Abstract
Painful conditions are among the leading causes of years lived with disability, and may increase following the coronavirus pandemic, which has led to temporary closure of some healthcare services for people with chronic pain. To reduce this burden, novel, cost-effective and accessible interventions are required. We propose that greenspace exposure may be one such intervention. Drawing on evidence from neuroscience, physiology, microbiology, and psychology, we articulate how and why exposure to greenspaces could improve pain outcomes and reduce the high global burden of pain. Greenspace exposure potentially provides opportunities to benefit from known or proposed health-enhancing components of nature, such as environmental microbiomes, phytoncides, negative air ions, sunlight, and the sights and sounds of nature itself. We review the established and potential links between these specific exposures and pain outcomes. While further research is required to determine possible causal links between greenspace exposure and pain outcomes, we suggest that there is already sufficient evidence to help reduce the global burden of pain by improving access and exposure to quality greenspaces.
Keywords: Greenspace, Microbiome, Pain, Public health
Highlights
-
•
We reviewed the associations between greenspace exposure and pain outcomes.
-
•
Greenspace exposure may reduce pain via phytoncides, negative air ions, and sunlight exposure.
-
•
Greenspaces also encourage social interaction and physical activity, which both reduce pain.
-
•
We identified environmental microbiomes as a new pathway of pain reduction from greenspace exposure.
-
•
Greenspace exposure may improve pain outcomes and reduce the burden of pain globally.
1. Introduction
Greenspace exposure typically brings with it exposure to components of nature, including biodiverse environmental microbiomes, phytoncides, negative air ions, sunlight, and the sights and sounds of nature itself. There is growing evidence of the benefits of exposure to greenspaces via these components for human health outcomes, including lower blood pressure, lower cortisol levels, improved diabetes, reduced all-cause mortality, and fewer adverse birth outcomes (Twohig-Bennett and Jones, 2018). These benefits may be enhanced with exposure to more biodiverse greenspaces (Aerts et al., 2018), with several proposed mechanisms (Kuo, 2015). The impact of any type of greenspace exposure on pain, however, is under-investigated (Twohig-Bennett and Jones, 2018).
Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage” (International Association for the Study of Pain (IASP), 2017). Painful conditions are among the leading causes of the global disease burden, with lower back pain, neck pain, ‘other’ musculoskeletal disorders, and migraines among the top 10 leading causes of years lived with disability (Vos et al., 2017). Indeed, lower back pain is the leading cause of years lived with disability in 65% of the 195 countries and territories investigated in the 2017 Global Burden of Disease study (James et al., 2018). This burden is likely to increase during and following the current coronavirus pandemic, because lockdowns and physical distancing has necessitated changes to healthcare services, including the closure of pain clinics (Eccleston et al., 2020), and the postponement or cancelation of elective surgeries (Chang Liang, 2020; Sarac, 2020).
Chronic pain is considered a condition in its own right, not simply a symptom, and is defined as “pain that persists or recurs for longer than 3 months” (World Health Organization, 2018). The prevalence of chronic pain is high. For example, the estimated prevalence of chronic pain, when defined as pain persisting for 3 months or longer, in the United Kingdom is 43.5% (Fayaz et al., 2016), and when defined as pain persisting for 6 months or longer the prevalence is estimated to be 15.4% in Australia (Miller et al., 2017), 20.4% in the United States of America (Dahlhamer et al., 2018), and 27.2% in France (Chenaf et al., 2018). The prevalence of chronic pain is similar in low-middle income countries (Jackson et al., 2016). For those with chronic pain in the United Kingdom, 10.4–14.3% report being moderately to severely disabled by their pain (Fayaz et al., 2016). To reduce this disease burden, safe, effective and timely management options for people with pain are required, both to reduce the risk of transitioning from acute to chronic pain, and also to reduce the prevalence and impact of chronic pain. While many existing interventions contribute to reducing the community burden of chronic pain, novel interventions that further help are sought-after, and this paper explores a possible new approach – exposure to greenspace.
In this narrative review we probe the question – can exposure to greenspace reduce the high global burden of pain? To answer this question, we first review the nature of pain, followed by an exploration of the possible mechanisms by which exposure to greenspace could lead to more positive outcomes. ‘Greenspace’ has been defined in various ways in the existing literature (Taylor and Hochuli, 2017). For the purposes of this review, we have followed a broad definition of ‘greenspace’ as any natural environment, including, but not limited to, parks, ovals, forests and gardens.
2. Pain mechanisms
Pain is a psychoneuroimmunoendocrinological process with three main types (nociceptive, neuropathic, nocipathic/nociplastic/algopathic; see Table 1 for descriptions), which can occur simultaneously in some people (Hainline et al., 2017). Pain processing occurs independent of pathology (Peppin and Schatman, 2016); hence, in this review, we discuss pain as a general condition, rather than focusing on pain from specific diseases or injuries (e.g. musculoskeletal, cancer, migraine).
Table 1.
Characteristics for the three main pain categories.
| Pain category | Characteristics |
|---|---|
| Nociceptive pain | Involves the stimulation of nociceptors (the peripheral nerve terminals that detect noxious stimuli, which may be mechanical, chemical or thermal) (Hainline et al., 2017; Loeser and Treede, 2008) Includes inflammatory pain (Loeser and Treede, 2008) Protective mechanism – the body's ‘first detection’ system (Hainline et al., 2017; Loeser and Treede, 2008) Activation of nociceptors does not necessarily result in pain (Hainline et al., 2017) The relationship between nociceptor activity and the pain experience is not linear (Hainline et al., 2017) |
| Neuropathic pain | Involves a lesion of the somatosensory nervous system (International Association for the Study of Pain (IASP), 2017; Kosek et al., 2016; Loeser and Treede, 2008) May result from trauma or disease (Vardeh et al., 2016), or repetitive mechanical loading or inflammatory irritation of the peripheral nerves (Hainline et al., 2017) |
| Nocipathic/nociplastic/algopathic pain | Also described as ‘dysfunctional pain’ (Nagakura, 2015) Occurs in the absence of tissue threat or damage, and without somatosensory nervous system lesions (Kosek et al., 2016) Pain may occur through altered nociceptive pathway function, pathological changes of nociception, or central sensitisation (Hainline et al., 2017; Kosek et al., 2016), which occurs when the central nervous system nociceptors become hypersensitive (Loeser and Treede, 2008) Thought to be the pain type associated with visceral pain disorders, fibromyalgia and Complex Region Pain Syndrome Type 1 (Kosek et al., 2016) |
Pain is not simply the result of damage, or even a sensory signal, but rather pain is a conscious event (Hainline et al., 2017). Pain is complex and varies widely between and within individuals, with a broad range of factors potentially playing a role, including neurophysiological, immunological, psychological, contextual, environmental, and social factors (Bushnell et al., 2013; Gatchel et al., 2007; Turk and Okifuji, 2002; Villemure and Bushnell, 2002). There are also many psychosocial factors associated with pain and poorer pain outcomes (e.g. transitioning from acute to chronic pain), such as stress, poorer mental health and lack of social coherence/support (see Box 1 ).
Box 1. Examples of psychosocial factors associated with pain outcomes.
Stress (Drake et al., 2018; Jayakumar et al., 2018)
Poorer mental health (e.g. anxiety, depression) (Drake et al., 2018; Hruschak and Cochran, 2018; Jayakumar et al., 2018; Liu et al., 2018)
Lack of social coherence (Jayakumar et al., 2018) and support (Fregoso et al., 2019; Jayakumar et al., 2018)
Sleep problems (Andreucci et al., 2017; Haack et al., 2020)
Beliefs about pain (Morton et al., 2019) and pain control (de Raaij et al., 2018)
Poorer expectations regarding pain (Hruschak and Cochran, 2018)
Catastrophisation (Fregoso et al., 2019; Hruschak and Cochran, 2018)
Kinesiophobia/ fear-avoidance beliefs (Drake et al., 2018; Hruschak and Cochran, 2018; Jayakumar et al., 2018; Morton et al., 2019)
Fear of surgery (Fregoso et al., 2019)
Perceived self-helplessness (Fregoso et al., 2019)
Poor self-resilience (Fregoso et al., 2019)
Poor self-efficacy (Fregoso et al., 2019)
Having non-adaptive pain thoughts (Jayakumar et al., 2018)
Alt-text: Box 1
The brain integrates information from various sources (e.g. sensory information, beliefs about pain ), and pain may or may not result. The modulation of pain is influenced by non-nociceptive sensory input (Moseley and Arntz, 2007), affective and cognitive factors (Bushnell et al., 2013), and contextual cues (Moseley and Arntz, 2007). Pain modulation occurs through anatomical or functional neurological changes (Hainline et al., 2017), and/or through various processes of the peripheral and central nervous systems (Bushnell et al., 2013).
There are several neural factors potentially involved in the experience of pain. These neural factors include the activation of nociceptors (that detect noxious stimuli (Hainline et al., 2017; Loeser and Treede, 2008)), and the descending pathways (that influence pain at the dorsal horn of the spinal cord (Guo et al., 2019; Zhuo, 2017)). Pain modulation may also be influenced by pro-inflammatory mediators, nerve growth factors, hormones (e.g. endorphins) and epigenetic modifications, and involves immune cells, mast cells, macrophages, and leukocytes (Guo et al., 2019). The activity of these cells is driven by several compounds, including short chain fatty acids and gamma-aminobutyric acid (GABA) (Guo et al., 2019). An awareness of the nature of pain is important for contextualising and interpreting the potential role of pain-reducing interventions. However, a further discussion regarding pain mechanisms is beyond the scope of this paper; interested readers are instead referred to other reviews for further information (e.g. Bushnell et al. (2013), Hainline et al. (2017), Fregoso et al. (2019), and Guo et al. (2019)).
3. How is pain currently treated?
Given the complex nature of pain, interventions can target various factors. Particularly in the acute phase, pain management may target nociception, including any underlying inflammation. In this acute phase, strategies to prevent the transition from acute to chronic pain may also be implemented, targeting any of the risk factors (Table 2 ). These factors may continue to be targeted in chronic pain management, although treatments aimed at reducing hypersensitivity may be added. Finally, surgical options may be considered to address underlying problems (e.g. joint replacement, spinal fusion, nerve decompression), as well as strategies to reduce hypersensitivity. Chronic pain treatment is typically multidisciplinary and may be provided by a range of health professionals including physiotherapists, psychologists, occupational therapists, dentists, podiatrists, general practitioners, pain physicians, neurologists, anaesthetists, and appropriate surgeons (e.g. neurosurgeons, orthopaedic surgeons).
Table 2.
Potential treatments for pain.
| Target | Examples of treatments |
|---|---|
| Reduce nociception & inflammation |
|
| Improving the emotional & cognitive factors |
|
| Reduce hypersensitivity |
|
The treatment of chronic pain can be complex, resource intensive, and have varying levels of success. Novel treatments to reduce the risk of transition from acute to chronic pain and to treat chronic pain itself are both required. These treatments need to be accessible in a timely manner, acceptable to the patient, safe, and cost-effective. While existing strategies contribute to managing pain, new strategies to manage pain should be explored to reduce the global burden further. Recent work on greenspace may provide an appropriate option to help reduce the high global burden of pain, particularly chronic pain.
4. Could exposure to greenspace help reduce the pain burden?
Greenspace exposure has been associated with a range of positive health outcomes, including conditions associated with pain (e.g. lower stress levels, and better mental health (Twohig-Bennett and Jones, 2018)), providing some indication that greenspace exposure may have a beneficial impact on pain. Despite this, the relationship between greenspace and pain outcomes or painful conditions (e.g. musculoskeletal disorders) have not been adequately investigated (Twohig-Bennett and Jones, 2018).
To our knowledge, only two studies (Ihlebæk et al., 2018; Maas et al., 2009) have investigated the possible association between greenspace exposure and pain or musculoskeletal outcomes, with mixed findings. Maas et al. (2009) investigated the relationship between the percentage of greenspace in circles with 1 or 3 km radii around the participants' places of residence, and health conditions reported in general practice notes in the 12 months prior. The health conditions targeted included musculoskeletal conditions such as neck/back complaints, severe back complaints, severe neck/back complaints, and severe elbow/wrist/hand complaints, osteoarthritis, and arthritis (Maas et al., 2009). Of these musculoskeletal conditions, there was a significant negative association between the percentage of greenspace in the 1 km radius circle and the number of neck/back complaints, severe back complaints, severe neck/back complaints, severe elbow/wrist/hand complaints (Maas et al., 2009). No such significant association was found for the 3 km radius (Maas et al., 2009). The study is directly relevant to the question we are asking, because ache, pain, or discomfort are generally used as proxy-measures of musculoskeletal disorders (Kuorinka et al., 1987), indicating that these symptoms can be pathognomonic of musculoskeletal disorders and that people diagnosed with musculoskeletal conditions are therefore likely to have experienced pain. However, one of the limitations of this study was that the patients with the musculoskeletal complaints studied might not necessarily present with pain.
In the second relevant study, Ihlebæk et al. (2018) investigated the association between the degree of “vegetation cover greenness” and “land use greenness” within the participants' residential ‘circuit’, and whether the participants reported pain and/or stiffness in their muscles/joints in the last four weeks in three or more (of six) body regions (although the body regions were not listed). No association between greenspace and pain for males was observed, but for females the prevalence of pain/stiffness was higher in those living in areas with more vegetation cover greenness and land use greenness (Ihlebæk et al., 2018). This unexpected finding should be interpreted with caution given a number of limitations. Firstly, the outcome measures employed were not tested for validity and reliability, and secondly there was no differentiation between pain and stiffness.
In both studies (Ihlebæk et al., 2018; Maas et al., 2009), the use of residential proximity to greenspace does not necessarily provide an accurate measure of a resident's greenspace exposure, owing to individual differences in exposure to greenspace.
We do however have additional corroborative evidence suggesting that a relationship is likely, and that further research in the area is worthwhile. There is evidence for example that forest therapy (Han et al., 2016; Kang et al., 2015), exercise in green areas (Huber et al., 2019) (not to be confused with ‘green prescriptions’ that refer to written advice to a patient regarding physical activity made by a health professional (New Zealand Ministry of Health, 2016)), and involvement in horticultural therapy (Kim et al., 2006; Verra et al., 2012) and conservation (Moore et al., 2007) are associated with better pain outcomes. However, these studies have not been designed with appropriate controls to ascertain whether greenspace exposure itself led to the benefits or whether these benefits could be due to other aspects, such as physical activity and/or social interaction. Furthermore, all used lower level study designs (National Health and Medical Research Council, 2009; Oxford Centre for Evidence-Based Medicine, 2011) (e.g. observational studies), and some studies of forest therapy and green exercise actually also included interventions (e.g. walking/hiking (Han et al., 2016; Huber et al., 2019; Kang et al., 2015), being residential (Han et al., 2016; Huber et al., 2019), music therapy (Han et al., 2016)), which were not provided to the comparison groups. As it stands, there is therefore some suggestion that greenspace exposure may assist in pain management, however the evidence to date is insufficient to determine whether the benefits are due to greenspace exposure per se.
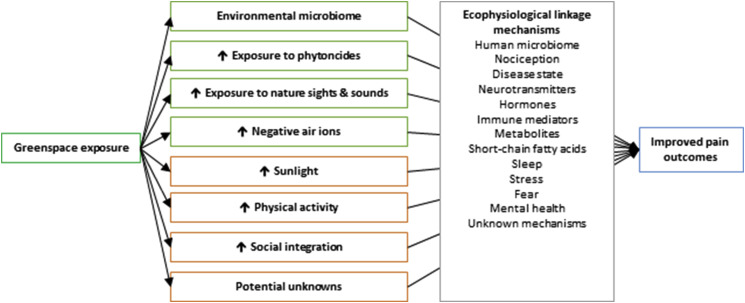
In the following sections, we explore the biological plausibility of greenspace exposure per se leading to an improvement in pain outcomes (see conceptual model in Fig. 1 ). These sections refer to the particular components of nature that greenspace exposure may provide, and we separately discuss those that are specific to greenspace (e.g. environmental microbiota, phytoncides, sights and sounds of greenspace) from those that are not greenspace-specific but are facilitated by greenspace exposure (e.g. sunlight, social integration and cohesion (Jennings and Bamkole, 2019), and physical activity (Keskinen et al., 2018)). We detail how these greenspace components could be linked to pain outcomes via various ecophysiological linkage mechanisms, some mechanisms of which are known, but including others that are not. Not represented in the conceptual model are additional intrinsic linkages within the ecophysiological linkage mechanisms, such as the influence of gut microbiome on mental health (Liu et al., 2019; Vaghef-Mehrabany, 2019; Yang et al., 2019). These added layers of complexity and unknowns must remain as open questions and are not discussed further in our study.
Fig. 1.
Conceptual model linking greenspace exposure to pain outcomes. Not shown are additional potential pathways joining different ecophysiological linkage mechanisms.
4.1. Environmental microbiomes
The ‘old friends’ hypothesis proposes that humans evolved alongside a diverse suite of environmental microbiota (collectively known as ‘microbiomes’), and that co-evolved symbiotic relationships developed (Rook et al., 2004). This co-evolution underpins our argument that exposure to greenspace (with its microbiome) may positively influence pain outcomes. It has recently been demonstrated that direct soil contact changes the human skin microbiome (Grönroos et al., 2019), and that exposure to different environments (and their respective microbiomes) changes the human nasal and skin microbiome (Lai et al., 2017). Importantly, the latter study was conducted indoors and is therefore not susceptible to some of the potential confounding exposures present outdoors (e.g. direct plant/soil/animal interactions, exposure to sunlight and phytoncides) that may also influence the human microbiome (as discussed below). The influence of the environmental microbiome on the human gut microbiome is not currently well understood (Blum et al., 2019; Tasnim et al., 2017), however animal studies indicate such an influence (Blum et al., 2019), even via indirect exposure to soil via the aerobiome only (Liddicoat et al., 2020).
The microbiome-gut-brain axis refers to the bidirectional communication between the gut microbiome, the gut and the brain, and is mediated by neurotransmitters, bacterial metabolites, cytokines, hormones and neural communication (Kelly et al., 2015; Mayer et al., 2014). Interest in the microbiome-gut-brain axis has increased dramatically since 2009, with over 500 papers published on the topic in 2018 alone (Zyoud et al., 2019). However, pain as an outcome has been relatively under-investigated, with studies predominantly focusing on visceral pain (Guo et al., 2019; Rea et al., 2019). The relationship between the human microbiome and pain outcomes has recently been comprehensively reviewed, hence we provide only a summary of the current evidence base, with interested readers referred to Guo et al. (2019) and Rea et al. (2019) for further detail.
Associations between the human microbiome and a range of painful conditions have been reported. These conditions include endometriosis (Leonardi et al., 2019), fibromyalgia (Malatji et al., 2017), myalgic encephalomyelitis/chronic fatigue syndrome (Nagy-Szakal et al., 2017), interstitial cystitis/bladder pain syndrome (Nickel et al., 2019), chronic prostatitis/chronic pelvic pain syndrome (Shoskes et al., 2016), dermatitis (Gulliver et al., 2018), and inflammatory bowel disease (Knights et al., 2013). Furthermore, there is emerging experimental evidence that changing the gut microbiome through probiotics (Lactobacillus casei Shirota (Lei et al., 2017), L. gasseri OLL2809 (Itoh et al., 2011), and combined L. acidophilus, L. plantarum, L. fermentum and L. gasseri (Khodaverdi et al., 2019)) reduces pain in people with knee osteoarthritis (Lei et al., 2017), and endometriosis (Itoh et al., 2011; Khodaverdi et al., 2019). Recently, faecal microbiota transplants have also been shown to reduce pain in those with fibromyalgia (Thurm et al., 2017) and Clostridium difficile infection (Alukal et al., 2019). Although these positive results could be due either to changes in the disease state or to changes in pain processing, they nonetheless suggest that exposure to greenspace – and its associated environmental microbiomes – may lead to reductions in pain, via changes in the human microbiome.
A recent study by Shiro et al. (2017) reported an association between stool consistency (a proxy measure of the gut microbiome) and pain intensity (initiated by mechanical stimulation of the inter-digital space between the second and third, and the fourth and fifth digits of the right hand). This study provides some evidence of the potential role of gut microbiome in pain perception, although the causal mechanisms are still hypothetical.
As outlined above, the gut microbiome can influence the brain via various microbially-mediated mechanisms, and those related to chronic pain have recently been reviewed elsewhere (Guo et al., 2019). Microbiota-derived mediators may decrease pain perception via peripheral and central mechanisms. For peripheral mechanisms, the mediators that reduce hypersensitivity include proteases, kynurenic acid, and GABA (Guo et al., 2019). Short-chain fatty acids regulate leucocyte functions, and one of these short-chain fatty acids, butyrate, reduces pain associated with nerve injury by inhibiting histone deacetylase (Guo et al., 2019). Bile acids are another type of mediator, that may reduce pain by activating release of endogenous opioids from macrophages (Guo et al., 2019). The bacteria that could be implicated in the production of the abovementioned mediators include Lactobacillus rhamnosus (Pokusaeva et al., 2017; Siragusa et al., 2007), L. brevis (Barrett et al., 2012), L. buchneri (Cho et al., 2007), L. paracasei (Komatsuzaki et al., 2005), L. plantarum (Siragusa et al., 2007), L. delbruekii subsp. bulgaricus (Siragusa et al., 2007), Monascus purpureus (Su et al., 2003), Streptococcus salivarius subsp. thermophilus (Yang et al., 2008), Clostridium butyricum (Liu et al., 2015; Rivière et al., 2016), Coprococcus eutactus (Rivière et al., 2016), C. comes (Rivière et al., 2016), Bifidobacterium spp. (Rivière et al., 2016), B. dentium (Barrett et al., 2012; Pokusaeva et al., 2017), B. infantis (Barrett et al., 2012), B. adolescentis (Barrett et al., 2012), Bacteroides fragilis (Strandwitz et al., 2019), Parabacteroides spp. (Strandwitz et al., 2019), Faecalibacterium prausnitzii (Rivière et al., 2016), Eubacterium hallii (Rivière et al., 2016), E. rectale (Rivière et al., 2016), Anaerostripes butyraticus (Rivière et al., 2016), A. caccae (Rivière et al., 2016), A. hadrus (Rivière et al., 2016), Butyricicoccus pullicaecorum (Rivière et al., 2016), Roseburia faecis (Rivière et al., 2016), R. inulinivorans (Rivière et al., 2016), R. intestinalis (Rivière et al., 2016), R. hominis (Rivière et al., 2016), and Escherichia spp. (Strandwitz et al., 2019), again supporting a potential association between gut microbiome and pain outcomes.
For central mechanisms, central sensitisation may be the result of glial activation which ultimately leads to decreased GABAergic synaptic neurotransmission and/or elevated glutamatergic synaptic neurotransmission, and the gut microbiome plays a role in microglial function, maturation and morphology (Guo et al., 2019). There is however no direct evidence, to our knowledge, linking the gut microbiome to central sensitisation, although GABA-producing bacteria could theoretically be implicated.
In addition to the abovementioned mechanisms linking the human microbiome and pain outcomes, the human microbiome influences mental health outcomes. Probiotics (e.g. Lactobacillus spp., Bacillus spp., Clostridium spp., Bifidobacterium spp.) can reduce anxiety (Liu et al., 2019) and depression (Liu et al., 2019; Vaghef-Mehrabany, 2019), and gut microbiome regulation (e.g. probiotics, dietary changes) can reduce anxiety (Yang et al., 2019). There is also an association between gut microbiome and sleep (Smith et al., 2019). Experimental sleep deprivation has been shown to influence the gut microbiome (Benedict et al., 2016; Poroyko et al., 2016), however to our knowledge no study has investigated whether changes to the microbiome influence sleep outcomes. By improving mental health and potentially sleep, due to the changes in gut microbiome, greenspace exposure may improve pain outcomes.
It has recently been demonstrated in a mouse study that a diverse gut microbiome is required for fear extinction learning to occur (Chu et al., 2019), which may have implications for chronic pain. There is some evidence to suggest that people with chronic pain have reduced differential learning (Harvie et al., 2017), and that fear-avoidance beliefs (Drake et al., 2018; Hruschak and Cochran, 2018; Jayakumar et al., 2018; Morton et al., 2019) are associated with chronic pain. Chu et al. (2019) suggested that interventions to reduce fear-avoidance (e.g. graded exposure) may have had limited success in those with lower gut microbiome diversity. These findings may also have implications for changing other cognitive elements of the pain experience such as pain beliefs, and expectations regarding pain and recovery.
Although the association between environmental microbiome and pain outcomes has not been investigated, we suggest that such an association is likely to exist owing to the influence of environmental microbiomes on human microbiomes, and the existence of multiple potential pathways linking the human microbiome and pain outcomes.
4.2. Sights and sounds of nature
The biophilia hypothesis – where humans have an innate and natural affiliation with nature (Wilson, 1986) – has traditionally been central to the proposed link between greenspace exposure and health outcomes, and relates to exposure to the sights and sounds of nature. Listening to pleasant nature sounds during elective Caesarean section has been shown to reduce post-operative pain severity (Farzaneh et al., 2019), and also resulted in lower pain for those undergoing mechanical ventilation (Saadatmand et al., 2015). Combined natural sounds and sights have resulted in lower pain severity compared with both city sounds and sights and with a control during bone marrow aspiration and biopsy (Lechtzin et al., 2010). Vincent et al. (2010) demonstrated differences in the effect of viewing an array of natural scenery on experimental pain sensation. They found that the combined prospect/refuge scenery resulted in lower pain sensation than prospect, refuge and hazard scenery and the control (a black screen). Listening to pleasant nature sounds has also been reported to improve sleep (Nasari et al., 2018), while a virtual nature experience reduced stress (Liszio et al., 2018), which may also lead to a reduction in pain. Greenspace exposure could therefore result in a reduction in pain due to exposure to natural sights and sounds.
4.3. Phytoncides
The antimicrobial volatile organic compounds emitted as a defence mechanism by plants are called phytoncides, and they permeate the air particularly in or near greenspace (Franco et al., 2017). To our knowledge no study has investigated the relationship between phytoncides and pain in humans, however an analgesic effect has been reported for mice (Cheng et al., 2009).
Given their antimicrobial properties (Franco et al., 2017), phytoncides may also influence the microbiome. To our knowledge, the impact of phytoncide exposure on the microbiome has not been examined, however the effect of dietary phytoncide supplements on gut Lactobacillus spp. and Escherichia coli counts has (Kim et al., 2018a; Li et al., 2015; Zhang et al., 2012). These studies of livestock found that dietary phytoncides supplements gave mixed results, with one study reporting no change (Zhang et al., 2018), and others reporting significantly higher Lactobacillus spp. counts (Kim et al., 2018a; Li et al., 2015; Zhang et al., 2012) and lower Escherichia coli counts (Kim et al., 2018a; Li et al., 2015) with the supplements. These alternations to the gut microbiome may influence pain perception, due to the mechanisms outlined above.
Phytoncides may also influence the human immune system, particularly natural killer cell function. In vitro studies have shown that phytoncides can enhance human natural killer cell function (Li et al., 2006). Natural killer cell function was enhanced for people walking in forests, but not in cities, and importantly phytoncides were only detected in the forest and not in the city (Li et al., 2008). This study did not, however, account for the potential impact of other forest exposures (e.g. environmental microbiome) that may have influenced the relationship. Nonetheless, greenspace exposure appears to improve natural killer cell activity, and natural killer cells have recently been proposed as a treatment for some types of pain (Davies et al., 2019).
Phytoncides have also been shown to improve sleep and reduce anxiety in animal studies (Cheng et al., 2009), providing further evidence of a potential link between greenspace exposure, phytoncides, and pain outcomes. Different anxiety responses have been observed with exposure to different tree species in forest bathing (Guan et al., 2017), which could be explained by differences in the phytoncides released. A recent randomised crossover study (Horiuchi et al., 2014) compared two forest bathing exposures; one where participants could see the forest and the other where they could not. There was a significant reduction in trait-anxiety, depression, confusion and fatigue when the forest could be viewed, but not when the view was occluded; however there were no significant differences in the outcomes between the two exposures post-exposure (Horiuchi et al., 2014). Horiuchi et al. (2014) indicated that phytoncides are unlikely to be the sole reason for changes in human health outcomes related to greenspace exposure, but supported the notion that greenspace exposure may improve pain outcomes.
4.4. Negative air ions
Negative air ions are generated by plants (see Jiang et al. (2018) for a list), shear forces of water, sunlight, atmospheric radiant or cosmic rays, and natural and artificial corona discharge (Jiang et al., 2018). They are less prevalent in urban settings compared with forests, places with moving water, and mountainous areas (Mao et al., 2012). There is some, albeit limited, evidence of negative air ion exposure altering pain outcomes (David et al., 1960; Minehart et al., 1961; Olivereau, 1970), through a range of potential effects on humans and other animals. These effects include decreased cyclic nucleotides, lower dopamine, activation of natural killer cells, and improved mental health (Jiang et al., 2018), all of which may reduce pain, including chronic pain (Davies et al., 2019; Drake et al., 2018; Hruschak and Cochran, 2018; Jayakumar et al., 2018; Li et al., 2019; Liu et al., 2018; Taylor et al., 2016).
Negative air ions have also been shown to kill or reduce a range of microbes, including Serratia marcescens, Staphylococcus albus, S. aureus, S. epidermidis, Pseudomonas veronii, P. fluorescens, Salmonella Enteriditis, Candida albicans, Escherichia coli, and Penicillum notatum, and have been shown to prevent Acinetobacter infections (Jiang et al., 2018). These antimicrobial effects indicate that negative air ions have the potential to alter the human microbiome, which may therefore influence pain outcomes.
4.5. Sunlight exposure
Sunlight exposure is the first of three generic factors that we propose may link greenspace exposure to pain outcomes. Depending on the weather, geographic location, canopy cover and time of day, spending time in greenspace is likely to lead to sunlight exposure. Sunlight exposure is perhaps most commonly associated with vitamin D production, but exposure to sunlight also leads to the production of beta-endorphin (an endogenous opioid peptide), melatonin, and nitric oxide (a vasodilator), as well as the release of carbon monoxide from haemoglobin (a vasodilator), and expression of the proopiomelanocortin gene (which results in the production of beta-endorphin and cortisol) (Holick, 2016).
Observational studies have identified an association between vitamin D levels and arthritis, muscle pain, chronic widespread pain (Wu et al., 2018), and low back pain (Zadro et al., 2017); however, studies investigating the impact of vitamin D supplementation on pain outcomes have generally shown that vitamin D supplementation is no better than placebo for people with lower back pain (Zadro et al., 2018) and non-specific musculoskeletal disorders (Gaikwad et al., 2017). However, there is some evidence of vitamin D lowering pain intensity for those with chronic widespread pain (Yong et al., 2017). The discrepancy between the observational and experimental evidence regarding the relationship vitamin D and pain may be the result of vitamin D acting as a proxy-measure of sunlight exposure. Sunlight exposure could lead to a change in pain through non-vitamin D pathways, including the release of beta-endorphins (Holick, 2016) and melatonin (Zhu et al., 2017), or indeed changes in the microbiome (Waterhouse et al., 2019). Furthermore, sunlight exposure (Düzgün and Durmaz Akyol, 2017) and vitamin D supplementation (Jamilian et al., 2019) have led to improved sleep including for people with chronic pain specifically (Huang et al., 2013). Vitamin D supplementation has also resulted in reduced inflammation and improvements in depression (Jamilian et al., 2019), which may in turn contribute to improved pain outcomes.
4.6. Physical activity
Exposure to greenspace reportedly facilitates physical activity (Keskinen et al., 2018); the second generic factor in our review. Physical activity is commonly prescribed by health professionals, particularly for patients in pain. Evidence in support of physical activity for reducing the prevalence and impact of pain include findings of physical activity being associated with a lower incidence of neck pain (Kim et al., 2018b), and lower prevalence of lower back pain (Alzahrani et al., 2019b), including frequent and chronic lower back pain (Shiri and Falah-Hassani, 2017). Furthermore, interventions to increase incidental physical activity lead to improved lower back pain-related disability (Alzahrani et al., 2019a). For those with musculoskeletal conditions in particular, physical activity may decrease nociception by improving the underlying musculoskeletal condition. Exercise reduces inflammation (Stigger et al., 2019; Zheng et al., 2019) and stress (Bischoff et al., 2019; Rodriguez-Ayllon et al., 2019), improves sleep (Banno et al., 2018; Kreutz et al., 2019; Lederman et al., 2019; Lowe et al., 2019; Stutz et al., 2019) and mental health (Béland, 2019; McDowell et al., 2019; Morres et al., 2019; Nakamura et al., 2019; Rodriguez-Ayllon et al., 2019), and changes the human microbiome (Mailing et al., 2019). The health improvements associated with exercise may also influence pain perception and the risk of transitioning from acute to chronic pain. Thus, physical activity, particularly when facilitated by greenspace exposure, is likely to also contribute to a reduction in the global burden of pain.
4.7. Social integration
Although social integration is not specific to greenspace, greenspace exposure is associated with a range of social benefits and has been identified as a facilitator of social integration and cohesion (Jennings and Bamkole, 2019), and would therefore be expected to improve social support. Social support has been associated with pain perception (Che et al., 2018b), including experimental pain (Che et al., 2018a), while low levels of social support are associated with a higher risk of transitioning from acute to chronic pain (Fregoso et al., 2019). In addition, higher levels of social support and integration are associated with lower levels of inflammation (Uchino et al., 2018), better sleep (Kent de Grey et al., 2018), and better mental health (Tengku Moud et al., 2019) which may all in turn influence pain perception. Of note, sleep may also influence the gut microbiome (Benedict et al., 2016; Poroyko et al., 2016) and thus potentially pain perception through that mechanism as well. We therefore suggest that greenspace exposure is likely to decrease both pain perception, and the transition from acute to chronic pain, via improvements in social integration and support.
5. Recommendations
Here we argue that exposure to greenspace may be an effective, safe and accessible strategy to help alleviate the global burden of pain. With the exception of those with compromised immune systems, exposure to greenspace should therefore be encouraged for those experiencing pain.
The association and potential therapeutic benefit of greenspace exposure for those with pain should be further explored, with a particular focus on the transition from acute to chronic pain, and the prevalence and burden of chronic pain. To do this we need valid and reliable measures of exposure to greenspace (e.g. time spent in greenspace, characteristics of the greenspace), which, to our knowledge, do not currently exist.
One of the advantages of greenspace exposure as an intervention for pain, particularly chronic pain, is that it is not reliant on medical intervention, and could be implemented while on waiting lists for specialist appointments – a particularly important consideration in the socially isolating conditions of a pandemic, with elective surgery and pain clinics closed down. It can take years for patients to gain access to these services (Anderson, 2016), during which time their nervous systems change and the burden of their pain increases. The caveat to this is, however, that appropriate greenspaces must be accessible to those who require them. Several general barriers to such greenspace access have been suggested, and include a lack of amenities (Cronin-de-Chavez et al., 2019; Sefcik et al., 2019), safety concerns (Boyd et al., 2018; Cronin-de-Chavez et al., 2019; Sefcik et al., 2019; Selby et al., 2019), proximity to greenspace (Selby et al., 2019), and issues with transport (Boyd et al., 2018; Cronin-de-Chavez et al., 2019; Fretwell and Greig, 2019; Sefcik et al., 2019). Perhaps more importantly, a lack of interest (Boyd et al., 2018; Fretwell and Greig, 2019) and time (Boyd et al., 2018; Fretwell and Greig, 2019; Holt et al., 2019; Selby et al., 2019), and debilitating health conditions (Boyd et al., 2018; Cronin-de-Chavez et al., 2019; Fretwell and Greig, 2019; Sefcik et al., 2019) have also been identified as barriers. Finally, in the current coronavirus pandemic situation, strict lockdowns in countries like Italy are likely to reduce greenspace exposure for many people. These barriers support the need to optimise opportunistic encounters with greenspace, such as advice to optimise private greenspaces to maximize benefits, as well as utilising verges and high-use areas (e.g. commuter paths, work place environments) for optimal greenspace, so that passive exposure to the aerobiome is achieved. Stakeholder engagement is also essential to improve usage of public greenspaces (Roberts et al., 2016).
To optimise greenspaces to improve pain outcomes we need to understand which elements of greenspace have the most influence on pain outcomes (e.g. phytoncides, microbiome), what the most advantageous greenspaces comprise of (e.g. the specific microbes that should be in relative abundance), and how to encourage people, particularly those in pain, into such greenspaces.
With specific reference to the environmental microbiome, further work is required to characterise the components of the environmental microbiome that directly influence pain. Such health outcome-environmental microbiome association studies have begun in non-pain related areas (e.g. anxiety-like behaviour (Liddicoat et al., 2020)), and are a required precursor to not only understanding the level of exposure to these potentially pain-mitigating microbiota from greenspaces, but also how to derive these pain management benefits via targeted changes to greenspaces.
6. Conclusions
Here we articulate how and why exposure to greenspaces is likely to reduce pain, particularly chronic pain. Greenspaces provide exposure to environmental microbiomes, phytoncides, negative air ions, natural sights and sounds, and sunlight, and may facilitate physical activity and social integration. We describe established or potential links between these specific exposures and pain outcomes. Further research is required to determine the mechanistic pathways that link greenspace and pain outcomes, as well as the nature and duration of specific exposures relevant to optimising pain outcomes. By making available public and private greenspaces accordingly, and reducing barriers to access, we are likely to see a reduction in the global burden of pain.
Data Accessibility Statement
There are no data used in this manuscript.
Author Contributions
J.S., M.F.B. and P.W. contributed to the conception of the article; J.S, M.F.B. and P.W. contributed to manuscript writing, revision, read and approved the submitted version.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Jessica Stanhope, Email: jessica.stanhope@adelaide.edu.au.
Martin F. Breed, Email: martin.breed@flinders.edu.au.
Philip Weinstein, Email: philip.weinstein@adelaide.edu.au.
References
- Aerts R. Biodiversity and human health: mechanisms and evidence of the positive health effects of diversity in nature and green spaces. Br. Med. Bull. 2018;127:5–22. doi: 10.1093/bmb/ldy021. [DOI] [PubMed] [Google Scholar]
- Alatawi S.F. Effectiveness of neural mobilization in the management of chronic low back pain with radiculopathy: a randomized controlled trial. Int. J. Physiother. 2019;6:217–223. [Google Scholar]
- Alukal J. Safety and efficacy of fecal microbiota transplant in 9 critically ill patients with severe and complicated Clostridium difficile infection with impending colectomy. J. Dig. Dis. 2019;20:301–307. doi: 10.1111/1751-2980.12750. [DOI] [PubMed] [Google Scholar]
- Alzahrani H. The effectiveness of incidental physical activity interventions compared to other interventions in the management of people with low back pain: a systematic review and meta-analysis of randomised controlled trials. Phys. Ther. Sport. 2019;36:34–42. doi: 10.1016/j.ptsp.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Alzahrani H. The association between physical activity and low back pain: a systematic review and meta-analysis of observational studies. Sci. Rep. 2019;9:8244. doi: 10.1038/s41598-019-44664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson T. Doctors lobby for better chronic pain management. Lancet. 2016;388:2856–2858. doi: 10.1016/S0140-6736(16)32514-4. [DOI] [PubMed] [Google Scholar]
- Andreucci A. Are sleep problems a risk factor for the onset of musculoskeletal pain in children and adolescents? a systematic review. Sleep. 2017;40:zsx093. doi: 10.1093/sleep/zsx093. [DOI] [PubMed] [Google Scholar]
- Baez S. Evaluation of cognitive behavioral interventions and psychoeducation implemented by rehabilitation specialists to treat fear-avoidance beliefs in patients with low back pain: a systematic review. Arch. Phys. Med. Rehabil. 2018;99:2287–2298. doi: 10.1016/j.apmr.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Bakker S.H. Selective intradural dorsal rhizotomy for persistent radicular leg pain: a contemporary series. Spinal J. 2019;19:306–313. doi: 10.1016/j.spinee.2018.06.364. [DOI] [PubMed] [Google Scholar]
- Ball E.F. Does mindfulness meditation improve chronic pain? A systematic review. Curr. Opin. Obstet. Gynecol. 2017;29:359–366. doi: 10.1097/GCO.0000000000000417. [DOI] [PubMed] [Google Scholar]
- Banno M. Exercise can improve sleep quality: a systematic review and meta-analysis. Peer J. 2018;6 doi: 10.7717/peerj.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. γ-Aminobutyric acid production by culturable bacteria from the human intestine. J. Appl. Microbiol. 2012;113:411–417. doi: 10.1111/j.1365-2672.2012.05344.x. [DOI] [PubMed] [Google Scholar]
- Béland M. Aerobic exercise alleviates depressive symptoms in patients with a major non-communicable chronic disease: a systematic review and meta-analysis. Br. J. Sports Med. 2019;54(5):272–278. doi: 10.1136/bjsports-2018-099360. [DOI] [PubMed] [Google Scholar]
- Benedict C. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metabol. 2016;5:1175–1186. doi: 10.1016/j.molmet.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binny J. Transcutaneous electric nerve stimulation (TENS) for acute low back pain: systematic review. Scand J. Pain. 2019;19:225–233. doi: 10.1515/sjpain-2018-0124. [DOI] [PubMed] [Google Scholar]
- Bischoff L.L. The effect of physical activity interventions on occupational stress for health personnel: a systematic review. Int. J. Nurs. Stud. 2019;97:94–104. doi: 10.1016/j.ijnurstu.2019.06.002. [DOI] [PubMed] [Google Scholar]
- Blum W.E.H. Does soil contribute to the human gut microbiome? Microorganisms. 2019;7:E287. doi: 10.3390/microorganisms7090287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd F. Who doesn't visit natural environments for recreation and why: a population representative analysis of spatial, individual and temporal factors among adults in England. Landsc. Urban Plann. 2018;175:102–113. [Google Scholar]
- Bushnell M.C. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K.V. Comparison of the effectiveness of suprascapular nerve block with physical therapy, placebo, and intra-articular injection in the management of chronic shoulder pain: a meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 2016;97:1366–1380. doi: 10.1016/j.apmr.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Chang Liang Z. Novel coronavirus and orthopaedic surgery: early experiences from Singapore. J. Bone Joint Surg. Am. 2020;102(9):745–749. doi: 10.2106/JBJS.20.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X. Investigating the influence of social support on experimental pain and related physiological arousal: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018;92:437–452. doi: 10.1016/j.neubiorev.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Che X. A systematic review of the processes underlying the main and the buffering effect of social support on the experience of pain. Clin. J. Pain. 2018;34:1061–1076. doi: 10.1097/AJP.0000000000000624. [DOI] [PubMed] [Google Scholar]
- Chenaf C. Prevalence of chronic pain with or without neuropathic characteristics in France using the capture-recapture method: a population-based study. Pain. 2018;159:2394–2402. doi: 10.1097/j.pain.0000000000001347. [DOI] [PubMed] [Google Scholar]
- Cheng W.-W. Neuropharmacological activities of phytoncide released from Cryptomeria japonica. J. Wood Sci. 2009;55:27–31. [Google Scholar]
- Cho Y.R. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 2007;17:104–109. [PubMed] [Google Scholar]
- Chu C. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574:543–548. doi: 10.1038/s41586-019-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter I.D. Manipulation and mobilization for treating chronic nonspecific neck pain: a systematic review and meta-analysis for an appropriateness panel. Pain Physician. 2019;22:E55–E70. [PMC free article] [PubMed] [Google Scholar]
- Cronin-de-Chavez A. Not a level playing field: a qualitative study exploring structural, community and individual determinants of greenspace use amongst low-income multi-ethnic families. Health Place. 2019;56:118–126. doi: 10.1016/j.healthplace.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Dahlhamer J. Prevalence of chronic pain and high-impact chronic pain among adults - United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67:1001–1006. doi: 10.15585/mmwr.mm6736a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David T.A. Polarized air as an adjunct in the treatment of burns. Am. J. Phys. Med. 1960;39:111–113. [PubMed] [Google Scholar]
- Davies A.J. Natural killer cells degenerate intact sensory afferents following nerve injury. Cell. 2019;176:716–728. doi: 10.1016/j.cell.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Raaij E.J. The association of illness perception and prognosis for pain and physical function in patients with noncancer musculoskeletal pain: a systematic literature review. J. Orthop. Sports Phys. Ther. 2018;48:789–800. doi: 10.2519/jospt.2018.8072. [DOI] [PubMed] [Google Scholar]
- Drake C. Psychosocial variables and presence, severity and prognosis of plantar heel pain: a systematic review of cross-sectional and prognostic associations. Muscoskel. Care. 2018;16:329–338. doi: 10.1002/msc.1246. [DOI] [PubMed] [Google Scholar]
- Düzgün G., Durmaz Akyol A. Effect of natural sunlight on sleep problems and sleep quality of the elderly staying in the nursing home. Holist. Nurs. Pract. 2017;31:295–302. doi: 10.1097/HNP.0000000000000206. [DOI] [PubMed] [Google Scholar]
- Eccleston C. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain. 2020;161:889–893. doi: 10.1097/j.pain.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh M. Comparative effect of nature-based sounds intervention and headphones intervention on pain severity after cesarean section: a prospective double-blind randomized trial. Anesthesiol. Pain Med. 2019;9 doi: 10.5812/aapm.67835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayaz A. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2015-010364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco L.S. A review of the benefits of nature experiences: more than meets the eye. Int. J. Environ. Res. Publ. Health. 2017;14:E864. doi: 10.3390/ijerph14080864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregoso G. Transition from acute to chronic pain: evaluating risk for chronic postsurgical pain. Pain Physician. 2019;22:479–488. [PubMed] [Google Scholar]
- Fretwell K., Greig A. Towards a better understanding of the relationship between individual's self-reported connection to nature, personal well-being and environmental awareness. Sustainability. 2019;11:1386. [Google Scholar]
- Gaikwad M. Does vitamin D supplementation alleviate chronic nonspecific musculoskeletal pain? A systematic review and meta-analysis. Clin. Rheumatol. 2017;36:1201–1208. doi: 10.1007/s10067-016-3205-1. [DOI] [PubMed] [Google Scholar]
- Gatchel R.J. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol. Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- Grönroos M. Short-term direct contact with soil and plant materials leads to an immediate increase in diversity of skin microbiota. Microbiologyopen. 2019;8 doi: 10.1002/mbo3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H. The tree-species-specific effect of forest bathing on perceived anxiety alleviation of young-adults in urban forests. Ann. For. Res. 2017;60:327–341. [Google Scholar]
- Gulliver W.P. Investigating the therapeutic potential of a probiotic in a clinical population with chronic hand dermatitis. Clin. Cosmet. Invest. Dermatol. 2018;11:265–271. doi: 10.2147/CCID.S164748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br. J. Anaesth. 2019;123:637–654. doi: 10.1016/j.bja.2019.07.026. [DOI] [PubMed] [Google Scholar]
- Haack M. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. 2020;45:205–216. doi: 10.1038/s41386-019-0439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainline B. Pain in elite athletes - neurophysiological, biomechanical and psychosocial considerations: a narrative review. Br. J. Sports Med. 2017;51:1259–1264. doi: 10.1136/bjsports-2017-097890. [DOI] [PubMed] [Google Scholar]
- Hajihasani A. The influence of cognitive behavioral therapy on pain, quality of life, and depression in patients receiving physical therapy for chronic low back pain: a systematic review. Pharm. Manag. PM R. 2019;11:167–176. doi: 10.1016/j.pmrj.2018.09.029. [DOI] [PubMed] [Google Scholar]
- Han J.W. The effects of forest therapy on coping with chronic widespread pain: physiological and psychological differences between participants in a forest therapy program and a control group. Int. J. Environ. Res. Publ. Health. 2016;13:E255. doi: 10.3390/ijerph13030255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvie D.S. Classical conditioning differences associated with chronic pain: a systematic review. J. Pain. 2017;18:889–898. doi: 10.1016/j.jpain.2017.02.430. [DOI] [PubMed] [Google Scholar]
- Hofmeister M. Effectiveness of neurostimulation technologies for the management of chronic pain: a systematic review. Neuromodulation. 2020;23(2):150–157. doi: 10.1111/ner.13020. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016;36:1345–1356. [PubMed] [Google Scholar]
- Holt E.W. Active and passive use of green space, health, and well-being amongst university students. Int. J. Environ. Res. Publ. Health. 2019;16:E424. doi: 10.3390/ijerph16030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M. Impact of viewing vs. not viewing a real forest on physiological and psychological responses in the same setting. Int. J. Environ. Res. Publ. Health. 2014;11:10883–10901. doi: 10.3390/ijerph111010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruschak V., Cochran G. Psychosocial predictors in the transition from acute to chronic pain: a systematic review. Psychol. Health Med. 2018;23:1151–1167. doi: 10.1080/13548506.2018.1446097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W. Improvement of pain, sleep, and quality of life in chronic pain patients with vitamin D supplementation. Clin. J. Pain. 2013;29:341–347. doi: 10.1097/AJP.0b013e318255655d. [DOI] [PubMed] [Google Scholar]
- Huber D. Green exercise and mg-ca-SO4 thermal balneotherapy for the treatment of non-specific chronic low back pain: a randomized controlled clinical trial. BMC Muscoskel. Disord. 2019;20:211. doi: 10.1186/s12891-019-2582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihlebæk C. Association between urban green space and self-reported lifestyle-related disorders in Oslo, Norway. Scand. J. Publ. Health. 2018;46:589–596. doi: 10.1177/1403494817730998. [DOI] [PubMed] [Google Scholar]
- International Association for the Study of Pain (IASP) International Association for the Study of Pain (IASP); Washington, D. C., USA: 2017. IASP Taxonomy. [Google Scholar]
- Itoh H. Lactobacillus gasseri OLL2809 is effective especially on the menstrual pain and dysmenorrhea in endometriosis patients: randomized, double-blind, placebo-controlled study. Cytotechnology. 2011;63:153–161. doi: 10.1007/s10616-010-9326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T. A systematic review and meta-analysis of the global burden of chronic pain without clear etiology in low- and middle-income countries: trends in heterogeneous data and a proposal for new assessment methods. Anesth. Analg. 2016;123:739–748. doi: 10.1213/ANE.0000000000001389. [DOI] [PubMed] [Google Scholar]
- James S.L. Global, regional, and national incidence, pervalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilian H. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: a systematic review and meta-analysis of randomized controlled trials. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2019;94:109651. doi: 10.1016/j.pnpbp.2019.109651. [DOI] [PubMed] [Google Scholar]
- Jayakumar P. What factors are associated with disability after upper extremity injuries? A systematic review. Clin. Orthop. Relat. Res. 2018;476:2190–2215. doi: 10.1097/CORR.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings V., Bamkole O. The relationship between social cohesion and urban green space: an avenue for health promotion. Int. J. Environ. Res. Publ. Health. 2019;16:E452. doi: 10.3390/ijerph16030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S.-Y. Negative air ions and their effects on human health and air quality improvement. Int. J. Mol. Sci. 2018;19:2966. doi: 10.3390/ijms19102966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B. Relief of chronic posterior neck pain depending on the type of forest therapy: comparison of the therapeutic effect of forest bathing alone versus forest bathing with exercise. Ann. Rehabil. Med. 2015;39:957–963. doi: 10.5535/arm.2015.39.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J.R. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent de Grey R.G. Social support and sleep: a meta-analysis. Health Psychol. 2018;37:787–798. doi: 10.1037/hea0000628. [DOI] [PubMed] [Google Scholar]
- Keskinen K.E. Nature as a facilitator for physical activity: defining relationships between the objective and perceived environment and physical activity among community-dwelling older people. Health Place. 2018;49:111–119. doi: 10.1016/j.healthplace.2017.12.003. [DOI] [PubMed] [Google Scholar]
- Khodaverdi S. Beneficial effects of oral Lactobacillus on pain severity in women suffering from endometriosis: a pilot placebo-controlled randomized clinical trial. Int. J. Fertil. Steril. 2019;13:178–183. doi: 10.22074/ijfs.2019.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. Effects of horticultural therapy program on serum cortisol, pain, anxiety and depression of the hospice patients. Korean J. Hortic. Sci. Technol. 2006;24:95–103. [Google Scholar]
- Kim M.J. Effects of dietary phytoncides extracted from Korean pine (Pinus koraiensis) cone on performance, egg quality, gut microflora, and immune response in laying hens. J. Anim. Physiol. Anim. Nutr. 2018;102:1220–1231. doi: 10.1111/jpn.12934. [DOI] [PubMed] [Google Scholar]
- Kim R. Identifying risk factors for first-episode neck pain: a systematic review. Musculoskelet Sci. Pract. 2018;33:77–83. doi: 10.1016/j.msksp.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Knights D. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut. 2013;62:1505–1510. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsuzaki N. Production of γ-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 2005;22:497–504. [Google Scholar]
- Kosek E. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157:1382–1386. doi: 10.1097/j.pain.0000000000000507. [DOI] [PubMed] [Google Scholar]
- Kreutz C. Effects of physical and mind-body exercise on sleep problems during and after breast cancer treatment: a systematic review and meta-analysis. Breast Canc. Res. Treat. 2019;176:1–15. doi: 10.1007/s10549-019-05217-9. [DOI] [PubMed] [Google Scholar]
- Kuo M. How might contact with nature promote human health? Promising mechanisms and a possible central pathway. Front. Psychol. 2015;6:1093. doi: 10.3389/fpsyg.2015.01093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuorinka I. Standardised Nordic questionnaires for the analysis of musculoskeletal symptoms. Appl. Ergon. 1987;18:233–237. doi: 10.1016/0003-6870(87)90010-x. [DOI] [PubMed] [Google Scholar]
- Lai P.S. Impact of environmental microbiota on human microbiome of workers in academic mouse research facilities: an observational study. PloS One. 2017;12 doi: 10.1371/journal.pone.0180969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtzin N. A randomized trial of nature scenery and sounds versus urban scenery and sounds to reduce pain in adults undergoing bone marrow aspirate and biopsy. J. Alternative Compl. Med. 2010;16:965–972. doi: 10.1089/acm.2009.0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman O. Does exercise improve sleep quality in individuals with mental illness? A systematic review and meta-analysis. J. Psychiatr. Res. 2019;109:96–106. doi: 10.1016/j.jpsychires.2018.11.004. [DOI] [PubMed] [Google Scholar]
- Lei M. The effect of probiotic Lactobacillus casei Shirota on knee osteoarthritis: a randomised double-blind, placebo-controlled clinical trial. Benef. Microbes. 2017;8:697–703. doi: 10.3920/BM2016.0207. [DOI] [PubMed] [Google Scholar]
- Leonardi M. Endometriosis and the microbiome: a systematic review. BJOG. 2019;127:239–249. doi: 10.1111/1471-0528.15916. [DOI] [PubMed] [Google Scholar]
- Li Q. Phytoncides (wood essential oils) induce human natural killer cell activity. Immunophatmacol. Immunotoxicol. 2006;28:319–333. doi: 10.1080/08923970600809439. [DOI] [PubMed] [Google Scholar]
- Li Q. Visiting a forest, but not a city, increases human natural killer activity and expression of anti-cancer proteins. Int. J. Immunopathol. Pharmacol. 2008;21:117–128. doi: 10.1177/039463200802100113. [DOI] [PubMed] [Google Scholar]
- Li H.L. Phytoncide, phytogenic feed additive as an alternative to conventional antibiotics, improved growth performance and decreased excreta gas emission without adverse effect on meat quality in broiler chickens. Livest. Sci. 2015;181:1–6. [Google Scholar]
- Li Z.H. Cyclic nucleotide signaling in sensory neuron hyperexcitability and chronic pain after nerve injury. Neurobiol. Pain. 2019;6:100028. doi: 10.1016/j.ynpai.2019.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddicoat C. Naturally-diverse airborne environmental microbial exposures modulate the gut microbiome and may provide anxiolytic benefits in mice. Sci. Total Environ. 2020;701:134684. doi: 10.1016/j.scitotenv.2019.134684. [DOI] [PubMed] [Google Scholar]
- Liszio S. The relaxing effect of virtual nature: immersive technology provides relief in acute stress situations. ARCTT. 2018;16:87–93. [Google Scholar]
- Liu J. Neuroprotective effects of Clostridium butyricum against vascular dementia in mice via metabolic butyrate. BioMed Res. Int. 2015;2015:412946. doi: 10.1155/2015/412946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. Association of depression/anxiety symptoms with neck pain: a systematic review and meta-analysis of literature in China. Pain Res. Manag. 2018:3259431. doi: 10.1155/2018/3259431. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R.T. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019;102:13–23. doi: 10.1016/j.neubiorev.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser J.D., Treede R.D. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137:473–477. doi: 10.1016/j.pain.2008.04.025. [DOI] [PubMed] [Google Scholar]
- López-de-Uralde-Villanueva I. A systematic review and meta-analysis on the effectiveness of graded activity and graded exposure for chronic nonspecific low back pain. Pain Med. 2016;17:172–188. doi: 10.1111/pme.12882. [DOI] [PubMed] [Google Scholar]
- Lowe H. Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clin. Psychol. Rev. 2019;68:1–12. doi: 10.1016/j.cpr.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Lucado A.M. Do joint mobilizations assist in the recovery of lateral elbow tendinopathy? A systematic review and meta-analysis. J. Hand Ther. 2019;32:262–276. doi: 10.1016/j.jht.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Maas J. Morbidity is related to a green living environment. J. Epidemiol. Community Health. 2009;63:967–973. doi: 10.1136/jech.2008.079038. [DOI] [PubMed] [Google Scholar]
- Mailing L.J. Exercise and the gut microbiome: a review of the evidence, potential mechanisms, and implications for human health. Exerc. Sport Sci. Rev. 2019;47:75–85. doi: 10.1249/JES.0000000000000183. [DOI] [PubMed] [Google Scholar]
- Malatji B.G. A diagnostic biomarker profile for fibromyalgia syndrome based on an NMR metabolomics study of selected patients and controls. BMC Neurol. 2017;17:88. doi: 10.1186/s12883-017-0863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G.X. Therapeutic effect of forest bathing on human hypertension in the elderly. J. Cardiol. 2012;60:495–502. doi: 10.1016/j.jjcc.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Mayer E.A. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 2014;34:15490–15496. doi: 10.1523/JNEUROSCI.3299-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell C.P. Physical activity and anxiety: a systematic review and meta-analysis of prospective cohort studies. Am. J. Prev. Med. 2019;57:545–556. doi: 10.1016/j.amepre.2019.05.012. [DOI] [PubMed] [Google Scholar]
- National Health. Medical Research Council . National Health and Medical Research Council Australia; 2009. NHMRC Additional Levels of Evidence and Grades for Recommendations for Developers of Guidelines. Stage 2 Consultation. [Google Scholar]
- Miller A. The prevalence of pain and analgesia use in the Australian population: findings from the 2011 to 2012 Australian National Health Survey. Pharmacoepidemiol. Drug Saf. 2017;26:1403–1410. doi: 10.1002/pds.4301. [DOI] [PubMed] [Google Scholar]
- Minehart J.R. The effect of artificially ionized air on post-operative discomfort. Am. J. Phys. Med. 1961;40:56–62. [PubMed] [Google Scholar]
- Moore M. Linking human and ecosystem health: the benefits of community involvement in conservation groups. EcoHealth. 2007;3:255–261. [Google Scholar]
- Morres I.D. Aerobic exercise for adult patients with major depressive disorder in mental health services: a systematic review and meta-analysis. Depress. Anxiety. 2019;36:39–53. doi: 10.1002/da.22842. [DOI] [PubMed] [Google Scholar]
- Morton L. Beliefs about back pain and pain management behaviours, and their associations in the general population: a systematic review. Eur. J. Pain. 2019;23:15–30. doi: 10.1002/ejp.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley G.L., Arntz A. The context of a noxious stimulus affects the pain it evokes. Pain. 2007;133:64–71. doi: 10.1016/j.pain.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Nagakura Y. Challenges in drug discovery for overcoming 'dysfunctional pain': an emerging category of chronic pain. Expet Opin. Drug Discov. 2015;10:1043–1045. doi: 10.1517/17460441.2015.1066776. [DOI] [PubMed] [Google Scholar]
- Nagy-Szakal D. Fecal metagenomic profiles in subgroups of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Microbiome. 2017;5:44. doi: 10.1186/s40168-017-0261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A. Physical activity during pregnancy and postpartum depression: systematic review and meta-analysis. J. Affect. Disord. 2019;246:29–41. doi: 10.1016/j.jad.2018.12.009. [DOI] [PubMed] [Google Scholar]
- Nasari M. Effects of nature sounds on sleep quality among patients hospitalized in coronary care units: a randomized controlled clinical trial. Nurs. Midwifery Stud. 2018;7:18–23. [Google Scholar]
- New Zealand Ministry of Health . New Zealand Government; Wellington: 2016. Green Prescriptions. [Google Scholar]
- Ngamkham S. A systematic review: mindfulness intervention for cancer-related pain. Asia Pac. J. Oncol. Nurs. 2019;6:161–169. doi: 10.4103/apjon.apjon_67_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel J.C. A culture-independent analysis of the microbiota of female interstitial cystitis/bladder pain syndrome participants in a MAPP Research Network. J. Clin. Med. 2019;8:E415. doi: 10.3390/jcm8030415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet G., Sehgal A. Pharmacology in the management of chronic pain. Anaesth. Intensive Care Med. 2019;20:555–558. [Google Scholar]
- Olivereau J.M. Effect of negative atmospheric ions on thermal algesia in mice. C. R. Seances Soc. Biol. Fil. 1970;164:287–291. [PubMed] [Google Scholar]
- Oxford Centre for Evidence-Based Medicine . Oxford University; Oxford: 2011. Oxford Centre for Evidence-Based Medicine 2011 Levels of Evidence. [Google Scholar]
- Peppin J.F., Schatman M.E. Terminology of chronic pain: the need to "level the playing field. J. Pain Res. 2016;9:23–24. doi: 10.2147/JPR.S99629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokusaeva K. GABA-producing Bifidobacterium dentium modulates visceral sensitivity in the intestine. Neuro Gastroenterol. Motil. 2017;29 doi: 10.1111/nmo.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poroyko V.A. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016;6:35405. doi: 10.1038/srep35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K. Pain bugs: gut microbiota and pain disorders. Curr. Opin. Physiol. 2019;11:97–102. [Google Scholar]
- Rivière A. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts H. Identifying effective behavior change techniques in built environment interventions to increase use of green space: a systematic review. Environ. Behav. 2016;50:28–55. [Google Scholar]
- Rodriguez-Ayllon M. Role of physical activity and sedentary behavior in the mental health of preschoolers, children and adolescents: a systematic review and meta-analysis. Sports Med. 2019;49:1383–1410. doi: 10.1007/s40279-019-01099-5. [DOI] [PubMed] [Google Scholar]
- Rook G.A.W. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin. Immunopathol. 2004;25:237–255. doi: 10.1007/s00281-003-0148-9. [DOI] [PubMed] [Google Scholar]
- Saadatmand V. Effects of natural sounds on pain: a randomized controlled trial with patients receiving mechanical ventilation support. Pain Manag. Nurs. 2015;16:483–492. doi: 10.1016/j.pmn.2014.09.006. [DOI] [PubMed] [Google Scholar]
- Sarac N.J. A review of state guidelines for elective orthopaedic procedures during the COVID-19 outbreak. J. Bone Joint Surg. Am. 2020 doi: 10.2106/JBJS.20.00510. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefcik J.S. Perceptions of nature and access to green space in four urban neighborhoods. Int. J. Environ. Res. Publ. Health. 2019;16:E2313. doi: 10.3390/ijerph16132313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby S. Facilitators and barriers to green exercise in chronic pain. Ir. J. Med. Sci. 2019;188:973–978. doi: 10.1007/s11845-018-1923-x. [DOI] [PubMed] [Google Scholar]
- Shiri R., Falah-Hassani K. Does leisure time physical activity protect against low back pain? Systematic review and meta-analysis of 36 prospective cohort studies. Br. J. Sports Med. 2017;51:1410–1418. doi: 10.1136/bjsports-2016-097352. [DOI] [PubMed] [Google Scholar]
- Shiro Y. Stool consistency is significantly associated with pain perception. PloS One. 2017;12 doi: 10.1371/journal.pone.0182859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoskes D.A. Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. J. Urol. 2016;196:435–441. doi: 10.1016/j.juro.2016.02.2959. [DOI] [PubMed] [Google Scholar]
- Siragusa V.A. Synthesis of gamma-aminobutyric acid by lactic acid bacteria isolated from a variety of Italian cheese. Appl. Environ. Microbiol. 2007;73:7283–7290. doi: 10.1128/AEM.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.P. Gut microbiome diversity is associated with sleep physiology in humans. PloS One. 2019;14 doi: 10.1371/journal.pone.0222394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigger F.S. Effects of exercise on inflammatory, oxidative, and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: a systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:616–624. doi: 10.1093/gerona/gly173. [DOI] [PubMed] [Google Scholar]
- Strandwitz P. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019;4:396–403. doi: 10.1038/s41564-018-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz J. Effects of evening exercise on sleep in healthy participants: a systematic review and meta-analysis. Sports Med. 2019;49:269–287. doi: 10.1007/s40279-018-1015-0. [DOI] [PubMed] [Google Scholar]
- Su Y.C. Production of the secondary metabolites gamma-aminobutyric acid and monacolin K by Monascus. J. Ind. Microbiol. Biotechnol. 2003;30:41–46. doi: 10.1007/s10295-002-0001-5. [DOI] [PubMed] [Google Scholar]
- Tasnim N. Linking the gut microbial ecosystem with the environment: does gut health depend on where we live? Front. Microbiol. 2017;8:1935. doi: 10.3389/fmicb.2017.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L., Hochuli D.F. Defining greenspace: multiple uses across multiple disciplines. Landsc. Urban Plann. 2017;158:25–38. [Google Scholar]
- Taylor A.M.W. Mesolimbic dopamine signaling in acute and chronic pain: implications for motivation, analgesia, and addiction. Pain. 2016;157:1194–1198. doi: 10.1097/j.pain.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegner H. Neurophyiological pain education for patients with chronic low back pain: a systematic review and meta-analysis. Clin. J. Pain. 2018;34:778–786. doi: 10.1097/AJP.0000000000000594. [DOI] [PubMed] [Google Scholar]
- Tengku Moud T.A.M. Social support and depression among community dwelling older adults in Asia: a systematic review. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm T. Fecal microbiota transplantation for fibromyalgia: a case report and review of the literature. Open J. Gastroenterol. 2017;7:131–139. [Google Scholar]
- Turk D.C., Okifuji A. Psychological factors in chronic pain: evolution and revolution. J. Consult. Clin. Psychol. 2002;70:678–690. doi: 10.1037//0022-006x.70.3.678. [DOI] [PubMed] [Google Scholar]
- Twohig-Bennett C., Jones A. The health benefits of the great outdoors: a systematic review and meta-analysis of greenspace exposure and health outcomes. Environ. Res. 2018;166:628–637. doi: 10.1016/j.envres.2018.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino B.N. Social support, social integration, and inflammatory cytokines: a meta-analysis. Health Psychol. 2018;37:462–471. doi: 10.1037/hea0000594. [DOI] [PubMed] [Google Scholar]
- Vaghef-Mehrabany E. Can psychobiotics “mood”ify gut? An update systematic review of randomized controlled trials in healthy and clinical subjects, on anti-depressant effects of probiotics, prebiotics, and synbiotics. Clin. Nutr. 2019;39(5):1395–1410. doi: 10.1016/j.clnu.2019.06.004. [DOI] [PubMed] [Google Scholar]
- Vardeh D. Toward a mechanism-based approach to pain diagnosis. J. Pain. 2016;17:T50–T69. doi: 10.1016/j.jpain.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verra M.L. Horticultural therapy for patients with chronic musculoskeletal pain: results of a pilot study. Alternative Ther. Health Med. 2012;18:44–50. [PubMed] [Google Scholar]
- Villemure C., Bushnell M.C. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Vincent E. The effects of nature images on pain in a simulated hospital patient room. HERD. 2010;3:42–55. doi: 10.1177/193758671000300306. [DOI] [PubMed] [Google Scholar]
- Vos T. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse M. Vitamin D and the gut microbiome: a systematic review of in vivo studies. Eur. J. Nutr. 2019;58:2895–2910. doi: 10.1007/s00394-018-1842-7. [DOI] [PubMed] [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge: 1986. Biophilia. [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2018. International Classification of Diseases. 11th revision (ICD-11) [Google Scholar]
- Wu Z. The association between vitamin D concentration and pain: a systematic review and meta-analysis. Publ. Health Nutr. 2018;21:2022–2037. doi: 10.1017/S1368980018000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Effects of therapeutic ultrasound for knee osteoarthritis; a systematic review and meta-analysis. Clin. Rehabil. 2019;33:1863–1875. doi: 10.1177/0269215519866494. [DOI] [PubMed] [Google Scholar]
- Xie E. Association between radiofrequency rhizotomy parameters and duration of pain relief in trigeminal neuralgia patients with recurrent pain. World Neurosurg. 2019;129:e128–e133. doi: 10.1016/j.wneu.2019.05.059. [DOI] [PubMed] [Google Scholar]
- Yang S.Y. Production of gamma-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids. 2008;34:473–478. doi: 10.1007/s00726-007-0544-x. [DOI] [PubMed] [Google Scholar]
- Yang B. Effects of regulating intestinal microbiota on anxiety symptoms: a systematic review. Gen. Psychiatr. 2019;32 doi: 10.1136/gpsych-2019-100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong W.C. Effect of vitamin D supplementation in chronic widespread pain: a systematic review and meta-analysis. Clin. Rheumatol. 2017;36:2825–2833. doi: 10.1007/s10067-017-3754-y. [DOI] [PubMed] [Google Scholar]
- Zadro J. Mapping the association between vitamin D and low back pain: a systematic review and meta-analysis of observational studies. Pain Physician. 2017;20:611–640. [PubMed] [Google Scholar]
- Zadro J.R. Is vitamin D supplementation effective for low back pain? A systematic review and meta-analysis. Pain Physician. 2018;21:121–145. [PubMed] [Google Scholar]
- Zhang S. Influences of phytoncide supplementation on growth performance, nutrient digestibility, blood profiles, diarrhea scores and fecal microflora shedding in weaning pigs. Asian-Australas. J. Anim. Sci. 2012;25:1309–1315. doi: 10.5713/ajas.2012.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.Y. Effects of dietary supplemental phytoncide instead of zinc oxide on growth performance, nutrient digestibility, blood profiles, and faecal microflora in growing pigs. J. Anim. Physiol. Anim. Nutr (Berl). 2018;103:269–275. doi: 10.1111/jpn.13030. [DOI] [PubMed] [Google Scholar]
- Zheng G. Effect of aerobic exercise on inflammatory markers in healthy middle-aged and older adults: a systematic review and meta-analysis of randomized controlled trials. Front. Aging Neurosci. 2019;11:98. doi: 10.3389/fnagi.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C.J. Exogenous melatonin in the treatment of pain: a systematic review and meta-analysis. Oncotarget. 2017;8:100582–100592. doi: 10.18632/oncotarget.21504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M. Descending facilitation: from basic science to the treatment of chronic pain. Mol. Pain. 2017;13 doi: 10.1177/1744806917699212. 1744806917699212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zyoud S.H. Global research trends in microbiome-gut-brain axis during 2009-2018: a bibliographic and visualized study. BMC Gastroenterol. 2019;19:158. doi: 10.1186/s12876-019-1076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no data used in this manuscript.