Abstract
Background
Surveillance of outdoor host-seeking malaria vectors is crucial to monitor changes in vector biting behaviour and evaluate the impact of vector control interventions. Human landing catch (HLC) has been considered the most reliable and gold standard surveillance method to estimate human-biting rates. However, it is labour-intensive, and its use is facing an increasing ethical concern due to potential risk of exposure to infectious mosquito bites. Thus, alternative methods are required. This study was conducted to evaluate the performance of human-odour-baited CDC light trap (HBLT) and human-baited double net trap (HDNT) for outdoor host-seeking malaria vector surveillance in Kenya and Ethiopia.
Methods
The sampling efficiency of HBLT and HDNT was compared with CDC light trap and HLC using Latin Square Design in Ahero and Iguhu sites, western Kenya and Bulbul site, southwestern Ethiopia between November 2015 and December 2018. The differences in Anopheles mosquito density among the trapping methods were compared using generalized linear model.
Results
Overall, 16,963 female Anopheles mosquitoes comprising Anopheles gambiae sensu lato (s.l.), Anopheles funestus s.l., Anopheles pharoensis, Anopheles coustani and Anopheles squamosus were collected. PCR results (n = 552) showed that Anopheles arabiensis was the only member of An. gambiae s.l. in Ahero and Bulbul, while 15.7% An. arabiensis and 84.3% An. gambiae sensu stricto (s.s.) constituted An. gambiae s.l. in Iguhu. In Ahero, HBLT captured 2.23 times as many An. arabiensis and 2.11 times as many An. funestus as CDC light trap. In the same site, HDNT yielded 3.43 times more An. arabiensis and 3.24 times more An. funestus than HBLT. In Iguhu, the density of Anopheles mosquitoes did not vary between the traps (p > 0.05). In Bulbul, HBLT caught 2.19 times as many An. arabiensis as CDC light trap, while HDNT caught 6.53 times as many An. arabiensis as CDC light trap. The mean density of An. arabiensis did not vary between HDNT and HLC (p = 0.098), whereas the HLC yielded significantly higher density of An. arabiensis compared to HBLT and CDC light trap. There was a significant density-independent positive correlation between HDNT and HLC (r = 0.69).
Conclusion
This study revealed that both HBLT and HDNT caught higher density of malaria vectors than conventional CDC light trap. Moreover, HDNT yielded a similar vector density as HLC, suggesting that it could be an alternative tool to HLC for outdoor host-seeking malaria vector surveillance.
Keywords: Malaria vectors, Outdoor host-seeking, Surveillance, Human-odour-baited CDC light trap, Human-baited double net trap, Kenya, Ethiopia
Background
Estimating the entomological inoculation rate (EIR), the number of infectious mosquito bites per person per unit time, is a key metric used to quantify malaria transmission intensity and evaluate the impact of vector control interventions [1, 2]. Estimating EIR requires sampling host-seeking Anopheles mosquitoes to determine human-biting rate (HBR) and sporozoite infection rate, the two components of the EIR [1, 3]. However, developing standardized methods for estimating the HBR that do not expose collectors to infectious mosquito bites has been a major challenge [4, 5], especially in settings where a substantial proportion of biting occurs outdoors [6–9].
The gold standard method to determine the HBR has been the human landing catch (HLC), which can be employed either indoors or outdoors to capture mosquitoes as they land to feed on a human host [4, 10–12]. However, HLC is a labour-intensive procedure requiring highly trained collectors and extensive supervision to obtain reliable results. Furthermore, there may be considerable differences between biting rates experienced by different collectors as a result of variability in individual attractiveness and skill in catching mosquitoes [13–15], thus it might be difficult to standardize the estimates based on biting catches. Last but not the least, conducting HLC raises ethical concerns associated with an increased risk of participants’ exposure to infectious mosquito bites if an appropriate anti-malarial chemoprophylaxis is not taken [4, 10, 16]. The increasing risk of arboviral infections further compounds its limitations [17]. Hence, it may not be practical to deploy HLC for routine malaria vector surveillance.
As an alternative to HLC, Centers for Disease Control and Prevention (CDC) miniature light trap has been widely employed for host-seeking mosquito collection [18–20]. The CDC light trap has been shown to have a good performance when used indoors due to its proximity to a sleeping person underneath a bed net [18, 21–23] and has been used as a proxy to estimate indoor-HBRs in different settings [24–26]. However, it may not be effective for the surveillance of outdoor biting malaria vectors in the absence of additional attractants that augment its trapping efficiency [20, 27, 28].
Consequently, efforts have been made to develop and evaluate alternative odour-baited trapping methods in a variety of settings for determining outdoor-HBRs. These include double bed-net traps [29–31], tent traps [32–35] and Mbita traps [36]. The double net traps have been shown to have good efficiency when compared to HLC in some settings [29, 30]. However, they do have their own drawbacks. In some studies, for instance, two persons are used to conduct a double net trap i.e. one individual acting as a bait and the other as collector, and such approach is almost as labour intensive as conducting HLC [30]. In another circumstance when one person is used both as bait and collector [29], there might be a possibility of exposure to infectious mosquito bites during the collection process. A similar concern related with operator’s exposure to mosquito bites has also been reported for the tent traps, despite their promising potential for monitoring host-seeking malaria vectors [32]. Although the Mbita trap is considered an exposure-free tool, it is less effective compared to both HLC and CDC light trap [36–38]. Hence, there is a need to look for an appropriate tool that is as effective as HLC outdoors, exposure-free and widely deployable.
The aim of this study was to evaluate the performance of two novel, exposure-free traps i.e. human-odour-baited CDC light trap (HBLT) and human-baited double net trap (HDNT) for outdoor host-seeking malaria vector surveillance. The HBLT consists of a CDC light trap baited with human-odour pumped from ordinary sleeping room, whereas the HDNT is a variant of previously designed double net trap [29] with an integrated CDC light trap. The trapping efficiency of the HBLT and HDNT was compared with conventional (unbaited) CDC light trap and HLC in western Kenya and southwestern Ethiopia. These study locations were chosen to evaluate the traps in diverse eco-epidemiological settings.
Methods
Study sites
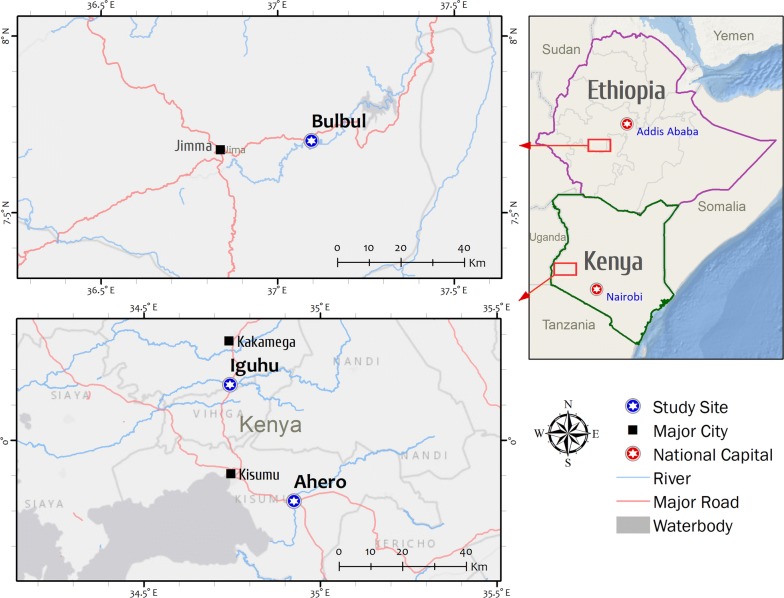
The study was conducted in two different eco-epidemiological settings of East Africa, western Kenya and southwestern Ethiopia (Fig. 1).
Fig. 1.
Map of the study sites
Western Kenya
The study was done in Ahero (0.13123° S, 34.93960° E, altitude 1162 m above sea level, asl) and Iguhu (0.15657° N; 34.74386° E, altitude 1430–1580 m asl) sites. Ahero is a lowland plain area located in Kisumu County while Iguhu is highland site characterized by undulating hills and valley bottoms located in Kakamega County [39, 40]. In both sites, most houses are mud-walled with roofs made of corrugated iron sheets. The inhabitants mainly depend on subsistence farming, with rice and maize being the main cultivated crops in the area. The sites have bimodal pattern of rainfall, with a long rainy season from April to June, which triggers the peak malaria transmission and short rains from October to November with minor transmission [41]. Plasmodium falciparum is the predominant malaria parasite species in the area and transmitted by Anopheles gambiae sensu stricto (s.s.), Anopheles arabiensis and Anopheles funestus [39, 42–44].
Southwestern Ethiopia
The study was carried out in Bulbul kebele (7.70285° N; 37.09592° E, altitude 1705 m asl), which is located in Kersa district, Oromia Region at 320 kms southwest of Addis Ababa. The majority of the houses are mud-walled with roofs made of corrugated iron sheets. The inhabitants mostly rely on subsistence farming. Maize and teff are the main cultivated crops. Malaria transmission is seasonal in Bulbul area. The transmission peaks from September to October, following the major rains from June to September. Minor transmission occurs in April and May, following the short rains of February to March. Plasmodium falciparum and Plasmodium vivax are the two predominant malaria parasite species in the area and are transmitted by An. arabiensis [45].
Description of trapping methods
Human-odour baited CDC light traps (HBLT)
The HBLT comprises a polyvinyl chloride (PVC) pipe that moves human odour from indoor (sleeping room) to outdoor mosquito catching station (Fig. 2a). The inner end of the pipe is wide (4-in. diameter) while its outer segment is narrow (2-in. diameter). A fan was installed into the inner end of the pipe to enhance outflow of the odour. A CDC light trap (John W. Hock Ltd, Gainesville, FL., USA) was set outdoor near the outer end of the pipe to capture mosquitoes attracted to the human odour. The pipe was connected from the sleeping room to the outdoor station through a small hole (2-in. diameter) made on the wall or window of selected houses. The length of the pipe from the wall of the house to its outer end was 2 m. The inner opening of the pipe was covered with untreated net to make sure that the pipe pumps odour only. The inner (wide section) of the pipe was connected with its outer (narrow) section using reducing bush so that the two parts could be easily disconnected when they were not in use. Outdoor host-seeking mosquito collection using the HBLT was done from 18:00 to 6:00 h during each collection night.
Fig. 2.

Vector sampling tools [human-odour-baited CDC light trap (a), human-baited double net trap (b), unbaited CDC light trap (c) and human landing catch (d)] used for outdoor host-seeking malaria vector surveillance in western Kenya and southwestern Ethiopia
Human-baited double net trap (HDNT)
The HDNT in this study consisted of two box nets (inner and outer nets) with a roof made of canvas. The inner net (97 cm high × 200 cm long × 100 cm wide) fully protects a human volunteer who rests on a mattress. The outer net (100 cm high × 250 cm long × 150 cm wide) is hung over the inner net and raised 30 cm off the ground. Mosquitoes attracted to the human-bait are collected by setting a CDC light trap between the two nets (Fig. 2b). The HDNT is an exposure-free tool since the lured mosquitoes are captured by the CDC light trap rather than by the person acting as a bait unlike the previously designed bed net traps [29]. Outdoor mosquito sampling using the HDNT was conducted from 18:00 to 6:00 h during each collection night.
CDC miniature light traps
Conventional CDC miniature light trap (Fig. 2c) was also set outdoor at about 2 m from each of the selected houses, at a height of 1.5 m from the ground from 18:00 to 06:00 h.
Human landing catch (HLC)
The HLC was performed by a male adult volunteer, who acted as both bait and collector (Fig. 2d). The collector seated outdoor on a chair with the legs exposed from foot to knee and captured mosquitoes as soon as they land on the exposed legs before they commence feeding using a flashlight and mouth aspirator [4, 10]. There were two collection shifts: one collector worked from 18:00 to 24:00 h during each collection night, followed by the second collector from 24:00 to 06:00 h. Each hour’s collection was kept separately in labelled paper cups. A supervisor was assigned to coordinate the collection activities and watch volunteers not to fall asleep during the collection nights. All collectors were provided with anti-malaria prophylaxis to avoid a risk of contracting malaria during the collection period. Mosquitoes were identified to species the next morning.
Experimental design
The study consisted of three consecutive experiments. The first experiment was conducted to compare HBLT with unbaited CDC light trap to test a hypothesis that the use of human-odour in HBLT could significantly improve its trapping efficiency as compared to the unbaited CDC light trap. In the second experiment, HDNT was compared with the HBLT. In the third experiment, the HBLT, HDNT and CDC light trap were compared with HLC, the gold standard method. Details of the experimental designs are presented as follows:
Human-odour-baited and unbaited CDC light trap comparison (experiment 1)
This experiment was carried out in Ahero and Iguhu sites, western Kenya. Each study site was classified into three clusters. Two houses with corresponding outdoor mosquito catching stations, about 2 m from each selected house, were selected from each cluster. The HBLT and unbaited CDC light trap were assigned to one of the two outdoor catching stations and swapped between the two houses daily in each cluster in both study sites. The experiment was conducted from November 2015 to February 2016. A total of 60 trapping nights were done for each trap in each study site.
Human-odour-baited CDC light trap and human-baited double net trap comparison (experiment 2)
Experiment 2 was conducted from June to July 2017 in the same study sites as experiment 1, using the same two houses in each cluster. The HBLT and HDNT were assigned to one of the two outdoor catching stations and swapped between the two houses daily in each cluster in both study sites. A total of 42 trapping nights were done for each trapping method in each study site.
Comparison of alternative outdoor traps with human landing catch (experiment 3)
The third experiment was conducted in Bulbul, southwestern Ethiopia. Four representative houses of similar size and design with corresponding outdoor catching stations were randomly selected. The HBLT, HDNT, CDC light trap and HLC were assigned to one of the four outdoor catching stations. The traps were rotated among the selected houses once monthly using 4 × 4 Latin Square Design. All traps were set simultaneously from 18:00 to 6:00 h. A total of 48 trapping nights were conducted for each trapping method. The experiment was conducted from January to December 2018.
Sample processing
All collected mosquito samples were identified morphologically to species or species complexes using morphological identification keys [46]. Adult female Anopheles mosquitoes were kept individually in labelled 1.5 ml Eppendorf tubes containing silica gel desiccant. Samples were stored at − 20 °C freezer at Climate and Human Health Research Laboratory of Kenya Medical Research Institute (KEMRI) or Jimma University Tropical and Infectious Diseases Research Center (TIDRC) Laboratory until used for further processing.
Identification of vector species complexes
All An. gambiae sensu lato (s.l.) samples collected from Iguhu, and sub-samples of An. gambiae s.l. randomly selected from each trap for Ahero and Bulbul sites were analysed by polymerase chain reaction (PCR) for identification of their sibling species, following the protocol developed by Scott et al. [47]. Moreover, sub-samples of An. funestus s.l. collected from Ahero and Iguhu sites were identified to sibling species by PCR following the protocol developed by Koekemoer et al. [48].
Detection of sporozoite infections
Dried head and thorax of the preserved Anopheles mosquito specimens were carefully separated from the abdomen and tested for P. falciparum and P. vivax circum-sporozoite protein (CSP) using sand-witch ELISA method [49, 50].
Data analysis
The difference in Anopheles mosquito density among different trapping methods was compared using generalized linear model based on a negative binomial distribution. Trap type was fitted as the main factor in the model. Experimental night was treated as a covariate for the first and second experiments, whereas sampling month was also considered as a covariate for the third experiment. The estimated marginal mean (EMM) density of Anopheles mosquitoes was determined for each trap using the negative binomial regression by adjusting for experimental night and month. Gini-Simpson’s diversity index (1-D) [51–53] was applied to evaluate mosquito species diversity for each trap. To determine the statistical significance of difference in species diversity among the traps, 95% confidence intervals (CI) were calculated [54]. The Simpson’s index of evenness (E) was computed to obtain a measure of the relative abundance of different mosquito species in each trapping method [51, 55].
Further analysis was conducted for the third experiment to determine whether each of the alternative outdoor trapping methods was correlated with the reference method i.e. HLC. Pearson correlation coefficient for the relationship among log-transformed catches for each Anopheles species was determined. To test if the sampling efficiency of each alternative trap (HBLT, HDNT or CDC light trap) relative to the HLC was affected by mosquito density, the ratios of the number of mosquitoes in each alternative trap to the number of mosquitoes in HLC [log(HLC + 1) − log(Alternative trap + 1)] were plotted against the average mosquito abundance, calculated as [log(HLC + 1) + log(Alternative trap + 1)]/2 [56]. Simple linear regression analysis was done for the relationship between the ratios and their average mosquito abundance [56]. The value of R-square (R2) derived from the analysis was then interpreted as an estimate of the proportion of deviation from perfect linear correlation due to density-dependence rather than random error, with a high and significant value indicating density-dependence.
The sporozoite rate was estimated as the proportion of mosquitoes positive for Plasmodium CSP over the total number tested. Data were analysed using SPSS version 20.0 (SPSS, Chicago, IL, USA) software package. p < 0.05 was considered statistically significant during the analysis.
Results
Mosquito species composition and abundance
Overall, 30,278 female mosquitoes (25,135 from Ahero, 1407 from Iguhu and 3736 from Bulbul) were collected outdoors over the course of 600 trapping nights. Of these, 16,963 (56.0%) were anophelines, with the remaining 13,315 (44.0%) being Culex species. 15,201 of the anophelines were collected from Ahero and Iguhu sites (5042 by HBLT, 1128 by CDC light trap and 9031 by HDNT). Anopheles gambiae s.l. was the predominant species, accounting for 57.3% of the anophelines collected from Ahero and Iguhu, followed by An. pharoensis (22.3%), An. coustani (15.5%) and An. funestus s.l. (4.9%). In Bulbul site, An. pharoensis was the most abundant species, accounting for 41.0% of the collected anophelines, followed by An. coustani (30.7%), An. gambiae s.l. (27.7%), An. squamosus (0.4%) and An. funestus s.l. (0.2%).
Composition of vector species complexes
A total of 602 An. gambiae s.l. specimens [258 from Ahero, 184 from Iguhu and 160 from Bulbul] and 90 An. funestus s.l. (from Ahero and Iguhu) were analysed for identification of sibling species. Of these, 552 An. gambiae s.l. and 84 An. funestus s.l. specimens were successfully amplified and identified to species by PCR. In Ahero, all of the amplified An. gambiae s.l. specimens were confirmed to be An. arabiensis. In Iguhu, An. arabiensis and An. gambiae s.s. accounted for 15.7 and 84.3% of the An. gambiae s.l., respectively. The sibling species composition of An. gambiae s.l. did not vary among the different trapping methods (χ2 = 0.086, df = 2, p = 0.958). Of the amplified An. funestus s.l. specimens, An. funestus s.s. and Anopheles leesoni accounted for 90.5 and 9.5%, respectively. Similar to Ahero, An. arabiensis was the only identified member species of the An. gambiae s.l. in Bulbul site.
Mosquito density and species diversity
Human-odour-baited and unbaited CDC light trap comparison (experiment 1)
Between November 2015 to February 2016, a total of 2783 female Anopheles mosquitoes were collected by HBLT and CDC light trap in Ahero and Iguhu sites. Overall, HBLT yielded 1.43 (95% CI 1.09–1.86, p = 0.009) times higher density of anophelines than CDC light trap (Table 1). In Ahero, HBLT caught 2.23 (95% CI 1.49–3.36, p < 0.001) times as many An. arabiensis per night as CDC light trap. Similarly, HBLT captured 2.11 (95% CI 1.28–3.47, p = 0.003) times higher number of An. funestus s.l. per night compared to CDC light trap. There was no significant difference between HBLT and CDC light trap in terms of the mean density of An. pharoensis and An. coustani (p > 0.05). In Iguhu site, the density of anophelines was low from both HBLT and CDC light trap (Table 1).
Table 1.
Estimates of a negative binomial regression for the comparison of outdoor host-seeking anopheline density between HBLT and CDC light trap in Ahero and Iguhu, western Kenya
| Site and species | Traps | Number collected | EMM (95% CI) | OR (95% CI) | p value |
|---|---|---|---|---|---|
| Ahero | |||||
| An. arabiensis | HBLT | 332 | 5.52 (4.19–7.26) | 2.23 (1.49–3.36) | < 0.001* |
| Light trap | 149 | 2.47 (1.83–3.33) | 1.0a | ||
| An. funestus s.l. | HBLT | 99 | 1.65 (1.20–2.27) | 2.11 (1.28–3.47) | 0.003* |
| Light trap | 47 | 0.78 (0.53–1.15) | 1.0a | ||
| An. pharoensis | HBLT | 554 | 8.21 (6.27–10.75) | 1.28 (0.87–1.87) | 0.213 |
| Light trap | 421 | 6.43 (4.89–8.46) | 1.0a | ||
| An. coustani | HBLT | 641 | 9.06 (6.93–11.86) | 1.16 (0.79–1.71) | 0.442 |
| Light trap | 497 | 7.80 (5.95–10.23) | 1.0a | ||
| Iguhu | |||||
| An. gambiae s.l. | HBLT | 15 | 0.22 (0.12–0.41) | 2.10 (0.79–5.57) | 0.137 |
| Light trap | 7 | 0.11 (0.05–0.24) | 1.0a | ||
| An. funestus group | HBLT | 10 | 0.16 (0.08–0.31) | 1.65 (0.56–4.87) | 0.360 |
| Light trap | 6 | 0.10 (0.04–0.22) | 1.0a | ||
| An. coustani | HBLT | 4 | 0.07 (0.02–0.18) | 4.0 (0.43–36.94) | 0.221 |
| Light trap | 1 | 0.02 (0.002–0.12) | 1.0a | ||
| Total anophelines | HBLT | 1655 | 12.74 (10.58–15.35) | 1.43 (1.09–1.86) | 0.009* |
| Light trap | 1128 | 8.92 (7.38–10.78) | 1.0a | ||
A total of 60 trap-nights were conducted for each trap in each study site
HBLT human odour-baited CDC light trap, EMM estimated marginal mean density, OR odds ratio, CI confidence interval
* Statistically significant
aReference value
The diversity of mosquito species captured was significantly higher for HBLT (Simpson diversity index ± 2SD = 0.63 ± 0.01) than for CDC light trap (0.59 ± 0.02). Moreover, the HBLT collected mosquitoes of different species more homogenously (Simpson evenness, E = 0.79 ± 0.02) than CDC light trap (0.71 ± 0.02).
Human-odour-baited CDC light trap and human-baited double net trap comparison (experiment 2)
A total of 12,418 Anopheles mosquitoes were collected by HBLT and HDNT in Ahero and Iguhu sites during the second experiment. Overall, HDNT yielded 2.75 (95% CI 2.01–3.74, p < 0.001) times higher density of anophelines compared to HBLT (Table 2). In Ahero, HDNT caught 3.43 (95% CI 2.22–5.30, p < 0.001) times as many An. arabiensis per night as HBLT. Likewise, HDNT captured 3.24 (95% CI 1.99–5.25, p < 0.001) times as many An. funestus s.l. and 3.55 (95% CI 2.25–5.61, p < 0.001) times as many An. coustani per night as HBLT. No significant difference was found in the mean density of An. pharoensis between the two traps (p = 0.183). In Iguhu site, the mean density of An. gambiae s.l. and An. funestus s.l. did not vary significantly between HDNT and HBLT (p > 0.05) (Table 2).
Table 2.
Estimates of a negative binomial regression for the comparison of outdoor host-seeking anopheline density between HDNT and HBLT in Ahero and Iguhu, western Kenya
| Site and species | Traps | Number collected | EMM (95% CI) | OR (95% CI) | p value |
|---|---|---|---|---|---|
| Ahero | |||||
| An. arabiensis | HDNT | 6188 | 148.83 (109.67–201.97) | 3.43 (2.22–5.30) | < 0.001* |
| HBLT | 1862 | 43.40 (31.90–59.04) | 1.0a | ||
| An. funestus s.l. | HDNT | 392 | 9.21 (6.67–12.71) | 3.24 (1.99–5.25) | < 0.001* |
| HBLT | 137 | 2.84 (1.99–4.06) | 1.0a | ||
| An. pharoensis | HDNT | 1386 | 32.91 (24.09–44.96) | 1.36 (0.87–2.13) | 0.183 |
| HBLT | 1016 | 24.25 (17.72–33.19) | 1.0a | ||
| An. coustani | HDNT | 895 | 21.30 (15.59–29.11) | 3.55 (2.25–5.61) | < 0.001* |
| HBLT | 252 | 6.00 (4.32–8.34) | 1.0a | ||
| Iguhu | |||||
| An. gambiae s.l. | HDNT | 92 | 2.17 (1.50–3.13) | 1.29 (0.75–2.20) | 0.353 |
| HBLT | 70 | 1.68 (1.14–2.47) | 1.0a | ||
| An. funestus s.l. | HDNT | 34 | 0.81 (0.52–1.27) | 1.42 (0.72–2.79) | 0.308 |
| HBLT | 24 | 0.57 (0.35–0.94) | 1.0a | ||
| An. pharoensis | HDNT | 6 | 0.13 (0.05–0.32) | 1.45 (0.38–5.58) | 0.587 |
| HBLT | 4 | 0.09 (0.03–0.26) | 1.0a | ||
| An. coustani | HDNT | 38 | 0.86 (0.55–1.34) | 1.65 (0.83–3.27) | 0.151 |
| HBLT | 22 | 0.52 (0.31–0.87) | 1.0a | ||
| Total anophelines | HDNT | 9031 | 108.69 (87.54–134.96) | 2.75 (2.01–3.74) | < 0.001* |
| HBLT | 3387 | 39.60 (31.84–49.25) | 1.0a | ||
A total of 42 trap-nights were conducted for each trap in each study site
HBLT human-odour-baited CDC light trap, HDNT human-baited double net trap, EMM estimated marginal mean density, OR odds ratio, CI confidence interval
* Statistically significant
aReference value
The diversity of mosquito species collected did not vary significantly between HDNT (Simpson diversity index = 0.66 ± 0.01) and HBLT (0.64 ± 0.01). Similarly, the species evenness did not vary significantly between the HDNT (E = 0.82 ± 0.01) and HBLT (0.81 ± 0.01).
Comparison of alternative outdoor traps with human landing catch (experiment 3)
A total of 1762 Anopheles mosquitoes were caught outdoors by HBLT, HDNT, CDC light trap and HLC in Bulbul site from January to December 2018. The EMM density of each anopheline species per trap is shown in Table 3. On average, HBLT caught 2.19 (95% CI 1.18–4.10, p 0.014) times as many An. arabiensis per night as CDC light trap, while HDNT caught 6.53 (95% CI 3.64–11.72, p < 0.001) times as many An. arabiensis per night as CDC light trap. The mean density of An. arabiensis did not vary between HDNT and HLC (p = 0.098), whereas the HLC caught 4.35 (95% CI 2.64–7.17, p < 0.001) times as many An. arabiensis as HBLT and 9.54 (95% CI 5.35–17.02, p < 0.001) times as many as CDC light trap.
Table 3.
Estimates of a negative binomial regression for comparison of outdoor host-seeking anopheline density between different traps in Bulbul, southwestern Ethiopia
| Site and species | Traps | Number collected | EMM (95% CI) | OR (95% CI) | p value |
|---|---|---|---|---|---|
| An. arabiensis | HBLT | 55 | 1.12 (0.76–1.65) | 0.23 (0.14–0.38) | < 0.001* |
| HDNT | 168 | 3.32 (2.40–4.59) | 0.69 (0.44–1.07) | 0.098 | |
| Light trap | 25 | 0.51 (0.31–0.83) | 0.11 (0.06–0.19) | < 0.001* | |
| HLC | 240 | 4.85 (3.56–6.63) | 1.0a | ||
| An. pharoensis | HBLT | 78 | 1.47 (1.02–2.12) | 0.20 (0.13–0.33) | < 0.001* |
| HDNT | 243 | 4.79 (3.51–6.55) | 0.66 (0.43–1.02) | 0.062 | |
| Light trap | 35 | 0.72 (0.46–1.12) | 0.10 (0.06–0.17) | < 0.001* | |
| HLC | 366 | 7.25 (5.35–9.81) | 1.0a | ||
| An. coustani | HBLT | 52 | 1.01 (0.67–1.51) | 0.15 (0.09–0.25) | < 0.001* |
| HDNT | 101 | 1.83 (1.29–2.61) | 0.28 (0.17–0.44) | < 0.001* | |
| Light trap | 26 | 0.48 (0.29–0.78) | 0.07 (0.04–0.13) | < 0.001* | |
| HLC | 362 | 6.62 (4.88–18.99) | 1.0a | ||
| Other anophelinesb | HBLT | 2 | 0.04 (0.01–0.16) | 0.35 (0.07–1.83) | 0.213 |
| HDNT | 3 | 0.06 (0.02–0.19) | 0.52 (0.12–2.21) | 0.372 | |
| Light trap | 0 | 0 | NA | NA | |
| HLC | 6 | 0.12 (0.04–0.27) | 1.0a | ||
| Total anophelines | HBLT | 187 | 3.63 (2.63–5.00) | 0.19 (0.12–0.29) | < 0.001* |
| HDNT | 515 | 10.02 (7.45–13.49) | 0.53 (0.35–0.80) | 0.003* | |
| Light trap | 86 | 1.74 (1.21–2.48) | 0.09 (0.06–0.15) | < 0.001* | |
| HLC | 974 | 18.99 (14.20–25.40) | 1.0a |
A total of 48 trap-nights were conducted for each trap in each study site
HBLT human-odour-baited CDC light trap, HDNT human-baited double net trap, HLC human landing catch, EMM estimated marginal mean density, OR odds ratio, CI confidence interval
* Statistically significant
aReference value
bOther anophelines include An. squamosus and An. funestus s.l.
The mean density of An. pharoensis captured by HBLT was 2.04 (95% CI 1.15–3.61, p = 0.015) times higher compared to CDC light trap, whereas the mean density of the same species collected by HDNT was 6.65 (95% CI 3.87–11.42, p < 0.001) times higher compared to the CDC light trap. No significant difference was found in the mean density of An. pharoensis between HDNT and HLC (p = 0.062), while the HLC collected 4.94 (95% CI 3.07–7.95, p < 0.001) times as many An. pharoensis per night as HBLT and 10.06 (95% CI 5.89–17.18, p < 0.001) times as many as CDC light trap (Table 3).
The mean density of An. coustani caught by HBLT was 2.11 (95% CI 1.12–3.99, p = 0.021) times higher compared to CDC light trap, while the mean density of An. coustani caught by HDNT was 3.84 (95% CI 2.10–7.02, p < 0.001) times higher compared to CDC light trap. The HLC captured 3.61 (95% CI 2.26–5.76, p < 0.001) times as many An. coustani per night as HDNT, 6.57 (95% CI 3.95–10.90) times as many as HBLT and 13.88 (95% CI 7.79–24.72, p < 0.001) times as many as CDC light trap. Very few An. squamosus and An. funestus s.l. were collected by HLC, HDNT and HBLT, whereas none of this species were collected by CDC light trap (Table 3).
The diversity of mosquito species collected in Bulbul was significantly higher for HDNT (Simpson diversity index = 0.70 ± 0.01) than for HBLT (0.63 ± 0.04), CDC light trap (0.50 ± 0.07) and HLC (0.63 ± 0.02). The diversity of mosquito species collected by HBLT was significantly higher than that of CDC light trap, whereas the HBLT and HLC collected mosquito of similar species diversity. The HDNT collected mosquitoes of different species more homogeneously (E = 0.85 ± 0.02) than HBLT (E = 0.76 ± 0.05), CDC light trap (E = 0.67 ± 0.09) and HLC (E = 0.75 ± 0.02).
Correlation of the alternative traps with human landing catch
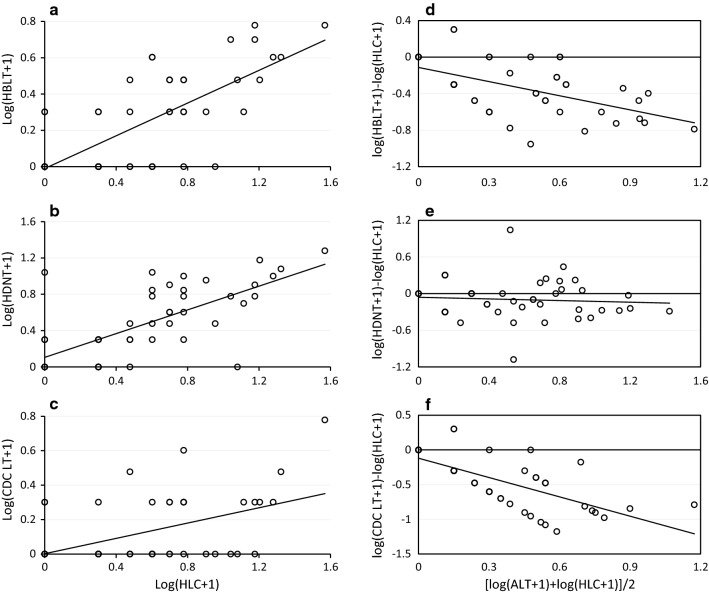
The correlation coefficients of alternative traps with HLC are shown in Table 4. There were significant positive correlations between HDNT and HLC in terms of the number of An. arabiensis (r = 0.691, p = < 0.001) and An. pharoensis (0.739, p < 0.001) (r = 0.691, p = < 0.001) captured, and R2 values did not deviate significantly from zero (Fig. 3; Table 4), which means that the relative sampling efficiency (RSE) of the HDNT was not dependent on mosquito density for these species. For An. coustani, a significant positive correlation was found between the HDNT and HLC (r = 0.655, p < 0.001), but the RSE was density-dependent. Significant positive correlations were also found between HBLT and HLC for An. arabiensis (r = 0.708, p < 0.001), An. pharoensis (r = 0.454, p = 0.001) and An. coustani (r = 0.664, p = 0.001), but the RSEs were dependent on mosquito density (Fig. 3; Table 4).
Table 4.
Correlation and density-dependence of the sampling efficiency of alternative outdoor trapping methods relative to human landing catch in Bulbul, southwestern Ethiopia
| Species | Alternative vs. HLC | Correlation coefficient | Density-dependence | |||
|---|---|---|---|---|---|---|
| R | p-value | R-square | T | p-value | ||
| An. arabiensis | HBLT | 0.708 | < 0.001 | 0.304 | 20.135 | < 0.001 |
| HDNT | 0.691 | < 0.001 | 0.006 | 0.284 | 0.597 | |
| Light trap | 0.469 | 0.001 | 0.461 | 39.408 | < 0.00 | |
| An. pharoensis | HBLT | 0.454 | 0.001 | 0.140 | 7.505 | 0.009 |
| HDNT | 0.739 | < 0.001 | 0.066 | 3.244 | 0.078 | |
| Light trap | 0.199 | 0.176 | 0.411 | 32.042 | < 0.001 | |
| An. coustani | HBLT | 0.664 | < 0.001 | 0.521 | 50.020 | < 0.001 |
| HDNT | 0.655 | < 0.001 | 0.233 | 13.973 | 0.001 | |
| Light trap | 0.569 | < 0.001 | 0.657 | 88.070 | < 0.001 | |
HBLT human-odour-baited CDC light trap, HDNT human-baited double net trap, HLC human landing catch
Fig. 3.
Correlation and density-dependence of the alternative outdoor trapping methods relative to human landing catch for catching Anopheles mosquitoes in Bulbul, southwestern Ethiopia [Correlation of human-odour-baited CDC light trap (a), human-baited double net trap (b) and unbaited CDC light trap (c) with human landing catch. The sampling efficiency (RSE) of human-odour-baited CDC light trap (d), human-baited double net trap (e) and unbaited CDC light trap (f) relative to human landing catch]. ALT represents alternative traps
Sporozoite rate
Overall, 7344 (43.3% of the total) Anopheles mosquitoes (5273 from Ahero, 309 from Iguhu and 1762 from Bulbul) were tested for P. falciparum and P. vivax CSP. Of these, 27 specimens (17 from Ahero, 4 from Iguhu and 6 from Bulbul) were positive for Plasmodium CSP.
Table 5 shows the sporozoite rates of anophelines collected from Ahero and Iguhu sites. In Ahero, the sporozoite rate of An. arabiensis was 0.12% from HBLT and 0.16% from HDNT. None of the tested An. arabiensis from CDC light trap were positive. In the same study site, the sporozoite rate of An. funestus s.l. was 2.1% from HBLT, 2.4% from HDNT and 2.1% from CDC light trap. In Iguhu, the sporozoite rate of An. gambiae s.s. was 1.5% from HBLT and 2.9% from HDNT, while the sporozoite rate of An. funestus s.l. from HDNT was 3.0%. No CSP was detected in An. funestus s.l. collected by HBLT and CDC light trap. Thus, the overall sporozoite rate of An. arabiensis, An. gambiae s.s. and An. funestus s.l. was 0.14, 2.1 and 2.2%, respectively. None of the tested An. pharoensis and An. coustani specimens were positive.
Table 5.
Plasmodium falciparum sporozoite rates of outdoor host-seeking Anopheles mosquitoes collected by different trapping methods in Ahero and Iguhu, western Kenya
| Study site and species | Parameters | Experiment 1 | Experiment 2 | Total | ||
|---|---|---|---|---|---|---|
| HBLT | Light trap | HBLT | HDNT | |||
| Ahero | ||||||
| An. arabiensis | No tested | 201 | 149 | 651 | 1929 | 2930 |
| Pf +ve (%) | 0 | 0 | 1 (0.15) | 3 (0.16) | 4 (0.14) | |
| An. funestus s.l. | No tested | 99 | 47 | 136 | 287 | 570 |
| Pf +ve (%) | 2 (2.0) | 1 (2.1) | 3 (2.2) | 7 (2.4) | 13 (2.3) | |
| An. pharoensis | No tested | 168 | 146 | 305 | 416 | 1035 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
| An. coustani | No tested | 193 | 150 | 125 | 270 | 738 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
| Iguhu | ||||||
| An. gambiae s.s. | No tested | 12 | 6 | 53 | 69 | 140 |
| Pf +ve (%) | 0 | 0 | 1 (1.9) | 2 (2.9) | 3 (2.1) | |
| An. funestus s.l. | No tested | 9 | 6 | 20 | 33 | 68 |
| Pf +ve (%) | 0 | 0 | 0 | 1 (3.0) | 1 (1.5) | |
| An. arabiensis | No tested | 2 | 1 | 11 | 12 | 26 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
| An. pharoensis | No tested | 0 | 0 | 4 | 6 | 10 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
| An. coustani | No tested | 4 | 1 | 22 | 38 | 65 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
HBLT: human-odour-baited CDC light trap; HDNT: human-baited double net trap; Pf +ve: number of P. falciparum positive Anopheles mosquitoes (rate in percent)
In Bulbul site, of the assayed anopheline specimens, 6 (2 An. arabiensis, 3 An. pharoensis and 1 An. coustani) were positive for Plasmodium CSP (four specimens for P. vivax and two for P. falciparum) (Table 6). The sporozoite rate of An. arabiensis was 0.6% from HDNT and 0.4% from HLC. No CSP was detected in An. arabiensis collected by HBLT and CDC light trap. The sporozoite rate of An. pharoensis was 1.3% from HBLT, 0.4% from HDNT and 0.3% from HLC. The sporozoite rate of An. coustani from HLC was 0.3%, whereas no CSP was detected in An. coustani collected by the other trapping methods. Hence, the overall sporozoite rate of An. arabiensis, An. pharoensis and An. coustani was 0.4, 0.3 and 0.2%, respectively.
Table 6.
Sporozoite rates of outdoor host-seeking Anopheles mosquitoes collected by different methods in Bulbul, southwestern Ethiopia
| Method | Species | No tested | Pf n (%) | Pv210 n (%) | Pv247 n (%) | Total n (%) |
|---|---|---|---|---|---|---|
| HBLT | An. arabiensis | 55 | 0 | 0 | 0 | 0 |
| An. pharoensis | 78 | 0 | 1 (1.3) | 0 | 1 (1.3) | |
| An. coustani | 52 | 0 | 0 | 0 | 0 | |
| An. squamosus | 1 | 0 | 0 | 0 | 0 | |
| An. funestus s.l. | 1 | 0 | 0 | 0 | 0 | |
| HDNT | An. arabiensis | 168 | 1 (0.6) | 0 | 0 | 1 (0.6) |
| An. pharoensis | 243 | 0 | 0 | 1 (0.4) | 1 (0.4) | |
| An. coustani | 101 | 0 | 0 | 0 | 0 | |
| An. squamosus | 2 | 0 | 0 | 0 | 0 | |
| An. funestus s.l. | 1 | 0 | 0 | 0 | 0 | |
| Light trap | An. arabiensis | 25 | 0 | 0 | 0 | 0 |
| An. pharoensis | 35 | 0 | 0 | 0 | 0 | |
| An. coustani | 26 | 0 | 0 | 0 | 0 | |
| HLC | An. arabiensis | 240 | 1 (0.4) | 0 | 0 | 0 (0.4) |
| An. pharoensis | 366 | 0 | 1 (0.3) | 0 | 1 (0.3) | |
| An. coustani | 362 | 0 | 1 (0.3) | 0 | 1 (0.3) | |
| An. squamosus | 4 | 0 | 0 | 0 | 0 | |
| An. funestus s.l. | 2 | 0 | 0 | 0 | 0 | |
| Overall | An. arabiensis | 488 | 2 (0.4) | 0 | 0 | 2 (0.4) |
| An. pharoensis | 722 | 0 | 2 (0.3) | 1 (0.1) | 3 (0.4) | |
| An. coustani | 541 | 0 | 1 (0.2) | 0 | 1 (0.2) | |
| An. squamosus | 7 | 0 | 0 | 0 | 0 | |
| An. funestus s.l. | 4 | 0 | 0 | 0 | 0 |
HBLT: human-odour-baited CDC light trap; HDNT: human-baited double net trap; HLC: human landing catch; Pf: P. falciparum; Pv: P. vivax; n: number positive (rate in percent)
Discussion
In this study, the potential of two human-odour baited traps, the HBLT and HDNT, to provide exposure-free alternatives to the HLC and CDC light trap for surveillance of outdoor host-seeking African malaria vectors was evaluated. The results showed that both HBLT and HDNT yielded significantly higher anopheline mosquito density compared to conventional CDC light trap. This suggests that the use of human-bait in HBLT and HDNT significantly enhanced the trapping efficiency of both traps. Moreover, the HDNT yielded a similar vector density as HLC. This indicates the usefulness of these tools for outdoor host-seeking vector surveillance.
The HBLT collected about twice as many An. arabiensis and An. funestus s.l. as unbaited CDC light trap. This indicates that the HBLT may also surpass the trapping efficiency of CO2-baited CDC light traps that have been compared with unbaited CDC light traps previously [57–60]. For instance, CO2-baited CDC light trap captured 1.39 times as many anophelines as unbaited CDC light trap in Thailand [57], whereas in other studies conducted in south-central Ethiopia and Suriname, synthetic CO2 did not improve the trapping efficiency of CDC light traps [28, 58]. The lower sampling efficiency of the CO2-baited CDC light traps in the previous studies might be due to a lower attraction of synthetic CO2 as compared to natural human odour. It was hypothesized that when synthetic CO2 is used in traps in isolation from other attractant stimuli produced by hosts, it could be considered as an artificial arrangement, and mosquitoes might not fly directly towards it but rather show an erratic behaviour [4]. Thus, the HBLT could represent a better outdoor vector surveillance tool than both unbaited and CO2-baited CDC light traps.
However, the HBLT yielded 4.35 times lower number of An. arabiensis compared to HLC, and 4.94 and 6.57 times lower for An. pharoensis and An. coustani, respectively. Similarly, the HBLT yielded significantly lower density of anophelines than HDNT. These variations are probably due to the difference in the location of persons used as bait. Although all traps were set outdoors in this study, a bait for HBLT was located indoor and odour was pumped-out through a pipe, while in the case of HLC and HDNT, human-baits were positioned outdoors on the actual mosquito catching stations. This means that the HBLT lacks thermal cues that may serve as supplementary short-range mosquito attractant [60], unlike the HLC and HDNT. On the other hand, HLC may also overestimate human-biting rates to some extent since the human-baits are relatively more available to host-seeking mosquitoes than under normal circumstance. Although it is habitual practice in Africa to spend evening and early-morning hours outdoors [61–63], people may not stay undisturbed in one place with legs exposed throughout the night unlike that of HLC.
The HDNT caught 6.53 times as many An. arabiensis and 6.65 times as many An. pharoensis as CDC light trap in Bulbul while the mean density of both An. arabiensis and An. pharoensis did not vary significantly between the HDNT and HLC, indicating the potential of the HDNT to substitute HLC. In previous studies in Africa, in which human served as both bait and collector in double net traps, the double net traps yielded significantly lower number of anophelines than HLC [31, 64]. The double net trap collected 7.5 times lower number of anophelines compared to HLC in Cameroon [31] and about four times lower number of anophelines in Nigeria [64]. The double net traps might have underestimated the density of anophelines in the previous studies since mosquitoes could escape the double net traps when they were unable to reach the bait [4]. While the probability of mosquitoes escaping the double net traps could be minimized by conducting hourly collections as described by Tangena et al. [29], such approach may also expose humans to infective mosquito bites when they get out of the inner net to perform mosquito collection. In the present study, the trapping efficiency of HDNT was enhanced by setting a CDC light trap between the double nets so that mosquitoes could be trapped as soon as they enter the HDNT. The HDNT could also provide a full protection since a person serving as bait in the HDNT is not involved in mosquito collection.
Moreover, HDNT showed significant positive correlation with HLC for sampling An. arabiensis and other secondary vectors, and its sampling efficiency did not depend on mosquito density. This suggests that the HDNT could represent an efficient alternative tool to HLC for surveillance of outdoor host-seeking malaria vectors. Furthermore, the HDNT collected higher mosquito species diversity compared to both CDC light trap and HLC. This makes the HDNT more useful for exploring outdoor mosquito species diversity.
The advantage of HDNT and HBLT is that they are not as labour intensive as HLC. In HDNT, a person acting as bait can rest throughout the night. Similarly, HBLT uses odours from human resting in ordinary sleeping room. In the case of HLC [65] and the previous design of double bed net traps [4, 29, 30, 66], the collectors have to remain active, and collect mosquitoes throughout the night. In addition, mosquito collections using HDNT and HBLT do not rely on the skill of collectors unlike that of HLC which is prone to bias due to interpersonal variation in the skill of the collectors.
Both HBLT and HDNT have limitations. The HBLT uses two batteries, one for a CDC light trap and the other for a pipe, hence may not be feasible in settings where there is no easy access to electricity. Using human odour in HBLT requires connecting a pipe from a sleeping room to outdoor mosquito catching station through a hole made on window or mud-wall of the room. Rooms with cement-plastered wall and without window are not appropriate to set the HBLT. Hence, further modification is needed to easily dispense human odour. Both HBLT and HDNT were set in the evening and trapped mosquitoes were collected from the traps once in the morning instead of hourly collection, hence hourly anopheline mosquito densities were not compared between these traps and HLC. Further modification using collection bottle rotator that allows automatic hourly collections may be needed to use them for monitoring vector biting times.
Conclusion
This study revealed that both HBLT and HDNT performed better than conventional CDC light traps to sample outdoor host-seeking malaria vectors. Moreover, the HDNT yielded a similar vector density as outdoor HLC, suggesting that it could represent an alternative tool to HLC for outdoor biting malaria vector surveillance. The HBLT could be used as an alternative when the HDNT cannot be used especially when there is flood that may affect a person resting under the net.
Acknowledgements
The authors would like to acknowledge all entomology technicians of Climate and Human Health Research, KEMRI-Kisumu and Jimma University TIDRC for their support in the field and laboratory. We would like to thank Ming-chie Lee for mapping the study sites. We are grateful to all data collectors, particularly Miftah Abagidi, Charles Otieno, Enock Onyango and Sally Mongoi for their technical support during the field work. We thank the communities of Ahero, Iguhu and Bulbul for their willingness to participate in the study. This study is published with the permission of the Directors of Kenya Medical Research Institute and Jimma University Research and Postgraduate Program.
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CI
Confidence interval
- CSP
Circumsporozoite protein
- EIR
Entomological inoculation rate
- ELISA
Enzyme-linked immunosorbent assay
- EMM
Estimated marginal mean density
- HBLT
Human-odour-baited CDC light trap
- HBR
Human-biting rate
- HDNT
Human-baited double net trap
- HLC
Human landing catch
- OR
Odds ratio
- PCR
Polymerase chain reaction
- RSE
Relative sampling efficiency
Authors’ contributions
TD, DY, AKG and GY designed the study protocol. TD involved in data collection, laboratory work and data analysis. GZ participated on data analysis. HA coordinated field work in Kenya. TD drafted the manuscript. DY, AKG and GY critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Grants from the National Institutes of Health (R01 AI050243, U19 AI129326 and D43 TW001505).
Availability of data and materials
Data supporting the conclusions of this article are included within the article. Raw data are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Ethical approval for the study was obtained from Ethical Review Board of Kenya Medical Research Institute (Protocol No. KEMRI/SERU/CGHR/0057/3363) and Jimma University Institutional Review Board (Ref No. IHRPGD/2075/18). Permission was sought from chief of each study site. Informed consent was obtained from heads of households.
Consent for publication
Not applicable.
Competing interests
We authors declare that we have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beier JC, Killeen GF, Githure JI. Entomologic inoculation rates and Plasmodium falciparum malaria prevalence in Africa. Am J Trop Med Hyg. 1999;61:109–113. doi: 10.4269/ajtmh.1999.61.109. [DOI] [PubMed] [Google Scholar]
- 2.Kelly-Hope LA, McKenzie FE. The multiplicity of malaria transmission: a review of entomological inoculation rate measurements and methods across sub-Saharan Africa. Malar J. 2009;8:19. doi: 10.1186/1475-2875-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, Internet access and review. Trans R Soc Trop Med Hyg. 2000;94:113–127. doi: 10.1016/S0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Service MW A critical review of procedures for sampling populations of adult mosquitoes. Bull Entomol Res. 1977;67:343–382. doi: 10.1017/S0007485300011184. [DOI] [Google Scholar]
- 5.Silver JB. Mosquito ecology: field sampling methods. Berlin: Springer Science & Business Media; 2007. [Google Scholar]
- 6.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyers JI, Pathikonda S, Popkin-Hall ZR, Medeiros MC, Fuseini G, Matias A, et al. Increasing outdoor host-seeking in Anopheles gambiae over 6 years of vector control on Bioko Island. Malar J. 2016;15:239. doi: 10.1186/s12936-016-1286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. Rijeka: Intech-Open; 2013. pp. 671–704. [Google Scholar]
- 10.WHO . Malaria entomology and vector control. Geneva: World Health Organization; 2013. [Google Scholar]
- 11.Lima JBP, Rosa-Freitas MG, Rodovalho CM, Santos F, Lourenço-de-Oliveira R. Is there an efficient trap or collection method for sampling Anopheles darlingi and other malaria vectors that can describe the essential parameters affecting transmission dynamics as effectively as human landing catches?—a review. Mem Inst Oswaldo Cruz. 2014;109:685–705. doi: 10.1590/0074-0276140134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO . Manual on practical entomology in malaria. Geneva: World Health Organization; 1995. [Google Scholar]
- 13.Lindsay S, Adiamah J, Miller J, Pleass R, Armstrong J. Variation in attractiveness of human subjects to malaria mosquitoes (Diptera: Culicidae) in The Gambia. J Med Entomol. 1993;30:368–373. doi: 10.1093/jmedent/30.2.368. [DOI] [PubMed] [Google Scholar]
- 14.Knols BG, de Jong R, Takken W. Differential attractiveness of isolated humans to mosquitoes in Tanzania. Trans R Soc Trop Med Hyg. 1995;89:604–606. doi: 10.1016/0035-9203(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 15.Qiu Y, Smallegange R, Van Loon J, Ter Braak C, Takken W. Interindividual variation in the ttractiveness of human odours to the malaria mosquito Anopheles gambiae s.s. Med Vet Entomol. 2006;20:280–287. doi: 10.1111/j.1365-2915.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 16.Kilama WL. Health research ethics in malaria vector trials in Africa. Malar J. 2010;9(Suppl 3):S3. doi: 10.1186/1475-2875-9-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simo FBN, Bigna JJ, Kenmoe S, Ndangang MS, Temfack E, Moundipa PF, et al. Dengue virus infection in people residing in Africa: a systematic review and meta-analysis of prevalence studies. Sci Rep. 2019;9:13626. doi: 10.1038/s41598-019-50135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lines J, Curtis C, Wilkes T, Njunwa K. Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res. 1991;81:77–84. doi: 10.1017/S0007485300053268. [DOI] [Google Scholar]
- 19.Mbogo C, Glass G, Forster D, Kabiru E, Githure J, Ouma J, et al. Evaluation of light traps for sampling anopheline mosquitoes in Kilifi, Kenya. J Am Mosq Control Assoc. 1993;9:260–263. [PubMed] [Google Scholar]
- 20.Costantini C, Sagnon N, Sanogo E, Merzagora L, Coluzzi M. Relationship to human biting collections and influence of light and bednet in CDC light-trap catches of West African malaria vectors. Bull Entomol Res. 1998;88:503–511. doi: 10.1017/S000748530002602X. [DOI] [Google Scholar]
- 21.Magbity E, Lines J, Marbiah M, David K, Peterson E. How reliable are light traps in estimating biting rates of adult Anopheles gambiae s.l. (Diptera: Culicidae) in the presence of treated bed nets? Bull Entomol Res. 2002;92:71–76. doi: 10.1079/BER2002200. [DOI] [PubMed] [Google Scholar]
- 22.Fornadel CM, Norris LC, Norris DE. Centers for disease control light traps for monitoring Anopheles arabiensis human biting rates in an area with low vector density and high insecticide-treated bed net use. Am J Trop Med Hyg. 2010;83:838–842. doi: 10.4269/ajtmh.2010.10-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis JR, Hall T, Chee EM, Majala A, Minjas J, Shiff CJ. Comparison of sampling anopheline mosquitoes by light-trap and human-bait collections indoors at Bagamoyo, Tanzania. Med Vet Entomol. 1995;9:249–255. doi: 10.1111/j.1365-2915.1995.tb00130.x. [DOI] [PubMed] [Google Scholar]
- 24.Drakeley C, Schellenberg D, Kihonda J, Sousa CA, Arez AP, Lopes D. An estimation of the entomological inoculation rate for Ifakara: a semi-urban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health. 2003;8:767–774. doi: 10.1046/j.1365-3156.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 25.Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors. 2013;6:114. doi: 10.1186/1756-3305-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Massebo F, Balkew M, Gebre-Michael T, Lindtjørn B. Entomologic inoculation rates of Anopheles arabiensis in Southwestern Ethiopia. Am J Trop Med Hyg. 2013;89:466–473. doi: 10.4269/ajtmh.12-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mboera L. Sampling techniques for adult Afrotropical malaria vectors and their reliability in the estimation of entomological inoculation rate. Tanzan J Health Res. 2005;7:117–124. doi: 10.4314/thrb.v7i3.14248. [DOI] [PubMed] [Google Scholar]
- 28.Kenea O, Balkew M, Tekie H, Gebre-Michael T, Deressa W, Loha E, et al. Comparison of two adult mosquito sampling methods with human landing catches in south-central Ethiopia. Malar J. 2017;16:30. doi: 10.1186/s12936-016-1668-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangena J-AA, Thammavong P, Hiscox A, Lindsay SW, Brey PT. The human-baited double net trap: an alternative to human landing catches for collecting outdoor biting mosquitoes in Lao PDR. PLoS ONE. 2015;10:e0138735. doi: 10.1371/journal.pone.0138735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q, Wang F, Lv X, Cao H, Zhou J, Su F, et al. Comparison of the human-baited double net trap with the human landing catch for Aedes albopictus monitoring in Shanghai, China. Parasit Vectors. 2018;11:483. doi: 10.1186/s13071-018-3053-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Goff G, Carnevale P, Fondjo E, Robert V. Comparison of three sampling methods of man-biting anophelines in order to estimate the malaria transmission in a village of south Cameroon. Parasite. 1997;4:75–80. doi: 10.1051/parasite/1997041075. [DOI] [PubMed] [Google Scholar]
- 32.Govella NJ, Chaki PP, Geissbuhler Y, Kannady K, Okumu F, Charlwood JD, et al. A new tent trap for sampling exophagic and endophagic members of the Anopheles gambiae complex. Malar J. 2009;8:157. doi: 10.1186/1475-2875-8-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Govella NJ, Chaki PP, Mpangile JM, Killeen GF. Monitoring mosquitoes in urban Dar es Salaam: evaluation of resting boxes, window exit traps, CDC light traps, Ifakara tent traps and human landing catches. Parasit Vectors. 2011;4:40. doi: 10.1186/1756-3305-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krajacich BJ, Slade JR, Mulligan RT, Labrecque B, Kobylinski KC, Gray M, et al. Design and testing of a novel, protective human-baited tent trap for the collection of anthropophilic disease vectors. J Med Entomol. 2014;51:253–263. doi: 10.1603/ME13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sikulu M, Govella NJ, Ogoma SB, Mpangile J, Kambi SH, Kannady K, et al. Comparative evaluation of the Ifakara tent trap-B, the standardized resting boxes and the human landing catch for sampling malaria vectors and other mosquitoes in urban Dar es Salaam, Tanzania. Malar J. 2009;8:197. doi: 10.1186/1475-2875-8-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathenge EM, Omweri GO, Irungu LW, Ndegwa PN, Walczak E, Smith TA, et al. Comparative field evaluation of the Mbita trap, the Centers for Disease Control light trap, and the human landing catch for sampling of malaria vectors in western Kenya. Am J Trop Med Hyg. 2004;70:33–37. doi: 10.4269/ajtmh.2004.70.33. [DOI] [PubMed] [Google Scholar]
- 37.Mathenge E, Killeen G, Oulo D, Irungu LW, Ndegwa P, Knols B. Development of an exposure-free bednet trap for sampling Afrotropical malaria vectors. Med Vet Entomol. 2002;16:67–74. doi: 10.1046/j.0269-283x.2002.00350.x. [DOI] [PubMed] [Google Scholar]
- 38.Laganier R, Randimby FM, Rajaonarivelo V, Robert V. Is the Mbita trap a reliable tool for evaluating the density of anopheline vectors in the highlands of Madagascar? Malar J. 2003;2:42. doi: 10.1186/1475-2875-2-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degefa T, Yewhalaw D, Zhou G, Lee M-C, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: implications for better understanding of residual transmission. Malar J. 2017;16:443. doi: 10.1186/s12936-017-2098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degefa T, Yewhalaw D, Zhou G, Lee M-C, Atieli H, Githeko AK, et al. Evaluation of the performance of new sticky pots for outdoor resting malaria vector surveillance in western Kenya. Parasit Vectors. 2019;12:278. doi: 10.1186/s13071-019-3535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munyekenye OG, Githeko AK, Zhou G, Mushinzimana E, Minakawa N, Yan G. Plasmodium falciparum spatial analysis, western Kenya highlands. Emerg Infect Dis. 2005;11:1571–1577. doi: 10.3201/eid1110.050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, Wamae P, et al. Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PLoS ONE. 2011;6:e20318. doi: 10.1371/journal.pone.0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Githeko AK, Ayisi JM, Odada PK, Atieli FK, Ndenga BA, Githure JI, et al. Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malar J. 2006;5:107. doi: 10.1186/1475-2875-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J. 2015;14:244. doi: 10.1186/s12936-015-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yewhalaw D, Legesse W, Van Bortel W, Gebre-Selassie S, Kloos H, Duchateau L, et al. Malaria and water resource development: the case of Gilgel-Gibe hydroelectric dam in Ethiopia. Malar J. 2009;8:21. doi: 10.1186/1475-2875-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gillies M, Coetzee M. A supplement to the anophelinae of Africa South of the Sahara. Publ S Afr Inst Med Res. 1987;55:1–143. [Google Scholar]
- 47.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 48.Koekemoer L, Kamau L, Hunt R, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 49.Beier J, Perkins P, Wirtz R, Whitmire RE, Muqambi M, Hockmeyer WT. Field evaluation of an enzyme-linked immunosorbent assay (ELISA) for Plasmodium falciparum sporozoite detection in anopheline mosquitoes from Kenya. Am J Trop Med Hyg. 1987;36:459–468. doi: 10.4269/ajtmh.1987.36.459. [DOI] [PubMed] [Google Scholar]
- 50.Wirtz RA, Burkot TR, Graves PM, Andre RG. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987;24:433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- 51.Simpson EH. Measurement of diversity. Nature. 1949;163:688. doi: 10.1038/163688a0. [DOI] [Google Scholar]
- 52.Peet RK. The measurement of species diversity. Annu Rev Ecol Syst. 1974;5:285–307. doi: 10.1146/annurev.es.05.110174.001441. [DOI] [Google Scholar]
- 53.Magurran AE. Ecological diversity and its measurement. Princeton: Princeton University Press; 1988. [Google Scholar]
- 54.Grundmann H, Hori S, Tanner G. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J Clin Microbiol. 2001;39:4190–4192. doi: 10.1128/JCM.39.11.4190-4192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwak TJ, Peterson JT. Community indices, parameters, and comparisons. In: Guy CS, Brown ML, editors. Analysis and interpretation of freshwater fisheries data. Bethesda: American Fisheries Society; 2007. pp. 677–763. [Google Scholar]
- 56.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. Statistician. 1983;32:307–317. doi: 10.2307/2987937. [DOI] [Google Scholar]
- 57.Sriwichai P, Karl S, Samung Y, Sumruayphol S, Kiattibutr K, Payakkapol A, et al. Evaluation of CDC light traps for mosquito surveillance in a malaria endemic area on the Thai–Myanmar border. Parasit Vectors. 2015;8:636. doi: 10.1186/s13071-015-1225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hiwat H, Andriessen R, Rijk MD, Koenraadt CJM, Takken W. Carbon dioxide baited trap catches do not correlate with human landing collections of Anopheles aquasalis in Suriname. Mem Inst Oswaldo Cruz. 2011;106:360–364. doi: 10.1590/S0074-02762011000300017. [DOI] [PubMed] [Google Scholar]
- 59.Chen YC, Wang CY, Teng HJ, Chen CF, Chang MC, Lu LC, et al. Comparison of the efficacy of CO2-baited and unbaited light traps, gravid traps, backpack aspirators, and sweep net collections for sampling mosquitoes infected with Japanese encephalitis virus. J Vector Ecol. 2011;36:68–74. doi: 10.1111/j.1948-7134.2011.00142.x. [DOI] [PubMed] [Google Scholar]
- 60.Service MW . Mosquito ecology: field sampling methods. London: Chapman & Hall; 1993. [Google Scholar]
- 61.Finda MF, Moshi IR, Monroe A, Limwagu AJ, Nyoni AP, Swai JK, et al. Linking human behaviours and malaria vector biting risk in south-eastern Tanzania. PLoS ONE. 2019;14:e0217414. doi: 10.1371/journal.pone.0217414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monroe A, Mihayo K, Okumu F, Finda M, Moore S, Koenker H, et al. Human behaviour and residual malaria transmission in Zanzibar: findings from in-depth interviews and direct observation of community events. Malar J. 2019;18:220. doi: 10.1186/s12936-019-2855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Monroe A, Moore S, Koenker H, Lynch M, Ricotta E. Measuring and characterizing night time human behaviour as it relates to residual malaria transmission in sub-Saharan Africa: a review of the published literature. Malar J. 2019;18:6. doi: 10.1186/s12936-019-2638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Service M The ecology of the mosquitos of the northern Guinea savannah of Nigeria. Bull Entomol Res. 1963;54:601–632. doi: 10.1017/S000748530004904X. [DOI] [Google Scholar]
- 65.Doolan DL. Malaria methods and protocols. Berlin: Springer Science & Business Media; 2002. [Google Scholar]
- 66.Akiyama J. Interpretation of the results of baited trap net collections. J Trop Med Hyg. 1973;76:283–284. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the conclusions of this article are included within the article. Raw data are available from the corresponding author upon reasonable request.