Abstract
Background.
Genital herpes simplex virus type 2 (HSV-2) infection causes recurrent lesions and frequent viral shedding. GEN-003 is a candidate therapeutic vaccine containing HSV-2 gD2∆TMR and ICP4.2, and Matrix-M2 adjuvant.
Methods.
Persons with genital herpes were randomized into 3 dose cohorts to receive 3 intramuscular doses 21 days apart of 10 µg, 30 µg, or 100 µg of GEN-003, antigens without adjuvant, or placebo. Participants obtained genital swab specimens twice daily for HSV-2 detection and monitored genital lesions for 28-day periods at baseline and at intervals after the last dose.
Results.
One hundred and thirty-four persons received all 3 doses. Reactogenicity was associated with adjuvant but not with antigen dose or dose number. No serious adverse events were attributed to GEN-003. Compared with baseline, genital HSV-2 shedding rates immediately after dosing were reduced with GEN-003 (from 13.4% to 6.4% for 30 μg [P < .001] and from 15.0% to 10.3% for 100 µg [P < .001]). Lesion rates were also significantly (P < .01) reduced immediately following immunization with 30 µg or 100 µg of GEN-003. GEN-003 elicited increases in antigen binding, virus neutralizing antibody, and T-cell responses.
Conclusions.
GEN-003 had an acceptable safety profile and stimulated humoral and cellular immune responses. GEN-003 at doses of 30 µg and 100 µg reduced genital HSV shedding and lesion rates.
Clinical Trials Registration.
NCT01667341 (funded by Genocea).
Keywords: Genital herpes, herpes simplex virus type 2 infection, therapeutic vaccine, GEN-003, immunotherapy.
(See the editorial commentary by Cohen on pages 844–6.)
Herpes simplex virus type 2 (HSV-2) is a common sexually transmitted pathogen that often causes recurrent genital lesions. Transmission occurs largely from asymptomatic genital tract virus shedding, making this a key target for a therapeutic HSV vaccine. The management of genital herpes currently includes episodic or daily suppressive therapy with nucleoside analogues, which abrogates most recurrences but only partly reduces viral shedding and transmission [1, 2].
GEN-003 contains a transmembrane deletion mutant of glycoprotein D (gD2ΔTMR), a primary target antigen for neutralizing antibody and T cells, combined with a large fragment of infected cell protein 4 (ICP4.2), an HSV-2 T-cell antigen prioritized through human T-cell screens. This antigen was selected by comparing T-cell responses of HSV-2–seropositive but asymptomatic and HSV-2–exposed seronegative individuals to individuals with recurrent disease, using high-throughput HSV-2 proteomic screens [3]. GEN-003 is a combination of these 2 proteins with a novel adjuvant, Matrix-M2™ [4]. Preclinical studies have demonstrated that GEN-003 elicits antibody and T-cell responses in mice and is protective in a guinea pig model of recurrent HSV-2 infection [5].
We conducted a double-blind, placebo-controlled, dose-escalation phase 1/2 study of GEN-003 to evaluate the safety, immunogenicity, and effect on viral shedding of this candidate immunotherapy (clinical trials registration NCT01667341).
METHODS
Study Participants
We recruited healthy, HSV-2–seropositive adults 18–50 years of age with a history of recurrent genital herpes for at least 1 year and 3–9 recurrences per year in the absence of antiviral suppressive therapy. HSV-2 infection was documented by Western blot, HSV-2 HerpeSelect 2 immunoglobulin G (IgG)–specific enzyme-linked immunosorbent assay (ELISA; an index value of >3.5 indicates a positive result), HSV-2 IgG Liaison assay, or type-specific culture or polymerase chain reaction (PCR), and findings were confirmed for all participants during screening by HSV-2 IgG Liaison assay. Exclusion criteria included immunocompromised state, antibody to human immunodeficiency virus or hepatitis C virus, presence of hepatitis B virus surface antigen, pregnant or nursing, history of genital HSV-1 infection, ocular HSV infection, HSV-related erythema multiforme, HSV meningitis or encephalitis, or prior receipt of HSV-2 vaccine. Effective contraception was required throughout the study.
Study Vaccine
GEN-003 is a purified protein subunit vaccine consisting of a transmembrane deletion mutant of gD (gD2ΔTMR) and a large fragment of infected cell protein 4 (ICP4.2) from HSV-2 (strain G) [5]. GEN-003 also contained Matrix-M2 (Novavax, Gaithersburg, MD) at a level of 50 µg per dose. Dulbecco’s phosphate-buffered saline was used as diluent (or as placebo) to achieve a volume of 0.5 mL for intramuscular administration. For the participants assigned to receive antigen only, Matrix-M2 was not added to the antigens.
Study Procedures
After obtaining informed consent but before randomization and dosing, participants obtained genital swab samples twice daily for 28 days and maintained a diary of genital lesions (baseline) [6]. To be eligible for randomization, participants were required to provide a minimum of 44 baseline shedding swab specimens. The swabbing procedure was repeated immediately after the third dose of vaccine and from weeks 29 to 33 and weeks 53 to 57 (referred to as 6 and 12 months after the last dose of vaccine, respectively). The initial protocol specified only the first postdosing shedding evaluation and was later amended to include 6- and 12-month collection periods.
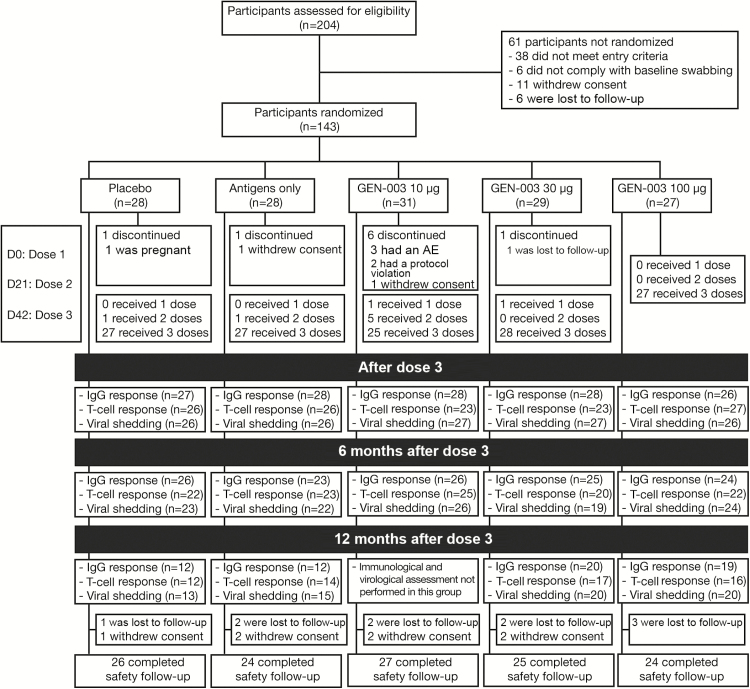
Participants were randomized sequentially into 3 cohorts defined by antigen dose (10, 30, or 100 µg of each antigen; Figure 1). Within each dose cohort, participants were randomized at a 3:1:1 ratio to receive either complete GEN-003, HSV-2 antigens without the adjuvant (antigens only), or placebo. Participants were randomized in blocks of 10 by a computer-based system to ensure an assignment to GEN-003, antigens only, and placebo at a ratio of 3:1:1. The study product was administered intramuscularly in the deltoid approximately 21 days apart. Study vaccine was prepared and administered by unblinded staff not involved in subsequent study procedures. Investigators and other clinic personnel, as well as participants, were blinded to treatment assignment. Clinical laboratory evaluation was performed prior to treatment and at intervals thereafter. Blood specimens were collected for assessment of cellular immune response at baseline, 7 days after each dose, and 6 and 12 months after the last dose. Antibody responses to each antigen were evaluated on the day of each dose and 6 and 12 months after the last dose.
Figure 1.
Consolidated Standard of Reporting Trials diagram of the study. Abbreviations: AE, adverse event; IgG, immunoglobulin G.
Participants were followed for safety until 12 months after the last scheduled dose. Adverse events (AEs) were captured from the first immunization until 28 days after the last dose. Solicited AEs, including those generally associated with immunization, were recorded by participants for 7 days after each immunization. Serious AEs and AEs of special interest, consisting of a predefined list of autoimmune disorders, were recorded through the end of the study. All AEs were graded by severity according to specified criteria [7].
Study Oversight
The study was designed collaboratively by investigators at Genocea Biosciences and the University of Washington in accordance with the ethical principles of the Declaration of Helsinki and the current principles of good clinical practice. An independent data and safety monitoring board reviewed the cumulative data by dose cohort at specified intervals. The efficacy analyses reported were conducted by external statisticians and independently at the University of Washington, according to a previously developed statistical plan. Both academic and industry authors contributed to manuscript drafts and approved the submission of the final manuscript. The study authors had full access to all data, verified their accuracy, and vouch for the fidelity of the study to the protocol and the completeness of the data presented. The study was approved by the institutional review board at each study center, and each participant provided written informed consent.
Laboratory Methods
HSV-2 DNA PCR
Genital swab specimens were tested for the presence of HSV-2 DNA, using a quantitative real-time PCR method [5]. Each plate included 4 negative controls, including 2 extraction and 2 PCR controls, and four 10-fold dilutions of HSV-2 strain 333 (positive controls). The limit of detection was 2 DNA copies per 20-µL reaction, with linearity (R2 = 0.98) over 5 logs of HSV-2 genomic DNA content.
Antibody Assays
IgG antibody responses directed against each of the GEN-003 antigens were measured by end point ELISA titer, as published previously [5]. HSV-2 neutralizing antibody titers were determined via a β-galactosidase colorimetric assay [5].
Cellular Immunity Assays
Gamma interferon enzyme-linked immunospot assays (ELISPOTs) were performed as previously described, with modifications for assays with human peripheral blood mononuclear cells [5].
Statistical Methods
The primary end point of the study was safety and tolerability, assessed by the frequency, nature, duration, and severity of AEs, by study group. The secondary end points included the reduction in HSV shedding rate in participants following immunization, compared with baseline. Other secondary end points included the humoral and cellular immune responses to GEN-003 and the ability of Matrix-M2 adjuvant to promote T-cell responses. Reduction in genital lesion rates was an exploratory end point.
Efficacy and safety analyses were conducted on a modified intent-to-treat population that included all participants who received at least 1 dose of study vaccine. Because of the small number (approximately 10 each) assigned to the unadjuvanted dose and placebo groups within each dose cohort, results from these groups were combined for all analyses except immune response analyses.
Shedding rate was calculated as the number of positive swabs divided by the total number of swab specimens tested. An analysis of the change in the shedding rate from that at baseline (ie, the rate ratio [RR]) was performed using a previously described longitudinal Poisson mixed model with a random intercept to test for differences between baseline and each postbaseline time point within treatment groups [6]. The model has the total number of HSV-2–positive swab specimens as the dependent variable and includes terms for treatment group, visit, treatment group by visit interaction, log of the total number of swab specimens collected (offset), and a random intercept. With this model, the sample size of 30 participants per treatment group was selected to detect a 30% reduction in the shedding rate of HSV-2 as compared to baseline, with a power of 80%, a 2-sided α of 0.05, and the assumption of a 20% shedding rate at baseline. More recently, the statistical model was modified to include an empirical variance estimate, because the type I error was found to be >5% by use of the previous method. A recent study by Magaret that compared available methods for evaluating crossover studies for viral detection indicated that a type I error of up to 28% was possible with previous methods but that type I error was controlled at 5% and power maximized by using the empirical variance structure [8]. Since these simulation results were not available either at the time of the writing of the statistical analysis plan or when the study data became available for analysis, we have included findings from the primary analysis, as well as findings of the updated analysis (Supplementary Results and Supplementary Table 1). Because few participants contributed data at 12 months and the Poisson model failed to converge, this time point was not included in the model, and results are presented as descriptive only.
The change between baseline and each of the follow-up visits was analyzed for antibody titers and T-cell responses, using a linear mixed model. The model included terms for treatment group, baseline log titer, visit, treatment by visit interaction, and participant identifier (random effect).
RESULTS
Study Population
The trial was conducted at 7 sites in the United States between July 2012 and April 2014. Of 204 persons screened, 143 (70%) were randomized and received at least 1 vaccination. The median age of participants was 37 years (range, 20–50 years); 62% were women (Table 1). One hundred and thirty-four participants (94%) received all 3 doses of the study product (Figure 1), and 130 provided genital swab specimens during the 28 days immediately following the scheduled third immunization and were included in the efficacy analyses. Some participants, including all participants in the 10-µg dose group, had completed the original protocol before an amendment to collect swab and blood samples 6 and 12 months after the last dose. Thus, 114 participants provided swab specimens at 6 months and 68 at 12 months following vaccination. Overall, the percentage of expected swab specimens collected was 96% (median value per person, 98%; range, 71%–100%).
Table 1.
Demographic and Clinical Characteristics of Study Participants, by Study Arm
| Characteristic | Placebo (n = 28) |
Antigen Only (n = 28) |
GEN–003 | ||
|---|---|---|---|---|---|
| 10 µg (n = 31) |
30 µg (n = 29) |
100 µg (n = 27) |
|||
| Age, y | 36.5 (23–50) | 37 (20–47) | 37 (24–49) | 38 (22–50) | 34 (24–50) |
| Female sex | 17 (61) | 17 (61) | 21 (68) | 18 (62) | 15 (56) |
| White | 17 (61) | 15 (54) | 23 (74) | 16 (55) | 17 (63) |
| African American | 10 (36) | 8 (29) | 5 (16) | 10 (35) | 8 (30) |
| HSV-1 positivity, proportion tested with conclusive results (%) | 11/25 (44) | 16/25 (64) | 15/26 (56) | 5/22 (22) | 14/25 (56) |
| Duration of genital herpes, y | 6.5 (1–26) | 6.0 (0–24)a | 6.0 (1–33) | 8.0 (1–25) | 6.0 (2–33) |
| Frequency of recurrences before study entry,b no./y | 5 (3–8) | 5 (3–9) | 5 (3–8) | 5 (3–9) | 5 (3–9) |
| Ever received suppressive therapy before study entry | 21 (75) | 19 (68) | 18 (58) | 18 (62) | 11 (41) |
| Swab specimens collected, no., by collection time | |||||
| Before immunization | |||||
| Overall, no. | 1527 | 1531 | 1707 | 1576 | 1483 |
| Per person, no., median (range) | 55 (50–56) | 56 (51–56) | 56 (50–56) | 56 (49–56) | 56 (50–56) |
| Immediately after immunization | |||||
| Overall, no. | 1373 | 1365 | 1361 | 1414 | 1346 |
| Per person, no., median (range) | 56 (37–56) | 56 (31–56) | 55 (13–56) | 54 (26–56) | 55 (27–56) |
| 6 mo after immunization | |||||
| Overall, no. | 1287 | 1183 | 1414 | 1017 | 1323 |
| Per person, no., median (range) | 56 (45–69) | 55 (41–64) | 56 (40–68) | 55 (46–57) | 56 (36–69) |
| 12 mo after immunization | |||||
| Overall, no. | 730 | 834 | … | 1080 | 1108 |
| Per person, no. | 56 (50–62) | 56 (49–60) | … | 56 (33–59) | 56 (51–58) |
Data are no. (%) of participants or median value (range), unless otherwise indicated.
Abbreviation: HSV, herpes simplex virus.
aOne participant had a formal diagnosis of genital HSV-2 infection only 6 months before randomization, rather than 1 year as required by the inclusion criteria, but had been symptomatic for much longer. Enrollment of this participant was approved by the sponsor.
bDuring the year before initiation of suppressive therapy for those receiving suppressive therapy.
Safety and Tolerability of GEN-003
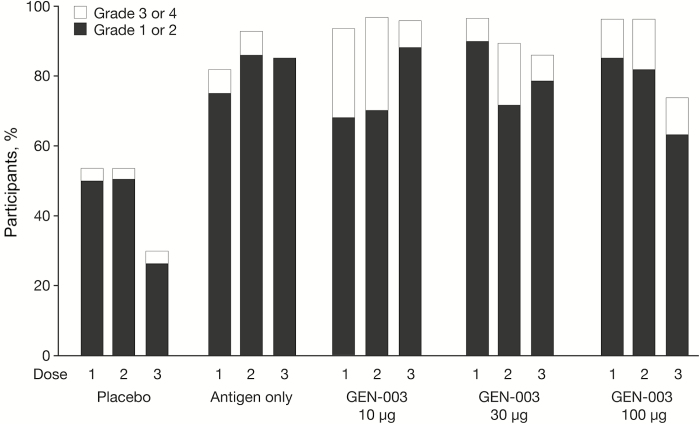
Local and systemic reactions within the first hour after immunization were infrequent, and the most common were pain, tenderness, induration, and redness at the injection site. These were most frequent among participants receiving GEN-003, but all were graded as mild or moderate (data not shown). In the first 7 days following receipt of any of the 3 administered doses, 75% of placebo recipients, 100% of antigen-only recipients, and 96%–100% of GEN-003 recipients reported local reactogenicity at the site of administration (Table 2). The most common reported systemic AEs for any of the 3 administered doses were myalgia and fatigue, with values of 43% and 46%, respectively, for placebo recipients, 54% and 61%, respectively, for antigen-only recipients, and 82% and 71%, respectively, for GEN-003 recipients, without significant differences across antigen dose levels. Neither local nor systemic reactogenicity increased with subsequent doses of the vaccine (Figure 2) or with the dose of the antigen (Table 2). Grade 3 or 4 solicited AEs were experienced by 11% of placebo recipients, 7% of participants in the antigen-only groups, and in 42%, 21%, and 22% of participants in the 10-, 30-, and 100-µg GEN-003 groups, respectively. The most common grade 3 and 4 local AEs were pain, tenderness, and induration, and the most common grade 3 and 4 systemic AEs were nausea, fatigue, and myalgias (Table 2). Participants who received GEN-003 had longer-lasting AEs and reported using more analgesics following immunization than the participants in the antigen-only or placebo groups. For example, pain was reported as a cumulative duration of 1.5 days (range, 1–2 days), 2 days (range, 1–7 days), and 3 days (range, 1–10 days) in the participants receiving placebo, antigen only, and GEN-003, respectively. Three of 31 participants in the GEN-003 10-µg dose group but none in the other GEN-003 groups discontinued dosing because of systemic reactogenicity. Five unrelated serious AEs were observed: femur fracture (GEN-003 10-µg group; day 185), suicide attempt (GEN-003 10-µg group; day 62), spontaneous miscarriage (placebo group; day 115), complex migraine (GEN-003 100-µg group; day 230), and myocardial infarction (GEN-003 100-µg group; day 370). No deaths or new-onset immune-mediated diseases were noted.
Table 2.
Adverse Events (AEs) Reported Following Receipt of Any Dose of Study Treatment
| AE | Placebo, No. (%) (n = 28) | Antigen Only, No. (%) (n = 28) | GEN-003, No. (%) | ||
|---|---|---|---|---|---|
| 10 µg (n = 31) |
30 µg (n = 29) |
100 µg (n = 27) |
|||
| Total solicited | 21 (75) | 28 (100) | 31 (100) | 28 (97) | 26 (96) |
| Grade 3 or 4 solicited | |||||
| Overall | 3 (11) | 2 (7) | 13 (42) | 6 (21) | 6 (22) |
| Injection site | |||||
| Pain | 0 | 0 | 5 (16) | 0 | 3 (11) |
| Tenderness | 0 | 1 (4) | 5 (16) | 3 (10) | 3 (11) |
| Induration | 0 | 0 | 1 (3) | 2 (7) | 3 (11) |
| Redness | 0 | 0 | 1 (3) | 1 (3) | 0 |
| Systemic | |||||
| Nausea | 1 (4) | 0 | 6 (19) | 2 (7) | 1 (4) |
| Vomiting | 0 | 0 | 3 (10) | 1 (3) | 0 |
| Diarrhea | 0 | 0 | 0 | 0 | 0 |
| Fatigue | 3 (11) | 2 (7) | 7 (23) | 2 (7) | 4 (15) |
| Myalgia | 1 (4) | 0 | 8 (26) | 3 (10) | 4 (15) |
| Fever | 0 | 0 | 3 (10) | 0 | 1 (4) |
| Other | |||||
| Any | 20 (71) | 21 (75) | 27 (87) | 18 (62) | 16 (59) |
| Grade 3 or 4 | 3 (11) | 1 (4) | 7 (23) | 3 (11) | 3 (11) |
| Serious | 1 (4) | 0 | 2 (6) | 0 | 2 (7) |
| Any leading to study discontinuation | 0 | 0 | 3 (10) | 0 | 0 |
All AEs were graded by severity according to criteria published elsewhere [7].
Figure 2.
Rates of local and systemic solicited adverse events within 7 days following vaccination, by treatment assignment and dose number.
Immunogenicity of GEN-003
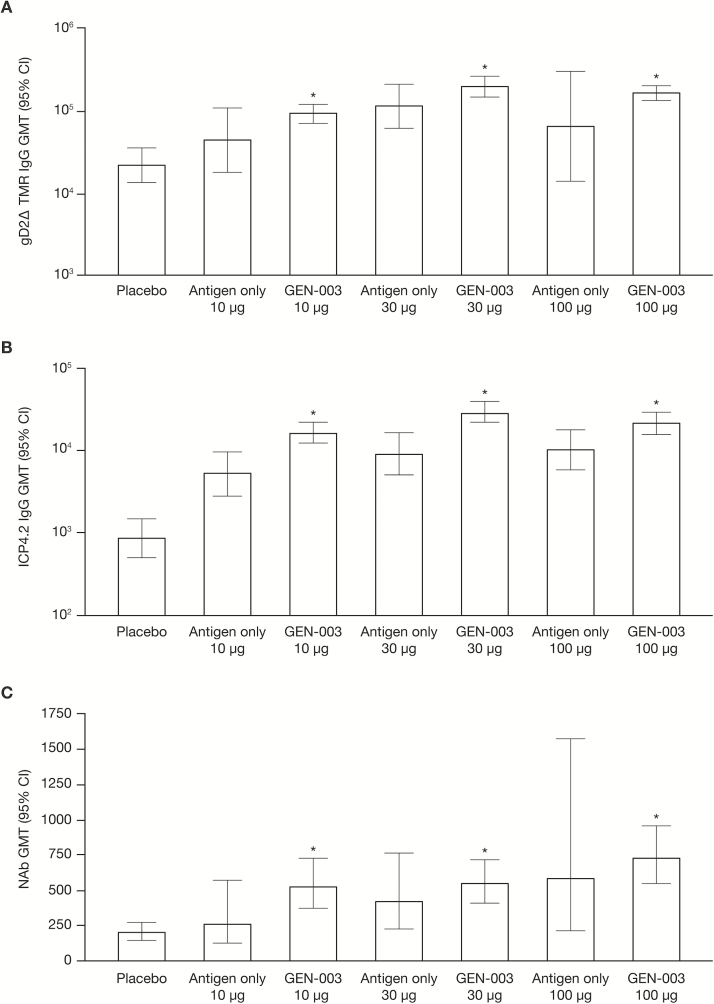
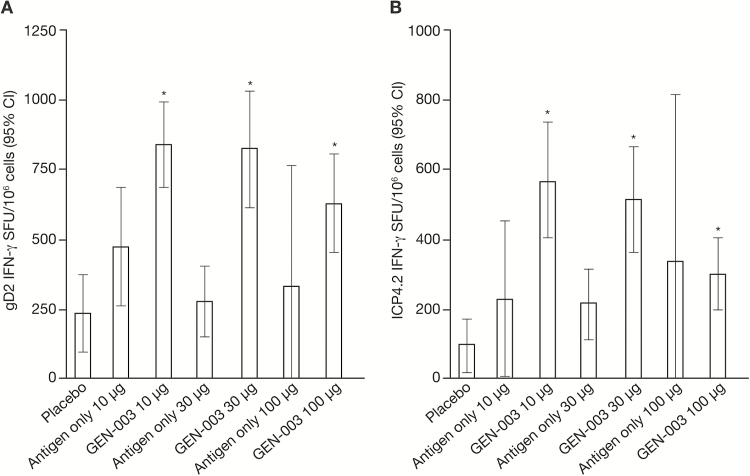
Immunization with GEN-003 elicited IgG and T-cell responses to gD2ΔTMR and ICP4.2 at all dose levels. IgG antibody responses were greatest with the 30-µg dose, while neutralizing antibody titers were the highest with the 100-µg dose. For each antigen dose, the addition of Matrix-M2 increased the responses (Figure 3). T-cell responses were highest for the lowest doses of GEN-003 (Figure 4). The IgG and T-cell responses observed for both antigens in the GEN-003 groups after the third immunization were significantly higher than those in the same groups on day 0 and those in the placebo group (P < .001). A detailed description of immune responses to GEN-003, including the data collected 6 and 12 months after immunization, has been published elsewhere [9].
Figure 3.
Humoral immune responses following the third immunization measured on day 63, 3 weeks after the third immunization. A, Immunoglobulin G (IgG) response to glycoprotein gD2ΔTMR, a transmembrane deletion mutant of glycoprotein D. B, IgG response to infected cell protein 4 (ICP4.2). C, Neutralizing antibody (NAb) response. Abbreviations: CI, confidence interval; GMT, geometric mean titer. *P < .001, compared with placebo and compared with day 0 in the same group.
Figure 4.
Cellular immune responses measured on day 49, 1 week after the third immunization. A, Mean level of interferon γ (IFN-γ) release in response to glycoprotein D2. B, Mean level of IFN-γ release in response to infected cell protein 4 (ICP4.2). Abbreviations: CI, confidence interval; gD2, glycoprotein D2; SFU, spot-forming units. *P < .001, compared with placebo and compared with day 0 in the same group.
Effect of GEN-003 on Viral Shedding
Among all participants, HSV was detected in 11.9% of swab specimens (933 of 7824) collected at baseline and ranged from 7.4% in the antigen-only group to 15.0% in the GEN-003 100-µg dose group (Table 3). In the placebo group, the RR for viral shedding was unchanged immediately after immunization but appeared to increase at 6 months (RR, 1.30; P = .012). In the antigen-only group, the shedding rate increased from 7.4% at baseline to 10.0% immediately after immunization (RR, 1.59; P = .017) and was 8.6% at 6 months (RR, 2.22; P < .001).
Table 3.
Genital Herpes Simplex Virus Type 2 (HSV-2) Shedding and Lesions Rates Before and After Immunization
| Characteristic | Placebo (n = 28) |
Antigen Only (n = 28) |
GEN-003 | ||
|---|---|---|---|---|---|
| 10 µg (n = 31) |
30 µg (n = 29) |
100 µg (n = 27) |
|||
| HSV shedding rate, proportion (%)a | |||||
| Before immunization | 190/1527 (12.4) |
114/1531 (7.4) |
185/1707 (10.8) |
221/1576 (13.4) |
223/1483 (15.0) |
| Immediately after immunization | 176/1373 (12.8) | 136/1365 (10.0) |
147/1361 (10.8) |
91/1414 (6.4) |
138/1346 (10.3) |
| 6 mo after immunization | 214/1287 (16.6) |
102/1183 (8.6) |
244/1414 (17.3) |
81/1017 (8.0) |
164/1323 (12.4) |
| 12 mo after immunization | 90/730 (12.3) |
120/834 (14.4) |
Not applicable | 133/1080 (12.3) |
123/1108 (11.1) |
| HSV shedding rate ratiob,c | |||||
| Immediately after immunization | 0.95 | 1.59d | 1.06 | 0.47e | 0.68e |
| 6 mo after immunization | 1.30d | 2.22e | 1.58e | 0.58e | 0.88 |
| Lesion rate, proportion (%)f | |||||
| Before immunization | 59/820 (7.2) |
76/804 (9.5) |
128/873 (14.7) |
79/816 (9.7) |
52/761 (6.8) |
| Immediately after immunization | 67/737 (9.1) |
48/721 (6.7) |
64/711 (9.0) |
38/760 (5.0) |
26/708 (3.7) |
| 6 mo after immunization | 61/665 (9.2) |
41/611 (6.7) |
82/733 (11.2) |
18/532 (3.4) |
32/691 (4.6) |
| 12 mo after immunization | 15/372 (4.0) |
11/427 (2.6) |
Not applicable | 31/556 (5.6) |
33/569 (5.8) |
| Lesion rate ratiob,g | |||||
| Immediately after immunization | 1.21 | 0.90 | 0.76 | 0.52h | 0.53h |
| 6 mo after immunization | 1.32 | 1.23 | 0.91 | 0.33e | 0.85 |
An analysis of the change from baseline in the shedding rate was performed using a previously described longitudinal Poisson mixed model with a random intercept to test for differences from baseline to each postbaseline time point within treatment groups [6]. The model has the total positive swabs as the dependent variable and includes terms for treatment group, visit, treatment group by visit interaction, log of total swab specimens collected (offset) and a random intercept.
aData are number of swab specimens testing positive for HSV/total number of swab specimens tested (%).
bA limited number of participants collected swab specimens and reported lesions during weeks 53–57, so the Poisson mixed model failed to converge for this period; thus, 12-month comparisons are not presented.
cFor each time point, rate ratios were calculated as the proportion of swab specimens testing positive for HSV/proportion testing positive before immunization.
d P < .05.
e P < .001.
fData are number of swabbing days on which a lesion was detected/total number of swabbing days (%).
gFor each time point, rate ratios were calculated as the proportion of swabbing days on which a lesion was detected/proportion of swabbing days before immunization during which a lesion was detected.
h P < .01.
The shedding rate for participants who received the 10-μg dose of GEN-003 was unchanged immediately following the immunizations but increased at 6 months (RR, 1.58; P < .001; Table 3). Data at 12 months are not available for this dose group (see “Methods”). In the GEN-003 30-µg group, the shedding rate was reduced by 52% immediately following immunizations (RR, 0.47; P < .001) and remained lower at 6 months (RR, 0.58; P < .001), returning to a value close to baseline at 12 months. Immunization with 100 µg of GEN-003 was also followed by a reduction in shedding immediately after immunizations (RR, 0.68; P < .001), which was not significant at 6 months (RR, 0.88; P = .224) and returned close to the baseline level at 12 months. As observed in similar studies of viral shedding [10, 11], some individuals did not shed in the initial period, because shedding is intermittent (Supplementary Figure 1).
Effect of GEN-003 on Genital Lesions
Lesion rates were similar before and after immunization for the placebo, antigen-only, and GEN-003 10-µg groups (Table 3). In the placebo group, a slight increase in the lesion rates at baseline was observed immediately after immunization, and they remained at this level at 6 months. Lesion rates then decreased at 12 months, but only a limited number of participants in the control group (n = 13) provided 12-month data. A similar pattern was observed in the antigen-only group, in which lesion rates remained stable at 3 and 6 months but decreased at 12 months, possibly because fewer participants (n = 15) provided data. The lesion rates decreased in the GEN-003 30-µg group, from 9.7% at baseline to 5.0% immediately after immunization (RR, 0.52; P < .01), and remained lower 6 and 12 months after immunization (3.4% and 5.6%, respectively; RR, 0.33 [P < .001] for the 6-month time point). The lesion rates in the GEN-003 100-µg dose group decreased from 6.8% at baseline to 3.7% immediately after immunization (RR, 0.53; P = .009) but not thereafter. As described in “Methods,” an alternative analysis of lesion and shedding rates, using a Poisson regression method with empirical variance estimation, can be found in Supplementary Table 1.
DISCUSSION
Our results of immunotherapy with GEN-003, a novel vaccine for genital herpes, composed of HSV-2 gD2ΔTMR and a fragment of ICP4 protein (ICP4.2) adjuvanted with Matrix-M2, demonstrate that it had an acceptable safety profile, was immunogenic, and reduced both recurrent viral shedding and the number of days with lesions at doses of 30 µg and 100 µg for each antigen. Despite the frequent mild-to-moderate AEs associated with injections and the percentage of grade 3 AEs, only 3 participants discontinued receipt of further dosing. The addition of the Matrix-M2 adjuvant was critical for boosting the cell-mediated response and was dose sparing for the antibody response to the vaccine.
Most of the reactogenicity of GEN-003 was likely due to the addition of the adjuvant, Matrix-M2, to the viral antigens and appeared acceptable for a therapeutic vaccine. Reassuringly, no AEs of special interest emerged during the study. While a few participants missed a day of work as a result of AEs, study discontinuation caused by these events was rare, further indicating that the safety profile, as observed in this trial, was acceptable to participants.
Prior attempts to develop a therapeutic vaccine for HSV-2 were unsuccessful. Some of the prior candidate vaccines focused on eliciting IgG and neutralizing antibody responses to surface antigens [12]. It had been hypothesized that effective T-cell responses are likely to be required to control intracellular pathogens such as HSV. However, in this study, the level of T cells, at least as measured by gamma interferon ELISPOT, did not correlate with the reduction in shedding or lesion rates; the frequencies of responding T cells were highest in the 10-µg group, which had little to no impact on viral shedding or lesion rates. Additionally, a replication defective (ie, gH-deleted) mutant virus failed to reduce recurrence rates or viral shedding in participants with genital HSV-2 in a double-blind, randomized study, although T-cell immune responses were identified in animal studies [13, 14]. The specific T-cell target may be critical to eliciting an effective immune response. We previously identified ICP4 as a potential T-cell target from HSV-2, using a high throughput antigen-screening method [3].
GEN-003 elicited both humoral and cellular immune responses. Antibody titers directed against gD2ΔTMR and ICP4.2 increased after each dose [9] and peaked with the 30-µg dose. Titers of neutralizing antibody were the highest in participants who received the 100-µg dose. By contrast, T-cell responses, measured by gamma interferon ELISPOT, were maximal after the first dose and appeared to be inversely related to antigen dose [9]. A similar effect was identified in preclinical studies of immunogenicity in mice [5]. Chronic infections may present high or chronic loads of antigen to the immune system, which can lead to T cells becoming anergized, becoming exhausted, or entering apoptosis after additional stimulation by vaccination [15]. Additional analyses are underway to explore the correlate of activity.
Viral shedding was used as the main efficacy outcome because it is an objective measure of mucosal HSV replication, its measurement has been previously used to demonstrate differences in efficacy between doses of antiviral therapies [6], and it is the primary mode for transmission [16]. Consequently, for dose selection of a therapeutic agent, shedding rates are most efficient and quantitative. There are inherent limitations of comparing results from across trials, owing to differences in study design, patient populations, exclusion/inclusion criteria, and end points measured. In the current study, the proportion of positive results among swab specimens from the 30-µg group after the last dose (6.4%) is similar to that observed in a study with valacyclovir (500 mg daily; 4%–6%) [6].
Immunization with GEN-003 at 30 and 100 µg significantly reduced viral shedding and clinical disease (as measured by the lesion rate) immediately after vaccination. At 6 months after immunization, significant reductions in viral shedding and lesion rates continued to be observed in the 30-µg group, while only a reduction in the lesion rate was observed in the 100-µg group. However, no such conclusion can be stated for the results at 12 months, as few participants provided data at this time. Changes between 6- and 12-month measurements may simply reflect this reduction in the number of participants, and further studies are required to assess the durability of response. Furthermore, although shedding is most certainly a requirement for transmission, no quantitative data exist to allow extrapolation from the observed reduction in shedding to a possible benefit on transmission. The durability of the response is encouraging, although the number of participants in the study at the 12-month evaluation period was small and may explain the apparent decrease in lesion rates observed in the control groups. Currently, the data indicate a durability of 6 months, an interval likely to be practical for maintenance delivery of therapeutic vaccine doses. Further studies are being conducted to better estimate the true durability of the effect.
In conclusion, GEN-003, a novel therapeutic vaccine for HSV-2 that is currently in clinical development, was shown to have an acceptable safety profile, reduce viral shedding, reduce the frequency of genital lesions, and boost the humoral and cellular immune responses to HSV-2. The optimal dose of the proteins and adjuvant and the durability of the effect will require further study.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. We thank the study volunteers, clinicians, nurses, and laboratory technicians at the study sites, for their contributions; and Deborah Long, Alexander Lee, Shane Larson, Mojca Skoberne, and Kym Raphino, from Genocea Biosciences, for laboratory support.
Financial support. This work was supported by Genocea Biosciences.
Potential conflicts of interest. D. I. B. receives funding from Genocea for preclinical studies of vaccines and has been a consultant to Genocea Biosciences, Vical, GlaxoSmithKline, and Merck for herpes virus vaccines. A. W. is a consultant for Aicuris, Amgen, and GlaxoSmithKline, received travel reimbursement from Admedus, and has received funds for sponsored projects for Genocea Biosciences and Vical. T. W. has served as the principal investigator on clinical trials related to herpes vaccines for Vical, Genocea Biosciences, GlaxoSmithKline, and Merck. K. F. receives research funding from Genocea Biosciences and Vical. S. T. has received research funding from Genocea for clinical studies on herpes simplex virus vaccines. P. L. has received research funding from Genocea Biosciences for clinical studies on herpes simplex virus vaccines. N. V. W. has been a consultant for Genocea Biosciences for herpes virus vaccines. A. M. consults for Immune Design Corporation and for AiCuris. J. B. F. is an employee and stock owner of Genocea Biosciences. Sybil Tasker was an employee of Genocea Biosciences at the time of the study. J. C. was an employee of Genocea Biosciences at the time of the study. A. M. is owner of IND2Results. S. H. is an employee and stock owner of Genocea Biosciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Workowski KA, Bolan GA; Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015; 64:1–137. [PMC free article] [PubMed] [Google Scholar]
- 2. Corey L, Wald A, Patel R, et al. ; Valacyclovir HSV Transmission Study Group. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N Engl J Med 2004; 350:11–20. [DOI] [PubMed] [Google Scholar]
- 3. Long D, Skoberne M, Gierahn TM, et al. Identification of novel virus-specific antigens by CD4⁺ and CD8⁺ T cells from asymptomatic HSV-2 seropositive and seronegative donors. Virology 2014; 464–465:296–311. [DOI] [PubMed] [Google Scholar]
- 4. Cox RJ, Pedersen G, Madhun AS, et al. Evaluation of a virosomal H5N1 vaccine formulated with Matrix M™ adjuvant in a phase I clinical trial. Vaccine 2011; 29:8049–59. [DOI] [PubMed] [Google Scholar]
- 5. Skoberne M, Cardin R, Lee A, et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in Guinea pigs. J Virol 2013; 87:3930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnston C, Saracino M, Kuntz S, et al. Standard-dose and high-dose daily antiviral therapy for short episodes of genital HSV-2 reactivation: three randomised, open-label, cross-over trials. Lancet 2012; 379:641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Food and Drug Administration. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm074775.htm. [DOI] [PubMed]
- 8. Magaret A. Models for HSV shedding must account for two levels of dispersion. UW biostatistics working paper series. Working paper 410 January 2016. http://biostats.bepress.com/uwbiostat/paper410/ Accessed 17 April 2016.
- 9. Flechtner JB, Long D, Larson S, et al. Immune responses elicited by the GEN-003 candidate HSV-2 therapeutic vaccine in a randomized controlled dose-ranging phase 1/2a trial. Vaccine 2016; 34:5314–20. [DOI] [PubMed] [Google Scholar]
- 10. Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women. Effect of acyclovir treatment. J Clin Invest 1997; 99:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med 1996; 124:8–15. [DOI] [PubMed] [Google Scholar]
- 12. Straus SE, Corey L, Burke RL, et al. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet 1994; 343:1460–3. [DOI] [PubMed] [Google Scholar]
- 13. de Bruyn G, Vargas-Cortez M, Warren T, et al. A randomized controlled trial of a replication defective (gH deletion) herpes simplex virus vaccine for the treatment of recurrent genital herpes among immunocompetent subjects. Vaccine 2006; 24:914–20. [DOI] [PubMed] [Google Scholar]
- 14. Farrell HE, McLean CS, Harley C, Efstathiou S, Inglis S, Minson AC. Vaccine potential of a herpes simplex virus type 1 mutant with an essential glycoprotein deleted. J Virol 1994; 68:927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leggatt GR. Peptide dose and/or structure in vaccines as a determinant of T cell responses. Vaccines (Basel) 2014; 2:537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mertz GJ, Benedetti J, Ashley R, Selke SA, Corey L. Risk factors for the sexual transmission of genital herpes. Ann Intern Med 1992; 116:197–202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.