Abstract
Introduction
We sought to examine the association of race/ethnicity with willingness to engage in studies that involve procedures typical of Alzheimer's disease (AD) clinical trials and determine whether any observed differences could be explained by research attitudes.
Methods
We studied 2749 adults aged ≥50 years who enrolled in a community‐based recruitment registry.
Results
Compared to non‐Hispanic (NH) whites (n = 2393, 87%), Hispanics (n = 191, 7%), NH Asians (n = 129, 5%) and NH blacks (n = 36, 1%) were 44%, 46%, and 64% less willing, respectively, to be contacted for studies that have requirements typical of AD prevention trials, namely: cognitive testing, brain imaging, blood draws, and investigational medications. Mediation by research attitudes was explored, but did not explain the observed differences.
Discussion
Our findings suggest that ethnoracial minorities are less willing to engage in studies that are typical of AD prevention trials. Future work should focus on understanding the factors that drive these differences.
Keywords: Alzheimer's disease, biomarker testing, prevention trials, race/ethnicity, recruitment, research attitudes, research participation
1. BACKGROUND
In the United States, ethnoracial minorities bear a disproportionate burden of Alzheimer's disease (AD) and related dementias. 1 , 2 , 3 Yet, they remain severely underrepresented in dementia studies, 4 particularly clinical trials. 5 , 6 Greater participation of minority groups is imperative because it leads to better generalization of trial findings and could enable identification of potential differences in treatment response between groups. 7 , 8 In addition to testing experimental medications, AD trials typically require participants to engage in potentially burdensome procedures. Recruitment challenges may worsen in the coming years as more AD trials focus on enrolling predementia populations, for whom sensitivities toward study risks may be heightened. 9
Online recruitment registries have emerged around the globe as a novel approach for accelerating enrollment of participants in clinical studies, including dementia research. 10 Eligible individuals are identified from the community through various outreach modalities and enrolled in a registry online. Researchers benefit by having access to a large research‐ready and often prescreened population. It remains unclear whether registries can improve enrollment of ethnically and racially diverse samples.
Some previous studies suggest that ethnoracial minorities are as willing as their non‐Hispanic (NH) white counterparts to participate in health research, 11 , 12 while others report a lower willingness among ethnoracial minorities. 13 , 14 , 15 Negative attitudes toward research, such as mistrust of clinical researchers, have been shown to be important predictors for lower willingness to participate in clinical research studies, 16 including in AD trials. 17
Improving our understanding of recruitment disparities by race/ethnicity is of paramount importance for generalizability of safety and efficacy of AD trials and is consistent with the goals set forth by the National Institute on Aging's National Strategy for Clinical Research Recruitment. 18 Accordingly, the present study leverages data from a local online recruitment registry in southern California to interrogate the relationship between race/ethnicity and older adults’ willingness to engage in research studies, and to explore whether this potential association is mediated by research attitudes.
2. METHODS
2.1. Study population
The Consent‐to‐Contact (C2C) Registry was developed and launched in August 2016 to accelerate enrollment of participants in clinical research studies at the University of California, Irvine (UCI), with a particular emphasis on preclinical AD trials. 19 C2C participants were aged 18 years and older and recruited from Orange County, CA during 2016–2019 through enrollment strategies including earned media, community outreach activities, postcard mailings, e‐mail, and social media. Upon enrolling through the registry website, participants provided informed consent electronically and completed structured questionnaires that ascertained information about research willingness, research attitudes, and sociodemographic and clinical characteristics. For the current study, we restricted analyses to participants who were aged 50 years or older. The Institutional Review Board at UCI approved this study.
2.2. Assessment of research willingness
The primary outcome was research willingness, which was assessed using separate items that asked participants about their willingness to be contacted for research studies that involved nine procedure types, namely: (1) modification of diet or physical activity, (2) cognitive testing, (3) blood draws, (4) magnetic resonance imaging (MRI), (5) positron emission tomography (PET) imaging, (6) approved and (7) investigational medications, (8) lumbar puncture (LP), and (9) autopsy. For analysis, we excluded any participant who failed to respond to at least one of these nine question items (n = 1).
HIGHLIGHTS
Ethnoracial minorities are underrepresented in Alzheimer's disease (AD) clinical trials.
We studied 2749 adults aged ≥50 years enrolled in a local online recruitment registry.
Willingness to be contacted for participation in AD trials was lower in minorities.
Research attitudes did not explain differences in willingness by race/ethnicity.
RESEARCH IN CONTEXT
Systematic Review: Numerous studies have reported severe disparities in the recruitment of ethnoracial minorities to clinical trials of Alzheimer's disease (AD). Less is known, however, about whether underrepresented minorities are willing to engage in procedures typically done in AD trials.
Interpretation: We found that, in a community‐based recruitment registry, ethnoracial minorities were less willing than non‐Hispanic whites to engage in studies involving cognitive testing, brain imaging, blood draws, and investigational medications. These differences were not explained by research attitudes.
Future Directions: Future work should focus on understanding the sociocultural and ethnicity‐specific factors that could potentially drive these differences.
2.3. Sociodemographic and clinical measures
Sociodemographic characteristics included self‐reported race/ethnicity, age, sex, and years of education. For our predictor of interest, we classified participants into four mutually exclusive ethnoracial categories of NH white, NH Asian, NH black, and Hispanics of any race. Due to small numbers, we excluded participants who self‐identified as American Indian or Alaskan Native (n = 3) and Native Hawaiian or other Pacific Islander (n = 2). We also excluded individuals who self‐identified with another race not specified (n = 36) and NH participants who were of multiple racial backgrounds (n = 17). Sensitivity analysis demonstrated that classifying multiracial participants into a single (majority) race or excluding them altogether did not change our findings. The final analytic sample was comprised of 2749 older adults.
We collected information about clinical characteristics from all participants and included self‐reported current medical conditions such as cancer, diabetes, coronary artery disease, congestive heart failure, kidney disease, liver disease, hypertension, hypercholesterolemia, and emphysema. In analysis, the number of current medical conditions or co‐morbidities were grouped into three categories (none, 1, and ≥2). We inquired about neurological diseases using the question, “Have you ever been diagnosed with a neurological disorder?” and offering 13 forced choice responses, including “other neurological disorder.” Subjective cognitive function was assessed using the 14‐item Cognitive Function Inventory (CFI). 20 Last, we collected information on the number of current prescription medications, which we grouped into four categories (none, 1–2, 3–4, and ≥5).
2.4. Assessment of research attitudes
Research attitudes, assessed using the 7‐item Research Attitude Questionnaire (RAQ) with 5‐point Likert‐type responses, 21 was a priori considered a potential mediator in the hypothesized relationship between ethnoracial status and research willingness. Among those who responded to at least six out of seven items, we operationalized the construct of research attitudes into a summary score that ranged from 7 to 35, with higher scores representing more positive research attitudes.
2.5. Statistical analysis
To investigate the association between race/ethnicity and research willingness, we fit separate logistic regression models for each of the nine procedure types. Each model controlled for potential confounding factors, which we identified a priori as age, sex, education, number of co‐morbidities, number of concomitant medications, and CFI scores. Estimated odds ratios (ORs) along with corresponding Wald‐based 95% confidence intervals were reported for the predictor of interest in all models. In secondary analyses, we assessed for potential mediation of each association 22 by first quantifying the relationship between: (1) race/ethnicity and research willingness, (2) race/ethnicity and research attitudes, and (3) research attitudes and research willingness adjusting for race/ethnicity. Next, we quantified the degree of attenuation of the association between race/ethnicity and research willingness when research attitudes (operationalized as RAQ summary score) was in the regression model. In sensitivity analysis, we assessed this degree of attenuation after adjustment for individual RAQ items rather than the summary measure.
In exploratory analyses, we assessed the association between race/ethnicity and research willingness in the context of AD prevention by creating a composite measure to simulate the eligibility requirements of an AD prevention trial. 9 The measure grouped five of the nine procedure types—namely (1) cognitive testing, (2) MRI scans, (3) PET scans, (4) blood draws, and (5) investigational medications—into a summary score that ranged from 0 to 5. Because relatively few participants scored 0–2 (n = 120) on the composite measure, we grouped these individuals together and created four levels of scores. Additionally, we excluded 460 participants who had a previous neurological disorder or non‐basal cell cancers. We used ordinal logistic regression to estimate cumulative ORs for having a higher versus a lower score on the composite measure. Score tests on each model were used to test the null hypothesis that the proportional odds assumption holds. In no case was there statistical evidence that the proportionality assumption was violated.
We had a minimal amount of missing data in our outcome measure of research willingness among those who responded to at least one of the nine items; missing responses ranged from 0.8% to 1.2% across items that had missing information. A total of 215 (7.8%) participants had two or more missing RAQ items and were excluded from mediation analyses. Excluded participants were less likely to have a college education (59% vs 74%, P < .0001), more likely to be using five or more concomitant medications (52% vs 31%, P < .0001), and more likely to have two or more co‐morbidities (44% vs 32%, P = .0006). In our multivariable regression models, we used established methods for multiple imputation, 23 with 20 imputed data sets, to impute missing information on key adjustment variables of educational attainment (n = 33, 1.2%), concomitant medications (n = 100, 3.6%), presence of a neurological disorder (n = 29, 1.1%), and CFI scores (n = 332, 12.1%). We also used a complete case approach and found that the results did not appreciably change. No adjustment for multiple comparisons were used for exploratory analyses. All statistical analyses were performed in SAS version 9.4 (SAS institute Inc., Cary, NC).
3. RESULTS
The distribution of our study population characteristics is depicted in Table 1. The sample was largely female (63%), had an average age of 66 years, and was highly educated (73% college or higher). Hispanics tended to be younger and more likely to report no comorbidities than NH whites (47% vs 33%). NH blacks were more likely than NH whites to have ≥2 co‐morbidities (42% vs 34%) and less likely to be taking concomitant medications (78% vs 87%). Hispanics and NH Asians had higher mean CFI scores than NH whites. Notably, the overall average RAQ summary score was 29 and did not differ by race/ethnicity. Roughly half of the sample enrolled through e‐mail outreach, with NH Asians more frequently having enrolled through community outreach.
TABLE 1.
Distribution of characteristics among C2C participants aged ≥50 years by race/ethnicity
| Overall sample | NH white | Hispanic (any race) | NH Asian | NH black | |
|---|---|---|---|---|---|
| Total N | 2,749 | 2,393 (87.1%) | 191 (7.0%) | 129 (4.7%) | 36 (1.3%) |
| Age, mean (SD) | 66.1 (9.2) | 66.7 (9.3) | 61.1 (7.7) | 64.4 (8.5) | 62.8 (9.9) |
| Female sex, n (%) | 1,694 (61.6%) | 1,480 (61.9%) | 117 (61.3%) | 74 (57.4%) | 23 (63.9%) |
| Educational attainment, n (%) | |||||
| Less than high school (<12 years) | 27 (1.0%) | 18 (0.8%) | 8 (4.3%) | 1 (0.8%) | 0 |
| High school (12 years) | 181 (6.7%) | 143 (6.0%) | 30 (16.0%) | 5 (4.0%) | 3 (8.3%) |
| Some college (13‐15 years) | 525 (19.3%) | 445 (18.8%) | 51 (27.3%) | 16 (12.7%) | 13 (36.1%) |
| College or higher (≥16 years) | 1,983 (73.0%) | 1,761 (74.4%) | 98 (52.4%) | 104 (82.5%) | 20 (55.6%) |
| Sources of enrollment | |||||
| Email outreach | 1410 (51.8%) | 1254 (53.0%) | 86 (45.0%) | 50 (39.4%) | 20 (55.6%) |
| Community talks | 281 (10.3%) | 241 (10.2%) | 17 (8.9%) | 21 (16.5%) | 2 (5.6%) |
| Postcard mailings | 231 (8.5%) | 192 (8.1%) | 22 (11.5%) | 14 (11.0%) | 3 (8.3%) |
| Other a | 798 (29.3%) | 679 (28.7%) | 66 (34.6%) | 42 (33.1%) | 11 (30.6%) |
| No. of current co‐morbidities a | |||||
| None | 942 (34.3%) | 798 (33.4%) | 90 (47.1%) | 43 (33.3%) | 11 (30.6%) |
| 1 | 895 (32.6%) | 794 (33.2%) | 41 (21.5%) | 50 (38.8%) | 10 (27.8%) |
| ≥2 | 912 (33.2%) | 801 (33.5%) | 60 (31.4%) | 36 (27.9%) | 15 (41.7%) |
| Neurological disorder diagnosis (yes), n (%) | 440 (16.2%) | 401 (16.9%) | 24 (12.8%) | 12 (9.5%) | 3 (8.6%) |
| No. of concomitant medications, n (%) | |||||
| None | 380 (14.4%) | 309 (13.4%) | 37 (20.4%) | 27 (21.6%) | 7 (21.9%) |
| 1‐2 | 753 (28.4%) | 644 (27.9%) | 58 (32.0%) | 41 (32.8%) | 10 (31.3%) |
| 3‐4 | 686 (25.9%) | 614 (26.6%) | 43 (23.8%) | 24 (19.2%) | 5 (15.6%) |
| ≥5 | 830 (31.3%) | 744 (32.2%) | 43 (23.8%) | 33 (26.4%) | 10 (31.3%) |
| Cognitive Function Inventory scores, mean (SD) | 2.8 (2.7) | 2.7 (2.7) | 3.4 (2.9) | 3.4 (3.2) | 2.9 (2.5) |
| Research attitude questionnaire scores, mean (SD) | 28.7 (4.4) | 28.7 (4.3) | 28.4 (4.9) | 28.6 (4.9) | 27.9 (4.4) |
Abbreviations: C2C, Consent‐to‐Contact; NH, non‐Hispanic; SD, standard deviation
aOther sources include earned media (newspaper [6.7%], television [<1%], radio [<1%]), social media (1.5%), online searches (3.6%), provider referral (4.0%), friend referral (5.2%), and other sources not specified (7.5%).
Comorbidities include self‐reported cancer, diabetes, coronary artery disease, congestive heart failure, kidney disease, liver disease, hypertension, hypercholesterolemia, and emphysema.
Table 2 compares the distribution of responses for each of the RAQ items across ethnoracial groups. Overall, roughly three quarters of participants agreed with each item, and relatively few (<5%) disagreed. Compared to NH whites, a higher proportion of NH blacks responded “neutral” to RAQ items that inquired about having a positive view of medical research (17% vs 6%), trust in medical researchers (35% vs 17%), viewing medical research as generally safe (41% vs 23%), confidence that personal information would be kept private and confidential (39% vs19%), and confidence that medical research would find cures to major diseases during their lifetimes (35% vs 21%). No appreciable differences in responses were observed between NH whites and other ethnoracial groups.
TABLE 2.
Distribution of research attitude responses by race/ethnicity
| Level of agreement n (%) | |||||
|---|---|---|---|---|---|
| Research Attitude Questionnaire itemsa a | NH white | Hispanic (any race) | NH Asian | NH black | |
| 1. I have a positive view about medical research in general. | Disagree | 30 (1.4%) | 3 (1.8%) | 3 (2.5%) | 0 |
| Neutral | 123 (5.6%) | 16 (9.5%) | 11 (9.1%) | 5 (17.2%) | |
| Agree | 2,061 (93.1%) | 149 (88.7%) | 108 (88.5%) | 24 (82.8%) | |
| 2. Medical researchers can be trusted to protect the interests of people who take part in their research studies | Disagree | 38 (1.7%) | 4 (2.4%) | 4 (3.3%) | 0 |
| Neutral | 374 (16.9%) | 33 (19.5%) | 22 (18.0%) | 10 (34.5%) | |
| Agree | 1,799 (81.4%) | 132 (78.1%) | 96 (78.7%) | 19 (65.5%) | |
| 3. We all have some responsibility to help others by volunteering for medical research. | Disagree | 783 (3.8%) | 7 (4.1%) | 4 (3.3%) | 0 |
| Neutral | 414 (18.7%) | 27 (16.0%) | 19 (15.7%) | 8 (27.6%) | |
| Agree | 1,716 (77.5%) | 135 (79.9%) | 98 (81.0%) | 21 (72.4%) | |
| 4. Society needs to devote more resources to medical research. | Disagree | 33 (1.5%) | 6 (3.6%) | 3 (2.5%) | 0 |
| Neutral | 245 (11.1%) | 16 (9.5%) | 17 (14.0%) | 3 (10.3%) | |
| Agree | 1,933 (87.4%) | 147 (87.0%) | 102 (83.6%) | 26 (89.7%) | |
| 5. Participating in medical research is generally safe. | Disagree | 55 (2.5%) | 6 (3.6%) | 5 (4.1%) | 0 |
| Neutral | 518 (23.4%) | 52 (31.1%) | 29 (24.0%) | 12 (41.4%) | |
| Agree | 1,637 (74.1%) | 109 (65.3%) | 87 (71.9%) | 17 (58.6%) | |
| 6. If I volunteer for medical research, I know my personal information will be kept private and confidential. | Disagree | 70 (3.2%) | 5 (3.0%) | 6 (4.9%) | 0 |
| Neutral | 419 (19.0%) | 29 (17.3%) | 24 (19.7%) | 11 (39.3%) | |
| Agree | 1,721 (77.9%) | 134 (79.8%) | 92 (75.4%) | 17 (60.7%) | |
| 7. Medical research will find cures for many major diseases during my lifetime. | Disagree | 105 (4.8%) | 9 (5.3%) | 2 (1.7%) | 2 (6.9%) |
| Neutral | 467 (21.1%) | 29 (17.2%) | 31 (25.6%) | 10 (34.5%) | |
| Agree | 1,640 (74.1%) | 131 (77.5%) | 88 (72.7%) | 17 (58.6%) | |
Abbreviation: NH, non‐Hispanic.
5‐point Likert‐type responses were strongly disagree, disagree, neutral, agree, and strongly agree. We collapsed strongly agree with agree and strongly disagree with disagree.
Figure 1 shows the absolute differences in willingness to be contacted for studies using various procedures by race/ethnicity. Across each ethnoracial group, we observed that the proportion of participants who were willing to be contacted for studies was >70% for all but two procedures—LP and autopsy—and >90% for studies involving diet/physical activity modification and cognitive testing. Fewer NH blacks than NH whites were willing to be contacted for studies that involved LP and autopsy (16% vs 42% and 47% vs 74%, respectively).
FIGURE 1.
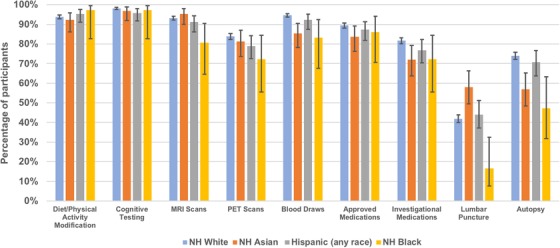
Percentage of participants by race/ethnicity willing to engage in studies of various research procedures
To further quantify these differences on a relative scale, Table 3 shows the association of race/ethnicity and willingness using logistic regression models. Hispanics exhibited lower odds for willingness relative to NH whites across all procedure types except for LP and diet/physical activity modification (ORs ranged from 0.44 to 0.85, unadjusted models). Adjustment for potential confounders (model 2) strengthened associations in most cases, and only reached statistical significance for studies involving PET scans and investigational medications (adjusted OR [aOR] = 0.56, 95% confidence interval [CI]: 0.36‐0.87 and aOR = 0.61, 95% CI: 0.40‐0.95, respectively). In NH Asians, we similarly observed lower odds of willingness compared to NH whites across a majority of procedure types, although the estimates were generally larger in magnitude than those of Hispanics, and reached statistical significance for studies involving blood draws, investigational medications, and autopsy in multivariable models. The one exception was LP; NH Asians had 84% higher odds of willingness to be contacted for studies involving LP than NH whites (aOR = 1.84, 95% CI: 1.28‐2.66). This association did not appreciably change after adjustment for sources of enrollment (not shown). For NH blacks, we observed a similar pattern of lower odds for willingness compared to NH whites across all but one procedure type, with the strongest associations observed for studies that involved MRI, blood draws, LP, and autopsy (aORs ranged from 0.26 to 0.31).
TABLE 3.
Association of race/ethnicity with willingness to be contacted for research studies
| Odds ratios (95% CI) for willingness to be contacted | |||||
|---|---|---|---|---|---|
| Type of procedure involved | NH white | Hispanic (any race) | NH Asian | NH black | |
| Diet/physical activity modification | Model 1a | 1.00 (ref) | 1.33 (0.67‐2.65) | 0.78 (0.40‐1.52) | 2.32 (0.32‐17.0) |
| Model 2 b | 1.00 (ref) | 1.08 (0.53‐2.21) | 0.70 (0.35‐1.38) | 1.96 (0.26‐14.5) | |
| Model 3 c | 1.00 (ref) | 1.21 (0.54‐2.67) | 0.65 (0.33‐1.29) | 1.51 (0.20‐11.4) | |
| Cognitive testing | Model 1a | 1.00 (ref) | 0.44 (0.20‐0.94 | 0.60 (0.21‐1.71) | 0.68 (0.09‐5.05) |
| Model 2 b | 1.00 (ref) | 0.52 (0.23‐1.18) | 0.55 (0.19‐1.58) | 0.74 (0.09‐5.70) | |
| Model 3 c | 1.00 (ref) | 0.50 (0.21‐1.19) | 0.51 (0.17‐1.50) | 0.62 (0.08‐5.00) | |
| MRI scans | Model 1a | 1.00 (ref) | 0.74 (0.44‐1.25) | 1.49 (0.65‐3.43) | 0.30 (0.13‐0.70) |
| Model 2 b | 1.00 (ref) | 0.68 (0.39‐1.17) | 1.37 (0.59‐3.19) | 0.29 (0.12‐0.68) | |
| Model 3 c | 1.00 (ref) | 0.67 (0.37‐1.19) | 1.26 (0.54‐2.94) | 0.32 (0.12‐0.88) | |
| PET scans | Model 1a | 1.00 (ref) | 0.73 (0.50‐1.05) | 0.84 (0.53‐1.33) | 0.50 (0.24‐1.05) |
| Model 2 b | 1.00 (ref) | 0.67 (0.46‐0.98) | 0.79 (0.50‐1.26) | 0.48 (0.22‐1.01) | |
| Model 3 c | 1.00 (ref) | 0.62 (0.42‐0.93) | 0.73 (0.45‐1.17) | 0.58 (0.24‐1.40) | |
| Blood draws | Model 1a | 1.00 (ref) | 0.66 (0.38‐1.16) | 0.33 (0.19‐0.55) | 0.28 (0.12‐0.69) |
| Model 2 b | 1.00 (ref) | 0.61 (0.34‐1.10) | 0.31 (0.18‐0.53) | 0.27 (0.11‐0.68) | |
| Model 3 c | 1.00 (ref) | 0.68 (0.36‐1.29) | 0.28 (0.16‐0.49) | 0.47 (0.14‐1.60) | |
| Approved medications | Model 1a | 1.00 (ref) | 0.82 (0.52‐1.28) | 0.61 (0.38‐0.99) | 0.74 (0.28‐1.91) |
| Model 2 b | 1.00 (ref) | 0.70 (0.43‐1.12) | 0.61 (0.37‐1.02) | 0.68 (0.25‐1.81) | |
| Model 3 c | 1.00 (ref) | 0.64 (0.39‐1.05) | 0.56 (0.33‐0.94) | 0.70 (0.23‐2.12) | |
| Investigational medications | Model 1a | 1.00 (ref) | 0.74 (0.52‐1.06) | 0.58 (0.39‐0.86) | 0.58 (0.28‐1.22) |
| Model 2 b | 1.00 (ref) | 0.62 (0.42‐0.90) | 0.54 (0.36‐0.83) | 0.52 (0.24‐1.12) | |
| Model 3 c | 1.00 (ref) | 0.58 (0.39‐0.87) | 0.49 (0.32‐0.75) | 0.43 (0.19‐0.99) | |
| Lumbar puncture | Model 1a | 1.00 (ref) | 1.09 (0.81‐1.46) | 1.92 (1.34‐2.76) | 0.28 (0.12‐0.67) |
| Model 2 b | 1.00 (ref) | 0.95 (0.70‐1.30) | 1.84 (1.28‐2.66) | 0.26 (0.11‐0.63) | |
| Model 3 c | 1.00 (ref) | 0.91 (0.66‐1.27) | 1.71 (1.17‐2.49) | 0.34 (0.13‐0.85) | |
| Autopsy | Model 1a | 1.00 (ref) | 0.85 (0.61‐1.17) | 0.47 (0.32‐0.70) | 0.31 (0.16‐0.61) |
| Model 2 b | 1.00 (ref) | 0.83 (0.59‐1.16) | 0.44 (0.30‐0.63) | 0.31 (0.16‐0.59) | |
| Model 3 c | 1.00 (ref) | 0.73 (0.51‐1.04) | 0.41 (0.28‐0.60) | 0.34 (0.16‐0.72) | |
Abbreviations: CFI, Cognitive Function Inventory; CI, confidence interval; MRI, magnetic resonance imaging; NH, non‐Hispanic; PET, positron emission tomography; RAQ, Research Attitude Questionnaire
Model 1 unadjusted.
Model 2 adjusted for age, sex, educational attainment, number of comorbidities, neurological disorder diagnosis, number of medications and CFI scores.
Model 3 further adjusted for RAQ scores.
When we considered potential mediation by research attitudes, we observed no appreciable attenuation of the main effect estimates in multivariable models that further adjusted for RAQ overall summary scores (Table 3, model 3) or when we attempted to decompose the total effect into the natural direct and indirect effects (not shown). Furthermore, we found no appreciable attenuation of the main effects in sensitivity analyses that adjusted for individual RAQ items rather than an overall RAQ summary score.
In exploratory analyses that grouped procedures according to requirements typical of AD prevention trials, we observed 44%, 46%, and 64% lower odds of willingness comparing Hispanics, NH Asians, and NH blacks with NH whites, respectively (Table 4, adjusted model). Other predictors for higher willingness in the multivariable model included younger age, being male, and higher CFI and RAQ scores.
TABLE 4.
Association of race/ethnicity with willingness to engage in studies involving requirements of AD prevention trials a
| Odds ratios (95% CI) for higher willingness to be contacted | ||||
|---|---|---|---|---|
| Unadjusted | P‐value | Adjusted b | P‐value | |
| Race/ethnicity | ||||
| NH white | 1.00 (ref) | 1.00 (ref) | ||
| Hispanic (any race) | 0.62 (0.44‐0.88) | .0070 | 0.56 (0.39‐0.83) | .0031 |
| NH Asian | 0.62 (0.42‐0.93) | .0195 | 0.54 (0.36‐0.82) | .0034 |
| NH black | 0.40 (0.19‐0.81) | .0117 | 0.36 (0.16‐0.80) | .0122 |
| Age (10‐year difference) | 0.95 (0.86‐1.05) | .3237 | 0.98 (0.97‐0.99) | .0008 |
| Sex (male vs female) | 1.56 (1.27‐1.91) | <.0001 | 1.72 (1.38‐2.13) | <.0001 |
| Educational attainment | ||||
| High school or less (≤12 years) | 0.95 (0.66‐1.37) | .7876 | 0.98 (0.65‐1.48) | .9266 |
| Some college (13‐15 years) | 1.28 (0.98‐1.66) | .0655 | 1.29 (0.97‐1.70) | .0752 |
| College or higher (≥16 years) | 1.00 (ref) | 1.00 (ref) | ||
| Number of comorbidities | ||||
| None | 1.00 (ref) | 1.00 (ref) | ||
| 1 | 1.19 (0.95‐1.49) | .1397 | 1.13 (0.88‐1.47) | .3345 |
| ≥2 | 1.35 (1.06‐1.70) | .0139 | 1.10 (0.82‐1.49) | .5188 |
| Number of medications | ||||
| None | 1.00 (ref) | 1.00 (ref) | ||
| 1‐2 | 1.31 (0.99‐1.74) | .0562 | 1.26 (0.94‐1.69) | .1209 |
| 3‐4 | 1.59 (1.18‐2.14) | .0024 | 1.41 (1.00‐1.97) | .0475 |
| >4 | 1.69 (1.26‐2.26) | .0005 | 1.46 (1.02‐2.09) | .0375 |
| CFI scores (3‐point difference) | 1.17 (1.03‐1.33) | .0146 | 1.06 (1.02‐1.14) | .0062 |
| RAQ scores (5‐point difference) | 1.25 (1.12‐1.39) | <.0001 | 1.05 (1.03‐1.08) | <.0001 |
Abbreviations: CFI, Cognitive Function Inventory; CI, confidence intervals; NH, non‐Hispanic; RAQ, Research Attitude Questionnaire
Excluded participants with neurological disorders and non‐squamous cell or non‐basal cell cancers.
Adjusted for age, sex, educational attainment, number of comorbidities, number of medications, CFI and RAQ scores.
4. DISCUSSION
In this cross‐sectional study of older adults participating in a local online recruitment registry, we found that ethnoracial minorities were less willing than their NH white counterparts to be contacted for studies that involved procedures typically required in AD prevention trials. These associations were robust, independent of potential confounders and consistent with previous reports of low participation rates among ethnoracial minorities in AD research. 4 , 5 , 6 Our findings underscore the pressing need to understand the factors that could improve recruitment of underrepresented older adults in research and the role that recruitment registries may play in addressing this disparity. 24 , 25
Recruitment registries aim to lower barriers to clinical trial enrollment by increasing awareness and improving access to available trials among enrollees. 26 Our findings suggest that, in contrast to previous reports, 11 access alone might not be sufficient for reducing recruitment disparities by race and ethnicity in AD research, particularly for interventional trials. It is likely that greater education and engagement will be needed to address shared barriers to participation among diverse minority groups, which can include mistrust of researchers and consequent fear of participation, stigma related to participation, and competing demands involving time and financial challenges. 27 For example, a recent intervention used an educational outreach program based upon social marketing principles and community engagement to improve African American participation in AD studies involving LP, PET, and MRI imaging. 28 Notably, this approach required many years of continuous effort, but ultimately yielded potentially critical discoveries related to disparities in AD biology. 29 Other interventions have shown promise in efforts to build trust with older members of minority communities and thereby reduce fears, particularly when researchers do not share ethnoracial membership with targeted enrollment groups. 30 For Hispanic elders, for example, culturally appropriate outreach materials may need to account for level of acculturation, immigration status and history, family values, health beliefs, and level of health literacy. 31 Indeed, future efforts to improve recruitment of multicultural populations in AD trials will likely need to use a range of approaches to community engagement that is tailored to each ethnoracial group.
While research attitudes—as measured by the RAQ—were an independent predictor of AD trial participation, consistent with previous studies, 32 , 33 RAQ score did not appear to mediate ethnoracial minorities’ lower willingness to participate. Thus, educational and engagement interventions targeting attitudes beyond those measured by the RAQ may be necessary to fully address disparities in participation. In particular, future research should aim to gain better insights into the role of ethnicity and culture in shaping attitudes about participation in AD trials. For example, cultural stigma associated with a diagnosis of dementia may pose a critical barrier for participation among Chinese American elders 34 and Mexican Americans at risk for AD. 35 Moreover, cross‐sectional studies about brain donation suggest an influence of religion and spirituality in minority groups’ research decisions. 36 , 37 , 38 , 39 For African Americans, reluctance to participate may be exacerbated by an acute awareness of past breaches of trust and the pervasive history of racism in clinical research. 14 , 38 , 40 More work is needed to understand how cultural values and experiences shape willingness to engage in potentially invasive AD biomarker testing.
Surprisingly, we found that NH Asians were more willing than NH whites to be contacted for studies involving LP. This contrasts with a 2‐year prospective study of 352 subjects who participated in AD research, which reported a 77% lower agreement rate for LP among Asians compared to whites. 41 Furthermore, those who viewed LP as a “frightening, invasive procedure” were 89% less likely to subsequently agree to the procedure than those who viewed it as a “standard medical procedure.” It is likely that relatively more NH Asian participants in this study, compared to the other ethnoracial groups, were exposed to educational outreach activities that included education about the LP through our ADRC as part of broader recruitment efforts of older Chinese adults. To corroborate this, we observed that a higher proportion of NH Asians compared to other groups enrolled in C2C through community events like public seminars. Future research will explore whether C2C participants who express a willingness to be contacted for studies involving LP actually participate.
We note several limitations in the present study. First, the cross‐sectional design does not allow us to infer a causal relationship between research attitudes and research willingness. For instance, it is possible that previous negative experiences with medical procedures may have shaped negative attitudes toward research. It is also possible that the differential source of enrollment among NH Asian participants introduced selection bias. Our ethnoracial categorizations were defined broadly, encompassing a wide range of countries of origin, diverse cultures, religious affiliations, and immigration statuses within each group, particularly for those of Hispanic origin and NH Asian backgrounds. Nonetheless, these ethnoracial classifications were based on self‐report, as is typically done in clinical trials. In addition, the educational attainment of our sample was higher than that reported at the national level, 42 thus supporting previous literature about the persistence of a “digital divide” by socioeconomic status among older adults. 43 For these reasons, our ability to generalize the findings is limited. How these results compare to other local registries, as well as larger national registries, should be the focus of future research.
In summary, we found that ethnoracial minorities were less willing to engage in some research studies than NH whites. We found no evidence to support mediation by research attitudes measured by the RAQ. Future work will focus on following these participants longitudinally to assess whether those who indicated willingness actually enroll in studies, including AD prevention trials.
CONFLICTS OF INTEREST
Authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors would like to acknowledge all participants in the C2C registry. This registry was made possible by a donation from HCP, Inc. and is supported by NIAAG016573 and NCATSUL1 TR001414. CRS is supported by a diversity supplement to NIA AG059407.
Salazar CR, Hoang D, Gillen DL, Grill JD. Racial and ethnic differences in older adults’ willingness to be contacted about Alzheimer's disease research participation. Alzheimer's Dement. 2020;6:e12023 10.1002/trc2.12023
REFERENCES
- 1. Steenland K, Goldstein FC, Levey A, Wharton W. A meta‐analysis of Alzheimer's disease incidence and prevalence comparing African‐Americans and Caucasians. J Alzheimers Dis. 2016;50:71‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimers Dement. 2016;12:216‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in United States race/ethnic populations. Alzheimers Dement. 2017;13:72‐83. [DOI] [PubMed] [Google Scholar]
- 4. Olin JT, Dagerman KS, Fox LS, Bowers B, Schneider LS. Increasing ethnic minority participation in Alzheimer disease research. Alzheimer Dis Assoc Disord. 2002;16(Suppl 2):S82‐S85. [DOI] [PubMed] [Google Scholar]
- 5. Canevelli M, Bruno G, Grande G, etal. Race reporting and disparities in clinical trials on Alzheimer's disease: a systematic review. Neurosci Biobehav Rev. 2019;101:122‐128. [DOI] [PubMed] [Google Scholar]
- 6. Faison WE, Schultz SK, Aerssens J, etal. Potential ethnic modifiers in the assessment and treatment of Alzheimer's disease: challenges for the future. Int Psychogeriatr. 2007;19:539‐558. [DOI] [PubMed] [Google Scholar]
- 7. Oh SS, Galanter J, Thakur N, etal. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brewster P, Barnes L, Haan M, etal. Progress and future challenges in aging and diversity research in the United States. Alzheimers Dement. 2019;15:995‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nuno MM, Gillen DL, Dosanjh KK, etal. Attitudes toward clinical trials across the Alzheimer's disease spectrum. Alzheimers Res Ther. 2017;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krysinska K, Sachdev PS, Breitner J, Kivipelto M, Kukull W, Brodaty H. Dementia registries around the globe and their applications: a systematic review. Alzheimers Dement. 2017;13:1031‐1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wendler D, Kington R, Madans J, etal. Are racial and ethnic minorities less willing to participate in health research?. PLoS Med. 2006;3:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katz RV, Green BL, Kressin NR, Claudio C, Wang MQ, Russell SL. Willingness of minorities to participate in biomedical studies: confirmatory findings from a follow‐up study using the Tuskegee Legacy Project Questionnaire. J Natl Med Assoc. 2007;99:1052‐1060. [PMC free article] [PubMed] [Google Scholar]
- 13. Webb FJ, Khubchandani J, Striley CW, Cottler LB. Black‐White Differences in Willingness to Participate and Perceptions About Health Research: results from the Population‐Based HealthStreet Study. J Immigr Minor Health. 2019;21:299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shavers VL, Lynch CF, Burmeister LF. Racial differences in factors that influence the willingness to participate in medical research studies. Ann Epidemiol. 2002;12:248‐256. [DOI] [PubMed] [Google Scholar]
- 15. Zhou Y, Elashoff D, Kremen S, Teng E, Karlawish J, Grill JD. African Americans are less likely to enroll in preclinical Alzheimer's disease clinical trials. Alzheimers Dement. 2017;3:57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Corbie‐Smith G, Thomas SB, St George DM. Distrust, race, and research. Arch Intern Med. 2002;162:2458‐2463. [DOI] [PubMed] [Google Scholar]
- 17. Snyder PJ, Papp KV, Bartkowiak J, Jackson CE, Doody RS. Commentary on "a roadmap for the prevention of dementia II. Leon Thal Symposium 2008." Recruitment of participants for Alzheimer's disease clinical trials: the role of trust in caregivers, clinical researchers, regulatory authorities, and industry sponsors. Alzheimers Dement. 2009;5:122‐124. [DOI] [PubMed] [Google Scholar]
- 18. Together We Make the Difference. National Strategy for Recruitment and Participation in Alzheimer’s and Related Dementias Clinical Research. Washington, DC: National Institutes of Health; 2018. https://www.nia.nih.gov/sites/default/files/2018-10/alzheimers-disease-recruitment-strategy-final.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grill JD, Hoang D, Gillen DL, etal. Constructing a local potential participant registry to improve Alzheimer's disease clinical research recruitment. J Alzheimers Dis. 2018;63:1055‐1063. [DOI] [PubMed] [Google Scholar]
- 20. Walsh SP, Raman R, Jones KB, Aisen PS, Alzheimer's Disease Cooperative Study G . ADCS Prevention Instrument Project: the Mail‐In Cognitive Function Screening Instrument (MCFSI). Alzheimer Dis Assoc Disord. 2006;20:S170‐S178. [DOI] [PubMed] [Google Scholar]
- 21. Rubright JD, Cary MS, Karlawish JH, Kim SY. Measuring how people view biomedical research: reliability and validity analysis of the Research Attitudes Questionnaire. J Empir Res Hum Res Ethics. 2011;6:63‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173‐1182. [DOI] [PubMed] [Google Scholar]
- 23. Little RJA, Rubin DB Estimation of Imputation Uncertainty . Statistical analysis with missing data, 2(75–89). Hoboken, NJ: John Wiley & Sons; 2002. [Google Scholar]
- 24. Babulal GM, Quiroz YT, Albensi BC, etal. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15:292‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Watson JL, Ryan L, Silverberg N, Cahan V, Bernard MA. Obstacles and opportunities in Alzheimer's clinical trial recruitment. Health Aff (Millwood). 2014;33:574‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aisen P, Touchon J, Andrieu S, etal. Registries and cohorts to accelerate early phase Alzheimer's trials. A report from the E.U./U.S. Clinical Trials in Alzheimer's disease Task Force. J Prev Alzheimers Dis. 2016;3:68‐74. [DOI] [PubMed] [Google Scholar]
- 27. George S, Duran N, Norris K. A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. Am J Public Health. 2014;104:e16‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams MM, Meisel MM, Williams J, Morris JC. An interdisciplinary outreach model of African American recruitment for Alzheimer's disease research. Gerontologist. 2011;51(Suppl 1):S134‐S141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morris JC, Schindler SE, McCue LM, etal. Assessment of racial disparities in biomarkers for Alzheimer disease. JAMA Neurol. 2019;76:264‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hughes TB, Varma VR, Pettigrew C, Albert MS. African Americans and clinical research: evidence concerning barriers and facilitators to participation and recruitment recommendations. Gerontologist. 2017;57:348‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bilbrey AC, Humber MB, Plowey ED, etal. The Impact of Latino values and cultural beliefs on brain donation: results of a pilot study to develop culturally appropriate materials and methods to increase rates of brain donation in this Under‐Studied Patient Group. Clin Gerontol. 2018;41:237‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grill JD, Zhou Y, Elashoff D, Karlawish J. Disclosure of amyloid status is not a barrier to recruitment in preclinical Alzheimer's disease clinical trials. Neurobiol Aging. 2016;39:147‐153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cary MS, Rubright JD, Grill JD, Karlawish J. Why are spousal caregivers more prevalent than nonspousal caregivers as study partners in AD dementia clinical trials?. Alzheimer Dis Assoc Disord. 2015;29:70‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hinton L, Guo Z, Hillygus J, Levkoff S. Working with culture: a qualitative analysis of barriers to the recruitment of Chinese‐American family caregivers for dementia research. J Cross Cult Gerontol. 2000;15:119‐137. [DOI] [PubMed] [Google Scholar]
- 35. Withers M, Sayegh P, Rodriguez‐Agudelo Y, etal. A mixed‐methods study of cultural beliefs about dementia and genetic testing among Mexicans and Mexican‐Americans at‐risk for autosomal dominant Alzheimer's disease. J Genet Couns. 2019;28:921‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boise L, Hinton L, Rosen HJ, etal. Willingness to be a brain donor: a survey of research volunteers from 4 Racial/Ethnic Groups. Alzheimer Dis Assoc Disord. 2017;31:135‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boise L, Hinton L, Rosen HJ, Ruhl M. Will My soul go to heaven if they take my brain? beliefs and worries about brain donation among four ethnic groups. Gerontologist. 2017;57:719‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lambe S, Cantwell N, Islam F, Horvath K, Jefferson AL. Perceptions, knowledge, incentives, and barriers of brain donation among African American elders enrolled in an Alzheimer's research program. Gerontologist. 2011;51:28‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jefferson AL, Lambe S, Cook E, Pimontel M, Palmisano J, Chaisson C. Factors associated with African American and White elders' participation in a brain donation program. Alzheimer Dis Assoc Disord. 2011;25:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Katz RV, Kegeles SS, Kressin NR, etal. Awareness of the Tuskegee Syphilis Study and the US presidential apology and their influence on minority participation in biomedical research. Am J Public Health. 2008;98:1137‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moulder KL, Besser LM, Beekly D, Blennow K, Kukull W, Morris JC. Factors influencing successful lumbar puncture in Alzheimer research. Alzheimer Dis Assoc Disord. 2017;31:287‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ryan C, Bauman K. U.S. Census Bureau. Educational attainment in the United States: 2015 Current Population Reports2016.
- 43. Mitchell UA, Chebli PG, Ruggiero L, Muramatsu N. The digital divide in health‐related technology use: the significance of race/ethnicity. Gerontologist. 2019;59:6‐14. [DOI] [PubMed] [Google Scholar]


