Abstract
Background
There is concern about the potential of an increased risk related to medications that act on the renin–angiotensin–aldosterone system in patients exposed to coronavirus disease 2019 (Covid-19), because the viral receptor is angiotensin-converting enzyme 2 (ACE2).
Methods
We assessed the relation between previous treatment with ACE inhibitors, angiotensin-receptor blockers, beta-blockers, calcium-channel blockers, or thiazide diuretics and the likelihood of a positive or negative result on Covid-19 testing as well as the likelihood of severe illness (defined as intensive care, mechanical ventilation, or death) among patients who tested positive. Using Bayesian methods, we compared outcomes in patients who had been treated with these medications and in untreated patients, overall and in those with hypertension, after propensity-score matching for receipt of each medication class. A difference of at least 10 percentage points was prespecified as a substantial difference.
Results
Among 12,594 patients who were tested for Covid-19, a total of 5894 (46.8%) were positive; 1002 of these patients (17.0%) had severe illness. A history of hypertension was present in 4357 patients (34.6%), among whom 2573 (59.1%) had a positive test; 634 of these patients (24.6%) had severe illness. There was no association between any single medication class and an increased likelihood of a positive test. None of the medications examined was associated with a substantial increase in the risk of severe illness among patients who tested positive.
Conclusions
We found no substantial increase in the likelihood of a positive test for Covid-19 or in the risk of severe Covid-19 among patients who tested positive in association with five common classes of antihypertensive medications.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (Covid-19), can infect host cells by means of interaction with membrane-bound angiotensin-converting enzyme 2 (ACE2) on respiratory epithelium.1 ACE2 is part of the renin–angiotensin–aldosterone system (RAAS) and its neurohormonal pathways; treatment with RAAS inhibitors can increase tissue expression of ACE2 and its presentation at the cell surface.2 For this reason, it has been suggested that treatment with ACE inhibitors or angiotensin-receptor blockers (ARBs) might increase the risk of Covid-19 after exposure to SARS-CoV-2.2-7 Some have suggested that calcium-channel blockers, which do not act on the RAAS, may be the preferred antihypertensive agents during the Covid-19 pandemic.8 Further fueling these concerns is the observation that hypertension may be associated with an increased risk of death among patients with Covid-19. A large, multicenter study on hypertension and risk of Covid-19 indicated that 24% of patients with severe disease had hypertension, as compared with 14% of patients with nonsevere disease, although that analysis was not adjusted for other clinical features.9
However, ACE2 is protective in animal models of acute lung injury, and pretreatment with ACE inhibitors, ARBs, or beta-blockers may reduce the extent of experimentally induced lung injury and improve outcomes, an effect mediated by inhibition of the RAAS.2,10-15 Thus, others have hypothesized that these medications could theoretically be beneficial, reducing the risk of severe disease among patients with Covid-19.2
Owing to the high global prevalence of hypertension (estimated to be 46% among adults in the United States), the relation between antihypertensive medications and Covid-19 outcomes is very important to public health.2,16 These considerations led the Heart Failure Society of America, the American College of Cardiology, and the American Heart Association to issue a joint statement calling for immediate research into this issue.17 We sought to estimate the association between the use of antihypertensive medications and the likelihood of a positive test for Covid-19 as well as the likelihood of severe Covid-19 (defined as intensive care, mechanical ventilation, or death) in a cohort of patients in a large health care network in New York City, an epicenter of the global Covid-19 pandemic.
Methods
Patient Population
We identified all the patients in the New York University (NYU) Langone Health electronic health record who had Covid-19 test results recorded from March 1 to April 15, 2020, including tests sent to commercial laboratories, tests performed at our local laboratory, and tests ordered by NYU Langone Health providers and conducted at the New York City or State Department of Health. Patients were deemed to be Covid-19–positive if any test was positive for SARS-CoV-2 RNA and Covid-19–negative if all tests were negative.
Medication and History Assessment
For each identified patient with Covid-19 test results, we extracted medical history from the chart on the basis of discretely documented diagnostic codes that had been entered into the medical history or problem list sections of the medical record or on the basis of encounter diagnoses, and we characterized the patient as having or not having a history of hypertension, heart failure, myocardial infarction, diabetes, chronic kidney disease, and obstructive lung disease (e.g., asthma and chronic obstructive pulmonary disease) on the basis of documentation present in the preceding 18 months, because these disorders are often treated with the medications of interest (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). We extracted medication data for each patient, on the basis of the history in the electronic health record, and defined previous treatment with an antihypertensive medication as use of the medication within the preceding 18 months, provided that there was no evidence that the medication was discontinued at least 1 month before the Covid-19 test. The criteria for medication use included clinical documentation in the chart of prescribed medications. Medication lists and problem lists are updated frequently in our electronic health record by clinicians, who are routinely prompted by the system to cross-reference the medication list with pharmacy fill records and to ask patients about adherence.
We report here on the following classes of antihypertensive medication — ACE inhibitors, ARBs, beta-blockers, calcium-channel blockers, and thiazide diuretics. We also analyzed the composite of treatment with ACE inhibitors or ARBs, because they act on the same system and are rarely used together. In this study, we considered sacubitril–valsartan to be an ARB.
Study Oversight
Our observational study was designed by the authors and approved with a waiver of authorization and informed consent by the institutional review board of the NYU Grossman School of Medicine. The data were analyzed by three of the authors, who vouch for the analysis. Four of the authors vouch for the accuracy of the data. The first author prepared the first draft of the manuscript. Four of the authors had full access to the data and were responsible for the decision to submit the manuscript for publication.
Analysis Overview
We calculated the percentages of patients who were Covid-19–negative and who were Covid-19–positive among tested patients, both overall and among patients with a history of hypertension as noted in the electronic health record. Patients with no medical record data other than the Covid-19 test result were excluded from the analysis. Among the patients with a positive Covid-19 test result, we identified patients with severe Covid-19, which was defined as admission to the intensive care unit (ICU), the use of invasive or noninvasive mechanical ventilation, or death. We computed the percentages of patients who met and those who did not meet this definition of severe illness as of April 15, 2020, among patients who tested positive for Covid-19.
To address confounding by indication and other sources of bias arising from the use of observational data, we estimated a propensity score for the likelihood of treatment with each medication class of interest, and we matched patients who had been treated with each medication class to those who had not been treated with that medication class using a 1:1 ratio without replacement, according to the estimated propensity scores.18 We assessed the percentage of patients who tested positive for Covid-19 and the percentage of patients with a positive test who had severe Covid-19, in relation to each medication class, using data before and after matching.
Statistical Analysis
For a given medication class, we used propensity-score models that adjusted for the following variables — age; sex; race; ethnic group; body-mass index; smoking history; history of hypertension, myocardial infarction, heart failure, diabetes, chronic kidney disease, and obstructive lung disease (e.g., asthma and obstructive pulmonary diseases); and other classes of medication. Propensity-score matching was implemented with the use of a nearest-neighbor strategy. The caliper for the matching was specified in the nearest-neighbor strategy if the unspecified approach did not result in satisfactory balance. For body-mass index, the mean value was imputed for patients with missing data. Our goal was to rule out a clinically meaningful difference in risk, which we defined a priori as an absolute difference of at least 10 percentage points in the likelihood of a positive or negative test for Covid-19 and in the likelihood of the presence or absence of severe Covid-19. Our primary analysis involved patients with hypertension. We also analyzed data from all patients, without regard to hypertension.
Before and after creating the matched sets for each medication class, we computed the difference in the likelihood of the outcome between patients who had been treated and those who had not been treated with each medication class, and we sampled from the posterior distribution of the difference in these proportions using the posterior probabilities conditional on the outcomes in each medication group and a prior distribution. As our prior distribution, we assumed a Beta (a,b) distribution, a conjugate to the binomial distribution, with both shape parameters a and b equal to 1. Because very few data are currently available, these uninformative parameters produce prior probabilities of outcomes that are distributed uniformly between 0 and 1. We estimated the difference as the median of the posterior samples, along with a 95% credible interval for the difference. Association between a medication class and an outcome was considered to be present when the 95% credible interval excluded zero. A substantial difference was considered to be present when the lower boundary of the 95% credible interval exceeded zero and the upper boundary exceeded 10 percentage points. We conducted sensitivity analyses using traditional frequentist approaches rather than Bayesian approaches and using adjusted logistic regression rather than propensity-score matching. Analyses were performed with the use of R statistical software, version 3.6.1 (with the libraries MatchIt and BayesianFirstAid).
Results
Characteristics of the Patients
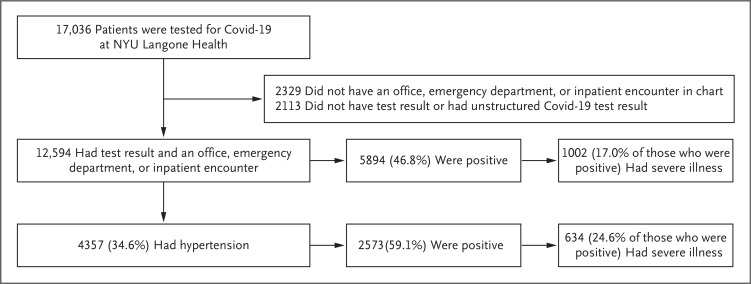
As of April 15, 2020, a total of 12,594 patients had a Covid-19 test result and had an office, emergency department, or inpatient encounter in the NYU Langone Health system. Among these patients, 5894 (46.8%) had a positive test result and 6700 (53.2%) had a negative test result (Figure 1). Among the 5894 patients with a positive test, 1002 (17.0%) had severe Covid-19, as indicated by ICU admission (in 726 patients), mechanical ventilation (in 311), or death (in 447) by April 15, 2020. The characteristics of the patients in the study population are shown in Table 1.
Figure 1. Patient Population.
Patients were deemed to be positive for coronavirus disease 2019 (Covid-19) if any test was positive for SARS-CoV-2 RNA and negative for Covid-19 if all tests were negative. An unstructured result meant that the data extraction algorithm could not identify the result, because it was scanned into the electronic health record rather than entered as discrete data. Severe Covid-19 was defined as admission to the intensive care unit, the use of noninvasive or invasive mechanical ventilation, or death. NYU denotes New York University.
Table 1. Characteristics of the Study Population of Patients Tested for Covid-19.*.
| Characteristic | Patients with Hypertension (N=4357) |
All Patients (N=12,594) |
|---|---|---|
| Median age (interquartile range) — yr | 64 (54–75) | 49 (34–63) |
| Female sex — no. (%) | 2143 (49.2) | 7365 (58.5) |
| Race — no. (%)† | ||
| Black | 875 (20.1) | 1895 (15.0) |
| Asian | 325 (7.5) | 1109 (8.8) |
| White | 2098 (48.2) | 5862 (46.5) |
| Other | 1059 (24.3) | 3728 (29.6) |
| Hispanic ethnic group — no. (%)† | 662 (15.2) | 2106 (16.7) |
| >1 Test for Covid-19 — no. (%) | 693 (15.9) | 1363 (10.8) |
| Smoking status — no. (%) | ||
| Current smoker | 231 (5.3) | 638 (5.1) |
| Former smoker | 1200 (27.5) | 2227 (17.7) |
| Never smoked | 2573 (59.1) | 8420 (66.9) |
| Missing data | 353 (8.1) | 1309 (10.4) |
| Median body-mass index (interquartile range)‡ | 28.7 (24.9–33.1) | 27.4 (24.7–23.9) |
| History of hypertension — no. (%) | 4357 (100) | 4357 (34.6) |
| History of heart failure — no. (%) | 703 (16.1) | 784 (6.2) |
| History of myocardial infarction — no. (%) | 460 (10.6) | 525 (4.2) |
| History of diabetes — no. (%) | 1730 (39.7) | 2271 (18.0) |
| History of chronic kidney disease — no. (%) | 1090 (25.0) | 1214 (9.6) |
| History of obstructive lung disease — no. (%) | 980 (22.5) | 1833 (14.6) |
| Medications — no. (%) | ||
| ACE inhibitor | 954 (21.9) | 1044 (8.3) |
| ARB | 1238 (28.4) | 1328 (10.5) |
| ACE inhibitor or ARB | 2141 (49.1) | 2319 (18.4) |
| Beta-blocker | 1381 (31.7) | 1686 (13.4) |
| Calcium-channel blocker | 1577 (36.2) | 1672 (13.3) |
| Thiazide diuretic | 903 (20.7) | 989 (7.9) |
Percentages may not total 100 because of rounding. ACE denotes angiotensin-converting enzyme, ARB angiotensin-receptor blocker, and Covid-19 coronavirus disease 2019.
Information on race and ethnic group was obtained from entries in the medical record, as reported by the patients.
The body-mass index is the weight in kilograms divided by the square of the height in meters. Data were available for 95.3% of the patient population, and the mean value was imputed in the remaining patients.
Medication Use and Covid-19 Test Results in Patients with Hypertension
Among the 12,594 patients who underwent testing for Covid-19, a total of 4357 (34.6%) had a history of hypertension; of these patients, 2573 (59.1%) were Covid-19–positive and 1784 (40.9%) were Covid-19–negative. The results of Covid-19 testing stratified according to previous treatment with each medication class in matched patients are shown in Table 2. We ruled out an absolute difference of at least 10 percentage points in the likelihood of a positive test with at least 97.5% certainty for ACE inhibitors, ARBs, beta-blockers, calcium-channel blockers, and thiazide diuretics.
Table 2. Likelihood of Positive Test for Covid-19, According to Treatment with Various Antihypertensive Agents, among Propensity-Score–Matched Patients, with Hypertension and Overall.*.
| Medication | Matched Patients with Hypertension | All Matched Patients | ||||
|---|---|---|---|---|---|---|
| Covid-19 in Patients Treated with Medication |
Covid-19 in Patients Not Treated with Medication |
Median Difference (95% CI) |
Covid-19 in Patients Treated with Medication |
Covid-19 in Patients Not Treated with Medication |
Median Difference (95% CI) |
|
| no./total no. (%) | percentage points | no./total no. (%) | percentage points | |||
| ACE inhibitor | 584/954 (61.2) | 583/954 (61.1) | 0.1 (−4.3 to 4.5) | 627/1044 (60.1) | 653/1044 (62.5) | −2.5 (−6.7 to 1.6) |
| ARB | 629/1057 (59.5) | 612/1057 (57.9) | 1.6 (−2.6 to 5.8) | 664/1137 (58.4) | 639/1137 (56.2) | 2.2 (−1.9 to 6.3) |
| ACE inhibitor or ARB | 1019/1692 (60.2) | 986/1692 (58.3) | 2.0 (−1.4 to 5.3) | 1110/1909 (58.1) | 1101/1909 (57.7) | 0.5 (−2.6 to 3.6) |
| Beta-blocker | 792/1381 (57.3) | 829/1381 (60.0) | −2.7 (−6.3 to 1.0) | 912/1686 (54.1) | 976/1686 (57.9) | −3.8 (−7.1 to −0.4) |
| Calcium-channel blocker | 950/1577 (60.2) | 930/1577 (59.0) | 1.3 (−2.2 to 4.7) | 992/1672 (59.3) | 976/1672 (58.4) | 0.9 (−2.3 to 4.3) |
| Thiazide diuretic | 515/903 (57.0) | 520/903 (57.6) | −0.6 (−5.1 to 3.9) | 549/986 (55.7) | 590/986 (59.8) | −4.2 (−8.5 to 0.2) |
Patients were propensity-score matched for age; sex; race; ethnic group; body-mass index; smoking history; history of hypertension, myocardial infarction, heart failure, diabetes, chronic kidney disease, and obstructive lung disease (e.g., asthma and obstructive pulmonary diseases); and other classes of medication. CI denotes credible interval.
The results of the unmatched analyses are shown in Table S2. In the unmatched analyses, several medication classes were associated with a higher likelihood of testing positive for Covid-19, probably owing to confounding by characteristics of the patients who were treated. Results of propensity-score–matching quality are shown in Tables S4 through S9. The standardized mean differences for each class of medication was greatly reduced after matching.
Medication Use and Covid-19 Severity in Patients with Hypertension
Among the 2573 patients with hypertension and positive results on Covid-19 testing, 634 (24.6%) had severe Covid-19, as indicated by ICU admission (in 422 patients), mechanical ventilation (in 165), or death (in 343) by April 15, 2020. The likelihood of severe Covid-19 among patients who tested positive, matched on propensity score for previous treatment with each medication class, is shown in Table 3. We ruled out a substantial difference (≥10 percentage points) in the likelihood of severe illness with at least 97.5% certainty for all the medication classes. There was a slightly higher likelihood of severe illness associated with the previous use of calcium-channel blockers (median difference between treated patients and untreated patients, 4.4 percentage points; 95% credible interval, 0.5 to 8.2). The results of unmatched analyses are provided in Table S3.
Table 3. Likelihood of Severe Covid-19, According to Treatment with Various Antihypertensive Agents, in Propensity-Score–Matched Patients with a Positive Test for Covid-19, with Hypertension and Overall.*.
| Medication | Matched Patients with Hypertension | All Matched Patients | ||||
|---|---|---|---|---|---|---|
| Severe Covid-19 in Patients Treated with Medication |
Severe Covid-19 in Patients Not Treated with Medication |
Median Difference (95% CI) |
Severe Covid-19 in Patients Treated with Medication |
Severe Covid-19 in Patients Not Treated with Medication |
Median Difference (95% CI) |
|
| no./total no. (%) | percentage points | no./total no. (%) | percentage points | |||
| ACE inhibitor | 139/584 (23.8) | 158/583 (27.1) | −3.3 (−8.2 to 1.7) | 150/627 (23.9) | 169/653 (25.9) | −1.9 (−6.6 to 2.8) |
| ARB | 161/629 (25.6) | 156/612 (25.5) | 0.1 (−4.8 to 4.9) | 162/664 (24.4) | 165/639 (25.8) | −1.4 (−6.1 to 3.3) |
| ACE inhibitor or ARB | 252/1019 (24.7) | 249/986 (25.3) | −0.5 (−4.3 to 3.2) | 275/1110 (24.8) | 274/1101 (24.9) | −0.1 (−3.7 to 3.5) |
| Beta-blocker | 210/792 (26.5) | 231/829 (27.9) | −1.4 (−5.7 to 3.0) | 230/912 (25.2) | 250/976 (25.6) | −0.4 (−4.3 to 3.6) |
| Calcium-channel blocker | 253/950 (26.6) | 207/930 (22.3) | 4.4 (0.5 to 8.2) | 263/992 (26.5) | 235/976 (24.1) | 2.4 (−1.4 to 6.2) |
| Thiazide diuretic | 116/515 (22.5) | 114/520 (21.9) | 0.6 (−4.5 to 5.7) | 120/549 (21.9) | 149/590 (25.3) | −3.4 (−8.3 to 1.6) |
Severe Covid-19 was defined as admission to the intensive care unit, the use of noninvasive or invasive mechanical ventilation, or death.
Analyses in All Propensity-Score–Matched Patients
The results of Covid-19 testing stratified according to previous treatment with each medication class in all propensity-score–matched patients, without regard to hypertension history, are shown in Table 2. We ruled out a substantial difference in the likelihood of severe Covid-19 with at least 97.5% certainty for all the medication classes. Patients who had been taking a beta-blocker had a marginally lower likelihood of a positive Covid-19 test than did matched patients who were not taking a beta-blocker (median difference, −3.8 percentage points; 95% credible interval, −7.1 to −0.4). The results of the unmatched analyses are shown in Tables S2 and S3, and the results of propensity-score–matching quality are shown in Tables S10 through S15.
The likelihood of severe Covid-19 among all the patients who tested positive, matched on propensity score for previous treatment with each medication class, is shown in Table 3. We ruled out a substantial difference in the likelihood of severe Covid-19 with at least 97.5% certainty for all the medication classes. Sensitivity analyses with the use of frequentist analyses of propensity-score–matched sets and multivariate logistic regression showed unchanged conclusions in every case (Tables S16 through S19).
Discussion
In this observational analysis in a cohort of more than 12,500 patients who were tested for Covid-19 in a large health network in New York City, previous treatment with medications acting on the RAAS was not associated with a higher risk of testing positive for Covid-19. There was also no substantially higher risk (by ≥10 percentage points) of severe Covid-19 associated with any of the medications studied among the patients with a positive test in our cohort. In most cases, the study sample size permitted us, with a high degree of confidence, to rule out that the risk was higher — even by as little as 6 percentage points — among treated patients than among untreated patients. An effect size smaller than 10 percentage points may be of clinical interest, but we elected to report our findings now before the accrual of a larger sample in order to provide actionable information to the medical community, given the urgency and the rapid progression of the pandemic. Our findings may help to allay concerns on the part of patients and providers regarding the continued use of these agents in patients undergoing testing or receiving treatment for Covid-19.
Our data suggested a modestly lower likelihood of a positive test for Covid-19 among patients taking beta-blockers that was of marginal significance in an analysis that included all matched patients. This finding could be attributable to effects of beta-blockers on the expression or presentation at the cell surface of ACE2, the viral receptor for SARS-CoV-2, or residual confounding in the observational study design. To be relevant in SARS-CoV-2 infection, the effect on ACE2 would need to be present on respiratory epithelium, but relevant studies in animals or humans are limited, so this proposed mechanism remains speculative. Furthermore, we did not observe a similar effect with ACE inhibitors or ARBs, which would be expected to have similar effects on ACE2. Because beta-blockers, ACE inhibitors, and ARBs act at different points in the RAAS, their effects on the risk of Covid-19 or the severity of Covid-19 could potentially differ. Our observation of a lack of association between beta-blocker use and the likelihood of severe Covid-19 argues against an antiviral effect.
Two small studies have shown consistent findings regarding the association between cardiac medications and Covid-19. In a multisite database study that included 78 patients with hypertension who were 65 years of age or older in China, there was no association between previous treatment with any cardiovascular medication class and severe Covid-19, but the risk of severe Covid-19 was lower in a subgroup of 46 patients who had received previous treatment with an ARB than among patients who had not received any of the five drug classes assessed in our study.19 A single-center study from China showed no significant difference in the likelihood of severe illness related to treatment with ACE inhibitors or ARBs in 42 patients.20
A limitation of our study is that variations in the diagnostic characteristics for the Covid-19 testing methods that were used in our cohort may have led to misclassification of Covid-19 status. The true sensitivity of testing remains unknown. Some patients in the cohort underwent multiple tests, which increased the likelihood of identifying disease. At this time, many persons with possible Covid-19 in New York City and elsewhere are not tested, particularly those who are not hospitalized. Thus, our study may include an overestimation of the proportion of cases of severe Covid-19. That said, it is unlikely that reduced access to testing would add systematic bias in assessment of the relation between use of antihypertensive medications and Covid-19.
There are several other caveats to consider in interpretation of our analysis. Ascertainment of medication use from the electronic health record may not reflect actual drug exposure. We did not account for socioeconomic status, insurance, or health care access in propensity scores. However, the medication classes that we studied are available as generic drugs, including within the New York City public hospital system, which serves a largely indigent population. Despite propensity-score matching, there may be additional unmeasured confounders affecting our results. Propensity-score matching aims to create overall balance between comparison groups. Our confidence in the evidence from the primary Bayesian analysis is also supported by the sensitivity analyses that used frequentist methods. Some patients in the NYU Langone Health system may have been tested at other health care systems, and we did not have access to those test results.
We identified no adverse effects of use of medications that act on RAAS pathways on the likelihood of a positive test for Covid-19 among patients who were tested or on the likelihood of severe Covid-19 among patients with a positive test.
Supplementary Appendix
Disclosure Forms
This article was published on May 1, 2020, and updated on May 6, 2020, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrario CM, Jessup J, Chappell MC, et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005;111:2605-2610. [DOI] [PubMed] [Google Scholar]
- 4.Walters TE, Kalman JM, Patel SK, Mearns M, Velkoska E, Burrell LM. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace 2017;19:1280-1287. [DOI] [PubMed] [Google Scholar]
- 5.Ramchand J, Patel SK, Srivastava PM, Farouque O, Burrell LM. Elevated plasma angiotensin converting enzyme 2 activity is an independent predictor of major adverse cardiac events in patients with obstructive coronary artery disease. PLoS One 2018;13(6):e0198144-e0198144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blumenfeld JD, Sealey JE, Mann SJ, et al. Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens 1999;12:451-459. [DOI] [PubMed] [Google Scholar]
- 7.Lezama-Martinez D, Flores-Monroy J, Fonseca-Coronado S, Hernandez-Campos ME, Valencia-Hernandez I, Martinez-Aguilar L. Combined antihypertensive therapies that increase expression of cardioprotective biomarkers associated with the renin-angiotensin and kallikrein-kinin systems. J Cardiovasc Pharmacol 2018;72:291-295. [DOI] [PubMed] [Google Scholar]
- 8.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med 2020;8(4):e21-e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L, Qiu HB, Yang Y, Wang L, Ding HM, Li HP. Losartan, an antagonist of AT1 receptor for angiotensin II, attenuates lipopolysaccharide-induced acute lung injury in rat. Arch Biochem Biophys 2009;481:131-136. [DOI] [PubMed] [Google Scholar]
- 11.Jin F. Losartan, a selective antagonist of AT1 receptor, attenuates acute lung injury induced by seawater inhalation in rats. Chest 2016;149(4):Suppl:A155-A155. [Google Scholar]
- 12.Yao S, Feng D, Wu Q, Li K, Wang L. Losartan attenuates ventilator-induced lung injury. J Surg Res 2008;145:25-32. [DOI] [PubMed] [Google Scholar]
- 13.He X, Han B, Mura M, et al. Angiotensin-converting enzyme inhibitor captopril prevents oleic acid-induced severe acute lung injury in rats. Shock 2007;28:106-111. [DOI] [PubMed] [Google Scholar]
- 14.Matsuishi Y, Jesmin S, Kawano S, et al. Landiolol hydrochloride ameliorates acute lung injury in a rat model of early sepsis through the suppression of elevated levels of pulmonary endothelin-1. Life Sci 2016;166:27-33. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Nie Y, Zheng Y, et al. Esmolol attenuates lung injury and inflammation in severe acute pancreatitis rats. Pancreatology 2016;16:726-732. [DOI] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics — 2019 update: a report from the American Heart Association. Circulation 2019;139(10):e56-e528. [DOI] [PubMed] [Google Scholar]
- 17.HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. American College of Cardiology, March 17, 2020. (https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19).
- 18.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-2281. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Huang F, Xu J, et al. Anti-hypertensive angiotensin II receptor blockers associated to mitigation of disease severity in elderly COVID-19 patients. MedRxiv. 2020. (https://www.medrxiv.org/content/10.1101/2020.03.20.20039586v1.full.pdf) (preprint).
- 20.Meng J, Xiao G, Zhang J, et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect 2020;9:757-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.