Abstract
Background
The disruption of the blood–brain barrier (BBB) plays a critical event in the pathogenesis of ischemia stroke. TGR5 is recognized as a potential target for the treatment for neurologic disorders.
Methods
This study investigated the roles of TGR5 activation in attenuating BBB damage and underlying mechanisms after middle cerebral artery occlusion (MCAO). Sprague-Dawley rats were subjected to model of MCAO and TGR5 agonist, INT777, was administered intranasally. Small interfering RNA (siRNA) for TGR5 and BRCA1 were administered through intracerebroventricular injection 48 h before MCAO. Infarct volumes, brain water content, BBB permeability, neurological scores, Western blot, immunofluorescence staining and co- immunoprecipitation were evaluated.
Results
Endogenous TGR5 and BRCA1 were upregulated in the injured hemisphere after MCAO and TGR5 expressed in endothelial cells. Treatment with INT777 alleviated brain water content and BBB permeability, reduced infarction volume and improved neurological scores at 24 h and 72 h after ischemia. INT777 administration increased BRCA1 and Sirt1 expression, as well as upregulated expressions of tight junction proteins. Ischemic damage induced interaction of TGR5 with BRCA1. TGR5 siRNA and BRCA1 siRNA significantly inhibited expressions of BRCA1 and Sirt1, aggravated BBB permeability and exacerbated stroke outcomes after MCAO. The protective effects of INT777 at 24 h after MCAO were also abolished by TGR5 siRNA or BRCA1 siRNA.
Conclusions
Our findings demonstrate that activating TGR5 could reduce BBB breakdown and improve neurological functions through BRCA1/Sirt1 signaling pathway after MCAO. TGR5 may serve as a potential new candidate to relieve brain injury after MCAO.
Keywords: TGR5, Blood-brain barrier, Neuroprotection, BRCA1, Sirt1, Middle cerebral artery occlusion
Background
Stroke is one of the most common causes of death and the main cause of long-term disability worldwide [1]. It has been recognized that disruption of the blood–brain barrier (BBB) is a critical event in the pathogenesis of stroke [2, 3]. During ischemia stroke, the disruption of BBB can lead to extravasation of solutes and fluids into the brain, resulting in vasogenic edema [4], which causes a poor clinical outcome. Therefore, the pharmacological targeting of protecting BBB may be a promising treatment strategy for cerebral infarction [5].
TGR5 is a plasma membrane-bound G protein-coupled bile acid receptor, which is present in various tissues, including in animal and human brain [6, 7]. TGR5 is recognized as a potential target for the treatment for hepatic disorders, metabolic disorders, and kidney disease, through anti-inflammation, anti-apoptosis and inhibition of oxidative stress [8, 9]. In central nervous system (CNS), studies have found that activating TGR5 alleviates brain damage and improves outcomes in a model of experimental autoimmune encephalomyelitis (EAE) and hepatic encephalopathy [10, 11]. Nevertheless, the effects of TGR5 on BBB integrity in brain injuries after ischemic stroke have not been investigated.
BRCA1, a tumor suppressor gene implicated in breast and ovarian cancers, is expressed by endothelial cells and can improve endothelial dysfunction, which may provide a protective role in neurological diseases [12–14]. Several researches have established that BRCA1 is a key regulator of sirtuin 1 (Sirt1) [15, 16]. Sirt1 is a nicotinamide adenine dinucleotide-dependent deacetylase, which is involved in the regulation of physiological functions, including cell senescence, gene transcription, energy balance, and oxidative stress. Studies have confirmed the protective role of sirt1 against BBB damage in CNS pathologies [17, 18].
In the present study, we hypothesized that (1) activating TGR5 protects BBB damage and attenuates brain insult after middle cerebral artery occlusion (MCAO) and (2) the protection of TGR5 on the BBB is mediated through a BRCA1/Sirt1-related signaling pathway.
Materials and methods
Animals
All experiments were approved by the Institutional Animal Care and Use Committee of Loma Linda University (approval no. 8170034) and Zhejiang University (approval no. 2016–193). All animal care and use were conducted according to the Guide for the Care and Use of Laboratory Animals (National Research Council). All procedures of experiments are reported in compliance with the ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines. Animals were housed in a 12 h light-dark cycle, temperature-controlled room. A total of 494 Sprague-Dawley male rats (2–3 months, weighing 250–300 g) were used in the study.
MCAO model
The transient MCAO model was induced as previously described [19]. Rats were anesthetized intraperitoneally with a mixture of ketamine (80 mg/kg) and xylazine (20 mg/kg). Briefly, the right common carotid artery (CCA), internal carotid artery (ICA) and external carotid artery (ECA) were surgically exposed. 4–0 nylon suture with silicon was inserted into the ICA through the ECA stump until the tip of the suture reached the origin of the anterior cerebral artery (ACA) (approximately 18 to 22 mm). After 2 h of occlusion, the suture was withdrawn to allow for reperfusion. During surgery, body temperature was maintained at a physiological level. Sham groups underwent the same procedure but without occluding the MCA.
Experimental design
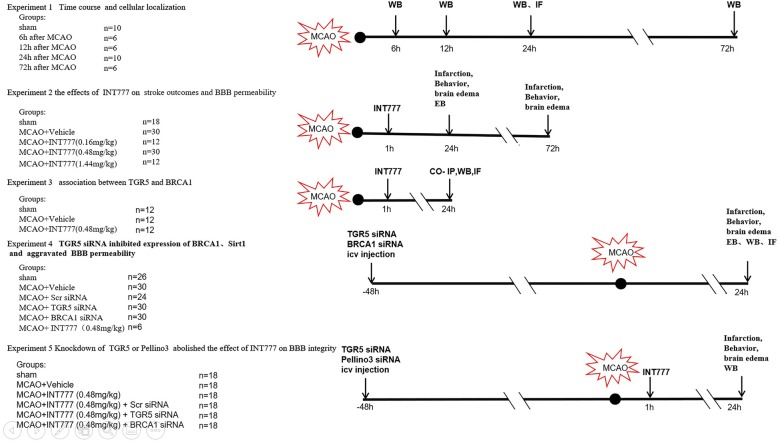
A schematic diagram of our research design was shown in Fig. 1.
Fig. 1.
Experimental design and animal group classification. IF, immunofluorescence; icv, intracerebral ventricular; MCAO, middle cerebral artery occlusion; Scr siRNA, Scramble small interfering RNA; WB, Western blot; Co-IP, Co- immunoprecipitation
Experiment 1
The time course of endogenous expressions of TGR5 and BRCA1 in right hemispheric tissue was evaluated by Western blot.30 rats were divided into 5 groups: Sham (n = 6), MCAO 6 h (n = 6), MCAO 12 h (n = 6), MCAO 24 h (n = 6), and MCAO 72 h (n = 6). An additional 8 rats, sham (n = 4) and MCAO 24 h (n = 4), were used for immunofluorescence staining to characterize the localization of TGR5 in endothelial cells (visualized using an antibody against von Willebrand factor (VWF)).
Experiment 2
One hundred two rats were used in the following groups: sham (n = 18), MCAO+vehicle (n = 30), MCAO+INT777 (0.16 mg/kg, n = 12), MCAO+INT777 (0.48 mg/kg, n = 30), MCAO+INT777 (1.44 mg/kg, n = 12). Infarction volume, neurobehavior scores, and brain water content were measured at 24 and 72 h after MCAO. Evans blue (EB) extravasation was evaluated at 24 h after MCAO and barrier function assessment in vitro was assessed by TEER. Based on neurological tests at 24 h and 72 h after MCAO, the middle dosage of INT777 (0.48 mg/kg) was chosen for further studies.
Experiment3
Thirty-six rats were divided into 3 groups for exploring the association between TGR5 and BRCA1 by co-immunoprecipitation: sham (n = 12), MCAO+vehicle (n = 12), MCAO+INT777 (n = 12). The immunofluorescence staining samples for co-labeling of TGR5 with BRCA1 were shared with experiment 1.
Experiment 4
To explore the effect of knocking down TGR5 and BRCA1 on stroke, 146 rats were randomly assigned to the following 5 groups: Sham (n = 26), MCAO+vehicle (n = 30), MCAO+Scramble siRNA (n = 24), MCAO+TGR5 siRNA (n = 30), MCAO+BRCA1siRNA (n = 30), MCAO++INT777 (n = 6). Infarction volume, neurobehavior scores, brain water content, EB extravasation, immunofluorescence staining and Western blots were measured. Four samples of sham for immunofluorescence staining were shared with experiment 1.
Experiment 5
One hundred eight rats were randomly assigned to 6 groups for mechanism study: sham (n = 18), MCAO+vehicle (n = 18), MCAO+INT777 (n = 18), MCAO+INT777 + scramble siRNA (n = 18), MCAO+INT777 + TGR5 siRNA (n = 18), and MCAO+INT777 + BRCA1 siRNA (n = 18). Neurobehavioral scores, brain infarction, brain water content and Western blot were evaluated.
Drug administration
Intranasal administration of INT777 (MedChemExpress,USA) was performed as previously described [20], with some modifications: rats were administered either saline, INT777 (0.16 mg/kg), INT777 (0.48 mg/kg) or INT777 (1.44 mg/kg) intranasally (5 μL/drop) over a period of 20 mins, alternating drops every 2 min between left and right nares. The total volume delivered was 50 μL at 1 h following MCAO.
Intracerebroventricular siRNA injection
Three different formats of TGR5-siRNA or BRCA1-siRNA (OriGene Technologies) were diluted with transfection reagent (entranser™,Engreen Biosystem) and were injected 48 h before MCAO by intracerebroventricular injection (ICV) as previously described [21, 22]. The ICV injection site was relative to location of bregma: anteroposterior 1 mm, right lateral 1.5 mm, depth 3.5 mm. The TGR5-siRNA, BRCA1-siRNA mixture or scramble-siRNA (100 pmol in 5 μL) was delivered into the ipsilateral ventricle with a Hamilton syringe (Microliter 701, Hamilton Company, Reno, NV) and administered over 5 min. The needle was left for 5 min after injection and was then slowly withdrawn over 5 min. After the needle was removed, the burr hole was sealed with bone wax.
Neurological scores
Neurobehavioral outcomes were assessed by a blinded investigator at 24 h and 72 h following MCAO [23]. The sensorimotor function scores were evaluated as following: spontaneous activity, symmetry in limb movement, symmetry of forelimb outstretching, climbing, body proprioception, response to vibrissae touch, and beam walking. The neurological scoring ranged from 3 (most severe deficits) to 21 (normal).
2.6. 2,3,5-Triphenyltetrazolium chloride (TTC) staining
Infarction volume was evaluated by TTC staining after MCAO [24]. standard methods were used to correct the possible interference of brain edema on infarct volume and infarction volume was expressed as a ratio of the whole brain volume, as previous report [25].
Brain water content
Brains were separated into left hemisphere, right hemisphere, cerebellum, and brain stem at 24 or 72 h after MCAO. Each brain samples were weighed immediately after removal (wet weight) and then dried in an oven at 105 °C for 72 h (dry weight). The percentage of brain water content was calculated as [(wet weight-dry weight)/wet weight] Χ100% [23].
BBB permeability
BBB permeability was evaluated by EB extravasation using spectrophotometry as previously described [26]. At 4 h before sacrifice, 2% EB dye in saline was injected intravenously as a BBB permeability tracer. A microplate fluorescence reader was used to determine EB dye fluorescence intensity. The amount of extravasated EB dye was quantified as micrograms per ischemic hemisphere.
Barrier function assessment of in vitro
The in vitro experiment was prepared as previously reported [27], with some modifications.bEnd.3 cells were purchased from the Bioleaf Biotech Co., Ltd. (Shanghai, China) and were cultured as previously described. After the bEnd.3 cells were treated with INT777 (30 μmol/l), OGD/R was induced in the cells for 6 h in a hypoxia chamber in RPMI 1640 culture medium without glucose, in an atmosphere of1% O2, 5% CO2, and 94% N2.Then the cells were cultured under normoxia conditions in normal culture medium after 2 h of OGD for 18 h. The integrity ofthe bEnd.3 cell monolayer was measured via the TEER assay.
Immunofluorescent staining
The method of double and triple immunofluorescence staining was performed as previously described [28, 29]. Rats were transcardially perfused with cold phosphate-buffered solution (PBS) followed by 10% paraformaldehyde after rats were deeply anesthetized at 24 h after MCAO. The whole brains were fixed in 10% paraformaldehyde for 24 h then in 30% sucrose solution for 72 h. Coronal frozen slices (10 μm) were obtained with a cryostat (CM3050S; Leica Microsystems, Wetzlar, Germany) and permeabilized with 0.3% Triton X-100 in PBS for 30 min. Sections were blocked with 5% donkey serum for 1 h and incubated at 4 °C overnight with primary antibodies: anti-TGR5 (1:100 Abcam), anti-BRCA1 (1:100 Santa Cruz Biotechnology), anti- vWF (1:100 Abcam) and anti- CD31 (1:100 Abcam). The slices were viewed with fluorescence microscope (DMi8; Leica Microsystems, Germany) or confocal LSM 710 microscope and fluorescence intensity was quantified using ImageJ.
Western blot analysis
Western blot analysis was performed as previously reported [30]. Proteins of the ipsilateral hemisphere were extracted by homogenizing in radio-immunoprecipitation assay lysis buffer. Equal amounts of a sample protein were loaded onto an SDS-PAGE gel. First, electrophoresis and transfer of the samples to a nitrocellulose membrane were performed. Second, the membrane was blocked for 2 h at room temperature and incubated overnight at 4 °C with the following primary antibodies: anti-TGR5 (1:1000, Abcam), anti-BRCA1 (1:1000, Santa Cruz Biotechnology), anti-Sirt1(1:1000, Abcam), anti-occludin (1:2000, Abcam, USA), anti-ZO-1(1:200, Santa Cruz Biotechnology) and anti-β-actin (1:5000, Santa Cruz Biotechnology). The secondary antibodies were all from Santa Cruz Biotechnology. Blot bands were visualized with an ECL reagent (Amersham Biosciences UK Ltd., PA, USA) and were quantified by densitometry using Image J software (Image J 1.4, NIH, USA).
Co-Immunoprecipitation (co-IP)
Co-IP was performed as previously described [20, 31].500 μg protein incubated with TGR5 antibody (1:50) or BRCA1 antibody (1:50) and agitated. Protein A/G agarose (20 μL; Sigma) was added to each sample and incubated overnight at 4 °C. Next, the mixture was precipitated by high-speed freezing centrifugation at 12000 rpm for 10 s. Then the sediment was washed three times with NP-40 buffer. Agarose-bound immunocomplexes were released using a denaturing solution. TGR5 and BRCA1 proteins in immunocomplex denaturing solution and total protein solution (for comparison) were analyzed by Western blot.
Statistical analysis
All data analyses were performed using SigmaPlot 11.0 and GraphPad Prism 6 (GraphPad software, San Diego, CA). Parametric data was expressed as mean ± SEM. Data from different groups were compared using one-way ANOVA followed by post hoc Tukey tests. Non-parametric data (neurological scores, beam walking) were analyzed with the Kruskal–Wallis test followed by Dunn’s post-hoc. In all statistical analysis, P < 0.05 was considered as significant.
Results
Mortality and exclusion
A total of 494 rats were used and 410 rats underwent MCAO induction. There were no deaths in sham group. For groups of MCAO, the mortality rate was 11.5% (47 of 410) (Supplementary Table 1). Seventeen animals were excluded if rats didn’t show signs of neurobehavioral deficits when waking up from MCAO (body twisting when lifted by the tail and walking in circles) or if subarachnoid hemorrhage was found during euthanasia.
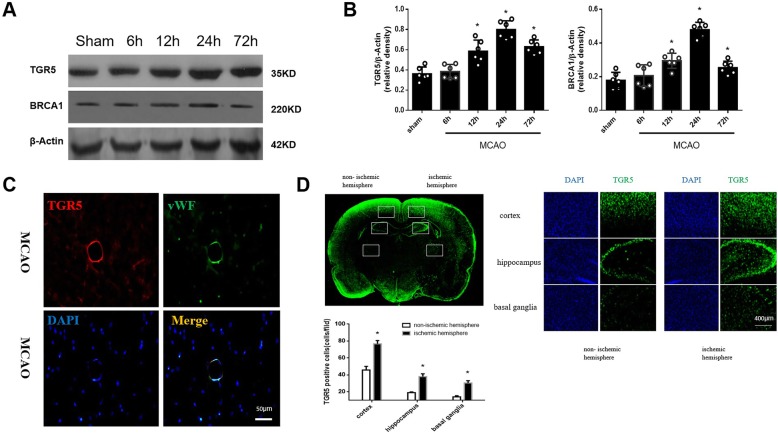
Endogenous TGR5 receptor and BRCA1 expression increased after MCAO
We investigated TGR5 and BRCA1 alterations after MCAO. In Fig. 2a and b, TGR5 and BRCA1 expressions significantly increased from12 hours to a peak at 24 h but declined at 72 h after MCAO (P < 0.05 versus Sham). Double immuno-fluorescence staining demonstrated that TGR5 was expressed in endothelial cells at 24 h after MCAO (Fig. 2c). Whole brain immunofluorescence staining showed that TGR5 expression was upregulated in the cortex, hippocampus and basal ganglia 24 h after MCAO when compared with contralateral non-ischemic hemisphere (Fig. 2d).
Fig. 2.
Expression of TGR5 and BRCA1 in the right hemisphere of the rat brain after MCAO. a, b Representative Western blot images and quantitative analyses of TGR5 and BRCA1 time-course expression after MCAO. n = 6 per group. c Double immunofluorescence staining revealed that both TGR5 (red) was predominantly expressed in vWF positive- endothelial cells (green) in penumbra at 24 h after MCAO. n = 4 per group. *P < 0.05 vs sham group. Bars represent mean ± SEM. Scale bar, 50 μm. vWF, Von Willebrand factor. d Whole brain immunofluorescence staining showed that TGR5 expression was upregulated in the cortex, hippocampus and basal ganglia 24 h after MCAO. n = 4 per group. *P < 0.05 vs contralateral non-ischemic hemisphere. Bars represent mean ± SEM. Scale bar, 400 μm
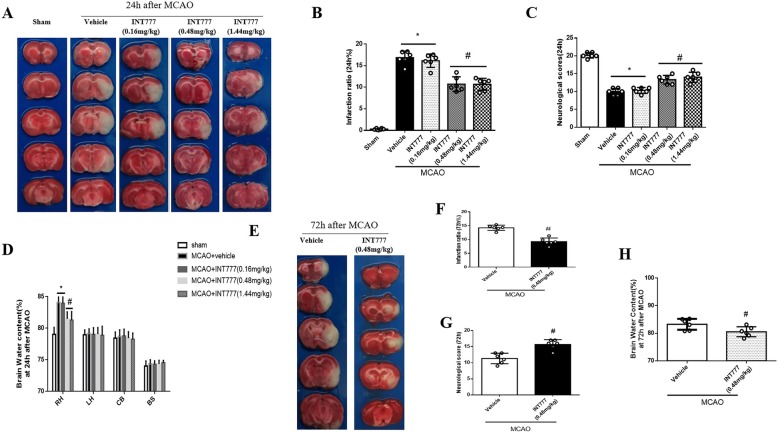
INT777 improved stroke outcomes and BBB permeability after MCAO
Treatment with 0.48 mg/kg and 1.44 mg/kg of INT777 significantly reduced infarct volume, improved neurological scores and reduced brain water content of right ischemic hemisphere at 24 h post-MCAO compared to MCAO+vehicle group (Fig. 3a-d) (P < 0.05). The administration of 0.48 mg/kg INT777 decreased cerebral infarction, restored neurological function and ameliorated brain water content at 72 h after injury (Fig. 3e-h) (P < 0.05 versus MCAO+vehicle). Based on the dose study, we chose to middle dosage of INT777 for all subsequent studies.
Fig. 3.
Exogenous TGR5 agonist INT777 ameliorated brain injury at 24 h and 72 h after MCAO. Representative TTC staining indicated brain infarction at 24 h and 72 h after MCAO (a, e);quantified infarct ratio(b, f), neurological scores (c, g) and brain water content(d, h) showed that INT777 decreased infarction and neurological deficits in medium dose as well as reduced brain edema of right ischemic hemisphere at 24 h and 72 h after MCAO . High dose was only analyzed at 24 h after MCAO. n = 6 for each group. *P < 0.05 vs sham, #P < 0.05 vs MCAO+ vehicle. Bars represent mean ± SEM. BS indicates brain stem; CB, cerebellum; LH, left hemisphere; RH, right hemisphere
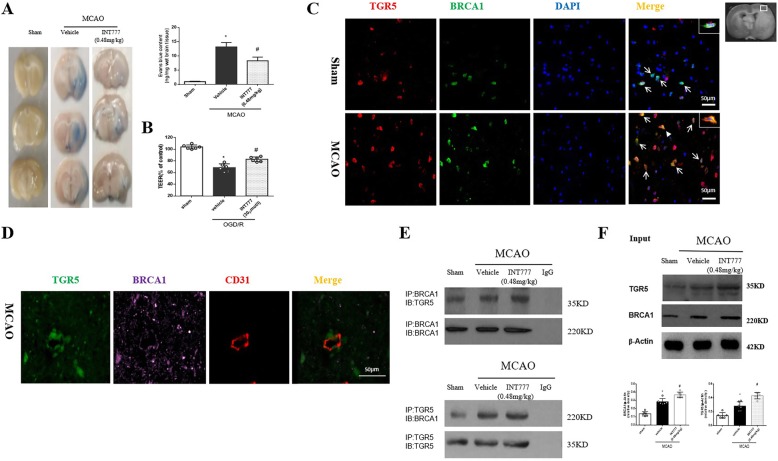
EB extravasation was markedly increased at 24 h post-MCAO (P < 0.05 versus sham), INT777 treatment significantly reduced EB dye leakage(P < 0.05 versus MCAO+vehicle) (Fig. 4a). INT777 also alleviated the TEER decrease in the in vitro model after OGD/R(P < 0.05 versus OGD/R + vehicle) (Fig. 4b).
Fig. 4.
INT777improved BBB permeability and TGR5 interacted with BRCA1 after MCAO. a INT777 treatment significantly reduced EB dye leakage, n = 6 per group; b INT777 alleviated the TEER decrease after OGD/R, n = 6 per group; c Double immunofluorescence staining showed that co-localization of TGR5 (red) and BRCA1 (green) was increased in penumbra 24 h after MCAO, d Triple-fluorescence staining showed that TGR5 and BRCA1colocalized in endothelial cell, n = 4 per group. Scale bar 50 μm. e Representative co-IP bands showed that interactions of TGR5 with BRCA1 occurred at 24 h after MCAO, n = 6 per group. f Expression of TGR5 and BRCA1 in total protein solution was detected by Western blot analysis and relative OD ratios were reported. n = 6 for each group. *P < 0.05 vs sham, #P < 0.05 vs MCAO+ vehicle. Bars represent mean ± SEM
MCAO induced interactions between TGR5 and BRCA1
In the sham group, double immunofluorescence staining showed that co-labeling of TGR5 with BRCA1 was detected in the brain. After ischemic injury, co-labeling of TGR5 with BRCA1 increased in the penumbra area (Fig. 4c). Triple-fluorescence staining also showed that TGR5 and BRCA1 co-localized in endothelial cell (Fig. 4d). Western blot showed that both TGR5 and BRCA1 expression increased at 24 h after MCAO (P < 0.05 versus sham), and INT777 further increased TGR5 and BRCA1 expression(P < 0.05 versus MCAO) (Fig. 4f). CO-IP showed that TGR5- BRCA1 interaction was found in the ischemic hemisphere (Fig. 4e).
TGR5 siRNA inhibited expression of BRCA1/Sirt1 and aggravated BBB permeability after MCAO
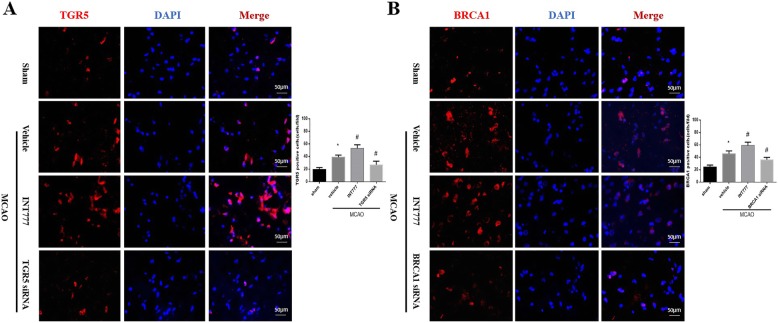
To further assess the role of TGR5 in stroke, TGR5 siRNA was administered by ICV injection to knockdown endogenous TGR5. Double immunofluorescence staining showed that both TGR5 and BRCA1 expressions increased in penumbra following MCAO while siRNA significantly reduced TGR5 or BRCA1 expression (Fig. 5a and b).
Fig. 5.
The effect of INT777 and siRNA on TGR5 and BRCA1 expression in penumbra after middle cerebral artery occlusion (MCAO). a, b Double immunofluorescence staining showed that expression of TGR5 or BRCA1 was upregulated in the penumbra area 24 h after MCAO; INT777 treatment increased the expressions while siRNA inhibited TGR5 or BRCA1 expression. n = 6 per group. *P < 0.05 vs sham, #P < 0.05 vs MCAO+ vehicle. Bars represent mean ± SEM. Scale bar, 50 μm
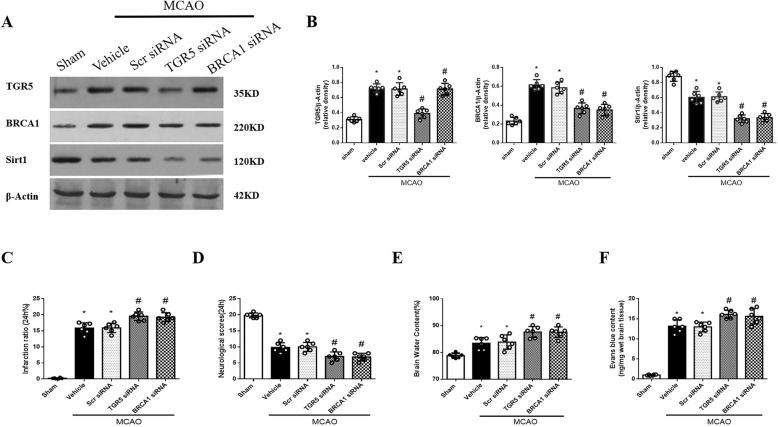
The results of Western blot staining showed that TGR5 expression was partially prevented by TGR5 siRNA (Fig. 6a and b). Compared with scramble siRNA group, TGR5 siRNA significantly inhibited expressions of BRCA1 and Sirt1 after MCAO (P < 0.05) (Fig. 6a and b). The knockdown efficacy of BRCA1 siRNA was also confirmed by Western blot and BRCA1 knockdown markedly decreased the Sirt1 expression and had no effect on TGR5 expression after MCAO (Fig. 6a and b).
Fig. 6.
The effect of knockdown TGR5 on BRCA1/Sirt1 expression and BBB permeability after MCAO. a The band of Western blot analysis; b The relative density of TGR5, BRCA1, Sirt1.n = 6 per group. TGR5 or BRCA1 knockout increased infarct volume (c), worsen neurobehavioral deficits (d), exacerbated brain water content (e) and BBB permeability (f). n = 6 per group. *P < 0.05 vs sham, #P < 0.05 vs MCAO+ Scr siRNA. Bars represent mean ± SEM. Scr siRNA, scramble siRNA
Both TGR5 siRNA or BRCA1 siRNA significantly exacerbated stroke outcomes and aggravated BBB permeability after MCAO (P < 0.05) (Fig. 6c-f), when compared with scramble siRNA group at 24 h after MCAO.
TGR5 or BRCA1 knockdown abolished the protective effects of INT777 on BBB integrity after MCAO
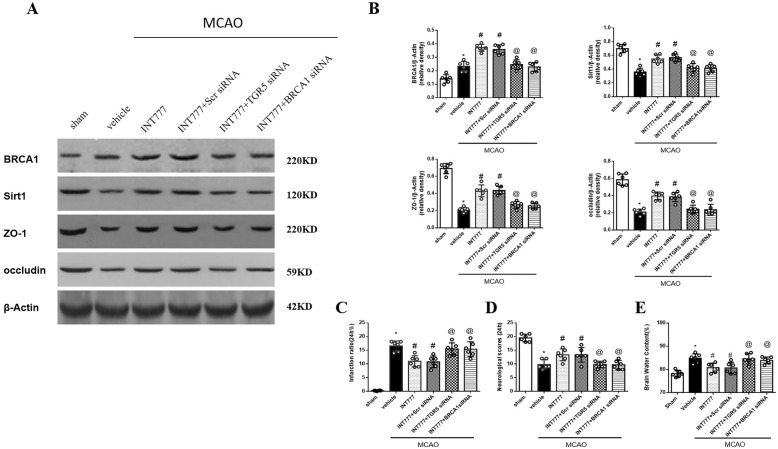
Decreased tight junction (TJ) protein expressions or variations are associated with alterations in BBB permeability [32]. As shown in Fig. 7a and b, Sirt1 and TJ proteins (ZO-1 and occludin) were remarkably decreased at 24 h after SAH, when compared with the sham group (P < 0.05). However, INT777 increased expressions of Sirt1, ZO-1 and occludin, compared with MCAO group (P < 0.05) (Fig. 7a and b). The results above demonstrated that INT777 alleviated disrupted BBB by increasing TJ proteins in ischemic brain.
Fig. 7.
Knockdown TGR5 or BRCA1 abolished the protective effects of INT777 on BBB permeability after MCAO. a Representative Western blots. b Quantitative analyses of TGR5, BRCA1, Sirt1, zo-1, occludin. Quantified infarct ratio (c), neurological scores (d) and brain water content (e), n = 6 per group. *P < 0.05 vs sham, #P < 0.05 vs MCAO+ vehicle,@P < 0.05 vs MCAO+INT777 + Scr siRNA group. Scr siRNA, scramble siRNA
When compared with INT777+ scramble siRNA group, TGR5 siRNA reversed the effect of INT777 on the expressions of TGR5, BRCA1, Sirt1, ZO-1and occludin at 24 h after MCAO (P < 0.05) (Fig. 7a and b). Western blot showed that BRCA1 siRNA also abolished the effects of INT777, leading to reduce expressions of Sirt1, ZO-1 and occludin (P < 0.05) (Fig. 7a and b).
Administration of TGR5 siRNA or BRCA1 siRNA significantly abolished the protective effect of INT777 on infarction volume, neurological deficits and brain edema at 24 h after MCAO (P < 0.05 versus MCAO+INT777 + Scramble siRNA) (Fig. 7c-e).
Discussion
In the present study, we first described the TGR5 mediated signaling pathway in BBB protection after MCAO in rats. Our data demonstrated that TGR5 and its essential downstream protein BRCA1 were upregulated in the injured hemisphere after MCAO. Exogenous TGR5 agonist INT777 reduced brain edema and BBB permeability and thereby alleviated stroke outcome after MCAO. In contrast, knockdown of endogenous TGR5 or BRCA1 by siRNA exacerbated brain edema, BBB disruption, infarction volume, and neurological deficits. INT777 increased TGR5, BRCA1 and Sirt1 expressions, as well as upregulated TJs. Furthermore, knockdown TGR5 or BRCA1 by siRNA abolished the beneficial effects of INT777, which were associated with reduced Sirt1, ZO-1 and occludin. Taking together, our study suggested that activating TGR5 may be involved in regulating BBB permeability after MCAO at least in part via a BRCA1 /Sirt1 signaling pathway.
Research have showed that Bile acids, such as tauroursodeoxycholic acid (TUDCA), play an important role of neuroprotection for brain pathologies which are mediated by TGR5 [33]. McMillin et al. found that TGR5 is present in the cortex of C57Bl/6 mice and is upregulated in the brain following azoxymethane induced acute liver failure. This up-regulation appears to be protective, as activating TGR5 reduces neurological decline [11]. In the model of EAE, mice treated with TGR5 agonists had significant reductions in the clinical score both at peak of disease and at the termination of the study [10]. In the present research, we observed that TRG5 was upregulated in the penumbra after MCAO and was expressed in endothelial cells. The administration of INT777 significantly diminished BBB disruption and improved stroke outcomes after MCAO, whereas silencing endogenous TGR5 by siRNA aggravated BBB breakdown and neurological deficits.
Although the exact mechanisms of TGR5–mediated BBB protection are not well clarified, BRCA1 may play an important role in the TGR5-mediated signaling pathway. BRCA1, a well-known tumor suppressor implicated in familial breast and ovarian cancers, provides a protective role in atherosclerosis and neurological diseases [12–14]. Noristani et al. found that BRCA1 is expressed by human microglia and is dysregulated in humans and an animal model of ALS [34]. Several studies demonstrated that BRCA1 deficiency contributes to neuronal injury in Huntington’s Disease and impairs cognitive function in mice [13, 14]. In vitro experiments, two major bile acids, deoxycholic acid and chenodeoxycholic acid, were found to increase BRCA1 expression relative to untreated control OVCAR3 ovarian cancer cells, through interaction with bile acid receptors [35]. In the present study, we observed that endogenous BRCA1 expression was increased at 24 h after MCAO and INT777 further augmented BRCA1 expression. Double immunofluorescence staining demonstrated an increased co-localization of TGR5 with BRCA1 after MCAO, and using CO-IP, we found an interaction between TGR5 and BRCA1 after MCAO. Furthermore, we observed that silencing TGR5 inhibited the expression of BRCA1 and reversed the protective effect of INT777 on BRCA1 expression. Taken together, these findings support that TGR5 is upstream to activate BRCA1, thereby alleviating BBB damage.
Several evidence have confirmed that BRCA1 is a key regulator of Sirt1 in cancer research. BRCA1 inactivation events (mutation, promoter methylation, or knockdown) are accompanied by decreased Sirt1 levels while overexpression of BRCA1 results in increased Sirt1 levels [15] by direction binding of BRCA1 to the Sirt1 promoter [16]. More studies showed that Sirt1 plays a major role in protecting against brain injuries during ischemia stroke [36]. Chen et al. reported that the activation of Sirt1 was associated with increased BBB permeability in vitro [37]. However, in most studies, increasing the Sirt1 level would benefit BBB damage after oxygen glucose deprivation, subarachnoid hemorrhage or sepsis-induced brain injury [17, 18, 38]. Our lab also found that Sirt1 was a key mediator of Hyperbaric Oxygen (HBO) protective effects in BBB damage after MCAO. Knockdown Sirt1 by Sirt1 siRNA reversed the protective effects of HBO [23].
In the current study, we found that INT777 increased the expression of Sirt1 after MCAO while TGR5 siRNA and BRCA1 siRNA inhibited the Sirt1 expression, reversed the effect of INT777 on Sirt1, which means TGR5 and BRCA1 can act as upstream regulators of Sirt1. Furthermore, our data demonstrated that TGR5 or BRCA1 knockdown significantly reverses the neuro-protection of INT777 on stroke outcomes, as well as decreasing ZO-1 and occludin expression. This finding supports the notion that the BRCA1/ Sirt1 signaling pathway plays a role in BBB protection induced by activation of TGR5 after MCAO.
There are some limitations in the present study. First, TGR5 produces pleiotropic effects via different signaling pathways, such as alleviating inflammation and attenuating apoptosis [39]. In this study, we only focused on the neuroprotective effects of TGR5 on BBB integrity after MCAO, but further studies are needed to explore other effects of TGR5 after MCAO and its underlying signaling mechanisms. Second, only young male rats were used. Following the STAIR recommendations, we need to repeat the key findings using aged males, as well as female rats.
Conclusions
As summarized in Fig. 8, we found that activating TGR5 could reduce BBB breakdown and improve neurological deficits after ischemic stroke. The results highlight TGR5/BRCA1/Sirt1 signaling as a critical contributor to alleviate BBB damage and as a novel target for brain edema in diseases characterized by BBB damage, such as stroke, inflammatory diseases, and neurodegenerative diseases.
Fig. 8.
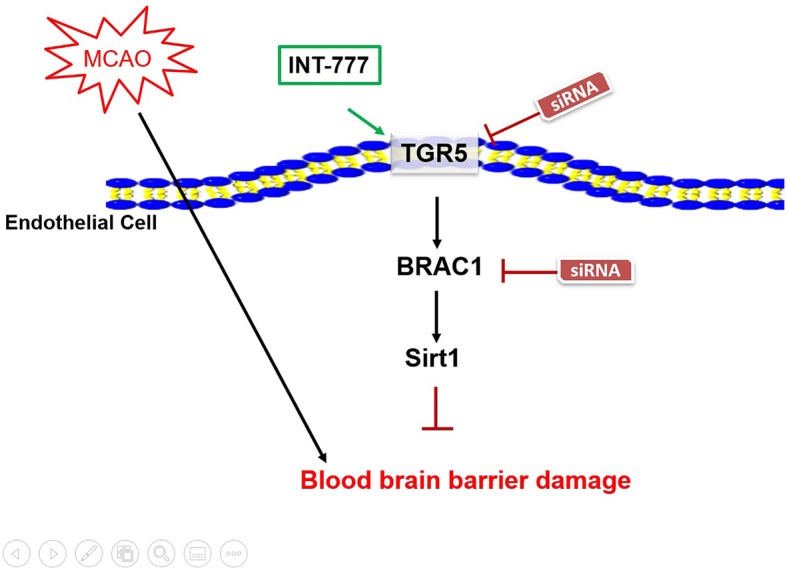
Proposed pathway in the present study. This study found that TGR5 agonist, INT777, could protect BBB and improve neurological outcomes after MCAO, which through BRCA1/Sirt1 signaling pathway after MCAO. Our findings suggest that TGR5 may serve as a potential new candidate to relieve brain injury after MCAO
Supplementary information
Acknowledgements
We thank Mr. Ruili Wei and Ms. Fangping He for excellent technical assistance.
Abbreviations
- ACA
Anterior cerebral artery
- BBB
Blood-brain barrier
- CCA
Common carotid artery
- CNS
Central nervous system
- Co-IP
Co-Immunoprecipitation
- EAE
Experimental autoimmune encephalomyelitis
- ECA
External carotid artery
- ICA
Internal carotid artery
- MCAO
Middle cerebral artery occlusion
- PBS
Phosphate-buffered solution
- siRNA
Small interfering RNA
- TJ
Tight junction
- TTC
Triphenyltetrazolium chloride
- TUDCA
Tauroursodeoxycholic acid
Authors’ contributions
HL participated in research design, experimental performances, Western blot, and immunohistochemistry, drafting the manuscript except neurobehavioral tests, and data analysis. NM and DWM gave technical assistant and manuscript preparation. YX and ZZ carried out intracerebroventricular injection, co-immunoprecipitation, behavioral tests and data analysis. JT participated in research design. BL and JHZ are the corresponding authors. All authors read and approved the final manuscript.
Funding
This study is supported partially by grants from National Institutes of Health (grantnumbers: NS081740 and NS082184) to Dr. Zhang and a grant from National Natural Science Foundation of China (81671143).
Availability of data and materials
All data used during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All animal protocols were approved by the Institutional Animal Care and Use Committee of Loma Linda University and Zhejiang University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Benyan Luo, Email: luobenyan@zju.edu.cn.
John H. Zhang, Email: johnzhang3910@yahoo.com
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12929-020-00656-9.
References
- 1.Broussalis E, Killer M, McCoy M, Harrer A, Trinka E, Kraus J. Current therapies in ischemic stroke. Part a. recent developments in acute stroke treatment and in stroke prevention. Drug Discov Today. 2012;17:296–309. doi: 10.1016/j.drudis.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Kassner A, Merali Z. Assessment of blood-brain barrier disruption in stroke. Stroke. 2015;46:3310–3315. doi: 10.1161/STROKEAHA.115.008861. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, Hu X. Pericytes in brain injury and repair after ischemic stroke. Transl Stroke Res. 2017;8:107–121. doi: 10.1007/s12975-016-0504-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Xiong XY, Liu L, Yang QW. Refocusing Neuroprotection in cerebral reperfusion era: new challenges and strategies. Front Neurol. 2018;9:249. doi: 10.3389/fneur.2018.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al. A G protein-oupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 7.Keitel V, Görg B, Bidmon HJ, Zemtsova I, Spomer L, Zilles K, Häussinger D. The bile acid receptor TGR5 (Gpbar-1) acts as a neurosteroid receptor in brain. Glia. 2010;58:1794–1805. doi: 10.1002/glia.21049. [DOI] [PubMed] [Google Scholar]
- 8.Duboc H, Taché Y, Hofmann AF. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang XX, Edelstein MH, Gafter U, Qiu L, Luo Y, Dobrinskikh E, Lucia S, Adorini L, D’Agati VD, Levi J, et al. G protein-coupled bile acid receptor TGR5 activation inhibits kidney disease in obesity and diabetes. J Am Soc Nephrol. 2016;27:1362–1378. doi: 10.1681/ASN.2014121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis ND, Patnaude LA, Pelletier J, Souza DJ, Lukas SM, King FJ, Hill JD, Stefanopoulos DE, Ryan K, Desai S, et al. A on myeloid cell activation in vitro and reduces experimental autoimmune encephalitis (EAE) in vivo. PLoS One. 2014;9:e100883. doi: 10.1371/journal.pone.0100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMillin M, Frampton G, Tobin R, Dusio G, Smith J, Shin H, Newell-Rogers K, Grant S, De Morrow S. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem. 2015;135:565–576. doi: 10.1111/jnc.13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh KK, Shukla PC, Quan A, Al-Omran M, Lovren F, Pan Y, Brezden-Masley C, Ingram AJ, Stanford WL, Teoh H, et al. BRCA1 is a novel target to improve endothelial dysfunction and retard atherosclerosis. J Thorac Cardiovasc Surg. 2013;146:949–960. doi: 10.1016/j.jtcvs.2012.12.064. [DOI] [PubMed] [Google Scholar]
- 13.Jeon GS, Kim KY, Hwang YJ, Jung MK, An S, Ouchi M, Ouchi T, Kowall N, Lee J, Ryu H. Deregulation of BRCA1 leads to impaired spatiotemporal dynamics of γ-H2AX and DNA damage responses in Huntington's disease. Mol Neurobiol. 2012;45:550–563. doi: 10.1007/s12035-012-8274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suberbielle E, Djukic B, Evans M, Kim DH, Taneja P, Wang X, Finucane M, Knox J, Ho K, Devidze N, et al. DNA repair factor BRCA1 depletion occurs in Alzheimer brains and impairs cognitive function in mice. Nat Commun. 2015;6:8897. doi: 10.1038/ncomms9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang RH, Zheng Y, Kim HS, Xu X, Cao L, Luhasen T, Lee MH, Xiao C, Vassilopoulos A, Chen W, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou YM, Li CY, Yang Q. A novel cross talk between BRCA1 and sirtuin 1 in ovarian cancer. Sci Rep. 2014;4:6666. doi: 10.1038/srep06666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou XM, Zhang X, Zhang XS, Zhuang Z, Li W, Sun Q, Li T, Wang CX, Zhu L, Shi JX, et al. SIRT1 inhibition by sirtinol aggravates brain edema after experimental subarachnoid hemorrhage. J Neurosci Res. 2014;92:714–722. doi: 10.1002/jnr.23359. [DOI] [PubMed] [Google Scholar]
- 18.Sun P, Bu F, Min JW, Munshi Y, Howe MD, Liu L, Koellhoffer EC, Qi L, McCullough LD, Li J, et al. Inhibition of calcium/calmodulin-dependent protein kinase kinase (CaMKK) exacerbates impairment of endothelial cell and blood–brain barrier after stroke. Eur J Neurosci. 2019;49:27–39. doi: 10.1111/ejn.14223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Popp A, Jaenisch N, Witte OW, Frahm C. Identification of ischemic regions in a rat model of stroke. PLoS One. 2009;4:e4764. doi: 10.1371/journal.pone.0004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Chen Y, Wu J, Manaenko A, Yang P, Tang J, Fu W, Zhang JH. Activation of dopamine D2 receptor suppresses Neuroinflammation through αB-crystalline by inhibition of NF-κB nuclear translocation in experimental ICH mice model. Stroke. 2015;46:2637–2646. doi: 10.1161/STROKEAHA.115.009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang X, Hu Q, Li B, McBride D, Bian H, Spagnoli P, Chen D, Tang J, Zhang JH. Follistatin-like 1 attenuates apoptosis via disco-interacting protein 2 homolog a /Akt pathway after middle cerebral artery occlusion in rats. Stroke. 2014;45:3048–3054. doi: 10.1161/STROKEAHA.114.006092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang B, Li H, Xu X, Shen H, Wang Y, Gao A, He W, Wang Z, Chen G. Cyclophilin a/cluster of differentiation 147 interactions participate in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2015;43:e369–e381. doi: 10.1097/CCM.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 23.Hu Q, Manaenko A, Bian H, Guo Z, Huang JL, Guo ZN, Yang P, Tang J, Zhang JH. Hyperbaric oxygen reduces infarction volume and hemorrhagic transformation through ATP/NAD+/Sirt1 pathway in hyperglycemic middle cerebral artery occlusion rats. Stroke. 2017;48:1655–1664. doi: 10.1161/STROKEAHA.116.015753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Y, Dempsey RJ, Sun D. Na+−K+-cl- cotransporter in rat focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:711–721. doi: 10.1097/00004647-200106000-00009. [DOI] [PubMed] [Google Scholar]
- 25.McBride DW, Klebe D, Tang J, Zhang JH. Correcting for brain Swelling’s effects on infarct volume calculation after middle cerebral artery. Transl Stroke Res. 2015;6:323–338. doi: 10.1007/s12975-015-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Cui X, Zacharek A, Chopp M. Increasing Ang1/Tie2 expression by simvastatin treatment induces vascular stabilization and neuroblast migration after stroke. J Cell Mol Med. 2009;13:1348–1357. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao G, Jiang N, Hu Y, Zhang Y, Wang G, Yin M, Ma X, Zhou K, Qi J, Yu B, et al. Ruscogenin attenuates cerebral ischemia-induced blood-brain barrier dysfunction by suppressing TXNIP/NLRP3 Inflammasome activation and the MAPK pathway. Int J Mol Sci. 2016;17:E1418. doi: 10.3390/ijms17091418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J, Hu Q, Chen Y, Liu F, Zheng Y, Tang J, Zhang J, Zhang JH. Neuroprotective role of an N-acetyl serotonin derivative via activation of tropomyosin-related kinase receptor B after subarachnoid hemorrhage in a rat model. Neurobiol Dis. 2015;78:126–133. doi: 10.1016/j.nbd.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu T, Stellmacher A, Znalesniak EB, Dieterich DC, Kalbacher H, Hoffmann W. Tff3 is expressed in neurons and microglial cells. Cell Physiol Biochem. 2014;34:1912–1919. doi: 10.1159/000366389. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Zhou F, Dou Y, Tian X, Liu C, Li H, Shen H, Chen G. Melatonin alleviates Intracerebral hemorrhage-induced secondary brain injury in rats via suppressing apoptosis, inflammation, oxidative stress, DNA damage, and mitochondria injury. Transl Stroke Res. 2018;9:74–91. doi: 10.1007/s12975-017-0559-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Y, Wang J, Song X, Qu L, Wei R, He F, Wang K, Luo B. RIP3 induces ischemic neuronal DNA degradation and programmed necrosis in rat via AIF. Sci Rep. 2016;6:29362. doi: 10.1038/srep29362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Yan J, Shi H. Role of hypoxia inducible factor 1 in hyperglycemia-exacerbated blood-brain barrier Disruptionin ischemic stroke. Neurobiol Dis. 2016;95:82–92. doi: 10.1016/j.nbd.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanguas-Casás N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramírez L. TUDCA: An agonist of the bile acid receptor GPBAR1/TGR5 with anti-inflammatory effects in microglial cells. J Cell Physiol. 2017;232:2231–2245. doi: 10.1002/jcp.25742. [DOI] [PubMed] [Google Scholar]
- 34.Noristani HN, Sabourin JC, Gerber YN, Teigell M, Sommacal A, Vivanco MD, Weber M, Perrin FE. Brca1 is expressed in human microglia and is dysregulated in human and animal model of ALS. Mol Neurodegener. 2015;10:34. doi: 10.1186/s13024-015-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Q, Noel O, Nguyen M, Sam L, Gerhard GS. Bile acids upregulate BRCA1 and downregulate estrogen receptor 1 gene expression in ovariancancer cells. Eur J Cancer Prev. 2018;27:553–556. doi: 10.1097/CEJ.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 36.Meng X, Tan J, Li M, Song S, Miao Y, Zhang Q. Sirt1: role under the condition of ischemia/hypoxia. Cell Mol Neurobiol. 2017;37:17–28. doi: 10.1007/s10571-016-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen T, Dai SH, Li X, Luo P, Zhu J, Wang YH, Fei Z, Jiang XF. Sirt1-Sirt3 axis regulates human blood-brain barrier permeability in response to ischemia. Redox Biol. 2018;14:229–236. doi: 10.1016/j.redox.2017.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao L, An R, Yang Y, Yang X, Liu H, Yue L, Li X, Lin Y, Reiter RJ, Qu Y. Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: the role of SIRT1 signaling. J Pineal Res. 2015;59:230–239. doi: 10.1111/jpi.12254. [DOI] [PubMed] [Google Scholar]
- 39.Yang H, Zhou H, Zhuang L, Auwerx J, Schoonjans K, Wang X, Feng C, Lu L. Plasma membrane-bound G protein-coupled bile acid receptor attenuates liver ischemia/reperfusion injury via the inhibition of toll-like receptor 4 signaling in mice. Liver Transpl. 2017;23:63–74. doi: 10.1002/lt.24628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used during the current study available from the corresponding author on reasonable request.