Abstract
Mild traumatic brain injuries (mTBI), accounting for more than 80% of TBIs, can cause cognitive and behavioral impairments, the severity and duration of which increase after additional mTBIs. While mTBI does not cause widespread neuronal death, the mechanisms underlying increased cellular susceptibility to subsequent head impacts remain unknown. To investigate the hypothesis that altered mitochondrial bioenergetics underlie cellular vulnerability to repeated insults, we employed a mouse model of mild closed head injury (CHI) to examine mitochondrial function and oxidative stress, because these mechanisms are often intertwined. Mitochondrial respiration was assayed (Seahorse XFe24 Flux Analyzer) from cortex and hippocampus collected at 6 h, 24 h, 48 h, and 96 h post-injury. State III (adenosine diphosphate [ADP]-mediated) respiration was significantly decreased in the hippocampal mitochondria of the CHI group compared with sham at 48 h post-injury. Further, cortex-derived mitochondria exhibited a decrease in State III respiration at 24 h and 48 h post-injury. No significant differences were observed at 6 h or 96 h post-injury in either region of interest. A second CHI repeated either 48 h or 96 h after the first did not worsen State III respiration at 48 h after the final injury compared with a single CHI, but CHI repeated at a 48 h interval prolonged cortical mitochondrial dysfunction to 96 h after the final injury. Markers of oxidative stress were significantly elevated after two CHIs delivered 48 h apart, but not after single CHI or two CHI delivered 96 h apart. This study establishes that mTBI results in early mitochondrial dysfunction, which may be a determinant for cellular vulnerability to repeated head impacts. Thus, therapies targeting mitochondrial impairment could improve outcomes after repeated mTBI.
Keywords: bioenergetics, closed head injury, concussion, oxidative stress, Seahorse
Introduction
Traumatic brain injuries (TBIs) are a major societal concern and can result in long-term consequences for affected individuals and their families. Of the many adverse neurological symptoms that can accompany TBIs, memory impairment is reported as the main predictive factor of impaired quality of life.1 The Centers for Disease Control and Prevention estimates that more than 80% of TBIs sustained in the United States are considered mild and these can potentially cause memory loss affecting the ability to work, resulting in lost productivity.2–5 The subtle symptomatic onset of mild TBI (mTBI) often belies long-lasting symptoms,6,7 including cognitive impairment, especially after multiple sustained mTBIs.8–12
Individuals who engage in “at-risk” activities, such as in theater combat or contact sports, are more likely to sustain multiple mTBIs13,14 leading to exacerbated or prolonged symptomology. The risk of worsened damage or dysfunction because of additional mTBI can be directly related to the amount of time after the initial injury and whether the cellular environment is given adequate recovery time. Although there is much pre-clinical and clinical data detailing prolonged and/or worsened outcomes after repeated head impacts,15–21 especially those close together, there is no definitive answer to the mechanism of cellular vulnerability or its time course.
Emerging literature focused on the cellular consequences of repeated mTBIs details metabolic dysfunction and oxidative stress.22–29 Clinical findings reveal a temporal window (15 days) of metabolic imbalance after a concussion during which a second concussion initially worsens and prolongs recovery of N-acetyl aspartate/creatine (NAA/Cre) levels to control levels.22 Interestingly, this window was longer than the conclusion of clinical symptoms, demonstrating the need for better diagnostic markers. Further, small animal studies detail temporal cerebral metabolite changes, with peak differences identified around two days after closed head injury (CHI) produced by weight drop.25,26,30
The effects of repeated concussive injuries on brain metabolism and NAA depend on the interval between injuries, supporting a “window of vulnerability” after a mTBI.26,30 Shorter intervals between injuries resulted in greater metabolic impairment with lower NAA and adenosine triphosphate-adenosine diphosphate (ATP-ADP) ratio.26,30 Biochemical indices of oxidative stress, a common byproduct of mitochondrial dysfunction, were higher after repeated mTBI at a three day interval compared with repeated mTBI at a five day interval.27
A common denominator of both cellular bioenergetics and oxidative stress resides at the level of the mitochondria. Therefore, the culmination of these results suggests that mitochondrial impairment is at the epicenter of cellular vulnerability after mTBI. The importance that mitochondria play in cellular vulnerability after repeated mTBI has also been implied by assessing cerebral metabolic rate of glucose (CMRglc).28,29 When there is a shorter interval between repeated injuries, the post-traumatic drop in CMRglc is exacerbated and prolonged.
It is well known that mitochondria are central regulators of cellular metabolism. Normally, the main role of mitochondria is production of ATP and buffering of calcium. After moderate-to-severe TBI, mitochondria are burdened with calcium influx causing ionic imbalances, loss of membrane potential, and reduced ATP production.31–36 In addition, reactive oxygen species (ROS) after TBI can contribute to and/or be amplified by mitochondrial impairment. Oxidative damage, including but not limited to lipid peroxidation and protein nitration, is well established after moderate-to-severe TBI in rodents37 and has been implicated recently in models of mTBI.38,39
Finally, while Lyons and associates40 found that no difference in mitochondrial bioenergetics exists at three days post-CHI,40 no study has directly evaluated mitochondrial function in the first 48 h after mTBI. In addition, there are no studies detailing the relationship between mitochondrial impairment and oxidative damage after mTBI.
In this study, we tested the hypotheses that mitochondrial bioenergetics are transiently altered after a single CHI and that mitochondrial dysfunction could be a determinant of post-injury cellular vulnerability to a repeated impact. In addition, we postulated that mitochondrial dysfunction would elevate markers of oxidative stress after CHI repeated at a short interval. The results of this study highlight therapeutic targets aimed at underlying cellular processes, not solely the resultant neuropathological outcomes, after mTBI.
Methods
Animals and experimental design
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Kentucky and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The Division of Laboratory Animal Resources at the University is accredited by the Association for the Assessment and Accreditation for Laboratory Animal Care, International, and all experiments were performed within its guidelines. All data were analyzed and reported according to ARRIVE guidelines.
Young adult (7–9 week old; 22–26 g) C57BL/6J male mice (Jackson Laboratories, Bar Harbor, ME) were acclimated for a one week period to the vivarium where they were housed (five per cage) in a 14 h/10 h light/dark cycle with food and water available ad libitum. Mice were then randomly assigned to two groups (CHI and sham) for single CHI experiments or three groups (CHI, repeated CHI at a 48 h interval, and repeated CHI at a 96 h interval) for the repeated CHI experiments.
For single CHI assessments, animals (n = 6/group) were euthanized before mitochondrial isolation at 6 h, 24 h, 48 h, or 96 h post-CHI. For repeated CHI experiments, animals (n = 8/group) were euthanized before mitochondrial isolation at 48 h or 96 h after the last CHI. An additional cohort (n = 2–3/group) was included, mirroring the repeated CHI experiments, to examine histopathological outcomes. No mice died as a result of CHI procedures, and none were removed from the study; 105 mice were utilized in total for all experiments. For all assays, technical triplicates were included for each sample. Data analysis was performed blinded to treatment groups.
CHI
Experimental CHI was induced according to a previously described procedure.41 Mice were anesthetized with 2.5% isofluorane delivered via a nose cone, and the head of each mouse was fixed between two zygomatic cuffs stabilized in a stereotaxic frame. The incision site was first cleaned with 70% ethanol and Betadine, and local analgesia was achieved by subcutaneous injection of 0.2 mL 1:200,000 epinephrine and 0.5% bupivacaine (Henry Schein Animal Health, Dublin, OH) in sterile, isotonic saline before scalp reflection. A pneumatically controlled cortical impact device (TBI-0310 Impactor, Precision Systems and Instrumentation, Fairfax Station, VA) with a 5 mm diameter, cushioned tip of 55 Shore A hardness was programmed to deliver a 2.0 mm impact at 3.5 m/sec with a 500 msec dwell time. The posterior edge of the tip was aligned at the lambda suture (approximately bregma level −5 mm). The diameter of the tip (5 mm) is such that the anterior edge of the tip meets the bregma suture (0 mm bregma level). If applicable, subsequent injuries were induced at the same midline location at the indicated time post-injury. This impact was characterized previously such that a single injury would result in minimal gliosis or cell death without resulting in skull fracture.21
Immediately after impact, mice were removed from the stereotaxic device and placed onto their backs on a heating pad. Apnea duration and the time to spontaneously right to a prone position (righting reflex) were assessed. On righting, mice were briefly re-anesthetized to suture the scalp using Vicryl sutures containing antibiotic agents (Ethicon, Cincinnati, OH). After suturing, 1 mL of sterile saline was delivered subcutaneously to increase hydration after the injury. Sham-injured animals underwent identical anesthesia and surgical procedures without receiving an impact. All animals received the same duration of surgical anesthesia.
All mice were monitored on a heating pad until they became ambulatory. In addition, mice were evaluated to 1–3 h and 24 h after each injury, followed by daily inspections. All mice were required to maintain 85% of their starting weight to receive repeated head injury. No mice needed to be removed from the study, however.
Isolation of mitochondria from brain tissue
The mitochondrial isolation protocol was adapted from previously described protocols with modifications.42–45 All the steps were performed at 4°C or on ice. After mice were euthanized with CO2 followed by rapid decapitation, the bilateral ventral cortex (containing entorhinal cortex) and bilateral hippocampus, vulnerable regions to mTBI,41 were dissected out quickly on a cold block and homogenized using a Teflon-glass dounce homogenizer containing isolation buffer (215 mM mannitol, 75 mM sucrose, 0.1% bovine serum albumin [BSA], 20 mM HEPES, 1 mM EGTA, adjusted to pH 7.2 with potassium hydroxide).
The homogenate was transferred to a 2 mL microcentrifuge tube and spun at 1,300 × g for 3 min. The supernatant was transferred to a fresh 2 mL microcentrifuge tube and spun at 13,000 × g for 10 min. The supernatant was discarded and the crude mitochondrial pellet was resuspended in 400 mL of isolation buffer. This suspension was added to a pressurized nitrogen cell disruptor at 1,200 psi for 10 min at 4°C to release the synaptic mitochondria from synaptosomes.
The suspension of total mitochondria was then transferred to 1.5 mL microcentrifuge tubes and pelleted at 10,000 × g for 10 min. The supernatants were discarded, and the total mitochondrial pellets were resuspended in EGTA-free isolation buffer to get ≥10 mg/mL approximate concentration of mitochondria. The absolute protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Pierce, Rockford, IL) by recording absorbance at 560 nm on a Biotek Synergy HT plate reader (Winooski, VT).
Mitochondrial bioenergetics measurements
Mitochondrial bioenergetic measurements were performed using a Seahorse XFe24 Extracellular Flux Analyzer (Agilent Technologies, Santa Clara, CA) to measure the oxygen consumption rates (OCR) during various states of respiration. The OCR were measured in the presence of different substrates, inhibitors, and uncouplers of the electron transport chain using previous methods with slight modifications.45–48 The stocks used for the assays were 500 mM pyruvate, 250 mM malate, and 30 mM ADP, and 1 M succinate (pH for all was adjusted to 7.2). Assay solutions of 1 mM oligomycin A, 1 mM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), and 1 mM rotenone were prepared in ethanol.
As per the instructions from the XFe24 Extracellular Flux kit, the sensor cartridge was hydrated and kept at 37°C overnight before the experiment. The injection ports A to D of the sensor cartridge were loaded with 75 μL of different combinations of the above substrates/inhibitors/uncouplers as follows: Before loading, the stocks were diluted appropriately in the respiration buffer (RB) (125 mM KCl [potassium chloride], 0.1% BSA, 20 mM HEPES, 2 mM magnesium chloride [MgCl2], and 2.5 mM monobasic potassium phosphate [KH2PO4]; pH 7.2) to get the final concentrations in the respiration chamber of 5 mM pyruvate, 2.5 mM malate, and 1 mM ADP (via port A), 1 μM oligomycin A (via port B), 4 μM FCCP (via port C), and 0.1 μM rotenone and 10 mM of succinate (via port D) starting with the initial volume of 525 μL RB in the chamber and diluting each substrate to 9X, 10X, 11X, and 12X with every injection through ports A to D, respectively.
Once loaded, the sensor cartridge was placed into the Seahorse XFe24 Flux Analyzer for automated calibration. Seahorse Standard XFe24 assay plates were used for loading mitochondria. Initially, total mitochondria were diluted to 10 μg/50 μL in RB, and 50 μL was loaded in each well resulting in 10 μg mitochondria/well. The assay plates were centrifuged at 3,000 rpm for 4 min at 4°C to adhere the mitochondria at the bottom of the wells. After centrifugation, 475 μL RB (pre-incubated to 37°C) was added without disturbing the mitochondrial layer to obtain a final volume of 525 μL per well. After the instrument calibration with the sensor cartridge was complete, the utility plate was replaced by the plate loaded with mitochondria for bioenergetics analysis.
The assays were performed under a previously optimized protocol.48 Briefly, it involved cyclic steps of mixing, sequential injections of substrates/inhibitors via ports A through D, mixing, equilibration, and measurement of the OCR through fluorimetric optical probes. The data output gives State III respiration in the presence of pyruvate and malate (PM) and ADP (port A) followed by State IV rate in the presence of oligomycin A (port B). Sequentially, it gives uncoupled respiration State (VPM) and State V succinate (Suc) (State V2) in the presence of FCCP (port C) and rotenone plus succinate (port D), respectively. Raw OCR values were used for analysis within a given experiment and reported in all figures. Plate-to-plate and day-to-day variation may result in different absolute OCR values between experiments.
Oxidative damage assays
Mitochondrial homogenate aliquots (unused during respiration assays) were stored at −20°C until utilization for oxidative stress blots. Three separate markers, 4-hydroxynonenal (HNE), 3-nitrotyrosine (3-NT), and protein carbonyls (PC), were assessed in these samples. Protein concentrations were determined using a BCA protein assay. For PC, 5 μL of sample, 5 μL of SDS (sodium dodecyl sulfate, 12% solution in H20), and 10 μL of 2,4- dinitrophenylhydrazine (DNPH) (1:10 dilution in phosphate buffered saline [PBS]) was added to a 0.6 mL Eppendorf tube. Then 7.5 μL of neutralization buffer was added to each sample assayed for PC. For HNE and 3-NT, 5 μL of sample, 5 μL of 12% SDS, and 10 μL of Laemmli buffer (0.125 M Trizma base, 4% SDS, and 20% glycerol) was added to a 0.6 mL Eppendorf tube without neutralization buffer. All samples were incubated at room temperature for 20 min. Protein concentration of the sample was titrated to 1 ng/μL in a 2 mL Eppendorf tube.
The BioRad Bio-Dot SF Microfiltration System was assembled by placing three filter papers in the lower Bio-Dot tray followed by the nitrocellulose membrane. The Bio-Dot system was then attached to a laboratory vacuum system and washed with 250 μL of PBS. After the PBS wash, each sample was vortexed and then loaded into the upper tray. Each sample was loaded into two “slots” in proximity. The samples were then processed onto the membrane by vacuum. The membrane was then removed, marked, and left to dry overnight. Blocking solution (25 mL of Wash Blot Solution/750 mg of BSA [Sigma]) was added to membranes placed in trays. The blocking solution was left on the membranes for 90 min and then removed.
For PC, 100 μL of polyclonal RbxDNP (from Oxy Blot Protein Oxidation Kit, Chemicon-Millipore, Billerica, MA, dilution 1:200; Cat No. S7150) was transferred into 20 mL of Wash-Blot Solution. For HNE, 4 μL of anti-protein-bound HNE pAb (Alpha Diagnostic International, San Antonio, TX; dilution 1: 500) was transferred into 20 mL of Wash-Blot Solution. For 3-NT, 8 μL of anti-3-NT pAb (Life Technologies, Carlsbad, CA; dilution 1:2500) was transferred into 20 mL of Wash-Blot Solution.
Previously, all antibodies were characterized using appropriate positive and negative controls. The appropriate antibody solutions were added to the trays and allowed to incubate for 120 min on the rocker. After 120 min, the antibody solutions were removed and the membranes were washed thrice 5 min each. The secondary antibody solution was made using 2.5 μL of Anti-Rabbit IgG alkaline phosphatase produced in goat (Sigma–Aldrich) added to 20 mL Wash-Blot Solution. The membranes were incubated with the secondary antibody for 60 min. The membranes were then washed. Developing solution was then prepared using 30 mL of alkaline phosphatase buffer, 99 μL of BCIP (5-bromo-4-chloro-3-indolyl phosphate), and 198 μL of nitroblue tetrazolium and added to trays. Membranes were allowed to develop and then washed with ddH2O water and allowed to dry overnight. The next day the membranes were scanned with a photo scanner (Epson Perfection V600, Long Beach, CA), and slot-blot line densities were quantified by the ImageQuant TL software package (GE Healthcare Bio-Sciences, Piscataway, NJ).
Tissue processing for histopathological analysis
A cohort of mice receiving either single CHI, repeated CHI at a 48 h interval, or repeated CHI at a 96 h interval were euthanized at 48 h after the final injury by intraperitoneal injection of Fatal Plus (130 mg/kg, Henry Schein Animal Health) before transcardial perfusion with cold, heparinized sterile saline followed by cold, 4% paraformaldehyde (PFA) for 10 min. After perfusion, mice were decapitated, and the brains were then removed from the skull and post-fixed in 4% PFA for an additional 24 h. After post-fixation, tissue was placed into 30% sucrose in 1X-Tris-buffered saline (TBS) for 48 h for cryoprotection. The brain tissue was frozen in −25 to −35°C isopentane before being cut into 40 μm thick coronal sections using a sliding microtome (Dolby-Jamison, Pottstown, PA).
For qualitative assessment of cell loss, sections spaced at 400 μm intervals were Nissl stained with 2.5% cresyl violet. Fluoro-Jade C (FJC) staining, as described previously,21 was used to identify degenerating neurons and axons in four sections selected at 400 μm intervals within the caudal hippocampus and entorhinal cortex.
To label for activated microglia, a series of free-floating tissue sections spaced 400 μm apart was used for immunohistochemistry, as performed previously.21,41 Tissues were blocked with 5% normal horse serum in 0.1%Triton X-100/1XTBS before incubation in anticluster of differentiation-68 (CD-68; 1:1000; MCA1957; Bio-Rad, Hercules, CA) overnight at 4°C. On the following day, tissue sections were rinsed and incubated in donkey anti-rat IgG, biotin-labeled (Jackson ImmunoResearch, West Grove, PA) for 1 h. The tissue was washed before incubating in Avidin-Biotin complex (Vector Laboratories, Burlingame, CA) for 1 h and then treated with 3,3’-diaminobenzidine as directed by the manufacturer (Vector Laboratories).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, La Jolla, CA). For all analyses, the significance of differences among groups was set at p < 0.05. For each measure, data were measured using interval/ratio scales. The Brown-Forsythe and Bartlett tests were performed to ensure homogeneity of variance. Further, the Shapiro-Wilk test was completed to ensure normality. As these criteria were met for all experimental data, parametric statistics were employed for data analyses. Statistical outliers, after averaging technical triplicates, were identified by internally studentized residuals as published previously.49
For the single CHI experiments, one statistical outlier was removed for all measures from the 48 h survival group. In the repeated CHI experiments, one statistical outlier was removed for all measures from each of two groups: the single CHI group and the group with repeated CHI at a 96 h interval euthanized at 96 h after the final CHI. For mitochondrial assessments and oxidative damage assays, data sets were evaluated using a one-way analysis of variance and, when appropriate, post hoc comparisons employing the Fisher least significant difference test. Power analysis was calculated for single mTBI mitochondrial experiments using the following assumptions: α = 0.05, 1-β = 0.8, and standard deviation 10% of mean for two groups.50 Similarly, power analysis was calculated for repeated mTBI mitochondrial experiments using the following assumptions: α = 0.05, 1-β = 0.8, and standard deviation 25% of mean for four groups.
Results
Mitochondrial bioenergetics in the hippocampus and ventral cortex after a single CHI
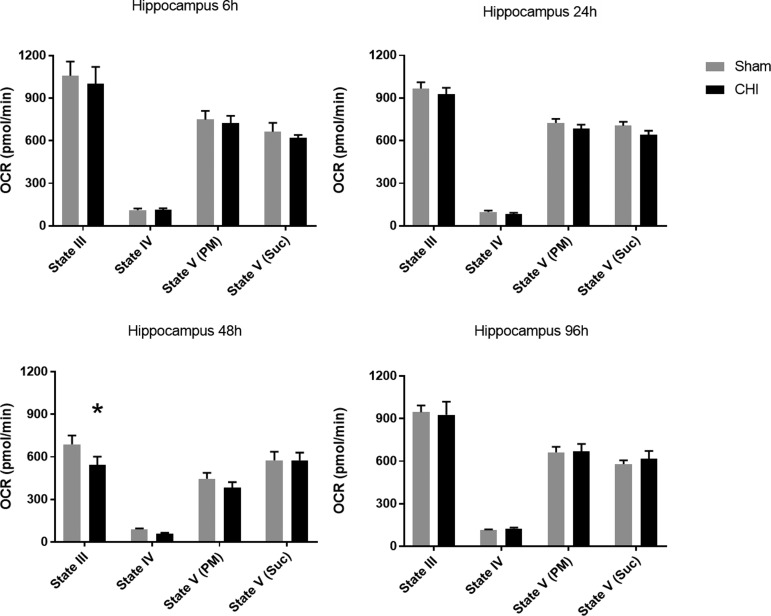
The hippocampus was chosen for analysis of acute changes in mitochondrial respiration because of its vulnerability after CHI21,41,51 and its role in cognitive function. The bioenergetic profile demonstrated a delayed dysfunction (20% decline in State III respiration) at 48 h after a single CHI with subsequent recovery at 96h (F(1, 36) = 3.22). At 48 h after injury, State III respiration was significantly decreased compared with sham (p = 0.0351), although no other respiration states were significantly different (Fig. 1).
FIG. 1.
A single closed head injury (CHI) results in delayed, transient mitochondrial dysfunction in the hippocampus. No significant difference was observed in oxygen consumption rate (OCR) for hippocampal mitochondria at 6, 24, or 96 h after a single CHI compared with sham injury. At 48 h post-CHI, mitochondria isolated from bilateral hippocampi demonstrated significantly lowered State III (adenosine diphosphate-mediated) respiration in the CHI group compared with sham. Other respiration states were not significantly different. State III OCR: 688.1 ± 62.9 (sham) vs. 544.7 ± 56.9 (CHI) at 48 h post-CHI. N = 6/group *p < 0.05. Bars + error bars correspond to mean + standard error of the mean.
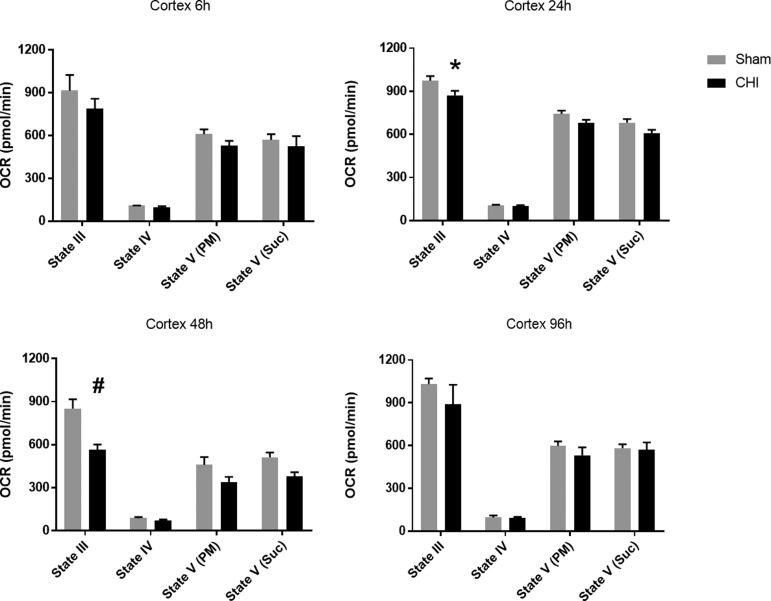
The ventral cortex, an area involved in the memory circuit, was also evaluated because of its vulnerability after CHI.21,41 Although no significant differences were observed at 6 h post-CHI, mitochondrial dysfunction was evident at 24 h (F(1, 40) = 14.00) and 48 h (F(1, 36) = 24.24) after a single CHI with subsequent recovery at 96 h (Fig. 2). At 24 h and 48 h post-CHI, State III respiration was significantly decreased (33% decline at 48 h) compared with sham (*p = 0.0134, and #p < 0.001, respectively). This region displays a longer duration, larger decline in mitochondrial bioenergetics compared with the hippocampus, suggesting greater cellular vulnerability.
FIG. 2.
A delayed, but early, impairment of mitochondrial bioenergetics is observed in the cortex after a single closed head injury (CHI). No significant difference was observed in oxygen consumption rate (OCR) for mitochondria from ventral cortex at 6 or 96 h after a single CHI when compared with sham injury. At 24 h and 48 h post-CHI, mitochondria isolated bilaterally from ventral cortex demonstrated significantly lowered State III (adenosine diphosphate-mediated) respiration in the CHI group compared with sham. Other respiration states were not significantly different. State III OCR: 973.8 ± 31.3 (sham) vs. 871.5 ± 28.7 (CHI) at 24 h post-CHI; 852.8 ± 56.7 (sham) vs. 565.3 ± 39.7 (CHI) at 48 h post-CHI. N = 6/group *p < 0.05 #p < 0.001. Bars + error bars correspond to mean + standard error of the mean.
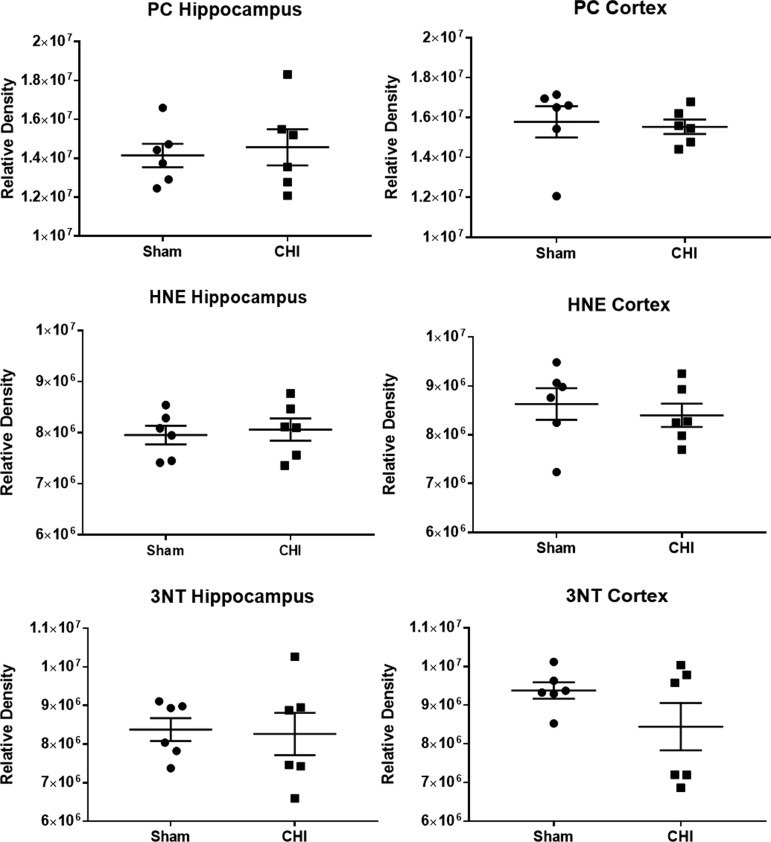
Markers for oxidative damage in mitochondria after a single CHI
After moderate-to-severe TBI, increased cytosolic free calcium promotes mitochondrial dysfunction and ROS production, leading to elevated oxidative stress in mitochondria. To examine whether mitochondrial oxidative stress occurs after mTBI, PC, 3-NT, and HNE were assayed in both hippocampus and ventral cortex at 48 h post-CHI. No differences were observed in oxidative damage markers in isolated mitochondria after a single CHI (Fig. 3), implicating sources other than ROS production in impaired State III mitochondrial respiration after a single CHI.
FIG. 3.
A single closed head injury (CHI) does not produce mitochondria-derived oxidative damage at 48 h post-injury. Oxidative damage levels quantified from slot blots for protein carbonyls (PC), 4-hydroxynonenal (HNE), and 3-nitrotyrosine (3-NT) in the hippocampus and ventral cortex 48 h after a single CHI. No difference was observed between injured and sham groups for any marker. N = 6/group. Values are reported as individual data points and lines correspond to mean ± standard error of the mean.
Mitochondrial bioenergetics after repeated CHI at 48 h and 96 h intervals
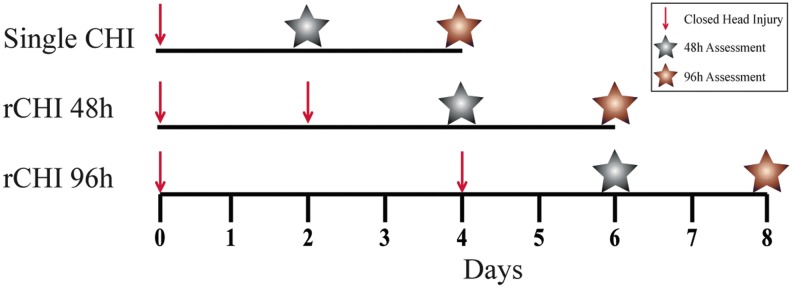
To establish mitochondrial impairment as a crucial determinant of cellular vulnerability after mTBI, animals were assigned randomly to one of three distinct injury groups: single CHI, repeated CHI at a 48 h interval, or repeated CHI at a 96h interval (Fig. 4). The interinjury intervals, coinciding with mitochondrial dysfunction at 48 h and mitochondrial recovery at 96 h, were chosen based on the mitochondrial bioenergetic time course after single CHI.
FIG. 4.
Experimental design of repeated closed head injury (CHI) studies. Red arrows correspond to time of CHI induction. Gray and copper stars correspond to time points for mitochondrial extraction at 48 h and 96 h after the final injury sustained, respectively. Color image is available online.
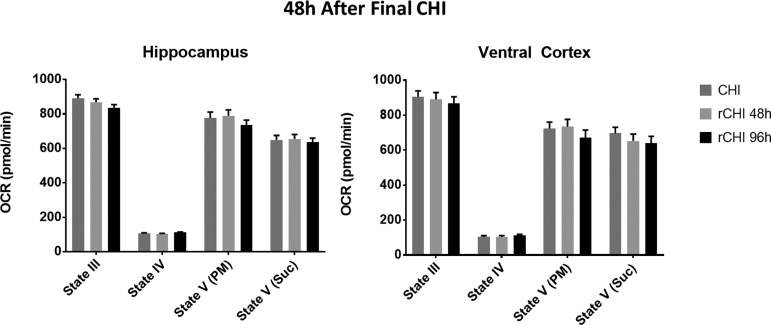
Mitochondrial function was first assessed at 48 h after the final CHI sustained (Fig. 4; gray stars) to determine whether repeated CHI exacerbates mitochondrial dysfunction observed after single CHI (Fig. 1, 2). In both the hippocampus and ventral cortex, however, there were no significant differences between either the repeated CHI group and single CHI (Fig. 5), demonstrating that all groups display similar level of mitochondrial impairment at 48 h.
FIG. 5.
Repeated closed head injury (CHI) does not exacerbate the mitochondrial deficit observed at 48 h after a single CHI. Oxygen consumption rate (OCR) for hippocampus and ventral cortex 48 h after a single CHI or two CHI (rCHI) was repeated at either a 48 h interval or a 96 h interval. No significant difference was observed among groups in either region at 48 h after the final CHI. N = 8/group. Bars + error bars correspond to mean + standard error of the mean.
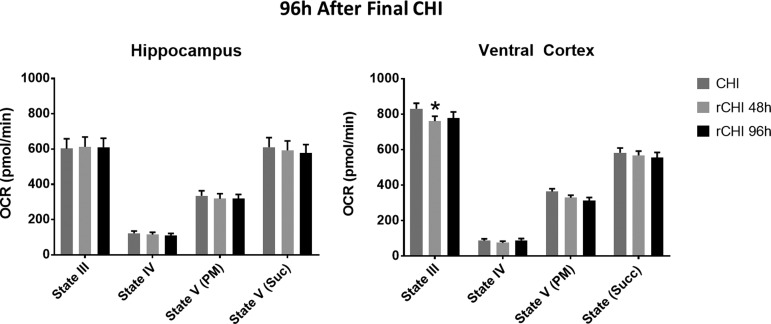
Mitochondrial bioenergetics were then evaluated at 96 h after the final CHI sustained (Fig. 4; copper stars) to determine whether repeated CHI prolongs mitochondrial dysfunction. In the hippocampus, there were no significant differences in any respiration state between single CHI and either repeated CHI group. In contrast, in the ventral cortex, State III respiration was significantly altered among groups (F(2, 72) = 2.86). State III OCR was lower at 96 h after CHIs repeated at a 48 h interval than after single CHI (p = 0.0242). When two CHIs were separated by a 96 h interval, however, mitochondrial function was similar to single CHI (Fig. 6). This finding points to prolonged cortical mitochondrial dysfunction after repeated CHI at a 48 h interval.
FIG. 6.
Repeated closed head injury (CHI) at a shorter interval produces prolonged mitochondrial dysfunction in the cortex compared with single CHI. Oxygen consumption rate (OCR) for hippocampal and cortical mitochondria measured 96 h after a single CHI or two CHI (rCHI) was repeated at either a 48 h interval or a 96 h interval. At 96 h after the final CHI, no significant difference was observed in the hippocampus. Mitochondria isolated from the cortex, however, displayed significantly lowered State III respiration in mice receiving rCHI at a 48 h interval compared with single CHI. State III OCR: 830.8 ± 31.1 (single CHI) vs. 760.7 ± 27.8 (rCHI with 48 h interval) vs. 779.0 ± 34.0 (rCHI with 96 h interval) at 96 h post-final CHI. N = 8/group. * p < 0.05 compared with single CHI. Bars + error bars correspond to mean + standard error of the mean.
Markers for oxidative damage in mitochondria after repeated CHI at a 48 h interval
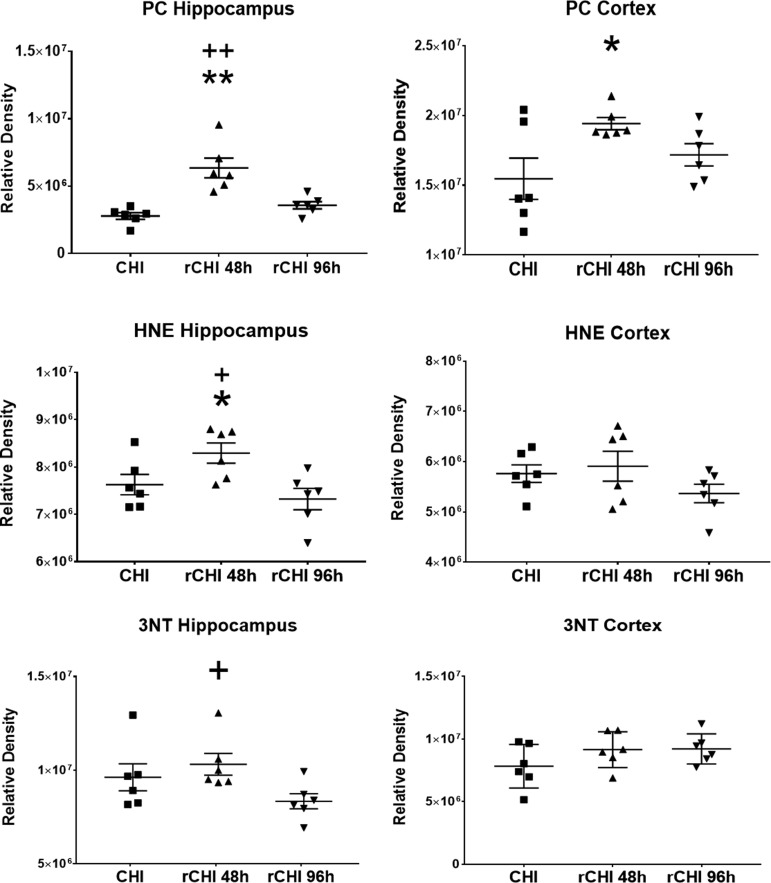
Markers of mitochondria-derived oxidative damage were assessed at 48 h after the final CHI. Compared with single CHI, oxidative damage markers were elevated after repeated CHI at a 48 h interval (Fig. 7). In the hippocampus, levels of PC (F(2, 15) = 15.69), HNE (F(2, 15) = 5.17), and 3-NT (F(2, 15) = 2.96) were altered among groups. The CHIs repeated at a 48 h interval resulted in significantly higher levels of PC (p < 0.0001) and HNE (p = 0.0476) compared with single CHI. In addition, repeated CHI at a 48 h interval resulted in significantly higher levels of PC (p = 0.0009), 3-NT (p = 0.0300), and HNE (p = 0.0067) compared with repeated CHI at a 96 h interval in the hippocampus.
FIG. 7.
Repeated closed head injury (CHI) at a shorter interval markedly increases oxidative damage compared with single CHI or CHI repeated at a longer interval. Protein carbonyls (PC), 4-hydroxynonenal (HNE), and 3-nitrotyrosine (3-NT) levels in mitochondrial homogenates from bilateral hippocampus and bilateral cortex at 48 h after single CHI or two CHI were repeated at a 48 h interval (rCHI 48 h) or a 96 h interval (rCHI 96 h). At 48 h after the final injury, mitochondria were isolated from these regions. Mice receiving rCHI at a 48 h interval had significantly higher levels of PC and HNE in the hippocampus compared with both mice with rCHI at a 96 h interval and mice with single CHI. Mice with rCHI at a 48 h interval had significantly higher levels of 3-NT in the hippocampus compared with mice with rCHI at a 96 h interval and of PC in the ventral cortex compared with mice with a single CHI. N = 8/group *p < 0.05 compared to CHI; **p < 0.001 compared with CHI; + p < 0.05 compared with rCHI at 96 h; ++ p < 0.001 compared with rCHI at 96 h. Values are reported as individual data points and lines correspond to mean ± SEM.
In the ventral cortex, levels of PC (F(2, 15) = 3.90) were different among groups. Repeated CHI at a 48 h interval resulted in significantly higher levels of PC (p = 0.0139) compared with single CHI. In general, two CHIs separated by a 48 h interval resulted in higher levels of acute mitochondria-derived oxidative damage compared with levels after single CHI and repeated CHI at a longer interval.
Repeated CHI does not result in neuron death or microgliosis in the hippocampus and entorhinal cortex
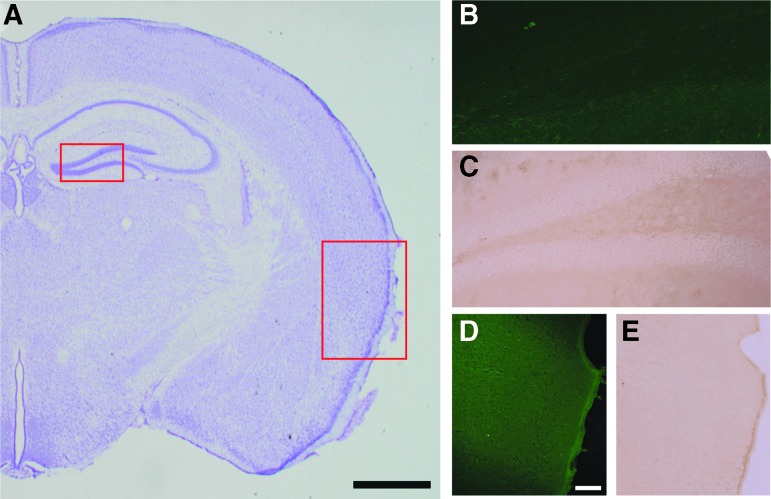
Brain sections stained for Nissl substance revealed no regions of notable neuron loss, gliosis, or hemorrhage after single or repeated CHI (Fig. 8A). Further, no neuronal degeneration was observed with FJC staining after single (not shown) or repeated CHI in either the hippocampus (Fig. 8B) or entorhinal cortex (Fig. 8D). After single or repeated CHI, no microgliosis was observed in the hippocampus (Fig. 8C). Two of three mice with single CHI exhibited few CD-68 positive microglia within the entorhinal cortex, but this was not observed in the repeated CHI groups (Fig. 8E). These results reiterate that this mild CHI is characterized by cellular bioenergetic dysfunction, rather than overt neuronal death and microgliosis. Further, the additive effects of mitochondrial impairment and oxidative damage after repeated CHI at a 48 h interval are not because of increased cell death or microgliosis compared with single CHI.
FIG. 8.
Repeated closed head injury (CHI) does not produce notable hippocampal or cortical pathology at 48 h after the final injury. (A) Representative image of Nissl staining shows a lack of morphological damage. Fluorojade-C staining in the hippocampus (B) or entorhinal cortex (D) reveals no neuron loss or death after CHI repeated at a 48 h interval. The CD-68 immunolabeling in the hippocampus (C) or entorhinal cortex (E) reveals no overt levels of microgliosis after CHI repeated at a 48 h interval. Scale bar in panel A represents 1 mm while scale bar in panel D represent 100 μm and applies to images B–E. Color image is available online.
Discussion
This study presents the first evidence of acute mitochondrial dysfunction after mild CHI in mice. The time course of mitochondrial function presented after a single mTBI provides a glimpse at the dynamic metabolic shifts after mTBI. Decreased State III respiration at 24 h or 48 h but not at 6 h post-injury demonstrates that mitochondrial dysfunction is delayed, suggesting interplay of secondary injury cascades rather than immediate physical damage. Impaired mitochondrial respiration at 24 h and 48 h in the ventral cortex and at 48 h in the hippocampus signifies a region-specific, as well as diffuse, response after mTBI induced by midline impact. Eventual stabilization of mitochondrial respiration in both tissues at 96 h may demonstrate cellular recovery.
A unique feature of CHI models is the diffuse, typically bilateral, nature of injury leading to widespread pathology without overt levels of cell death (Fig. 8).16,17,21 While mitochondrial dysfunction is observed typically in the contusion and its penumbra in the more severe controlled cortical impact (CCI) model,32,33,46,49 we observe bioenergetic impairment in regions remote from the impact location validating the diffuse, bilateral profile of this injury paradigm. Diffuse alterations in mitochondrial respiration are also observed after rapid non-impact rotational brain injury in a pediatric swine model, highlighting that this bioenergetic profile can be replicated in larger animal species.52
After CCI, mitochondrial impairment in the contused cortex starts immediately after injury, peaks within 24 h, and gradually resolves by 72 h after injury, accompanied by mitochondria-mediated cell death.31 Mild TBI produces little to no overt cellular death (Fig. 8), so mitochondrial impairment does not escalate to induce apoptosis as in CCI models.21,29 Rather, surviving cells become dysfunctional, displaying acute alterations in mitochondrial respiration. With a recent report by Lyons and colleagues,40 it is also hypothesized that there can be loss of mitochondrial bioenergetics at a chronic stage after mTBI because of the pathological cellular environment. The time course of mitochondrial respiration observed in this study is unique to mTBI and determines the therapeutic window for targeting this impairment acutely.
Impaired mitochondrial function after single mTBI could play a role in determining cellular vulnerability to subsequent mTBIs. This idea was evaluated with the repeated CHI experiments at intervals of 48 h and 96 h (Fig. 4). Our hypothesis was that repeating mTBI at a 48 h interval, a time point when mitochondrial bioenergetics are compromised, would worsen and/or prolong mitochondrial dysfunction after the second mTBI. While two CHI repeated at a 48 h interval did not exacerbate mitochondrial dysfunction at 48 h after the second injury (Fig. 5), multiple oxidative damage markers (PC, 3-NT, and HNE) in the hippocampus and PC in the ventral cortex were elevated compared with either single CHI or CHI repeated at a 96 h interval. Because levels of oxidative damage usually correlate to decreases in mitochondrial respiration, there may be other aspects of cellular dysregulation at play to explain this disconnect after repeated mTBI.
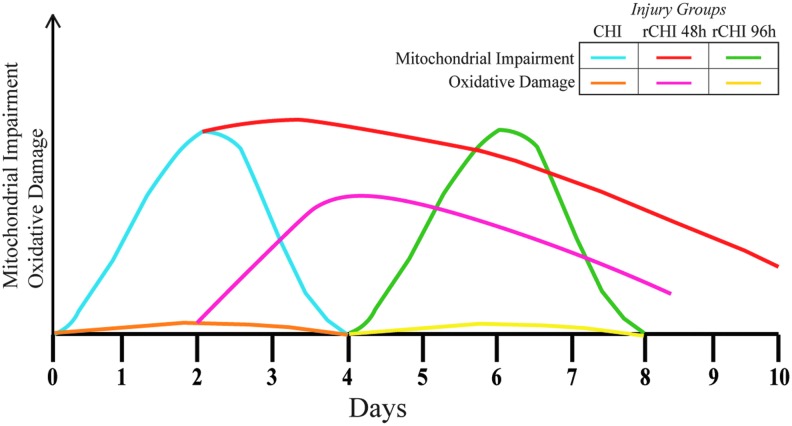
The second experiment (Fig. 4; copper stars) evaluated whether a second mTBI induced at a time when mitochondrial bioenergetics were altered (48 h after first CHI) would prolong mitochondrial dysfunction. Indeed, State III OCR of mitochondria in the ventral cortex derived from mice receiving CHI repeated at a 48 h interval showed a more protracted decline than that from mice receiving only single CHI, although no change from single CHI was observed in the hippocampus. Graphical representation of the temporal mitochondrial and oxidative responses after CHI and repeated CHI at differing intervals illustrates the dynamic metabolic activity in response to mTBI (Fig. 9).
FIG. 9.
Graphical representation of the dynamic mitochondrial and oxidative damage responses after single closed head injury (CHI) and repeated injury (rCHI) at either a 48 h or 96 h interinjury interval. Color image is available online.
One common hallmark found after moderate-to-severe TBI is mitochondrial Ca2+ overloading.35,36,53,54 The Ca2+ overload in mitochondria can reduce mitochondrial respiration44 and sometimes lead to cell death under pathological conditions such as TBI.55,56 While Ca2+ overloading could be a mechanism at play after mTBI, mitochondrial dysfunction more likely is dictated by in vivo biochemical/metabolite alterations26,29,40,57 that cannot be ascertained in isolated mitochondria. The lack of measurable changes in oxidative damage after single mTBI points to a lack of ROS-induced burden on the mitochondria.
In all mitochondrial respiration assays detailed in this article, the only respiration state affected is State III, which specifically measures ATP-mediated respiration. This seems to be a unique aspect of this injury model and may represent either dysfunction in the ATP synthase or the adenine nucleotide transporter or some other as yet unknown modulator of mitochondrial ATP production. Impaired State III levels allude to impaired cellular ATP levels in vivo, potentially leading to a transient metabolic crisis. Future studies are planned for elucidating mechanisms underlying the selective State III respiration changes after mTBI. For example, using metabolomics could allow for the identification of underlying players of mitochondrial disruption after mTBI.
Free radical production is a byproduct of ATP generation in mitochondria via the electron transport chain. After a cellular insult, hydroxyl radicals rapidly attack unsaturated fatty acids in membranes, causing lipid peroxidation and the production of HNE that conjugates to membrane proteins, further impairing mitochondrial function.58 Aberrant levels of ROS, induction of lipid peroxidation, and protein oxidation products may be particularly important after TBI.59,60 Specifically, in the CCI model, the timing of mitochondrial dysfunction coincides with elevation of HNE levels.61 After two mTBIs repeated at a 24 h interval, but not after single mTBI, Yates and coworkers38 found a significant increase in the numbers of HNE+ cortical neurons compared with sham at 48 h after the final injury.38 In addition, elevated ROS in the brain has been observed 3 weeks after repetitive mTBI,62 supporting the idea of persistent oxidative damage after repeated mTBI.
In the current study, State III OCR was reduced in isolated mitochondria from both ventral cortex and hippocampus after single mTBI without differences in oxidative damage. In contrast, mTBIs repeated at a 48 h interval resulted in elevated levels of several oxidative markers. This highlights the role of the dysfunctional environment after the first mTBI and potential of mitochondria-induced aberrant ROS production. We propose that mitochondrial dysfunction is prolonged after repeated mTBIs at 48 h intervals inducing concurrent oxidative damage mechanisms.
Kim and associates63 recently have reviewed existing data to argue for a role of mitochondrial dysfunction after mTBI. This review focused on indirect outcomes surrounding mitochondrial dysfunction and cited several models of moderate-to-severe TBI. As mentioned previously, many articles have shown data related to indirect mitochondrial influence on metabolic consequences such as CMRglc and presence of neurometabolites.26,29 Direct evidence of mitochondrial dysfunction acutely after mild (concussion-type) TBI, however, had not been established previously, although long-term impairment has been reported recently.40 Further, the hippocampus has been shown to have an altered metabolic profile, as determined using MRS, acutely (3–5 days) up to 30 days after mTBI.40,57 A recent study of gene and protein expression of markers related to mitochondrial fusion and fission after severe and mild TBI demonstrated a transitory mitochondrial fusion response in whole brain, indicating cellular recovery, up to five days after mTBI.64
Our results build on previous neuropathological findings in our CHI model of repetitive mTBI. Although we observe a lack of neuropathology after two repeated mTBIs in the current study, five repeated mTBIs at a 24 h interval have been shown to produce measurable gliosis and neuronal death. With five mTBIs, this 24 h interval resulted in exacerbated acute gliosis and neuron death in the entorhinal cortex while a longer interinjury interval of 48 h produced no additive neuropathology compared with single mTBI.21 A subsequent study, however, found comparable levels of neurobehavioral deficits, neuroinflammation, and neurodegeneration in white matter tracts 10 weeks after five mTBIs repeated at either 24 h or 48 h intervals.41 These findings suggest that a 48 h interinjury interval is within the window of cellular vulnerability for long-lasting pathology in the brain, corroborating the data in the current study. Based on our current findings, acute mitochondrial impairment could be upstream of long-term neuropathology and separating mTBIs by at least 96 h may allow for more complete cellular recovery.
One limitation of this study is that all experiments were performed in male mice. Because growing evidence shows that there are sex-based differences in the cellular and pathological response to TBI,65–69 additional studies of female mice are warranted to observe whether acute mitochondrial bioenergetics have a sex-based difference after single and/or repeated mTBI.
While mitochondrial dysfunction is well established after moderate-to-severe contusion brain injuries,31–33,53,60 here we show the first direct evidence of acute mitochondrial dysregulation after mild CHI. Single CHI produces a delayed but early (24 h) decrease in State III respiration in the ventral cortex that resolves by 96 h post-injury, while in the hippocampus, State III respiration decreases at 48 h and resolves by 96 h post-injury. A second CHI induced 48 h after the first, while mitochondria are bioenergetically compromised, results in more prolonged depression of State III respiration and notable oxidative damage (Fig. 9).
This study provides more evidence on the benefits of sufficient cellular recovery time after mTBI to prevent additive cellular dysfunction. Because much is known about the resultant neuropathology after single and repetitive mTBIs,17,21,70,71 these data highlight mitochondrial impairment as an underlying cellular mechanism and potential therapeutic target to mitigate secondary pathological outcomes after mTBI.
Acknowledgments
WBH was supported in part by a Kentucky Spinal Cord and Head Injury Research Trust fellowship. The authors would like to thank the Redox Metabolism-Shared Resource Facility (RM-SRF) at the University of Kentucky for their technical assistance with slot blotting and acknowledge the NCI Cancer Center Support Grant (P30 CA177558). This project was supported by NSF EPSCoR Seed Grant 4978/111315 (National Science Foundation Grant No. 1539068) (WBH), VA Merit Award 1I01BX003405-01A1 (PGS) and Kentucky Spinal Cord and Head Injury Research Trust (KSCHIRT) Grant 14-13A (KES).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Ghroubi S., Alila S., Feki I., and Elleuch M.H. (2016). Quality of life after traumatic brain injury. Ann. Phys. Rehabil. Med. 59S, e135 [Google Scholar]
- 2. Warden D. (2006). Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 21, 398–402 [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. (2015). Report to congress on traumatic brain injury in the united states: Epidemiology and rehabilitation. National Center for Injury Prevention and Control; Division of Unintentional Injury Prevention: Atlanta, GA [Google Scholar]
- 4. Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 5. McAllister T.W., Sparling M.B., Flashman L.A., Guerin S.J., Mamourian A.C., and Saykin A.J. (2001). Differential working memory load effects after mild traumatic brain injury. Neuroimage 14, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 6. Rimel R.W., Giordani B., Barth J.T., Boll T.J., and Jane J.A. (1981). Disability caused by minor head injury. Neurosurgery 9, 221–228 [PubMed] [Google Scholar]
- 7. McMahon P., Hricik A., Yue J.K., Puccio A.M., Inoue T., Lingsma H.F., Beers S.R., Gordon W.A., Valadka A.B., Manley G.T., Okonkwo D.O.; TRACK-TBI Investigators. (2014). Symptomatology and functional outcome in mild traumatic brain injury: Results from the prospective TRACK-TBI study. J. Neurotrauma 31, 26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gronwall D., and Wrightson P. (1975). Cumulative effect of concussion. Lancet 2, 995–997 [DOI] [PubMed] [Google Scholar]
- 9. Slobounov S., Slobounov E., Sebastianelli W., Cao C., and Newell K. (2007). Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery 61, 338–344 [DOI] [PubMed] [Google Scholar]
- 10. McAllister T.W., Ford J.C., Ji S., Beckwith J.G., Flashman L.A., Paulsen K., and Greenwald R.M. (2012). Maximum principal strain and strain rate associated with concussion diagnosis correlates with changes in corpus callosum white matter indices. Ann Biomed Eng 40, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Harding H.P., Jr., Matthews A., Mihalik J.R., and Cantu R.C. (2007). Recurrent concussion and risk of depression in retired professional football players. Med. Sci. Sports Exerc. 39, 903–909 [DOI] [PubMed] [Google Scholar]
- 12. Covassin T., Elbin R., Kontos A., and Larson E. (2010). Investigating baseline neurocognitive performance between male and female athletes with a history of multiple concussion. J. Neurol. Neurosurg. Psychiatry 81, 597–601 [DOI] [PubMed] [Google Scholar]
- 13. Marar M., McIlvain N.M., Fields S.K., and Comstock R.D. (2012). Epidemiology of concussions among United States high school athletes in 20 sports. Am. J. Sports Med. 40, 747–755 [DOI] [PubMed] [Google Scholar]
- 14. Guskiewicz K.M., Weaver N.L., Padua D.A., and Garrett W.E., Jr. (2000). Epidemiology of concussion in collegiate and high school football players. Am. J. Sports Med. 28, 643–650 [DOI] [PubMed] [Google Scholar]
- 15. Uryu K., Laurer H., McIntosh T., Pratico D., Martinez D., Leight S., Lee V.M., and Trojanowski J.Q. (2002). Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 22, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shitaka Y., Tran H.T., Bennett R.E., Sanchez L., Levy M.A., Dikranian K., and Brody D.L. (2011). Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exp. Neurol. 70, 551–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mouzon B., Chaytow H., Crynen G., Bachmeier C., Stewart J., Mullan M., Stewart W., and Crawford F. (2012). Repetitive mild traumatic brain injury in a mouse model produces learning and memory deficits accompanied by histological changes. J. Neurotrauma 29, 2761–2773 [DOI] [PubMed] [Google Scholar]
- 18. Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 19. Aungst S.L., Kabadi S.V., Thompson S.M., Stoica B.A., and Faden A.I. (2014). Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J. Cereb. Blood Flow Metab. 34, 1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mountney A., Boutte A.M., Cartagena C.M., Flerlage W.F., Johnson W.D., Rho C., Lu X.C., Yarnell A., Marcsisin S., Sousa J., Vuong C., Zottig V., Leung L.Y., Deng-Bryant Y., Gilsdorf J., Tortella F.C., and Shear D.A. (2017). Functional and molecular correlates after single and repeated rat closed-head concussion: indices of vulnerability after brain injury. J. Neurotrauma 34, 2768–2789 [DOI] [PubMed] [Google Scholar]
- 21. Bolton A.N., and Saatman K.E. (2014). Regional neurodegeneration and gliosis are amplified by mild traumatic brain injury repeated at 24-hour intervals. J. Neuropathol. Exp. Neurol. 73, 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vagnozzi R., Signoretti S., Tavazzi B., Floris R., Ludovici A., Marziali S., Tarascio G., Amorini A.M., Di Pietro V., Delfini R., and Lazzarino G. (2008). Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes—part III. Neurosurgery 62, 1286–1295 [DOI] [PubMed] [Google Scholar]
- 23. Harris J.L., Yeh H.W., Choi I.Y., Lee P., Berman N.E., Swerdlow R.H., Craciunas S.C., and Brooks W.M. (2012). Altered neurochemical profile after traumatic brain injury: (1)H-MRS biomarkers of pathological mechanisms. J. Cereb. Blood Flow Metab. 32, 2122–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schuhmann M.U., Stiller D., Skardelly M., Bernarding J., Klinge P.M., Samii A., Samii M., and Brinker T. (2003). Metabolic changes in the vicinity of brain contusions: a proton magnetic resonance spectroscopy and histology study. J. Neurotrauma 20, 725–743 [DOI] [PubMed] [Google Scholar]
- 25. Pascual J.M., Solivera J., Prieto R., Barrios L., Lopez-Larrubia P., Cerdan S. and Roda J.M. (2007). Time course of early metabolic changes following diffuse traumatic brain injury in rats as detected by (1)H NMR spectroscopy. J. Neurotrauma 24, 944–959 [DOI] [PubMed] [Google Scholar]
- 26. Vagnozzi R., Tavazzi B., Signoretti S., Amorini A.M., Belli A., Cimatti M., Delfini R., Di Pietro V., Finocchiaro A., and Lazzarino G. (2007). Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment—part I. Neurosurgery 61, 379–388 [DOI] [PubMed] [Google Scholar]
- 27. Tavazzi B., Vagnozzi R., Signoretti S., Amorini A.M., Belli A., Cimatti M., Delfini R., Di Pietro V., Finocchiaro A., and Lazzarino G. (2007). Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses—part II. Neurosurgery 61, 390–395 [DOI] [PubMed] [Google Scholar]
- 28. Prins M., Greco T., Alexander D., and Giza C.C. (2013). The pathophysiology of traumatic brain injury at a glance. Dis. Model. Mech. 6, 1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Prins M.L., Alexander D., Giza C.C., and Hovda D.A. (2013). Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma 30, 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vagnozzi R., Signoretti S., Tavazzi B., Cimatti M., Amorini A.M., Donzelli S., Delfini R., and Lazzarino G. (2005). Hypothesis of the postconcussive vulnerable brain: experimental evidence of its metabolic occurrence. Neurosurgery 57, 164–171 [DOI] [PubMed] [Google Scholar]
- 31. Singh I.N., Sullivan P.G., Deng Y., Mbye L.H., and Hall E.D. (2006). Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J. Cereb. Blood Flow Metab. 26, 1407–1418 [DOI] [PubMed] [Google Scholar]
- 32. Sullivan P.G., Keller J.N., Mattson M.P., and Scheff S.W. (1998). Traumatic brain injury alters synaptic homeostasis: implications for impaired mitochondrial and transport function. J. Neurotrauma 15, 789–798 [DOI] [PubMed] [Google Scholar]
- 33. Sullivan P.G., Thompson M.B., and Scheff S.W. (1999). Cyclosporin a attenuates acute mitochondrial dysfunction following traumatic brain injury. Exp. Neurol. 160, 226–234 [DOI] [PubMed] [Google Scholar]
- 34. Lewen A., Fujimura M., Sugawara T., Matz P., Copin J.C., and Chan P.H. (2001). Oxidative stress-dependent release of mitochondrial cytochrome c after traumatic brain injury. J. Cereb. Blood Flow Metab. 21, 914–920 [DOI] [PubMed] [Google Scholar]
- 35. Robertson C.L. (2004). Mitochondrial dysfunction contributes to cell death following traumatic brain injury in adult and immature animals. J. Bioenerg. Biomembr. 36, 363–368 [DOI] [PubMed] [Google Scholar]
- 36. Xiong Y., Gu Q., Peterson P.L., Muizelaar J.P., and Lee C.P. (1997). Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J. Neurotrauma 14, 23–34 [DOI] [PubMed] [Google Scholar]
- 37. Hall E.D. (2015). The contributing role of lipid peroxidation and protein oxidation in the course of cns injury neurodegeneration and neuroprotection: An overview, in: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Kobeissy F.H. (ed): CRC Press/Taylor & Francis: Boca Raton, FL: [PubMed] [Google Scholar]
- 38. Yates N.J., Lydiard S., Fehily B., Weir G., Chin A., Bartlett C.A., Alderson J., and Fitzgerald M. (2017). Repeated mild traumatic brain injury in female rats increases lipid peroxidation in neurons. Exp. Brain Res. 235, 2133–2149 [DOI] [PubMed] [Google Scholar]
- 39. Cho H.J., Sajja V.S., Vandevord P.J., and Lee Y.W. (2013). Blast induces oxidative stress, inflammation, neuronal loss and subsequent short-term memory impairment in rats. Neuroscience 253, 9–20 [DOI] [PubMed] [Google Scholar]
- 40. Lyons D.N., Vekaria H., Macheda T., Bakshi V., Powell D.K., Gold B.T., Lin A.L., Sulllivan P., and Bachstetter A.D. (2018). A mild traumatic brain injury in mice produces lasting deficits in brain metabolism. J. Neurotrauma 35, 2435–2447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bolton Hall A.N., Joseph B., Brelsfoard J.M., and Saatman K.E. (2016). Repeated closed head injury in mice results in sustained motor and memory deficits and chronic cellular changes. PLoS One 11, e0159442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brown M.R., Sullivan P.G., Dorenbos K.A., Modafferi E.A., Geddes J.W., and Steward O. (2004). Nitrogen disruption of synaptoneurosomes: an alternative method to isolate brain mitochondria. J. Neurosci. Methods 137, 299–303 [DOI] [PubMed] [Google Scholar]
- 43. Nukala V.N., Singh I.N., Davis L.M., and Sullivan P.G. (2006). Cryopreservation of brain mitochondria: a novel methodology for functional studies. J. Neurosci. Methods 152, 48–54 [DOI] [PubMed] [Google Scholar]
- 44. Pandya J.D., Nukala V.N., and Sullivan P.G. (2013). Concentration dependent effect of calcium on brain mitochondrial bioenergetics and oxidative stress parameters. Front. Neuroenergetics 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vekaria H.J., Talley Watts L., Lin A.L., and Sullivan P.G. (2017). Targeting mitochondrial dysfunction in CNS injury using Methylene Blue; still a magic bullet? Neurochem. Int. 109, 117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pandya J.D., Sullivan P.G., Leung L.Y., Tortella F.C., Shear D.A., and Deng-Bryant Y. (2016). Advanced and high-throughput method for mitochondrial bioenergetics evaluation in neurotrauma. Methods Mol. Biol. 1462, 597–610 [DOI] [PubMed] [Google Scholar]
- 47. Patel S.P., Sullivan P.G., Pandya J.D., Goldstein G.A., VanRooyen J.L., Yonutas H.M., Eldahan K.C., Morehouse J., Magnuson D.S., and Rabchevsky A.G. (2014). N-acetylcysteine amide preserves mitochondrial bioenergetics and improves functional recovery following spinal trauma. Exp. Neurol. 257, 95–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sauerbeck A., Pandya J., Singh I., Bittman K., Readnower R., Bing G., and Sullivan P. (2011). Analysis of regional brain mitochondrial bioenergetics and susceptibility to mitochondrial inhibition utilizing a microplate based system. J. Neurosci. Methods 198, 36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hubbard W.B., Harwood C.L., Geisler J.G., Vekaria H.J., and Sullivan P.G. (2018). Mitochondrial uncoupling prodrug improves tissue sparing, cognitive outcome, and mitochondrial bioenergetics after traumatic brain injury in male mice. J. Neurosci. Res. 96, 1677–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Charan J., and Kantharia N.D. (2013). How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 4, 303–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Creed J.A., DiLeonardi A.M., Fox D.P., Tessler A.R., and Raghupathi R. (2011). Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J. Neurotrauma 28, 547–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kilbaugh T.J., Karlsson M., Duhaime A.C., Hansson M.J., Elmer E., and Margulies S.S. (2016). Mitochondrial response in a toddler-aged swine model following diffuse non-impact traumatic brain injury. Mitochondrion 26, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hubbard W.B., Davis L.M., and Sullivan P.G. (2018). Mitochondrial damage in traumatic CNS injury, in: Acute neuronal injury: The Role of Excitotoxic Programmed Cell Death Mechanisms. Fujikawa D.G. (ed). Springer International Publishing: New York, NY, pps. 63–81 [Google Scholar]
- 54. Sullivan P.G., Rabchevsky A.G., Waldmeier P.C., and Springer J.E. (2005). Mitochondrial permeability transition in CNS trauma: cause or effect of neuronal cell death? J. Neurosci. Res. 79, 231–239 [DOI] [PubMed] [Google Scholar]
- 55. Duchen M.R. (2000). Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 529, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trump B.F., and Berezesky I.K. (1995). Calcium-mediated cell injury and cell death. FASEB J. 9, 219–228 [DOI] [PubMed] [Google Scholar]
- 57. Singh K., Trivedi R., Verma A., D'souza M.M., Koundal S., Rana P., Baishya B., and Khushu S. (2017). Altered metabolites of the rat hippocampus after mild and moderate traumatic brain injury - a combined in vivo and in vitro (1) H-MRS study. NMR Biomed. 30. [DOI] [PubMed] [Google Scholar]
- 58. Singh I.N., Gilmer L.K., Miller D.M., Cebak J.E., Wang J.A., and Hall E.D. (2013). Phenelzine mitochondrial functional preservation and neuroprotection after traumatic brain injury related to scavenging of the lipid peroxidation-derived aldehyde 4-hydroxy-2-nonenal. J. Cereb. Blood Flow Metab. 33, 593–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hall E.D., and Sullivan P.G. (2004). Preserving function in acute nervous system injury, in: Neuroscience, Molecular Medicine and the Therapeutic Transfomation of Neurology. Waxman S.G. (ed). Elsevier/Academic Press: San Diego [Google Scholar]
- 60. Lifshitz J., Sullivan P.G., Hovda D.A., Wieloch T., and McIntosh T.K. (2004). Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondria 4, 705–713 [DOI] [PubMed] [Google Scholar]
- 61. Hill R.L., Singh I.N., Wang J.A., and Hall E.D. (2017). Time courses of post-injury mitochondrial oxidative damage and respiratory dysfunction and neuronal cytoskeletal degradation in a rat model of focal traumatic brain injury. Neurochem. Int. 111, 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mei Z., Zheng P., Tan X., Wang Y., and Situ B. (2017). Huperzine a alleviates neuroinflammation, oxidative stress and improves cognitive function after repetitive traumatic brain injury. Metab. Brain Dis. 32, 1861–1869 [DOI] [PubMed] [Google Scholar]
- 63. Kim S., Han S.C., Gallan A.J., and Hayes J.P. (2017). Neurometabolic indicators of mitochondrial dysfunction in repetitive mild traumatic brain injury. Concussion 2, CNC48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Di Pietro V., Lazzarino G., Amorini A.M., Signoretti S., Hill L.J., Porto E., Tavazzi B., Lazzarino G., and Belli A. (2017). Fusion or fission: the destiny of mitochondria in traumatic brain injury of different severities. Sci. Rep. 7, 9189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bazarian J.J., Blyth B., Mookerjee S., He H., and McDermott M.P. (2010). Sex differences in outcome after mild traumatic brain injury. J. Neurotrauma 27, 527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tucker L.B., Fu A.H., and McCabe J.T. (2016). Performance of male and female C57BL/6J mice on motor and cognitive tasks commonly used in pre-clinical traumatic brain injury research. J, Neurotrauma 33, 880–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Doran S.J., Ritzel R., Glaser E., Henry R., Faden A., and Loane D.J. (2018). Sex differences in acute neuroinflammation after experimental traumatic brain injury are mediated by infiltrating myeloid cells. J. Neurotrauma [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ferguson S.A., Mouzon B.C., Lynch C., Lungmus C., Morin A., Crynen G., Carper B., Bieler G., Mufson E.J., Stewart W., Mullan M., and Crawford F. (2017). Negative impact of female sex on outcomes from repetitive mild traumatic brain injury in hTau mice is age dependent: a chronic effects of neurotrauma consortium study. Front. Aging Neurosci. 9, 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Broshek D.K., Kaushik T., Freeman J.R., Erlanger D., Webbe F., and Barth J.T. (2005). Sex differences in outcome following sports-related concussion. J. Neurosurg. 102, 856–863 [DOI] [PubMed] [Google Scholar]
- 70. Weil Z.M., Gaier K.R., and Karelina K. (2014). Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 70, 108–116 [DOI] [PubMed] [Google Scholar]
- 71. Longhi L., Saatman K.E., Fujimoto S., Raghupathi R., Meaney D.F., Davis J., McMillan B.S., Conte V., Laurer H.L., Stein S., Stocchetti N., and McIntosh T.K. (2005). Temporal window of vulnerability to repetitive experimental concussive brain injury. Neurosurgery 56, 364–374 [DOI] [PubMed] [Google Scholar]