To the Editor.
The basolateral amygdala (BLA) forms a triad with the medial prefrontal cortex (mPFC) and the hippocampus (HPC) to process incoming stimuli and orchestrate emotional responses[1]. In post-traumatic stress disorder (PTSD), a dysfunction in this triad is characterized by a deficit of mPFC inhibition of the BLA[2]. As a result, metabolic hyperactivity of the BLA characterize symptomatic episodes in PTSD patients[3]. We are using deep brain stimulation (DBS) in PTSD subjects to modulate the activity of the BLA and promote fear extinction[4]. Targeted focal neuromodulation offers a unique opportunity to gain new insights into the circuitry of emotion in PTSD patients. In this study, we included two patients with refractory PTSD who underwent bilateral BLA-DBS as part of our clinical trial with the aim of mapping the behavioral responses to acute amygdala stimulation. Unlike rodent or non-human primate models, patients can effectively communicate their feelings and emotions in real-time allowing us to link a psychological percept to a specific focal stimulation.
All procedures were performed under approval of the institutional board review of the VAGLAHS. Patients provided informed consent for all parts of the study including surgery and data collection. To identify the behavioral responses of stimulation of the amygdala, we tested each of the four contacts of the DBS electrode increasing the amplitude to a maximum of 5V (66 stimulation trials in the two patients). The stimulated region encompassed the BLA (basal, basal accessory, and lateral nucleus of the amygdala), central nucleus of the amygdala, and HPC. Since the behavioral responses in PTSD patients can have unique features, these were captured by an experienced psychiatrist who monitored the patients while they underwent telemetry and video-EEG monitoring one month after implantation. These responses were categorized as positive (including happiness, euphoria, relaxation, or any other positive states), negative (anxiety, anger, or any other distressing responses), and autonomic symptoms. The anxiety was rated in each stimulation trial using the subjective units of distress scale (SUDS).
To identify stimulation ‘hotspots’ associated with behavioral responses within the whole stimulated area, we first modelled the electrical field of each stimulation trial by generating volumes of tissue activated (VTA) based on the stimulation parameters. These VTAs were generated in the standard MNI space. Secondly, we performed a non-parametric permutation approach of the VTAs to find clusters associated with each behavioral response in each subject. We used a single group-average general linear model with an additional covariate (each behavioral response). We found significant clusters associated with positive, negative, and autonomic responses in each patient along the entire stimulated area.
To identify the amygdala subregions associated with the behavioral responses, we performed histological segmentation of the amygdala on the ICBM2009 template using Freesurfer software (Boston, MA)[5]. The clusters were then overlaid with the amygdala segmentation. The clusters associated with positive responses mainly involved the basal nucleus, whereas the involvement of the lateral nucleu and the HPC was associated with negative responses (Fig 1). These negative responses, mainly anger, were also associated with relatively higher voltages. Anxiety was particularly associated with involvement of the subiculum, presubiculum, and the CA1. The CA1 hippocampal sub-field has been reported to influence the retrieval of contextual memories and the acquisition of fear extinction[6]. We found that anxiety levels were higher when stimulating the hippocampal area and this can be explained by a possible worsening of retrieval of contextual information caused by the electrical stimulation. This is in line with experimental evidence suggesting that reciprocal connections between the BLA and the HPC are crucial in anxiety-related behaviors[6,7]. The processing of fear requires the coordination of network activity, specifically within the emotional triad (i.e. the BLA, the HPC and the mPFC). Each module of the triad can exert its influence on the network when a stimulus is processed[1]. On the other hand, the autonomic responses were located dorsoventrally along the entire amygdala involving the basolateral complex but also the accessory basal nucleus, and central nucleus of the amygdala.
Figure 1: Structural mapping of the behavioral responses after DBS of the basolateral amygdala.
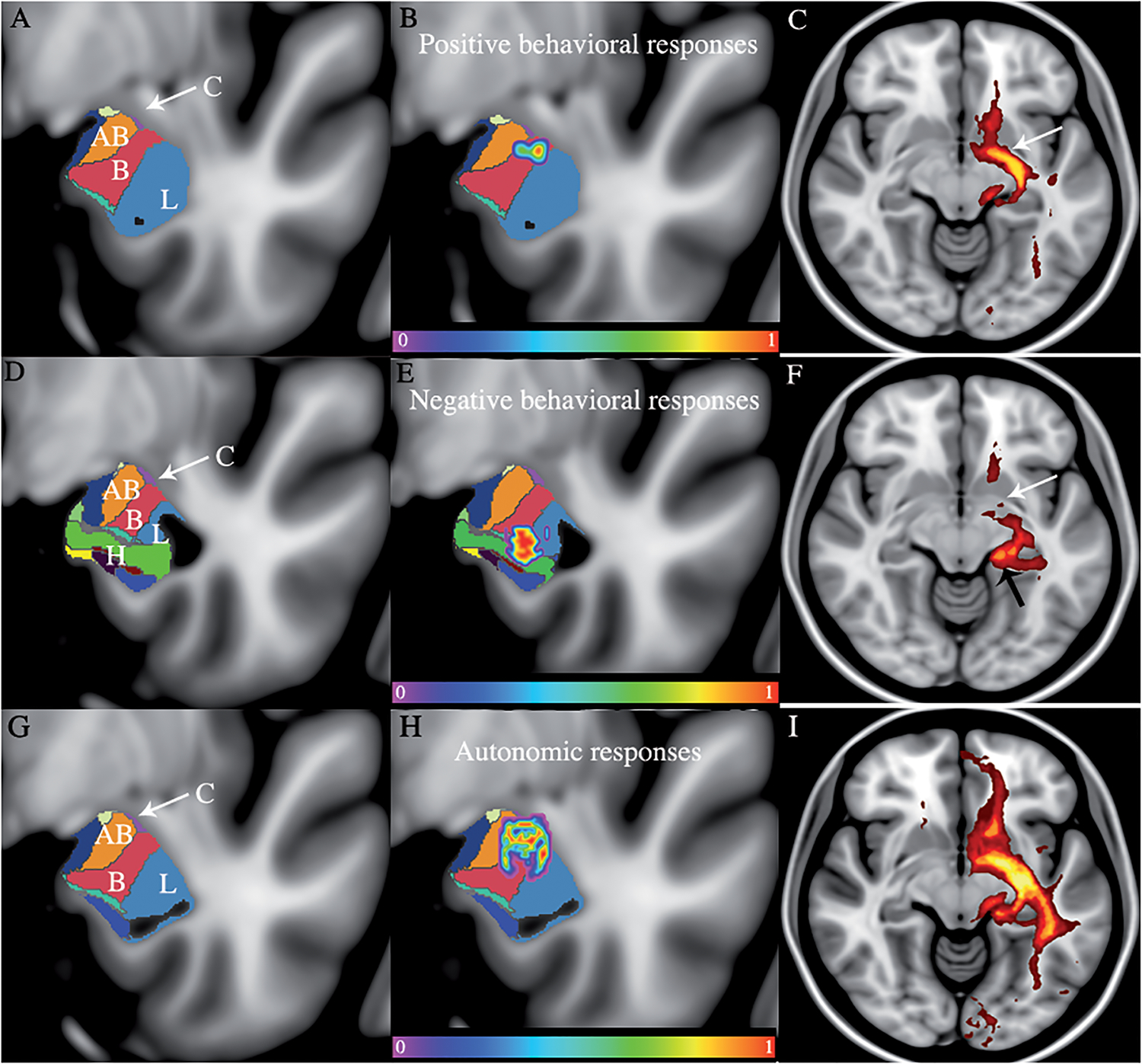
The averaged clusters of both subjects were overlaid with the histological segmentation of the amygdala. A-D-G. The entire stimulated area included the accessory basal nucleus (AB), the basal nucleus (B), the lateral nucleus (L), the central nucleus (C with arrow), and the hippocampus (H). B. The averaged cluster associated with the positive behavioral responses is located in the basal nucleus. C. The positive behavioral responses were associated with involvement of the ventral amygdalofugal pathway (VAF) E. The averaged cluster associated with negative behavioral responses is mainly located in the lateral nucleus and the hippocampal area. F. The negative responses mainly involved the stria terminalis (ST) H. The averaged cluster associated with autonomic responses is located from the most dorsal to the central part of the amygdala involving the white matter dorsal to the amygdala, the basal, accessory basal, lateral, and the central nucleus of the amygdala. I. The autonomic responses involved the ST and the VAF. The MNI coordinates of the coronal slices are as follows: Positive behavioral responses B: y=−5. Negative behavioral responses E: y=−8. Autonomic behavioral responses H: y=−6.
Since one of the mechanism of actions of DBS is the modulation of upstream and downstream axonal pathways, we investigated the pathways involved by each of the significant clusters associated with the behavioral responses. We used the imaging data of ten randomly-selected subjects from the Nathan-Klein Institute neuroimaging repository. This analysis revealed that the behavioral responses were mainly segregated along two distinct pathways. Positive emotions were mainly associated with involvement of the ventral amygdalofugal (VAF) pathway. Negative emotions and in particular worsened anxiety, were mainly associated with the involvement of the stria terminalis (ST). This finding is consistent with findings from other authors where the ST was inadvertently stimulated chronically[8]. Autonomic symptoms were associated with involvement of both the VAF and the ST (Supplementary Fig). The VAF pathway originates in the dorsomedial part of the amygdala and travels through the substantia innominata and anterior perforated substance. This pathway is then distributed to the mPFC, lateral hypothalamus, and mediodorsal thalamus, nucleus accumbens (Nacc), among other targets. On the other hand, the ST emerges in the caudomedial aspect of the amygdala and it sends efferents to the ventromedial hypothalamus and the bed nucleus of the stria terminalis.
In this work, we found that PTSD patients experienced happiness and anxiety reduction in response to acute stimulation of the basal nucleus. We saw the emergence of two pathways with distinct emotional responses. Stimulation of regions connected to the ST are more likely to worsen anxiety and fear. Conversely, stimulation of the VAF pathway, which is heavily connected to the mPFC, is more likely to produce pleasant sensations and fear extinction. This mPFC-BLA connection is known to be critical in regulating fear. In particular, theta-gamma phase amplitude coupling (PAC) between mPFC and BLA has been identified as a potential competitive switch in the fear network[9]. The region with the dominant theta rhythm can predict the outcome of a stimulus with the mPFC-theta signaling extinction and the BLA theta leading to fear. BLA neuromodulation may play a role in desynchronizing the underlying theta-gamma PAC that orchestrates fear, leading to anxiety reduction and happiness in PTSD patients. Also, connections between the BLA and the Nacc, through the VAF pathway, have been implicated in positive reinforcement and the formation of cue-reward associations[10].
In summary, stimulation of the basal nucleus may induce positive emotional responses in patients with PTSD. This nucleus is connected to the mPFC through the VAF pathway. Spread of stimulation to adjacent areas of this nucleus and the involvement of the ST pathway may induce negative effects such as anxiety or anger.
Supplementary Material
Acknowledgements:
The authors would like to thank the financial support from Casa Colina Centers for Rehabilitation. The authors would also like to acknowledge the support from NIH funding under brain initiative/UH3 program, award: 1UH3NS107673-01A1.
Footnotes
Conflict of Interests
Dr. Bari disclosed being consultant of Medtronic. All other authors declare no biomedical financial interests or potential conflicts of interest.
References
- [1].Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Groblewski PA, Stafford JM. When the Medial Prefrontal Cortex Fails: Implications for Extinction and Posttraumatic Stress Disorder Treatment. J Neurosci 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 2005. [DOI] [PubMed] [Google Scholar]
- [4].Koek RJ, Langevin J-P, Krahl SE, Chen JWY, Kulick AD, Schwartz HN, et al. Amygdala DBS for PTSD: 2 years of observations on the first case. Brain Stimul 2017. [Google Scholar]
- [5].Saygin ZM, Kliemann D, Iglesias JE, van der Kouwe AJW, Boyd E, Reuter M, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ji J, Maren S. Differential roles for hippocampal areas CA1 and CA3 in the contextual encoding and retrieval of extinguished fear. Learn Mem 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Piacentini S, Romito L, Franzini A, Granato A, Broggi G, Albanese A. Mood disorder following DBS of the left amygdaloid region in a dystonia patient with a dislodged electrode. Mov Disord 2008. [DOI] [PubMed] [Google Scholar]
- [9].Stujenske JM, Likhtik E, Topiwala MA, Gordon JA. Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beyeler A, Namburi P, Glober GF, Simonnet C, Calhoon GG, Conyers GF, et al. Divergent Routing of Positive and Negative Information from the Amygdala during Memory Retrieval. Neuron 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


