Abstract
KRAS mutation is a negative predictive biomarker of anti-EGFR agents in patients with metastatic colorectal cancer (mCRC), and remains an elusive target. Pelareorep, a double-stranded RNA virus selectively replicates in KRAS mutated cells, and is synergistic with irinotecan. A dose escalation trial of FOLFIRI/bevacizumab (irinotecan (150–180 mg/m2) and pelareorep (1x1010 TCID50-3x1010 TCID50) was implemented in adult patients with oxaliplatin refractory/intolerant, KRAS mutant mCRC. Pelareorep was administered intravenously over 1 hour on days 1–5 every 4 weeks. Additional studies included pharmacokinetics, tumor morphology, and immune responses. Among FOLFIRI naïve patients, the highest dose of FOLFIRI/bevacizumab (180mg/m2 irinotecan) and pelareorep (3x1010 TCID50) was well tolerated, without a DLT. At the RPTD, three of six patients (50%) had a partial response; the median progression free and overall survival (PFS, OS) were 65.6 weeks and 25.1 months, respectively. Toxicities included myelosuppression, fatigue, and diarrhea. Transmission electron microscopy revealed viral factories (viral collections forming vesicular structures), at various stages of development. Immunogold staining against viral capsid σ−1 protein demonstrated viral “homing” in the tumor cells. The nucleus displayed sufficient euchromatin regions suggestive of active transcription. Flow cytometry revealed rapid dendritic cell maturation (48 hours) with subsequent activation of cytotoxic T cells (7 days). The combination of pelareorep with FOLFIRI/bevacizumab is safe. The PFS and OS data are encouraging and deserve further exploration. Pelareorep leads to a clear recurrent immune stimulatory response with cytotoxic T cell activation, and homes and replicates in the tumor.
Keywords: Colorectal, Kras, Pelareorep, T lymphocyte, Dendritic cell
INTRODUCTION AND BACKGROUND
Colorectal cancer (CRC) pathogenesis is marked by a series of somatic alterations, among which an oncogenic mutation in the KRAS gene (40–45% of patients) is common and one of the best described (1,2). The outlook for patients with metastatic CRC (mCRC) has seen dramatic improvements over the past 20 years. While 5-FU remained the only drug with any clinical benefit for 4 preceding decades, there are now 14 drugs that are USFDA approved and in active use (3). The median overall survival (OS) has improved from a dismal 12 months to close to 30 months since the availability of these agents (4,5). An essential arsenal in the struggle against this scourge remains the combination chemotherapy of folinic acid (FOL), infusion 5-FU (F) and irinotecan (IRI)(FOLFIRI), frequently used as a second line chemotherapy combination from among the chemotherapy armamentarium in patients with mCRC.
The presence of a KRAS mutation in a patient’s tumor is now a well-accepted predictive biomarker of exclusion of epidermal growth factor receptor (EGFR) directed therapy in mCRC (1,6). In patients with a KRAS wild type (WT) tumor, both cetuximab and panitumumab offer valuable clinical benefit. In spite of tremendous investment, both financial and intellectual, over the past decades, targeting KRAS has remained an elusive goal (7,8). In the 40–45% of patients with mCRC with a KRAS mutation, treatment options are limited once they are refractory to oxaliplatin and irinotecan based regimens (5,9,10). There is therefore an urgent unmet need to develop novel interventions that can be made available as a meaningful part of the treatment paradigm.
Pelareorep (Reolysin®, Oncolytics Biotech Inc., Calgary, Alberta, Canada) is Reovirus Type 3 Dearing strain, a naturally occurring, ubiquitous, non-enveloped human double stranded (ds) RNA virus. It is purported to replicate selectively in transformed cells with a EGFR pathway induction or KRAS mutation (11,12), or with a v-erb B oncogene (13). In non-transformed cells, the early viral transcripts lead to the auto-phosphorylation of dsRNA activated protein kinase R (PKR). The activated phosphorylated PKR (pPKR) in turn phosphorylates and activates the alpha subunit of the eukaryotic translation initiation factor 2 (EIF2 alpha) and subsequently inhibits viral protein synthesis (14). In transformed cells, the active Ras-signaling pathway inhibits the auto-phosphorylation of PKR, and thereby permits the synthesis of viral proteins, facilitating the uninhibited replication of the pelareorep (15).
We have previously elucidated in in vitro models that pelareorep is selectively efficient in its cytotoxic effect in KRAS mutant conditions, in contrast to KRAS WT, and is synergistic with irinotecan (12). We carried over these observations, and confirmed them in in vivo xenograft models of CRC cell lines. Subsequently, we launched a phase I dose-escalation open-label clinical trial of pelareorep combined with standard FOLFIRI, and bevacizumab, in patients with KRAS mutant oxaliplatin refractory/intolerant mCRC.
PATIENTS AND METHODS
Animal studies
Approval was obtained by the Albert Einstein College of Medicine Institutional Animal Care and Use Committee (IACUC). Athymic nude mice (Harlan Laboratories # nu69), 8–9 weeks old were injected with 5 million HCT116 cells mixed with matrigel (1:1) into their right flank, and monitored daily. Drug intervention was begun when tumor volume reached 100 mm3. Mice were randomized into four groups (12–14 animals each); pelareorep at a daily dose of 10 million tissue culture infective dose-50 (TCID50) administered intra-tumorally (IT), and placebo intra-peritoneally (IP), or irinotecan at 1.25 mg/kg body weight twice weekly IP and placebo IT, the combination of both active agents, or placebo in both. The animals were monitored for ulceration, bleeding or infection of the skin and tumor, and for decreased mobility or moribund status. The tumor length and width were measured every 72 hours with digital calipers and the tumor volume (width X length2) calculated. The animals were euthanized by carbon dioxide inhalation when the tumor size reached 2000 mm3. At the end of 6 weeks, all surviving animals were euthanized, once the final tumor volume measurements were taken. All experiments were performed in triplicate.
Patients and drug administration
This was a three center open label phase I dose escalation clinical trial. The study enrolled patients between 2011 and 2017 with mCRC who had progressed on or were intolerant to prior oxaliplatin based therapy in the metastatic setting, or relapsed within 6 months of adjuvant oxaliplatin. Patients were adults ≥18 years; with a pathological diagnosis of KRAS mutated mCRC. They were required to have an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, and with measurable or evaluable disease. Prior bevacizumab was permitted. Patients with history of brain metastases were permitted if the tumors were treated and controlled. A washout period of 28 days was required after cessation of prior radiotherapy or chemotherapy or major surgery. Additional active cancer(s) were excluded. All prior toxicities had to have resolved to grade 1 or less. Key laboratory parameters required were absolute neutrophil count (ANC) ≥1500/uL, platelets ≥100,000/uL, bilirubin/ALT/AST within normal limits, creatinine clearance ≥50 ml/minute, and urine proteinuria <30 mg% (once bevacizumab was added). Pregnant and breast-feeding women were excluded.
The primary objective was to determine the dose limiting toxicity (DLT), maximum tolerate dose (MTD), and the recommended phase two dose (RPTD). Only cycle 1 toxicities were considered in identifying a DLT. The latter was defined as a grade ≥3 non-hematologic toxicity (except sub-optimally treated nausea/vomiting/diarrhea), grade 4 thrombocytopenia, or grade 3 thrombocytopenia with bleeding, grade 4 neutropenia ≥5 days, and grade ≥3 febrile neutropenia. Use of growth factors was not allowed in the first two cycles. Secondary objectives included pharmacokinetics (PK), pharmacodynamics (PD), immunological response, and clinical efficacy.
Patients received escalating doses of irinotecan in the FOLFIRI regimen (irinotecan 150–180 mg/m2 intravenous (IV) over 90 minutes, leucovorin 400 mg/m2 IV over 2 hours, followed by 5-FU 400 mg/m2 bolus, and 5-FU 2400 mg/m2 continuous IV over 46 hours) and repeated every 14 days. Standard drug preparation and administration guidelines, including anti-emetic and atropine premeditations were followed. Pelareorep was supplied by Oncolytics Inc. in vials containing 7.2X1011 tissue culture infective dose (TCID50) per ml of pelareorep in a phosphate-buffered solution and stored at −70°C. Escalating doses of pelareorep ranging from 1X1010 to 3X1010 TCID50 were administered IV over 1 hour on days 1–5 every 28 days (with alternate doses of FOLFIRI chemotherapy).
Prior irinotecan was permitted, until DLTs were observed in two patients at the highest irinotecan dose of 180 mg/m2. The protocol was amended to exclude prior irinotecan chemotherapy exposure. Furthermore, as clinical data and practice evolved with the survival benefit of continuing bevacizumab (16), it was added to the FOLFIRI regimen. Pelareorep was also moved from cycle 1 to cycle 2, to allow for distinction of FOLFIRI related from FOLFIRI/pelareorep related toxicities. The study protocol was approved by all local institutional review boards (IRB) and ethics committees. All procedures were performed after obtaining such approval. All patients gave written informed consent prior to any study related procedure.
Assessments
Pretreatment evaluation included medical history, physical examination laboratory analyses (CBC, basic metabolic panel, liver function tests, coagulation tests, urine for proteinuria), within 14 days of first dose. All patients had evaluable disease documented by imaging, including a CT scan of chest, and CT or MRI of abdomen, and pelvis. All laboratory tests were repeated at each treatment cycle, while imaging studies were repeated at 8 weeks, irrespective of dose delays or skipped doses. Response was assessed according to RECIST (Response Evaluation Criteria in Solid Tumors), version 1.1 (17). Patients remained on study until documented progression or intolerable toxicities or patient choice. They were followed every 8 weeks for survival, until death or withdrawal of consent. As there was no expectation of drug-drug interaction, and to minimize patient discomfort and blood draws, limited PK sampling was performed for irinotecan, SN-38 and 5-FU in cycles 1 and 2, both with and without pelareorep (18). Plasma samples were drawn at 0, and 90 minutes, and 4, 24, and 48 hours from time of start of irinotecan. The collection and analysis of samples have been previously described by our group (19).
Flow Cytometry (FACS)
We also sought to assess the immune-modulating effects of pelareorep, once the MTD was established. Blood was drawn into cell preparation tubes (CPT) (BD Vacutainer® CPT™, Mononuclear Cell Preparation Tubes, (manufacturer # 362753) to isolate peripheral blood mononuclear cells (PBMC) at 0, 3, 24, 48, 72, and 96 hours, and 8 and 15 days post D1 of pelareorep containing and non-pelareorep cycles. Fluorescence activated cell sorting (FACS) assay was performed using fluorophore labeled antibody staining for T helper lymphocyte (FITC-CD4; catalog # 11–0049; Thermofisher-eBiosciences), cytotoxic T lymphocyte (PE-CD8; catalog # 12–0088; Thermofisher-eBiosciences), activated cytotoxic T lymphocyte (CD70-eFluor 660; catalog # 50–0709; Thermofisher-eBiosciences), mature dendritic cell (CD123-PE-Cy7; catalog # 25–1239; Thermofisher-eBiosciences) and Natural killer (NK) cells (CD56-eFluor 450 catalog # 48–0566; Thermofisher-eBiosciences) along with live dead marker( FVD-eFluor 780; catalog # 65–0865 Thermofisher-eBiosciences). The staining and data acquisition was performed within 3 hours of sample collection. Flo Jo software (version 9.8.1) was used for all analysis and gating was maintained unaltered throughout the entire analysis.
Analysis of Tumor Tissue
Pre-treatment tissue, if available, was subjected to molecular analysis by next-generation sequencing (NGS) using the platform by Foundation One. On-study tumor biopsies were optional and were performed on consenting patients. These were analyzed by electron microscopy and immune histochemistry (IHC).
Transmission Electron Microscopy (TEM)
The biopsy samples were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer, post-fixed with 1% osmium tetroxide followed by 1% uranyl acetate, dehydrated through a graded series of ethanol and embedded in LX112 resin (LADD Research Industries, Burlington VT). Ultrathin (80 nm) sections were cut on a Reichert Ultra-cut UCT, stained with uranyl acetate followed by lead citrate. For the immunogold staining, sections were etched with saturated sodium metaperiodate for 1 hour, followed by preheated 0.01M sodium citrate buffer, pH of 6.0 for 10 minutes, with intermittent microwave heating. Sections were washed with PBS, blocked with 1%BSA and incubated with primary antibody to viral capsid σ−1 protein (sourced from Oncolytics) overnight at 4°C followed by goat anti-rabbit 10nm gold particles (https://aurion.nl/) attached secondary antibody for three hours at room temperature. Sections were stained with uranyl acetate. All EM images were viewed on a JEOL 1200EX transmission electron microscope at 80kv.
Statistical Analysis
The study followed a standard 3+3 dose escalation design. Once the protocol defined highest dose was reached, a total of six patients were enrolled, to confirm safety and tolerability. Descriptive statistics were used to characterize patient demographics, response and toxicity (including laboratory abnormalities) rates. Time-to-event parameters were analyzed according to the Kaplan-Meier method. GraphPad Prism version 7.0 (GraphPad Software, LaJolla, CA) was used for all statistical analyses.
RESULTS
Human derived cell line xenograft study
Twelve to fourteen mice were treated in each of the four conditions. We observed a rapid tumor growth in the control mice, and all control mice were sacrificed before 10 days from start of drug intervention (Figure I). As single agents, irinotecan and pelareorep exhibited a similar degree of tumor growth control (52% and 60%). The mice that received both drugs clearly had the best outcome, with some mice showing complete tumor regressions (p=0.0005). The mean tumor volume reduction in the combination group was 77%, 52%, and 42% compared to control, irinotecan and pelareorep injected tumors, respectively. All mice were sacrificed and euthanized when the tumor volume reached 2000 mm3 or at the end of the experiment at 6 weeks (Supplementary figure 1).
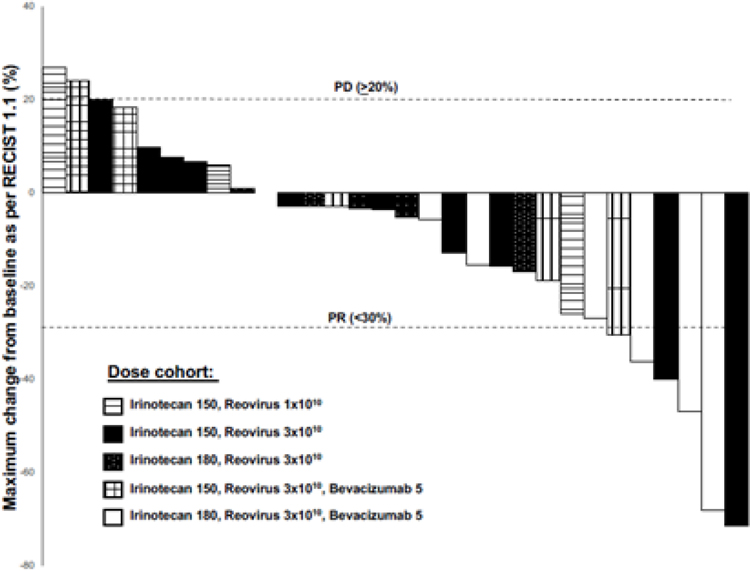
Figure 1.
Waterfall plot of the 30 patients evaluable for tumor response by RECIST 1.1, depicting the best change in overall tumor dimensions. Six patients had a PR as the best response, and 22 had SD.
Patient characteristics
The study enrolled 36 patients with a median age of 59 (range 30–77) years, of which 23 (64%) were women (Table I). All patients had received prior oxaliplatin chemotherapy, and except one, all patients received it in the metastatic setting, and all had progression of disease as the reason for discontinuation. Thirteen (41%) had prior radiation therapy and 13 (41%) had prior FOLFIRI chemotherapy. Seventy % had colonic, while the other 30% had rectal primary tumors. More than half the patients had either a G12V or a G12D mutation (exon 2, codon 12, KRAS gene). At the RPTD, two patients withdrew from study after the first dose of FOLFIRI/bevacizumab, did not receive pelareorep, and were not evaluable for toxicity. An additional four patients did not receive the planned 4 cycles of chemotherapy prior to their first disease assessment, due to toxicity or patient choice, leading to 30 patients assessable for response.
Table 1.
This table highlights the important clinical characteristics of all the patients who consented and were enrolled in the clinical trial.
| Baseline characteristics (n=36) | ||
|---|---|---|
| Age in years: median (range) | 59 (30–77) | |
| Sex | Female | 23 (64%) |
| Male | 13 (36%) | |
| Race | Non-Hispanic White | 19 (53%) |
| Non-Hispanic Black | 12 (33%) | |
| Hispanic | 4 (11%) | |
| Asian | 1 (3%) | |
| ECOG Performance Status | 0 | 3 (8%) |
| 1 | 32 (89%) | |
| 2 | 1 (3%) | |
| Prior treatment | Surgery | 32 (89%) |
| Chemotherapy | 36 (100%) | |
| (FOLFIRI) | 13 (37%) | |
| Bevacizumab | 9 (25%) | |
| Radiotherapy | 13 (37%) | |
| Primary site | Right colon | 10 (28%) |
| Left colon | 15 (42%) | |
| Rectal | 11 (30) | |
| Kras mutation status | G12V | 12 (33%) |
| G12D | 10 (28%) | |
| G12C | 4 (11%) | |
| G12A | 2 (6%) | |
| G13D | 2 (6%) | |
| G12’X’ | 3 (8%) | |
| G12R | 1 (3%) | |
| Q61’X’ | 1 (3%) | |
| K117N | 1 (3%) | |
“X” = unknown/not reported
Safety, Dose Limiting Toxicities, Maximum Tolerated Dose, and Recommended Phase Two Dose
Table 2 lists the grade ≥ 2 toxicities experienced by all the patients in the trial. The initial design of the trial permitted entry of patients with prior irinotecan treatment Among them, at the irinotecan dose of 180 mg/m2 (cohort 3), two of six patients experienced a DLT; one patient experienced grade 4 thrombocytopenia, and another developed febrile neutropenia and urosepsis. The latter patient was a 65 year old male with colon cancer with extensive liver metastases, who had been treated with both FOLFOX and FOLFIRI, both with bevacizumab. He suffered a significant myelosuppressive episode with the first dose of FOLFIRI/pelareorep, developed urosepsis and finally succumbed to it. This dose was considered to have exceeded the MTD, and the next lower dose cohort of irinotecan at 150 mg/m2 (cohort 2), was tested and was well tolerated, with no DLT observed among the 12 patients treated. At this time, the protocol was amended to include bevacizumab, and to exclude prior FOLFIRI (cohorts 4–5). No further DLT was observed, and cohort 5 was declared the RPTD - full dose of FOLFIRI (irinotecan at 180 mg/m2, every 14 days), and the highest single agent dose of pelareorep (3X1010 TCID50 on days 1–5 every 28 days).
Table 2.
Summary of all grades (2–5) toxicities observed in > 10% of patients that were considered possibly, probably, or definitely related to study related intervention.
| Treatment related (possible, probable, or definite) toxicities that occurred in > 10% of patients | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Toxicity | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Cohort 5 | |||||||||||
| Irinotecan | 150 mg/m2 | 150 mg/m2 | 180 mg/m2 | 150 mg/m2 | 180 mg/m2 | |||||||||||
| Reovirus | 1 X 1010 TCID50 | 3 X 1010 TCID50 | 3 X 1010 TCID50 | 3 X 1010 TCID50 | 3 X 1010 TCID50 | |||||||||||
| Bevacizumab | None | None | None | 5 mg/kg | 5 mg/kg | |||||||||||
| n | 3 | 12 | 6 | 7 | 8 | |||||||||||
| Grade | 2 | 3 | 4 | 2 | 3 | 4 | 2 | 3 | 4 | 5 | 2 | 3 | 4 | 2 | 3 | 4 |
| Myelosuppression | ||||||||||||||||
| Neutropenia | 1 | 5 | 1 | 1 | 3 | 3 | 1 | 3 | 3 | |||||||
| Anemia | 3 | 1 | 1 | 2 | 1 | 2 | 3 | |||||||||
| Thrombocytopenia | 1 | 1 | 3 | 1** | 1 | |||||||||||
| Infection | 2 | 1 | 1** | 1 | ||||||||||||
| GI Symptoms | ||||||||||||||||
| Diarrhea | 4 | 2 | 1 | 1 | 3 | 2 | ||||||||||
| Constipation | 1 | 1 | 2 | |||||||||||||
| Nausea | 1 | 2 | 1 | 2 | ||||||||||||
| Vomiting | 2 | 1 | 1 | 2 | 1 | |||||||||||
| Stomatitis# | 1 | 1 | ||||||||||||||
| Anorexia | 1 | 2 | 2 | 1 | ||||||||||||
| Dehydration | 1 | |||||||||||||||
| Flu like symptoms | ||||||||||||||||
| Fatigue | 2 | 5 | 2 | 3 | 3 | 3 | 2 | |||||||||
| Pyrexia | 1 | 1 | 1 | |||||||||||||
| Dehydration | 1 | |||||||||||||||
| Musculoskeletal$ | 2 | 4 | 1 | |||||||||||||
| Generalized | ||||||||||||||||
| Dizziness | 3 | 2 | ||||||||||||||
| Insomnia | 4 | 2 | 1 | 2 | ||||||||||||
| Dyspnea | 1 | 1 | ||||||||||||||
| DVT | 1 | 1 | 2 | |||||||||||||
| HTN | 1 | 1 | 1 | 1 | 3 | |||||||||||
| Epistaxis | 1 | 1 | 1 | 3 | 1 | |||||||||||
| Proteinuria | 2 | 3 | 2 | |||||||||||||
includes oropharyngeal pain, mucositis
includes arthralgias, back pain, myalgias, extremity pain, headaches
Dose limiting toxicity
The most common grade ≥ 2 toxicities experienced by the patients can be grouped into three groups, including myelosuppression [neutropenia, n=21 {58%; (grade ≥ 3, 50%)}; and anemia, n=13 {36%; (grade ≥ 3, 25%)}]; gastrointestinal [diarrhea {n=13 (36%; (grade ≥ 3, 8%)}]; and constitutional [fatigue, n=20 {36%; (grade ≥ 3, 11%)}]. Fever was observed in 3 patients, clearly attributable to the pelareorep administration, similar to prior experience (20). Two patients suffered from grade 3 proteinuria, attributable to the bevacizumab. No overlapping toxicities of concern were observed in this study.
Response and Clinical Benefit
All patients underwent imaging every 8 weeks, irrespective of dose delays or skipping of therapy. Images were reviewed by an independent radiologist, and response assessed as per RECIST 1.1 (17). At the RPTD, three of six patients experienced a partial response (PR) (Figure 1). Overall, of the 30 evaluable patients, 6 (20%) had a partial response (PR), and 22 patients (73.3%) had stable disease (SD) as their best response, with a clinical benefit rate (PR +SD) of 93.3%. Among the 6 patients with a PR, 4 had liver only metastases, 1 had metastases in lung, liver, and abdominal masses, and the sixth patient had pelvic masses and lymph nodes (Supplementary table 1). Two patients had progression of disease at their first post therapy initiation imaging. The median progression free survival (PFS) was 65.6 weeks at the RPTD, and 22.2 weeks for all patients. The OS was 25.1 months at the RPTD, and 11.7 months for all patients (Supplementary Figure 1). Of note, 1 patient had to discontinue the study due to inability to keep up with the 5 days of pelareorep administration, and was continued on FOLFIRI/bevacizumab. Encouragingly, she stayed on this regimen for 19.5 additional months after withdrawal of study, until she experienced disease progression.
Across all doses, 13 patients were treated with FOLFIRI prior to study entry and their PFS and OS was 18.5 weeks and 9.7 months, respectively. In contrast, among patients who were FOLFIRI naïve, the PFS and OS was 25.7 weeks and 19.5 months, respectively. Not surprisingly, the clinical outcome was numerically better for patients that were FOLFIRI naïve, however, this study was clearly not designed for studying this question.
Pharmacokinetics
Consistent with our expectation, there was no evidence of any pharmacokinetic drug-drug interaction between pelareorep and chemotherapy (Supplementary Table 1). As expected, the mean AUC (exposure) of irinotecan was higher at the 180 mg/m2 cohort than the 150 mg/m2. There was no discernible difference in the AUC, or clearance in the presence or absence of pelareorep. Similarly, no differences were observed in the PK of SN-38 or 5-FU.
In depth analysis of tumor mutations
All 36 enrolled patients carried a mutation in KRAS (details in Table 1). Among them, 22 had available tissue that was assayed by next generation sequencing. Additionally, one patient’s tissue was assayed for BRAF mutations. Of the 22 patients, 8 had a mutation in the PI3K pathway, with a mutation in in PIK3CA or PTEN. As expected, 18 of the 22 had an APC mutation and 10 had a p53 mutation. Among the 23 patients in whom the BRAF status was known, only 1 had a mutation. This lower rate of BRAF mutations is consistent with prior observations that there is almost a “mutual exclusivity” or RAS and BRAF mutations (Supplementary table 1).
Pharmacodynamics
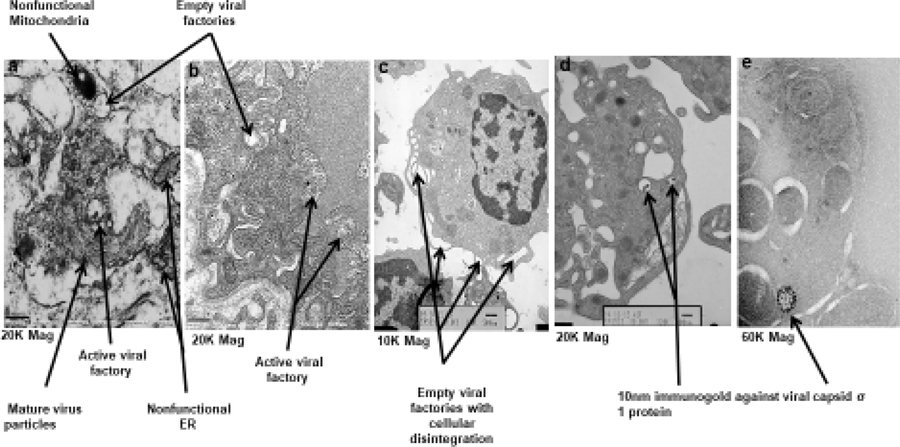
On study biopsies were obtained from 3 patients, of which 1 was subject to NGS, 1 to TEM and IHC staining, and the third for both studies. TEM showed distinct morphology relating to pelareorep growth and propagation. The viruses home themselves into a vesicular structure indicated as viral factories (figure 3a and 3b), where new virions assemble [(Figure 2a–b, Figure 2d (immunogold staining)]. At a magnification of 20,000, in the cells containing viral factories, the nonfunctional endoplasmic reticulum, mitochondria, and the mature viral particles are evident. Upon dissemination of virus, the empty factories disintegrate causing overall cytoplasmic disintegration. This is clear at a lower magnification of 10,000 X (figure 3c). The presence of multiple viral factories engaged at various stages of virus development in a single tumor cell (figures 3a–c) indicate simultaneous infection by several viruses. The tumor cell nucleus has sufficient euchromatin regions supporting the notion that transcription is active within the cell while virus mediated cytoplasmic degeneration is in progress (figure 3c). Another distinct cytoplasmic feature is the presence of multivesicular bodies indicating that the cell is in distress. As further evidence of viral homing into the tumor, we detected the viral particles embedded within tumor cells, using immune gold staining for the pelareorep capsid σ−1 protein (figure 3d).
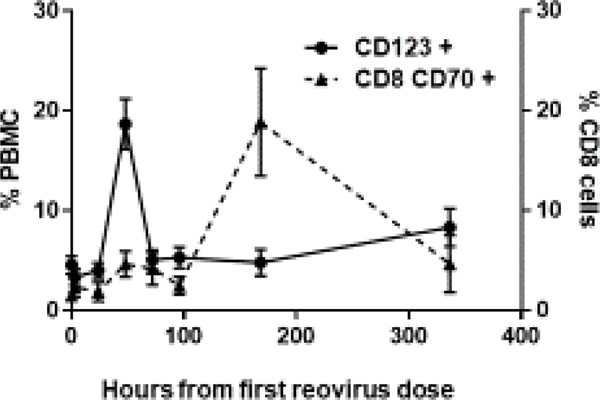
Figure 3a.
The figure represents the FACS analysis of changes in circulating immune cells. Samples were collected from 5 patients treated at the RPTD over 14 cycles. The graphs depict mean and SEM. A rapid peak of the dendritic cell maturation was observed at 48 hours, followed by the activation of CD8 T cells at 7 days.
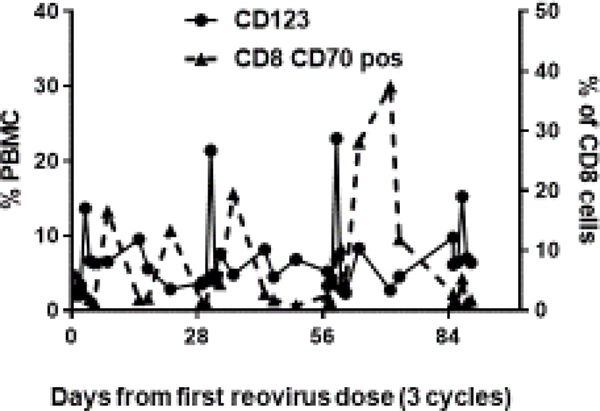
Figure 3b.
The figure represents FACS analysis of changes in circulating immune cells from a single patient at the RPTD over 3 cycles. In each cycle, samples were drawn on days 1, 3, 8, 15, 17, 29 (day 1 of next cycle), and ended on day 85 (day 1 of cycle 4). A peak in the dendritic cell maturation was observed at 48 hours (days 3, 31, 59, 87), followed by activation of CD8 T cells after 7 days (days 8, 36, 64), a process repeated with a dose of pelareorep delivered every 28 days.
Figure 2.
This figure depicts the key findings from electron micrographs representing the process of virus propagation. Figure 3a shows empty and active viral factories as well as nonfunctional subcellular organelles including mitochondria and endoplasmic reticulum confirming that the cell is dying. Figure 3b shows several active viral factories. Figure 3c indicates the process of cellular disintegration at points of empty viral factories. Figure 3d and 3e is immunogold staining of the tumor sample obtained by biopsy of a patient using 10nm gold particles to detect the localization of pelareorep sigma 1 capsid protein again seen confined to viral factories (60X mag).
With regard to NGS assay, one patient had a KRAS G12D, APC R1450*, IDH1R132C, both in the pre and post pelareorep sample, but gained a PIK3CA R357Q mutation in the post pelareorep sample. The second patient had a KRAS G12V and SMAD4 G419R mutation on the pre- and KRAS G12V, ERRFI1 loss, APC Y622fs*7, ARID1A Q1584*, DNMT3A R882H (subclonal) on the post-pelareorep specimen. With this limited dataset, it appears that the nature of the KRAS mutation remains intact with pelareorep therapy.
Immune Response
We performed an in depth study using FACS to isolate and quantify lymphocytes from PBMC at the RPTD. Among the six patients entered, five consented to PBMC sampling for FACS. Samples were collected at protocol specified times points in one cycle in one patient, and in multiple cycles in four patients.
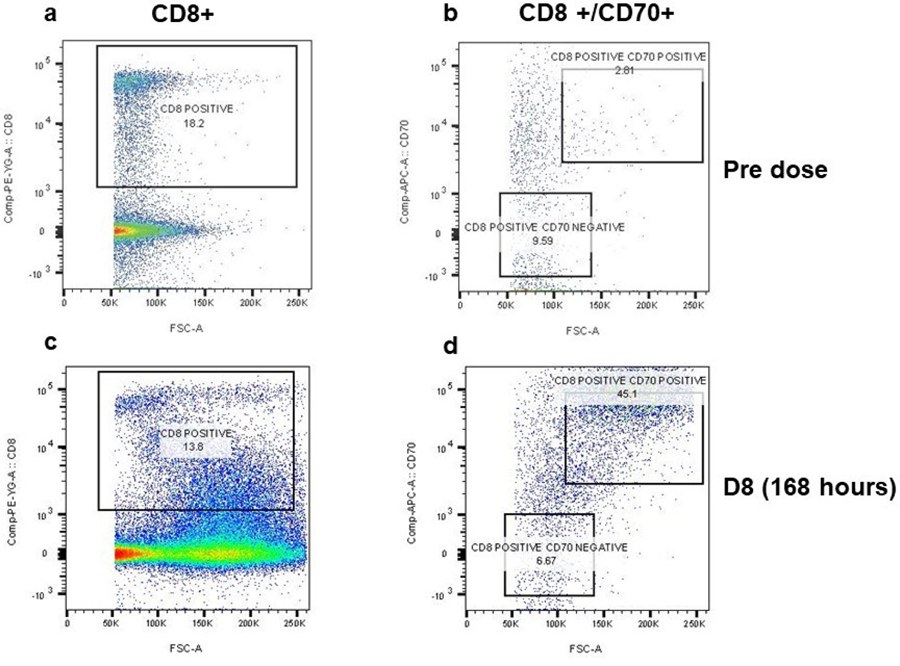
A very rapid maturation of the dendritic cells was observed at 48 hours, from a baseline mean of 4.5% to a mean of 18.6% (4.1 fold change, p=0.000016). This was followed by an increase in absolute CD8 counts (2.4 fold change, p=000015), and CD4 counts (3.5 fold change, p=000015), on day 4. The most important observation was the activation of CD8 cells (CD8+CD70+) on day 8, from a baseline mean of 1.5% to a mean of 18.8% (12.9 fold change, p=0.0009); (Figure 3a). A simultaneous drop in non-activated CD8 cells (CD8+ CD70-) accompanied this increase in activated CD8 cells. Samples were also drawn at 48 hours and 7 days post FOLFIRI chemotherapy infused without pelareorep. Not surprisingly, a similar dendritic cell maturation and CD8 activation were not replicated. This unique pattern of dendritic cell maturation followed by CD8 activation was observed repeatedly with each dose of pelareorep (representative patient data shown in Figure 3b). The details of the change in CD8 and CD70 cells in a representative patient are shown in Figure 4. We did not observe any obvious common pattern of the NK cells in response to pelareorep.
Figure 4.
Flow cytometry analysis of bivariate (CD8+ CD70+) cell population in a representative patient’s PBMC at pre (day 1 prior to pelareorep administration) and day 8 (168 hours post dose). Cells were labelled with PE-CD8 (eBioscience #12–0088-80) and efluro660-CD70 (e Biosciences # 50–0709) antibodies post live dead (FVD eFluro780 # 65–0865) staining. The CD8 positive cell population were gated out of the live cell population. The CD8+ cells were further selected for CD8 CD70+ and CD8 CD70− population (indicated in square boxes). The CD8 positive cell population with low CD70 expression was carefully excluded from the analysis. The D8 CD8CD70+ population increases from 2.8% to 45.1%.
DISCUSSION
The idea of using viruses to target cancer dates back to over a century (21). More recently, there has been a renewed interest in the use of infectious agents, such as viruses, as adjuncts in the treatment of cancer. Single agent use of pelareorep has been supplanted by convincing pre-clinical and clinical evidence that chemotherapy facilitates better delivery of pelareorep into the tumor (15,22–24). Pelareorep has been tested in multiple clinical trials to date, both, as an intra-tumoral injection, and intravenously (15). These trials have each demonstrated unique toxicities depending on the cytotoxic agent employed. (25,26). These unique findings highlight the need to conduct careful well-planned phase 1 combination dose escalation studies, when studying two agents with widely differing mechanisms of action.
Over the past decade, an oncogenic mutation in KRAS in a patient’s tumor with mCRC is entrenched as a biomarker of exclusion of anti-EGFR therapy (3,27).. The urgent need for novel approaches for this specific patient population cannot be overemphasized. Our group has previously proven synergistic anti-cancer activity using in vitro model systems of the combination of pelareorep with irinotecan (12). We demonstrated that pelareorep preferentially induced apoptosis in the KRAS mutant rather than KRAS WT genotype in a panel of CRC cell lines.
In this multi-center, biomarker driven, phase I study, the combination of FOLFIRI/bevacizumab chemotherapy with pelareorep was well tolerated; the highest doses of each individual drug was easily deliverable; strong efficacy signals were observed at the RPTD; and correlative science demonstrated a unique immune enhancing phenomenon, and electron microscopic characteristics. No DLT was observed in the first 28 days of assessment. The only DLT events observed were in the patients with prior exposure to FOLFIRI therapy, and were related to myelosuppression, attributable to the chemotherapy component of the regimen, rather than the pelareorep. Overall, grade 3–5 neutropenia was seen in 18 (50%) patients, which is higher than previously seen with FOLFIRI/anti-VEGF agent combination studies (16,28,29); however, this may be explained by cumulative neutropenia from the prolonged therapy. Grade 3–4 diarrhea was experienced by 8% of patients, similar to historical data.
Recent progress in second line therapy for mCRC has included 3 anti-VEGF agents, bevacizumab (16), aflibercept (28), and ramucirumab (29), improving OS by 1.4–1.6 months compared to FOLFIRI, to a median of 11.2 to 13.5 months. By comparison, in the current study, at the RPTD, the median PFS of the six patients was 65.6 weeks, and median OS was 25.1 months. Such a dramatic improvement compared to historic data is encouraging, warranting further testing. Anecdotally, one patient withdrew consent as she was not able to keep up with the rigorous schedule of pelareorep administration, and continued on FOLFIRI/bevacizumab alone, well beyond 19 months.
This is not the first time that pelareorep underwent evaluation in mCRC. In a separate randomized phase two study in front line patients; it was combined with standard FOLFOX chemotherapy (30). The primary endpoint was PFS, and surprisingly the pelareorep arm was inferior, although the response rates were higher, and OS did not differ. In a randomized phase II study in men with castrate resistant prostate cancer, patients received docetaxel/prednisone with or without pelareorep (31). Overall, the study had no efficacy improvement observed. Although the 12 week “lack of progression” was improved with pelareorep, the median OS was not different. These two studies highlight the difficulty of translating pre-clinical science into clinical results. There is no data on the combination of pelareorep and oxaliplatin specifically in CRC models, and on the combination of pelareorep and docetaxel in prostate cancer models. It is quite well established that pre-clinical success is no guarantee of clinical success while pre-clinical failure or lack of successful data is a sure road to clinical failure. Second, the patients were not selected based on Ras status in either study. The lack of a predictive biomarker may have contributed to the disappointing results too. The current study, on the other hand, is built on robust in vitro data showing preferential susceptibility in Ras mutant conditions, and in vitro and in vivo data demonstrating synergy with irinotecan.
Correlative science involved detailed FACS based assays for immune markers. A very rapid maturation of the dendritic cells that are potent antigen presenting cells (APC) was observed at 48 hours, and subsequently, a peak in the CD8 activation was seen after 7 days. This significant activation of the host adaptive immune system is a likely contributor to the efficacy of the pelareorep. More importantly, such dendritic cell maturation and activation of CD8 was observed with every cycle of pelareorep dosing, suggesting that the host immune response (neutralizing anti-pelareorep antibody, NARA) was insufficient in suppressing viral replication, allowing for sustained clinical benefit. It is well established that NARA is detectable in patients prior to first dose of pelareorep, and its titer increases by hundreds of fold upon exposure to pelareorep (20,32). On the same theme, a prior report argued that pelareorep is carried within dendritic cells, and able to evade host immunity (33).
Our electron microscopy findings are consistent with the fact that that the virus exerts dual mode of tumor cell killing. While being a potent immune stimulator it also physically destroys the tumor cells by forming active viral factories. In such events, the subcellular organelles like mitochondria and rough endoplasmic reticulum lose structural architecture, and several multicellular bodies are formed indicating progression towards cellular death. Interestingly the obvious chromosomal condensation is not observed until the very end, probably because the virus controls the transcriptional activities and continues to help maximize the synthesis of active virions. Perhaps for the first time, we have elucidated, by electron microscopy the chronology of the intra cellular morphologic changes in the structure and organelles taking place because of pelareorep infection.
The further development of pelareorep could take many shapes and forms. One interesting approach under study is the utilization of viruses to convert “cold” tumors into “hot” or “inflamed” tumors. With the advent of immunotherapy, there is tremendous interest in combining viruses with immune checkpoint inhibitors (34–37). Early results from in vivo models of syngeneic mice from our laboratory have shown synergistic activity with anti-mouse PD-1 monoclonal antibody specifically in KRAS mutant microsatellite stable (MSS) CRC, but not so in KRAS WT microsatellite unstable (MSI) mice (36). Specifically, the combination of pelareorep and checkpoint inhibitor has been studied in patients with pancreatic ductal adenocarcinoma, with encouraging signs of activity (37). This is of particular interest in the patients with tumors harboring a KRAS mutation that tend to have a higher prevalence of MSS tumors (2).
Supplementary Material
ACKNOWLEDGEMENTS
The authors deeply appreciate the willingness of the patients to participate in the trial and to their families who supported them all along. This study would not have been possible without such contribution. We further want to extend our sincere thanks to Dr. Lydia Tesfa, ex-Associate Director, Flow Cytometry core facility and Ms.Leslie Gunther-Cummins, Electron Microscope Specialist, Analytical Imaging Facility (AIF) of Albert Einstein College of Medicine for their constant support and guidance throughout the execution of the project.
Research Support and Conflict of Interest statement: SG was supported by the Advanced Clinical Research Award (ACRA) in Colorectal Cancer from the Conquer Cancer Foundation, of the American Society of Clinical Oncology (2010–2013) and from Oncolytics. AO received funding from Oncolytics to conduct this trial. MC is an employee and stock owner in Oncolytics Inc. The other authors have no COI to declare.
Footnotes
Prior presentation: Presented in part at the European Society of Clinical Oncology Congress in 2018
REFERENCES
- 1.Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091–6 [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.www.nccn.org. Accessed July 16, 2019.
- 4.Goldberg RM, Rothenberg ML, Van Cutsem E, Benson AB 3rd, Blanke CD, Diasio RB, et al. The continuum of care: a paradigm for the management of metastatic colorectal cancer. Oncologist 2007;12:38–50 [DOI] [PubMed] [Google Scholar]
- 5.Aparo S, Goel S. Evolvement of the treatment paradigm for metastatic colon cancer. From chemotherapy to targeted therapy. Crit Rev Oncol Hematol 2012;83:47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merla A, Goel S. Novel drugs targeting the epidermal growth factor receptor and its downstream pathways in the treatment of colorectal cancer: a systematic review. Chemother Res Pract 2012;2012:387172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porru M, Pompili L, Caruso C, Biroccio A, Leonetti C. Targeting KRAS in metastatic colorectal cancer: current strategies and emerging opportunities. J Exp Clin Cancer Res 2018;37:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCormick F Targeting KRAS Directly. Annual Review of Cancer Biology 2018;2:81–90 [Google Scholar]
- 9.Grothey A, Cutsem EV, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. The Lancet 2013;381:303–12 [DOI] [PubMed] [Google Scholar]
- 10.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19 [DOI] [PubMed] [Google Scholar]
- 11.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science 1998;282:1332–4 [DOI] [PubMed] [Google Scholar]
- 12.Maitra R, Seetharam R, Tesfa L, Augustine TA, Klampfer L, Coffey MC, et al. Oncolytic reovirus preferentially induces apoptosis in KRAS mutant colorectal cancer cells, and synergizes with irinotecan. Oncotarget 2014;5:2807–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strong JE, Lee PW. The v-erbB oncogene confers enhanced cellular susceptibility to reovirus infection. J Virol 1996;70:612–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strong JECM, Tang D, Sabinin P, Lee PW. . The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J 1998;17:3351–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitra R, Ghalib MH, Goel S. Reovirus: a targeted therapeutic--progress and potential. Mol Cancer Res 2012;10:1514–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. The Lancet Oncology 2013;14:29–37 [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47 [DOI] [PubMed] [Google Scholar]
- 18.Mick R, Gupta E, Vokes EE, Ratain MJ. Limited-sampling models for irinotecan pharmacokinetics-pharmacodynamics: prediction of biliary index and intestinal toxicity. J Clin Oncol 1996;14:2012–9 [DOI] [PubMed] [Google Scholar]
- 19.Goel S, Desai K, Karri S, Gollamudi R, Chaudhary I, Bulgaru A, et al. Pharmacokinetic and safety study of weekly irinotecan and oral capecitabine in patients with advanced solid cancers. Invest New Drugs 2007;25:237–45 [DOI] [PubMed] [Google Scholar]
- 20.Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs 2010;28:641–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webb HE, Smith CE. Viruses in the treatment of cancer. Lancet 1970;1:1206–8 [DOI] [PubMed] [Google Scholar]
- 22.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res 2008;14:259–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong J, Sachdev E, Mita AC, Mita MM. Clinical development of reovirus for cancer therapy: An oncolytic virus with immune-mediated antitumor activity. World J Methodol 2016;6:25–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips MB, Stuart JD, Rodriguez Stewart RM, Berry JT, Mainou BA, Boehme KW. Current understanding of reovirus oncolysis mechanisms. Oncolytic Virother 2018;7:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinemann L, Simpson GR, Boxall A, Kottke T, Relph KL, Vile R, et al. Synergistic effects of oncolytic reovirus and docetaxel chemotherapy in prostate cancer. BMC Cancer 2011;11:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res 2011;17:581–8 [DOI] [PubMed] [Google Scholar]
- 27.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–34 [DOI] [PubMed] [Google Scholar]
- 28.Van Cutsem E, Tabernero J, Lakomy R, Prenen H, Prausova J, Macarulla T, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506 [DOI] [PubMed] [Google Scholar]
- 29.Tabernero J, Yoshino T, Cohn AL, Obermannova R, Bodoky G, Garcia-Carbonero R, et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): a randomised, double-blind, multicentre, phase 3 study. The Lancet Oncology 2015;16:499–508 [DOI] [PubMed] [Google Scholar]
- 30.Jonker DJ, Tang PA, Kennecke H, Welch SA, Cripps MC, Asmis T, et al. A Randomized Phase II Study of FOLFOX6/Bevacizumab With or Without Pelareorep in Patients With Metastatic Colorectal Cancer: IND.210, a Canadian Cancer Trials Group Trial. Clin Colorectal Cancer 2018;17:231–9 e7 [DOI] [PubMed] [Google Scholar]
- 31.Eigl BJ, Chi K, Tu D, Hotte SJ, Winquist E, Booth CM, et al. A randomized phase II study of pelareorep and docetaxel or docetaxel alone in men with metastatic castration resistant prostate cancer: CCTG study IND 209. Oncotarget 2018;9:8155–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res 2008;14:7127–37 [DOI] [PubMed] [Google Scholar]
- 33.Adair RA, Scott KJ, Fraser S, Errington-Mais F, Pandha H, Coffey M, et al. Cytotoxic and immune-mediated killing of human colorectal cancer by reovirus-loaded blood and liver mononuclear cells. Int J Cancer 2013;132:2327–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, et al. Combination Therapy With Reovirus and Anti-PD-1 Blockade Controls Tumor Growth Through Innate and Adaptive Immune Responses. Mol Ther 2016;24:166–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samson A, Scott KJ, Taggart D, West EJ, Wilson E, Nuovo GJ, et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Science Translational Medicine 2018;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titto A Augustine RM, Peter John, and Sanjay Goel. Abstract 3917: Potentiating effect of reovirus in anti-PD1 therapy in colorectal cancer. 2018.
- 37.Mahalingam D, Wilkinson GA, Eng KH, Fields P, Raber P, Moseley JL, et al. Pembrolizumab in Combination with the Oncolytic Virus Pelareorep and Chemotherapy in Patients with Advanced Pancreatic Adenocarcinoma: A Phase Ib Study. Clin Cancer Res 2020;26:71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.