Abstract
Introduction:
Smokers benefit from ongoing cessation support upon leaving the hospital and returning to their home environment. This study examined the impact of telephone-delivered care coordination on utilization of and adherence to cessation pharmacotherapy after hospital discharge.
Methods:
Inpatient smokers (n = 606) were randomized to receive counseling with care coordination (CCC) or counseling alone (C) for smoking cessation. Both groups received written materials and telephone-based cessation counseling during hospitalization and postdischarge. CCC recipients received help in selecting, obtaining, and refilling affordable pharmacotherapy prescriptions during and after hospitalization. Study outcomes included self-reported utilization, duration of use, and type of medication during the 3 months postdischarge.
Results:
Of the 487 (80%) of participants completing 3-month follow-up, 211 (43.3%) reported using cessation pharmacotherapy postdischarge; this did not differ by study arm (CCC: 44.7%, C: 42.0%,p = .55). Use of pharmacotherapy postdischarge was associated with smoking at least 20 cigarettes/day at baseline (odds ratio [OR]: 1.48; 95% confidence interval [CI]: 1.00–2.19) and receipt of pharmacotherapy during hospitalization (OR: 4.00; 95% CI: 2.39–6.89). Smokers with Medicaid (OR: 2.29; 95% CI: 1.32–4.02) or other insurance (OR: 1.69; 95% CI: 1.01–2.86) were more likely to use pharmacotherapy postdischarge than those with no health care coverage. Less than one in four (23.8% of CCC; 22.2% of C) continued pharmacotherapy beyond 4 weeks.
Conclusions:
Supplemental care coordination did not improve use of postdischarge pharmacotherapy beyond that of inpatient treatment and behavioral counseling. Insurance coverage and use of medications during the hospitalization are associated with higher use of evidence-based treatment postdischarge.
Implications:
Many hospitalized smokers do not receive the benefits of cessation pharmacotherapy postdischarge and telephone quitline programs often fail to help smokers procure pharmacotherapy. Thus, effective strategies are needed to improve utilization and adherence to evidence-based cessation therapies when smokers leave the hospital. We found that use of postdischarge pharmacotherapy was strongly associated with receipt of pharmacotherapy during the hospitalization and with the availability of insurance to cover the costs of treatment. Additional efforts to coordinate pharmacotherapy services did not improve either utilization or adherence to therapy.
Introduction
An estimated 6.5 million smokers are hospitalized each year in the United States.1 Hospitalization provides a unique opportunity to address smoking cessation.2,3 Clinical practice guidelines for the treatment of tobacco use specifically recommend treatment for hospitalized smokers, including counseling, pharmacotherapy, unless contraindicated, and follow-up postdischarge.4 Inpatient counseling that extends for at least 1 month after discharge is key to supporting smoking abstinence.3 With guidance from the Joint Commission (formerly the Joint Commission on Accreditation of Healthcare Organizations),5 hospitals are now being measured on their ability to address tobacco treatment by implementing comprehensive evidence-based cessation treatment during hospitalization and upon discharge.6,7 Many are now turning to telephone quitlines to provide follow-up treatment after discharge.8–10
Smoking cessation pharmacotherapy is an effective but underutilized tool for helping hospitalized smokers quit.11–13 In outpatient settings, pharmacotherapy can double or triple the chances of successfully quitting smoking,4,14,15 and in hospitalized patients, pharmacotherapy increases the odds of quitting by 50%.16,17 Nevertheless, pharmacotherapy appears to be underutilized during hospitalization and postdischarge.18–20 Furthermore, when pharmacotherapy is utilized, adherence to treatment is suboptimal, with 50%–90% of smokers failing to complete a standard course of therapy.21–23
Smokers are generally abstinent and may initiate cessation treatment during their hospitalization, but care plans initiated during the hospitalization do not always carry over to the outpatient setting.24 These transitions of care may be particularly problematic for smoking cessation where there is often confusion about insurance coverage for treatment25–27 and over-the-counter cessation treatment might not be included among a patient’s discharge prescriptions, even when insurance covers the cost of these key medications.
Counseling services may also be disconnected from pharmacotherapy treatment. Although tobacco quitlines can provide critical follow-up counseling to patients postdischarge, the state tobacco quitlines in the United States do not coordinate services with health care providers. Better care coordination, integrated with smoking cessation counseling, might improve the uptake and adherence to smoking cessation pharmacotherapy. The purpose of this study was to investigate the impact of counseling with care coordination (CCC) on the utilization of and adherence to cessation pharmacotherapy compared to standard smoking cessation counseling (C) alone. Furthermore, we aimed to identify other factors predictive of postdischarge pharmacotherapy use.
Methods
Setting and Participants
Screening, consent, and counseling procedures for both arms were conducted centrally at the University of Kansas Medical Center (KUMC). All study procedures were reviewed and approved by the KUMC institutional review board. The trial was registered atwww.clinicaltrials.gov, #NCT01063972.
Participants were recruited from 31 hospitals across the state of Kansas from April 2010 to October 2013. Staff at each participating hospital screened inpatients for smoking status, and asked smokers if they would be willing to be contacted by a smoking cessation counselor about a research project for hospitalized smokers. If a smoker gave permission to be contacted, hospital staff provided the patient with written information about the study, including a consent form, and faxed the smoker’s hospital contact information to KUMC study staff.
A research assistant then contacted potential study participants either in the hospital or within 2 business days postdischarge. The research assistant provided further details about the study, reviewed the consent form, and conducted an eligibility assessment. Participants were considered eligible if they were not less than 18 years of age, smoked cigarettes on not less than 25 of the last 30 days, had a home address and telephone, and were willing to participate in phone assessments. Smokers were excluded if they had a terminal medical condition with a life expectancy less than 1 year or were pregnant. Participation was not contingent upon willingness to quit. If participants were found to be eligible and were interested in participating, informed consent was obtained verbally via telephone. After being consented, participants completed a baseline assessment and were given a written smoking cessation guide. Participants were then randomly assigned either to C or to CCC for smoking cessation. Randomization occurred centrally at the University of Kansas Medical Center after receiving the referral from the hospital staff. Hospital staff were not made aware of the participant’s allocation in the study until after randomization was complete and the study intervention was underway. To minimize potential confounding by hospital, separate blocked randomization lists were developed for each hospital.
Interventions
Smoking Cessation Guide
All participants received a 50-page smoking cessation guide written by the study team at an eighth grade reading level. The guide covered health benefits of quitting and multiple cognitive and behavioral strategies for staying smoke-free after hospital discharge. Topics included getting support for quitting, coping with withdrawal and triggers, creating a personalized quit plan, managing stress, and preventing relapse. The guide provided 16 pages of information about first-line pharmacotherapies to aid quitting (nicotine replacement, bupropion, varenicline), including cost, potential side effects and symptom management, and frequently asked questions. Information also included descriptions of medication assistance programs from GlaxoSmithKline, Pfizer, and Together RX for those smokers who did not have insurance coverage.
The guide also provided specific information about how participants could work with their health care provider to obtain a prescription for cessation pharmacotherapy. Variation in insurance coverage and changes in coverage over time precluded us from developing a comprehensive guide to pharmacotherapy coverage for all insurers in the state of Kansas.
Counseling
In the C group, counselors delivered individualized, telephone-based counseling following protocols similar to those used by tobacco treatment quitline programs. Counseling calls were made proactively at enrollment, 2 days postenrollment, and at 1, 3, and 6 weeks postenrollment for a total of five counseling calls. During each call, counselors addressed the participant’s readiness to quit smoking and used motivation-enhancing strategies to encourage a quit attempt. All participants were advised to use nicotine replacement therapy (NRT) during their hospitalization to alleviate withdrawal symptoms regardless of their current interest in quitting. At each counseling session, counselors helped participants who were interested in quitting develop a personalized quit plan that incorporated cognitive–behavioral strategies, environmental changes, and pharmacotherapy. For participants in this group who developed a quit plan, counselors provided advice to use pharmacotherapy to help them quit, a menu of pharmacotherapy options, and guidance on working with their health care provider for help in procuring cessation medications. Medication use was assessed in every counseling session, and adherence and side effect management were addressed as needed during any session in which participants reported pharmacotherapy use. If the participant was not thinking about quitting or not ready to set a quit plan, medication use was not discussed unless the participant requested more information. Similar to the independent nature of quitline counseling, after participants in this group enrolled in the study, there was no further communication between the study counselor and the hospital or the participant’s health care provider.
Counseling With Care Coordination
The same cessation counselors delivered the CCC intervention. Participants in the CCC arm were contacted at the same timepoints as C and received similar individualized, telephone-based counseling, as described above. In contrast, in the CCC arm, counselors’ recommendations for pharmacotherapy use were delivered using an “opt-out” approach. In this approach, counselors identified pharmacotherapy alternatives based on the participant’s insurance coverage and potential contraindications. Participants were not asked to declare an interest in making a quit plan before being presented with a menu of pharmacotherapy choices and asked to choose among these treatment options. This style of opt-out treatment changes the default for smoking cessation treatment and was designed to mimic the presentation of treatment choices for other chronic conditions, such as diabetes or hypertension.28–30 If the participant in CCC “opted-out” and expressed a desire not to use any medications, the counselor addressed concerns, but switched focus to behavioral strategies to support a quit attempt or motivational strategies to encourage a quit attempt. If the participant selected a medication, the counselor faxed treatment recommendations to the participant’s health care provider, including a summary of the counseling progress notes and a prescription request. Faxes were sent to either the hospital or the doctor’s office depending on whether or not the patient had been discharged at the time of the call. If during the course of follow-up calls the counselors identified the need for a prescription refill or a change in medication due to side effects or a lack of efficacy, a follow-up counseling report and prescription request was faxed to the physician’s office.
Counseling Training, Supervision, and Treatment Fidelity
Counselors were trained in providing comprehensive tobacco treatment from the Tobacco Treatment Specialist Training and Certification Program from University of Massachusetts. They passed proficiency exams, completed additional in-house training in providing study treatment protocols, and participated in ongoing clinical supervision from a licensed psychologist for fidelity monitoring. Supervision included reviewing recordings of counseling sessions. The supervisor completed a fidelity rating form to evaluate global counseling skills and compliance with treatment arm counseling protocols. Fidelity monitoring focused on ensuring that only the CCC arm included the “opt-out” approach for pharmacotherapy use and subsequent care coordination.
Measures
Study staff collected participant self-reported data at baseline and 3 months postdischarge. At baseline, participants provided demographic data, as well as information on insurance coverage and their health status. Study staff also assessed smoking history and prior and current use of smoking cessation pharmacotherapy. Motivation and confidence to quit were assessed using single-item 10-point Likert scales. Nicotine dependence was assessed using the Heavy Smoking Index.31 Health insurance coverage was assessed and was divided into three categories: Medicaid (the prescription program for the poor in the United States), Other insurance, and Uninsured. At 3 months postdischarge, all participants, regardless of their smoking status, were asked to report on the type and duration of use of postdischarge cessation pharmacotherapy. Utilization of pharmacotherapy was assessed by asking participants which medication they used and for how long. Further, because prescriptions are often written with a 1-month supply, we examined medication adherence based on a dichotomous variable of use of pharmacotherapy for either more or less than 4 weeks. Pharmacotherapy use was classified as use of varenicline, bupropion, nicotine patch, short-acting NRT (gum, lozenge, nasal spray, or nicotine inhaler), or use of more than one agent.
Data Analysis
All data were summarized using frequencies and descriptive statistics. We examined differences between those who completed the 3-month assessment versus those who did not using chi-square and independent samplest tests where applicable (Supplementary Table 1). We also examined differences between study arms of those who completed the 3-month assessment versus those who did not. We examined bivariate relationships between participant characteristics and use of pharmacotherapy and use of pharmacotherapy for more than 4 weeks (Supplementary Table 2). We then used multiple logistic regression to further delineate the role of insurance coverage on pharmacotherapy use postdischarge and adherence (use >4 weeks). Because we were looking at the role of a specific variable (insurance) and were not trying to develop an overall predictive model, we sought to avoid problems with multicollinearity by omitting certain variables from the model, such as “prior use of pharmacotherapy.”32 We prespecified the inclusion “receipt of smoking cessation medications during the hospitalization” as a marker of the participants interest in use of pharmacotherapy. We also prespecified inclusion of gender, age, and treatment group in the model and added cigarettes smoked per day based on its strong bivariate relationship with the outcome. Analyses were completed using SAS v. 9.4 (SAS Institute Inc, Cary, NC).
Results
Study Sample
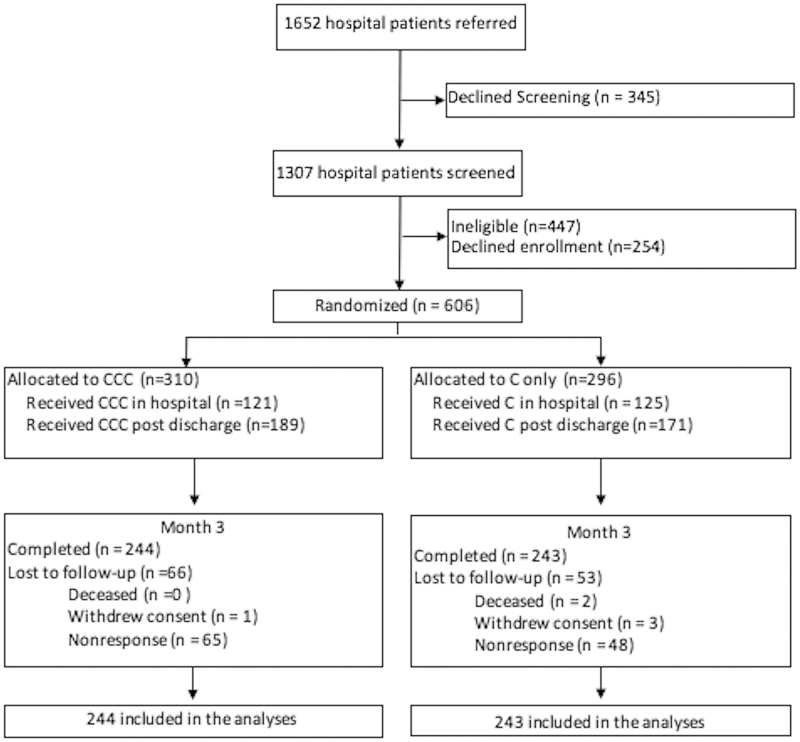
A total of 1652 potential participants were referred by hospital personnel. Of these, 345 declined screening, 447 were ineligible, and 254 otherwise eligible participants declined enrollment. Of the 606 participants enrolled in the study, 487 (80.4%) completed the 3-month follow-up assessment and were included in the subsequent analysis (Figure 1). Follow-up rates did not differ between CCC (244, 78.7%) and C (243, 82.1%) (p = .29). The two treatment groups were similar at baseline, but compared to all participants, those completing follow-up were older (p = .005) and more likely to have health insurance (p = .006); they also completed more counseling sessions (p ≤ .0001) and were less likely to have young children in the home (p = .007) (Supplementary Table 1). Due to these differences in study attrition, participants in the C and CCC arms completing the 3-month follow-up differed in level of education and motivation to quit smoking and confidence in their ability to quit smoking (Table 1). Of the 487 participants completing the 3-month follow-up, 201 (41.3%) received their first counseling call while they were in the hospital, while the remaining 58.7% did not get their first counseling call until after discharge. The majority of participants were female (63.2%) and white (90.3%); 41.9% were less than 100% of the federal poverty level. Participants smoked on average 19.8 (SD = 10.6) cigarettes/day and were highly motivated to quit. About one-third reported believing their current hospitalization was related to smoking. Nearly two-thirds (65.3%) had used smoking cessation pharmacotherapy in the past and 41.5% reported use of cessation pharmacotherapy during the hospitalization. Participants completed similar numbers of counseling calls in both the C and CCC arms.
Figure 1.
Flow diagram of participants completing 3-mo follow-up.
Table 1.
Baseline Characteristics of 487 Study Participants Completing 3-mo Assessments
| Characteristics | Total (n = 487) | Counseling (n = 243) | CCC (n = 244) | p |
|---|---|---|---|---|
| Age, mean (SD), y | 51.6 (11.7) | 51.5 (11.5) | 51.7 (11.9) | .84 |
| Female,N (%) | 308 (63.2) | 154 (63.4) | 154 (63.1) | .95 |
| Race,N (%) | .51 | |||
| White | 440 (90.3) | 224 (92.2) | 216 (88.5) | |
| African American | 21 (4.3) | 9 (3.7) | 12 (4.9) | |
| American Indian | 19 (3.9) | 8 (3.3) | 11 (4.5) | |
| Other/not reported | 7 (1.4) | 2 (0.8) | 5 (2.0) | |
| Latino,N (%) | 26 (5.3) | 8 (3.3) | 18 (7.4) | .05 |
| High school graduate,N (%) | 258 (53.0) | 116 (47.7) | 142 (58.2) | .02 |
| <100% of federal poverty level,N (%) | 204 (41.9) | 96 (39.5) | 108 (44.3) | .29 |
| Have health insurance,N (%) | 382 (78.4) | 190 (78.2) | 192 (78.7) | .89 |
| Medicaid,N (%) | 141 (29.0) | 72 (29.6) | 69 (28.3) | .52 |
| Prescription insurance,N (%) | 353 (72.5) | 178 (73.3) | 175 (71.7) | .52 |
| Smoke within 30min of waking,N (%) | 227 (46.6) | 114 (46.9) | 113 (46.3) | .77 |
| No. of cigarettes per day, mean (SD) | 19.8 (10.6) | 20.0 (11.1) | 19.7 (10.2) | .76 |
| Heaviness of Smoking Index ≥4,N (%)a | 226 (46.4) | 112 (46.1) | 114 (46.7) | .89 |
| Living statusb | .79 | |||
| Lives alone,N (%) | 109 (22.4) | 42 (17.3) | 67 (27.5) | |
| Other smokers in home,N (%) | 223 (45.8) | 119 (49.0) | 104 (42.6) | |
| Only nonsmokers in home,N (%) | 154 (31.6) | 81 (33.3) | 73 (29.9) | |
| Children age <12 in home,N (%) | 103 (21.1) | 51 (21.0) | 52 (21.3) | .93 |
| Motivation to quit, mean (SD)c | 9.3 (1.5) | 9.2 (1.6) | 9.4 (1.3) | <.0001 |
| Confidence to quit, mean (SD)c | 6.9 (2.5) | 6.7 (2.4) | 7.1 (2.6) | <.0001 |
| Planning to quit in next 30 d,N (%) | 472 (96.9) | 233 (95.9) | 239 (98.0) | .19 |
| Relate current hospitalization to their smoking,N (%)d | 162 (33.3) | 79 (32.5) | 83 (34.0) | .72 |
| Length of hospital stay, mean (SD) | 4.1 (3.5) | 4.2 (3.4) | 4.0 (3.5) | .64 |
| Previous use of cessation pharmacotherapy,N (%) | 318 (65.3) | 161 (66.3) | 157 (64.3) | .66 |
| Receipt of smoking medications during hospitalization,N (%) | 202 (41.5) | 99 (40.7) | 103 (42.2) | .74 |
| Received counseling call during the hospitalization,N (%) | 201 (41.3) | 105 (43.2) | 96 (39.5) | .39 |
| Counseling calls completed posthospitalization, mean (SD) | 3.1 (1.7) | 3.1 (1.6) | 3.2 (1.7) | .58 |
CCC = counseling with care coordination.
aHeaviness of Smoking Index ranges from 0 to 6. Scores of 4 or higher indicate greater levels of nicotine dependence.
b n = 1 participant did not respond to living status question.
cMotivation and confidence to quit smoking scores range from 0 to 10.
dRate agreement based on a response of 6 or 7 on a 7-point Likert scale.
Utilization of Pharmacotherapy Postdischarge
Table 2 presents data on use of pharmacotherapy postdischarge. During the 3 months after hospital discharge, 43.3% of study participants used some form of pharmacotherapy to stop smoking; 35.5% used only one medication while 7.8% tried two or more agents. The most commonly used pharmacotherapy was the nicotine patch (28.3% of participants), followed by the short-acting NRT (15.2%), varenicline (9.9%), and bupropion (9.0%).
Table 2.
Participants Using Smoking Cessation Pharmacotherapy During the 3 mo After Hospital Discharge
| Pharmacotherapy | Any use of pharmacotherapy | Use of pharmacotherapy >4 wk | ||||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 487),N (%) | Counseling (n = 243),N (%) | CCC (n = 244),N (%) | p | Total (n = 487),N (%) | Counseling (n = 243),N (%) | CCC (n = 244),N (%) | p | |
| Any pharmacotherapy | 211 (43.3) | 102 (42.0) | 109 (44.7) | 0.55 | 112 (23.0) | 54 (22.2) | 58 (23.8) | .68 |
| Nicotine patch | 138 (28.3) | 78 (32.1) | 60 (24.6) | 0.07 | 47 (9.7) | 29 (11.9) | 18 (7.4) | .09 |
| Short-acting NRT | 74 (15.2) | 34 (14.0) | 40 (16.4) | 0.46 | 13 (2.7) | 3 (1.2) | 10 (4.1) | .05 |
| Bupropion | 44 (9.03) | 21 (8.6) | 23 (9.4) | 0.76 | 30 (6.2) | 16 (6.6) | 14 (5.7) | .70 |
| Varenicline | 48 (9.86) | 20 (8.2) | 28 (11.5) | 0.23 | 22 (4.5) | 8 (3.3) | 14 (5.7) | .19 |
| More than one | 38 (7.80) | 17 (7.0) | 21 (8.6) | 0.51 | 10 (2.1) | 4 (1.7) | 6 (2.5) | .52 |
CCC = counseling with care coordination; NRT = nicotine replacement therapy.
Use of pharmacotherapy postdischarge did not differ significantly between study arms (44.7% of CCC recipients and 42.0% of C recipients reporting use of one or more medications,p = .55). In bivariate analyses, use of pharmacotherapy was positively associated with older age, smoking 20 or more cigarettes/day, prior use of pharmacotherapy, and receipt of pharmacotherapy during the hospitalization (Supplementary Table 2). While 125 (61.9%) of the 202 smokers who initiated pharmacotherapy during the hospital continued to use it after discharge, only 86 (30.2%) of the 285 smokers who did not receive medication during the hospitalization initiated it after discharge (p < .001). Among the 285 smokers who did not receive pharmacotherapy during the hospitalization, 48 (16.8%) of CCC participants and 38 (13.3%) of C recipients initiated pharmacotherapy after discharge (p = .16). In a multivariate analysis, 20 or more cigarettes/day (odds ratio [OR]: 1.48; 95% confidence interval [CI]: 1.00–2.19), pharmacotherapy use during hospitalization (OR: 4.00; 95% CI: 2.39–6.89), and having health insurance (either Medicaid [OR: 2.29; 95% CI: 1.32–4.02] or other insurance [OR: 1.69; 95% CI: 1.01–2.86]) were significantly associated with use of pharmacotherapy postdischarge (Table 3).
Table 3.
Independent Predictors of Pharmacotherapy Utilization and Extended Use of Pharmacotherapy After Hospital Dischargea
| Factor | Any pharmacotherapy | Pharmacotherapy >4 wk |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| CCC | 1.01 (0.75–1.61) | 1.07 (0.67–1.67) |
| Female | 1.23 (0.83–1.83) | 1.34 (0.84–2.16) |
| ≥50 y old | 1.44 (0.97–2.16) | 1.38 (0.86–2.25) |
| ≥20 cigarettes/d | 1.48 (1.00–2.19) | 1.05 (0.66–1.66) |
| Receipt of smoking medications during hospitalization | 4.00 (2.39–6.89) | 4.23 (2.54–7.06) |
| Insurance | ||
| Medicaid | 2.29 (1.32–4.02) | 1.78 (0.93–3.54) |
| Other insurance | 1.69 (1.01–2.86) | 1.49 (0.80–2.85) |
CCC = counseling with care coordination; CI = confidence interval; OR = odds ratio.
aBased on multivariate logistic regression model.
Adherence to Pharmacotherapy Postdischarge
Nearly one-fourth of participants reported using pharmacotherapy for more than 4 weeks, as presented inTable 2. Use of pharmacotherapy for more than 4 weeks was similar across study arms, with the exception of short-acting NRT. Participants receiving CCC were more likely to use short-acting NRT for more than 4 weeks (p = .05). Among those using pharmacotherapy, the mean number of weeks of use postdischarge was 6.0 weeks (SD = 5.0) for the CCC group and 5.2 weeks (SD = 4.9) for the C group (p = .87). Weeks of therapy varied according to the type of medication, ranging from a mean of 8.6 weeks (SD = 3.7) for recipients of bupropion to 1.6 weeks (SD = 2.3) for recipients of short-acting NRT. In bivariate analyses, use of pharmacotherapy was positively associated with older age, prior use of pharmacotherapy, a quit attempt in the past 6 months, and receipt of pharmacotherapy during the hospitalization (Supplementary Table 2). In multivariate analysis, only pharmacotherapy use during the hospitalization was significantly associated with use for more than 4 weeks postdischarge (OR: 4.32; 95% CI: 2.54–7.06) (Table 3).
Discussion
This study examined the impact of adding care coordination to standard smoking cessation counseling to facilitate use of pharmacotherapy following hospital discharge. Findings failed to demonstrate a significant treatment effect of this additional medication care coordination above and beyond any impact that might have been provided by counseling alone. Specifically, despite directive recommendations to use pharmacotherapy to support abstinence and offers to assist smokers with obtaining cessation medication, additional care coordination did not increase pharmacotherapy utilization in the current sample.
Our findings are in sharp contrast to a 2014 study by Rigotti and colleagues that tested the impact of a “sustained care intervention” on cessation pharmacotherapy use and cessation outcomes.33 That study showed that a free 30-day supply of medication, refillable for up to a 90-day supply, supported by automated interactive voice response telephone calls, was associated with high rates of postdischarge use of pharmacotherapy (86.9%)—significantly higher use than seen in their control condition. Several factors could explain the differences in our study findings. All of the participants in the Rigotti study received inpatient counseling and a higher percentage (67%) received pharmacotherapy during their hospitalization. The most obvious difference, however, is how the medication management was addressed. While our study relied on prescriptions from providers and traditional pharmacies to provide medications, in the Rigotti study, all of the smokers in the “sustained care intervention” received free medications directly without the need to coordinate care with their physician or procure prescriptions from the pharmacy. Whereas free pharmacotherapy distributed directly by quitlines can have a positive impact on both utilization of services and cessation success, our study suggests that efforts by quitlines to coordinate prescriptions with providers and insurance coverage would be unlikely to have a comparable impact on use of effective cessation treatment.
The high rate of pharmacotherapy use seen by Rigotti when medications are provided free, highlights the potential importance of cost on use of treatment. The 2010 Patient Protection and Affordable Care Act mandates new insurance plans to provide coverage for evidence-based tobacco treatment, as of 2014.34 We showed that insurance coverage, particularly Medicaid, is associated with higher uptake of smoking cessation pharmacotherapy. In the United States, Medicaid provides insurance for individuals who meet certain eligibility criteria and are too poor to get insurance by any other means. Nevertheless, 22% of the hospitalized smokers in this study were still uninsured. Kansas is one of 21 states in the United States that has not expanded Medicaid coverage as part of the Affordable Care Act.35 In our study, these uninsured smokers were significantly less likely to receive cessation pharmacotherapy. Our study suggests that expansion of Medicaid coverage in the United States could provide an important opportunity to increase access to cessation pharmacotherapy.
Our study identified a strong relationship between use of pharmacotherapy in the hospital and continuation of this evidence-based treatment after discharge. Our finding is similar to the relationship that Regan et al. demonstrated on the relationship between inpatient and subsequent postdischarge use of NRT.36 Together, these findings highlight the potential importance of the Joint Commission performance standards that advocate both counseling and medication use for hospitalized smokers.37
In our study, only 23% of smokers continued cessation treatment beyond 4 weeks despite the proactive delivery of telephone-based cessation counseling and support. Of note, Rigotti et al. demonstrated that 69% of those receiving free medications and automated telephone calls continued treatment for at least 4 weeks after discharge.33 This threefold difference in adherence suggests the need for a much closer examination of how we provide medications and counseling support to smokers after discharge. Indeed, the telephone-based care coordination used in our study may not be nearly as effective as the proactive provision of a 30-day supply of treatment.
Design considerations and study limitations influence the interpretation of the findings of this study. The sample in this study consisted of inpatient smokers primarily in critical access hospitals in rural Kansas communities, which may limit generalizability to urban settings or to states or countries with different insurance structure, or different systems in place to treat tobacco. Participants were referred to the program by hospital personnel; these smokers may differ in important ways from those who were not referred, and we do not know how many smokers were not referred. Further, we could not control for any counseling or education the participants may have received while in the hospital or from other sources, which could have contributed to the strong effect of receiving medication in hospital in the multivariate analysis. We were unable to assess the use or impact of the written smoking cessation guide, but the clinical supervision and monitoring of treatment fidelity throughout the intervention rules make it unlikely that failure to find a statistically significant treatment effect could be attributed to counseling contamination across treatment arms. To assess the impact of insurance coverage on pharmacotherapy use, we prespecified specific variables to include; we eliminated other variables that we felt might be causally linked between insurance coverage and pharmacotherapy use and create problems with multicollinearity. Alternative methods of model construction might lead to different results.
Many state quitlines do not offer free pharmacotherapy to all participants. This study is among the first to examine uptake of cessation medications in an environment in which free pharmacotherapy is not readily available through a single third party. Although this study failed to show a significant impact of additional pharmacotherapy care coordination, future research should examine variations in pharmacotherapy uptake associated with different quitline providers and state insurance coverage policies.
Conclusions
Despite clinical practice recommendations for identification and treatment of hospitalized smokers and clear evidence that pharmacotherapy supports smoking abstinence, the majority of our smokers from predominantly rural critical access hospitals failed to either receive or continue to use smoking cessation pharmacotherapy following hospitalization. While smoking counseling and education may have provided broad support to smokers, supplemental medication care coordination did not further enhance the use of postdischarge pharmacotherapy beyond that achieved by inpatient treatment and postdischarge smoking cessation counseling. Importantly, smokers who have insurance coverage and initiated medication use during the hospitalization were significantly more likely to use pharmacotherapy, highlighting the need of initiating pharmacotherapy during hospitalization. Maximizing evidence-based therapy postdischarge may benefit from expansion of insurance coverage to all poor smokers and establishing systems that proactively offer cessation therapy to all hospitalized smokers.
Supplementary Material
Supplementary Tables 1 and 2 can be found online at http://www.ntr.oxfordjournals.org
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health (R01CA101963). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. This trial was registered onclinicaltrials.gov (NCT01063972).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
The authors would like to thank the hospitals throughout the state of Kansas that participated in this study and the volunteers who participated in this research.
References
- 1. Benowitz NL.Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment.Prog Cardiovasc Dis.2003;46(1):91–111. doi:10.1016/S0033-0620(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 2. Hennrikus DJ, Lando HA, McCarty MC, et al. The TEAM project: the effectiveness of smoking cessation intervention with hospital patients.Prev Med.2005;40(3):249–258. doi:10.1016/j.ypmed.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 3. Rigotti NA, Munafo MR, Stead LF.Smoking cessation interventions for hospitalized smokers: a systematic review.Arch Intern Med.2008;168(18):1950–1960. doi:10.1001/archinte.168.18.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiore MC, Jaén CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary.Respir Care.2008;53(9):1217–1222.http://rc.rcjournal.com/content/53/9/1217.full.pdf+html. Accessed December 2015. [PubMed] [Google Scholar]
- 5. Fiore MC, Goplerud E, Schroeder SA.The Joint Commission’s new tobacco-cessation measures—will hospitals do the right thing? N Engl J Med.2012;366(13):1172–1174. doi:10.1056/NEJMp1115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US Department of Health and Human Services.Hospital Compare 2015.www.hospitalcompare.hhs.gov AccessedJune 2015. [Google Scholar]
- 7. Rollow W, Lied TR, McGann P, et al. Assessment of the Medicare quality improvement organization program.Ann Intern Med.2006;145(5):342–353. doi:10.7326/0003-4819-145-5-200609050-00134. [DOI] [PubMed] [Google Scholar]
- 8. North American Quitline Consortium.A Practical Guide to Promising Approaches.Phoenix, AZ:North American Quitline Consortium;2005https://c.ymcdn.com/sites/www.naquitline.org/resource/resmgr/reports-naqc/quitline_approaches.pdf AccessedDecember 2015. [Google Scholar]
- 9. Perry RJ, Keller PA, Fraser D, Fiore MC.Fax to quit: a model for delivery of tobacco cessation services to Wisconsin residents.WMJ.2005;104(4):37–40, 44.www.wisconsinmedicalsociety.org/_WMS/publications/wmj/pdf/104/4/37.pdf AccessedDecember 2015. [PubMed] [Google Scholar]
- 10. Bernstein SL, Jearld S, Prasad D, Bax P, Bauer U.Rapid implementation of a smokers’ quitline fax referral service in an urban area.J Health Care Poor Underserved.2009;20(1):55–63. doi:10.1353/hpu.0.0112. [DOI] [PubMed] [Google Scholar]
- 11. Wadland WC, Holtrop JS, Weismantel D, Pathak PK, Fadel H, Powell J.Practice-based referrals to a tobacco cessation quit line: assessing the impact of comparative feedback vs general reminders.Ann Fam Med.2007;5(2):135–142. doi:10.1370/afm.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bentz CJ, Bayley KB, Bonin KE, Fleming L, Hollis JF, McAfee T.The feasibility of connecting physician offices to a state-level tobacco quit line.Am J Prev Med.2006;30(1):31–37. doi:10.1016/j.amepre.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 13. Willett JG, Hood NE, Burns EK, et al. Clinical faxed referrals to a tobacco quitline: reach, enrollment, and participant characteristics.Am J Prev Med.2009;36(4):337–340. doi:10.1016/j.amepre.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 14. Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial.JAMA.2006;296(1):56–63. doi:10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 15. Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial.JAMA.2006;296(1):47–55. [DOI] [PubMed] [Google Scholar]
- 16. Rigotti NA, Clair C, Munafo MR, Stead LF.Interventions for smoking cessation in hospitalised patients.Cochrane Database Syst Rev.2012;5:CD001837. doi:10.1002/14651858.CD001837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rigotti NA, Thorndike AN, Regan S, et al. Bupropion for smokers hospitalized with acute cardiovascular disease.Am J Med.2006;119(12):1080–1087. doi:10.1016/j.amjmed.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention.Quitting smoking among adults—United States, 2001–2010.MMWR.2011;60(44):1513–1519.www.cdc.gov/mmwr/preview/mmwrhtml/mm6044a2.htm AccessedDecember 2015. [PubMed] [Google Scholar]
- 19. Ferketich AK, Khan Y, Wewers ME.Are physicians asking about tobacco use and assisting with cessation? Results from the 2001–2004 National Ambulatory Medical Care Survey (NAMCS).Prev Med.2006;43(6):472–476. doi:10.1016/j.ypmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 20. Thorndike AN, Regan S, Rigotti NA.The treatment of smoking by US physicians during ambulatory visits: 1994 2003.Am J Public Health.2007;97(10):1878–1883. doi:10.2105/AJPH.2006.092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A.Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler.Arch Int Med.1999;159(17):2033–2038. doi:10.1001/archinte.159.17.2033. [DOI] [PubMed] [Google Scholar]
- 22. Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, van Schayck CP.Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease.Arch Int Med.2005;165(19):2286–2292. doi:10.1001/archinte.165.19.2286. [DOI] [PubMed] [Google Scholar]
- 23. Fossati R, Apolone G, Negri E, et al. A double-blind, placebo-controlled, randomized trial of bupropion for smoking cessation in primary care.Arch Int Med.2007;167(16):1791–1797. doi:10.1001/archinte.167.16.1791. [DOI] [PubMed] [Google Scholar]
- 24. Snow V, Beck D, Budnitz T, et al. Transitions of Care Consensus policy statement: American College of Physicians, Society of General Internal Medicine, Society of Hospital Medicine, American Geriatrics Society, American College of Emergency Physicians, and Society for Academic Emergency Medicine.J Hosp Med.2009;4(6):364–370. doi:10.1002/jhm.510. [DOI] [PubMed] [Google Scholar]
- 25. State Medicaid coverage for tobacco-dependence treatments—United States, 2005.MMWR.2006;55(44):1194–1197.www.cdc.gov/mmwr/preview/mmwrhtml/mm5544a2.htm AccessedDecember 2015. [PubMed] [Google Scholar]
- 26. McMenamin SB, Halpin HA, Ibrahim JK, Orleans CT.Physician and enrollee knowledge of Medicaid coverage for tobacco dependence treatments.Am J Prev Med.2004;26(2):99–104. doi:10.1016/j.amepre.2003.10.017 [DOI] [PubMed] [Google Scholar]
- 27. Murphy JM, Mahoney MC, Hyland AJ, Higbee C, Cummings KM.Disparity in the use of smoking cessation pharmacotherapy among Medicaid and general population smokers.J Public Health Manag Pract.2005;11(4):341–345. [DOI] [PubMed] [Google Scholar]
- 28. Richter KP, Ellerbeck EF.It’s time to change the default for tobacco treatment.Addiction.2015;110(3):381–386. doi:10.1111/add.12734. [DOI] [PubMed] [Google Scholar]
- 29. Whitworth JA, Chalmers J.World Health Organisation-International Society of Hypertension (WHO/ISH) hypertension guidelines.Clin Exp Hypertens.2004;26:747–752. [DOI] [PubMed] [Google Scholar]
- 30. American Diabetes Association.7. Approaches to glycemic treatment.Diabetes Care 2015;28(suppl 1):S41–S48. [DOI] [PubMed] [Google Scholar]
- 31. Chabrol H, Niezborala M, Chastan E, de Leon J.Comparison of the Heavy Smoking Index and of the Fagerstrom Test for Nicotine Dependence in a sample of 749 cigarette smokers.Addict Behav.2005;30(7):1474–1477. doi:10.1016/j.addbeh.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 32. McDonald JH. Handbook of Biological Statistics.2nd ed.Baltimore, MD:Sparky House Publishing;2009. [Google Scholar]
- 33. Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial.JAMA.2014;312(7):719–728. doi:10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Centers for Disease Control.Coverage for Tobacco Use Cessation Treatments.2014www.cdc.gov/tobacco/quit_smoking/cessation/coverage/pdfs/coverage-508–1019.pdf AccessedNovember 2015. [Google Scholar]
- 35. Cardwell A, Sheedy K. Map: Where States Stand on Medicaid Expansion Decisions.www.statereforum.org/Medicaid-Expansion-Decisions-Map AccessedNovember 2015. [Google Scholar]
- 36. Regan S, Reyen M, Richards AE, Lockhart AC, Liebman AK, Rigotti NA.Nicotine replacement therapy use at home after use during a hospitalization.NTR.2012;14(7):885–889. doi:10.1093/ntr/ntr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. The Joint Commission.Specifications Manual for National Hospital Inpatient Quality Measures.2015www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_measures.aspx AccessedNovember 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.