Abstract
Background:
Cardiovascular disease has its origins in adolescents. Endothelial dysfunction, arterial stiffness, and decreased endocardial oxygen supply: demand ratios are early functional markers of cardiovascular risk. The goal of this study was to determine the relationships of these markers to physical, inflammatory, and metabolic markers in healthy non-Hispanic, white adolescents.
Methods:
Thirty-four of the 75 subjects were female. Mean age was 15.0 ± 1.7 years and mean body mass index (BMI) was 22.0 ± 5.8 kg/m2 (mean ± SD). Reactive hyperemia was measured using venous occlusion plethysmography. Arterial tonometry was used to measure the augmentation index (AIx75) and the Buckberg subendocardial viability ratio. Blood samples were taken to measure inflammatory and lipid markers and oral glucose tolerance test was used to assess insulin sensitivity.
Results:
Reactive hyperemia decreased as body mass and fat mass increased. It also decreased with increasing neutrophil count. The Buckberg index was higher in males and was positively related to insulin sensitivity even when accounting for age, sex, and resting heart rate. AIx75 was not related to any of the other variables.
Conclusions:
These results demonstrate that increased fat mass and decreased insulin sensitivity are related to poorer vascular function and cardiac risk in adolescents before the development of actual cardiovascular disease.
Keywords: adolescents, arterial stiffness, inflammation, insulin sensitivity, reactive hyperemia
1 |. INTRODUCTION
While cardiometabolic diseases such as coronary artery disease, atherosclerosis, hypertension, and type 2 diabetes, usually do not produce significant mortality and morbidity until adulthood, there is clear evidence that these diseases have their origins in childhood and adolescence. Studies of soldiers killed in Korea at average age of 22 years showed 77% had significant atherosclerosis.1 Autopsy studies of 15–34 year old’s who died from accidental or non-accidental trauma demonstrated raised coronary artery lesions in individuals as young as 15 years with significantly increasing prevalence over time.2 With the rising incidence of obesity associated with poorer diet and less physical activity in children and adolescents, it is important that we study these diseases early in their course if we are to prevent future cardiometabolic morbidity and mortality.
In healthy adults insulin resistance has been closely linked to impaired cardiac function, increased arterial stiffness,3,4 and decreased endothelial function.5–7 In addition, hyperinsulinism has been shown to impair endothelial function.8,9 In adolescents, where cardiovascular disease has its origins, less is known. Most articles report decreased endothelial function and/or increased arterial stiffness in obese adolescents10–15 although there may be potential racial differences.16,17 One study reported increased flow mediated dilation with increased adiposity in this age group.18 These studies have found variable relationships to insulin sensitivity and other measures of cardiovascular risk. Diastolic dysfunction in obese adolescents has been related to increased insulin resistance.19 We, first, hypothesized that adiposity and insulin resistance would be associated with impaired vascular and cardiac functional markers in adolescents. Second, as adolescent females have been shown to have lower insulin sensitivity than males of similar body mass index (BMI) and Tanner stage,20 we hypothesized that they should, also, have reduced cardiac and vascular function compared to adolescent males. Thus, the goal of this study was to investigate the relationships of vascular and cardiac functional risk markers to physical, inflammatory, and metabolic risk markers in healthy adolescents.
2 |. METHODS
2.1 |. Subjects
Seventy-five, white, non-Hispanic identifying adolescents between the ages of 12 to 17 years 11 months were recruited to participate. Subjects were medication free for at least 2 weeks except for oral contraceptives in females. Subjects with a history of autoimmune endocrine, connective tissue disease, hematologic disease, renal disease, or malignancy were excluded. The protocol was approved by the Nationwide Children’s Hospital Institutional Review Board and written informed assent from the subject and informed consent from a parent or guardian were obtained.
2.2 |. Protocol
The protocol was registered on ClinicalTrials.gov, NCT02821104.
2.2.1 |. Study visit
Subjects arrived at the Clinical Research Center of the Wexner Medical Center at The Ohio State University at 08:00 after overnight fast beginning at 22:00 the night before the study. Upon arrival, a brief history was taken and physical examination was performed.
2.2.2 |. Anthropometrics
Height (Stadiometer: 216 Accu-Hite Measuring Device, Seca North America, Chino, California), weight (SECA 1360 Wireless scale) were measured. Waist circumference was measured to the nearest 0.1 cm at the narrowest place between the lowest rib and the iliac crest at the end of normal exhalation with a spring-loaded, inelastic measuring tape.21 Body fat percentage was measured using air displacement plethysmography in the BODPOD (COSMED USA Inc., Concord, California). Fat mass index (FMI) was calculated as total body fat weight/height2 and fat free mass index (FFMI) as total non-fat weight/height.2
2.2.3 |. Arterial tonometry
Arterial tonometry (SphygmoCor, AtCor Medical, Inc., Itasca, Illinois) was used to determine the augmentation index, as a measure of vascular stiffness. Results were corrected to heart rate of 75 (AIx75). The software, also, calculates the Buckberg subendocardial viability ratio, which is a measure of cardiac oxygen supply: demand ratio.3,22
2.2.4 |. Endothelial function
Endothelial function was measured using postocclusion, shear stressinduced, endothelially mediated vasodilation (reactive hyperemia) after a 20-minute rest period. Strain gauge venous occlusion plethysmography (Hokanson AI6 plethysmograph, DE Hokanson, Inc., Bellevue, Washington) was used to measure forearm blood flow (FBF) and forearm vascular resistance (FVR) before and after upper arm occlusion. This method of testing endothelial function assesses resistance vessel function.17
The reactive hyperemia response was quantified as percent decrease in FVR using the following protocol. Two minutes of baseline FBF were measured after which the upper arm cuff was inflated to 200 mmHg pressure for 5 minutes to occlude arterial inflow. It was then released and FBF was measured for 1 minute. FVR were calculated by dividing mean arterial blood pressure by FBF. Arterial blood pressure was measured using automated sphygmomanometer. Mean intra-observer coefficient of variation (CV) for FBF before upper arm occlusion is 5.1% and 7.4% after upper arm occlusion.
2.2.5 |. Blood sampling
After completion of the test of endothelial function, an intravenous catheter was placed in one arm for initial blood sampling and for oral glucose tolerance testing. Twenty-five milliliters of blood was taken from each study subject for measurement of fasting plasma glucose, insulin, lipid, interleukin-6 (IL6) and endothelin 1 concentrations, and high sensitivity c-reactive protein (CRP), and plasminogen activator inhibitor-1 (PAI1) levels. White blood cell and neutrophil counts and IL6 concentrations and CRP levels are markers of inflammation, which are clearly increased in individuals at increased cardiometabolic risk.23,24 PAI1 was used to assess clotting risk. Endothelin 1 is an endothelially-produced vasoconstrictor and has been shown to be a biochemical marker for endothelial dysfunction.25
2.2.6 |. Insulin sensitivity and secretion
Subjects were given 1.75 g/kg of oral glucose (up to a maximum of 75 g). Blood samples for measurement of plasma glucose and insulin concentrations were drawn every 30 minutes for 120 minutes. Insulin sensitivity was assessed using the Matsuda Index (MAT = 10 000/√[fasting glucose × fasting insulin] × [mean glucose × mean insulin]) while insulin secretion was assessed as the change in insulin divided by the change in glucose from 0 to 30 minutes or insulinogenic indexe(IGI = ΔI0–30 /ΔG0–30).26 Disposition index (DI) was calculated by multiplying both these values (DI = IS × IGI).26 Disposition index is an important predictor of future type 2 diabetes.27
2.2.7 |. Cardiovascular measures, glucose, and insulin
Cell differentials (neutrophils, total white blood cells) from EDTA-peripheral blood were measured by a Sysmex Kx-21 N cell counter (Sysmex, Lincolnshire, Illinois). Plasma lipids, glucose, insulin, IL6, CRP, endothelin 1, fibrinogen, plasminogen activator inhibitor antigen (PAI1), were measured in the Clinical Research Center CORE laboratory.
2.2.8 |. Statistical analysis
All analysis was done using non-parametric testing. Mann Whitney U test was used for comparison between sexes. The relationships of cardiovascular function measures (reactive hyperemia, Aix75, Buckberg index) to measures of body habitus and physical (heart rate, blood pressure), biochemical (lipids, inflammatory markers, endothethelin-1, PAI-1) and carbohydrate metabolism (fasting insulin, insulin sensitivity, insulin secretion, disposition index) cardiometabolic risk factors were assessed using robust, rank order regression analysis with age and sex included in all models. When the cardiovascular function measures were significantly related to both body habitus and other cardiometabolic risk factors additional models were constructed to include both measures in the model. The cut-off point for outliers was 3 and 100 iterations were performed. Results are presented as 95% confidence intervals (CI). All analysis was performed using Systat 13 (Systat, Inc., Evanston, Illinois).
3 |. RESULTS
3.1 |. Demographics
Thirty-four of the 75 subjects were female. Mean age was 15.0 ± 1.7 years and mean BMI was 22.0 ± 5.8 kg/m2 (mean ± SD). Mean BMI percentile was 53.8 ± 28.7. Sixty-two subjects were lean (BMI percentile<85) and nine were considered obese (BMI percentile≥95). Table 1 shows values for male and female subjects. Males had increased waist circumference and FFMI compared to females and females had increased percent body fat and FMI.
TABLE 1.
Demographic and body habitus values by sex (mean ± SD)
| Girls (n = 34) | Boys (n = 41) | |
|---|---|---|
| Age (years) | 15.4 ± 1.6 | 15.0 ± 1.7 |
| BMI (kg/m2) | 22.3 ± 5.8 | 21.7 ± 5.9 |
| BMI percentile | 53.9 ± 30.6 | 53.2 ± 27.4 |
| Waist circumference (cm) | 70.2 ± 13.1* | 74.6 ± 13.0* |
| Body fat (%) | 28.4 ± 8.2* | 20.2 ± 11.0* |
| FMI (kg/m2) | 6.70 ± 3.10* | 4.86 ± 4.61* |
| BMI (kg/m2) | 15.5 ± 2.2* | 16.9 ± 2.5* |
Abbreviations: BMI, body mass index; FMI, fat mass index.
P < .05 for sex difference.
3.2 |. Cardiovascular measures
Table 2 shows the cardiovascular measures in each sex. Females, for the most part, had increased cardiometabolic risk compared to males as indicated by lower Buckberg and Matsuda indices, higher neutrophil counts, and PAI-1 levels although they, also, had higher HDL concentrations and disposition index.
TABLE 2.
Cardiovascular measures according to sex (mean ± SE)
| Girls(n = 34) | Boys (n = 41) | |
|---|---|---|
| Heart rate (beats/min) | 69.7 ± 2.2 | 63.7 ± 2.1 |
| SBP (mmHg) | 113 ± 2 | 116 ± 2 |
| DBP (mmHg) | 65 ± 1 | 64 ± 1 |
| Reactive hyperemia (%) | −80.8 ± 1.3 | −82.5 ± 1.3 |
| AIx75 (%) | −1.03 ± 3.71 | −5.29 ± 3.32 |
| Buckberg index (%) | 145 ± 7* | 175 ± 7* |
| White blood cell (cell/μl) | 7.42 ± 0.30 | 7.02 ± 0.36 |
| Neutrophil (cell/μl) | 4.35 ± 0.27* | 3.74 ± 0.26* |
| IL-6 (mg/ml) | 0.572 ± 0.061 | 0.776 ± 0.132 |
| CRP (mg/L) | 2.40 ± 0.72 | 1.04 ± 0.37 |
| HDL (mg/dl) | 55 ± 3* | 49 ± 2* |
| LDL (mg/dl) | 80 ± 4 | 71 ± 3 |
| Triglycerides (mg/dl) | 81 ± 15 | 64 ± 5 |
| Insulin sensitivity | 3.67 ± 0.31* | 4.78 ± 0.41* |
| Insulin secretion (μU dl/mg ml) | 1.80 ± 0.19* | 0.96 ± 0.10* |
| Disposition index | 5.74 ± 0.59* | 4.33 ± 0.56* |
| Endothelin 1 (pg/ml) | 2.11 ± 0.09 | 2.02 ± 0.08 |
| PAI-1 (ng/ml) | 4.44 ± 0.54* | 3.19 ± 0.35* |
P < .05 for sex difference.
3.3 |. Cardiovascular measures and body habitus
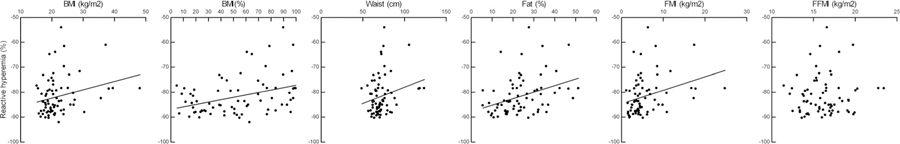
The reactive hyperemic response was not related to age or sex and worsened as BMI, BMI percentile, percent body fat, waist circumference and FMI increased (Figure 1). It was not related to FFMI. AIx75 was not related to age, sex or any measure of body habitus. The Buckberg index decreased with increasing percent body fat (β = −1.03; 95% CI: −2.04 to −0.02). Interestingly, including percent body fat in the regression analysis eliminated the sex difference in MAT.
FIGURE 1.
Relationships between reactive hyperemia and measures of body habitus in healthy, non-Hispanic white adolescents. BMI: β = 0.32, 95%CI: 0.05–0.58; BMI (%): β = 0.071, 95%CI: 0.027–0.12; waist circumference β = 0.14, 95% CI: 0.02–0.27; Fat (%) β = 0.22, 95% CI: 0.05–0.40; FMI: β = 0.45, 95% CI: 0.07–0.84; FFMI: β = 0.54, 95% CI = −0.27–1.34. BMI, body mass index
3.4 |. Cardiovascular measures and risk factors
Reactive hyperemia worsened with increased neutrophil count and fasting insulin concentration (Table 3: Models 1 and 2) but was not related to white blood cell count, CRP, IL6, lipids, MAT, IGI, DI, endothelin 1, or PAI-1. When percent body fat was included in the equation with either neutrophil count or fasting insulin concentration all relationships approached significance (Table 3: Models 3 and 4). If percent body fat, neutrophil count and fasting insulin were included, the relationship to neutrophil count was significant and the other relationships approached significance (Model 5). AIx75 was not related to any of the measures.
TABLE 3.
Beta coefficients for the relationship of Reactive Hyperemia and Buckberg subendocardial viability ratio to age, sex, and cardiovascular risk factors in healthy, non-Hispanic white adolescents
| Reactive hyperemia | Buckberg index | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Intercept | Independent variable | β | 95% confidence interval | Model | Intercept | Independent variable | β | 95% confidence interval |
| 1 | −81.7 | Age | −0.43 | −1.46–0.581 | 6 | 59.2 | Age | 5.53 | −0.32–11.4 |
| … | Sex | −0.60 | −4.06–2.86 | … | Sex | 23.2 | 2.44–43.9 | ||
| … | Neutrophils | 1.41 | 0.33–2.49 | … | %Body fat | −1.01 | −2.00–0.024 | ||
| 2 | −76.3 | Age | −0.44 | −1.45–0.58 | 7 | 4.46 | Age | 4.88 | −1.25–11.0 |
| … | Sex | −1.70 | −5.14–1.74 | … | Sex | 33.6 | 13.0–54.2 | ||
| … | Fasting insulin | 0.17 | 0.008–0.33 | … | Insulin sensitivity | 5.89 | 1.22–10.6 | ||
| 3 | −90.6 | Age | −0.27 | −1.36–0.82 | 8 | 244 | Age | 2.57 | −1.24–6.38 |
| … | Sex | 1.43 | −2.80–5.66 | … | Sex | 11.6 | −0.91–24.0 | ||
| … | % Body fat | 0.11 | −0.004–0.40 | … | Heart rate | −2.25 | −2.74–1.76 | ||
| … | Neutrophils | 0.58 | −0.012–2.32 | … | … | … | … | ||
| 4 | −86.6 | Age | −0.22 | −1.29–0.85 | 9 | 9.67 | Age | 5.24 | −1.16–11.6 |
| … | Sex | 0.54 | −3.66–4.75 | … | Sex | 28.3 | 4.34–52.3 | ||
| … | % Body Fat | 0.18 | −0.049–0.41 | … | %Body fat | −0.087 | −1.51–1.33 | ||
| … | Fasting insulin | 0.15 | −0.047–0.34 | … | Insulin sensitivity | 6.54 | 0.76–12.3 | ||
| 5 | −91.1 | Age | −0.26 | −1.34–0.82 | 10 | 252 | Age | 2.26 | −1.19–5.71 |
| … | Sex | 1.33 | −2.86–5.52 | … | Sex | 6.61 | −4.31–17.5 | ||
| … | % Body fat | 0.12 | −0.074–0.39 | … | Insulin sensitivity | 3.49 | 1.03–5.95 | ||
| … | Neutrophils | 1.17 | 0.018–2.33 | … | Heart Rate | −2.36 | −2.87–1.86 | ||
| … | Fasting insulin | 0.16 | −0.035–0.35 | … | … | … | … | ||
The Buckberg index was negatively related to WBC (β = −4.80; 95% CI: −9.53–0.07) and was positively related to MAT (Figure 2) but was not related to WBC, neutrophil, count, CRP, IL-6, fasting insulin, IGI, endothelin 1 or PAI-1. Sex remained a significant predictor of the Buckberg index when either percent body fat or MAT was individually included in the regression analysis (Table 3, Models 6 and 7). As both MAT and the Buckberg index were significantly related to percent body fat modeling was, also, done with both included as independent variables (Model 9). Interestingly, the relationship to MAT remained significant while the relationship to percent fat was no longer significant. Sex remained significant.
FIGURE 2.
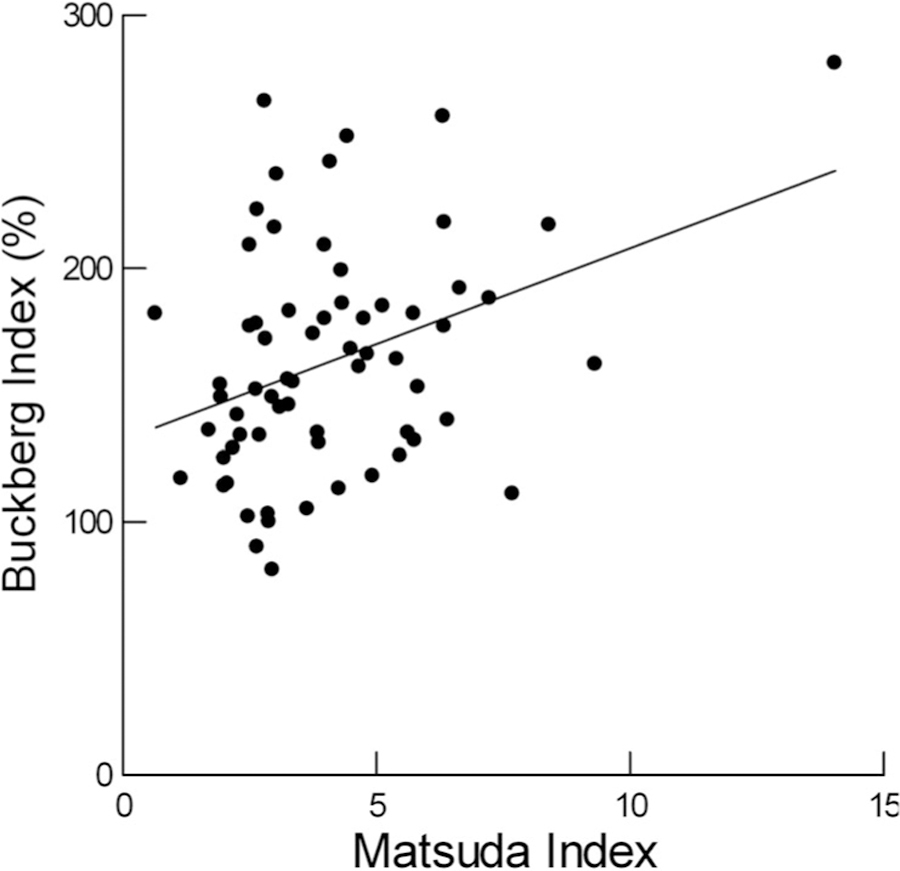
Relationship of Buckberg index to insulin sensitivity in healthy, non-Hispanic white adolescents. β = 5.89, 95% CI: 1.22–10.57
3.5 |. Cardiovascular risk factors and pulse and blood pressure
Reactive hyperemia and AIx75 were not significantly related to heart rate or blood pressure. The Buckberg index was negatively related to heart rate (Model 8) and inclusion of heart rate in the model eliminated the sex effect. When heart rate was included in the model for the Buckberg index both heart rate and MAT were significant predictors but sex was not (Model 10). Buckberg was not related to blood pressure.
4 |. DISCUSSION
If cardiovascular disease is to be prevented, it is important that we understand the early pathophysiology of the process in adolescents before overt disease and its complications may confound potentially mechanistic relationships. The current study adds to our understanding regarding the relationships of vascular and cardiac perfusion risk factors to traditional and non-traditional metabolic risk factors.
Endothelial dysfunction is thought to be one of the earliest predictors of future atherosclerotic disease, as the endothelium plays an important role in all phases of atherosclerosis, ranging from affecting tissue blood supply to clotting and inflammation.28 Our results demonstrate that the primary association of endothelial function in this age group is with obesity as we found consistent decreases in the reactive hyperemic response with increasing body size. Increasing fat mass appears to be the primary culprit for this relationship as the reactive hyperemic response was closely related to FMI, but not FFMI. In adults, the presence of insulin resistance in obesity may mediate this relationship.5–7 The relationship of endothelial function to insulin sensitivity in adolescents is less clear as a relationship has been found in some,29,30 but not all studies,17 and may be dependent on how endothelial function and insulin sensitivity are assessed.31 In the current study we did not find a relationship between endothelial function and insulin sensitivity. The Matsuda index has been found to closely correlate with results from hyperinsulinemic clamp in adolescents.32 Our small number of extremely obese, insulin resistant subjects may minimize the relationship between insulin sensitivity and endothelial function.
Interestingly, subjects with higher fasting insulin levels had poorer reactive hyperemic responses in spite of the lack of relationship to either MAT or IGI from the OGTT. This relationship continued to approach significance even when adiposity was included in the model. Potential reasons for this include the fact that fasting insulin is more closely related to hepatic than peripheral or total body insulin sensitivity33 and that chronic high fasting insulin may have a more deleterious effect on endothelial function than increased postload responses. In addition, we only studied non-Hispanic white subjects. We, previously, found a significant relationship between insulin secretion in response to intravenous glucose and endothelial function in black, but not white, subjects.17
The decreasing reactive hyperemic response with increasing neutrophil count in adolescents is an important new finding. This relationship continued to approach significance even when percent body fat was included in the model, indicating the relationship is not solely due to obesity. Increased white cell counts have been shown to predict arterial stiffness in adults in a variety of conditions34 and increased neutrophil counts predict future cardiovascular disease in adults.35 The increased neutrophil counts are thought to be evidence of increased endothelial inflammatory damage. The relationship between reactive hyperemia and neutrophils in the current study indicates that increased neutrophil count in adolescents is an indicator of increased cardiovascular risk although we did not find a relationship to AIx75. We did not find relationships between reactive hyperemia or AIx75 and either of the two inflammatory biochemical markers measured, IL-6 and CRP. This may indicate that we used the wrong markers. Tomosa et al.,30 however, found no relationship between reactive hyperemia and tissue necrosing factor-α, but did find a relationship for AIx75. It is, also, possible that these relationships may not develop until a later age. Both of the measured markers in our study are significantly related to arterial stiffness markers in adults.34 Kapiotis et al.10 found higher CRP and IL-6 in obese and morbidly obese children. It is, again, possible these relationships might have been found had we included a larger number of obese subjects.
Another important new finding is the significant relationship between the Buckberg subendocardial viability ratio and insulin sensitivity, as measured using OGTT and the Matsuda Index. The Buckberg index assesses the ratio of oxygen supply to oxygen demand by the myocardium22 with increasing ratios indicating reduced cardiac risk. Koshdel et al.3 found reduced Buckberg indices in insulin resistant adult men with the metabolic syndrome, but did not report a direct relationship between the Buckberg index and insulin sensitivity. Franssen et al.19 reported a direct relationship of insulin resistance and/or hyperinsulinemia to several echocardiographic parameters of diastolic dysfunction in a mixed group of obese and lean adolescence and also a relationship to delayed heart rate recovery following exercise.
Importantly, the relationship between the Buckberg index and insulin sensitivity remained significant even when percent body fat was included in the equation. In fact, the relationship to percent body fat was no longer significant in this model. The reasons for the relationship between the Buckberg index and insulin sensitivity are not immediately clear. One possibility might be both being indicators of increased physical fitness which we did not directly measure. The Buckberg index was closely and negatively related to heart rate which might support this possibility as reduced resting heart rate is associated with improved physical fitness.36 Peterson et al.37 found a significant relationship between physical inactivity and increased cardiometabolic risk in adolescents. However, we found that both insulin sensitivity and resting heart rate significantly predicted the Buckberg index when both were included as independent variables. This suggests that the association between insulin sensitivity and the Buckberg index are, at least partially, independent of physical fitness.
Previous studies have found varied sex differences in cardiovascular risk factors in adolescents consistent with those found in this study.17,37–39 Peterson et al.37 compiled an overall metabolic risk score, however, and found no sex difference in spite of differences in individual factors. This is consistent with our findings of differences suggestive of both increased and decreased risk in both sexes. Our study adds to this by demonstrating lower cardiac oxygen supply: demand ratio in females. Differences in physical fitness may be partially responsible as the sex difference was no longer significant when heart rate was included in the model. This is unlikely to be the only cause, however, as the 95% confidence intervals were still strongly positive for a sex effect. When both heart rate and insulin sensitivity were included in the model the sex effect was clearly lost. This may be due to loss of statistical power, although as mentioned the relationships to heart rate and insulin sensitivity remained significant. The fact that some females were using oral contraceptives may confound our findings regarding insulin sensitivity although previous studies have demonstrated lower insulin sensitivity in adolescent females without the use oral contraceptives.20 No sex differences in vascular function were found.
Cross-sectional relationships do not demonstrate causality and our study was limited to non-Hispanic white adolescents, so the results may not be applicable to other racial groups. Importantly, our results do demonstrate the adverse effects of increased fat mass, inflammation, and insulin resistance on functional cardiovascular risk, which are present even in adolescents before the development of cardiovascular disease.
ACKNOWLEDGEMENTS
The project was supported by a grant from the Great Rivers Affiliate of the American Heart Association and Award Number Grant UL1TR001070 from the National Center for Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health. The authors thank the nursing staff at the Clinical Research Center at the Wexner Medical Center of The Ohio State University and the staff in the Section of Endocrinology at Nationwide Children’s Hospital for their support in this project.
Funding information
American Heart Association; National Center for Advanced Translational Sciences, Grant/Award Number: UL1TR001070
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest.
REFERENCES
- 1.Enos WF, Holmes RH, Beyer J. Landmark article, July 18, 1953: coronary disease among United States soldiers killed in action in Korea. Preliminary report. By William F. Enos, Robert H. Holmes and James Beyer. JAMA 1986;256(20):2859–2862. [DOI] [PubMed] [Google Scholar]
- 2.McGill HC Jr, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr 2000;72(5 Suppl):1307S–1315S. [DOI] [PubMed] [Google Scholar]
- 3.Khoshdel AR, Eshtiaghi R. Assessment of arterial stiffness in metabolic syndrome related to insulin resistance in apparently healthy men. Metab Syndr Relat Disord 2019;17(2):90–96. [DOI] [PubMed] [Google Scholar]
- 4.Yki-Jarvinen H, Westerbacka J. Insulin resistance, arterial stiffness and wave reflection. Adv Cardiol 2007;44:252–260. [DOI] [PubMed] [Google Scholar]
- 5.Ardigo D, Franzini L, Valtuena S, Monti LD, Reaven GM, Zavaroni I. Relation of plasma insulin levels to forearm flow-mediated dilatation in healthy volunteers. Am J Cardiol 2006;97(8):1250–1254. [DOI] [PubMed] [Google Scholar]
- 6.Jonkers IJAM, van de Ree MA, Smelt AHM, et al. Insulin resistance but not hypertriglyceridemia per se is associated with endothelial dysfunction in chronic hypertriglyceridemia. Cardiovasc Res 2002;53(2): 496–501. [DOI] [PubMed] [Google Scholar]
- 7.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation 2005;112(1):32–38. [DOI] [PubMed] [Google Scholar]
- 8.Arcaro G, Cretti A, Balzano S, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation 2002;105(5): 576–582. [DOI] [PubMed] [Google Scholar]
- 9.Campia U, Sullivan G, Bryant MB, Waclawiw MA, Quon MJ, Panza JA. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol Heart Circ Physiol 2004; 286(1):H76–H82. [DOI] [PubMed] [Google Scholar]
- 10.Kapiotis S, Holzer G, Schaller G, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol 2006; 26(11):2541–2546. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Gungor N, Bacha F, Arslanian S. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 2007;30(8):2091–2097. [DOI] [PubMed] [Google Scholar]
- 12.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics 2006;117 (5):1560–1567. [DOI] [PubMed] [Google Scholar]
- 13.Lentferink YE, Kromwijk LAJ, van der Aa MP, Knibbe CAJ, van der Vorst MMJ. Increased arterial stiffness in adolescents with obesity. Glob Pediatr Health 2019;6:2333794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mikola H, Pahkala K, Niinikoski H, et al. Cardiometabolic determinants of carotid and aortic Distensibility from childhood to early adulthood. Hypertension 2017;70(2):452–460. [DOI] [PubMed] [Google Scholar]
- 15.Joo Turoni C, Maranon RO, Felipe V, et al. Arterial stiffness and endothelial function in obese children and adolescents and its relationship with cardiovascular risk factors. Horm Res Paediatr 2013;80(4): 281–286. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman RP. Effect of adolescent obesity on cardiometabolic risk in African-Americans and Caucasians. ISRN Obes 2012;2012 https://www.hindawi.com/journals/isrn/2012/603205/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duck MM, Hoffman RP. Impaired endothelial function in healthy African-American adolescents compared with Caucasians. J Pediatr 2007;150(4):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryder JR, Dengel DR, Jacobs DR Jr, Sinaiko AR, Kelly AS, Steinberger J. Relations among adiposity and insulin resistance with flow-mediated dilation, carotid intima-media thickness, and arterial stiffness in children. J Pediatr 2016;168:205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franssen WMA, Beyens M, Hatawe TA, et al. Cardiac function in adolescents with obesity: cardiometabolic risk factors and impact on physical fitness. Int J Obes 2019;43(7):1400–1410. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman RP, Vicini P, Sivitz WI, Cobelli C. Pubertal adolescent male-female differences in insulin sensitivity and glucose effectiveness determined by the one compartment minimal model. Pediatr Res 2000;48(3):384–388. [DOI] [PubMed] [Google Scholar]
- 21.Hinriksdottir G, Tryggvadottir A, Olafsdottir AS, Arngrimsson SA. Fatness but not fitness relative to the fat-free mass is related to C-reactive protein in 18 year-old adolescents. PLoS ONE 2015;10(6): e0130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman JI, Buckberg GD. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc 2014;3(1):e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Onat A, Uzunlar B, Hergenc G, et al. Cross-sectional study of complement C3 as a coronary risk factor among men and women. Clin Sci 2005;108(2):129–135. [DOI] [PubMed] [Google Scholar]
- 24.Wei JN, Li HY, Sung FC, et al. Obesity and clustering of cardiovascular disease risk factors are associated with elevated plasma complement C3 in children and adolescents. Pediatr Diabetes 2012;13(6): 476–483. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez C, Rodriguez B, Losada E, Corraliza L, Garcia-Ramirez M, Simo R. Normoalbuminuric type 1 diabetic patients with retinopathy have an impaired tubular response to desmopressin: its relationship with plasma endothelin-1. J Clin Endocrinol Metab 2009;94(6):2060–2065. [DOI] [PubMed] [Google Scholar]
- 26.Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care 2009;32(2):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman RN, Stefanovski D, Kim SP. Systems analysis and the prediction and prevention of type 2 diabetes mellitus. Curr Opin Biotechnol 2014;28:165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuzawa Y, Lerman A. Endothelial dysfunction and coronary artery disease: assessment, prognosis, and treatment. Coron Artery Dis 2014;25(8):713–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miniello VL, Faienza MF, Scicchitano P, et al. Insulin resistance and endothelial function in children and adolescents. Int J Cardiol 2014; 174(2):343–347. [DOI] [PubMed] [Google Scholar]
- 30.Tomsa A, Klinepeter Bartz S, Krishnamurthy R, Krishnamurthy R, Bacha F. Endothelial function in youth: a biomarker modulated by adiposity-related insulin resistance. J Pediatr 2016;178:171–177. [DOI] [PubMed] [Google Scholar]
- 31.Brar PC, Patel P, Katz S. The relationship between insulin resistance and endothelial dysfunction in obese adolescents. J Pediatr Endocrinol Metab 2017;30(6):635–642. [DOI] [PubMed] [Google Scholar]
- 32.Henderson M, Rabasa-Lhoret R, Bastard JP, et al. Measuring insulin sensitivity in youth: how do the different indices compare with the gold-standard method? Diabetes Metab 2011;37(1):72–78. [DOI] [PubMed] [Google Scholar]
- 33.Hoffman RP. Indices of insulin action calculated from fasting glucose and insulin reflect hepatic, not peripheral, insulin sensitivity in African-American and Caucasian adolescents. Pediatr Diabetes 2008; 9(3 Pt 2):57–61. [DOI] [PubMed] [Google Scholar]
- 34.Mozos I, Malainer C, Horbanczuk J, et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol 2017;8: 1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah AD, Denaxas S, Nicholas O, Hingorani AD, Hemingway H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: a caliber cohort study. J Am Coll Cardiol 2017;69(9):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarganas G, Schaffrath Rosario A, Neuhauser HK. Resting heart rate percentiles and associated factors in children and adolescents. J Pediatr 2017;187:174–181.e173. [DOI] [PubMed] [Google Scholar]
- 37.Peterson MD, Saltarelli WA, Visich PS, Gordon PM. Strength capacity and cardiometabolic risk clustering in adolescents. Pediatrics 2014; 133(4):e896–e903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyai N, Arita M, Miyashita K, Morioka I, Takeda S. The influence of obesity and metabolic risk variables on brachial-ankle pulse wave velocity in healthy adolescents. J Hum Hypertens 2009;23(7): 444–450. [DOI] [PubMed] [Google Scholar]
- 39.Orri JC, Carter SR, Howington EB. Gender comparison of C-reactive protein and cardiovascular disease risk in college students and intercollegiate athletes. J Sports Med Phys Fitness 2010;50(1):72–78. [PubMed] [Google Scholar]