Abstract
Bipolar disorder (BD) is a neuropsychiatric mood disorder characterized by recurrent episodes of mania and depression in addition to disruptions in sleep, energy, appetite, and cognitive functions-rhythmic behaviors that typically change on daily cycles. BD symptoms can also be provoked by seasonal changes, sleep, and/or circadian disruption, indicating that chronobiological factors linked to the circadian clock may be a common feature in the disorder. Research indicates that BD exists on a clinical spectrum, with distinct subtypes often intersecting with other psychiatric disorders. This heterogeneity has been a major challenge to BD research and contributes to problems in diagnostic stability and treatment outcomes. To address this heterogeneity, we propose that chronobiologically related biomarkers could be useful in classifying BD into objectively measurable phenotypes to establish better diagnoses, inform treatments, and perhaps lead to better clinical outcomes. Presently, we review the biological basis of circadian time keeping in humans, discuss the links of BD to the circadian clock, and present recent studies that evaluated chronobiological measures as a basis for establishing BD phenotypes. We conclude that chronobiology may inform future research using other novel techniques such as genomics, cell biology, and advanced behavioral analyses to establish new and more biologically based BD phenotypes.
Keywords: Bipolar disorder, Circadian rhythm, Sleep, Genetics, Chronobiology
Introduction
Bipolar disorder (BD) is a common, severe, and debilitating mental illness that affects approximately 1–3% of the population [1, 2]. The negative impact associated with BD is immense. This chronic illness currently ranks as the sixth leading cause of disability worldwide [3] and is estimated to be the world's fourth largest cause of disability among young adults aged 15–44 years [4]. Among psychiatric disorders, BD has one of the highest suicide rates, with estimates that 10–20% of affected patients ultimately take their own lives [5]. Even though the vast majority of individuals suffering from affective episodes experience significant degrees of psychosocial impairment [6], many patients suffering from BD go untreated [1].
BD is defined primarily by recurrent manic and depressive episodes. Additional diagnostic criteria reveal underlying chronobiological disruptions in BD. BD is marked by fluctuations and disturbances in activity and energy, appetite, attention, subjective speeds of thought, and sleep − all processes that commonly show diurnal variation in healthy subjects. Additionally, some specifiers used to describe the illness, such as rapid cycling and seasonality, also suggest that rhythm disturbances are a core feature of the disorder [7]. Rhythm disruptions are a hallmark of BD [7, 8]. Variations in intrinsic circadian periods [9, 10], phase shifts [11, 12, 13, 14, 15, 16, 17, 18, 19], and less stable biological rhythms [20, 21, 22, 23, 24, 25] have all been noted in association with the illness. Disturbances in lifestyle regularity [26, 27, 28], sleep disturbances [29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42], variations in melatonin secretion [17, 43], and disruptions in rhythmic locomotor activity [24, 25, 44, 45] have all been reported.
Among the challenges in studying and treating BD is the heterogeneity, defined as the variability in the clinical presentation, of the disorder. The illness is not fully characterized by existing diagnostic and classification systems, and many individuals with the same diagnosis show considerable differences in illness course and treatment response [46]. This heterogeneity in clinical presentation may reflect the presence of multiple, distinct pathophysiological mechanisms underlying the development and/or progression of BD [47]. While considerable progress has been made in understanding the biological mechanisms underlying BD, much remains to be known, and phenotypic heterogeneity has undoubtedly contributed to the challenges in identifying pathophysiological mechanisms associated with BD. One proposed method to address the issue of heterogeneity is to identify phenotypes, or observable traits and characteristics, for the illness [48]. A plausible phenotype should be supported by empirical evidence, be comprised of measurable characteristics, and be directly applicable to the disorder in question [48]. For BD, differences in the expression of chronobiological characteristics like circadian rhythm disturbances, sleep abnormalities, and seasonality among others may meet these criteria.
In this review we examine the heterogeneity of BD from a chronobiological perspective. We provide a background for the genetic and neurobiological foundations of the circadian clock system and evaluate evidence linking BD patients and chronobiological disturbances. Finally, the evidence supporting distinct chronobiological phenotypes in BD is reviewed, with discussion of the directions for future research in the field.
Structure and Function of the Circadian Timing System
Circadian rhythms play an essential role in life. They are self-sustained, ∼24-h rhythms that are present in nearly every organism, including humans. The circadian timing system directly or indirectly influences the timing of nearly all rhythmic physiological activity in humans, including sleep and activity cycles as well as seasonal rhythms [49]. Additional physiological functions under the regulation of the circadian timing system include temperature regulation, feeding and metabolism, hormone secretion, and inflammation. In mammals, the suprachiasmatic nucleus of the hypothalamus functions as the master pacemaker [49]. However, many brain regions besides the suprachiasmatic nucleus contain circadian clocks, including areas that have been implicated in mood regulation and mood disorders, such as the frontal cortex, hippocampus, amygdala, and striatum [50, 51]. Recent estimates in nonhuman primate sampling from 64 tissues across the body indicates that >80% of the genome is rhythmically expressed in at least one tissue and that genes involved in critical cellular processes are typically rhythmic in the relevant tissue for that function [52]. Moreover, rhythms in the brain are widespread and show anatomically distinct profiles comprised of distinct ensembles of rhythmic genes in different brain regions [52]. Accordingly, behaviors and neurophysiological processes affected by mood disorders, such as cognitive function, reward processing, motivation, and mood regulation, are under the regulation of the circadian clock [50, 51].
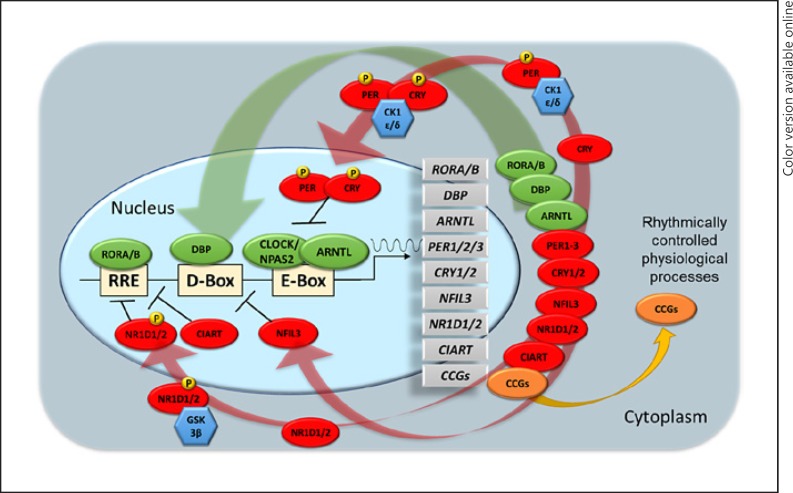
At the core of the circadian timing system are endogenous molecular clocks comprised of transcriptional/translational feedback loops made up of circadian genes [53, 54] (Fig. 1). The positive feedback loop consists of heterodimeric transcriptional activator complexes (CLOCK/NPAS2-ARNTL) that bind to CACGTG E-box or related E-box-like sequences to regulate transcription of core clock genes (PER1/2, CRY1/2, CIART, NR1D1/2, and DBP) [55, 56]. The CLOCK/NPAS2-ARNTL complex regulates the expression of its own transcriptional repressors PER1/2/3 and CRY1/2 that gradually inhibit their own expression over ∼24-h cycles to sustain a rhythmic circadian oscillator. Negative feedback is achieved upon accumulation of PER and CRY proteins in the cytoplasm, where they dimerize to form a PER-CRY repressor complex that translocate back to the nucleus upon phosphorylation by CSNK1D/E to negatively regulate their own transcription. CLOCK/NPAS2-ARNTL heterodimers also activate the expression of transcription factors NR1D1/2, CIART, and RORA/B, which form a second feedback loop and activate and repress ARNTL, NFIL3, and CRY1 transcription at ROR response elements containing a 5′-AGGTCA-3′ motif. D-box elements (5′-TTAYGTAA-3′) are activated and repressed by DBP and NFIL3, respectively, and regulate circadian transcriptional oscillations of PER1/2/3, NR1D1/2, and RORA/B. DBP and NFIL3 proteins are critical for determining the period length of the circadian oscillator [57] and have been implicated in phase resetting of the circadian clocks [58].
Fig. 1.
Transcriptional regulation of the human molecular clock. Transcriptional/translational feedback loops of molecular clocks are comprised of core circadian genes consisting of transcriptional activators (green) and repressors (red) responsible for the rhythmic expression of core clock genes and clock-controlled genes. Kinases (blue) are responsible for post-translation modification via phosphorylation (P) of key proteins within the cytoplasm required for translocation of proteins and protein complexes into the nucleus. ARNTL, aryl hydrocarbon receptor nuclear translocator-like; CCGs, clock-controlled genes; CIART, circadian-associated repressor of transcription; CK1ε/δ, casein kinase 1 epsilon/delta; CLOCK, clock circadian regulator; CRY1/2, cryptochrome circadian regulator 1/2; DBP, D-box-binding PAR BZIP transcription factor; GSK3β, glycogen synthase kinase 3 beta; NFIL3, nuclear factor, interleukin 3-regulated; NPAS2, neuronal PAS domain protein 2; NR1D1/2, nuclear receptor subfamily 1 group D member 1/2; PER1/2/3, period circadian regulator 1/2/3; RORA/B, RAR-related orphan receptor A/B; RRE, ROR response elements.
Virtually every cell in the body has an autonomous circadian clock [59, 60]. It is the expression of clock genes that results in the ability of cells to maintain time keeping rhythms in a cell-autonomous manner. In addition to governing molecular clock functions, clock genes also regulate the expression of clock-controlled output genes, i.e., genes that do not have a direct time keeping function but are involved in temporal regulation of tissue-specific, physiological processes in which timing plays an important role, including many implicated in mood regulation [61, 62]. The majority of rhythmically expressed genes in the body fall into this latter category of clock-controlled genes.
Effects of Circadian Misalignment on Health
One of the important functions of the circadian timing system is to coordinate physiological processes and behaviors across systems. It is believed that stable organization of biological rhythms is an indicator of good health and well-being [63]. Chronobiological disturbances are now widely recognized as a general health concern influencing a wide array of diseases [64, 65, 66, 67], including psychiatric illnesses [68, 69, 70, 71, 72, 73]. Circadian misalignment, or misalignment between the circadian pacemaker and behavioral or environmental cues, is associated with health problems [74] and with adverse physiological [75, 76] and mental sequelae [63, 76, 77].
Evidence has demonstrated that there is individual variability in the suscepti bility towards temporal disorganization and the propensity to experience symptoms of circadian misalignment [78, 79, 80]. In BD, this includes abnormalities in circadian phase [16, 18, 81, 82, 83], low-amplitude rhythms/rhythm fragmentation [83, 84, 85, 86], and sleep disturbances [87, 88]. It may, therefore, be the case that a subset of BD patients suffer to a greater degree from chronobiological disturbances. If this is the case, chronobiological phenotyping may play a significant role in identifying BD groups that are most vulnerable or resistant to the adverse health effects of circadian misalignment and/or desynchronization.
Impact of Chronotype on BD
Circadian rhythm disorders represent the extreme ends of a broader spectrum of morning versus evening preferences that extends into the healthy population. This circadian phenotype is commonly called chronotype [89]. Chronotype, or the diurnal preference for daily activities, is often used to obtain a measure of interindividual variations in circadian rhythms and appears to be a relatively stable trait [90] likely associated with genetic markers [90]. People with different chronotypes can differ dramatically in responses to shift work [91], homeostatic sleep regulation [92, 93, 94], activity phase [95, 96], responses to sleep fragmentation [97, 98], total sleep deprivation [99, 100], and circadian phase [101, 102]. Chronotype is estimated to be about 50% heritable [103] and varies across populations and developmental stage [104, 105, 106, 107]. Resting on a continuum, chronotype is likely to be polygenic in origin [90, 108].
It has been suggested that in BD patients, chronotype is a stable trait characteristic [16, 109]. BD patients consistently exhibit a significantly higher preference for eveningness compared to control subjects [16, 81, 110, 111, 112, 113]. Chronotypic traits may impact the clinical presentation and course of bipolar illness. A greater degree of eveningness has been associated with rapid mood swings [82], higher recurrence rates [82, 114], and an earlier age of illness onset [82] and lithium response [115]. Chronotype has also been associated with physiological parameters [116, 117], including variations in body temperature [101, 116, 118], catecholamine secretion [116, 119], sleep patterns [116, 120, 121, 122], subjective activation and arousal [116, 119, 123], and circadian rhythms of hormone secretion [118] in healthy controls that may be important to the underlying pathophysiology of BD and/or phenotypic expression.
Physiological markers have been associated with BD. For example, an evening chronotype has been associated with insomnia [124], longer sleep latency [113], a higher percentage of total body fat and obesity [125, 126], higher homocysteine levels [127], increased atherogenic index of plasma [128], and a higher level of triglycerides [128]. Chronotype may also have clinical implications in BD. For example, an evening chronotype has been associated with higher response rates to the antidepressant response of total sleep deprivation plus light therapy [129].
Genome-Wide Associations of Clock Genes with Chronotype and BD
While genome-wide association studies (GWAS) have not identified core clock gene associations with BD; the sample size of the most recent BD GWAS [130] is still relatively underpowered compared to chronobiologically related GWAS studies with samples sizes surpassing 100,000 individuals [108, 131, 132, 133, 134, 135]. Variants in the core clock genes ARNTL [131, 136], NPAS2 [131], PER2 [108, 131, 132, 135, 136], PER3 [135, 136], CRY1 [131, 132], and RORB [131] have all shown significant GWAS association with chronotype. Other clock genes including ARNTL [56], NFIL3 [137], and CRY2 [136] demonstrated weaker associations with BD and chronotype that did not reach the threshold for genome-wide significance. Other GWAS chronotype-associated genes − MEIS1/2 [131, 132] and VIP [131, 132, 136] − have previously well-established roles in regulating circadian rhythms [135, 136].
There is some indication that genetic markers of BD overlap with chronobiological phenotypes [138, 139]. For instance, a chronotype-associated locus lies in proximity to the clock gene CIART [56, 140] and overlaps with a newly reported risk locus (rs7544145) for BD [130]. Similarly, a variant in the clock gene ARNTL was among a small number of markers that differentiated polygenic risk for schizophrenia from BD [141]. Genetic variation in clock genes may not be limited to BD only. In addition to chronotype, differences in other chronobiological phenotypes also have a genetic basis [142]. Looking at related traits, CRY1, PER2, and PER3 variants are associated with ease of getting up in the morning [132]. Markers on the genes NFASC, SLC25A17, and MEIS with roles in regulating circadian rhythms are associated with lower relative amplitude in locomotor activity [143] and relative amplitude of rest-activity cycles [143]. RORB and MEIS1 variants are associated with insomnia [132, 133, 134, 144], and MEIS1 variants are also related to sleep duration [145] and other sleep-related traits [133]. These data indicate that many chronotype-associated markers identified by GWAS are not well functionally characterized, and there are likely to be numerous pathways and genes that influence rhythmic behaviors both within and beyond the core circadian clock. It is not yet clear to what degree the genes identified may demonstrate pleiotropic effects on other systems or what the impact of these identified markers are on chronobiological aspects of BD. For instance, NR1D2 variants exhibit robust GWAS associations with intelligence [146, 147, 148, 149] and cognitive ability [150, 151, 152], traits that have been previously linked to BD [153, 154, 155, 156, 157].
Cellular Models of Circadian Clocks in BD
In recent years, novel techniques have been developed to assess the cellular function of molecular clocks in healthy human subjects. These studies have begun to establish that there are significant interindividual differences in the functioning of molecular clocks [158, 159] and that this variation is associated with differences in behavior, chronotype, and physiology [158, 159, 160]. Brown et al. [158] characterized the expression of molecular clocks in fibroblasts of 19 individuals. While the average period of the sample was similar within the normal range previously reported for humans, the investigators noted a greater than expected variability in circadian phase. These investigators also found that period and phase of cellular clocks were associated with the chronotypes of human cell donors [159]. These findings were partially replicated by Hida et al. [160], who found a relationship between the period of molecular clocks and chronotype. While these observations have been made in the general population, similar chronobiological heterogeneity may exist in BD.
While GWAS are well suited to capture information from relatively large population cohorts, they typically cannot inform researchers about the functional consequences of a genetic variant as it relates to a time keeping function. Therefore, a complementary approach is to assess molecular clock functioning and chronobiological phenotypes in cells from BD patients. Since circadian clocks are cell-autonomous and present throughout the body, peripheral cell types like fibroblasts may be one useful model. These cells have the advantage of being relatively accessible and easy to grow. In one early study by Yang et al. [161] the expression of 12 clock genes was examined using PCR and protein analyses in fibroblast cultures in a time series over 72 h. Lower amplitude expression rhythms for BMAL1 (ARNTL), REV-ERBα (NR1D1), and DBP as well as decreased phosphorylation of GSK3B were found in the BD compared to the control cells. Later studies again studied fibroblasts, but this time using a bioluminescent reporter gene (PER2-luc), which allowed for more frequent sampling over longer times. In this study, cells from BD patients were found to have a longer circadian period and abnormal amplitude response to treatment of the cells with lithium [162]. In follow-up studies the same authors again studied fibroblast cultures, but this time from cells obtained in the course of a prospective, multicenter, clinical trial of lithium monotherapy [115]. In lithium responder samples, cells had a shorter period compared to samples from non-responders. Moreover, there were other rhythm characteristics that differed between groups, with a linear relationship between period and phase and a period shortening effect in lithium responders, but not in cells from non-responders. These cellular models have provided some important functional context to genetic studies. However, since fibroblasts lack key features of neurons (e.g., neurotransmitters, electrical activity, synaptic connections), there may be additional BD-related chronobiological functions that are better examined in neuronal cells.
Sleep Abnormalities and BD
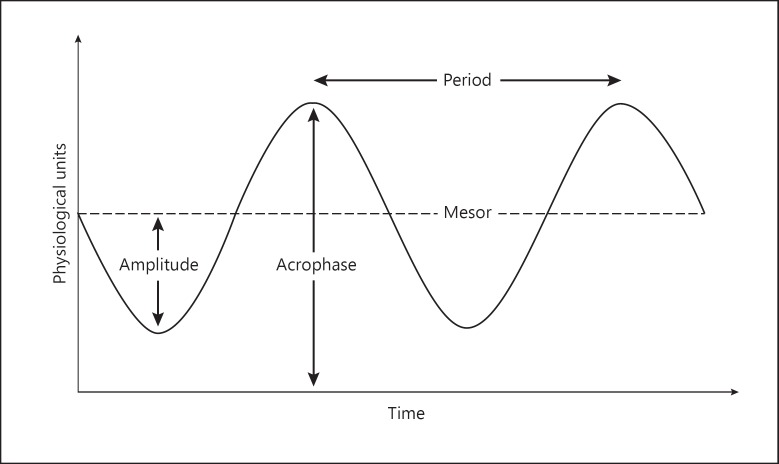
The two-factor theory of sleep predicts that circadian rhythms have a major impact on sleep behavior [163]. The American Academy of Sleep Medicine currently recognizes four intrinsic circadian rhythm sleep-wake disorders (CRSWDs) [164]. These include advanced sleep phase disorder, delayed sleep phase disorder, irregular sleep-wake rhythm disorder, and non-24-h sleep-wake disorder, each disorder being marked by a particular characteristic. Advanced sleep phase disorder has been associated with shorter circadian acrophase and period (Fig. 2), while delayed sleep phase disorder has been associated with longer circadian acrophase and period. Irregular sleep-wake rhythm disorder is characterized by fragmented and pattern-lacking sleep-wake cycles. Non-24-h sleep-wake disorder presents with a progressive lengthening of circadian period.
Fig. 2.
Cosinor curve frequently used to represent circadian rhythms. In cosinor modeling, period is the duration of one cycle, amplitude is the peak value from a wave's mean, acrophase is a measure of the time of overall high values recurring in each cycle, and mesor is the rhythm-adjusted mean.
Takaesu and colleagues [165, 166, 167] have conducted a series of studies examining the presence of CRSWD in BD. These investigators found that approximately one-third of bipolar patients met the criteria for a CRSWD [165, 166]. They further demonstrated that these comorbidities had clinical implications. BD patients with co-occurring CRSWD were associated with higher suicide rates [165], greater recurrence rates [166, 167], antidepressant-related manic switch [166], and higher rates of family history of psychiatric disorders [165].
Sleep characteristics may also prove to be important phenotypic signatures of BD and overlap with chronobiological mechanisms. Scott et al. [168] studied the relationship between sleep and BD in families. In this study, individuals with a family history of BD and subjects without a history of familial mood disorders were compared using actigraphy measures and Pittsburgh Sleep Quality Index scores. They found that the family history-positive group differed in mean nighttime sleep duration, variability in waking after sleep onset, sleep disturbances, and daytime dysfunction, indicating that sleep problems cosegregate with the genetic risk for BD in families. Studies also suggest that sleep phenotypes exist in BD. For example, short sleep duration has demonstrated association with more severe symptoms [42], while both short and long sleep duration have been associated with poor functioning and quality of life [42]. A worse course of illness [30, 169], increased symptom severity [30, 42, 169], and impairments in functioning and quality of life [30, 42, 169] have also been related to sleep disruption. Specific types of sleep disturbances may also be associated with specific mood states. Variability in sleep latency has been associated with depressive symptoms [169], and lower and more variable sleep efficiency has been associated with more lifetime depressive episodes [169]. Decreased sleep efficiency [169] and the duration of REM and slow-wave sleep [30] have also been associated with mania. Disturbances of sleep could potentially predispose a subset of BD patients towards disruptions in such circadian components as phase and acrophase, thus leading to a misalignment of circadian rhythms in BD.
Seasonal Rhythms and BD
Seasonality, including recurrent mood episodes and the level of functioning associated with seasonal changes, is another potential BD phenotype with chronobiological mechanisms. While seasonal rhythms occur over longer time intervals than the circadian rhythm (i.e., months versus hours), the suprachiasmatic nucleus and circadian clock genes are also critically involved in regulating these long cycles, in conjunction with melatonin and effects on thyroid hormones in the pars tuberalis of the anterior pituitary gland [170]. Therefore, circadian variation in BD patients may also be associated with seasonal mood changes [171]. A subpopulation of BD patients present with a seasonal pattern to their mood episodes [172, 173, 174, 175, 176]. For those with seasonal patterns, mania appears to peak in the early spring with a nadir in the late fall [172], mixed mania peaks in the late summer with a nadir in the late fall [172], and depression appears in the autumn to winter months [173].
Chronobiological Phenotypes and Clinical Features
The clinical characteristics of BD are heterogeneous, with differences in the course of illness, comorbidity of medical and psychiatric conditions, and treatment responses [177]. This variability is likely the result of biological and environmental variability across several etiological mechanisms [47]. Unfortunately, it is this heterogeneity that may have hindered discoveries of pathophysiological mechanisms associated with the illness. Better-defined BD phenotypes may unravel some of the diagnostic complexity and help to identify more coherent categorization schemes [48]. This in turn may yield better diagnostic approaches and improve treatment selection. Based on the work reviewed herein, we propose that variability in circadian and chronobiological measurements may be objective and quantifiable traits that could be used to more precisely organize BD patients into coherent phenotypes.
Humans show significant interindividual differences in a wide variety of chronobiological characteristics [90]. Individual differences in the free-running circadian period (tau) [178, 179, 180], circadian amplitude [102, 181], and circadian phase [101, 102, 179, 181] have all been reported. There is mounting evidence suggesting that chronobiological disturbances are not only associated with BD, but that specific clinical and physiological signatures point to circadian disruptions as potential chronobiological phenotypes in the illness. As with individuals intolerant to shift work [63], subsets of patients with BD may differ in susceptibility to the disruption of biological rhythms [182]. People who demonstrate an inability to adapt to shift work demonstrate alterations in sleep such as insomnia, short sleep duration, poor sleep quality, and mood alterations including irritability and mood lability [63, 183, 184, 185, 186, 187, 188]. The question arises whether a similar mechanism may apply to BD and whether specific chronobiological phenotypes are present within the larger diagnostic category.
Pagani et al. [189] examined actigraphy-based phenotypes in 26 Costa Rican and Colombian pedigrees. The study included 136 euthymic BD individuals and 422 unaffected relatives. BD subjects expressed fragmented rhythms overall compared to unaffected controls (i.e., lower activity, longer sleep times, and low amplitude). Forty-nine activity-related phenotypes exhibited significant heritability, and 12 of these overlapped with heritability for BD. Using linkage analysis, the study identified a genome-wide significant locus on chromosome 12 for inter-daily stability of activity and suggestive linkage in the same region for the mean number of sleep bouts in the awake period and amplitude. Taken together, these studies indicate that a wide variety of rhythmic processes governing sleep and activity are altered in BD and that some of the factors underlying this variability may also overlap with the risk for BD.
Conclusion
In mammals, the circadian timing system keeps physiological rhythms synchronized with each other and the environment. Organism-wide coordination of rhythms influences multiple physiological systems, brain regions, and behaviors that are germane to both healthy mood regulation and mood disorders, including BD. Given the important and fundamental nature of the circadian clock, it is not surprising that rhythm disturbances are associated with detrimental mental health sequelae.
Humans show significant interindividual differences in a wide variety of chronobiological characteristics, suggesting that these traits lie on a continuum even in healthy subjects. As with many other human traits, this variability lends itself to potential dysfunction when located at extreme ends of the spectrum. It is now widely recognized that chronobiological disturbances predispose individuals experiencing them to health problems and disease states including psychiatric disorders. As in other complex trait disorders, individuals are not all prone to the development of adverse consequences related to chronobiological disruption. This may be particularly relevant in BD.
Rhythm disruptions are a hallmark of BD. While generally associated with the illness, chronobiological disturbances may be enriched in particular BD subgroups, suggesting they may be markers of certain illness chronobiological phenotypes, possibly with distinct etiological factors, illness course, and treatment response. Recent research has begun to support this notion. It is estimated that as many as one-third of bipolar patients may suffer from an independent CRSWD. These comorbid conditions may be related to a worse illness course. BD patients with significant chronobiological disturbances have been shown to have higher suicide rates, recurrence rates, and antidepressant-related manic switch rates. Another example is chronotype where a greater degree of eveningness has been associated with rapid mood swings, greater recurrence rates, and an earlier age of illness onset in the disorder, and less response to treatment with lithium.
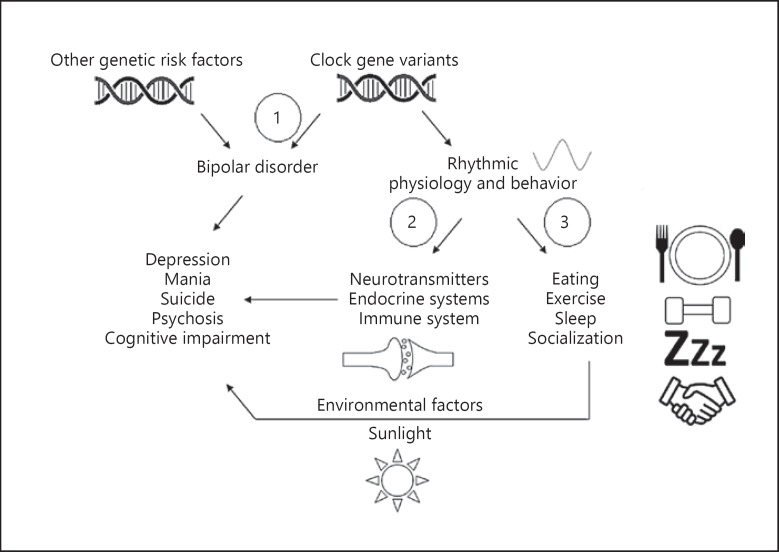
The effects of chronobiology interacting with BD could arise in several different ways, none of which are mutually exclusive (Fig. 3). First, disruption of clock genes could be a primary factor that directly contributes to BD in some subjects. Next, inherited chronobiological disturbances could disturb rhythms in physiological processes that worsen BD through secondary effects on the illness (e.g., globally increased stress, decreased sleep). Finally, chronobiological disturbances could lead to unhealthy interactions with environmental factors (e.g., light, diet, social contacts) that provoke symptoms or negatively affect illness course. Given the widespread involvement of the circadian clock in multiple organ systems and physiological processes, multiple mechanisms may be involved in each individual. It will be of interest in the future to determine whether BD patients who differ in circadian disruption differ in outcomes to chronobiologically based treatments such as melatonin or orexin receptor agonists, bright light therapy, partial sleep deprivation/phase advance, social rhythms therapy, and others.
Fig. 3.
Pathways by which clock gene variation could affect the illness progression of BD. (1) Clock gene variants may be causally related to BD and directly lead to the emergence of symptoms and mediate some aspects of the illness. (2) Clock genes may act in a pleiotropic manner to alter rhythms in biological processes like neurotransmission, endocrine systems, immune response, and others that affect relevant physiology and modulate the course BD. (3) Chronobiological factors also affect behaviors that affect interactions with environmental cofactors such as sunlight, diet, and socialization. These environmental factors interact with genetic and other biological substrates (including BD-specific risk alleles) to affect the course and progression of BD. It is unclear whether these environmental effects are mediators, modulators, or both. Of note, the three pathways outlined above are not mutually exclusive and could in principle run concurrently. BD, bipolar disorder.
While initial studies are promising, additional research is required to better define these putative chronobiological phenotypes in BD. New methods allow researchers to investigate the function of molecular clocks in living cells. The establishment of induced pluripotent stem cell-derived neurons may allow us to examine molecular rhythms in human neurons for the first time, perform transcriptome analysis over the circadian time course, and use integrative approaches for transcriptome-wide association studies to identify significant gene expression-trait associations. Using these approaches, it will be useful to identify the intersections of clock outputs with other biological risk factors for BD. Similarly, long-term sleep and activity measurements in human subjects are becoming more accessible and feasible in clinical populations. More refined analysis of larger cohorts with detailed clinical examination and collection of biomarkers will undoubtedly help to further resolve the target populations. It will be important to determine the clinical, course of illness, physiological/cellular/molecular mechanisms, and genetic differences that distinguish these groups. Finally, it also will be of considerable interest to correlate molecular and genetic factors with rhythmic behaviors in human subjects and to identify clinical features of BD that may differ as a function of chronobiological features. It is of vital importance to understand how the molecular gears of the ticking clock interlock with other biological and clinical factors underlying BD. These exciting new avenues for chronobiologically based research will continue to bring us closer to a bridge from the bench to the bedside.
Most importantly, research in this important area marks a step toward personalized medicine. Further research on chronobiological profiles in BD may improve diagnostic and classification criteria, inform research of the underlying pathophysiology of the disorder, and clarify the relationships with other clinical characteristics of the illness. Moreover, chronobiological phenotypes may help to identify subgroups of BD patients with distinct etiological factors and/or responses to treatment which may aid in the development of novel and specifically directed interventions [48, 190]. As activity and sleep rhythms are increasingly measured reliably and passively with electronic devices, future research could develop methods for monitoring the illness, allowing patients and clinicians to more effectively intervene when necessary. We firmly believe that the identification and characterization of chronobiological phenotypes will represent a large step toward improving the characterization, management, and treatment of this debilitating disorder.
Disclosure Statement
M.J. McCarthy has served as a consultant for Janssen Pharmaceuticals. The other authors have no relevant disclosures.
Funding Sources
M.J. McCarthy is funded by a Merit Award BX003431 from the US Department of Veterans Affairs.
Author Contributions
R. Gonzalez, S.D. Gonzalez, and M.J. McCarthy all contributed to the research and writing of the manuscript.
References
- 1.Merikangas KR, Jin R, He JP, Kessler RC, Lee S, Sampson NA, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011 Mar;68((3)):241–51. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen CB, Mors O, Bertelsen A, Waltoft BL, Agerbo E, McGrath JJ, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiatry. 2014 May;71((5)):573–81. doi: 10.1001/jamapsychiatry.2014.16. [DOI] [PubMed] [Google Scholar]
- 3.Kleinman L, Lowin A, Flood E, Gandhi G, Edgell E, Revicki D. Costs of bipolar disorder. Pharmacoeconomics. 2003;21((9)):601–22. doi: 10.2165/00019053-200321090-00001. [DOI] [PubMed] [Google Scholar]
- 4.Insel TR, Scolnick EM. Cure therapeutics and strategic prevention: raising the bar for mental health research. Mol Psychiatry. 2006 Jan;11((1)):11–7. doi: 10.1038/sj.mp.4001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tondo L, Hennen J, Baldessarini RJ. Lower suicide risk with long-term lithium treatment in major affective illness: a meta-analysis. Acta Psychiatr Scand. 2001 Sep;104((3)):163–72. doi: 10.1034/j.1600-0447.2001.00464.x. [DOI] [PubMed] [Google Scholar]
- 6.Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RM, Petukhova M, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007 May;64((5)):543–52. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Psychiatric Association . American Psychiatric Association. DSM-5 Task Force.: Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington (VA): American Psychiatric Association; 2013. [Google Scholar]
- 8.Gonzalez R. The relationship between bipolar disorder and biological rhythms. J Clin Psychiatry. 2014 Apr;75((4)):e323–31. doi: 10.4088/JCP.13r08507. [DOI] [PubMed] [Google Scholar]
- 9.Wehr TA, Sack DA, Duncan WC, Mendelson WB, Rosenthal NE, Gillin JC, et al. Sleep and circadian rhythms in affective patients isolated from external time cues. Psychiatry Res. 1985 Aug;15((4)):327–39. doi: 10.1016/0165-1781(85)90070-8. [DOI] [PubMed] [Google Scholar]
- 10.Kripke DF, Mullaney DJ, Atkinson M, Wolf S. Circadian rhythm disorders in manic-depressives. Biol Psychiatry. 1978 Jun;13((3)):335–51. [PubMed] [Google Scholar]
- 11.Wehr TA, Muscettola G, Goodwin FK. Urinary 3-methoxy-4-hydroxyphenylglycol circadian rhythm. Early timing (phase-advance) in manic-depressives compared with normal subjects. Arch Gen Psychiatry. 1980 Mar;37((3)):257–63. doi: 10.1001/archpsyc.1980.01780160027002. [DOI] [PubMed] [Google Scholar]
- 12.Salvatore P, Ghidini S, Zita G, De Panfilis C, Lambertino S, Maggini C, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008 Mar;10((2)):256–65. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 13.Linkowski P, Mendlewicz J, Leclercq R, Brasseur M, Hubain P, Golstein J, et al. The 24-hour profile of adrenocorticotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985 Sep;61((3)):429–38. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- 14.Linkowski P, Van Cauter E, Leclercq R, Desmedt D, Brasseur M, Golstein J, et al. ACTH, cortisol and growth hormone 24-hour profiles in major depressive illness. Acta Psychiatr Belg. 1985 Sep-Oct;85((5)):615–23. [PubMed] [Google Scholar]
- 15.Linkowski P, Kerkhofs M, Van Onderbergen A, Hubain P, Copinschi G, L'Hermite-Balériaux M, et al. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch Gen Psychiatry. 1994 Aug;51((8)):616–24. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- 16.Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, Monk K, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009 Apr;166((2-3)):201–9. doi: 10.1016/j.psychres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nurnberger JI, Jr, Adkins S, Lahiri DK, Mayeda A, Hu K, Lewy A, et al. Melatonin suppression by light in euthymic bipolar and unipolar patients. Arch Gen Psychiatry. 2000 Jun;57((6)):572–9. doi: 10.1001/archpsyc.57.6.572. [DOI] [PubMed] [Google Scholar]
- 18.Moon JH, Cho CH, Son GH, Geum D, Chung S, Kim H, et al. Advanced Circadian Phase in Mania and Delayed Circadian Phase in Mixed Mania and Depression Returned to Normal after Treatment of Bipolar Disorder. EBioMedicine. 2016 Sep;11:285–95. doi: 10.1016/j.ebiom.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nováková M, Praško J, Látalová K, Sládek M, Sumová A. The circadian system of patients with bipolar disorder differs in episodes of mania and depression. Bipolar Disord. 2015 May;17((3)):303–14. doi: 10.1111/bdi.12270. [DOI] [PubMed] [Google Scholar]
- 20.Pflug B, Martin W. [Analysis of circadian temperature rhythm in endogenous depressive illness (author's transl)] Arch Psychiatr Nervenkr (1970) 1980;229((2)):127–43. doi: 10.1007/BF00343078. [DOI] [PubMed] [Google Scholar]
- 21.Tsujimoto T, Yamada N, Shimoda K, Hanada K, Takahashi S. Circadian rhythms in depression. Part II: circadian rhythms in inpatients with various mental disorders. J Affect Disord. 1990 Mar;18((3)):199–210. doi: 10.1016/0165-0327(90)90037-9. [DOI] [PubMed] [Google Scholar]
- 22.Pflug B, Erikson R, Johnsson A. Depression and daily temperature. A long-term study. Acta Psychiatr Scand. 1976 Oct;54((4)):254–66. doi: 10.1111/j.1600-0447.1976.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 23.Pflug B, Johnsson A, Ekse AT. Manic-depressive states and daily temperature. Some circadian studies. Acta Psychiatr Scand. 1981 Mar;63((3)):277–89. doi: 10.1111/j.1600-0447.1981.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 24.Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005 Apr;7((2)):176–86. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 25.Krane-Gartiser K, Henriksen TE, Morken G, Vaaler A, Fasmer OB. Actigraphic assessment of motor activity in acutely admitted inpatients with bipolar disorder. PLoS One. 2014 Feb;9((2)):e89574. doi: 10.1371/journal.pone.0089574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashman SB, Monk TH, Kupfer DJ, Clark CH, Myers FS, Frank E, et al. Relationship between social rhythms and mood in patients with rapid cycling bipolar disorder. Psychiatry Res. 1999 Apr;86((1)):1–8. doi: 10.1016/s0165-1781(99)00019-0. [DOI] [PubMed] [Google Scholar]
- 27.Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D, et al. Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: a preliminary investigation. Arch Gen Psychiatry. 1998 Aug;55((8)):702–7. doi: 10.1001/archpsyc.55.8.702. [DOI] [PubMed] [Google Scholar]
- 28.Malkoff-Schwartz S, Frank E, Anderson BP, Hlastala SA, Luther JF, Sherrill JT, et al. Social rhythm disruption and stressful life events in the onset of bipolar and unipolar episodes. Psychol Med. 2000 Sep;30((5)):1005–16. doi: 10.1017/s0033291799002706. [DOI] [PubMed] [Google Scholar]
- 29.Hudson JI, Lipinski JF, Keck PE, Jr, Aizley HG, Lukas SE, Rothschild AJ, et al. Polysomnographic characteristics of young manic patients. Comparison with unipolar depressed patients and normal control subjects. Arch Gen Psychiatry. 1992 May;49((5)):378–83. doi: 10.1001/archpsyc.1992.01820050042006. [DOI] [PubMed] [Google Scholar]
- 30.Eidelman P, Talbot LS, Gruber J, Hairston I, Harvey AG. Sleep architecture as correlate and predictor of symptoms and impairment in inter-episode bipolar disorder: taking on the challenge of medication effects. J Sleep Res. 2010 Dec;19((4)):516–24. doi: 10.1111/j.1365-2869.2010.00826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitaram N, Gillin JC, Bunney WE., Jr The switch process in manic-depressive illness. Circadian variation in time of switch and sleep and manic ratings before and after switch. Acta Psychiatr Scand. 1978 Sep;58((3)):267–78. doi: 10.1111/j.1600-0447.1978.tb06938.x. [DOI] [PubMed] [Google Scholar]
- 32.Bunney W. The switch process in manic-depressive psychosis. Ann Intern Med. 1977 Sep;87((3)):319–35. doi: 10.7326/0003-4819-87-3-319. [DOI] [PubMed] [Google Scholar]
- 33.Bunney WE, Jr, Goodwin FK, Murphy DL, House KM, Gordon EK. The “switch process” in manic-depressive illness. II. Relationship to catecholamines, REM sleep, and drugs. Arch Gen Psychiatry. 1972 Sep;27((3)):304–9. doi: 10.1001/archpsyc.1972.01750270014002. [DOI] [PubMed] [Google Scholar]
- 34.Duffy A, Alda M, Hajek T, Sherry SB, Grof P. Early stages in the development of bipolar disorder. J Affect Disord. 2010 Feb;121((1-2)):127–35. doi: 10.1016/j.jad.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Skjelstad DV, Malt UF, Holte A. Symptoms and signs of the initial prodrome of bipolar disorder: a systematic review. J Affect Disord. 2010 Oct;126((1-2)):1–13. doi: 10.1016/j.jad.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Duffy A. The early course of bipolar disorder in youth at familial risk. J Can Acad Child Adolesc Psychiatry. 2009 Aug;18((3)):200–5. [PMC free article] [PubMed] [Google Scholar]
- 37.Faedda GL, Baldessarini RJ, Glovinsky IP, Austin NB. Pediatric bipolar disorder: phenomenology and course of illness. Bipolar Disord. 2004 Aug;6((4)):305–13. doi: 10.1111/j.1399-5618.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 38.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manic-depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994 Aug;31((4)):281–94. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 39.Egeland JA, Hostetter AM, Pauls DL, Sussex JN. Prodromal symptoms before onset of manic-depressive disorder suggested by first hospital admission histories. J Am Acad Child Adolesc Psychiatry. 2000 Oct;39((10)):1245–52. doi: 10.1097/00004583-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic-depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003 Feb;64((2)):161–74. [PubMed] [Google Scholar]
- 41.Rucklidge JJ. Retrospective parent report of psychiatric histories: do checklists reveal specific prodromal indicators for postpubertal-onset pediatric bipolar disorder? Bipolar Disord. 2008 Feb;10((1)):56–66. doi: 10.1111/j.1399-5618.2008.00533.x. [DOI] [PubMed] [Google Scholar]
- 42.Gruber J, Harvey AG, Wang PW, Brooks JO, 3rd, Thase ME, Sachs GS, et al. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) J Affect Disord. 2009 Apr;114((1-3)):41–9. doi: 10.1016/j.jad.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pacchierotti C, Iapichino S, Bossini L, Pieraccini F, Castrogiovanni P. Melatonin in psychiatric disorders: a review on the melatonin involvement in psychiatry. Front Neuroendocrinol. 2001 Jan;22((1)):18–32. doi: 10.1006/frne.2000.0202. [DOI] [PubMed] [Google Scholar]
- 44.Rock P, Goodwin G, Harmer C, Wulff K. Daily rest-activity patterns in the bipolar phenotype: A controlled actigraphy study. Chronobiol Int. 2014 Mar;31((2)):290–6. doi: 10.3109/07420528.2013.843542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez R, Tamminga CA, Tohen M, Suppes T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014 Apr;75((4)):e317–22. doi: 10.4088/JCP.13m08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benvenuti A, Miniati M, Callari A, Giorgi Mariani M, Mauri M, Dell'Osso L. Mood Spectrum Model: evidence reconsidered in the light of DSM-5. World J Psychiatry. 2015 Mar;5((1)):126–37. doi: 10.5498/wjp.v5.i1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Craddock N, Sklar P. Genetics of bipolar disorder. Lancet. 2013 May;381((9878)):1654–62. doi: 10.1016/S0140-6736(13)60855-7. [DOI] [PubMed] [Google Scholar]
- 48.Hasler G, Drevets WC, Gould TD, Gottesman II, Manji HK. Toward constructing an endophenotype strategy for bipolar disorders. Biol Psychiatry. 2006 Jul;60((2)):93–105. doi: 10.1016/j.biopsych.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends Neurosci. 2005 Mar;28((3)):152–7. doi: 10.1016/j.tins.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci USA. 2013 Jun;110((24)):9950–5. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, Zhan J, Singer JH, Kirkwood A, Zhao H, Berson DM, Hattar S. Light Affects Mood and Learning through Distinct Retina-Brain Pathways. Cell. 2018 Sep;175((1)):71–84.e18. doi: 10.1016/j.cell.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mure LS, Le HD, Benegiamo G, Chang MW, Rios L, Jillani N, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018 Mar;359((6381)):359. doi: 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edery I. Circadian rhythms in a nutshell. Physiol Genomics. 2000 Aug;3((2)):59–74. doi: 10.1152/physiolgenomics.2000.3.2.59. [DOI] [PubMed] [Google Scholar]
- 54.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002 Aug;418((6901)):935–41. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 55.Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000 Mar;14((6)):679–89. [PMC free article] [PubMed] [Google Scholar]
- 56.Goriki A, Hatanaka F, Myung J, Kim JK, Yoritaka T, Tanoue S, et al. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol. 2014 Apr;12((4)):e1001839. doi: 10.1371/journal.pbio.1001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamajuku D, Shibata Y, Kitazawa M, Katakura T, Urata H, Kojima T, et al. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett. 2011 Jul;585((14)):2217–22. doi: 10.1016/j.febslet.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 58.Yoshitane H, Asano Y, Sagami A, Sakai S, Suzuki Y, Okamura H, et al. Functional D-box sequences reset the circadian clock and drive mRNA rhythms. Commun Biol. 2019 Aug;2((1)):300. doi: 10.1038/s42003-019-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007 May;129((3)):605–16. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zool Sci. 2004 Apr;21((4)):359–68. doi: 10.2108/zsj.21.359. [DOI] [PubMed] [Google Scholar]
- 61.Hühne A, Welsh DK, Landgraf D. Prospects for circadian treatment of mood disorders. Ann Med. 2018 Dec;50((8)):637–54. doi: 10.1080/07853890.2018.1530449. [DOI] [PubMed] [Google Scholar]
- 62.Ketchesin KD, Becker-Krail D, McClung CA. Mood-related central and peripheral clocks. Eur J Neurosci. 2020 Jan;51((1)):326–45. doi: 10.1111/ejn.14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reinberg AE, Ashkenazi I, Smolensky MH. Euchronism, allochronism, and dyschronism: is internal desynchronization of human circadian rhythms a sign of illness? Chronobiol Int. 2007;24((4)):553–88. doi: 10.1080/07420520701534624. [DOI] [PubMed] [Google Scholar]
- 64.Hsu PK, Ptáček LJ, Fu YH. Genetics of human sleep behavioral phenotypes. Methods Enzymol. 2015;552:309–24. doi: 10.1016/bs.mie.2014.10.046. [DOI] [PubMed] [Google Scholar]
- 65.Stewart J, Bachman G, Cooper C, Liu L, Ancoli-Israel S, Alibiglou L. Circadian dysfunction and fluctuations in gait initiation impairment in Parkinson's disease. Exp Brain Res. 2018 Mar;236((3)):655–64. doi: 10.1007/s00221-017-5163-5. [DOI] [PubMed] [Google Scholar]
- 66.Sobolewska-Włodarczyk A, Włodarczyk M, Szemraj J, Stec-Michalska K, Fichna J, Wiśniewska-Jarosińska M. Circadian rhythm abnormalities - Association with the course of inflammatory bowel disease. Pharmacol Rep. 2016 Aug;68((4)):847–51. doi: 10.1016/j.pharep.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Vargas PA, Flores M, Robles E. Sleep quality and body mass index in college students: the role of sleep disturbances. J Am Coll Health. 2014;62((8)):534–41. doi: 10.1080/07448481.2014.933344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colten HR, Altevogt BM editors, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): 2006. [PubMed] [Google Scholar]
- 69.Eyre H, Baune BT. Neuroimmunomodulation in unipolar depression: a focus on chronobiology and chronotherapeutics. J Neural Transm (Vienna) 2012 Oct;119((10)):1147–66. doi: 10.1007/s00702-012-0819-6. [DOI] [PubMed] [Google Scholar]
- 70.Cho CH, Lee T, Kim MG, In HP, Kim L, Lee HJ. Mood Prediction of Patients With Mood Disorders by Machine Learning Using Passive Digital Phenotypes Based on the Circadian Rhythm: Prospective Observational Cohort Study. J Med Internet Res. 2019 Apr;21((4)):e11029. doi: 10.2196/11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cox RC, Tuck B, Olatunji BO. The role of eveningness in obsessive-compulsive symptoms: cross-sectional and prospective approaches. J Affect Disord. 2018 Aug;235:448–55. doi: 10.1016/j.jad.2018.04.060. [DOI] [PubMed] [Google Scholar]
- 72.Smagula SF, Boudreau RM, Stone K, Reynolds CF, 3rd, Bromberger JT, Ancoli-Israel S, et al. Osteoporotic Fractures in Men (MrOS) Research Group Latent activity rhythm disturbance sub-groups and longitudinal change in depression symptoms among older men. Chronobiol Int. 2015;32((10)):1427–37. doi: 10.3109/07420528.2015.1102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boland EM, Ross RJ. Recent Advances in the Study of Sleep in the Anxiety Disorders, Obsessive-Compulsive Disorder, and Posttraumatic Stress Disorder. Psychiatr Clin North Am. 2015 Dec;38((4)):761–76. doi: 10.1016/j.psc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci. 2013;119:283–323. doi: 10.1016/B978-0-12-396971-2.00010-5. [DOI] [PubMed] [Google Scholar]
- 75.Shea SA, Hilton MF, Hu K, Scheer FA. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res. 2011 Apr;108((8)):980–4. doi: 10.1161/CIRCRESAHA.110.233668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009 Mar;106((11)):4453–8. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burke TM, Scheer FA, Ronda JM, Czeisler CA, Wright KP., Jr Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J Sleep Res. 2015 Aug;24((4)):364–71. doi: 10.1111/jsr.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Georgas K, Rumballe B, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, et al. Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol. 2009 Aug;332((2)):273–86. doi: 10.1016/j.ydbio.2009.05.578. [DOI] [PubMed] [Google Scholar]
- 79.Reinberg A, Ashkenazi I. Concepts in human biological rhythms. Dialogues Clin Neurosci. 2003 Dec;5((4)):327–42. doi: 10.31887/DCNS.2003.5.4/areinberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reinberg AE, Bicakova-Rocher A, Gorceix A, Ashkenazi IE, Smolensky MH. Placebo effect on the circadian rhythm period tau of temperature and hand-grip strength rhythms: interindividual and gender-related difference. Chronobiol Int. 1994 Feb;11((1)):45–53. doi: 10.3109/07420529409057230. [DOI] [PubMed] [Google Scholar]
- 81.Kim KL, Weissman AB, Puzia ME, Cushman GK, Seymour KE, Wegbreit E, et al. Circadian Phase Preference in Pediatric Bipolar Disorder. J Clin Med. 2014 Mar;3((1)):255–66. doi: 10.3390/jcm3010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mansour HA, Wood J, Chowdari KV, Dayal M, Thase ME, Kupfer DJ, et al. Circadian phase variation in bipolar I disorder. Chronobiol Int. 2005;22((3)):571–84. doi: 10.1081/CBI-200062413. [DOI] [PubMed] [Google Scholar]
- 83.Carr O, Saunders KE, Tsanas A, Bilderbeck AC, Palmius N, Geddes JR, et al. Variability in phase and amplitude of diurnal rhythms is related to variation of mood in bipolar and borderline personality disorder. Sci Rep. 2018 Jan;8((1)):1649. doi: 10.1038/s41598-018-19888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nudell V, Wei H, Nievergelt C, Maihofer AX, Shilling P, Alda M, et al. Entrainment of Circadian Rhythms to Temperature Reveals Amplitude Deficits in Fibroblasts from Patients with Bipolar Disorder and Possible Links to Calcium Channels. Mol Neuropsychiatry. 2019 Apr;5((2)):115–24. doi: 10.1159/000497354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McGowan NM, Goodwin GM, Bilderbeck AC, Saunders KE. Actigraphic patterns, impulsivity and mood instability in bipolar disorder, borderline personality disorder and healthy controls. Acta Psychiatr Scand. 2020 Jan;:acps.13148. doi: 10.1111/acps.13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Banks FD, Lobban F, Fanshawe TR, Jones SH. Associations between circadian rhythm instability, appraisal style and mood in bipolar disorder. J Affect Disord. 2016 Oct;203:166–75. doi: 10.1016/j.jad.2016.05.075. [DOI] [PubMed] [Google Scholar]
- 87.Sylvia LG, Chang WC, Kamali M, Tohen M, Kinrys G, Deckersbach T, et al. Sleep disturbance may impact treatment outcome in bipolar disorder: A preliminary investigation in the context of a large comparative effectiveness trial. J Affect Disord. 2018 Jan;225:563–8. doi: 10.1016/j.jad.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 88.Bradley AJ, Webb-Mitchell R, Hazu A, Slater N, Middleton B, Gallagher P, et al. Sleep and circadian rhythm disturbance in bipolar disorder. Psychol Med. 2017 Jul;47((9)):1678–89. doi: 10.1017/S0033291717000186. [DOI] [PubMed] [Google Scholar]
- 89.Van Dongen HP. In: Molecular Biology of Circadian Rhythms. Sehgal A editor, editor. New York (NY): John Wiley & Sons; 2004. pp. pp. 255–69. [Google Scholar]
- 90.Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–90. doi: 10.1016/B978-0-12-396971-2.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Juda M, Vetter C, Roenneberg T. Chronotype modulates sleep duration, sleep quality, and social jet lag in shift-workers. J Biol Rhythms. 2013 Apr;28((2)):141–51. doi: 10.1177/0748730412475042. [DOI] [PubMed] [Google Scholar]
- 92.Mongrain V, Carrier J, Dumont M. Chronotype and sex effects on sleep architecture and quantitative sleep EEG in healthy young adults. Sleep. 2005 Jul;28((7)):819–27. doi: 10.1093/sleep/28.7.819. [DOI] [PubMed] [Google Scholar]
- 93.Mongrain V, Carrier J, Dumont M. Difference in sleep regulation between morning and evening circadian types as indexed by antero-posterior analyses of the sleep EEG. Eur J Neurosci. 2006 Jan;23((2)):497–504. doi: 10.1111/j.1460-9568.2005.04561.x. [DOI] [PubMed] [Google Scholar]
- 94.Mongrain V, Carrier J, Paquet J, Bélanger-Nelson E, Dumont M. Morning and evening-type differences in slow waves during NREM sleep reveal both trait and state-dependent phenotypes. PLoS One. 2011;6((8)):e22679. doi: 10.1371/journal.pone.0022679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, et al. A marker for the end of adolescence. Curr Biol. 2004 Dec;14((24)):R1038–9. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 96.Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007 Dec;11((6)):429–38. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 97.Mongrain V, Noujaim J, Blais H, Dumont M. Daytime vigilance in chronotypes: diurnal variations and effects of behavioral sleep fragmentation. Behav Brain Res. 2008 Jun;190((1)):105–11. doi: 10.1016/j.bbr.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 98.Mongrain V, Dumont M. Increased homeostatic response to behavioral sleep fragmentation in morning types compared to evening types. Sleep. 2007 Jun;30((6)):773–80. doi: 10.1093/sleep/30.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taillard J, Philip P, Claustrat B, Capelli A, Coste O, Chaumet G, et al. Time course of neurobehavioral alertness during extended wakefulness in morning- and evening-type healthy sleepers. Chronobiol Int. 2011 Jul;28((6)):520–7. doi: 10.3109/07420528.2011.590623. [DOI] [PubMed] [Google Scholar]
- 100.Killgore WD. Effects of sleep deprivation and morningness-eveningness traits on risk-taking. Psychol Rep. 2007 Apr;100((2)):613–26. doi: 10.2466/pr0.100.2.613-626. [DOI] [PubMed] [Google Scholar]
- 101.Kerkhof GA, Van Dongen HP. Morning-type and evening-type individuals differ in the phase position of their endogenous circadian oscillator. Neurosci Lett. 1996 Nov;218((3)):153–6. doi: 10.1016/s0304-3940(96)13140-2. [DOI] [PubMed] [Google Scholar]
- 102.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000 Jun;9((2)):117–27. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 103.Barclay NL, Gregory AM. Quantitative genetic research on sleep: a review of normal sleep, sleep disturbances and associated emotional, behavioural, and health-related difficulties. Sleep Med Rev. 2013 Feb;17((1)):29–40. doi: 10.1016/j.smrv.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 104.Hur YM. Stability of genetic influence on morningness-eveningness: a cross-sectional examination of South Korean twins from preadolescence to young adulthood. J Sleep Res. 2007 Mar;16((1)):17–23. doi: 10.1111/j.1365-2869.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 105.Vink JM, Groot AS, Kerkhof GA, Boomsma DI. Genetic analysis of morningness and eveningness. Chronobiol Int. 2001 Sep;18((5)):809–22. doi: 10.1081/cbi-100107516. [DOI] [PubMed] [Google Scholar]
- 106.Barclay NL, Eley TC, Parsons MJ, Willis TA, Gregory AM. Monozygotic twin differences in non-shared environmental factors associated with chronotype. J Biol Rhythms. 2013 Feb;28((1)):51–61. doi: 10.1177/0748730412468698. [DOI] [PubMed] [Google Scholar]
- 107.Klei L, Reitz P, Miller M, Wood J, Maendel S, Gross D, et al. Heritability of morningness-eveningness and self-report sleep measures in a family-based sample of 521 hutterites. Chronobiol Int. 2005;22((6)):1041–54. doi: 10.1080/07420520500397959. [DOI] [PubMed] [Google Scholar]
- 108.Jones SE, Tyrrell J, Wood AR, Beaumont RN, Ruth KS, Tuke MA, et al. Genome-Wide Association Analyses in 128,266 Individuals Identifies New Morningness and Sleep Duration Loci. PLoS Genet. 2016 Aug;12((8)):e1006125. doi: 10.1371/journal.pgen.1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seleem MA, Merranko JA, Goldstein TR, Goldstein BI, Axelson DA, Brent DA, et al. The longitudinal course of sleep timing and circadian preferences in adults with bipolar disorder. Bipolar Disord. 2015 Jun;17((4)):392–402. doi: 10.1111/bdi.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boudebesse C, Lajnef M, Geoffroy PA, Bellivier F, Nieto I, Gard S, et al. French Academic Centres of Expertise for Bipolar Disorders (FACE-BD) Collaborators Chronotypes of bipolar patients in remission: validation of the French version of the circadian type inventory in the FACE-BD sample. Chronobiol Int. 2013 Oct;30((8)):1042–9. doi: 10.3109/07420528.2013.798330. [DOI] [PubMed] [Google Scholar]
- 111.Ahn YM, Chang J, Joo YH, Kim SC, Lee KY, Kim YS. Chronotype distribution in bipolar I disorder and schizophrenia in a Korean sample. Bipolar Disord. 2008 Mar;10((2)):271–5. doi: 10.1111/j.1399-5618.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 112.Baek JH, Kim JS, Kim MJ, Ryu S, Lee K, Ha K, et al. Lifetime Characteristics of Evening-Preference and Irregular Bed-Rise Time Are Associated With Lifetime Seasonal Variation of Mood and Behavior: Comparison Between Individuals With Bipolar Disorder and Healthy Controls. Behav Sleep Med. 2016;14((2)):155–68. doi: 10.1080/15402002.2014.974179. [DOI] [PubMed] [Google Scholar]
- 113.Giglio LM, Magalhães PV, Andersen ML, Walz JC, Jakobson L, Kapczinski F. Circadian preference in bipolar disorder. Sleep Breath. 2010 Jun;14((2)):153–5. doi: 10.1007/s11325-009-0301-3. [DOI] [PubMed] [Google Scholar]
- 114.Subramanian K, Sarkar S, Kattimani S. Bipolar disorder in Asia: illness course and contributing factors. Asian J Psychiatr. 2017 Oct;29:16–29. doi: 10.1016/j.ajp.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 115.McCarthy MJ, Wei H, Nievergelt CM, Stautland A, Maihofer AX, Welsh DK, et al. Chronotype and cellular circadian rhythms predict the clinical response to lithium maintenance treatment in patients with bipolar disorder. Neuropsychopharmacology. 2019 Feb;44((3)):620–8. doi: 10.1038/s41386-018-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kudielka BM, Federenko IS, Hellhammer DH, Wüst S. Morningness and eveningness: the free cortisol rise after awakening in “early birds” and “night owls”. Biol Psychol. 2006 May;72((2)):141–6. doi: 10.1016/j.biopsycho.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 117.Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness-eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001 Aug;115((4)):895–9. doi: 10.1037//0735-7044.115.4.895. [DOI] [PubMed] [Google Scholar]
- 118.Bailey SL, Heitkemper MM. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol Int. 2001 Mar;18((2)):249–61. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- 119.Akerstedt T, Fröberg JE. Interindividual differences in circadian patterns of catecholamine excretion, body temperature, performance, and subjective arousal. Biol Psychol. 1976 Dec;4((4)):277–92. doi: 10.1016/0301-0511(76)90019-3. [DOI] [PubMed] [Google Scholar]
- 120.Carrier J, Monk TH, Buysse DJ, Kupfer DJ. Sleep and morningness-eveningness in the ‘middle’ years of life (20-59 y) J Sleep Res. 1997 Dec;6((4)):230–7. doi: 10.1111/j.1365-2869.1997.00230.x. [DOI] [PubMed] [Google Scholar]
- 121.Ishihara K, Miyasita A, Inugami M, Fukuda K, Miyata Y. Differences in sleep-wake habits and EEG sleep variables between active morning and evening subjects. Sleep. 1987 Aug;10((4)):330–42. doi: 10.1093/sleep/10.4.330. [DOI] [PubMed] [Google Scholar]
- 122.Rosenthal L, Day R, Gerhardstein R, Meixner R, Roth T, Guido P, et al. Sleepiness/alertness among healthy evening and morning type individuals. Sleep Med. 2001 May;2((3)):243–8. doi: 10.1016/s1389-9457(00)00047-2. [DOI] [PubMed] [Google Scholar]
- 123.Adan A, Guàrdia J. Circadian variations of self-reported activation: a multidimensional approach. Chronobiologia. 1993 Jul-Dec;20((3-4)):233–44. [PubMed] [Google Scholar]
- 124.Kanagarajan K, Gou K, Antinora C, Buyukkurt A, Crescenzi O, Beaulieu S, et al. Morningness-Eveningness questionnaire in bipolar disorder. Psychiatry Res. 2018 Apr;262:102–7. doi: 10.1016/j.psychres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 125.Soreca I, Fagiolini A, Frank E, Goodpaster BH, Kupfer DJ. Chronotype and body composition in bipolar disorder. Chronobiol Int. 2009 May;26((4)):780–8. doi: 10.1080/07420520902929060. [DOI] [PubMed] [Google Scholar]
- 126.Melo MC, Garcia RF, Araújo CF, Luz JH, Bruin PF, Bruin VM. Chronotype in bipolar disorder: an 18-month prospective study. Br J Psychiatry. 2020 Jan-Feb;42((1)):68–71. doi: 10.1590/1516-4446-2019-0489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ozdogan MG, Aydin EF, Ustundag MF, Ceyhun HA, Oral E, Bakan E. Homocysteine, chronotype and clinical course in bipolar disorder patients. Nord J Psychiatry. 2020 Jan;•••:1–6. doi: 10.1080/08039488.2019.1710250. [DOI] [PubMed] [Google Scholar]
- 128.Godin O, Henry C, Leboyer M, Azorin JM, Aubin V, Bellivier F, et al. Sleep quality, chronotype and metabolic syndrome components in bipolar disorders during the remission period: results from the FACE-BD cohort. Chronobiol Int. 2017;34((8)):1114–24. doi: 10.1080/07420528.2017.1332071. [DOI] [PubMed] [Google Scholar]
- 129.Dallaspezia S, Suzuki M, Clara L, Colombo C, Benedetti F. Chronotype influences response to antidepressant chronotherapeutics in bipolar patients. Chronobiol Int. 2018 Sep;35((9)):1319–25. doi: 10.1080/07420528.2018.1469034. [DOI] [PubMed] [Google Scholar]
- 130.Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. eQTLGen Consortium BIOS Consortium Bipolar Disorder Working Group of the Psychiatric Genomics Consortium Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019 May;51((5)):793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jones SE, Lane JM, Wood AR, van Hees VT, Tyrrell J, Beaumont RN, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019 Jan;10((1)):343. doi: 10.1038/s41467-018-08259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jansen PR, Watanabe K, Stringer S, Skene N, Bryois J, Hammerschlag AR, et al. 23andMe Research Team Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019 Mar;51((3)):394–403. doi: 10.1038/s41588-018-0333-3. [DOI] [PubMed] [Google Scholar]
- 133.Lane JM, Liang J, Vlasac I, Anderson SG, Bechtold DA, Bowden J, et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet. 2017 Feb;49((2)):274–81. doi: 10.1038/ng.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lane JM, Jones SE, Dashti HS, Wood AR, Aragam KG, van Hees VT, et al. HUNT All In Sleep Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019 Mar;51((3)):387–93. doi: 10.1038/s41588-019-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lane JM, Vlasac I, Anderson SG, Kyle SD, Dixon WG, Bechtold DA, et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat Commun. 2016 Mar;7((1)):10889. doi: 10.1038/ncomms10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun. 2016 Feb;7((1)):10448. doi: 10.1038/ncomms10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Avramopoulos D, Pearce BD, McGrath J, Wolyniec P, Wang R, Eckart N, et al. Infection and inflammation in schizophrenia and bipolar disorder: a genome wide study for interactions with genetic variation. PLoS One. 2015 Mar;10((3)):e0116696. doi: 10.1371/journal.pone.0116696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Buniello A, MacArthur JA, Cerezo M, Harris LW, Hayhurst J, Malangone C, et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 2019 Jan;47(D1):D1005–12. doi: 10.1093/nar/gky1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McCarthy MJ. Missing a beat: assessment of circadian rhythm abnormalities in bipolar disorder in the genomic era. Psychiatr Genet. 2019 Apr;29((2)):29–36. doi: 10.1097/YPG.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 140.Anafi RC, Lee Y, Sato TK, Venkataraman A, Ramanathan C, Kavakli IH, et al. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014 Apr;12((4)):e1001840. doi: 10.1371/journal.pbio.1001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics Consortium Electronic address drve, Bipolar D, Schizophrenia Working Group of the Psychiatric Genomics C: Genomic Dissection of Bipolar Disorder and Schizophrenia, Including 28 Subphenotypes. Cell. 2018 Jun;173((7)):1705–15.e16. doi: 10.1016/j.cell.2018.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang L, Ptáček LJ, Fu YH. Diversity of human clock genotypes and consequences. Prog Mol Biol Transl Sci. 2013;119:51–81. doi: 10.1016/B978-0-12-396971-2.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ferguson A, Lyall LM, Ward J, Strawbridge RJ, Cullen B, Graham N, et al. Genome-Wide Association Study of Circadian Rhythmicity in 71,500 UK Biobank Participants and Polygenic Association with Mood Instability. EBioMedicine. 2018 Sep;35:279–87. doi: 10.1016/j.ebiom.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hammerschlag AR, Stringer S, de Leeuw CA, Sniekers S, Taskesen E, Watanabe K, et al. Genome-wide association analysis of insomnia complaints identifies risk genes and genetic overlap with psychiatric and metabolic traits. Nat Genet. 2017 Nov;49((11)):1584–92. doi: 10.1038/ng.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Doherty A, Smith-Byrne K, Ferreira T, Holmes MV, Holmes C, Pulit SL, et al. GWAS identifies 14 loci for device-measured physical activity and sleep duration. Nat Commun. 2018 Dec;9((1)):5257. doi: 10.1038/s41467-018-07743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018 Jul;50((7)):912–9. doi: 10.1038/s41588-018-0152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hill WD, Marioni RE, Maghzian O, Ritchie SJ, Hagenaars SP, McIntosh AM, et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol Psychiatry. 2019 Feb;24((2)):169–81. doi: 10.1038/s41380-017-0001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coleman JR, Bryois J, Gaspar HA, Jansen PR, Savage JE, Skene N, et al. Biological annotation of genetic loci associated with intelligence in a meta-analysis of 87,740 individuals. Mol Psychiatry. 2019 Feb;24((2)):182–97. doi: 10.1038/s41380-018-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JR, Krapohl E, et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet. 2017 Jul;49((7)):1107–12. doi: 10.1038/ng.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. 23andMe Research Team COGENT (Cognitive Genomics Consortium) Social Science Genetic Association Consortium Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018 Jul;50((8)):1112–21. doi: 10.1038/s41588-018-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun. 2018 May;9((1)):2098. doi: 10.1038/s41467-018-04362-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lam M, Trampush JW, Yu J, Knowles E, Davies G, Liewald DC, et al. Large-Scale Cognitive GWAS Meta-Analysis Reveals Tissue-Specific Neural Expression and Potential Nootropic Drug Targets. Cell Rep. 2017 Nov;21((9)):2597–613. doi: 10.1016/j.celrep.2017.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Smeland OB, Bahrami S, Frei O, Shadrin A, O'Connell K, Savage J, et al. Genome-wide analysis reveals extensive genetic overlap between schizophrenia, bipolar disorder, and intelligence. Mol Psychiatry. 2019 doi: 10.1038/s41380-018-0332-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Porter RJ, Inder M, Douglas KM, Moor S, Carter JD, Frampton CM, et al. Improvement in cognitive function in young people with bipolar disorder: results from participants in an 18-month randomised controlled trial of adjunctive psychotherapy. Aust N Z J Psychiatry. 2019 Nov;•••:4867419887794. doi: 10.1177/0004867419887794. [DOI] [PubMed] [Google Scholar]
- 155.Castaño Ramírez OM, Gómez Montoya SM, Lemos Buitrago R, Valderrama Sánchez A, Castro Navarro JC. Relationship Between Cognitive Function and Clinical Features in Patients With Bipolar I Disorder. Rev Colomb Psiquiatr. 2018 Oct-Dec;47((4)):204–10. doi: 10.1016/j.rcp.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 156.Deng M, Pan Y, Zhou L, Chen X, Liu C, Huang X, et al. Resilience and Cognitive Function in Patients With Schizophrenia and Bipolar Disorder, and Healthy Controls. Front Psychiatry. 2018 Jun;9:279. doi: 10.3389/fpsyt.2018.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vreeker A, Abramovic L, Boks MP, Verkooijen S, van Bergen AH, Ophoff RA, et al. The relationship between brain volumes and intelligence in bipolar disorder. J Affect Disord. 2017 Dec;223:59–64. doi: 10.1016/j.jad.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brown SA, Fleury-Olela F, Nagoshi E, Hauser C, Juge C, Meier CA, et al. The period length of fibroblast circadian gene expression varies widely among human individuals. PLoS Biol. 2005 Oct;3((10)):e338. doi: 10.1371/journal.pbio.0030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A, et al. Molecular insights into human daily behavior. Proc Natl Acad Sci USA. 2008 Feb;105((5)):1602–7. doi: 10.1073/pnas.0707772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hida A, Kitamura S, Ohsawa Y, Enomoto M, Katayose Y, Motomura Y, et al. In vitro circadian period is associated with circadian/sleep preference. Sci Rep. 2013;3((1)):2074. doi: 10.1038/srep02074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang S, Van Dongen HP, Wang K, Berrettini W, Bućan M. Assessment of circadian function in fibroblasts of patients with bipolar disorder. Mol Psychiatry. 2009 Feb;14((2)):143–55. doi: 10.1038/mp.2008.10. [DOI] [PubMed] [Google Scholar]
- 162.McCarthy MJ, Wei H, Marnoy Z, Darvish RM, McPhie DL, Cohen BM, et al. Genetic and clinical factors predict lithium's effects on PER2 gene expression rhythms in cells from bipolar disorder patients. Transl Psychiatry. 2013 Oct;3((10)):e318. doi: 10.1038/tp.2013.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Borbély AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1((3)):195–204. [PubMed] [Google Scholar]
- 164.American Academy of Sleep Medicine . International classification of sleep disorders. ed 3. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 165.Takaesu Y, Inoue Y, Murakoshi A, Komada Y, Otsuka A, Futenma K, et al. Prevalence of Circadian Rhythm Sleep-Wake Disorders and Associated Factors in Euthymic Patients with Bipolar Disorder. PLoS One. 2016 Jul;11((7)):e0159578. doi: 10.1371/journal.pone.0159578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takaesu Y, Inoue Y, Ono K, Murakoshi A, Futenma K, Komada Y, et al. Circadian rhythm sleep-wake disorders as predictors for bipolar disorder in patients with remitted mood disorders. J Affect Disord. 2017 Oct;220:57–61. doi: 10.1016/j.jad.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 167.Takaesu Y, Inoue Y, Ono K, Murakoshi A, Futenma K, Komada Y, et al. Circadian Rhythm Sleep-Wake Disorders Predict Shorter Time to Relapse of Mood Episodes in Euthymic Patients With Bipolar Disorder: A Prospective 48-Week Study. J Clin Psychiatry. 2018 Jan-Feb;79((1)):79. doi: 10.4088/JCP.17m11565. [DOI] [PubMed] [Google Scholar]
- 168.Scott J, Naismith S, Grierson A, Carpenter J, Hermens D, Scott E, et al. Sleep-wake cycle phenotypes in young people with familial and non-familial mood disorders. Bipolar Disord. 2016 Dec;18((8)):642–9. doi: 10.1111/bdi.12450. [DOI] [PubMed] [Google Scholar]
- 169.Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter-episode bipolar disorder. J Behav Ther Exp Psychiatry. 2010 Jun;41((2)):145–9. doi: 10.1016/j.jbtep.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tournier BB, Dardente H, Simonneaux V, Vivien-Roels B, Pévet P, Masson-Pévet M, et al. Seasonal variations of clock gene expression in the suprachiasmatic nuclei and pars tuberalis of the European hamster (Cricetus cricetus) Eur J Neurosci. 2007 Mar;25((5)):1529–36. doi: 10.1111/j.1460-9568.2007.05421.x. [DOI] [PubMed] [Google Scholar]
- 171.Kripke DF, Elliott JA, Welsh DK, Youngstedt SD. Photoperiodic and circadian bifurcation theories of depression and mania. F1000 Res. 2015 May;4:107. doi: 10.12688/f1000research.6444.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cassidy F, Carroll BJ. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disord. 2002 Feb;68((1)):25–31. doi: 10.1016/s0165-0327(00)00325-6. [DOI] [PubMed] [Google Scholar]
- 173.Silverstone T, Romans S, Hunt N, McPherson H. Is there a seasonal pattern of relapse in bipolar affective disorders? A dual northern and southern hemisphere cohort study. Br J Psychiatry. 1995 Jul;167((1)):58–60. doi: 10.1192/bjp.167.1.58. [DOI] [PubMed] [Google Scholar]
- 174.Shin K, Schaffer A, Levitt AJ, Boyle MH. Seasonality in a community sample of bipolar, unipolar and control subjects. J Affect Disord. 2005 May;86((1)):19–25. doi: 10.1016/j.jad.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 175.Thompson C, Stinson D, Fernandez M, Fine J, Isaacs G. A comparison of normal, bipolar and seasonal affective disorder subjects using the Seasonal Pattern Assessment Questionnaire. J Affect Disord. 1988 May-Jun;14((3)):257–64. doi: 10.1016/0165-0327(88)90043-2. [DOI] [PubMed] [Google Scholar]
- 176.Hakkarainen R, Johansson C, Kieseppä T, Partonen T, Koskenvuo M, Kaprio J, et al. Seasonal changes, sleep length and circadian preference among twins with bipolar disorder. BMC Psychiatry. 2003 Jun;3((1)):6. doi: 10.1186/1471-244X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Alda M. The phenotypic spectra of bipolar disorder. Eur Neuropsychopharmacol. 2004 May;14(Suppl 2):S94–9. doi: 10.1016/j.euroneuro.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 178.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999 Jun;284((5423)):2177–81. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 179.Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS One. 2009 Jun;4((6)):e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hasan S, Santhi N, Lazar AS, Slak A, Lo J, von Schantz M, et al. Assessment of circadian rhythms in humans: comparison of real-time fibroblast reporter imaging with plasma melatonin. FASEB J. 2012 Jun;26((6)):2414–23. doi: 10.1096/fj.11-201699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008 Aug;3((8)):e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Souêtre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989 Jun;28((3)):263–78. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 183.Reinberg A, Andlauer P, De Prins J, Malbecq W, Vieux N, Bourdeleau P. Desynchronization of the oral temperature circadian rhythm and intolerance to shift work. Nature. 1984 Mar;308((5956)):272–4. doi: 10.1038/308272a0. [DOI] [PubMed] [Google Scholar]
- 184.Reinberg A, Motohashi Y, Bourdeleau P, Andlauer P, Lévi F, Bicakova-Rocher A. Alteration of period and amplitude of circadian rhythms in shift workers. With special reference to temperature, right and left hand grip strength. Eur J Appl Physiol Occup Physiol. 1988;57((1)):15–25. doi: 10.1007/BF00691232. [DOI] [PubMed] [Google Scholar]
- 185.Reinberg A, Motohashi Y, Bourdeleau P, Touitou Y, Nouguier J, Nouguier J, et al. Internal desynchronization of circadian rhythms and tolerance of shift work. Chronobiologia. 1989 Jan-Mar;16((1)):21–34. [PubMed] [Google Scholar]
- 186.Motohashi Y. Alteration of circadian rhythm in shift-working ambulance personnel. Monitoring of salivary cortisol rhythm. Ergonomics. 1992 Nov;35((11)):1331–40. doi: 10.1080/00140139208967396. [DOI] [PubMed] [Google Scholar]
- 187.Reinberg A, Andlauer P, Guillet P, Nicolai A, Vieux N, Laporte A. Oral temperature, circadian rhythm amplitude, ageing and tolerance to shift-work. Ergonomics. 1980 Jan;23((1)):55–64. doi: 10.1080/00140138008924718. [DOI] [PubMed] [Google Scholar]
- 188.Andlauer P, Reinberg A, Fourré L, Battle W, Duverneuil G. Amplitude of the oral temperature circadian rhythm and the tolerance to shift-work. J Physiol (Paris) 1979;75((5)):507–12. [PubMed] [Google Scholar]
- 189.Pagani L, St Clair PA, Teshiba TM, Service SK, Fears SC, Araya C, et al. Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc Natl Acad Sci USA. 2016 Feb;113((6)):E754–61. doi: 10.1073/pnas.1513525113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.First MB, Pincus HA, Levine JB, Williams JB, Ustun B, Peele R. Clinical utility as a criterion for revising psychiatric diagnoses. Am J Psychiatry. 2004 Jun;161((6)):946–54. doi: 10.1176/appi.ajp.161.6.946. [DOI] [PubMed] [Google Scholar]