Abstract
The Lipid A family of glycolipids, found in the outer membranes of all Gram-negative bacteria, exhibits considerable structural diversity in both lipid and glycan moieties. The lack of facile methods to prepare analogues of these natural products represents a major roadblock in understanding the relationship between their structure and immunomodulatory activities. Here we present a modular, cell-free multienzymatic platform to access these structure–activity relationships. By individually purifying 19 Escherichia coli proteins and reconstituting them in vitro in the presence of acetyl-CoA, UDP-N-acetylglucosamine, NADPH, and ATP, we have developed a system capable of synthesizing Lipid IVA, the first bioactive intermediate in the Lipid A pathway. Our reconstituted multienzyme system revealed considerable promiscuity for orthologs with distinct substrate specificity, as illustrated by swapping enzymes from distantly related cyanobacterial and Pseudomonas species. Analysis of the agonistic and antagonistic activities of the resulting products against the THP-1 human monocytic cell line revealed hitherto unrecognized trends, while opening the door to harnessing the potent biological activities of these complex glycolipid natural products.
Lipid A, the hydrophobic anchor of bacterial lipopolysaccharide (LPS, also known as endotoxin), is not only an essential component of the outer membrane of all Gram-negative bacteria, but also one of the most potent small-molecule elicitors of the eukaryotic innate immune response. Humans have evolved multiple proteins to recognize this glycolipid with high affinity, including lipopolysaccharide binding protein (LBP), CD14, the TLR4-MD2 complex, caspase 4, and caspase 5.1 Notwithstanding impressive advances in the chemical- and biosynthesis of these natural products,2–4 the availability of facile methods to prepare structurally defined analogues remains a major roadblock in our understanding of their remarkable biological properties. Here we present a novel approach to access these structure–activity relationships.
Our target for this study was Lipid IVA, the first bioactive intermediate in the Lipid A biosynthetic pathway (Figure 1). In humans, Lipid IVA is an antagonist of Lipid A by virtue of its ability to bind to the transmembrane receptor TLR4-MD2 without eliciting a response. Synthetic analogues of Lipid IVA have been investigated as potential treatments of severe sepsis,5 viral infections,6 myocardial and renal ischemia-reperfusion injury,7 chronic LPS-mediated airway disease,8 contact lens-associated corneal infection,9 and liver diseases.10 Further, the TRIF-biased TLR4 agonist monophosphoryl Lipid A is clinically approved for use as a vaccine adjuvant (Cervarix, Fendrix).11
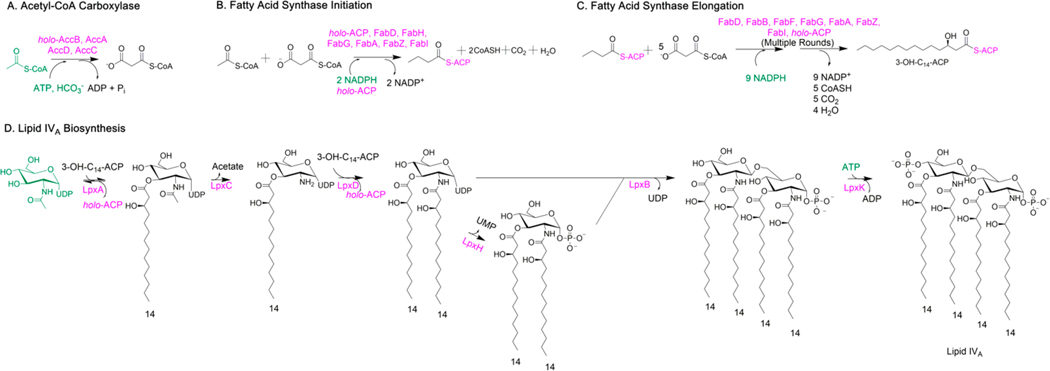
Figure 1.
Enzymatic synthesis of E. coli Lipid IVA. Modules A, B, and C synthesize 3-hydroxymyristoyl-ACP (3-OH-C14-ACP), the acyl-ACP substrate for Lipid IVA biosynthesis, in situ. Module D synthesizes Lipid IVA. Protein components are in pink, while substrates and cofactors are in green.
The building blocks for Lipid IVA biosynthesis are acetyl-CoA and UDP-N-acetylglucosamine (UDP-GlcNAc) with NADPH and ATP as essential cofactors. By individually purifying 19 E. coli proteins and reconstituting them in vitro along with these substrates and cofactors (see Supporting Information), we have developed a chemically defined system capable of synthesizing Lipid IVA at yields and purity required for a cell culture-based investigation of structure–activity relationships.
The biosynthetic pathway of Lipid IVA (Figure 1) can be operationally divided into four modules. Module A, comprised of AccC, AccA, AccD, and holo-AccB (with a tethered biotin cofactor), converts acetyl-CoA into malonyl-CoA. In the presence of NADPH, acetyl-CoA and malonyl-CoA initiate synthesis of a fatty acyl chain via the collaborative activity of FabH, FabD, FabG, FabA or FabZ, FabI, and holo-ACP (which harbors a tethered phosphopantetheine arm) (Module B). Additional malonyl-CoA and NADPH equivalents are consumed by FabB or FabF, FabD, FabG, FabA or FabZ, FabI, and holo-ACP (Module C) to elongate the butyryl-ACP thioester product of module B. Whereas this pathway generates fatty acyl chains of varying lengths and degrees of reduction, a fourth module (Module D) composed of six enzymes (LpxA, LpxC, LpxD, LpxH, LpxB, and LpxK) intercepts the 3-hydroxymyristoyl-ACP intermediate to yield Lipid IVA.
To ascertain the activity of purified enzymatic components of the Lipid A biosynthetic pathway, 5 μM each of LpxA, LpxC, LpxD, LpxH, LpxB, and LpxK were mixed with 10 μM holo-AccB, 10 μM holo-ACP, and 1 μM each of all of the other enzymes shown in Figure 1. Control reactions individually lacking LpxK, LpxK/B, LpxK/B/H, LpxK/B/H/D, and LpxK/B/H/D/C were also performed. As shown in Figure S1, high-resolution LC-ESI-MS analysis verified formation of the expected products, including Lipid IVA, in each reaction mixture. Importantly, the desired peak was the major product of each assay mixture; prior intermediates from the Lipid IVA biosynthetic pathway were undetectable.
To facilitate kinetic analysis of the reconstituted Lipid IVA system, we sought to develop a continuous assay in which NADPH consumption by FabG and FabI could be measured by UV spectrometry (Figures S2–S3). In a control experiment utilizing acyl-ACP thioesterase TesA from E. coli as a release mechanism, Modules A–C exhibited a collective turnover rate of 40 min−1 (Figure 2). When TesA was replaced with equimolar ratios of LpxA, LpxC, LpxD, LpxH, LpxB, and LpxK, we anticipated that 3-hydroxymyristoyl-ACP would be redirected by LpxA, LpxC, and LpxD at some frequency. However, two questions arose. First, to what extent would NADPH consumption capture the activity of LpxA/C/D, given that holo-ACP was present in substantial stoichiometric excess? Second, was the LpxA-catalyzed acyl transfer reaction thermodynamically unfavorable, as had been previously proposed?12
Figure 2.
Effect of sequential addition of LpxA-LpxK on overall turnover rate. The assay mixtures (A–E) incrementally build the Lipid IVA biosynthesis pathway by sequentially introducing 2 μM LpxA/C/D/H/B/K to Modules A–C, shown in Figure 1. (A) No Lpx enzymes, (B) 2 μM LpxA, (C) 2 μM LpxA/C, (D) 2 μM LpxA/C/D, and (E) 2 μM LpxA/C/D/H/B/K. As controls, (F) is the assay mixture, (E) lacking holo-ACP, and (G) corresponds to sample (A) plus 10 μM TesA. Turnover rate is normalized to the concentration of FabG. For representative progress curves, see Figure S3.
In experiments containing LpxA/C/D, modules A–C exhibited a turnover rate of 99 min−1 for at least 20 turnovers (Figures 2 and S3). LpxC was confirmed as the first committed step toward Lipid IVA synthesis (Figure 2 and S3). In the absence of a release mechanism, a turnover rate of 26 min−1 was observed, presumably due to the presence of excess ACP intended to mimic protein concentrations observed in E. coli.13 The excess ACP supported NADPH consumption for approximately two ACP elongations, where one elongation is defined as conversion of holo-ACP to C18-ACP (Figure S3).
It is known that the reconstituted fatty acid synthase from E. coli produces saturated as well as cis-(ω−7) monounsaturated fatty acyl-ACP species.11 Since biosynthetically derived Lipid A in E. coli is exclusively derived from saturated fatty acids, at the outset of this study we expected that unsaturated fatty acyl-ACP species would be dead-end products in our reconstituted system for enzymatic Lipid IVA synthesis. Remarkably, however, Lipid IVA analogues with one or more monounsaturated 3-OH-C14 fatty acyl chains were observed in substantial amounts in addition to the fully saturated natural product (Figure S1). This observation suggested that both LpxA and LpxD have tolerance for unsaturated as well as saturated fatty acyl chains, prompting us to revisit the structure of Lipid A synthesized by E. coli. E. coli DH5α was grown under standard conditions (37 °C), and Lipid A was extracted using an established protocol.14 Consistent with earlier reports, only the fully saturated natural product was observed (Figure S4), suggesting that E. coli harbors a regulatory mechanism to suppress incorporation of monounsaturated acyl chain substrates into the natural product. The precise nature of this regulatory mechanism remains to be elucidated, but we hypothesize it relates to the cocistronic expression of the genes encoding LpxA, LpxD and FabZ in E. coli (Figure S5). Other observations also suggest the activities of FabZ and the enzymes responsible for Lipid A synthesis may be coregulated.15 Therefore, we sought a means of enriching for fully saturated Lipid IVA analogues through rational modifications to the platform’s enzymatic components, as outlined below.
In previous studies with reconstituted fatty acid synthases, we had also observed that, when E. coli FabZ and FabA were replaced with FabZ from the cyanobacterium Synechococcus sp. PCC7002, saturated fatty acids were the dominant products.16 We therefore attempted to engineer the composition of enzymatically synthesized Lipid IVA by an analogous manipulation. As shown in Figure 3A, fully saturated Lipid IVA was by far the most abundant product when E. coli FabZ and FabA were replaced by Synechococcus FabZ. Unexpectedly, however, the product of this reconstituted system predominantly harbored C12 hydroxyacyl chains in lieu of C14 acyl chains. With increasing concentrations of the FabB ketosynthase, it was expected that unsaturated C14 analogues of Lipid IVA would be the dominant products in the presence of E. coli FabA and FabZ;17 indeed, this was found to be the case (Figure 3B).
Figure 3.
Engineering Lipid IVA structure by rational modification of the in vitro enzymatic system. The reaction mixture yielding Product A contained FabZ from Synechococcus sp. PCC 7002 in lieu of both E. coli FabA and FabZ. Product mixture B was synthesized in the presence of E. coli FabA and FabB at a ratio 1.5:10. Product mixture C was synthesized in the presence of LpxA/C/D from P. aeruginosa in lieu of E. coli LpxA/C/D. The reported values of relative abundance were calculated using area under extracted ion chromatograms. Dashed lines (red) indicate potential locations of double bonds. Abundance is normalized to the compound denoted by an asterisk. All reaction products are mixtures, and only major products are shown. For supporting data, see Figures S6–104.
To further test structure modification strategies, E. coli LpxA/C/D was replaced with P. aeruginosa LpxA/C/D. LpxA and LpxD orthologs from P. aeruginosa are known to be selective for C10 and C12 hydroxyacyl chains, respectively.18 While a major reaction product harbored the expected acyl chains, several unanticipated products were also observed (Figure 3C). These side reactions can be explained by the ability of P. aeruginosa LpxD to incorporate C14 hydroxyacyl chain.18 Together, these findings suggest that, while the role of LpxA and LpxD as “hydrocarbon rulers” in selecting acyl chains with appropriate lengths has been explored in isolation,19–23 the fully reconstituted system may shine new light on the control of chain length in vivo.
To demonstrate the promise of this multienzyme system for the synthesis of novel immunomodulatory glycolipids, product mixtures A–C (Figure 3) were tested against the multipotent human monocytic cell line THP-1, which is widely used to verify the ability of LPS to elicit the pro-inflammatory cytokine IL-6.24 In each case the reaction products were further separated from residual quantities of contaminant LPS using mixed mode (anion exchange/normal phase) chromatography (Figure S105, Table S12).
Anti-inflammatory activities of product mixtures A–C were measured by coadministration with 1 ng/mL LPS (Figure S106) to cells that had been freshly differentiated into macrophage-like cultures by pretreatment with phorbol 12-myristate 13-acetate (PMA) (Figure 4A; see Supporting Information for experimental details). On the other hand, pro-inflammatory activities were measured by adding product mixtures alone to PMA-differentiated THP-1 cells (Figure 4B). All three products of enzymatic synthesis showed comparable LPS-antagonistic and TLR4-agonistic activity to a Lipid IVA standard. In previous studies, Lipid IVA showed modest agonistic activity at high concentrations.25 Because glycolipids with dual antagonist–agonist properties are of considerable pharmacological interest (e.g., monophosphoryl Lipid A), we sought to study the immunomodulatory properties of product A in greater depth at a higher concentration (e.g., 100 ng/mL).
Figure 4.
Immunomodulatory activity of enzymatically synthesized Lipid IVA analogues. (A) Antagonistic and (B) agonistic activities of product mixtures A–C (Figure 3) were measured by quantifying IL-6 secreted by macrophage-like cells derived from the human THP-1 monocytic cell line. (C) Product A (100 ng/mL) appears to be a biased TLR4 agonist. For experimental details, see Supporting Information. Data shown is the mean of four or more biological replicates with error bars representing 95% confidence interval. Concentration values are order of magnitude estimates.
Using a multiplexed cytokine quantification assay (Figure S107, Table S13), we compared the agonistic activity of Product A to that of LPS derived from E. coli 0111:B4 strain and synthetic Lipid IVA. As shown in Figure 4C and Figure S107, this product has a remarkable bias toward eliciting IL-22 and, to a lesser extent, TNF-α, compared to IL-6. Thus, Lipid IVA can regulate immune response through cytokine modulation and merits careful analysis in future structure–activity studies. Forthcoming efforts to scale up these multienzyme synthetic systems and to improve the separation of close structural analogues synthesized within the same reaction mixture will aid in further analysis of their unique biological activities.
Not with standing these limitations, the stage is set for a structure–activity analysis of Lipid IVA analogues. Beyond this application, the tool offers unprecedented access to probing the Lipid A biosynthetic pathway; studies are underway in the authors’ laboratory to mechanistically understand the lack of unsaturation in the natural product. Further, as an essential, conserved pathway in many Gram-negative bacteria, these enzymes are an attractive antibiotic target. A long-term goal is to discover novel molecules that interfere with this biosynthetic pathway. Finally, Lipid A-like molecules have been discovered in plants26 and are presumably involved in plant defense responses.27 An enzymatic platform opens the door to investigating Lipid A-like molecules in organisms where genetic modifications are currently intractable.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by grants from the NIH (U19 AI 109662 and R01 GM 087934) to C.K. We thank Dr.Yael Rosenberg-Hasson and Stanford Human Immune Monitoring Center for conducting the multiplex cytokine immunoassay.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b03066.
Materials, methods, and supporting figures (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Xiao X; Sankaranarayanan K; Khosla C. Biosynthesis and Structure-Activity Relationships of the Lipid A Family of Glycolipids. Curr. Opin. Chem. Biol 2017, 40, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kusumoto S; Fukase K; Fujimoto Y. Chemical Synthesis of Bacterial Lipid A In Microbial Glycobiology; Holst O, Brennan PJ, Itzstein M. v., A P, Eds.; Academic Press: San Diego, 2010; Chapter 23, pp 413–427. [Google Scholar]
- (3).Needham BD; Carroll SM; Giles DK; Georgiou G; Whiteley M; Trent MS Modulating the Innate Immune Response by Combinatorial Engineering of Endotoxin. Proc. Natl. Acad. Sci. U. S. A 2013, 110 (4), 1464–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Mamat U; Wilke K; Bramhill D; Schromm AB; Lindner B; Kohl TA; Corchero JL; Villaverde A; Schaffer L; Head SR; Souvignier C; Meredith TC; Woodard RW Detoxifying Escherichia coli for Endotoxin-Free Production of Recombinant Proteins. Microb. Cell Fact 2015, DOI: 10.1186/s12934-015-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Opal SM; Laterre P-F; Francois B; LaRosa SP; Angus DC; Mira J-P; Wittebole X; Dugernier T; Perrotin D; Tidswell M; Jauregui L; Krell K; Pachl J; Takahashi T; Peckelsen C; Cordasco E; Chang CS; Oeyen S; Aikawa N; Maruyama T; Schein R; Kalil AC; Van Nuffelen M; Lynn M; Rossignol DP; Gogate J; Roberts MB; Wheeler JL; Vincent JL Effect of Eritoran, an Antagonist of MD2-TLR4, on Mortality in Patients with Severe Sepsis: The ACCESS Randomized Trial. JAMA 2013, 309 (11), 1154–1162. [DOI] [PubMed] [Google Scholar]
- (6).Shirey KA; Lai W; Scott AJ; Lipsky M; Mistry P; Pletneva LM; Karp CL; McAlees J; Gioannini TL; Weiss J; Chen WH; Ernst RK; Rossignol DP; Gusovsky F; Blanco JCG; Vogel SN The TLR4 Antagonist, Eritoran, Protects Mice from Lethal Influenza Infection. Nature 2013, 497 (7450), 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Akira S; Chong Albert J; Masaki Y; Shin S; Takayama H; Fleisig Ani J; Agnew Matthew L; Hampton Craig R; Rothnie Christine L; Spring Denise J; Pohlman TH; Shimpo H; Verrier ED Inhibition of Toll-like Receptor 4 With Eritoran Attenuates Myocardial Ischemia-Reperfusion Injury. Circulation 2006, 114, I-270–I-274. [DOI] [PubMed] [Google Scholar]
- (8).Savov JD; Brass DM; Lawson BL; McElvania-Tekippe E; Walker JKL; Schwartz DA Toll-like Receptor 4 Antagonist (E5564) Prevents the Chronic Airway Response to Inhaled Lipopolysaccharide. Am. J. Physiol.-Lung Cell. Mol. Physiol 2005, 289 (2), L329–L337. [DOI] [PubMed] [Google Scholar]
- (9).Sun Y; Pearlman E. Inhibition of Corneal Inflammation by the TLR4 Antagonist Eritoran Tetrasodium (E5564). Invest. Ophthalmol. Vis. Sci 2008, 50 (3), 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kitazawa T; Tsujimoto T; Kawaratani H; Fukui H. Therapeutic Approach to Regulate Innate Immune Response by Toll-like Receptor 4 Antagonist E5564 in Rats with D-Galactosamine-Induced Acute Severe Liver Injury. J. Gastroenterol. Hepatol 2009, 24 (6), 1089–1094. [DOI] [PubMed] [Google Scholar]
- (11).Mata-Haro V; Cekic C; Martin M; Chilton PM; Casella CR; Mitchell TC The Vaccine Adjuvant Monophosphoryl Lipid A as a TRIF-Biased Agonist of TLR4. Science 2007, 316 (5831), 1628–1632. [DOI] [PubMed] [Google Scholar]
- (12).Anderson MS; Bull HG; Galloway SM; Kelly TM; Mohan S; Radika K; Raetz CR UDP-N-Acetylglucosamine Acyltransferase of Escherichia coli. The First Step of Endotoxin Biosynthesis Is Thermodynamically Unfavorable. J. Biol. Chem 1993, 268 (26), 19858–19865. [PubMed] [Google Scholar]
- (13).Yu X; Liu T; Zhu F; Khosla C. In Vitro Reconstitution and Steady-State Analysis of the Fatty Acid Synthase from Escherichia coli. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (46), 18643–18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Yi EC; Hackett M. Rapid Isolation Method for Lipopolysaccharide and Lipid A from Gram-Negative Bacteria. Analyst 2000, 125 (4), 651–656. [DOI] [PubMed] [Google Scholar]
- (15).Mohan S; Kelly TM; Eveland SS; Raetz CR; Anderson MS An Escherichia coli Gene (FabZ) Encoding (3R)-Hydroxymyristoyl Acyl Carrier Protein Dehydrase. Relation to FabA and Suppression of Mutations in Lipid A Biosynthesis. J. Biol. Chem 1994, 269 (52), 32896–32903. [PubMed] [Google Scholar]
- (16).Kuo J; Khosla C. The Initiation Ketosynthase (FabH) Is the Sole Rate-Limiting Enzyme of the Fatty Acid Synthase of Synechococcus Sp. PCC 7002. Metab. Eng 2014, 22, 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Xiao X; Yu X; Khosla C. Metabolic Flux Between Unsaturated and Saturated Fatty Acids Is Controlled by the FabA:FabB Ratio in the Fully Reconstituted Fatty Acid Biosynthetic Pathway of E. coli. Biochemistry 2013, 52 (46), 8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Williamson JM; Anderson MS; Raetz CR Acyl-Acyl Carrier Protein Specificity of UDP-GlcNAc Acyltransferases from Gram-Negative Bacteria: Relationship to Lipid A Structure. J. Bacteriol 1991, 173 (11), 3591–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Williamson JM; Anderson MS; Raetz CR Acyl-Acyl Carrier Protein Specificity of UDP-GlcNAc Acyltransferases from Gram-Negative Bacteria: Relationship to Lipid A Structure. J. Bacteriol 1991, 173 (11), 3591–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Anderson MS; Raetz CR Biosynthesis of Lipid A Precursors in Escherichia coli. A Cytoplasmic Acyltransferase That Converts UDP-N-Acetylglucosamine to UDP-3-O-(R-3-Hydroxymyristoyl)-N-Acetylglucosamine. J. Biol. Chem 1987, 262 (11), 5159–5169. [PubMed] [Google Scholar]
- (21).Bartling CM; Raetz CRH Crystal Structure and Acyl Chain Selectivity of Escherichia coli LpxD, the N-Acyltransferase of Lipid A Biosynthesis. Biochemistry 2009, 48 (36), 8672–8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Dotson GD; Kaltashov IA; Cotter RJ; Raetz CR Expression Cloning of a Pseudomonas Gene Encoding a Hydroxydecanoyl-Acyl Carrier Protein-Dependent UDP-GlcNAc Acyltransferase. J. Bacteriol 1998, 180 (2), 330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Raetz CRH; Reynolds CM; Trent MS; Bishop RE Lipid A Modification Systems in Gram-Negative Bacteria. Annu. Rev. Biochem 2007, 76, 295–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Chanput W; Mes JJ; Wichers HJ THP-1 Cell Line: An in Vitro Cell Model for Immune Modulation Approach. Int. Immunopharmacol 2014, 23 (1), 37–45. [DOI] [PubMed] [Google Scholar]
- (25).Kovach NL; Yee E; Munford RS; Raetz CR; Harlan JM Lipid IVA Inhibits Synthesis and Release of Tumor Necrosis Factor Induced by Lipopolysaccharide in Human Whole Blood Ex Vivo. J. Exp. Med 1990, 172 (1), 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Li C; Guan Z; Liu D; Raetz CRH Pathway for Lipid A Biosynthesis in Arabidopsis Thaliana Resembling That of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (28), 11387–11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Newman MA; Daniels MJ; Dow JM The Activity of Lipid A and Core Components of Bacterial Lipopolysaccharides in the Prevention of the Hypersensitive Response in Pepper. Mol. Plant-Microbe Interact 1997, 10 (7), 926–928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.