Abstract
Background: Liothyronine (LT3) has limited short-term clinical applications, all of which aim at suppressing thyrotropin (TSH) secretion. A more controversial application is chronic administration along with levothyroxine in the treatment of hypothyroidism. Long-term treatment with LT3 is complicated by its unique pharmacokinetics that result in a substantial triiodothyronine (T3) peak in the blood three to four hours after oral dosing. This is a significant problem, given that T3 levels in the blood are normally stable, varying by <10% throughout the day.
Methods: A metal coordinated form of LT3 (Zn[T3][H2O])n, known as poly-zinc-liothyronine (PZL), was synthesized and loaded into coated gelatin capsules for delivery to the duodenum where sustained release of T3 from PZL occurs. Male Wistar rats were made hypothyroid by feeding on a low iodine diet and water containing 0.05% methimazole for five to six weeks. Rats were given a capsule containing 24 μg/kg PZL or equimolar amounts of LT3. Blood samples were obtained multiple times from the tail vein during the first 16 hours, and processed for T3 and TSH serum levels. Some animals were treated daily for eight days, and blood samples were collected daily.
Results: Rats given LT3 exhibited the expected serum T3 peak (about fivefold baseline) at 3.5 hours, followed by a rapid decline, with serum levels almost returning to baseline values by 16 hours. In contrast, serum T3 in PZL-treated rats exhibited about a 30% lower T3 peak at nine hours. Furthermore, the plateau time, that is, the time-span during which the serum T3 concentration is at least half of T3 peak, increased from 4.9 to 7.7 hours in LT3- versus PZL-treated rats, respectively. Serum TSH dropped in both groups, but PZL-treated rats exhibited a more gradual decrease, which was delayed by about four hours compared to LT3-treated rats. Chronic treatment with either LT3 or PZL restored growth, lowered serum cholesterol, and stimulated hepatic expression of the Dio1 mRNA and other T3-dependent markers in the central nervous system.
Conclusion: Capsules of PZL given orally restore T3-dependent biological effects while exhibiting a reduced and delayed serum T3 peak after dosing, thus providing a longer period of relatively stable serum T3 levels compared to capsules of LT3.
Keywords: : hypothyroidism, liothyronine, thyroid hormone, serum T3, serum TSH, slow-release
Introduction
Treatment with levothyroxine (LT4) is the standard of care for hypothyroidism, a condition that affects >10 million Americans and hundreds of millions worldwide (1). Hypothyroidism occurs when the thyroid gland fails to produce normal amounts of thyroid hormones (2). Thyroxine (T4) is the main secretory product of the thyroid gland, and LT4 is the most widely used pharmaceutical form of T4. Thus, the rationale for replacing hypothyroid patients with LT4 is sound. However, T4 is only minimally active. In multiple tissues outside the thyroid gland, T4 is activated to triiodothyronine (T3) after one atom of iodine is removed from the molecule (3). On a daily basis, about 25 μg T3 exits the cells and enters the circulation, mixing with the approximately 5 μg T3 that is secreted directly from the thyroid gland. Serum T3 levels are maintained at fairly stable levels throughout the day via feedback mechanisms at the hypothalamus and pituitary gland, the HPT axis. These mechanisms react to changes in circulating levels of T4 and T3 by adjusting thyrotropin (TSH) secretion, accelerating or slowing down thyroidal activity. Maintaining fairly stable serum T3 levels is a priority for the HPT axis, given that T3 levels are kept quite stable within the normal reference range, even in animals that are genetically modified not to convert T4 to T3 (4–6).
In the years following the introduction of LT4, it was well accepted that LT4-treated hypothyroid patients maintained normal serum levels of T3 via the deiodination process. However, studies of thousands of LT4-treated patients in Europe and the United States revealed that such patients exhibit about 10% lower serum levels of T3 (6,7). This is due to uneven ubiquitination of the type 2 deiodinase (D2) in the hypothalamus versus peripheral tissues, as shown in a thyroidectomized rat model (8). It is unclear whether the minimally reduced serum T3 levels are responsible for any residual symptoms in LT4-treated hypothyroid patients. In rats, however, preclinical data indicate that normalization of serum cholesterol and tissue markers of hypothyroidism in the brain, skeletal muscle, and liver can only be achieved by combining levothyroxine and liothyronine (LT3), the pharmaceutical form of T3 (8).
Indeed, between 10% and 15% of patients on LT4 exhibit residual symptoms, mostly related to weight management, fatigue/energy levels, mood, and memory, despite adherence to treatment and normalization of serum TSH (9,10). For example, hypothyroid patients who are adequately treated with LT4 exhibit a lower metabolic rate (11,12). Furthermore, unbiased analyses utilizing data from the National Health and Nutrition Examination Survey revealed that LT4-treated individuals weigh about 10 pounds more, despite reporting lower calorie intake corrected by body weight (13). They also report 35% lower physical activity levels and are 45–60% more likely to be taking statins, beta-blockers, or antidepressants. Additionally, LT4-treated subjects reported an increased frequency of episodes of memory problems/confusion and were less likely to report being in excellent/good health (13).
To address these residual symptoms, more and more physicians are treating hypothyroid patients with combination therapy (i.e., LT4 plus LT3). It is estimated that about 5% of hypothyroid patients in America use some form of combination therapy (9). However, it is unclear whether combination therapy is superior to monotherapy in managing hypothyroidism. A series of double-blind placebo-controlled trials provided conflicting results, and most indicated that both therapies are effective (1). A recent survey of approximately 12,000 hypothyroid individuals revealed that patients on combination therapy report superior quality of life and are happier with their physicians than patients on LT4 (14).
A complicating factor in treating patients with LT3 is its unique pharmacokinetic (PK) properties (i.e., rapid absorption and clearance rates). Patients taking LT3 exhibit a substantial serum T3 peak (about 40% above baseline) three to four hours after dosing (15). This is significant, given that serum T3 levels are normally stable, not varying by more than 5–10% throughout the day (16). Long-term studies indicate that having even minimally elevated thyroid hormone levels places patients at a significantly greater risk for cardiovascular events (17,18) and hip fractures (19). Indeed, some patients on combination therapy with LT4 and LT3 might complain of palpitations, chest tightness, and/or sweating, but not all double-blinded studies reveal cardiovascular symptoms in such patients (20). Accordingly, substitution of the full replacement dose of LT4 with LT3 typically results in increased “thyrotoxic-like” symptoms (21), and these safety concerns, especially in older adult patients, have reduced the enthusiasm for combination therapy (1). Many have looked for alternatives, and because the peak of T3 is short-lived, some recommend splitting the LT3 tablets in two or three doses daily. However, even smaller, more frequent peaks and valleys of T3 are typically not well tolerated. Thus, despite patient preference and positive results in some clinical trials, due to the lack of safety data and the uncertainty around possible long-term side effects, combination therapy for hypothyroidism has not yet been universally embraced. Notably, in the last revision of the American Thyroid Association guidelines for the treatment of hypothyroidism, the Task Force issued a number of recommendations for future directions, including “development of a sustained release T3 preparation that can then be prospectively tested in clinical trials” (1).
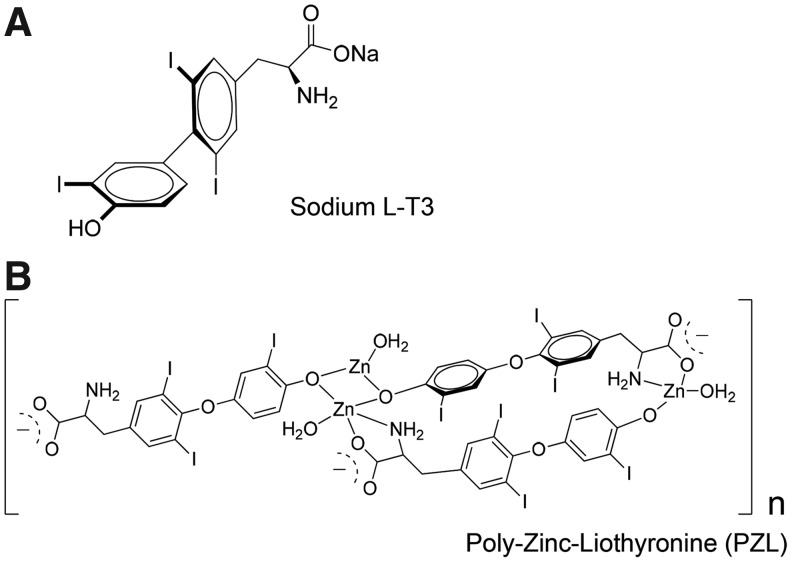
A study utilizing in-house slow-release preparation of LT3 (SR-T3) demonstrated that by extending the absorption time, one could reduce serum T3 peaks (22). SR-T3 used in combination with LT4 improved serum T4 and T3 values, the T4:T3 ratio, and serum TSH compared to treatment with LT4-only (22). Along the same lines, a number of compounding pharmacies offer capsules of LT3 marketed as “slow-release” formulations, but performance and utility in vivo have not been rigorously tested (23). This article describes the preclinical application of poly-zinc-liothyronine (PZL), a metal coordinated LT3 (Fig. 1). Metal coordination involves attaching a pharmaceutically acceptable metal (e.g., zinc, bismuth, or magnesium) to an active pharmaceutical agent in order to modify its PK properties (24). When administered orally to rats, PZL-derived T3 exhibits delayed intestinal absorption and provides relatively stable levels of circulating T3 over time.
FIG. 1.
Schematic representation of (A) sodium liothyronine (LT3) and (B) poly-zinc-liothyronine (PZL).
Methods
Reagents
LT3 or PZL ([Zn(T3)(H2O)]n) was loaded into size #9 gelatin capsules (Torpac, Inc., Fairfield, NJ) coated with Eudragit® L100-55 (Evonik Industries, Parsippany, NJ), which provides protective and sustained-release performance of drugs targeted to the duodenum (Fig. 1). Capsules were prepared by Synthonics, Inc. (Blacksburg VA) and delivered to Rush University Medical Center for testing.
Animals
All experiments were approved by the Rush University Medical Center Institutional Animal Care and Use Committee (IACUC). Male, adult, Wistar rats (10 weeks old) weighing about 320 g were purchased from Envigo (Huntingdon, United Kingdom) and kept two animals per cage under controlled light (12 h/12 h light/dark cycle) and temperature (22°C). Control animals were kept on a standard diet (TD 97350; Envigo) and had access to drinking water ad libitum. Hypothyroidism was induced by feeding animals a low-iodine diet (TD 95007; Envigo) and water containing 0.05% methimazole for five to six weeks. Capsules containing 25 μg/kg PZL or equimolar amounts of LT3 were administered individually through gavage using a stainless steel dosing syringe (Torpac, Inc.). In some experiments, gavage was repeated daily for eight days as indicated. At the indicated times, approximately 250 μL blood was collected from the lateral tail vein of conscious animals in a Microtainer® (BD, Franklin Lakes, NJ; no additive) tubes, incubated at 4°C overnight, centrifuged at 1000 g for 10 min at 4°C, and serum collected. Serum was separated by centrifugation and stored at −20°C.
Experimental design
On the morning of the experiments (9–10am) a baseline blood sample was collected followed by administration of capsules containing the active ingredient (LT3 or PZL). Blood samples were obtained at multiple time points after gavage as indicated. In the eight-day experiment, a baseline blood sample was collected daily between 9am and 10am, before administration of the capsules. Capsules were administered and blood samples collected as indicated. In all experiments, animals were killed by CO2 inhalation immediately after the last blood sample was obtained. Their livers were dissected, snap frozen, and stored at −80°C.
Analytical procedures
Serum TSH (Millipore, Burlington, MA) and T3 (CUSABIO, Wuhan, China) were measured by immunoassays utilizing standard curves prepared with stripped rodent serum, as described previously (25,26). TSH was detected in 4 μL of serum using a multiplex platform where α-TSH is attached to magnetic beads. Immunofluorescence data were obtained in a MAGPIX reader, plotted, and calculated using a five-parameter logistic (5-PL) curve fit. T3 was detected in 35 μL of serum by enzyme-linked immunosorbent assay. Optical density data were obtained in a plate reader, plotted, and calculated using a 4-PL curve fit.
RNA was extracted using the RNeasy Kit (Qiagen, Hilden, Germany) and quantified with a NanoDrop spectrophotometer. cDNA synthesis was performed with the First-Strand cDNA Synthesis Kit for RT-PCR (Roche, Basel, Switzerland) using 100 ng total RNA. Genes of interest were measured by reverse transcription polymerase chain reaction (StepOnePlus real-time PCR system; Applied Biosciences, Beverly Hills, CA) using SYBR Green Supermix (Quanta Biosciences, Inc., Beverly, MA). Standard curves consisting of four or five points of serially diluted mixed experimental and control group cDNA were included. The coefficient of correlation was consistently >0.98, with an amplification efficiency of 80–110%. Primers were used to measure target genes mRNA levels, and cyclophilin A served as an internal control (Table 1). Amplicon specificity was assessed by the melting curve. For serum cholesterol, blood was collected into BD Microtainer (no additive) tubes, incubated at 4°C overnight, centrifuged at 1000 g for 10 min at 4°C, and serum collected. Serum cholesterol was measured with a enzymatic colorimetric assay Cholesterol/Cholesteryl Ester Assay Kit—Quantitation (ab65359; Abcam, Cambridge, United Kingdom).
Table 1.
List of Primers Used in the qRT-PCR Experiments
| Name | Sequence |
|---|---|
| Cyclophilin A (CycloA) F | TTA GGC AGC AAG GGC TTT T |
| Cyclophilin A (CycloA) R | CCA TCC CTC TGG TGA AGC G |
| Iodothyronine deiodinase 1 (Dio1) F | GCA AGA CAG GCT TTC CAG A |
| Iodothyronine deiodinase 1 (Dio1) R | CTG CCT CCT AAA TCC GAC A |
| Iodothyronine deiodinase 2 (Dio2) F | GCC ATT CCC CTG CTG TAA CT |
| Iodothyronine deiodinase 2 (Dio2) R | CCG TCA GTC CAA AGC CAT CT |
| Hairless F | GCC TAC AAA CAG GTT AAA TTG G |
| Hairless R | CCG TCT TCT CTG AGG CAC AAT |
| Myosin heavy chain 7 (Myh7) | TTT GCT GAA GGA CAC TCA AAT C |
| Myosin heavy chain 7 (Myh7) | TTC TGG TTG ATG AGG CTG GT |
qRT-PCR, quantitative reverse transcription polymerase chain reaction.
PK parameters
Simple non-compartmental methods were applied to serum profile data after a single oral dose to estimate basic PK parameters of PZL-derived T3 and to contrast them with those of LT3. The following parameters were calculated for each animal: (i) area under the curve (AUC; based on the trapezoidal method); (ii) time of the maximum serum concentration (tmax); (iii) maximum serum concentration (Cmax); (iv) the plateau time (t1/2), the time-span of one dosing cycle during which the serum concentration is at least half of Cmax (≥1/2 Cmax), as determined by measuring the distance along the time axis at 1/2 Cmax on individual serum profile curves for each rat. Cmax, tmax, AUC, and t1/2 were obtained from averaging the calculated parameters of each individual rat serum T3 profile, as opposed to calculating from a serum profile generated from averaged T3 values. Parameters were calculated using raw data or after baseline correction. The latter was performed by subtracting baseline serum T3 values from all subsequent T3 serum levels.
Statistical analysis
Results are presented as mean ± standard deviation or mean ± standard error of the mean when indicated. As indicated, some graphs are presented utilizing the box and whiskers format. Differences between groups were considered statistically significant when p-values were <0.05. Two groups were compared using Student's t-test, whereas analysis of variance followed by the Newman–Keuls test was used for comparisons between more than two groups.
Results
Single-dose administration of LT3 or PZL to hypothyroid rats: 16 h PK and serum TSH
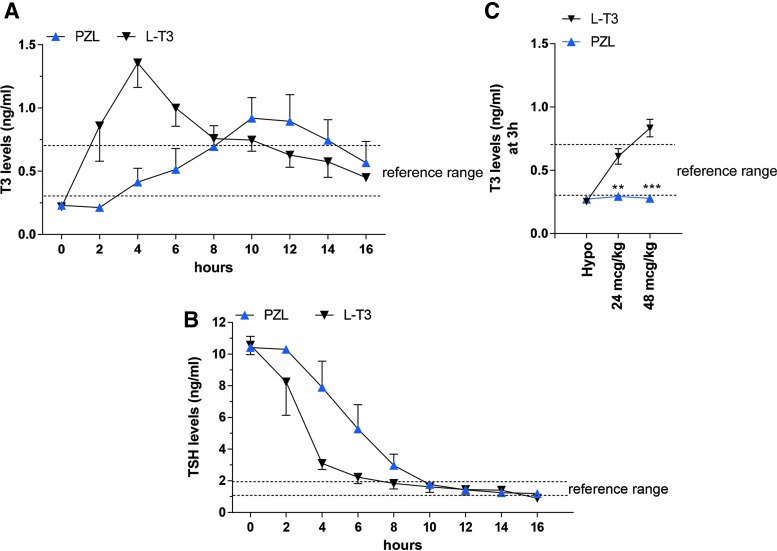
Reference values for serum T3 (0.5 ± 0.1 ng/mL) and TSH (1.5 ± 0.2 ng/mL) levels were obtained from eight intact rats. In the single-dose experiment, the baseline serum T3 concentration was 0.23 ± 0.02 ng/mL, within the expected range for a hypothyroid rat (Fig. 2A). Serum T3 increased rapidly after administration of the 24 μg LT3/kg capsule, reaching a peak by four hours, followed by a progressive decline (Fig. 2A). In the animals that received capsules containing equimolar amounts of PZL, serum T3 concentration rose slowly from baseline, reaching a peak between 10 and 12 hours, subsequently declining at a similar rate as seen in the LT3-treated rats. After 16 h of gavage, serum T3 levels were down to around 0.45 ng/mL in both groups of animals (Fig. 2A). Analyses of the curves confirmed distinct PK parameters for LT3- and PZL-treated rats, whether using raw data or baseline corrected data (Table 2). In the PZL-derived T3 curve, Cmax was 26–29% lower, tmax was 5.5–6.6 hours later, and t1/2 was 2.8–3.3 hours longer. The AUC for serum T3 was around 31% smaller in PZL-treated rats compared to LT3-treated rats, but it is likely that the addition of later time points would have minimized these differences (Table 2).
FIG. 2.
Serum (A) triiodothyronine (T3) and (B) thyrotropin (TSH) levels in hypothyroid rats given 24 μg/kg body weight LT3 (body weight: 300 ± 9.6 g) or equimolar amounts of PZL (body weight: 309 ± 9.3 g) through gavage. (C) Dose–response curve of T3 at three hours in the serum of hypothyroid rats given 24 μg/kg and 48 μg/kg LT3 or PZL. Blood samples were obtained through caudal vein sampling and processed for hormone quantification. Values are the mean ± standard deviation (SD) of four independent samples. An unpaired Student's t-test was used for all comparisons. **p < 0.01; ***p < 0.001. Color images available online at www.liebertpub.com/thy
Table 2.
Pharmacokinetic Parameters Calculated Based on Serum T3 Data Points Obtained from LT3- or PZL-Treated Rats
| Drug | Cmax(ng/mL) | tmax(h) | AUC (ng-h/mL) | t1/2(h) |
|---|---|---|---|---|
| Raw data | ||||
| LT3 | 1.5 ± 0.30 | 3.4 ± 0.94 | 11.5 ± 2.0 | 4.9 ± 0.70 |
| PZL | 1.1 ± 0.25# | 9.0 ± 3.6** | 9.2 ± 2.1## | 7.7 ± 0.85*** |
| Baseline corrected data | ||||
| LT3 | 1.22 ± 0.30 | 3.5 ± 0.69 | 8.4 ± 2.0 | 4.9 ± 0.70 |
| PZL | 0.87 ± 0.24# | 9.0 ± 3.6* | 6.0 ± 1.9# | 7.7 ± 0.85** |
Entries are the mean ± SD of four animals. Parameters were calculated by averaging values obtained for each individual animal.
p ≤ 0.05 vs. LT3; **p ≤ 0.01 vs. LT3; ***p ≤ 0.001 vs. LT3; #p = 0.06 vs. LT3; ##p < 0.08.
LT3, liothyronine; PZL, poly-zinc-liothyronine; Cmax, maximum serum concentration; tmax, time of maximum serum concentration; AUC, area under the curve; t1/2, plateau time; SD, standard deviation.
Serum TSH levels at baseline were about 10 ng/mL, compatible with primary hypothyroidism (Fig. 2B). Administration of LT3 resulted in rapid decline of serum TSH levels that by four hours were about 3.0 ng/mL (70% of baseline) and by 16 hours were about 1.0 ng/mL. In contrast, the serum TSH versus time curve shifted to the right in the animals given PZL between the two- and eight-hour time points. In the animals given PZL, the calculated time for serum TSH to reach 3 ng/mL was around eight hours. By 10 hours, serum TSH levels were similar in LT3- and PZL-treated rats (Fig. 2B).
To test whether the unique PK exhibited by PZL was affected by the dose administered, groups of rats similarly prepared as above were given two doses of PZL, 24 or 48 μg/kg, or equimolar doses of LT3, and killed three hours later. Remarkably, serum T3 levels in the PZL-treated rats remained unaffected, despite doubling of the PZL dose (Fig. 2C). In contrast, LT3-treated rats exhibited further elevation in serum T3 levels, already above the reference range by three hours (Fig. 2C).
Multidose administration of LT3 or PZL to hypothyroid rats: eight-day PK, serum TSH, and biological effects in the liver
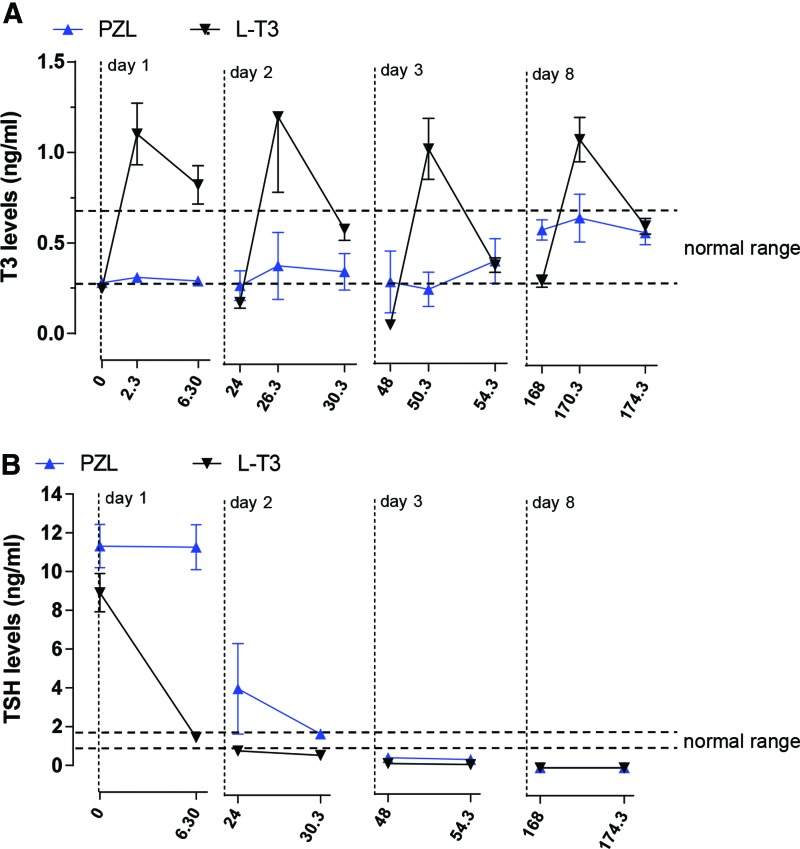
At baseline day 1, serum T3 levels were about 0.25 ng/mL and, during the first six hours after gavage, evolved with different profiles, depending on whether animals were given capsules containing 24 μg LT3/kg or equimolar amounts of PZL (Fig. 3A). In the animals given LT3, serum T3 levels increased sharply by about fourfold and decreased during the next 24 hours to near baseline. This profile was reproduced on days 2, 3, 5, and 8, so that by the end of the experiment, the morning of day 8, baseline T3 levels were indistinguishable from those on day 1 (Fig. 3A).
FIG. 3.
(A, B) As Figure 2, except that animals were dosed daily for eight days. Body weights are shown in the legend to Figure 4. Blood samples at two time points were obtained on the indicated days (i.e., days 1, 2, 3, and 8) before dosing and 6.5 hours later. Values are the mean ± SD of four independent samples. Color images available online at www.liebertpub.com/thy
A very different serum T3 profile was observed in PZL-treated rats. On day 1, baseline T3 levels were around 0.28 ng/mL, with a positive trend after PZL administration (Fig. 3A). The baseline level on day 2 was about 0.4 ng/mL, with minimal fluctuation compared to LT3-treated rats. The buildup continued, and on day 3, baseline levels were around 0.5 ng/mL. On subsequent days, baseline values varied between 0.4 and 0.5 ng/mL (Fig. 3A).
On day 1, serum TSH levels at baseline were around 10 ng/mL (Fig. 3B), and administration of LT3 resulted in a rapid decline that reached around 2.0 ng/mL by six hours. In contrast, serum TSH levels remained unchanged in the animals given PZL up until six hours after gavage (Fig. 3B). On day 2, PZL-treated rats exhibited lower baseline serum TSH, but levels were still higher than in T3-treated rats. Later in the day, six hours after gavage, serum TSH was similar in both T3- and PZL-treated rats (Fig. 3B). On days 3 and 5, serum TSH remained suppressed (Fig. 3B).
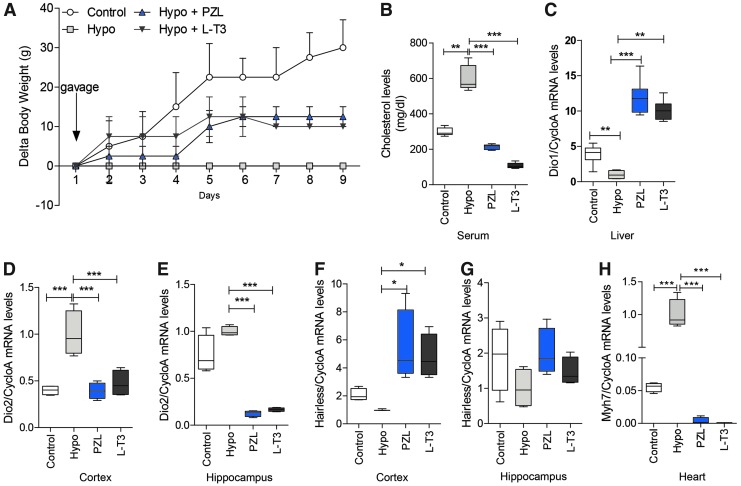
To evaluate systemic T3-dependent processes, the study looked at known T3-dependent parameters, such as changes in body weight (which in this case reflects growth rate), serum cholesterol, and hepatic Dio1 mRNA levels (27,28). Growth (body weight gain) was unchanged in hypothyroid animals but responded dramatically to either LT3 or PZL treatment (Fig. 4A). This is expected, given the positive effects of T3 on growth hormone and insulin growth factor 1 secretion (28). At the same time, serum cholesterol was elevated twofold in hypothyroid rats, and treatment with LT3 or PZL reduced levels to well below the reference range (Fig. 4B), which is compatible with the suppressed serum TSH levels. Notably, both T3- and PZL-treated rats exhibited a five- to sixfold elevation in Dio1 mRNA levels in the liver, a sensitive marker of systemic T3 effects (Fig.4C).
FIG. 4.
Changes in (A) body weight, (B) serum cholesterol, (C) hepatic Dio1 mRNA levels, (D and E) central nervous system Dio2 mRNA levels, (F and G) Hr mRNA levels, and (H) heart Myh7 mRNA levels in all animals studied on Figure 3. Initial body weights were: control, 523 ± 19 g; hypothyroid, 328 ± 13 g; hypothyroid + LT3, 310 ± 12 g; and hypothyroid + PZL, 303 ± 22 g. Animals were killed on the morning of day 9, and tissue samples were prepared for the indicated measurements. There were four animals in each group. Values are the mean ± SD or a box and whiskers format. Gene abbreviations are as shown in Table 2. Analysis of variance followed by the Newman–Keuls test was used for all comparisons. *p < 0.05; **p < 0.01; ***p < 0.001. Color images available online at www.liebertpub.com/thy
The study also analyzed T3-responsive genes in the central nervous system (CNS) and the heart: Dio2 and hairless (Hr) in the cerebral cortex and hippocampus, and myosin heavy chain 7 (Myh7) in the heart. Dio2 mRNA levels were markedly increase in the cerebral cortex, which were normalized by treatment with either LT3 or PZL (Fig. 4D). In the hippocampus, Dio2 mRNA was not affected by hypothyroidism but was markedly reduced by either LT3 or PZL (Fig. 4E). In contrast to Dio2, Hr is positively regulated by T3 in the CNS. Its mRNA levels were reduced in the cerebral cortex and responded positively to both LT3 and PZL (Fig. 4F). In the hippocampus, however, changes in Hr mRNA levels did not reach statistical significance (Fig. 4G). These data confirm that different areas of the CNS respond differently to hypothyroidism and treatment with thyroid hormone. In the heart, Myh7 is negatively regulated by T3. As expected, its mRNA levels were markedly increased in hypothyroid rats but greatly reduced by treatment with either T3 or PZL (Fig. 4H). Most importantly, the effects of PZL-derived T3 on T3-responsive genes in the liver, CNS, and heart mimicked those of LT3.
Discussion
LT3 is used in the clinical setting of investigating suspected thyroid autonomy, in patients with thyroid cancer being prepared for total body scans, or in the differential diagnosis between TSH-secreting tumors and resistance to thyroid hormone syndrome (29). In all these cases, treatment is short term, lasting one to two weeks, and intended to cause transient systemic thyrotoxicosis to suppress TSH secretion. LT3 can also be given chronically to manage hypothyroid patients, but in this case its utilization is controversial. The commonly observed peak of T3 in serum three to four hours after LT3 dosing may be associated with undesirable side effects that are primarily cardiac in nature (15). This article reports the development of PZL, a novel metal coordinated T3 molecule that slowly releases bioavailable T3 in the duodenum for systemic absorption. Using the hypothyroid rat model, it is shown that oral administration of PZL minimizes post-dose peaks in circulating T3 typically seen with LT3 administration (Fig. 2A), without interfering with T3-dependent biological effects (Fig. 4). That the unique PK of PZL-derived T3 results in beneficial pharmacodynamics (PD) is illustrated by a four-hour delay in TSH suppression (Fig. 2B) and in sustained fasting serum T3 levels after eight days of daily doses of PZL (Fig. 3A). The latter indicates that PZL-derived T3 is absorbed at such a reduced rate that even 24 hours after PZL dosing, serum T3 is higher than baseline. After a few daily doses of PZL, T3 levels in the circulation are even higher, within the reference range, reflecting less fluctuation during the 24-hour period between doses, much closer to normal T3 homeostasis compared to dosing with LT3.
The search for a thyroid hormone replacement strategy that restores physiologic serum T3 levels is not new. This pursuit became particularly relevant several years ago after the demonstration in rodents that LT4 therapy alone is incapable of normalizing serum TSH and T3 levels in all tissues (30,31). Later, it was verified that LT4-treated hypothyroid rats with a normal serum TSH exhibit signs of hypothyroidism in the liver, skeletal muscle, and brain, which are normalized with therapy containing both LT4 and LT3 (8). Delivery routes such as through the skin, as commonly used for steroids, seem inviable, given the rapid D3-mediated thyroid hormone catabolism in this organ (32). Other studies have focused on oral LT3 capsules coated with insoluble matrixes or other formulations to create “slow-release” versions of LT3, with promising results in vitro (23) and in patients (22). In addition, taking advantage of the fact that liver contains desulfatase enzymes, an ingenious strategy was devised in which an excess of the biologically inactive T3 sulfate was given orally to patients with hypothyroidism (33). The goal was for the liver to become a steady-state depot/source of endogenous T3 production defined by the velocity of hepatic desulfatization. Indeed, in hypothyroid patients, administration of T3 sulfate resulted in an increase in serum T3 concentration at one hour post dose that increased further at two and four hours and remained steady up to 48 hours in initial studies.
Despite all efforts and experimental approaches, however, a clinically acceptable and consistent oral formulation for sustained release of LT3 is still lacking today.
PZL is a polymeric complex composed of divalent zinc ions bound to a tridentate dianion of T3. This tridentate form of T3 possesses two ionizable functional groups—amino acid and phenol—capable of forming two coordinate covalent bonds with a metal. Due to the phenol group, tyrosine and T3 are the only natural amino acids that can act as tridentate ligands, which favors formation of supramolecular or polymeric structures (34). The supramolecular structure of PZL possesses many characteristics that promote intestinal mucoadhesion, including its novel polymeric nature, an ability to form non-covalent bonds with the negatively charged mucosal surface, and electrostatic interactions between Zn2+ and the mucosa (35). Both the slower rate of T3 absorption (Fig. 2A) and experience with other metal coordinated molecules support a mechanism by which T3 molecules separate from the metal complex by exchange with endogenous ligands before absorption into the bloodstream. The combination of PZL's mucoadhesive properties and the ligand exchange rate contributes to the slower rate of T3 delivery and the extended period of T3 absorption.
The present results obtained after acute administration of PZL to hypothyroid rats are encouraging. Compared to rats acutely treated with LT3, PZL-treated rats exhibit lower Cmax, later tmax, and longer t1/2 (plateau time), while exhibiting a similar (slightly smaller) AUC (Table 2). In other words, when equimolar amounts of LT3 are delivered as PZL (vs. LT3), serum T3 levels do not exhibit a rapid peak. Rather, serum T3 levels are sustained over time with a delayed but more desirable biological response. The latter is illustrated by the more gradual drop in serum TSH levels, which was delayed by about four hours in PZL-treated rats compared to LT3-treated rats (Fig. 2B).
It is notable that due to the relatively fast T3 kinetics, there is little buildup of T3 in the circulation over time in rats chronically treated with LT3 (Fig. 3A). Baseline T3 levels were barely affected, remaining below the reference range, even after eight days of daily administration of LT3 capsules. In contrast, chronic administration of PZL resulted in doubling of baseline serum T3 levels in the morning of the eighth day, resulting in levels that were within the normal reference range (Fig. 3A). These data indicate that sustained absorption of PZL-delivered T3 attenuates unwanted serum peaks and prolongs T3 bioavailability over time, permitting elevated baseline serum T3 levels. As a result, the biological effects observed with L-T3 and PZL-derived T3 were similar, that is, body weight gain (Fig. 4A), serum cholesterol (Fig. 4B), and hepatic Dio1 mRNA levels (Fig. 4C), despite the absence of serum T3 peak observed in the LT3-treated rats. The same was true for markers of T3 action, that is, Dio2 and Hr mRNA levels in the CNS (Fig. 4D-G), and Myn7 mRNA levels in the heart (Fig. 4H).
PZL contains LT3, a pharmaceutical approved by the Food and Drug Administration, and Zn, an essential mineral for catalytic, structural, and regulatory physiological functions, with a recommended dietary allowance (RDA) of 10 mg/day. Both T3 and Zn are present in PZL at a 1:1 ratio. The chemistry and drug product manufacturing of PZL present negligible potential for human risk due to the substantial overlap with processes and substances associated with the reference product, LT3. Furthermore, the expected daily dose of Zn in PZL is <1/1000 of the RDA for the metal.
While the objective benefit of adding LT3 to therapy with LT4 remains controversial, one could argue that the intrinsically unfavorable PK and PD of conventional LT3 have limited the effectiveness of all clinical trials to date, whether prospective or retrospective in nature, and thus have frustrated attempts to address this issue. It is also known that normalization of serum TSH with LT4 monotherapy to hypothyroid patients results in 5–10% lower serum T3 (7,13). Similar results were obtained in rats (8,30,31). By adding LT3 to the LT4 regimen, it is expected that normalization of serum TSH will occur with a slightly lower serum T4 and slightly higher serum T3, as seen in humans (20) and rats (8,30,31). Although clinical data are not available, a normalization of serum T3 in preclinical studies utilizing thyroidectomized rats normalized a series of T3-dependent parameters in the liver, skeletal muscle, and CNS (8). This is probably because in a number of tissues, including the liver and kidney, most T3 bound to nuclear thyroid receptors originates from circulating T3, as opposed to the brain, pituitary gland, and brown adipose tissue where about half of the T3 is produced within the tissue from local T4 activation (36,37). Thus, plasma remains an important source of tissue T3, even in those tissues that express D2 and are capable of activating T4 locally.
The current results carry significant potential implications for patients who suffer from hypothyroidism. The continued development of PZL or PZL-like molecules that provide stable serum T3 levels will reveal the clinical relevance if any of normalizing serum T3 in LT4-treated patients. It will allow for more measured comparisons through future clinical studies between monotherapy with LT4 and combination therapies with LT4 and a true sustained release formulation of LT3 (PZL or PZL-like molecules) in the treatment of hypothyroidism.
Acknowledgments
The authors are grateful to Synthonics, Inc., for kindly making PZL available for testing, and to Drs. Thomas Piccariello, Scott Palmer, and John Price for invaluable advice and assistance in planning, performing, and interpreting these studies.
Author Disclosure Statement
A.C.B. is a consultant for Sentier Therapeutics LLC and Synthonics, Inc. For all other authors, no competing financial interests exist.
References
- 1.Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, Cooper DS, Kim BW, Peeters RP, Rosenthal MS, Sawka AM; American Thyroid Association Task Force on Thyroid Hormone Replacement 2014. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association Task Force on Thyroid Hormone Replacement. Thyroid 24:1670–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaker L, Bianco AC, Jonklaas J, Peeters RP. 2017. Hypothyroidism. Lancet 390:1550–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 4.Christoffolete MA, Arrojo e Drigo R, Gazoni F, Tente SM, Goncalves V, Amorim BS, Larsen PR, Bianco AC, Zavacki AM. 2007. Mice with impaired extrathyroidal thyroxine to 3,5,3′-triiodothyronine conversion maintain normal serum 3,5,3′-triiodothyronine concentrations. Endocrinology 148:954–960 [DOI] [PubMed] [Google Scholar]
- 5.Galton VA, Schneider M, Clark AS, Germain DL. 2009. Life without T4 to T3 conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology 150:2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdalla SM, Bianco AC. 2014. Defending plasma T3 is a biological priority. Clin Endocrinol 81:633–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gullo D, Latina A, Frasca F, Le Moli R, Pellegriti G, Vigneri R. 2011. Levothyroxine monotherapy cannot guarantee euthyroidism in all athyreotic patients. PLoS One 6:e22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werneck de Castro JP, Fonseca TL, Ueta CB, McAninch EA, Abdalla S, Wittmann G, Lechan RM, Gereben B, Bianco AC. 2015. Differences in hypothalamic type 2 deiodinase ubiquitination explain localized sensitivity to thyroxine. J Clin Invest 125:769–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAninch EA, Bianco AC. 2016. The history and future of treatment of hypothyroidism. Ann Intern Med 164:50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAninch EA, Bianco AC. 2015. New insights into the variable effectiveness of levothyroxine monotherapy for hypothyroidism. Lancet Diabetes Endocrinol 3:756–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorman CA, Jiang NS, Ellefson RD, Elveback LR. 1979. Comparative effectiveness of dextrothyroxine and levothyroxine in correcting hypothyroidism and lowering blood lipid levels in hypothyroid patients. J Clin Endocrinol Metab 49:1–7 [DOI] [PubMed] [Google Scholar]
- 12.Samuels MH, Kolobova I, Smeraglio A, Peters D, Purnell JQ, Schuff KG. 2016. Effects of levothyroxine replacement or suppressive therapy on energy expenditure and body composition. Thyroid 26:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peterson SJ, McAninch EA, Bianco AC. 2016. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab 101:4964–4973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterson SJ, Cappola AR, Castro MR, Dayan CM, Farwell AP, Hennessey JV, Kopp PA, Ross DS, Samuels MH, Sawka AM, Taylor PN, Jonklaas J, Bianco AC. 2018. An online survey of hypothyroid patients captured predominantly dissatisfied individuals. Thyroid 28:707–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saravanan P, Siddique H, Simmons DJ, Greenwood R, Dayan CM. 2007. Twenty-four hour hormone profiles of TSH, free T3 and free T4 in hypothyroid patients on combined T3/T4 therapy. Exp Clin Endocrinol Diabetes 115:261–267 [DOI] [PubMed] [Google Scholar]
- 16.Russell W, Harrison RF, Smith N, Darzy K, Shalet S, Weetman AP, Ross RJ. 2008. Free triiodothyronine has a distinct circadian rhythm that is delayed but parallels thyrotropin levels. J Clin Endocrinol Metab 93:2300–2306 [DOI] [PubMed] [Google Scholar]
- 17.Biondi B, Fazio S, Palmieri EA, Tremalaterra R, Angellotti G, Bone F, Riccio G, Cittadini A, Lombardi G, Sacca L. 1999. Effects of chronic subclinical hyperthyroidism from levothyroxine on cardiac morphology and function. Cardiologia 44:443–449 [PubMed] [Google Scholar]
- 18.Sawin CT, Geller A, Wolf PA, Belanger AJ, Baker E, Bacharach P, Wilson PW, Benjamin EJ, D'Agostino RB. 1994. Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. New Engl J Med 331:1249–1252 [DOI] [PubMed] [Google Scholar]
- 19.Bauer DC, Ettinger B, Nevitt MC, Stone KL. 2001. Risk for fracture in women with low serum levels of thyroid-stimulating hormone. Ann Intern Med 134:561–568 [DOI] [PubMed] [Google Scholar]
- 20.Jonklaas J. 2016. Risks and safety of combination therapy for hypothyroidism. Expert Rev Clin Pharmacol 9:1057–1067 [DOI] [PubMed] [Google Scholar]
- 21.Jonklaas J, Burman KD. 2016. Daily administration of short-acting liothyronine is associated with significant triiodothyronine excursions and fails to alter thyroid-responsive parameters. Thyroid 26:770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennemann G, Docter R, Visser TJ, Postema PT, Krenning EP. 2004. Thyroxine plus low-dose, slow-release triiodothyronine replacement in hypothyroidism: proof of principle. Thyroid 14:271–275 [DOI] [PubMed] [Google Scholar]
- 23.Hamid B, Clayton C, Gilzad K, Shabnam S. 2017. Kinetic analysis of drug release from compounded slow-release capsules of liothyronine sodium (T3). Int J Pharm Compd 21:418–425 [PubMed] [Google Scholar]
- 24.Price JD, Piccariello T, Palmer S. 2014. Metal-coordinated pharmaceuticals. Drug Dev Deliv 14:34–39 [Google Scholar]
- 25.Fonseca TL, Correa-Medina M, Campos MP, Wittmann G, Werneck-de-Castro JP, Arrojo e Drigo R, Mora-Garzon M, Ueta CB, Caicedo A, Fekete C, Gereben B, Lechan RM, Bianco AC. 2013. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest 123:1492–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nascimento BPP, Bocco B, Fernandes GW, Fonseca TL, McAninch EA, Cardoso CV, Bondan EF, Nassif RJ, Cysneiros RM, Bianco AC, Ribeiro MO. 2018. Induction of type 2 iodothyronine deiodinase after status epilepticus modifies hippocampal gene expression in male mice. Endocrinology 159:3090–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zavacki AM, Ying H, Christoffolete MA, Aerts G, So E, Harney JW, Cheng SY, Larsen PR, Bianco AC. 2005. Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology 146:1568–1575 [DOI] [PubMed] [Google Scholar]
- 28.Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, Refetoff S, Sharlin DS, Simonides WS, Weiss RE, Williams GR. 2014. American Thyroid Association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid 24:88–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen PR, Davies T, Schlumberger M, Hay I. 2007. Thyroid physiology and diagnostic evaluation of patients with thyroid disorders. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS. (eds) Williams' Textbook of Endocrinology. Saunders, New York, NY, pp 299–332 [Google Scholar]
- 30.Escobar-Morreale HF, Rey F, Obregon MJ, Escobar GM. 1996. Only the combined treatment with thyroxine and triiodothyronine ensures euthyroidism in all tissues of the thyroidectomized rat. Endocrinology 137:2490–2502 [DOI] [PubMed] [Google Scholar]
- 31.Escobar-Morreale HF, Obregon MJ, Escobar del Rey F, Morreale de Escobar G. 1995. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest 96:2828–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santini F, Vitti P, Chiovato L, Ceccarini G, Macchia M, Montanelli L, Gatti G, Rosellini V, Mammoli C, Martino E, Chopra IJ, Safer JD, Braverman LE, Pinchera A. 2003. Role for inner ring deiodination preventing transcutaneous passage of thyroxine. J Clin Endocrinol Metab 88:2825–2830 [DOI] [PubMed] [Google Scholar]
- 33.Santini F, Giannetti M, Ricco I, Querci G, Saponati G, Bokor D, Rivolta G, Bussi S, Braverman LE, Vitti P, Pinchera A. 2014. Steady state serum T3 concentrations for 48 hours following the oral administration of a single dose of 3,5,3′-triiodothyronine sulfate (T3S). Endocr Pract 20:680–689 [DOI] [PubMed] [Google Scholar]
- 34.Li DQ, Zhou J, Liu X. 2007. The one-dimensional chain polymer of aqua(tyrosinato)zinc(II). Acta Crystallogr C 63:m371–373 [DOI] [PubMed] [Google Scholar]
- 35.Smart JD. 2005. The basics and underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev 57:1556–1568 [DOI] [PubMed] [Google Scholar]
- 36.Bianco AC, Silva JE. 1987. Nuclear 3,5,3′-triiiodothyronine (T3) in brown adipose tissue: receptor occupancy and sources of T3 as determined by in vivo techniques. Endocrinology 120:55–62 [DOI] [PubMed] [Google Scholar]
- 37.Silva JE, Dick TE, Larsen PR. 1978. The contribution of local tissue thyroxine monodeiodination to the nuclear 3,5,3′-triiodothyronine in pituitary, liver, and kidney of euthyroid rats. Endocrinology 103:1196–1207 [DOI] [PubMed] [Google Scholar]