Abstract
The ongoing wreaking global outbreak of the novel human beta coronavirus (CoV) pathogen was presumed to be from a seafood wholesale market in Wuhan, China, belongs to the Coronaviridae family in the Nidovirales order. The virus is highly contagious with potential human-human transmission which was named as the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), has spread across six continents and emerged as a global pandemic in short span with alarming levels of spread and severity. This virus associated symptoms and infectious respiratory illness is designated as coronavirus disease 19 (COVID-19). The SARS-CoV-2 possesses enveloped club-like spike protein projections with positive-sense large RNA genome and has a unique replication strategy. This virus was believed to have zoonotic origin with genetical identity to bat and pangolin CoV. In the current review, we introduce a general overview about the human CoVs and the associated diseases, the origin, structure, replication and key clinical events that occur in the COVID-19 pathogenicity. Furthermore, we focused on possible therapeutic options such as repurposing drugs including antimalarials, antivirals, antiparasitic drugs, and anti-HIV drugs, as well as monoclonal antibodies, vaccines as potential treatment options. Also we have summarized the latest research progress on the usage of stem cell therapy, human convalescent serum, interferon's, in the treatment of COVID-19.
Keywords: SARS-CoV-2, COVID-19, Zoonotic origin, Spike proteins, Repurposing drugs
Graphical abstract
1. Introduction to viruses
Viruses are the non-cellular infectious particles that contain either RNA or DNA as their genetic material which may be single or double-stranded. These viral particles are obligatory in nature, which cannot replicate on their own, hence depends upon the host cellular machinery to reproduce and increase viral particles [1]. A virion is a complete functional virus that includes the genetic material, the envelope, the protein part capsid, and the membrane proteins that allow the virus to infect living tissue, bind to its host cells and gain the access. Viruses are mostly contagious in nature and infect all most of living organisms. The viral particles are submicron in size with size range of 150–200 nm, which cannot be observed under normal microscope [2].
2. Corona viruses and the genomic features
Corona viruses (CoVs) are the largest group of viruses that can infect mammals and various other species. The name “coronavirus” is derived from Latin, where the meaning is “crown” or “wreath”. The name infers the distinctive appearance of virions that have a lipid envelope studded with club-shaped fringe projections creating an image resembling solar corona [3]. These viruses belong to the order Nidovirales which include the family Coronaviridae, and subfamily Orthocoronavirinae. The Orthocoronavirinae are further subdivided into four groups based on the genera that include the alpha, beta, gamma, and delta CoVs. The viruses from Nidovirales order possess a 5′ capped, enveloped, non-segmented, single-strand positive-sense RNA viruses with unusually large RNA genome ∼ 30 kb with typical sizes ranging from 80 to 120 nm [4]. These viruses are generally characterized by their spikes that protrude from their surface and follow unique replication strategy [5]. The RNA genome in CoVs is composed of six to ten open reading frames (ORFs) that encode the replicase proteins and four main structural proteins that include spike, membrane protein, envelope protein, and nucleocapsid proteins, all of which are encoded within the 3′ end of the viral genome. Where the spike proteins are involved in the viral entry and in determining host range, both membrane and envelope proteins are crucial for virus assembly. Besides, some CoVs also contain a gene which encodes hemagglutinin esterase (HE), which is a glycoprotein of approximately 60–65 kDa. This enzyme possesses an acetyl-esterase activity that disrupts sialic acid receptors that present in the host cell surface and help in the invasion and attachment that enhance the infectious properties of the virion. However, the more precise function of HE is still enigmatic [6,7]. The presence of helically symmetrical nucleocapsid is a distinct feature of CoVs, which is infrequent among positive-sense RNA viruses, but far more usual for negative-sense RNA viruses [8]. CoVs infect a substantial number of species and cause wide diseases such as pneumonia, hepatitis, enteritis, encephalomyelitis, nephritis, reproductive diseases, sialodacryoadenitis, polyserositis, and various other disorders [9]. The first member of CoV strain was identified in poultry flocks that caused infectious bronchitis and it was reported in 1930s [10].
3. The emergence of new human CoVs and diseases associated with CoVs
Human CoVs were first identified in mid-1960s, where the first CoV (HCoV-229E) was isolated from people suffering from the common cold in 1965. Later, HCoV-OC43 type was identified, which was thought to be responsible for 5 to 30% of human respiratory tract infections in infants, and older adults and immunocompromised individuals [11,12]. Almost 40 years later, in November 2002, a newly emerged CoV was identified as the causative reason for the severe acute respiratory syndrome (SARS) and named as the SARS coronavirus (SARS-CoV). It represents the first human-human transmissible pandemic disease in the 21st century. The World Health Organization (WHO) declared this ailment as “a worldwide health threat.” The outbreak of SARS started in the southern People's Republic of China, where 5300 people were infected and killed 349 nationwide [13]. Surprisingly, SARS-CoV has no genetic relatedness to any known human CoVs known by that time [14]. SARS-CoV was eradicated from the human population by global public health response. Thereafter, the other human CoV viruses including, HCoV-NL63 and HCoVHKU1 were identified in 2004 and 2005 [9]. On the other side, another lethal zoonotic beta CoV was identified in Saudi Arabia in 2012 and named as Middle East Respiratory Syndrome (MERS-CoV) which is different from SARS-CoV, which was responsible for 858 deaths out of total 2494 confirmed cases in 27 countries according to the WHO [15]. In late December 2019, the world has witnessed another deadliest outbreak for the first time in Wuhan, China, as cluster of pneumonia cases and various degrees of respiratory illness with unknown etiology and it was identified as a novel betacoronavirus, first called as 2019 novel coronavirus (2019-nCov) and it was renamed as SARS-CoV-2 on the basis of its close resemblance with 2002 SARS-CoV, the disease it caused was termed as coronavirus disease 2019 (COVID-19) [16], which had become pandemic and evolved all around the globe and spread to >203 countries and territories, by strong human-human community transmission. For the viral entry into target cells both SARS-CoV and SARS-CoV-2 use similar mechanism as both of these viruses contain variable receptor-binding domain (RBD) that bind to angiotensin-converting enzyme-2 (ACE-2) receptors. The SARS-CoV-2 was suspected to have originated from bats and pangolins [17]. The different human CoV strains and associated diseases are listed in Table 1 .
Table 1.
Different human CoVs and their symptoms.
| S. no | CoV Strain | Types | Symptoms associated with CoVs | Year | References |
|---|---|---|---|---|---|
| 1 | Human CoV OC43 | Beta | Fever, cough upper respiratory tract infections and fatal encephalitis | 1960 | [18] |
| 2 | Human CoV HKU1 | Beta | Respiratory tract illness, pneumonia and flu-like symptoms | 2005 | [19] |
| 3 | Human CoV 229E | Alpha | Common cold | 1960 | [20] |
| 4 | Human CoV NL63 | Alpha | Bronchiolitis and pneumonia | 2004 | [21] |
| 5 | Human SARS-CoV | Beta | Respiratory illness, shortness of breath, fever, chills, malaise and dry cough | 2003 | [22] |
| 6 | Human MERS-CoV | Beta | Fever, chills, cough, pneumonia, shortness of breath, and GIT symptoms includes diarrhea | 2012 | [23] |
| 7 | COVID-19 (SARS-CoV-2) | Beta | Flu-like symptoms, nausea, respiratory illness, pneumonia, shortness of breath and diarrhea | 2019 | [24] |
4. Structure of SARS-CoV-2, entry mechanisms and replication of viral particles
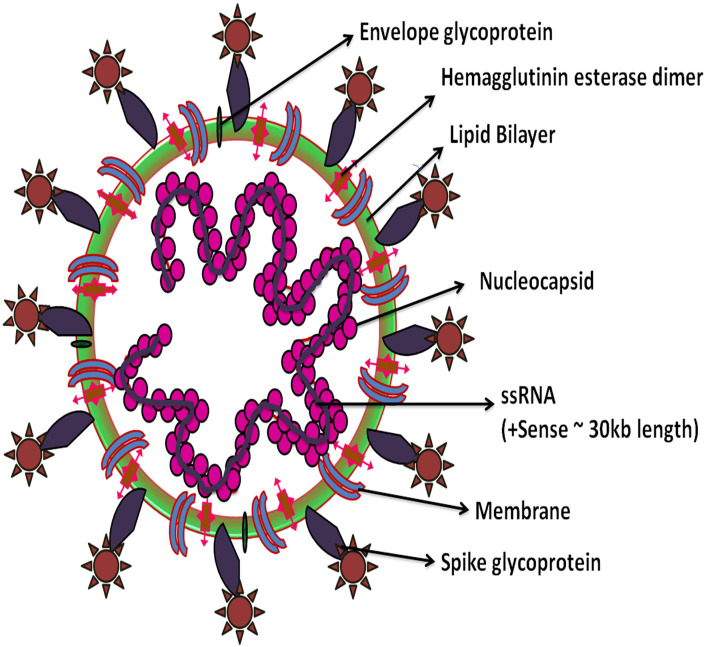
The viral strain was isolated from a patient's bronchoalveolar lavage (BAL) fluid and further identified as SARS-CoV-2 by the metagenomic RNA sequencing. The phylogenetic analysis revealed that the SARS-CoV-2 virion is spherical with 60 to 140 nm diameter in size has club-shaped glycoprotein spikes with 8 to 12 nm length. The virion contains a single positive-sense RNA genome with a total of 29,903 nucleotides (nt) with the G+C content of 38% [25]. SARS-CoV-2 genome has 10 open reading frames (ORFs), the essential genes that make complete viral genome from 5′ to 3′ are as follows 1) ORF1ab that encodes replicase polyprotein 1ab, the ORFs 2–10 encodes viral structural proteins which include 2) spike 3) envelope 4) membrane 5) nucleocapsid and 6) other auxiliary proteins (Fig. 1 ).
Fig. 1.
Structure of human SARS-CoV-2.
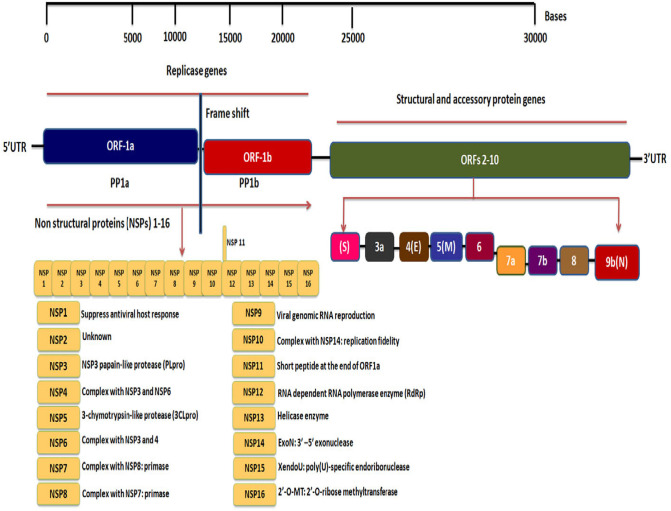
The spike and membrane proteins create a viral envelope which consists of a double layer of lipids and nucleocapsid holds the RNA genome which gives the virus a crown-like or solar corona appearance [26]. Phylogenetic analysis by Fan et al. revealed that the complete viral genome has ~29,903 nucleotides that were more closely related to a group of SARS-like Betacoronaviruses, and subgenus Sarbecovirus with 89.1% nucleotide similarity. SARS-CoV-2 has 5′ and 3′ terminal sequences that are typical for beta CoVs with 265 nt at the 5′ end and 229 nt at the 3′ terminal end. The predicted replicase ORF1a and ORF1b genes are 21,291 nt in length and comprise about two thirds of the viral genome that codes 16 predicted non-structural proteins (NSPs1–16). There is A-1 frame shift between ORF1a and ORF1b, leading to production of two polypeptides (pp1a and pp1ab). The NSP containing amino acids (aa) and functions are 1) NSP-1 (180 aa) mediates RNA replication and processing, 2) NSP2 (638 aa) function is unknown, 3) NSP3 (1945 aa) contains multiple domains, including a segment of SARS unique domain and papain-like protease (PLpro), 4) NSP4 (500 aa) complexes with NSP3 and 6, that in protein-protein interactions, 5) NSP5 (306 aa) is a 3-chymotrypsin-like protease (3CLpro), 6) NSP6 (290 aa) complexes with NSP3 and 4, 7) NSP7 (83 aa) complexes with NSP8: primase, 8) NSP 8 (198 aa) complexes with NSP7: primase, 9) NSP 9 (113 aa) involves in viral genomic RNA reproduction, 10) NSP 10 (139 aa) complexes with NSP14: involves in replication fidelity, 11) NSP 11 (13 aa) is a short peptide at the end of ORF1a, 12) NSP 12 (932 aa) is a RNA dependent RNA polymerase enzyme (RdRp), 13) NSP 13 (601 aa) is a Helicase enzyme, 14) NSP 14 (527 aa), is an Exon: 3′–5′ exonuclease, 15) NSP 15 (346 aa) is an XendoU: poly(U)-specific endoribonuclease, and 16) NSP 16 (298 aa) is an 2′-O-MT: 2′-O-ribose methyltransferase. The predicted nt in structural proteins (spike, envelope, membrane and nucleocapsid) are 3822, 228, 669 and 1260 nt in length. The other gene clusters such as ORF3a, ORF6, ORF7a, and ORF8 (828, 186, 366, 366 nt) appear only in SARS-CoV-2, bat-SL-CoVZC45 and the SARS-CoV-1 viruses. The ORF10 gene cluster is found to be unique to SARS-CoV-2 [[26], [27], [28]] (Fig. 2 ).
Fig. 2.
Genome of SARS CoV-2. The virion contains a single positive-sense RNA genome with a total of ~30,000 nucleotides (nt). The genome includes a 5′-cap, 5′-untranslated region (UTR), a large number of transcripts encoding 10 open reading frames(ORFs), ORFs with fusion, deletion, and/or frameshift and 3′-UTR, the essential genes that make complete viral genome from 5′ to 3′ are as follows 1) ORF1ab that encodes replicase polyprotein 1a and 1b, the ORFs 2–10 encodes viral structural proteins which include spike (S), envelope (E), membrane (M), nucleocapsid (N) and other accessory proteins. The ORF1ab gene constitutes a total of 16 non-structural proteins (NSPs1–16) within the pp1ab gene.
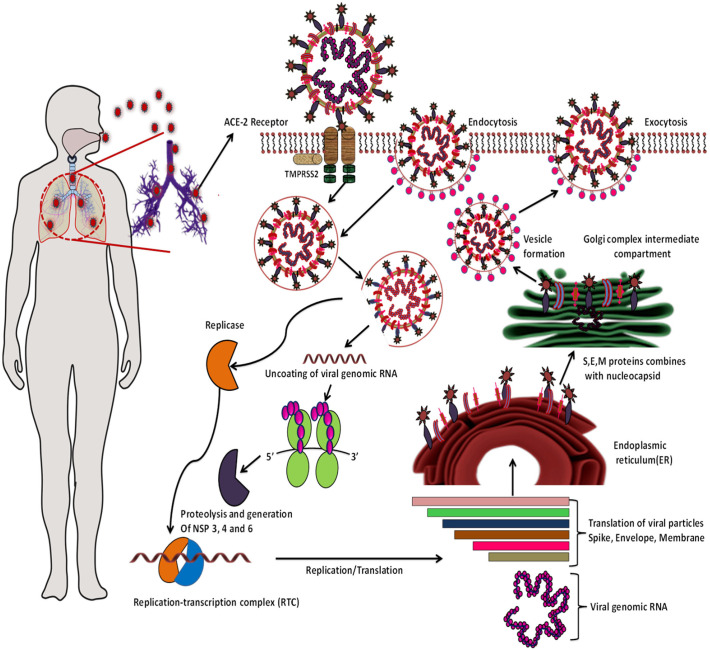
The life cycle of SARS-CoV-2 and viral replication begins in ACE-2 receptor over expressed cells, which is critical in the pathogenesis. The spike glycoprotein is located on the surface of CoVs is crucial for viral infection and pathogenesis, which are subdivided into S1 and S2 subunits. S1 has a RBD which constitutes 394 glutamine residues and this is recognized by lysine 31 residues on the human ACE2 receptor that determines the cellular tropism and virus-host range, while S2 subunit mediates virus-cell membrane fusion. After receptor binding, S protein is subjected to enzymatic modification by the transmembrane serine protease 2 (TMPRSS2), which is present at the vicinity of ACE2, induces conformational changes and fuses with the plasma membrane or enter by endocytosis [28]. The HE protein facilitates the cell entry of S protein through the mucosa [3]. For the SARS-CoV-2 entry through endocytosis, where 1-phosphatidylinositol-3-phosphate 5-kinase (PIKfyve), two-pore channel subtype 2 (TPC2), and cathepsin L are considered crucial. The availability of proteases in the host such as furin, TMPRSS2, trypsin, cathepsins and human airway trypsin-like protease (HAT) cleave spike proteins that largely determine whether CoVs enter cells through plasma membrane or endocytosis [29].
For fusion and the release of viral RNA genome into host cytoplasm, the virion undergoes various modifications by lysosomal cysteine proteases, cathepsin L and cathepsin B in the endo-lysosomes and this step is found to be highly pH-dependent. Highly acidic endosomal pH is needed for the fusion and eventually uncoating of the envelope [29]. Further, the viral genomic RNA is released into the cytoplasm and translates into two viral replicase polyproteins, pp1a and pp1ab that encode non-structural proteins, and form replication-transcription complex (RTC) [30]. Continuously the polymerase produces a nested set of subgenomic mRNAs and finally translates into relevant viral proteins. Furthermore, endoplasmic reticulum and Golgi together assemble the newly formed genomic RNA, envelope, nucleocapsid and glycoproteins in vesicles thereby the virion fuses with the plasma membrane to release outside the cell. The envelope protein E has conserved the functional features, such as ion-channels and a PDZ-binding motif (PBM) which is required to induce virulence [31]. The virus can infect ACE2-bearing cells in organs such as lung, esophagus, mouth, heart, intestines, bladder, and kidney [32] (Fig. 3 ).
Fig. 3.
The life cycle of SARS-CoV-2 in human host cells; begins its life cycle when spike protein binds to the angiotensin converting enzyme 2 (ACE-2) present on the outer surface of the cells in lungs, arteries, heart, kidney, and intestines. SARS-CoV-2 also enters the cells via pH dependent endocytosis. The newly formed viral particles will be transported outside by exocytosis.
5. Epidemiology of SARS-CoV-2
SARS infections in humans have first emerged as a cluster of pneumonia cases with mysterious etiology in Wuhan, Hubei, China in December 2019. A high-throughput sequencing from respiratory tract samples disclosed a novel virus as the causative pathogen belonging to the Coronaviridae, beta coronavirus genera, and Sarbecovirus subgenus and an official name was given as SARS-CoV-2 by the International Committee on Taxonomy of Viruses (ICTV) and World Health Organization on February 11, 2020, announced it as COVID-19 and on 30 January 2020, WHO officially declared it as a public health emergency of international concern [33,34], and has now ravaged the world in almost all countries and emerged as a global pandemic with serious hazards to global health in 2020. According to the Johns Hopkins Corona Virus Resource center as of 8 April 2020, this viral infection has spread to 6 continents with 1,430,141 confirmed cases and 82,133 deaths worldwide. In China, the epicenters of the SARS-CoV-2 outbreak, there were 82,783 confirmed cases with 3337 deaths but did not exhibit an exponential increase in the cases from March 7, 2020. On the other side, the epidemics are creating wreaking havoc in the USA, Spain and Italy with an exponential increase in confirmed cases 399,081, 141,942, 135,586 and with death toll of 12,907, 14,045, 17,127 respectively [35].
6. The origin of SARS-CoV-2
The phylogenetic data of SARS-CoV-2 implicate that origin could be zoonotic probably from the Huanan seafood market in Wuhan that has played a role in the early spreading but this observation remains uncertain, as the first documented patient was not linked to this Seafood market [17]. Hence, the origin of the SARS-CoV-2 is still ambiguous. Li et al., studied the molecular dating estimate by Bayesian time-scaled phylogenetic analysis using the tip-dating method based on the genomic sequences, where it was estimated that the time to the most recent common ancestor (TMRCA) and evolutionary rate of SARS-CoV-2, ranged from 22 to 24 November 2019 and 1.19 to 1.31 × 10−3 substitutions per site per year, respectively [36,37]. Recent pieces of evidence suggest that bats could be the possible primary reservoir but its origin is still under debate [38]. A study by Roujian Lu et al., performed next-generation sequencing from BAL or throat swab from nine patient samples and found interesting observations that the SARS-CoV-2 genome sequences exhibited >99·98% sequence similarity among different patient samples tested, which shows that the smaller degree of diversification. They also found an 88% similarity to bat derived SARS like CoVs including bat-SL-CoVZC45 and bat-SL-CoVZXC21. Interestingly, this study also compared the similarity to SARS-CoV and MERS-CoV, where it was observed a 79 and 50% similarity which indicates that these viruses are distinct to SARS-CoV-2. Surprisingly, in late December Wuhan bat species were in hibernation and were not available for sale in the seafood market. This report also speculates that the initial original reservoir might be the bats transmitted to the intermediate host which were found in the market, where human is the terminal host [39]. On the other hand, Zhou et al. reported a genome sequencing of bat CoV RaTG13, and found 96.2% similarity compared with SARS-CoV-2, although its spike diverges in the RBD, hence pointed that the bats could be the natural host or reservoir [40]. Another group of researchers found evidence of CoV by genomic sequencing in dead Malayan pangolins and named it as Pangolin-CoV and found that 91.02% similarity to SARS-CoV-2, and 90.55% with Bat CoV RaTG13, have provided evidence based on serological, virological, and histopathology, and also found that there is a close relation with RBD of S1 protein and of SARS-CoV-2 which is important to interact with the ACE2 protein and indicate that pangolins could be the natural reservoirs [41]. Besides, the systematic comparison and analysis approach predicted the possibilities that the interaction between the key amino acids of S protein and ACE2 indicates that other than pangolins, snakes, and turtles could also be the potential intermediate hosts in transmitting SARS-CoV-2 to humans [42]. Furthermore, Huang et al. have shown that the spike protein in bat CoV RaTGl3 showed 97.43% similarity with SARS-CoV-2 suggesting that this might be the closest strain and pangolin is the most possible intermediate reservoir to humans. Their findings propose the aptitude for evolution with higher chances of homologous recombination between bat and pangolin CoVs and emerged as SARS-CoV-2 recombinant virus with mutations in coding regions of 125 SARS-CoV-2 genomes [43,44]. Although differences arise in the observations, these evidences would be helpful to unravel the origin and probable intermediate host of SARS-CoV-2. The origin of virus and various evidences has been described in Table 2 .
Table 2.
Important findings on the origin of SARS-CoV-2.
| S. no | SARS-CoV-2 | Findings | Reference |
|---|---|---|---|
| 1 | Higher sequence identity between SARS-CoV-2 and RaTG13 | SARS-CoV-2 from BAL fluid represented 96.2% sequence similarity between SARS-CoV-2 and bat RaTG13. As per the accumulating evidence, bats could be the closest relative. Also, this study further confirms SARS-CoV-2 spike protein utilizes ACE2 receptor to enter the cells. | [40] |
| 2 | Pangolin-CoV is identical to SARS-CoV-2 at the whole-genome level | Zhang et al. has reported that Pangolin-CoV exhibited 91.02% similarity to SARS-CoV-2. Where, the S1 spike subunit of Pangolin-CoV is exhibited much more similarity to SARS-CoV-2 than RaTG13. This data suggest that there are five key amino acid residues involved in binding with ACE2 receptors, which are entirely consistent between Pangolin-CoV and SARS-CoV-2. Also, this data further tells that some of the Pangolin-CoV genes depicted higher amino acid sequence identity to SARS-CoV-2 genes than to RaTG13 genes include ORF1b (73.4%/72.8%), ORF7a (96.9%/93.6%), and ORF10 (97.3%/94.6%) as well as the spike protein (97.5%/95.4%). | [41] |
| 3 | SARS-CoV-2 might be a recombinant virus, with its genome evolved from Yunnan bat virus–like SARSr-CoVs and its RBD region acquired from pangolin virus-like SARSr-CoVs. | Lau et al. evidences that SARS-CoV-2 genomes exhibited different percentage genome identities such as 96.1% of SARSr-Ra-BatCoV-RaTG13, 87.8% of SARSr-Rp-BatCoV-ZC45, 87.6% of SARSr-Rp-BatCoV-ZXC21, and 85.3% of pangolin-SARSr CoV/P4L/Guangxi/2017. Additionally, these findings suggest that SARS-CoV-2 showed high amino acid sequence identities with that of SARSr-Ra-BatCoV RaTG13, except the receptor RBD region while RBD is closest to that of pangolin-SARSr-CoV/MP789/Guangdong/2019 infers that SARS-CoV-2 might be a recombinant virus. | [45] |
| 4 | SARS-CoV-2 was likely constructed via laboratory recombination | James Lyons-Weiler claimed that SARS-CoV-2 having a unique sequence (1378 bp), which is located in the middle of its spike glycoprotein gene that had no similarity with other coronaviruses. Furthermore, this report also claims that this sequence was much similar to that of a common expression vector named pShuttle-SN commonly used in research. | [46] |
| 5 | SARS-CoV-2 is not a laboratory origin | Hao et al. opposed the above mentioned James Lyons-Weiler's conclusion and claimed that sequence (1378 bp) from SARS-CoV-2 was also found in other coronavirus and which exists as naturally. The vector system pShuttle-SN was built in year of 2005 as an expression plasmid carrying a fragment sequence of spike gene from SARS-CoV, which caused the similarity match between plasmid and the SARS-CoV-2 spike gene sequence. | [47] |
| 6 | SARS-CoV-2 is not a purposefully manipulated virus or laboratory construct. | Andersen et al. claims that SARS-CoV-2 spike protein has six RBD amino acids that are critical for binding to human or human-like ACE2, is different from SARS-CoV, as a reason, the virus might be the most likely the result of natural selection. | [48] |
7. SARS-CoV-2 transmission, symptoms, incubation, and clinical characteristics
SARS-CoV-2 is highly contagious with powerful transmissibility. The current evidence suggest that human-human transmission of SARS-CoV-2 is mediated by coughing or sneezing from the infected individual through respiratory droplets (droplet particles > 5–10 μm in diameter) in close contact (within 1 m) or as droplet nuclei (particles < 5 μm in diameter), where the virus will be remain stable for long time and it can be easily spread to others with the gap of 1 m distance. The transmission is also possible with the indirect contact with surfaces in the immediate environment exposes and virus will enter through nose, mouth or conjunctiva [49]. Recent evidence suggest that gastrointestinal symptoms are frequently associated with this viral RNA or live infectious virus appear in faeces, which infers that faecal–oral transmission also other possible route of spread [50]. Interestingly, the possibility of vertical transmission of COVID-19 infection was not observed in two case studies reported so far [51,52].
During an emerging pandemic outbreak of an infectious disease, it is essential to know the level of transmission and the contagiousness which can be estimated by “R naught” (R0), a mathematical term which is the basic reproduction number that determines the average number secondary cases who are previously free of infection gets infected by one sick host. If the R0 is less than one the existing infection causes less than one infection, in such cases the spread declines and disappears. If R0 value equals 1, each existing infection causes one new infection. If R0 is >1, each existing infection may spread on an average more than one individual which may lead to dramatic increase in transmission and may result in epidemic [53]. The R0 value of SARS was ranging from 1 and 2.75, while the R0 of MERS-CoV was <1. The R0 of SARS-CoV-2 in Wuhan, China was estimated to be in the range of 2.24 to 3.58 in the early phases [54]. Steven Sanche et al. found that R0 of SARS-CoV-2 is likely to be 5.7 [55]. Data from six different studies estimated that R0 could range from 1.5 to 6.49, with an average of 4.2. However, WHO estimates R0 from 1.4 to 2.5 [56]. COVID-19 affected people patients serum show SARS-CoV-2 nucleic acid viral load (RNAaemia) [57] and exhibits the symptoms which include fever, coughing, sneezing, throat pain, anorexia, chest pain, diarrhea, nausea, vomiting dyspnea, the other characteristics most commonly exhibited are prolonged prothrombin time and lymphopenia. However, there are documented additional fatal complications include pneumonia, acute respiratory distress syndrome (ARDS) and acute cardiac injury as well as the incidence of grand-glass opacities, which defines as an area of increased attenuation in the lung on computed tomography (CT) with preserved bronchial and vascular markings in the periphery of the lungs with haziness overlying an area of the lung [58] found in some cases, which lead to increased mortality rate [59]. Recent evidence suggest that 97.5% of infected individuals develop symptoms within 11.5 days and the median incubation period to develop symptoms was estimated to be 5.1 days and 101 out of every 10,000 cases were found to develop symptoms after 14 days of active monitoring or quarantine [60].
By Jan 2, 2020, SARS-CoV2 complications were studied in a case series on initially hospitalized 41 COVID-19 confirmed patients. Among the cases 73% of the infected patients were men, 20% diabetes, 15% had hypertension and 15% of cases had other cardiovascular diseases. All patients in common had pneumonia. The other common symptoms were fever, myalgia or fatigue, cough, and the less common symptoms were hemoptysis, sputum production, lymphopenia, dyspnea, diarrhea, and the other complications are ARDS and secondary superinfections [61].
Cytokine storm refers to an aberrant or uncontrolled release of proinflammatory cytokines that lead to acute inflammation and pyrexia, which begins at a local site and spreads throughout the body via the systemic circulation. The previous out breaks of SARS-CoV and MERS-CoV have witnessed acute lung injury (ALI), ARDS and death which is accompanied by rapid virus replication that led to excessive inflammatory cell infiltration and cytokine storm [62]. Similarly, now in COVID-19 outbreak, elevated cytokine levels are observed in critically ill patients. A case report by Chen et al., has observed a markedly higher levels of TNF-α, IL-10, IL-2R, and IL-6, with remarkable reduction in CD4+ and CD8+ T lymphocytes, nearly in all 11 severe cases tested as compared to 10 moderate cases. The reduction in T lymphocyte number may further reduce the production of IFN-γ [63]. These findings are in line with Pedersen et al. and Diao et al. which demonstrated that the depletion of T cells and dysfunction in COVID-19, which can be implicated with the cytokine storm through the activation of highly inflammatory macrophages that produce the inflammatory cytokines and may dampen adaptive immunity against SARS-CoV-2 infection. Also, an increase in the inflammatory indicators such as D-dimer and procalcitonin were found [64,65]. Based on the clinical data by Hui Li many critically ill COVID-19 patients developed typical sepsis symptoms such as cold extremities, shock weak peripheral pulses and severe metabolic acidosis and hypothesized that the viral sepsis could be one of the critical clinical manifestations in cytokine storm. However, very little is known about the involvement of Toll like receptors (TLR) [66]. In another case study, the COVID-19 patients plasma samples were analyzed for cytokines by multiplex assay, where IL-1β, IL-7, IL-10, IL-8, IL-9, basic FGF, IL1-RA, GM-CSF, G-CSF, IP-10, MIP-1A, MIP-1B, MCP-1, PDGF, TNF-α, VEGF, and IFN-γ were found to be elevated in both (Intensive care unit) ICU and non-ICU patients, while no apparent changes were found in the levels of IL-15, IL12p70, IL-5, Eotaxin, and RANTES when compared to healthy adults. On the other side, the higher plasma levels of IL-7, IL-2, IL-10, GSCF, TNF-α, MCP-1, MIP-1A, and IP-10 were observed in ICU patients in comparison with non-ICU patients. This data suggest that the disease severity might be through the involvement of cytokine storm with SARS-CoV-2 infected critically ill patients [61]. Another case series was on 138 hospitalized patients, where the symptoms are consistent with the previously mentioned case report, all of the 138 patients showed bilateral involvement, critical illness was seen in older patients with co-morbidities. Apart from these symptoms, prolonged prothrombin and elevated lactate dehydrogenase levels were observed in 80 and 55 patients, respectively. Another observation was a gradual increase in blood urea, creatinine levels, neutrophils and lymphocytes counts before death. The other major complications during hospitalization are shock, arrhythmia, ARDS, and acute kidney injury [67]. Chen and colleagues based on a retrospective, single-center study on 99 cases [25], subsequently, Qian and colleagues reported that in 67.03% infected patients had multilobe infiltration in 53.85% patient population and an elevated levels of C-reactive protein (CRP) was seen, further in 15.39% patients elevated levels of procalcitonin was detected. Interestingly, in one case report COVID-19 had been detected from the rectal swab, who suffered from abdominal symptoms but tested twice negative by throat swab [68]. Therefore, along with the antiviral therapy it may be crucial to use anti- inflammatory agents in treating COVID-19 patients. Theoretically, corticosteroids may suppress lung inflammation, and they have been prescribed in 72.2% of ICU patients [69] but its efficacy in treating the cytokine storm in COVID-19 patients is still controversial as the concerns centered around their short-and long-term adverse effects as well as promotion of viral rebound and ARDS [70].Therefore, it is important to identify the cytokine storm in response to SARS-CoV-2 infection and the mechanism behind the cytokine storm. It would also be important to elucidate the role of anti-inflammatory agents against SARS-CoV-2 induced inflammation.
8. Prevention of COVID-19 entry and its transmission
WHO Director General constitutes a Public Health Emergency of International Concern for the outbreak of COVID-19, recommends the options to prevent the disease in new areas and possible reduction in human-to-human transmission to curtail its further spread that can be achieved by the strict quarantine, which involves the movement restriction or separation from the rest of the population with sustained and intense hygiene measures. Avoiding mass gatherings in enclosed spaces, maintaining the distance of at least 1 meter from any person with respiratory symptoms (e.g. coughing, sneezing); wearing a medical mask for an adequate level of protection combined with hand hygiene frequently by using an alcohol-based hand rub or soap is recommended. The people who develop respiratory complications during quarantine period should be specially treated and managed as a suspected case of COVID-19 and further tests should be required to confirm whether positive or negative. The front-line healthcare personnel must use the personal protective equipment (PPE) includes N95 or FFP2 standard masks, gowns, gloves, eye protection shields to protect themselves, patients, and others when providing the care [71].
9. The current update and possible treatment options for management of COVID-19
9.1. Renin-angiotensin system (RAS) inhibitors in SARS-CoV-2 patients
RAS and ACE2 inhibitors are widely used in treating patients with hypertension. As described earlier ACE2 is the target receptor for SARS-CoV-2 and is highly expressed in epithelial cells in the oral mucosa [72]. Additionally, this protein is widely expressed in immune reactive cells such as macrophages, lungs, blood vessels and intestine [73]. Clinical findings suggest that circulating ACE2 levels were significantly increased in diabetic and cardiovascular patients, however, this protein is aberrantly expressed with ACE inhibitors Lisinopril alone [74].
But the ACE2 activity is not altered correspondingly, while cardiac ACE2 mRNA expression was increased with RAS receptor inhibitor Losartan. Combination of Losartan and Lisinopril did not affect the ACE2 activity compared with Losartan alone, but on other side, ACE2 mRNA was highly expressed with Losartan alone [75]. Thus there is a lack of correlation between up and down expression of ACE2 mRNA with ACE2 activity. However, there is a debate on the use of RAS and ACE inhibitors in SARS-CoV-2 pneumonia infections and few clinical trials are going on for the usage of Losartan among patients who have not previously administered with RAS inhibitors and are either hospitalized (NCT04312009) or not hospitalized (NCT04311177) [76,77]. The selective ACE2 inhibitor DX600 might show beneficial results in SARS-CoV-2 infections; however its clinical significance in COVID19 has not evaluated [78]. Further, in one promising study, circulating recombinant human soluble ACE2 protein upon intravenous administration produced significant blockade of initial stages of SARS-CoV-2 viral entry and infections by preventing the binding of viral spike protein onto cell host cell surface ACE2 receptors [79].
9.2. Antimalarial drugs inhibit the SARS-CoV-2 infections
Chloroquine and Hydroxychloroquine are the class of quinoline derivatives and widely used to treat malaria caused by Plasmodium vivax, P. malariae, and P. ovale [80]. It is the active constituent of the bark of Cinchona (Cinchona officinalis) plant. Apart from malaria, Chloroquine and Hydroxychloroquine can be used to treat amebiasis [81] and other autoimmune disorders such as rheumatoid arthritis [82] and lupus erythematosus syndrome [83]. Chloroquine and its derivative Hydroxychloroquine inhibit the heme polymerase in malarial trophozoites, resisting the conversion of heme to hemozoin. Plasmodium species continue to accumulate toxic heme, killing the parasite and exhibits antimalarial activity [84]. Whereas the antiviral activity of Chloroquine is exerted by diffusing into the host cells and accumulating in endosomes, lysosomes and Galgi complexes, where it is converted into protonated form, then this moiety is deposited in organelles and further involve in raising the surrounding pH [85]. The raised pH in endosomes and lysosomes prevent viral fusion and inhibits the viral entry by endocytosis into the cells [86]. ACE2 is the target receptor for SARS-CoV and SARS-CoV-2 for viral fusion, however, Chloroquine does not affect the ACE2 levels but downregulated the terminal glycosylation of ACE2. Insufficient glycosylation of ACE2 offers inefficiency to bind with SARS-CoV-2 viral spike proteins and inhibits viral fusion [87]. Hu et al. demonstrated that Chloroquine inhibited the endocytosis mediated cell uptake of SARS-CoV-2 by suppressing the phosphatidylinositol binding clathrin assembly protein (PICALM) [88]. In the field of nanomedicine, Chloroquine/Hydroxychloroquine has been used extensively to understand the mechanism of nanoparticles uptake into cells. It was used as inhibitor of nanoparticles uptake via endocytosis pathway. Similar kind of viral particle entry through endocytosis route is proposed to be inhibited by Chloroquine [88]. The combination of Chloroquine or its derivative Hydroxychloroquine with drugs like Remdesivir or Azithromycin demonstrated to produce beneficial effects against this novel virus. However, its use in SARS-CoV-2 therapy has not been approved by US FDA and there are multiple clinical trials going on to evaluate the efficacy of Chloroquine/Hydroxychloroquine in COVID-19 (NCT04328272, NCT04323631, NCT04315896, NCT04318444, NCT04318015, NCT04303507, NCT04321278, NCT04322123, NCT04325893, and NCT04261517). Apart from therapeutic benefits, Chloroquine exerts various side effects such as fever, chills, loss of appetite, blurred vision and insomnia. Additionally, few reports indicated that Chloroquine exerts proarrhythmic effects by increasing the QT interval in the ECG patterns and decreasing the heart rate. This drug cannot be indicated for the patients who suffer from retinopathy and porphyria [89]. Based on the promising evidences, Chloroquine may become suitable drug for SARS-CoV-2 disease. Further, to improve the efficacy, Hydroxychloroquine may be used in place of Chloroquine. There were reports suggested the superior antiviral effects of Hydroxychloroquine compared to Chloroquine with better safety profiles [83]. Further, the combinational approach along with other antiviral drugs may be designed to evaluate the efficacy. Other antimalarial drugs such as Artesunate and Artemisone found to be effective against human cytomegaloviruses, but effect of these compounds on SARS-CoV-2 needs to be evaluated [90,91]. Moreover, the severe inflammatory cytokine storm observed in multiple organs may be curtailed by Chloroquine due to its immunomodulatory effects.
9.3. Antibacterial and antihelmintic drugs
Azithromycin is a renowned antibacterial drug belongs to the class of macrolide antibiotics. It shows antibacterial activity by binding to bacterial 50s ribosomal subunit and represses the protein synthesis. It is commonly used in the treatment of pneumonia, sinusitis, Lyme disease, skin infections and sexually transmitted diseases [92]. Apart from antibacterial activity, Azithromycin has antiviral activity observed in bronchial epithelial cells infected with rhinovirus, where it increased the production of interferon-stimulated genes [93]. Additionally, its antiviral activity was reported against Zika virus in human glial cells, where it prevented the virus induced alterations in fetal brain [94]. Also, the combination of Hydroxychloroquine and Azithromycin was found to be effective in SARS-CoV-2 associated pneumonia, where this combination significantly decreased the virus load and involved in the elimination of virus [95]. Energetics based modeling suggests that this drug combination might show the effect on SARS-CoV-2 spike-ACE2 complex. Recently Pfizer has reported the necessary data of Azithromycin for SARS-CoV-2 clinical trials (NCT04321278). Another antibiotic and anti-tuberculosis drug Carrimycin being tested to treat the SARS-CoV-2 patients, currently, it is under clinical trials (NCT04286503), however, its safety and efficacy need to be established in COVID-19 patients.
The fluoroquinolone antibacterial drug Ciprofloxacin is used to treat respiratory tract infections. Apart from its antibacterial activity, it can reduce the replication of polyoma BK virus. Ciprofloxacin reduced the viral load with IC50 value (50% virus inhibitory concentration) of 216.67 ± 16.7 μg/ml, whereas, Coumermycin showed the IC50 value of 10.6 ± 3.9 μg/ml [96]. The aminoglycoside antibiotics including Neomycin, Kasugamycin, and Streptomycin inhibited herpes simplex, influenza A and Zika virus replication by upregulating the interferon-stimulated genes (ISGs) [97]. The polyether antibiotic CP-44161 is recommended to treat varicella-zoster virus infections and also it inhibited the proliferation of herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) [97,98]. However, these antibiotics were not tested on SARS-CoV-2. Few antibiotics which exhibit antiviral properties are described in Table 3 .
Table 3.
List of antibiotics, which have the antiviral activity.
| S. no. | Antibiotic | Mode of action | Viruses get affected | Ref |
|---|---|---|---|---|
| 1. | Distamycin A | The reduction of DNA-dependent RNA synthesis | Vaccinia, herpes simplex, herpes zoster, myxoma, and adenoviruses | [99] |
| 2. | Netropsin | Inhibition of DNA-dependent DNA- and RNA syntheses | Vaccinia and Rauscher virus | [99] |
| 3. | Ehrlichin | Suppressing the formation of viral hemagglutinin | Influenza B virus | [100] |
| 4. | Teicoplanin | Inhibits the cathepsin L in the Late Endosome/Lysosome and suppress the viral entry | Ebola, MERS-CoV, and SARS-CoV viruses | [101] |
| 5. | Rifampicin | Inhibition of late stage viral protein synthesis, virion assembly and also suppresses the de novo synthesized viral polymerase. | Poxviruses and vaccinia virus | [102] |
| 6. | Doxycycline | Inhibits the viral replication | Chikungunya virus | [103] |
The US-FDA approved antihelmintic drug Niclosamide is also explored as an anticancer, antibacterial and antiviral agent. Also, it was found to be effective as a protease inhibitor in shedding the SARS-CoV and MERS-CoV replication and viral antigen synthesis was suppressed by Niclosamide at 1.56 μM [104]. It might be a good pharmacological agent to treat SARS-CoV-2 infections as well and studies may be designed to evaluate the same in vitro and clinical conditions [105]. The FDA approved antiparasitic drug Ivermectin is widely used as an essential medicine to treat lymphatic filariasis, strongiloidiasis, ascariasis, head lice, scabies, and river blindness etc. [106]. Ivermectin was tested for its repurposing antiviral activity against SARS-CoV-2, where it has shown excellent antiviral activity. The in vitro results suggest that Ivermectin reduced viral RNA load >5000 fold with 5 μM concentration at 48 h [107]. It might be a good repurposing drug in treating SARS-CoV-2 infections and further studies are required for this specific use.
9.4. Antiviral drugs
An American biotechnology company Gilead Sciences developed the antiviral drug Remdesivir (GS-5734) against Ebola virus. Remdesivir exerts broad-spectrum antiviral activity and showed beneficial results in SARS-CoV and MERS-CoV induced respiratory infections. It has been recommended to administer to SARS-CoV-2 infected patients in United States of America, Europe, and Japan, where physicians found the beneficial results [108]. But it is not yet approved by the drug regulators for treating the patients who are suffering from SARS-CoV-2. Remdesivir is a prodrug and is metabolised to its active form GS-441524, which is a nucleotide analogue inhibitor of RNA-dependent RNA polymerases [109]. It interferes with the viral RNA polymerase activity and further inhibits the viral exoribonuclease involved in proofreading and ultimately causes the deprivation of viral RNA production [110]. Remdesivir was tested in clinical isolates of SARS-CoV-2 in vitro, where it exhibited significant antiviral activity. Further, it was also tested in combination with Chloroquine and found to be effective against COVID-19 [85]. However, the data available is limited on Remdesivir and further studies need to be conducted to prove its essential role in treating COVID-19 along with its drug-drug interactions and contraindications.
Oseltamivir is recommended to treat and prevention of Influenza A and Influenza B viruses. It belongs to the class of neuraminidase inhibitors and acts as a competitive inhibitor of neuraminidase enzyme present in influenza virus and prevents the respiratory tract infections. This enzyme involved in the cleavage of the sialic acid which is an important component of glycoproteins present on the surface of human cells and helps new virions to exit the cells [111]. The therapeutic potential of Oseltamivir on SARS-CoV-2 infections needs to be evaluated. Few of the combinational drugs such as ASC09F and Ritonavir are being evaluated together with Oseltamivir, these are under the clinical trials now (NCT04261270).
Coronaviruses are associated with different types of proteases such as main protease (Mpro) also known as 3 chymotrypsin-like protease (3CLpro), papain-like proteases (PLpro) and transmembrane protease, serine 2 (TMPRSS2). Mpro involved in viral replication and plays a pivotal role in processing the polyproteins, which are translated from its RNA. Linlin Zhang et al. developed broad-spectrum inhibitors such as peptidomimetic α-ketoamides against main proteases of beta and alphacoronavirus. Among all, pyridone-containing α-ketoamide compound 13b inhibited the SARS-CoV-2 Mpro with IC50 value of 0.67 ± 0.18 μM and showed improved safety and lung tropism as well as suitable for inhalational route of administration [112]. In another hand, Chen et al. performed the virtual screening for approved drugs using 3D model of SARS-CoV-2 Mpro with the help of the crystal structure of SARS-CoV, which is having almost 96% similarity. They proposed and considered nearly 16 candidates and among Ledipasvir and Velpatasvir are attractive and might be effective in treating COVID-19 with minimal side effects [113]. Alméciga-Díaz et al. performed a virtual screening using molecular docking and proposed plausible drugs include Cefuroxime, Cepharanthine, Ergoloid mesylates, Lumacaftor, Nimorazole, Nilotinib, and Pictilisib which are having higher binding affinity for SARS-CoV-2 Mpro [114]. Ramaprasad et al. screened 9 natural compounds against SARS-CoV-2 Mpro, among them carlinoside and quercetin 3-O-sophoroside showed higher binding affinity [115]. Apart from these, Remdesivir, Saquinavir and Darunavir, as well as flavone and coumarin derivatives were found to be effective against SARS-CoV-2 Mpro [116].Another SARS-CoV-2 protease PLpro plays an important role in viral replication and survival, which mainly involved in cleaving the viral polyproteins a/b. Goswami et al. proposed that compounds isolated from Alpinia officinarum and ginger having SARS-CoV-2 PLpro inhibitory activity confirmed by molecular docking studies [117]. Also virtual docking studies support that Chloroquine and formoterol also found to be effective against SARS-CoV-2 PLpro [118]. In contrast to others, Bagherzadeh et al. proposed that dual protease Mpro and PLpro inhibitors Valaganciclovir, Nelfinavir, Merimepodib, Remdesivir, Inarigivir, Taribavirine and TAS106-106 are suitable in COVID-19 therapy, among all Remdesivir and Inarigivir showed highest binding affinity [119]. Camostat mesylate is TMPRSS2 inhibitor, is involved in proteolytic processing of spike proteins and it suppressed the SARS-2-S-driven fusion into Caco-2 and Vero-TMPRSS2 cells and might be effective in COVID-19 therapy [120].
Other protease inhibitors include Lopinavir and Ritonavir were found to be effective in SARS-CoV infections and also these drugs are currently under the clinical trials for testing the efficacy and safety against SARS-CoV-2 [121]. Investigators performed an open-label randomized trial at Wuhan hospital, China. They have given the treatment to adult SARS-CoV-2 patients with oral Lopinavir and Ritonavir (400 and100 mg, respectively) twice daily for 14 days. They found that Lopinavir and Ritonavir combination was significantly effective against SARS-CoV-2 [122]. However, further studies are needed to draw a clear conclusion on the usage of the combination of Lopinavir and Ritonavir in SARS-CoV-2.
The nucleoside analogue Ribavirin has extensive activity against DNA and RNA viruses and it is used to treat SARS-CoV patients [123]. The exact antiviral mechanism of Ribavirin has been studied for decades and it is still unclear. Ribavirin usage is associated the major side effect of anaemia which is found in 27–59% of patients. The importance of Ribavirin for fighting against SARS-CoV-2 was evaluated in combination with protease inhibitors and corticosteroids [124]. This drug has been showing significant results with Sofosbuvir and this combination is under the clinical trials (NCT01497366).
The nucleotide analogue Sofosbuvir is used to treat hepatitis C and it is recommended in combination with Ribavirin, Velpatasvir, and Voxilaprevir [125]. Molecular docking studies of RNA dependent RNA polymerase model suggest that Sofosbuvir in combination with Ribavirin and Remdesivir could exhibit possible therapeutic effects in SARS-CoV-2. Basically, Sofosbuvir is a prodrug, which converts into active metabolite GS-461203 (2′-deoxy-2′-α-fluoro-β-C-methyluridine-5′-triphosphate) and serve as a defective substrate for the non-structural protein 5B (NS5B) of RNA dependent RNA polymerase and further inhibits RNA synthesis [126]. Sofosbuvir and other combinations will be tested in the view of adverse effects such as pruritis, upper respiratory tract infections, and lymphopenia [127]. The antiviral drugs which are under the clinical trials for treating COVID-19 are described in Table 4 .
Table 4.
Antiviral drugs which are under the clinical trials for treating SARS-CoV-2 viral infections.
| S. no. | Antiviral drug | Nature of the intervention | Clinical trials |
|---|---|---|---|
| 1. | Remdesivir (RDV; GS-5734) | Class of nucleotide analogues and developed for Ebola virus and Marburg virus infections | Phase 3 (NCT04292899, NCT04292730, NCT04257656, NCT04252664) Early phase (NCT04323761, NCT04302766) |
| 2. | Lopinavir/Ritonavir | Protease inhibitors used in HIV/AIDS | Phase 3 (NCT04304053) |
| 3. | Abidol hydrochloride (Umifenovir) | It shows the virucidal effects on influenza virus | Phase 4 (NCT04255017) |
| 4. | ASC09F + Oseltamivir | Antiviral drugs | Phase 3 (NCT04261270) |
| Ritonavir + Oseltamivir | |||
| Oseltamivir | |||
| 5. | Combination of Oseltamivir, Favipiravir, and Hydroxychloroquine | Protease inhibitors used as antiviral drugs | Phase 3 (NCT04303299) |
| 6. | Ganovo + Ritonavir ± Interferon nebulization | Protease inhibitors used in hepatitis C | Phase 4 (NCT04291729) |
| 7. | Camostat | It is a serine protease inhibitor | Phase 1/Phase 2 (NCT04321096) |
| 8. | Darunavir and Cobicistat | Protease inhibitor and CYP3A inhibitor, respectively used in HIV/AIDS | Phase 3 (NCT04252274) |
The short term anti-coagulant, antiviral and antibacterial drug Nafamostat mesylate inhibits Spike protein induced membrane fusion in MERS-CoV and also researchers suggested that using Nafamostat alone or with combination of antiviral drugs could be a safe approach in treating COVID-19 [128,129].
9.5. Effect of interferons on SARS-CoV-2
The 166 amino acid sequence of Interferon alfacon-1 is produced by recombinant DNA technology, which is the class of non-naturally occurring type-I interferon [130]. Interferon alfacon-1 acts as anticancer and antiviral agent. The therapeutic effect of Interferon alfacon-1 was observed in leukemia, melanoma, HIV/AIDS related Kaposi's sarcoma, and hepatitis C [131]. It is also found to be effective in SARS-CoV and also tested in SARS-CoV-2 in combination with corticosteroids [132,133]. Interferon alfacon-1 shows antiviral activity by binding to interferon receptors type 1 including IFNAR1 and IFNAR2c. Further, it initiates the dimerization and activates the Janus kinase 1 (Jak1) and tyrosine kinase 2 (Tyk2) phosphorylation. The signal transducers and activators of transcription 1 and 2 (STAT1 and STAT2) bind to the phosphorylated IFNAR and initiate the expression of immunomodulators and antiviral protein expression including protein kinase R (PKR) and 2′-5′ oligoadenylate synthase (2′-5′ OAS) [134]. Additional clinical studies are required to approve this drug for SARS-CoV-2 therapy. There are multiple reports on the antiviral activity of IFN-α and IFN-β, and combinations of IFN-α/β, IFN-β 1a and IFN-γ against SARS-CoV and these agents might be also effective against SARS-CoV-2 too, which need to be evaluated in suitable studies [135,136].
9.6. Therapeutic importance of amniotic fluid cells and convalescent plasma in SARS-CoV-2 ARDS infections
Amniotic fluid cells are the possible source of stem cells for clinical purposes such as fetal therapies and regenerative medicine, where they proliferate rapidly can differentiate into different cells [137]. The human amniotic fluid is approved by US-FDA for tissue injury and also used to treat inflammatory and fibrotic diseases. The researchers from the University of Utah tested the nebulized and/or intravenous purified amniotic fluid in SARS-CoV-2 patients, where it reduced the respiratory inflammatory responses observed with this novel pandemic virus. Recently they received the approvals from regulatory bodies to perform the clinical trials (NCT04319731).
The Chinese scientific group Chenguang Shen and coworkers have collected 400 ml of convalescent plasma from patients who are recovered from SARS-CoV-2 infection and had been symptom-free for at least 10 days and transfused to five critically ill patients of SARS-CoV-2, who were under the ventilation and have ARDS. Following infusion, these patients have started to recover from day one and SARS-CoV-2 viral load was gradually decreased and the body temperatures were significantly decreased within three days. At day 9, it was reported that patients have started to take own breath without ventilation support. All the patients were symptomless, became stable and 3 out of 5 patients were discharged too [138]. Recently, the US-FDA allowed medical practitioners and physicians to use convalescent plasma in emergency to treat critical ill SARS-CoV-2 patients and few of the clinical studies are also initiated for the use of anti-SARS-CoV-2 and convalescent Plasma (NCT04321421, NCT04323800, NCT04325672).
9.7. Monoclonal antibodies to treat SARS-CoV-2 infection
Monoclonal antibodies are currently used for diagnostic and therapeutic purposes. Various monoclonal antibodies are approved by US-FDA to treat cancer and autoimmune disorders. Additionally, few of monoclonal antibodies are tried to treat SARS-CoV-2 infections such as Bevacizumab (NCT04305106), Tocilizumab (NCT04317092), and Meplazumab (NCT04275245) etc. Xiaolong Tian et al. reported that SARS-CoV-specific human monoclonal antibody, CR3022, which has the ability to bind with SARS-CoV-2 RBD (KD of 6.3 nM). CR3022 epitope was not overlapped with the ACE2 binding site within SARS-CoV-2 RBD [139]. This novel monoclonal antibody might be effective clinically for treating SARS-CoV-2 associated pneumonia. Zhongyu Zhu et al. tested the human monoclonal antibody m396 for treating the SARS-CoV, which competes with the SARS-CoV receptor, ACE2 and inhibits the interaction between SARS-CoV and ACE2 [140]. Chunyan Wang and coworkers tested human monoclonal antibody 47D11 on SARS-CoV and SARS-CoV-2 strains, where it binds with conserved epitope present on the spike RBD and further it cross-neutralized SARS-CoV and SARS-CoV-2, and this mode of action is independent to receptor binding inhibition [141]. Anjeanette Roberts and colleagues developed monoclonal antibody MAb 201 and tested on the golden Syrian hamster model of SARS-CoV infection, where it inhibited the viral replication and decreased the interstitial pneumonitis [142]. Human-to-human transmission of SARS-CoV-2 is possible due to interaction of spike protein with human ACE2, thus spike protein is the main target for antibody-mediated neutralization. Various neutralizing monoclonal antibodies are tested against SARS-CoV including 80R, CR3014, CR3022, m396, B1, 201, 68, 1F8, and 5E9, these antibodies might play an important therapeutic role in SARS-CoV-2 infections [143]. Collectively, spike proteins, ACE2 and their interactions are the main targets for developing new therapeutic monoclonal antibodies for treating SARS-CoV-2 infections.
Tocilizumab is used in autoimmune diseases such as rheumatoid arthritis and multiple myoloma and it is a human recombinant IL-6 receptor (IL-6R) antibody [144]. It is associated with major side effects such as allergy, liver toxicity and hyperlipidaemia [145]. IL-6 is involved in the activation of various immunological and inflammatory mediators, which are responsible for respiratory collapse observed in SARS-CoV-2 infected patients. Tocilizumab is under phase II clinical trials and tested for SARS-CoV-2 induced pneumonia (NCT04317092).
Another IL-6R monoclonal antibody Sarilumab is under clinical trials for SARS-CoV-2 (NCT04315298). It is widely used in the treatment of rheumatoid arthritis and it suppresses the IL6R mediated inflammation [146]. Cytokine storm is responsible for the pneumonia condition in SARS-CoV-2 infected patients. Clinical trials on Sarilumab and Remdesivir have been initiated to test the therapeutic efficacy against SARS-CoV-2 [147]. The potential therapeutic role of Sarilumab in COVID-19 need to be further confirmed in clinical conditions. The monoclonal antibodies which are under the clinical trials are represented in Table 5 .
Table 5.
Monoclonal antibodies under the clinical trials.
| S. no. | Intervention/treatment | Nature of the intervention | Clinical trials |
|---|---|---|---|
| 1. | Emapalumab | IL-6 inhibitor used for RA | Phase 2/Phase 3 (NCT04324021) |
| Anakinra | Anti-interferon-gamma antibody used for the treatment of hemophagocytic lymphohistiocytosis | ||
| 2. | Siltuximab | IL-6 inhibitor used for cancer therapy | NCT04322188 |
| 3. | Tocilizumab | IL-6 inhibitor is used to treat rheumatoid arthritis | Phase 2 (NCT04317092) |
| 4. | Sarilumab | Monoclonal antibody against IL-6R used in RA | Phase 2 (NCT04315298) |
| 5. | PD-1 blocking antibody | It Inhibits the T cell depletion in sepsis patients | Phase 2 (NCT04268537) |
| 6. | Bevacizumab | It is VEGR inhibitor and used to treat cancers | (NCT04305106) |
| 7. | Tocilizumab | IL-6R inhibitor is used treat RA | Phase 3 (NCT04320615) |
| 8. | CD24Fc | Innate checkpoint against the inflammatory response and immunomodulator | Phase 3 (NCT04317040) |
| 9. | Recombinant human interferon Alpha-1b | Immunomodulator | Phase 3 (NCT04320238) |
| Thymosin alpha 1 | Immunomodulator | ||
| 10. | Recombinant human interferon α1β | Antiviral activity | Early Phase 1 (NCT04293887) |
| 11. | Meplazumab | Humanized anti-CD147 antibody | Phase 1/Phase 2 (NCT04275245) |
| 12. | Intravenous immunoglobulin therapy | Activates innate and adaptive immunity | Phase 2/Phase 3 (NCT04261426) |
9.8. Natural products and dietary supplements
Our diet contains a lot of vitamins, minerals, carbohydrates, proteins, fats, and lipids and they play an important role in maintaining the homeostasis in our body and play important role in maintaining the immunity. Additionally, our diet contains various biological active natural products and they exert multiple pharmacological properties such as anticancer, antioxidant, anti-inflammatory, anti-diabetic, antibacterial, antiviral and antifungal properties etc. The battery of pro-inflammatory cytokines are responsible for the cytokine storm, which is involved in manifestation of severe acute lung injury, this is the main cause for SARS-CoV-2 induced morbidity and mortality. The active constituent of turmeric, Curcumin exhibits wider pharmacological activities such as anti-bacterial, antiviral, anticancer, anti-diabetic properties. Curcumin inhibited pro-inflammatory cytokines IL-1β, IL-6, and TNF-α level and suppressed the cytokine storm in Ebola virus infected experimental models [148]. Additionally, Curcumin also inhibited the SARS-CoV replication and the effective concentration (EC50 value) was found to be around 10 μM and also acted as a protease inhibitor [149]. Thus it might be effective against SARS-CoV-2 infections, where intravenous (i.v.) route of administration in suitable formulation form may enhance the better bioavailability. Similarly, a thorough search across all the natural products needs to be evaluated for their direct antiviral effects and their potential role in controlling severe inflammatory responses observed in COVID-19 induced pneumonia.
The Indian traditional plant Neem (Azadirachta indica) and its parts such as leaves, seeds, flowers, barks and routes are widely using in various diseases. Ancient people believe that it is a Sarva roga nivarini (the cure for all diseases). Methanolic extract of neem leaves exhibited antiviral activity against HSV-1 infections by glycoprotein mediated viral fusion [150]. Nimbolide is an active constituent of Neem tree explored as a pharmacological modulator in treating various diseases such as cancer, diabetes, and inflammatory diseases [151]. TNF-α is a pleiotropic cytokine involved in activation of various signaling cascade and this cytokine might be playing role in respiratory failure associated with COVID-19 mediated pneumonia. Nimbolide is found to be TNF-α inhibitor and also suppresses the nuclear translocation of p65 NF-κB and HDAC-3 and inhibited the cytokine storm observed in ARDS experimental model [152]. Thus it might show beneficial effects in SARS-CoV-2 infections by direct antiviral activity or indirect supportive therapy by controlling the inflammatory cytokine storm. This natural product may have clinical significance in inflammation associated with viral diseases. The other natural product Withaferin A is isolated from Ashwagandha (Withania somnifera) and widely used to treat various diseases such as cancer, fibrosis, and inflammatory disorders. It has shown the antiviral activity against Herpes Simplex virus 1 and 2 [153], which may show plausible effects against COVID-19.
Andrographolide is a diterpenoid isolated from Andrographis paniculata, which has been employed in treating various diseases such as cancer and inflammatory diseases [154]. Apart from anti-inflammatory activity, Andrographolide exhibits immunomodulatory effect by increasing the level of cytotoxic T cells, NK cells, phagocytic cells, and shows the broad spectral antiviral activity against various deadliest viruses [155]. Recent computational analysis data suggest that Andrographolide and its derivatives chrysin-7-O-β-glucuronide might produce therapeutic effects against SARS-CoV-2 pneumonia infections.
Quercetin is widely present in leaf vegetables, red onions, seeds, and grains, which is a plant flavonol from the flavonoid group of polyphenols and employed as a pharmacological agent in treating inflammation and cancer [156]. It has shown the antiviral activity against Dengue virus by inhibiting the viral replication without affecting its attachment and entry process [157]. Also Quercetin inhibited the SARS-CoV entry into the host cells and exhibited the antiviral activity against HIV-luc/SARS with EC50 value of 83.4 μM [158]. The US-FDA approved drug ingredient, Quercetin might show therapeutic activity against SARS-CoV-2.
Saikosaponins belong to the class of triterpenoids and found as essential components of various plant extracts including Scrophularia scorodonia, Heteromorpha spp., and Bupleurum spp., exert antiviral activity against Corona virus 229E by inhibiting the viral attachment and penetration [159]. The molecular docking studies suggested that Saikosaponin B4 can be employed as spike protein inhibitors and could become potential therapeutic molecule against SARS-CoV-2 [160].
The water soluble vitamin C (ascorbic acid) is profoundly available in lemon, oranges, amla, kiwi, guava, grapes, broccoli, cauliflower, and capsicum etc. Vitamin C is well-known as an antioxidant and inhibits the ageing process by enhancing the collagen expression, further, it involved in the wound healing process [161]. Apart from the antioxidant effects, Vitamin C involved in chemotaxis, phagocytosis and increases the reactive oxygen species to kill the microorganisms and also acts as an antibacterial agent. It also protects cells by clearing the non-functional organelles by apoptosis mechanisms. Also, it involved in proliferation and differentiation of B- and T-cells, which play a pivotal role in immune mediated reactions [162]. Recent scientific reports suggest that vitamin C inhibits the LPS induced TNF-α and IL-1β expression ad reduced the MDA levels and increased the GSH levels and acts as an anti-inflammatory agent [163]. Additionally, Vitamin C inhibits the lung fibrosis by reducing the IL-17 secretion and induced SOD levels [164]. Vitamin C also has a direct virucidal effect at higher concentrations and decreased the virus load of Ebstein-Barr virus (EBV) infected cells [165,166]. In general, vitamin C is considered as strong immune booster and must able to protect wide array of microbial infections. However, some reports suggest that ≥1 g per day vitamin C supplementation had no significant effect on common cold incidence. But, some evidence infers that vitamin C could have moderate preventive effects in low dietary intake groups or acute physical stress suffering people [167]. Based on the anti-inflammatory and antiviral properties, Vitamin C infusion was tried in SARS-CoV-2 infected pneumonia patients and numerous clinical trials (NCT04323514, NCT04264533, NCT03680274, and NCT04326725) are ongoing for its therapeutic usage.
The crude extracts and active constituents of Heteromorpha spp., Bupleurum spp., Lycoris radiata, Artemisia annua, Scrophularia scorodonia, Lindera aggregata, Pyrrosia lingua, Isatis indigotica, Houttuynia cordata, and Torreya nucifera were found to be effective in treating SARS-CoV and these agents may also have effects in curing SARS-CoV-2 infections [168]. Few of the natural products and dietary supplements having antiviral activity are enlisted in Table 6 .
Table 6.
Antiviral and immune-modulating natural products and dietary supplements.
| S. no. | Natural product/dietary supplement | Mechanism | Viruses evaluated | Reference |
|---|---|---|---|---|
| 1. | Garlic (Allium sativum) | Inhibits the viral adsorption or penetration | Human rhinovirus-2, vaccinia virus, HSV-1, HSV-2, parainfluenza viru-3, and vesicular stomatitis virus | [169] |
| 2. | Fresh ginger (Zingiber officinale) | Suppresses viral attachment and internalization | Human respiratory syncytial virus | [170] |
| 3. | Cinnamon (Cinnamomum cassia) | Inhibits the viral replication | H7N3 influenza A Virus | [171] |
| 4. | Tulsi (Ocimum sanctum) | Shows the virucidal activity | H9N2 virus | [172] |
| 5. | Ginseng (Panax ginseng) | Inhibits the viral replication | SARS-CoV | [173] |
| 6. | Glycyrrhizin (Liquorice roots; Glycyrrhiza glabra) | Induces the nitric oxide synthase and produces the nitrosative stress in macrophages and exhibit the virucidal activity | SARS-CoV, Japanese encephalitis virus, HIV-1 and chronic hepatitis C virus | [174] |
| 7. | Theaflavin-3,3′-digallate (TF3) (Black tea) | Inhibits the 3C-like protease (3CL(Pro)) and suppresses the viral replication | SARS-CoV | [175] |
| 8. | Resveratrol | Suppresses the viral replication | MERS-CoV | [176] |
| 9. | Welsh onion (Allium fistulosum L.) | Improves the host-immune system | Influenza A | [177] |
| 10. | Vitamin D | Immunomodulator | Influenza virus | [178] |
| 11. | Zinc | Inhibits the RNA polymerase activity and suppress the viral replication | SARS-CoV and equine arteritis virus (EAV) | [179] |
| 12. | Baicalein (Scutellaria baicalensis and Scutellaria lateriflora) | Exhibits virucidal activity and suppresses the viral adsorption and replication | Dengue virus | [180] |
| 13. | Raoulic (Raoulia australis) | Broad spectrum antiviral agent | Picornaviruses | [181] |
| 14. | Ladanein (Marrubium peregrinum) | Suppresses viral entry | Hepatitis C virus | [182] |
9.9. The pharmacological interventions used in treating SARS-CoV and MERS-CoV
As described earlier, SARS-CoV-2 is different from SARS-CoV and MERS-CoV. However clinical features seem close to SARS-CoV and MERS-CoV but vary in fatality rate, transmission, reproductive number etc. [183]. Due to this pandemic situation, physicians are recommending the drugs which are used to treat SARS-CoV and MERS-CoV to treat COVID-19 such as Chloroquine diphosphate, Remdesivir, Lopinavir, Ritonavir and Ribavirin etc. IFNs include α and β (Type 1), γ (Type II), and λ (Type III) showed broad-spectrum antiviral activity against SARS-CoV, where IFN-β exhibits more potent activity compared with IFN-α as well as IFN-γ. Here, MERS-CoV is more sensitive to IFN-α in contrast to SARS-CoV. The immunosuppressant Mycophenolic acid is ineffective against SARS-CoV, but it was showed promising results against MERS-CoV, when it is given by oral route of administration and exhibited significant results when it was administrated along with IFN-β and thiopurine analogues 6-Mercaptopurine/6-Thioguanine [184]. The other immunosuppressant Cyclosporin A was found to be effective in reducing viral replication against SARS-CoV and MERS-CoV and might be effective in COVID-19 by reducing cytokine storm [185]. Omrani et al. reported that Ribavirin and IFN-α2a showed improved results in MERS-CoV infected patients and found to be effective in improving survival at 14 days rather than 28 days [186].
Tyrosine kinase inhibitors such as Imatinib mesylate (Inhibitor of Abelson tyrosine-protein kinase 2 (Abl2), which is involved in SARS-CoV and MERS-CoV replication) and Dasatinib inhibit both MERS-CoV and SARS-CoV, whereas Nilotinib inhibits only SARS-CoV. The microtubule polymerization inhibitor Nocodazole showed higher activity against MERS-CoV and suppressed the viral replication, but it was ineffective in SARS-CoV. The ion channel inhibitors including Monensin and Salinomycin sodium showed effective results against MERS-CoV but these drugs were insensitive towards SARS-CoV [187]. SSYA10-001 is a 1,2,4 triazole reported as replication inhibitor that prevents the helicase activity of SARS-CoV and MERS-CoV [188]. These interventions which are used to treat SARS-CoV and MERS-CoV might be useful in treating COVID-19. Apart from these, few more interventions are illustrated by Dyall et al. about their anti-SARS-CoV and anti-MERS-CoV activity [189].
9.10. Vaccine for SARS-CoV-2
The biological preparation of vaccine is useful in attaining the acquired immunity against specific infectious diseases. Vaccines are prepared from dead or inactivated microorganisms or purified products derived from the respective organisms. Vaccines are available as prophylactic as well as therapeutic agents. Apart from the traditional live attenuating method, Novavax is constructing the recombinant vaccine, where the antigens stably expressed from the spike protein in baculovirus system [190]. In this method, they are isolating the genetic code for the protein spike on the surface of SARS-CoV-2, which is involved to provoke an immune reaction in humans, and this sequence is pasted into the genome of a bacterium or yeast, where it will produce the large quantity of protein of interest. The mRNA-1273 vaccine against SARS-COV-2 was developed by National Institute of Allergy and Infectious Diseases (NIAID) scientists and their collaborators at the biotechnology company Moderna, Inc., based in Cambridge, Massachusetts. The Coalition for Epidemic Preparedness Innovations (CEPI) supported the manufacturing of the vaccine candidate for the clinical trials. Here, the mRNA vaccine is injected to the people through intra muscular route, where the immunogens are produced in human cells instead of recombinant system and exhibit the higher immunocompatibility [191]. In this mRNA vaccine system, the uridines are modified into pseudouridines in order to prevent the immune-mediated reactions exerted by toll-like receptors such as TLR7 and TLR8, where they recognize the uridines and further activate the interferon response [192,193]. This vaccine strategy is currently under phase I clinical trials, where they are working on two primary goals, the first goal is to study the safety and reactogenicity of a 2-dose vaccination schedule of mRNA-1273, given 28 days apart, across 3 dosages in healthy adults. The secondary goal is to determine the immunogenicity by ELISA against SARS-CoV-2 spike protein levels following a 2-dose vaccination schedule of mRNA-1273 at Day 57 [194]. However, it will take some more time to evaluate its full efficacy and safety potential before it is allowed for patient application by regulatory bodies. Similar type mRNA vaccine BNT162 was developed by BioNTech and Pfizer against COVID-19, which is under the clinical trials [195].
Shenzhen Geno-Immune Medical Institute has developed synthetic minigene vaccine LV-SMENP-DC and antigen-specific CTLs and artificial antigen-presenting cells (aAPC) for SARS-CoV-2. This vaccine is under the clinical trials, where they are going to test safety and efficacy of innovative SARS-CoV-2 minigenes, engineered based on multiple viral genes, developed by lentiviral vector system (LV) to express viral proteins and immune-modulatory genes to modify dendritic cells (DCs) and aAPC to activate cytotoxic T cells [196]. Additionally, Chinese based company CanSino Biologics has developed the recombinant novel CoVs vaccine (adenovirus type 5 vector) candidate in alliance with Beijing Institute of Biotechnology (BIB), based on the viral vector vaccine technology platform, which was previously used to develop Ebola vaccine. This vaccine strategy has given significant results in preclinical studies, now they have received the approval to initiate clinical trials and Phase 1 clinical trials are ongoing on.
University of Oxford researchers have developed the ChAdOx1 nCoV-19 vaccine and this vaccine was tested on 18–55 years old UK healthy adult volunteers. Further studies and data are needed to get clearance from regulatory authorities, which might need significant amount of time [197].
Repurposing of vaccine is a hot topic in SARS-CoV-2 therapy. The tuberculosis vaccine Bacillus Calmette–Guérin (BCG) is primarily used to prevent tuberculosis and other diseases such as leprosy and Buruli ulcers [198]. The recent epidemiological study suggests that universal BCG vaccination might play an important role in controlling the morbidity and mortality associated with SARS-CoV-2 infections. The SARS-CoV-2 affected countries mortality is different in various countries; it may be due to temperature, elder population, humidity, cultural differences, migration, and health systems. New York Institute of Technology (NYIT) scientists proposed that the countries that do not opt the universal BCG vaccination were affected mainly by SARS-CoV-2 infections such as Italy, Netherlands, and USA etc. as compared to the counties who follow the universal BCG vaccination [199]. BCG vaccination offers broad-spectrum activity against the prevention of respiratory infections and it might reduce the SARS-CoV-2 oriented mortalities. Currently, Australia, Netherlands, Germany and the United Kingdom (UK) have started the clinical trials first on the front line health workers [200]. If it works effectively in SARS-CoV-2, it will be a game-changer in vaccine research in the current overwhelming situation.
Various Pharmaceutical and Biotechnological companies such as Sanofi, Zydus Cadila, Codagenix, Serum Institute of India, Bharath Biotech, Inovio Pharmaceuticals & Beijing Advaccine Biotechnology, Sanofi & Translate Bio, Pfizer, Johnson & Johnson, GlaxoSmithKline (GSK), Commonwealth Serum Laboratories (CSL) and Seqirus have started the vaccine development programs in association with academic institutes, research centers and hospitals. Few of the novel vaccines showed positive results in preclinical trials and they are trying to push their products for clinical trials in the current year 2020 [201]. The list of the vaccines under clinical trials is represented in Table 7 .
Table 7.
Vaccines under the clinical trials.
| S. no. | Vaccine | Clinical trials |
|---|---|---|
| 1. | LV-SMENP-DC vaccine and antigen-specific CTLs | Phase 1/Phase 2 (NCT04276896) |
| 2. | Pathogen-specific artificial antigen presenting cells (aAPC) | Phase 1 (NCT04299724) |
| 3. | mRNA-1273 vaccine | Phase 1 (NCT04283461) |
| 4. | ChAdOx1 nCoV-19 vaccine | Phase 1/Phase 2 (NCT04324606) |
| 5. | Recombinant novel CoV vaccine (adenovirus type 5 vector) | Phase 1 (NCT04313127) |
| 6. | Bacillus Calmette–Guérin (BCG) vaccine | Phase 1 (EudraCT: 2020-000919-69) |
9.11. Stem cell therapy and natural killer (NK) cells
Stem cells are differentiated into different cells and widely used in treating various diseases such as cancer and organ failure diseases. Apart from traditional drug therapy, researchers are trying to treat patients who suffer from SARS-CoV-2 infections with mesenchymal stem cells (MSCs). MSCs are the type of multipotent stromal cells that can able to differentiate into various types of cells such as myocytes, adipocytes, osteoblasts and chondrocytes [202]. SARS-CoV-2 enhances the pro-inflammatory cytokine levels such as IL-2, IL-6, IL-7, and TNF-α and further causes the terrible cytokine storm in the lungs followed by edema, alveolar dysfunction for the air exchange, ARDS, acute cardiac injury and the systemic infection, which may ultimately lead to death [61]. MSCs are having a powerful immunomodulating effects and may involve in preventing and reducing the cytokine storm caused by SARS-CoV-2.
Zikuan Leng et al. treated seven patients who are suffering from SARS-CoV-2 pneumonia with MSCs in Beijing You'an Hospital, China. They have evaluated the inflammation and immune-related parameters for 14 days after a single infusion of MSCs, where they found that all the patients recovered from all the symptoms without any adverse effects. Where MSCs inhibited the cytokine storm by upregulating the cytotoxic cells and suppressed the TNF-α levels. Thus, the intravenous administration of MSCs will be a safe and effective approach for treating severe SARS-CoV-2 pneumonia patients [203]. Various scientific groups are working on MSCs to treat SARS-CoV-2 and few of the studies are under the clinical trials (Table 8 ).
Table 8.
List of stem cell research works on SARS-CoV-2 therapy.
| S. no. | Stem cells | Mechanism | Clinical trials |
|---|---|---|---|
| 1. | Wharton's Jelly-mesenchymal stem cells (WJ-MSCs) | It involves in immunomodulatory properties and maintains both innate and adaptive immune responses. | Phase 1 (NCT04313322) |
| 2. | Mesenchymal stem cells | Protects against SARS-CoV-2 induced organ failure | Phase 1 (NCT04252118) |
| 3. | Stem cell educator-treated mononuclear cells apheresis | It is used to reverse the autoimmune response in Type 1 diabetes, Alopecia Areata | Phase 2 (NCT04299152) |
| 4. | Umbilical cord (UC)-derived mesenchymal stem cells (MSCs) | Protects against organ failure | Phase 2 (NCT04273646) |
| 5. | MSCs-derived exosomes | Shows anti-inflammatory properties and inhibit organ failure | Phase 1 (NCT04276987) |
| 6. | NestCell® mesenchymal stem cells | Inhibits the cytokine storm | Phase 1 (NCT04315987) |
| 7. | Dental pulp mesenchymal stem cells | Suppress the inflammatory mediated signaling | Early Phase 1 (NCT04302519) |
Cytotoxic lymphocytes mainly available as cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, these are essential for limiting the dangerous viral infections. Meijuan Zheng et al. reported that CD8+ T-cells and NK cells were significantly decreased, while NKG2A expression was dramatically increased in SARS-CoV-2 infected patients [204]. NK cells recognize the NKG2D, which is an activating receptor of NK cells involved in clearing the virus infected cells. Chimeric antigen receptors (CAR) exhibit an important role in modifying the NK cells and play an essential role in cell-based immunotherapy for treating cancer and viral diseases [205]. Actually, NK cells have shorter half-life and their activity was improved by IL-15 super agonist (sIL-15/IL-15Rɑ chimeric protein) up to 20 times more and ultimately increases the target cytotoxicity [206]. In general Granulocyte-macrophage colony-stimulating factor (GM-CSF) neutralizes the CAR-T cell-mediated cytokine release syndrome (CRS) and neurotoxicity [207]. Chongqing Public Health Medical Center has constructed and prepared the universal off-the-shelf IL-15 super agonist- and GM-CSF neutralizing scFv-secreting NKG2D-ACE2 CAR-NK derived from cord blood. This developed vaccine system inhibited expression of the spike protein of SARS-CoV-2 and suppressed the NKG2DL on the surface of infected cells with ACE2. Additionally, IL-15 super agonist enhances the NK cells activity and CRS is prevented by GM-CSF neutralizing scFv. Further, ACE2 CAR-NK cells inhibited SARS-CoV-2 infection safely and effectively. These advanced targeted cell therapy strategies need to be evaluated under controlled clinical trials, which is already initiated currently (NCT04280224 and NCT04324996).
10. Conclusion and future perspective
Over the past 50 years, in the pre-SARS era, many different CoVs have been identified that caused a wide variety of human and veterinary diseases. In particular, the human CoVs were found to cause mild respiratory diseases. In November 2002, SARS-CoV infection was emerged in Guangdong Province of China, identified to be fatal resulting in ARDS, which became a pandemic in 37 countries with over 8000 reported cases and 800 deaths [208]. This deadly SARS virus was found to be originated from horseshoe bats that transmitted to pangolins before reaching humans. At that time no specific drugs or vaccines were available and only preventive measures were implemented to limit the transmission rate by maintaining social distance, quarantine, travel restrictions, patient isolation. In 2012, another zoonotic CoV were identified from Saudi Arabia postulated to have originated from the dromedary camels which infected 2494 people and 858 deaths were reported [209], In late December 2019, a recent global outbreak, a novel CoV strain has been evolved which is linked to a seafood market in Wuhan, Hubei Province, China with the symptoms varying from mild respiratory illness and pneumonia in most of the patients, to fatal consequences that progresses to ARDS by the induction of a pro-inflammatory cytokine storm. As of 8 April 2020, approximately 1.43 million cases of COVID-19 has been confirmed worldwide, and this pandemic is rapidly escalating all around the globe and most of the countries implemented the most restrictive mass quarantines that led to severe global socioeconomic disruption which has become the greatest concern of WHO [210]. As of now, there are no specific approved drugs for treating SARS-CoV-2 and currently, physicians are using the repurposing drugs such as Chloroquine/Hydroxychloroquine, Remdesivir, Tocilizumab, Azithromycin, Dexamethasone, Acetaminophen etc., and they are showing plausible results, however more studies are required for specific use against SARS-CoV-2. Moreover, immune boosters such as Vitamin C, Zinc and other natural products are suggested in preventing the viral infections. Additionally, scientists are developing the new antiviral drugs and vaccines, and few are under the clinical trials. Apart from traditional drug therapy, medical practitioners are transfusing the convalescent plasma to rescue the critically ill patients with successful outcome in viral load reduction; Also, MSCs were effective for treatment with encouraging improvement with fewer side effects. Thus there is a lot of scope to develop antiviral drugs and vaccines against SARS-CoV-2; there is a need for vigorous effort on the vaccine development and exploration of potential antiviral treatment regimens. Simultaneously, the primary and intermediate host and mechanism of its cross-species spread and human interface needs to be investigated. Owing to the fact that new CoV viruses will continue to evolve on the basis of their ability to undergo frequent recombination's of their genomes, mutations, the propensity to infect multiple species and the increasing human-animal interface, the strict legislations should be made to prohibit the consumption and farming of terrestrial wild animals to prevent the future outbreaks.
Declaration of competing interest
Authors declare there is no conflict of interest.
Acknowledgments
Acknowledgement
Authors sincerely acknowledge Director, NIPER-Hyderabad, India and Department of Pharmaceuticals (DoP), Ministry of Chemicals and Fertilizers, Government of India for financial support.
Author contribution
V.P. and S.T. conceptualized the review, collected the data and written the manuscript. C.G. provided technical support for the work and reviewed the manuscript.
References
- 1.SpringerLink; Viruses in biology. https://link.springer.com/article/10.1007/s12052-012-0441-y (n.d.)
- 2.Lodish H., Berk A., Zipursky S.L., Matsudaira P., Baltimore D., Darnell J. 4th edition. 2000. Viruses: Structure, Function, and Uses, Molecular Cell Biology.https://www.ncbi.nlm.nih.gov/books/NBK21523/ [Google Scholar]
- 3.Masters P.S. Advances in Virus Research. Academic Press; 2006. The molecular biology of coronaviruses; pp. 193–292.http://www.sciencedirect.com/science/article/pii/S0065352706660053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4:1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol. Mol. Biol. Rev. 2005;69:635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desforges M., Desjardins J., Zhang C., Talbot P.J. The acetyl-esterase activity of the hemagglutinin-esterase protein of human coronavirus OC43 strongly enhances the production of infectious virus. Journal of Virology. 2013;87:3097–3107. doi: 10.1128/JVI.02699-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.PNAS; Structure of coronavirus hemagglutinin-esterase offers insight into corona and influenza virus evolution. https://www.pnas.org/content/105/26/9065.short (n.d.) [DOI] [PMC free article] [PubMed]
- 8.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacLachlan N.J., Dubovi E.J., editors. Fenner's Veterinary Virology. fifth edition. Academic Press; Boston: 2017. Chapter 24 - Coronaviridae; pp. 435–461.http://www.sciencedirect.com/science/article/pii/B9780128009468000246 [Google Scholar]
- 10.Science; Infection of the Cloaca with the virus of infectious bronchitis. https://science.sciencemag.org/content/76/1958/34.2 (n.d.) [DOI] [PubMed]
- 11.Killerby M.E., Biggs H.M., Haynes A., Dahl R.M., Mustaquim D., Gerber S.I., Watson J.T. Human coronavirus circulation in the United States 2014–2017. J. Clin. Virol. 2018;101:52–56. doi: 10.1016/j.jcv.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau S.K.P., Lee P., Tsang A.K.L., Yip C.C.Y., Tse H., Lee R.A., So L.-Y., Lau Y.-L., Chan K.-H., Woo P.C.Y., Yuen K.-Y. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J. Virol. 2011;85:11325–11337. doi: 10.1128/JVI.05512-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y. National Academies Press; US: 2004. The SARS Epidemic and Its Aftermath in China: A Political Perspective.https://www.ncbi.nlm.nih.gov/books/NBK92479/ [Google Scholar]
- 14.Wang L.-F., Shi Z., Zhang S., Field H., Daszak P., Eaton B.T. Review of bats and SARS. Emerg. Infect. Dis. 2006;12:1834–1840. doi: 10.3201/eid1212.060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO WHO; Middle East respiratory syndrome coronavirus (MERS-CoV) http://www.who.int/emergencies/mers-cov/en/ (n.d.)
- 16.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16:1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morfopoulou S., Brown J.R., Davies E.G., Anderson G., Virasami A., Qasim W., Chong W.K., Hubank M., Plagnol V., Desforges M., Jacques T.S., Talbot P.J., Breuer J. Human coronavirus OC43 associated with fatal encephalitis. N. Engl. J. Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 19.Kanwar A., Selvaraju S., Esper F. Human coronavirus-HKU1 infection among adults in Cleveland, Ohio. Open Forum Infect Dis. 2017;4:ofx052. doi: 10.1093/ofid/ofx052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ScienceDirect Topics; Human Coronavirus 229E - an overview. https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/human-coronavirus-229e (n.d.)
- 21.Pyrc K., Berkhout B., van der Hoek L. The novel human coronaviruses NL63 and HKU1. J. Virol. 2007;81:3051–3057. doi: 10.1128/JVI.01466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): a review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernheim A., Mei X., Huang M., Yang Y., Fayad Z.A., Zhang N., Diao K., Lin B., Zhu X., Li K., Li S., Shan H., Jacobi A., Chung M. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. 2020;200463 doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ASM.org; 2019 novel coronavirus (2019-nCoV) update: uncoating the virus. https://asm.org/Articles/2020/January/2019-Novel-Coronavirus-2019-nCoV-Update-Uncoating (n.d.)
- 27.Wang C., Liu Z., Chen Z., Huang X., Xu M., He T., Zhang Z. The establishment of reference sequence for SARS-CoV-2 and variation analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., Yuan M.-L., Zhang Y.-L., Dai F.-H., Liu Y., Wang Q.-M., Zheng J.-J., Xu L., Holmes E.C., Zhang Y.-Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020;11 doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Wilde A.H., Snijder E.J., Kikkert M., van Hemert M.J. Host factors in coronavirus replication. Curr. Top. Microbiol. Immunol. 2018;419:1–42. doi: 10.1007/82_2017_25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jimenez-Guardeño J.M., Nieto-Torres J.L., DeDiego M.L., Regla-Nava J.A., Fernandez-Delgado R., Castaño-Rodriguez C., Enjuanes L. The PDZ-binding motif of severe acute respiratory syndrome coronavirus envelope protein is a determinant of viral pathogenesis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burchfield J. Renin-angiotensin-aldosterone system: double-edged sword in COVID-19 infection. 2020. https://www.preprints.org/manuscript/202003.0365/v1
- 33.Li B., Yang J., Zhao F., Zhi L., Wang X., Liu L., Bi Z., Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls. StatPearls Publishing; Treasure Island (FL): 2020. Features, evaluation and treatment coronavirus (COVID-19)http://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 35.Johns Hopkins Coronavirus Resource Center; COVID-19 map. https://coronavirus.jhu.edu/map.html (n.d.)
- 36.Li X., Zai J., Zhao Q., Nie Q., Li Y., Foley B.T., Chaillon A. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2. J. Med. Virol. 2020 doi: 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X., Wang W., Zhao X., Zai J., Zhao Q., Li Y., Chaillon A. Transmission dynamics and evolutionary history of 2019-nCoV. J. Med. Virol. 2020;92:501–511. doi: 10.1002/jmv.25701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G., Shi Z.-L. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv. 2020 doi: 10.1101/2020.01.22.914952. [DOI] [Google Scholar]
- 39.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang T., Wu Q., Zhang Z. Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Current Biology. 2020;30:1346–1351.e2. doi: 10.1016/j.cub.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z., Xiao X., Wei X., Li J., Yang J., Tan H., Zhu J., Zhang Q., Wu J., Liu L. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS-CoV-2. J. Med. Virol. 2020 doi: 10.1002/jmv.25726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang J.-M., Jan S.S., Wei X., Wan Y., Ouyang S. Evidence of the recombinant origin and ongoing mutations in severe acute respiratory syndrome 2 (SARS-COV-2) bioRxiv. 2020 doi: 10.1101/2020.03.16.993816. [DOI] [Google Scholar]
- 44.Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.-J., Li N., Guo Y., Li X., Shen X., Zhang Z., Shu F., Huang W., Li Y., Zhang Z., Chen R.-A., Wu Y.-J., Peng S.-M., Huang M., Xie W.-J., Cai Q.-H., Hou F.-H., Liu Y., Chen W., Xiao L., Shen Y. Isolation and characterization of 2019-nCoV-like coronavirus from Malayan pangolins. bioRxiv. 2020 doi: 10.1101/2020.02.17.951335. [DOI] [Google Scholar]
- 45.Lau S.K.P., Luk H.K.H., Wong A.C.P., Li K.S.M., Zhu L., He Z., Fung J., Chan T.T.Y., Fung K.S.C., Woo P.C.Y. Early release - possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases Journal. July 2020;26(7) doi: 10.3201/eid2607.200092. (CDC, n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jameslyonsweiler.com; 2020. Moderately strong confirmation of a laboratory origin of 2019-nCoV. https://jameslyonsweiler.com/2020/02/02/moderately-strong-confirmation-of-a-laboratory-origin-of-2019-ncov/
- 47.Hao P., Zhong W., Song S., Fan S., Li X. Is SARS-CoV-2 originated from laboratory? A rebuttal to the claim of formation via laboratory recombination. Emerg Microbes Infect. 2020;9:545–547. doi: 10.1080/22221751.2020.1738279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations (n.d.)
- 50.Hindson J. COVID-19: faecal–oral transmission? Nat. Rev. Gastroenterol. Hepatol. 2020:1. doi: 10.1038/s41575-020-0295-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., Liao J., Yang H., Hou W., Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.C. Fan, D. Lei, C. Fang, C. Li, M. Wang, Y. Liu, Y. Bao, Y. Sun, J. Huang, Y. Guo, Y. Yu, S. Wang, Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry?, Clin Infect Dis. (n.d.). doi: 10.1093/cid/ciaa226. [DOI] [PMC free article] [PubMed]
- 53.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22:69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W., Lou Y., Gao D., Yang L., He D., Wang M.H. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanche S., Lin Y.T., Xu C., Romero-Severson E., Hengartner N., Ke R. Early release - high contagiousness and rapid spread of severe acute respiratory syndrome coronavirus 2. Emerging Infectious Diseases Journal. July 2020;26(7) doi: 10.3201/eid2607.200282. (CDC, n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J., Li F. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.02.29.20029520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mirtcheva R.M., Vazquez M., Yankelevitz D.F., Henschke C.I. Bronchioloalveolar carcinoma and adenocarcinoma with bronchioloalveolar features presenting as ground-glass opacities on CT. Clin. Imaging. 2002;26:95–100. doi: 10.1016/s0899-7071(01)00372-2. [DOI] [PubMed] [Google Scholar]
- 59.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109:102433. doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lauer S.A., Grantz K.H., Bi Q., Jones F.K., Zheng Q., Meredith H.R., Azman A.S., Reich N.G., Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann. Intern. Med. 2020 doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang X., Chen H., Yu H., Zhang X., Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130 doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pedersen S.F., Ho Y.-C. SARS-CoV-2: a storm is raging. J. Clin. Invest. 2020 doi: 10.1172/JCI137647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Wu Y., Chen Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) medRxiv. 2020 doi: 10.1101/2020.02.18.20024364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: observations and hypotheses. The Lancet. 2020;0 doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.G.-Q. Qian, N.-B. Yang, F. Ding, A.H.Y. Ma, Z.-Y. Wang, Y.-F. Shen, C.-W. Shi, X. Lian, J.-G. Chu, L. Chen, Z.-Y. Wang, D.-W. Ren, G.-X. Li, X.-Q. Chen, H.-J. Shen, X.-M. Chen, Epidemiologic and clinical characteristics of 91 hospitalized patients with COVID-19 in Zhejiang, China: a retrospective, multi-centre case series, QJM. (n.d.). doi: 10.1093/qjmed/hcaa089. [DOI] [PMC free article] [PubMed]
- 69.Liu B., Li M., Zhou Z., Guan X., Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J. Autoimmun. 2020;102452 doi: 10.1016/j.jaut.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chihrin S., Loutfy M.R. Overview of antiviral and anti-inflammatory treatment for severe acute respiratory syndrome. Expert Rev. Anti-Infect. Ther. 2005;3:251–262. doi: 10.1586/14787210.3.2.251. [DOI] [PubMed] [Google Scholar]
- 71.Advice for public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public (n.d.)
- 72.Xu H., Zhong L., Deng J., Peng J., Dan H., Zeng X., Li T., Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science. 2020;12:1–5. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anguiano L., Riera M., Pascual J., Valdivielso J.M., Barrios C., Betriu A., Mojal S., Fernández E., Soler M.J., Castro E., María V., Molí T., Soria M., Regidor A., José M., Jaume A., Esther P., Coloma A., Rubio B., Rodríguez B., Sara B.-G., Jordi B.S., Josep B.A., Romero C., Juan B., Salomé M.C., Jesús C.V., Pilar C.A., Jordi C.B., Aleix C.A., Elisabet M.J., Jesús C.P., Secundino C.G., Saray L.P., Lourdes C.M., Isabel C., Teresa C.J. Ma, Marta C.I., de Fernando Á., Covadonga H.O., de la F. Gabriel A., del P. y P. Ma Dolores, Rafael D.-T.I., Marta D., Verónica D., Sara E.T., José F.R. Ma, Loreto F.R. Ma, Guillermina F., Antonio G.S., Cesar G.C., Herrera G., Antonio L., Mercedes G.M., Luis G.S., Maria A., Luis G.J., Emma H.L., Luis L.J., Antonio L.C., Álvarez M., Pedro J., Nàdia M.A., Jesús M.G., Alberto M.C., María M.V., Isabel M., Iñigo M.E., Silvia M.L.H., Ricardo M.M., Antonia M.V., Beatriz M.D. Ana, González N., Juan F., Javier N., Agustín C., Enrique N.F., Alberto O., Beatriz F., Vicente P., Miguel P.F., Ana P.D., Celestino P.H., Dolores P.G. Ma, Mario P.V., Carmina P.M., Maite R.G., Esther R., Pilar R., Mercedes S.L., Puerto M., Isabel A., Tomero S., Antonio J., Emilio S.J., Ramon S.L., Ramon S., Maria S., Oreto P., Fernando S., Daniel T., Fernando T.M., Carrasco U., Javier J., Ildefonso V.C., del P. Ma Merce V., Ruiz V., Rafael C. Circulating angiotensin-converting enzyme 2 activity in patients with chronic kidney disease without previous history of cardiovascular disease. Nephrol Dial Transplant. 2015;30:1176–1185. doi: 10.1093/ndt/gfv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hristova M., Stanilova S., Miteva L. Serum concentration of renin-angiotensin system components in association with ACE I/D polymorphism among hypertensive subjects in response to ACE inhibitor therapy. Clin. Exp. Hypertens. 2019;41:662–669. doi: 10.1080/10641963.2018.1529782. [DOI] [PubMed] [Google Scholar]
- 75.Li G., Hu R., Zhang X. Antihypertensive treatment with ACEI/ARB of patients with COVID-19 complicated by hypertension. Hypertens. Res. 2020:1–3. doi: 10.1038/s41440-020-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fang L., Karakiulakis G., Roth M. Antihypertensive drugs and risk of COVID-19? – authors' reply. The Lancet Respiratory Medicine. 2020;0 doi: 10.1016/S2213-2600(20)30159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. New England Journal of Medicine. 2020;0 doi: 10.1056/NEJMsr2005760. (null) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang L., Sexton D.J., Skogerson K., Devlin M., Smith R., Sanyal I., Parry T., Kent R., Enright J., Wu Q., Conley G., DeOliveira D., Morganelli L., Ducar M., Wescott C.R., Ladner R.C. Novel peptide inhibitors of angiotensin-converting enzyme 2. J. Biol. Chem. 2003;278:15532–15540. doi: 10.1074/jbc.M212934200. [DOI] [PubMed] [Google Scholar]
- 79.News-Medical.net; 2020. Experimental drug APN01 prevents COVID-19 infection in the lab. https://www.news-medical.net/news/20200406/Experimental-drug-APN01-prevents-COVID-19-infection-in-the-lab.aspx
- 80.Goel P., Gerriets V. StatPearls. StatPearls Publishing; Treasure Island (FL): 2020. Chloroquine.http://www.ncbi.nlm.nih.gov/books/NBK551512/ [Google Scholar]
- 81.Knight R. The chemotherapy of amoebiasis. J. Antimicrob. Chemother. 1980;6:577–593. doi: 10.1093/jac/6.5.577. [DOI] [PubMed] [Google Scholar]
- 82.Freedman A., Steinberg V.L. Chloroquine in rheumatoid arthritis. Ann. Rheum. Dis. 1960;19:243–250. doi: 10.1136/ard.19.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6:1–4. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coronado L.M., Nadovich C.T., Spadafora C. Malarial hemozoin: from target to tool. Biochim. Biophys. Acta. 2014;1840:2032–2041. doi: 10.1016/j.bbagen.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fredericksen B.L., Wei B.L., Yao J., Luo T., Garcia J.V. Inhibition of endosomal/lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu T.Y., Frieman M., Wolfram J. Insights from nanomedicine into chloroquine efficacy against COVID-19. Nat. Nanotechnol. 2020:1–3. doi: 10.1038/s41565-020-0674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.e Braga C.B., Martins A.C., Cayotopa A.D.E., Klein W.W., Schlosser A.R., da Silva A.F., de Souza M.N., Andrade B.W.B., Filgueira-Júnior J.A., de J. Pinto W., da Silva-Nunes M. Side effects of chloroquine and primaquine and symptom reduction in malaria endemic area (Mâncio Lima, Acre, Brazil) Interdiscip Perspect Infect Dis. 2015;2015 doi: 10.1155/2015/346853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chou S., Marousek G., Auerochs S., Stamminger T., Milbradt J., Marschall M. The unique antiviral activity of artesunate is broadly effective against human cytomegaloviruses including therapy-resistant mutants. Antivir. Res. 2011;92:364–368. doi: 10.1016/j.antiviral.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 91.Oiknine-Djian E., Weisblum Y., Panet A., Wong H.N., Haynes R.K., Wolf D.G. The artemisinin derivative artemisone is a potent inhibitor of human cytomegalovirus replication. Antimicrobial Agents and Chemotherapy. 2018;62 doi: 10.1128/AAC.00288-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parnham M.J., Erakovic Haber V., Giamarellos-Bourboulis E.J., Perletti G., Verleden G.M., Vos R. Azithromycin: mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014;143:225–245. doi: 10.1016/j.pharmthera.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Gielen V., Johnston S.L., Edwards M.R. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur. Respir. J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 94.Iannetta M., Ippolito G., Nicastri E. Azithromycin shows anti-zika virus activity in human glial cells. Antimicrobial Agents and Chemotherapy. 2017;61 doi: 10.1128/AAC.01152-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Randhawa P.S. Anti-BK virus activity of ciprofloxacin and related antibiotics. Clin. Infect. Dis. 2005;41:1366–1367. doi: 10.1086/497080. (author reply 1367) [DOI] [PubMed] [Google Scholar]
- 97.Gopinath S., Kim M.V., Rakib T., Wong P.W., van Zandt M., Barry N.A., Kaisho T., Goodman A.L., Iwasaki A. Topical application of aminoglycoside antibiotics enhances host resistance to viral infections in a microbiota-independent manner. Nat. Microbiol. 2018;3:611–621. doi: 10.1038/s41564-018-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamagishi Y., Ueno M., Ueno C., Kato A., Kanasaki R., Sato B., Takakura K., Fujie A., Nakajima H., Fujii T., Hino M., Tsujii E. Anti-herpes virus activity of polyether antibiotic CP-44161 in vivo. The Journal of Antibiotics. 2009;62:95–98. doi: 10.1038/ja.2008.18. [DOI] [PubMed] [Google Scholar]
- 99.Grunicke H., Puschendorf B., Werchau H. Reviews of Physiology, Biochemistry and Pharmacology. vol. 75. Springer Berlin Heidelberg; Berlin, Heidelberg: 1976. Mechanism of action of distamycin A and other antibiotics with antiviral activity; pp. 69–96. [DOI] [PubMed] [Google Scholar]
- 100.Groupé V., Frankel J.W., Lechevalier M.P., Waksman S.A. Antiviral properties of ehrlichin, an antibiotic produced by Streptomyces lavendulae. J. Immunol. 1951;67:471–482. [PubMed] [Google Scholar]
- 101.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moshkowitz A., Goldblum N., Heller E. Studies on the antiviral effect of rifampicin in volunteers. Nature. 1971;229:422–424. doi: 10.1038/229422a0. [DOI] [PubMed] [Google Scholar]
- 103.Rothan H.A., Bahrani H., Mohamed Z., Teoh T.C., Shankar E.M., Rahman N.A., Yusof R. A combination of doxycycline and ribavirin alleviated chikungunya infection. PLoS One. 2015;10 doi: 10.1371/journal.pone.0126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu J., Shi P.-Y., Li H., Zhou J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis. 2020 doi: 10.1021/acsinfecdis.0c00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeon S., Ko M., Lee J., Choi I., Byun S.Y., Park S., Shum D., Kim S. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. bioRxiv. 2020 doi: 10.1101/2020.03.20.999730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.González Canga A., Sahagún Prieto A.M., Diez Liébana M.J., Fernández Martínez N., Sierra Vega M., García Vieitez J.J. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 2008;10:42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antivir. Res. 2020;104787 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hundreds of virus patients allowed to try Gilead's Ebola drug. https://news.bloomberglaw.com/pharma-and-life-sciences/hundreds-of-corona-patients-allowed-to-try-gileads-ebola-drug (n.d.)
- 109.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020 doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moscona A. Neuraminidase inhibitors for influenza. N. Engl. J. Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 112.Zhang L., Lin D., Sun X., Curth U., Drosten C., Sauerhering L., Becker S., Rox K., Hilgenfeld R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. 2020;368:409–412. doi: 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Y.W., Yiu C.-P., Wong K.-Y. 2020. Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like Protease (3CLpro) Structure: Virtual Screening Reveals Velpatasvir, Ledipasvir, and Other Drug Repurposing Candidates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alméciga-Díaz C.J., Pimentel-Vera L.N., Caro A., Mosquera A., Moreno C.A.C., Rojas J.P.M., Díaz-Tribaldos D.C. 2020. Virtual Screening of Potential Inhibitors for SARS-CoV-2 Main Protease. [DOI] [Google Scholar]
- 115.Ettayapuram Ramaprasad A.S., La Merrill M., Durkin K.A., Smith M.T. 2020. Structure-based Virtual Screening of a Natural Product Database to Identify Several Possible SARS-CoV-2 Main Protease Inhibitors. [DOI] [Google Scholar]
- 116.Khan S.A., Zia K., Ashraf S., Uddin R., Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J. Biomol. Struct. Dyn. 2020;0:1–10. doi: 10.1080/07391102.2020.1751298. [DOI] [PubMed] [Google Scholar]
- 117.Goswami D., Kumar M., Ghosh S.K., Das A. 2020. Natural Product Compounds in Alpinia officinarum and Ginger Are Potent SARS-CoV-2 Papain-like Protease Inhibitors. [DOI] [Google Scholar]
- 118.Arya R., Das A., Prashar V., Kumar M. 2020. Potential Inhibitors Against Papain-like Protease of Novel Coronavirus (SARS-CoV-2) From FDA Approved Drugs. [DOI] [Google Scholar]
- 119.Bagherzadeh K., Daneshvarnejad K., Abbasinazari M., Azizian H. 2020. In Silico Repositioning for Dual Inhibitor Discovery of SARS-CoV-2 (COVID-19) 3C-like Protease and Papain-like Peptidase. [DOI] [Google Scholar]
- 120.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chu C.M., Cheng V.C.C., Hung I.F.N., Wong M.M.L., Chan K.H., Chan K.S., Kao R.Y.T., Poon L.L.M., Wong C.L.P., Guan Y., Peiris J.S.M., Yuen K.Y., HKU/UCH SARS Study Group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neil M.D., Ampel M. Lopinavir-ritonavir was not effective for COVID-19. NEJM Journal Watch. 2020;2020 doi: 10.1056/nejm-jw.NA51172. [DOI] [Google Scholar]
- 123.Koren G., King S., Knowles S., Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ. 2003;168:1289–1292. [PMC free article] [PubMed] [Google Scholar]
- 124.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020;248:117477. doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bourlière M., Pietri O., Castellani P., Oules V., Adhoute X. Sofosbuvir, velpatasvir and voxilaprevir: a new triple combination for hepatitis C virus treatment. One pill fits all? Is it the end of the road? Therap Adv Gastroenterol. 2018;11 doi: 10.1177/1756284818812358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fung A., Jin Z., Dyatkina N., Wang G., Beigelman L., Deval J. Efficiency of incorporation and chain termination determines the inhibition potency of 2′-modified nucleotide analogs against hepatitis C virus polymerase. Antimicrob. Agents Chemother. 2014;58:3636–3645. doi: 10.1128/AAC.02666-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bhatia H.K., Singh H., Grewal N., Natt N.K. Sofosbuvir: a novel treatment option for chronic hepatitis C infection. J. Pharmacol. Pharmacother. 2014;5:278–284. doi: 10.4103/0976-500X.142464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yamamoto M., Matsuyama S., Li X., Takeda M., Kawaguchi Y., Inoue J., Matsuda Z. Identification of nafamostat as a potent inhibitor of Middle East respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob. Agents Chemother. 2016;60:6532–6539. doi: 10.1128/AAC.01043-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Drug Target Review; D.T.T. says, Nafamostat can inhibit SARS-CoV-2 infection. https://www.drugtargetreview.com/news/58915/nafamostat-inhibits-sars-cov-2-infection-preventing-covid-19-transmission/ (n.d.)
- 130.Melian E.B., Plosker G.L. Interferon alfacon-1: a review of its pharmacology and therapeutic efficacy in the treatment of chronic hepatitis C. Drugs. 2001;61:1661–1691. doi: 10.2165/00003495-200161110-00009. [DOI] [PubMed] [Google Scholar]
- 131.Antibiotics Manual. John Wiley & Sons, Ltd; 2017. Intron A (interferon alpha) infergen (interferon alfacon-1) injection intron A (interferon alfa-2a) injection; pp. 198–200. [DOI] [Google Scholar]
- 132.Paragas J., Blatt L.M., Hartmann C., Huggins J.W., Endy T.P. Interferon alfacon1 is an inhibitor of SARS-corona virus in cell-based models. Antivir. Res. 2005;66:99–102. doi: 10.1016/j.antiviral.2005.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Loutfy M.R., Blatt L.M., Siminovitch K.A., Ward S., Wolff B., Lho H., Pham D.H., Deif H., LaMere E.A., Chang M., Kain K.C., Farcas G.A., Ferguson P., Latchford M., Levy G., Dennis J.W., Lai E.K.Y., Fish E.N. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 134.Zorzitto J., Galligan C.L., Ueng J.J., Fish E.N. Characterization of the antiviral effects of interferon-α against a SARS-like coronoavirus infection in vitro. Cell Res. 2006;16:220–229. doi: 10.1038/sj.cr.7310030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuri T., Zhang X., Habjan M., Martínez-Sobrido L., García-Sastre A., Yuan Z., Weber F. Interferon priming enables cells to partially overturn the SARS coronavirus-induced block in innate immune activation. J Gen Virol. 2009;90:2686–2694. doi: 10.1099/vir.0.013599-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hensley L.E., Fritz E.A., Jahrling P.B., Karp C., Huggins J.W., Geisbert T.W. Interferon-β 1a and SARS coronavirus replication. Emerg. Infect. Dis. 2004;10:317–319. doi: 10.3201/eid1002.030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dziadosz M., Basch R.S., Young B.K. Human amniotic fluid: a source of stem cells for possible therapeutic use. Am. J. Obstet. Gynecol. 2016;214:321–327. doi: 10.1016/j.ajog.2015.12.061. [DOI] [PubMed] [Google Scholar]
- 138.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Y., Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., Lu L., Jiang S., Yang Z., Wu Y., Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhu Z., Chakraborti S., He Y., Roberts A., Sheahan T., Xiao X., Hensley L.E., Prabakaran P., Rockx B., Sidorov I.A., Corti D., Vogel L., Feng Y., Kim J.-O., Wang L.-F., Baric R., Lanzavecchia A., Curtis K.M., Nabel G.J., Subbarao K., Jiang S., Dimitrov D.S. Potent cross-reactive neutralization of SARS coronavirus isolates by human monoclonal antibodies. PNAS. 2007;104:12123–12128. doi: 10.1073/pnas.0701000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang C., Li W., Drabek D., Okba N.M.A., van Haperen R., Osterhaus A.D.M.E., van Kuppeveld F.J.M., Haagmans B.L., Grosveld F., Bosch B.-J. A human monoclonal antibody blocking SARS-CoV-2 infection. bioRxiv. 2020 doi: 10.1101/2020.03.11.987958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Roberts A., Thomas W.D., Guarner J., Lamirande E.W., Babcock G.J., Greenough T.C., Vogel L., Hayes N., Sullivan J.L., Zaki S., Subbarao K., Ambrosino D.M. Therapy with a severe acute respiratory syndrome–associated coronavirus–neutralizing human monoclonal antibody reduces disease severity and viral burden in golden Syrian hamsters. J. Infect. Dis. 2006;193:685–692. doi: 10.1086/500143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38:10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 144.Scott L.J. Tocilizumab: a review in rheumatoid arthritis. Drugs. 2017;77:1865–1879. doi: 10.1007/s40265-017-0829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jones G., Ding C. Tocilizumab: a review of its safety and efficacy in rheumatoid arthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2010;3:81–89. doi: 10.4137/CMAMD.S4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pelechas E., Voulgari P.V., Drosos A.A. Clinical evaluation of the safety, efficacy and tolerability of sarilumab in the treatment of moderate to severe rheumatoid arthritis. Ther. Clin. Risk Manag. 2019;15:1073–1079. doi: 10.2147/TCRM.S167452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Drug Discovery from Technology Networks; Remdesivir and sarilumab – COVID-19 clinical trials begin. https://www.technologynetworks.com/drug-discovery/blog/remdesivir-and-sarilumab-covid-19-clinical-trials-begin-332627 (n.d.)
- 148.Sordillo P.P., Helson L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo. 2015;29:1–4. [PubMed] [Google Scholar]
- 149.Wen C.-C., Kuo Y.-H., Jan J.-T., Liang P.-H., Wang S.-Y., Liu H.-G., Lee C.-K., Chang S.-T., Kuo C.-J., Lee S.-S., Hou C.-C., Hsiao P.-W., Chien S.-C., Shyur L.-F., Yang N.-S. Specific plant terpenoids and lignoids possess potent antiviral activities against severe acute respiratory syndrome coronavirus. J. Med. Chem. 2007;50:4087–4095. doi: 10.1021/jm070295s. [DOI] [PubMed] [Google Scholar]
- 150.Tiwari V., Darmani N.A., Yue B.Y.J.T., Shukla D. In vitro antiviral activity of neem (Azardirachta indica L.) bark extract against herpes simplex virus type-1 infection. Phytother Res. 2010;24:1132–1140. doi: 10.1002/ptr.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Elumalai P., Gunadharini D.N., Senthilkumar K., Banudevi S., Arunkumar R., Benson C.S., Sharmila G., Arunakaran J. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol. Lett. 2012;215:131–142. doi: 10.1016/j.toxlet.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 152.Pooladanda V., Thatikonda S., Bale S., Pattnaik B., Sigalapalli D.K., Bathini N.B., Singh S.B., Godugu C. Nimbolide protects against endotoxin-induced acute respiratory distress syndrome by inhibiting TNF-α mediated NF-κB and HDAC-3 nuclear translocation. Cell Death Dis. 2019;10:81. doi: 10.1038/s41419-018-1247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grover A., Agrawal V., Shandilya A., Bisaria V.S., Sundar D. Non-nucleosidic inhibition of Herpes simplex virus DNA polymerase: mechanistic insights into the anti-herpetic mode of action of herbal drug withaferin A. BMC Bioinformatics. 2011;12:S22. doi: 10.1186/1471-2105-12-S13-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rajagopal S., Kumar R.A., Deevi D.S., Satyanarayana C., Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J. Exp. Ther. Oncol. 2003;3:147–158. doi: 10.1046/j.1359-4117.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- 155.Gupta S., Mishra K.P., Ganju L. Broad-spectrum antiviral properties of andrographolide. Arch. Virol. 2017;162:611–623. doi: 10.1007/s00705-016-3166-3. [DOI] [PubMed] [Google Scholar]
- 156.Jeong J.-H., An J.Y., Kwon Y.T., Rhee J.G., Lee Y.J. Effects of low dose quercetin: cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009;106:73–82. doi: 10.1002/jcb.21977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zandi K., Teoh B.-T., Sam S.-S., Wong P.-F., Mustafa M.R., AbuBakar S. Antiviral activity of four types of bioflavonoid against dengue virus type-2. Virol. J. 2011;8:560. doi: 10.1186/1743-422X-8-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78:11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cheng P.-W., Ng L.-T., Chiang L.-C., Lin C.-C. Antiviral effects of saikosaponins on human coronavirus 229e in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33:612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Goswami T., Bagchi B. 2020. Molecular Docking Study of Receptor Binding Domain of SARS-CoV-2 Spike Glycoprotein With Saikosaponin, a Triterpenoid Natural Product. [DOI] [Google Scholar]
- 161.Pullar J.M., Carr A.C., Vissers M.C.M. The roles of vitamin C in skin health. Nutrients. 2017;9 doi: 10.3390/nu9080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9 doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang X.-Y., Xu Z.-P., Wang W., Cao J.-B., Fu Q., Zhao W.-X., Li Y., Huo X.-L., Zhang L.-M., Li Y.-F., Mi W.-D. Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018;65:438–447. doi: 10.1016/j.intimp.2018.10.020. [DOI] [PubMed] [Google Scholar]
- 164.da Silva M.R., Schapochnik A., Leal M.P., Esteves J., Hebeda C.B., Sandri S., Pavani C., Horliana A.C.R.T., Farsky S.H.P., Lino-dos-Santos-Franco A. Beneficial effects of ascorbic acid to treat lung fibrosis induced by paraquat. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Biancatelli R.M.L.C., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev. Anti-Infect. Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 166.Uesato S., Kitagawa Y., Kaijima T., Tokuda H., Okuda M., Mou X.Y., Mukainaka T., Nishino H. Inhibitory effects of 6-O-acylated L-ascorbic acids possessing a straight- or branched-acyl chain on Epstein-Barr virus activation. Cancer Lett. 2001;166:143–146. doi: 10.1016/s0304-3835(01)00444-x. [DOI] [PubMed] [Google Scholar]
- 167.Hemilä H. Vitamin C, respiratory infections and the immune system. Trends Immunol. 2003;24:579–580. doi: 10.1016/j.it.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin L.-T., Hsu W.-C., Lin C.-C. Antiviral natural products and herbal medicines. J. Tradit. Complement. Med. 2014;4:24–35. doi: 10.4103/2225-4110.124335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Weber N.D., Andersen D.O., North J.A., Murray B.K., Lawson L.D., Hughes B.G. In vitro virucidal effects of Allium sativum (garlic) extract and compounds. Planta Med. 1992;58:417–423. doi: 10.1055/s-2006-961504. [DOI] [PubMed] [Google Scholar]
- 170.Aboubakr H.A., Nauertz A., Luong N.T., Agrawal S., El-Sohaimy S.A.A., Youssef M.M., Goyal S.M. In vitro antiviral activity of clove and ginger aqueous extracts against feline calicivirus, a surrogate for human norovirus. J. Food Prot. 2016;79:1001–1012. doi: 10.4315/0362-028X.JFP-15-593. [DOI] [PubMed] [Google Scholar]
- 171.Fatima M., Zaidi N.-U.-S.S., Amraiz D., Afzal F. In vitro antiviral activity of Cinnamomum cassia and its nanoparticles against H7N3 influenza a virus. J. Microbiol. Biotechnol. 2016;26:151–159. doi: 10.4014/jmb.1508.08024. [DOI] [PubMed] [Google Scholar]
- 172.Ghoke S.S., Sood R., Kumar N., Pateriya A.K., Bhatia S., Mishra A., Dixit R., Singh V.K., Desai D.N., Kulkarni D.D., Dimri U., Singh V.P. Evaluation of antiviral activity of Ocimum sanctum and Acacia arabica leaves extracts against H9N2 virus using embryonated chicken egg model. BMC Complement. Altern. Med. 2018;18:174. doi: 10.1186/s12906-018-2238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wu C.-Y., Jan J.-T., Ma S.-H., Kuo C.-J., Juan H.-F., Cheng Y.-S.E., Hsu H.-H., Huang H.-C., Wu D., Brik A., Liang F.-S., Liu R.-S., Fang J.-M., Chen S.-T., Liang P.-H., Wong C.-H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen C.-N., Lin C.P.C., Huang K.-K., Chen W.-C., Hsieh H.-P., Liang P.-H., Hsu J.T.-A. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3,3′-digallate (TF3) Evid. Based Complement. Alternat. Med. 2005;2:209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin S.-C., Ho C.-T., Chuo W.-H., Li S., Wang T.T., Lin C.-C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect Dis. 2017;17 doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee J.-B., Miyake S., Umetsu R., Hayashi K., Chijimatsu T., Hayashi T. Anti-influenza A virus effects of fructan from Welsh onion (Allium fistulosum L.) Food Chem. 2012;134:2164–2168. doi: 10.1016/j.foodchem.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yamshchikov A.V., Desai N.S., Blumberg H.M., Ziegler T.R., Tangpricha V. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr. Pract. 2009;15:438–449. doi: 10.4158/EP09101.ORR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.te Velthuis A.J.W., van den Worm S.H.E., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zandi K., Teoh B.-T., Sam S.-S., Wong P.-F., Mustafa M.R., AbuBakar S. Novel antiviral activity of baicalein against dengue virus. BMC Complement. Altern. Med. 2012;12:214. doi: 10.1186/1472-6882-12-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Choi H.J., Lim C.H., Song J.H., Baek S.H., Kwon D.H. Antiviral activity of raoulic acid from Raoulia australis against Picornaviruses. Phytomedicine. 2009;16:35–39. doi: 10.1016/j.phymed.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 182.Haid S., Novodomská A., Gentzsch J., Grethe C., Geuenich S., Bankwitz D., Chhatwal P., Jannack B., Hennebelle T., Bailleul F., Keppler O.T., Poenisch M., Bartenschlager R., Hernandez C., Lemasson M., Rosenberg A.R., Wong-Staal F., Davioud-Charvet E., Pietschmann T. A plant-derived flavonoid inhibits entry of all HCV genotypes into human hepatocytes. Gastroenterology. 2012;143:213–222.e5. doi: 10.1053/j.gastro.2012.03.036. [DOI] [PubMed] [Google Scholar]
- 183.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clinical Microbiology and Infection. 2020 doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mo Y., Fisher D. A review of treatment modalities for Middle East Respiratory Syndrome. J. Antimicrob. Chemother. 2016;71:3340–3350. doi: 10.1093/jac/dkw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sanchez-Pernaute O., Romero-Bueno F.I., O'Callaghan A.S. Why choose cyclosporin A as first-line therapy in COVID-19 pneumonia. Reumatol Clin. 2020 doi: 10.1016/j.reuma.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Omrani A.S., Saad M.M., Baig K., Bahloul A., Abdul-Matin M., Alaidaroos A.Y., Almakhlafi G.A., Albarrak M.M., Memish Z.A., Albarrak A.M. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect. Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dyall J., Coleman C.M., Hart B.J., Venkataraman T., Holbrook M.R., Kindrachuk J., Johnson R.F., Olinger G.G., Jahrling P.B., Laidlaw M., Johansen L.M., Lear-Rooney C.M., Glass P.J., Hensley L.E., Frieman M.B. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Adedeji A.O., Singh K., Kassim A., Coleman C.M., Elliott R., Weiss S.R., Frieman M.B., Sarafianos S.G. Evaluation of SSYA10-001 as a replication inhibitor of severe acute respiratory syndrome, mouse hepatitis, and Middle East respiratory syndrome coronaviruses. Antimicrob. Agents Chemother. 2014;58:4894–4898. doi: 10.1128/AAC.02994-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dyall J., Gross R., Kindrachuk J., Johnson R.F., Olinger G.G., Hensley L.E., Frieman M.B., Jahrling P.B. Middle East respiratory syndrome and severe acute respiratory syndrome: current therapeutic options and potential targets for novel therapies. Drugs. 2017;77:1935–1966. doi: 10.1007/s40265-017-0830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Novavax Inc. - IR Site; Novavax advances development of novel COVID-19 vaccine. https://ir.novavax.com/news-releases/news-release-details/novavax-advances-development-novel-covid-19-vaccine (n.d.)
- 191.Spinney L. The Guardian; 2020. Coronavirus vaccine: when will it be ready? https://www.theguardian.com/world/2020/mar/28/coronavirus-vaccine-when-will-it-be-ready
- 192.Moderna charts fast track of SARS-CoV-2 vaccine to clinical trials, clinical OMICs - molecular diagnostics in precision medicine. 2020. https://www.clinicalomics.com/topics/patient-care/moderna-charts-fast-track-of-sars-cov-2-vaccine-to-clinical-trials/
- 193.ServickMar K. AAAS; 2020. 25, 2020, 12:20 Pm, Meet the company that has just begun testing a coronavirus vaccine in the United States, Science. https://www.sciencemag.org/news/2017/02/mysterious-2-billion-biotech-revealing-secrets-behind-its-new-drugs-and-vaccines
- 194.Safety and immunogenicity study of 2019-nCoV vaccine (mRNA-1273) to prevent SARS-CoV-2 infection - full text view - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04283461 (n.d.)
- 195.Clinical Trials Arena; 2020. BioNTech and Pfizer get German nod for Covid-19 vaccine trial. https://www.clinicaltrialsarena.com/news/biontech-pfizer-covid-19-vaccine-trial/
- 196.Function and safety study of SARS-CoV-2 synthetic minigene vaccines. https://www.smartpatients.com/trials/NCT04276896 (n.d.)
- 197.Ahmedabad Mirror; I.| U. Mar 28, 2020, 18:56 Ist, COVID-19 vaccine trial: Oxford University to recruit 500 volunteers. https://ahmedabadmirror.indiatimes.com/news/world/covid-19-vaccine-trial-oxford-university-to-recruit-500-volunteers/articleshow/74864262.cms (n.d.)
- 198.Phillips R.O., Phanzu D.M., Beissner M., Badziklou K., Luzolo E.K., Sarfo F.S., Halatoko W.A., Amoako Y., Frimpong M., Kabiru A.M., Piten E., Maman I., Bidjada B., Koba A., Awoussi K.S., Kobara B., Nitschke J., Wiedemann F.X., Kere A.B., Adjei O., Löscher T., Fleischer B., Bretzel G., Herbinger K.-H. Effectiveness of routine BCG vaccination on buruli ulcer disease: a case-control study in the Democratic Republic of Congo, Ghana and Togo. PLoS Negl Trop Dis. 2015;9:e3457. doi: 10.1371/journal.pntd.0003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced morbidity and mortality for COVID-19: an epidemiological study. medRxiv. 2020 doi: 10.1101/2020.03.24.20042937. [DOI] [Google Scholar]
- 200.ScienceAlert; AFP, Australia's trialing a TB vaccine against COVID-19, and health workers get it first. https://www.sciencealert.com/australia-is-trialling-a-tb-vaccine-for-coronavirus-and-health-workers-get-it-first (n.d.)
- 201.Coronavirus vaccine: Zydus, Serum Institute among 43 global firms in race. https://www.businesstoday.in/bt-buzz/news/coronavirus-india-zydus-cadila-serum-institute-among-43-global-firms-working-on-covid-19-vaccine/story/399546.html (n.d.)
- 202.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zikuan Leng R.Z., Zikuan Leng R.Z. Transplantation of ACE2− mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging and Disease. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & Molecular Immunology. 2020:1–3. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu D., Tian S., Zhang K., Xiong W., Lubaki N.M., Chen Z., Han W. Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV. Protein Cell. 2017;8:861–877. doi: 10.1007/s13238-017-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Guo Y., Luan L., Patil N.K., Sherwood E.R. Immunobiology of the IL-15-IL-15Rα complex as an antitumor and antiviral agent. Cytokine Growth Factor Rev. 2017;38:10–21. doi: 10.1016/j.cytogfr.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sterner R.M., Sakemura R., Cox M.J., Yang N., Khadka R.H., Forsman C.L., Hansen M.J., Jin F., Ayasoufi K., Hefazi M., Schick K.J., Walters D.K., Ahmed O., Chappell D., Sahmoud T., Durrant C., Nevala W.K., Patnaik M.M., Pease L.R., Hedin K.E., Kay N.E., Johnson A.J., Kenderian S.S. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood. 2019;133:697–709. doi: 10.1182/blood-2018-10-881722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fung S.-Y., Yuen K.-S., Ye Z.-W., Chan C.-P., Jin D.-Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chung J.S., Ling M.L., Seto W.H., Ang B.S.P., Tambyah P.A. Debate on MERS-CoV respiratory precautions: surgical mask or N95 respirators? Singapore Med J. 2014;55:294–297. doi: 10.11622/smedj.2014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.WHO Director-General's opening remarks at the media briefing on COVID-19 - 16 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---16-march-2020 (n.d.)