Abstract
Background
In December 2019, a novel coronavirus-associated pneumonia, now known as coronavirus disease 2019 (COVID-19), was first detected in Wuhan, China. To prevent the rapid spread of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and treat patients with mild symptoms, sports stadiums and convention centers were reconstructed into mobile hospitals.
Research Question
It is unknown whether a mobile cabin hospital can provide a safe treatment site for patients with mild COVID-19 symptoms.
Study Design and Methods
This study retrospectively reviewed the medical records of 421 patients with COVID-19 admitted to a mobile cabin hospital in Wuhan from February 9, 2020, to March 5, 2020. Clinical data comprised patient age, sex, clinical presentation, chest imaging, nucleic acid testing, length of hospitalization, and outcomes.
Results
Of the patients who were discharged from the cabin hospital, 362 (86.0%) were categorized as recovered; 14.0% developed severe symptoms and were transferred to a designated hospital. The most common presenting symptoms were fever (60.6%) and cough (52.0%); 5.2% exhibited no obvious symptoms. High fever (> 39.0°C) was more common in severe cases than in recovered cases (18.6% vs 6.6%). The distribution of lung lesions was peripheral in 85.0% of patients, multifocal in 69.4%, and bilateral in 68.2%. The most common pattern was ground-glass opacity (67.7%), followed by patchy shadowing (49.2%). The incidence of patchy shadowing was higher in patients with severe disease (66.1%) than in those who recovered (31.8%, P < .0001). The median length of hospitalization was 17 days (interquartile range, 14-19 days), and the median time taken for positive real-time reverse transcriptase polymerase chain reaction results to become negative in recovered patients was 8 days (interquartile range, 6-10 days).
Interpretation
Mobile cabin hospitals provide a safe treatment site for patients with mild COVID-19 symptoms and offer an effective isolation area to prevent the spread of severe acute respiratory syndrome coronavirus 2.
Key Words: clinical characteristics, COVID-19, mobile cabin hospital, outcomes, SARS-CoV-2
Abbreviations: COVID-19, coronavirus disease 2019; RT-PCR, real-time reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
FOR EDITORIAL COMMENT, SEE PAGE 839
In December 2019, a novel coronavirus-associated pneumonia, now known as coronavirus disease 2019 (COVID-19), was first detected in Wuhan, Hubei Province, China.1 The pneumonia was caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a novel beta coronavirus.2, 3, 4 Since then, the number of patients infected with COVID-19 has been increasing rapidly throughout China.5 As of March 24, 2020, a total of 372,757 confirmed cases had been reported globally.6 The rapid spread and person-to-person transmission of COVID-19 has been confirmed.7 , 8 In early February 2020, the construction of the first mobile cabin hospitals in Wuhan began as part of the effort to contain and treat patients with SARS-CoV-2 who had mild symptoms. Sports stadiums and convention centers were turned into mobile hospitals with a total of > 20,000 beds to compensate for the limited number of designated hospitals and enable the admission of every patient testing positive who had mild symptoms. Many of the patients with mild symptoms who were admitted to this treatment facility may be managed at home in other countries, but such an approach makes it hard to prevent familial clusters. Following the introduction of mobile hospitals, the number of daily new cases in Wuhan decreased substantially from early March 2020.9
The current descriptive study was conducted to investigate the clinical characteristics and prognosis of 421 patients with COVID-19 who were treated in Dongxihu Cabin Hospital, the largest mobile cabin hospital in Wuhan. We believe that our findings will provide helpful information for the control of the global COVID-19 pandemic.
Patients and Methods
Patient Population
The current retrospective study included 421 patients who were diagnosed with COVID-19 by using nucleic acid testing. All patients were admitted to Cabin C of Dongxihu Cabin Hospital between February 9, 2020, and March 5, 2020. Patients were only admitted if they met all of the following criteria: (1) mild or moderate symptoms; (2) normal activities of daily living; (3) no important organ dysfunction; (4) no psychiatric history; and (5) resting pulse oxygen saturation > 95%. This study was conducted in accordance with the amended Declaration of Helsinki. The ethics committee of Zhongnan Hospital of Wuhan University approved the protocol (No. 2020075K), and written informed consent was obtained from all patients. The clinical data analyzed in the present study comprised patient age, sex, clinical manifestations, CT findings, nucleic acid test results, duration of hospitalization, and outcome.
Mobile Cabin Hospital Characteristics
Mobile cabin hospitals were constructed to deal with a shortage of medical space and equipment needed to treat the COVID-19 outbreak in Wuhan, Hubei Province. One of the first mobile hospitals created was the Dongxihu Cabin Hospital at the Wuhan Parlor Convention Center in Dongxihu District, Wuhan, with a capacity of 2,000 beds. A medical team from Zhongnan Hospital of Wuhan University was dispatched to this mobile cabin hospital to care for patients who had tested positive for the virus but exhibited no severe symptoms. If a patient's symptoms worsened, he or she was transferred in a timely manner to Zhongnan Hospital of Wuhan University or Jinyintan Hospital, the city’s designated hospitals for the admission of patients with COVID-19. Daily nutritious meals were provided to patients in the mobile hospital. Medical staff in the mobile hospital provided entertainment activities with Chinese characteristics, such as square dancing and Tai Chi, to improve the patients’ physical and mental conditions. The mobile hospital was equipped with a mobile CT machine, oxygen cylinders, pulse oximeters, ED, and a mobile P3 laboratory capable of performing nucleic acid tests. All patients in the mobile hospital received mainly oral medications.
Nucleic Acid Assay and Chest CT Imaging
Throat swab samples were collected for the extraction of COVID-19 RNA from patients with suspected COVID-19. Real-time reverse transcription polymerase chain reaction (RT-PCR) assays were performed in accordance with the protocol established by the World Health Organization.10 For patients with multiple RT-PCR assays, repeated testing was performed at least 24 h following the initial test. All images were obtained by using one of two CT systems (uCT 528; Shanghai United Imaging Healthcare Co., Ltd.; ScintCare CT 16; MinFound) with the patients in the supine position. All images were reviewed and graded by a chest radiologist and a respiratory physician with > 10 years of experience each (C. Xie and W. W.). All chest CT images were analyzed independently, and final decisions were reached by consensus. CT imaging was performed on admission to the mobile hospital and when a patient’s condition changed during hospitalization. The final chest CT scan was performed after the final negative RT-PCR result.
Discharge Protocol
Patients were discharged from the mobile cabin hospital in accordance with the following conditions: (1) body temperature had been normal for > 3 days; (2) respiratory symptoms had substantially improved; (3) pulmonary imaging showed marked improvement; and (4) two consecutive nucleic acid test results of throat swab samples with a sampling interval of at least 1 day were negative. Patients were transferred to designated hospitals if they had any of the following conditions: (1) respiratory rate ≥ 30 breaths/min; (2) resting pulse oxygen saturation ≤ 93%; (3) Pao 2/Fio 2 ≤ 300 mm Hg; and (4) chest CT imaging showed lesion progression of > 50% in 24 to 48 h.
Statistical Analysis
Statistical analyses were performed by using SPSS 22.0 software (IBM SPSS Statistics, IBM Corporation). Normally distributed continuous data are presented as the mean ± SD; nonnormally distributed data are presented as the median (interquartile range). Classification data are presented as number (%). Categorical variables were analyzed by using the χ2 test or Fisher exact test. Normally distributed continuous data were analyzed by using the two-sample Student t test.
Results
Patient Demographic Characteristics
A total of 421 patients with COVID-19 were admitted to the mobile hospital from February 9, 2020, to March 5, 2020, and were included in the current study. Of these patients, 49.2% were female. The median patient age was 52 (39-61) years, 13.3% were aged > 65 years, and 99.5% were living in Wuhan. The percentage of patients with a history of contact with patients with COVID-19 was 51.3%, and 20.2% had a history of contact with patients with fever. The most common presenting symptom was fever, which was recorded in 255 patients (60.6%) on admission, followed by cough (219 patients; 52.0%). Among the 421 study patients, 5.2% showed no obvious symptoms. Comorbid conditions were present in 20.9% of all patients.
Of the patients who were discharged from the cabin hospital, 362 (86.0%) were categorized as recovered, whereas 59 (14.0%) were categorized as severe and were transferred to designated hospitals. The patients in severe cases were older than those in recovered cases by a median of 5 years. High fever (> 39.0°C), dyspnea, and diarrhea were more common in severe cases than in recovered cases (18.6% vs 6.6% [P = .002], 45.8% vs 3.9% [P < .0001], and 15.3% vs 0.6% [P < .0001], respectively). All data are shown in Table 1 .
Table 1.
Overview of Patient Characteristics
| Characteristic | All Patients (N = 421) | Recovered (n = 362) | Severe (n = 59) | P Value |
|---|---|---|---|---|
| Age, median (IQR), y | 52 (39-61) | 51 (38-60) | 56 (45-63) | … |
| Age group, y | ||||
| 0-18 | 2 (0.5%) | 2 (0.6%) | 0 | .981 |
| 19-49 | 176 (41.8%) | 158 (43.6%) | 18 (30.5%) | .058 |
| 50-64 | 187 (44.4%) | 156 (43.1%) | 31 (52.5%) | .176 |
| ≥ 65 | 56 (13.3%) | 46 (12.7%) | 10 (16.9%) | .374 |
| Sex | ||||
| Female | 207 (49.2%) | 176 (48.6%) | 31 (52.5%) | .576 |
| Male | 214 (50.8%) | 186 (51.4%) | 28 (47.5%) | .576 |
| Days from illness onset to admission | ||||
| ≤ 7 | 195 (46.3%) | 170 (47.0%) | 25 (42.4%) | .512 |
| 8-14 | 100 (23.8%) | 89 (24.6%) | 11 (18.6%) | .320 |
| ≥ 15 | 126 (29.9%) | 103 (28.5%) | 23 (39.0%) | .101 |
| Exposure history within past 14 d | ||||
| Living in Wuhan | 419 (99.5%) | 361 (99.7%) | 58 (98.3%) | .654 |
| Recently visit Wuhan | 2 (0.5%) | 1 (0.3%) | 1 (1.7%) | .654 |
| Contact with infected patients | 216 (51.3%) | 192 (53.0%) | 24 (40.7%) | .078 |
| Contact with wildlife | 1 (0.2%) | 0 | 1 (1.7%) | .140 |
| Contact with patients with fever | 85 (20.2%) | 82 (22.7%) | 3 (50.8%) | .003 |
| Symptoms and signs | ||||
| Fever | 255 (60.6%) | 217 (59.9%) | 38 (64.4%) | .515 |
| Highest temperature (°C) | ||||
| < 37.3 | 166 (39.4%) | 145 (40.1%) | 21 (35.6%) | .515 |
| 37.3-38.0 | 81 (19.2%) | 74 (20.4%) | 7 (11.9%) | .121 |
| 38.1-39.0 | 139 (33.0%) | 119 (32.9%) | 20 (33.9%) | .877 |
| > 39.0 | 35 (8.3%) | 24 (6.6%) | 11 (18.6%) | .002 |
| Cough | 219 (52.0%) | 183 (50.6%) | 36 (61.0%) | .136 |
| Sore throat | 102 (24.2%) | 95 (26.2%) | 7 (11.9%) | .017 |
| Nasal congestion | 24 (5.7%) | 23 (6.4%) | 1 (1.7%) | .259 |
| Sputum production | 32 (7.6%) | 26 (7.2%) | 6 (10.2%) | .442 |
| Headache | 44 (10.4%) | 37 (10.2%) | 7 (11.9%) | .702 |
| Myalgia | 96 (22.8%) | 82 (22.7%) | 14 (23.7%) | .855 |
| Chills | 97 (23.0%) | 86 (23.8%) | 11 (18.6%) | .387 |
| Dyspnea | 41 (9.7%) | 14 (3.9%) | 27 (45.8%) | < .0001 |
| Diarrhea | 11 (2.6%) | 2 (0.6%) | 9 (15.3%) | < .0001 |
| No symptoms | 22 (5.2%) | 22 (6.1%) | 0 | .056 |
| Comorbidity | ||||
| Any | 88 (20.9%) | 73 (20.2%) | 15 (25.4%) | .357 |
| Hypertension | 44 (10.5%) | 36 (9.9%) | 8 (13.6%) | .400 |
| Diabetes | 13 (3.1%) | 10 (2.8%) | 3 (5.1%) | .582 |
| COPD | 18 (4.3%) | 17 (4.7%) | 1 (1.7%) | .478 |
| Cerebrovascular disease | 8 (1.9%) | 6 (1.7%) | 2 (3.4%) | .697 |
| TB | 4 (1.0%) | 4 (1.1%) | 0 | .9996 |
| Cancer | 4 (1.0%) | 4 (1.1%) | 0 | .9996 |
| Others | 16 (3.8%) | 12 (3.3%) | 4 (6.8%) | .356 |
IQR = interquartile range.
Imaging Findings
Chest CT imaging showed no abnormalities in 24 patients (5.7%), whereas 105 patients (24.9%) had involvement of two lobes. The lung lesion distribution was peripheral in 85.0% of patients, multifocal in 69.4%, and bilateral in 68.2%. The lower lung lobes were more susceptible to infection lesions than the upper lobes. Lesions were present in the right lower lung lobe in 80.0% of patients, the left lower lobe in 71.0%, and both lower lobes in 62.7%. The most common lesion pattern was ground-glass opacity (67.7%), followed by patchy shadowing (49.2%); uncommon patterns were consolidation (15.0%) and nodular lesions (1.2%). Radiographic findings are shown in Table 2 .
Table 2.
Radiographic Findings
| Characteristic | All Patients (N = 421) | Recovered (n = 362) | Severe (n = 59) | P Value |
|---|---|---|---|---|
| No. of lobes involved | ||||
| 0 | 24 (5.7%) | 24 (6.6%) | 0 | .035 |
| 1 | 76 (18.1%) | 75 (20.7%) | 1 (1.7%) | .001 |
| 2 | 105 (24.9%) | 101(27.9%) | 4 (6.8%) | .011 |
| 3 | 79 (18.8%) | 74 (20.4%) | 5 (8.5%) | .029 |
| 4 | 70 (16.6%) | 54 (14.9%) | 16 (27.1%) | .020 |
| 5 | 67 (15.9%) | 34 (9.4%) | 33 (55.9%) | < .0001 |
| Distribution | ||||
| Periphery | 358 (85.0%) | 301 (83.1%) | 57 (96.6%) | .013 |
| Bilateral | 287 (68.2%) | 230 (63.5%) | 57 (96.6%) | < .0001 |
| Multifocal | 292 (69.4%) | 235 (64.9%) | 57 (96.6%) | < .0001 |
| Unifocal | 100 (23.8%) | 98 (27.1%) | 2 (3.4%) | < .0001 |
| Frequency of lobe distribution | ||||
| Left upper lobe | 175 (41.6%) | 125 (34.5%) | 50 (84.7%) | < .0001 |
| Left lower lobe | 299 (71.0%) | 243 (67.1%) | 56 (94.9%) | < .0001 |
| Right upper lobe | 200 (47.5%) | 148 (40.9%) | 52 (88.1%) | < .0001 |
| Right middle lobe | 102 (24.2%) | 69 (19.1%) | 33 (55.9%) | < .0001 |
| Right lower lobe | 337 (80.0%) | 280 (77.3%) | 57 (96.6%) | .001 |
| Bilateral upper lobes | 134 (31.8%) | 85 (23.5%) | 49 (83.1%) | < .0001 |
| Bilateral lower lobes | 264 (62.7%) | 209 (57.7%) | 55 (93.2%) | < .0001 |
| Features of the lesion | ||||
| Ground-glass opacity | 285 (67.7%) | 253 (69.9%) | 32 (54.2%) | .017 |
| Patchy shadowing | 154 (49.2%) | 115 (31.8%) | 39 (66.1%) | < .0001 |
| Consolidation | 63 (15.0%) | 43 (11.9%) | 20 (33.9%) | < .0001 |
| Interstitial abnormalities | 96 (22.8%) | 73 (20.2%) | 23 (39.0%) | .001 |
| Nodular lesions | 5 (1.2%) | 4 (1.1%) | 1 (1.7%) | .795 |
| Others | 17 (4.0%) | 13 (3.6%) | 4 (6.8%) | .425 |
Involvement of all five lobes was found in 55.9% of severe cases and 9.4% of recovered cases (P < .0001). Unifocal distribution was more common in the recovered cases (27.1%) than in the severe cases (3.4%; P < .0001). In addition, patchy shadowing was more common in the severe cases (66.1%) than in the recovered cases (31.8%; P < .0001).
Treatment and Outcomes Until Discharge or Transfer From Cabin Hospital
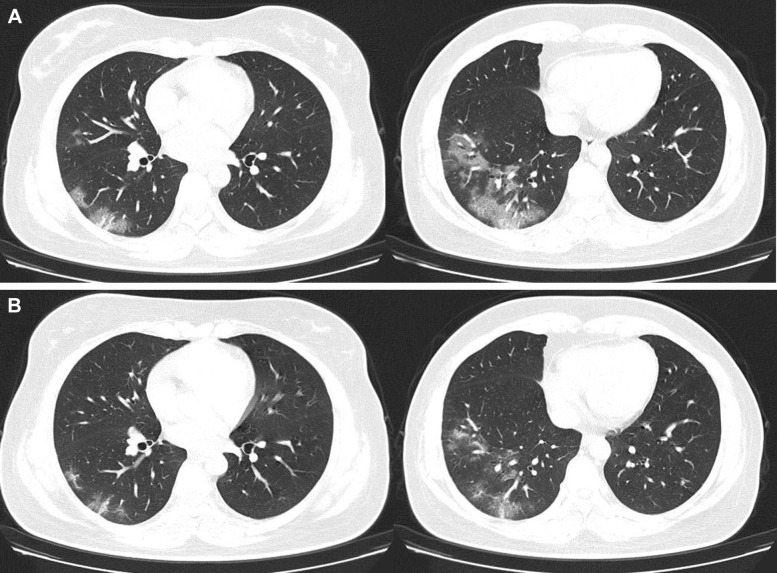
Table 3 summarizes the treatment data and clinical outcomes. Among the 421 patients, 87.6% received oral antibiotics, 90.5% received umifenovir, and 100.0% received traditional Chinese antivirus herbs. Oxygen therapy was administered in 13.6% of the severe cases and 8.6% of the recovered cases. The median number of nucleic acid tests performed in all patients was 2 (2-3), and the median length of cabin hospital stay was 17 (14-19) days. The duration of hospitalization was 18 (14-19) days in recovered cases and 15 (11-19) days in severe cases. The median duration between positive and negative RT-PCR results in recovered patients was 8 (6-10) days. A typical case of a patient who recovered and was discharged is presented in Figure 1 .
Table 3.
Treatments and Outcomes Until Discharge or Transfer From Mobile Cabin Hospital
| Characteristic | All Patients (N = 421) | Recovered (n = 362) | Severe (n = 59) | P value |
|---|---|---|---|---|
| Treatments | ||||
| Antibiotics | 369 (87.6%) | 315 (87.0%) | 54 (91.5%) | .633 |
| Arbidol | 381 (90.5%) | 327 (90.3%) | 54 (91.5%) | .772 |
| Chinese herbs | 421 (100.0%) | 362 (100.0%) | 59 (100.0%) | … |
| Oxygen therapy | 39 (9.3%) | 31 (8.6%) | 8 (13.6%) | .220 |
| Outcomes | ||||
| Median no. of nucleic acid tests performed | 2 (2-3) | 2 (2-3) | 2 (2-3) | … |
| Duration between positive and negative test results (RT-PCR) | … | 8 (6-10) | … | … |
| Length of cabin hospital stay | 17 (14-19) | 18 (14-19) | 15 (11-19) | … |
Data are median (interquartile range) unless otherwise indicated. RT-PCR = reverse transcription polymerase chain reaction.
Figure 1.
A 38-year-old woman infected with acute respiratory syndrome coronavirus 2. A, Chest CT scan performed at the time of admission to the mobile hospital shows ground-glass opacity in the right lower lung. B, CT scan performed 1 week following admission shows that the lesion has shrunk substantially. The patient was discharged from the mobile hospital after two consecutive negative nucleic acid test results with an interval of 1 day.
Discussion
In the early stage of the SARS-CoV-2 outbreak, medical resources were overwhelmed at ground zero in Wuhan, the capital of Hubei Province. In a previous retrospective study from our hospital, hospital-related transmission of SARS-CoV-2 was suspected in 41% of patients, 26% were treated in the ICU, and the mortality was 4.3%.11 The novel SARS-CoV-2 virus frequently causes only mild symptoms similar to the common cold, and asymptomatic carriers are difficult to identify.12, 13, 14 SARS-CoV-2 has been detected in the GI tract, saliva, and urine, which carries potential transmission risks.1 To avoid hospital-related transmission, traditional inpatient settings should not be allowed until all negative results, including chest CT imaging, nucleic acid, and blood IgM of SARS-Cov2, have been confirmed.
Because family clusters are one of the outbreak characteristics, home quarantine failed to effectively prevent the spread of the SARS-CoV-2 virus, and the number of patients with SARS-CoV-2 confirmed by nucleic acid testing increased rapidly in the first month of the outbreak. Based on the demand for medical management, large indoor stadiums and convention centers were reconstructed as mobile cabin hospitals with a capacity of > 20,000 beds for patients who had COVID-19 with mild symptoms. The establishment of mobile hospitals not only relieves the shortage of medical resources but also promotes the mental health of medical staff and the public.15 , 16
The most common symptom of SARS-CoV-2 in the current study was fever (60.6%), but this incidence of fever is less than that in patients with SARS-CoV (99%) and Middle East respiratory syndrome-related coronavirus (98%).17 Other common symptoms at the onset of illness due to SARS-CoV-2 were cough, sore throat, chills, and myalgia. However, asymptomatic patients with COVID-19 represented a small portion of patients in the current study, posing a challenge in the prevention of the epidemic.7 , 13 The most common comorbidities of patients with SARS-CoV-2 were hypertension, COPD, and diabetes mellitus. Most of these comorbidities were effectively controlled by the administration of oral medications in the mobile cabin hospital.
Chest CT imaging revealed no abnormalities in 24 (5.7%) of the 421 patients. Among the patients with abnormal findings on chest CT imaging, there was no predilection for the number of lung lobes affected. However, the right lower lobe was most commonly affected of all the lung lobes. This might be caused by the anatomic structure of the trachea and bronchi; because the right bronchus is short and straight, SARS-CoV-2 might be more likely to affect this location.18 Lower respiratory tract symptoms were more common than upper respiratory tract symptoms, indicating that the cells targeted by the virus might be located in the lower lobes.19 We speculate that the higher incidence of lesions in the lower airways seen in the current study may be related to false-negative results obtained from throat swab samples, as reported in a recent study.5 In the current study, 67.7% of patients had a ground-glass opacity pattern on chest CT imaging. The typical ground-glass opacity pattern may be related to alveolar exudation.12 Pathologic findings revealed prominent nucleoli in the intra-alveolar spaces of patients with COVID-19, indicating viral cytopathic-like changes.20 , 21 Peripheral lesion distribution was another characteristic of the current study patients, similar to the findings of a previous clinical study.22
In the current study, the incidence of patients with COVID-19 who had severe symptoms (14.0%) was similar to that reported in an earlier study.1 In the mobile cabin hospital, the most common reasons for transfer were a resting pulse oxygen saturation ≤ 93% and respiratory rate ≥ 30 breaths/min, as pulse oximeters were widely used and the respiratory rate was easy to monitor. Recovery from COVID-19 was recorded in 86.0% of the study patients, as confirmed by two consecutive negative nucleic acid test results with a sampling interval of at least 1 day and substantial improvement on chest CT imaging. The median duration of hospitalization was 17 days, and the median duration between positive and negative RT-PCR results was 8 days. The median number of nucleic acid tests performed was two, indicating that the mobile hospital enabled the effective observation and treatment of patients while minimizing the medical costs.
The current study had several limitations. First, blood tests were unable to be performed for every patient, partially because blood testing was not routine in patients with COVID-19 with mild symptoms, and partially due to the imbalance between the large number of patients and a serious shortage of medical staff. Second, at the time of manuscript writing, the available outcomes of the included patients had an end point of discharge or transfer from the cabin hospital. Thus, the current study mainly describes our experience regarding the diagnosis and treatment process of patients with COVID-19 in a mobile cabin hospital. Third, the results of follow-up nucleic acid tests performed following hospital discharge had not been obtained at the time of manuscript writing because of the limited data collection time. Positive RT-PCR results have reportedly been obtained from patients who have recovered from COVID-19.23
Interpretation
Mobile hospitals can be used to effectively treat patients with COVID-19 who have mild symptoms and to prevent the spread of the virus. Of 421 patients with confirmed SARS-CoV-2 infection who were admitted to the mobile hospital, 86% recovered, and 14% developed severe symptoms and were transferred to designated hospitals.
Acknowledgments
Author contributions: J. C. and W. W. conceived of the study and were guarantor of the paper. Z. X. and C. Xin. prepared the first draft and finalized the manuscript based on comments from all other authors. X. Y., Y. C., K. Z. and C. Xie. helped data analysis and interpretation of the results. T. Z., X. W., K. L. and Z. L. participated in data preparation and analysis and provided comments on the manuscript. All authors contributed to the analysis and reviewed the manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors : The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
Drs Wang, Xin and Xiong contributed equally and should be regarded as co-first authors.
FUNDING/SUPPORT: This work was supported by the National Natural Science Foundation of China [Project Number 81771280].
References
- 1.Guan W.J., Ni Z.Y., Hu Y. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ai T., Yang Z., Hou H. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology. 2020:200642. doi: 10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization Coronavirus disease (COVID-19) pandemic. https://www.who.int Accessed February 15, 2020.
- 7.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan L.T., Nguyen T.V., Luong Q.C. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Health Commission of the People’s Republic of China home page. http://www.nhc.gov.cn. Accessed March 12, 2020. [DOI] [PMC free article] [PubMed]
- 10.World Health Organization Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Accessed February 5, 2020.
- 11.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J., Wu X., Zeng W. Chest CT findings in patients with coronavirus disease 2019 and its relationship with clinical features. Invest Radiol. 2020;55(5):257–261. doi: 10.1097/RLI.0000000000000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang L., Li Y., Hu S. The mental health of medical workers in Wuhan, China dealing with the 2019 novel coronavirus. Lancet Psychiatry. 2020;7(3):e14. doi: 10.1016/S2215-0366(20)30047-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Y.T., Yang Y., Li W. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20(4):425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu X., Yu C., Qu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47(5):1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan L., Xu D., Ye G. PositiveRT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]