Abstract
Background
Limited information exists regarding the cost-effectiveness of rehabilitation strategies for individuals with knee osteoarthritis (OA).
Objective
The study objective was to compare the cost-effectiveness of 4 different combinations of exercise, manual therapy, and booster sessions for individuals with knee OA.
Design
This economic evaluation involved a cost-effectiveness analysis performed alongside a multicenter randomized controlled trial.
Setting
The study took place in Pittsburgh, Pennsylvania; Salt Lake City, Utah; and San Antonio, Texas.
Participants
The study participants were 300 individuals taking part in a randomized controlled trial investigating various physical therapy strategies for knee OA.
Intervention
Participants were randomized into 4 treatment groups: exercise only (EX), exercise plus booster sessions (EX+B), exercise plus manual therapy (EX+MT), and exercise plus manual therapy and booster sessions (EX+MT+B).
Measurements
For the 2-year base case scenario, a Markov model was constructed using the United States societal perspective and a 3% discount rate for costs and quality-adjusted life years (QALYs). Incremental cost-effectiveness ratios were calculated to compare differences in cost per QALY gained among the 4 treatment strategies.
Results
In the 2-year analysis, booster strategies (EX+MT+B and EX+B) dominated no-booster strategies, with both lower health care costs and greater effectiveness. EX+MT+B had the lowest total health care costs. EX+B cost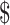 1061 more and gained 0.082 more QALYs than EX+MT+B, for an incremental cost-effectiveness ratio of
1061 more and gained 0.082 more QALYs than EX+MT+B, for an incremental cost-effectiveness ratio of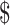 12,900/QALY gained.
12,900/QALY gained.
Limitations
The small number of total knee arthroplasty surgeries received by individuals in this study made the assessment of whether any particular strategy was more successful at delaying or preventing surgery in individuals with knee OA difficult.
Conclusions
Spacing exercise-based physical therapy sessions over 12 months using periodic booster sessions was less costly and more effective over 2 years than strategies not containing booster sessions for individuals with knee OA.
Osteoarthritis is a leading cause of disability in the United States, with annual patient and payer expenditures exceeding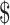 186 billion.1,2 Because the knee is the most commonly affected joint,3 it is imperative to identify knee osteoarthritis (OA) treatments that are both clinically effective and cost-effective. A recent systematic review concluded that evidence regarding cost-effectiveness of surgical knee OA treatments is limited.4 An economic analysis found various combinations of exercise and manual therapy were all more cost-effective than usual care (eg, physician visits, pharmaceuticals, knee injections) among New Zealanders with osteoarthritis.5 However, it is unclear which treatment combination is most cost-effective for knee OA in the United States.
186 billion.1,2 Because the knee is the most commonly affected joint,3 it is imperative to identify knee osteoarthritis (OA) treatments that are both clinically effective and cost-effective. A recent systematic review concluded that evidence regarding cost-effectiveness of surgical knee OA treatments is limited.4 An economic analysis found various combinations of exercise and manual therapy were all more cost-effective than usual care (eg, physician visits, pharmaceuticals, knee injections) among New Zealanders with osteoarthritis.5 However, it is unclear which treatment combination is most cost-effective for knee OA in the United States.
To reduce knee OA–related pain and disability, exercise is an effective first-line intervention endorsed by professional organizations including the American Academy of Orthopaedic Surgeons, American College of Rheumatology, Osteoarthritis Research Society International, and European League Against Rheumatism.6–9 A recent systematic review suggested that greater improvements may be achieved with an individually supervised exercise program rather than a group- or home-based program.10
Evidence regarding manual therapy for individuals with knee OA is mixed,6–9,11,12 but recent studies suggest that it likely provides at least short-term benefits in pain and physical function and may be cost-saving compared to usual care.5,13,14 Recently published clinical effectiveness results of a randomized controlled trial (RCT) also support the presence of short-term benefits of manual therapy for knee OA.15
Current evidence is conflicting regarding whether booster physical therapy sessions sustain rehabilitation benefits over longer periods. Booster sessions are supervised sessions occurring weeks or months following the initial formal supervised program and may aid in progression of an independent home program and motivate the patient to continue the program.14,16 We recently found similar clinical effectiveness at 1-year follow-up between physical therapy strategies that did and did not include booster sessions.15 Other recent evidence is conflicting, with 2 studies noting positive clinical benefits of booster sessions for those with knee OA and a third study finding no benefit.14,16 The cost-effectiveness of booster sessions has not been studied.
The clinical effects of the 4 physical therapy strategies studied in the present RCT were similarly positive, further supporting the effectiveness of exercise but providing conflicting information regarding whether manual therapy and/or booster sessions enhance the magnitude or persistence of benefits from exercise therapy.15 These findings warrant further investigation to determine whether different physical therapy strategies are equally effective, but 1 strategy costs substantially less. Dissemination and implementation of that strategy may provide substantial cost savings and inform payer and provider policies regarding delivery of physical therapy services for knee OA. In the present economic evaluation conducted alongside a 2-year RCT,15 we evaluated the cost-effectiveness of 4 combinations of exercise therapy, manual therapy, and booster sessions provided by physical therapists.
Methods
Design Overview
We adopted a societal perspective to compare the relative cost-effectiveness of 4 different physical therapy strategies for individuals with knee OA over a 2-year period. The economic evaluation was conducted alongside an RCT investigating the clinical effectiveness of the 4 physical therapy strategies.15 Economic outcomes were described in incremental cost-effectiveness ratios (ICERs), briefly described as the difference in costs between 2 physical therapy strategies divided by the difference in effectiveness.
Setting and Participants
Data were collected from 300 RCT participants who were 40 years old or older and who met American College of Rheumatology criteria for knee OA.17 Participants were recruited from sites in Pittsburgh, Pennsylvania; Salt Lake City, Utah; and San Antonio, Texas. Local institutional review boards approved the study, and all participants provided informed consent. Table1 outlines the baseline characteristics of the participants; additional details are described elsewhere.15
Table 1.
Baseline Characteristics of Study Participants.a
| Treatment Groupb | ||||
|---|---|---|---|---|
| Characteristic | Ex (n = 75) | Ex+B (n = 76) | Ex+MT (n = 75) | Ex+MT+B (n = 74) |
| Age, y, X,(SD) | 58.3 (10.0) | 58.4 (8.7) | 58.0 (9.8) | 58.5 (9.4) |
| Sex | ||||
| Men | 23 (31) | 25 (33) | 26 (35) | 27 (36) |
| Women | 52 (69) | 51 (67) | 49 (65) | 47 (64) |
| Body mass index, X, (SD) | 30.1 (6.5) | 31.4 (7.2) | 31.1 (5.7) | 31.7 (5.6) |
| Bilateral involvement | 45 (60) | 46 (61) | 46 (61) | 44 (59) |
| Duration of knee symptoms, y | ||||
| <1 | 8 (10.7) | 9 (11.8) | 9 (12.0) | 8 (10.8) |
| 1–2 | 12 (16.0) | 10 (13.2) | 7 (9.3) | 8 (10.8) |
| 3–5 | 14 (18.7) | 19 (25.0) | 13 (17.3) | 14 (18.9) |
| 5–10 | 25 (33.3) | 18 (23.7) | 27 (36.0) | 20 (27.0) |
| <10 | 16 (21.3) | 20 (26.3) | 19 (25.3) | 24 (32.4) |
Data are reported as number (percentage) of participants unless otherwise indicated.
EX = exercise only, EX+B = exercise plus booster sessions, EX+MT = exercise plus manual therapy, EX+MT+B = exercise plus manual therapy and booster sessions.
Randomization and Interventions
Participants were randomized into 4 physical therapy treatment groups: exercise only (EX), exercise plus booster sessions (EX+B), exercise plus manual therapy (EX+MT), and exercise plus manual therapy and booster sessions (EX+MT+B). All groups received similar exercise interventions focusing on strength and flexibility of hip and knee musculature. The manual therapy groups additionally received stretching and nonthrust knee joint mobilizations. Hip and ankle treatments were used if clinical examination indicated the presence of impairment in these joints. Additional details of the interventions are described elsewhere.15
Individuals receiving booster sessions received 8 visits over 9 weeks, followed by 4 additional booster sessions spaced across 1 year. Booster sessions were periodic face-to-face appointments with the treating physical therapist. At each booster session, the physical therapist reviewed the home exercise program with the participant, discussed problems, and made recommendations for progression or modification of the program. Although individuals receiving booster sessions had a reduced frequency of initial physical therapy (8 visits over 9 weeks) compared to the 1 to 3 visits per week commonly used in outpatient physical therapy care, the overall dosage of physical therapy was equal across both groups (12 total visits). Individuals not receiving booster sessions received 12 physical therapy sessions across 9 weeks.
Outcomes and Follow-Up
Clinical outcome measures, including the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), were measured at baseline, 9 weeks, 1 year, and 2 years. Health care utilization and quality-of-life data were measured at baseline, 1 year, and 2 years.15
Direct medical and direct nonmedical costs were obtained and calculated from a combination of participant self-report and publicly available databases. Using the Osteoarthritis Cost and Consequences Questionnaire,18 participants reported 12-month health care utilization at baseline (looking back over the 12 months prior to study enrollment), 1 year, and 2 years. Utilization variables included surgeries because of osteoarthritis, corticosteroid or hyaluronan injections, imaging, medication related to osteoarthritis, outpatient services specific to knee OA, durable medical equipment and home modifications, use of community services, cost of transportation to/from medical appointments for knee OA, and emergency room and inpatient services specific to knee OA (eTab. 1; available athttps://academic.oup.com/ptj). Unit costs for services covered by health insurance were obtained from the Medicare Physician Fee Schedule and the Nationwide Inpatient Sample.19,20 Costs for services not covered by health insurance (eg, acupuncture, massage, house cleaner directly related to knee OA, transportation costs to and from medical visits) were self-reported. These costs were aggregated by health state (eg, poor function, good function; Fig.1) and by treatment group. For example, monthly cost of pain medication for participants in the EX+MT group averaged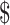 117.29 per person who was functioning poorly according to the WOMAC; for participants in the EX+MT group who were functioning well, monthly cost of pain medication averaged
117.29 per person who was functioning poorly according to the WOMAC; for participants in the EX+MT group who were functioning well, monthly cost of pain medication averaged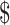 37.40.
37.40.
Figure 1.
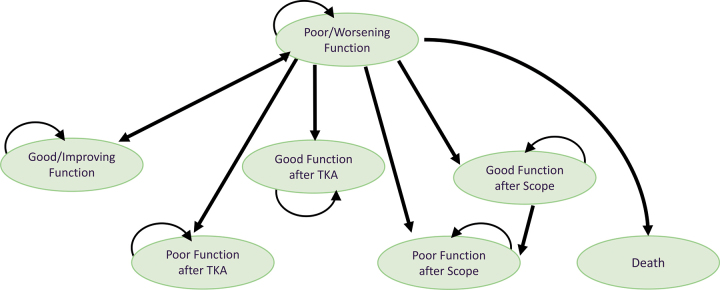
Schematic depiction of Markov model. Participants in all 4 treatment groups (exercise only, exercise plus manual therapy, exercise plus booster sessions, and exercise plus manual therapy and booster sessions) entered the model in “Poor/Worsening Function.” Model health states are shown as ovals. During monthly model cycles, transitions between health states or remaining in the same health state could occur and are represented by arrows. Transitions to different states depended upon whether a participant underwent surgery and whether the Western Ontario and McMaster Universities Osteoarthritis Index score changed beyond the minimum clinically important difference. Death, while possible in our model, is not depicted because all participants were alive at the 2-year follow-up. Scope = arthroscopy, TKA = total knee arthroplasty.
To capture risk and associated costs of relatively rare events such as surgical complications, we used publicly available databases and data from large studies. Further detail regarding health resource utilization and cost data is located ineTable 1.
Effectiveness was measured in quality-adjusted life years (QALYs). Quality-of-life utility values were elicited using the US version of the EuroQol-5-Dimension tool, which queries an individual's perceived limitations related to mobility, self-care, usual activities, pain/discomfort, and anxiety/depression.21 Each participant's ratings were transformed into a utility score using published preferences based on the US population. Utility values are a measure of preference for health states, anchored at scores of 0 = death and 1 = perfect health.
Data Analysis
Model construction
Markov state-transition modeling was used to estimate the cost-effectiveness of the 4 physical therapy strategies. Primary physical function data collected from each study participant over the 2-year period were entered into the model to depict the functional and surgical status of each study participant over time. Although a trial-based cost-effectiveness model would provide extremely similar results to modeling for the base case analysis, modeling was selected in favor of a trial-based cost-effectiveness analysis method for 2 primary reasons. First, modeling allows robust sensitivity analysis, with systematic variation of each parameter over empiric ranges, including the probability of adverse events not observed in the sample. Second, modeling allows projection of data beyond the observation period.
There are several possible Markov health states that a participant could be in at any point in time (see Fig.1 for a schematic depiction of health states). All study participants were assumed to be in a state of “poor/worsening function” upon entry to the study. Transitions between states were dependent upon whether each participant underwent surgery of the affected knee and whether the WOMAC score improved or declined beyond the published minimum clinically important difference (16-point improvement or 33-point decline on the 240-point version of the scale).22 Transitions between states were averaged across each time period. For example, if 12 individuals had “good function” at the 1-year follow-up and “poor function” at the 2-year follow-up, then the model assumed that 1 individual per month had transitioned from good function to poor function. Individuals whose baseline WOMAC score was too low to improve beyond the minimum clinically important difference (ie, the participant was too highly functioning) were excluded from the analysis. Otherwise, missing data (which totaled 7.5% of data because of dropouts during the study) was handled by imputing the mean value for the missing variable. Death was assumed possible only as a complication from surgery.
The model does not depict the possibility of a person moving directly from “good/improving function” to any of the surgical states. Logically, a person who is functioning well would not undergo surgery. However, since our WOMAC data were only collected at baseline, 9 weeks, 1 year, and 2 years, a few individuals did indeed progress directly from good/improving function to arthroscopy or total knee arthroplasty (TKA). The probability of doing so varied from 0 to 0.043; this direct transition was accounted for in the model but is not depicted in Figure1 or Table2 for the sake of simplicity.
Table 2.
Parameter Values and Ranges Used in the Sensitivity Analysis.a
| Description | Base Case | Minimum | Maximum | Source of Data |
|---|---|---|---|---|
| Probabilities (%)b | ||||
| Total knee arthroplasty (TKA) | ||||
| Probability of TKA | 6.2 | 13.2 | Observed | |
| EX | 7.6 (5 TKAs) | |||
| EX+B | 6.5 (4 TKAs) | |||
| EX+MT | 13.2 (9 TKAs) | |||
| EX+MT+B | 6.2 (4 TKAs) | |||
| Mortality from TKA | 0.26 | 0.001 | 0.26 | Bozic et al35; Singh et al36 |
| Complications from TKA | 3.1 | 1.8 | 9.0 | Bozic et al35; Singh et al36 |
| Arthroscopy | ||||
| Probability of arthroscopy | 0 | 3.3 | Observed | |
| EX | 0 (0 arthroscopies) | |||
| EX+B | 3.3 (2 arthroscopies) | |||
| EX+MT | 0 (0 arthroscopies) | |||
| EX+MT+B | 1.5 (1 arthroscopy) | |||
| Mortality from arthroscopy | 0.008 | 0.0004 | 0.008 | Martin et al27; Salzler et al28 |
| Complications from arthroscopy | 1.6 | 0 | 2.8 | Martin et al27; Salzler et al28 |
| Non–knee surgery related | ||||
| Complications from medical treatment (eg, gastrointestinal bleed) | 0 | 0 | 3.22 | Observed37 |
Medical Costs (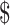 )c )c
| ||||
| TKA | ||||
| TKA surgery and hospitalization (1-time cost) | 14,028 | 13,984 | 14,097 | HCUP19 |
| TKA surgery with complications (1-time cost) | 19,595 | 19,335 | 19,732 | HCUP19 |
| Good function after TKA (monthly cost) | 355 | 613 | Observed | |
| EX | 471 | |||
| EX+B | 355 | |||
| EX+MT | 498 | |||
| EX+MT+B | 613 | |||
| Poor function after TKA (monthly cost) | 359 | 624 | Observed | |
| EX | 359 | |||
| EX+B | N/A | |||
| EX+MT | 469 | |||
| EX+MT+B | 624 | |||
| Arthroscopy | ||||
| Knee arthroscopy surgery (1-time cost) | 6310 | 4732 | 7888 | Lubowitz and Appleby38 |
| Good function after knee arthroscopy (monthly cost) | 81 | 225 | Observed | |
| EX | N/A | |||
| EX+B | N/A | |||
| EX+MT | N/A | |||
| EX+MT+B | 225 | |||
| Poor function after knee arthroscopy (monthly cost) | 130 | 530 | Observed | |
| EX | N/A | |||
| EX+B | 530 | |||
| EX+MT | N/A | |||
| EX+MT+B | N/A | |||
| Costs of remaining in nonsurgical health states | ||||
| Good function (monthly cost) | 81 | 164 | Observed | |
| EX | 108 | |||
| EX+B | 140 | |||
| EX+MT | 113 | |||
| EX+MT+B | 89 | |||
| Poor function (monthly cost) | 130 | 264 | Observed | |
| EX | 154 | |||
| EX+B | 180 | |||
| EX+MT | 218 | |||
| EX+MT+B | 161 | |||
| Other medical costs | ||||
| Total cost of providing study physical therapy treatment | 1204 | 1440 | Observed, Medicare fee schedule | |
| EX, EX+B | 1204 | |||
| EX+MT, EX+MT+B | 1440 | |||
| Complications from medical treatment (eg, hospital stay for gastrointestinal bleed; 1-time cost) | 6837 | 5101 | 10,770 | HCUP19 |
| Utility Values by Health Stated | ||||
| Poor function | 0.647 | 1.0 | Observed | |
| EX | 0.833 | |||
| EX+B | 0.828 | |||
| EX+MT | 0.821 | |||
| EX+MT+B | 0.811 | |||
| Good function | 0.659 | 1.0 | Observed | |
| EX | 0.862 | |||
| EX+B | 0.888 | |||
| EX+MT | 0.880 | |||
| EX+MT+B | 0.883 | |||
| Good function after TKA | 0.678 | 1.0 | Observed | |
| EX | 0.899 | |||
| EX+B | 0.836 | |||
| EX+MT | 0.822 | |||
| EX+MT+B | 0.913 | |||
| Poor function after TKA | 0.738 | 0.861 | Observed | |
| EX | 0.8 | |||
| EX+B | 0.8 | |||
| EX+MT | 0.782 | |||
| EX+MT+B | 0.795 | |||
| Good function after arthroscopy | 0 | 1.0 | Observed | |
| EX | 1.0 | |||
| EX+B | 1.0 | |||
| EX+MT | 1.0 | |||
| EX+MT+B | 1.0 | |||
| Poor function after arthroscopy | 0.756 | 0.861 | Observed | |
| EX | 0.8 | |||
| EX+B | 0.809 | |||
| EX+MT | 0.8 | |||
| EX+MT+B | 0.8 | |||
EX = exercise only, EX+B = exercise plus booster sessions, EX+MT = exercise plus manual therapy, EX+MT+B = exercise plus manual therapy and booster sessions, HCUP = Healthcare Cost and Utilization Project, N/A = not applicable.
Observed cumulative probabilities over the 2-year study period; in the model, these probabilities varied at each study time point.
Base case costs of surgery, hospitalization, and study-related physical therapy were based upon information from the literature and nationally available databases; the minimum and maximum values reflected the confidence intervals. Annual costs of each health state reflected the sum of all costs for knee osteoarthritis–related health care utilization, averaged for each treatment group.
For base case utility values, we used the average utility score for each group across all study time points. The minimum and maximum values reflected the highest and lowest utility scores reported by any individual in any treatment group at any time point.
Table2 provides the values of model parameters used in the base case scenario and the variable ranges used in sensitivity analyses. We used a societal perspective, as defined by the Panel on Cost-Effectiveness in Health and Medicine.23 Year 2011 US dollars were used, and an annual discount rate of 3% was applied for future costs and effectiveness in accordance with the panel's recommendation.23 ICERs were calculated to compare the relative cost-effectiveness of the 4 treatment strategies, by dividing the difference in costs between 2 strategies by the difference in effectiveness between 2 strategies. There is no agreed-upon criterion in the United States regarding an ICER that denotes whether a strategy should be considered cost-effective. For the present study, we chose to use a criterion of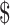 100,000/QALY gained, in accordance with recent literature recommendations.24,25
100,000/QALY gained, in accordance with recent literature recommendations.24,25
Sensitivity analysis
In 1-way sensitivity analyses, each parameter was individually varied over the ranges outlined in Table2. In addition, a probabilistic sensitivity analysis (PSA) was performed to account for sampling and parameter uncertainty.26 All parameter values were varied simultaneously over distributions 5000 times. Triangular distributions were used for most variables, and uniform distributions were used when data were sparse. A cost-effectiveness acceptability curve was constructed from the results of the PSA.
Short-term reductions in utility were expressed for 5 scenarios (arthroscopy with or without complication, TKA with or without complication, and hospitalization because of complications from medical treatment of knee OA). For each scenario, the number of days of disutility were expressed as the median length of stay; minimum and maximum values were the median length of stay plus/minus the standard error.19,27,28 We assumed those days were worth zero utility and were essentially QALYs lost; in a sensitivity analysis, we varied this over a utility range of 0.0 to 1.0.
Our base case scenario had a 2-year time horizon, based on the duration of study data collection. The Markov cycle length was 1 month (a participant must remain in a health state for 1 month; at the end of the monthly cycle, the participant may move into a different health state depending upon functional and surgical status). State transition probabilities were averaged over time to account for the fact that data were not collected monthly.
Secondary analysis
n an exploratory aim, a secondary analysis projected costs and effectiveness to 5 years, to allow exploration of potential benefits of rehabilitation that were not captured within the 2-year study follow-up period. This analysis continued the same probability values used in the second year of the model. Because most participants somewhat declined from year 1 to year 2 while they were not receiving active treatment, continuation of these probabilities models the continued decline that would be expected with a chronic degenerative disease such as knee OA. TreeAge Pro version 2015 (TreeAge Software Inc, Williamstown, MA) was used for model construction and analyses.
Role of the Funding Source
This study was supported by a grant from the Agency for Healthcare Research and Quality. The study sponsors did not play a role in study design; collection, analysis, or interpretation of the data; writing of the manuscript; or manuscript publication decisions.
Results
A total of 270 participants completed all study follow-up visits (EX: n = 66; EX+B: n = 59; EX+MT: n = 69; EX+MT+B: n = 64). However, 6 participants were excluded from analysis because of a ceiling effect on the baseline WOMAC score (1 in EX+B, 1 in EX, 2 in EX+MT+B, and 2 in EX+MT). Thirty participants dropped out during the course of the study (EX: n = 7; EX+B: n = 13; EX+MT: n = 3; EX+MT+B: n = 7); reasons for attrition have been described elsewhere.15
Average costs were, predictably, higher for participants who underwent TKA surgery because of increased utilization of rehabilitation, durable medical equipment, home health services, and imaging in the perioperative period (Tab.2;eTab. 1). Surgical utilization was relatively low, with a total of 22 TKAs and 3 knee arthroscopies over 2 years (Tab.2). Among participants who did not undergo surgery, monthly direct costs were higher for those reporting poor function than those reporting good function (Tab.2).
Table3 provides 2-year base case cost-effectiveness results. Lowest costs were observed for the EX+MT+B strategy, while greatest effectiveness (most QALYs gained) was in the EX+B strategy. When EX+MT+B is compared to EX+B, EX+B gains 0.08 QALY while costing an additional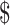 1062. The result is an ICER of
1062. The result is an ICER of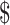 12,900/QALY gained, which falls well within the
12,900/QALY gained, which falls well within the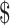 100,000/QALY threshold.24,29 Strategies that did not contain boosters were dominated (higher costs and lower effectiveness) by booster strategies.
100,000/QALY threshold.24,29 Strategies that did not contain boosters were dominated (higher costs and lower effectiveness) by booster strategies.
Table 3.
Base Case and 5-Year Projected Model Results Listed in Order of Increasing Cost, From Least Costly to Most Costly.a
| Strategy | Cost (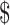 ) ) |
Incremental Cost (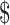 )b )b
|
Effectiveness (QALYs) | Incremental Effectiveness (QALYs)b | ICERb |
|---|---|---|---|---|---|
| Base Case Results | |||||
| EX+MT+B | 5283 | 1.32 | |||
| EX | 5437 | 154 | 1.22 | −0.10 | Dominated |
| EX+B | 6344 | 1062 | 1.40 | 0.08 |
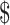 12,900 12,900 |
| EX+MT | 8249 | 1904 | 1.05 | −0.35 | Dominated |
| 5-Year Projected Model | |||||
| EX+MT+B | 12,997 | 3.14 | |||
| EX | 13,310 | 312 | 3.21 | 0.062 |
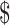 5059 5059 |
| EX+B | 15,275 | 1965 | 3.30 | 0.09 |
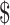 21,548 21,548 |
| EX+MT | 21,299 | 6024 | 2.48 | −0.81 | Dominated |
EX = exercise only, EX+B = exercise plus booster sessions, EX+MT = exercise plus manual therapy, EX+MT+B = exercise plus manual therapy and booster sessions, ICER = incremental cost-effectiveness ratio, QALYs = quality-adjusted life years.
Incremental costs (in US dollars), incremental effectiveness, and ICERs were in relation to the least costly strategy (EX+MT+B).
Table3 also provides results of the 5-year projected model. The EX+MT+B strategy remains the least expensive strategy, while the EX and EX+B strategies fall within a cost-effective range, with EX+B remaining the favored strategy when a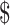 50,000 or
50,000 or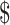 100,000/QALY threshold is used. The EX+MT strategy remains dominated by the booster strategies.
100,000/QALY threshold is used. The EX+MT strategy remains dominated by the booster strategies.
In 1-way sensitivity analyses performed to test the robustness of the model, variation across the ranges outlined in Table2 did not change the results of the cost-effectiveness analysis except in a few unlikely scenarios. In the EX+MT+B and EX+B treatment groups, if the probability of remaining in poor function (for EX+MT+B) or good function (EX+B) is varied to a substantial degree, then the EX+MT+B strategy becomes dominant over all other strategies (Tab.4). In other words, the preferred strategy becomes even more strongly preferred.
Table 4.
One-Way Sensitivity Analysis.a
| Parameter | Base Case Table Multiplierb | Threshold Multiplier Valueb | Favored Strategy Below Threshold |
|---|---|---|---|
| Probability of continuing poor function | |||
| EX | 1.0 | 0.5 | EX dominated all other strategies |
| EX+MT+B | 1.0 | 0.7 | EX+MT+B, EX+B dominatedc |
| Probability of continuing good function | |||
| EX+B | 1.0 | 0.9 | EX+MT+B, EX+B dominatedc |
EX = exercise only, EX+B = exercise plus booster sessions, EX+MT+B = exercise and manual therapy plus booster sessions.
Applied to time-based, strategy-specific values in Appendix Table2 (eg, a multiplier of 0.5 would take all of that table's listed time-based probabilities for that strategy and cut them by half).
In this case, the preferred strategy (EX+MT+B) would become even more strongly preferred.
Only 1 parameter was revealed to potentially change the preferred strategy in a 1-way sensitivity analysis. Within the EX group, substantially altering the probability of a participant in poor function remaining in poor function makes the EX strategy dominant over the other 3 strategies. However, this time-based probability (eTab. 2, row 1) would need to be halved (Tab.4; threshold multiplier value of 0.5) compared to the observed probability in the study; this is extremely unlikely.
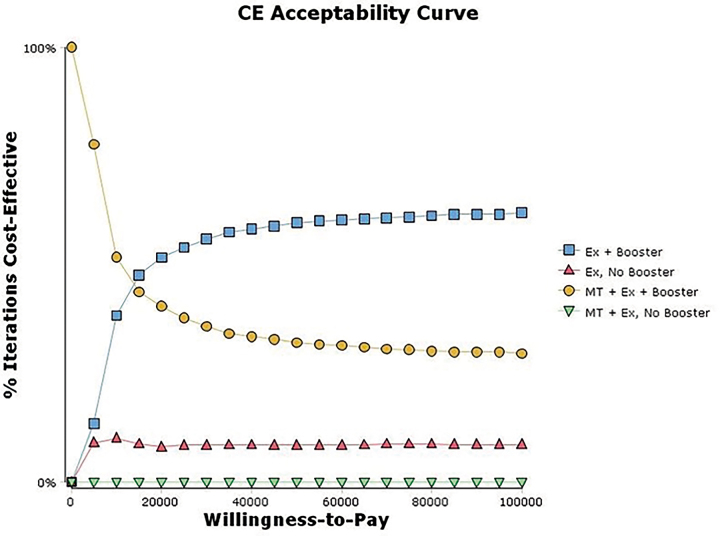
A PSA varying all parameters simultaneously over distributions in the 2-year model showed that the EX+B strategy was most likely to be cost-effective when the willingness-to-pay threshold was greater than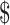 15,000/QALY gained (Fig.2); below that threshold, the EX+MT+B strategy is the most likely to be cost-effective. Strategies not containing boosters (EX+MT and EX) were less likely than booster strategies (EX+MT+B and EX+B) to be cost-effective across the range of willingness-to-pay values. At willingness-to-pay thresholds of
15,000/QALY gained (Fig.2); below that threshold, the EX+MT+B strategy is the most likely to be cost-effective. Strategies not containing boosters (EX+MT and EX) were less likely than booster strategies (EX+MT+B and EX+B) to be cost-effective across the range of willingness-to-pay values. At willingness-to-pay thresholds of 50,000 and
50,000 and 100,000 per QALY gained, EX+MT+B was favored in approximately 33% and 30% of model iterations; EX+B was favored in approximately 60% and 63% of model iterations, respectively.
100,000 per QALY gained, EX+MT+B was favored in approximately 33% and 30% of model iterations; EX+B was favored in approximately 60% and 63% of model iterations, respectively.
Figure 2.
Cost-effectiveness (CE) acceptability curve. Below a willingness-to-pay threshold of 13,000, the exercise (EX) plus manual therapy (MT) and booster sessions strategy was preferred more often. Above a willingness-to-pay threshold of
13,000, the exercise (EX) plus manual therapy (MT) and booster sessions strategy was preferred more often. Above a willingness-to-pay threshold of 13,000, the EX plus booster sessions strategy was preferred more often. Strategies not containing booster sessions were never the most likely option to be cost-effective at any willingness-to-pay value.
13,000, the EX plus booster sessions strategy was preferred more often. Strategies not containing booster sessions were never the most likely option to be cost-effective at any willingness-to-pay value.
In a post hoc analysis, we calculated incremental costs and effectiveness between the 4 strategies after including the 6 participants who had been excluded from the base case calculations because of a ceiling effect on the WOMAC. This did not change the preferred strategy; the EX+MT+B strategy remained the least costly and was dominant over nonbooster strategies.
Discussion
Our results indicate physical therapy strategies using booster sessions, where physical therapy sessions are distributed over 1 year, result in greater effectiveness and lower health care utilization than nonbooster strategies (all allotted sessions delivered within 9 weeks). In nearly every plausible scenario, the preferred strategy in our cost-effective analysis was a combination of exercise and booster sessions, with or without manual therapy.
Clinical effectiveness results of this trial (published elsewhere) indicated that all 4 treatment strategies were associated with marked improvement in physical function, but no single strategy resulted in superior WOMAC scores compared to the others.15 Because it may be argued that all 4 strategies may result in similar clinical improvement, determining cost-effectiveness is important because both clinicians and insurers may want to select the strategy that is least costly or provides the greatest improvement in quality of life. Therefore, these results support the adoption of booster sessions in the physical therapy management of knee OA.
A strength of this study is that data were collected directly from participants over 2 years. It was important to collect primary data for this analysis because little information is available regarding the use of booster sessions for patients in the United States; therefore, reliance upon existing literature would have significant limitations. We did refer to existing literature to estimate probabilities and costs when appropriate. Because of the breadth of information needed to perform a cost-effectiveness analysis, it is relatively rare to have a data set based on actual measurements of most parameters as opposed to estimation from the literature or expert opinion.
Sensitivity analysis strongly supported the results of the study, as shown by the stability of the model. We varied all parameters across plausible ranges and/or 95% confidence intervals. Results indicate that all variables were stable across all plausible ranges. Preferred strategies changed only when varying parameters well beyond what was observed within the study. The PSA further supported the model's stability, indicating that strategies containing booster sessions are superior to those without booster sessions.
Current clinical practice is influenced by payment models that encourage short episodes of physical therapy care with a specific number of visits over a discrete period. Our findings indicate spacing visits across longer periods may result in sustained improvements in function and decreased health care utilization. These sustained improvements would have a substantial impact on health and well-being for patients with knee OA. However, widespread adoption of booster sessions in physical therapy would require health insurers to consider innovative payment models.
Literature examining the cost-effectiveness of physical therapy strategies to treat knee OA is scarce.4 One well-known study in the United Kingdom, the ESCAPE knee pain trial, found that an exercise-based program consisting of 12 physical therapy visits was cost-effective over 30 months compared to usual care across willingness-to-pay thresholds up to £9750.30 This analysis, however, was reported from the third-party payer perspective only while our base case analysis was performed from the societal perspective as recommended by the Panel on Cost-Effectiveness in Health and Medicine.30,31
One study examined the cost-effectiveness of a physical therapy strategy including physical activity promotion and booster sessions over 1 year compared to traditional guideline-based physical therapy for people with hip and/or knee OA.32 Surprisingly, this study found large ICERs favoring usual physical therapy care. These results were largely driven by a lack of difference in effect between the 2 strategies. That study did not include manual therapy, and thus is not directly comparable to the strategies used in our study. In addition, that study was performed in the Netherlands; its information regarding costs and health care utilization are not generalizable to the United States.
In the recent Management of Osteoarthritis (MOA) trial, Abbott et al13 found that either exercise therapy or manual therapy was superior to usual care for individuals with hip and knee OA. The combination of exercise and manual therapy was beneficial, but less effective than exercise or manual therapy alone.13 Cost-effectiveness analysis revealed similar results: manual therapy was cost-saving; exercise therapy was cost-effective, while combination therapy and usual care were not as cost-effective.5 Our results within nonbooster strategies are fairly consistent with the MOA trial; the EX+MT group had higher costs and lower effectiveness than the EX group. However, results for the 2 booster strategies in the present trial are somewhat inconsistent with the findings of Abbott et al (the EX+MT+B group in the present study had lower costs and slightly lower effectiveness than the EX+B group). It is difficult to directly compare the 2 studies because we did not study manual therapy alone, and the MOA trial did not investigate booster sessions. However, dosage may play a role in the relative cost-effectiveness of different strategies. In the MOA trial, participants receiving combined manual and exercise therapy tended to receive less of each (eg, participants may receive 60 minutes of manual therapy, 60 minutes of exercise therapy, or 30 minutes of each). In our trial, participants who received combined manual therapy and exercise had longer physical therapy visits.
Limitations
A primary limitation in our study was the small number of TKA surgeries. This resulted in limited power to detect differences between strategies in the number of cases that progressed to TKA. If 1 strategy is superior in its ability to delay or eliminate the need for TKA, then that strategy would likely be the least costly. The study was not powered to detect differences in the number of TKAs undergone by participants in each group. A larger sample size and longer follow-up would be needed to investigate this issue. In addition, our estimates of utility scores for individuals with TKA are likely imprecise because of the small number of TKAs. However, we did vary utility scores from 0 to 1 in additional sensitivity analyses, and the preferred treatment strategy remained the same.
We relied on participant self-report for utilization of common health care procedures such as knee radiographs, injections, and medications. Although our cost data were derived from publicly available databases and thus were accurate, it is possible that recall bias regarding utilization impacted the overall calculation of health care costs.19,20 However, the Osteoarthritis Cost and Consequences Questionnaire has been validated against reference-standard provider databases over 3 months and similar cost questionnaires have been validated over longer periods of time.18,33,34
To capture potential benefits of physical therapy strategies used beyond the 2-year follow-up, we used our Markov model to perform secondary analyses projected to a 5-year time horizon using the same probabilities observed over the 2-year period. It is possible that these probabilities would not have remained steady from year 2 to year 5, so the results of the 5-year projected analysis should be interpreted with caution.
We followed guidelines for cost-effectiveness analysis that recommend using the societal perspective.23,31 We did not include lost productivity, as it was not recommended by the Panel on Cost-Effectiveness in Health and Medicine at the time this study was conducted. Because our analysis is from a societal perspective, our analysis is not generalizable to individual health care consumers or payers. These results may change if the analysis is performed from an individual or payer perspective.
The trial on which this analysis was based did not include a usual care group because the trial's purpose was to compare different strategies of physical therapy for individuals with knee OA. This precludes us from comparing cost-effectiveness between usual care and structured rehabilitation. However, because most clinical practice guidelines recommend physical therapy or supervised exercise as a first-line treatment for knee OA, we feel that the present study provides policy-relevant information to the field of physical therapy.
Future Directions
Our findings suggested that people with knee OA may benefit from a policy shift allowing longer episodes of physical therapy care with periodic boosters to promote long-term maintenance of improvements. Innovative payment models, including bundled care and pay-for-performance, may be the first step toward adoption of cost-effective nonsurgical management of knee OA. Future research should comprehensively study progression to TKA or other costly surgery in a larger sample, as well as the potential influence of various physical therapy strategies on this progression.
Conclusions
Spacing exercise-based physical therapy sessions over 12 months using periodic booster sessions was cost-effective over 2 years compared to strategies not utilizing boosters.
Supplementary Material
Author Contributions and Acknowledgments
Concept/idea/research design: A.M. Bove, K.J. Smith, C.G. Bise, J.M. Fritz, G.P. Brennan, J.H. Abbott, G.K. Fitzgerald
Writing: A.M. Bove, K.J. Smith, C.G. Bise, J.M. Fritz, J.D. Childs, G.K. Fitzgerald
Data collection: A.M. Bove, J.M. Fritz, J.D. Childs, G.P. Brennan
Data analysis: A.M. Bove, K.J. Smith, C.G. Bise, G.P. Brennan
Project management: A.M. Bove, J.M. Fritz, J.D. Childs, G.P. Brennan, G.K. Fitzgerald
Fund procurement: G.P. Brennan, J.H. Abbott, J.M. Fritz, G.K. Fitzgerald
Providing participants: J.D. Childs, G.P. Brennan, J.M. Fritz, G.K. Fitzgerald
Providing facilities/equipment: J.D. Childs, G.P. Brennan, J.M. Fritz, G.K. Fitzgerald
Providing institutional liaisons: G.P. Brennan, G.K. Fitzgerald
Consultation (including review of manuscript before submitting): A.M. Bove, K.J. Smith, C.G. Bise, J.M. Fritz, J.D. Childs, G.P. Brennan, J.H. Abbott, G.K. Fitzgerald
Ethics Approval
Local institutional review boards approved the study, and all participants provided informed consent.
Funding
This study was funded by a grant from the Agency for Healthcare Research and Quality (grant no. AHRQ 1R01HS019642-02).
Clinical Trial Registration
This trial was registered on ClinicalTrials.gov (identifier: NCT 01314183).
Disclosures
The authors completed the ICJME Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
J.D. Childs was funded by a grant from the Henry M. Jackson Foundation; J.H. Abbott was supported, in part, by a Sir Charles Hercus Health Research Fellowship.
References
- 1. Guccione AA,Felson DT,Anderson JJ et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health.1994;84:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kotlarz H,Gunnarsson CL,Fang H,Rizzo JA. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data.Arthritis Rheum.2009;60:3546–3553. [DOI] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention.Osteoarthritis.http://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed October 4,2017.
- 4. Pinto D,Robertson MC,Hansen P,Abbott JH. Cost-effectiveness of nonpharmacologic, nonsurgical interventions for hip and/or knee osteoarthritis: systematic review.Value Health.2012;15:1–12. [DOI] [PubMed] [Google Scholar]
- 5. Pinto D,Robertson MC,Abbott JH,Hansen P,Campbell AJ,Trial Team MOA. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee, 2: economic evaluation alongside a randomized controlled trial.Osteoarthritis Cartilage.2013;21:1504–1513. [DOI] [PubMed] [Google Scholar]
- 6. American Academy of Orthopedic Surgeons.Treatment of Osteoarthritis of the Knee: Evidence-Based Guideline.2nd ed2013.http://www.aaos.org/research/guidelines/TreatmentofOsteoarthritisoftheKneeGuideline.pdf. Published 2013. Accessed October 4,2017. [Google Scholar]
- 7. Jordan KM,Arden NK,Doherty M et al. EULAR recommendations 2003: an evidence based approach to the management of knee osteoarthritis—report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis.2003;62:1145–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang W,Moskowitz RW,Nuki G et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines.Osteoarthritis Cartilage.2008;16:137–162. [DOI] [PubMed] [Google Scholar]
- 9. Hochberg MC,Altman RD,April KT et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee.Arthritis Care Res (Hoboken).2012;64:465–474. [DOI] [PubMed] [Google Scholar]
- 10. Fransen M,McConnell S,Harmer AR,Van der Esch M,Simic M,Bennell KL. Exercise for osteoarthritis of the knee: a Cochrane systematic review.Br J Sports Med.2015;49:1554–1557. [DOI] [PubMed] [Google Scholar]
- 11. Jansen MJ,Viechtbauer W,Lenssen AF,Hendriks EJ,de Bie RA. Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review.J Physiother.2011;57:11–20. [DOI] [PubMed] [Google Scholar]
- 12. French HP,Brennan A,White B,Cusack T. Manual therapy for osteoarthritis of the hip or knee: a systematic review.Man Ther.2011;16:109–117. [DOI] [PubMed] [Google Scholar]
- 13. Abbott JH,Robertson MC,Chapple C et al. Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee: a randomized controlled trial.1: Clinical effectiveness. Osteoarthritis Cartilage.2013;21:525–534. [DOI] [PubMed] [Google Scholar]
- 14. Abbott JH,Chapple CM,Fitzgerald GK et al. The incremental effects of manual therapy or booster sessions in addition to exercise therapy for knee osteoarthritis: a randomized clinical trial.J Orthop Sports Phys Ther.2015;45:975–983. [DOI] [PubMed] [Google Scholar]
- 15. Fitzgerald GK,Fritz JM,Childs JD et al. Exercise, manual therapy, and use of booster sessions in physical therapy for knee osteoarthritis: a multi-center, factorial randomized clinical trial.Osteoarthritis Cartilage.2016;24:1340–1349. [DOI] [PubMed] [Google Scholar]
- 16. Bennell KL,Kyriakides M,Hodges PW,Hinman RS.. Effects of two physiotherapy booster sessions on outcomes with home exercise in people with knee osteoarthritis: a randomized controlled trial.Arthritis Care Res (Hoboken).2014;66:1680–1687. [DOI] [PubMed] [Google Scholar]
- 17. Altman R,Asch E,Bloch D et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee.Arthritis Rheum.1986;29:1039–1049. [DOI] [PubMed] [Google Scholar]
- 18. Pinto D,Robertson MC,Hansen P,Abbott JH. Good agreement between questionnaire and administrative databases for health care use and costs in patients with osteoarthritis.BMC Med Res Methodol.2011;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Databases HCUP.Healthcare Cost and Utilization Project (HCUP).Rockville, MD:Agency for Healthcare Research and Quality;2017.www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed October 4,2017. [PubMed] [Google Scholar]
- 20. Centers for Medicare & Medicaid Services.Overview: physician fee schedule search.CMS.gov website.https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed October 4,2017.
- 21. Shaw JW,Johnson JA,Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model.Med Care.2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 22. Angst F,Aeschlimann A,Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities.Arthritis Rheum.2001;45:384–391. [DOI] [PubMed] [Google Scholar]
- 23. Weinstein MC,Siegel JE,Gold MR,Kamlet MS,Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA.1996;276:1253–1258. [PubMed] [Google Scholar]
-
24.
Braithwaite RS,Meltzer DO,King JT,Leslie D,Roberts MS. What does the value of modern medicine say about the
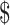 50,000 per quality-adjusted life-year decision rule? Med Care.2008;46:349–356. [DOI] [PubMed] [Google Scholar]
50,000 per quality-adjusted life-year decision rule? Med Care.2008;46:349–356. [DOI] [PubMed] [Google Scholar] -
25.
Neumann PJ,Cohen JT,Weinstein MC. Updating cost-effectiveness: the curious resilience of the
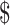 50,000-per-QALY threshold.N Engl J Med.2014;371:796–797. [DOI] [PubMed] [Google Scholar]
50,000-per-QALY threshold.N Engl J Med.2014;371:796–797. [DOI] [PubMed] [Google Scholar] - 26. Briggs AH,Weinstein MC,Fenwick EA et al. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making.2012;32:722–732. [DOI] [PubMed] [Google Scholar]
- 27. Martin CT,Pugely AJ,Gao Y,Wolf BR. Risk factors for thirty-day morbidity and mortality following knee arthroscopy: a review of 12,271 patients from the National Surgical Quality Improvement Program database.J Bone Joint Surg Am.2013;95:e98 1–e98 10. [DOI] [PubMed] [Google Scholar]
- 28. Salzler MJ,Lin A,Miller CD,Herold S,Irrgang JJ,Harner CD. Complications after arthroscopic knee surgery.Am J Sports Med.2014;42:292–296. [DOI] [PubMed] [Google Scholar]
- 29. Ubel PA,Hirth RA,Chernew ME,Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med.2003;163:1637–1641. [DOI] [PubMed] [Google Scholar]
- 30. Hurley MV,Walsh NE,Mitchell H,Nicholas J,Patel A. Long-term outcomes and costs of an integrated rehabilitation program for chronic knee pain: a pragmatic, cluster randomized, controlled trial.Arthritis Care Res.2012;64:238–247. [DOI] [PubMed] [Google Scholar]
- 31. Siegel JE,Weinstein MC,Russell LB,Gold MR. Recommendations for reporting cost-effectiveness analyses.JAMA.1996;276:1339–1341. [DOI] [PubMed] [Google Scholar]
- 32. Coupé VM,Veenhof C,van Tulder MW,Dekker J,Bijlsma JW,Van den Ende CH. The cost effectiveness of behavioural graded activity in patients with osteoarthritis of hip and/or knee.Ann Rheum Dis.2007;66:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Longobardi T,Walker JR,Graff LA,Bernstein CN. Health service utilization in IBD: comparison of self-report and administrative data.BMC Health Serv Res.2011;11:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Brink M,van den Hout WB,Stiggelbout AM,Putter H,van de Velde CJ,Kievit J. Self-reports of health-care utilization: diary or questionnaire? Int J Technol Assess Health Care.2005;21:298–304. [DOI] [PubMed] [Google Scholar]
- 35. Bozic KJ,Grosso LM,Lin Z et al. Variation in hospital-level risk-standardized complication rates following elective primary total hip and knee arthroplasty.J Bone Joint Surg Am.2014;96:640–647. [DOI] [PubMed] [Google Scholar]
- 36. Singh JA,Kwoh CK,Boudreau RM,Lee GC,Ibrahim SA. Hospital volume and surgical outcomes after elective hip/knee arthroplasty: a risk-adjusted analysis of a large regional database.Arthritis Rheum.2011;63:2531–2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ramey DR,Watson DJ,Yu C,Bolognese JA,Curtis SP,Reicin AS. The incidence of upper gastrointestinal adverse events in clinical trials of etoricoxib vs. non-selective NSAIDs: an updated combined analysis.Curr Med Res Opin.2005;21:715–722. [DOI] [PubMed] [Google Scholar]
- 38. Lubowitz JH,Appleby D. Cost-effectiveness analysis of the most common orthopaedic surgery procedures: knee arthroscopy and knee anterior cruciate ligament reconstruction.Arthroscopy.2011;27:1317–1322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.