Abstract
Paenibacillus larvae, a Gram-positive bacterium, causes American foulbrood (AFB) in honey bee larvae (Apis mellifera Linnaeus [Hymenoptera: Apidae]). P. larvae spores exit dormancy in the gut of bee larvae, the germinated cells proliferate, and ultimately bacteremia kills the host. Hence, spore germination is a required step for establishing AFB disease. We previously found that P. larvae spores germinate in response to l-tyrosine plus uric acid in vitro. Additionally, we determined that indole and phenol blocked spore germination. In this work, we evaluated the antagonistic effect of 35 indole and phenol analogs and identified strong inhibitors of P. larvae spore germination in vitro. We further tested the most promising candidate, 5-chloroindole, and found that it significantly reduced bacterial proliferation. Finally, feeding artificial worker jelly containing anti-germination compounds to AFB-exposed larvae significantly decreased AFB infection in laboratory-reared honey bee larvae. Together, these results suggest that inhibitors of P. larvae spore germination could provide another method to control AFB.
Keywords: American foulbrood, spore germination, indoles
Honey bees are vital pollinators of agricultural and horticultural crops (Matheson 1993, Morse and Calderone 2000). However, recent losses of managed honey bee colonies have compromised pollination of crops (Neumann and Carreck 2010). The decline in honey bees results from a combination of factors, including exposure to pesticides, viruses, parasites, and bacterial diseases (Cox-Foster et al. 2007, Dainat et al. 2012). American foulbrood (AFB) is a bacterial disease that is lethal to bee larvae. The loss of larvae impacts the fitness of AFB-infected colonies and ultimately leads to their collapse (White 1920, Genersch 2010).
P. larvae, the causative agent of AFB, is a Gram-positive, spore forming, obligate pathogen that requires honey bee larvae to complete its life cycle (White 1920, Genersch 2010). P. larvae exists as either a dormant spore or a vegetative cell depending on environmental conditions. Under nutrient deprivation, P. larvae cells form resistant spores that remain viable for as long as 70 yr (Shimanuki and Knox 2000, Forsgren et al. 2008). P. larvae spores are only known to germinate or exit dormancy in the gut of a honey bee larva host. AFB disease occurs in first or second instar larvae as newly germinated P. larvae cells proliferate resulting in extreme bacteremia and death several days after infection (Yue et al. 2008). Once the larvae are consumed, P. larvae cells re-sporulate, forming billions of spores. The infectious spores are then transmitted within the colony or among colonies by bees and beekeeping practices (Sturtevant 1932, Lindström et al. 2008).
Eradication of AFB is difficult because spores are resistant to high temperatures, desiccation, UV irradiation, and harsh chemicals (Dobbelaere et al. 2001). Moreover P. larvae spores can remain dormant throughout the hive, including honey, pollen, wax, adult bees, and hive surfaces (Fries et al. 2006, Lindström et al. 2008, Adjlane et al. 2014). Although terramycin and other antibiotics have been used to treat active infections and prevent AFB disease, spores are not affected by antibiotics and can remain dormant long after the antibiotic treatment (Peng et al. 1992, Lodesani and Costa 2005, Alippi et al. 2007). Furthermore, overuse of antibiotics has led to resistance in some P. larvae strains (Alippi et al. 2007).
Because P. larvae spore germination is the first step of infection, preventing spore germination is a promising avenue to prevent AFB. Inhibition of spore germination for the prevention of anthrax and Clostridium difficile infection has been used effectively (Alvarez et al. 2010; Howerton et al. 2011; Howerton et al. 2013a,b). Previous research in our laboratory identified uric acid and l-tyrosine as co-germinants necessary for P. larvae spore germination. Both compounds are unique as agonists in spore germination and have not been observed to trigger germination in other sporulating bacteria (Salas and Ellar 1985, Smith and Sullivan 1989, Alvarado et al. 2013).
The P. larvae genome encodes putative Ger receptors similar to those found in Bacillus and Clostridium species. However, because direct interactions between Ger receptors and their cognate germinants have not been determined in any species, molecular probes have been used to map germinant-receptor interactions (Woese et al. 1958, Cortezzo et al. 2004, Abel-Santos and Dodatko 2007, Dodatko et al. 2009, Luu et al. 2011, Howerton et al. 2011). Using these approaches, we found that indole and phenol acted as antagonists of P. larvae spore germination (Alvarado et al. 2013).
The goal of the research reported here was to assess the efficiency of germination inhibition as a treatment strategy for AFB in vitro. A series of indole and phenol analogs were tested as inhibitors of uric acid/l-tyrosine-mediated germination of P. larvae spores. The half-maximal inhibitory concentration (IC50) of the best inhibitory indole analogs was calculated. We also tested the effect of indole analogs on P. larvae vegetative replication. Finally, indole inhibitors were tested for their ability to protect laboratory-reared honey bee larvae from AFB disease without toxic effects. These tests identified 5-chloroindole as a strong non-toxic inhibitor of spore germination, bacterial growth, and AFB disease development.
Materials and Methods
Materials
Chemicals were purchased from Sigma-Aldrich Corporation (St Louis, MO) and VWR International (Radnor, PA). Dehydrated culture media components including yeast extract, Mueller–Hinton broth, and tryptic soy agar were purchased from BD Difco (Franklin Lakes, NJ) and Amresco (Solon, OH). P. larvae ERIC IV strain B-3685/ATCC 49843 was obtained from the American Tissue Culture Collection (ATCC, Manassas, VA). Lyophilized royal jelly was obtained from GloryBee Foods (Eugene, OR) and stored at -20°C until needed. Honey bee larvae food was prepared as described previously (Peng et al. 1992, Crailsheim et al. 2013).
P. larvae Spore Preparation
P. larvae strain ATCC 49843 was grown on BD tryptic soy agar plates for 7 d under 5% CO2 at 37°C. The resulting bacterial lawns were collected by flooding with ice-cold deionized water and scraping plates using a cell spreader. The spores/vegetative cell mixture was pelleted by centrifugation 8,820 x g and resuspended in fresh deionized water. After three washing and centrifugation steps, spores were separated from vegetative cells and partially sporulated forms by centrifugation at 18,200 × g through a 20–50% HistoDenz density gradient. The resulting spore pellet was washed five times with water as earlier and stored at 4°C for the duration of the experiment. Spore preparations were over 90% pure as determined by microscopic observation of Schaeffer–Fulton-stained samples (Schaeffer and Fulton 1933, Alvarado et al. 2013).
Testing for Antagonists of P. larvae Spore Germination
The decrease in optical density (OD) of a bacterial suspension is inversely proportional to spore germination (Powell 1950). To monitor spore germination in real time, changes in light diffraction intensity were monitored at 580 nm (OD at 580 nm) on a BioMate 5 (Thermo Electron Corporation, Waltham, MA) or a Tecan Infinite M200 (Tecan Group, Männedorf, Switzerland) spectrophotometer. Experiments were carried out in 96-well plates (200 µl/well).
In preparation for germination assays, P. larvae spore suspensions were washed three times with water, as earlier. Purified spores were then heat activated at 70°C for 30 min. Heat-activated spore aliquots were diluted with 0.1 M sodium phosphate buffer (pH 7) to an average OD at 580 nm [OD580] of 1.0. Spores were monitored for auto-germination for 30 min. Germination experiments were performed with spores that did not auto-germinate. Experiments were performed in triplicate with at least two different cultures. Relative OD values were derived by dividing each OD580 reading by its initial OD580 reading.
To test for antagonists of P. larvae spore germination, spore samples were first individually supplemented with 400 µM of individual indole or phenol analogs dissolved in dimethyl sulfoxide. Spore suspensions were incubated for 15 min at room temperature while monitoring changes in OD580. If no germination was detected, l-tyrosine and uric acid were added to final concentrations of 3,000 µM, and germination was monitored for 120 min. Germination percentage for each treatment was determined by dividing the final measured relative OD by the final measured relative OD of untreated controls.
The best inhibitors were further tested to determine their relative efficacy as anti-germinants. Spore samples were individually supplemented with individual indole analogs at concentrations of 0–1,600 µM. Germination rates were determined by linear fitting of changes in relative OD during the early time points. Germination rates for all conditions were divided by the uninhibited maximum germination rate obtained by treating spores with only germinants and are reported as percent germination. Percent germination was plotted against the log of inhibitor concentrations. The resulting sigmoidal curves were fitted using the four-parameter logistic function in SigmaPlot v.12 to calculate the half-maximal germination inhibitory concentrations (IC50s).
Testing for Indole Analogs Activity Against Proliferation of P. larvae Vegetative Cells
Overnight P. larvae cultures were diluted to an OD580 of ~0.2, and 200 µl of the cultures were placed in 96-well plates. Indole analogs were added to P. larvae cells to a final concentration of 0, 50, 100, 150, 200, 500, 1,000, 1,500, or 2,000 µM. Cells were incubated at 37°C with shaking at 225 rpm for a 12-h period. OD was measured every 2 h on a BioMate 5 (Thermo Electron Corporation, Waltham, MA) or a Tecan Infinite M200 (Tecan Group, Männedorf, Switzerland) spectrophotometer. Experiments were performed in triplicate with two different cultures. Relative OD values were derived by dividing each OD580 reading by its initial OD580 reading.
Laboratory Rearing of Honey Bee Larvae
Experiments were conducted on larvae obtained from two colonies headed by naturally mated queens kept in the University of Nevada Las Vegas apiary according to standard industry practices (Honey Bee Genetics, Vacaville, CA). Artificial worker jelly (AWJ) was prepared an hour prior to use for larval rearing. d-glucose, d-fructose, and yeast extract were dissolved in warm autoclaved double-distilled water. This solution was mixed with lyophilized royal jelly thoroughly with a vortex mixer. To accommodate protein and sugar requirements of developing larvae, the composition and amounts of AWJ was changed based on larval stage (Supp Table 1 [online only]) (Crailsheim et al. 2013).
First instar, 1-d-old honey bee larvae used in our experiments were collected from worker brood cells. Larval age was controlled by confining queens in a cage on empty frames. The queen was released after 24 h and the eggs were left in the hive until first instar larvae could be collected 3 d later. A group of 40 similarly aged first instar, 1-d-old larvae were transferred to a six-well tissue culture plate containing 2.5 ml of the AWJ per well with a beekeeper’s grafting tool (Amazon, Seattle, WA). Plated larvae were grown at 35°C and 95% relative humidity following the published methods (Segur 1953, Crailsheim et al. 2013). The desired relative humidity and temperature were verified with an iButton data logger (Maxim Integrated, San Jose, CA). Two days after collection, honey bee larvae were transferred to individual wells on new 48-well plates containing 250 μl of fresh AWJ. From Day 3 until defecation at the pre-pupal stage, larvae were not relocated to reduce handling-related mortality.
Every 24 h, larvae were monitored for survival under a dissecting microscope. Larvae were classified as dead by the absence of movement or respiration, edema development, color change, and/or failure to pupate after 7 d (Evans 2004, Genersch et al. 2005). Dead honey bee larvae were immediately removed from their wells. In our hands, larvae fed AWJ reared from the first larval instar to the pre-pupal defecation had mortality below 3%.
Analog Toxicity Assay
Chronic exposure to indole analogs was performed to assess toxicity on honey bee larvae. Indole analogs were individually dissolved in autoclaved water to a 500 µM final concentration. Each indole analog stock solution was supplemented with d-glucose, d-fructose, yeast extract, and royal jelly (Supp Table 1 [online only]). Indole-supplemented AWJ was prepared an hour prior to use for larval testing. Larvae were placed on the indole-supplemented artificial diet as described earlier. Larval survival was determined every 24 h under a stereo microscope as described earlier. Each experiment contained three replicate trials with 30 larvae each, for a total of 90 larvae per treatment (Crailsheim et al. 2013).
AFB Exposure Model
A stock spore suspension with an OD580 of 1.0 was prepared in water (4.4 × 108 spores per milliliter based on a microscopic count with a counting chamber). The stock spore suspension was diluted 1:100 with water, and 100 µl of the dilution (4.4 × 105 spores) was added to 5 ml of AWJ. The spore-containing diet was prepared 1 h before use. Second instar (2-d-old larvae) were challenged with 2.2 × 105 spores in 2.5 ml final volume (Supp Table 1 [online only]) of spore-containing diet. After spore exposure, third instar (3 d old) larvae were removed from wells containing the remaining spore-containing larval diets, cleaned using Kimwipes, and then moved to individual wells in 48-well plates containing fresh, sterile 250 μl larval diets. Larval survival was determined daily as described earlier. Each experiment contained three replicate trials with 30 larvae, for a total of 90 larvae per treatment (Crailsheim et al. 2013).
AFB Prevention by Indole Analogs
The ability of indole analogs to prevent AFB infection was assayed by combining the toxicity and AFB exposure model protocols. Honey bee larvae were fed diets containing indole analogs at a 500 µM final concentration. The spore-containing diet was prepared 1 h before use and fed to second instar larvae (2 d old) for a 24-h period. After spore exposure, third instar (3 d old) larvae were removed from wells containing the remaining spore-containing larval diets, cleaned using Kimwipes, and then moved to individual wells in 48-well plates containing fresh sterile 250 μl larval diets. Larval survival was determined daily as described earlier. Each experiment contained three replicate trials with 30 larvae, for a total of 90 larvae per treatment (Crailsheim et al. 2013).
Survival Analysis
Larval survival data were analyzed using Kaplan–Meier Survival plots. The log-rank test from SigmaPlot v.12 was used to test for statistically significant differences between survival curves. The Holm–Šidák statistic was used to identify pairs of survival curves that are statistically significantly different from other pairs (P < 0.05).
Results
Inhibition of P. larvae Spore Germination by Indole and Phenol Analogs
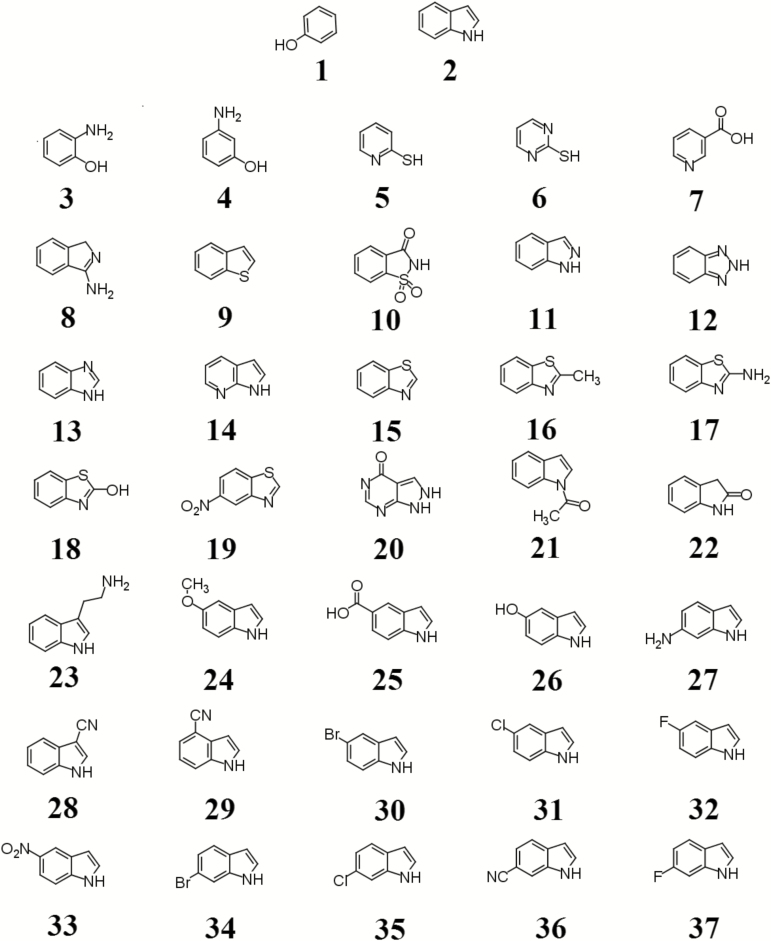
We have previously shown that indole and phenol inhibited P. larvae spore germination (Alvarado et al. 2013). However, both indole and phenol (compounds 1 and 2) were weak inhibitors with sub-millimolar values of IC50. To identify stronger inhibitors, the effect of 35 indole and phenol structural analogs (Fig. 1) on P. larvae spore germination in vitro was tested (Table 1) at a single concentration.
Fig. 1.
Compounds tested as antagonists of P. larvae spore germination. 1. phenol; 2. indole; 3. 2-aminophenol; 4. 3-aminophenol; 5. 2-mercaptopyridine; 6. 2-mercaptopyrimidine; 7. nicotinic acid; 8. 1H-isoindol-3amine; 9. thionaphthene; 10. saccharin; 11. indazole; 12. 1H-benzotriazole; 13. benzimidazole; 14. 7-azaindole; 15. benzothiazole; 16. 2-methylbenzothiazole; 17. 2-aminobenzothiazole; 18. 2-hydroxybenzothiazole; 19. 6-nitrobenzothiazole; 20. allopurinol; 21. 1-acetylindole; 22. 2-oxoindole; 23. 3-ethanamineindole; 24. 5-methoxyindole; 25. 5-carboxyindole; 26. 5-hydroxyindole; 27. 6-aminoindole; 28. 3-cyanoindole; 29. 4-cyanoindole; 30. 5-bromoindole; 31. 5-chloroindole; 32. 5-fluoroindole; 33. 5-nitroindole; 34. 6-bromoindole; 35. 6-chloroindole; 36. 6-cyanoindole; 37. 6-fluoroindole.
Table 1.
Percent germination of P. larvae spores (±SE) after treatment with indole and phenol analogs
| Compound | Germination (%) | SE | Compound | Germination (%) | SE |
|---|---|---|---|---|---|
| Control | 100.0 | 1.9 | 19 | 98.4 | 4.1 |
| 01 | 56.8 | 2.9 | 20 | 97.3 | 3.2 |
| 02 | 47.6 | 3.6 | 21 | 77.3 | 6.4 |
| 03 | 97.6 | 2.7 | 22 | 87.1 | 6.9 |
| 04 | 99.2 | 1.6 | 23 | 93.6 | 5.0 |
| 05 | 100.4 | 1.4 | 24 | 70.3 | 2.5 |
| 06 | 98.9 | 1.9 | 25 | 99.0 | 1.0 |
| 07 | 100.1 | 1.5 | 26 | 81.4 | 8.8 |
| 08 | 97.7 | 2.3 | 27 | 101.7 | 5.7 |
| 09 | 91.3 | 8.9 | 28 | 24.8 | 9.8 |
| 10 | 96.3 | 2.0 | 29 | 37.1 | 14.4 |
| 11 | 91.7 | 3.9 | 30 | 1.5 | 0.5 |
| 12 | 94.0 | 4.7 | 31 | 0.0 | 0.5 |
| 13 | 93.9 | 5.5 | 32 | 50.2 | 10.3 |
| 14 | 101.3 | 2.6 | 33 | 0.3 | 0.2 |
| 15 | 92.8 | 3.1 | 34 | 1.2 | 0.5 |
| 16 | 62.5 | 9.3 | 35 | 0.6 | 0.1 |
| 17 | 93.7 | 3.6 | 36 | 0.0 | 0.3 |
| 18 | 89.1 | 1.9 | 37 | 17.4 | 1.4 |
P. larvae spores were incubated with 400 µM of an indole or phenol analog (Fig. 1) for 15 min prior to the addition of 3,000 µM l-tyrosine and 3,000 µM uric acid. Germination rates for all conditions were divided by the uninhibited maximum germination rate obtained by treating spores with 3,000 µM l-tyrosine plus 3,000 µM uric acid (control) and are reported as percent germination. Standard errors (SE) were calculated from at least six independent measurements.
All phenol analogs (compounds 3–7) were weaker inhibitors of P. larvae spore germination than phenol. In fact, any tested modification to the benzene ring compromised inhibitory activity. In a similar manner, modification to the five-member ring of indole reduced the efficacy of the inhibitor on spore germination. Substitution of the N1 of indole for carbon (compound 8), sulfur (compound 9), or sulfonyl (compound 10) produced compounds that were unable to inhibit spore germination. Substitution of carbons for nitrogens at positions 2 (compound 11), 2,3 (compound 12), 3 (compound 13), or 7 (compound 14) of indole also abolished the inhibitory effect on spore germination. Benzothiazole (compound 15) only differs from indole by the addition of a sulfur group to position 3. However, compound 15 failed to prevent P. larvae spore germination. No increase in germination inhibition was observed with any benzothiazole derivatives tested (compounds 16–19). Allopurinol (compound 20), a purine analog that has functional groups found in both indole and uric acid, did not inhibit P. larvae spore germination as indole.
We had previously shown that methylation of indole did not increase anti-germination activity (Alvarado et al. 2013). Addition of acetyl, carbonyl, ethanamine, methoxy, carboxyl, hydroxyl, or amino functional group (compounds 21–27) reduced the inhibitory effect of indole on spore germination.
In contrast, indole analogs with electron-withdrawing groups (compounds 28–37) had increased inhibitory effects on P. larvae spore germination (Fig. 2 and Table 1). Quantitative analysis to compare the effectiveness of germination inhibition showed that the maximum inhibitory effect on P. larvae spore germination was obtained for indole analogs with halide and nitro group substituents (Table 2). Halide- and nitro-containing compounds were approximately four times as potent as indole. Additionally, cyano-containing compounds were approximately two to four times more potent than indole.
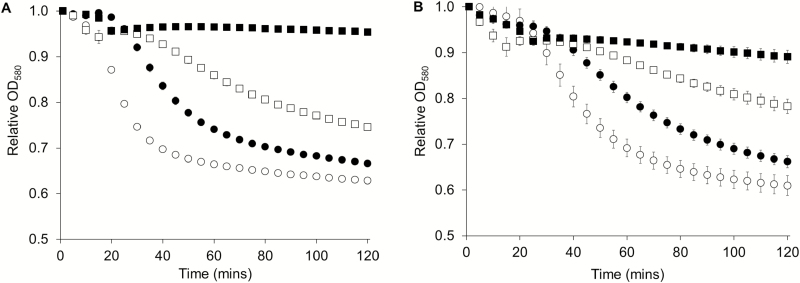
Fig. 2.
P. larvae spore germination in the presence of 5-bromoindole and 5-chloroindole. (A) P. larvae spores were incubated with germinants and supplemented with 0 µM (open circle), 40 µM (filled circle), 80 µM (open square), and 200 µM (filled square) of 5-bromoindole. (B) P. larvae spores were incubated with germinants and supplemented with 0 µM (open circle), 20 µM (filled circle), 100 µM (open square), and 200 µM (filled square) of 5-chloroindole. Data are shown every 5 min for clarity. Spore germination was measured by a decrease in the relative OD over time. Error bars represent standard error obtained from at least six independent measurements.
Table 2.
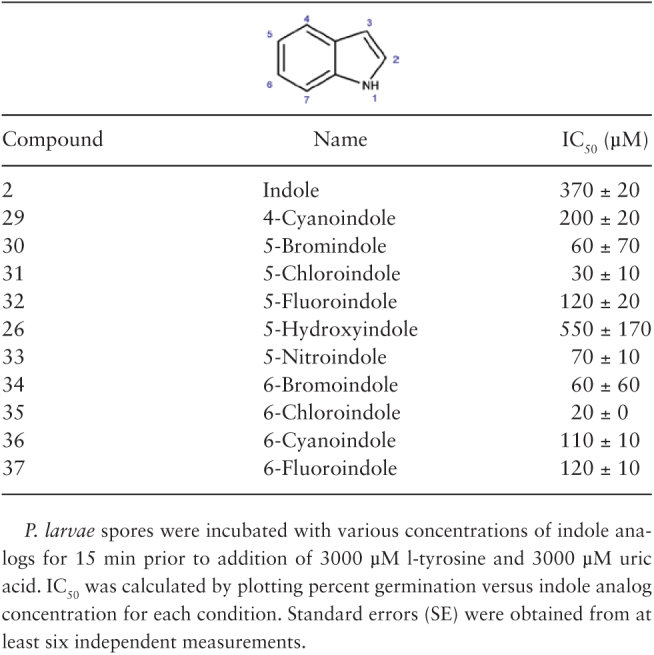
Concentration of indole analogs that reduces P. larvae spore germination by half (IC50s ± SE)
Antimicrobial Activity of Indole Analogs on P. larvae Cells
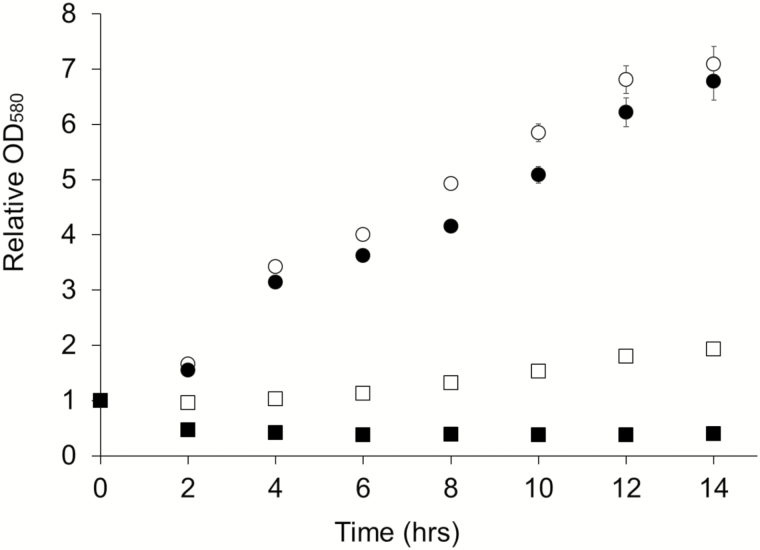
Tests of antimicrobial activity were performed with various concentrations (from 50 to 2000 µM) of 5-bromoindole, 5-chloroindole, 5-nitroindole, 6-bromoindole, and 6-chloroindole at. As shown with 5-bromoindole, indole analogs at final concentrations between 50 and 500 µM did not alter P. larvae cellular growth (Fig. 3). However, P. larvae growth was affected by higher concentrations of indole analogs. The most potent antimicrobial activity against P. larvae cells was observed in the presence of 6-bromoindole. The minimum inhibitory concentration at which no visible growth of P. larvae occurred in medium was 2000 µM for all indole analogs tested. Antibacterial activity was greatly diminished at concentrations below 500 µM.
Fig. 3.
Bacteriostatic activity of 5-bromoindole on P. larvae cells. P. larvae vegetative cells were grown in medium supplemented with 0 µM (open circle), 500 µM (filled circle), 1000 µM (open square), and 2000 µM (filled square) of 5-bromoindole. Bacterial growth was monitored over a 14-h period. Relative OD580 values were obtained by dividing each data point by the initial OD.
Indole Analogs do not Alter Honey Bee Larval Development
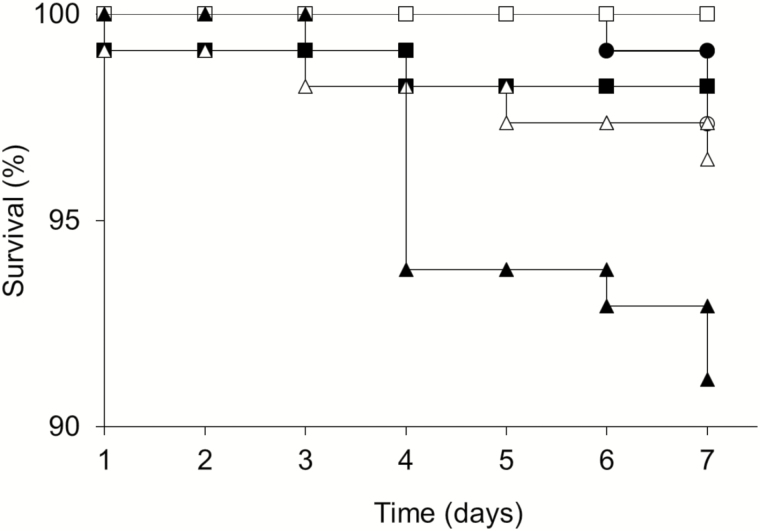
To test for toxicity, laboratory-reared larvae were fed AWJ supplemented with 500 µM of 5-bromoindole, 5-chloroindole, 5-nitroindole, 6-bromoindole, or 6-chloroindole (Fig. 4) from the first instar (1 d old) through the pre-pupation defecation period. Addition of 1500 µM of indole analogs resulted in a slight but statistically significant increase (Holm–Šidák method, P < 0.05) in larvae mortality in our hands. This was especially true for 6-chloroindole. However, even for 6-chloroindole, the survival rate was 92%, which is within the range of survival reported when larvae are simply manipulated during rearing (Peng et al. 1992, Huang 2009). Sub-lethal exposure to chemicals can in certain cases interfere with larvae behavior. However, under the conditions tested, larvae mobility, size, respiration, and time to pupation were not affected (data not shown).
Fig. 4.
Toxicity of indole analogs on honey bee larvae. Honey bee larvae were reared in larval diet supplemented with water (open circle), 500 µM of 5-bromoindole (filled circle), 500 µM of 5-cholorindole (open square), 500 µM of 5-nitroindole (filled square), 500 µM of 6-bromoindole (open triangle), or 500 µM of 6-cholorindole (filled triangle). Surviving larvae were counted daily. Differences between survival curves were determined using Kaplan–Meier analysis, log-rank test (χ2=19.422, df = 5, P < 0.002), and the Holm–Šidák method (P < 0.05).
Indole Analogs Protect Honey Bee Larvae from AFB Disease
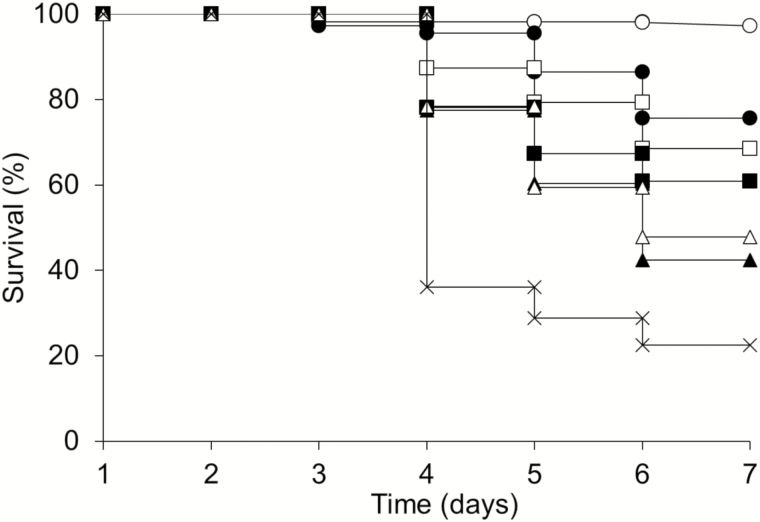
Second instar larvae-fed P. larvae spores started dying rapidly 4 d after challenge (Fig. 5). Only 22% of challenged untreated larvae survived to pupation. Honey bee larval survival was significantly higher (Holm–Šidák method, P < 0.05) for larvae that were fed with indole analogs after spore challenge. The highest survival percentages were observed in challenged larvae treated with 5-bromoindole (75%) or 5-chloroindole (65%).
Fig. 5.
Effects of indole analogs on AFB disease. Honey bee larvae were challenged with P. larvae spores in larval diet supplemented with water (crosses), 500 µM of 5-bromoindole (filled circles), 500 µM of 5-cholorindole (open squares), 500 µM of 5-nitroindole (filled squares), 500 µM of 6-bromoindole (open triangles), or 500 µM of 6-cholorindole (filled triangles). Unchallenged larvae (open circles) were used as experimental control and showed >95% survival. Differences between survival curves were determined using Kaplan–Meier analysis, log-rank test (χ2=194.484, df = 6, P < 0.001), and the Holm–Šidák method (P < 0.05).
Discussion
Previous work from our laboratory has shown the importance of using chemical probes to explore germination requirements that permit spores to exit their dormant stage (Dodatko et al. 2009; Alvarez et al. 2010; Howerton et al. 2011; Alvarado et al. 2013; Howerton et al. 2013a,b). We have identified germination inhibitors for B. anthracis, B. cereus, C. perfringens, C. sordellii, C. difficile, and P. larvae (Akoachere et al. 2007; Alvarez and Abel-Santos 2007; Ramirez and Abel-Santos 2010; Dodatko et al. 2010; Howerton et al. 2011; Luu et al. 2011; Liggins et al. 2011; Howerton et al. 2013a,b). In this work, we expanded our search for indole analogs that would prevent P. larvae spore germination in vitro and reduced AFB disease in laboratory-reared larvae.
Most compounds tested in this study did not significantly reduce P. larvae spore germination in vitro. Changing the nitrogen or carbon atoms in the indole ring reduced the inhibitory effect of the molecule. Indeed, 13 out of the 35 compounds tested differed from indole only by two atoms but were unable to inhibit P. larvae spore germination. These results suggest that an intact indole ring is essential for binding at the putative germinant binding site.
The strongest inhibitors of P. larvae spore germination were indole analogs with electron-withdrawing groups (halide, cyano, and nitro). We hypothesize that indole analogs compete with the germinant uric acid for binding to the putative germination receptors. Both indole and uric acid structures are composed of two heteroaromatic rings. Electron-withdrawing groups in the most active indole analogs may have improved interaction with the putative germination receptor by mimicking the carbonyl groups found in uric acid.
Uric acid is present at saturating concentrations inside the gut of honey bee larvae, resulting in the formation of large crystals (Winston 1987, Yadav 2003). Similarly, the concentration of tyrosine in the larval gut has been estimated to be in the millimolar range (Liming et al. 2009). Indeed, the concentration of uric acid plus l-tyrosine used to identify P. larvae germination inhibitors in this study was 3000 µM. We identified five indole analogs (5-bromoindole, 5-chloroindole, 5-nitroindole, 6-bromoindole, and 6-chloroindole) that had IC50 values ~5–20 times lower than indole is spore germination inhibition assays while having very low antibiotic activity. These results are encouraging because utilizing antibiotic compounds for AFB treatment would certainly alter the microbiome and honey bee health.
The five indole analogs selected not only prevented P. larvae spore germination in vitro but protected honey bee larvae from AFB disease at concentrations below the toxicity threshold. These five indole analogs are similar in their protective properties due to their common structural features. Thus, as the previous studies suggest, indole and indole analogs can serve as protective compounds against infectious organisms (Ueno et al. 2005, Kim et al. 2011, Alvarado et al. 2013). Because of our intervention, these larvae entered their pupation stage despite AFB infection.
Conclusion
AFB, a bacterial disease of honey bee larvae, is particularly troublesome because the infectious agent is the bacterial spore of P. larvae (Genersch 2010). Bacterial spores are resistant to extreme temperatures, antibiotics, and disinfectants. Spores can remain dormant for years until they can revert to vegetative cells (Alippi 1995, Dobbelaere et al. 2001, Lodesani and Costa 2005). In this work, we tested the hypothesis that inhibiting P. larvae spore germination using indole analogs will result in decreased AFB disease in laboratory-reared honey bee larvae. We found that halide-substituted indoles prevented P. larvae spore germination in vitro and AFB disease in laboratory-reared larvae, suggesting that compounds like 5-chloroindole can be tested to develop a cheap, easily administered, and non-toxic treatment.
Five indole analogs were identified that were potent inhibitors of spore germination in vitro, non-toxic to vegetative cells, non-toxic to larvae, and able to protect honey bee larvae from AFB disease. In every case, active indole analogs contained an electron-withdrawing group that made the indole ring electron-poor. We hypothesize that indole analogs interact with a putative electron-rich binding site that normally recognizes the carbonyl-rich uric acid germinant.
The larvae gut contains bacteria known to inhibit P. larvae vegetative growth (Evans 2004, Evans and Armstrong 2006, Yoshiyama and Kimura 2009, Forsgren et al. 2010). These bacteria should be preserved to avoid complications due to antimicrobial treatment with tetracycline (Raymann et al. 2017). Tetracycline treatment reduces the size and diversity of the microbiome observed in healthy honey bees. Furthermore, there is a correlation between the tetracycline use and a reduction in survival of bees exposed to opportunistic pathogens. The results of our work suggest that substituted indole analogs have weak antibiotic activity and should not affect the gut microbiome of the larvae.
It is improbable that P. larvae will develop resistance to indole analog treatments. One reason is that the dormant stage of the microorganism is targeted, rather than vegetative cells. Furthermore, if P. larvae alters the germination receptors, they might never exit dormancy or would germinate in an environment not suited for this fastidious organism. Thus, we will continue to identify compounds that block P. larvae germination receptors.
Developing a suitable inhibitor delivery system for honey bee colonies is the final step toward a potential disease treatment. Ideally, the treatment substances would be ingested, be effective at low concentrations, and degrade prior to contaminating honey stores. Honey bees are often fed sugar–water mixtures, sugar powder, or patties infused with treatments (Elzen et al. 2002, Yoder et al. 2014). In the colony, both nectar and pollen are eaten by bees performing larval care (nurse) duties (Crailsheim et al. 1992, Grüter and Farina 2007). Once ingested by the nurse bees, the compounds can be delivered to individual honeycomb cells. Hence, nurse bees, normally a vector for the spread of P. larvae spores, could be used to prevent AFB (Gillard et al. 2008, Lindström et al. 2008, Garbian et al. 2012). The compounds identified need to work at low concentrations so that they cannot accumulate in the hive and the AFB treatment remains cost effective. Similarly, the half-life of the compounds needs to be limited to days or weeks at most so that no residue remains in wax, honey, or pollen (Frison et al. 1999, Bogdanov 2006, Lopez et al. 2008). To see if P. larvae spore germination can be further inhibited, indole analogs with multiple electron-withdrawing atoms at positions 2, 5, and 7 should be tested. The results presented here suggest that developing a new, inexpensive, non-toxic, and effective AFB treatment based on indole analogs is possible.
Supplementary Data
Supplementary data is available at Journal of Insect Science online.
Supplementary Material
Acknowledgments
We thank Rodney Mehring for beekeeping assistance. This project was supported by the Agriculture and Food Research Initiative competitive grant 011-67013-30169 from the USDA National Institute of Food and Agriculture. I.A. was supported by UNLV through a DGR award and the Hermsen Fellowship. Participation by MME in this work was supported by the National Science Foundation while serving at the NSF. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the NSF.
References Cited
- Abel-Santos E., and Dodatko T.. 2007. Differential nucleoside recognition during Bacillus cereus 569 (ATCC 10876) spore germination. New J. Chem. 31: 748–755. [Google Scholar]
- Adjlane N., Haddad N., and Kechih S.. 2014. Comparative study between techniques for the diagnosis of American Foulbrood (Paenibacillus larvae) in honeybee colony. J. Anim. Vet. Adv. 13: 970–973. [Google Scholar]
- Akoachere M., Squires R. C., Nour A. M., Angelov L., Brojatsch J., and Abel-Santos E.. 2007. Identification of an in vivo inhibitor of Bacillus anthracis Sterne spore germination. J. Biol. Chem. 282: 12112–12118. [DOI] [PubMed] [Google Scholar]
- Alippi A. 1995. Detection of Bacillus larvae spores in Argentinian honeys by using a semi-selective medium. Microbiol. Semin. 11: 343–350. [PubMed] [Google Scholar]
- Alippi A. M., López A. C., Reynaldi F. J., Grasso D. H., and Aguilar O. M.. 2007. Evidence for plasmid-mediated tetracycline resistance in Paenibacillus larvae, the causal agent of American Foulbrood (AFB) disease in honeybees. Vet. Microbiol. 125: 290–303. [DOI] [PubMed] [Google Scholar]
- Alvarado I., Phui A., Elekonich M. M., and Abel-Santos E.. 2013. Requirements for in vitro germination of Paenibacillus larvae spores. J. Bacteriol. 195: 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Z., and Abel-Santos E.. 2007. Potential use of inhibitors of bacteria spore germination in the prophylactic treatment of anthrax and Clostridium difficile-associated disease. Expert Rev. Anti. Infect. Ther. 5: 783–792. [DOI] [PubMed] [Google Scholar]
- Alvarez Z., Lee K., and Abel-Santos E.. 2010. Testing nucleoside analogues as inhibitors of Bacillus anthracis spore germination in vitro and in macrophage cell culture. Antimicrob. Agents Chemother. 54: 5329–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanov S. 2006. Contaminants of bee products. Apidologie 37: 1–18. [Google Scholar]
- Cortezzo D. E., Setlow B., and Setlow P.. 2004. Analysis of the action of compounds that inhibit the germination of spores of Bacillus species. J. Appl. Microbiol. 96: 725–741. [DOI] [PubMed] [Google Scholar]
- Cox-Foster D. L., Conlan S., Holmes E. C., Palacios G., Evans J. D., Moran N. A., Quan P. L., Briese T., Hornig M., Geiser D. M., et al. 2007. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 318: 283–287. [DOI] [PubMed] [Google Scholar]
- Crailsheim K., Schneider L., Hrassnigg N., Bühlmann G., Brosch U., Gmeinbauer R., and Schöffmann B.. 1992. Pollen consumption and utilization in worker honeybees (Apis mellifera carnica): dependence on individual age and function. J. Insect Physiol. 38: 409–419. [Google Scholar]
- Crailsheim K., Brodschneider R., Aupinel P., Behrens D., Genersch E., Vollmann J., and Riessberger-Gallé U.. 2013. Apis mellifera larvae. J. Apic. Res. 52: 1. [Google Scholar]
- Dainat B., Evans J. D., Chen Y. P., Gauthier L., and Neumann P.. 2012. Predictive markers of honey bee colony collapse. PLoS One 7: e32151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere W., De Graaf D. C., Reybroeck W., Desmedt E., Peeters J. E., and Jacobs F. J.. 2001. Disinfection of wooden structures contaminated with Paenibacillus larvae subsp. larvae spores. J. Appl. Microbiol. 91: 212–216. [DOI] [PubMed] [Google Scholar]
- Dodatko T., Akoachere M., Muehlbauer S., Helfrich F., Howerton A., Ross C., Wysocki V., Brojatsch J., and Abel-Santos E.. 2009. Bacillus cereus spores release alanine that synergizes with inosine to promote germination. PLoS One 4: e6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodatko T., Akoachere M., Jimenez N., Alvarez Z., and Abel-Santos E.. 2010. Dissecting interactions between nucleosides and germination receptors in Bacillus cereus 569 spores. Microbiology 156: 1244–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzen P., Westervelt D., Causey D., Ellis J., Hepburn H., and Neumann P.. 2002. Method of application of tylosin, an antibiotic for American Foulbrood control, with effects on small hive beetle (Coleoptera: Nitidulidae) populations. J. Econ. Entomol. 95: 1119–1122. [DOI] [PubMed] [Google Scholar]
- Evans J. D. 2004. Transcriptional immune responses by honey bee larvae during invasion by the bacterial pathogen, Paenibacillus larvae. J. Invertebr. Pathol. 85: 105–111. [DOI] [PubMed] [Google Scholar]
- Evans J., and Armstrong T.. 2006. Antagonistic interactions between honey bee bacterial symbionts and implications for disease. BMC Ecol. 6: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren E., Stevanovic J., and Fries I.. 2008. Variability in germination and in temperature and storage resistance among Paenibacillus larvae genotypes. Vet. Microbiol. 129: 342–349. [DOI] [PubMed] [Google Scholar]
- Forsgren E., Olofsson T. C., Vasquez A., and Fries I.. 2010. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie 41: 99–108. [Google Scholar]
- Fries I., Lindström A., and Korpela S.. 2006. Vertical transmission of American foulbrood (Paenibacillus larvae) in honey bees (Apis mellifera). Vet. Microbiol. 114: 269–274. [DOI] [PubMed] [Google Scholar]
- Frison S., Breitkreitz W., Currie R., Nelson D., and Sporns P.. 1999. The analysis of fluvalinate in beeswax using GC/MS. Food Res. Int. 32: 35–41. [Google Scholar]
- Garbian Y., Maori E., Kalev H., Shafir S., and Sela I.. 2012. Bidirectional transfer of RNAi between honey bee and Varroa destructor: Varroa gene silencing reduces Varroa population. PLoS Pathog. 8: e1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genersch E. 2010. American Foulbrood in honeybees and its causative agent, Paenibacillus larvae. J. Invertebr. Pathol. 103: S10–S19. [DOI] [PubMed] [Google Scholar]
- Genersch E., Ashiralieva A., and Fries I.. 2005. Strain- and genotype-specific differences in virulence of Paenibacillus larvae subsp larvae, a bacterial pathogen causing American Foulbrood disease in honeybees. Appl. Environ. Microbiol. 71: 7551–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard M., Charriere J. D., and Belloy L.. 2008. Distribution of Paenibacillus larvae spores inside honey bee colonies and its relevance for diagnosis. J. Invertebr. Pathol. 99: 92–95. [DOI] [PubMed] [Google Scholar]
- Grüter C., and Farina W.. 2007. Nectar distribution and its relation to food quality in honeybee (Apis mellifera) colonies. Insectes Soc. 54: 87–94. [Google Scholar]
- Howerton A., Ramirez N., and Abel-Santos E.. 2011. Mapping interactions between germinants and Clostridium difficile spores. J. Bacteriol. 193: 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton A., Patra M., and Abel-Santos E.. 2013a. Fate of ingested Clostridium difficile spores in mice. PLoS One 8: e72620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howerton A., Patra M., and Abel-Santos E.. 2013b. A new strategy for the prevention of Clostridium difficile infection. J. Infect. Dis. 207: 1498–1504. [DOI] [PubMed] [Google Scholar]
- Huang Z. 2009. A standardized procedure for the in vitro rearing of honey bee larvae. http://www.cdpr.ca.gov/docs/registration/reevaluation/larval_protocol.pdf (accessed 16 September 2017). [Google Scholar]
- Kim Y., Lee J., Cho M., and Lee J.. 2011. Indole and 3-indolylacetonitrile inhibit spore maturation in Paenibacillus alvei. BMC Microbiol. 11: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liggins M., Ramirez N., Magnuson N., and Abel-Santos E.. 2011. Progesterone analogs influence germination of Clostridium sordellii and Clostridium difficile spores in vitro. J. Bacteriol. 193: 2776–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liming W., Jinhui Z., Xiaofeng X., Yi L., and Jing Z.. 2009. Fast determination of 26 amino acids and their content changes in royal jelly during storage using ultra-performance liquid chromatography. J. Food Compost. Anal. 22: 242–249. [Google Scholar]
- Lindström A., Korpela S., and Fries I.. 2008. The distribution of Paenibacillus larvae spores in adult bees and honey and larval mortality, following the addition of American Foulbrood diseased brood or spore-contaminated honey in honey bee (Apis mellifera) colonies. J. Invertebr. Pathol. 99: 82. [DOI] [PubMed] [Google Scholar]
- Lodesani M., and Costa M.. 2005. Limits of chemotherapy in beekeeping: development of resistance and the problem of residues. Bee World 86: 102–109. [Google Scholar]
- Lopez M. I., Pettis J. S., Smith I. B., and Chu P.. 2008. Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS. J. Agric. Food Chem. 56: 1553–1559. [DOI] [PubMed] [Google Scholar]
- Luu H., Akoachere M., Patra M., and Abel-Santos E.. 2011. Cooperativity and interference of germination pathways in Bacillus anthracis spores. J. Bacteriol. 193: 4192–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson A. 1993. World bee health report. Bee World 74: 179–212. [Google Scholar]
- Morse R.A. and Calderone N.W. 2000. The value of honey bees as pollinators of U.S. crops. Bee Culture 128: 1–15. [Google Scholar]
- Neumann P., and Carreck N. L.. 2010. Honey bee colony losses. J. Apic. Res. 49: 1–6. [Google Scholar]
- Peng Y. C., Mussen E., Fong A., Montague M. A., and Tyler T.. 1992. Effects of chlortetracycline of honey bee worker larvae reared in vitro. J. Invertebr. Pathol. 60: 127–133. [Google Scholar]
- Powell J. F. 1950. Factors affecting the germination of thick suspensions of Bacillus subtilis spores inl-alanine solution. J. Gen. Microbiol. 4: 330–338. [DOI] [PubMed] [Google Scholar]
- Ramirez N., and Abel-Santos E.. 2010. Requirements for germination of Clostridium sordellii spores in vitro. J. Bacteriol. 192: 418–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymann K., Shaffer Z., and Moran N. A.. 2017. Antibiotic exposure perturbs the gut microbiota and elevates mortality in honeybees. PLoS Biol. 15: e2001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas J., and Ellar D.. 1985. Uric acid and allantoin uptake by Bacillus fastidiosus spores. Febs. Lett. 183: 256–259. [Google Scholar]
- Schaeffer A. B., and Fulton M. D.. 1933. A simplified method of staining endospores. Science 77: 194–194. [DOI] [PubMed] [Google Scholar]
- Segur J. 1953. Physical properties of glycerol and its solutions. Glycerol 2:347–348. [Google Scholar]
- Shimanuki H., and Knox D.. 2000. Diagnosis of honey bee diseases. U.S. Department of Agriculture, Beltsville, MD. [Google Scholar]
- Smith M., and Sullivan C.. 1989. Germination of Clostridium cylindrosporum spores on medium containing uric acid. Appl. Environ. Microbiol. 55: 1380–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturtevant A. P. 1932. Relation of commercial honey to the spread of American Foulbrood. J. Agric. Res. 45: 257. [Google Scholar]
- Ueno M., Kihara J., Honda Y., and Arase S.. 2005. Effects of some indole-related compounds on the infection behavior of Magnaporthe grisea. J. Gen. Plant. Pathol. 71: 196–199. [Google Scholar]
- White G. F. 1920. American foulbrood. U.S. Dept of Agriculture, Washington, DC. [Google Scholar]
- Winston M. L. 1987. The biology of the honey bee. Harvard University Press, Cambridge, MA. [Google Scholar]
- Woese C. R., Morowitz H. J., and Hutchison C. A.. 1958. Analysis of action ofl-alanine analogues in spore germination. J. Bacteriol. 76: 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M. 2003. Physiology of insects. Discovery Publishing House Pvt. Limited, New Delhi, India. [Google Scholar]
- Yoder J. A., Jajack A. J., Cornacchione W. S., Dunn A. L., Cunningham E. G., Matchett C. L., and Rosselot A. E.. 2014. In vitro evaluation of sugar syrups, antibiotics, and miticides on growth of honey bee pathogen, Ascosphaera apis: emphasis for chalkbrood prevention is on keeping bees healthy. Apidologie 45: 568–578. [Google Scholar]
- Yoshiyama M., and Kimura K.. 2009. Bacteria in the gut of Japanese honeybee, Apis cerana japonica, and their antagonistic effect against Paenibacillus larvae, the causal agent of American Foulbrood. J. Invertebr. Pathol. 102: 91–96. [DOI] [PubMed] [Google Scholar]
- Yue D., Nordhoff M., Wieler L. H., and Genersch E.. 2008. Fluorescence in situ hybridization (FISH) analysis of the interactions between honeybee larvae and Paenibacillus larvae, the causative agent of American foulbrood of honeybees (Apis mellifera). Environ. Microbiol. 10: 1612–1620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.