DOI] [PubMed] [Google Scholar]
- 41.Kaiser-Kupfer MI, Kupfer C, Rodrigues MM. Tamoxifen retinopathy: a clinicopathologic report. Ophthalmology. 1981;88(1):89-93. doi: 10.1016/S0161-6420(81)35071-4 [DOI] [PubMed] [Google Scholar]
- 42.Makridakis N, Reichardt JK. Pharmacogenetic analysis of human steroid 5 alpha reductase type II: comparison of finasteride and dutasteride. J Mol Endocrinol. 2005;34(3):617-623. doi: 10.1677/jme.1.01725 [DOI] [PubMed] [Google Scholar]
- 43.Li J, Ding Z, Wang Z, et al. Androgen regulation of 5α-reductase isoenzymes in prostate cancer: implications for prostate cancer prevention. PLoS One. 2011;6(12):e28840. doi: 10.1371/journal.pone.0028840 [DOI] [PMC free article] [PubMed] [Google Scholar]