Abstract
Introduction
Genetic-guided selection of non-oncologic medications is not commonly practiced in general, and at University of California, San Francisco (UCSF) Health, specifically. Understanding the unique position of clinicians with respect to clinical pharmacogenetics (PG) at a specific institution or practice is fundamental for implementing a successful PG consult service.
Objectives
To assess clinicians’ current practices, needs, and interests with respect to clinical PG at UCSF Health, a large tertiary academic medical center.
Methods
A list of 42 target medications with clinical PG recommendations was complied. Clinical specialties that routinely used the target medications were identified. A 12-question survey focused on practice of PG for target medications was developed. Pharmacists and physicians were surveyed anonymously in several clinical specialties. Survey results were analyzed using descriptive statistics.
Results
Of the 396 clinicians surveyed, 76 physicians and 59 pharmacists participated, resulting in 27% and 50% average response rates, respectively. The current use of PG in clinical practice for physicians and pharmacists was 29% and 32%, respectively, however this number varied across clinical specialties from 0% to 80%. Of clinicians whom reported they do not currently apply PG, 63% of physicians and 54% of pharmacists expressed interest in integrating PG. However, the level of interest varied from 20% to 100% across specialties. Of the respondents, 64% of physicians and 56% of pharmacists elected to provide contact information to investigators to further discuss their interest related to clinical PG.
Conclusions
While PG is not uniformly practiced at UCSF Health, there is considerable interest in utilizing PG by the respondents. Our approach was successful at identifying clinicians and services interested in PG for specific drug-gene pairs. This work has set a foundation for next steps to advance PG integration at UCSF Health. Clinicians can adopt our approach as preliminary work to build a clinical PG program at their institutions.
Keywords: Pharmacogenetics, genetic testing, clinical pharmacy service, pharmacists, physicians, surveys and questionnaires
There are well over 200 medications, oncologic and non-oncologic, with inherited pharmacogenetic (PG) biomarkers discussed in their official United States (US) Food and Drug Administration approved package inserts.(1) Of these, at least 49 medications have specific dosing guidelines established by expert panels in both the US (Clinical Pharmacogenetic Implementation Consortium [CPIC])(2) and Europe (Royal Dutch Association for the Advancement of Pharmacy – Pharmacogenetics Working Group [DPWG]).(3) Despite the availability of translational data necessary for clinical implementation, clinicians in the US do not routinely practice PG.(4, 5) There are only a handful of large research based institutions across the country with organized and centralized clinical PG programs.(6–11) While a seemingly obvious theoretical framework for the implementation of PG is easy to discuss, the practical steps necessary for integrating PG into routine clinical practice are challenging.
Scientific, financial, regulatory, ethical, and process challenges to clinical implementation of PG exist, with limited or lack of payer reimbursement (4, 12) prominent among them. Other specific challenges include limited education of clinicians, slow turnaround time of PG testing, inadequate electronic clinical decision support tools, ineffective integration of PG into the electronic health record (EHR), and limited or lack of cost-effectiveness and clinical utility data in support of PG.(2, 12–18) While these barriers are a general representation of the field of clinical PG, they may not equally contribute to lack or limited practice of PG at specific institutions across the US. For example, for clinicians at Vanderbilt University, electronic decision support may not be a barrier, given that they have an extensive system in place.(10) Similarly, at the University of Chicago, preemptive genotyping of patients prior to the acute need for treatment is used to overcome the barrier associated with slow turnaround time of PG tests.(9) Thus for any institution interested in initiating a PG program, it is important to assess (i.e., “personalize”) the current climate of that institution with respect to PG.
While numerous published studies focus on knowledge and attitudes of clinicians on clinical PG,(5, 17–19) there is a gap in approach for assessment of current practices, needs, and interests of clinicians about clinical PG across specialties. A current assessment of needs and interests of clinicians and an understanding of challenges to the routine application of PG testing across services is essential to integrating PG as a standard of care in general, and at University of California, San Francisco (UCSF) Health, specifically.
The overarching goal of this exploratory study was to better understand current practice surrounding clinical PG in a large tertiary medical center and to identify clinical faculty with a specific interest in the application of PG in their practice (PG Champions). The primary objective of this study was to develop and implement a systematic process and validated survey tool to identify current practices, needs, and interests of clinicians at a large tertiary medical center.
Methods
Study Design
This is a cross-sectional, exploratory, and qualitative study of clinicians at UCSF Health to determine their current practices and needs related to clinical PG.
Medication Selection for Clinician’s Needs Assessment
A list of medications with actionable PG data supported by a strong level of recommendation for drug-gene interactions based on clinical guidelines published by the CPIC and the DPWG as of November 2016 was assembled. Next, we identified the utilization of our targeted medications over a finite period (January 2015 – September 2016) at UCSF Health. To be sure that all of these medications were prescribed by providers at UCSF Heath, for each medication on this list, counts of orders, counts of administration (inpatient only), and counts of distinct patient encounters were extracted for inpatient and outpatient from Epic, the UCSF Health EHR system (called APeX at UCSF Health). The clinicians in the team discussed each medication for inclusion in the survey.
Target Services for Needs Assessment
The target medications selected for the needs assessment were the basis for identifying clinical services that would commonly prescribe these medications. These services were selected based on discussion with clinicians in our team. The following eight services were the target of the needs assessment survey: 1) cardiology, 2) psychiatry, 3) pain management, 4) infectious diseases, 5) oncology, 6) transplant (solid and bone marrow), 7) neurology, and 8) primary care (i.e., internal medicine and family practice).
Survey Development
A 12-question survey was developed to assess clinicians’ current practices related to PG or interest in integrating PG into their practice at UCSF Health (Appendix I). Specifically, the survey included three questions about clinician and practice history (i.e., degree[s] completed, number of years in practice, and area of practice[s]; note: a clinician could select more than one area of practice), followed by a fourth question that specifically asked if they have used PG in their practice within the past 12 months. Clinicians who responded “yes” to question 4 were then presented in questions 5 and 6 with drug-gene pairs populated based on their response to question 3, and asked to identify drug-gene interaction(s) used in the past 12 months and frequency of use (in question 5) and drug-gene interaction(s) that they would like to add to their service, respectively. In both questions 5 and 6, clinicians had the opportunity to add drug-gene interaction(s) not listed in the choices provided. Clinicians, who responded “no” to question 4, were then directed to question 7, where they were asked if they would like to integrate PG into their clinical practice. A “yes” response for question 7 led to question 8, where clinicians were presented with drug-gene pair(s) most appropriate for their area of practice that they could select to add to their clinical service. The clinicians also had the opportunity to free-text drug-gene interactions that were not available in the choices provided, but that they were interested in adding to their clinical practice.
While the survey participants could choose to remain anonymous, given our ultimate project goal of initiation of a clinical PG service, it was important to determine clinical services and practitioners currently using or interested in using PG in their current practice. As such, all clinicians in question 9 of the survey were given an opportunity to provide their name, service, and preferred method of contact as an option. Clinicians who completed question 9 were entered into a raffle to win one of three iPad minis. Regardless of their response to questions 1 through 9, all clinicians were asked to respond to questions 10, 11, and 12. In question 10, clinicians were asked to select from a list of barriers to clinical PG for their practice, including “no barriers”. In question 11, clinicians were asked if observational studies in support of clinical PG were sufficient evidence for making a decision related to PG. Question 12 was a free-text response where clinicians had an opportunity to provide any additional comments on this survey and its goal.
PGPG
Survey Validation
The survey questions were assessed for their clarity and dependability by having them reviewed and piloted by physicians (N=4) and pharmacists (N=8) at UCSF Health who were not part of the study population.
Study Population
A Qualtrics™ (www.qualtrics.com)-based survey platform was used to disseminate and collect the survey data. It is important to acknowledge that our survey can be administered to all health care providers involved in making decisions related to the use of medications. At our institution, PG-guided prescribing is most likely to be performed by either a physician or clinical pharmacist. Therefore, only physicians and pharmacists in targeted specialty services were invited to participate in the survey. To ensure widespread dissemination, we obtained the support of the chief medical officer and the director of pharmacy at UCSF Health. The chief medical officer provided the names of department heads to contact for survey dissemination within the different targeted specialties. The UCSF Committee on Human Subjects Research approved this study and clinicians’ consent was obtained prior to survey participation.
Physician Recruitment
A link to the survey was emailed to the head of nine departments. The department heads were given the following three options. First, to have the head of the department directly redistribute the sent email containing the Qualtrics link to physicians in their department. Second, to provide the email list of physicians in the department to the principal investigator (PI) to email the survey to them directly. The third option was to have a department group meeting with investigators to introduce the survey prior to emailing the link.
Pharmacist Recruitment
The department chair of Clinical Pharmacy in the UCSF School of Pharmacy and director of Pharmaceutical Services at UCSF Health were asked to help with dissemination of the survey to pharmacists at UCSF Health. They were provided with the same options listed above for survey distribution.
Data Analysis
The survey response data were exported to Microsoft® Excel (Microsoft Corporation, Redmond, Washington) for analysis. Contingency tables were created for descriptive statistical analysis of participant demographics and responses to survey questions. All data were summarized by descriptive statistics for physicians and pharmacists separately,
Results
Medication Selection
A list of 49 medications with actionable PG data supported by a strong level of recommendation for drug-gene interactions based on clinical guidelines published by the CPIC and the DPWG as of November 2016 was assembled (Supplementary Table 1). Of these 49 medications, 7 medications were excluded from the survey either because the medication was not prescribed at UCSF Health in 2015 and 2016 (e.g., boceprevir and trimipramine), shifting clinical guidelines limited their use (i.e., peginterferon: 28 patients in 2016; ribavirin: 15 patients in 2016; thioguanine: 7 patients in 2016) and clinical judgment of our team (i.e., metoprolol, haloperidol, carvedilol).
Survey Distribution
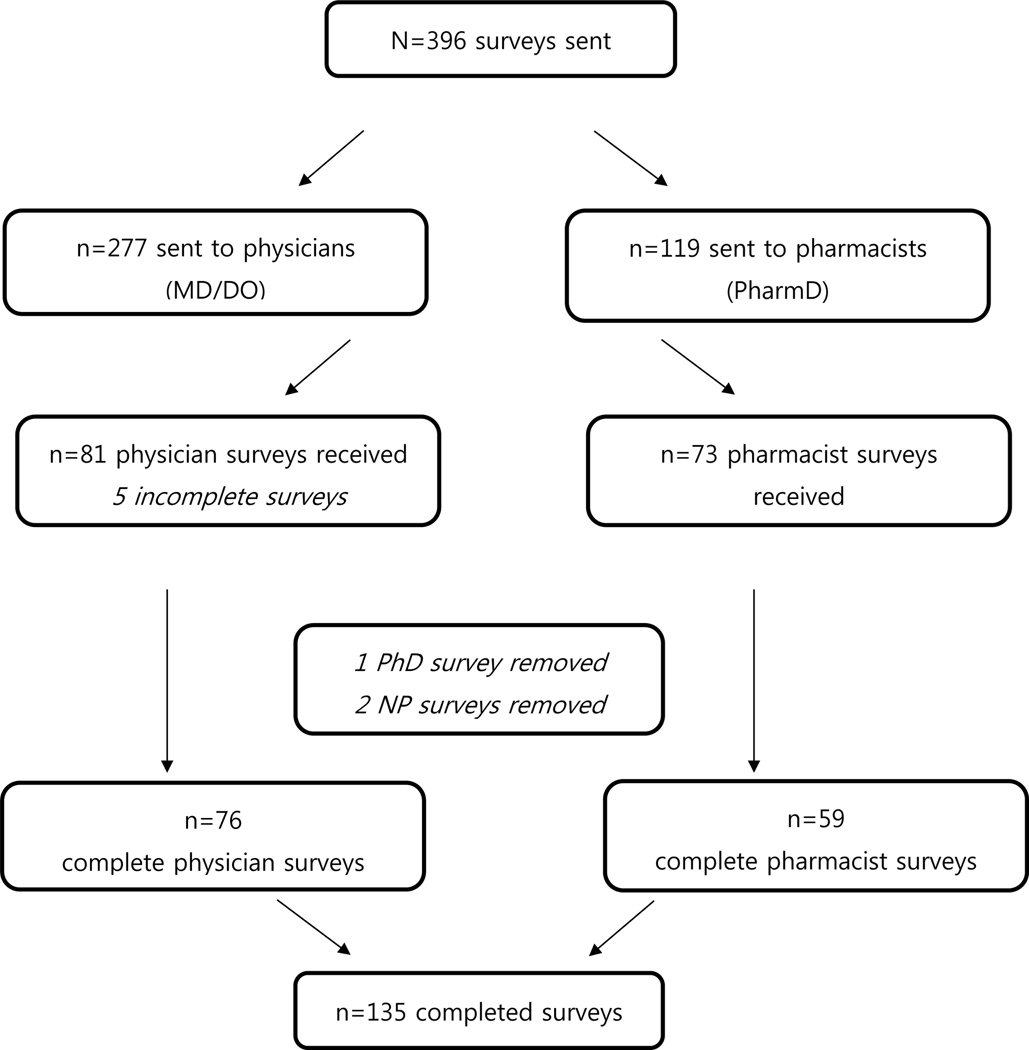
Surveys were sent to 396 clinicians (277 physicians and 119 pharmacists) from January 2017 to June 2017 (Figure 1). The process of reaching the clinicians across different services was not uniform and differed by service. Family practice requested more guidance on PG testing practice prior to sending the survey link out to clinicians in that service and a brief document summarizing CPIC guidelines on medications commonly used in that service was provided to physicians. Two of the provider groups, cardiology and family practice, preferred the PI to introduce the survey to clinicians via a short presentation followed by a question and answer session at their department faculty meeting. For all other departments (n=7), the department head or division chief distributed the survey link through a survey introduction email. The survey link was sent to all physicians within a department a maximum of three times over the study period.
Figure 1.
Flow chart of the number of surveys sent, received, and completed by physicians and pharmacists.
The survey was distributed to the pharmacists by providing the link for the survey along with introductory information in two separate issues of a weekly newsletter from the office of the director of Pharmaceutical Services at UCSF Medical Center to pharmacists. Additionally, the PI was invited to present at a UCSF Health pharmacy managers’ meeting on two occasions prior to distribution of the newsletter.
Survey Results
Of the 396 clinicians to whom a link for the survey was provided, we received 166 responses, of which 135 were complete (Figure 1). A total of 76 physicians and 59 pharmacists completed the survey yielding 27% and 50% response rates, respectively. The number of years in practice for physicians varied, with the majority of physicians (64%) being in practice over 10 years, while 32% of pharmacists had a practice history of over 10 years (Table 1).
Table 1.
Summary of Survey Participants (N=135) and Responses
| Physician Respondents (n=76) (%) | Pharmacist† Respondents (n=59) (%) | |
|---|---|---|
| Number of years in practice | ||
| Less than 5 years | 11 (14) | 20 (34) |
| 5–10 years | 16 (21) | 20 (34) |
| More than 10 years | 49 (64) | 19 (32) |
| Survey response rate | 27% | 50% |
| Medical specialty (response rate) | ||
| Cardiology | 9 (20) | 9 |
| Infectious diseases | 5 (ND‡) | 12 |
| Neurology | 4 (ND‡) | 7 |
| Oncology | 9 (47) | 7 |
| Pain | 11 (48) | 9 |
| Primary care | 16 (27) | 18 |
| Psychiatry | 14 (ND‡) | 5 |
| Transplant | 11 (35) | 17 |
| Currently using PG testing in practice | 22 (29) | 19 (32) |
| Interest in starting PG testing in practice | 48 (63) | 32 (54) |
| Currently not using PG and not interested in integrating PG | 6 (8) | 8 (14) |
| Clinicians indicating interest in discussing their interest in clinical PG with us | 49 (64) | 33 (56) |
| Observational studies sufficient evidence for PG testing | 45 (59) | 35 (59) |
ND = not determined; PG = pharmacogenetics.
Response rate for pharmacists across medical specialties is not determined given that all pharmacists selected more than one clinical specialty in the survey.
Unable to calculate response rate because we did not know the number of physicians who received the survey.
The current reported use of PG testing in clinical practice for physicians and pharmacists was 29% and 32%, respectively (Table 1), however this number varied across services from 0% to 80% (Table 2). Of the clinicians who reported they were not currently using PG in their practice, on average, 63% of physicians and 54% of pharmacists were interested in integrating PG testing into their practice, however their interest varied from a low of 20% (i.e., infectious diseases) to a high of 100% (i.e., cardiology) (Table 2). For example, of eight cardiologists who completed the survey, none reported current use of PG in their practice; however, all were interested in starting PG testing for their practice (Table 2). More than half of the survey respondents (64% of physicians and 56% of pharmacists) self-identified and provided contact information to further discuss their interest related to clinical PG for their service. On average, 59% of physicians and pharmacists were comfortable with evidence obtained through observational studies in support of clinical PG; however, this number varied across services and professions (Table 2). For example, while all cardiologists who completed the survey were interested in clinical PG, only half would consider observational studies as sufficient evidence for integration of PG. However, PG evidence from observational study was sufficient for all oncologists.
Table 2.
Summary of Survey Responses Sorted by Clinical Service†
| Cardiology | Psychiatry | Pain | Infectious Diseases | Oncology | Transplant | Primary Care | Neurology | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PharmD (n=9) (%) | MD (n=8) (%) | PharmD (n=5) (%) | MD (n=9) (%) | PharmD (n=9) (%) | MD (n=11) (%) | PharmD (n=12) (%) | MD (n=5) (%) | PharmD (n=7) (%) | MD (n=9) (%) | PharmD (n=16) (%) | MD (n=11) (%) | PharmD (n=12) (%) | MD (n=16) (%) | PharmD (n=7) (%) | MD (n=4) (%) | |
| Currently using PG testing in practice | 3 (37) | 0 (0) | 2 (40) | 2 (22) | 3 (33) | 3 (27) | 6 (50) | 4 (80) | 2 (29) | 7 (78) | 7 (44) | 3 (27) | 4 (33) | 3 (19) | 1 (17) | 1 (25) |
| Interest in starting PG testing in practice | 5 (62) | 8 (100) | 3 (60) | 7 (78) | 6 (67) | 8 (73) | 6 (50) | 1 (20) | 5 (71) | 2 (22) | 9 (56) | 8 (73) | 8 (67) | 13 (81) | 5 (83) | 3 (75) |
| Observational studies sufficient evidence for PG testing | 8 (89) | 4 (50) | 4 (80) | 3 (33) | 4 (44) | 9 (82) | 8 (67) | 4 (80) | 4 (57) | 9 (100) | 13 (81) | 7 (64) | 10 (83) | 9 (56) | 7 (100) | 3 (75) |
All the pharmacists whom responded to the survey selected more than one clinical specialty in the survey.
PG = pharmacogenetics.
Table 3 summarizes the responses received from eight services for clinicians who currently (within the last 12 months) use PG-based prescribing and clinicians who are not currently using PG but are interested in integrating PG testing for specific drugs-gene pairs. Several agents, such as warfarin or simvastatin, were included in the list of medications for more than one service while others, like phenytoin, were only included in one survey from a neurology service. Current use and interest in specific drug-gene pairs varied across services. For example, none of the cardiologists and primary care physicians reported use of PG for guiding initial warfarin dosing in the past 12 months, but 1 of 4 neurologists reports having used PG to guide warfarin dosing in the past 12 months. However, 38% (n=3) of cardiologists and 50% (n=8) of primary care physicians were interested in integrating warfarin PG in their practice while none of the 3 remaining neurologists were interested in integrating warfarin PG in their practice.
Table 3.
Summary of Survey Results for Clinicians Who Are Currently Using (First Number) PG and Clinicians Who Are Not Currently Using PG But Are Interested in Integration of PG into Clinical Practice (Second Number) for Specific Drug-Gene Pairs Across 8 Different Services
| Gene | Medication | Cardiology | Psychiatry | Pain | Infectious Diseases | Oncology | Transplant | Primary Care | Neurology | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PharmD (n=9) | MD (n=8) | PharmD (n=5) | MD (n=9) | PharmD (n=9) | MD (n=11) | PharmD (n=12) | MD (n=5) | PharmD (n=7) | MD (n=9) | PharmD (n=16) | MD (n=11) | PharmD (n=12) | MD (n=16) | PharmD (n=6) | MD (n=4) | ||
| CYP2C9/ VKORC1 | Warfarin | 0/3 | 0/3 | 1/6 | 0/8 | 0/3 | 1/0 | ||||||||||
| CYP2C9/ HLA-B | Phenytoin | 1/4 | 1/2 | ||||||||||||||
| CYP2C19/CYP2D6 | Sertraline/Citalopram/ Escitalopram/Paroxetine/ Fluoxetine/Fluvoxamine | 1/2 | 2/6 | 1/5 | 0/10 | ||||||||||||
| Imipramine/Doxepin/ Amitriptyline/Clomipramine | 0/3 | 0/6 | 0/4 | 2/6 | 0/6 | 0/5 | |||||||||||
| CYP2C19 | Clopidogrel | 0/3 | 0/4 | 0/4 | 0/0 | ||||||||||||
| Voriconazole | 4/5 | 3/1 | 0/3 | 1/1 | |||||||||||||
| Pantoprazole/Omeprazole/ Lansoprazole | 0/3 | 0/8 | |||||||||||||||
| CYP2D6 | Codeine/Oxycodone | 0/4 | 3/7 | 0/3 | 1/1 | 2/5 | 2/3 | 1/5 | 0/9 | ||||||||
| Venlafaxine | 1/2 | 0/6 | 0/4 | 2/7 | |||||||||||||
| Tramadol | 0/2 | 1/6 | 0/1 | 0/1 | 1/5 | 0/7 | |||||||||||
| Tamoxifen | 0/2 | 0/0 | |||||||||||||||
| Risperidone/Aripiprazole/ Haloperidol | 1/3 | 1/5 | |||||||||||||||
| Propafenone/Flecainide | 0/3 | 0/3 | |||||||||||||||
| Mirtazapine | 0/3 | 0/6 | 0/5 | 0/6 | |||||||||||||
| Atomoxetine | 0/2 | 0/5 | |||||||||||||||
| Ondansetron | 0/4 | 2/6 | 0/3 | 1/2 | 0/5 | 0/3 | |||||||||||
| TPMT | Mercaptopurine/ Azathioprine | 0/2 | 6/1 | ||||||||||||||
| DPYD | Fluorouracil/Capecitabine | 0/4 | 0/0 | ||||||||||||||
| CYP3A5 | Tacrolimus | 0/3 | 0/0 | 5/7 | 2/7 | ||||||||||||
| SLCO1B1 | Simvastatin | 0/1 | 0/2 | 0/6 | 0/7 | ||||||||||||
| G6PD | Rasburicase | 0/2 | 2/1 | ||||||||||||||
| HLA-B | Carbamazepine | 1/3 | 1/2 | 1/4 | 1/2 | ||||||||||||
| Abacavir | 4/3 | 4/1 | 1/4 | 3/0 | |||||||||||||
| Allopurinol | 0/2 | 1/1 | 0/5 | 1/0 | |||||||||||||
| UGT1A1 | Atazanavir | 0/2 | 1/1 | 0/5 | 0/8 | ||||||||||||
| Irinotecan | 1/4 | 1/2 | |||||||||||||||
Tacrolimus-CYP3A5 and voriconazole-CYP2C19 appear to be the most commonly used drug-gene pair by pharmacists in the past 12 months with 31% and 33% of pharmacists having reported the use of PG for these medications, respectively (Table 3). In the physician group, the most commonly used drug-gene pair was mercaptopurine/azathioprine-TPMT with 67% (n=6) of oncologists having reported current use of this PG test. It is clear from these data, with the exception of a few medications in certain services (e.g., warfarin and neurology), that there is interest in integrating PG into clinical practice for most medications on this list by clinicians across services.
Table 4 reports the barriers identified by clinicians in this survey for adoption of PG. The most common barriers (i.e., identified by more than 50% of clinicians) reported by physicians and pharmacists for integrating PG are a lack of established and clear guidelines/protocols for translating test results (68%), limited professional education in PG (59%), and cost/payer restrictions on reimbursement for PG testing (59%).
Table 4.
Barriers to PG Testing Identified by UCSF Health Physicians and Pharmacists Sorted From Highest to Lowest Number of Times Selected by Both Clinicians (i.e., Combined)
| Barriers | Physicians n=76 (%) | Pharmacists n=59 (%) | Combined N=135 (%) |
|---|---|---|---|
| Lack of established and clear guidelines/protocols for translating test results | 51 (67) | 41 (69) | 92 (68) |
| Cost/payer’s restrictions on reimbursement for PG testing | 48 (63) | 32 (54) | 80 (59) |
| Limited professional education in PG | 43 (57) | 37 (63) | 80 (59) |
| Limited internal UCSF PG testing options | 32 (42) | 27 (46) | 59 (44) |
| Ordering PG testing is not easy | 36 (47) | 22 (37) | 58 (43) |
| Turnaround time on PG testing is not practical | 21 (28) | 33 (56) | 54 (40) |
| Lack of a UCSF PG consultation service | 26 (34) | 23 (39) | 49 (36) |
| Lack of point-of-care electronic clinical decision support to utilize PG tests | 28 (37) | 19 (32) | 47 (34) |
| Limited scientific evidence linking test results to health outcomes | 24 (32) | 18 (31) | 42 (31) |
| Diagnostic tests are not FDA-approved | 13 (17) | 6 (10) | 19 (14) |
| There are no barriers for my practice/service. We are using the test | 7 (9) | 3 (5) | 10 (7) |
| Patients do not want PG testing | 1 (1) | 0 (0) | 1 (1) |
FDA = Food and Drug Administration; PG = pharmacogenetic; UCSF = University of California, San Francisco.
Discussion
Given the potential benefits of reducing cost and adverse outcomes, there is a shortage of organized translation of PG research to clinical practice in general,(4) and at UCSF Health, specifically. This lack of routine clinical application may contribute to suboptimal treatment outcomes. To overcome this challenge, our goal is to develop a service that facilitates seamless integration of PG into clinical practice. The first step towards this goal is to obtain a deeper understanding of current practices, needs, interests, and challenges of clinicians about PG. A survey of clinicians across services provided valuable insight on current clinician practices and needs about PG. This information is instrumental in developing a system for organized translation of this science. Indeed, our results indicate that despite the existence of considerable interest in using PG in clinical practice, it is not optimally integrated and uniformly practiced across services. Importantly, given the diverse interest of clinicians for PG testing, the work presented in this study is leading to development of an array-based PG testing capability at UCSF Health.
While numerous published studies focus on knowledge and attitudes of clinicians on clinical PG (5, 17–19), this study focused primarily on PG in clinical practice. To our knowledge, the questions related to clinician needs, practices, and interests have not previously been reported in general, and certainly not for UCSF Health. This study uniquely engaged both physicians and pharmacists within UCSF Health to gain insight about their practice needs and interests related to specific drug-gene combinations across specialties.
The bioinformatics and non-bioinformatics challenges limiting widespread use of clinical PG in practice reported by clinicians at UCSF Health are not novel.(2, 12, 20–22) However, an important limitation, often not documented in literature, is related to the level of evidence that clinicians feel is needed for clinical application of PG. Given that the majority of recommendations published to date related to clinical PG are driven from observational studies, some clinicians see that as insufficient evidence. While a discussion related to level of evidence for practice of clinical PG is beyond the scope of this paper, all efforts targeted towards initiation of such services should determine the willingness of both pharmacists and physicians to adopt PG based on evidence from observational studies.
There are limitations with our approach that need to be acknowledged. First, there was a potential of sampling bias. Although the majority of participants received the survey through an email link, two groups (cardiologists and family practice physicians) received an in person introduction to the survey before it was sent to the team. However, given that the response rate for these services was similar to other services, sampling bias does not appear to have affected these results. Second, calculation of the survey response rate was subject to several limitations. First, the investigators were not in control of whom the survey was sent to, and as such, it was challenging to determine the response rate. Furthermore, the use of email lists may not have included the current group of clinicians in a service. Finally, the overall survey response rate among the physicians and pharmacists is considered below the desired 60% threshold.(23) Survey fatigue of clinicians in a large medical center may have contributed to low response rates and increasing risk of participation bias or non-response bias. We attempted to compensate for survey fatigue by focusing the survey on current practices and limiting the number of questions such that a clinician could complete the survey in less than 10 minutes.
Despite these limitations, we have learned a significant amount about current practices and interests of physicians and pharmacists across several services at UCSF Health. Our survey identified 22 physicians and 19 pharmacists who have used PG in the past 12 months, another 48 physicians and 32 pharmacists who were interested in adopting PG in their services, and lastly, the contact information of 49 physicians and 33 pharmacists that were interested in discussing clinical PG with the authors. This work has given us the basis for planning the next stages of our endeavors to initiate and optimize clinical PG at UCSF Health. Given the exploratory nature of this study, these results are helpful for assessment of clinician needs, interest, and challenges that have hindered wide use of PG at UCSF Health. These finding have informed our subsequent plans for initiation and optimization of clinical PG practices at UCSF Health and we believe that such data can help other institutions with their plans in initiating or optimizing PG practices
In conclusion, as a first step, this qualitative approach for needs assessment was appropriate and sufficient for identifying clinicians and services interested in PG at UCSF Health. These results have set the foundation for next steps towards an organized approach to further PG integration as standard of care for our patients. While this quantitative method is imprecise and the results obtained are limited to practices in services surveyed at UCSF Health, this approach is sufficient to explore the interest and practice of clinicians in other institutions looking to initiate a clinical PG program.
Supplementary Material
Acknowledgments:
We would like to express appreciation for all of the physicians and pharmacists who participated in this survey study. We are also grateful for the department heads, the chief medical officer at UCSF (Dr. Adrienne Greene), the director of pharmaceutical services (Dr. Daniel Wandres), and the PG Pharmacy & Therapeutic subcommittee for supporting this work. We thank the UCSF Medical Center Chancellor’s strategic initiative for funding this study.
Funding: UCSF Medical Center Chancellor’s strategic initiative funds. Kathryn A. Phillips was supported in part by National Cancer Institute R01-CA-221870.
Footnotes
Conflict of interest: The authors have no conflict of interest to disclose.
Contributor Information
Angela Zakinova, School of Pharmacy, University of California San Francisco, San Francisco, CA..
Janel R. Long-Boyle, Department of Clinical Pharmacy, University of California San Francisco, San Francisco, CA..
Deborah French, Department of Laboratory Medicine, University of California San Francisco, San Francisco, CA..
Rhiannon Croci, Department of Health Informatics, University of California San Francisco, San Francisco, CA..
Leslie Wilson, Department of Clinical Pharmacy, University of California San Francisco, San Francisco, CA..
Kathryn A. Phillips, UCSF Center for Translational and Policy Research on Personalized Medicine (TRANSPERS), Department of Clinical Pharmacy, University of California San Francisco, San Francisco, CA..
Deanna L. Kroetz, Department of Bioengineering and Therapeutic Sciences, University of California San Francisco, San Francisco, CA..
Jaekyu Shin, Department of Clinical Pharmacy, University of California San Francisco, San Francisco, CA..
Bani Tamraz, Department of Clinical Pharmacy, University of California San Francisco, San Francisco, CA.
References
- 1.U.S. Food & Drug Administration. Table of pharmacogenomic biomarkers in drug labeling. Available from http://www.fda.gov/drugs/scienceresearch/Researchareas/pharmacogenetics/ucm083378.htm. Accessed January 10, 2018.
- 2.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther 2011;89(3):464–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swen JJ, Wilting I, de Goede AL, et al. Pharmacogenetics: from bench to byte. Clin Pharmacol Ther 2008;83(5):781–7. [DOI] [PubMed] [Google Scholar]
- 4.Stanek EJ, Sanders CL, Taber KA, et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther 2012;91(3):450–8. [DOI] [PubMed] [Google Scholar]
- 5.Haga SB, Burke W, Ginsburg GS, Mills R, Agans R. Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin Genet. 2012;82(4):388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielinski SJ, Olson JE, Pathak J, et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc 2014;89(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldspiel BR, Flegel WA, DiPatrizio G, et al. Integrating pharmacogenetic information and clinical decision support into the electronic health record. J Am Med Inform Assoc 2014;21(3):522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet 2014;166C(1):45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell PH, Bush A, Spitz J, et al. The 1200 patients project: creating a new medical model system for clinical implementation of pharmacogenomics. Clin Pharmacol Ther 2012;92(4):446–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pulley JM, Denny JC, Peterson JF, et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther 2012;92(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weitzel KW, Elsey AR, Langaee TY, et al. Clinical pharmacogenetics implementation: approaches, successes, and challenges. Am J Med Genet C Semin Med Genet 2014;166C(1):56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott SA. Clinical pharmacogenomics: opportunities and challenges at point of care. Clin Pharmacol Ther 2013;93(1):33–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips KA, Ann Sakowski J, Trosman J, Douglas MP, Liang SY, Neumann P. The economic value of personalized medicine tests: what we know and what we need to know. Genet Med 2014;16(3):251–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan J, Wordsworth S, Schuh A. Issues surrounding the health economic evaluation of genomic technologies. Pharmacogenomics 2013;14(15):1833–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Sauver JL, Bielinski SJ, Olson JE, et al. Integrating pharmacogenomics into clinical practice: promise vs reality. Am J Med. 2016;129(10):1093–9 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plumpton CO, Roberts D, Pirmohamed M, Hughes DA. A systematic review of economic evaluations of pharmacogenetic testing for prevention of adverse drug reactions. Pharmacoeconomics 2016;34(8):771–93. [DOI] [PubMed] [Google Scholar]
- 17.Formea CM, Nicholson WT, McCullough KB, et al. Development and evaluation of a pharmacogenomics educational program for pharmacists. Am J Pharm Educ 2013;77(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen Taber KA, Dickinson BD. Pharmacogenomic knowledge gaps and educational resource needs among physicians in selected specialties. Pharmgenomics Pers Med 2014;7:145–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson JF, Field JR, Shi Y, et al. Attitudes of clinicians following large-scale pharmacogenomics implementation. Pharmacogenomics J 2016;16(4):393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JP. Overcoming regulatory and economic challenges facing pharmacogenomics. N Biotechnol 2012;29(6):751–6. [DOI] [PubMed] [Google Scholar]
- 21.Rinke ML, Mikat-Stevens N, Saul R, Driscoll A, Healy J, Tarini BA. Genetic services and attitudes in primary care pediatrics. Am J Med Genet A 2014;164A(2):449–55. [DOI] [PubMed] [Google Scholar]
- 22.Selkirk CG, Weissman SM, Anderson A, Hulick PJ. Physicians’ preparedness for integration of genomic and pharmacogenetic testing into practice within a major healthcare system. Genet Test Mol Biomarkers 2013;17(3):219–25. [DOI] [PubMed] [Google Scholar]
- 23.McLeod CC, Klabunde CN, Willis GB, Stark D. Health care provider surveys in the United States, 2000–2010: a review. Eval Health Prof 2013;36(1):106–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.