Abstract
Background/Aim
Uniform treatment of hepatocellular carcinoma (HCC) with molecular targeted drugs (e.g., sorafenib) results in a poor overall tumor response when tumor subtyping is absent. Patient stratification based on actionable gene expression is a method that can potentially improve the effectiveness of these drugs. Here we aimed to identify the clinical application of actionable genes in predicting response to sorafenib.
Methods
Through quantitative real-time reverse transcription PCR, we analyzed the expression levels of seven actionable genes (VEGFR2, PDGFRB, c-KIT, c-RAF, EGFR, mTOR, and FGFR1) in tumors versus noncancerous tissues from 220 HCC patients treated with sorafenib. Our analysis found that 9 responders did not have unique clinical features compared to nonresponders. A receiver operating characteristic curve evaluated the predictive performance of the treatment benefit score (TBS) calculated from the actionable genes.
Results
The responders had significantly higher TBS values than the nonresponders. With an area under the curve of 0.779, a TBS combining mTOR with VEGFR2, c-KIT, and c-RAF was the most significant predictor of response to sorafenib. When used alone, sorafenib had a 0.7–3% response rate among HCC patients, but when stratifying the patients with actionable genes, the tumor response rate rose to 15.6%. Furthermore, actionable gene expression is significantly correlated with tumor response.
Conclusions
Our findings on patient stratification based on actionable molecular subtyping potentially provide a therapeutic strategy for improving sorafenib's effectiveness in treating HCC.
Keywords: Sorafenib, Biomarker, Gene signature, Hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is a lethal malignancy that is notoriously resistant to chemotherapy [1], leading to a search for alternative treatment strategies. Among these, molecular targeted drugs are designed to antagonize “oncogenic-actionable genes” that are specifically altered in tumors [2]. Actionable molecules have been successfully applied to patient stratification in various cancers [3]. Lung cancer can be classified into 18 subtypes based on specific genetic aberrations (e.g., overexpression and mutations) [4], while melanoma has 11 subtypes associated with targeted treatment [5]. Unfortunately, the highly heterogeneous HCC lacks molecular predictors of treatment response. Although many molecular targeted agents have been tested in HCC, only sorafenib and lenvatinib have been approved as first-line agents for advanced stages of this cancer [6, 7, 8]. The lack of a second-line option after lenvatinib [9], however, means that sorafenib is the recommended standard treatment for advanced HCC [9], despite its poor therapeutic response (0.7–3%) [6, 7]. New therapeutic strategies are necessary to improve sorafenib's efficacy in treating HCC.
Currently, there is no clinically applicable biomarker for predicting HCC response to sorafenib [10], although VEGFA [11] and FGF3/4 [12] are more frequently amplified in sorafenib responders. Previously, we investigated the mRNA expression of five actionable genes (VEGFR2, PDGFRB, EGFR, mTOR, and FGFR1) in HCC tumors and surrounding noncancerous tissues, and then used the results to stratify HCC patients. Additionally, we found evidence suggesting that c-RAF and PDGFRB expression could predict sorafenib susceptibility [13].
In this study, we aimed to confirm whether actionable gene expression can predict response to sorafenib in HCC tissues, and if so, identify those with the best diagnostic performance. We compared the mRNA expression of seven actionable genes in responder and nonresponder tumor tissues from 220 HCC patients treated with sorafenib. We then calculated and compared treatment benefit scores (TBS) from relative mRNA levels per gene.
Subjects and Methods
Patients and Tissue Samples
Patients with histologically confirmed HCC were enrolled if they met the following criteria: (1) age ≥20 years; (2) diagnosed with unresectable advanced HCC; (3) Eastern Cooperative Oncology Group performance status ≤2; (4) Child-Pugh class A; and (5) receiving sorafenib as a palliative first-line systemic treatment. Individuals were excluded if they required combination therapy (including chemotherapy, radiotherapy, hepatic arterial chemoembolization, and radiofrequency ablation) or possessed severe, uncontrolled medical conditions. In total, 390 patients were enrolled from 7 medical institutions; all provided written informed consent. Sorafenib treatment occurred during 2014–2018. Inoperable patients were subjected to ultrasound-guided needle biopsy before sorafenib treatment. For patients experiencing recurrence within 3 months after surgical resection with curative intent, needle biopsy was omitted. Instead, tumor tissues frozen at the time of resection were used for analysis.
Complete clinical information was available for all cases. Radiologic analyses (computed tomography [CT] and magnetic resonance imaging [MRI]) evaluated tumor response to sorafenib, following the modified Response Evaluation Criteria in Solid Tumors for HCC [14]. Immediately after needle biopsy, HCC tissue samples were snap-frozen in liquid nitrogen and stored at −80°C. Patient staging information was obtained from CT or MR images, and conventional TNM (Tumor, Node, and Metastasis) classification (American Joint Committee on Cancer, 7th edition), along with BCLC (Barcelona Clinic Liver Cancer) staging, was used.
Measurement of Clinical Outcomes
The primary endpoint was tumor response to sorafenib, assessed 3 and 6 months after drug administration.
RNA Extraction and cDNA Synthesis
Published methods were used for RNA extraction and cDNA synthesis [13]. Total RNA was extracted from both tumor and surrounding noncancerous frozen tissues using the RNeasy Mini Kit (Qiagen, Hilden, Germany) with DNase I treatment (Qiagen). Total RNA integrity was verified using a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). Next, 4 μg of RNA was incubated with 2 μL of 10 μM oligo(dT)18 primer (GenoTech, Daejeon, South Korea) at 70°C for 7 min, before being cooled on ice for 5 min. Reverse transcriptase enzyme mix was added to the annealed total RNA sample, and the reaction was incubated for 90 min at 42°C. Reverse transcriptase was then heat-inactivated at 80°C for 10 min. Diethylpyrocarbonate-treated water was added to bring the final volume of the cDNA samples to 400 μL.
Quantitative Real-Time PCR
Quantitative real-time PCR was performed as described previously [13], using an ABI PRISM 7900HT instrument (Applied Biosystems, Foster City, CA, USA). The total reaction volume was 10 μL. The thermocycling conditions were as follows: 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. The primer and probe sequences were designed in Primer Express 3.0 (Applied Biosystems); all probes were labeled with TAMRA at the 3′ end and FAM at the 5′ end. The target genes were mTOR, VEGFR2, PDGFRB, FGFR1, c-KIT, EGFR, and c-RAF. The internal control was the average expression of five reference genes (β2-microglobulin [B2M], glyceraldehyde 3-phosphate dehydrogenase [GAPDH], hydroxymethylbilane synthase [HMBS], hypoxanthine phosphoribosyltransferase 1 [HPRT1], and succinate dehydrogenase complex, subunit A, flavoprotein variant [SDHA]). The 2−ΔCt method was used to determine target gene expression levels.
Treatment Benefit Score
The TBS is the summation of all log2-transformed target gene expression multiplied by its corresponding regression coefficient, as follows: TBS = (0.118910 × mTOR) + (0.138561 × VEGFR2) + (0.258877 × c-KIT) + (0.147012 × c-RAF). The regression coefficients of each gene were calculated by logistic regression analysis with the R package (version 3.3.3; R Development Core Team; https://www.r-project.org/).
Statistical Analysis
Receiver operating characteristic curve analysis was used to determine the accuracy of the threshold values separating tumor responders and nonresponders using the TBS. Relationships between tumor response and clinicopathologic variables or gene expression were evaluated using either χ2 tests or Fisher's exact tests. Gene expression data were log2 transformed and tested for normality with the Shapiro-Wilk test. As the data did not meet the normality assumptions, significant differences between responders and nonresponders were evaluated using the Mann-Whitney U test. Every tested gene from the 220 patients was analyzed using logistic regression to understand the relationships between response to sorafenib treatment, TBS classification, and clinicopathologic variables. Significance was set at p < 0.05 (two-tailed). All statistics were performed in R version 3.3.3.
Results
Clinicopathologic Characteristics of the Sorafenib Responders
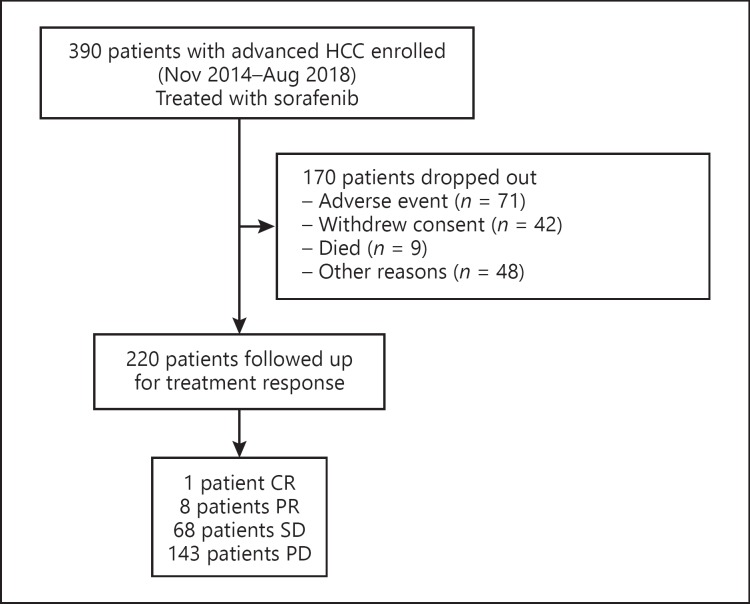
Of the 390 sorafenib-treated patients, 220 were retained for follow-up treatments. The remainder dropped out due to adverse events (n = 71), withdrawal of consent (n = 42), death (n = 9), or other reasons (n = 48) (Fig. 1).
Fig. 1.
Flowchart of treatment enrollment and follow-up. HCC, hepatocellular carcinoma; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
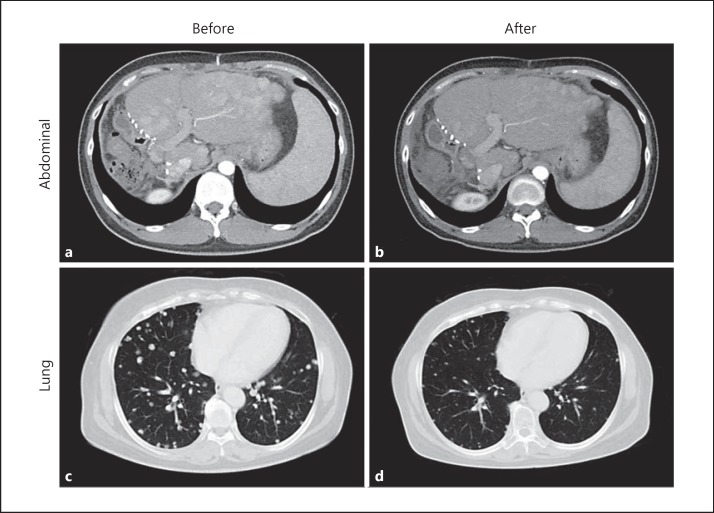
The results of the CT and MRI scans revealed 1 patient with a complete response, 8 with a partial response, 68 with stable disease, and the remaining 143 with progressive disease. The overall tumor response rate was 4.1%. Representative tumors presented a dramatic disappearance of nodules in the lung and liver after sorafenib treatment (Fig. 2). An analysis of clinicopathologic features associated with response to sorafenib revealed variable α-fetoprotein levels among responders, ranging from 1 to 14,046 ng/mL (Table 1). A comparison between responders and nonresponders did not reveal any clinical features unique to responders (Table 2).
Fig. 2.
Abdominal (a, b) and lung (c, d) images from representative responders. CT images taken before (a, c) and after (b, d) sorafenib treatment, showing changes in multiple nodular lesions spread in the organs.
Table 1.
Clinicopathologic features of sorafenib responders
| Patient No. | Age, years | Sex | Viral status | TNM stage (AJCC 7th ed.) | BCLC stage | AFP, ng/mL | Metastasis | Tumor response |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 | F | HBV | IV | C | 1.6 | X | PR |
| 2 | 46 | F | HBV | IV | C | 489.7 | O | PR |
| 3 | 52 | F | HBV | IV | B | 6,519 | O | PR |
| 4 | 52 | M | HBV | III | C | 14,046.0 | X | PR |
| 5 | 56 | M | HBV | IV | B | 10.1 | O | PR |
| 6 | 38 | M | HBV | III | C | 7.6 | X | PR |
| 7 | 43 | M | HBV | IV | C | 617.0 | O | PR |
| 8 | 56 | M | HBV | IV | C | 3 | X | CR |
| 9 | 62 | M | HBV | IV | C | 3.9 | X | PR |
AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; HBV, hepatitis B virus; CR, complete response; PR, partial response.
Table 2.
Baseline characteristics and comparison of clinical factors between responders and nonresponders
| Clinicopathologic parameters | Responders (n = 9) | Nonresponders (n = 211) | p value1 |
|---|---|---|---|
| Age | (-1) | 0.7384 | |
| <55 years | 3 | 88 | |
| ≥55 years | 6 | 122 | |
| Gender | (-1) | 0.4148 | |
| Male | 6 | 165 | |
| Female | 3 | 45 | |
| HBV | (-1) | 0.3627 | |
| Absent | 0 | 37 | |
| Present | 9 | 173 | |
| HCV | (-2) | 1 | |
| Absent | 9 | 200 | |
| Present | 0 | 9 | |
| Tumor TNM stage (AJCC | 7th ed.) | (-6) | 0.7607 |
| I | 0 | 0 | |
| II | 0 | 10 | |
| III | 2 | 29 | |
| IV | 7 | 166 | |
| BCLC stage | (-2) | 0.4223 | |
| A | 0 | 3 | |
| B | 2 | 23 | |
| C | 7 | 181 | |
| D | 0 | 2 | |
| AFP | (-14) | 0.7363 | |
| <100 ng/mL | 5 | 91 | |
| ≥100 ng/mL | 4 | 106 | |
| Tumor response | |||
| Complete response | 1 | 0 | |
| Partial response | 8 | 0 | |
| Stable disease | 0 | 68 | |
| Progressive disease | 0 | 143 |
HBV, hepatitis B virus; HCV, hepatitis C virus; TNM, Tumor, Node, and Metastasis classification; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein. 1 p values were calculated using Fisher's exact test.
Comparison of Actionable Gene Expression between Responders and Nonresponders
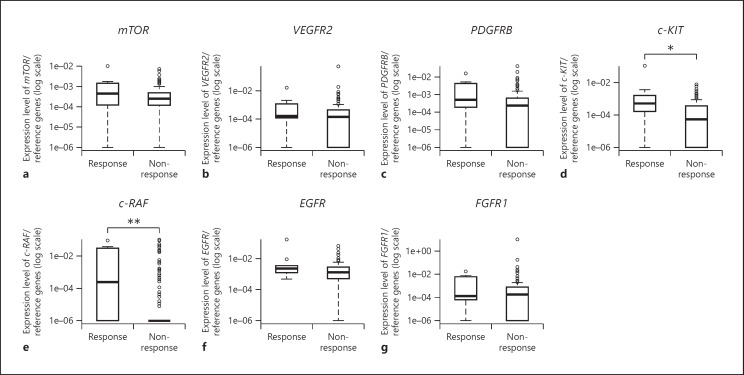
In a comparison across responders and nonresponders to sorafenib, we found that five genes (mTOR: p = 0.309; PDGFRB: p = 0.119; c-KIT: p = 0.014; c-RAF: p = 0.002; and EGFR: p = 0.137) had a higher mean expression in the responders than in the nonresponders (Fig. 3a, c–f). However, the mean VEGFR2 (p = 0.224) and FGFR1 (p = 0.475) expression was lower in the responders than in the nonresponders (Fig. 3b, g).
Fig. 3.
Actionable gene expression in responders versus nonresponders. Relative expression of seven actionable genes in 9 responders and 211 nonresponders. * p < 0.05, ** p < 0.01. amTOR. bVEGFR2. cPDGFRB. dc-KIT. ec-RAF. fEGFR. gFGFR1.
Comparing TBS between Responders and Nonresponders
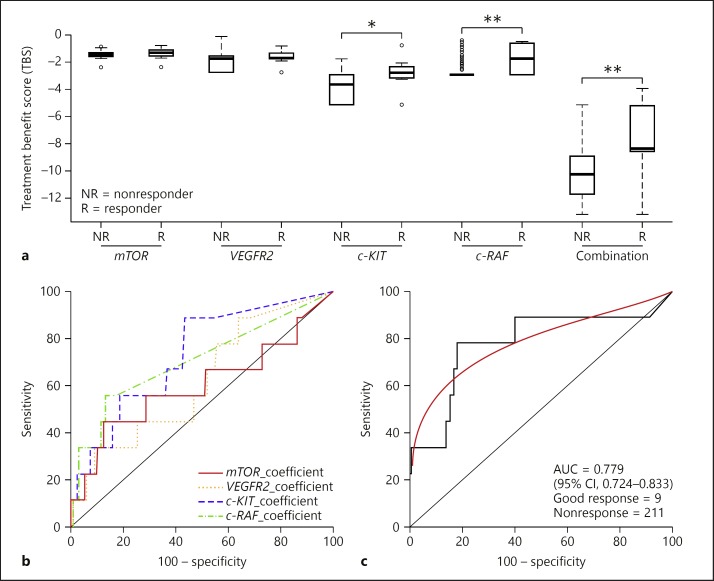
We found that combinations of mTOR, VEGFR2, c-KIT, and c-RAF were most effective in discriminating responders from nonresponders. Compared with the TBS of a single gene (mTOR, VEGFR2, c-KIT, or c-RAF), the TBS of mTOR/VEGFR2 and c-KIT/c-RAF combinations differed significantly between the responders and the nonresponders (Fig. 4a). The mean TBS of mTOR/VEGFR2 and c-KIT/c-RAF was −10.110464 in the nonresponders and −7.805714 in the responders, respectively (p < 0.001).
Fig. 4.
Treatment benefit score (TBS) of seven actionable genes in responders versus nonresponders. a Relative TBS of seven actionable genes compared across 9 responders and 211 nonresponders. Scores were calculated as individual values for mTOR, VEGFR2, c-KIT, and c-RAF, as well as paired values from mTOR with VEGFR2 and c-KIT with c-RAF. The thick line in each box is the median. Circles extend to the most extreme data point, no more than 1.5 times the interquartile range. * p < 0.05, ** p < 0.01. b, c Receiver operating characteristic curve analysis to predict the response to sorafenib (TBS) per gene (b) and per pair (c). cmTOR_VEGFR2_c-KIT_c-RAF.
Performance of Gene Classifiers in Predicting Tumor Response to Sorafenib
Multiple actionable genes were differentially expressed in tumor tissues between responders and nonresponders. We performed a receiver operating characteristic curve analysis of genes in combination; the number of genes combined was from 1 to 7. Among all the gene combinations, 9 of the classifiers revealed over 70% in sensitivity and specificity. The performance of the best classifier (combinations of mTOR, VEGFR2, c-KIT, and c-RAF) was at the threshold value at −8.6237; the prediction sensitivity in separating responders from nonresponders was 77.8% (95% CI: 40.0–97.2), while the specificity was 82.0% (95% CI: 76.1–86.9) (Fig. 4c). Additionally, the area under the curve was 0.779 (95% CI: 0.724–0.833), which was statistically significant (p < 0.001; Table 3).
Table 3.
Classifiers of tumor response to sorafenib
| Rank | Gene combination | Combination | Sensitivity, % | Specificity, % | AUC | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| 1 | mTOR_VEGFR2_c-KlT_c-RAF | 4 | 77.78 | 81.99 | 0.779 | 81.82 | 15.56 | 98.86 |
| 2 | mTOR_c-KlT_c-RAF | 3 | 77.78 | 81.52 | 0.778 | 81.36 | 15.22 | 98.85 |
| 3 | mTOR_VEGFR2_c-KlT_c-RAF_FGFR1 | 5 | 77.78 | 81.52 | 0.770 | 81.36 | 15.22 | 98.85 |
| 4 | VEGFR2_c-KlT_c-RAF | 3 | 77.78 | 80.57 | 0.781 | 80.45 | 14.58 | 98.84 |
| 5 | mTOR_c-KlT_c-RAF_FGFR1 | 4 | 77.78 | 80.09 | 0.764 | 80.00 | 14.29 | 98.83 |
| 6 | c-KlT_c-RAF | 2 | 77.78 | 77.73 | 0.791 | 77.73 | 12.96 | 98.80 |
| 7 | c-KlT_c-RAF_FGFR1 | 3 | 77.78 | 77.73 | 0.769 | 77.73 | 12.96 | 98.80 |
| 8 | VEGFR2_c-KlT_c-RAF_FGFR1 | 4 | 77.78 | 77.25 | 0.765 | 77.27 | 12.73 | 98.79 |
| 9 | mTOR_VEGFR2_c-KlT_c-RAF_EGFR | 5 | 77.78 | 73.46 | 0.769 | 73.46 | 11.11 | 98.73 |
| mTOR | 1 | 44.44 | 87.68 | 0.600 | 85.91 | 13.33 | 97.37 | |
| VEGFR2 | 1 | 88.89 | 36.02 | 0.618 | 38.18 | 5.59 | 98.70 | |
| c-KlT | 1 | 88.89 | 56.87 | 0.731 | 58.18 | 8.08 | 99.17 | |
| c-RAF | 1 | 55.56 | 86.73 | 0.706 | 85.45 | 15.15 | 97.86 | |
| mTOR_VEGFR2_PDGFRB_c-KlT_EGFR_FGFR1 | 6 | 55.56 | 82.94 | 0.730 | 81.82 | 12.20 | 97.77 | |
| mTOR_VEGFR2_PDGFRB_c-KlT_c-KlT_EGFR_FGFR1 | 7 | 88.89 | 58.29 | 0.747 | 59.55 | 8.33 | 99.19 | |
AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value; mTOR, mammalian target of rapamycin; VEGFR2, vascular endothelial growth factor receptor 2; c-KIT, KIT proto-oncogene receptor tyrosine kinase; c-RAF, Raf-1 proto-oncogene serine/threonine kinase; FGFR1, fibroblast growth factor receptor 1; EGFR, epidermal growth factor receptor; PDGFRB, platelet-derived growth factor receptor beta.
TBS-Based Classifiers and Clinicopathologic Characteristics Both Predict Response to Sorafenib Treatment
Logistic regression demonstrated that TBS-based classifiers stemming from mTOR, VEGFR2, c-KIT, and c-RAF combinations significantly predicted tumor response to sorafenib treatment (p < 0.001; Table 4). Logistic regression also showed that none of the measured clinicopathologic variables (age: p = 0.611; sex: p = 0.404; hepatitis B virus: p = 0.993; hepatitis C virus: p = 0.994; TNM stage: p = 0.812; BCLC stage: p = 0.399; and α-fetoprotein levels: p = 0.373) significantly predicted response to sorafenib (Table 4).
Table 4.
Logistic regression analysis of tumor response to sorafenib and variables
| Variables | Odds ratio | 95% CI | p value |
|---|---|---|---|
| mTOR_VEGFR2_c-KIT_c-RAF (low vs. high) | 15.93 | 3.18–79.73 | 0.002 |
| Age (<55 vs. ≥55 years) | 1.44 | 0.35–5.93 | 0.611 |
| Gender (male vs. female) | 1.83 | 0.44–7.62 | 0.404 |
| HBV (absence vs. presence) | Inf. | 0.00–Inf. | 0.993 |
| HCV (absence vs. presence) | 0.00 | 0.00–Inf. | 0.994 |
| TNM stage (II–III vs. IV) | 0.82 | 0.16–4.11 | 0.812 |
| BCLC stage (AB vs. CD) | 0.50 | 0.10–2.52 | 0.399 |
| AFP level (<100 vs. ≥100 ng/mL) | 0.52 | 0.12–2.21 | 0.373 |
CI, confidence interval; mTOR, mammalian target of rapamycin; VEGFR2, vascular endothelial growth factor receptor 2; c-KIT, KIT proto-oncogene receptor tyrosine kinase; c-RAF, Raf-1 proto-oncogene serine/threonine kinase; HBV, hepatitis B virus; HCV, hepatitis C virus; TNM, Tumor, Node, and Metastasis classification; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein.
Improving the Predictability of TBS-Based Classifiers
Thus far, our findings have indicated that TBS can distinguish HCC patients with a complete response or partial response from those with stable disease or progressive disease, indicating that TBS-based patient selection should improve sorafenib's efficacy compared with the lack of any classification. When we examined 45 HCC patients with TBS >–8.6237 for mTOR, VEGFR2, c-KIT, and c-RAF combinations, we found 7 responders (response rate 15.6%; Table 5). The correlation between TBS and tumor responses to sorafenib was significant (p < 0.001).
Table 5.
Response rates based on gene classifier
| Classifier | Criterion | Responders | Nonresponders | Response rate, % |
|---|---|---|---|---|
| mTOR_VEGFR2_c-KlT_c-RAF | >-8.6237 | 7 | 38 | 15.6 |
| ≤-8.6237 | 2 | 173 | 1.16 |
mTOR, mammalian target of rapamycin; VEGFR2, vascular endothelial growth factor receptor 2; c-KIT, KIT proto-oncogene receptor tyrosine kinase; c-RAF, Raf-1 proto-oncogene serine/threonine kinase.
Discussion
In this study, we found that TBS based on actionable gene expression successfully predict HCC tumor response to sorafenib. When the TBS was used for patient selection, the rate of response to sorafenib increased from 4.1 to 15.6%. Our findings suggest that patient stratification based on actionable molecular subtyping is a useful therapeutic strategy for improving sorafenib's effectiveness in HCC, supporting our previous research [13].
An increased expression of actionable molecules (PDGFRB and c-KIT) in tumor cell lines may elevate tumor sensitivity to molecular targeted drugs. Based on the previous study, we investigated the mRNA expression of five actionable genes (VEGFR2, PDGFRB, EGFR, mTOR, and FGFR1) and two additional genes (c-KIT and c-RAF) in HCC tumors and surrounding noncancerous tissues (online suppl. Fig. 1, 2; for all online suppl. material, see www.karger.com/doi/10.1159/000504548). Then we conducted a seven-actionable-gene-based study. Here, we observed a significantly higher mean expression of three actionable genes (PDGFRB, c-KIT, and c-RAF) in responders than in nonresponders. This outcome is consistent with the fact that these three genes are direct sorafenib targets and associated with an enhanced response to sorafenib treatment [15]. However, mTOR and EGFR confer resistance to sorafenib through Akt activation [16, 17], and, indeed, their expression did not differ significantly between responders and nonresponders, although the mean values were slightly higher in the responders (Fig. 3). Overall, our results suggest that mRNA levels of individual actionable genes are insufficient for predicting therapeutic response to sorafenib.
Although individual actionable genes were not useful biomarkers, our results demonstrated that the TBS of gene combinations were significantly higher in responders (Fig. 4a), with mTOR/VEGFR2 and c-KIT/c-RAF achieving 77.8% sensitivity and 82.0% specificity (Fig. 4c; Table 3). These data are in line with the observation that multiple biomarkers usually outperform single biomarkers in diagnosis.
Furthermore, we demonstrated that adding VEGFR2, c-KIT, and c-RAF to the mTOR classifier could maximize predictions of response to sorafenib and thus improve treatment response rates. However, although the TBS raised the rates of response to sorafenib treatment from 4.1 to 15.6%, a rate of 15.6% is still low in absolute terms. We believe that this low overall response rate is largely due to the small number of responders and heterogeneity in HCC patients (in contrast to the uniformity of HCC cell lines). Nevertheless, TBS based on actionable gene combinations exhibited a promising level of diagnostic performance in predicting response to sorafenib.
Given that sorafenib is the standard therapy for advanced HCC, excluding potential nonresponders might be the best approach to maximizing the drug's benefits. We therefore recommend against sorafenib treatment for patients below our suggested threshold values from our mTOR/VEGFR2 and c-KIT/c-RAF classifiers.
In conclusion, we have demonstrated the potential of actionable gene expression in predicting clinical responses to sorafenib treatment in HCC. Future research should aim to replicate these results using a larger gene pool. Nonetheless, our findings contribute to efforts aimed at improving diagnostic approaches that personalize sorafenib treatment of HCC.
Statement of Ethics
The protocol was approved by the Institutional Review Boards of Ajou University Medical Center, Asan Medical Center, Gachon University Gil Medical Center, Korea University Medical Center, and Catholic University Medical Center. All patients provided written informed consent. The Declaration of Helsinki and Good Clinical Practice guidelines were followed.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (Grant No. NRF-2013R1A1A4A01009053) and the Gil Medical Center, Gachon University College of Medicine (Grant No. CBS2013-10), South Korea.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
The research was supported in part by the Korea Cancer Biomarker Consortium.
References
- 1.Shin JW, Chung YH. Molecular targeted therapy for hepatocellular carcinoma: current and future. World J Gastroenterol. 2013 Oct;19((37)):6144–55. doi: 10.3748/wjg.v19.i37.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ocana A, Pandiella A, Siu LL, Tannock IF. Preclinical development of molecular-targeted agents for cancer. Nat Rev Clin Oncol. 2010 Dec;8((4)):200–9. doi: 10.1038/nrclinonc.2010.194. [DOI] [PubMed] [Google Scholar]
- 3.Sokolenko AP, Imyanitov EN. Molecular Diagnostics in Clinical Oncology. Front Mol Biosci. 2018 Aug;5:76. doi: 10.3389/fmolb.2018.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004 May;350((21)):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Tsao H, Chin L, Garraway LA, Fisher DE. Melanoma: from mutations to medicine. Genes Dev. 2012 Jun;26((11)):1131–55. doi: 10.1101/gad.191999.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. SHARP Investigators Study Group Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008 Jul;359((4)):378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009 Jan;10((1)):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 8.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018 Mar;391((10126)):1163–73. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 9.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018 Mar;391((10127)):1301–14. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 10.Marisi G, Cucchetti A, Ulivi P, Canale M, Cabibbo G, Solaini L, et al. Ten years of sorafenib in hepatocellular carcinoma: are there any predictive and/or prognostic markers? World J Gastroenterol. 2018 Sep;24((36)):4152–63. doi: 10.3748/wjg.v24.i36.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horwitz E, Stein I, Andreozzi M, Nemeth J, Shoham A, Pappo O, et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 2014 Jun;4((6)):730–43. doi: 10.1158/2159-8290.CD-13-0782. [DOI] [PubMed] [Google Scholar]
- 12.Arao T, Ueshima K, Matsumoto K, Nagai T, Kimura H, Hagiwara S, et al. FGF3/FGF4 amplification and multiple lung metastases in responders to sorafenib in hepatocellular carcinoma. Hepatology. 2013 Apr;57((4)):1407–15. doi: 10.1002/hep.25956. [DOI] [PubMed] [Google Scholar]
- 13.Kwon JH, Lee N, Park JY, Yu YS, Kim JP, Shin JH, et al. Actionable gene expression-based patient stratification for molecular targeted therapy in hepatocellular carcinoma. PLoS One. 2013 Jun;8((6)):e64260. doi: 10.1371/journal.pone.0064260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet J. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010 Feb;30((1)):52–60. doi: 10.1055/s-0030-1247132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008 Oct;7((10)):3129–40. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuda M, Chen WY, Miyanaga A, Nakamura Y, Kawasaki K, Sakuma T, et al. Alternative mammalian target of rapamycin (mTOR) signal activation in sorafenib-resistant hepatocellular carcinoma cells revealed by array-based pathway profiling. Mol Cell Proteomics. 2014 Jun;13((6)):1429–38. doi: 10.1074/mcp.M113.033845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ezzoukhry Z, Louandre C, Trécherel E, Godin C, Chauffert B, Dupont S, et al. EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. Int J Cancer. 2012 Dec;131((12)):2961–9. doi: 10.1002/ijc.27604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data