Abstract
Background
Our study aims to evaluate the current perinatal registry, analyze national childbirth outcomes and study ethnic disparities in middle-income country Suriname, South America.
Methods
A nationwide birth registry study was conducted in Suriname. Data were collected for 2016 and 2017 from the childbirth books of all five hospital maternity wards, covering 86% of all births in the country. Multinomial regression analyses were used to assess ethnic disparities in outcomes of maternal deaths, stillbirths, teenage pregnancy, cesarean delivery, low birth weight and preterm birth with Hindustani women as reference group.
Results
18.290 women gave birth to 18.118 (98%) live born children in the five hospitals. Hospital-based maternal mortality ratio was 112 per 100.000 live births. Hospital-based late stillbirth rate was 16 per 1000 births. Stillbirth rate was highest among Maroon (African-descendent) women (25 per 1000 births, aOR 2.0 (95%CI 1.3–2.8) and lowest among Javanese women (6 stillbirths per 1000 births, aOR 0.5, 95%CI 0.2–1.2). Preterm birth and low birthweight occurred in 14 and 15% of all births. Teenage pregnancy accounted for 14% of all births and was higher in Maroon women (18%) compared to Hindustani women (10%, aOR 2.1, 95%CI 1.8–2.4). The national cesarean section rate was 24% and was lower in Maroon (17%) than in Hindustani (32%) women (aOR 0.5 (95%CI 0.5–0.6)). Cesarean section rates varied between the hospitals from 17 to 36%.
Conclusion
This is the first nationwide comprehensive overview of maternal and perinatal health in a middle income country. Disaggregated perinatal health data in Suriname shows substantial inequities in outcomes by ethnicity which need to be targetted by health professionals, researchers and policy makers.
Keywords: Perinatal registry, Maternal mortality, Stillbirths, Middle-income country, Suriname, Ethnicity, Racial, Disaggregate
Plain English summary
In middle-income country Suriname we studied all hospital births to describe childbirth outcomes and to discover inequities by ethnicity. In 2016 and 2017, 18.290 women gave birth to a baby in either of the five hospitals, which represents 86% of all national births in Suriname. There were 20 maternal deaths, resulting in a maternal mortality ratio of 112 per 100.000 live births. There were 285 stillbirths beyond 28 weeks of gestation, resulting in a late stillbirth rate of 16 per 1000 births. Stillbirth rate was highest in Maroon women (25 per 1000 births) and lowest in Javanese women (6 per 1000 births). Teenage pregnancies accounted for 14% of all births, was highest in Indigenous (21%) and Maroon (18%) women, and lowest in Hindustani (10%) and Chinese (3%) women. Babies with low birth weight accounted for 15% of all births and were most frequently seen in Hindustani women (18%). The cesarean section rate was 24%, but varied from 17 to 36% between the five hospitals. Hindustani women were almost twice more likely to receive a cesarean section than Maroon women (32 vs. 17%). Disaggregating perinatal health data is encouraged to identify and help target inequity within the health system. In conclusion, there are substantial inequities by ethnicity with Maroon women experiencing the highest risk on adverse outcomes (maternal mortality, stillbirth, increased preterm birth, low apgar score). The inequitable access to care experienced by women of African-descent requires policy makers to review possible interventions.
Background
Maternal and perinatal vital registration systems are essential to monitor outcomes of pregnant women and their offspring, identify inequities in service provision and health outcomes and facilitate quality control in perinatal care [1, 2]. Following lessons learned from the Millennium Development Goals (MDGs), the Sustainable Development Goals (SDGs) call for statistics “disaggregated by income, gender, age, race, ethnicity, migratory status, disability, geographic location and other characteristics relevant in national contexts” to monitor progress and identified inequities in health outcomes [2–4].
The Global Strategy for Women’s, Children’s and Adolescents’ Health, 2016–2030 is a roadmap for ending all preventable maternal and newborn deaths (including stillbirths) and is central to the achievement of the SDGs [5]. This strategy urgently calls for the extension and strengthening of health information systems to generate high quality data and evidence to measure progress and be able to reach the target of a global maternal mortality ratio (MMR) under 70 per 100.000 live births and stillbirth rate (SBR) under 12 stillbirths per 1000 births [2, 6].
National childbirth registries have been established in several high income countries [7–9]. Low and middle-income countries (LMIC) are increasingly investing in robust national information on maternal and perinatal health indicators for SDG monitoring. However, given the complexity of establishing a well-functioning registry system, data collection in these countries is often not uniform, lack important indicators and data are frequently missing [1]. In many (currently, 34) Latin American and Caribbean countries, the Perinatal Information System (SIP), a digital clinical record and local management software standard has been implemented [10]. Suriname, an upper middle-income country, is one of the countries where SIP will be re-launched after a previous attempt in 2014, which failed for unknown reasons [11].
Ethnic disparities in birth outcomes have modestly been studied compared to other social determinants of health, such as wealth, education, age and place of residence. Several studies in high-income countries have shown that women of African descent experience a two to six times higher risk for severe maternal outcomes compared to Caucasian women (often linked to socio-economic factors) [12–16]. Suriname is particularly of interest as it has multiple ethnic groups without one great majority [17].
To promote an efficient and adequate implementation process of the new perinatal data system in Suriname and subsequently evaluate its effect, this study provides a baseline assessment of perinatal data from all hospitals in the country. Our study aims to evaluate the current perinatal registry, analyze national maternal and perinatal characteristics and study ethnic disparities in childbirth outcomes in Suriname.
Methods
Study design
A two-year registry-based nationwide study of all hospital births was conducted, using the childbirth books of the five maternity wards between January 1st, 2016 and December 31st, 2017.
Study context and ethnicities
Suriname is a multi-ethnical, upper middle-income country on the northeast coast of South-America. With an estimated population of 598,000 people, it is one of the least populous countries in the Americas [18, 19]. Ethnical distribution among the general Surinamese population in 2013 was: Hindustani (27%), Maroon (22%), Creole (16%), Javanese (14%), Mixed (13%), Indigenous (4%), Chinese (1%) and Other (3%) [19, 20]. Diversity in Suriname is a reflection of the country’s history. Indigenous people, also known as Amerindians, are the original inhabitants of the country. Maroon and Creoles are African-descendants who were enslaved and brought to Suriname in the seventeenth and eighteenth century. Maroon people, in contrast to Creoles, escaped into the interior of the country. Creoles gained their freedom in 1863 when slavery was abolished in Suriname and often have mixed African - European (Dutch and British) ancestry. Asian-descendants: Hindustani (from East-India), Javanese (from Indonesia, then a Dutch-ruled colony) and Chinese people, came to Suriname in the late nineteenth century as contract workers. Mixed people are the result of interchanging identities between almost all ethnicities. Other ethnicities include mostly Brazilians, Caucasians (descendants of Dutch colonists) and few Lebanese [17].
Study setting
The study was conducted in all hospitals in Suriname: four hospitals located in the capital Paramaribo and one hospital in Nickerie (West coast). Institutions perform approximately 92% of all births in Suriname with approximately 86% in hospitals and 6% in primary health care centers. Information regarding the primary health care births, home births (4%) and births of unknown location (4%) were not available [19, 20].
The Multiple Indicator Cluster Survey (MICS) of 2018, estimated that 13% of women did not receive antenatal care, 56% received their first antenatal care visit within the first 3 months of pregnancy, 62–80% received at least four antenatal care visits and 95–98% of births were attended by skilled birth attendants [20]. Maternal mortality has dropped 43%, from 226 to 130 per 100.000 live births, between 1990 and 2015, as demonstrated by two Reproductive Age Mortality Surveys [21, 22].
Participants and data collection
All hospital births with babies born by at least 22 weeks of gestation or with a birth weight of at least 500 g, were eligible for inclusion [23]. Paper childbirth books provide manually written data on every birth in all hospitals. Birth attendants are responsible for the information to be registered in the books. Hospital personnel digitalized this data in Microsoft Excel and IBM SPSS with instructions and prespecified definitions. All variables were entered in the secured database anonymously. Three of the authors (KV, ZP, RP) cross-checked the digital data with the paper childbirth book data. Medical files were assessed for stillbirths and maternal deaths to validate the death.
Variables
The digitalization, software used and reported variables per hospital can be found in Table 1 and an elaborated version in Additional file 1. Ethnicity was self-reported similar to the MICS [20]. Teenage pregnancy was defined as childbirth below the age of 20 years [24]. Severe anemia was defined according to the WHO definition: a hemoglobin level below 70 g/L (4.3 mmol/L) was considered severe and below 100 g/L (6.2 mmol/L) was moderate [25]. Sickle cell anemia is assessed during antenatal care in each woman in Suriname, but not reported in the childbirth book and could therefore not be studied. Outcome variables were coded into categories, based on the Dutch Perinatal data registry data [26]. Preterm birth was defined as childbirth before 37 weeks of gestation [26]. Low birth weight was defined as a newborn with a birth weight below 2500 g [26]. APGAR score was considered low when the 5 min APGAR score was below 7 [26]. Postpartum hemorrhage (PPH) was defined as blood loss of at least 500 mL and severe PPH at least 1000 mL [27]. Stillbirth was defined as a fetus born with no signs of life. Late stillbirth was defined, according to the International Classification of Diseases – Perinatal Mortality (ICD-PM), as stillbirth after 28 weeks of gestation or, if gestational age was unknown, birth weight of 1000 g or more [28]. Stillbirth rate (SBR) was calculated as the number of late stillbirths per 1000 births beyond 28 weeks of gestation (or > 1000 g) [28]. Maternal death was defined according to the International Classification of Diseases – Maternal Mortality (ICD-MM), as death of a woman while pregnant or within 42 days of termination of pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes [29]. The national MMR was calculated as the number of maternal deaths per 100.000 live births beyond 22 weeks of gestation (or > 500 g) [29]. Data on social economic status such as income and level of education, and data on obesity and smoking were not available.
Table 1.
Overview of maternal and perinatal data registration per hospital in Suriname
| Hospital | |||||
|---|---|---|---|---|---|
| I | II | III | IV | V | |
| Childbirth registry | On paper | On paper | On paper | On paper | On paper |
| Digitalizing of paper parturition books | Special secretary for this task | General secretary, with other responsibilities | General secretary, with other responsibilities | No one; students used for this study | Responsible midwife doing the delivery |
| Software used | SPSS | Excel | Access | Excel | Excel |
| Common reported variables | Maternal age, ethnicity, gravidity, parity, gestational age, singleton or twin, data and time of delivery, presentation, mode of delivery, indication for cesarean section, APGAR score after 1 and 5 min, sex, birth weight, length, head circumference, stillbirth, weight placenta, length of umbilical cord, rupture type, blood loss | ||||
| Other variables reported per hospital | |||||
| HIV | Yes | – | – | Yes | Yes |
| Hepatitis B | – | – | – | Yes | – |
| Syphilis | – | – | – | Yes | – |
| 1st antenatal care visit | Yes | – | – | Yes | – |
| Amount of ANC visits | – | – | Yes | – | – |
| 1st ultrasound | Yes | – | – | – | – |
| Hemoglobin level | Yes | – | – | Yes | Yes |
| Blood type and rhesus | Yes | Yes | – | Yes | Yes |
| Induction of labor | Yes | – | – | – | – |
| Augmentation of labor | Yes | – | – | – | – |
| Duration 3rd stage of labor | Yes | – | – | – | – |
| Active 3rd stage of labor | – | Yes | Yes | Yes | Yes |
Statistical methods
Descriptive statistics were used for baseline characteristics analysis using frequencies and percentages for categorical data. Differences between groups were tested with chi-square test for significance (p < 0.05). Missing data on ethnicity was negligible (< 5%) and no data imputation was performed. To assess the relation between ethnic groups and health outcomes, multinomial logistic regression was performed. Hindustani ethnicity was used as reference group as they represent the largest proportion of the general population. Odds ratios (OR) were obtained, which were interpreted as relative risks in the case that the probability of the disease was less than 10% [30]. Possible confounders were selected by constructing causal diagrams and adjusted odds ratios (aOR) were obtained [31]. IBM SPSS version 25 was used for statistical analyses.
Ethical approval
This research was performed according to the Declaration of Helsinki and has been approved by the ethical review board of the Surinamese Committee on Research Involving Human Subjects (#VG11–18).
Results
There were 18.290 hospital births, 9202 in 2016 and 9088 in 2017. A total of 18.504 babies were born, 97.9% (n = 18.118) were live births.
The approach to maternal and perinatal data registration by the different hospitals is shown in Table 1. Additional file 2 illustrates maternal and neonatal characteristics and outcomes per hospital. The largest differences between the hospitals are teenage pregnancy rates (range from 7.3 to 17.9%), ethnical distribution, grand multiparous births (range from 4.6 to 18.5%), low birth weight rates (range from 11.3 to 20.8%), cesarean section rates (range from 17.4 to 36.2%) and stillbirth rates (range from 7.3 to 25.6 per 1000 births).
Table 2 presents maternal and perinatal characteristics. Median age for nulliparous women to give birth was 22 (IQR 19–27) years. Teenage pregnancies occurred in 13.8% (n = 2518) of births. The majority of women giving birth were African descendants: Maroon (27.6%) and Creole (23.5%) women (see Fig. 1). Preterm birth occurred in 14.0% (n = 2529) and birthweight was below 2500 g in 15.1% (n = 2774). Cesarean section was performed in 24.1% of all births. In primiparous women the cesarean section rate was 27.0% (n = 1683). Repeat cesareans (n = 1321) contributed to 30% of all cesarean sections. Post partum hemorrhage of 500 mL or more occurred in 7.9% of births (n = 1287/16348). In cesarean sections the incidence of post partum hemorrhage was 17.9% (n = 501/2838, missings n = 1571) and in vaginal births 5.8% (n = 786/13510, missings n = 371).
Table 2.
Maternal characteristics of all hospital births in Suriname in 2016 and 2017
| Total n = (%) |
|
|---|---|
| Total deliveries | 18,290 |
| Hospitals | |
| I | 4380 (23.9) |
| II | 5070 (27.7) |
| III | 5089 (27.8) |
| IV | 3034 (16.6) |
| V | 717 (3.9) |
| Age (years) | |
| < 20 | 2518 (13.8) |
| 20–35 | 13,646 (74.8) |
| > 36 | 2087 (11.4) |
| Missings n = 39 | |
| Ethnicity | |
| Maroon | 4950 (27.6) |
| Creole | 4217 (23.5) |
| Hindustani | 3395 (18.9) |
| Mixed | 2254 (12.5) |
| Javanese | 1948 (10.9) |
| Indigenous / Amerindian | 681 (3.8) |
| Chinese | 381 (2.1) |
| Othera | 137 (0.8) |
| Missings n = 327 | |
| Parity | |
| 0 | 6243 (34.3) |
| 1–3 | 9553 (52.4) |
| > 4 | 2428 (13.3) |
| Missings n = 66 | |
| Gestational age (GA) | |
| < 28 weeks | 217 (1.2) |
| 28–36 weeks | 2312 (12.8) |
| > 37 weeks | 15,569 (86.0) |
| Missings n = 192 | |
| Anemia | |
| Moderate | 2571 (39.9) |
| Severe | 153 (2.4) |
| Missings n = 11.851 | |
| Multiple pregnancy | |
| Twin | 208 (1.1) |
| Triplet | 3 (−) |
| Mode of delivery | |
| Spontaneous | 13,650 (74.6) |
| Instrumental | 231 (1.3) |
| Cesarean section | 4409 (24.1) |
| Presentation | |
| Cephalic | 17,671 (96.6) |
| Breech | 560 (3.1) |
| Transverse | 59 (0.3) |
| Post partum hemorrhage | |
| 500–999 mL | 1031 (6.3) |
| > 1000 mL | 256 (1.6) |
| Missings n = 1942 | |
| Total babies born | 18,504 |
| Live births | 18,118 (97.9) |
| Stillbirths, > 22 weeks | 386 (2.1) |
| Sex | |
| Girls | 8915 (48.2) |
| Missings n = 17 | |
| Birth weight | |
| < 2500 g | 2774 (15.1) |
| 2500–4000 g | 15,069 (81.9) |
| > 4000 g | 558 (3.0) |
| Missings n = 103 | |
| Apgar-score 5 min | |
| Below 7 | 648 (3.9) |
| Missings n = 1704 | |
| Late stillbirth | |
| n= | 285 |
| SBR per 1000 hospital births b | 15.6 |
| Maternal deaths | |
| n= | 25c |
| National MMR per 100.000 LB d | 127 |
| n= | 20 |
| Hospital-based MMR per 100.000 LB d | 110 |
aEthnicity other: Brazilian (n = 101, 0.5%), Caucasian (n = 25, 0.1%), Caribbean (n = 11)
bSBR: stillbirth rate = per 1000 births > 28 weeks or > 1000 g
cTwo maternal death occurred at home, one during transport, two in primary health care services. Four deaths occurred antepartum, three of which in the hospitals
dMMR: maternal mortality rate, maternal deaths per 100.000 live births (LB)
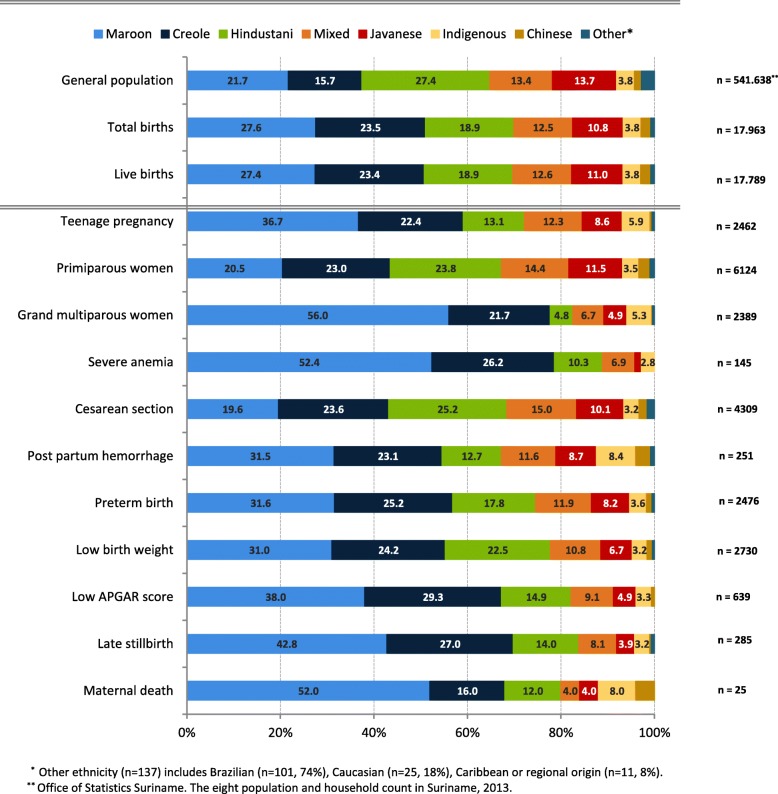
Fig. 1.
Ethnic distribution in percentages among the pregnant population
Twenty maternal deaths occurred in the hospitals, resulting in a hospital-based maternal mortality ratio (MMR) of 112 per 100.000 live births. Among all babies with a gestational age of > 22 weeks or more (or if unknown, > 500 g), 386 stillbirths (2.1%) occurred. Of these, 285 were late stillbirths (gestational age > 28 or if unknown > 1000 g), resulting in a hospital-based stillbirth rate of 15.6 per 1000 births.
Table 3 and Fig. 1 show ethnic disparities in maternal and perinatal characteristics. Table 4 presents associations between ethnic group and outcomes, adjusted for confounders and with Hindustani women as reference group. Teenage pregnancy was twice higher among Maroon women (18.3%, n = 904) compared to Hindustani women (9.5%, n = 322), (aOR 2.1, 95%CI 1.8–2.4). Compared to Hindustani women, Maroon women had a higher risk for severe anemia (4.9%, aOR 2.4, 95%CI 1.4–4.3). Maroon and Indigenous women were most often grand multiparous women (27.1%, aOR 14.4 (95%CI 11.9–18.1) and 19%, aOR 9.9 (95%CI 7.4–13.4)) compared to Hindustani (3.4%). Women of other ethnicities had a significantly lower risk of giving birth to low birth weight infants compared to Hindustani women (aOR range of 0.4–0.7). The cesarean section rate was lower in Maroon women (17.1%) compared to Hindustani women (32.1%) (aOR 0.7 (95%CI 0.6–0.8)). Post-partum hemorrhage occurred most frequently in Indigenous women (8.1%, aOR 3.0, 95%CI 1.7–5.3).
Table 3.
Ethnic disparities in maternal and perinatal characteristics and outcomes in Suriname, 2016–2017
| Total n = (%) |
Maroon n = (%) |
Creole n = (%) |
Hindustani n = (%) |
Mix n = (%) |
Javanese n = (%) |
Indigenous n = (%) |
Chinese n = (%) |
Other a n = (%) |
p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total births | 18,290 | 4950 (27.6) | 4217 (23.5) | 3395 (18.9) | 2254 (12.5) | 1948 (10.8) | 681 (3.8) | 381 (2.1) | 137 (0.8) | |
| Maternal age | ||||||||||
| < 20 years | 2462 (13.7) | 904 (18.3) | 552 (13.1) | 322 (9.5) | 303 (13.5) | 211 (10.9) | 146 (21.4) | 11 (2.9) | 13 (9.5) | |
| 20–35 years | 13,420 (74.9) | 3383 (68.4) | 3164 (75.1) | 2774 (82.0) | 1704 (75.8) | 1504 (77.4) | 465 (68.3) | 326 (85.6) | 101 (73.7) | < 0.05 |
| > 36 years | 2043 (11.4) | 654 (13.2) | 496 (11.8) | 286 (8.5) | 241 (10.7) | 229 (11.8) | 70 (10.3) | 44 (11.5) | 23 (16.8) | |
| Parity | ||||||||||
| 0 | 6124 (34.2) | 1253 (25.4) | 1411 (33.6) | 1456 (43.0) | 883 (39.3) | 704 (36.2) | 212 (31.3) | 148 (38.8) | 57 (41.6) | |
| 1–3 | 9387 (52.5) | 2346 (47.5) | 2267 (54.0) | 1812 (53.6) | 1204 (53.6) | 1122 (57.7) | 338 (49.9) | 228 (59.8) | 71 (51.8) | < 0.05 |
| > 4 | 2389 (13.3) | 1338 (27.1) | 519 (12.4) | 115 (3.4) | 159 (7.1) | 117 (6.0) | 127 (18.8) | 5 (1.3) | 9 (6.6) | |
| Gestational age | ||||||||||
| < 28 weeks | 210 (1.2) | 72 (1.5) | 64 (1.5) | 35 (1.0) | 21 (0.9) | 10 (0.5) | 7 (1.0) | 1 (0.3) | – | |
| 28–37 weeks | 2266 (12.7) | 711 (14.6) | 561 (13.4) | 405 (12.0) | 274 (12.3) | 192 (9.9) | 82 (12.2) | 29 (7.7) | 13 (9.7) | < 0.05 |
| Term | 15,299 (86.1) | 4104 (84.0) | 3548 (85.1) | 2933 (87.0) | 1933 (86.8) | 1728 (89.5) | 583 (86.8) | 349 (92.1) | 121 (90.3) | |
| Anemiaantepartum | ||||||||||
| Moderate | 2258 (36.4) | 808 (51.8) | 667 (43.8) | 372 (33.5) | 146 (20.9) | 147 (19.0) | 71 (37.6) | 36 (13.9) | 11 (11.7) | < 0.05 |
| Severe | 145 (2.3) | 76 (4.9) | 38 (2.5) | 15 (1.4) | 10 (1.4) | 2 (0.3) | 4 (2.1) | – | – | |
| Mode of delivery | ||||||||||
| Spontaneous | 13,428 (74.8) | 4074 (82.3) | 3174 (75.3) | 2223 (65.5) | 1575 (69.9) | 1482 (76.1) | 531 (78.0) | 301 (79.0) | 69 (50.4) | < 0.05 |
| Vacuum | 225 (1.3) | 31 (0.6) | 28 (0.7) | 84 (2.5) | 34 (1.5) | 31 (1.6) | 12 (1.8) | 4 (1.0) | 1 (0.7) | |
| Cesarean | 4309 (24.0) | 845 (17.1) | 1015 (24.1) | 1088 (32.1) | 645 (28.7) | 435 (22.3) | 138 (20.3) | 76 (19.9) | 67 (48.9) | |
| Presentation | ||||||||||
| Cephalic | 17,359 (96.7) | 4787 (96.7) | 4093 (97.1) | 3252 (95.8) | 2193 (97.3) | 1873 (96.1) | 661 (97.1) | 367 (96.3) | 134 (97.8) | 0.03 |
| Post partum hemorrhage | ||||||||||
| 500–999 mL | 1012 (6.3) | 288 (6.3) | 239 (6.3) | 140 (4.8) | 144 (7.3) | 121 (6.9) | 50 (8.1) | 21 (6.2) | 9 (9.0) | < 0.05 |
| > 1000 mL | 251 (1.6) | 79 (1.7) | 58 (1.5) | 32 (1.1) | 29 (1.5) | 22 (1.3) | 21 (3.4) | 8 (2.4) | 2 (2.0) | |
| Total babies born | 18,504 | 5035 (27.7) | 4268 (23.5) | 3422 (18.8) | 2277 (12.5) | 1965 (10.8) | 687 (3.8) | 382 (2.1) | 139 (0.8) | |
| Live Births | 17,789 (97.9)b | 4877 (96.9) | 4157 (97.4) | 3370 (98.5) | 2240 (98.4) | 1951 (99.3) | 674 (98.1) | 381 (99.7) | 139 (98.6) | < 0.05 |
| Stillbirths (> 22 weeks or 1000 g) | 386 (2.1) | 158 (3.1) | 111 (2.6) | 52 (1.5) | 37 (1.6) | 14 (0.7) | 13 (1.9) | 1 (0.3) | 0 (0) | |
| Sex | ||||||||||
| Girl | 8767 (48.3) | 2485 (49.4) | 2083 (48.9) | 1637 (47.9) | 1064 (46.8) | 926 (47.1) | 321 (46.9) | 181 (47.4) | 70 (50.4) | 0.39 |
| Birth weight | ||||||||||
| < 2500 g | 2730 (15.1) | 847 (16.9) | 662 (15.6) | 614 (18.0) | 294 (13.0) | 183 (9.4) | 87 (12.6) | 32 (8.4) | 11 (8.0) | |
| 2500–4000 g | 14,805 (81.9) | 4047 (80.8) | 3449 (81.4) | 2710 (79.6) | 1887 (83.2) | 1690 (86.4) | 568 (83.4) | 334 (87.9) | 120 (86.1) | < 0.05 |
| > 4000 g | 542 (3.0) | 115 (2.3) | 127 (3.0) | 81 (2.4) | 88 (3.9) | 83 (4.2) | 26 (3.8) | 14 (3.7) | 8 (5.8) | |
| APGAR 5 min’ | ||||||||||
| < 7 | 639 (3.8) | 243 (5.3) | 187 (4.8) | 95 (3.0) | 58 (2.8) | 31 (1.7) | 21 (3.5) | 4 (1.1) | – | < 0.05 |
| Late stillbirth | ||||||||||
| n= | 285 | 122 | 77 | 40 | 23 | 11 | 9 | 1 | 2 | < 0.05 |
| SBR per 1000 births c | 15.6 | 25.0 | 18.6 | 11.9 | 10.3 | 5.7 | 13.4 | – | 14.6 | |
| Maternal deaths | ||||||||||
| Nationwide maternal deaths, n= | 25 | 13 | 4 | 3 | 1 | 1 | 2 | 1 | – | |
| MMR per 100.000 national live births d | 127 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | |
| Hospital maternal deathsd, n= | 20 | 9 | 4 | 3 | 1 | 1 | 1 | 1 | – | 0.59 |
| MMR per 100.000 hospital live births d | 112 | 184 | 96 | 89 | 45 | 51 | 147 | 262 | – | |
aEthnicity other: Brazilian (n = 101, 73.7%), Caucasian (n = 25, 18.2%), Afro-Caribbean (n = 11). Missing ethnicities, n = 327 (1.8%)
bMissing ethnicities n = 328 with no significant differences within the variables
cSBR: stillbirth rate = per 1000 births > 28 weeks or > 1000 g
dMMR: maternal mortality rate, maternal deaths per 100.000 live births (LB), of 20 hospital maternal deaths two occurred antepartum
Table 4.
Childbirth outcomes in different ethnicities, with Hindustani as reference group
| Outcome | Odds ratio (95% confidence interval) | Adjusted Odds ratio (95% confidence interval) |
|---|---|---|
| Teenage pregnancy | a | |
| Maroon | 2.1 (1.9–2.5) | 2.1 (1.8–2.4) |
| Creole | 1.4 (1.2–1.7) | 1.4 (1.2–1.6) |
| Mixed | 1.5 (1.3–1.8) | 1.5 (1.3–1.8) |
| Javanese | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) |
| Indigenous | 2.7 (2.1–3.3) | 2.6 (2.1–3.2) |
| Chinese | 0.3 (0.2–0.5) | 0.3 (0.2–0.6) |
| Other | 1.0 (0.6–1.8) | 1.1 (0.6–1.9) |
| Primiparous women | a | |
| Maroon | 0.5 (0.4–0.5) | 0.5 (0.4–0.5) |
| Creole | 0.7 (0.6–0.7) | 0.7 (0.6–0.8) |
| Mixed | 0.9 (0.8–1.0) | 0.8 (0.8–0.9) |
| Javanese | 0.8 (0.7–0.8) | 0.7 (0.7–0.8) |
| Indigenous | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) |
| Chinese | 0.8 (0.7–1.1) | 0.8 (0.6–1.0) |
| Other | 1.0 (0.7–1.3) | 1.0 (0.6–1.3) |
| Grand multiparous women | a | |
| Maroon | 10.6 (8.7–12.8) | 14.7 (11.9–18.1) |
| Creole | 4.0 (3.3–4.9) | 4.1 (3.3–5.1) |
| Mixed | 2.2 (1.7–2.8) | 2.2 (1.7–2.9) |
| Javanese | 1.8 (1.4–2.4) | 1.7 (1.3–2.3) |
| Indigenous | 6.6 (5.0–8.6) | 9.9 (7.4–13.4) |
| Chinese | 0.4 (0.2–0.9) | 0.3 (0.1–0.8) |
| Other | 2.0 (1.0–4.0) | 1.6 (0.7–3.4) |
| Severe anemia | a | |
| Maroon | 3.7 (2.1–6.5) | 2.4 (1.4–4.3) |
| Creole | 1.9 (1.0–3.4) | 1.7 (0.9–3.0) |
| Mixed | 1.1 (0.5–2.4) | 1.8 (0.8–4.2) |
| Javanese | 0.2 (0.1–0.8) | 0.3 (0.1–1.3) |
| Indigenous | 1.6 (0.5–4.8) | 1.1 (0.4–3.5) |
| Chinese | N/A | N/A |
| Other | N/A | N/A |
| Cesarean section | b | |
| Maroon | 0.4 (0.4–0.5) | 0.7 (0.6–0.8) |
| Creole | 0.7 (0.6–0.7) | 0.8 (0.7–0.9) |
| Mixed | 0.9 (0.7–1.0) | 0.9 (0.8–1.0) |
| Javanese | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) |
| Indigenous | 0.5 (0.4–0.7) | 0.8 (0.6–0.9) |
| Chinese | 0.5 (0.4–0.7) | 0.4 (0.3–0.5) |
| Other | 2.0 (1.4–2.7) | 1.7 (1.2–2.4) |
| Post partum hemorrhage | c | |
| Maroon | 1.6 (1.1–2.4) | 1.5 (1.0–2.4) |
| Creole | 1.4 (0.9–2.2) | 1.4 (0.9–2.2) |
| Mixed | 1.4 (0.8–2.3) | 1.2 (0.7–1.9) |
| Javanese | 1.2 (0.7–2.0) | 1.1 (0.7–2.0) |
| Indigenous | 3.2 (1.8–5.5) | 3.0 (1.7–5.3) |
| Chinese | 2.2 (1.0–4.8) | 2.1 (0.9–4.6) |
| Other | 1.9 (0.4–7.8) | 1.4 (0.3–6.0) |
| Preterm birth | a | |
| Maroon | 1.3 (1.1–1.4) | 1.2 (1.1–1.4) |
| Creole | 1.2 (1.0–1.3) | 1.1 (1.0–1.3) |
| Mixed | 1.0 (0.8–1.3) | 1.0 (0.9–1.2) |
| Javanese | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) |
| Indigenous | 1.0 (0.8–1.3) | 1.0 (0.8–1.3) |
| Chinese | 0.6 (0.4–0.8) | 0.6 (0.4–0.9) |
| Other | 0.8 (0.5–1.4) | 0.8 (0.4–1.4) |
| Low birth weight | b | |
| Maroon | 0.9 (0.8–1.0) | 0.7 (0.6–0.8) |
| Creole | 0.8 (0.7–0.9) | 0.6 (0.5–0.7) |
| Mixed | 0.7 (0.6–0.8) | 0.6 (0.5–0.7) |
| Javanese | 0.5 (0.4–0.5) | 0.4 (0.3–0.5) |
| Indigenous | 0.7 (0.5–0.8) | 0.5 (0.4–0.7) |
| Chinese | 0.4 (0.3–0.6) | 0.5 (0.3–0.8) |
| Other | 0.4 (0.2–0.8) | 0.6 (0.3–1.1) |
| Low APGAR score | c | |
| Maroon | 1.8 (1.4–2.3) | 1.6 (1.2–2.0) |
| Creole | 1.6 (1.3–2.1) | 1.4 (1.0–2.0) |
| Mixed | 0.9 (0.7–1.3) | 1.0 (0.7–1.6) |
| Javanese | 0.6 (0.4–0.9) | 0.7 (0.4–1.2) |
| Indigenous | 1.2 (0.7–1.9) | 1.1 (0.6–2.0) |
| Chinese | 0.4 (0.1–1.0) | 0.5 (0.1–1.7) |
| Other | N/A | N/A |
| Late stillbirth | c | |
| Maroon | 2.1 (1.5–3.0) | 1.7 (1.1–2.5) |
| Creole | 1.5 (1.1–2.3) | 1.4 (0.9–2.1) |
| Mixed | 0.9 (0.6–1.6) | 1.0 (0.6–1.8) |
| Javanese | 0.5 (0.2–0.9) | 0.4 (0.2–0.9) |
| Indigenous | 1.1 (0.5–2.3) | 1.1 (0.5–2.5) |
| Chinese | 0.2 (0.0–1.6) | 0.3 (0.0–2.4) |
| Other | N/A | N/A |
| Maternal death | d | |
| Maroon | 3.4 (0.8–15.7) | 3.3 (0.7–16.1) |
| Creole | 1.2 (0.2–7.2) | 1.2 (0.2–7.1) |
| Mixed | N/A | N/A |
| Javanese | 1.7 (0.2–12.4) | 1.8 (0.2–12.5) |
| Indigenous | 2.5 (0.2–27.5) | 2.5 (0.2–28.5) |
| Chinese | 4.5 (0.4–49.4) | 4.7 (0.4–53.4) |
| Other | N/A | N/A |
Bold = significant results.N/A not available
aAdjusted for maternal age and hospital
bAdjusted for maternal age, parity, hospital, birth weight, gestational age
cAdjusted for maternal age, parity, hospital, cesarean section, birth weight, gestational age
dAdjusted for maternal age, parity and hospital
MMR was highest in Maroon women with 184 (n = 9/4877) per 100.000 live births. In Hindustani, Javanese and mixed women MMRs were 89 (n = 3/3370), 51 (n = 1/1951) and 45 (n = 1/2240). Stillbirth rates were significantly higher for African-descendent Maroon women (25.0 per 1000 births, aOR 1.7, 95%CI 1.1–2.5) and significantly lower for Javanese women (5.7 per 1000 births, aOR 0.4 95%CI 0.2–0.9) compared to Hindustani women (11.9 per 1000 births).
Discussion
This study provides a national overview of maternal and perinatal health in multi-ethnic, middle-income country Suriname, where no formal national perinatal registry is in place yet. Disaggregation of the perinatal data shows substantial inequities in maternal and perinatal health for specific ethnic groups.
Although Suriname is classified as an upper middle-income country, it is among countries in the Latin America and the Caribbean region with the highest maternal mortality ratio and stillbirth rate. The maternal mortality ratio of 127 maternal deaths per 100.000 live births is comparable to previous studies conducted in Suriname the past years [22, 32]. Suboptimal quality of care plays an important role in the high maternal mortality ratio in Suriname and led to different quality improvement projects such as maternal death and morbidity audits, obstetric guidelines and obstetric skills team training [22, 32, 33]. The stillbirth rate, 15.6 stillbirths per 1000 births, is second highest of the region, preceded only by Haiti (SBR 24.9), and followed by Paraguay (13.4) and Bolivia (12.9) [34]. Timing and underlying causes of stillbirths are unknown and audit and in-depth case review is necessary to understand why these babies die.
Inequity in maternal and perinatal health within countries are as great as or greater than those between countries [3–5, 35]. In the SDGs, equity in health, i.e. available and affordable high-quality health services to all, is emphasized as a priority, and this was further stressed in the 2019 Report of the Commission of the Pan American Health Organization (PAHO) on Equity and Health Inequalities in the Americas [4, 5]. Disaggregating perinatal health data can identify and target inequity within the health system. Ethnicity or race is a non-modifiable risk factor to adverse maternal and perinatal outcomes, and an important social determinant of health [4, 35]. The causal pathway of ethnical disparities is influenced partly by biological factors, such as genetic predisposition, but mostly by environmental and socio-economic mediators, such as wealth, culture, nutrition and socio-economic situation – often a reflection of underlying structural and historical drivers [3, 4]. While place of residence (urban vs. rural) is an important proxy of environmental and socio-economic factors as well as access to health care, place of residence has a smaller role in Suriname, where most pregnant women (temporarily) reside in urban areas and give birth in hospitals [20].
Biological factors of ethnical disparities seem to contribute quite strongly to infants born small for gestational age. In our study in Suriname, Hindustani women generally have more favorable socio-economic status than women of other ethnical backgrounds, yet their babies are significantly smaller [17]. An explanation for this finding is lacking, as Hindustani women are not prone to severe anemia, as seen in this study, and are generally known to have a high dietary diversity [36]. A WHO study confirms that significant differences in fetal weight are seen between ten countries, with the lowest median birth weight among Indian women, also after adjustment for maternal characteristics, gestational age and fetal sex [37]. In contrast, INTERGROWTH-21 found that when mothers’ nutritional and health needs are met and there are few environmental constraints on growth, only 3.5% of the total variability of growth was due to differences between populations [38]. It is therefore controversial to locally adjust growth charts to increase predictive performance, as they can potentially deprive smaller babies of their needs for intensified health care given that most have impaired fetal growth due to malnutrition or other environmental factors.
Giving birth during adolescence is not only a risk for adverse outcomes, but also has a negative impact on the future well-being of the mother and infant, leading to stigmatism and socio-economic consequences with school drop-out, lower employment opportunities, and a higher risk of poverty and intergenerational transmission of inequities [4, 39]. High numbers of pregnancies among teenagers were seen in Maroon (18.3%) and Indigenous women (21.4%). This results in a threefold higher adolescent birth rate for Maroons and Indigenous girls (79 and 88 per 1000 girls 15–19 years) compared to Hindustani girls (27 per 1000 girls 15–19 years). In a recently conducted nationwide survey, the adolescent birth rates for Maroon and Indigenous girls are reported even higher (124 and 99 per 1000 girls 15–19 years) [20]. While the national teenage pregnancy rate (13.8%) in Suriname is somewhat lower than in many Latin American countries (16–22%), ethnic disparities within the country are significant [40, 41]. Tailored health care services for teenagers should be made available, including prevention of teenage pregnancy with free contraception, especially geared towards the groups most at risk [24, 42].
Ethnic disparities for cesarean section rates have been observed in many low- and middle income countries [43]. This is similar to findings in Suriname, where Maroon women have the lowest cesarean section rate despite increased risks of adverse pregnancy outcomes. Hospital differences in cesarean section rates may partially reflect ethnic distributions between the hospitals.
Multiple studies in high-income countries with Caucasian majorities such as the United Kingdom, the United States, the Netherlands and different Latin American countries, demonstrated an increased risk of maternal deaths, maternal morbidity and stillbirths in ethnic minority women, such as women from African, Asian or Indigenous descent, compared to Caucasian women [12–15, 44, 45]. African-descendant Maroon women in Suriname are at two- to four-fold higher risk of stillbirth (25 stillbirths per 1000 births) compared to Asian-descendant women (12 and 6 stillbirths per 1000 births in Hindustani and Javanese women). Despite low numbers of maternal death, similar trends are found with MMRs of 184 in Maroon women compared to 89 per 100.000 live births in Hindustani women, though not statistically significant due to absolute low number of deaths.
Women from ethnic minorities, women of low socio-economic status, adolescents, migrant women and women living with HIV are particularly likely to not only have increased adverse pregnancy outcomes, but also more likely to experience disrespectful or even neglect during pregnancy and childbirth [46]. Respecful maternity care, i.e. effective communication and equal engagement of health care workers to all women, is essential in reducing disparities in pregnancy care and outcomes [47]. Dialogue, research into disparities of health care outcomes and advocacy of safe motherhood is an important public health and human rights issue.
Limitations
A number of limitations need to be considered. First, this study only covers hospital births. Although these comprise majority of all live births in the country, women who delivered in the more deprived interior settings (mostly maroons and indigenous women), may be underrepresented. As a result, the national teenage pregnancy rate or stillbirth rate could be higher than reported in this study. Second, important explanatory or risk factors such as body mass index, smoking, level of education, level of income, residency, number of antenatal care visits and medical and obstetric history were not available. Other important indicators for quality of care, such as early neonatal mortality, timing of stillbirths and indications of caesarean sections for classification according to Robson criteria were not provided by the childbirth books [48, 49]. It is recommended that these factors are included in perinatal registries in the future.
Recommendations
While achievement of health equity requires overarching structural changes that promote social, economic and political equality, there are specific strategies policymakers could prioritize to achieve equity in reproductive, maternal and perinatal health [3, 4]. In connection to our discussion, recommendations can be made in the following direction:
develop a nationwide perinatal registry that includes primary health care centers and allows for disaggregated analysis by groups at risk of inequities in health outcomes;
ensure that quality obstetric care along the continuum from preconception and antenatal to postpartum, including safe abortion services, is accessible to all equitably;
monitor the quality of care, including auditing of maternal and perinatal mortality, stillbirths, severe maternal morbidity and caesarean sections;
provide free contraception and adolescent programs for sexual and reproductive health;
strenghten community based outreach and improve health literacy of women; and
address the structural drivers and conditions of daily life which determine equity and a dignified life.
Conclusion
This is the a nationwide comprehensive overview of maternal and perinatal health status in Suriname, a middle-income country in South America and shows substantial inequities in maternal and perinatal health by ethnicity. African descendent Maroon women experienced the highest risk of adverse outcomes (maternal mortality, stillbirth, increased preterm birth, low apgar score). Hindustani women have lower risk on adverse outcomes, yet give birth to smaller babies and give birth by cesarean section most frequently. Disaggregating perinatal health data can identify and help target inequity within the health system.
Supplementary information
Additional file 1. Visual summary of data availability per hospital.
Additional file 2. Differences in maternal and neonatal characteristics between hospitals in Suriname.
Acknowledgements
The authors would firstly like to thank the hospital directors, secretaries and for the availability of the data and childbirth books. The authors would kindly thank the Surinamese medical students who contributed to the data curation, especially Rubinah Paidin and Raïz Boerleider and Dutch medical students Nicole Schenkelaars, Rosemarijn Ettema and Stephanie Thierens.
Abbreviations
- ANC
Antenatal Care
- LB
Live birth
- MDG
Millennium Development Goals
- MICS
Multiple Indicator Cluster Survey
- MMR
Maternal mortality ratio
- (a)OR
(Adjusted) odds ratio
- PAHO
Pan American Health Organization
- PPH
Post-partum hemorrhage
- SIP
Perinatal Information System
- SBR
Stillbirth Rate
- SDG
Sustainable Development Goals
- WHO
World Health Organization
Authors’ contributions
All authors approved the final version of the manuscript. KV conceptualized the study, performed data curation and analysis and wrote the first and final draft. ZP performed data curation, data analysis and helped write the first and final draft. RP helped in data analysis and helped writing the first draft. LK supervised the project locally and reviewed the final version of the manuscript. MR and KB reviewed and edited the final version of the manuscript. JB supervised the project, validated data analysis and reviewed and edited the first and final version of the manuscript.
Funding
The study was not funded.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study has been approved by the ethical review board of the Surinamese Committee on Research Involving Human Subjects (#VG11–18).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12978-020-0902-7.
References
- 1.Bose CL, Bauserman M, Goldenberg RL, et al. The Global Network Maternal Newborn Health Registry: a multi-national, community-based registry of pregnancy outcomes. Reprod Health. 2015;12(Suppl 2):S1. doi: 10.1186/1742-4755-12-S2-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkema L, Chou D, Hogan D, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN maternal mortality estimation inter-agency group. Lancet. 2016;387(10017):462–474. doi: 10.1016/S0140-6736(15)00838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNICEF . Health Equity Report 2016: analysis of reproductive, maternal, newborn, child and adolescent health inequities in Latin America and the Caribbean to inform policymaking. 2016. [Google Scholar]
- 4.Just Societies . Report of the Commission of the Pan American Health Organization on Equity and Health Inequalities in the Americas. Washington, DC: PAHO; 2019. Health Equity and Dignified Lives. [Google Scholar]
- 5.WHO, UNAIDS, UNFPA, UNICEF, UN Women, The World Bank Group. Survive, Thrive, Transform . Global Strategy for Women’s, Children’s and Adolescents’ Health: 2018 report on progress towards 2030 targets. Geneva: World Health Organization; 2018. [Google Scholar]
- 6.Susannah B, Leisher H, Lawn JE, et al. Stillbirths: Investment in ending preventable stillbirths by 2030 will yield multiple returns and help achieve multiple Sustainable Development Goals. Brief for GSDR. 2016. [Google Scholar]
- 7.Bliddal M, Broe A, Pottegård A, et al. The Danish medical birth register. Eur J Epidemiol. 2018;33(1):27–36. doi: 10.1007/s10654-018-0356-1. [DOI] [PubMed] [Google Scholar]
- 8.Skulstad SM, Igland J, Johannessen A, et al. Validation of maternal reported pregnancy and birth characteristics against the Medical Birth Registry of Norway. PLoS One. 2017;12(8). 10.1371/journal.pone.0181794. [DOI] [PMC free article] [PubMed]
- 9.Perined. Perinatale Zorg in Nederland 2016. Utrecht; 2018. https://assets.perined.nl/docs/7935f9c6-eaac-4f59-a150-307ae04efa27.pdf. Accessed 5 Nov 2019.
- 10.World Health Organization . History of the Perinatal Information System (SIP): A newsletter of worldwide activity. 2010. pp. 1–8. [Google Scholar]
- 11.Government of the Republic of Suriname . MGD Progress Report 2014. 2014. pp. 1–174. [Google Scholar]
- 12.Howell E. Reducing disparities in severe maternal morbidity and mortality. Clin Obstet Gynecol. 2018;61(2):1–13. doi: 10.1097/GRF.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sow M, Racape J, Schoenborn C, et al. Is the socioeconomic status of immigrant mothers in Brussels relevant to predict their risk of adverse pregnancy outcomes? BMC Pregnancy Childbirth. 2018;18(1):1–11. doi: 10.1186/s12884-018-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coleman H, Pemu A, Rankin J, et al. Perinatal health outcomes and care among asylum seekers and refugees: a systematic review of systematic reviews. BMC Med. 2018;16(1):1–25. doi: 10.1186/s12916-018-1064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandes KG, Sousa MH, Cecatti JG. Skin color and maternal near miss: exploring a demographic and health survey in Brazil. Rev Bras Ginecol Obstet. 2017;39(5):209–216. doi: 10.1055/s-0037-1603498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwart JJ, Jonkers MD, Richters A, et al. Ethnic disparity in severe acute maternal morbidity: a nationwide cohort study in the Netherlands. Eur J Pub Health. 2011;21(2):229–234. doi: 10.1093/eurpub/ckq046. [DOI] [PubMed] [Google Scholar]
- 17.Stell G. Sociolinguistic Indexicalities in ethnic diversity: perceptions of ethnicity and language in Suriname. NWIG New West Indian Guid. 2018;92(1–2):35–61. doi: 10.1163/22134360-09201054. [DOI] [Google Scholar]
- 18.The World Bank. World Development Indicators Suriname 1990–2013. http://databank.worldbank.org/data/views/reports/tableview.aspx. Accessed 17 May 2015.
- 19.General Bureau of Statistics . Demographic Data Suriname 2013-2016. 2017. [Google Scholar]
- 20.Ministry of Social Affairs and, Housing, General Bureau of Statistics . Suriname Multiple Indicator Cluster Survey 2018, Final Report. 2019. [Google Scholar]
- 21.Mungra A, Van Bokhoven SC, Florie J, et al. Reproductive age mortality survey to study under-reporting of maternal mortality in Surinam. Eur J Obstet Gynecol Reprod Biol. 1998. 10.1016/S0301-2115(97)00224-8. [DOI] [PubMed]
- 22.Kodan LR, Verschueren KJC, van Roosmalen J, et al. Maternal mortality audit in Suriname between 2010 and 2014, a reproductive age mortality survey. BMC Pregnancy Childbirth. 2017;17(1). 10.1186/s12884-017-1466-6. [DOI] [PMC free article] [PubMed]
- 23.O’Malley EG, Popivanov P, Fergus A, et al. Maternal near miss: what lies beneath? Eur J Obstet Gynecol Reprod Biol. 2017;199:116–120. doi: 10.1016/j.ejogrb.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 24.Pan American Health Organization (PAHO), UNFPA, UNICEF . Accelerating Progress toward the Reduction of Adolescent Pregnancy in Latin America and the Caribbean. Washington, DC: Report of a Technical Consultation; 2016. [Google Scholar]
- 25.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva; 2011. https://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed 11 Nov 2019.
- 26.PeriNed . Perinatale Zorg in Nederland 2017. Utrecht: PeriNed; 2019. [Google Scholar]
- 27.World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. Geneva; 2012. https://apps.who.int/iris/bitstream/handle/10665/75411/9789241548502_eng.pdf?sequence=1. Accessed 11 Nov 2019. [PubMed]
- 28.World Health Organization . The WHO Application of ICD-10 to Deaths during the Perinatal Period. Geneva: ICD-PM; 2016. [Google Scholar]
- 29.World Health Organization. ICD-10 Application to deaths during pregnancy, childbirth and the puerperium. Geneva; 2012. https://apps.who.int/iris/bitstream/handle/10665/70929/9789241548458_eng.pdf. Accessed 11 Nov 2019.
- 30.Grimes DA, Schulz KF. Making sense of odds and odds ratios. Obstet Gynecol. 2008;111:423–426. doi: 10.1097/01.AOG.0000297304.32187.5d. [DOI] [PubMed] [Google Scholar]
- 31.Groenwold RHH, Klungel OH, Grobbee DE, et al. Selection of confounding variables should not be based on observed associations with exposure. Eur J Epidemiol. 2011;26(8):589–593. doi: 10.1007/s10654-011-9606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodan LR, Verschueren KJC, Kanhai HHH, et al. The golden hour of sepsis : An in-depth analysis of sepsis-related maternal mortality in middle-income country Suriname. PLoS One. 2018;27(7):1–14. doi: 10.1371/journal.pone.0200281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verschueren KJC, Kodan LR, Brinkman TK, et al. Bottom-up development of national obstetric guidelines in middle-income country Suriname. BMC Health Serv Res. 2019;19:651. doi: 10.1186/s12913-019-4377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pingray V, Althabe F, Vazquez P, et al. Stillbirth rates in 20 countries of Latin America: an ecological study. BJOG. 2018;125(10):1263–1270. doi: 10.1111/1471-0528.15294. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. State of Inequality: Reproductive, maternal, newborn and childhealth. Geneva; 2015. https://www.who.int/docs/default-source/gho-documents/health-equity/state-of-inequality/state-of-inequality-reproductive-maternal-new-born-and-child-health.pdf?sfvrsn=f4034289_2. Accessed 11 Nov 2019.
- 36.Van Wijk E, Jager L, Van Der Kroon . Leven Om Te Eten: Surinaamse En Antilliaanse Vrouwen over Eten, Bewegen En Overgewicht. 2010. [Google Scholar]
- 37.Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization fetal growth charts: a multinational longitudinal study of ultrasound biometric measurements and estimated fetal weight. PLoS Med. 2017;14(1):e1002220. doi: 10.1371/journal.pmed.1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papageorghiou AT, Kennedy SH, Salomon LJ, et al. The INTERGROWTH-21st fetal growth standards: toward the global integration of pregnancy and pediatric care. Am J Obstet Gynecol. 2018;218(2):S630–S640. doi: 10.1016/j.ajog.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 39.Ganchimeg T, Ota E, Morisaki N, et al. Pregnancy and childbirth outcomes among adolescent mothers: a World Health Organization multicountry study. BJOG. 2014;121(Suppl):40–48. doi: 10.1111/1471-0528.12630. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Pan American Health Organization. Health Situation in the Americas: Core Indicators 2016. Washington, DC; 2016. https://iris.paho.org/bitstream/handle/10665.2/31289/CoreIndicators2016-eng.pdf?sequence=1&isAllowed=y. Accessed 11 Nov 2019.
- 41.Neal S, Harvey C, Chandra-Mouli V, et al. Trends in adolescent first births in five countries in Latin America and the Caribbean: disaggregated data from demographic and health surveys. Reprod Health. 2018;15(1):1–10. doi: 10.1186/s12978-018-0578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. Recommendations on Adolescent Sexual and Reproductive Health and Rights. Washington, DC; 2018. https://apps.who.int/iris/bitstream/handle/10665/275374/9789241514606-eng.pdf?ua=1. Accessed 9 Nov 2019.
- 43.Boerma T, Ronsmans C, Melesse DY, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392(10155):1341–1348. doi: 10.1016/S0140-6736(18)31928-7. [DOI] [PubMed] [Google Scholar]
- 44.Penn N, Oteng-Ntim E, Oakley LL, et al. Ethnic variation in stillbirth risk and the role of maternal obesity: analysis of routine data from a London maternity unit. BMC Pregnancy Childbirth. 2014;14(1):1–9. doi: 10.1186/s12884-014-0404-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesenburg MA, Restrepo-Mendez MC, Amigo H, et al. Ethnic group inequalities in coverage with reproductive, maternal and child health interventions: cross-sectional analyses of national surveys in 16 Latin America and the Caribbean countries. Lancet Glob Health. 2018:e902–13. 10.1016/S2214-109X(18)30300-0. [DOI] [PMC free article] [PubMed]
- 46.Bowser D, Hill K. Exploring evidence for disrespect and abuse in facility-based childbirth: report of a landscape analysis. USAID / TRAction Project 2010. https://www.ghdonline.org/uploads/Respectful_Care_at_Birth_9-20-101_Final1.pdf. Accessed 18 Mar 2020.
- 47.World Health Organization. The prevention and elimination of disrespect and abuse during facility-based childbirth: WHO statement, vol. 4. Geneva; 2015. https://apps.who.int/iris/bitstream/handle/10665/134588/WHO_RHR_14.23_eng.pdf;jsessionid=84F2CEDC71B19F1DE0FBAAAA98B1E981?sequence=1. Accessed 18 Mar 2020.
- 48.Vogel JP, Betrán AP, Vindevoghel N, et al. Use of the robson classification to assess caesarean section trends in 21 countries: a secondary analysis of two WHO multicountry surveys. Lancet Glob Health. 2015;3(5):e260–e270. doi: 10.1016/S2214-109X(15)70094-X. [DOI] [PubMed] [Google Scholar]
- 49.Miller S, Abalos E, Chamillard M, et al. Beyond too little, too late and too much, too soon: a pathway towards evidence-based, respectful maternity care worldwide. Lancet. 2016;388(10056):2176–2192. doi: 10.1016/S0140-6736(16)31472-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Visual summary of data availability per hospital.
Additional file 2. Differences in maternal and neonatal characteristics between hospitals in Suriname.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.