Abstract
Background
The pneumonia severity index (PSI) and the CURB-65 (confusion, urea, respiratory rate, blood pressure, age ≥ 65 years) score have been shown to predict mortality in community-acquired pneumonia. Their ability to predict influenza-related pneumonia, however, is less well-established.
Methods
A total of 693 laboratory-confirmed FluA-p patients diagnosed between Jan 2013 and Dec 2018 and recruited from five teaching hospitals in China were included in the study. The sample included 494 patients in the derivation cohort and 199 patients in the validation cohort. The prediction rule was established based on independent risk factors for 30-day mortality in FluA-p patients from the derivation cohort.
Results
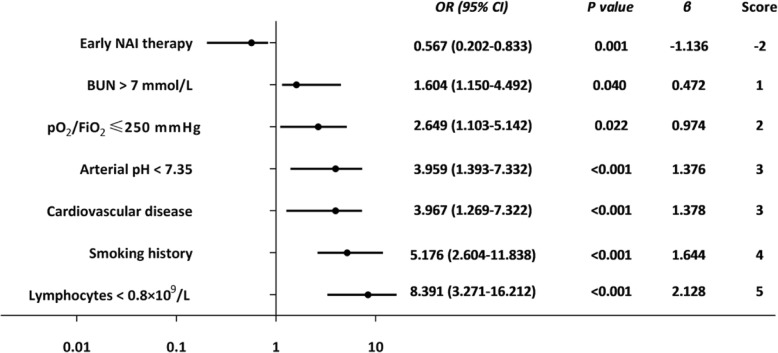
The 30-day mortality of FluA-p patients was 19.6% (136/693). The FluA-p score was based on a multivariate logistic regression model designed to predict mortality. Results indicated the following significant predictors (regression statistics and point contributions toward total score in parentheses): blood urea nitrogen > 7 mmol/L (OR 1.604, 95% CI 1.150–4.492, p = 0.040; 1 points), pO2/FiO2 ≤ 250 mmHg (OR 2.649, 95% CI 1.103–5.142, p = 0.022; 2 points), cardiovascular disease (OR 3.967, 95% CI 1.269–7.322, p < 0.001; 3 points), arterial PH < 7.35 (OR 3.959, 95% CI 1.393–7.332, p < 0.001; 3 points), smoking history (OR 5.176, 95% CI 2.604–11.838, p = 0.001; 4 points), lymphocytes < 0.8 × 109/L (OR 8.391, 95% CI 3.271–16.212, p < 0.001; 5 points), and early neurominidase inhibitor therapy (OR 0.567, 95% CI 0.202–0.833, p = 0.005; − 2 points). Seven points was used as the cut-off value for mortality risk stratification. The model showed a sensitivity of 0.941, a specificity of 0.762, and overall better predictive performance than the PSI risk class (AUROC = 0.908 vs 0.560, p < 0.001) and the CURB-65 score (AUROC = 0.908 vs 0.777, p < 0.001).
Conclusions
Our results showed that a FluA-p score was easy to derive and that it served as a reliable prediction rule for 30-day mortality in FluA-p patients. The score could also effectively stratify FluA-p patients into relevant risk categories and thereby help treatment providers to make more rational clinical decisions.
Keywords: Influenza, Pneumonia, Mortality, Prediction rule
Background
Influenza is a common contagious respiratory disease and influenza-related epidemics and pandemics have occurred all over the world [1, 2]. Despite advances in medical technology and greater economic development in many countries, influenza still causes numerous hospitalizations and is associated with considerable mortality [3–5]. Each year, 10–20% of the global population experiences symptomatic influenza, including 3–5 million cases of severe illness and 290–650 thousand deaths [6]. For these reasons, influenza is regarded as the greatest threat to global health in the twenty-first century [7].
Patients infected with influenza may exhibit a broad spectrum of clinical symptoms, ranging from self-limited upper respiratory tract illness to severe pneumonia [8, 9]. Influenza-related pneumonia (Flu-p), including primary viral pneumonia and secondary bacterial pneumonia, is the major cause of influenza-associated hospitalizations and deaths [10]. Primary influenza pneumonia and post-influenza secondary bacterial pneumonia are distinct pathologies but difficult to distinguish clinically. The pathogenesis of primary influenza pneumonia shows diffuse alveolar damage associated with haemorrhage and necrotising bronchiolitis, and the secondary bacterial pneumonia presents with neutrophil influx, loss of alveolar architecture and consolidation [10]. When the diagnosis of pneumonia is confirmed, the first priority is to assess the degree of disease severity. Several prediction rules have been established to help clinicians predict the mortality rate of patients with pneumonia. Scores on the CURB-65 (confusion, urea, respiratory rate, blood pressure, age ≥ 65 years) and the pneumonia severity index (PSI) are the most widely used indices to predict 30-day mortality rates for patients diagnosed with community-acquired pneumonia [11, 12]. However, the validity of these two measures for use with Flu-p patients is questionable [13, 14]. Some variables that might be more useful in predicting severe influenza include PO2/FiO2 and lymphocyte counts [15, 16]. But to our knowledge, standard decision rules using these (and perhaps other) variables to predict the extent of Flu-p severity have yet to be developed.
In an effort to remedy this situation we conducted a multicenter, retrospective study with the principal aim being to develop an easy-to-use and accurate severity assessment tool to predict the 30-day mortality rate of patients with influenza A-related pneumonia (FluA-p). Our assessment tool is designed to have greater predictive power than either CURB-65 or PSI scores.
Methods
Study design and patient recruitment
Hospitalised patients who tested positive for influenza A virus RNA at the Microbiology Labs of five tertiary hospitals in China from 1st Jan 2013 to 31st Dec 2018 were screened for inclusion (the information for the participating centers is contained in Supplementary material 1). Patients with laboratory-confirmed Flu-p were included. Exclusion criteria for the patients were as follows [17]: (i) Younger than 14 years old; (ii) pneumonia whose onset was not in the community (i.e., pneumonia onset ≥48 h after admission and hospitalised within the last 28 days); and (iii) immunocompromised status.
Disease and treatment definitions
Patients with influenza-related pneumonia experienced disease onset during the influenza season and manifested with respiratory symptoms along with newly developed pulmonary infiltrates on chest radiographs. In addition, patients with influenza-related pneumonia tested positive for influenza virus RNA by reverse-transcription polymerase chain reaction (RT-PCR). The biological samples subjected to RT-PCR were respiratory specimens (i.e., nasal/nasopharyngeal swabs, sputum, bronchial aspirates or branchoalveolar lavage fluid). Community-acquired respiratory co-infections resulting from coinfected pathogens were identified using standard microbiologic procedures within the first 48 h after admission [18]. Early neuraminidase inhibitor (NAI) treatment was defined as any NAI (oseltamivir, zanamivir or peramivir) administered within 48 h after illness onset [19]. Systemic corticosteroid use was defined as at least one dose of any systemic corticosteroid administered during hospitalisation.
Data collection
Data were retrospectively collected and included demographic information, chronic medical conditions (Supplementary material 2), baseline clinical characteristics (clinical symptoms, vital signs, laboratory and radiological findings), illness severity of pneumonia on admission (CURB-65 and PSI scores), community-acquired respiratory coinfections (Supplementary material 3), clinical management (administration of NAI, systemic corticosteroid use, invasive and non-invasive mechanical ventilation, admittance to the intensive care unit (ICU)), and 30-day mortality rate.
Statistical analysis
All of 693 FluA-p patients were divided into a derivation cohort (494 patients from 2013 to 2016) and a validation cohort (199 patients from 2017 to 2018). The derivation cohort was used to establish the statistical model, and the validation cohort was used to validate the model.
According to the survival status at 30 days post-admission, the 693 patients were divided into surviving and deceased groups. Baseline characteristics of these two groups were compared. Variables with p-values < 0.1 in the univariate analyses were entered into a backward stepwise logistic regression model to explore risk factors for 30-day mortality. For pragmatic reasons, the score for each predictor was assigned an integer value relative to the regression coefficient (β). A cut-off point was designated following Youden’s index from the receiver operating characteristic (ROC) curve. A Kaplan-Meier analysis was performed to compare the difference in 30-day mortality rates between the low-risk and high-risk groups according to the designated cut-off value. Performance of the cut-off score was assessed by measuring the area under the ROC curve (AUROC) and by calculating measures of sensitivity and specificity.
The data were analysed for normality using a Kolmogorov–Smirnov test. In presenting our results, variables with a normal distribution are shown as the mean ± standard deviation. Those variables with a non-normal distribution are expressed as medians. Categorical variables were analysed using either the Chi-square test or Fisher’s exact test. Continuous variables were analysed using either Student’s t test or the Mann–Whitney U test. For all analyses, a two-tailed P-value < 0.05 was considered statistically significant. All statistical analyses were performed using either SPSS version 22.0 or MedCalc version 19.0.
Results
Screening process
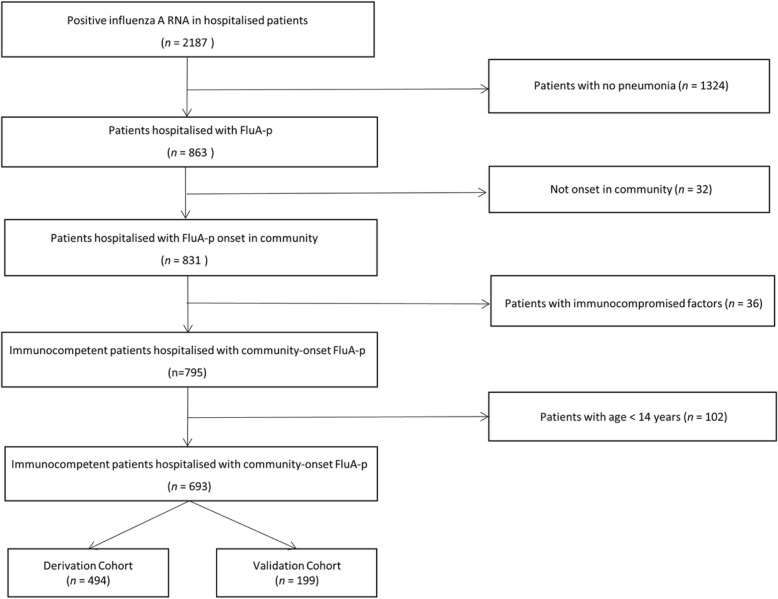
We screened 2187 hospitalised patients who tested positive for influenza A RNA. Overall, 693 immunocompetent adult and adolescent patients hospitalised with FluA-p were included in the final analysis (Fig. 1). Among these patients, 38.1% (264/693) were infected with A(H1N1)pdm09 and 11.0% (76/693) were infected with A(H3N2). In addition, 50.9% (353/693) of patients were infected with an unclassified influenza subtype. Because not all of the five hospitals in our study carried out influenza subtype tests, to identify the subtypes of influenza A using RT-PCR, subtype-specific primers were developed.
Fig. 1.
Patient screening algorithm for FluA-p
Overview of FluA-p patients
Overall, 39.2% (272/693) of patients were above 65 years old and 66.5% (461/693) of patients were male. 35.1% (243/693) of patients had a history of smoking. Cardiovascular disease (19.6%), diabetes mellitus (13.3%) and cerebrovascular disease (10.4%) were the most common chronic medical conditions. Respiratory rates ≥30 beats/min and mental confusion could be seen in 17.5% (121/693) and 4.6% (32/693) of patients, respectively. Only 1.2% (8/693) of patients had SBP < 90 mmHg. 26.9% (172/639) of patients had pO2/FiO2 ≤ 250 mmHg (Table 1).
Table 1.
Comparison of clinical features between deceased and survival patients
| Variable | Total (n = 693) |
Deceased group (n = 136) |
Survival group (n = 557) |
p-value |
|---|---|---|---|---|
| Male (n, %) | 461 (66.5) | 92 (67.6) | 369 (66.2) | 0.757 |
| Age ≥ 65 years (n, %) | 272 (39.2) | 60 (44.1) | 212 (38.1) | 0.195 |
| Obesity (n, %)a | 48 (6.9) | 0 (0.0) | 48 (8.6) | < 0.001 |
| Pregnancy (n, %) | 8 (1.2) | 0 (0.0) | 8 (1.4) | 0.338 |
| Smoking history (n, %)a | 243 (35.1) | 68 (50.0) | 175 (31.4) | < 0.001 |
| Comorbidities (n, %) | ||||
| Cardiovascular diseasea | 136 (19.6) | 48 (35.3) | 88 (15.8) | < 0.001 |
| Diabetes mellitus | 92 (13.3) | 14 (10.3) | 78 (14.0) | 0.253 |
| Cerebrovascular disease | 72 (10.4) | 10 (7.4) | 62 (11.1) | 0.195 |
| COPDa | 40 (5.8) | 3 (2.2) | 37 (6.6) | 0.047 |
| Asthma | 19 (2.7) | 2 (1.5) | 17 (3.1) | 0.222 |
| Chronic kidney disease | 16 (2.3) | 6 (4.4) | 10 (1.8) | 0.139 |
| Malignant solid tumor | 16 (2.3) | 0 (0.0) | 16 (2.9) | 0.193 |
| Clinical and radiologic characteristics (n, %) | ||||
| Respiratory rates ≥30 times/min | 121 (17.5) | 25 (18.4) | 96 (17.2) | 0.752 |
| Mental confusiona | 32 (4.6) | 32 (23.5) | 0 (0.0) | < 0.001 |
| SBP < 90 mmHg | 8 (1.2) | 0 (0.0) | 8 (1.4) | 0.338 |
| Leukocytes > 10 × 109/La | 118 (17.0) | 42 (30.9) | 76 (13.6) | < 0.001 |
| Lymphocytes < 0.8 × 109/La | 299/677 (44.2) | 120 (88.2) | 179/541 (33.1) | < 0.001 |
| Hb < 100 g/La | 69 (10.0) | 34 (25.0) | 35 (6.3) | < 0.001 |
| ALB < 35 g/La | 58/639 (9.1) | 12/131 (9.2) | 46/508 (9.1) | 0.970 |
| BUN > 7 mmol/La | 183/685 (26.7) | 97 (71.3) | 86/549 (15.7) | < 0.001 |
| BG > 14 mmol/L | 8 (1.2) | 0 (0.0) | 8 (1.4) | 0.288 |
| Arterial PH < 7.35a | 120/639 (18.8) | 60 (44.1) | 60/503 (11.9) | < 0.001 |
| pO2/FiO2 ≤ 250 mmHga | 172/639 (26.9) | 28 (20.6) | 144/503 (28.6) | 0.061 |
| Multilobar infiltratesa | 546 (78.8) | 120 (88.2) | 426 (76.5) | 0.003 |
| Pleural effusiona | 120 (17.3) | 36 (26.5) | 84 (15.1) | < 0.001 |
| Coinfections (n, %)a | 265 (38.2) | 84 (61.8) | 181 (32.5) | < 0.001 |
| Early NAI use (n, %)a | 232 (33.5) | 60 (43.4) | 172 (30.9) | 0.003 |
| Systemic corticosteroid use (n, %)a | 132 (19.0) | 60 (44.1) | 72 (12.9) | < 0.001 |
| Noninvasive ventilation (n,%) | 159 (22.9) | 71 (52.2) | 88 (15.8) | < 0.001 |
| Invasive ventilation (n,%) | 158 (22.8) | 86 (63.2) | 72 (12.9) | < 0.001 |
| Admittance to ICU (n,%) | 176 (25.4) | 92 (67.6) | 84 (15.1) | < 0.001 |
The bolded values are p-values < 0.05, which represented significant differences between survival group and deceased group
COPD chronic obstructive pulmonary disease, SBP systolic blood pressure, Hb hemoglobin, ALB albumin, BUN blood urea nitrogen, BG blood glucose, pO2/FiO2 arterial pressure of oxygen/fraction of inspiration oxygen, NAI neuraminidase inhibitor
avariables cited in the table above were the candidates which were entered into the multivariate logistic regression model
Almost 40 % (38.2%, 265/693) of patients were coinfected with other community-acquired pathogens. Streptococcus pneumoniae (33.2%) was the most common coinfection, followed by Klebsiella pneumoniae (30.6%) and Staphylococcus aureus (20.4%) (Supplementary material 4).
All patients received antibiotic treatment within 48 h after admission (Supplementary material 5), and NAI therapy during the course of the disease. Early NAI therapy and systemic corticosteroid use were administered in 33.5% (232/693) and 19.0% (132/693) of patients, respectively. 22.8% (158/693) of patients received invasive ventilation, 25.4% (176/693) of patients were admitted to the ICU, and the 30-day mortality rate was 19.6% (136/693) (Table 1).
There were no significant differences in the demographic characteristics, clinical features, approach to clinical management, and treatment outcomes between patients in the derivation and validation cohorts (Supplementary material 6).
Predicted and actual mortality in FluA-p patients stratified by CURB-65 score and PSI risk class
Supplemental material 7 shows the actual and predicted mortality rates stratified by PSI risk class and CURB-65 scores. For the 136 deceased patients, the proportions of patients with PSI risk I ~ V were 38.2% (52/136), 8.8% (12/136), 5.9% (8/136), 47.1% (64/136) and 0% (0/136), respectively; the proportions of patients with CURB-65 scores 0–5 were 0% (0/136), 66.9% (91/136), 12.5% (17/136), 0% (0/136) and 0% (0/136), respectively.
Risk factors for 30-day mortality
Following the procedures described in the Statistical Analysis section, the following variables were entered into a backward stepwise logistic regression analysis: obesity, smoking history, cardiovascular disease, chronic pulmonary disease (COPD), altered mental status, leukocytes > 10 × 109/L, lymphocytes < 0.8 × 109/L, hemoglobin (Hb) < 100 g/L, albumin (ALB) < 35 g/L, blood urea nitrogen (BUN) > 7 mmol/L, arterial PH < 7.35, pO2/FiO2 ≤ 250 mmHg, multilobar infiltrates, pleural effusion, early NAI therapy, systemic corticosteroid use, and coinfections.
A multivariate logistic regression model indicated that the following variables were significantly associated with 30-day mortality (see Fig. 2): BUN > 7 mmol/L (OR 1.604, 95% CI 1.150–4.492, p = 0.040), pO2/FiO2 ≤ 250 mmHg (OR 2.649, 95% CI 1.103–5.142, p = 0.022), cardiovascular disease (OR 3.967, 95% CI 1.269–7.322, p < 0.001), arterial PH < 7.35 (OR 3.959, 95% CI 1.393–7.332, p < 0.001), smoking history (OR 5.176, 95% CI 2.604–11.838, p < 0.001), lymphocytes < 0.8 × 109/L (OR 8.391, 95% CI 3.271–16.212, p < 0.001) and early NAI therapy (OR 0.567, 95% CI 0.202–0.833, p = 0.001).
Fig. 2.
Multivariate analysis associated with mortality of FluA-p patients
Comparison of severity scores for mortality prediction
In order to develop a simple and useful clinical predicting tool, relative weights were assigned according to the regression coefficient (β) of each categorical variable. Supplementary material 8 shows that the AUROC of the derivation cohort was 0.934 (95% CI 0.906–0.957), which was higher than the CURB-65 score (AUC = 0.813, 95% CI 0.772–0.850, p < 0.001) and the PSI risk class (AUC = 0.577, 95% CI 0.527–0.625, p < 0.001) (Supplemental Figure 1). Supplementary material 9 shows that the AUROC of the validation cohort was 0.846 (95% CI 0.781–0.897), which was higher than the CURB-65 score (AUC = 0.681, 95% CI 0.604–0.752, p < 0.001) and the PSI risk class (AUC = 0.525, 95% CI 0.445–0.604, p < 0.001) (Supplemental Figure 2). For the full sample of 693 patients, the AUROC was 0.908 (95% CI 0.881–0.931), which was higher than the CURB-65 score (AUC = 0.777, 95% CI 0.740–0.811, p < 0.001) and the PSI risk class (AUC = 0.560, 95% CI 0.518–0.602, p < 0.001) (Table 2 and Fig. 3). Table 3 shows the sensitivity, specificity and actual mortality associated with the FluA-p score (in the full sample of 693 patients). In accordance with the cut-score approach described earlier, patients were divided into high-risk and low-risk groups based on a cut-off value of 7. The Kaplan-Meier survival curves showed that 30-day mortality was significantly higher in patients with high-risk than for patients at low-risk (52.9% vs 2.1%, log rank test, p < 0.001) (Fig. 4).
Table 2.
AUC for mortality predictions in FluA-p patients
| Variable | AUC | SE | 95% CI | Z statistic | p value |
|---|---|---|---|---|---|
| FluA-p score | 0.908 | 0.016 | 0.881–0.931 | – | Reference |
| PSI risk class | 0.560 | 0.035 | 0.518–0.602 | 10.875 | < 0.001 |
| CURB-65 score | 0.777 | 0.020 | 0.740–0.811 | 6.041 | < 0.001 |
AUC area under the curve, SE standard error, CI confidence interval
Fig. 3.
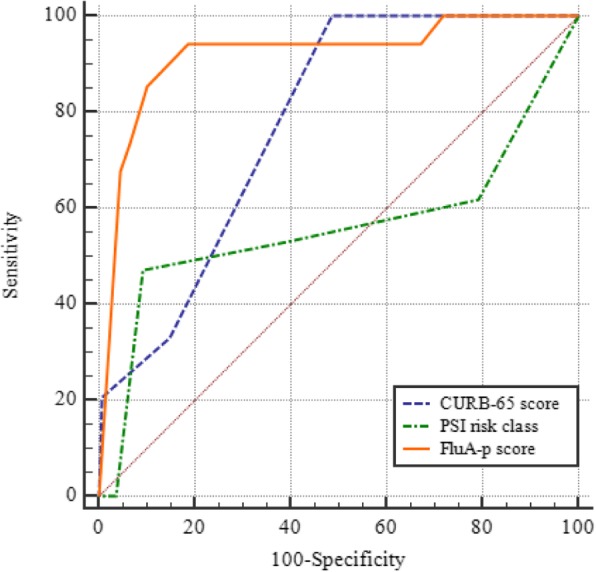
ROCs for mortality prediction of three severity scores in FluA-p patients
Table 3.
FluA-p score and actual mortality
| Score |
Actual 30-day Mortality (n, %) |
Sensitivity | 95% CI | Specificity | 95% CI | +LR | -LR |
|---|---|---|---|---|---|---|---|
| -2 ~ 1 | 0/120 (0.0) | 100.00 | 97.3–100.0 | 0.00 | 0.0–0.8 | 1.00 | |
| 2 | 8/16 (50.0) | 100.00 | 97.3–100.0 | 25.05 | 21.2–29.2 | 1.33 | 0.00 |
| 3 ~ 6 | 0/237 (0.0) | 94.12 | 88.7–97.4 | 26.72 | 22.8–30.9 | 1.28 | 0.22 |
| 7 | 20/82 (24.4) | 94.12 | 88.7–97.4 | 76.20 | 72.1–79.9 | 3.95 | 0.077 |
| 8 | 8/32 (25.0) | 79.41 | 71.6–85.9 | 89.14 | 86.0–91.8 | 7.32 | 0.23 |
| 9 | 40/56 (71.4) | 73.53 | 65.3–80.7 | 94.15 | 91.7–96.1 | 12.58 | 0.28 |
| 10 | 8/8 (100.0) | 44.12 | 35.6–52.9 | 97.49 | 95.7–98.7 | 17.61 | 0.57 |
| 11 | 52/64 (81.3) | 38.24 | 30.0–47.0 | 97.49 | 95.7–98.7 | 15.26 | 0.63 |
| 12 | NA | 0.00 | 0.0–2.7 | 100.00 | 99.2–100.0 | 1.00 |
+LR positive likelihood ratio, −LR negative likelihood ratio
Fig. 4.
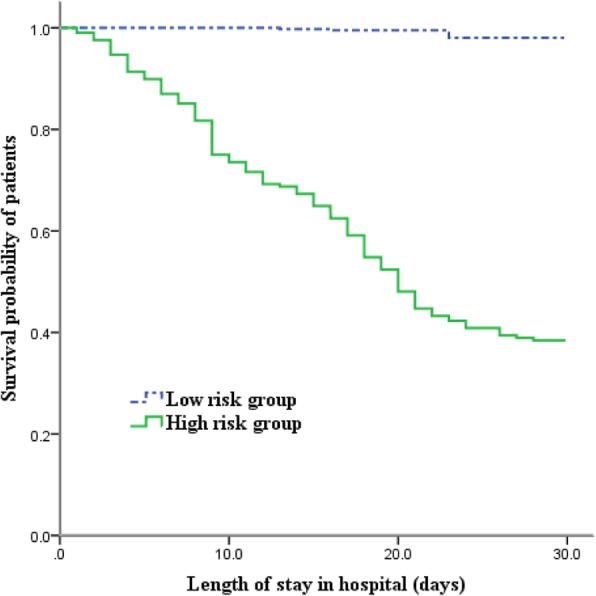
Survival of FluA-p patients by different levels of FluA-p scores. For 30-day mortality: FluA-p score < 7: Low risk; FluA-p score ≥ 7: High risk
Discussion
Our study not only assessed several risk factors, but also developed a simple and reliable prediction tool for predicting mortality in patients with FluA-p. Our method showed greater predictive validity than did the common pneumonia severity scores of PSI and CURB-65.
PSI and CURB-65 scores are recommended by the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) and the British Thoracic Society (BTS) for the assessment of disease severity of CAP [20, 21]. Numerous studies have found that PSI and CURB-65 scores accurately predict the 30-day mortality rates of CAP and are applicable for use in many clinical settings [22–24]. Recently, however, some studies suggested that they were insufficient for predicting mortality in settings involving influenza pneumonia [13–16]. Our results likewise suggested that PSI and CURB-65 scores underestimated the mortality of FluA-p patients. More than half of the deceased patients were classified as low death risk (CURB-65 score 0–2 and PSI risk class I~III). Both CURB-65 and PSI were heavily weighted by age and comorbidities. But many Flu-p patients were young and previously healthy individuals. In our study cohort, 60% of patients were younger than 65 years of age. During the H1N1 influenza A pandemic in 2009, a large proportion of severe cases were young patients who experienced acute respiratory failure [25, 26]. Another issue to consider is that the current severity tool that relies on PSI and CURB-65 scores was possibly derived from patients diagnosed primarily with bacterial and atypical bacterial pneumonia rather than influenza pneumonia [20, 27]. In fact, Guo et al. reported that CURB-65 scores were not powerful predictors of mortality in the context of viral pneumonia [28].
Several studies have reported lymphocytopenia in severe influenza [27, 29, 30]. Shi et al. suggested that lymphocytopenia was an early and reliable predictor of mortality in patients diagnosed with influenza A(H1N1)pdm09 pneumonia [27]. Although the mechanisms of lymphocytopenia in severe influenza are not well elucidated, it is believed that the reduction of T lymphocytes (including CD8 + T effector and central memory cells, CD4 + T, and/or NK cells), rather than B lymphocytes, in the peripheral blood might be the causes of lymphocytopenia [31, 32]. Lymphocytopenia also plays a role in suppressive cellular immunity and the delayed clearance of viruses [33].
Smoking history was another pedictor of FluA-p mortality in our study, which is a finding commensurate with some previous reports [34–36]. Wong and colleagues, for example, found that influenza-related mortality for all-causes and for cardiovascular and respiratory diseases was greater in current and ex-smokers than in never smokers [34]. A case-control study by Hennessy et al. found that smoking (OR 3.03, 95% CI 1.01–9.23) was a significant risk factor for death in patients with A(H1N1) pdm09 [35]. Although the precise nature of the association between smoking and influenza-related mortality has yet to be determined, some potential mechanisms suggest the possibility of biological associations. Smoking could disrupt the normal defenses of the respiratory tract by causing peribronchiolar inflammation, slowing mucociliary clearance, and/or damaging respiratory epithelial cells [37]. Animal studies using mouse models have shown that smoking induces inflammatory mediators and suppresses innate immunity against influenza infection [38]. Smoking could increase viral replication by directly suppressing epithelial antiviral pathways, facilitating cytokine release in mucosal innate immunity and increasing deoxyribonucleic acid (DNA) methylation for viral infection [39].
BUN, pO2/FiO2, and arterial PH were parameters in calculating PSI and/or CURB-65 scores. Our study showed that these parameters were valuable predictors of mortality in FluA-p patients. Early administration of NAI therapy is associated with better outcomes in severe influenza [40, 41]. Old age, obesity, pregnancy and chronic medical conditions, such as COPD, diabetes mellitus, and chronic kidney disease, have been associated with poorer outcomes in patients with influenza [35, 42, 43]. However, in our study only cardiovascular disease was identified as a risk factor for mortality in FluA-p patients. Other studies have shown that coinfections can worsen illness severity and increase mortality in severe influenza [44, 45]. In our univariate analyses, coinfections were associated with increased mortality for FluA-p patients, but coinfections were not significant predictors in the multivariate analysis.
FluA-p score is a very simple severity assessment tool containing only seven parameters and it serves as a reliable prediction rule. ROC showed better predictive validity compared to PSI risk class and the CURB-65 score. Although the specificity of score 2 is not good (only 25%), judging from the performance of score − 2 ~ 1 and score 3 ~ 6, we believe it is mainly because patients with score 2 were scarce (only 16 cases) in our study. Larger subgroup sample sizes would allow for stronger inferences Using a cutoff value of 7, the new FluA-p score could stratify patients into two groups with significantly different death risks. We believe this novel assessment tool is suitable for use in clinical settings with FluA-p patients. In addition, the parameters include indicators widely used in clinics, even in small and perhaps less equipped hospitals. Consequently, we think the assessment tool has a great practical value.
Some limitations of our study should be noted. First, despite our respectable sample size and comprehensive statistical approach, the retrospective research design meant some unavoidable selection bias. For example, the nucleic acid tests were performed based on the subjective judgement of the attending physicians. It was possible that more severe (or milder) patients were inclined to be tested; thus, not all respiratory cases were eligible for swabbing and there was likely some type of selection. Second, due to the retrospective study design, we were unable to retrieve and evaluate vaccination data, and the incomplete data might have lowered the accuracy of our results. Finally, some studies have suggested that the clinical characteristics and prognosis of immunocompromised patients with influenza is not the same as that for immunocompetent hosts [46, 47]. Thus, it is important to further assess our influenza prediction model in immunocompromised patients.
Conclusions
We developed a simple and reliable prediction rule for 30-day mortality in patients hospitalised with FluA-p. The prediction rule could help clinicians to more accurately assess influenza disease severity. Our recommendation is that clinicians should pay particular attention to patients with FluA-p scores ≥7, as such individuals have an increased risk for death.
Supplementary information
Additional file 1. Supplementary material 1: Details of participating centers. Supplementary material 2 Definition of underlying diseases. Supplementary material 3 Definition of microbiological criteria of coinfected with other pathogens. Supplementary material 4 coinfections with other community-acquired pathogens. Supplemental material 5 Empirical antibiotics therapy regimes. Supplementary material 6 Comparison of baseline clinical characteristics and outcomes between the derivation and validation cohort. Supplementary material 7 Predicted and actual mortality rates in FluA-p patients stratified by two common severity scores. Supplementary material 8 AUC for mortality prediction in FluA-p patients from derivation cohort. Supplementary material 9 AUC for mortality prediction in FluA-p patients from validation cohort. Figure S1. ROCs for mortality prediction of three severity scores in FluA-p patients from derivation cohort. Figure S2. ROCs for mortality prediction of three severity scores in FluA-p patients from validation cohort.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.com/) for the expert linguistic services provided.
Abbreviations
- Flu-p
Influenza-related pneumonia
- FluA-p
Influenza A-related pneumonia
- PSI
Pneumonia severity index
- CURB-65
Confusion, urea, respiratory rate, blood pressure, age ≥ 65 years
- NAI
Neuraminidase inhibitor
- OR
Odds ratio
- IC
Interval confidence
- ROC
Receiver operating characteristic
- AUROC
Area under the ROC curve
- COPD
Chronic obstructive pulmonary disease
- SBP
Systolic blood pressure
- Hb
Hemoglobin
- ALB
Albumin
- BUN
Blood urea nitrogen
- PH
Hydrogen ion index
- pO2/FiO2
Arterial pressure of oxygen/fraction of inspiration oxygen
- ICU
Intensive care unit
- IDSA/ATS
Infectious Diseases Society of America/American Thoracic Society
- BTS
British Thoracic Society
- DNA
deoxyribonucleic acid
Authors’ contributions
Study concept and design: LC, XdH. Acquisition of data: LC, XdH, YlL, CxZ, XqX. Statistical analysis of data: LC. Drafting of the manuscript: LC. Critical revision of the manuscript for important intellectual content: XdH, XqX. All authors agree with the article submission. All authors read and approved the final manuscript.
Funding
This study is founded by Beijing JST research (ZR − 201921).
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information materials].
Ethics approval and consent to participate
The study was carried out according to Declaration of Helsinki and obtained the approval of the local Ethics Committee (Beijing Jishuitan Hospital). Protocol number 201911–15. Given the retrospective nature of the study, the Ethics Committee determined that an informed consent was not necessary.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no confict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12931-020-01379-z.
References
- 1.Neuzil KM, Bresee JS, de la Hoz F, Johansen K, Karron RA, Krishnan A, et al. Data and product needs for influenza immunization programs in low- and middle-income countries: rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine. 2017;35:5734–5747. doi: 10.1016/j.vaccine.2017.08.088. [DOI] [PubMed] [Google Scholar]
- 2.Spreeuwenberg P, Kroneman M, Paget J. Reassessing the global mortality burden of the 1918 influenza pandemic. Am J Epidemiol. 2018;187:2561–2567. doi: 10.1093/aje/kwy191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nielsen J, Vestergaard LS, Richter L, Schmid D, Bustos N, Asikainen T, et al. European all-cause excess and influenza-attributable mortality in the 2017/18 season: should the burden of influenza B be reconsidered? Clin Microbiol Infect. 2019;25:1266–1276. doi: 10.1016/j.cmi.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Vestergaard LS, Nielsen J, Krause TG, Espenhain L, Tersago K, Bustos Sierra N, et al. Excess all-cause and influenza-attributable mortality in Europe, December 2016 to February 2017. Euro Surveill. 2017;22:30506. doi: 10.2807/1560-7917.ES.2017.22.14.30506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010-2016. Pediatrics. 2018;141:e20172918. doi: 10.1542/peds.2017-2918. [DOI] [PubMed] [Google Scholar]
- 6.Ly S, Arashiro T, Ieng V, Tsuyuoka R, Parry A, Horwood P, et al. Establishing seasonal and alert influenza thresholds in Cambodia using the WHO method: implications for effective utilization of influenza surveillance in the tropics and subtropics. Western Pac Surveill Response J. 2017;8:22–32. doi: 10.5365/wpsar.2017.8.1.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gentile A, Paget J, Bellei N, Torres JP, Vazquez C, Laguna-Torres VA, et al. Influenza in Latin America: a report from the global influenza initiative (GII) Vaccine. 2019;37:2670–2678. doi: 10.1016/j.vaccine.2019.03.081. [DOI] [PubMed] [Google Scholar]
- 8.Peteranderl C, Herold S, Schmoldt C. Human influenza virus infections. Semin Respir Crit Care Med. 2016;37:487–500. doi: 10.1055/s-0036-1584801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kumar V. Influenza in Children. Indian J Pediatr. 2017;84:139–143. doi: 10.1007/s12098-016-2232-x. [DOI] [PubMed] [Google Scholar]
- 10.Almond MH, McAuley DF, Wise MP, Griffiths MJ. Influenza-related pneumonia. Clin Med (Lond) 2012;12:67–70. doi: 10.7861/clinmedicine.12-1-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim WS, van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 13.Commons RJ, Denholm J. Triaging pandemic flu: pneumonia severity scores are not the answer. Int J Tuberc Lung Dis. 2012;16:670–673. doi: 10.5588/ijtld.11.0446. [DOI] [PubMed] [Google Scholar]
- 14.Mulrennan S, Tempone SS, Ling IT, William SH, Gan GC, Murray RJ, et al. Pandemic influenza (H1N1) 2009 pneumonia: CURB-65 score for predicting severity and nasopharyngeal sampling for diagnosis are unreliable. PLoS One. 2010;5:e12849. doi: 10.1371/journal.pone.0012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha BA, Pherez FM, Schoch P. Diagnostic importance of relative lymphopenia as a marker of swine influenza (H1N1) in adults. Clin Infect Dis. 2009;49:1454–1456. doi: 10.1086/644496. [DOI] [PubMed] [Google Scholar]
- 16.Ho YC, Wang JL, Wang JT, Wu UI, Chang CW, Wu HS, et al. Prognostic factors for fatal adult influenza pneumonia. J Inf Secur. 2009;58:439–445. doi: 10.1016/j.jinf.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Chen L, Zhou F, Li H, Xing X, Han X, Wang Y, et al. Disease characteristics and management of hospitalised adolescents and adults with community-acquired pneumonia in China: a retrospective multicentre survey. BMJ Open. 2018;8:e018709. doi: 10.1136/bmjopen-2017-018709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–1575. doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- 20.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community-acquired pneumonia in adults. Clin Infect Diss. 2007;44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim WS, Baudouin SV, George RC, Hill AT, Jamieson C, Le Jeune I, et al. BTS guidelines for the management of community acquired pneumonia in adults: update 2009. Thorax. 2009;64(Suppl 3):iii1–ii55. doi: 10.1136/thx.2009.121434. [DOI] [PubMed] [Google Scholar]
- 22.Chalmers JD, Singanayagam A, Akram AR, Mandal P, Short PM, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Thorax. 2010;65:878–883. doi: 10.1136/thx.2009.133280. [DOI] [PubMed] [Google Scholar]
- 23.Buising KL, Thursky KA, Black JF, MacGregor L, Street AC, Kennedy MP, et al. A prospective comparison of severity scores for identifying patients with severe community-acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61:419–424. doi: 10.1136/thx.2005.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capelastegui A, Espana PP, Quintana JM, Areitio I, Gorordo I, Egurrola M, et al. Validation of a predictive rule for the management of community-acquired pneumonia. Eur Respir J. 2006;27:151–157. doi: 10.1183/09031936.06.00062505. [DOI] [PubMed] [Google Scholar]
- 25.Riquelme R, Jimenez P, Videla AJ, Lopez H, Chalmers J, Singanayagam A, et al. Predicting mortality in hospitalised patients with 2009 H1N1 influenza pneumonia. Int J Tuberc Lung Dis. 2011;15:542–546. doi: 10.5588/ijtld.10.0539. [DOI] [PubMed] [Google Scholar]
- 26.Singanayagam A, Singanayagam A, Wood V, Chalmers JD. Factors associated with severe illness in pandemic 2009 influenza a (H1N1) infection: implications for triage in primary and secondary care. J Inf Secur. 2011;63:243–251. doi: 10.1016/j.jinf.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Shi SJ, Li H, Liu M, Liu YM, Zhou F, Liu B, et al. Mortality prediction to hospitalised patients with influenza pneumonia: PO2 /FiO2 combined lymphocyte count is the answer. Clin Respir J. 2017;11:352–360. doi: 10.1111/crj.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo L, Wei D, Zhang X, Wu Y, Li Q, Zhou M, et al. Clinical features predicting mortality risk in patients with viral pneumonia: the MuLBSTA score. Front Microbiol. 2019;10:2752. doi: 10.3389/fmicb.2019.02752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Chen J, Huang JA. Persistence of lymphocytopenia with CT abnormalities among patients with critical H7N9 swine-origin influenza A virus infection. Jpn J Radiol. 2015;33:657–662. doi: 10.1007/s11604-015-0476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellelli V, d'Ettorre G, Celani L, Borrazzo C, Ceccarelli G, Venditti M. Clinical significance of lymphocytopenia in patients hospitalised with pneumonia caused by influenza virus. Crit Care. 2019;23:330. doi: 10.1186/s13054-019-2608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez Y, Juárez E, Carranza C, Sada E, Pedraza-Sánchez S, Torres M. Diminished effector and memory CD8+ circulating T lymphocytes in patients with severe influenza caused by the AH1N1 pdm09 virus. Virology. 2017;500:139–148. doi: 10.1016/j.virol.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Fox A, Le NM, Horby P, van Doorn HR, Nguyen VT, Nguyen HH, et al. Severe pandemic H1N1 2009 infection is associated with transient NK and T deficiency and aberrant CD8 responses. PLoS One. 2012;7:e31535. doi: 10.1371/journal.pone.0031535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.To KK. Hung IF, Li IW, Lee KL, Koo CK, Yan WW, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50:850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong CM, Yang L, Chan KP, Chan WM, Song L, Lai HK, et al. Cigarette smoking as a risk factor for influenza-associated mortality: evidence from an elderly cohort. Influenza Other Respir Viruses. 2013;7:531–539. doi: 10.1111/j.1750-2659.2012.00411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hennessy TW, Bruden D, Castrodale L, Komatsu K, Erhart LM, Thompson D, et al. A case-control study of risk factors for death from 2009 pandemic influenza A(H1N1): is American Indian racial status an independent risk factor? Epidemiol Infect. 2016;144:315–324. doi: 10.1017/S0950268815001211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han L, Ran J, Mak YW, Suen LK, Lee PH, Peiris JSM, et al. Smoking and influenza-associated morbidity and mortality: a systematic review and meta-analysis. Epidemiology. 2019;30:405–417. doi: 10.1097/EDE.0000000000000984. [DOI] [PubMed] [Google Scholar]
- 37.Kulkarni R, Rampersaud R, Aguilar JL, Randis TM, Kreindler JL, Ratner AJ. Cigarette smoke inhibits airway epithelial cell innate immune responses to bacteria. Infect Immun. 2010;78:2146–2152. doi: 10.1128/IAI.01410-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amatngalim GD, Broekman W, Daniel NM, van der Vlugt LE, van Schadewijk A, Taube C, et al. Cigarette smoke modulates repair and innate immunity following injury to airway epithelial cells. PLoS One. 2016;11:e0166255. doi: 10.1371/journal.pone.0166255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu W, Zhang W, Booth JL, Hutchings DC, Wang X, White VL, et al. Human primary airway epithelial cells isolated from active smokers have epigenetically impaired antiviral responses. Respir Res. 2016;17:111. doi: 10.1186/s12931-016-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Lim WS, Al Mamun A, et al. Impact of neuraminidase inhibitors on influenza A(H1N1)pdm09-related pneumonia: an individual participant data meta-analysis. Influenza Other Respir Viruses. 2016;10:192–204. doi: 10.1111/irv.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Louie JK, Yang S, Acosta M, Yen C, Samuel MC, Schechter R, et al. Treatment with neuraminidase inhibitors for critically ill patients with influenza A (H1N1)pdm09. Clin Infect Dis. 2012;55:1198–1204. doi: 10.1093/cid/cis636. [DOI] [PubMed] [Google Scholar]
- 42.Mertz D, Geraci J, Winkup J, Gessner BD, Ortiz JR, Loeb M. Pregnancy as a risk factor for severe outcomes from influenza virus infection: a systematic review and meta-analysis of observational studies. Vaccine. 2017;35:521–528. doi: 10.1016/j.vaccine.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coleman BL, Fadel SA, Fitzpatrick T, Thomas SM. Risk factors for serious outcomes associated with influenza illness in high- versus low- and middle-income countries: systematic literature review and meta-analysis. Influenza Other Respir Viruses. 2018;12:22–29. doi: 10.1111/irv.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.MacIntyre CR, Chughtai AA, Barnes M, Ridda I, Seale H, Toms R, et al. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect Dis. 2018;18:637. doi: 10.1186/s12879-018-3548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luyt CE, Rice TW. Co-infection in severe influenza: a new epidemiology? Intensive Care Med. 2017;43:107–109. doi: 10.1007/s00134-016-4597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kossyvakis A, Mentis AA, Tryfinopoulou K, Pogka V, Kalliaropoulos A, et al. Antiviral susceptibility profile of influenza A viruses; keep an eye on immunocompromised patients under prolonged treatment. Eur J Clin Microbiol Infect Dis. 2017;36:361–371. doi: 10.1007/s10096-016-2809-3. [DOI] [PubMed] [Google Scholar]
- 47.Ison MG. Influenza prevention and treatment in transplant recipients and immunocompromised hosts. Influenza Other Respir Viruses Suppl. 2013;3:60–66. doi: 10.1111/irv.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary material 1: Details of participating centers. Supplementary material 2 Definition of underlying diseases. Supplementary material 3 Definition of microbiological criteria of coinfected with other pathogens. Supplementary material 4 coinfections with other community-acquired pathogens. Supplemental material 5 Empirical antibiotics therapy regimes. Supplementary material 6 Comparison of baseline clinical characteristics and outcomes between the derivation and validation cohort. Supplementary material 7 Predicted and actual mortality rates in FluA-p patients stratified by two common severity scores. Supplementary material 8 AUC for mortality prediction in FluA-p patients from derivation cohort. Supplementary material 9 AUC for mortality prediction in FluA-p patients from validation cohort. Figure S1. ROCs for mortality prediction of three severity scores in FluA-p patients from derivation cohort. Figure S2. ROCs for mortality prediction of three severity scores in FluA-p patients from validation cohort.
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information materials].