Abstract
The degree of normal fibroglandular tissue that enhances on breast MRI, known as background parenchymal enhancement (BPE), was initially described as an incidental finding that could affect interpretation performance. While BPE is now established to be a physiologic phenomenon that is affected by both endogenous and exogenous hormone levels, evidence supporting the notion that BPE frequently masks breast cancers is limited. However, compelling data have emerged to suggest BPE is an independent marker of breast cancer risk and breast cancer treatment outcomes. Specifically, multiple studies have shown that elevated BPE levels, measured qualitatively or quantitatively, are associated with a greater risk of developing breast cancer. Evidence also suggests that BPE could be a predictor of neoadjuvant breast cancer treatment response and overall breast cancer treatment outcomes. These discoveries come at a time where breast cancer screening and treatment have moved toward an increased emphasis on targeted and individualized approaches, of which the identification of imaging features that can predict cancer diagnosis and treatment response is an increasingly recognized component. Historically, researchers have primarily studied quantitative tumor imaging features in pursuit of clinically useful biomarkers. However, the need to segment less well-defined areas of normal tissue for quantitative BPE measurements presents its own unique challenges. Furthermore, there is no consensus on the optimal timing on dynamic contrast enhanced MRI for BPE quantitation. This article comprehensively reviews BPE with a particular focus on its potential to increase precision approaches to breast cancer risk assessment, diagnosis, and treatment. It also describes areas of needed future research, such as applicability of BPE to women at average risk, the biological underpinnings of BPE, and the standardization of BPE characterization.
Keywords: breast MRI, breast cancer risk, background parenchymal enhancement, fibroglandular tissue, biomarkers, precision medicine
Introduction
Over the past decade, there has been a paradigm shift within the breast oncology community from a singular, one-size-fits-all approach to breast cancer screening and treatment to those that are tailored to patients’ genetic, environmental, and lifestyle factors. The introduction of established tumor biomarkers to the standard TNM Classification of Malignant Tumors in the most recent edition of the American Joint Committee on Cancer Staging Manual for breast cancer (1) illustrates the ongoing movement towards precision diagnostic and therapeutic approaches. Breast imagers have the opportunity to align behind this shift within the broader oncologic community to help determine optimal approaches for breast cancer screening based on an individual’s lifetime risk.
To this end, it has been established that the amount of fibroglandular breast tissue as measured by mammographic breast density is associated with breast cancer risk (2–4), and several studies have evaluated the addition of mammographic density as a factor in risk prediction models (5,6). However, dense breasts are present in approximately half of screening-aged women (7), most of whom will never develop breast cancer, and thus density has provided only a marginal incremental benefit for predicting who will develop breast cancer (5,6). Recently, it has been suggested that MRI features of normal breast tissue may provide a more precise breast cancer risk assessment than mammographic density. It has long been known that normal fibroglandular breast tissue on MRI can enhance at variable levels after the administration of gadolinium-based contrast material, which has been termed background parenchymal enhancement (BPE). Although BPE was initially considered an incidental finding that could decrease breast MRI sensitivity, recent data suggest it has only a mild impact on breast MRI interpretation performance. Interestingly, BPE levels are now more strongly linked to breast cancer risk and treatment outcomes.
Since King and colleagues’ initial report of BPE corresponding to increased risk of breast cancer diagnosis in 2011 (8), over 25 studies have been published evaluating BPE and breast cancer risk. Given the rapid increase in interest in the potential prognostic value of BPE and the growing number of publications on the topic, this article aims to comprehensively review BPE. Specifically, we describe the typical MRI appearance of BPE, factors influencing BPE levels, BPE effect on MRI diagnostic performance, and the association of BPE levels with breast cancer risk and outcomes. Finally, we discuss the limitations of current studies, challenges in BPE quantitation, and the promise of radiomics for identifying normal tissue phenotypes to predict meaningful outcomes.
Typical MRI Appearances of BPE
Among other standard sequences, current breast MRI protocols routinely include dynamic contrast enhanced (DCE) T1-weighted images comprised of a pre-contrast and variable number of post-contrast sequences to identify suspicious enhancing lesions and assess lesion enhancement kinetic features. Although typically to a lesser degree than malignant lesions, normal fibroglandular tissue also exhibits enhancement at variable levels, which is qualitatively assessed by the interpreting radiologist on the early phase post-contrast sequence as minimal, mild, moderate, or marked (Figure 1) according to the Breast Imaging Reporting and Data System (BI-RADS) MR imaging lexicon (9). While there are various methods to quantitatively assess BPE, which are described in more detail below, algorithms vary from institution to institution and no standard method of quantifying BPE is currently used in clinical practice (10–13).
Figure 1:
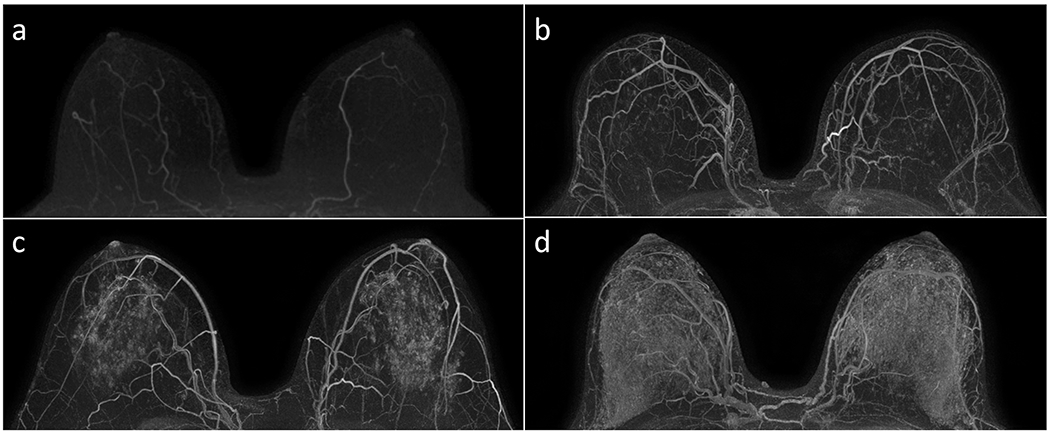
Examples of qualitative BPE assessments of minimal (A), mild (B), moderate (C), and marked (D) on subtracted post-contrast maximum intensity projection images in accordance with the BI-RADS Atlas. Each case was in a unique patient, and all four MRIs revealed no suspicious enhancement in either breast.
BPE commonly occurs in a “picture framing” or “cortical” pattern, where enhancement occurs initially at the periphery of the fibroglandular tissue, likely due to the pattern of blood inflow from the internal mammary and lateral thoracic artery branches into the breast tissue (14). However, not all patients exhibit peripheral BPE, with other patterns of central, nodular, regional, and diffuse foci of enhancement also described (15). In general, BPE is not directly affected by mammographic breast density or amount of fibroglandular tissue, as women with extremely dense breasts may exhibit the full range of qualitative BPE assessments. To this effect, several authors have shown that BPE does not correlate with mammographic breast density when breast MRI is timed to the first half of the menstrual cycle in pre-menopausal women (16–18). However, women with less fibroglandular tissue relative to breast volume may on average exhibit lower qualitative levels of BPE since there is a smaller amount of parenchyma that can enhance relative to adipose tissue and overall breast volume. Accordingly, a few reports did find that less-dense breasts were associated with less BPE when imaging was not restricted to a certain phase of the menstrual cycle (19,20).
BPE can also be described as symmetrical or asymmetrical, owing to a variety of patient factors. Most women exhibit symmetrical BPE; those with asymmetrical BPE commonly have asymmetric amounts of fibroglandular tissue (e.g. accessory breast tissue). Similarly, a history of prior breast conservation therapy can affect BPE pattern and symmetry (21), as there will often be reduced BPE in the breast that has undergone a lumpectomy due to both a reduced volume of tissue and sequela from radiation therapy.
Timing of the DCE sequences also has a strong effect on BPE and lesion visibility. BPE increases with time after contrast injection, with one study demonstrating BPE peaking around 210 seconds after injection and plateauing by 320 seconds (22). It is recommended that BPE be assessed during the early phase of DCE series where cancers typically exhibit peak enhancement. This timepoint with k-space centered one to two minutes after contrast injection should result in malignancies to exhibit maximal enhancement while BPE has not reached peak enhancement, lessening the risk that BPE could limit cancer detection (Figure 2).
Figure 2:
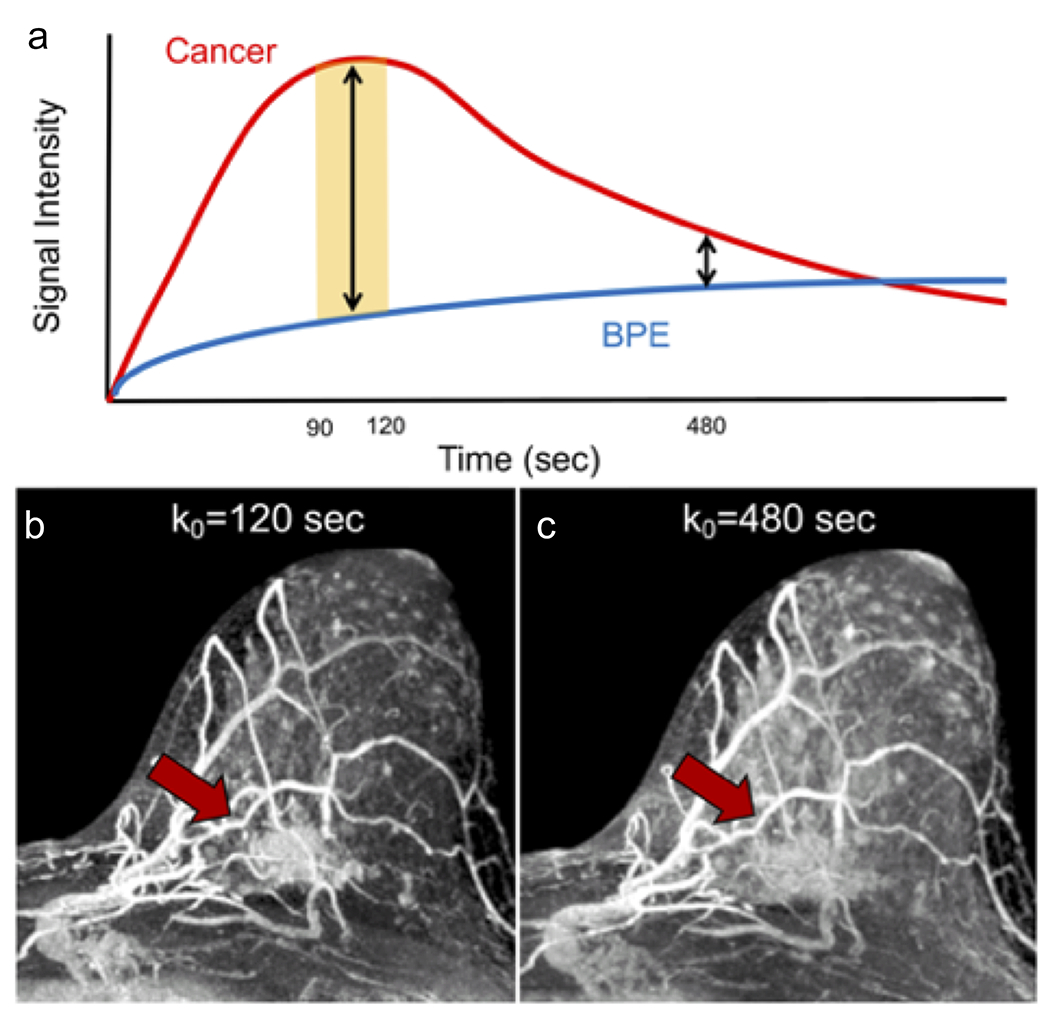
Graphical and selected image timepoints from a DCE series representing the signal intensity of BPE vs. a cancer (invasive lobular carcinoma, red arrow) over time after injection of gadolinium contrast. The signal intensity time curve (A) of the cancer and BPE illustrate that the malignancy demonstrates rapid initial phase increase in signal peaking in the range of k0 = 90 to 120s (yellow shaded zone) and then decreases in signal over time (washes out) while normal tissue enhancement demonstrates slow initial phase increase in signal with persistent increase in signal. On subtracted maximum intensity projections (MIPs) of the left breast, the cancer (red arrow) is readily distinguished from the moderate BPE at time point k0 = 120s (B) but becomes less discrete at time point k0 = 480s (C), at which time the BPE has reached close to maximal signal intensity.
Hormonal and metabolic influences on BPE
BPE levels are known to be affected by sex hormones. In premenopausal women, several studies have demonstrated that BPE levels vary throughout the menstrual cycle (Figure 3), with the lowest amount of BPE typically during the 2nd week of the menstrual cycle (15,23–25). Additional indirect evidence of influences on BPE have been observed in the context of BPE changes after risk-reducing salpingo-oophorectomies (RRSO) and menopause. It has been observed that BRCA 1 or 2 mutation carriers who underwent RRSO exhibited a greater decrease in BPE than fibroglandular tissue volume on subsequent MRIs than women who did not undergo RRSO, suggesting the amount of endogenous estrogens can affect BPE (26). Furthermore, in a study of 28 women who underwent breast MRIs before and after menopause, a significant number of women exhibited relatively lower BPE levels on postmenopausal MRIs compared to premenopausal MRIs (27). More recently, direct evidence of endogenous hormone levels and BPE has been uncovered, with a study demonstrating higher serum concentrations of estrone and estradiol present in postmenopausal women with elevated BPE levels (28).
Figure 3:
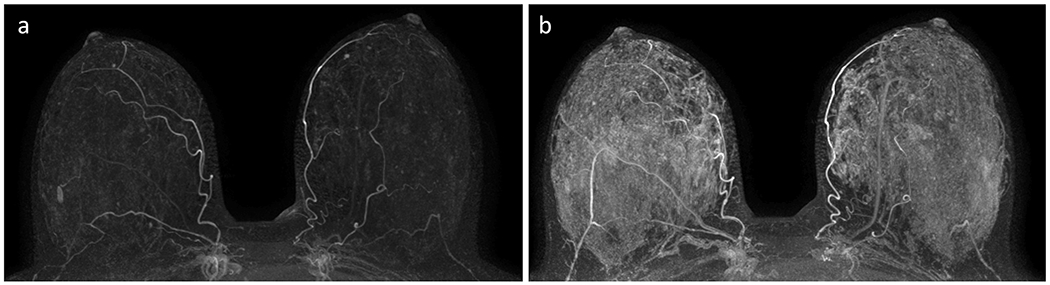
Example of varying background parenchymal enhancement (BPE) due to menstrual cycle variations in a premenopausal woman. This patient presented for a baseline screening breast MRI at age 31 (A), which demonstrated “minimal” BPE on the subtracted maximal intensity projection (MIP) and no suspicious enhancement. Two years later, subtracted MIP from a screening MRI demonstrated “marked” BPE (B) and no suspicious enhancement. This variation in BPE levels from examination to examination in the same premenopausal woman is presumed to be related to menstrual cycle variation.
From a standpoint of exogenous hormone effects, BPE has been shown to increase in the setting of hormone replacement therapy, typically exhibiting bilateral symmetric enhancement without suspicious kinetic features (29). In patients undergoing endocrine therapy for breast cancer, the anti-estrogenic effects of both aromatase inhibitors and selective estrogen receptor modulators (SERM) such as tamoxifen have been associated with decreased BPE (30–35) though to a lesser degree with aromatase inhibitors (35). Finally, cessation of tamoxifen therapy may result in a rebound increase in BPE (Figure 4) as endogenous hormones’ effects on the fibroglandular tissue resume (14). As a result, careful review of clinical and medication history is important when evaluating breast MRIs in women undergoing anti-estrogen therapies.
Figure 4:
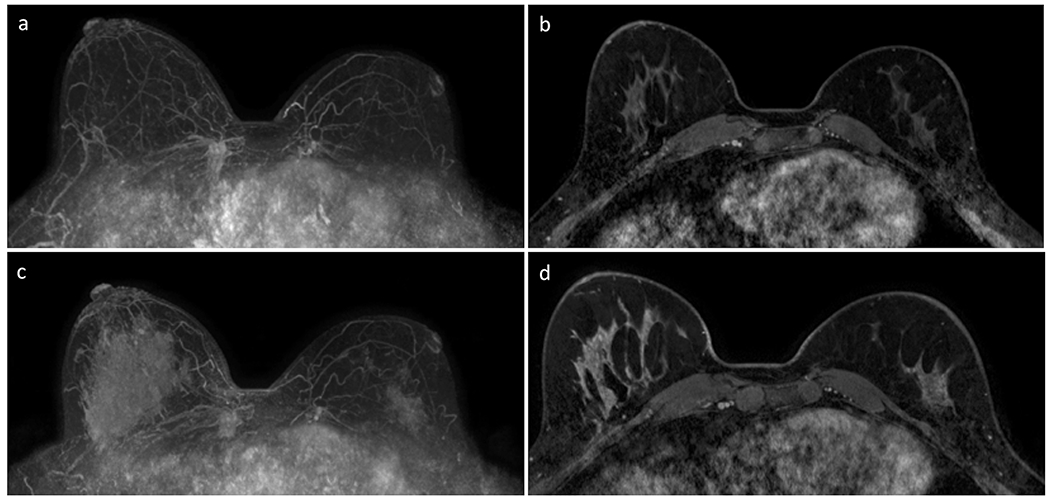
Example of asymmetric BPE and rebound phenomenon after cessation of anti-estrogen therapy (tamoxifen) in a 41-year-old woman with history of treated left breast cancer (invasive ductal carcinoma and DCIS spanning 20 mm total) undergoing surveillance with breast MRI. Subtracted post-contrast maximum intensity projection (MIP, A) and initial phase post-contrast T1-weighted series with fat suppression (B) obtained three years after left lumpectomy and radiation therapy demonstrates asymmetric breast sizes and symmetrical minimal BPE. MIP (C) and initial phase post-contrast T1-weighted series with fat suppression (D) obtained two years later after cessation of anti-estrogen therapy demonstrates asymmetric BPE, with right breast exhibiting marked BPE and left breast showing mild BPE. The increase in BPE in both breasts is due to cessation of the anti-estrogen therapy, while the asymmetry in BPE is due to both a smaller volume of normal tissue in the left breast from prior lumpectomy as well as effects from prior radiation therapy.
Finally, a few studies have also suggested that BPE may reflect increased metabolic activity within the normal tissue, as represented by increased uptake of 18F-fluorodeoxyglucose (FDG) on positron emission tomography (PET) examinations (13,36,37). For example, in a study of 298 consecutive premenopausal women with breast cancer who underwent both breast MRI and FDG-PET, the level of BPE in the contralateral breast without cancer correlated with both SUVmax and SUVmean values of the whole volume of fibroglandular tissue (36). Another retrospective analysis of 327 women with breast cancer demonstrated associations between SUVmax and both quantitative and qualitative measurements of BPE in the contralateral breast (13).
While the data correlating BPE with hormonal influences and metabolic activity are compelling, there are sparse data assessing the pathophysiology of BPE directly. This is due to the challenge of obtaining normal tissue to evaluate pathologically during a phase of peak enhancement. A recent study of 80 pre-menopausal women newly diagnosed with breast cancer who underwent contralateral prophylactic mastectomy found that higher levels of microvessel density, CD34 (measure of endothelial density), glandular components, and vascular endothelial growth factor (VEGF, a factor that promotes blood vessel growth) were present in the fibroglandular tissue within contralateral breasts assessed as having higher qualitative BPE levels (38). Thus, although the exact biological mechanisms responsible for elevated BPE still require further research, there are preliminary data suggesting that BPE is higher in women in whom there is a greater concentration of glandular tissue that has greater vascularity, higher metabolic activity, and is sensitive to hormone level variations.
Variability of Qualitative BPE Assessments and Effect on Diagnostic Performance
Because BPE is qualitatively and subjectively assessed clinically, it has been often assumed to suffer from wide inter-reader variability, similar to mammographic density assessments. The few studies to date that have evaluated inter-reader agreement of BPE assessments have found that agreement ranges from “fair” to “substantial,” though the populations of these studies also have varied considerably. Scaranelo and colleagues were one of the first to report inter-reader agreement in a pilot study of 147 women who underwent preoperative breast MRI, where two readers independently assessed BPE for all examinations, finding fair agreement between the two readers (weighted κ = 0.37) (39). Melsaether and colleagues subsequently assessed variability in qualitative BPE assessments but excluded women newly diagnosed with breast cancer or with a history of breast cancer so as to not lead to confounding effects on BPE assessments. They also found that inter-reader agreement was “fair” (κ = 0.36), but with training, agreement improved to “moderate” levels (κ = 0.45 to 0.48) (40). Finally, in a study evaluating BPE in the setting of neoadjuvant chemotherapy, Preibsch et al found that inter-reader agreement was “substantial” prior to initiation of therapy (κ = 0.73 to 0.77) and “moderate” after therapy (κ = 0.43 to 0.60) (41).
It also has been hypothesized that increased BPE could “mask” malignant lesions on breast MRI in a manner analogous to breast density on mammography. However, in practice, several studies examining the effect of BPE on diagnostic performance have not identified a significant decrease in sensitivity and cancer detection rate in women with higher BPE (19,20,42,43). These studies did suggest that increasing BPE may cause higher abnormal interpretation rates, with one study also demonstrating a higher biopsy rate in women with higher BPE (43). Furthermore, moderate and marked BPE have also been shown to be associated with false-positive MRIs leading to canceled MR-guided biopsies due to lesion non-visualization at time of biopsy (44). Due to the uncertain performance benefit of attempting to “time” breast MRIs in pre-menopausal women to minimize BPE and the challenges such a small window imposes on patient access, the authors generally advise against restricting MRI scheduling based on menstrual cycle phase, particularly for women who have a newly diagnosed breast cancer. This is further supported by a study by Dontchos and colleagues that found that “timing” of MRIs neither led to improved performance nor a significantly lower BPE level (45). Regardless of whether menstrual cycle timing is employed, it is essential that DCE sequences include an early “peak” post-contrast T1 weighted image where malignancies typically exhibit maximal enhancement against a relatively lower BPE to maximize conspicuity (Figure 2). In fact, emerging data suggest that “ultrafast” approaches that include many high temporal resolution (~4–5 seconds) images within the first one to two minutes after injecting contrast can further improve MRI performance by allowing rapid identification of cancers that enhance much earlier than BPE and decrease MRI false-positives by excluding areas of BPE that may mimic non-mass enhancement (NME) (Figure 5) (46).
Figure 5:
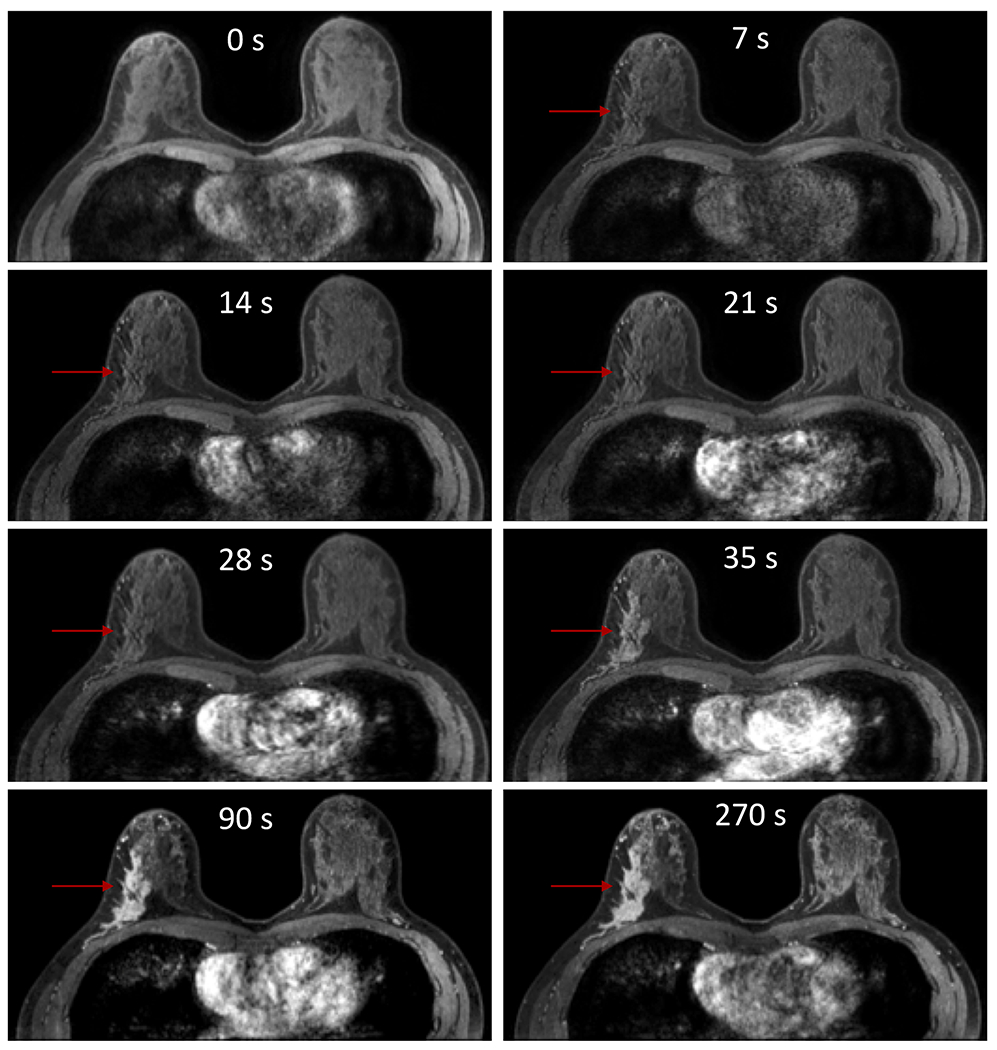
Example of an ultrafast dynamic contrast enhanced (DCE) series of images that depicts a malignancy demonstrating much earlier enhancement than BPE, which could be useful in both discriminating a cancer from BPE and reducing false-positives. In this example using a temporal resolution of 7s for the initial early phase of MRI acquisition, the invasive lobular carcinoma in the right breast (arrow) demonstrates subtle enhancement (segmental non-mass enhancement) at 7s, 14s, 21s, and 28s while the surrounding normal breast tissue and the contralateral left breast exhibits no discernible BPE. At 35s, the malignancy is clearly enhancing and detectable, while there still remains no visually detectable BPE. At 90s and 270s, the malignancy has peaked in enhancement, but the BPE also has progressively increased. Although at these time points the malignancy is still distinct from the BPE, portions of the BPE could be mistaken for unique lesions and lead to other false-positive lesions.
Finally, BPE may affect accuracy in determining extent of disease of some newly diagnosed breast cancers, particularly those that present as NME. One study found that moderate or marked BPE adversely affected the accuracy of total span of newly diagnosed cancers, and that this was particularly problematic for NMEs when compared to masses (47). A similar effect was also seen in a study examining ductal carcinoma in situ (DCIS) lesions only – Baek and colleagues found that the span of DCIS lesions presenting as masses on MRI with minimal and mild BPE were more accurately assessed on preoperative MRI than NMEs with moderate or marked BPE (48). It is interesting to note that these findings were not corroborated by Preibsch et al, who found no association with BPE and DCIS measurement accuracy (49). Finally, there are also mixed data on BPE’s effect on margin status at breast conservation therapy: one study demonstrated elevated BPE was an independent risk factor for positive margins (50) while another demonstrated no effect on reoperation rates for invasive lobular carcinomas (51). Given the limited number of studies to date and mixed results, further research on the effect of BPE on preoperative breast MRI extent of disease accuracy is warranted.
Approaches for BPE quantitation
As described above, the most common way to categorize BPE in clinical practice is to use the four qualitative categories (minimal, mild, moderate, marked) defined by the BI-RADS Atlas (9). Quantitative measurement of BPE may provide a less subjective and more reproducible method of BPE assessment (10–13); however, imaging protocols vary widely and algorithms to calculate BPE vary from institution to institution. Although there is currently no standard method of quantifying BPE in clinical practice, a brief description of the most prevalent approaches described in the literature follow below. In this section, we will review ROI based measures first, followed by segmentation techniques.
Two-dimensional (2D) region of interest (ROI) based measures are the most common form of quantitative BPE measures described in the literature. In this approach, an ROI or series of ROIs is manually placed in an area of enhancement. The ROIs are then propagated to the pre-contrast and all the post contrast images in the study. The ROI is typically small, consisting of a set number of pixels (16,52,53) or a target diameter ranging from a few millimeters to a few centimeters (35,54,55). A few studies have utilized larger ROIs, encompassing as much as the normal fibroglandular tissue as possible (56–58). Following ROI placement, the percent enhancement (PE) is calculated as:
Where Spre is the mean signal intensity of the ROI in the precontrast image and Spost is the mean signal intensity in the post contrast image. Spost is typically measured on the first post contrast image. PE may also be measured on later phase post contrast images. The signal enhancement ratio (SER) has also been used in addition to or in place of PE (55,59). SER is defined as:
Where Searly is the mean signal intensity of the first post contrast image and Sdelayed is the mean signal intensity from a delayed phase image. In most cases, the ROI is intentionally placed in an area of normal appearing breast parenchymal tissue free of any obvious fat or large vessels and far from any enhancing lesions. However, in some studies the ROIs have intentionally been placed near the tumor boundary. For example, Kim MY et al. (60), Kim SA et al. (59), and Park et al. (61) performed a series of studies looking at BPE in the context of recurrence rates where a series of ROIs were placed at varying distances from the tumor boundary.
One drawback of such 2D ROI-based measures is the inherent variability among readers in determining where an ROI is placed to represent normal tissue and the size of the ROI drawn, which likely contributes to the variability in the results in studies using quantitative BPE measurements. A number of studies have sought to reduce this reliance on manual ROI placement by using automated or semi-automated methods to quantify parenchymal tissue as a whole with the potential benefit of more accurately replicating BI-RADS categorization without the inter-reader variability. This workflow typically involves a number of post processing segmentation steps. Typically, the operator segments the breast tissue to remove the skin, chest wall, air, and any other non-mammary structure included in the field of view. Next, segmentation of the fibroglandular tissue from the breast adipose tissue and vessels is performed, followed by selection of the voxels displaying enhancement and calculation of the final quantitative parameter(s).
Segmentation of the breast from the chest wall and other non-mammary structures and segmentation of fibroglandular tissue from adipose tissue and vessels is challenging, and there is no universally accepted method to complete these tasks (Figure 7). Groups have addressed this challenge in multiple ways, including manual segmentation of the tissue of interest (24,58,62,63), which can be prohibitively time consuming (64). Efficiency may improve through the use of interpolation between manually segmented slices(63) or use of an ROI encircling the fibroglandular tissue and applying a thresholding operation to define the fibroglandular tissue (24,58). Automated or semiautomated segmentation methods have also been employed to remove the skin (55,63,65–68) and to define and remove the chest wall (55,66–72), typically using a form of edge detection or gradient tracing with appropriate constraints. Template or atlas based methods have also been applied (55). Several approaches for separating the fibroglandular tissue of interest from the surrounding fat tissue or non-enhancing fibroglandular tissue have also been described. For example, simple thresholding operations have been described in applications where the T1 images are fat suppressed or subtracted (24,58,73–75). The most common automated or semi-automated method for identifying the fibroglandular tissue uses K-means or fuzzy-C means clustering (55,65–67,70,76–79). In these types of approaches, an algorithm attempts to identify different classes of tissue based on their intensity differences (69), and can be applied to protocols with and without fat saturation. Due to the signal variation present in many MR breast images from the coil sensitivity profile, intensity correction typically must be applied to avoid misclassification errors in the clustering algorithm (55,63,65,66,70,77,79). Other novel approaches to define the fibroglandular tissue have also been proposed and are being actively investigated (64,80), including chemical shift encoded MRI techniques (81–83) and the application of artificial intelligence (11,84).
Figure 7:
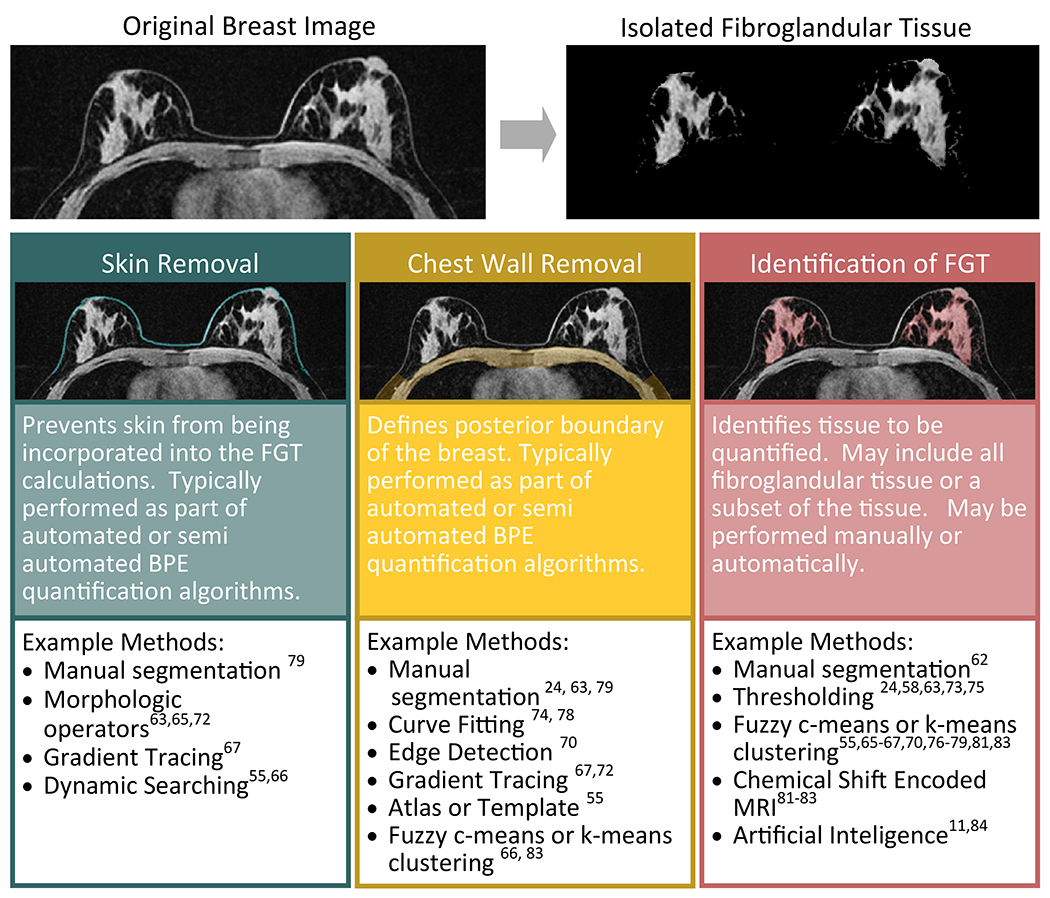
Diagram of the potential steps involved in isolation of the fibroglandular tissue (FGT) for quantification of BPE. Many different approaches have been taken to isolate the FGT ranging from pure manual segmentation to fully automatic algorithms. Typical steps, illustrated here, include skin removal, chest wall removal, and final isolation of the FGT itself. These steps may be performed on a single representative slice of the breast or on the entire breast volume.
Once the fibroglandular tissue of interest has been identified, similar quantitative measures to those used in the quantitative ROI analysis scan be employed. In addition to the mean PE calculated in most of the ROI based methods (16,32,35,52–57,59), additional measures can be calculated such as volume and percentage of the enhancing fibroglandular tissue and statistics on the characteristics of all the voxels or subsets such as the most and least enhanced voxels (12,58,67,70,73,78). When BPE is reported as the volume or percentage of overall fibroglandular tissue, a threshold is typically applied to the voxel-wise calculation of PE to define the voxels considered “enhanced”. The definition of this threshold varies from study to study but choices have included thresholds selected based on the noise characteristics of the images (62,67), a fraction of the maximum enhancement seen in an enhancing lesion (78), or thresholds based on analysis of the enhancement patterns seen in the images assigned to different qualitative BPE categories (73). While a quantitative value derived in some manner from the calculation of PE is most commonly described, other values have also been calculated, including SER (55,77), model based fitting parameters (62), and features related to texture analysis (85).
BPE and Breast Cancer Risk
Multiple retrospective case-control and cohort studies have evaluated the association of increased qualitative BPE levels and the risk of breast cancer diagnosis (Table 1) (8,58,67,70,86–89). The potential association of BPE with breast cancer risk was first reported by King et al. in a 2011 study of 1275 women undergoing breast MRI for high-risk screening (8). Breast MRIs from 39 women diagnosed with breast cancer matched to both negative and false positive controls demonstrated that moderate or marked BPE was associated with increased odds of breast cancer diagnosis. One limitation of this study was that all BPE assessments were made retrospectively, and BPE was only correlated with a contemporaneous diagnosis of breast cancer and not future breast cancer diagnoses.
Table 1.
Studies evaluating association of qualitative background parenchymal enhancement (BPE) levels with breast cancer risk.
| Study Design | Patient Population | Endpoint | BPE threshold | Findings | |
|---|---|---|---|---|---|
| King, et al., 2011 (8) | Retrospective case control, 38 cancers matched 1:2 to negative control and 1:1 to false positive control | High risk screening | Risk of breast cancer diagnosis at time of MRI | ≥ moderate threshold at first time point (k0 not reported) | Increased BPE associated with cancer |
| Dontchos, et al., 2015 (87) | Retrospective case control, 23 cancers matched 1:1 to negative control | High risk screening | Risk of either cancer diagnosis at time of MRI or in future | ≥ mild threshold at first time point (k0 = 90–110s) | Increased BPE associated with cancer |
| Telegrafo, et al., 2015 (89) | Retrospective cohort | High risk screening and diagnostic (problem solving and extent of disease) | Risk of breast cancer diagnosis at time of MRI | ≥ moderate threshold at third time point (k0 = 180s) | Increased BPE associated with cancer |
| Bennani-Baiti, et al., 2016 (86) | Retrospective cohort | Diagnostic (problem solving and extent of disease) | Risk of breast cancer diagnosis at time of MRI | ≥ moderate threshold (k0 = 120s) | No association of BPE with cancer |
| Grimm, et al., 2018 (88) | Retrospective case control, 61 cancers matched 1:2 to negative control | High risk screening | Risk of future breast cancer diagnosis | ≥ mild threshold (k0 not reported) | Increased BPE associated with breast cancer diagnosis |
| Arasu, et al., 2019 (90) | Retrospective cohort | Screening and Diagnostic | Risk of future breast cancer diagnosis | ≥ mild threshold (k0 not reported due to numerous institutions included) | Increased BPE associated with breast cancer diagnosis; effect greater for invasive breast cancers than DCIS |
BPE = background parenchymal enhancement; DCIS = ductal carcinoma in situ
However, a number of subsequent studies have since corroborated the association of elevated BPE levels with the likelihood of a future cancer diagnosis in high risk women (58,87,88). Dontchos and colleagues found in a 1:1 matched case-control of 46 women with elevated breast cancer risk, those qualitatively assessed to have at least mild BPE were nine times as likely as those with minimal BPE to be diagnosed with breast cancer (87). Grimm et al corroborated these findings in a larger subsequent study of 61 patients, demonstrating a two to three times greater likelihood of future diagnosis of breast cancer in high risk women with mild or greater BPE (88). Finally, a large retrospective study using data from the numerous community and academic practices in the Breast Cancer Surveillance Consortium (4,247 women, 176 of whom developed breast cancer at least 3 months after index MRI) demonstrated that women with mild or greater BPE levels had an increased risk of future breast cancer diagnosis, and that BPE predicted breast cancer risk independent of breast density and more strongly predicted future diagnoses of invasive breast cancer than of DCIS (90).
Because BPE is subjective with fair to moderate levels of agreement, there is strong interest in identifying optimal quantitative BPE metrics that may be less prone to inter-operator variability and could serve as a more robust marker of risk. Indeed, three pilot studies examining quantitative BPE assessments’ associations with breast cancer risk have shown potential in developing a quantitative BPE biomarker; however, these studies have used different patient populations and have varied in quantitation approaches (Table 2). A follow-up analysis of the Dontchos et al study cohort using quantitative assessments of BPE demonstrated that women who ultimately developed breast cancer were more likely to have larger two-dimensional areas of BPE and higher BPE signal intensity (58). Hu and colleagues (67) also utilized a case-control design (101 normal MRIs, 101 benign lesions, and 101 malignancies), but instead performed an automated segmentation of the fibroglandular tissue of the bilateral whole breasts to determine a “background parenchymal enhancement rate” or “BPER,” which was defined as the volume ratio of enhancing fibroglandular tissue to the entire volume of fibroglandular tissue. They found that higher levels of BPER were associated with increased odds of being in the cancer cohort for both pre-menopausal and post-menopausal women. Interestingly, they also found that BPER was associated with breast cancer morbidity, though the effect was less pronounced. Finally, Wu et al also utilized an automated fibroglandular tissue segmentation tool to determine quantitative whole breast BPE measurements at the first and third post-contrast time points in women with BRCA 1 or 2 mutations who also underwent RRSO. Using their software, the authors calculated an absolute total volume of BPE by summing the number of voxels demonstrating an increase in signal from pre-contrast to post contrast sequences as well as a relative ratio of BPE vs. total volume of fibroglandular tissue (BPE%) for each patient. Their study found that those who did not subsequently develop cancer after RRSO had both significantly decreased absolute total volume of BPE and BPE% on post-RRSO breast MRIs compared to pre-RRSO examinations (70).
Table 2.
Studies evaluating association of quantitative BPE features with breast cancer risk
| Study Design | Patient Population | Endpoint | BPE parameters | Findings | |
|---|---|---|---|---|---|
| Wu, et al., 2015 (70) | Retrospective cohort | BRCA1/2 mutation carriers who underwent RRSO | Risk of future breast cancer diagnosis | Absolute and relative whole breast volumes of BPE above a predefined enhancement threshold | Decreased BPE measurements after RRSO associated with no cancer development. Increased BPE post-RRSO associated with cancer development. |
| Hu, et al, 2017 (67) | Retrospective cohort (malignant, benign, negative) | Not disclosed | Risk of breast cancer diagnosis at time of MRI | Ratio of whole breast volume of BPE to total FGT at varying thresholds | Increased BPE associated with cancer development. |
| Lam, et al., 2018 (58) | Retrospective case control, 23 cancers matched 1:1 to no cancer control | High risk screening | Risk of either cancer diagnosis at time of MRI or in future | Variable thresholds of percent enhancement for each voxel within 2D area of FGT | Greater BPE area and increased intensity parameters associated with cancer development. |
BPE = background parenchymal enhancement, RRSO = risk-reducing salpingo-oopherectomy, FGT = fibroglandular tissue
Since MRI is primarily indicated in women already at high risk for breast cancer, typically defined as greater than 20–25% lifetime risk (as determined by genetic factors or a risk-model that incorporates family history), most studies linking BPE with breast cancer risk have been performed only in high risk women. However, two studies explored the use of BPE to predict breast cancer development in women at average risk of breast cancer - one study demonstrated a positive correlation between BPE and breast cancer (89) while the other study found no correlation (86). A significant limitation of these studies was that breast MRI was performed in symptomatic women (e.g. bloody nipple discharge) or to evaluate the extent of disease for current diagnosis of cancer; therefore, it is uncertain whether the findings in these studies can extend to the general population of asymptomatic women with an average lifetime risk of breast cancer. However, given the interest in using abbreviated MR techniques to expand breast MRI screening to a wider patient population (91,92), there likely will be ample opportunity to further explore the value of BPE in average to intermediate risk women to further refine breast cancer risk assessments.
BPE as a predictor of treatment outcomes
Existing evidence suggests that BPE could be a predictor of neoadjuvant treatment response and varying BPE metrics may be associated with treatment outcomes (Table 3). There are several retrospective cohort studies examining the association of BPE and changes in BPE with response to neoadjuvant chemotherapy (NAC) (41,66,93–96) (Figure 6). To date, all published studies evaluating changes in BPE from pre-therapy to post-therapy breast MRI found an association between decreased BPE and pathologic complete response (pCR) (41,66,94–96). This effect can be seen as early as after the 2nd cycle of NAC (95,96) and includes tumors undergoing neoadjuvant human epidermal growth factor receptor-2 (HER-2)-targeted therapy (95). In one study, this association was only demonstrated in women less than 55 years of age, which could be due to typically lower levels of baseline BPE in postmenopausal women (66). A few studies examined the relationship between baseline (pre-NAC) BPE and varying metrics of tumor response and found no significant association (66,94,96). One study examined the association between baseline BPE and response to neoadjuvant endocrine therapy for estrogen receptor (ER) positive and HER-2 negative tumors and found an inverse association – lower BPE (minimal or mild) was associated with better response, defined as stable or reduced tumor size on clinical or ultrasound examination (93).
Table 3.
Studies evaluating association of qualitative BPE parameters with breast cancer treatment outcomes
| Study Design | Patient Population | Endpoint | BPE parameters | Findings | |
|---|---|---|---|---|---|
| Choi, et al., 2016 (97) | Retrospective cohort, 93 patients underwent pre- and post-NAC MRI | Unilateral IBC undergoing NAC | RFS | Contralateral BPE categorized as high (moderate/marked) or low (minimal/mild) | High BPE on pre-NAC MRI associated with worse RFS |
| Preibsch, et al., 2016 (41) | Retrospective cohort, 73 patients underwent pre- and post-NAC MRI | IBC undergoing NAC | Morphologic and histopathological tumor response ranging from pCR to PD | Bilateral BPE category change post-NAC (specific series used or k0 not specified) | Degree of BPE reduction correlated with tumor response |
| Lim, et al., 2017 (98) | Retrospective cohort, 804 patients | IBC | RFS and various other clinicopathological variables | Qualitative BPE (bilateral vs. unilateral assessment not specified) on first post-contrast series (k0 = 90s) | Increased BPE associated with PR+, LVI, close surgical margin Increased BPE associated with worse RFS in post-menopausal women by one reader |
| Vreeman, et al, 2018 (100) | Retrospective cohort, 76 patients | IBC, DCIS | Tumor subtypes | Contralateral qualitative BPE on first post-contrast subtraction series (k0 = 90s) | Increased BPE associated with lower tumor grade and PR+ status |
| Shin, et al, 2018 (55) | Retrospective cohort, 289 patients | Unilateral IBC, ER+, HER2− > 5 mm in size | RFS and DFS | Contralateral qualitative BPE and quantitative BPE | BPE not associated with outcome |
| You, et al., 2018 (95) | Retrospective cohort, 71 patients underwent baseline MRI and MRI after 2nd cycle of NAC | Invasive HER2 positive breast cancer undergoing NAC with trastuzumab | pCR | Contralateral qualitative BPE on “early phase” (k0 = 90s) | No association between baseline BPE and pCR Decreased BPE after 2nd cycle of NAC associated with pCR |
| Oh, et al, 2018 (94) | Retrospective cohort of 186 patients who under breast MRI before and after NAC | IBC undergoing NAC | pCR, RFS, and Tumor subtypes | Contralateral qualitative BPE on first post-contrast phase (k0 =61 s) | No association between baseline BPE and pCR Decreased BPE after NAC greater in pCR group than non-pCR group No association between BPE and RFS or tumor molecular subtypes |
| Hilal, et al., 2018 (93) | Retrospective cohort, 21 patients underwent baseline MRI | IBC, ER+, HER2− undergoing NAC with endocrine therapy | Stable disease or reduced tumor size on clinical or ultrasound examination | Qualitative BPE (specific phase not specified) | Minimal/mild BPE associated with response (stable to reduced tumor size) |
BPE = background parenchymal enhancement, IBC = invasive breast cancer, NAC = neoadjuvant chemotherapy, pCR = pathologic complete response, ER = estrogen receptor, DFS = disease-free survival, HER2 = human epidermal growth factor receptor 2, RFS=recurrence free survival, PD = progressive disease, PR = progesterone receptor, LVI = lymphovascular invasion, DCIS = ductal carcinoma in situ, FGT=fibroglandular tissue, MIP = maximum intensity projection
Figure 6:
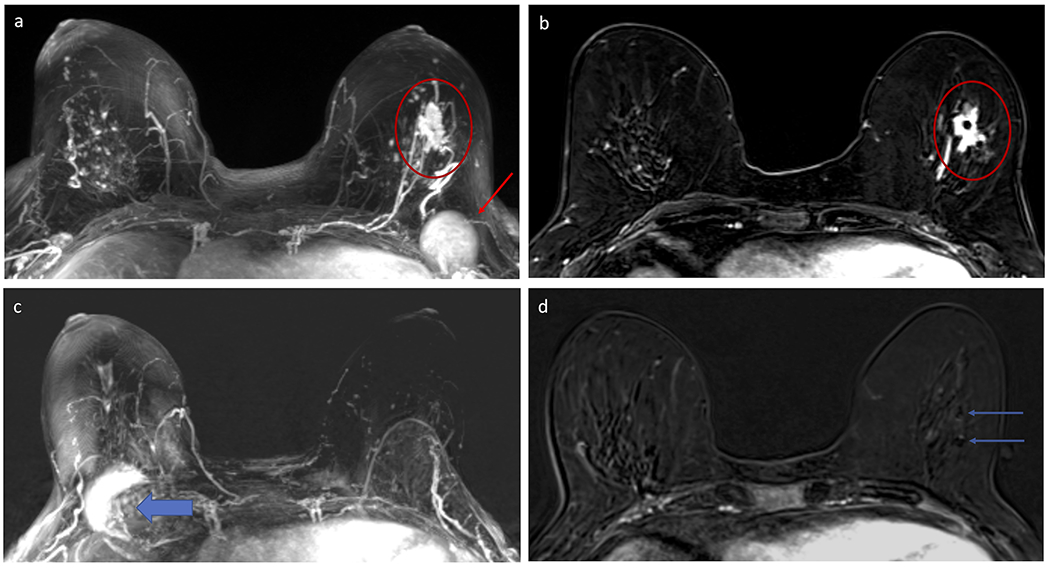
Example of decrease in background parenchymal enhancement (BPE) in a 59 year-old woman who underwent neoadjuvant chemotherapy (NAC) for a poorly differentiated invasive ductal carcinoma (IDC) in the left breast. Subtracted maximum intensity projection (MIP) from the pre-NAC breast MRI (A) demonstrates moderate BPE in both breasts, with the left breast IDC (red circle) distinct from the BPE and an enlarged left level I axillary lymph node (red arrow) also evident. Selected post-contrast T1-weighted subtracted axial slice centered at the level of the left breast IDC demonstrates the mass with two clips within (red circle). Subtracted MIP from the post-NAC MRI performed five months later (B) demonstrates decrease in BPE, now minimal, with resolution of both the mass and axillary lymphadenopathy (note artifact on the MIP from the port-a-catheter in the right chest for chemotherapy, wide blue arrow). Patient was confirmed to have pathologic complete response (pCR) on surgery. Several papers have suggested that a reduction in BPE after NAC could help identify which patients have better clinical outcomes, including pCR.
The data supporting a consistent association between BPE and prognosis and survival are less clear. In a retrospective cohort study of extent-of-disease MRIs conducted among 531 women with invasive unilateral breast cancer, increased BPE was independently associated with improved overall survival among women with ER positive, HER-2 negative breast cancer (65). Alternatively, in a study evaluating both premenopasual and postmenopausal women undergoing NAC, increased baseline qualitative BPE greater than “mild” was associated with worse RFS (97). In a different study of women not undergoing NAC found that increased BPE was associated with worse RFS only in postmenopausal women (98); however, this finding was only significant for one of the two independent readers, which highlights the subjectivity and variability inherent in qualitative assessments of BPE. In addition, two other retrospective cohort studies of 186 and 289 patients each demonstrated no association between pre-treatment BPE and recurrence free survival (RFS) for invasive breast cancer of various molecular subtypes (55,94).
In support of precision therapy, BPE could be associated with other clinicopathologic factors that may help determine optimal treatment. In a retrospective case-control study of patients with unilateral ductal carcinoma in situ, higher quantitative mean BPE on pre-treatment breast MR imaging was associated with disease recurrence following treatment (99). These findings indicate that BPE could be helpful in determining which patients require adjuvant radiation or other therapies. In patients with invasive breast cancer, one study demonstrated an association between increased BPE and hormone (estrogen and progesterone) receptor positivity, presence of lymphovascular invasion, and close surgical margins (98). Another study demonstrated that increased BPE was associated with lower tumor grade, as well progesterone receptor (PR) positivity (100). While these studies need to be further verified in larger, prospective studies, their findings point to the potential that BPE on pre-operative breast MRI could provide prognostic information to help determine the need for neoadjuvant and/or adjuvant therapy and tailor optimal surgical approaches.
Radiomic analysis of BPE
Recent advances in the fields of artificial intelligence and medical image analysis have led to the development of the new field of “radiomics,” which aims to extract high throughput, quantitative imaging features from medical images to complement conventional biomarkers and thus aid in clinical decision making (101,102). These features allow for the objective and quantitative characterization of the morphology, texture, and pharmacokinetic behavior of breast tumors and the surrounding parenchyma as depicted by DCE. In applications for breast cancer risk assessment, radiomic features have emerged as surrogate measures for breast parenchymal patterns in mammographic and tomographic images (103). With the expanding use of DCE-MRI for breast cancer screening, recent studies have suggested associations between radiomic characterizations of BPE and breast cancer risk (73,104,105). Specifically, in each study, radiomic features characterizing the morphology, texture, and kinetics of BPE as well as features quantifying breast density, demonstrated a high correlation with qualitative phenotypic characteristics determined by a radiologist, emphasizing the complementary value of radiomics for breast cancer risk assessment.
With respect to personalized breast cancer diagnosis and treatment response monitoring, studies have demonstrated that increasing the scope of radiomic analysis to include surrounding BPE within the peritumoral region can result in improved diagnostic and predictive performance (106–109). Mazurowski et al. (106) demonstrated that radiomic features characterizing the texture and pharmacokinetic behavior of breast lesions and the surrounding parenchyma were highly associated with the luminal B subtype, suggesting that quantitative characterization of BPE could provide personalized diagnoses. Similar conclusions were reported by Wang et. al (107), where features characterizing texture were extracted from pharmacokinetic maps generated from DCE images of the tumor and surrounding parenchyma. To identify the molecular basis and prognostic value of imaging characteristics of BPE, Wu et al. extracted 10 radiomic features describing kinetic behavior and texture from the background parenchyma of 60 women diagnosed with invasive breast cancer (110). The authors found that one feature characterizing BPE heterogeneity was associated with RFS. Additionally, the tumor necrosis signaling factor was found to be the most enriched molecular pathway associated with the radiomic features, suggesting that heterogeneous BPE was associated with tumor necrosis and poor survival.
Augmenting a classification model of lesion texture features with BPE texture features also further improved performance for the identification of triple negative breast cancers (TNBC). The added predictive value of BPE radiomic characterization was recently suggested by Braman et. al (108), who showed that radiomic features extracted from intratumoral and peritumoral regions can predict pathologic complete response (pCR) to neoadjuvant chemotherapy with areas under the ROC curve ranging from 0.83 to 0.93. The predictive value of radiomic features extracted from background parenchyma were also assessed by Fan et. al. (111) Here, authors extracted 158 radiomic features characterizing the morphology, dynamics, and texture of breast lesions and background parenchyma from the abnormal and contralateral breasts to evaluate their ability in predicting response to neoadjuvant chemotherapy. Of the final 12 features selected after extensive feature selection, six features were associated with the background parenchyma of the abnormal breast, and five were associated with the contralateral breast. The authors concluded that features characterizing BPE were the most representative for prediction, and that this behavior extended to the contralateral, unaffected breast as well. Taken together, this emerging evidence suggests that radiomic analysis has the potential to provide novel prognostic and predictive biomarkers, as well as quantitative insight into parenchymal enhancement patterns to further facilitate the role of BPE in personalized clinical decision making.
Limitations and Directions for Future Work
Despite existing evidence implicating BPE as a risk factor for developing future breast cancer, a number of limitations and gaps in knowledge remain. First, the applicability of BPE to women of average or lower risk levels remains unclear primarily because study populations in nearly all existing studies have focused on women with high breast cancer risk undergoing high risk screening with breast MRI. Additionally, findings from studies that evaluated non-high risk women were not always consistent and the association between BPE and breast cancer risk may vary based on individual patient risk level. Future work should therefore continue to explore the utility of BPE as a marker for average risk women.
Complementary work to further elucidate the biological underpinnings of BPE – for example, evaluating the relationship between BPE and biomarkers captured in pathological tissue specimens – would provide important additional insight on the manner by which BPE affects tumorigenesis or recurrence rates. This work could lead to novel medical prevention strategies that could be monitored with imaging for efficacy in preventing breast cancer development. Furthermore, it could lead to novel therapeutics for patients with newly diagnosed breast cancers that could help decrease recurrence rates.
Second, DCE protocols used and the patient populations included in existing studies vary considerably (112). Specifically, the timing of the post-contrast sequences and which sequence or how many sequences are used to determine BPE are widely inconsistent across studies. In premenopausal patients, fluctuating BPE during the menstrual cycle may confound some findings. Furthermore, it may be that the prognostic effect and the BPE thresholds of value could differ depending on patient features such as menopause status, hormone replacement therapy, or history of prior radiation or medical therapy. Accordingly, the value of artificial intelligence to further refine the effect of BPE on risk assessment should be explored in larger datasets to help control for such variables.
Third, evidence should be interpreted in view of the variation in BPE calculation and scoring. There is inherent subjectivity in qualitative BPE assessments (minimal to marked), with interpretations likely varying somewhat between radiologists and studies (40,63). While quantitative measures are more reproducible within a particular study, there are wide variations in the algorithms used to calculate BPE among the different studies and the methods and thresholds used (112). More studies are needed to identify reliable and reproducible methods for quantitative BPE assessment. Future studies could support the ability to deliver personalized care by testing optimal methods for both quantitation and qualitative assessment, as well as determining the best thresholds for predicting risk and treatment outcomes.
Conclusion
BPE reflects varying levels of physiologic enhancement of normal fibroglandular tissue that appears to have a mild effect on breast MRI interpretation performance but has recently become a highly studied imaging marker of breast cancer risk and treatment outcomes. Emerging data demonstrate that, in general, higher levels of BPE may reflect breast tissue environment more likely to develop breast cancer, and reductions in BPE in women with breast cancer undergoing neoadjuvant chemotherapy may have better treatment outcomes. While existing evidence for BPE is compelling, its clinical value is limited since data from the many single site studies suffer from small cohort sizes, variations in patient populations included, and disparate methods for qualitative and quantitative BPE assessment. Thus, additional research is needed to clarify how to best capture, quantify, and operationalize BPE to translate this emerging marker of breast cancer risk into clinical practice.
Table 4.
Studies evaluating association of quantitative BPE parameters with breast cancer treatment outcomes
| Study Design | Patient Population | Endpoint | BPE parameters | Findings | |
|---|---|---|---|---|---|
| Chen, et al., 2015 (66) | Retrospective cohort, 46 patients underwent pre-treatment MRI and two follow-up MRI exams | IBC undergoing NAC | pCR | Average % increase in bilateral BPE signal intensity over 12 post-contrast sequences | Decrease in BPE correlated with pCR in patients with ER− tumors |
| van der Velden, et al., 2015 (65) | Retrospective cohort, 531 patients | Unilateral IBC | Overall survival and invasive DFS | Late phase mean top 10% enhancement in contralateral breast | Increased BPE associated with better overall survival for ER+, HER2− tumor subtypes, especially for those who underwent endocrine therapy |
| You, et al, 2017 (96) | Retrospective cohort, 90 patients underwent baseline MRI and MRI after 2nd, 4th, and 6th cycles of NAC | Unilateral IBC and DCIS | Tumor size pCR | Ratio of enhanced FGT volume to total FGT volume and mean BPE of the 4 times points (k0= 90, 180, 270, and 360s) in contralateral breast | BPE and changes of BPE at each monitoring point associated with tumor size |
| Luo, et al., 2017 (99) | Retrospective case control, 11 cases with recurrence matched (1:1) to cases without recurrence | Unilateral DCIS | Ipsilateral breast cancer recurrence > 6 months after definitive surgery | Ipsilateral BPE using a 10% threshold on first post-contrast series (k0= 90–120s) | Patients with DCIS recurrence more likely to have greater mean BPE |
BPE = background parenchymal enhancement, IBC = invasive breast cancer, NAC = neoadjuvant chemotherapy, pCR = pathologic complete response, ER = estrogen receptor, DFS = disease-free survival, HER2 = human epidermal growth factor receptor 2, RFS=recurrence free survival, PD = progressive disease, PR = progesterone receptor, LVI = lymphovascular invasion, DCIS = ductal carcinoma in situ, FGT=fibroglandular tissue, MIP = maximum intensity projection
Acknowledgements:
Grant Support: R01 CA203883 (Rahbar), R01 CA207290 (Partridge), R01 CA160620 (Moy), P41EB017183 (Moy), P30 CA1520 (Strigel)
References
- 1.Hortobagyi GN, Connolly JL, D’Orsi CJ, et al. AJCC cancer staging manual: Breast In: Amin MB, editor. AJCC cancer staging manual. Eight edition / editor-in-chief, Amin Mahul B. ; editors, Edge Stephen B. and 16 others ; Gress Donna M. - Technical editor ; Meyer Laura R. - Managing editor. ed. Chicago IL: American Joint Committee on Cancer, Springer; 2017. p. xvii, 1024 pages. [Google Scholar]
- 2.Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. J Natl Cancer Inst 1995;87(9):670–675. [DOI] [PubMed] [Google Scholar]
- 3.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med 2007;356(3):227–236. [DOI] [PubMed] [Google Scholar]
- 4.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000;92(13):1081–1087. [DOI] [PubMed] [Google Scholar]
- 5.Barlow WE, White E, Ballard-Barbash R, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst 2006;98(17):1204–1214. [DOI] [PubMed] [Google Scholar]
- 6.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med 2008;148(5):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst 2014;106(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011;260(1):50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris EA; Comstock CE; Lee CH; Lehman CD; Ikeda DM ACR BI-RADS® Magnetic Resonance Imaging ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 10.Eyal E, Badikhi D, Furman-Haran E, Kelcz F, Kirshenbaum KJ, Degani H. Principal component analysis of breast DCE-MRI adjusted with a model-based method. J Magn Reson Imaging 2009;30(5):989–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha R, Chang P, Mema E, et al. Fully Automated Convolutional Neural Network Method for Quantification of Breast MRI Fibroglandular Tissue and Background Parenchymal Enhancement. J Digit Imaging 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha R, Mema E, Guo X, et al. Three-Dimensional Quantitative Validation of Breast Magnetic Resonance Imaging Background Parenchymal Enhancement Assessments. Curr Probl Diagn Radiol 2016;45(5):297–303. [DOI] [PubMed] [Google Scholar]
- 13.Mema E, Mango VL, Guo X, et al. Does breast MRI background parenchymal enhancement indicate metabolic activity? Qualitative and 3D quantitative computer imaging analysis. J Magn Reson Imaging 2018;47(3):753–759. [DOI] [PubMed] [Google Scholar]
- 14.Giess CS, Yeh ED, Raza S, Birdwell RL. Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics 2014;34(1):234–247. [DOI] [PubMed] [Google Scholar]
- 15.Kuhl CK, Bieling HB, Gieseke J, et al. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology 1997;203(1):137–144. [DOI] [PubMed] [Google Scholar]
- 16.Cubuk R, Tasali N, Narin B, Keskiner F, Celik L, Guney S. Correlation between breast density in mammography and background enhancement in MR mammography. Radiol Med 2010;115(3):434–441. [DOI] [PubMed] [Google Scholar]
- 17.Hansen NL, Kuhl CK, Barabasch A, Strobel K, Schrading S. Does MRI breast “density” (degree of background enhancement) correlate with mammographic breast density? J Magn Reson Imaging 2014;40(2):483–489. [DOI] [PubMed] [Google Scholar]
- 18.Ko ES, Lee BH, Choi HY, Kim RB, Noh WC. Background enhancement in breast MR: correlation with breast density in mammography and background echotexture in ultrasound. Eur J Radiol 2011;80(3):719–723. [DOI] [PubMed] [Google Scholar]
- 19.Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol 2011;196(1):218–224. [DOI] [PubMed] [Google Scholar]
- 20.Uematsu T, Kasami M, Watanabe J. Should breast MRI be performed with adjustment for the phase in patients’ menstrual cycle? Correlation between mammographic density, age, and background enhancement on breast MRI without adjusting for the phase in patients’ menstrual cycle. Eur J Radiol 2012;81(7):1539–1542. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Dershaw DD, Lee CH, Joo S, Morris EA. Breast MRI after conservation therapy: usual findings in routine follow-up examinations. AJR Am J Roentgenol 2010;195(3):799–807. [DOI] [PubMed] [Google Scholar]
- 22.Melsaether A, Pujara AC, Elias K, et al. Background parenchymal enhancement over exam time in patients with and without breast cancer. J Magn Reson Imaging 2017;45(1):74–83. [DOI] [PubMed] [Google Scholar]
- 23.Delille JP, Slanetz PJ, Yeh ED, Kopans DB, Garrido L. Physiologic changes in breast magnetic resonance imaging during the menstrual cycle: perfusion imaging, signal enhancement, and influence of the T1 relaxation time of breast tissue. Breast J 2005;11(4):236–241. [DOI] [PubMed] [Google Scholar]
- 24.Jung Y, Jeong SK, Kang DK, Moon Y, Kim TH. Quantitative analysis of background parenchymal enhancement in whole breast on MRI: Influence of menstrual cycle and comparison with a qualitative analysis. Eur J Radiol 2018;103:84–89. [DOI] [PubMed] [Google Scholar]
- 25.Muller-Schimpfle M, Ohmenhauser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology 1997;203(1):145–149. [DOI] [PubMed] [Google Scholar]
- 26.DeLeo MJ 3rd, Domchek SM, Kontos D, Conant E, Chen J, Weinstein S Breast MRI fibroglandular volume and parenchymal enhancement in BRCA1 and BRCA2 mutation carriers before and immediately after risk-reducing salpingo-oophorectomy. AJR Am J Roentgenol 2015;204(3):669–673. [DOI] [PubMed] [Google Scholar]
- 27.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 2012;22(12):2641–2647. [DOI] [PubMed] [Google Scholar]
- 28.Brooks JD, Sung JS, Pike MC, et al. MRI background parenchymal enhancement, breast density and serum hormones in postmenopausal women. Int J Cancer 2018;143(4):823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pfleiderer SO, Sachse S, Sauner D, et al. Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res 2004;6(3):R232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King V, Goldfarb SB, Brooks JD, et al. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology 2012;264(3):670–678. [DOI] [PubMed] [Google Scholar]
- 31.King V, Kaplan J, Pike MC, et al. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J 2012;18(6):527–534. [DOI] [PubMed] [Google Scholar]
- 32.Mousa NA, Eiada R, Crystal P, Nayot D, Casper RF. The effect of acute aromatase inhibition on breast parenchymal enhancement in magnetic resonance imaging: a prospective pilot clinical trial. Menopause 2012;19(4):420–425. [DOI] [PubMed] [Google Scholar]
- 33.Oksa S, Parkkola R, Luukkaala T, Maenpaa J. Breast magnetic resonance imaging findings in women treated with toremifene for premenstrual mastalgia. Acta Radiol 2009;50(9):984–989. [DOI] [PubMed] [Google Scholar]
- 34.Tan-Chiu E, Wang J, Costantino JP, et al. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. J Natl Cancer Inst 2003;95(4):302–307. [DOI] [PubMed] [Google Scholar]
- 35.Schrading S, Schild H, Kuhr M, Kuhl C. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast-enhanced breast MR imaging: a longitudinal intraindividual cohort study. Radiology 2014;271(1):45–55. [DOI] [PubMed] [Google Scholar]
- 36.An YS, Jung Y, Kim JY, et al. Metabolic Activity of Normal Glandular Tissue on (18)F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography: Correlation with Menstrual Cycles and Parenchymal Enhancements. J Breast Cancer 2017;20(4):386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leithner D, Baltzer PA, Magometschnigg HF, et al. Quantitative Assessment of Breast Parenchymal Uptake on 18F-FDG PET/CT: Correlation with Age, Background Parenchymal Enhancement, and Amount of Fibroglandular Tissue on MRI. J Nucl Med 2016;57(10):1518–1522. [DOI] [PubMed] [Google Scholar]
- 38.Sung JS, Corben AD, Brooks JD, et al. Histopathologic characteristics of background parenchymal enhancement (BPE) on breast MRI. Breast Cancer Res Treat 2018;172(2):487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scaranelo AM, Carrillo MC, Fleming R, Jacks LM, Kulkarni SR, Crystal P. Pilot study of quantitative analysis of background enhancement on breast MR images: association with menstrual cycle and mammographic breast density. Radiology 2013;267(3):692–700. [DOI] [PubMed] [Google Scholar]
- 40.Melsaether A, McDermott M, Gupta D, Pysarenko K, Shaylor SD, Moy L. Inter- and intrareader agreement for categorization of background parenchymal enhancement at baseline and after training. AJR Am J Roentgenol 2014;203(1):209–215. [DOI] [PubMed] [Google Scholar]
- 41.Preibsch H, Wanner L, Bahrs SD, et al. Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: correlation with tumour response. Eur Radiol 2016;26(6):1590–1596. [DOI] [PubMed] [Google Scholar]
- 42.DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol 2012;198(4):W373–380. [DOI] [PubMed] [Google Scholar]
- 43.Ray KM, Kerlikowske K, Lobach IV, et al. Effect of Background Parenchymal Enhancement on Breast MR Imaging Interpretive Performance in Community-based Practices. Radiology 2018;286(3):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brennan SB, Sung JS, Dershaw DD, Liberman L, Morris EA. Cancellation of MR imaging-guided breast biopsy due to lesion nonvisualization: frequency and follow-up. Radiology 2011;261(1):92–99. [DOI] [PubMed] [Google Scholar]
- 45.Dontchos BNDW, Rahbar H, Peacock S, Lehman CD. Influence of Menstrual Cycle Timing on Screening Breast MRI Performance in Pre-Menopausal Women. Presented (oral) at the Radiological Society of North America (RSNA) Annual Meeting, 2012, Chicago, IL. . [Google Scholar]
- 46.van Zelst JCM, Vreemann S, Witt HJ, et al. Multireader Study on the Diagnostic Accuracy of Ultrafast Breast Magnetic Resonance Imaging for Breast Cancer Screening. Invest Radiol 2018;53(10):579–586. [DOI] [PubMed] [Google Scholar]
- 47.Baek JE, Kim SH, Lee AW. Background parenchymal enhancement in breast MRIs of breast cancer patients: impact on tumor size estimation. Eur J Radiol 2014;83(8):1356–1362. [DOI] [PubMed] [Google Scholar]
- 48.Baek SH, Choi WJ, Cha JH, Kim HH, Shin HJ, Chae EY. Comparison of mammography, ultrasound, and MRI in size assessment of ductal carcinoma in situ with histopathologic correlation. Acta Radiol 2017;58(12):1434–1441. [DOI] [PubMed] [Google Scholar]
- 49.Preibsch H, Beckmann J, Pawlowski J, et al. Accuracy of Breast Magnetic Resonance Imaging Compared to Mammography in the Preoperative Detection and Measurement of Pure Ductal Carcinoma In Situ: A Retrospective Analysis. Acad Radiol 2018. [DOI] [PubMed] [Google Scholar]
- 50.Park SY, Kang DK, Kim TH. Does background parenchymal enhancement on MRI affect the rate of positive resection margin in breast cancer patients? Br J Radiol 2015;88(1046):20140638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Preibsch H, Richter V, Bahrs SD, et al. Repeated surgeries in invasive lobular breast cancer with preoperative MRI: Role of additional carcinoma in situ and background parenchymal enhancement. Eur J Radiol 2017;90:181–187. [DOI] [PubMed] [Google Scholar]
- 52.Kajihara M, Goto M, Hirayama Y, et al. Effect of the menstrual cycle on background parenchymal enhancement in breast MR imaging. Magn Reson Med Sci 2013;12(1):39–45. [DOI] [PubMed] [Google Scholar]
- 53.Tomida T, Urikura A, Uematsu T, Shirata K, Nakaya Y. Contrast Enhancement in Breast Cancer and Background Mammary-Gland Tissue During the Super-Early Phase of Dynamic Breast Magnetic Resonance Imaging. Academic radiology 2017;24(11):1380–1386. [DOI] [PubMed] [Google Scholar]
- 54.Kang SS, Ko EY, Han BK, Shin JH, Hahn SY, Ko ES. Background parenchymal enhancement on breast MRI: influence of menstrual cycle and breast composition. J Magn Reson Imaging 2014;39(3):526–534. [DOI] [PubMed] [Google Scholar]
- 55.Shin GW, Zhang Y, Kim MJ, et al. Role of dynamic contrast-enhanced MRI in evaluating the association between contralateral parenchymal enhancement and survival outcome in ER-positive, HER2-negative, node-negative invasive breast cancer. J Magn Reson Imaging 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho GY, Moy L, Kim SG, et al. Comparison of contrast enhancement and diffusion-weighted magnetic resonance imaging in healthy and cancerous breast tissue. Eur J Radiol 2015;84(10):1888–1893. [DOI] [PubMed] [Google Scholar]
- 57.Kim JY, Kim SH, Kim YJ, et al. Enhancement parameters on dynamic contrast enhanced breast MRI: do they correlate with prognostic factors and subtypes of breast cancers? Magn Reson Imaging 2015;33(1):72–80. [DOI] [PubMed] [Google Scholar]
- 58.Lam DL, Hippe DS, Kitsch AE, Partridge SC, Rahbar H. Assessment of Quantitative Magnetic Resonance Imaging Background Parenchymal Enhancement Parameters to Improve Determination of Individual Breast Cancer Risk. J Comput Assist Tomogr 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SA, Cho N, Ryu EB, et al. Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology 2014;270(3):699–707. [DOI] [PubMed] [Google Scholar]
- 60.Kim MY, Cho N, Koo HR, et al. Predicting local recurrence following breast-conserving treatment: parenchymal signal enhancement ratio (SER) around the tumor on preoperative MRI. Acta Radiol 2013;54(7):731–738. [DOI] [PubMed] [Google Scholar]
- 61.Park VY, Kim EK, Kim MJ, Yoon JH, Moon HJ. Breast parenchymal signal enhancement ratio at preoperative magnetic resonance imaging: association with early recurrence in triple-negative breast cancer patients. Acta Radiol 2016;57(7):802–808. [DOI] [PubMed] [Google Scholar]
- 62.Pineda FD, Medved M, Wang S, et al. Ultrafast Bilateral DCE-MRI of the Breast with Conventional Fourier Sampling: Preliminary Evaluation of Semi-quantitative Analysis. Academic radiology 2016;23(9):1137–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pujara AC, Mikheev A, Rusinek H, et al. Comparison between qualitative and quantitative assessment of background parenchymal enhancement on breast MRI. J Magn Reson Imaging 2018;47(6):1685–1691. [DOI] [PubMed] [Google Scholar]
- 64.Wu S, Weinstein SP, Conant EF, Kontos D. Automated fibroglandular tissue segmentation and volumetric density estimation in breast MRI using an atlas-aided fuzzy C-means method. Med Phys 2013;40(12):122302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Velden BH, Dmitriev I, Loo CE, Pijnappel RM, Gilhuijs KG. Association between Parenchymal Enhancement of the Contralateral Breast in Dynamic Contrast-enhanced MR Imaging and Outcome of Patients with Unilateral Invasive Breast Cancer. Radiology 2015;276(3):675–685. [DOI] [PubMed] [Google Scholar]
- 66.Chen JH, Yu HJ, Hsu C, Mehta RS, Carpenter PM, Su MY. Background Parenchymal Enhancement of the Contralateral Normal Breast: Association with Tumor Response in Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Transl Oncol 2015;8(3):204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu X, Jiang L, Li Q, Gu Y. Quantitative assessment of background parenchymal enhancement in breast magnetic resonance images predicts the risk of breast cancer. Oncotarget 2017;8(6):10620–10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang L, Hu X, Xiao Q, Gu Y, Li Q. Fully automated segmentation of whole breast using dynamic programming in dynamic contrast enhanced MR images. Medical physics 2017;44(6):2400–2414. [DOI] [PubMed] [Google Scholar]
- 69.Nie K, Chen JH, Chan S, et al. Development of a quantitative method for analysis of breast density based on three-dimensional breast MRI. Med Phys 2008;35(12):5253–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu S, Weinstein SP, DeLeo MJ 3rd,, et al. Quantitative assessment of background parenchymal enhancement in breast MRI predicts response to risk-reducing salpingo-oophorectomy: preliminary evaluation in a cohort of BRCA1/2 mutation carriers. Breast Cancer Res 2015;17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu S, Weinstein SP, Conant EF, Schnall MD, Kontos D. Automated chest wall line detection for whole-breast segmentation in sagittal breast MR images. Medical physics 2013;40(4):042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson JM, Henze Bancroft LC, Hernando D, Zea R, Reeder SB, Strigel RM. Gradient tracing for semiautomatic full breast segmentation of low resolution, low contrast breast MR images. Proceedings of the 25th Annual Meeting of ISMRM. Honolulu, HI: Proceedings of the 25th Annual Meeting of ISMRM; 2017 p. 2131. [Google Scholar]
- 73.Tagliafico A, Bignotti B, Tagliafico G, Tosto S, Signori A, Calabrese M. Quantitative evaluation of background parenchymal enhancement (BPE) on breast MRI A feasibility study with a semi-automatic and automatic software compared to observer-based scores. The British journal of radiology 2015;88(1056):20150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang Q, Li L, Zhang J, Shao G, Zheng B. A new quantitative image analysis method for improving breast cancer diagnosis using DCE-MRI examinations. Medical physics 2015;42(1):103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Q, Li L, Zhang J, Shao G, Zhang C, Zheng B. Computer-aided diagnosis of breast DCE-MRI images using bilateral asymmetry of contrast enhancement between two breasts. Journal of digital imaging 2014;27(1):152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng L, Lo G, Moshonov H, Liang J, Hodgson D, Crystal P. Breast Background Parenchymal Enhancement on Screening Magnetic Resonance Imaging in Women Who Received Chest Radiotherapy for Childhood Hodgkin’s Lymphoma. Academic radiology 2016;23(2):168–175. [DOI] [PubMed] [Google Scholar]
- 77.Wu J, Sun X, Wang J, et al. Identifying relations between imaging phenotypes and molecular subtypes of breast cancer: Model discovery and external validation. J Magn Reson Imaging 2017;46(4):1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mazurowski MA, Grimm LJ, Zhang J, et al. Recurrence-free survival in breast cancer is associated with MRI tumor enhancement dynamics quantified using computer algorithms. Eur J Radiol 2015;84(11):2117–2122. [DOI] [PubMed] [Google Scholar]
- 79.Klifa C, Suzuki S, Aliu S, et al. Quantification of background enhancement in breast magnetic resonance imaging. J Magn Reson Imaging 2011;33(5):1229–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lewin AA, Kim SG, Babb JS, et al. Assessment of Background Parenchymal Enhancement and Lesion Kinetics in Breast MRI of BRCA 1/2 Mutation Carriers Compared to Matched Controls Using Quantitative Kinetic Analysis. Acad Radiol 2016;23(3):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Clendenen TV, Zeleniuch-Jacquotte A, Moy L, Pike MC, Rusinek H, Kim S. Comparison of 3-point Dixon imaging and fuzzy C-means clustering methods for breast density measurement. J Magn Reson Imaging 2013;38(2):474–481. [DOI] [PubMed] [Google Scholar]
- 82.Strigel RM, Henze Bancroft LC, Hernando D, Reeder SB. Proton Density Water Fraction as a Measurement of Breast Fibroglandular Tissue Volume and Concentration. Proceedings of the 24th ISMRM Scientific Meeting Singapore: Proceedings of the 24th ISMRM Scientific Meeting; 2016. [Google Scholar]
- 83.Ding J, Stopeck AT, Gao Y, et al. Reproducible automated breast density measure with no ionizing radiation using fat-water decomposition MRI. J Magn Reson Imaging 2018;48(4):971–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Chen JH, Chang KT, et al. Automatic Breast and Fibroglandular Tissue Segmentation in Breast MRI Using Deep Learning by a Fully-Convolutional Residual Neural Network U-Net. Academic radiology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amano Y, Woo J, Amano M, Yanagisawa F, Yamamoto H, Tani M. MRI Texture Analysis of Background Parenchymal Enhancement of the Breast. Biomed Res Int 2017;2017:4845909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bennani-Baiti B, Dietzel M, Baltzer PA. MRI Background Parenchymal Enhancement Is Not Associated with Breast Cancer. PLoS One 2016;11(7):e0158573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dontchos BN, Rahbar H, Partridge SC, et al. Are Qualitative Assessments of Background Parenchymal Enhancement, Amount of Fibroglandular Tissue on MR Images, and Mammographic Density Associated with Breast Cancer Risk? Radiology 2015;276(2):371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grimm LJ, Saha A, Ghate SV, et al. Relationship between Background Parenchymal Enhancement on High-risk Screening MRI and Future Breast Cancer Risk. Acad Radiol 2018. [DOI] [PubMed] [Google Scholar]
- 89.Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G, Moschetta M. Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magnetic resonance imaging 2016;34(2):173–176. [DOI] [PubMed] [Google Scholar]
- 90.Arasu VA, Miglioretti DL, Sprague BL, et al. Population-Based Assessment of the Association Between Magnetic Resonance Imaging Background Parenchymal Enhancement and Future Primary Breast Cancer Risk. J Clin Oncol 2019:JCO1800378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kuhl CK. Abbreviated breast MRI for screening women with dense breast: the EA1141 trial. Br J Radiol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol 2014;32(22):2304–2310. [DOI] [PubMed] [Google Scholar]
- 93.Hilal T, Covington M, Kosiorek HE, et al. Breast MRI phenotype and background parenchymal enhancement may predict tumor response to neoadjuvant endocrine therapy. Breast J 2018. [DOI] [PubMed] [Google Scholar]
- 94.Oh SJ, Chae EY, Cha JH, Shin HJ, Choi WJ, Kim HH. Relationship between background parenchymal enhancement on breast MRI and pathological tumor response in breast cancer patients receiving neoadjuvant chemotherapy. Br J Radiol 2018;91(1088):20170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.You C, Gu Y, Peng W, et al. Decreased background parenchymal enhancement of the contralateral breast after two cycles of neoadjuvant chemotherapy is associated with tumor response in HER2-positive breast cancer. Acta Radiol 2018;59(7):806–812. [DOI] [PubMed] [Google Scholar]
- 96.You C, Peng W, Zhi W, et al. Association Between Background Parenchymal Enhancement and Pathologic Complete Remission Throughout the Neoadjuvant Chemotherapy in Breast Cancer Patients. Transl Oncol 2017;10(5):786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Choi JS, Ko ES, Ko EY, Han BK, Nam SJ. Background Parenchymal Enhancement on Preoperative Magnetic Resonance Imaging: Association With Recurrence-Free Survival in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy. Medicine (Baltimore) 2016;95(9):e3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lim Y, Ko ES, Han BK, et al. Background parenchymal enhancement on breast MRI: association with recurrence-free survival in patients with newly diagnosed invasive breast cancer. Breast Cancer Res Treat 2017;163(3):573–586. [DOI] [PubMed] [Google Scholar]
- 99.Luo J, Johnston BS, Kitsch AE, et al. Ductal Carcinoma in Situ: Quantitative Preoperative Breast MR Imaging Features Associated with Recurrence after Treatment. Radiology 2017;285(3):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vreemann S, Gubern-Merida A, Borelli C, Bult P, Karssemeijer N, Mann RM. The correlation of background parenchymal enhancement in the contralateral breast with patient and tumor characteristics of MRI-screen detected breast cancers. PLoS One 2018;13(1):e0191399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambin P, Rios-Velazquez E, Leijenaar R, et al. Radiomics: extracting more information from medical images using advanced feature analysis. European journal of cancer 2012;48(4):441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2015;278(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gastounioti A, Conant EF, Kontos D. Beyond breast density: a review on the advancing role of parenchymal texture analysis in breast cancer risk assessment. Breast Cancer Research 2016;18(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mendel KR, Li H, Giger ML. Quantitative breast MRI radiomics for cancer risk assessment and the monitoring of high-risk populations. Medical Imaging 2016: Computer-Aided Diagnosis. Volume 9785: International Society for Optics and Photonics; 2016. p. 97851W. [Google Scholar]
- 105.Sutton EJ, Huang EP, Drukker K, et al. Breast MRI radiomics: comparison of computer-and human-extracted imaging phenotypes. European radiology experimental 2017;1(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mazurowski MA, Zhang J, Grimm LJ, Yoon SC, Silber JI. Radiogenomic analysis of breast cancer: luminal B molecular subtype is associated with enhancement dynamics at MR imaging. Radiology 2014;273(2):365–372. [DOI] [PubMed] [Google Scholar]
- 107.Wang J, Kato F, Oyama-Manabe N, et al. Identifying triple-negative breast cancer using background parenchymal enhancement heterogeneity on dynamic contrast-enhanced MRI: a pilot radiomics study. PLoS One 2015;10(11):e0143308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Braman NM, Etesami M, Prasanna P, et al. Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MRI. Breast Cancer Research 2017;19(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wu S, Berg WA, Zuley ML, et al. Breast MRI contrast enhancement kinetics of normal parenchyma correlate with presence of breast cancer. Breast Cancer Research 2016;18(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu J, Li B, Sun X, et al. Heterogeneous enhancement patterns of tumor-adjacent parenchyma at MR imaging are associated with dysregulated signaling pathways and poor survival in breast cancer. Radiology 2017;285(2):401–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan M, Wu G, Cheng H, Zhang J, Shao G, Li L. Radiomic analysis of DCE-MRI for prediction of response to neoadjuvant chemotherapy in breast cancer patients. European journal of radiology 2017;94:140–147. [DOI] [PubMed] [Google Scholar]
- 112.Bignotti B, Signori A, Valdora F, et al. Evaluation of background parenchymal enhancement on breast MRI: a systematic review. Br J Radiol 2017;90(1070):20160542. [DOI] [PMC free article] [PubMed] [Google Scholar]


