This cohort study characterizes drusen and hyperreflective foci in patients with age-related macular degeneration using artificial intelligence algorithms and spatiotemporal atlas methods.
Key Points
Question
Are there characteristic morphologic patterns on optical coherence tomography in eyes with age-related macular degeneration that develop macular atrophy and macular neovascularization?
Findings
In this cohort study of 8529 optical coherence tomographic volumes of 512 patients, eyes progressing to advanced age-related macular degeneration had higher drusen and hyperreflective foci volume and an accelerated increase in drusen volume and hyperreflective foci activity.
Meaning
Drusen and hyperreflective foci distribution, time course, and volumes may differ among distinct progression pathways in patients with age-related macular degeneration.
Abstract
Importance
The morphologic changes and their pathognomonic distribution in progressing age-related macular degeneration (AMD) are not well understood.
Objectives
To characterize the pathognomonic distribution and time course of morphologic patterns in AMD and to quantify changes distinctive for progression to macular neovascularization (MNV) and macular atrophy (MA).
Design, Setting, and Participants
This cohort study included optical coherence tomography (OCT) volumes from study participants with early or intermediate AMD in the fellow eye in the HARBOR (A Study of Ranibizumab Administered Monthly or on an As-needed Basis in Patients With Subfoveal Neovascular Age-Related Macular Degeneration) trial. Patients underwent imaging monthly for 2 years (July 1, 2009, to August 31, 2012) following a standardized protocol. Data analysis was performed from June 1, 2018, to January 21, 2020.
Main Outcomes and Measures
To obtain topographic correspondence between patients and over time, all scans were mapped into a joint reference frame. The time of progression to MNV and MA was established, and drusen volumes and hyperreflective foci (HRF) volumes were automatically segmented in 3 dimensions using validated artificial intelligence algorithms. Topographically resolved population means of these markers were constructed by averaging quantified drusen and HRF maps in the patient subgroups.
Results
Of 1097 patients enrolled in HARBOR, 518 (mean [SD] age, 78.1 [8.2] years; 309 [59.7%] female) had early or intermediate AMD in the fellow eye at baseline. During the 24-month follow-up period, 135 (26%) eyes developed MNV, 50 eyes (10%) developed MA, and 333 (64%) eyes did not progress to advanced AMD. Drusen and HRF had distinct topographic patterns. Mean drusen thickness at the fovea was 29.6 μm (95% CI, 20.2-39.0 μm) for eyes progressing to MNV, 17.2 μm (95% CI, 9.8-24.6 μm) for eyes progressing to MA, and 17.1 μm (95% CI, 12.5-21.7 μm) for eyes without disease progression. At 0.5-mm eccentricity, mean drusen thickness was 25.8 μm (95% CI, 19.1-32.5 μm) for eyes progressing to MNV, 21.7 μm (95% CI, 14.6-28.8 μm) for eyes progressing to MA, and 14.4 μm (95% CI, 11.2-17.6 μm) for eyes without disease progression. The mean HRF thickness at the foveal center was 0.072 μm (95% CI, 0-0.152 μm) for eyes progressing to MNV, 0.059 μm (95% CI, 0-0.126 μm) for eyes progressing to MA, and 0.044 μm (95% CI, 0.007-0.081) for eyes without disease progression. At 0.5-mm eccentricity, the largest mean HRF thickness was seen in eyes progressing to MA (0.227 μm; 95% CI, 0.104-0.349 μm) followed by eyes progressing to MNV (0.161 μm; 95% CI, 0.101-0.221 μm) and eyes without disease progression (0.085 μm; 95% CI, 0.058-0.112 μm).
Conclusions and Relevance
In this study, drusen and HRF represented imaging biomarkers of disease progression in AMD, demonstrating distinct topographic patterns over time that differed between eyes progressing to MNV, eyes progressing to MA, or eyes without disease progression. Automated localization and precise quantification of these factors may help to develop reliable methods of predicting future disease progression.
Introduction
Age-related macular degeneration (AMD) is highly prevalent among the elderly population.1 Because age-associated retinal changes are abundantly present, it is difficult to distinguish between healthy retinal aging and AMD disease in clinical practice. For instance, in patients with drusen and hyperpigmentation, determining the individual risk of progression to advanced AMD within a given time remains challenging.2 This challenge results in an unmet need to counsel patients regarding their individual risk even despite recent advances in imaging, genetic analysis, and artificial intelligence (AI)–based prediction models.3,4,5 For instance, an AI model based on optical coherence tomography (OCT) and genetic factors was limited to an accuracy of 0.68 to 0.80 area under the receiver operating characteristic curve (ROC AUC) when predicting the individual onset of neovascular and nonneovascular AMD.3 Thus, further insight into the pathophysiology of AMD progression may be helpful when such modeling attempts are made.
The main hallmarks of AMD are drusen and pigmentary abnormalities. Both of these markers are accessible to in vivo analysis by OCT.6 However, although OCT provides a detailed representation of the macula, clinical practice has thus far only allowed the qualitative assessment of drusen and hyperreflective foci (HRF), the OCT correlate of hyperpigmentation.7,8 The advent of AI now enables the autonomous and reproducible quantification of these changes.9 The advancement in AI provides the opportunity to find information on the natural history of AMD by investigating the distribution and time course of these features in large patient populations.
The aim of our study was to characterize drusen and HRF in a post hoc analysis of patients from a clinical trial with AMD by using AI algorithms and spatiotemporal atlas methods. Using these tools, we searched for patterns in the quantitative spatial and temporal development of HRF and drusen.
Methods
This cohort study was a post hoc analysis of imaging data collected during the HARBOR (A Study of Ranibizumab Administered Monthly or on an As-needed Basis in Patients With Subfoveal Neovascular Age-Related Macular Degeneration) randomized clinical trial. The trial design has been described in detail previously.10 HARBOR investigated the efficacy and safety of ranibizumab for neovascular AMD in 1097 patients who underwent OCT (Cirrus OCT; Zeiss) following a standardized protocol and monthly follow-up for 24 months to determine when an as-needed regimen had noninferior visual acuity outcomes compared with monthly treatment. In this study, we focused on patients in the HARBOR trial with early or intermediate AMD in their fellow eye. This study was conducted in compliance with the Declaration of Helsinki.11 Written informed consent was obtained from all patients on enrollment into the HARBOR clinical trial to allow post hoc analyses of deidentified images as was performed in this investigation; permission of individual clinical sites or other investigators of HARBOR to conduct this post hoc analysis was not obtained or required according to Genentech, the owner of the HARBOR data. The Ethics Committee at the Medical University of Vienna, Austria, approved the presented post hoc analysis. No compensation or incentive was offered to patients for participation in the study.
Diagnosis of AMD Progression
All OCT images of fellow eyes were screened by 2 independent, masked retina specialists (including one of us [S.R.]) to diagnose AMD and the onset of advanced AMD (ie, macular neovascularization [MNV] and macular atrophy [MA]) in accordance with a grading procedure proposed previously.12 The diagnostic criteria for MNV were occurrence of a fibrovascular pigment epithelial detachment and/or subretinal hyperreflective material and intraretinal and/or subretinal fluid.12 MA was diagnosed when choroidal hypertransmission was observed with evidence of loss of the photoreceptor layers and retinal pigment epithelium (RPE) in the absence of a scrolled RPE layer.13 Of 1095 fellow eyes, 29 were discarded from grading because most scans were missing. After the grading, 452 eyes were excluded because they had bilateral advanced AMD from the onset of the study. A total of 72 eyes without disease progression were discarded because of absence of drusen. Finally, 24 eyes were eliminated because of poor imaging quality.
Quantification of Retinal Morphologic Features by AI
Previously developed and validated segmentation algorithms were used to quantify the AMD-related pathologic features in the OCT data set. The outer retina–drusen complex and the Bruch membrane were segmented using the Iowa reference algorithm14 with modified smoothness constraints as published previously.15 HRF were quantified using a custom-built deep learning algorithm described in detail elsewhere and validated previously.16 Both algorithms segmented drusen and HRF in full 3-dimensional volumes, providing pixel-level information about these biomarkers. Both algorithms were deployed as fully trained models as reported in the previous publications,15,16 without further training or modifications. Exemplary OCT images with segmentations are provided in the eFigure in the Supplement.
Spatiotemporal Atlas for Analysis of Morphometric Patterns
As described elsewhere, a spatiotemporal atlas was developed to enable the analysis of all imaging data in a joint reference frame (W.-D. Vogl, unpublished data, March 26, 2020). In brief, all OCT data sets were centered on the foveal center and rotated such that the optic nerve head center was located at an angle of 5.6°.17 Right eyes were mirrored to conform to left eyes. This procedure results in all images being normalized with regard to the spatial orientation and enabled a joint analysis of topographic features and analysis of identical retinal loci over time.
Statistical Analysis
To analyze the topographic distribution of AMD features, we cross-sectionally compared patients whose eyes progressed to MNV (1 month before progression) vs those whose eyes progressed to MA (1 month before progression) vs those without disease progression (last available visit in study) using the following metrics. Drusen thickness at a particular position in the macula was measured as the vertical distance between the apical RPE surface and the Bruch membrane. HRF thickness was defined as the cumulative height of all HRFs within a vertical 1 × 1 pixel column at a particular position in the macula. Across all eyes in each group, mean thickness maps were calculated for the thickness data mapped into the reference frame and truncated at 3-mm eccentricity from the fovea. The mean thickness values were plotted as a function of eccentricity resulting in topographic profiles. Values at each location were compared using the Mann-Whitney test. Adjustment for multiple testing by controlling false discovery rate of 5% was performed using the Benjamini-Hochberg method.
Furthermore, drusen and HRF volumes were computed for different fixed eccentricities: 0 to 0.5 mm (circle), 0.5 to 1.5 mm, and 1.5 to 3 mm (rings) from the fovea. Significant differences in group means were evaluated by a Kruskal-Wallis test. Pairwise comparison of means was performed using a Mann-Whitney test with Bonferroni correction for multiple testing.
Longitudinal changes of retinal morphologic features were analyzed by calculating the mean feature volume in the central foveal area within a 3-mm diameter and the mean values plotted using LOESS curves. The change over time between the groups was compared using joint models based on longitudinal mixed-effects models that captured the evaluation over time and a proportional hazards survival model that accounted for right-censoring that occurred in this time-to-event data. We used a bayesian joint model provided by R package JMBayes, version 0.8.83 (R Project for Statistical Computing).18
To evaluate the predictive value of the obtained quantitative data, logistic regression was performed with age and sex as covariates (baseline), and separate models were obtained for each baseline volume compartment (drusen volume, HRF volume, and HRF not above drusen volume for 0-0.5 mm, 0.5-1.5 mm, and 1.5-3 mm) plus age and sex. The prediction target was disease progression within the 2-year observation period (July 1, 2020, to August 31, 2012) vs nonprogression. Data analysis was performed from June 1, 2018, to January 21, 2020. We reported ROC AUC as the performance metric. Evaluation was performed in a 5-fold, stratified, cross-validation setup of MNV vs nonprogression and MA vs nonprogression. A 2-sided P < .05 was considered statistically significant.
Results
Study Patients
Of 1097 patients enrolled in HARBOR, 518 (mean [SD] age, 78.1 [8.2] years; 309 [59.7%] female) had early or intermediate AMD in the fellow eye at baseline. During the 24-month follow-up period, 135 eyes developed MNV (mean [SD] patient age, 78.5 [8.5] years; 94 [69.6%] female), 50 eyes developed MA (mean [SD] patient age, 81.2 [6.4] years; 29 [58.0%] female), and 333 eyes did not progress to advanced AMD (mean [SD] patient age, 77.4 [8.2] years; 186 [55.9%] female). A total of 9683 spectral-domain OCT volumes were available for analysis. A total of 198 volumes were removed because of segmentation errors that result from poor image quality, and 956 volumes were removed during intrapatient registration because of motion artifacts disturbing the vessel structure necessary for a proper alignment or because of low contrast. A total of 8529 spectral-domain OCT volumes were successfully mapped into the spatiotemporal atlas for statistical evaluation.
Characteristics of Drusen
The mean spatial distribution of drusen in the macula followed a characteristic pattern (Figure 1). In eyes developing MNV, an increased mean drusen thickness of 29.6 μm (95% CI, 20.2-39.0 μm) was detected at the foveal center. At 0.5-mm eccentricity, mean drusen thickness decreased with 25.8 μm (95% CI, 19.1-32.5 μm). In contrast, in eyes developing MA, mean drusen thickness at the foveal center was 17.2 μm (95% CI, 9.8-24.6). The largest mean drusen thickness was found at 0.5-mm eccentricity (21.7 μm; 95% CI, 14.6-28.8 μm). Eyes that did not develop advanced AMD within 24 months had an overall lower mean drusen thickness, which peaked at the foveal center (17.1 μm; 95% CI, 12.5-21.7 μm) and was 14.4 μm (95% CI, 11.2-17.6 μm) at 0.5-mm eccentricity. For all 3 groups, the mean drusen thickness observed at 1.5-mm eccentricity and outside was negligible (MNV: 8.9 μm; 95% CI, 6.8-11.0 μm; MA: 6.5 μm; 95% CI, 5.4-7.5; no disease progression: 5.4 μm; 95% CI, 4.7-6.0 μm). The Table provides quantitative measures of mean drusen volume in 0- to 0.5-mm, 0.5- to 1.5-mm, and 1.5- to 3-mm eccentricity from the fovea.
Figure 1. Spatial Distribution of Drusen and Hyperreflective Foci (HRF).
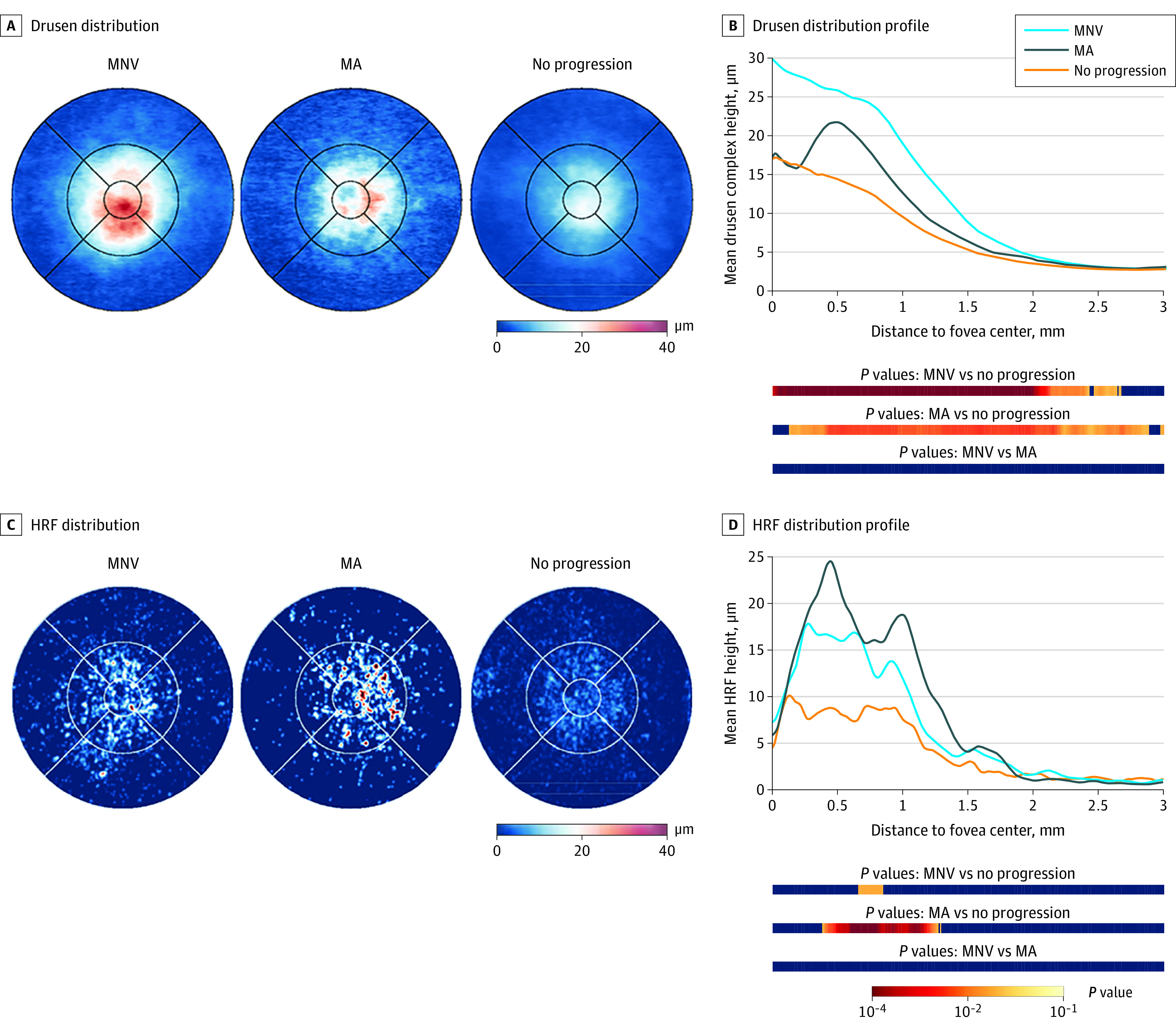
A and B, Eyes progressing to macular neovascularization (MNV) had a peak of drusen accumulation in the foveal center, whereas eyes developing macular atrophy (MA) had the highest drusen volume at 0.5-mm eccentricity from the fovea. C and D, HRF occurred predominantly in the 0.5- to 1.0-mm eccentricity zone, whereas fewer HRF were found at the foveal center. Eyes developing MA had the highest mean HRF thickness, with a preponderance temporal to the fovea. In the distribution profiles, the statistical significance of the difference between the curves at each point of distance to the fovea is color coded in the bars below the graph. Red denotes lower P values, whereas blue represents P > .05. P values are adjusted for multiple testing.
Table. Measured Drusen and Hyperreflective Foci Volume in the Different Groups of Eccentricity to the Fovea.
| Eccentricity | MNV, mean (SD) | MA, mean (SD) | No disease progression mean (SD) | P valuesa | |||
|---|---|---|---|---|---|---|---|
| Overall | MNV vs no disease progression | MA vs no disease progression | MNV vs MA | ||||
| Drusen | |||||||
| 0.0-0.5 mm | 21.07 (31.30) × 106 μm3 | 15.26 (14.38) × 106 μm3 | 12.04 (26.14) × 106 μm3 | <.001 | <.001 | .01 | .46 |
| 0.5-1.5 mm | 107.06 (125.42) × 106 μm3 | 75.08 (62.18) × 106 μm3 | 55.77 (78.80) × 106 μm3 | <.001 | <.001 | .002 | .02 |
| 1.5-3.0 mm | 84.78 (64.49) × 106 μm3 | 76.70 (25.56) × 106 μm3 | 67.87 (42.87) × 106 μm3 | <.001 | <.001 | .004 | >.99 |
| Hyperreflective foci | |||||||
| 0.0-0.5 mm | 125.95 (242.90) × 103 μm3 | 162.85 (384.73) × 103 μm3 | 68.25 (176.60) × 103 μm3 | .003 | .02 | .01 | .74 |
| 0.5-1.5 mm | 558.70 (842.50) × 103 μm3 | 790.29 (916.25) × 103 μm3 | 365.88 (776.63) × 103 μm3 | <.001 | .01 | <.001 | .04 |
| 1.5-3.0 mm | 340.01 (595.58) × 103 μm3 | 279.80 (343.96) × 103 μm3 | 289.74 (557.69) × 103 μm3 | .68 | .86 | .67 | >.99 |
Abbreviations: MA, macular atrophy; MNV, macular neovascularization.
To test for significant differences between group means, a Kruskal-Wallis test was performed to compare all groups and a Mann-Whitney test with Bonferroni correction for multiple testing to compare groups pairwise.
Longitudinal modeling of the drusen volume within 1.5-mm eccentricity revealed that eyes progressing to MNV featured a faster increase in drusen volume in the months before conversion (2.1 × 106 μm3 per month; 95% CI, 0.9-3.3 × 106 μm3 per month) compared with eyes developing MA (−0.7 × 106 μm3; 95% CI, −2.3 to 0.9 × 106 μm3 per month) and eyes not progressing (0.6 × 106 μm3; 95% CI, 0.3-1.0 × 106 μm3 per month), both showing a slow increase of drusen volume over time (Figure 2).
Figure 2. Longitudinal Analysis of Drusen and Hyperreflective Foci (HRF) Volume.
A, Eyes progressing to macular neovascularization (MNV) had a faster increase in drusen volume than eyes in the other patients. B, In contrast, a continuous increase in HRF volume was observed in eyes developing MNV, whereas the HRF volume was fluctuating in patients developing MA. Dashed lines represent 95% CIs.
Characteristics of Hyperreflective Foci
As opposed to drusen, HRF were mainly located juxtafoveally at 0.5-mm eccentricity regardless of the progression status (Figure 1). Conversely, a low mean HRF thickness was detected at the foveal center (MNV: 0.072 μm; 95% CI, 0-0.152 μm; MA: 0.059 μm; 95% CI, 0-0.126 μm; no disease progression: 0.044 μm; 95% CI, 0.007-0.081 μm). At 0.5-mm eccentricity, the largest mean HRF thickness was seen in eyes progressing to MA (0.227 μm; 95% CI, 0.104-0.349 μm) followed by eyes progressing to MNV (0.161 μm; 95% CI, 0.101-0.221 μm) and eyes with no disease progression (0.085 μm; 95% CI, 0.058-0.112). In terms of topographic distribution, the highest mean HRF thickness was detected temporally to the fovea (MA: superior parafovea: 0.150 μm; 95% CI, 0.146-0.154 μm; temporal parafovea: 0.201 μm; 95% CI, 0.196-0.207 μm; inferior parafovea: 0.095 μm; 95% CI, 0.091-0.098 μm; nasal parafovea: 0.053 μm; 95% CI, 0.050-0.055 μm) (Figure 3). Similar to drusen, a lower mean HRF thickness was detected at 1.5-mm eccentricity and outside (MNV: 0.041 μm; 95% CI, 0.021-0.061 μm; MA: 0.041 μm; 95% CI, 0.022-0.060 μm; no disease progression: 0.030 μm; 95% CI, 0.021-0.038). The Table summarizes the mean HRF volume at set eccentricities.
Figure 3. Topographic Analysis of Hyperreflective Foci (HRF) .
A, HRF overlying drusen. B, HRF not overlying drusen. Most HRF were detected in the direct neighborhood of drusen; however, eyes progressing to macular atrophy (MA) also had HRF not associated with drusen. The statistical significance of the difference between the curves at each point of distance to the fovea is color coded in the bars below the graphs. Red denotes lower P values, whereas blue represents P > .05. P values are adjusted for multiple testing. MNV, macular neovascularization.
During longitudinal analysis, the mean volume of HRF within the central 3-mm diameter fluctuated slightly during the months before conversion (Figure 2). In eyes progressing to MNV, a slight steady increase of mean HRF volume was observed over time (MNV: 23.0 × 103 μm per month; 95% CI, 10.2-35.5 103 μm per month; MA: 5.1 × 103 μm; 95% CI, −11.3 to 20.5 103 μm per month; no disease progression: 9.2 × 103 μm; 95% CI, 4.7-13.2 103 μm per month).
Topographic Association Between HRF and Drusen
To investigate the topographic association between HRF and drusen, we differentiated between HRF overlying drusen and HRF not overlying drusen. The mean HRF thickness was found to be larger when overlying drusen (Figure 3). However, in eyes progressing to MA, the mean HRF thickness was also increased in areas unaffected by drusen (mean HRF not over drusen height at 0.5-mm eccentricity: MNV: 0.025 μm; 95% CI, 0.015-0.035 μm; MA: 0.043 μm; 95% CI, 0.021-0.065 μm; no disease progression: 0.020 μm; 95% CI, 0.015-0.025 μm).
Predictive Value of HRF and Drusen Volume
Results of the logistic regression model are given in the eTable in the Supplement. For the development of MNV, the largest mean (SD) ROC AUC (0.66 [0.07]) was obtained using drusen volume at 0.5- to 1.5-mm eccentricity, with a similar mean (SD) ROC AUC (0.65 [0.06]) for the central 0- to 0.5-mm area. Regarding the development of MA, HRF volume at 0.5- to 1.5-mm eccentricity had the largest mean (SD) ROC AUC (0.73 [0.11]).
Discussion
This study was undertaken to characterize the typical manifestations of early and intermediate AMD in a large cohort from a randomized clinical trial. We used AI to quantify retinal morphologic features and a spatiotemporal atlas to analyze morphometric information. Our results suggest that the signs of AMD follow a characteristic distribution. Eyes progressing to MNV had substantial drusen height at the foveal center and HRF, as a surrogate of focal hyperpigmentation, overlying these drusen as well as peaking at 0.5-mm eccentricity. In contrast, eyes with imminent onset of MA did not exhibit drusen or HRF at the foveal center, but these signs were found at 0.5-mm eccentricity (ie, at the edge of the foveal pit). Eyes that did not progress to advanced AMD had lower amounts of drusen and HRF, following the distribution patterns seen in the eyes progressing to MNV.
Small drusen can accumulate over time to form confluent large drusen.19 Histologically, drusen constitute the main feature of AMD as sub-RPE accumulation of apolipoproteins and oxidized proteins.20 Measurement of drusen volume is already feasible in clinical practice because tools performing such measurements are available in some OCT devices.12 However, the spatial distribution of drusen in the macula was previously unclear. Because we were able to quantify drusen volumetrically and map these across the macula, we could demonstrate how drusen height builds up in the foveal center of eyes progressing to neovascular AMD. The natural history of drusen formation may follow a strictly fovea-centric rule and may, for instance, be associated with retinal cone density or RPE metabolic activity. However, drusen can also acutely collapse.21 A previous study15 found that AMD progression was associated with preceding drusen regression. Our study found that eyes with imminent onset of MA had low amounts of drusen in the foveal center, which could be a sign of previous drusen regression having occurred as a first step in the disease path that leads to photoreceptor, RPE, and choroidal atrophy. However, whether drusen regression is the cause or consequence of MA remains to be elucidated.
The second main sign of AMD investigated in our study was HRF as a surrogate for focal hyperpigmentation. HRF may represent migratory RPE cells that disengage from the RPE monolayer and move toward the inner retina, whereas they transdifferentiate to express macrophage markers.20,22 OCT-based research has found that HRF migrate toward the retinal surface and increase over time as AMD progresses.7 Other hypotheses for anatomical HRF correlates include lipid exudation and inflammatory aggregates.23,24,25 Because AI technology enabled us to quantify HRF autonomously,16 our study had the opportunity to analyze such subclinical biomarkers, which does not seem to be feasible using manual grading or in current routine clinical practice.
The mean distribution of HRF across the macula differed markedly from the typical distribution of drusen. The largest amounts of HRF were detected at 0.5-mm eccentricity from the foveal center, which represents the slope of the foveal pit. Moreover, HRF were not distributed uniformly according to eccentricity but had a preponderance to occur at the temporal side of the fovea (Figure 3), most pronounced in eyes developing MA. Of interest, if HRF represent migratory RPE cells, it is conceivable that these cells travel along the main direction of retinal cell axons (ie, the fibers of the Henle layer). Because these fibers are directed centripetally around the foveal center, a distribution following the Henle fibers may lead to an accumulation of cells around the fovea. This occurrence would, however, not explain the concentration of HRF in the temporal region. However, we also found that most HRF occurred directly overlying drusen. Although this finding supports the hypothesis that HRF represent RPE cells having disengaged from their monolayer after the epithelium has begun to degenerate (eg, in drusen), a low HRF density was found at the foveal center, where most of the drusen are located. Our study supports an association between HRF and the development of MA as reported previously.3,26,27
AI represents a paradigm-shifting tool in retinal image analysis.9,28 However, the application of AI methods also results in enormous amounts of quantitative data being generated. We used a spatiotemporal atlas that enabled us to analyze the pixel-level information of drusen and HRF in OCT scans of a large cohort.29 The atlas allowed the mapping of the structural information into a common reference frame, supporting spatial and temporal alignment and enabling joint analysis of all patients and time points, while maintaining a more refined analysis than conventional grids. In future research, a mathematical model of physiological and pathological changes of the aging retina may become available, which would allow the assessment of similarities and differences of individual patients with regard to the typical disease trajectories in the general population. Such a model would potentially provide a more precise risk assessment of patients with AMD in the future because it would result in a normative database against which individual retinal morphologic fingerprints could be compared.
Limitations
Our study is mainly limited by the population included in the analysis (ie, study participants of HARBOR who had neovascular AMD in one eye and early or intermediate AMD in the other eye). It is unknown how our findings translate to patients with bilateral early or intermediate AMD, patients with unilateral advanced atrophic AMD, or patients who otherwise meet the eligibility criteria of HARBOR in the clinical practice setting. Currently, studies are underway to provide the needed data sets of patients with AMD who are continuously followed up by imaging during an extended period.30 In addition, our study did not include OCT angiography to detect subclinical MNV, an important factor in determining the risk of disease progression in patients with drusen.31 Additional imaging biomarkers, such as reticular pseudodrusen, which were not analyzed in our study, may be of relevance in creating an atlas of AMD in the future.32 In addition, the quantitative metrics used in our study, in particular HRF measurements, are currently not available to most practitioners, and our findings have not been corroborated in any other independent data set or by other investigators.
Conclusions
In this study, drusen and HRF distribution, time course, and volumes differed among the progression pathways in AMD. Although drusen were most frequently occurring in the foveal center of eyes progressing to MNV, parafoveal drusen and HRF were seen in eyes developing MA. Our results may support the development of an atlas of retinal changes in the aging eye to advance personalized risk counseling of patients and better understanding of the pathologic features of AMD.
eFigure. Representative Examples of the Quantified Imaging Biomarkers
eTable. Logistic Regression Model
References
- 1.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. doi: 10.1016/S0140-6736(12)60282-7 [DOI] [PubMed] [Google Scholar]
- 2.Ferris FL III, Wilkinson CP, Bird A, et al. ; Beckman Initiative for Macular Research Classification Committee . Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844-851. doi: 10.1016/j.ophtha.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt-Erfurth U, Waldstein SM, Klimscha S, et al. Prediction of individual disease conversion in early AMD using artificial intelligence. Invest Ophthalmol Vis Sci. 2018;59(8):3199-3208. doi: 10.1167/iovs.18-24106 [DOI] [PubMed] [Google Scholar]
- 4.Seddon JM, Rosner B. Validated prediction models for macular degeneration progression and predictors of visual acuity loss identify high-risk individuals. Am J Ophthalmol. 2019;198:223-261. doi: 10.1016/j.ajo.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleiman K, Veerappan M, Winter KP, et al. ; Age-Related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group . Optical coherence tomography predictors of risk for progression to non-neovascular atrophic age-related macular degeneration. Ophthalmology. 2017;124(12):1764-1777. doi: 10.1016/j.ophtha.2017.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res. 2016;50:1-24. doi: 10.1016/j.preteyeres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 7.Christenbury JG, Folgar FA, O’Connell RV, Chiu SJ, Farsiu S, Toth CA; Age-related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group . Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology. 2013;120(5):1038-1045. doi: 10.1016/j.ophtha.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folgar FA, Chow JH, Farsiu S, et al. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci. 2012;53(8):4626-4633. doi: 10.1167/iovs.12-9813 [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Erfurth U, Sadeghipour A, Gerendas BS, Waldstein SM, Bogunović H. Artificial intelligence in retina. Prog Retin Eye Res. 2018;67:1-29. doi: 10.1016/j.preteyeres.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 10.Busbee BG, Ho AC, Brown DM, et al. ; HARBOR Study Group . Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120(5):1046-1056. doi: 10.1016/j.ophtha.2012.10.014 [DOI] [PubMed] [Google Scholar]
- 11.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 12.Abdelfattah NS, Zhang H, Boyer DS, et al. Drusen volume as a predictor of disease progression in patients with late age-related macular degeneration in the fellow eye. Invest Ophthalmol Vis Sci. 2016;57(4):1839-1846. doi: 10.1167/iovs.15-18572 [DOI] [PubMed] [Google Scholar]
- 13.Sadda SR, Guymer R, Holz FG, et al. Consensus definition for atrophy associated with age-related macular degeneration on OCT: classification of atrophy report 3. Ophthalmology. 2018;125(4):537-548. doi: 10.1016/j.ophtha.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garvin MK, Abramoff MD, Kardon R, Russell SR, Wu X, Sonka M. Intraretinal layer segmentation of macular optical coherence tomography images using optimal 3-D graph search. IEEE Trans Med Imaging. 2008;27(10):1495-1505. doi: 10.1109/TMI.2008.923966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogunovic H, Montuoro A, Baratsits M, et al. Machine learning of the progression of intermediate age-related macular degeneration based on OCT imaging. Invest Ophthalmol Vis Sci. 2017;58(6):BIO141-BIO150. doi: 10.1167/iovs.17-21789 [DOI] [PubMed] [Google Scholar]
- 16.Schlegl T, Bogunovic H, Klimscha S, et al. Fully automated segmentation of hyperreflective foci in optical coherence tomography images. Preprint. Posted May 8, 2018. arXiv 180503278.
- 17.Rohrschneider K. Determination of the location of the fovea on the fundus. Invest Ophthalmol Vis Sci. 2004;45(9):3257-3258. doi: 10.1167/iovs.03-1157 [DOI] [PubMed] [Google Scholar]
- 18.Rizopoulos D. The R Package JMbayes for fitting joint models for longitudinal and time-to-event data. J Stat Softw. 2016;72(7):46. doi: 10.18637/jss.v072.i07 [DOI] [Google Scholar]
- 19.Ferris FL, Davis MD, Clemons TE, et al. ; Age-Related Eye Disease Study (AREDS) Research Group . A simplified severity scale for age-related macular degeneration: AREDS report No. 18. Arch Ophthalmol. 2005;123(11):1570-1574. doi: 10.1001/archopht.123.11.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanzottera EC, Ach T, Huisingh C, Messinger JD, Spaide RF, Curcio CA Visualizing retinal pigment epithelium phenotypes in the transition to geographic atrophy in age-related macular degeneration. Retina 2016;36(suppl 1):S26-S39. [DOI] [PMC free article] [PubMed]
- 21.Schlanitz FG, Baumann B, Kundi M, et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br J Ophthalmol. 2017;101(2):198-203. doi: 10.1136/bjophthalmol-2016-308422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curcio CA, Zanzottera EC, Ach T, Balaratnasingam C, Freund KB. Activated retinal pigment epithelium, an optical coherence tomography biomarker for progression in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58(6):BIO211-BIO226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolz M, Schmidt-Erfurth U, Deak G, Mylonas G, Kriechbaum K, Scholda C; Diabetic Retinopathy Research Group Vienna . Optical coherence tomographic hyperreflective foci: a morphologic sign of lipid extravasation in diabetic macular edema. Ophthalmology. 2009;116(5):914-920. doi: 10.1016/j.ophtha.2008.12.039 [DOI] [PubMed] [Google Scholar]
- 24.Uji A, Murakami T, Nishijima K, et al. Association between hyperreflective foci in the outer retina, status of photoreceptor layer, and visual acuity in diabetic macular edema. Am J Ophthalmol. 2012;153(4):710-717.e1. doi: 10.1016/j.ajo.2011.08.041 [DOI] [PubMed] [Google Scholar]
- 25.Ogino K, Murakami T, Tsujikawa A, et al. Characteristics of optical coherence tomographic hyperreflective foci in retinal vein occlusion. Retina. 2012;32(1):77-85. doi: 10.1097/IAE.0b013e318217ffc7 [DOI] [PubMed] [Google Scholar]
- 26.Fragiotta S, Rossi T, Cutini A, Grenga PL, Vingolo EM. Predictive factors for development of neovascular age-related macular degeneration: a spectral-domain optical coherence tomography study. Retina. 2018;38(2):245-252. doi: 10.1097/IAE.0000000000001540 [DOI] [PubMed] [Google Scholar]
- 27.Nassisi M, Fan W, Shi Y, et al. Quantity of intraretinal hyperreflective foci in patients with intermediate age-related macular degeneration correlates with 1-year progression. Invest Ophthalmol Vis Sci. 2018;59(8):3431-3439. doi: 10.1167/iovs.18-24143 [DOI] [PubMed] [Google Scholar]
- 28.Ting DSW, Peng L, Varadarajan AV, et al. Deep learning in ophthalmology: the technical and clinical considerations. Prog Retin Eye Res. 2019;72:100759. doi: 10.1016/j.preteyeres.2019.04.003 [DOI] [PubMed] [Google Scholar]
- 29.Vogl WD, Waldstein SM, Gerendas BS, Schmidt-Erfurth U, Langs G. Predicting macular edema recurrence from spatio-temporal signatures in optical coherence tomography images. IEEE Trans Med Imaging. 2017;36(9):1773-1783. doi: 10.1109/TMI.2017.2700213 [DOI] [PubMed] [Google Scholar]
- 30.Finger RP, Schmitz-Valckenberg S, Schmid M, et al. ; on behalf of the MACUSTAR consortium . MACUSTAR: development and clinical validation of functional, structural, and patient-reported endpoints in intermediate age-related macular degeneration. Ophthalmologica. 2019;241(2):61-72. doi: 10.1159/000491402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Oliveira Dias JR, Zhang Q, Garcia JMB, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125(2):255-266. doi: 10.1016/j.ophtha.2017.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naysan J, Jung JJ, Dansingani KK, Balaratnasingam C, Freund KB. Type 2 (subretinal) neovascularization in age-related macular degeneration associated with pure reticular pseudodrusen phenotype. Retina. 2016;36(3):449-457. doi: 10.1097/IAE.0000000000000758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Representative Examples of the Quantified Imaging Biomarkers
eTable. Logistic Regression Model




