SUMMARY
Background
Abdominal ultrasound fails to detect over one-fourth of hepatocellular carcinoma (HCC) at an early stage in patients with cirrhosis. Identifying patients in whom ultrasound is of inadequate quality can inform interventions to improve surveillance effectiveness.
Aim
To evaluate and identify predictors of ultrasound quality in patients with cirrhosis.
Methods
We performed a retrospective cohort study among patients who underwent ultrasound examination for a cirrhosis-related indication between April 2015 and October 2015. Three fellowship-trained abdominal radiologists collectively reviewed all ultrasound exams and categorised exam quality as definitely adequate, likely adequate, likely inadequate and definitely inadequate to exclude liver lesions. We performed multivariable logistic regression to determine characteristics associated with inadequate ultrasound quality.
Results
Among 941 patients, 191 (20.3%) ultrasounds were inadequate for excluding HCC- 134 definitely inadequate and 57 likely inadequate. In multivariable analysis, inadequate quality was associated with male gender (OR 1.68, 95% CI 1.14–2.48), body mass index category (OR 1.67, 95% CI 1.45–1.93), Child–Pugh B or C cirrhosis (OR 1.93, 95% CI 1.32–2.81), alcohol-related cirrhosis (OR 2.11, 95% CI 1.33–3.37), NASH cirrhosis (OR 2.87, 95% CI1.71–4.80), and in-patient status (OR 1.55, 95% CI 1.01–2.37). Ultrasounds were inadequate in over one-third of patients with Child–Pugh C cirrhosis, BMI >35, or NASH cirrhosis.
Conclusions
One in five ultrasounds in patients with cirrhosis are inadequate for exclusion of HCC, which can contribute to surveillance failure. Alternative surveillance modalities are needed in subgroups prone to inadequate ultrasounds including obese patients, those with Child Pugh B or C cirrhosis, and those with alcohol- or NASH-related cirrhosis.
INTRODUCTION
Ultrasound serves as the backbone of hepatocellular carcinoma (HCC) surveillance and is recommended every 6 months in patients with cirrhosis to improve early tumour detection and overall survival.1, 2 Ultrasound can be efficacious for early HCC detection, with a meta-analysis reporting a pooled sensitivity of 63% for detecting HCC at an early stage.3 Several prospective cohort studies have demonstrated patients undergoing ultrasound based surveillance have earlier stages of disease as well as improved survival, even after adjusting for lead time bias, than those who had not undergone surveillance.4–6
A pillar of achieving this survival benefit in clinical practice is having effective surveillance tools, and ultrasound’s effectiveness is variable with some studies reporting a sensitivity as low as 32% in clinical practice.7 Inadequate ultrasound sensitivity is the most common reason for late stage tumour detection in patients followed in tertiary-care centres.8 This gap between ultrasound’s efficacy and effectiveness can be related to several factors including differences in imaging protocols, differences in patient populations, and the operator dependent nature of ultrasound.9 Enthusiasm for alternate radiological modalities, such as computerised tomography and magnetic resonance imaging, in all patients with cirrhosis is hampered by concerns regarding radiation exposure and cost.10, 11
Characterising reasons for ultrasound failure and identifying patients in whom ultrasound is inadequate for evaluation of HCC is important for informing interventions to improve surveillance effectiveness. Therefore, the aim of our study was to evaluate ultrasound quality and identify clinical predictors of inadequate ultrasound quality among a large cohort of patients with cirrhosis undergoing HCC surveillance.
METHODS
Study setting and patient population
We conducted a retrospective cohort study among 941 patients who underwent at least one abdominal ultrasound for a cirrhosis-related indication at Parkland Health and Hospital System between 1 April 2015 and 31 October 2015. Parkland is the integrated safety-net health system of Dallas County comprised of twelve primary care clinics, a Hepatology out-patient clinic, a multidisciplinary HCC clinic, and a tertiary hospital – all sharing one electronic medical record system.12 Parkland currently provides out-patient and in-patient care for over 2000 patients with cirrhosis in Dallas. Parkland utilises 18 in-patient and out-patient ultrasound scanners and performs >3500 ultrasound examinations per month. Ultrasounds are performed by one of 29 ultrasound technologists using a standard protocol and interpreted by one of 26 subspecialty radiologists. All examinations were performed on an iU22 or Epiq7 ultrasound system (Philips Healthcare, Cleveland, OH, USA), utilising a C5–1 or C9–2 curvilinear probe for deep imaging, and an L12–5 or L12–3 linear probe for superficial imaging. The liver ultrasound protocol involves sequential longitudinal and transverse imaging through the left and right hepatic lobes; high-resolution imaging of the hepatic capsule; Doppler interrogation of the main portal vein; evaluation of the gall-bladder and biliary system; measurement of spleen length and volume; and assessment for ascites.
Patients were identified by an electronic search of all patients who completed abdominal ultrasound exams. One author (O.S.) adjudicated cases to confirm they met diagnostic criteria for cirrhosis, including stage 4 fibrosis on liver biopsy, any non-invasive marker of fibrosis suggesting cirrhosis, or a cirrhotic-appearing liver on abdominal imaging. This study was approved by the Institutional Review Board of UT Southwestern Medical Center and conducted in compliance with Health Information and Privacy Accountability Act.
Data collection
Patient demographics, clinical history, and laboratory data were obtained through review of computerised medical records using a standardised collection form. Patient age, gender, race, ethnicity and body mass index (BMI) at the time of the ultrasound were recorded. BMI was categorised using the International classification schema as: normal (BMI <25), overweight (BMI 25–29.99), obese class I (BMI 30–34.99), obese class II (BMI 35–39.99), and morbid obesity (BMI ≥40). Patients were classified according to aetiology of liver disease using laboratory data and clinical notes as follows: hepatitis C virus (positive HCV antibody or viral load), hepatitis B virus (positive HBsAg or viral load), alcohol-related liver disease (as determined by clinic provider), non-alcoholic steatohepatitis (presence of metabolic syndrome in the absence of other causes of chronic liver disease or as determined by clinic provider), and other. Data regarding presence of decompensation (ascites or hepatic encephalopathy) were abstracted from clinical notes and classified as none, mild or controlled, and severe or uncontrolled. Laboratory data of interest included platelet count, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, international normalised ratio (INR) and alpha fetoprotein (AFP). All laboratory data were within 6 months of ultrasound examination. For patients with multiple laboratory results, we used those values closest to the ultrasound date.
We recorded the location (in-patient vs. out-patient), intent (surveillance vs. diagnostic exam), and quality for each ultrasound exam. For patients with more than one ultrasound exam, we selected the first ultrasound during the study period. Intent of ultrasound imaging was determined through review of ultrasound reports and orders. Indications including ‘surveillance’, ‘screening’, ‘rule out HCC’ and ‘cirrhosis’ were classified as surveillance indications. Ultrasound exams performed for diagnostic reasons, for example, abdominal pain or elevated liver enzymes, were classified as nonsurveillance exams.
One of three fellowship-trained abdominal radiologists, experienced in ultrasound (D.F., T.Y. or T.B.), who were blinded to the radiology clinical report and patient characteristics, reviewed an equal number of ultrasound exams. Ultrasound quality was categorised as definitely adequate, likely adequate, likely inadequate or definitely inadequate according to the radiologist’s confidence in visualisation of the entire liver and ability to exclude any liver lesions including HCC. All three radiologists reviewed the first 50 exams and quality scores were determined by consensus to calibrate quality assessment, after which time there was felt to be sufficiently high inter-rater reliability for independent review of subsequent exams. The remaining ultrasounds were then reviewed by one of the three radiologists. Quality assessment was based on an overall impression of overall exam quality based on combination of anatomical coverage (less than 2/3 of liver visualised), visual clarity of the liver parenchyma including heterogeneity and nodularity, depth of penetration and any other exam limitations such as obstruction from ribs, lungs or bowel gas. Given the lack of accepted quality benchmarks for ultrasound exam adequacy, these criteria were developed as the minimum needed for an ultrasound exam to be regarded as adequate for HCC surveillance.
Statistical analysis
Our primary outcome of interest was adequacy of the ultrasound exam for exclusion of liver lesions including HCC; this was defined as a composite outcome of definite or likely adequate. Demographics and clinical features were compared between patients with adequate and inadequate ultrasound quality using the Student’s t-test and chi square test for continuous and categorical variables, respectively. We used univariate and multivariable logistic regression analyses to identify patient-factors associated with adequate vs. inadequate ultrasound quality. Multivariable analysis included variables of a priori clinical importance (e.g. obesity and Child Pugh class) and predictor variables with P < 0.05 in univariate analysis. Statistical significance was defined as P < 0.05 for multivariable analyses.
RESULTS
Study population
Baseline characteristics of the 941 eligible patients are detailed in Table 1. Mean age was 56.5 ± 9.9 years and 63.7% were men. The cohort was racially/ethnically [notdef] diverse with 25.7% non-Hispanic Caucasian, 31.8% Black, and 38.0% Hispanic. Over 39.1% were obese with a BMI >30, and 9.0% were classified as having morbid obesity with BMI >40. The most common aetiologies of cirrhosis were HCV infection (47.6%), alcohol-induced (18.0%), and NASH (11.7%). Most patients had compensated cirrhosis, with 68.5% having Child Pugh A cirrhosis, 24.1% Child Pugh B cirrhosis, and 7.3% Child Pugh C cirrhosis. Ascites was present in 31.1% of patients, and 16.9% had hepatic encephalopathy. Most ultrasounds were done as an out-patient, although 21.9% of exams were performed while an in-patient.
Table 1 |.
Patient characteristics
| Characteristic | Adequate ultrasound quality (n = 750) | Inadequate ultrasound quality (n = 191) | P-value |
|---|---|---|---|
| Age (years) | 56.5 ± 9.8 | 56.9 ± 10.2 | 0.60 |
| Gender (% male) | 466 (62.1%) | 133 (70.0%) | 0.04 |
| Race/ethnicity | |||
| Non-Hispanic Caucasian | 192 (25.6%) | 50 (26.3%) | 0.15 |
| Black | 260 (34.7%) | 39 (20.5%) | |
| Hispanic | 261 (34.8%) | 96 (50.5%) | |
| Other/unknown | 37 (4.9%) | 5 (2.7%) | |
| BMI | |||
| Normal (BMI <25) | 243 (32.5%) | 25 (13.2%) | <0.001 |
| Overweight (BMI 25–29.99) | 242 (32.4%) | 53 (28.0%) | |
| Obesity class II (BMI 30–34.99) | 152 (20.3%) | 45 (23.8%) | |
| Obesity class II (BMI 35–39.99) | 60 (8.0%) | 33 (17.5%) | |
| Morbid obesity (BMI ≥40) | 51 (6.8%) | 33 (17.5%) | |
| Aetiology of liver disease | |||
| Hepatitis C | 383 (51.15%) | 64 (33.7%) | 0.08 |
| Hepatitis B | 44 (5.9%) | 8 (4.2%) | |
| Alcohol-related | 116 (15.5%) | 53 (27.9%) | |
| Non-alcoholic steatohepatitis | 72 (9.6%) | 38 (20.0%) | |
| Cryptogenic | 99 (13.2%) | 23 (12.0%) | |
| Other* | 36 (4.8%) | 4 (2.1%) | |
| Child Pugh class | |||
| Child Pugh A | 541 (72.1%) | 104 (54.5%) | <0.001 |
| Child Pugh B | 167 (22.3%) | 60 (31.4%) | |
| Child Pugh C | 42 (5.6%) | 27 (14.1%) | |
| Presence of hepatic encephalopathy (%) | 113 (15.1%) | 46 (24.2%) | 0.003 |
| Presence of ascites (%) | 211 (28.1%) | 81 (42.6%) | <0.001 |
| In-patient status (%) | 152 (20.3%) | 54 (28.3%) | 0.02 |
| Platelet count (*109/L) | 158.2 ± 82.7 | 138.5 ± 89.1 | 0.004 |
| Creatinine (mg/dL) | 1.1 ± 1.2 | 0.9 ± 0.5 | 0.05 |
| AST (U/L) | 63.7 ± 58.4 | 60.5 ± 52.4 | 0.54 |
| ALT (U/L) | 56.1 ± 73.6 | 44.9 ± 36.8 | 0.04 |
| Albumin (g/dL) | 3.7 ± 0.7 | 3.5 ± 0.8 | <0.001 |
| Bilirubin (mg/dL) | 1.1 ± 1.5 | 1.2 ± 3.2 | <0.001 |
| INR | 1.2 ± 0.4 | 1.3 ± 0.5 | 0.01 |
BMI, body mass index; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalised ratio.
Other aetiology category includes 22 patients with autoimmune hepatitis, seven primary biliary cirrhosis, five cardiac cirrhosis, three primary sclerosing cholangitis, one hemochromatosis, one sarcoidosis, and one parasitic infection.
Ultrasounds were determined to be inadequate in 191(20.3%) of cases – 134 definitely inadequate and 57 likely inadequate (Figure 1). The most common reasons for inadequate ultrasound exams were rib shadowing and inadequate beam penetration allowing visualisation of less than two thirds of the hepatic parenchyma. There were 26 patients with poor beam penetration, 33 with rib shadowing, and 82 patients with both quality issues. Liver heterogeneity and bowel gas were uncommon reasons for inadequate ultrasounds, reported in only 20 and eight patients respectively. Illustrative examples are shown in Figure 2.
Figure 1 |.
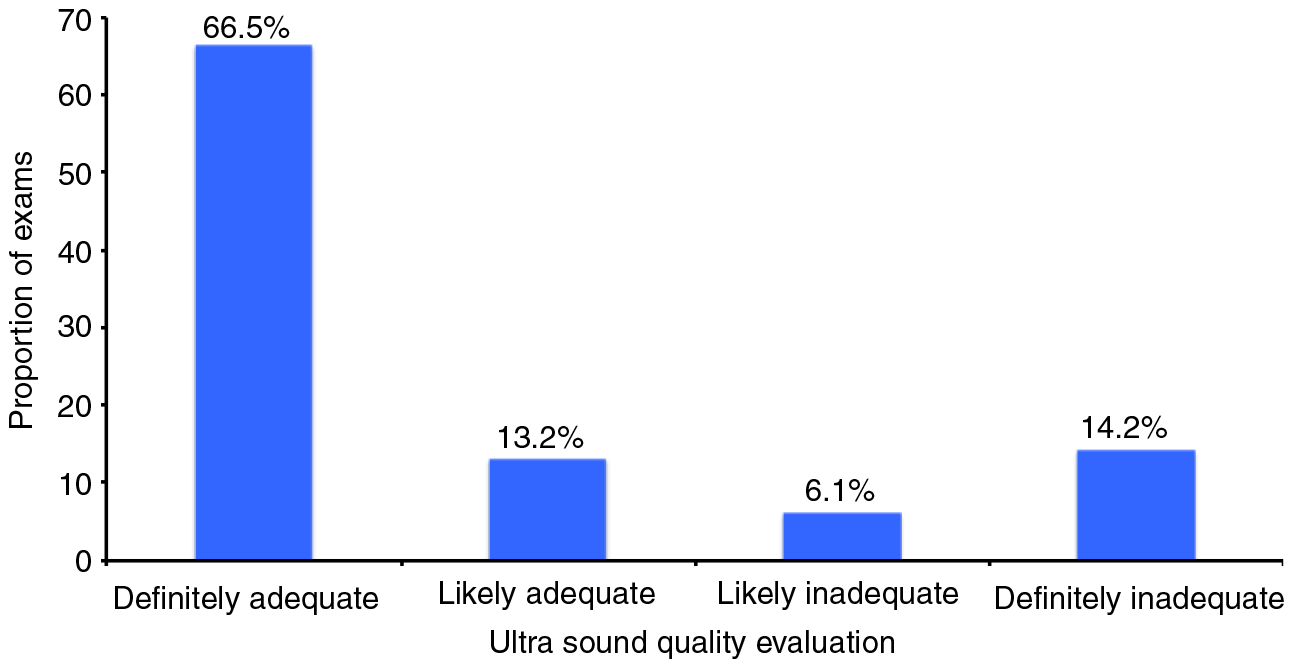
Quality of ultrasound exams for exclusion of liver masses. Among 941 patients, ultrasounds were inadequate in 191 (20.3%) of cases – 134 definitely inadequate and 57 likely inadequate.
Figure 2 |.
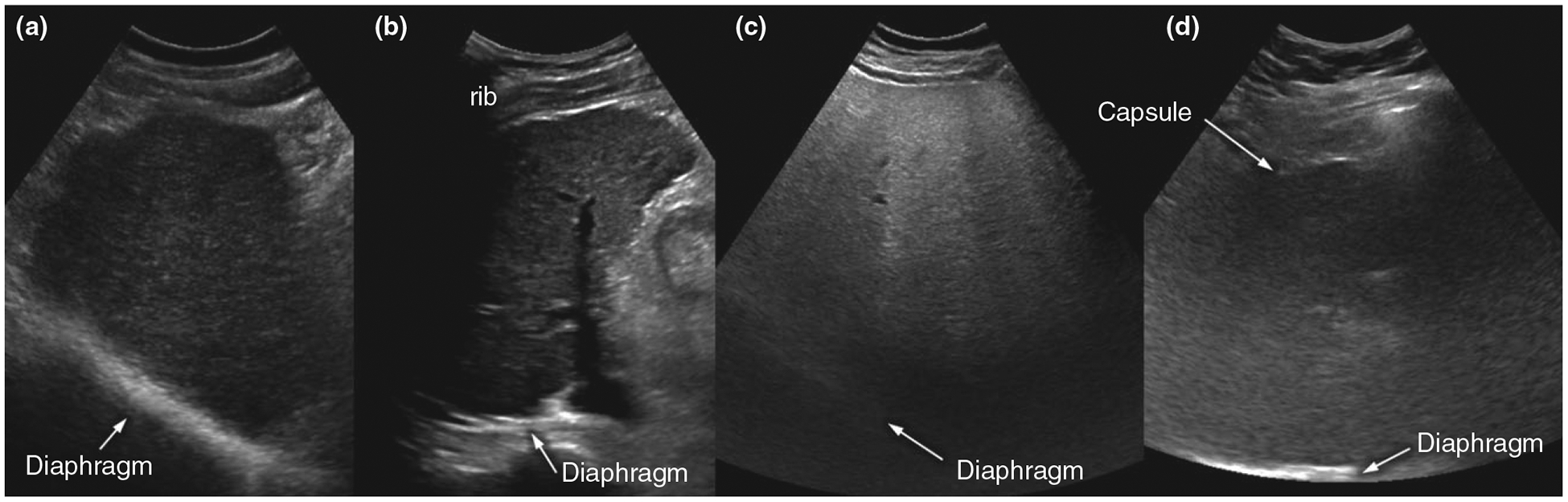
Illustrative examples of adequate and inadequate ultrasound quality. (a) Adequate-quality exam: Although diffusely heterogeneous, liver parenchyma is clearly visualised and focal liver lesions were ruled out with high confidence. (b) Inadequate-quality exam: Right hepatic dome could not be visualised due to extensive rib shadowing. (c) Inadequate-quality exam: Posterior half of the liver could not be visualised due to severe parenchymal fatty liver disease. (d) Inadequate-quality exam: Liver parenchyma is poorly visualised throughout due to morbid obesity and thick subcutaneous/visceral fat, in addition to probable severe underlying parenchymal disease.
In univariate analyses, inadequate ultrasound quality was significantly associated with male gender, Child Pugh B or C cirrhosis, BMI category, alcohol or NASH aetiology of liver disease, elevated ALT level, and in-patient status (Table 2). Although the individual components of Child Pugh score were associated with ultrasound quality, they were not included in multivariable analysis given clinical collinearity. In multivariable analysis, inadequate ultrasound quality was directly associated with male gender, increasing BMI category, in-patient status, Child Pugh B or C cirrhosis, alcohol-related cirrhosis and NASH-related cirrhosis. Ultrasounds were inadequate in 16.1% of patients with Child Pugh A cirrhosis, 26.4% of those with Child Pugh B cirrhosis, and 39.1% of patients with Child Pugh C cirrhosis. Similarly, inadequate quality was observed in 9.3% of normal weight patients, 18.0% of overweight patients, 22.8% of patients with obesity class I, 35.5% of patients with obesity class II, and 39.3% of patients with morbid obesity. Ultrasound exams were inadequate in 31.4% of patients with alcohol-related cirrhosis and 34.6% of patients with NASH-related cirrhosis, compared to only 15.0% of patients with other aetiologies of cirrhosis. Among the 55 patients with BMI >30, alcohol or NASH aetiology, and Child Pugh B or C cirrhosis, 25 (45.5%) had ultrasounds of inadequate quality. In contrast, ultrasound was inadequate in only 4.4% of patients without any of these characteristics, that is, normal-weight patients with Child Pugh A cirrhosis due to aetiologies other than alcohol or NASH.
Table 2 |.
Factors associated with inadequate ultrasound quality (N = 941)
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Characteristic | OR | 95% CI | OR | 95% CI |
| Male gender | 1.42 | 1.01–2.01 | 1.68 | 1.14–2.48 |
| Child Pugh B or C cirrhosis | 2.17 | 1.56–3.00 | 1.93 | 1.32–2.81 |
| BMI category | ||||
| Normal (BMI <25) | Ref | Ref | Ref | Ref |
| Overweight (BMI 25–29.99) | 2.12 | 1.28–3.54 | 2.29 | 1.35–3.88 |
| Obesity class II (BMI 30–34.99) | 2.88 | 1.70–4.89 | 2.95 | 1.67–5.20 |
| Obesity class II (BMI 35–39.99) | 5.35 | 2.96–9.66 | 6.37 | 3.35–12.12 |
| Morbid obesity (BMI ≥40) | 6.29 | 3.45–11.47 | 8.22 | 4.30–15.73 |
| Aetiology of liver disease | ||||
| Hepatitis C | Ref | Ref | Ref | Ref |
| Hepatitis B | 1.09 | 0.49–2.42 | 1.87 | 0.79–4.39 |
| Alcohol-related | 2.73 | 1.80–4.16 | 2.11 | 1.33–3.37 |
| Non-alcoholic steatohepatitis | 3.16 | 1.97–5.07 | 2.87 | 1.71–4.80 |
| Other | 0.66 | 0.23–1.93 | 0.67 | 0.22–2.04 |
| ALT >40 U/L | 0.70 | 0.50–0.97 | 0.93 | 0.64–1.34 |
| In-patient status | 1.55 | 1.08–2.23 | 1.55 | 1.01–2.37 |
There were 625 patients with definite signs of cirrhosis – 98 by biopsy and 527 by imaging showing a cirrhotic appearing liver with signs of portal hypertension. Among this subset, ultrasound was determined to be inadequate in 141 (22.6%) of cases – 98 definitely inadequate and 43 likely inadequate. As above, the most common reasons for inadequate ultrasound exams were rib shadowing and inadequate beam penetration allowing visualisation of less than two-thirds of the hepatic parenchyma. Although parenchyma heterogeneity was reported more often among the subset of patients with definite cirrhosis, this still accounted for less than 13% of patients. Factors associated with inadequate ultrasound in multivariable analysis were the same as the primary cohort (Table 3). In a sensitivity analysis excluding patients with Child C cirrhosis, 121 (20.8%) patients had inadequate quality ultrasound exams. Male gender, increasing BMI category, in-patient status, and Child Pugh B cirrhosis continued to be associated with inadequate ultrasound quality; however, alcohol- and NASH-related cirrhosis were no longer statistically significant on multivariable analysis (data not shown).
Table 3 |.
Factors associated with inadequate ultrasound quality in subset of patients with definite features of cirrhosis on imaging (N = 625)
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Characteristic | OR | 95% CI | OR | 95% CI |
| Male gender | 1.51 | 0.99–2.29 | 1.72 | 1.07–2.77 |
| Child Pugh B or C cirrhosis | 1.99 | 1.36–2.90 | 1.65 | 1.06–2.57 |
| BMI category | ||||
| Normal (BMI <25) | Ref | Ref | Ref | Ref |
| Overweight (BMI 25–29.99) | 2.15 | 1.16–3.99 | 2.60 | 1.36–4.97 |
| Obesity class II (BMI 30–34.99) | 2.79 | 1.46–5.30 | 3.47 | 1.74–6.94 |
| Obesity class II (BMI 35–39.99) | 4.22 | 2.04–8.74 | 5.59 | 2.54–12.30 |
| Morbid obesity (BMI ≥40) | 6.39 | 3.07–13.32 | 8.86 | 4.02–19.51 |
| Aetiology of liver disease | ||||
| Hepatitis C | Ref | Ref | Ref | Ref |
| Hepatitis B | 1.23 | 0.44–3.45 | 2.02 | 0.67–6.10 |
| Alcohol-related | 2.28 | 1.43–3.61 | 1.84 | 1.09–3.09 |
| Non-alcoholic steatohepatitis | 2.47 | 1.37–4.44 | 2.48 | 1.30–4.75 |
| Other | 0.64 | 0.22–1.89 | 0.73 | 0.24–2.28 |
| ALT >40 U/L | 0.68 | 0.46–1.01 | 0.92 | 0.59–1.43 |
| In-patient status | 2.14 | 1.40–3.27 | 2.11 | 1.27–3.51 |
DISCUSSION
Current guidelines from the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL) recommend ultrasound alone for HCC surveillance, but there have been few studies characterising ultrasound quality and defining populations in whom ultrasound is prone to failure.1, 13 We found that 20% of all ultrasound exams in our cohort of patients with cirrhosis were categorised as inadequate quality for HCC surveillance. The most common reasons for inadequate quality were rib shadowing and inadequate ultrasound beam penetration. Inadequate ultrasound quality was significantly associated with in-patient status, male gender, obesity, Child Pugh B or C cirrhosis, and alcohol or NASH aetiology of liver disease.
It is becoming increasingly evident that ultrasound limitations contribute to deficiencies in HCC surveillance effectiveness in clinical practice. A prior study from Italy demonstrated ultrasound failed to detect 30% of HCC at an early stage, while another study from Canada reported ultrasound failure in approximately 25% of cases.14, 15 The authors postulated this was due to a combination of aggressive tumour biology and surveillance ultrasound’s imperfect sensitivity; however, they could not determine the relative contribution of each factor. Our study helps further evaluate this issue, finding ultrasound quality may be inadequate, predisposing to surveillance failure in approximately 20% of patients.
To the best of our knowledge, our study is the first to demonstrate that surveillance ultrasound exam quality is impaired in obese patients and those with alcohol or NASH-related cirrhosis. The association between BMI and inadequate ultrasound quality is likely mediated by attenuation of the ultrasound beam by subcutaneous fat, impairing the ability to obtain high-quality images of the entire liver.16 Although some techniques, such as repositioning, increased pressure on the transducer, or use of a lower frequency transducer, can overcome some of these effects, it is unclear how often this is done in high volume clinical practices. Similarly, alcohol- and NASH related cirrhosis are both steatosis-mediated conditions, a tissue property that can exacerbate attenuation of the ultrasound pulse and impair visualisation of deep structures, including liver masses.17 Unlike subcutaneous fat, there are fewer well-described techniques to overcome this issue. Although the association between ultrasound quality and alcohol- or NASH-related cirrhosis was no longer significant on sensitivity analysis when excluding Child Pugh C cirrhosis, this may have been due to limited sample size.
We also found inadequate ultrasound quality was associated with Child Pugh B or C cirrhosis and male gender – two factors previously reported to be associated with surveillance ultrasound failure.14 Child Pugh B or C cirrhosis may intuitively pre-dispose to inadequate ultrasound quality given increased liver nodularity and parenchymal heterogeneity, impairing the ability of radiologists to distinguish focal liver lesions, including HCC. A severely shrunken liver in Child Pugh B or C cirrhosis is also more difficult to visualise as most of the liver is retracted under the rib cage, even at deep-inspiration. However, the reason for the association between male gender and inadequate ultrasound quality is less clear. Del Poggio and colleagues postulated this association may be driven by higher rates of obesity and steatosis14; however, male gender continued to be associated with inadequate ultrasound quality after adjustment for these factors in our study. We found male patients were significantly more likely to have rib shadowing (62.4% vs. 43.9%, P = 0.02) in exploratory post hoc analyses, but this association requires validation in future studies.
Although several factors associated with ultrasound adequacy were immutable, in-patient status was significantly associated with worse ultrasound quality. This association may be due to differences in ultrasound operator experience, patient difficulty with exam cooperation while acutely ill, or clinical deterioration (e.g. increased ascites) that might hamper exam quality. Although it might be convenient for patients to have HCC surveillance performed while being seen as an in-patient, our study highlights that this approach might hinder obtaining a high-quality exam and limit surveillance effectiveness in the long term.
The issue of compromised ultrasound quality and surveillance failure may become more problematic as the epidemiology of HCC shifts from primarily HCV-mediated to NASH-mediated, highlighting the need for improvements in ultrasound image acquisition and/or evaluation of alternative surveillance modalities.18, 19 Use of cross-sectional modalities such as CT or MRI may increase sensitivity for HCC; however, there are no data evaluating their performance as a surveillance strategy. Furthermore, the increased cost and adverse effect profile, such as radiation exposure, limit their use as a primary surveillance modality in all-comer patients with cirrhosis. Studies are needed to determine if this strategy could be cost effective if limited to a subset of patients who are both high risk for HCC and prone to ultrasound failure.20 Despite a lack of alternative imaging modalities, there is hope that HCC biomarkers can increase early tumour detection rates in clinical practice. Using AFP, the best studied biomarker to date, in combination with surveillance ultrasound may increase sensitivity for early HCC in clinical practice.7, 21 Adding AFP may be particularly beneficial in patients with alcohol- or NASH-related cirrhosis; not only is ultrasound prone to failure in these patients but AFP also has higher specificity and overall accuracy for early HCC detection as compared to its performance in patients with HCV-related cirrhosis.22 Novel biomarkers are also being explored through multi centre efforts such as the Early Detection Research Network (EDRN).23
Our study had a few limitations. Our study was conducted at a single centre with a high volume of cirrhotic patients and surveillance ultrasound exams using fellowship-trained abdominal radiologists, and our results may not be generalised to all practice settings, particularly given the operator dependent nature of ultrasound. Second, our study had a small number of HBV patients so interpretability of results in this subgroup is more limited. Third, we cannot exclude possible unmeasured confounders or measurement bias given the retrospective nature of our study. For example, we used BMI as a measure of obesity given lack of data regarding factors such as truncal obesity or degree of visceral fat. Fourth, ultrasound quality was determined using static images reviewed by a radiologist, which may not be representative of the entire ultrasound examination as performed by the technologist. However, we feel this is reflective of how ultrasound exams are typically performed in the USA and interpreted so our results should reflect ultrasound quality in clinical practice. Finally, our outcome was a subjective assessment of ultrasound quality and adequacy for HCC surveillance; although inadequate ultrasound quality would intuitively predispose to HCC surveillance failure, further studies are needed to firmly establish this association.
In summary, we found that one in 5 ultrasound exams in our cohort of patients with cirrhosis were of inadequate quality for HCC surveillance. The most common reasons for inadequate quality were rib shadowing and inadequate ultrasound beam penetration. Obesity, Child Pugh B or C cirrhosis, and alcohol or NASH-related cirrhosis are associated with inadequate ultrasound quality, with these patients having inadequate exams in over one-third of cases. Alternative surveillance strategies may be needed, particularly for subgroups prone to surveillance ultrasound failure.
ACKNOWLEDGEMENTS
Declaration of personal interests: None.
Declaration of funding interests: This study was conducted with support from AHRQ Center for Patient-Centered Outcomes Research (R24 HS022418) and CPRIT (CPRIT R15-MIRA-2). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Cancer Society.
Footnotes
LINKED CONTENT
This article is linked to Simmons et al and Patel papers. To view these articles visit https://doi.org/10.1111/apt.13910 and https://doi.org/10.1111/apt.13891.
REFERENCES
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2010; 53: 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001; 35: 421–30. [DOI] [PubMed] [Google Scholar]
- 3.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther 2009; 30: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med 2014; 11: e1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trevisani F, De NS, Rapaccini G,et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience). Am J Gastroenterol 2002; 97: 734–44. [DOI] [PubMed] [Google Scholar]
- 6.van Meer S, de Man RA, Coenraad MJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in the Netherlands. J Hepatol 2015; 63: 1156–63. [DOI] [PubMed] [Google Scholar]
- 7.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol Biomarkers Prev 2012; 21: 793–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singal AG, Nehra M, Adams-Huet B,et al. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol 2013; 108: 425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finberg HJ. Whither (wither?) the ultrasound specialist? J Ultrasound Med 2004; 23: 1543–7. [DOI] [PubMed] [Google Scholar]
- 10.Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008; 6: 1418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brenner DJ, Hall EJ. Computed tomography–an increasing source of radiation exposure. N Engl J Med 2007; 357: 2277–84. [DOI] [PubMed] [Google Scholar]
- 12.Yopp AC, Mansour JC, Beg MS, et al. Establishment of a multidisciplinary hepatocellular carcinoma clinic is associated with improved clinical outcome. Ann Surg Oncol 2014; 21: 1287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llovet JM, Ducreaux M, Lencioni R, et al. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56: 908–43. [DOI] [PubMed] [Google Scholar]
- 14.Del Poggio P, Olmi S, Ciccarese F,et al. Factors that affect efficacy of ultrasound surveillance for early-stage hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014; 12: 1927–33. [DOI] [PubMed] [Google Scholar]
- 15.Khalili K, Menezes R, Kim TY, Yazdi LK, et al. The effectiveness of ultrasound surveillance for hepatocellular carcinoma in a Canadian centre and determinants of its success. Can J Gastroenterol Hepatol 2015; 29: 267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uppot RN, Sahani DV, Hahn PF, Kalra MK, Saini SS, Mueller PR. Effect of obesity on image quality: fifteen-year longitudinal study for evaluation of dictated radiology reports. Radiology 2006; 240: 435–9. [DOI] [PubMed] [Google Scholar]
- 17.Tchelepi H, Ralls PW, Radin R, GrantE. Sonography of diffuse liver disease. J Ultrasound Med 2002; 21: 1023–32; quiz 1033–4. [DOI] [PubMed] [Google Scholar]
- 18.Sanyal AJNASH. A global health problem. Hepatol Res 2011; 41: 670–4. [DOI] [PubMed] [Google Scholar]
- 19.El-Serag HB. Epidemiology ofviral hepatitis and hepatocellular carcinoma. Gastroenterology 2012; 142: 1264–73 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singal AG, Mukherjee A, Joseph Elmunzer B, et al. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol 2013; 108: 1723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrero JA, El-Serag HB. Alpha fetoprotein should be included in the hepatocellular carcinoma surveillance guidelines of the American Association for the Study of Liver Diseases. Hepatology 2011; 53: 1060–1; author reply 1061–2. [DOI] [PubMed] [Google Scholar]
- 22.Gopal P, Yopp AC, Waljee AK, et al. Factors that affect accuracy of alpha fetoprotein test in detection of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2014; 12: 870–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology 2009; 137: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


