Objectives
This is a protocol for a Cochrane Review (intervention). The objectives are as follows:
To evaluate the benefits and harms of anti‐angiogenesis agents for the treatment of metastatic and recurrent cervical cancer.
Background
Historically, women with metastatic or recurrent cervical cancer have had limited treatment options with poor prognosis. New anti‐angiogenesis therapies act by inhibiting the growth of new blood vessels and thereby restricting tumour growth by cutting off their nutrient supply. These agents offer an alternative strategy to conventional chemotherapy in the clinical management of these women.
Description of the condition
Cervical cancer is the second most commonly diagnosed cancer to affect women worldwide and the third‐leading cause of cancer death amongst women in low‐income countries (Ferlay 2019; Siegel 2019; Torre 2015). Since the introduction of cytology‐based cervical screening, cervical cancer incidence and mortality rates have been reduced by approximately 75% in countries with co‐ordinated population vaccination and screening programmes (Cancer Research UK 2014; ISD Scotland 2011; Siegel 2019; Welsh Cancer Intelligence and Surveillance 2011).
The majority of cervical cancer cases (60%) are associated with inadequate screening and are therefore potentially preventable, or may be diagnosed at a very early stage, whilst still easily curable with surgery (Alldredge 2016; Eskander 2014; Rebolj 2019). However, even in women diagnosed at a relatively early stage who have undergone treatment with either surgery or radical chemoradiotherapy, the risk of recurrent disease is 10% to 20% for stages IB to IIA, and 28% to 64% in women with locally advanced FIGO stages IIB to IV, as per International Federation of Obstetrics and Gynaecology (FIGO) classification (see Figure 1 for FIGO cervical cancer staging) (Bhatla 2018; Bray 2018; Cancer Research UK 2014).
1.
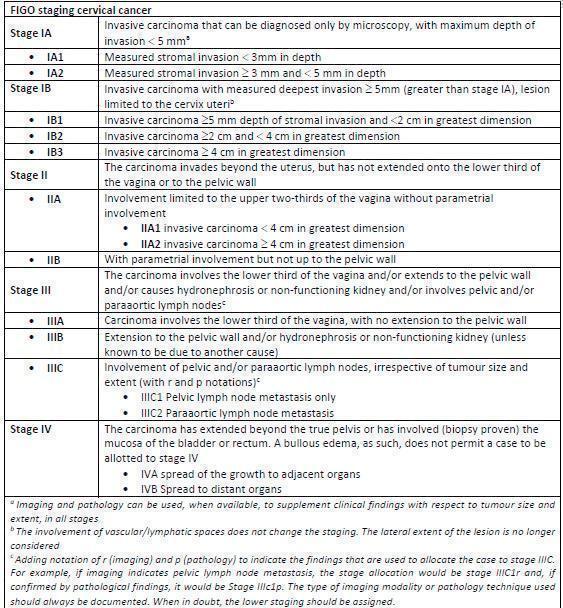
FIGO stage 2018.
Very advanced cervical cancer (FIGO stage IVB), where disease has spread to areas outside of the pelvis at diagnosis (metastatic disease), has a very poor prognosis. Treatment options are limited and are unlikely to be curative, therefore management is aimed at prolonging life and managing symptoms. Similarly, in recurrent disease, unless exenterative surgery is possible, women are usually treated with palliative chemotherapy. Chemotherapy aims to prolong survival, provide symptom control, and improve quality of life. In patients not medically fit enough for chemotherapy, treatment options in both metastatic and recurrent disease may be limited to best supportive care, with the option of palliative radiotherapy, if appropriate (Eleje 2019; Fanfani 2016; Greer 2010; Matsuo 2015; Waggoner 2003).
Three phase III randomised trials (GOG‐204, GOG‐240, and Japanese Clinical Oncology Group 0505 (JCOG‐0505) have examined chemotherapy regimens for women with advanced and recurrent cervical cancer (Monk 2009; Saito 2010; Tewari 2014). The main concern was acquired resistance to platinum in patients with recurrent cervical cancer previously treated with chemotherapy. However, the regimens that did not include a platinum agent were not superior to cisplatin‐based treatments, therefore cisplatin plus paclitaxel remains the chemotherapy standard for recurrent and metastatic disease (Eleje 2019; Monk 2014).
Angiogenesis inhibitors are a class of promising biological agents that target the process by which a tumour forms new blood vessels to supply it with oxygen and nutrients. This process is key to cervical cancer growth, both locally and for metastatic deposits (Willmott 2009).
In 1971, Dr Folkman proposed a tumour angiogenesis‐dependent doctrine, which suggested that development of tumours was due not only to the ability of the individual cancer cells to grow without normal controls, but was also dependent on nutrient and oxygen supply via blood vessels (Folkman 1971). The transition of a tumour from an avascular to a vascular phase was termed the 'angiogenic switch' and was thought to be an important factor in the clinical progression from tumour dormancy to cancer invasion (Hanahan 1996). The angiogenic switch occurs through a dynamic process involving pro‐angiogenic and anti‐angiogenic factors that are normally maintained in balance (physiological homeostasis). In cancer there is an up‐regulation of pro‐angiogenic factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), platelet‐derived growth factor (PDGF), and angiopoietins, and a down‐regulation of anti‐angiogenic factors, such as thrombospondin, angiostatin, and endostatin, leading to the development of new blood vessels (neovascularisation) and tumour progression (Hicklin 2005). Neovascularisation is therefore a vital process in metastatic spread to allow malignant cells to enter into the circulation (Falzone 2019; Folkman 1971; Folkman 1990; Pfaendler 2016).
Activation of VEGF promotes the proliferation and migration of endothelial cells, which line blood vessels, and the development of new blood vessels. Moreover, VEGF activation enhances the leakiness (permeability) of existing blood vessels and, as a consequence, causes increased leakage of multiple plasma proteins that are involved in the angiogenesis process (Nagy 2007; Turajlic 2018). These proteins are ligands to three VEGF receptors (VEGFR 1 to 3) and are responsible for initiating a cascade of reactions that promote migration, proliferation, and survival of endothelial cells, leading to the formation of new blood vessels. VEGFR‐1 and VEGFR‐2 are responsible for regulation of angiogenesis, whilst VEGFR‐3 plays a secondary role in angiogenesis, but it is essential for lymphangiogenesis (development of new lymphatic vessels) and is therefore involved in the production of ascites and lymphatic dissemination of metastatic factors (Ellis 2008).
Angiogenesis is present in pre‐malignant conditions of the cervix, as well as in invasive disease, which can be seen at colposcopy with the occurrence of vascular changes. Studies have shown that microvessel (small vessels) density increases progressively with grades of cervical intraepithelial neoplasia (CIN), suggesting that angiogenesis may be an early event in the development of cervical pre‐cancer and cancer (Dobbs 1997; Guidi 1994; Krill 2015; Smith‐McCune 1994). Cervical cancer has been shown to be more dependent on angiogenesis than most other solid cancers. This is because human papillomavirus (the cause of almost all cervical cancers) and hypoxia, with increased hypoxia‐inducible factor 1 alpha, are associated with higher levels of VEGF (Monk 2010 (a)). In addition, increased intratumoural microvessel density during histological examination and enhanced expression of proteins, such as the endothelial antigen CD31, have been associated with poor prognosis (Dellas 1997; Dobbs 1997; Tjalma 1999). Targeting the cancer micro‐environment, specifically new blood vessel formation, using VEGF pathway inhibition is the main goal of angiogenesis inhibition.
Description of the intervention
Anti‐angiogenesis factors
Anti‐angiogenic factors include inhibitors targeting either single or multiple angiogenic pathways. VEGF acts by binding to its receptor, which then signals within the cell via tyrosine kinase enzyme activity (tyrosine kinases). VEGF receptor‐1 (Flt‐1 or fms‐like‐tyrosine kinase) and VEGFR‐2 (KDR/flk‐1) belong to the best characterised angiogenesis signalling pathway and can be blocked by inhibitors, such as antibodies (bevacizumab) or soluble receptors, which bind to VEGF and prevent it from binding to its receptor on cells. VEGFR‐2 is thought to be the dominant receptor responsible to translate and regulate angiogenesis (Bellone 2007).
In 1993, it was shown that anti‐VEGF monoclonal antibodies exerted a potent inhibitory effect on the growth of three tumour cell lines injected subcutaneously into mice(Kim 1993). Interestingly, the antibody had no effects on the cell lines in vitro. However, several parallel studies confirmed in vivo growth inhibition, which correlated with decreased tumour microvessel density and inhibition of tumour angiogenesis (Borgstrom 1996; Borgstrom 1998; Borgstrom 1999).
Bevacizumab is a humanised monoclonal antibody directed against all major isoforms of VEGF (VEGF‐A, VEGF‐B, VEGF‐C, VEGF‐D, placental growth factor (PIGF), VEGF‐E, and VEGF‐F members), binding to VEGF and blocking its binding to the receptor VEGFR. This inhibits endothelial cell proliferation and the development of vessels (Ferrara 2004). Its use has been evaluated in many solid cancers. It is used extensively in the USA and is the most studied biological agent in gynaecological cancer (Grothey 2009; Tewari 2015 (a)). Several randomised controlled trials (RCTs) and non‐randomised trials have been published reporting the use of the binding and inactivation of VEGF by clinical use of bevacizumab as single agent (Monk 2009; Monk 2009 (a); Monk 2010; Monk 2010 (a); Schefter 2012; Schefter 2014; Takano 2013; Tan 2010; Tewari 2005; Tewari 2014; Tewari 2014 (a); Wright 2006).
Tyrosine kinase inhibitors
Tyrosine kinase inhibitors (TKI) bind to the ATP‐binding catalytic site of the tyrosine kinase domain of VEGF receptors (VEGFRs) and directly inhibit the activity of VEGFR.
Sorafenib is an inhibitor of VEGFR‐1, VEGFR‐2, VEGFR‐3, platelet‐derived growth factor receptor (PDGFR‐beta), and Raf‐1 tyrosine kinase activity.
Cediranib blocks VEGF signalling by inhibiting VEGFR1‐2‐3 tyrosine kinases as well as PDGFR‐alpha and stem cell factor receptor (c‐Kit) (Tewari 2015; Tewari 2015 (a); Tewari 2015 (b)).
Sunitinib blocks tumour proliferation and angiogenesis by acting on the intracellular kinase domains of the VEGF receptor isoforms (VEGFR‐1, VEGFR‐2, and VEGFR‐3) and on the PDGFR, c‐Kit, and class III tyrosine kinase (RTK) and fms‐inhibitor like tyrosine kinase III (FLT3) (Mendel 2003).
Pazopanib is a multi‐target TKI of VEGFR‐1, ‐2, and ‐3; PDGFR‐alpha and ‐beta; and c‐Kit (Hurwitz 2009).
How the intervention might work
VEGF‐targeted therapy can have an effect on number of blood vessels and their function as well as directly on tumour cells. It has been reported that vessel beds of normal adult tissues are quiescent and in a VEGF‐independent state. In contrast, the immature vessels in tumours are more susceptible to VEGF‐targeted therapy (Kerbel 2008).
Structural irregularities and insufficient vascular supply are common in tumour vasculature, which is thought to be due mainly to lack of oxygen, which then causes increased expression of hypoxia‐inducible factor‐1. This factor induces the expression of a larger number of genes that are involved in tumour progression and metastasis (Forsythe 1996), the most important of which is VEGF (Pennacchietti 2003). Over‐expression of VEGF is responsible for vascular hyperpermeability (leakiness), which may render the cancer less sensitive to therapies (Carpini 2010; Seebacher 2019;
Some animal studies support the idea that treatment with VEGF‐targeted agents encourages intratumoural delivery of therapeutic agents by causing vasoconstriction and flow dynamic changes (Wildiers 2003). The findings demonstrated that anti‐VEGF therapy changes not only vascular function, but also inhibits classical angiogenesis (Wildiers 2003).
Activation of VEGFR‐2 by VEGF also leads to expression of vasodilator factors, hence blocking VEGF signalling, which leads to a reduction in vasodilator factors This hypothesis of vascular normalisation fits well with the observation that bevacizumab monotherapy has produced only modest symptomatic release clinical benefits, but that it increases survival when used in combination with chemotherapy (Tewari 2014).
Finally, VEGF‐targeted therapy may have direct inhibitory effects on tumour cells. Studies on breast cancer cell lines reported a decrease in chemotactic migration when the VEGF was blocked (Nasarre 2003; Nasarre 2005). Studies in colon cancer and pancreatic cancer cell lines have shown that the activation of VEGFR‐1 leads to an increase in tumour cell invasion and migration, which was blocked by treatment with antibodies to VEGFR‐1 (Fan 2005; Liu 2015; Wey 2005). (See also Table 1 for drug actions.)
1. Actions of included drugs.
| Name of drug | Actions |
| Bevacizumab | VEGF‐A‐B‐C‐D‐E‐F PIGF |
| Sorafenib | VEGFR 1‐2‐3 PDGFR‐beta Raf‐1 |
| Cediranib | VEGFR 1‐2‐3 PDGFR‐alpha c‐Kit |
| Sunitinib | VEGFR 1‐2‐3 PDGFR c‐Kit RTK FLT3 |
| Pazopanib | VEGFR 1‐2‐3 PDGFR‐alpha and ‐beta c‐kit |
| VEGFR: vascular endothelial growth factor receptors; VEGF: vascular endothelial growth factor; PDGFR: platelet‐derived growth factor receptor; c‐Kit: tyrosine kinase; RTK: tyrosine kinase; FLT3: tyrosine kinase; Raf 1: tyrosine kinase; PIGF: placental growth factor | |
Why it is important to do this review
Increasing knowledge about the molecular biology of gynaecological cancers has led to the development of biological therapies. Treatment options for metastatic and recurrent cervical cancer are limited, and other agents have not shown a survival advantage compared with standard therapy. However, a phase III clinical trial showed that the addition of bevacizumab to chemotherapy improved survival from 13.3 months to 16.8 months (hazard ratio for death 0.71, 98% confidence interval 0.54 to 0.95; P = 0.004 in a one‐sided t test) (Penson 2015; Tewari 2014; Tewari 2017). Since publication of the early results of Tewari 2014, bevacizumab has become the standard of care in addition to chemotherapy for individuals with metastatic and recurrent cervical cancer in some high income countries Other anti‐angiogenic agents have been studied to a lesser degree than bevacizumab.
We aim to perform a systematic review to assess the benefits and harms of angiogenesis inhibitors for the treatment of cervical cancer with the goal of assisting clinicians and patients in the decision‐making process.
Objectives
To evaluate the benefits and harms of anti‐angiogenesis agents for the treatment of metastatic and recurrent cervical cancer.
Methods
Criteria for considering studies for this review
Types of studies
RCTs that evaluate the use of anti‐angiogenesis therapy for the treatment of recurrent or metastatic cervical cancer. We will exclude observational studies such as case reports, case series, case‐control and case‐cohort and non‐RCTs. If no RCTs are identified, we will summarise the outcomes of non‐randomised studies in a narrative review and in tabular format.
Types of participants
We will include adult women over 18 years of age, diagnosed with recurrent or metastatic cervical cancer of any stage.
Types of interventions
Anti‐angiogenic therapies, single agent or combined with other chemotherapeutic agents, such as platinum‐based chemotherapy, taxane and/or topotecan for the treatment of advanced cervical cancer versus other platinum‐based or non‐platinum chemotherapy regimens (see Table 1).
Types of outcome measures
We will consider overall survival, progression‐free survival, adverse events, and quality of life.
Primary outcomes
Overall survival (OS)
Progression‐free survival (PFS)
Secondary outcomes
Response rates assessed using Response Evaluation Criteria in Solid Tumours (RECIST) v 1.1 cervical cancer
Disease‐specific survival
Adverse events during treatment such as dry mouth, cough, voice changes, loss of appetite, mouth sores, headache, back pain, flu‐like symptoms (stuffy nose, sneezing, sore throat), dry or watery eyes, dry or flaky skin, hair loss, and change of taste classified according to World Health Organization (WHO) or National Cancer Institute Common Toxicity Criteria (NCI‐CTC) (www.ctep.cancer.gov).
Serious adverse events and side effects of treatment (i.e. hypertension, fistula formation, etc.)
Quality of life (QoL) on treatment and post‐treatment when reported, and measured by the Functional Assessment of Cancer Therapy‐Cervix Trial Outcome Index (FACT‐Cx TOI) (http://www.facit.org/facitorg/) to assess physical and functional well‐being specific to cervical cancer. European Organisation for Research and Treatment of Cancer Quality Questionnaire Core 30 Items (EORTC QLQ‐C30), the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System, the Rotterdam Symptom Checklist (RSCL), and the Symptom Distress Scale (SDS) (Tamburini 2001).
Search methods for identification of studies
We will identify all relevant RCTs regardless of language and publication status (published, unpublished, in press, or in progress). The search date will commence in 1990 as we do not expect to find any trials investigating anti‐angiogenesis inhibition before this date (Rodriguez‐Freixinos 2015).
Electronic searches
We will search the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL; latest issue) in the Cochrane Library;
MEDLINE via Ovid (1946 to present);
Embase via Ovid (1980 to present).
We will also search online registers of clinical trials, abstracts of scientific meetings, and reference lists of included studies, and we will use the 'related articles' feature in PubMed of the studies we are including to avoid missing studies. See search strategies designed for MEDLINE (Appendix 1), Embase (Appendix 2), and CENTRAL (Appendix 3).
Searching other resources
We will search for study records from different registries: the ISRCTN registry (www.isrctn.com) and from the National Institute for Health Research www.isrctn.com) US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the National Cancer Institute (www.nci.nih.gov), for ongoing trials. We will search abstracts from the American Society of Clinical Oncology (ASCO), European Society Gynaecological Oncology (ESGO), International Gynaecologic Cancer Society (IGCS), British Gynaecological Cancer Society (BGCS) and Society of Gynecologic Oncology (SGO). We will also review other relevant websites: the WCG Center Watch (www.isrctn.com), the European Organisation for Research and Treatment of Cancer (www.isrctn.com) and the Cochrane library (www.cochranelibrary.com),
Data collection and analysis
Selection of studies
We will download all titles and abstracts retrieved by electronic searching to the EndNote database version X9 (EndNote X9)and remove duplicates. Two review authors (IR and SO) will examine the remaining articles to identify potentially relevant trials. Any disagreements will be resolved by discussion or by consulting a third review author (RB) if necessary. We will exclude studies that clearly do not meet the inclusion criteria and obtain the full texts of potentially relevant trials. We will document the reasons for exclusion of any excluded studies.
Data extraction and management
Two review authors will independently extract data (IR and RB) onto a pre‐designed data collection form. We will assess the risk of bias of the included studies according to the Cochrane Handbook for Systematic Reviews of Interventions and analyse data using Review Manager 5 (Review Manager 2014).
We will collect the following data for the included studies when reported.
Author, year of publication and journal citation, and country where the trial was performed.
Inclusion and exclusion criteria of the study.
Study design and methodology.
Study population including: total number of women enrolled, women characteristics, age, previous therapy, and comorbidities.
Cervical cancer characteristics such as FIGO stage, histological type, tumour grade, and extent of disease.
Total number of intervention groups.
Intervention details such as the anti‐angiogenetic factor studied, dose, regimen, frequency and number of cycles.
Details about the comparison when applicable such as type of control and dose, regimen, frequency and number of cycles.
Proportion of participants who received all/part/none of the intended treatment.
Duration of follow‐up.
Outcomes such as overall survival, PFS, QoL, symptom control, and adverse events.
In the case that cluster‐randomised trials are eligible, we will carry out analysis using the inverse‐variance method, and appropriately choose intraclass correlations. If there are women with more than one recorded time‐to‐recurrence interval, we will include only the first interval where possible.
We will extract data on outcomes as follows.
For time‐to‐event data (OS and PFS), we will abstract the hazard ratio (HR), log of the hazard ratio (log(HR)) and its standard error (SE) from trial reports where possible. If these are not reported, we will attempt to estimate them from other reported statistics using the methods of Parmar 1998 (Parmar 1998) (e.g. number of events in each arm and log‐rank P value comparing the relevant outcomes in each arm). If it is not possible to estimate the HR, we will abstract the number of women in each treatment arm who experienced the outcome of interest at a specific time point, in order to estimate a risk ratio (RR).
For dichotomous outcomes (e.g. adverse events), we will abstract the number of women in each treatment arm who experienced the outcome of interest and the number of women assessed at endpoint, in order to estimate an RR.
For continuous outcomes (e.g. QoL measures), we will abstract the mean difference (MD) and standard deviation (SD) between the final value of the outcome measure in each treatment arm at the end of follow‐up. If the SDs of final values are not available, we will use change scores if their SDs are available. If no SDs are available, we will omit these trials from the analyses and include them only in a narrative analysis with a table.
Where possible, we will extract data relevant to an intention‐to‐treat analysis (ITT), analysing participants in the groups to which they were assigned. Where time‐to‐event outcomes are assessed by more than one method (e.g. radiology reviews, investigator assessment or oncology review), we will use the radiology review data. We will collect the time points at which outcomes are collected and reported. Where data from several time points are reported, we will use the data from the last assessment in our meta‐analyses if appropriate. Where a trial evaluates the same drug in two or more different doses versus the use of anti‐angiogenic factor, we will collect the combined data and the individual data of the most efficacious regimen versus the intervention.
Two review authors (IR and SO) will independently collect data using a piloted data extraction form designed specifically for this review.
Assessment of risk of bias in included studies
We will assess risk of bias of the included RCTs using the Cochrane 'Risk of bias' tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This will include an assessment of the following.
Selection bias (random sequence generation and allocation concealment)
Performance bias (blinding of participants and personnel)
Detection bias (blinding of outcome assessment)
Attrition bias (incomplete outcome data; we will record the proportion of participants whose outcomes are not reported at the end of the study, considering greater than 20% attrition to be at high risk of bias)
Reporting bias (selective reporting of outcomes)
Other possible sources of bias.
Two review authors (IR and RB) will apply the 'Risk of bias' tool, resolving any differences by discussion or by consultation with a third review author (SO) if necessary. We will present results in a 'Risk of bias' summary graph, and interpret the results of the meta‐analyses in light of the findings with respect to risk of bias.
Measures of treatment effect
We will use the following measures of the effect of treatment.
For time‐to‐event data, we will use HR and 95% confidence interval (CI).
For dichotomous outcomes, we will use RR and 95% CI.
For continuous outcomes, we will use MD between treatment arms and the 95% CI if outcomes are measured on the same scale; otherwise we will calculate standardised mean difference (SMD).
For studies where there is no control,we will perform subgroup analysis according to FIGO stage..
Unit of analysis issues
We do not expect any unit of analysis issues.
Dealing with missing data
We will attempt to contact study authors to obtain missing data (participant, outcome, or summary data). For participant data, we will conduct analysis on an intention‐to‐treat basis where possible; otherwise we will analyse data as reported. We will not impute missing outcome data.
Assessment of heterogeneity
We will assess heterogeneity between trials by visual inspection of forest plots, estimation of the percentage of heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2011), and a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there is evidence of moderate heterogeneity, we will investigate the possible reasons for this and report this information.
Assessment of reporting biases
We will evaluate the potential small‐study effects such as publication bias using funnel plots if more than 10 studies are included in the review.
Data synthesis
If sufficient clinically similar trials are available, we will pool their results and perform meta‐analysis. For time‐to‐event data we will use HRs and analyse the data using the generic inverse‐variance method (Review Manager 2014).
For dichotomous outcomes and for continuous outcomes, we will pool RR. We will use the random‐effects method for the meta‐analysis, as a degree of heterogeneity is expected. In the case of multi‐arm studies, we will combine all relevant interventional groups into a single group and compare to the standard treatment as recommended in Section 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions.
We will assess the overall certainty of the evidence using the GRADE approach, employing GRADEpro GDT (GRADEpro; Schunemann 2011), and we will distinguish:
high certainty: further research is very unlikely to change our confidence in the estimate of effect;
moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate;
low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate;
very low certainty: we are very uncertain about the estimate.
We will group studies according to the type of drug used in the 'Summary of findings' tables.
We will only include studies reporting data on the use of bevacizumab, sorafenib, cediranib, sunitinib, pazopanib as single agent or in combination for the treatment of metastatic and recurrent cervical cancer.
'Summary of findings' table
We will present the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Langendam 2013; Schnemann 2011). We will create a 'Summary of findings' table (Table 2) based on the methods described in the Cochrane Handbook for Systematic Reviews, (Higgins 2011a), employing GRADEpro GDT (GRADEpro). We will use the GRADE checklist and GRADE Working Group definitions for certainty of the evidence. We will downgrade the evidence from 'high' certainty by one level for serious (or by two levels for very serious) concerns for each limitation.
2. Draft 'Summary of findings' table.
| Vascular endothelial growth factor (VEGF) inhibitors for the treatment of metastatic and recurrent cervical cancer | ||||||
|
Patient or population: metastatic or recurrent cervical cancer Settings: hospital Interventions: bevacizumab plus standard chemotherapy Comparison: standard chemotherapy | ||||||
| Outcomes | Illustrative comparative risks* | Relative effect (95% CI) | No. of participants (studies) | Certainty of evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Overall survival | ||||||
| Median progression‐free survival | ||||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and it's 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
|
GRADE: Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are uncertain about the estimate. | ||||||
High certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of the effect.
We will include the following outcomes in the 'Summary of findings' table:
overall survival;
median progression‐free survival.
If meta‐analysis is not possible, we will present the results in a narrative 'Summary of findings' table format (Chan 2011).
Subgroup analysis and investigation of heterogeneity
We will group the RCTs by type of intervention, and subgroup analysis will include advanced cervical cancer and single use of anti‐angiogenesis therapy and different drug combinations.
Sensitivity analysis
We will exclude studies at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
See Table 2 for an example of the summary of findings example (Table 2)
What's new
| Date | Event | Description |
|---|---|---|
| 25 May 2021 | Amended | Merged with Vascular endothelial growth factor (VEGF) targeting therapy for persistent, recurrent, or metastatic cervical cancer. See www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013348.pub2/full. |
History
Protocol first published: Issue 5, 2020
Notes
Merged with Vascular endothelial growth factor (VEGF) targeting therapy for persistent, recurrent, or metastatic cervical cancer. See www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD013348.pub2/full.
Acknowledgements
We thank Jo Morrison, Co‐ordinating Editor of the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group, for clinical and editorial advice; Jo Platt, Information Specialist for designing the search strategy; and Managing Editor Gail Quinn for her contribution to the editorial process.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
We would like to thank all of our external peer reviewers, which include Andrew Bryant, Jennifer Cove, Philipp Harter, Emma Hudson, and Monique Spillman.
Appendices
Appendix 1. MEDLINE search strategy
1. Uterine Cervical Neoplasms/ 2. (cervi* adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma*)).mp. 3. 1 or 2 4. exp Angiogenesis Inhibitors/ 5. (angiogene* adj5 (inhibit* or antagoni*)).mp. 6. (anti‐angiogene* or antiangiogene*).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub‐heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 7. (bevacizumab or sorafenib or cediranib or avastin or sunitinib or sutent or pazopanib or votrient or brivanib or "BMS‐582664").mp. 8. exp Vascular Endothelial Growth Factors/ 9. (vascular endothelial growth factor* or VEGF*).mp. 10. 4 or 5 or 6 or 7 or 8 or 9 11. 3 and 10
Key:
[mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
Appendix 2. Embase search strategy
1. exp uterine cervix tumor/ 2. (cervi* adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma*)).mp. 3. 1 or 2 4. exp angiogenesis inhibitor/ 5. (angiogene* adj5 (inhibit* or antagoni*)).mp. 6. (anti‐angiogene* or antiangiogene*).mp. 7. (bevacizumab or sorafenib or cediranib or avastin or sunitinib or sutent or pazopanib or votrient or brivanib or "BMS‐582664").mp. 8. exp vasculotropin/ 9. (vascular endothelial growth factor* or VEGF*).mp. 10. 4 or 5 or 6 or 7 or 8 or 9 11. 3 and 10
Key:
[mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
Appendix 3. CENTRAL search strategy
#1 MeSH descriptor: [Uterine Cervical Neoplasms] explode all trees #2 cervi* near/5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma*) #3 #1 or #2 #4 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #5 angiogene* near/5 (inhibit* or antagoni*) #6 anti‐angiogene* or antiangiogene* #7. bevacizumab or sorafenib or cediranib or avastin or sunitinib or sutent or pazopanib or votrient or brivanib or "BMS‐582664" #8 MeSH descriptor: [Vascular Endothelial Growth Factors] explode all trees #9 vascular endothelial growth factor* or VEGF* #10 #4 or #5 or #6 or #7 or #8 or #9 #11 #3 and #10
Contributions of authors
IR wrote the first draft. SO, RB, and AS edited the draft of the protocol.
Sources of support
Internal sources
-
None to declare, Other
Not applicable
External sources
-
New source of support, UK
Ipswich library who provided the papers needed to complete the protocol
Declarations of interest
Ivana Rizzuto: nothing to declare Sophie Otter: nothing to declare Rasiah Bharathan: nothing to declare Alexandra Stweart: nothing to declare
Edited (no change to conclusions)
References
Additional references
Alldredge 2016
- Alldredge JK, Tewari KS. Clinical trials of antiangiogenesis therapy in recurrent/persistent and metastatic cervical cancer. Oncologist 2016;21:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellone 2007
- Bellone S, Frera G, Landolfi G, Romani C, Bandiera E, Tognon G, et al. Overexpression of epidermal growth factor type-1 receptor (EGF-R1) in cervical cancer: implications for Cetuximab-mediated therapy in recurrent/metastatic disease. Gynecologic Oncology 2007;106:513-20. [DOI] [PubMed] [Google Scholar]
Bhatla 2018
- Bhatla N, Denny L. FIGO Cancer report 2018. International Journal of Gynaecology and Obstetrics 2019;145(1):129-135. [DOI: 10.1002/ijgo.12608] [DOI] [PubMed] [Google Scholar]
Borgstrom 1996
- Borgstrom P, Hillan K, Sriramarao P, Ferrara N. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Research 1996;56:4032-9. [PubMed] [Google Scholar]
Borgstrom 1998
- Borgstrom P, Bourdon M, Hillan K, Sriramarao P, Ferrara N. Neutralizing anti-vascular endothelial growth factor antibody completely inhibits angiogenesis and growth of human prostate carcinoma micro tumours in vivo. Prostate 1998;35:1-10. [DOI] [PubMed] [Google Scholar]
Borgstrom 1999
- Borgstrom P, Gold D, Hillan K, Ferrara N. Importance of VEGF for breast cancer angiogenesis in vivo: implications from intravital microscopy of combination treatments with an anti-VEGF neutralizing monoclonal antibody and doxorubicin. Anticancer Research 1999;19:4203-14. [PubMed] [Google Scholar]
Bray 2018
- Bray F, Ferlay J, Soerjomataram I, Siegel RI, Torre LA, Jernal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
Cancer Research UK 2014
- Cancer Research UK . Incidence of cervical cancer. www.cruk.org.uk.
Carpini 2010
- Carpini JD, Karam AK, Montgomery L. Vascular endothelial growth factor and its relationship to the prognosis and treatment of breast, ovarian and cervical cancer.. Angiogenesis 2010;13:43-50. [DOI] [PubMed] [Google Scholar]
Cochrane Handbook for Systematic Reviews
- Higgins J, Thomas J. Cochrane Handbook for Systematic reviews of Interventions. Vol. 6. Cochrane group, 2019. [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
Dellas 1997
- Dellas A, Moch H, Schultheiss E, Feichter G, Almendral A, Gudat F, et al. Association of tumour induced vascularization with clinicopathological parameters in cervical neoplasm. International Journal of Oncology 1997;11(1):105-9. [PubMed] [Google Scholar]
Dobbs 1997
- Dobbs SP, Hewett PW, Johnson IR, Carmichael J, Murray JC. Angiogenesis is associated with vascular endothelial growth factor expression in cervical intraepithelial neoplasia. British Journal of Cancer 1997;76(11):1410-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eleje 2019
- Eleje GU, Eke AC, Igberase GO, Igwegbe AO, Eleje LI. Palliative interventions for controlling vaginal bleeding in advanced cervical cancer (Review). Cochrane Database of Systematic Reviews 2019;3:1-30. [CD011000] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 2008
- Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Reviews. Cancer 2008;8:579-91. [DOI] [PubMed] [Google Scholar]
EndNote X9 [Computer program]
- EndNote. Clarivate Analytics, 2018.
Eskander 2014
- Eskander RN, Tewari KS. Targeting angiogenesis in advanced cervical cancer. Therapeutic Advances in Medical Oncology 2014;6(6):280-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Falzone 2019
- Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Frontiers in Pharmacology 2018;9:1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2005
- Fan F, Wey JS, McCarty MF, Belcheva A, Liu W, Bauer TW, et al. Expression and function of endothelial growth factor receptor-1 on human colorectal cancer cells. Oncogene 2005;24:2647-53. [DOI] [PubMed] [Google Scholar]
Fanfani 2016
- Fanfani F, Vizza E, Landoni F, Iaco P, Ferrandina G, Corrado G, et al. Radical hysterectomy after chemoradiation in FIGO stage III cervical cancer patients versus chemoradiation and brachytherapy: complications and 3 years survival. European Journal of Surgical Oncology 2016;24:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Ferlay 2019
- Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019;144:1941-53. [DOI] [PubMed] [Google Scholar]
Ferrara 2004
- Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nature Reviews. Drug Discovery 2004;3:391-400. [DOI] [PubMed] [Google Scholar]
Folkman 1971
- Folkman J. Tumor angiogenesis: therapeutic implications. New England Journal of Medicine 1971;285:1182-6. [DOI] [PubMed] [Google Scholar]
Folkman 1990
- Folkman J. What is the evidence that tumors are angiogenesis dependent? Journal of the National Cancer Institute 1990;82:4-6. [DOI] [PubMed] [Google Scholar]
Forsythe 1996
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor-1. Molecular and Cellular Biology 1996;16:4604-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University and Evidence Prime Inc GRADEpro GDT. McMaster University and Evidence Prime Inc, 2015.
Greer 2010
- Greer B, Koh W, Abu-Rustum N, Apte S, Campos S, Chan J, et al. Cervical cancer. Journal of the National Cancer Institute 2010;8:1388-416. [DOI] [PubMed] [Google Scholar]
Grothey 2009
- Grothey A, Galanis E. Targeting angiogenesis: progress with anti-VEGF treatment with large molecules. Nature Reviews. Clinical Oncology 2009;6:507-18. [DOI] [PubMed] [Google Scholar]
Guidi 1994
- Guidi AJ, Fischer L, Harris JR, Schmitt SJ. Microvessel density and distribution in ductal carcinoma in situ of the breast. Journal of the National Cancer Institute 1994;86:614-9. [DOI] [PubMed] [Google Scholar]
Hanahan 1996
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996;86(3):353-64. [DOI] [PubMed] [Google Scholar]
Hicklin 2005
- Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of Clinical Oncology 2005;23:1011-27. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hurwitz 2009
- Hurwitz HI, Dowlati A, Saini S. Phase 1 trial of pazopanib in patients with advanced cancer. Clinical Cancer Research 2009;15(12):4220-7. [DOI] [PubMed] [Google Scholar]
ISD Scotland 2011
- ISD Scotland. Cancer incidence. www.idsscotland.org/health-topics/cancer/publications/index.asp (accessed July 2014).
Kerbel 2008
- Kerbel R. Tumor angiogenesis. New England Journal of Medicine 2008;358:2039-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 1993
- Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993;29(362):6423. [DOI] [PubMed] [Google Scholar]
Krill 2015
- Krill LS, Tewari KS. Exploring the therapeutic rationale for angiogenesis blockade in cervical cancer. Clinical Therapeutics 2015;37(1):9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2015
- Liu FW, Cripe J, Tewari KS. Anti-angiogenesis therapy in gynecologic malignancies. Oncology 2015;29(5):350-68. [PubMed] [Google Scholar]
Matsuo 2015
- Matsuo K, Mabuchi S, Okazawa M, Kawano M, Kuroda H, Kamiura S, et al. Clinical implication of surgically treated early-stage cervical cancer with multiple high-risk factors. Journal of Gynecologic Oncology 2015;26(1):3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendel 2003
- Mendel DB, Laird AD, Xin X, Louie SG, Schreck RE, Abrams TJ, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clinical Cancer Research 2003;9:327-37. [PubMed] [Google Scholar]
Monk 2009
- Monk BJ, Sill MW, McMekin S, Cohn DE, Ramodetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent or persistent cervical carcinoma: a Gynaecological Oncology Group study. Journal of Clinical Oncology 2009;27(28):4649-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monk 2009 (a)
- Monk BJ, Sill M, Burger R, Gray H, Buekers T, Roman L. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2009;27:1069-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Monk 2010
- Monk BJ, Willmott LJ, Sumner DA. Anti-angiogenesis agents in metastatic or recurrent cervical cancer. Gynecologic Oncology 2010;116:181-6. [DOI] [PubMed] [Google Scholar]
Monk 2010 (a)
- Monk BJ, Mas Lopez L, Zarba J, Oaknin A, Tarpin C, Termrungruanglert W, et al. Phase II open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. Journal of Clinical Oncology 2010;28(22):3562-9. [DOI] [PubMed] [Google Scholar]
Monk 2014
- Monk BJ. Evidence-based therapy for recurrent cervical cancer. J Clin Oncol 2014;32(25):2687-90. [DOI] [PubMed] [Google Scholar]
Nagy 2007
- Nagy JA, Dvorak AM, Dvorak HF. VEGF-A and the induction of pathological angiogenesis. Annual Review of Pathology 2007;2:251-75. [DOI] [PubMed] [Google Scholar]
Nasarre 2003
- Nasarre P, Constantin B, Rouhaud L, Harnois T, Raymond G, Drabkin HA, et al. Semaphorin SEMA3F and VEGF have opposing effects on cell attachment and spreading. Neoplasia 2003;5:83-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nasarre 2005
- Nasarre P, Kusy S, Constantin B, Castellani V, Drabkin HA, Bagnard D. Semaphorin SEMA3F has a repulsing activity on breast cancer cells and inhibits E-cadherin mediated cell adhesion. Neoplasia 2005;7:180-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints.. Stat Med 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Pennacchietti 2003
- Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S, Comoglio PM. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell 2003;3:347-61. [DOI] [PubMed] [Google Scholar]
Penson 2015
- Penson RT, Huang HQ, Wenzel LB, Monk BJ, Stockman S, Long HJ, et al. Patient reported outcomes in a practice changing randomized trial of bevacizumab in the treatment of advanced cervical cancer: an NGR Oncology/Gynecologic Oncology Group Study. Lancet Oncology 2015;16(3):301-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfaendler 2016
- Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Expert Reviews Gynecology 2016;214(1):23-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rebolj 2019
- Rebolj M, Rimmer J, Denton K, Tidy J, Mathews C, Ellis K, et al. Primary cervical screening with high risk human papillomavirus testing: observational study. BMJ 2019;364:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodriguez‐Freixinos 2015
- Rodriguez-Freixinos V, Mackay HJ. Breaking down the evidence for bevacizumab in advanced cervical cancer: past, present and future. Gynecologic Oncology Research and Practice 2015;2(8):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saito 2010
- Saito I, Kitagawa R, Fukuda H, Shibata T, Katsumata N, Konishi I, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505). Japanese Journal of Clinical Oncology 2010;40(1):90-3. [DOI] [PubMed] [Google Scholar]
Schefter 2012
- Schefter T, Winter K, Kwon J, Stuhr K, Balaraj K, Yaremko B, et al. A phase II study of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: preliminary results of RTOG 0417. International Journal of Radiation Oncology, Biology, Physics 2012;83:1179-84. [DOI] [PubMed] [Google Scholar]
Schefter 2014
- Schefter T, Winter K, Kwon JS, Stuhr K, Balaraj K, Yaremko BP, et al. RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. International Journal of Radiation Oncology, Biology, Physics 2014;88:101-5. [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Seebacher 2019
- Seebacher NA, Stacy AE, Porter GM, Merlot AM. Clinical development of targeted and immune based anti-cancer therapies. Journal of Experimental & Clinical Cancer Research 2019;38(156):1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siegel 2019
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2019. CA: A Cancer Journal for Clinicians 2019;69:7-34. [DOI] [PubMed] [Google Scholar]
Smith‐McCune 1994
- Smith-McCune KK, Weidner N. Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Research 1994;54:800-4. [PubMed] [Google Scholar]
Takano 2013
- Takano M, Ikeda Y, Kudoh K, Kita T, Sasaki N, Kikuchi Y. Complete remission of recurrent ovarian clear cell carcinoma by chemotherapy with bevacizumab, trabectedin and oxaliplatin. Journal of Obstetrics and Gynaecology 2013;39(4):872-5. [DOI] [PubMed] [Google Scholar]
Tamburini 2001
- Tamburini M. Health-related quality of life measures in cancer. Annals of Oncology 2001;12(Suppl 3:S7):10. [DOI] [PubMed] [Google Scholar]
Tan 2010
- Tan SJ, Juan YH, Fu PT, Yu MH, Lai HC. Chemotherapy with low-dose bevacizumab and carboplatin in the treatment of a patient with recurrent cervical cancer. European Journal of Gynaecological Oncology 2010;31(3):350-3. [PubMed] [Google Scholar]
Tewari 2005
- Tewari K, Monk B. Gynecologic Oncology Group trials of chemotherapy for metastatic and recurrent cervical cancer. Current Oncology Reports 2005;7:419-34. [DOI] [PubMed] [Google Scholar]
Tewari 2014
- Tewari K, Sill M, Long H, Penson R, Huang H, Ramondetta L, et al. Improved survival with bevacizumab in advanced cervical cancer. New England Journal of Medicine 2014;370:734-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tewari 2014 (a)
- Tewari KS, Monk BJ. New strategies in advanced cervical cancer: from angiogenesis blockade to immunotherapy. Clinical Cancer Research 2014;20(21):5349-58. [DOI] [PubMed] [Google Scholar]
Tewari 2015
- Tewari KS, Sill MW, Monk BJ, Penson RT, long HJ, Poveda A, et al. Factors in women with advanced cervical cancer treated with chemotherapy with/without bevacizumab: NRG Oncology/GOG Study. Clinical Cancer Research 2015;21(24):5480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tewari 2015 (a)
- Tewari KS, Sill MW, Monk BJ, Penson RT, Long HJ, Poveda A, et al. Prospective validation of pooled prognostic factors in women with advanced cervical cancer treated with chemotherapy with/without bevacizumab: NRG Oncology/GOG Study. Clinical Cancer Research 2014;21:5480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tewari 2015 (b)
Tewari 2017
- Tewari KS, Sill MW, Penson RT, Huang H, Ramodetta LM, Landrum LM, et al. Final overall survival of the phase III randomised trial of chemotherapy with and without bevacizumab for advanced cervical cancer: an NRG Oncology/Gynecologic Oncology Group study. Lancet 2017;390:1654-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tjalma 1999
- Tjalma W, Sonnemans H, Weyler J, Van Marck E, Van Daele A, Van Dam P. Angiogenesis in cervical intraepithelial neoplasia and the risk of recurrence. American Journal of Obstetrics and Gynecology 1999;181(3):554-9. [DOI] [PubMed] [Google Scholar]
Torre 2015
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics. CA: A Cancer Journal for Clinicians 2015;65:87-108. [DOI] [PubMed] [Google Scholar]
Turajlic 2018
- Turajlic S, Swanton C, Boshoff C. Kidney cancer: the next decade. Journal of Experimental Medicine 2018;215(10):2477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waggoner 2003
- Waggoner SE. Cervical cancer. Lancet 2003;361:2217-25. [DOI] [PubMed] [Google Scholar]
Welsh Cancer Intelligence and Surveillance 2011
- Welsh Cancer Intelligence and Surveillance Unit. Cancer incidence 2001-2017. www.wcisu.wales.nhs.uk.
Wey 2005
- Wey JS, Fan F, Gray MJ, Bauer TW, McCarty MF, Somcio R, et al. Vascular endothelial growth factor receptor-1 promotes migration and invasion in pancreatic carcinoma cell lines. Cancer 2005;104:427-38. [DOI] [PubMed] [Google Scholar]
Wildiers 2003
- Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, et al. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. British Journal of Cancer 2003;88(12):1979-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 2006
- Wright J, Viviano D, Powell M, Gibb R, Mutch D, Grigsby P, et al. Bevacizumab combination therapy in heavily pretreated, recurrent cervical cancer. Gynecologic Oncology 2006;103:489-93. [DOI] [PubMed] [Google Scholar]


