Abstract
The identification of Parkinson’s disease (PD)-associated genes has created a powerful platform to begin to understand and nominate pathophysiological disease mechanisms. Herein, we discuss the genetic and experimental evidence supporting endolysosomal dysfunction as a major pathway implicated in PD. Well-studied familial PD-linked gene products, including LRRK2, VPS35, and α-synuclein, demonstrate how disruption of different aspects of endolysosomal sorting pathways by disease-causing mutations may manifest into PD-like phenotypes in many disease models. Newly-identified PD-linked genes, including auxilin, synaptojanin-1 and Rab39b, as well as putative risk genes for idiopathic PD (endophilinA1, Rab29, GAK), further support endosomal sorting deficits as being central to PD. LRRK2 may represent a nexus by regulating many distinct features of endosomal sorting, potentially via phosphorylation of key endocytosis machinery (i.e. auxilin, synaptojanin-1, endoA1) and Rab GTPases (i.e. Rab29, Rab8A, Rab10) that function within these pathways. In turn, LRRK2 kinase activity is critically regulated by Rab29 at the Golgi complex and retromer-associated VPS35 at endosomes. Taken together, the known functions of PD-associated gene products, the impact of disease-linked mutations, and the emerging functional interactions between these proteins points to endosomal sorting pathways as a key point of convergence in the pathogenesis of PD.
Keywords: Parkinson’s disease, endolysosomal sorting, VPS35, LRRK2, α-synuclein, synaptic vesicle endocytosis, auxilin, GAK, synaptojanin-1, endophilin, Rab GTPases
Genetics lead the way to understanding Parkinson’s disease pathogenesis
As the global population grows and ages, neurological disorders have become the largest source of disability and the second largest cause of death worldwide (Collaborators., 2019). Of these disorders Parkinson’s disease (PD) is one of the fastest growing with nearly 22% increase in age-standardized prevalence between 1990 and 2016 (Collaborators., 2018). PD is the most common neurodegenerative movement disorder affecting 10 million people worldwide and projected to affect nearly 1 million in North America alone by 2020 (Marras et al., 2018, Dorsey and Bloem, 2018, Dorsey et al., 2018). Clinically, PD is largely diagnosed via cardinal motor symptoms, including resting tremor, bradykinesia, rigidity and postural instability (Lang and Lozano, 1998a, Lang and Lozano, 1998b). Pathologically, PD is characterized by the relatively selective loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) resulting in dopamine depletion of the nigrostriatal pathway and brainstem Lewy pathology (Lang and Lozano, 1998a, Lang and Lozano, 1998b). Despite urgent clinical need and impending societal health burden, current therapies are purely palliative and often lose efficacy as disease progresses. The lack of tractable therapeutic success is likely due to our nascent understanding of disease etiology. Over the past 200 years since PD was originally described, many pathogenic mechanisms have been proposed based on genetic, epidemiological, experimental and pathological evidence including; endolysosomal dysfunction, mitochondrial deficits, synaptic dysfunction, protein aggregation, and inflammation, to name a few (Meissner et al., 2011, Moore et al., 2005). Yet today, it remains uncertain what causes the selective loss of nigral dopaminergic neurons and other neuronal populations in people with PD.
Although the majority of PD is idiopathic, PD can also occur in a familial manner due to the Mendelian inheritance of distinct genetic mutations, with the identification of at least 15 genes to date. While familial PD due to autosomal dominant inheritance (i.e. LRRK2, VPS35, SNCA) is usually similar to idiopathic disease with typical late-onset, clinical symptoms and neuropathology, autosomal recessive PD (i.e. Parkin, PINK1, DJ-1) tends to be early-onset, slowly progressive, and most often lacks Lewy pathology (Hernandez et al., 2016, Blauwendraat et al., 2019). Genome-wide association studies (GWAS) for idiopathic PD have also highlighted common genetic variants (~90 SNPs) in loci involved in PD risk, that include genes linked to familial PD (i.e. SNCA, LRRK2) as well as within candidate genes that cluster in similar biological pathways (Nalls et al., 2014, Chang et al., 2017, Nalls et al., 2019). The genes linked to familial PD or nominated by GWAS provide key insight into the molecular and cellular mechanisms that regulate age-related neuronal vulnerability and Lewy pathology in PD, and collectively implicate the endolysosomal system as an important subcellular location and pathway for disease pathogenesis (Table 1). In this review, we will discuss the most well-studied familial PD genes involved in the endolysosomal system; LRRK2, VPS35 and α-synuclein, and their connections to other, recently identified PD-linked endosomal genes. Currently, human genetic and cell biological studies provide the strongest evidence for disease pathogenesis through endolysosomal dysfunction (Abeliovich and Gitler, 2016).
Table 1:
PD-associated genes and their functions within endosomal pathways.
| Gene/ locus name |
Protein name | Phenotype | Function | References |
|---|---|---|---|---|
| SNCA (PARK1) | α-synuclein | AD LO PD | Synaptic vesicle exocytosis; SNARE chaperone | (Polymeropoulos et al., 1997, Kruger et al., 1998, Zarranz et al., 2004, Appel-Cresswell et al., 2013, Lesage et al., 2013) |
| SNCA (PARK4) | α-synuclein | AD EO PD | (Singleton et al., 2003, Chartier-Harlin et al., 2004) | |
| LRRK2 (PARK8) | LRRK2 | AD LO PD | Kinase, GTPase | (Paisan-Ruiz et al., 2013, Zimprich et al., 2004) |
| PARK16 | Rab29 (Rab7L1) | PD risk, GWAS | Rab GTPase | (Nalls et al., 2014, Tucci et al., 2010, MacLeod et al., 2013) |
| VPS35 (PARK17) | VPS35 | AD LO PD | Endosomal cargo sorting - retromer | (Deng et al., 2012, Vilarino-Guell et al., 2011, Sharma et al., 2012, Zimprich et al., 2011) |
| DNAJC6 (PARK19) | Auxilin | AR Juvenile PD | Clathrin uncoating | (Edvardson et al., 2012, Song et al., 2017) |
| SYNJ1 (PARK20) | Synaptojanin-1 | AR EO PD | Lipid phosphatase | (Krebs et al., 2013, Quadri et al., 2013, Olgiati et al., 2014, Chen et al., 2015, Kirola et al., 2016) |
| RAB32 | Rab32 | AD, LO PD | Rab GTPase | (Gustavsson E, 2017) |
| RAB39B | Rab39B | X-linked EO PD | Rab GTPase | (Lesage et al., 2015, Wilson et al., 2014) |
| GAK (DNAJC26) | GAK | PD risk, GWAS | Clathrin uncoating | (Chen et al., 2013, Beilina et al., 2014) |
Key: AD, autosomal dominant; AR, autosomal recessive; LO, late-onset; EO, early-onset; PD, Parkinson’s disease; GWAS, genome-wide association study.
Role of familial PD genes in endosomal sorting pathways
The endolysosomal system:
In order to understand how alterations in familial PD genes cause disease, an understanding of how they play roles within, or interact with, the endosomal system is required. Briefly, proteins enter the endosomal system from two main directions, either via endocytosis at the plasma membrane or from the trans-Golgi network (TGN) (Figure 1). Endocytic vesicles from either direction fuse with endosomes, adding their contents and/or membrane-bound “cargo” proteins into the endosomal lumen. Once in the endosomal system, cargo can be recycled back to the plasma membrane or TGN, transported to a new destination or remain in the endosome as it acidifies and matures into a lysosome for eventual degradation. At this stage the lysosome may fuse with other organelles such as autophagosomes for organelle or protein degradation and clearance. Perturbations to this system can result in cargo that abnormally remains in the maturing endosome and is eventually degraded in the lysosome. It is hypothesized that the accumulation of protein cargo or pathogenic proteins with time may eventually contribute to lysosome dysfunction and cell death. A number of PD models support this concept as perturbation of PD-linked proteins have been shown to consistently disrupt endolysosomal functions resulting in the accumulation and/or swelling of different vesicular structures.
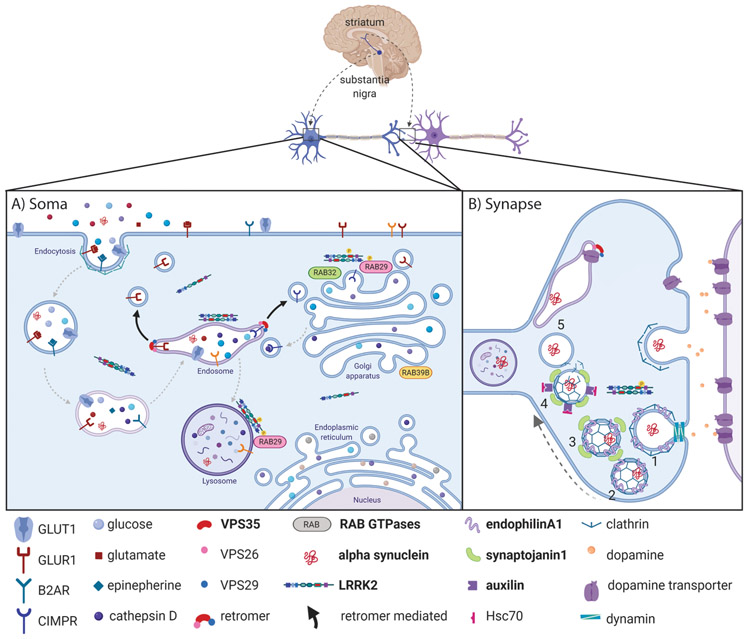
Figure 1:
PD-related proteins (bolded) in endolysosomal sorting pathways. A) In the neuronal soma ligands, receptors and transporters are taken up via endocytosis at the plasma membrane. As endosomes mature, lysosomal hydrolases, including cathepsin D, are delivered to the late endosome and lysosome from the trans-Golgi (TGN) as vesicles become more acidic (indicated by deepening purple). The retromer associates with early endosomes to identify cargo for retrograde transport (bold arrows) to the TGN or plasma membrane. Cargo that fail to be retrieved from endosomes accumulate and eventually undergo degradation in lysosomes. LRRK2 is cytoplasmic but is enriched upon different vesicular membranes in a dimeric, active conformation. Certain Rab GTPases are phosphorylated by LRRK2 with Rab29 regulating LRRK2 recruitment to the TGN and stressed lysosomes. PD-linked Rab32 and Rab39b may play a role within the Golgi complex. B) At the synapse, PD-associated proteins are responsible for clathrin-mediated synaptic vesicle endocytosis. 1) EndophilinA1 regulates membrane curvature and recruits dynamin-1 for constriction of plasma membrane buds and 2) eventual fission of clathrin-coated vesicles (CCV). 3) Synaptojanin-1 is recruited by endophilinA1 to dephosphorylate membrane lipids and release adaptor proteins (not shown), allowing auxilin to bind. 4) Auxilin and its cofactor Hsc70 remove the clathrin coat to produce synaptic vesicles that can fuse with endosomes or be loaded with neurotransmitters. Endocytosis represents one potential route for intake of misfolded or aggregated α-synuclein. Created using BioRender.com.
For retrieval and recycling from the endosome, cargo must be recognized and sorted to other compartments. Recognition of cargo in the endosomal membrane is performed by three main complexes, including the retromer, retriever and ESCRT (endosomal sorting complex required for transport) (Seaman, 2012, McNally and Cullen, 2018, Vietri et al., 2020). Briefly, both retromer and retriever select cargo from early and recycling endosomes for retrograde transport, subsequently preventing cargo from undergoing lysosomal degradation. Although retromer and retriever are functionally and structurally similar (i.e. heterotrimers that include a VPS29 subunit), each complex recognizes a specific set of protein cargo for recycling (Seaman, 2012, McNally and Cullen, 2018, McNally et al., 2017). In contrast, the ESCRT complex is less cargo-selective and instead gathers ubiquitinated proteins upon late endosomes for inclusion into intra-luminal vesicles (ILV). Cargo-enriched ILVs are contained in multivesicular bodies for future degradation by the lysosome (Wollert and Hurley, 2010, Christ et al., 2017). Here, we focus on VPS35 and the retromer due to their importance to endosomal sorting and familial PD (Williams et al., 2017).
Vacuolar protein sorting-associated protein 35 (VPS35):
Retromer is a heteropentameric complex responsible for selecting cargo in the early endosome for retrograde transport to the plasma membrane or TGN. VPS35, VPS26, and VPS29 form the cargo-selective complex (CSC) which is required for the identification of cargo for recycling (Seaman, 2012). VPS35 is the largest component of the CSC consisting of an α-solenoid that makes up a central backbone that binds to VPS26 at its N-terminus and VPS29 at its C-terminus (Hierro et al., 2007, Kovtun et al., 2018). The CSC trimer associates with a sorting nexin (SNX) dimer, consisting of either SNX1 or SNX2 and SNX5 or SNX6, to form a pentamer (Cullen and Korswagen, 2011). Although the CSC is required for cargo recognition, it is unable to associate with the endosomal membrane on its own and requires membrane recruitment via the SNX dimer and Rab7A (Seaman, 2012). A proportion of the retromer associates with FAM21, a component of the pentameric Wiscott-Aldrich syndrome protein and SCAR homolog (WASH) complex, to recruit WASH to the endosomal membrane where it mediates F-actin polymerization and the formation of patches required for the partitioning of cargo into endosomal tubules for sorting (Seaman et al., 2013, Seaman and Freeman, 2014).
One classical retromer cargo is the cation-independent mannose-6 phosphate receptor (CIMPR) that binds newly synthesized acid hydrolases in the TGN, including cathepsin D, for transport to the maturing endolysosome (Arighi et al., 2004, Seaman, 2004). Note that the mechanism involved in sorting proteins to the endosome does not involve the retromer. As the endolysosome matures and acidifies, the cathepsin D ligand is released into the lumen. CIMPR is subsequently identified by the retromer and recycled to the TGN for reuse. Similarly, cargo that undergo endocytosis at the plasma membrane often consist of receptors and ion channels, including the glucose transporter (GLUT1), AMPA receptor subunits (GluR1/R2), or the β2-adrenergic receptor, although in neurons there is also a large degree of specialized endocytosis at synapses including pre- and post-synaptic compartments (Choy et al., 2014, Steinberg et al., 2013, Temkin et al., 2011). In both locations, clathrin-coated pits form at the plasma membrane to remove receptors and transporters following ligand binding and/or activation. Following endocytosis, receptor cargo are sequestered into early endosomes where they remain until being recycled back to the cell surface through recognition and sorting by the retromer and WASH complexes, or sorted for degradation via the lysosome. These examples indicate that cargo retrieval is important for protein recycling and efficient sorting but also to maintain normal lysosomal health.
Mutations in VPS35 were first identified to cause autosomal dominant, familial PD less than a decade ago, but this and other discoveries have promoted great interest into the role of endosomal sorting in PD (Zimprich et al., 2011, Vilarino-Guell et al., 2011). Several putative mutations in VPS35 have been identified to date, but only a single missense mutation (p.Asp620Asn, D620N, c.1858G>A) has been confirmed as pathogenic based upon segregation with disease in multiple PD families (Deng et al., 2012, Kumar et al., 2012, Sharma et al., 2012, Zimprich et al., 2011, Vilarino-Guell et al., 2011, Williams et al., 2017). Familial PD due to VPS35 mutations is clinically similar to idiopathic disease with late-onset, slow progression, and a good response to levodopa therapy. Only a single VPS35-linked PD subject has been assessed at autopsy albeit incompletely without assessment of the brainstem, and therefore the neuropathological hallmarks associated with VPS35 mutations are not yet clear (Wider et al., 2008, Struhal et al., 2014). PET imaging of the caudate putamen in VPS35-linked PD subjects is consistent with degeneration of the nigrostriatal dopaminergic pathway (Wider et al., 2008, Deng et al., 2013). A dominant pattern of inheritance combined with a lack of familial mutations that produce deleterious truncations, rearrangements or exonic deletions, may suggest that heterozygous VPS35 mutations most likely cause disease through a toxic gain-of-function or dominant-negative mechanism (Williams et al., 2017). However, the pathogenic effects of the D620N mutation in different cells and models are still being investigated.
At the present time, the only known molecular defect induced by the D620N mutation is a reduced interaction of VPS35 with the WASH complex via impaired binding to FAM21 (McGough et al., 2014, Zavodszky et al., 2014). The D620N mutation does not alter the protein stability of VPS35, alter retromer assembly or distribution in cells or brain, and does not induce global defects in retromer cargo sorting including sortilin or sorLA (Tsika et al., 2014). However, altered sorting of CIMPR, AMPA receptors or ATG9A have been reported in certain cell types expressing D620N VPS35 (Follett et al., 2016, MacLeod et al., 2013, Munsie et al., 2015, Zavodszky et al., 2014). The effects of the D620N mutation on the interaction of VPS35 with other retromer cargo or accessory proteins is not yet clear, whereas it is not known whether the reduction in WASH complex recruitment contributes to cellular damage (Zavodszky et al., 2014). While D620N VPS35 retains many of the functions of its wild-type counterpart (Williams et al., 2017), there are examples where the D620N protein may act in a loss-of-function manner depending on the specific phenotypic or cellular context (Linhart et al., 2014, MacLeod et al., 2013, Zavodszky et al., 2014). Together, these studies indicate that D620N VPS35 may produce subtle functional deficits most consistent with a partial loss-of-function effect but how or whether these effects are sufficient to manifest neurodegeneration remains unknown.
Rodent models have further helped to understand the pathogenic mechanism of the D620N mutation. The viral-mediated overexpression of human D620N VPS35 within the nigrostriatal pathway of adult rats is sufficient to induce dopaminergic neurodegeneration and axonal pathology (Tsika et al., 2014), consistent with a gain-of-function or partial dominant-negative mechanism. Furthermore, the recent development of D620N VPS35 knockin mice indicate that homozygous D620N mice exhibit normal development, survival and motor function (Chen et al., 2019, Ishizu et al., 2016, Cataldi et al., 2018). This contrasts with the embryonic lethality of VPS35 knockout mice (Chen et al., 2019, Wen et al., 2011), demonstrating that the D620N VPS35 protein is largely functional and unlikely to manifest via a loss-of-function. In addition, both heterozygous and homozygous D620N VPS35 knockin mice display equivalent and progressive dopaminergic neuronal degeneration, axonal damage and somatodendritic tau pathology with advanced age (Chen et al., 2019). A recent study provides evidence for LRRK2 hyperactivation in tissues from D620N VPS35 knockin mice as indicated by increased phosphorylation of the LRRK2 substrate Rab10 as well as LRRK2 autophosphorylation (i.e. at Ser1292) (Mir et al., 2018). LRRK2 hyperactivation is equivalent between heterozygous and homozygous knockin mice (Mir et al., 2018), which together with neuropathological observations in these mice (Chen et al., 2019), support a toxic gain-of-function mechanism for the D620N mutation and exclude a loss-of-function mechanism.
In general, the loss of VPS35 expression is known to be detrimental in different cells and animal models (Williams et al., 2017), indicating a critical requirement of VPS35 function for normal cellular health and viability. Earlier studies have focused on the role of VPS35 loss-of-function in Alzheimer’s disease (AD) since vulnerable brain regions of AD subjects exhibit decreased VPS35 protein levels (Small et al., 2005, Wen et al., 2011, Muhammad et al., 2008). In PD, studies have explored a potential functional interaction between PD-linked LRRK2 and VPS35, and have shown that PD-linked LRRK2 mutants can phenocopy the effects of VPS35 depletion in Drosophila and primary cortical neuronal models (Linhart et al., 2014, MacLeod et al., 2013). Further evidence that these two proteins may operate in a common pathway is supported by observations that the overexpression of wild-type VPS35 (but not the D620N mutant) can rescue mutant LRRK2-induced neuronal phenotypes in these models (Linhart et al., 2014, MacLeod et al., 2013). Moreover, endogenous VPS35 protein is reduced by PD-linked LRRK2 mutants in cells or brains of LRRK2 transgenic mice (MacLeod et al., 2013), however, there are conflicting data concerning whether VPS35 levels are also reduced in human brains from idiopathic or LRRK2 mutant PD subjects (Tsika et al., 2014, Zhao et al., 2018). The concept that PD-linked LRRK2 mutations may exert their pathogenic effects in PD by decreasing VPS35 expression and impairing retromer function is attractive since, 1) this would indicate that LRRK2 and VPS35 interact and operate in a common cellular pathway relevant to PD that manifests in retromer deficiency, and 2) that targeting this interaction could provide a single therapeutic strategy for different familial forms of PD, and potentially be relevant to idiopathic PD as well as AD. Additional studies are now warranted to further validate this potential LRRK2/VPS35 disease mechanism, especially using rodent models of PD and human brain tissue.
Due to the relevance of VPS35 loss to AD pathophysiology (Small et al., 2005, Small and Petsko, 2015, Li et al., 2019), there have been significant efforts to develop therapeutic compounds that can boost VPS35 levels and/or stabilize the retromer (Berman et al., 2015). While such compounds have been developed with AD in mind, it is not yet clear whether this approach could also be used in PD subjects, especially those harboring VPS35 mutations. R33 and R55 are small molecule chemical chaperones that stabilize the interaction between VPS35 and VPS29 to increase retromer stability and boost its expression (Mecozzi et al., 2014, Berman et al., 2015). While these compounds are effective at increasing retromer and reducing Aβ peptide accumulation in cultured hippocampal neurons (Mecozzi et al., 2014), their pharmacokinetic properties and brain permeability for neuroprotective studies in rodent models of AD or PD are being evaluated (Li et al., 2020). While PD-linked LRRK2 mutations may drive retromer deficiency in some simple models (Linhart et al., 2014, MacLeod et al., 2013), studies in D620N VPS35 knockin rodent models do not necessarily suggest a loss-of-function mechanism (Chen et al., 2019). Therefore, the utility of retromer-stabilizing compounds for PD awaits further evaluation and mechanistic studies in PD-linked VPS35 rodent models.
The homozygous deletion of VPS35 in mice results in early embryonic lethality whereas heterozygous null (VPS35+/−) mice exhibit normal development and lifespan (Chen et al., 2019, Tang et al., 2015a, Wen et al., 2011). Therefore, VPS35 is critically required for normal embryonic development and viability. VPS35+/− mice develop chronic and progressive nigrostriatal degeneration resulting in the modest loss of dopaminergic neurons (~20% loss) by 12 months of age but without overt neuronal loss in the hippocampus (Tang et al., 2015a). Furthermore, the conditional homozygous deletion of VPS35 selectively in DAT-positive dopaminergic neurons (VPS35DAT-Cre mice) induces early dopaminergic neuronal and axonal loss (~30%) by 2-3 months of age (Tang et al., 2015b), although it is unclear whether neuronal loss is progressive in this model. These studies indicate that VPS35 depletion in mice is sufficient to produce age-dependent PD-like neuropathology, and demonstrate that VPS35 is critically required for the normal maintenance and survival of dopaminergic neurons. However, what remains uncertain is whether VPS35 deletion selectively impacts dopaminergic neurons of the substantia nigra (A9 population), versus other dopaminergic (i.e. A10 ventral tegmental area) or non-dopaminergic neuronal populations, and how these effects specifically relate to the pathogenic mechanisms induced by the PD-linked D620N mutation. Recent studies have reported the conditional deletion of VPS35 selectively in microglia or forebrain neurons of mice. Deletion of VPS35 in microglial cells (VPS35CX3CR1-CreER) results in normal survival but increased microglial density and activity in the hippocampus, increased neuronal progenitor proliferation but decreased neuronal differentiation (Appel et al., 2018). Curiously, VPS35 deletion in CamKIIα-positive forebrain neurons results in glial activation (Qureshi et al., 2019), whereas deletion in embryonic pyramidal neurons (VPS35neurod6-Cre) impairs axonal and dendritic terminal differentiation, and induces cortical atrophy and reactive gliosis (Tang et al., 2020). Together, these studies suggest that different brain cells are vulnerable to VPS35 loss, including midbrain and forebrain neurons, although selective deletion in microglia is not sufficient alone to produce PD-like pathology implying that dopaminergic neuronal loss is likely to be a cell-autonomous mechanism (Tang et al., 2015a, Tang et al., 2015b). Whether VPS35 depletion is relevant to the mechanisms underlying PD-linked mutations requires additional investigation, especially given the difference in survival between homozygous VPS35 knockout and D620N VPS35 knockin mice (Chen et al., 2019, Wen et al., 2011), and the limited evidence for reduced VPS35 in PD brains (MacLeod et al., 2013, Tsika et al., 2014, Zhao et al., 2018).
While VPS35 plays a distinct role in retromer-dependent endosomal sorting and is directly linked to familial PD, it is also known to physically or functionally interact with proteins implicated in endosomal pathways and PD pathophysiology including LRRK2, α-synuclein, tau, parkin and Rab29, which will be addressed in later sections (Chen et al., 2019, Linhart et al., 2014, MacLeod et al., 2013, Miura et al., 2014, Williams et al., 2018, Dhungel et al., 2015).
Leucine-rich repeat kinase 2 (LRRK2):
LRRK2 is reported to influence a number of cellular processes or pathways and its precise function within the endosomal system is incompletely understood. LRRK2 is enriched upon multiple intracellular membranes and vesicular compartments including early and late endosomal membranes (Biskup et al., 2006, Berger et al., 2010, Alegre-Abarrategui et al., 2009, Stafa et al., 2014). Furthermore, LRRK2 is reported to interact with a number of PD-linked gene products that can reside and/or function within the endosomal system, including VPS35, α-synuclein, auxilin/DNAJC6, synaptojanin-1 (SYNJ1), endophilinA1 (EndoA1) and Rab GTPases (i.e. Rab29, Rab32). LRRK2 can also regulate endocytosis and the sorting of receptors or cargo for recycling or lysosomal degradation in different mammalian cells (Gomez-Suaga et al., 2014, MacLeod et al., 2013, Wallings et al., 2019, Xiong et al., 2010). Together, these observations suggest LRRK2 may serve as a nexus in the endosomal system in PD, although the specific molecular mechanisms involved have not been elucidated.
Mutations in the LRRK2 gene cause late-onset, autosomal dominant PD and represent the most common cause of familial PD (Biskup and West, 2009, Healy et al., 2008, Paisan-Ruiz et al., 2004, Zimprich et al., 2004). Common genetic variation at the LRRK2 locus is also associated with increased risk for idiopathic PD (Nalls et al., 2014), indicating that LRRK2 can serve as a pleomorphic risk locus. Similar to VPS35-linked PD, familial PD due to LRRK2 mutations is clinically indistinguishable from idiopathic disease although mutations can produce pleomorphic neuropathology (Biskup and West, 2009, Healy et al., 2008, Zimprich et al., 2004). LRRK2 is a large multi-domain protein that is notable for containing two distinct enzymatic domains, including a Ras-of-Complex (Roc) GTPase domain and a serine/threonine-directed protein kinase domain (Islam and Moore, 2017). LRRK2 belongs to the ROCO protein family that is defined by containing a Roc domain in tandem with a C-terminal-of-Roc (COR) linker domain. At least seven missense mutations in LRRK2 have been identified that segregate with disease in families with PD, including N1437H, R1441C/G/H, Y1699C, G2019S, and I2020T (Hernandez et al., 2016, Islam and Moore, 2017). These pathogenic mutations cluster within the central Roc-COR-kinase catalytic domains and share the capacity to enhance LRRK2 kinase activity in cells via direct effects on the kinase activation loop (G2019S, I2020T) or indirectly by impairing GTP hydrolysis and prolonging the GTP-bound ‘on’ state (Islam and Moore, 2017, Sheng et al., 2012, Steger et al., 2017, Steger et al., 2016, West et al., 2005, West et al., 2007, Xiong et al., 2010). These observations have supported the development of LRRK2 kinase inhibitors as a potential therapeutic strategy for PD. Structurally-distinct LRRK2 kinase inhibitors can produce on-target pathology in rodents, resembling the phenotypes of LRRK2 knockout models (Baptista et al., 2013, Herzig et al., 2011, Tong et al., 2012, Tong et al., 2010), including pigmented and enlarged kidneys with lysosomal accumulation or lamellar bodies in lung pneumocytes (Andersen et al., 2018, Fuji et al., 2015). Such pathology is dose-dependent, reversible after a short washout period, and does not impact kidney or lung function following chronic inhibitor administration (Andersen et al., 2018).
Despite a general consensus that familial LRRK2 mutations increase kinase activity in different cells (Steger et al., 2017, Steger et al., 2016), the mechanisms by which LRRK2 activation induces neuronal damage are still being explored (Islam and Moore, 2017). Originally, most studies relied upon in vitro kinase assays to identify LRRK2 substrates, which include ArfGAP1, RPS15, MARK1 and β-tubulin (Gillardon, 2009, Islam and Moore, 2017, Krumova et al., 2015, Martin et al., 2014, Stafa et al., 2012, Xiong et al., 2012), in addition to LRRK2 autophosphorylation at Ser1292 (Sheng et al., 2012, Kluss et al., 2018). With the exception of pSer1292-LRRK2, many of these earlier substrates have not been extensively validated in mammalian cells or brain tissue and await the development of phospho-specific substrate antibodies. Recent proteome-wide mass spectrometry studies using embryonic fibroblast cells derived from G2019S LRRK2 knockin mice have identified at least 14 Rab GTPases as the first bona fide cellular substrates of LRRK2, including Rab8A, Rab10, Rab12 and Rab29 (Steger et al., 2017, Steger et al., 2016). Rab phosphorylation occurs on a highly conserved threonine or serine residue with the Switch II catalytic motif (i.e. Thr73 in Rab10). These Rabs are discreetly distributed across intracellular membranes within the endolysosomal system where they are thought to play roles in vesicular membrane recognition, sorting and fission/fusion events (Bonet-Ponce and Cookson, 2019). Phospho-specific Rab antibodies and PhosTag methodology have been developed as important tools for monitoring LRRK2 activity in different cells and tissues and for evaluating the efficacy of LRRK2 kinase inhibitors (Ito et al., 2016, Kluss et al., 2018, Lis et al., 2018). While increased LRRK2-dependent Rab phosphorylation has been detected in different tissues from PD-linked mutant LRRK2 knockin and transgenic rodent models (Ito et al., 2016, Lis et al., 2018, Steger et al., 2017, Steger et al., 2016), the significance of Rab phosphorylation for mediating cellular processes such as endolysosomal sorting as well as neuronal damage are still emerging. Whether Rabs represent the only or most relevant substrates of LRRK2 kinase activity, especially within brain cells, is not yet known as detection of robust Rab phosphorylation in the brain has proven challenging (Steger et al., 2016, Kelly et al., 2018). Nevertheless, roles for the LRRK2-mediated phosphorylation of certain Rabs (i.e. Rab8A, Rab10, Rab29) have recently emerged in mediating ciliogenesis and centrosomal cohesion deficits, the response to lysosomal stress, and the recruitment and activation of LRRK2 at the TGN (Liu et al., 2018, Purlyte et al., 2018, Steger et al., 2017, Dhekne et al., 2018, Eguchi et al., 2018, Gomez et al., 2019, Lara Ordonez et al., 2019, Beilina et al., 2014).
Although LRRK2 has been implicated in myriad cellular processes, the removal of LRRK2 does not clearly indicate a required function in vivo. LRRK2 knockout rodents are viable and do not exhibit PD-related neuropathology or behavioral deficits (Andres-Mateos et al., 2009, Hinkle et al., 2012, Baptista et al., 2013, Pellegrini et al., 2018, Herzig et al., 2011, Tong et al., 2010). This is consistent with the idea that familial mutations act via a gain-of-function mechanism, given that PD-linked mutations commonly enhance kinase activity and mutant LRRK2 overexpression promotes neuronal damage in primary culture models and certain transgenic or viral-mediated rodent models (Blauwendraat et al., 2018, Steger et al., 2016, West et al., 2005, West et al., 2007, Greggio et al., 2006, Smith et al., 2006, Ramonet et al., 2011, Dusonchet et al., 2011, Lee et al., 2010, Tsika et al., 2015). However, LRRK2 knockout rodents consistently develop enlarged, darkly pigmented kidneys with lysosomal accumulation, and type II pneumocytes in lung bearing lamellar bodies, though without an obvious impact on organ function (Hinkle et al., 2012, Herzig et al., 2011, Baptista et al., 2013, Ness et al., 2013, Andersen et al., 2018, Tong et al., 2012, Tong et al., 2010, Boddu et al., 2015). The accumulation of lysosomal-like vesicles in knockout rodents supports an important role for LRRK2 in regulating the endolysosomal pathway.
Further cementing a role for LRRK2 in endosomal sorting pathways is the intriguing connection with the PD-related proteins, Rab29 and VPS35. Rab29 (a.k.a. Rab7L1) is normally enriched upon the TGN where it plays a role in the recruitment of LRRK2 (Beilina et al., 2014, Liu et al., 2018, Purlyte et al., 2018). This recruitment is dependent on LRRK2 kinase activity, with increased relocalization of PD-linked LRRK2 mutants that are kinase hyperactive, and requires membrane- and GTP-bound Rab29 (Gomez et al., 2019, Liu et al., 2018). In turn, Rab29 binding to the N-terminal region of LRRK2 is required for kinase activation including Ser1292 autophosphorylation and Rab phosphorylation (Purlyte et al., 2018). Rab29 itself also serves as a substrate of LRRK2 (Liu et al., 2018, Purlyte et al., 2018). Rab29 recruitment and activation of LRRK2 to the TGN may serve to stabilize membrane and GTP-bound Rabs and regulate their interactions with effector proteins (Liu et al., 2018). This process has been implicated in the clearance of Golgi-derived vesicles via the autophagy-lysosomal system (Beilina et al., 2014). A similar Rab29-dependent mechanism has also been suggested to occur at the lysosomal membrane that recruits and activates LRRK2 in response to lysosomal stress to maintain their homeostasis via lysosomal secretion (Eguchi et al., 2018). A recent study suggests that Rab29-mediated membrane association is the most important determinant for LRRK2 recruitment and activation rather than the membrane type or subcellular location per se (Gomez et al., 2019).
Distinct from Rab29 function, VPS35 and the retromer mediate endosomal cargo recycling to the TGN (Seaman, 2012). As discussed earlier, familial LRRK2 mutants are reported to induce a retromer deficiency by interacting with and decreasing VPS35 protein levels in cell lines or brains of R1441C LRRK2 transgenic mice via an unknown mechanism (MacLeod et al., 2013). G2019S LRRK2 can disrupt endosomal sorting of the retromer cargo CIMPR in primary neurons, and this can be rescued by VPS35 overexpression (MacLeod et al., 2013). Similarly, VPS35 overexpression can rescue the pathogenic effects of G2019S LRRK2 on neurite outgrowth in primary cultures and dopaminergic neuronal loss in Drosophila (MacLeod et al., 2013). In Drosophila models, VPS35 or VPS26 overexpression can rescue eye phenotypes, reduced lifespan and locomotor deficits induced by I2020T LRRK2, whereas VPS35 gene silencing can phenocopy I2020T LRRK2 in flies (Linhart et al., 2014). These observations support the idea that PD-linked LRRK2 mutations and VPS35 loss-of-function may act through similar mechanisms. Lastly, PD brains harboring G2019S LRRK2 mutations reveal decreased VPS35 protein levels in frontal and occipital cortex relative to idiopathic PD or control brains (Zhao et al., 2018), although an earlier study found no difference in VPS35 levels in frontal cortex of G2019S PD brains (Tsika et al., 2014). These studies provide initial support for a functional interaction of LRRK2 and VPS35 in vivo and further suggest that PD-linked LRRK2 mutations may exert their pathogenic effects via retromer disruption. Additional validation of this mechanism is now required in rodent models of PD, potentially including neuroprotection studies in mutant LRRK2 models with retromer-stabilizing chemical chaperones (i.e. R33/R55) (Mecozzi et al., 2014).
Further complicating our understanding of a LRRK2/VPS35 molecular pathway is the recent observation that D620N VPS35 knockin mice unexpectedly display robust LRRK2 hyperactivation in cells and tissues as indicated by increased Rab phosphorylation (Rab8a, Rab10, Rab12) and LRRK2 Ser1292 autophosphorylation (Mir et al., 2018). Notably, LRRK2 activation in these D620N VPS35 mice is greater (~6-fold) than that produced in R1441C or G2019S LRRK2 knockin mice (~4- or 2-fold, respectively), and surprisingly endogenous VPS35 is even required in part for the increased LRRK2 kinase activity in R1441C LRRK2 mice (Mir et al., 2018). Rab phosphorylation in VPS35 knockin mice was shown to be dependent on LRRK2 using the potent and selective kinase inhibitor MLi-2 (Mir et al., 2018). LRRK2 hyperactivation could also be detected in human primary neutrophils and monocytes derived from PD subjects harboring a heterozygous D620N VPS35 mutation (Mir et al., 2018). This new study supports a previously unappreciated role for the retromer in critically regulating LRRK2 activity and supports a putative model where VPS35 may lie upstream of LRRK2. The mechanism by which D620N VPS35 activates LRRK2 is not yet known, and whether this occurs through a direct interaction, via an interaction with an intermediate regulatory protein or complex, and/or by altering LRRK2 membrane occupancy or subcellular localization, awaits further study. Strategies to correct the pathogenic effects associated with the D620N VPS35 protein or LRRK2 kinase inhibition may provide therapeutic opportunities for treating both VPS35- and LRRK2-linked familial PD. It will be important in future studies to confirm and reconcile the observations that familial LRRK2 mutations can induce retromer deficiency whereas VPS35 mutations can induce LRRK2 activation (MacLeod et al., 2013, Mir et al., 2018), although both potential mechanisms are not necessarily mutually exclusive. Also, it will be important to address how the LRRK2/VPS35 pathway impacts upon specific endolysosomal functions and its relevance to disease pathogenesis.
SNCA / α-synuclein:
Missense mutations and multiplications of the SNCA gene encoding the α-synuclein protein cause autosomal dominant PD (Polymeropoulos et al., 1997, Kruger et al., 1998, Singleton et al., 2003, Chartier-Harlin et al., 2004, Zarranz et al., 2004, Appel-Cresswell et al., 2013, Lesage et al., 2013). α-Synuclein fibrils are a major component of Lewy bodies and neurites, one of the pathological hallmarks of PD (Spillantini et al., 1997, Spillantini et al., 1998). GWAS have identified common variation at the SNCA locus as a risk factor for idiopathic PD (Simon-Sanchez et al., 2009, Zhang et al., 2018, Nalls et al., 2014), indicating that SNCA serves as a pleomorphic risk gene similar to LRRK2. α-Synuclein mutations were first linked to familial PD in 1997 (Polymeropoulos et al., 1997), making α-synuclein the most widely studied PD-related protein, yet the primary function of α-synuclein and how it contributes to the molecular pathogenesis of PD is still a matter of some debate. While the process of α-synuclein fibrillization and aggregation into Lewy pathology, and the propagation of α-synuclein species between cells, are intrinsically linked to disease (Burre et al., 2018), interference with these events in humans is eagerly awaited to confirm their importance as key pathogenic mechanisms (McFarthing and Simuni, 2019).
α-Synuclein is a small 140 residue protein lacking enzymatic domains that is enriched at presynaptic nerve terminals and interacts with proteins involved in the synaptic vesicle machinery suggesting a role in synaptic vesicle trafficking (Burre et al., 2018, Burre, 2015). Overexpression of α-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis leading to a reduction in the synaptic vesicle recycling pool (Nemani et al., 2010), whereas α-synuclein knockout mice have limited alterations in synaptic transmission (Abeliovich et al., 2000). Multimeric forms of α-synuclein have been reported to assemble upon membranes where they can act as a SNARE complex chaperone at the presynaptic terminal (Burre et al., 2014). Familial PD-linked mutations in α-synuclein result in an increased propensity to aggregate into oligomeric and/or fibrillar species that promote neurotoxicity (Burre et al., 2018). Therefore, in terms of endosomal sorting, α-synuclein aggregation impairs its normal endocytic function at synapses while aggregates themselves can serve as endocytic cargo and in turn interfere with endosomal sorting (Burre, 2015). α-Synuclein species can propagate from neuron-to-neuron in a “prion-like” manner in experimental models, and this process is reported to involve both exocytosis and endocytosis (Ma et al., 2019).
Intriguingly, recent studies have linked α-synuclein-induced neuronal degeneration to both LRRK2 and VPS35. The genetic deletion or pharmacological kinase inhibition of LRRK2 was shown to rescue dopaminergic neuronal loss induced by the AAV-mediated delivery of human α-synuclein in rats (Daher et al., 2015, Daher et al., 2014). These studies suggest that endogenous LRRK2 is critical for mediating the pathogenic effects of α-synuclein in this model. It is less clear whether endogenous LRRK2 plays a similar role in α-synuclein pathology induced by preformed fibrils in rodents, although G2019S LRRK2 overexpression is reported to worsen α-synuclein pathology and propagation in these models (Bieri et al., 2019, Henderson et al., 2019, Volpicelli-Daley et al., 2016). VPS35 is also reported to genetically interact with α-synuclein where VPS35 deletion in yeast and C.elegans models exacerbates the toxic effects of human α-synuclein (Dhungel et al., 2015). In addition, the viral-mediated overexpression of wild-type VPS35 was shown to attenuate hippocampal neuronal loss, gliosis and α-synuclein pathology that develop in a well-established transgenic mouse model expressing human wild-type α-synuclein (Dhungel et al., 2015). These observations suggest a potentially critical role for VPS35 and the retromer in mediating α-synuclein pathology and neurotoxicity, although it is not yet clear whether α-synuclein-induced toxicity manifests via a retromer deficiency similar to the effects of familial LRRK2 mutations. It is of interest that D620N VPS35 knockin mice do not develop α-synuclein pathology within their lifespan, but instead develop somatodendritic tau pathology (Chen et al., 2019), and it will be important to determine whether Lewy pathology is a feature of VPS35-linked PD brains (Wider et al., 2008). Furthermore, the D620N VPS35 mutation is not sufficient to alter the lethal neurodegenerative phenotype that develops in human A53T α-synuclein transgenic mice (Chen et al., 2019). These studies suggest that the interaction between α-synuclein and VPS35 is likely complex and/or context-dependent and now awaits additional studies further clarifying the nature of their interaction. In addition to interactions within the endosomal pathway, α-synuclein is also reported to be selectively translocated into lysosomes for degradation by the chaperone-mediated autophagy (CMA) pathway (Cuervo et al., 2004). Aggregation-prone mutant α-synuclein can bind to the LAMP2a receptor on lysosomal membranes and serve to block uptake by CMA and inhibit degradation (Cuervo et al., 2004). LRRK2 is also reported to inhibit CMA by interfering with the LAMP2a translocation complex (Orenstein et al., 2013). Together, these studies suggest roles for α-synuclein in endolysosomal pathways, including interactions with PD-linked LRRK2 and VPS35, and such pathways are important for mediating the pathogenic effects of α-synuclein aggregates.
Emerging endosomal evidence in PD: synaptic vesicle endocytosis and Rab GTPases
Synaptic vesicle endocytosis (SVE):
Synaptic vesicle endocytosis (SVE) plays an important role in the uptake of clathrin-coated vesicles at the presynaptic terminal for delivery to the endosomal system (Dittman and Ryan, 2009, Saheki and De Camilli, 2012). In neurons, this is critical for recycling neurotransmitter receptors and other proteins required for synaptic transmission, for example the dopamine receptor. After SVE these proteins are sorted and trafficked by the endosomal system in a process identical to that in the soma (Figure 1). First, endophilin is recruited to the plasma membrane by adaptor proteins to regulate membrane curvature and for recruitment of the membrane scission protein, dynamin. Once vesicle fission occurs, endophilin recruits the dual lipid phosphatase synaptojanin-1 to clathrin-coated vesicles (CCVs) to dephosphorylate membrane lipids (i.e. phosphoinositides), which serves to facilitate the release of adaptor proteins and allows auxilin/Hsc70 to bind to CCVs and mediate clathrin uncoating. Notably, a number of PD-linked mutations have recently been identified in genes involved in SVE and endosomal sorting, including auxilin (DNAJC6) and synaptojanin-1 (SYNJ1), whereas cyclin-G-associated kinase (GAK) and endophilinA1 (EndoA1) have been nominated as PD risk loci by GWAS (Table 1) (Nguyen et al., 2019).
Auxilin/DNAJC6:
Mutations in the DNAJC6 gene encoding auxilin were first discovered in two Palestinian brothers resulting in juvenile-onset, autosomal recessive PD (Edvardson et al., 2012). Additional mutations have since been identified in other PD families that cause mRNA splicing defects or exonic deletions that consequently decrease auxilin protein levels and act via a loss-of-function mechanism. While PD patients with DNAJC6 mutations display cardinal motor symptoms, some also develop epilepsy and intellectual disability (Koroglu et al., 2013, Olgiati et al., 2016). The phenotypic variation indicates widespread neurological problems beyond the nigrostriatal pathway indicating that DNAJC6 mutations can manifest atypical forms of PD.
Auxilin is part of the evolutionarily conserved DNAJ/Hsp40 family that stimulates the ATPase activity of Hsc70 to mediate the uncoating of CCVs following endocytosis (Saheki and De Camilli, 2012). Clathrin coat removal is required for vesicles to fuse and enter the endosomal pathway for cargo sorting. Auxilin knockout models display defects in clathrin-mediated endocytosis, including the accumulation of CCVs and empty clathrin cages, and exhibit dopaminergic neurodegeneration (Yim et al., 2010, Song et al., 2017, Hirst et al., 2008). Auxilin reduction in Drosophila results in climbing deficits, reduced lifespan, and dopaminergic neurodegeneration that phenocopies, and is exacerbated by, α-synuclein overexpression (Song et al., 2017). Interestingly, auxilin knockout mice display a 3-fold increase in the auxilin homolog GAK, that also serves as a co-chaperone for Hsc70 in clathrin uncoating (Yim et al., 2010). Auxilin is further tied to PD pathogenesis via its interaction with LRRK2. Auxilin can be phosphorylated by LRRK2 at Ser627 that results in differential binding to clathrin and impaired SVE (Nguyen and Krainc, 2018). This results in the accumulation of oxidized dopamine in iPSC-derived dopaminergic neurons from PD subjects harboring LRRK2 R1441C, R1441G or G2019S mutations (Nguyen and Krainc, 2018). Auxilin R857G knockin mice have recently been developed as a model of PD that develop motor deficits, impaired synaptic recycling, dystrophic Golgi morphology and lipid accumulation (Roosen DA, 2019). Similar to the effects of LRRK2 phosphorylation, PD-linked mutations in auxilin selectively impair the interaction with clathrin but not clathrin adaptor proteins (Roosen DA, 2019).
Cyclin-G-associated kinase (GAK):
GAK is an auxilin homolog and performs similar functions in SVE, including recruitment of Hsc70 to mediate clathrin uncoating. In contrast to auxilin, which is primarily expressed in neurons, GAK is ubiquitously expressed and its deletion selectively in the brain or germline is lethal (Lee et al., 2008). In auxilin knockout mice, GAK can compensate for auxilin in some capacities indicating some functional redundancy (Yim et al., 2010), but auxilin does not appear to be sufficient to rescue GAK knockout in vivo. GAK has gained attention in PD since common variation at the GAK/TMEM175 locus has been linked to increased PD risk by GWAS (Nalls et al., 2014, Yu et al., 2015, Nalls et al., 2019). GAK can form a functional complex with LRRK2 and Rab29 in cells and mouse brain, that participates in the clearance of Golgi-derived vesicles (Beilina et al., 2014). Carriers of PD risk variants at the GAK locus are associated with the increased expression of α-synuclein in the frontal cortex (Dumitriu et al., 2011). Although GAK represents an interesting and compelling risk gene for PD directly involved in the endosomal system, it is not clear whether PD risk variants at this locus impact the expression of GAK, TMEM175 or both genes (Nalls et al., 2019, Nalls et al., 2014). TMEM175 encodes a lysosomal potassium pump that would also fit with pathogenic mechanisms implicated in PD (Jinn et al., 2019, Krohn et al., 2020). Future studies of risk-associated variants will attempt to understand how the GAK/TMEM175 locus contributes to PD.
Synaptojanin-1 (SYNJ1):
Similar to auxilin, SYNJ1 mutations cause early-onset, autosomal recessive Parkinsonism with some cases also displaying seizures or other atypical symptoms (Krebs et al., 2013, Quadri et al., 2013, Chen et al., 2015, Kirola et al., 2016). Synaptojanin-1 is a lipid phosphatase that dephosphorylates membrane lipids (i.e. phosphatidylinositol-4,5-bisphosphate) to initiate the release of adaptor proteins from CCVs (Drouet and Lesage, 2014). Once adaptor proteins are removed from CCVs, auxilin/Hsc70 can bind and mediate clathrin uncoating. Mutations in SYNJ1 (i.e. R258Q, R459P) are rare with those located in the Sac 1 domain leading to decreased phosphatase activity (Drouet and Lesage, 2014). Although synaptojanin-1 and auxilin have different functions, their sequential roles in SVE may explain why knockout of either gene causes similar phenotypes. Both SYNJ1 and auxilin knockout mice similarly display neurological deficits, motor abnormalities and accumulate CCVs at the synaptic terminal (Yim et al., 2010, Cremona et al., 1999, Kim et al., 2002). R258Q SYNJ1 knockin mice also display SVE deficits, the accumulation of clathrin-coated intermediates, a compensatory elevation of auxilin, parkin and endoA1 proteins, and dystrophic dopaminergic axonal terminals (Cao et al., 2017). Alternatively, Drosophila models harboring an R258Q SYNJ1 knockin display normal synaptic vesicle cycling but instead exhibit deficits in synaptic autophagosome maturation and modest dopaminergic neuronal loss (Vanhauwaert et al., 2017), indicating that SYNJ1 mutations may impact both endocytosis and autophagy pathways. Synaptojanin-1 was first identified by phospho-proteome profiling as a LRRK2 substrate in Drosophila brain and human synaptojanin-1 can be phosphorylated by LRRK2 at Thr1173 in vitro (Islam et al., 2016). Functionally, synaptojanin-1 phosphorylation by LRRK2 at a second site (Thr1205) was reported to disrupt its interaction with endoA1 that is required for SVE (Pan et al., 2017). Furthermore, SYNJ1 haploinsufficiency in G2019S LRRK2 transgenic mice impaired sustained exocytosis in midbrain neurons and caused mild alterations in motor function (Pan et al., 2017). Therefore, similar to auxilin, synaptojanin-1 interactions and function can also be modulated by LRRK2. Auxilin and SYNJ1 further highlight the importance of SVE and the endosomal pathway in the development of early-onset PD, whereas the interaction with LRRK2 also links both SVE proteins to typical late-onset PD.
EndophilinA1 (EndoA1):
Common variation at the SH3GL2 locus that encodes endoA1 has been identified as a risk factor for idiopathic PD by GWAS (Chang et al., 2017). Prior to this, endoA1 has long been of interest in PD due to its proximity to other PD-linked proteins including synaptojanin-1, LRRK2, and parkin (Islam et al., 2016, Trempe et al., 2009, Matta et al., 2012). In SVE, endoA1 plays an intermediary role between initial vesicle invagination and dynamin-dependent scission, and the recruitment of synaptojanin-1 and auxilin to initiate CCV uncoating (Figure 1). Similar to auxilin and synaptojanin-1, endoA1 can also be phosphorylated by LRRK2 at Ser75, which alters its association with and the tubulation of vesicle membranes that result in SVE deficits in Drosophila (Matta et al., 2012, Ambroso et al., 2014). The connection of endoA1 to PD further supports the concept that multiple steps in the SVE pathway are important to the pathophysiological mechanisms underlying PD.
Rab GTPases:
The role of Rab GTPases in PD has gained recent interest following their identification as LRRK2 kinase substrates, human genetic evidence linking certain Rabs to PD, and due to their roles in the endolysosomal pathway (Bonet-Ponce and Cookson, 2019). Mutations in RAB32 and RAB39B have recently been identified to cause familial PD whereas common variation at or near the RAB29 (PARK16) locus may contribute to the risk of idiopathic PD. There is also emerging evidence that these Rabs may functionally interact with other PD-linked gene products, namely LRRK2, VPS35 or α-synuclein. Herein, we briefly summarize evidence for a role of Rab GTPases in PD pathophysiology, but for a more thorough review please refer to (Gao et al., 2018, Bonet-Ponce and Cookson, 2019).
Rab39B:
The function of Rab39B is largely uncharacterized, although it is enriched in the brain and highly expressed in neurons, and localizes to the secretory network including the endoplasmic reticulum and cis-Golgi interface, the TGN and recycling endosomes (Giannandrea et al., 2010, Gambarte Tudela et al., 2019). Mutations in RAB39B were first identified to cause early-onset, X-linked dominant PD in Australian and Wisconsin kindreds due to a ~45 kb deletion encompassing the entire RAB39B gene or a T168K mutation, respectively (Wilson et al., 2014). Although affected family members exhibit typical PD symptoms and Lewy pathology, they also develop intellectual disability. These findings were later confirmed through the identification of a nonsense mutation (Trp186Stop) in RAB39B in a French subject, and a missense mutation (G192R) in seven affected members of a US family with PD (Lesage et al., 2015, Mata et al., 2015). While most mutations decrease Rab39B levels due to reduced protein stability, gene deletions or truncations (Gao et al., 2020), the G192R mutation induces Rab39B mislocalization in cells (Mata et al., 2015). PD-linked mutations in Rab39B therefore manifest disease through a loss-of-function mechanism. Rab39B gene silencing in cultured primary neurons decreased neurite branching and the number of growth cones (Giannandrea et al., 2010), and surprisingly reduced the steady-state levels of α-synuclein and the density of α-synuclein-positive puncta in dendritic processes (Wilson et al., 2014). These findings suggest that Rab39B depletion can dysregulate α-synuclein homeostasis, and this effect now awaits confirmation in rodent models with Rab39B deletion.
Rab32:
A missense mutation (S71R) in RAB32 has been suggested to cause familial PD (Gustavsson E, 2017). Rab32 is also connected to PD via its direct interaction with the N-terminal armadillo domain of LRRK2, where they colocalize upon recycling endosomes and transport vesicles (Waschbusch et al., 2014, McGrath et al., 2019). The putative PD-linked S71R mutation in Rab32 disrupts a highly conserved residue within the catalytic Switch II region, that in related Rabs is known to serve as a robust site of phosphorylation by LRRK2 (Steger et al., 2016). Rab32 was recently shown to interact with SNX6, one of the components of the retromer-associated SNX dimer, to influence retromer-dependent sorting of CIMPR to the TGN (Waschbusch et al., 2019). Rab32 therefore provides an interesting new link to PD that may cooperate with LRRK2 and the retromer to regulate endosomal sorting.
Rab29 (Rab7L1):
Common variation at the PARK16 locus is associated with an increased risk for idiopathic PD (Satake et al., 2009, Simon-Sanchez et al., 2009, Chang et al., 2017, Nalls et al., 2014). Although five genes are nominated within the PARK16 locus, RAB29 is of special interest due to its genetic and functional interaction with LRRK2 and its known role in endosomal sorting (Beilina et al., 2014, Liu et al., 2018, MacLeod et al., 2013, Purlyte et al., 2018). Genetic studies initially suggested that PD risk variants at the PARK16 and LRRK2 loci are highly interrelated in terms of their transcriptional impact in brain and risk associations (MacLeod et al., 2013). RAB29 was nominated as the critical risk gene at the PARK16 locus with PD risk variants suggested to alter mRNA splicing and lower Rab29 expression (MacLeod et al., 2013). Accordingly, Rab29 silencing in cultured primary neurons was shown to phenocopy the effects of G2019S LRRK2 on inhibition of neurite outgrowth, the accumulation of enlarged lysosomes, and altered sorting of CIMPR (MacLeod et al., 2013, Wang et al., 2014). Rab29 overexpression could oppositely rescue these G2019S LRRK2-induced phenotypes in neurons (MacLeod et al., 2013). Similarly, in Drosophila, G2019S LRRK2 overexpression and silencing of the Rab29 ortholog Lightoid produce similar phenotypes, including reduced survival and dopaminergic neuronal loss, whereas Rab29 overexpression can rescue the effects of G2019S LRRK2 in flies (MacLeod et al., 2013). Lastly, mice lacking LRRK2, RAB29 or both genes exhibit similar phenotypes including enlarged and pigmented kidneys and the accumulation of lysosome-related pathology in kidneys and lungs (Kuwahara et al., 2016). These phenotypes were slightly less severe in RAB29 knockout animals but were not additive in the LRRK2/RAB29 double knockout mice, suggesting that LRRK2 and Rab29 may function in the same pathway (Kuwahara et al., 2016).
In agreement with a common LRRK2/Rab29 pathway, Rab29 is now known to interact with and recruit LRRK2 to the TGN where it becomes phosphorylated, a process that is enhanced by PD-linked LRRK2 mutations and requires membrane- and GTP-bound Rab29 (Beilina et al., 2014, Liu et al., 2018, MacLeod et al., 2013). The Rab29 interaction with LRRK2 stimulates its kinase activity including LRRK2 autophosphorylation and Rab phosphorylation (i.e. Rab8A, Rab10) (Liu et al., 2018, Purlyte et al., 2018). Rab29 therefore serves as a master regulator of LRRK2 activity and localization in cells (Purlyte et al., 2018). Rab29 and LRRK2 function together in the endolysosomal pathway that may be important for understanding the pathophysiological mechanisms driving PD.
Conclusion and future directions
Substantial human genetic and experimental evidence now points to endosomal sorting pathways as important to the pathophysiology of PD (Abeliovich and Gitler, 2016, Bandres-Ciga et al., 2019, Gao et al., 2018, Shahmoradian et al., 2019, Bonet-Ponce and Cookson, 2019). As discussed, LRRK2 may function as a nexus within these pathways, where it can influence many distinct functions of endosomal sorting via protein interactions with the key endosomal machinery and phosphorylation of Rabs that decorate these intracellular vesicles and membranes (Islam et al., 2016, Matta et al., 2012, Nguyen and Krainc, 2018, Steger et al., 2017, Steger et al., 2016). LRRK2 may also regulate α-synuclein-induced neurotoxicity (Bieri et al., 2019, Daher et al., 2014, Daniel et al., 2015, Volpicelli-Daley et al., 2016), potentially via the convergence of these proteins upon lysosomal function (Cuervo et al., 2004, Orenstein et al., 2013), and may regulate VPS35 levels and retromer function (MacLeod et al., 2013). LRRK2 activity can in turn be regulated by these sorting factors, with Rab29 serving as a master regulator to recruit and activate LRRK2 at the TGN and potentially lysosomes (Beilina et al., 2014, Eguchi et al., 2018, Liu et al., 2018, Purlyte et al., 2018), and PD-linked mutations in VPS35 leading to LRRK2 hyperactivation (Mir et al., 2018). It is unclear at this juncture how these functional interactions will translate into identifying new therapeutic targets and strategies, since endosomal sorting pathways are dynamic, multifaceted and increasingly complex . It may not be sufficient to simply improve bulk output, such as by improving lysosomal function, as many cargo require sorting and recycling from the endosome to other compartments to prevent their inappropriate lysosomal degradation. Such a strategy may be appropriate for the removal of neurotoxic proteins, such as oligomeric or aggregated α-synuclein, as suggested by studies that improve CMA by LAMP2a overexpression (Xilouri et al., 2013). Restoring or improving the function of single endosomal protein components, such as auxilin, synaptojanin-1 or endoA1, may not lead to general improvements in endosomal function.
What is perhaps needed is a key focus on the consequences of disease-causing mutations in PD-linked gene products that are likely to impact endosomal function more broadly. For example, in the case of LRRK2, this could generally involve inhibiting or normalizing overactive kinase activity, or interfering with key downstream kinase substrates (i.e. Rab8A or Rab10) or master regulators of LRRK2 activity (i.e. Rab29) (Liu et al., 2018, Purlyte et al., 2018, Steger et al., 2017, Steger et al., 2016). Additional general strategies might include inhibition of LRRK2 activity via modulation of GTPase activity, disrupting dimerization or downregulation by antisense oligonucleotides (Nguyen and Moore, 2017). Likewise, approaches for normalizing VPS35 and retromer function might include retromer-stabilizing chemical chaperones (i.e. R33/R55), improving retromer membrane recruitment (by inhibition of TBC1D5 that leads to enhanced GTP- and membrane-bound Rab7A) or by restoration of specific retromer cargo depletion or accumulation (Follett et al., 2016, Mecozzi et al., 2014, Seaman et al., 2018, Zavodszky et al., 2014). Some of these retromer-targeted approaches may also serve to attenuate LRRK2 kinase activity (Mir et al., 2018). Much will now depend on establishing whether PD-linked VPS35 mutations act via a loss- or gain-of-function mechanism to induce neurodegeneration in PD (Chen et al., 2019, Williams et al., 2017). An improved molecular and cellular understanding of how PD-linked gene products regulate endosomal sorting pathways in different brain and peripheral cells, and the functional interactions or points of convergence between these proteins, will prove pivotal in the design of effective therapeutics and for biomarker development for PD. Endosomal sorting pathways are likely to provide a critical point of convergence for understanding the pathophysiological mechanisms driving familial and sporadic PD.
Acknowledgments
The authors work is supported by funding from the National Institutes of Health (R01 NS091719, R01 NS105432, R21 AG058241), Michael J. Fox Foundation for Parkinson’s Research, and the Van Andel Institute.
REFERENCES
- ABELIOVICH A & GITLER AD 2016. Defects in trafficking bridge Parkinson's disease pathology and genetics. Nature, 539, 207–216. [DOI] [PubMed] [Google Scholar]
- ABELIOVICH A, SCHMITZ Y, FARINAS I, CHOI-LUNDBERG D, HO WH, CASTILLO PE, SHINSKY N, VERDUGO JM, ARMANINI M, RYAN A, HYNES M, PHILLIPS H, SULZER D & ROSENTHAL A 2000. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron, 25, 239–52. [DOI] [PubMed] [Google Scholar]
- ALEGRE-ABARRATEGUI J, CHRISTIAN H, LUFINO MM, MUTIHAC R, VENDA LL, ANSORGE O & WADE-MARTINS R 2009. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet, 18, 4022–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMBROSO MR, HEGDE BG & LANGEN R 2014. Endophilin A1 induces different membrane shapes using a conformational switch that is regulated by phosphorylation. Proc Natl Acad Sci U S A, 111, 6982–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSEN MA, WEGENER KM, LARSEN S, BADOLO L, SMITH GP, JEGGO R, JENSEN PH, SOTTY F, CHRISTENSEN KV & THOUGAARD A 2018. PFE-360-induced LRRK2 inhibition induces reversible, non-adverse renal changes in rats. Toxicology, 395, 15–22. [DOI] [PubMed] [Google Scholar]
- ANDRES-MATEOS E, MEJIAS R, SASAKI M, LI X, LIN BM, BISKUP S, ZHANG L, BANERJEE R, THOMAS B, YANG L, LIU G, BEAL MF, HUSO DL, DAWSON TM & DAWSON VL 2009. Unexpected lack of hypersensitivity in LRRK2 knock-out mice to MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). J Neurosci, 29, 15846–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APPEL-CRESSWELL S, VILARINO-GUELL C, ENCARNACION M, SHERMAN H, YU I, SHAH B, WEIR D, THOMPSON C, SZU-TU C, TRINH J, AASLY JO, RAJPUT A, RAJPUT AH, JON STOESSL A & FARRER MJ 2013. Alpha-synuclein p.H50Q, a novel pathogenic mutation for Parkinson's disease. Mov Disord, 28, 811–3. [DOI] [PubMed] [Google Scholar]
- APPEL JR, YE S, TANG F, SUN D, ZHANG H, MEI L & XIONG WC 2018. Increased Microglial Activity, Impaired Adult Hippocampal Neurogenesis, and Depressive-like Behavior in Microglial VPS35-Depleted Mice. J Neurosci, 38, 5949–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARIGHI CN, HARTNELL LM, AGUILAR RC, HAFT CR & BONIFACINO JS 2004. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol, 165, 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANDRES-CIGA S, SAEZ-ATIENZAR S, BONET-PONCE L, BILLINGSLEY K, VITALE D, BLAUWENDRAAT C, GIBBS JR, PIHLSTROM L, GAN-OR Z, COOKSON MR, NALLS MA & SINGLETON AB 2019. The endocytic membrane trafficking pathway plays a major role in the risk of Parkinson's disease. Mov Disord, 34, 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAPTISTA MA, DAVE KD, FRASIER MA, SHERER TB, GREELEY M, BECK MJ, VARSHO JS, PARKER GA, MOORE C, CHURCHILL MJ, MESHUL CK & FISKE BK 2013. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS One, 8, e80705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEILINA A, RUDENKO IN, KAGANOVICH A, CIVIERO L, CHAU H, KALIA SK, KALIA LV, LOBBESTAEL E, CHIA R, NDUKWE K, DING J, NALLS MA, INTERNATIONAL PARKINSON'S DISEASE GENOMICS C, NORTH AMERICAN BRAIN EXPRESSION C, OLSZEWSKI, HAUSER DN, KUMARAN R, LOZANO AM, BAEKELANDT V, GREENE LE, TAYMANS JM, GREGGIO E & COOKSON MR 2014. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci U S A, 111, 2626–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER Z, SMITH KA & LAVOIE MJ 2010. Membrane localization of LRRK2 is associated with increased formation of the highly active LRRK2 dimer and changes in its phosphorylation. Biochemistry, 49, 5511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERMAN DE, RINGE D, PETSKO GA & SMALL SA 2015. The use of pharmacological retromer chaperones in Alzheimer's disease and other endosomal-related disorders. Neurotherapeutics, 12, 12–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIERI G, BRAHIC M, BOUSSET L, COUTHOUIS J, KRAMER NJ, MA R, NAKAYAMA L, MONBUREAU M, DEFENSOR E, SCHULE B, SHAMLOO M, MELKI R & GITLER AD 2019. LRRK2 modifies alpha-syn pathology and spread in mouse models and human neurons. Acta Neuropathol, 137, 961–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISKUP S, MOORE DJ, CELSI F, HIGASHI S, WEST AB, ANDRABI SA, KURKINEN K, YU SW, SAVITT JM, WALDVOGEL HJ, FAULL RL, EMSON PC, TORP R, OTTERSEN OP, DAWSON TM & DAWSON VL 2006. Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol, 60, 557–69. [DOI] [PubMed] [Google Scholar]
- BISKUP S & WEST AB 2009. Zeroing in on LRRK2-linked pathogenic mechanisms in Parkinson's disease. Biochim Biophys Acta, 1792, 625–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAUWENDRAAT C, NALLS MA & SINGLETON AB 2019. The genetic architecture of Parkinson's disease. Lancet Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLAUWENDRAAT C, REED X, KIA DA, GAN-OR Z, LESAGE S, PIHLSTROM L, GUERREIRO R, GIBBS JR, SABIR M, AHMED S, DING J, ALCALAY RN, HASSIN-BAER S, PITTMAN AM, BROOKS J, EDSALL C, HERNANDEZ DG, CHUNG SJ, GOLDWURM S, TOFT M, SCHULTE C, BRAS J, WOOD NW, BRICE A, MORRIS HR, SCHOLZ SW, NALLS MA, SINGLETON AB & COOKSON MR 2018. Frequency of Loss of Function Variants in LRRK2 in Parkinson Disease. JAMA Neurol, 75, 1416–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODDU R, HULL TD, BOLISETTY S, HU X, MOEHLE MS, DAHER JP, KAMAL AI, JOSEPH R, GEORGE JF, AGARWAL A, CURTIS LM & WEST AB 2015. Leucine-rich repeat kinase 2 deficiency is protective in rhabdomyolysis-induced kidney injury. Hum Mol Genet, 24, 4078–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONET-PONCE L & COOKSON MR 2019. The role of Rab GTPases in the pathobiology of Parkinson' disease. Curr Opin Cell Biol, 59, 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURRE J 2015. The Synaptic Function of alpha-Synuclein. J Parkinsons Dis, 5, 699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURRE J, SHARMA M & SUDHOF TC 2014. alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci U S A, 111, E4274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURRE J, SHARMA M & SUDHOF TC 2018. Cell Biology and Pathophysiology of alpha-Synuclein. Cold Spring Harb Perspect Med, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAO M, WU Y, ASHRAFI G, MCCARTNEY AJ, WHEELER H, BUSHONG EA, BOASSA D, ELLISMAN MH, RYAN TA & DE CAMILLI P 2017. Parkinson Sac Domain Mutation in Synaptojanin 1 Impairs Clathrin Uncoating at Synapses and Triggers Dystrophic Changes in Dopaminergic Axons. Neuron, 93, 882–896.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATALDI S, FOLLETT J, FOX JD, TATARNIKOV I, KADGIEN C, GUSTAVSSON EK, KHINDA J, MILNERWOOD AJ & FARRER MJ 2018. Altered dopamine release and monoamine transporters in Vps35 p.D620N knock-in mice. NPJ Parkinsons Dis, 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG D, NALLS MA, HALLGRIMSDOTTIR IB, HUNKAPILLER J, VAN DER BRUG M, CAI F, KERCHNER GA, AYALON G, BINGOL B, SHENG M, HINDS D, BEHRENS TW, SINGLETON AB, BHANGALE TR & GRAHAM RR 2017. A meta-analysis of genome-wide association studies identifies 17 new Parkinson's disease risk loci. Nat Genet, 49, 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARTIER-HARLIN MC, KACHERGUS J, ROUMIER C, MOUROUX V, DOUAY X, LINCOLN S, LEVECQUE C, LARVOR L, ANDRIEUX J, HULIHAN M, WAUCQUIER N, DEFEBVRE L, AMOUYEL P, FARRER M & DESTEE A 2004. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet, 364, 1167–9. [DOI] [PubMed] [Google Scholar]
- CHEN KH, WU RM, LIN HI, TAI CH & LIN CH 2015. Mutational analysis of SYNJ1 gene (PARK20) in Parkinson's disease in a Taiwanese population. Neurobiol Aging, 36, 2905.e7–8. [DOI] [PubMed] [Google Scholar]
- CHEN X, KORDICH JK, WILLIAMS ET, LEVINE N, COLE-STRAUSS A, MARSHALL L, LABRIE V, MA J, LIPTON JW & MOORE DJ 2019. Parkinson's disease-linked D620N VPS35 knockin mice manifest tau neuropathology and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A, 116, 5765–5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN YP, SONG W, HUANG R, CHEN K, ZHAO B, LI J, YANG Y & SHANG HF 2013. GAK rs1564282 and DGKQ rs11248060 increase the risk for Parkinson's disease in a Chinese population. J Clin Neurosci, 20, 880–3. [DOI] [PubMed] [Google Scholar]
- CHOY RW, PARK M, TEMKIN P, HERRING BE, MARLEY A, NICOLL RA & VON ZASTROW M 2014. Retromer mediates a discrete route of local membrane delivery to dendrites. Neuron, 82, 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRIST L, RAIBORG C, WENZEL EM, CAMPSTEIJN C & STENMARK H 2017. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem Sci, 42, 42–56. [DOI] [PubMed] [Google Scholar]
- COLLABORATORS GN 2019. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 18, 459–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLABORATORS GPSD 2018. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol, 17, 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CREMONA O, DI PAOLO G, WENK MR, LUTHI A, KIM WT, TAKEI K, DANIELL L, NEMOTO Y, SHEARS SB, FLAVELL RA, MCCORMICK DA & DE CAMILLI P 1999. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell, 99, 179–88. [DOI] [PubMed] [Google Scholar]
- CUERVO AM, STEFANIS L, FREDENBURG R, LANSBURY PT & SULZER D 2004. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science, 305, 1292–5. [DOI] [PubMed] [Google Scholar]
- CULLEN PJ & KORSWAGEN HC 2011. Sorting nexins provide diversity for retromer-dependent trafficking events. Nat Cell Biol, 14, 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHER JP, ABDELMOTILIB HA, HU X, VOLPICELLI-DALEY LA, MOEHLE MS, FRASER KB, NEEDLE E, CHEN Y, STEYN SJ, GALATSIS P, HIRST WD & WEST AB 2015. Leucine-rich Repeat Kinase 2 (LRRK2) Pharmacological Inhibition Abates alpha-Synuclein Gene-induced Neurodegeneration. J Biol Chem, 290, 19433–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHER JP, VOLPICELLI-DALEY LA, BLACKBURN JP, MOEHLE MS & WEST AB 2014. Abrogation of alpha-synuclein-mediated dopaminergic neurodegeneration in LRRK2-deficient rats. Proc Natl Acad Sci U S A, 111, 9289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL G, MUSSO A, TSIKA E, FISER A, GLAUSER L, PLETNIKOVA O, SCHNEIDER BL & MOORE DJ 2015. alpha-Synuclein-induced dopaminergic neurodegeneration in a rat model of Parkinson's disease occurs independent of ATP13A2 (PARK9). Neurobiol Dis, 73, 229–43. [DOI] [PubMed] [Google Scholar]
- DENG H, GAO K & JANKOVIC J 2013. The VPS35 gene and Parkinson's disease. Mov Disord, 28, 569–75. [DOI] [PubMed] [Google Scholar]
- DENG H, XU H, DENG X, SONG Z, ZHENG W, GAO K, FAN X & TANG J 2012. VPS35 mutation in Chinese Han patients with late-onset Parkinson's disease. Eur J Neurol, 19, e96–7. [DOI] [PubMed] [Google Scholar]
- DHEKNE HS, YANATORI I, GOMEZ RC, TONELLI F, DIEZ F, SCHULE B, STEGER M, ALESSI DR & PFEFFER SR 2018. A pathway for Parkinson's Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. Elife, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHUNGEL N, ELEUTERI S, LI LB, KRAMER NJ, CHARTRON JW, SPENCER B, KOSBERG K, FIELDS JA, STAFA K, ADAME A, LASHUEL H, FRYDMAN J, SHEN K, MASLIAH E & GITLER AD 2015. Parkinson's disease genes VPS35 and EIF4G1 interact genetically and converge on alpha-synuclein. Neuron, 85, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMAN J & RYAN TA 2009. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol, 25, 133–60. [DOI] [PubMed] [Google Scholar]
- DORSEY ER & BLOEM BR 2018. The Parkinson Pandemic-A Call to Action. JAMA Neurol, 75, 9–10. [DOI] [PubMed] [Google Scholar]
- DORSEY ER, SHERER T, OKUN MS & BLOEM BR 2018. The Emerging Evidence of the Parkinson Pandemic. J Parkinsons Dis, 8, S3–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROUET V & LESAGE S 2014. Synaptojanin 1 mutation in Parkinson's disease brings further insight into the neuropathological mechanisms. Biomed Res Int, 2014, 289728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMITRIU A, PACHECO CD, WILK JB, STRATHEARN KE, LATOURELLE JC, GOLDWURM S, PEZZOLI G, ROCHET JC, LINDQUIST S & MYERS RH 2011. Cyclin-G-associated kinase modifies alpha-synuclein expression levels and toxicity in Parkinson's disease: results from the GenePD Study. Hum Mol Genet, 20, 1478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUSONCHET J, KOCHUBEY O, STAFA K, YOUNG SM JR., ZUFFEREY R, MOORE DJ, SCHNEIDER BL & AEBISCHER P 2011. A rat model of progressive nigral neurodegeneration induced by the Parkinson's disease-associated G2019S mutation in LRRK2. J Neurosci, 31, 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDVARDSON S, CINNAMON Y, TA-SHMA A, SHAAG A, YIM YI, ZENVIRT S, JALAS C, LESAGE S, BRICE A, TARABOULOS A, KAESTNER KH, GREENE LE & ELPELEG O 2012. A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS One, 7, e36458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGUCHI T, KUWAHARA T, SAKURAI M, KOMORI T, FUJIMOTO T, ITO G, YOSHIMURA SI, HARADA A, FUKUDA M, KOIKE M & IWATSUBO T 2018. LRRK2 and its substrate Rab GTPases are sequentially targeted onto stressed lysosomes and maintain their homeostasis. Proc Natl Acad Sci U S A, 115, E9115–e9124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLLETT J, BUGARCIC A, YANG Z, ARIOTTI N, NORWOOD SJ, COLLINS BM, PARTON RG & TEASDALE RD 2016. Parkinson Disease-linked Vps35 R524W Mutation Impairs the Endosomal Association of Retromer and Induces alpha-Synuclein Aggregation. J Biol Chem, 291, 18283–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJI RN, FLAGELLA M, BACA M, BAPTISTA MA, BRODBECK J, CHAN BK, FISKE BK, HONIGBERG L, JUBB AM, KATAVOLOS P, LEE DW, LEWIN-KOH SC, LIN T, LIU X, LIU S, LYSSIKATOS JP, O'MAHONY J, REICHELT M, ROOSE-GIRMA M, SHENG Z, SHERER T, SMITH A, SOLON M, SWEENEY ZK, TARRANT J, URKOWITZ A, WARMING S, YAYLAOGLU M, ZHANG S, ZHU H, ESTRADA AA & WATTS RJ 2015. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med, 7, 273ra15. [DOI] [PubMed] [Google Scholar]
- GAMBARTE TUDELA J, BUONFIGLI J, LUJAN A, ALONSO BIVOU M, CEBRIAN I, CAPMANY A & DAMIANI MT 2019. Rab39a and Rab39b Display Different Intracellular Distribution and Function in Sphingolipids and Phospholipids Transport. Int J Mol Sci, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAO Y, MARTINEZ-CERDENO V, HOGAN KJ, MCLEAN CA & LOCKHART PJ 2020. Clinical and Neuropathological Features Associated With Loss of RAB39B. Mov Disord. [DOI] [PubMed] [Google Scholar]
- GAO Y, WILSON GR, STEPHENSON SEM, BOZAOGLU K, FARRER MJ & LOCKHART PJ 2018. The emerging role of Rab GTPases in the pathogenesis of Parkinson's disease. Mov Disord, 33, 196–207. [DOI] [PubMed] [Google Scholar]
- GIANNANDREA M, BIANCHI V, MIGNOGNA ML, SIRRI A, CARRABINO S, D'ELIA E, VECELLIO M, RUSSO S, COGLIATI F, LARIZZA L, ROPERS HH, TZSCHACH A, KALSCHEUER V, OEHL-JASCHKOWITZ B, SKINNER C, SCHWARTZ CE, GECZ J, VAN ESCH H, RAYNAUD M, CHELLY J, DE BROUWER AP, TONIOLO D & D'ADAMO P 2010. Mutations in the small GTPase gene RAB39B are responsible for X-linked mental retardation associated with autism, epilepsy, and macrocephaly. Am J Hum Genet, 86, 185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLARDON F 2009. Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability--a point of convergence in parkinsonian neurodegeneration? J Neurochem, 110, 1514–22. [DOI] [PubMed] [Google Scholar]
- GOMEZ-SUAGA P, RIVERO-RIOS P, FDEZ E, BLANCA RAMIREZ M, FERRER I, AIASTUI A, LOPEZ DE MUNAIN A & HILFIKER S 2014. LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum Mol Genet, 23, 6779–96. [DOI] [PubMed] [Google Scholar]
- GOMEZ RC, WAWRO P, LIS P, ALESSI DR & PFEFFER SR 2019. Membrane association but not identity is required for LRRK2 activation and phosphorylation of Rab GTPases. J Cell Biol, 218, 4157–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGGIO E, JAIN S, KINGSBURY A, BANDOPADHYAY R, LEWIS P, KAGANOVICH A, VAN DER BRUG MP, BEILINA A, BLACKINTON J, THOMAS KJ, AHMAD R, MILLER DW, KESAVAPANY S, SINGLETON A, LEES A, HARVEY RJ, HARVEY K & COOKSON MR 2006. Kinase activity is required for the toxic effects of mutant LRRK2/dardarin. Neurobiol Dis, 23, 329–41. [DOI] [PubMed] [Google Scholar]
- GUSTAVSSON E FJ, RAMIREZ CM, TRINH J, FOX J, AASLY E, POURCHER E, HENTATI F, FARRER M RAB32 as a cause for familial Parkinson's disease. In International Congress of Parkinson's Disease and Movement Disorders, 2017. British Columbia, Canada. [Google Scholar]
- HEALY DG, FALCHI M, O'SULLIVAN SS, BONIFATI V, DURR A, BRESSMAN S, BRICE A, AASLY J, ZABETIAN CP, GOLDWURM S, FERREIRA JJ, TOLOSA E, KAY DM, KLEIN C, WILLIAMS DR, MARRAS C, LANG AE, WSZOLEK ZK, BERCIANO J, SCHAPIRA AH, LYNCH T, BHATIA KP, GASSER T, LEES AJ, WOOD NW & INTERNATIONAL LC 2008. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study. Lancet Neurol, 7, 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDERSON MX, SENGUPTA M, MCGEARY I, ZHANG B, OLUFEMI MF, BROWN H, TROJANOWSKI JQ & LEE VMY 2019. LRRK2 inhibition does not impart protection from alpha-synuclein pathology and neuron death in non-transgenic mice. Acta Neuropathol Commun, 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ DG, REED X & SINGLETON AB 2016. Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J Neurochem, 139 Suppl 1, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZIG MC, KOLLY C, PERSOHN E, THEIL D, SCHWEIZER T, HAFNER T, STEMMELEN C, TROXLER TJ, SCHMID P, DANNER S, SCHNELL CR, MUELLER M, KINZEL B, GREVOT A, BOLOGNANI F, STIRN M, KUHN RR, KAUPMANN K, VAN DER PUTTEN PH, ROVELLI G & SHIMSHEK DR 2011. LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum Mol Genet, 20, 4209–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIERRO A, ROJAS AL, ROJAS R, MURTHY N, EFFANTIN G, KAJAVA AV, STEVEN AC, BONIFACINO JS & HURLEY JH 2007. Functional architecture of the retromer cargo-recognition complex. Nature, 449, 1063–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKLE KM, YUE M, BEHROUZ B, DACHSEL JC, LINCOLN SJ, BOWLES EE, BEEVERS JE, DUGGER B, WINNER B, PROTS I, KENT CB, NISHIOKA K, LIN WL, DICKSON DW, JANUS CJ, FARRER MJ & MELROSE HL 2012. LRRK2 knockout mice have an intact dopaminergic system but display alterations in exploratory and motor co-ordination behaviors. Mol Neurodegener, 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRST J, SAHLENDER DA, LI S, LUBBEN NB, BORNER GH & ROBINSON MS 2008. Auxilin depletion causes self-assembly of clathrin into membraneless cages in vivo. Traffic, 9, 1354–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZU N, YUI D, HEBISAWA A, AIZAWA H, CUI W, FUJITA Y, HASHIMOTO K, AJIOKA I, MIZUSAWA H, YOKOTA T & WATASE K 2016. Impaired striatal dopamine release in homozygous Vps35 D620N knock-in mice. Hum Mol Genet, 25, 4507–4517. [DOI] [PubMed] [Google Scholar]
- ISLAM MS & MOORE DJ 2017. Mechanisms of LRRK2-dependent neurodegeneration: role of enzymatic activity and protein aggregation. Biochem Soc Trans, 45, 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISLAM MS, NOLTE H, JACOB W, ZIEGLER AB, PUTZ S, GROSJEAN Y, SZCZEPANOWSKA K, TRIFUNOVIC A, BRAUN T, HEUMANN H, HEUMANN R, HOVEMANN B, MOORE DJ & KRUGER M 2016. Human R1441C LRRK2 regulates the synaptic vesicle proteome and phosphoproteome in a Drosophila model of Parkinson's disease. Hum Mol Genet, 25, 5365–5382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO G, KATSEMONOVA K, TONELLI F, LIS P, BAPTISTA MA, SHPIRO N, DUDDY G, WILSON S, HO PW, HO SL, REITH AD & ALESSI DR 2016. Phos-tag analysis of Rab10 phosphorylation by LRRK2: a powerful assay for assessing kinase function and inhibitors. Biochem J, 473, 2671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JINN S, BLAUWENDRAAT C, TOOLAN D, GRETZULA CA, DROLET RE, SMITH S, NALLS MA, MARCUS J, SINGLETON AB & STONE DJ 2019. Functionalization of the TMEM175 p.M393T Variant as a risk factor for Parkinson Disease. Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY K, WANG S, BODDU R, LIU Z, MOUKHA-CHAFIQ O, AUGELLI-SZAFRAN C & WEST AB 2018. The G2019S mutation in LRRK2 imparts resiliency to kinase inhibition. Exp Neurol, 309, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM WT, CHANG S, DANIELL L, CREMONA O, DI PAOLO G & DE CAMILLI P 2002. Delayed reentry of recycling vesicles into the fusion-competent synaptic vesicle pool in synaptojanin 1 knockout mice. Proc Natl Acad Sci U S A, 99, 17143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIROLA L, BEHARI M, SHISHIR C & THELMA BK 2016. Identification of a novel homozygous mutation Arg459Pro in SYNJ1 gene of an Indian family with autosomal recessive juvenile Parkinsonism. Parkinsonism Relat Disord, 31, 124–128. [DOI] [PubMed] [Google Scholar]
- KLUSS JH, CONTI MM, KAGANOVICH A, BEILINA A, MELROSE HL, COOKSON MR & MAMAIS A 2018. Detection of endogenous S1292 LRRK2 autophosphorylation in mouse tissue as a readout for kinase activity. NPJ Parkinsons Dis, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOROGLU C, BAYSAL L, CETINKAYA M, KARASOY H & TOLUN A 2013. DNAJC6 is responsible for juvenile parkinsonism with phenotypic variability. Parkinsonism Relat Disord, 19, 320–4. [DOI] [PubMed] [Google Scholar]
- KOVTUN O, LENEVA N, BYKOV YS, ARIOTTI N, TEASDALE RD, SCHAFFER M, ENGEL BD, OWEN DJ, BRIGGS JAG & COLLINS BM 2018. Structure of the membrane-assembled retromer coat determined by cryo-electron tomography. Nature, 561, 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREBS CE, KARKHEIRAN S, POWELL JC, CAO M, MAKAROV V, DARVISH H, DI PAOLO G, WALKER RH, SHAHIDI GA, BUXBAUM JD, DE CAMILLI P, YUE Z & PAISAN-RUIZ C 2013. The Sac1 domain of SYNJ1 identified mutated in a family with early-onset progressive Parkinsonism with generalized seizures. Hum Mutat, 34, 1200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROHN L, OZTURK TN, VANDERPERRE B, OULED AMAR BENCHEIKH B, RUSKEY JA, LAURENT SB, SPIEGELMAN D, POSTUMA RB, ARNULF I, HU MTM, DAUVILLIERS Y, HOGL B, STEFANI A, MONACA CC, PLAZZI G, ANTELMI E, FERINI-STRAMBI L, HEIDBREDER A, RUDAKOU U, COCHEN DE COCK V, YOUNG P, WOLF P, OLIVA P, ZHANG XK, GREENBAUM L, LIONG C, GAGNON JF, DESAUTELS A, HASSIN-BAER S, MONTPLAISIR JY, DUPRE N, ROULEAU GA, FON EA, TREMPE JF, LAMOUREUX G, ALCALAY RN & GAN-OR Z 2020. Genetic, Structural, and Functional Evidence Link TMEM175 to Synucleinopathies. Ann Neurol, 87, 139–153. [DOI] [PubMed] [Google Scholar]
- KRUGER R, KUHN W, MULLER T, WOITALLA D, GRAEBER M, KOSEL S, PRZUNTEK H, EPPLEN JT, SCHOLS L & RIESS O 1998. Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson's disease. Nat Genet, 18, 106–8. [DOI] [PubMed] [Google Scholar]
- KRUMOVA P, REYNIERS L, MEYER M, LOBBESTAEL E, STAUFFER D, GERRITS B, MULLER L, HOVING S, KAUPMANN K, VOSHOL J, FABBRO D, BAUER A, ROVELLI G, TAYMANS JM, BOUWMEESTER T & BAEKELANDT V 2015. Chemical genetic approach identifies microtubule affinity-regulating kinase 1 as a leucine-rich repeat kinase 2 substrate. FASEB J, 29, 2980–92. [DOI] [PubMed] [Google Scholar]
- KUMAR KR, WEISSBACH A, HELDMANN M, KASTEN M, TUNC S, SUE CM, SVETEL M, KOSTIC VS, SEGURA-AGUILAR J, RAMIREZ A, SIMON DK, VIEREGGE P, MUNTE TF, HAGENAH J, KLEIN C & LOHMANN K 2012. Frequency of the D620N mutation in VPS35 in Parkinson disease. Arch Neurol, 69, 1360–4. [DOI] [PubMed] [Google Scholar]
- KUWAHARA T, INOUE K, D'AGATI VD, FUJIMOTO T, EGUCHI T, SAHA S, WOLOZIN B, IWATSUBO T & ABELIOVICH A 2016. LRRK2 and RAB7L1 coordinately regulate axonal morphology and lysosome integrity in diverse cellular contexts. Sci Rep, 6, 29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG AE & LOZANO AM 1998a. Parkinson's disease. First of two parts. N Engl J Med, 339, 1044–53. [DOI] [PubMed] [Google Scholar]
- LANG AE & LOZANO AM 1998b. Parkinson's disease. Second of two parts. N Engl J Med, 339, 1130–43. [DOI] [PubMed] [Google Scholar]
- LARA ORDONEZ AJ, FERNANDEZ B, FDEZ E, ROMO-LOZANO M, MADERO-PEREZ J, LOBBESTAEL E, BAEKELANDT V, AIASTUI A, LOPEZ DE MUNAIN A, MELROSE HL, CIVIERO L & HILFIKER S 2019. RAB8, RAB10 and RILPL1 contribute to both LRRK2 kinase-mediated centrosomal cohesion and ciliogenesis deficits. Hum Mol Genet, 28, 3552–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE BD, SHIN JH, VANKAMPEN J, PETRUCELLI L, WEST AB, KO HS, LEE YI, MAGUIRE-ZEISS KA, BOWERS WJ, FEDEROFF HJ, DAWSON VL & DAWSON TM 2010. Inhibitors of leucine-rich repeat kinase-2 protect against models of Parkinson's disease. Nat Med, 16, 998–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE DW, ZHAO X, YIM YI, EISENBERG E & GREENE LE 2008. Essential role of cyclin-G-associated kinase (Auxilin-2) in developing and mature mice. Mol Biol Cell, 19, 2766–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LESAGE S, ANHEIM M, LETOURNEL F, BOUSSET L, HONORE A, ROZAS N, PIERI L, MADIONA K, DURR A, MELKI R, VERNY C & BRICE A 2013. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol, 73, 459–71. [DOI] [PubMed] [Google Scholar]
- LESAGE S, BRAS J, CORMIER-DEQUAIRE F, CONDROYER C, NICOLAS A, DARWENT L, GUERREIRO R, MAJOUNIE E, FEDEROFF M, HEUTINK P, WOOD NW, GASSER T, HARDY J, TISON F, SINGLETON A & BRICE A 2015. Loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol Genet, 1, e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI JG, CHIU J & PRATICO D 2019. Full recovery of the Alzheimer's disease phenotype by gain of function of vacuolar protein sorting 35. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- LI JG, CHIU J, RAMANJULU M, BLASS BE & PRATICO D 2020. A pharmacological chaperone improves memory by reducing Abeta and tau neuropathology in a mouse model with plaques and tangles. Mol Neurodegener, 15, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINHART R, WONG SA, CAO J, TRAN M, HUYNH A, ARDREY C, PARK JM, HSU C, TAHA S, PETERSON R, SHEA S, KURIAN J & VENDEROVA K 2014. Vacuolar protein sorting 35 (Vps35) rescues locomotor deficits and shortened lifespan in Drosophila expressing a Parkinson's disease mutant of Leucine-Rich Repeat Kinase 2 (LRRK2). Mol Neurodegener, 9, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIS P, BUREL S, STEGER M, MANN M, BROWN F, DIEZ F, TONELLI F, HOLTON JL, HO PW, HO SL, CHOU MY, POLINSKI NK, MARTINEZ TN, DAVIES P & ALESSI DR 2018. Development of phospho-specific Rab protein antibodies to monitor in vivo activity of the LRRK2 Parkinson's disease kinase. Biochem J, 475, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU Z, BRYANT N, KUMARAN R, BEILINA A, ABELIOVICH A, COOKSON MR & WEST AB 2018. LRRK2 phosphorylates membrane-bound Rabs and is activated by GTP-bound Rab7L1 to promote recruitment to the trans-Golgi network. Hum Mol Genet, 27, 385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA J, GAO J, WANG J & XIE A 2019. Prion-Like Mechanisms in Parkinson's Disease. Front Neurosci, 13, 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD DA, RHINN H, KUWAHARA T, ZOLIN A, DI PAOLO G, MCCABE BD, MARDER KS, HONIG LS, CLARK LN, SMALL SA & ABELIOVICH A 2013. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson's disease risk. Neuron, 77, 425–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARRAS C, BECK JC, BOWER JH, ROBERTS E, RITZ B, ROSS GW, ABBOTT RD, SAVICA R, VAN DEN EEDEN SK, WILLIS AW & TANNER CM 2018. Prevalence of Parkinson's disease across North America. NPJ Parkinsons Dis, 4, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN I, KIM JW, LEE BD, KANG HC, XU JC, JIA H, STANKOWSKI J, KIM MS, ZHONG J, KUMAR M, ANDRABI SA, XIONG Y, DICKSON DW, WSZOLEK ZK, PANDEY A, DAWSON TM & DAWSON VL 2014. Ribosomal protein s15 phosphorylation mediates LRRK2 neurodegeneration in Parkinson's disease. Cell, 157, 472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATA IF, JANG Y, KIM CH, HANNA DS, DORSCHNER MO, SAMII A, AGARWAL P, ROBERTS JW, KLEPITSKAYA O, SHPRECHER DR, CHUNG KA, FACTOR SA, ESPAY AJ, REVILLA FJ, HIGGINS DS, LITVAN I, LEVERENZ JB, YEAROUT D, INCA-MARTINEZ M, MARTINEZ E, THOMPSON TR, CHOLERTON BA, HU SC, EDWARDS KL, KIM KS & ZABETIAN CP 2015. The RAB39B p.G192R mutation causes X-linked dominant Parkinson's disease. Mol Neurodegener, 10, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTA S, VAN KOLEN K, DA CUNHA R, VAN DEN BOGAART G, MANDEMAKERS W, MISKIEWICZ K, DE BOCK PJ, MORAIS VA, VILAIN S, HADDAD D, DELBROEK L, SWERTS J, CHAVEZ-GUTIERREZ L, ESPOSITO G, DANEELS G, KARRAN E, HOLT M, GEVAERT K, MOECHARS DW, DE STROOPER B & VERSTREKEN P 2012. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron, 75, 1008–21. [DOI] [PubMed] [Google Scholar]
- MCFARTHING K & SIMUNI T 2019. Clinical Trial Highlights: Targetting Alpha-Synuclein. J Parkinsons Dis, 9, 5–16. [DOI] [PubMed] [Google Scholar]
- MCGOUGH IJ, STEINBERG F, JIA D, BARBUTI PA, MCMILLAN KJ, HEESOM KJ, WHONE AL, CALDWELL MA, BILLADEAU DD, ROSEN MK & CULLEN PJ 2014. Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Curr Biol, 24, 1670–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGRATH E, WASCHBUSCH D, BAKER BM & KHAN AR 2019. LRRK2 binds to the Rab32 subfamily in a GTP-dependent manner via its armadillo domain. Small GTPases, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNALLY KE & CULLEN PJ 2018. Endosomal Retrieval of Cargo: Retromer Is Not Alone. Trends Cell Biol, 28, 807–822. [DOI] [PubMed] [Google Scholar]
- MCNALLY KE, FAULKNER R, STEINBERG F, GALLON M, GHAI R, PIM D, LANGTON P, PEARSON N, DANSON CM, NAGELE H, MORRIS LL, SINGLA A, OVERLEE BL, HEESOM KJ, SESSIONS R, BANKS L, COLLINS BM, BERGER I, BILLADEAU DD, BURSTEIN E & CULLEN PJ 2017. Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat Cell Biol, 19, 1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MECOZZI VJ, BERMAN DE, SIMOES S, VETANOVETZ C, AWAL MR, PATEL VM, SCHNEIDER RT, PETSKO GA, RINGE D & SMALL SA 2014. Pharmacological chaperones stabilize retromer to limit APP processing. Nat Chem Biol, 10, 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEISSNER WG, FRASIER M, GASSER T, GOETZ CG, LOZANO A, PICCINI P, OBESO JA, RASCOL O, SCHAPIRA A, VOON V, WEINER DM, TISON F & BEZARD E 2011. Priorities in Parkinson's disease research. Nat Rev Drug Discov, 10, 377–93. [DOI] [PubMed] [Google Scholar]
- MIR R, TONELLI F, LIS P, MACARTNEY T, POLINSKI NK, MARTINEZ TN, CHOU MY, HOWDEN AJM, KONIG T, HOTZY C, MILENKOVIC I, BRUCKE T, ZIMPRICH A, SAMMLER E & ALESSI DR 2018. The Parkinson's disease VPS35[D620N] mutation enhances LRRK2-mediated Rab protein phosphorylation in mouse and human. Biochem J, 475, 1861–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIURA E, HASEGAWA T, KONNO M, SUZUKI M, SUGENO N, FUJIKAKE N, GEISLER S, TABUCHI M, OSHIMA R, KIKUCHI A, BABA T, WADA K, NAGAI Y, TAKEDA A & AOKI M 2014. VPS35 dysfunction impairs lysosomal degradation of alpha-synuclein and exacerbates neurotoxicity in a Drosophila model of Parkinson's disease. Neurobiol Dis, 71, 1–13. [DOI] [PubMed] [Google Scholar]
- MOORE DJ, WEST AB, DAWSON VL & DAWSON TM 2005. Molecular pathophysiology of Parkinson's disease. Annu Rev Neurosci, 28, 57–87. [DOI] [PubMed] [Google Scholar]
- MUHAMMAD A, FLORES I, ZHANG H, YU R, STANISZEWSKI A, PLANEL E, HERMAN M, HO L, KREBER R, HONIG LS, GANETZKY B, DUFF K, ARANCIO O & SMALL SA 2008. Retromer deficiency observed in Alzheimer's disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci U S A, 105, 7327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUNSIE LN, MILNERWOOD AJ, SEIBLER P, BECCANO-KELLY DA, TATARNIKOV I, KHINDA J, VOLTA M, KADGIEN C, CAO LP, TAPIA L, KLEIN C & FARRER MJ 2015. Retromer-dependent neurotransmitter receptor trafficking to synapses is altered by the Parkinson's disease VPS35 mutation p.D620N. Hum Mol Genet, 24, 1691–703. [DOI] [PubMed] [Google Scholar]
- NALLS MA, BLAUWENDRAAT C, VALLERGA CL, HEILBRON K, BANDRES-CIGA S, CHANG D, TAN M, KIA DA, NOYCE AJ, XUE A, BRAS J, YOUNG E, VON COELLN R, SIMON-SANCHEZ J, SCHULTE C, SHARMA M, KROHN L, PIHLSTROM L, SIITONEN A, IWAKI H, LEONARD H, FAGHRI F, GIBBS JR, HERNANDEZ DG, SCHOLZ SW, BOTIA JA, MARTINEZ M, CORVOL JC, LESAGE S, JANKOVIC J, SHULMAN LM, SUTHERLAND M, TIENARI P, MAJAMAA K, TOFT M, ANDREASSEN OA, BANGALE T, BRICE A, YANG J, GAN-OR Z, GASSER T, HEUTINK P, SHULMAN JM, WOOD NW, HINDS DA, HARDY JA, MORRIS HR, GRATTEN J, VISSCHER PM, GRAHAM RR, SINGLETON AB, ANDME RESEARCH T, SYSTEM GENOMICS OF PARKINSON'S DISEASE C & INTERNATIONAL PARKINSON'S DISEASE GENOMICS C 2019. Identification of novel risk loci, causal insights, and heritable risk for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet Neurol, 18, 1091–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NALLS MA, PANKRATZ N, LILL CM, DO CB, HERNANDEZ DG, SAAD M, DESTEFANO AL, KARA E, BRAS J, SHARMA M, SCHULTE C, KELLER MF, AREPALLI S, LETSON C, EDSALL C, STEFANSSON H, LIU X, PLINER H, LEE JH, CHENG R, IKRAM MA, IOANNIDIS JP, HADJIGEORGIOU GM, BIS JC, MARTINEZ M, PERLMUTTER JS, GOATE A, MARDER K, FISKE B, SUTHERLAND M, XIROMERISIOU G, MYERS RH, CLARK LN, STEFANSSON K, HARDY JA, HEUTINK P, CHEN H, WOOD NW, HOULDEN H, PAYAMI H, BRICE A, SCOTT WK, GASSER T, BERTRAM L, ERIKSSON N, FOROUD T & SINGLETON AB 2014. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson's disease. Nat Genet, 46, 989–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEMANI VM, LU W, BERGE V, NAKAMURA K, ONOA B, LEE MK, CHAUDHRY FA, NICOLL RA & EDWARDS RH 2010. Increased expression of alpha-synuclein reduces neurotransmitter release by inhibiting synaptic vesicle reclustering after endocytosis. Neuron, 65, 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NESS D, REN Z, GARDAI S, SHARPNACK D, JOHNSON VJ, BRENNAN RJ, BRIGHAM EF & OLAHARSKI AJ 2013. Leucine-rich repeat kinase 2 (LRRK2)-deficient rats exhibit renal tubule injury and perturbations in metabolic and immunological homeostasis. PLoS One, 8, e66164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN AP & MOORE DJ 2017. Understanding the GTPase Activity of LRRK2: Regulation, Function, and Neurotoxicity. Adv Neurobiol, 14, 71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN M & KRAINC D 2018. LRRK2 phosphorylation of auxilin mediates synaptic defects in dopaminergic neurons from patients with Parkinson's disease. Proc Natl Acad Sci U S A, 115, 5576–5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NGUYEN M, WONG YC, YSSELSTEIN D, SEVERINO A & KRAINC D 2019. Synaptic, Mitochondrial, and Lysosomal Dysfunction in Parkinson's Disease. Trends Neurosci, 42, 140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLGIATI S, DE ROSA A, QUADRI M, CRISCUOLO C, BREEDVELD GJ, PICILLO M, PAPPATA S, QUARANTELLI M, BARONE P, DE MICHELE G & BONIFATI V 2014. PARK20 caused by SYNJ1 homozygous Arg258Gln mutation in a new Italian family. Neurogenetics, 15, 183–8. [DOI] [PubMed] [Google Scholar]
- OLGIATI S, QUADRI M, FANG M, ROOD JP, SAUTE JA, CHIEN HF, BOUWKAMP CG, GRAAFLAND J, MINNEBOO M, BREEDVELD GJ, ZHANG J, VERHEIJEN FW, BOON AJ, KIEVIT AJ, JARDIM LB, MANDEMAKERS W, BARBOSA ER, RIEDER CR, LEENDERS KL, WANG J & BONIFATI V 2016. DNAJC6 Mutations Associated With Early-Onset Parkinson's Disease. Ann Neurol, 79, 244–56. [DOI] [PubMed] [Google Scholar]
- ORENSTEIN SJ, KUO SH, TASSET I, ARIAS E, KOGA H, FERNANDEZ-CARASA I, CORTES E, HONIG LS, DAUER W, CONSIGLIO A, RAYA A, SULZER D & CUERVO AM 2013. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci, 16, 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAISAN-RUIZ C, JAIN S, EVANS EW, GILKS WP, SIMON J, VAN DER BRUG M, LOPEZ DE MUNAIN A, APARICIO S, GIL AM, KHAN N, JOHNSON J, MARTINEZ JR, NICHOLL D, MARTI CARRERA I, PENA AS, DE SILVA R, LEES A, MARTI-MASSO JF, PEREZ-TUR J, WOOD NW & SINGLETON AB 2004. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron, 44, 595–600. [DOI] [PubMed] [Google Scholar]
- PAISAN-RUIZ C, LEWIS PA & SINGLETON AB 2013. LRRK2: cause, risk, and mechanism. J Parkinsons Dis, 3, 85–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN PY, LI X, WANG J, POWELL J, WANG Q, ZHANG Y, CHEN Z, WICINSKI B, HOF P, RYAN TA & YUE Z 2017. Parkinson's Disease-Associated LRRK2 Hyperactive Kinase Mutant Disrupts Synaptic Vesicle Trafficking in Ventral Midbrain Neurons. J Neurosci, 37, 11366–11376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELLEGRINI L, HAUSER DN, LI Y, MAMAIS A, BEILINA A, KUMARAN R, WETZEL A, NIXON-ABELL J, HEATON G, RUDENKO I, ALKASLASI M, IVANINA N, MELROSE HL, COOKSON MR & HARVEY K 2018. Proteomic analysis reveals co-ordinated alterations in protein synthesis and degradation pathways in LRRK2 knockout mice. Hum Mol Genet, 27, 3257–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLYMEROPOULOS MH, LAVEDAN C, LEROY E, IDE SE, DEHEJIA A, DUTRA A, PIKE B, ROOT H, RUBENSTEIN J, BOYER R, STENROOS ES, CHANDRASEKHARAPPA S, ATHANASSIADOU A, PAPAPETROPOULOS T, JOHNSON WG, LAZZARINI AM, DUVOISIN RC, DI IORIO G, GOLBE LI & NUSSBAUM RL 1997. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science, 276, 2045–7. [DOI] [PubMed] [Google Scholar]
- PURLYTE E, DHEKNE HS, SARHAN AR, GOMEZ R, LIS P, WIGHTMAN M, MARTINEZ TN, TONELLI F, PFEFFER SR & ALESSI DR 2018. Rab29 activation of the Parkinson's disease-associated LRRK2 kinase. Embo j, 37, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QUADRI M, FANG M, PICILLO M, OLGIATI S, BREEDVELD GJ, GRAAFLAND J, WU B, XU F, ERRO R, AMBONI M, PAPPATA S, QUARANTELLI M, ANNESI G, QUATTRONE A, CHIEN HF, BARBOSA ER, OOSTRA BA, BARONE P, WANG J & BONIFATI V 2013. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum Mutat, 34, 1208–15. [DOI] [PubMed] [Google Scholar]
- QURESHI YH, BERMAN DE, KLEIN RL, PATEL VM, SIMOES S, KANNAN S, COX R, WAKSAL SD, STEVENS B, PETSKO GA & SMALL SA 2019. Retromer repletion with AAV9-VPS35 restores endosomal function in the mouse hippocampus. bioRxiv, 618496. [Google Scholar]
- RAMONET D, DAHER JP, LIN BM, STAFA K, KIM J, BANERJEE R, WESTERLUND M, PLETNIKOVA O, GLAUSER L, YANG L, LIU Y, SWING DA, BEAL MF, TRONCOSO JC, MCCAFFERY JM, JENKINS NA, COPELAND NG, GALTER D, THOMAS B, LEE MK, DAWSON TM, DAWSON VL & MOORE DJ 2011. Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS One, 6, e18568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOSEN DA NL, MELISSA CONTI, NATHAN SMITH, SARA SAEZ-ATIENZAR, JINHUI DING, ALEKSANDRA BEILINA, RAVINDRAN KUMARAN, ALICE KAGANOVICH, JOHANN DU HOFFMANN, CHAD D WILLIAMSON DAVIDCGERSHLICK, LUIS BONET-PONCE, LUCIANA SAMPIERI, CHRISTOPHER KEBLECK, CHENGYU LIU, BONIFACINO JUANS, YAN LI, LEWIS PATRICKA, COOKSON MARKR 2019. Mutations in Auxilin cause parkinsonism via impaired clathrin-mediated trafficking at the Golgi apparatus and synapse. bioRxiv. [Google Scholar]
- SAHEKI Y & DE CAMILLI P 2012. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol, 4, a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATAKE W, NAKABAYASHI Y, MIZUTA I, HIROTA Y, ITO C, KUBO M, KAWAGUCHI T, TSUNODA T, WATANABE M, TAKEDA A, TOMIYAMA H, NAKASHIMA K, HASEGAWA K, OBATA F, YOSHIKAWA T, KAWAKAMI H, SAKODA S, YAMAMOTO M, HATTORI N, MURATA M, NAKAMURA Y & TODA T 2009. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat Genet, 41, 1303–7. [DOI] [PubMed] [Google Scholar]
- SEAMAN M & FREEMAN CL 2014. Analysis of the Retromer complex-WASH complex interaction illuminates new avenues to explore in Parkinson disease. Commun Integr Biol, 7, e29483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN MN 2004. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol, 165, 111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN MN 2012. The retromer complex - endosomal protein recycling and beyond. J Cell Sci, 125, 4693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN MN, GAUTREAU A & BILLADEAU DD 2013. Retromer-mediated endosomal protein sorting: all WASHed up! Trends Cell Biol, 23, 522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN MNJ, MUKADAM AS & BREUSEGEM SY 2018. Inhibition of TBC1D5 activates Rab7a and can enhance the function of the retromer cargo-selective complex. J Cell Sci, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAHMORADIAN SH, LEWIS AJ, GENOUD C, HENCH J, MOORS TE, NAVARRO PP, CASTANO-DIEZ D, SCHWEIGHAUSER G, GRAFF-MEYER A, GOLDIE KN, SUTTERLIN R, HUISMAN E, INGRASSIA A, GIER Y, ROZEMULLER AJM, WANG J, PAEPE A, ERNY J, STAEMPFLI A, HOERNSCHEMEYER J, GROSSERUSCHKAMP F, NIEDIEKER D, EL-MASHTOLY SF, QUADRI M, VAN IWFJ, BONIFATI V, GERWERT K, BOHRMANN B, FRANK S, BRITSCHGI M, STAHLBERG H, VAN DE BERG WDJ & LAUER ME 2019. Lewy pathology in Parkinson's disease consists of crowded organelles and lipid membranes. Nat Neurosci, 22, 1099–1109. [DOI] [PubMed] [Google Scholar]
- SHARMA M, IOANNIDIS JP, AASLY JO, ANNESI G, BRICE A, BERTRAM L, BOZI M, BARCIKOWSKA M, CROSIERS D, CLARKE CE, FACHERIS MF, FARRER M, GARRAUX G, GISPERT S, AUBURGER G, VILARINO-GUELL C, HADJIGEORGIOU GM, HICKS AA, HATTORI N, JEON BS, JAMROZIK Z, KRYGOWSKA-WAJS A, LESAGE S, LILL CM, LIN JJ, LYNCH T, LICHTNER P, LANG AE, LIBIOULLE C, MURATA M, MOK V, JASINSKA-MYGA B, MELLICK GD, MORRISON KE, MEITNGER T, ZIMPRICH A, OPALA G, PRAMSTALLER PP, PICHLER I, PARK SS, QUATTRONE A, ROGAEVA E, ROSS OA, STEFANIS L, STOCKTON JD, SATAKE W, SILBURN PA, STROM TM, THEUNS J, TAN EK, TODA T, TOMIYAMA H, UITTI RJ, VAN BROECKHOVEN C, WIRDEFELDT K, WSZOLEK Z, XIROMERISIOU G, YOMONO HS, YUEH KC, ZHAO Y, GASSER T, MARAGANORE D & KRUGER R 2012. A multi-centre clinico-genetic analysis of the VPS35 gene in Parkinson disease indicates reduced penetrance for disease-associated variants. J Med Genet, 49, 721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHENG Z, ZHANG S, BUSTOS D, KLEINHEINZ T, LE PICHON CE, DOMINGUEZ SL, SOLANOY HO, DRUMMOND J, ZHANG X, DING X, CAI F, SONG Q, LI X, YUE Z, VAN DER BRUG MP, BURDICK DJ, GUNZNER-TOSTE J, CHEN H, LIU X, ESTRADA AA, SWEENEY ZK, SCEARCE-LEVIE K, MOFFAT JG, KIRKPATRICK DS & ZHU H 2012. Ser1292 autophosphorylation is an indicator of LRRK2 kinase activity and contributes to the cellular effects of PD mutations. Sci Transl Med, 4, 164ra161. [DOI] [PubMed] [Google Scholar]
- SIMON-SANCHEZ J, SCHULTE C, BRAS JM, SHARMA M, GIBBS JR, BERG D, PAISAN-RUIZ C, LICHTNER P, SCHOLZ SW, HERNANDEZ DG, KRUGER R, FEDEROFF M, KLEIN C, GOATE A, PERLMUTTER J, BONIN M, NALLS MA, ILLIG T, GIEGER C, HOULDEN H, STEFFENS M, OKUN MS, RACETTE BA, COOKSON MR, FOOTE KD, FERNANDEZ HH, TRAYNOR BJ, SCHREIBER S, AREPALLI S, ZONOZI R, GWINN K, VAN DER BRUG M, LOPEZ G, CHANOCK SJ, SCHATZKIN A, PARK Y, HOLLENBECK A, GAO J, HUANG X, WOOD NW, LORENZ D, DEUSCHL G, CHEN H, RIESS O, HARDY JA, SINGLETON AB & GASSER T 2009. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet, 41, 1308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGLETON AB, FARRER M, JOHNSON J, SINGLETON A, HAGUE S, KACHERGUS J, HULIHAN M, PEURALINNA T, DUTRA A, NUSSBAUM R, LINCOLN S, CRAWLEY A, HANSON M, MARAGANORE D, ADLER C, COOKSON MR, MUENTER M, BAPTISTA M, MILLER D, BLANCATO J, HARDY J & GWINN-HARDY K 2003. alpha-Synuclein locus triplication causes Parkinson's disease. Science, 302, 841. [DOI] [PubMed] [Google Scholar]
- SMALL SA, KENT K, PIERCE A, LEUNG C, KANG MS, OKADA H, HONIG L, VONSATTEL JP & KIM TW 2005. Model-guided microarray implicates the retromer complex in Alzheimer's disease. Ann Neurol, 58, 909–19. [DOI] [PubMed] [Google Scholar]
- SMALL SA & PETSKO GA 2015. Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat Rev Neurosci, 16, 126–32. [DOI] [PubMed] [Google Scholar]
- SMITH WW, PEI Z, JIANG H, DAWSON VL, DAWSON TM & ROSS CA 2006. Kinase activity of mutant LRRK2 mediates neuronal toxicity. Nat Neurosci, 9, 1231–3. [DOI] [PubMed] [Google Scholar]
- SONG L, HE Y, OU J, ZHAO Y, LI R, CHENG J, LIN CH & HO MS 2017. Auxilin Underlies Progressive Locomotor Deficits and Dopaminergic Neuron Loss in a Drosophila Model of Parkinson's Disease. Cell Rep, 18, 1132–1143. [DOI] [PubMed] [Google Scholar]
- SPILLANTINI MG, CROWTHER RA, JAKES R, HASEGAWA M & GOEDERT M 1998. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proc Natl Acad Sci U S A, 95, 6469–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPILLANTINI MG, SCHMIDT ML, LEE VM, TROJANOWSKI JQ, JAKES R & GOEDERT M 1997. Alpha-synuclein in Lewy bodies. Nature, 388, 839–40. [DOI] [PubMed] [Google Scholar]
- STAFA K, TRANCIKOVA A, WEBBER PJ, GLAUSER L, WEST AB & MOORE DJ 2012. GTPase activity and neuronal toxicity of Parkinson's disease-associated LRRK2 is regulated by ArfGAP1. PLoS Genet, 8, e1002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAFA K, TSIKA E, MOSER R, MUSSO A, GLAUSER L, JONES A, BISKUP S, XIONG Y, BANDOPADHYAY R, DAWSON VL, DAWSON TM & MOORE DJ 2014. Functional interaction of Parkinson's disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum Mol Genet, 23, 2055–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEGER M, DIEZ F, DHEKNE HS, LIS P, NIRUJOGI RS, KARAYEL O, TONELLI F, MARTINEZ TN, LORENTZEN E, PFEFFER SR, ALESSI DR & MANN M 2017. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEGER M, TONELLI F, ITO G, DAVIES P, TROST M, VETTER M, WACHTER S, LORENTZEN E, DUDDY G, WILSON S, BAPTISTA MA, FISKE BK, FELL MJ, MORROW JA, REITH AD, ALESSI DR & MANN M 2016. Phosphoproteomics reveals that Parkinson's disease kinase LRRK2 regulates a subset of Rab GTPases. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG F, GALLON M, WINFIELD M, THOMAS EC, BELL AJ, HEESOM KJ, TAVARE JM & CULLEN PJ 2013. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol, 15, 461–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRUHAL W, PRESSLAUER S, SPIELBERGER S, ZIMPRICH A, AUFF E, BRUECKE T, POEWE W & RANSMAYR G 2014. VPS35 Parkinson's disease phenotype resembles the sporadic disease. J Neural Transm (Vienna), 121, 755–9. [DOI] [PubMed] [Google Scholar]
- TANG FL, ERION JR, TIAN Y, LIU W, YIN DM, YE J, TANG B, MEI L & XIONG WC 2015a. VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for alpha-Synuclein Degradation and Prevention of Pathogenesis of Parkinson's Disease. J Neurosci, 35, 10613–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG FL, LIU W, HU JX, ERION JR, YE J, MEI L & XIONG WC 2015b. VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function. Cell Rep, 12, 1631–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG FL, ZHAO L, ZHAO Y, SUN D, ZHU XJ, MEI L & XIONG WC 2020. Coupling of terminal differentiation deficit with neurodegenerative pathology in Vps35-deficient pyramidal neurons. Cell Death Differ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEMKIN P, LAUFFER B, JAGER S, CIMERMANCIC P, KROGAN NJ & VON ZASTROW M 2011. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol, 13, 715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG Y, GIAIME E, YAMAGUCHI H, ICHIMURA T, LIU Y, SI H, CAI H, BONVENTRE JV & SHEN J 2012. Loss of leucine-rich repeat kinase 2 causes age-dependent bi-phasic alterations of the autophagy pathway. Mol Neurodegener, 7, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONG Y, YAMAGUCHI H, GIAIME E, BOYLE S, KOPAN R, KELLEHER RJ 3RD & SHEN J 2010. Loss of leucine-rich repeat kinase 2 causes impairment of protein degradation pathways, accumulation of alpha-synuclein, and apoptotic cell death in aged mice. Proc Natl Acad Sci U S A, 107, 9879–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREMPE JF, CHEN CX, GRENIER K, CAMACHO EM, KOZLOV G, MCPHERSON PS, GEHRING K & FON EA 2009. SH3 domains from a subset of BAR proteins define a Ubl-binding domain and implicate parkin in synaptic ubiquitination. Mol Cell, 36, 1034–47. [DOI] [PubMed] [Google Scholar]
- TSIKA E, GLAUSER L, MOSER R, FISER A, DANIEL G, SHEERIN UM, LEES A, TRONCOSO JC, LEWIS PA, BANDOPADHYAY R, SCHNEIDER BL & MOORE DJ 2014. Parkinson's disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum Mol Genet, 23, 4621–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSIKA E, NGUYEN AP, DUSONCHET J, COLIN P, SCHNEIDER BL & MOORE DJ 2015. Adenoviral-mediated expression of G2019S LRRK2 induces striatal pathology in a kinase-dependent manner in a rat model of Parkinson's disease. Neurobiol Dis, 77, 49–61. [DOI] [PubMed] [Google Scholar]
- TUCCI A, NALLS MA, HOULDEN H, REVESZ T, SINGLETON AB, WOOD NW, HARDY J & PAISAN-RUIZ C 2010. Genetic variability at the PARK16 locus. Eur J Hum Genet, 18, 1356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHAUWAERT R, KUENEN S, MASIUS R, BADEMOSI A, MANETSBERGER J, SCHOOVAERTS N, BOUNTI L, GONTCHARENKO S, SWERTS J, VILAIN S, PICILLO M, BARONE P, MUNSHI ST, DE VRIJ FM, KUSHNER SA, GOUNKO NV, MANDEMAKERS W, BONIFATI V, MEUNIER FA, SOUKUP SF & VERSTREKEN P 2017. The SAC1 domain in synaptojanin is required for autophagosome maturation at presynaptic terminals. EMBO J, 36, 1392–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIETRI M, RADULOVIC M & STENMARK H 2020. The many functions of ESCRTs. Nat Rev Mol Cell Biol, 21, 25–42. [DOI] [PubMed] [Google Scholar]
- VILARINO-GUELL C, WIDER C, ROSS OA, DACHSEL JC, KACHERGUS JM, LINCOLN SJ, SOTO-ORTOLAZA AI, COBB SA, WILHOITE GJ, BACON JA, BEHROUZ B, MELROSE HL, HENTATI E, PUSCHMANN A, EVANS DM, CONIBEAR E, WASSERMAN WW, AASLY JO, BURKHARD PR, DJALDETTI R, GHIKA J, HENTATI F, KRYGOWSKA-WAJS A, LYNCH T, MELAMED E, RAJPUT A, RAJPUT AH, SOLIDA A, WU RM, UITTI RJ, WSZOLEK ZK, VINGERHOETS F & FARRER MJ 2011. VPS35 mutations in Parkinson disease. Am J Hum Genet, 89, 162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLPICELLI-DALEY LA, ABDELMOTILIB H, LIU Z, STOYKA L, DAHER JP, MILNERWOOD AJ, UNNI VK, HIRST WD, YUE Z, ZHAO HT, FRASER K, KENNEDY RE & WEST AB 2016. G2019S-LRRK2 Expression Augments alpha-Synuclein Sequestration into Inclusions in Neurons. J Neurosci, 36, 7415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLINGS R, CONNOR-ROBSON N & WADE-MARTINS R 2019. LRRK2 interacts with the vacuolar-type H+-ATPase pump a1 subunit to regulate lysosomal function. Hum Mol Genet, 28, 2696–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG S, MA Z, XU X, WANG Z, SUN L, ZHOU Y, LIN X, HONG W & WANG T 2014. A role of Rab29 in the integrity of the trans-Golgi network and retrograde trafficking of mannose-6-phosphate receptor. PLoS One, 9, e96242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASCHBUSCH D, HUBEL N, OSSENDORF E, LOBBESTAEL E, BAEKELANDT V, LINDSAY AJ, MCCAFFREY MW, KHAN AR & BARNEKOW A 2019. Rab32 interacts with SNX6 and affects retromer-dependent Golgi trafficking. PLoS One, 14, e0208889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASCHBUSCH D, MICHELS H, STRASSHEIM S, OSSENDORF E, KESSLER D, GLOECKNER CJ & BARNEKOW A 2014. LRRK2 transport is regulated by its novel interacting partner Rab32. PLoS One, 9, e111632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEN L, TANG FL, HONG Y, LUO SW, WANG CL, HE W, SHEN C, JUNG JU, XIONG F, LEE DH, ZHANG QG, BRANN D, KIM TW, YAN R, MEI L & XIONG WC 2011. VPS35 haploinsufficiency increases Alzheimer's disease neuropathology. J Cell Biol, 195, 765–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEST AB, MOORE DJ, BISKUP S, BUGAYENKO A, SMITH WW, ROSS CA, DAWSON VL & DAWSON TM 2005. Parkinson's disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A, 102, 16842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEST AB, MOORE DJ, CHOI C, ANDRABI SA, LI X, DIKEMAN D, BISKUP S, ZHANG Z, LIM KL, DAWSON VL & DAWSON TM 2007. Parkinson's disease-associated mutations in LRRK2 link enhanced GTP-binding and kinase activities to neuronal toxicity. Hum Mol Genet, 16, 223–32. [DOI] [PubMed] [Google Scholar]
- WIDER C, SKIPPER L, SOLIDA A, BROWN L, FARRER M, DICKSON D, WSZOLEK ZK & VINGERHOETS FJ 2008. Autosomal dominant dopa-responsive parkinsonism in a multigenerational Swiss family. Parkinsonism Relat Disord, 14, 465–70. [DOI] [PubMed] [Google Scholar]
- WILLIAMS ET, CHEN X & MOORE DJ 2017. VPS35, the Retromer Complex and Parkinson's Disease. J Parkinsons Dis, 7, 219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS ET, GLAUSER L, TSIKA E, JIANG H, ISLAM S & MOORE DJ 2018. Parkin mediates the ubiquitination of VPS35 and modulates retromer-dependent endosomal sorting. Hum Mol Genet, 27, 3189–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON GR, SIM JC, MCLEAN C, GIANNANDREA M, GALEA CA, RISELEY JR, STEPHENSON SE, FITZPATRICK E, HAAS SA, POPE K, HOGAN KJ, GREGG RG, BROMHEAD CJ, WARGOWSKI DS, LAWRENCE CH, JAMES PA, CHURCHYARD A, GAO Y, PHELAN DG, GILLIES G, SALCE N, STANFORD L, MARSH AP, MIGNOGNA ML, HAYFLICK SJ, LEVENTER RJ, DELATYCKI MB, MELLICK GD, KALSCHEUER VM, D'ADAMO P, BAHLO M, AMOR DJ & LOCKHART PJ 2014. Mutations in RAB39B cause X-linked intellectual disability and early-onset Parkinson disease with alpha-synuclein pathology. Am J Hum Genet, 95, 729–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLERT T & HURLEY JH 2010. Molecular mechanism of multivesicular body biogenesis by ESCRT complexes. Nature, 464, 864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XILOURI M, BREKK OR, LANDECK N, PITYCHOUTIS PM, PAPASILEKAS T, PAPADOPOULOU-DAIFOTI Z, KIRIK D & STEFANIS L 2013. Boosting chaperone-mediated autophagy in vivo mitigates alpha-synuclein-induced neurodegeneration. Brain, 136, 2130–46. [DOI] [PubMed] [Google Scholar]
- XIONG Y, COOMBES CE, KILARU A, LI X, GITLER AD, BOWERS WJ, DAWSON VL, DAWSON TM & MOORE DJ 2010. GTPase activity plays a key role in the pathobiology of LRRK2. PLoS Genet, 6, e1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIONG Y, YUAN C, CHEN R, DAWSON TM & DAWSON VL 2012. ArfGAP1 is a GTPase activating protein for LRRK2: reciprocal regulation of ArfGAP1 by LRRK2. J Neurosci, 32, 3877–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YIM YI, SUN T, WU LG, RAIMONDI A, DE CAMILLI P, EISENBERG E & GREENE LE 2010. Endocytosis and clathrin-uncoating defects at synapses of auxilin knockout mice. Proc Natl Acad Sci U S A, 107, 4412–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU WJ, CHENG L, LI NN, WANG L, TAN EK & PENG R 2015. Interaction between SNCA, LRRK2 and GAK increases susceptibility to Parkinson's disease in a Chinese population. eNeurologicalSci, 1, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZARRANZ JJ, ALEGRE J, GOMEZ-ESTEBAN JC, LEZCANO E, ROS R, AMPUERO I, VIDAL L, HOENICKA J, RODRIGUEZ O, ATARES B, LLORENS V, GOMEZ TORTOSA E, DEL SER T, MUNOZ DG & DE YEBENES JG 2004. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol, 55, 164–73. [DOI] [PubMed] [Google Scholar]
- ZAVODSZKY E, SEAMAN MN, MOREAU K, JIMENEZ-SANCHEZ M, BREUSEGEM SY, HARBOUR ME & RUBINSZTEIN DC 2014. Mutation in VPS35 associated with Parkinson's disease impairs WASH complex association and inhibits autophagy. Nat Commun, 5, 3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Y, SHU L, SUN Q, PAN H, GUO J & TANG B 2018. A Comprehensive Analysis of the Association Between SNCA Polymorphisms and the Risk of Parkinson's Disease. Front Mol Neurosci, 11, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO Y, PERERA G, TAKAHASHI-FUJIGASAKI J, MASH DC, VONSATTEL JPG, UCHINO A, HASEGAWA K, JEREMY NICHOLS R, HOLTON JL, MURAYAMA S, DZAMKO N & HALLIDAY GM 2018. Reduced LRRK2 in association with retromer dysfunction in post-mortem brain tissue from LRRK2 mutation carriers. Brain, 141, 486–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMPRICH A, BENET-PAGES A, STRUHAL W, GRAF E, ECK SH, OFFMAN MN, HAUBENBERGER D, SPIELBERGER S, SCHULTE EC, LICHTNER P, ROSSLE SC, KLOPP N, WOLF E, SEPPI K, PIRKER W, PRESSLAUER S, MOLLENHAUER B, KATZENSCHLAGER R, FOKI T, HOTZY C, REINTHALER E, HARUTYUNYAN A, KRALOVICS R, PETERS A, ZIMPRICH F, BRUCKE T, POEWE W, AUFF E, TRENKWALDER C, ROST B, RANSMAYR G, WINKELMANN J, MEITINGER T & STROM TM 2011. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet, 89, 168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMPRICH A, BISKUP S, LEITNER P, LICHTNER P, FARRER M, LINCOLN S, KACHERGUS J, HULIHAN M, UITTI RJ, CALNE DB, STOESSL AJ, PFEIFFER RF, PATENGE N, CARBAJAL IC, VIEREGGE P, ASMUS F, MULLER-MYHSOK B, DICKSON DW, MEITINGER T, STROM TM, WSZOLEK ZK & GASSER T 2004. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron, 44, 601–7. [DOI] [PubMed] [Google Scholar]