Abstract
Background
Erythropoiesis‐stimulating agents (ESAs) reduce anemia in cancer patients and may improve quality of life, but there are concerns that ESAs might increase mortality.
Objectives
Our objectives were to examine the effect of ESAs and identify factors that modify the effects of ESAs on overall survival, progression free survival, thromboembolic and cardiovascular events as well as need for transfusions and other important safety and efficacy outcomes in cancer patients.
Search methods
We searched the Cochrane Library, Medline, Embase and conference proceedings for eligible trials. Manufacturers of ESAs were contacted to identify additional trials.
Selection criteria
We included randomized controlled trials comparing epoetin or darbepoetin plus red blood cell transfusions (as necessary) versus red blood cell transfusions (as necessary) alone, to prevent or treat anemia in adult or pediatric cancer patients with or without concurrent antineoplastic therapy.
Data collection and analysis
We performed a meta‐analysis of randomized controlled trials comparing epoetin alpha, epoetin beta or darbepoetin alpha plus red blood cell transfusions versus transfusion alone, for prophylaxis or therapy of anemia while or after receiving anti‐cancer treatment. Patient‐level data were obtained and analyzed by independent statisticians at two academic departments, using fixed‐effects and random‐effects meta‐analysis. Analyses were according to the intention‐to‐treat principle. Primary endpoints were on study mortality and overall survival during the longest available follow‐up, regardless of anticancer treatment, and in patients receiving chemotherapy. Tests for interactions were used to identify differences in effects of ESAs on mortality across pre‐specified subgroups. The present review reports only the results for the primary endpoint.
Main results
A total of 13933 cancer patients from 53 trials were analyzed, 1530 patients died on‐study and 4993 overall. ESAs increased on study mortality (combined hazard ratio [cHR] 1.17; 95% CI 1.06‐1.30) and worsened overall survival (cHR 1.06; 95% CI 1.00‐1.12), with little heterogeneity between trials (I2 0%, p=0.87 and I2 7.1%, p=0.33, respectively). Thirty‐eight trials enrolled 10441 patients receiving chemotherapy. The cHR for on study mortality was 1.10 (95% CI 0.98‐1.24) and 1.04; 95% CI 0.97‐1.11) for overall survival. There was little evidence for a difference between trials of patients receiving different cancer treatments (P for interaction=0.42).
Authors' conclusions
ESA treatment in cancer patients increased on study mortality and worsened overall survival. For patients undergoing chemotherapy the increase was less pronounced, but an adverse effect could not be excluded.
Plain language summary
Anti‐anemia drugs shorten survival for some cancer patients
People with cancer may develop a blood problem called anemia, due to the treatment or from the disease itself. They will have very low levels of healthy red blood cells, causing additional health problems. For years, doctors have tried to prevent or treat anemia with injections of erythropoiesis stimulating agents (ESAs) in order to spare cancer patients the many serious harms associated with a red‐blood cell transfusion (such as hepatitis, transfusion‐related acute lung injury, infection). Earlier reviews of the research showed that ESA treatment reduces the need for transfusion but, in recent years, several studies have shown that ESAs themselves cause harm. The drug may, for example, stimulate tumor growth and cause potentially fatal blood clots. In 2007, new studies reported that ESAs shortens survival in people with breast, non‐small cell lung, head and neck, lymphoid and cervical cancers.
A new systematic review was needed to evaluate the old and the new evidence together and determine the impact of ESAs on survival in cancer patients to see if there are groups of patients who are at increased or decreased risk compared to the average. To accomplish this the authors of this meta‐analysis conducted an in‐depth assessment of the individual patient data generated by the care of nearly 14,000 patients from 53 trials conducted worldwide. Data on each of these patients were provided by three companies that make ESAs: Amgen, Johnson & Johnson, and Roche, and by several independent researchers. (The drug companies, however, had no role in conducting the meta‐analysis.) The trials investigated one of two types of ESAs, epoetin or darbepoetin, and compared the use of one of these drugs plus red blood cell transfusion (as needed), with red blood cell transfusion alone (as needed). Most patients were given their treatment while undergoing anti‐cancer therapy (chemotherapy and/or radiotherapy); but others received the treatment after they had completed their anti‐cancer therapy. Some patients already had anemia; others were treated in order to prevent it. The patients had many different forms of cancer and many different anti‐cancer treatments.
The authors of this new meta‐analysis concluded that ESA treatment shortens survival. They could not identify with certainty any subgroup of patients at either increased or decreased risk of dying when taking ESAs. With their doctors' help, cancer patients should consider the risks of taking ESA against the risks of a blood transfusion. Be aware, however, that uncertainties remain about the magnitude of each.
Background
Description of the condition
Tumor anemia
Anemia is defined as a deficiency in red blood cells (RBC) and is a widely prevalent complication among cancer patients (Ludwig 2004). A commonly used classification of anemia according to hemoglobin levels (National Cancer Institute) is shown in the following table (Groopman 1999):
| Category | Women | Men |
| Grade 0 (normal) | 12.0 to 16.0 g/dl | 14.0 to 18.0 g/dl |
| Grade 1 (mild) | 10.0 to <12.0 g/dl | 10.0 to <14.0 g/dl |
| Grade 2 (moderate) | 8.0 to <10.0 g/dl | 8.0 to <10.0 g/dl |
| Grade 3 (severe) | 6.5 to <8.0 g/dl | 6.5 to <8.0 g/dl |
| Grade 4 (life threatening) | <6.5 g/dl | <6.5 g/dl |
The pathophysiology of tumor anemia is multifactorial (Spivak 2005). Tumor‐associated factors such as tumor bleeding, hemolysis, deficiency in folic acid and vitamin B12, can be acute or chronic. In the advanced stages of hematological malignancies, bone marrow involvement often leads to progressive anemia. In addition, interaction between tumor‐cell populations and the immune system can lead to the release of cytokines, especially interferon‐gamma, interleukin‐1 and tumor necrosis factor. This disrupts endogenous erythropoietin synthesis in the kidney and suppresses differentiation of erythroid precursor cells in the bone marrow. As a result, patients with tumor anemia may have relatively low levels of erythropoietin for the grade of anemia observed (Spivak 2005). Moreover, activation of macrophages can lead to a shorter erythrocyte half‐life and a decrease in iron utilization. Cytostatic therapy and radiation further aggravates anemia in cancer patients. Platinum‐based chemotherapy regimens may diminish endogenous erythropoietin production by damaging renal tubular cells (Wood 1995) and myelotoxic anticancer drugs can compromise erythroid precursor cells. As a consequence, dose‐intensified treatment regimens or shortened treatment intervals as well as multimodal therapies are associated with a higher degree of anemia. Mild or moderate (grade 1 and 2) anemia in patients with solid cancers may affect about 60% of patients after platinum‐based chemotherapy (Groopman 1999). Severe (grade 3) anemia in elderly patients with hematological malignancies may occur in up to 74% in patients with Non‐Hodgkin lymphoma after standard CHOP treatment (Groopman 1999). In addition, some of the newer chemotherapeutic agents such as taxanes or vinorelbine are strongly myelosuppressive and frequently cause severe anemia (Groopman 1999).
The clinical manifestation and severity of anemia can vary considerably among individual patients. Mild to moderate anemia can typically cause signs and symptoms such as headache, palpitations, tachycardia and shortness of breath. Chronic anemia can result in severe organ damage affecting the cardiovascular system, immune system, lungs, kidneys and the central nervous system (Ludwig 2001). In addition to physical symptoms, the subjective impact of cancer‐related anemia on quality of life (QoL), mental health and social activities may be substantial. Clinical studies have reported correlations between hemoglobin (Hb) levels and QoL (Cella 1997; Holzner 2002; Lind 2002). A common anemia‐related problem is fatigue, which impairs the patient’s ability to perform normal daily activities (Ludwig 2001; Vogelzang 1997; Cramp 2008).
Another aspect of anemia in patients with malignant disease is the effect on the tumor itself. For several cancers, including cervical carcinoma, head and neck, prostate, bladder and lung cancer as well as lymphoma, anemia is known to be a factor associated with a worse prognosis (Caro 2001). This is partly due to confounding factors because advanced cancers usually present with lower Hb levels at diagnosis compared with early‐stage cancers and also have poorer survival outcomes. Besides this, one causal explanation might be the improved oxygenation of tumor tissue at higher Hb levels. Since tumor cells become resistant by tumor hypoxia, improved oxygenation may prevent hypoxia maintaining tumor cells sensitive to radiation and most cytostatic drugs. Due to an abnormal microenvironment, solid tumor tissue is often hypoxic. Hypoxia may be more prevalent in anemic patients than in patients with normal Hb levels (Vaupel 2005). Tumor hypoxia may impair the effectiveness of radiotherapy and oxygen‐dependent chemotherapies (Vaupel 2005; Schrijvers 1999; Hockel 1993). Anemia is associated with a poor outcome in patients treated with radiotherapy, most likely because anemia results in poor tumor oxygenation (Vaupel 2001). Radiobiological studies have shown that tumor hypoxia leads to less radiation induced cytotoxic free radicals resulting in less radiation‐induced DNA damage and tumor cell kill. Tumor oxygenation is also impaired by hemoglobin levels >14 g/dl in women and >15 g/dl in men which result in increased viscosity and a drop in nutritive perfusion (Vaupel 2002). It was therefore suggested to keep the hemoglobin levels during radiotherapy within a potentially optimal range of 12‐14 g/dl for women and 13‐15 g/dl for men in order to achieve maximum tumor oxygenation (Vaupel 2002). These observations generated the hypothesis that strategies to diminish cancer‐related anemia might not only alleviate anemia‐related symptoms but also improve tumor response and overall survival.
Description of the intervention
Recombinant human erythropoietins
Conventionally, blood transfusions are used to treat severe cancer‐related anemia. Homologous blood transfusion is the fastest method to alleviate symptoms. Potential complications include transmission of infectious diseases, transfusion reactions, alloimmunization, lung injury, over‐transfusion and immune modulation with possible adverse effects on tumor growth (Goodnough 2005; Toy 2005). The risks of transfusion‐related transmissions are 1:180,000 per units of blood transfused for hepatitis B virus, 1:1,600,000 for hepatitis C virus and 1:1,900,000 for HIV in the US (Goodnough 2003).
Short and long‐acting preparations of recombinant human erythropoietins (ESAs) offer an alternative treatment option. Human erythropoietin is an acidic glycoprotein hormone and the primary regulator of human erythropoiesis. Human erythropoietin is mainly synthesized in the kidney and to a minor degree in the liver (Lai 1986; Koury 1991; Koury 1988). Tissue hypoxia triggers the synthesis and release of erythropoietin into the plasma. The effects of erythropoietin in the bone marrow are mediated by a specific surface erythropoietin receptor located mainly on RBC precursor cells (D'Andrea 1989). Erythropoietin has two major functions: stimulating proliferation of erythroid progenitor cells and maintaining their viability (Koury 1990). Recombinant human erythropoietin was first approved for the treatment of anemia in patients with chronic renal disease. In 1993, the use of erythropoietin was approved by the FDA for the treatment of anemia in cancer patients. Three different recombinant erythropoietins are available to date: epoetin alfa (Procrit®, OrthoBiotech; Epogen®, Amgen), epoetin beta (NeoRecormon®, Roche) and darbepoetin alfa (Aranesp®, Amgen). All three erythropoietins have similar clinical efficacy (Halstenson 1991; Storring 1998; Glaspy 2005). Another substance called CERA® (Continuous Erythropoietin Receptor Activator, Roche) is currently being investigated in phase I and II clinical trials. Epoetin delta (Shire plc) differs from recombinant erythropoietins as it is produced in a human cell line using gene‐activation technology. A randomized controlled trial of epoetin delta was recently presented (Zajda 2007).
How the intervention might work
Efficacy and safety
Multiple studies and subsequent meta‐analyses have demonstrated that ESA treatment increases hemoglobin (Hb) levels and reduces the proportion of patients receiving red blood cell transfusions in cancer patients (Seidenfeld 2001; Bottomley 2002; Clark 2002; Bohlius 2006; Sehata 2007). In our previous meta‐analysis including 42 studies with 6,510 patients the relative risk to receive RBC transfusions was 0.67 [95% confidence interval (CI) 0.60, 0.68] (Bohlius 2006).
Concern regarding the impact of ESAs on survival has been raised by several studies in oncology and hematology patients that have reported increased mortality in patients treated with ESAs (Leyland‐Jones 2003; Henke 2003; Smith 2008; Hedenus 2003; Overgaard 2007; Wright 2007; Goss 2005. Three clinical studies reported increased tumor progression or death due to tumor progression in patients receiving ESAs (Henke 2003; Leyland‐Jones 2003; Overgaard 2007). However, this effect was not consistently observed and several studies did not show an increased risk for tumor progression for patients receiving ESAs (Machtay 2007; Chang 2005; EPO‐GBR‐7; Moebus 2007; Hedenus 2003). In addition, an increased risk for thromboembolic events has been consistently observed in various patient populations (Leyland‐Jones 2003; Henke 2003; Thomas 2008; Goss 2005; Rosenzweig 2004; Smith 2008).
However, because erythropoietin receptors have been detected in numerous cancers (Arcasoy 2003; Arcasoy 2005; Dagnon 2005; McBroom 2005; Leo 2006), it is also possible that endogenously produced or exogenously administered erythropoietin promotes the proliferation and survival of erythropoietin receptor expressing cancer cells (Feldman 2006; Yasuda 2003; Mohyeldin 2005; Henke 2006). There is an ongoing debate about the validity of those studies, because the antibodies used most often lacked EPO‐R specificity (Elliott 2006; Osterborg 2007). Thus, the interpretation of the observations made in many of those studies is questionable.
Besides this, other researchers have postulated an anti‐apoptotic effect of ESAs on other tissues including neural (Brines 2004; Brines 2000) and cancer cells (Um 2007). In addition, it has been proposed that there is a link between endogenous erythropoietin and angiogenesis in vivo (Ribatti 2007b; Ribatti 2007a; Hardee 2007). Possibly, endogenous erythropoietin is needed to promote tumor angiogenesis and to maintain the viability of endothelial cells. However, the clinical implications of these findings have not been clarified to date. Apart from the direct tumor growth stimulation, a pathophysiological relationship between thromboembolic events and cancer has been described. Studies have implicated the tumor‐mediated activation of the hemostatic system in both the formation of tumor stroma and in tumor metastasis (Francis 1998; Levine 2003).
In summary, a direct relationship between the presence of erythropoietin receptors on tumor cells and tumor proliferation in response to exogenous ESAs has not been established to date. Overall, the evidence from both in vitro and in vivo studies as well as clinical trials is insufficient to draw firm conclusions whether ESAs promote tumor proliferation or not.
Three Oncologic Drugs Advisory Committee (ODAC) hearings took place to discuss the safety of erythropoietins in cancer patients. After the first hearing in May 2004 the FDA concluded the Hb target for ESA treatment should not be higher than Hb 12 g/dL (Luksenburg 2004). Package inserts in the USA were amended to include this recommendation. Since then, another two randomized controlled trials showed detrimental effects for patients receiving ESAs. One study was conducted in patients with head and neck cancer undergoing radiotherapy (Overgaard 2007), another study was conducted with palliative intent for patients with advanced stage cancers not receiving chemotherapy (Smith 2008). The second ODAC hearing was held on May 10th 2007. In March 2007 a black box warning was added to the package inserts in the USA. This warning recommends that 1) ESAs should be used at the lowest dose that will gradually increase the Hb concentration to the lowest level sufficient to avoid the need for RBC transfusions, 2) ESAs should not be used in patients with active malignant disease not receiving chemotherapy or radiotherapy and 3) the target Hb should be 12 g/dL and not higher. In November 2007 another warning was released, declaring that “the risks of shortened survival and tumor progression have not been excluded when ESAs are dosed to target hemoglobin of < 12 g/dL.” Following the release of study data from two additional studies (Thomas 2008; Untch 2008), a third ODAC hearing was held in March 2008. At that meeting it was discussed whether the indication for ESAs in cancer patients receiving chemotherapy should be withdrawn, whether the drugs should not be used in cancer patient who are likely to be cured, which suggests the drugs should only be used as part of a best‐supportive care regimen in patients with advanced cancer. It was also discussed that the drug should not be used in advanced or metastatic breast cancer as well as patients with head and neck cancer.
Why it is important to do this review
Rationale
We previously conducted a Cochrane Review on the effectiveness of ESAs which included trials published through 2001. This analysis suggested a survival benefit for patients receiving ESAs compared to patients only receiving red blood cell transfusions (hazard ratio (HR) 0.81, 95% confidence interval (CI) 0.67 to 0.99, n = 2865) (Bohlius 2005). This review was subsequently updated with studies published through April 2005. The updated review included 57 trials with 9353 patients (Bohlius 2006). In contrast to our previous findings, the results of the updated review suggested detrimental survival effects in patients receiving erythropoietin or darbepoetin compared to patients only receiving red blood cell transfusions (HR 1.08; 95%‐CI 0.99‐1.18; 42 trials, n = 8167) (Bohlius 2006). In addition, use of ESAs was statistically significantly associated with an increased risk for thromboembolic events (relative risk 1.67, 95%‐CI 1.35‐2.06; 35 trials, n = 6769) (Bohlius 2006).
However, to date it has not been convincingly shown whether ESA treatment increases or decreases tumor progression and overall survival. Risk factors to develop TEEs (thromboembolic events) under ESA treatment have not been identified yet.
The need for an individual patient data meta‐analysis
The meta‐analyses conducted so far are limited to published data aggregated across trials at the level of randomized groups (active treatment versus control). Pooled time‐to‐event analyses allow the examination of potential confounding and interaction, and are generally more efficient than analyses based on aggregated data. We therefore expanded our prior aggregated data meta‐analysis to individual patient data (IPD). This will allow us to assess the associations between ESA treatment and risk for thromboembolic events, disease progression, quality of life and deaths in cancer patients and would provide a unique opportunity to shed light on the important questions discussed above.
Objectives
1. To examine the effect of ESAs on overall survival, progression free survival, thromboembolic and cardiovascular events as well as need for transfusions and other important safety and efficacy outcomes in cancer patients.
2. To identify factors that modify the effect of ESAs on overall survival, progression free survival, thromboembolic and cardiovascular events, need for transfusions and other important safety and efficacy outcomes in cancer patients.
Methods
Criteria for considering studies for this review
Types of studies
In accordance with best practice in reviews of the effects of interventions, we included all eligible randomized controlled trials (Higgins 2006), for which individual patient data were available. Studies were included regardless of publication status, i.e. unpublished studies were included as well. We considered only studies that were planned to include at least 50 patients per study arm or at least 100 patients in total. Studies that were terminated prematurely and did therefore not reach the planned study size were included as well. A sensitivity analysis was conducted to test the influence of prematurely terminated studies. Placebo control was not required for inclusion but was recorded in the context of trial quality (see below). For the endpoints overall survival we included any eligible trial, regardless whether the study was designed for the endpoint survival or not.
Studies that did not collect or report data for any of the primary and secondary outcomes of this project (see below) were excluded. Ongoing studies, i.e. studies that were not completed according to the study specific protocol (e.g. complete follow‐up for primary outcome), were included if the following criteria were met: recruiting phase completed, interim analyses conducted with in depth validation of the data, all initially randomized patients included in the interim analysis. Any other ongoing study was excluded from the present analysis but will be included in a later update of this analysis (e.g. German Hodgkin Study Group HD 15). Some studies offered ESA treatment to patients in the control arm after a defined period, e.g. after 12 weeks of study duration and allowed cross‐over to the treatment arm after this defined period. For those studies we evaluated only the trial phase, where patients allocated to the control arm did not receive ESAs and patients allocated to the treatment arm received ESAs. For on study mortality we analyzed only data while the patient was on trial treatment plus a short follow‐up period (four weeks or 28 days). For overall survival we collected the longest follow‐up available, including the time after the end of study drug treatment.
Types of participants
Pediatric and adult, male and female patients with a clinically or histologically confirmed diagnosis of cancer receiving or not receiving chemotherapy or radiotherapy or combined modality treatment were included. Both patients with solid and hematological malignancies were eligible.
Studies on high‐dose myeloablative chemotherapy regimens followed by bone marrow or peripheral blood stem cell transplantation, myelodysplastic syndromes or acute leukemia as well as trials using ESAs for short‐term preoperative treatment were excluded. Studies were excluded if more than 20% of the entire patient population presents with an ineligible condition. However, if the respective study was randomized using a stratification technique and includes single strata that do fulfill the inclusion criteria, these strata were included in the analysis.
Types of interventions
Cancer patients in the experimental group must have received short or long acting ESAs to prevent or reduce anemia, given singly or concomitantly with chemotherapy, radiotherapy, combination therapy or no therapy. ESAs had to be administered subcutaneously or intravenously. No minimum treatment duration or minimum ESA dosage was required for inclusion. Patients in both the control group and the experimental group(s) were to receive red blood cell transfusions if necessary. Studies with active controls i.e. head‐to‐head comparisons of different ESA types or dosages were excluded. Supportive care such as iron given either as necessary or following a fixed schedule was allowed. Apart from administration of ESAs, participants in experimental and control groups must have intended to receive identical care. For purposes of this analysis, patients receiving chemotherapy were considered to be receiving identical care, even if the regimens they received may have included different chemotherapy drugs. In the protocol we had stated that there was to be one exception: studies that compared ESA plus iron compared to no ESA and no iron were included. However, in the present review we also included two studies with different start of radiotherapy in the ESA and the control arm (Strauss 2008) and different transfusion trigger in the ESA and the control arm (Thomas 2008). The impact of these studies on the overall analysis was explored in a sensitivity analysis.
Types of outcome measures
Primary outcomes
On study mortality
Populations of interest, defined at study level (see below: Other definitions, Population of interest):
cancer patients receiving chemotherapy or combined modality treatment regardless of Hb level
all cancer patients receiving chemotherapy/combined modality treatment, radiotherapy/radio‐chemotherapy or no anticancer treatment regardless of Hb level
Type of information: time‐to‐event, definition of event: death from any cause, starting time point: date of randomization, date of last follow‐up to be considered: see Statistics section. A minimal follow‐up time was not required for inclusion.
Overall survival
Populations of interest, defined at study level (see below: Other definitions, Population of interest):
cancer patients receiving chemotherapy or combined modality treatment regardless of Hb level
all cancer patients receiving chemotherapy/combined modality treatment, radiotherapy/radio‐chemotherapy or no anticancer treatment regardless of Hb level
Type of information: time‐to‐event, definition of event: death from any cause, starting time point: date of randomization, date of last follow‐up to be considered: longest follow‐up available. A minimal follow‐up time was not required for inclusion.
Secondary outcomes
On study mortality and overall survival
Populations of interest, defined at study level (see below: Other definitions, Population of interest):
cancer patients receiving radiotherapy/radio‐chemotherapy treatment regardless of Hb level
cancer patients receiving no anticancer treatment regardless of Hb level
Note: these and all other secondary outcomes (not listed here) reported in the protocol (Bohlius 2008) were postponed and are not part of the present report. For details see protocol.
Other time points of interest
In addition to the time points specified above, we specifically examined the following points in time: 4, 8, 12, 24, 36, 60 months after randomization. These time points were calculated for the overall population as well as separately for the populations chemotherapy, radio(chemo)therapy, “mixed” and none.
Other definitions
Populations of interest
Highest priority was given to the analyses of cancer patients receiving concomitant chemotherapy and cancer patients receiving ESAs irrespective of concomitant anticancer treatment. The respective treatment strategies (chemotherapy/combined modality treatment versus radiotherapy/radiochemotherapy versus “mixed” versus no treatment) were explored in subset analyses. Note: the no treatment and the radio(chemo)therapy populations have not been analyzed separately.
Definitions of anticancer treatment populations: The definition of anticancer treatment populations was referring to the anticancer treatment at study level and not to the anticancer treatment an individual patient had actually received. A cut of 70% was chosen to define the different anticancer treatment populations at study level. I.e. if in a given study 70% of the patients had received chemotherapy, the study was classified as “chemotherapy population”. “Chemotherapy“ refers to patients receiving a myelosuppressive chemotherapy. Combined modality treatment was defined as chemotherapy followed by radiotherapy. Radiochemotherapy was defined as treatment strategy where radiotherapy and chemotherapy were given at the same time. Radiotherapy was defined as population of patients receiving mainly radiotherapy only. “None” was defined as patients population were more than 70% of patients did not receive a myelosuppressive chemo/and or radiotherapy. Of note: “none” does not mean, that these patients did not receive any anticancer treatment. Patients in this population did receive corticosteroids, hormonal therapies, low dose chemotherapies and radiotherapies and other substances. However, this information is only available from the clinical study reports and the specific treatment per patient is not available.
Baseline variables
Hb and Hct
Baseline Hb and Hct were defined as Hb or Hct measurement up to 30 days before date of randomization or up to seven days after randomization.
Baseline age
Baseline age refers to age at date of randomization calculated based on the birth date provided per patient. For two studies (Thomas 2008; Machtay 2007) birth dates were not reported; age at randomization or age at study entry was provided instead.
Other baseline variables
All other baseline values refer to the baseline as provided by the investigators.
Terminology
Subgroup” and “subset” analyses
Any analyses that relate to information on the individual patient level are termed “subgroup analyses”. Any analyses that relate to information at study level are termed “subset analyses”.
“Missing” and “not reported” data
“Missing” means that the data were not provided in the requested standardized data format for this analysis, however, the data might be on file at the investigators´ site. “Not reported” means that the data are not on file at the investigators’ site.
Study numbers
A five digit study number was assigned randomly to each trial. A complete list of corresponding study numbers, study protocols and publications is on file and is not provided in this report.
Search methods for identification of studies
For the first and the updated version of this review (01/1985 to 12/2001 and 1/2002 to 04/2005) we identified relevant trials through electronic searches of the Cochrane Library, MEDLINE and EMBASE. For the planned IPD meta‐analysis the same databases were searched for 2005 until December 2007. The first search was conducted in March 2007. The update search was conducted in January 2008. In addition, we searched relevant trials through searches of the conference proceedings of the American Society of Clinical Oncology, American Society of Hematology and European Society of Medical Oncology. Searches of conference proceedings were either done online, with CD‐ROMs or by handsearching. For the present IPD meta‐analysis we searched abstracts in the conference proceedings reported above for the years 2005 to end of 2007.
Reference lists of identified guidelines, systematic reviews and clinical trials were checked for additional information. Documents posted for the ODAC hearings in 2004 and 2007 were evaluated, documents posted for the ODAC hearing in March 2008 were not evaluated. Data bases of ongoing studies were searched as well. Previous searches of ongoing studies were updated to June 2007. Any accidentally identified trials were evaluated as well. Lists of identified studies were sent to the pharmaceutical companies who manufacture ESAs. Companies were asked to review and complete these lists. For a detailed description of the literature searches see below.
Electronic searches
For the individual patient data (IPD) meta‐analysis on the effects of erythropoiesis‐stimulating agents in cancer patients the results of electronic database search from two previous published reviews (Bohlius 2004; Bohlius 2006) which include the period 01/1985 to 12/2001 and 01/2002 to 9/2004 and an additional search which gives an update of published studies up to 12/2007 were used. A total of potential relevant hits 5546 (including duplicates caused by an overlap of these three searches) identified from these literature databases. For search strategies see Appendix 1.
Cochrane Review 2004
The first version of the Cochrane Review (Bohlius 2004) based on a main search period from 01/1985 to 12/2001.
Following databases are used:
Cochrane Central Register of Controlled Trials Register (CENTRAL)
MEDLINE (01/1985 to 12/2001)
Cancer Lit (01/1985 to 12/2001)
EMBASE (01/1985 to 12/2001)
Medikat (01/1985 to 12/2001)
Russmed Articles (01/1988 to 12/2001)
SOMED (01/1985 to 12/2001)
Toxline (01/1985 to 12/2001)
BIOSIS Previews (01/1985 to 12/2001)
LILACS (01/1986 to 12/2001)
The initial literature search in March 2002 retrieved 1,592 references.
Update Cochrane Review 2006
For the first update of the Cochrane Review (Bohlius 2006) the search strategy for epoetin alpha and beta was adapted from the previous Cochrane search strategy and run from 2000 until September 2004. In the case of darbepoetin alpha the search ran from 1996, the year before phase I studies were initiated on it. Searches ended in September 2004.
The following bibliographic databases were searched:
Cochrane Central Register of Controlled Trials Register (CENTRAL) (01/2002 to 9/2004)
MEDLINE (01/2002 to 9/2004)
EMBASE (01/2002 to 9/2004)
Science Citation Index (01/2002 to 9/2004)
In addition, all PubMed was screened on a daily basis by one reviewer (JB) until April 2005; all studies identified up to April 2005 were included in this review.
In addition to the initial literature search from March 2002, which retrieved 1,592 references, 1,859 references have been identified and screened.
Literature search update for the IPD meta‐analysis
For this IPD meta‐analysis additional database searches were performed for two periods.
The first search performed in March 2007 included all studies published later than 2000 until February 2007 (date of Index in database). The second search completed in January 2008 ensures an update of the information about available publications up to end of 2007.
The following bibliographic databases were searched:
Cochrane Central Register of Controlled Trials Register (CENTRAL 01/2000 to 01/2008)
MEDLINE (01/2000 to 12/2007)
EMBASE (01/2005 to 12/2007)
Science Citation Index (01/2000 to 12/2007)
This literature search retrieved 1,851 references for search conducted in March 2007 and 244 for the update search up to end of 2007 conducted in January 2008.
A total of 5546 hits (including duplicates caused by an overlap of these three searches) were identified from the literature databases. Out of the 5546 references identified 447 full text publications were retrieved for assessment.
Studies identified by database search
Thirty‐two studies included in the IPD meta‐analysis were identified by the database search: Aapro 2008; Abels 1993; Boogaerts 2003; Case 1993; Cazzola 1995; Chang 2005; Charu 2007; Dammacco 2001; Grote 2005; Hedenus 2003; Henke 2003; Henry 1995; Kotasek 2003; Leyland‐Jones 2003; Littlewood 2001; Machtay 2007; O'Shaugnessy 2005; Oberhoff 1998; Osterborg 1996; Osterborg 2002; Pirker 2008; Razzouk 2006; Savonije 2005; Smith 2008; Strauss 2008; Ten Bokkel Huinink 1998; Thatcher 1999; Thomas 2008; Vansteenkiste 2002; Wilkinson 2006; Witzig 2005; Wright 2007.
The other publications are additional references to already included or excluded studies (see 'Studies and references' table).
Searching other resources
Conference proceedings
For the first and the updated version of of the previously published Cochrane review (Bohlius 2006) we identified relevant studies through searches of the conference proceedings of the American Society of Clinical Oncology, American Society of Hematology and European Society of Medical Oncology (01/1985 to 12/2001 and 1/2002 to 04/2005). Searches of conference proceedings were either done online, with CD‐ROMs or by handsearching.
For the IPD meta‐analysis, we have searched the same conferences for the years 2005 to end of June 2007. The search was updated during the project in January 2008, extending the search to end of December 2007.
Handsearching was performed for the conference proceedings:
European Hematology Association (2001 to 2007)
American Society of Clinical Oncology (1989 to 1996)
European Society of Medical Oncology (1989 to 2008)
American Society of Hematology (1989 to 1997)
Electronic searching of the conference proceedings:
Annual Meeting of the American Society of Clinical Oncology (1997 to 2008)
Annual Meeting of the American Society of Hematology (1998 to 2008)
Out of 96 potential relevant abstracts from RCTs 21 studies fulfill the inclusion criteria of the IPD meta‐analysis were published until December 2007 and were identified by systematic screening of conference proceedings (ASCO, ASH, EHA and ESMO). The other abstract publications are additional references to already included or excluded studies (see 'Studies and references' section).
Thirteen studies are published as abstract only and eligible for the IPD meta‐analysis: Gordon 2006; Goss 2005; Huddart 2002; Kotasek 2002; Moebus 2007; Pronzato 2002, Quirt 1996; Ray‐Coquard 2006; Rose 1994; Taylor 2005; Thomas 2008; Untch 2008; Vadhan‐Raj 2004.
Reference lists
The reference lists from following evidence based guidelines, systematic reviews and HTA reports were checked to identify potential relevant clinical studies:
Guidelines
ASCO / ASH 2007: Rizzo 2008
FNLCC 2007: Fédération nationale des centres de lutte contre le cancer. Recommandations pour la pratique clinique: Standards, Options et Recommandations 2007 pour l’indication de l’agent stimulant l’érythropoïèse (ASE: époétine alpha, époétine bêta et darbepoétine) dans la prise en charge de l’anémie en cancérologie (Available: http://www.fnclcc.fr/sor/structure/index‐sorspecialistes.html)
HTA Reports
Reviews
There was no additional relevant study identified.
ODAC documents
Documents posted for the ODAC hearings in 2004 and 2007 were evaluated. These documents include briefing document plus additional power point presentation prepared by medical reviewers of the Food and Drug Administration (FDA) and the companies Roche, Johnson & Johnson and Amgen. All of these documents are publicly available through the FDA briefing document at ODAC hearing 2004, briefing documents from FDA, Roche, Johnson & Johnson and Amgen:
Slides: http://www.fda.gov/ohrms/dockets/ac/04/slides/4037s2.htm,
Briefing documents: http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2.htm
(Last time URL checked: 27 March 2009)
ODAC hearing 2007, briefing documents from FDA, Johnson & Johnson and Amgen
Slides: http://www.fda.gov/ohrms/dockets/ac/07/slides/2007‐4301s2‐00‐index.htm
Briefing documents: http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007‐4301b2‐00‐index.htm (last time URL checked: 27 March 2009)
Following nine eligible studies primarily identified by screening of the FDA web sites:
EPO‐GBR‐7; EPO‐CAN‐15 (Goss 2005) ; EPO‐CAN‐20 (Wright 2007); GOG‐191 (Thomas 2008); EPO‐INT‐1; EPO‐INT‐3; N93 004 (Grote 2005); CC2574‐P‐174; EPO‐GER‐22 (Debus 2006).
Five of them are published in meantime and also identified by systematic search of databases and abstracts:
(EPO‐CAN‐15, 2004) (Goss 2005)
(EPO‐CAN‐20, 2004) (Wright 2007)
(EPO‐GER‐22, 2007) (Debus 2006)
(GOG‐191, 2004) (Thomas 2008)
(N93 004, 2004) (Grote 2005)
Register of ongoing studies
Further potential relevant studies and ongoing trials identified by using the metaRegister of Controlled Trials (mRCT) http://www.controlled‐trials.com/‐ which include information of eight active registers. The last search was done June 30 2008 to allow an current status of the identified studies.The electronic search using the terms (epo* OR darb* OR erythrop* OR aranesp OR nesp* results in 671 hits, 95 of them are studies investigate ESAs in cancer patients. Forty‐five studies fulfill the inclusion criteria for the IPD meta‐analysis and 50 studies investigate ESA in cancer do not fulfill the inclusion criteria (intervention / control or disease). Out of the 45 studies which are potential eligible 22 can be assigned to at least one publication and 15 studies can not associated to any publication, 3 of 15 are stated as terminated. Further eight studies are declared as ongoing. For two trials interim results were published in local conferences (Debus 2006; Pronzato 2002).
Accidentally identified studies
Accidentally identified studies were evaluated as well.
Press release
One study (Untch 2008) was identified with a press release (Amgen 2007)
Contact with companies
Lists of identified completed and ongoing studies were sent to the pharmaceutical companies who manufacture ESAs. The three responsible companies Amgen, Hoffmann‐LaRoche, Johnson & Johnson were asked to review and complete these lists:
One additional reference (Milroy 2003) was identified in a list of trials conducted by the companies.
Two previously not identified studies were also identified: (EPO‐GER‐20; OBE/EPO‐INT‐03)
Contact to authors
All authors of published RCTs were contacted to clarify the potential eligibility for the IPD meta‐analysis (esp. the criterion on number of patients planned to be randomized).
Studies included in the IPD meta‐analysis
Out of the different searches a total of 53 studies can be included in the meta‐analysis of the effects of erythropoiesis‐stimulating agents in cancer patients based on individual patient data.
Individual patient data are available and used from following 53 studies: (EPO‐GBR‐7; EPO‐INT‐1; EPO‐INT‐3; CC2574‐P‐174; EPO‐GER‐20; OBE/EPO‐INT‐03; Aapro 2008; Abels 1993; Boogaerts 2003; Case 1993; Cazzola 1995; Chang 2005; Charu 2007; Dammacco 2001; Debus 2006; Gordon 2006; Goss 2005; Grote 2005; Hedenus 2003; Henke 2003; Henry 1995; Huddart 2002; Kotasek 2002; Kotasek 2003; Leyland‐Jones 2003; Littlewood 2001; Machtay 2007; Milroy 2003; Moebus 2007; O'Shaugnessy 2005; Oberhoff 1998; Osterborg 1996; Osterborg 2002; Pirker 2008; Pronzato 2002; Quirt 1996; Ray‐Coquard 2006; Razzouk 2006; Rose 1994; Savonije 2005; Smith 2008; Strauss 2008; Taylor 2005; Ten Bokkel Huinink 1998; Thatcher 1999; Thomas 2008; Thomas 2002; Untch 2008; Vadhan‐Raj 2004; Vansteenkiste 2002; Wilkinson 2006; Witzig 2005; Wright 2007)
Data collection and analysis
Selection of studies
Trials identified through the update literature searches were screened independently by two reviewers (JB, OW) for the eligibility criteria stated previously. If eligibility could not be assessed satisfactorily from the title and abstract, a full text version was obtained for assessment. Studies that appeared to meet the inclusion criteria in the initial screening were further assessed for eligibility with the following questions:
Q1. Is the study described as randomized?
Q2. Did the participants in the study have a previously treated or untreated malignant disease?
Q3. Was one group given Epoetin‐alfa or Epoetin‐beta or Epoetin‐delta or Darbepoetin‐alfa or any other erythropoiesis‐stimulating agent subcutaneously or intravenously?
Q4. Did the control group receive the same care (e.g. chemotherapy and supportive therapies) with or without placebo? Exception: iron, see Types of studies.
Q5. Did the study document any of the following outcomes: overall survival or thromboembolic / cardiovascular events or tumor progression or a similar endpoint or QoL measured with a validated instrument?
Q6. Did the study plan to include at least 50 patients per treatment arm or at least 100 patients in total?
Q7. Is the study completed by its own study protocol definition or has the study been terminated prematurely? For ongoing studies: is patient recruitment terminated and has a validated interim analysis been done? (see 'Criteria for considering studies for this review')
To be eligible, studies had to meet all of the criteria stated above. If there was insufficient information to judge eligibility, the first author of the report was contacted for clarification.
Studies excluded from the previous Cochrane Reviews were reassessed, because the eligibility criteria for the present IPD meta‐analysis were not identical to those of the Cochrane Review. For example, studies with iron supplementation in one study arm only had been excluded from the previous Cochrane Reviews. Eligibility of these studies had to be reassessed for the present analysis. To assess Q6 (Did the study plan to include at least 50 patients per treatment arm or at least 100 patients in total?) we contacted the sponsoring companies and independent investigators of studies that had evaluated less than 100 patients to clarify whether they had intended to include more than 100 patients. Lists of eligible studies were sent to the companies/investigators for confirmation of study eligibility. Studies evaluating less than 50 patients were excluded from the analysis. This criterion was discussed with the Steering Committee in January 2008 but had not been included in the final version of the protocol. If the two reviewers (JB, OW) could not reach consensus the principal investigator (AE) and the Steering Committee were involved. Any disagreements between the reviewers regarding eligibility were resolved by discussion.
Data extraction and management
Materials
The following documents were requested for each of the included studies
Study protocol
Clinical study report
Case report form including Quality of Life instruments used
Publications
Individual patient data
Data sets had to include the individual patient data as defined for this project of all patients initially randomized.
Data Extraction and Compilation
Data submitted by the sponsors/investigators
Information were collected both at the level of the trial and at the patient level. The following study level characteristics were requested from the sponsors/independent investigators:
Study level information
Components of methodological quality, source of funding, completion of study, planned follow‐up duration, duration of study, ESAs (type, dose, frequency and route of administration, criteria for stopping study drug), Hb/ hematocrit (Hct) target, policy regarding iron supplementation, planned and administered anticancer treatment.
Individual patient level information
Age, sex, type of tumor, type of antineoplastic therapy received (chemotherapy during ESA study yes/no/not reported, radiotherapy during ESA study yes/no/not reported), ESA dose received, red blood cell transfusions received, Hb and Hct values at baseline and during follow‐up, date of death or date last time seen alive.
Based on these information additional variables were derived. A detailed list of variables including the coding scheme for each variable is on file.
Data extraction from available study documents
The investigators of the studies provided protocols, clinical study reports and case report forms for the included studies. For information at study level that was not provided by the investigators two reviewers (JB, SK) independently extracted the information from study protocols, clinical study reports, case report forms and publications if necessary. Data extractions were compared and inconsistencies discussed until consensus was reached. If necessary, the sponsor or independent investigator submitting the data was contacted for clarification.
The following study characteristics were extracted:
Was the study designed for long‐term follow‐up (defined as follow‐up of at least 12 months after end of study phase)?
Did the study have a prespecified cancer treatment protocol?
Treatment category: chemotherapy, combined modality treatment, radiotherapy, radiochemotherapy, none or mixed.
“cross‐over”, i.e. whether patients in the control group were allowed to receive ESAs after a specified study period.
Data extraction not in duplicate
Data that were used for descriptive purposes in tables only and that were not used in any of the statistical analysis were extracted by one person only (JB).
Coding of the variable “metastatic disease”
For the present analysis we had requested two variables to describe the disease stage of the patients, i.e. whether the patient had extensive disease or metastatic disease or neither extensive nor metastatic disease. This simplified scheme did not work for the majority of trials and cancer types included and as a result for about 80% of patients we had no structured information on disease stage as requested. In addition, we had requested a free text entry describing the disease stage for each individual patient. Based on the free text entries and the available clinical study reports, for each patient the information “metastatic solid cancer or advanced hematological malignancies” yes versus no or not reported/unclear was assigned. The assignment was done by one reviewer (JB). The assigned categories were checked for consistency across trials in conjunction with the clinical study reports (JB).
The general coding rules were as follows:
Patients with solid cancers and metastatic disease or stage IV were coded as “metastatic”, all other patients were categorized as “non metastatic”. Patients with hematological malignancies in Ann Arbor stage III or IV were categorized as “advanced”; all other patients were categorized as “not advanced”. For patients with small cell lung cancer we differentiated “extensive disease” versus ”limited disease”. If for a given study no information was available at patient level, but the clinical study report stated that for example all patients included in the study had metastatic disease, each patient of that particular study was coded as “metastatic”.
This procedure included several limitations; the main limitation is the inconsistency of tumor coding between trials. For some studies we received only the data entry “metastatic” and “non‐metastatic” without specification of the TNM stages. In this case “metastatic” was classified “metastatic” for the coding system for the present analysis and “non‐metastatic” was classified “other than metastatic for solid cancers”. For hematological malignancies “metastatic” was classified “advanced stage” and “non‐metastatic” was classified “not advanced”. For other studies we received only TNM stages, e.g. stage I, II, III, or IV. However, not in all tumor types stage “IV” and “metastatic” are identical, i.e. only patients in stage IVB are metastatic whereas patients in stage IVA are not. Only for few cancer entities this problem does not exist, e.g. in breast cancer all patients with stage IV are metastatic. This inconsistency between the coding in the different studies is a limitation of the current data set. However, the variables “metastatic” versus “non metastatic” serves as a proxy to see whether baseline imbalances or interaction between disease stage and study drug with effect on the outcome mortality exist.
Data management
Data were entered in a dedicated database. The format of the data requested is on file. Data were checked for accuracy, consistency, and completeness of follow‐up (Stewart 1995). We used descriptive statistics to describe baseline characteristics of patients in each trial and to identify outliers. Accepted ranges for continuous variables were defined in advance. All data identified as missing, implausible or inconsistent were listed and sent to the investigators or company providing the data for the respective trial for clarification where possible. Overall survival and on study mortality of the different treatment groups in each trial were derived using the Kaplan‐Meier method and standard Cox regression analysis and compared with published survival estimates. Any discrepancies between published data and provided individual patient data was reported to and discussed with the original investigator or company providing the data. A detailed report of the data management is provided on file.
Monitoring
The following step described in the protocol was considered not feasible and has not been done:
“To assess the quality of the coding we will review investigator comments and investigator texts as reported in the case report forms of approximately 200 patients experiencing thromboembolic events, 200 patients not experiencing thromboembolic events, and 200 patients who died. Once absolute numbers of thromboembolic events and deaths are available percentages of events to be reviewed will be calculated. Patients will be selected by random stratified by company. Which discrepancy rate will be accepted and which measures will be taken if the discrepancy rates is exceeded requires further discussion. In general, error rates during the process of data collection and data entry tend to be low. For example, error rates during data collection were estimated to be between 0.5% to 1.0% (Eisenstein 2005). In randomized controlled trials with blinding of study participants and study personnel, errors during data collection and data entry will be distributed randomly between groups and are unlikely to affect point estimates of difference between comparison groups. Computer simulations of analyses of moderate to large randomized controlled trials with real‐time validation checks during data entry have found that error rates up to 10% had little effect (Mcentegart 1999). If and to which extend data submitted not by sponsoring companies but by independent investigators are monitored requires further discussion with the independent investigators."
Assessment of risk of bias in included studies
The quality of the study data was assessed in the context of the individual patient data, study protocols, clinical study reports and available publications. For assessment of study quality and patient data level. Since all analyses were performed based on the intention‐to‐treat principle (analyzed in the allocated treatment arm); intention‐to‐treat was not assessed as a quality parameter.
The following quality components, which are part of the CONSORT statement, were assessed based on available study protocols, clinical study reports, publications or individual patient data:
Was treatment allocation sequence randomized? (assessed with study documents in duplicate, JB, SK)
Was treatment allocation concealed? (assessed with study documents in duplicate, JB, SK)
Were clinicians / care givers blinded (masked) to the allocated treatment? (assessed with study documents in duplicate, JB, SK)
Were patients blinded (masked) to the allocated treatment? (assessed with study documents in duplicate, JB, SK)
Were outcome assessors blinded (masked) to the allocated treatment? (assessed with study documents in duplicate, JB, SK)
What proportion of patients was excluded from the analysis and what was the ratio of exclusions between arms? This criterion has to be assessed for each endpoint separately (assessed with IPD data set)
Were the number and reason of patient withdrawals, dropouts and losses to follow‐up in each group documented? (assessed with study documents, JB)
The quality assessment for the parameter 1 to 5 and 7 outlined above refer to the quality of the studies as reported in the available documents. These parameters therefore primarily reflect the reporting of these variables in the available documents. Data were extracted in duplicate and compared. Inconsistencies were discussed until consensus was reached. For any parameter that was “unclear” after assessment we did not contact the sponsors/investigators for clarification. Because of time constraints we did not send questionnaires concerning the study design to the investigators to collect additional information as had been stated in the protocol. Specific coding rules used to assess the outlined study quality parameter are on file.
Measures of treatment effect
Organizational issues
Data management including data cleaning processes and derivation of new variables was done at the University of Cologne (CB). Main outcome variables (on study mortality and overall survival) were independently re‐coded in duplicate at the Institute of Social and Preventive Medicine (ISPM) in Bern (KS). Main statistical analyses were done independently at the ISPM at the University of Bern (KS), Switzerland and the Institute of Medical Biometry and Medical Informatics (IMBI) at the University of Freiburg, Germany (GS). Any discrepancies were resolved in discussion during two meetings at the ISPM in Bern.
Results tables and graphs were provided to members of the Steering Committee and the Advisory Board and discussed during meetings or telephone conferences.
It was prespecified in the protocol to provide the following minimum set of tables and graphs (additional tables and graphs might be provided):
Baseline table: summary of each included trial for the variables (continuous variables are presented as means and medians with accompanying standard deviations; dichotomous variables are presented as proportions) (note: on file, not provided in this review).
Kaplan‐Meier curves: standard Kaplan‐Meier curves for each time‐to‐event outcome plus the number of patients under observation at specified time points for each trial (note: on file, not provided in this report). Reverse Kaplan‐Meier curves: to assess time to censoring for each trial (note: on file, not provided in this review).
Event tables: for each time‐to‐event outcome a listing of the number of events, the number of patients included in the analysis, the patient‐years of follow‐up, and the mean observation time all separately for each trial (note: on file, not provided in this report).
Analyses tables: for each regression analysis a listing of hazard ratios of coefficients and interaction terms, accompanying 95% confidence intervals (derived from Wald test P values), and relevant P values from likelihood ratio tests (separately for each step of the respective analysis)
Forest plots: standard forests plots for each outcome separately
Funnel plots: standard funnel plots for each outcome separately
Dealing with missing data
Analysis set, missing data and losses to follow‐up
All analyses were performed based on the intention‐to‐treat principle: analyses included all randomized patients and patients were analyzed in the group they were allocated to, regardless of the treatment received or other protocol violations.
In patients lost to follow up, time was censored at the date of last official study visit according to the respective study protocol.
For patients censored on day one of randomization, 0.1 days was utilized as censoring time for technical reasons.
On study mortality
In the protocol we had defined on study mortality as time from randomization until 28 days after last planned ESA/placebo dose. In the statistical analysis plan we had specified two different methods for the generation of on study mortality:
Administrative censoring: each patient will be censored at a preplanned point in time, i.e. planned duration of ESA study plus 28 days follow‐up.
Informative censoring: each patient will be censored at the last study visit during study period plus 28 days follow‐up.
Ad 1: due to the complexity of the ESA studies this was not feasible. One difficulty was the different study designs of the ESA studies included. In about 32 studies there was a prespecified duration of ESA treatment. In 20 studies the duration of ESA administration was dependent on the duration of chemotherapy, i.e. ESA was given during the duration of chemotherapy. The duration of chemotherapy itself was variable, i.e. it was recommended to give additional 4 to 6 cycles of chemotherapy with a cycle length of 21 to 28 days. Therefore, it was not possible to set an administrative point of censoring based on the study information. In turn, using the duration of chemotherapy of the individual patient depends on the clinical course of the patient and can therefore not be regarded as “administrative”.
Ad 2: in the present study we analyzed the study data for on study mortality as provided by the companies and investigators, i.e. for each patient the companies and independent investigators had submitted a date of “end of study”, (variable ENDSTUDDT_ in DISPOSIT table of data set), i.e. the last official study visit of the patient during active ESA study phase. In some of the studies, this “end of study date” included already a follow‐up of 28 days, in other studies the date provided reflected the last visit and 28 days of follow had to be added. (Details of the programming of “on study mortality” on file, not provided in this review.)
Complete‐case analyses
Main analyses were conducted based on complete‐case analyses i.e. on patients with all data available for the relevant analysis. However, in the data sets received data were often not missing scattered across trials. In contrast, there were several trials which did not report specific variables for the entire study population. In the protocol we had stated the following: “The imputation of missing data (independent variables and continuous efficacy outcomes) using multiple imputation methods will be explored for sensitivity analyses.” Given the unbalanced pattern of missing data across studies we preferred not to impute any data.
Assessment of heterogeneity
Between‐trial heterogeneity was visually examined in forest plots and assessed by calculating the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance (Higgins 2002; Whitehead 2002). Standard tests of heterogeneity were also done. We examined small study effects in funnel plots of log hazard ratios or effect sizes against their standard error.
Assessment of reporting biases
Asymmetry of the funnel plot was assessed by the asymmetry coefficient (the difference in log hazard ratio or effect size per unit increase in standard error) (Sterne 2001) and tests for small study biases (Sterne 2001; Egger 2001; Egger 1997).
Data synthesis
Overview of statistical approaches
All analyses took into account the original randomization in each trial: no comparisons of patients from one trial with patients from another trial were made. Stratified Cox analyses were conducted in fixed‐effects models. All other meta‐analyses were conduced in both fixed‐ and random‐effect models. The fixed‐effect analysis was considered the primary analysis; the random‐effects analysis was used to examine the robustness of the results.
We used pre‐specified and exploratory variables; all variables were prespecified in the protocol for this analysis. The ‘main set’ of variables include variables that were defined for subset analyses in our first Cochrane Protocol in 2002 (Langensiepen 2002). We consider these variables to be truly pre‐specified because they were documented before the first trials with detrimental effects on survival were published. All variables that were proposed later are influenced by the observations made when detrimental study results became available. These variables were considered as ‘exploratory’, see Appendix 2.
Two different approaches for individual patient data meta‐analyses can be distinguished (Simmonds 2005). In the two‐stage method the available IPD are analyzed separately for each trial and then combined using standard meta‐analysis. The method is relatively simple to apply in practice and well suited to assess between trial heterogeneity caused by study level characteristics. It is, however, less suitable to identify prognostic factors and interactions of patient level characteristics. A meta‐analysis of IPD can also be seen as a multilevel model, with essentially two levels, the first level being the patients and the second level being the studies. This framework therefore allows estimating effects of interest in relation to both study‐level covariates and patient‐level covariatess.
Analysis to address objective 1: effects of ESAs
Meta‐analyses were based on a Cox regression analysis stratified by trial with treatment as the only factor in the model. This approach is a fixed‐effect model which allows for different baseline hazard functions in each trial (Smith 2007). Log rank estimates were calculated for each study and meta‐analyzed based on the fixed and the random‐effects models. We also calculated (log)‐hazard ratios for each trial separately using standard Cox regression analysis, which were then combined using fixed‐effects and the DerSimonian‐Laird random‐effects model (DerSimonian 1986). The assumption of proportional hazards was examined on the basis of Schoenfeld residuals and graphically using log‐log plots for each trial included.
Baseline imbalances
We assessed whether baseline imbalances could explain any effects seen on time‐to‐event outcomes. Bivariate Cox regression analysis stratified by trial was used. The variables that were considered as independent variables besides treatment are listed in Appendix 2. All variables with a corresponding P value of less then 0.10 were included in a multivariate Cox regression analysis stratified by trial. The following procedure was stated in the protocol: “Model selection was based on a standard stepwise selection procedure with 5% inclusion/exclusion criteria based on the likelihood ratio test.” Since we had many missing data and the missing data were not distributed evenly across trials (data were often missing for entire studies), the selection for variables was based on P value of the Likelihood Ratio (LR) test as stated above and number of cases reported per variable. We also planned to explore the possibility to implement a Cox regression model stratified by trial with random treatment effects (Smith 2005). However, since the heterogeneity between trials was low and the results of the log‐rank based meta‐analyses for both fixed and random‐effects models were model identical, this was not considered a priority.
Methodological characteristics of trials
The following method was stated in the protocol: “Univariable fixed‐effect meta‐regression based on the (log)‐hazard ratios of effect sizes of individual trials were used to examine whether treatment effects vary by trial level characteristics. The variables that will be considered as independent variables are listed in the Appendix 2. All variables with a corresponding P value of less than 0.10 will be included in a multivariate fixed‐effect meta‐regression analysis. For the survival analysis only variables 1 to 3 and 5 to 8 outlined in Appendix 2 will be included in the model. Random‐effects meta‐regression will be used to explore the robustness of the results.” Instead the study level parameters were assessed in the Cox model by using interaction terms. Meta‐regression analyses were used for exploration of effect modifiers at study level (exploratory analysis).
Continuous independent variables
The following step was planned but considered to be not feasible: “Non‐linear effects of continuous variables were examined by comparing linear models with models with quadratic terms using the Akaike Information Criterion (Akaike 1974). Alternative methods of analyzing continuous variables will be explored (Boucher 1998; Royston 1999).” The following procedure was done: continuous variables were included in the multivariate models based on categories that had been outlined in the protocol for this analysis.
Hematological response
Analysis of hematological response and other time dependent explanatory variables was postponed.
Assessment of eligible studies not included in the present analysis
To assess the impact of eligible studies with no available individual patient data, these studies were included in the analyses based on the aggregated results reported in the literature or provided by the investigators, see 'Results' section.
Numbers needed to treat
We calculated numbers needed to treat for one additional harmful outcome (NNTH) (Altman 1999; Altman 1998).
Sample size considerations
The sample size was determined by the number and size of trials for which individual patient data were available as well as the event rates in these trials. We had previously updated the literature based Cochrane Review (including studies up to end of June 2007) and identified 53 studies including 12353 patients that did fulfill the eligibility criteria outlined above. These studies reported approximately 4400 deaths from all causes. These numbers were preliminary estimates. Based on these estimates we assumed that the combined data set was to provide sufficient statistical power to detect clinically relevant adverse effects of ESA treatment, although power was expected to be insufficient to exclude small effects. Also, power was expected to be more limited for analyses of interactions. For number of studies, patients and events reported in the present analysis see 'Results' section.
Limitations
Multiple testing
This is an exploratory study and several hypotheses tests were performed. No adjustments for multiple testing were made and no higher confidence levels for confidence intervals were applied. The multiplicity of analyses, however, has to be considered when interpreting the result.
Comparison of different drug formulations
No separate analysis by ESAs (epoetin alpha, epoetin beta and darbepoetin alpha) nor any comparisons between the different ESAs was made upon specific request of the companies providing data for this study.
Organizational structure
All study centers that conducted ESA studies were invited to join the collaborative group and submit their individual patient data. Data were held securely and treated confidentially. Analyses, results and their interpretation were discussed with the collaborators.
Secretariat
The secretariat for this project was located at the Editorial Base of the Cochrane Haematological Malignancies Group in Cologne, Germany. The secretariat coordinated the project.
Statistical Analyses and Data Management
All data were anonymized and sent encrypted to the data center at the University of Cologne. Statistical analyses were done independently at the Institute of Social and Preventive Medicine (ISPM) at the University of Bern, Switzerland and the Institute of Medical Biometry and Medical Informatics (IMBI) at the University of Freiburg, Germany.
Steering Committee
The steering committee for this project consists of an international group of experts for hematology, oncology, radiotherapy, clinical epidemiology/biostatistics and a consumer representative. The steering committee gave advice on strategic issues and analyses. Final decisions concerning inclusion and exclusion of studies, statistical analyses and interpretation of findings were made by the Steering Committee. The tasks of the Steering Committee are documented in the Steering Committee Charter (on file, not provided in this review).
Advisory Board
Trialists and pharmaceutical companies who provided data for the analysis joined the Advisory Board. All data analyses were presented to the Advisory Board. The Advisory Board could give advice to the secretariat and the steering committee, but had no decision‐making authority. The tasks of the Advisory Board are documented in the Advisory Board Charter (on file, not provided in this review).
Protocol amendments
Protocol changes were avoided whenever possible. If nonetheless changes became necessary they were documented in an amendment. Any substantial change or addition to this protocol required a written protocol amendment that had to be approved by the Steering Committee and the Advisory Board. There was not substantial change to the protocol.
Subgroup analysis and investigation of heterogeneity
Analysis to address objective 2: analysis of effect modification (treatment by covariate interaction)
The focus of this analysis was on first order multiplicative interactions of independent variables with allocated treatment. The variables that were considered as independent variables are listed in Appendix 2. Bivariate Cox regression analyses with factor and treatment allocation stratified by trial and including the respective factor‐treatment interaction term (treatment by independent variable) were used. Models with and without the respective interaction term were compared using the likelihood ratio test. The possibility to implement a model with multiple interaction terms was reported in the protocol but not explored in the current analysis. Methodological characteristics of the studies (e.g. concealment of allocation, placebo controlled) were assessed using interaction terms. In addition, the following exploratory analyses were done: Meta‐regression analyses were conducted for study level variables with statistically significant effect modifications in the bivariate analyses. Meta‐regression was based on unadjusted and adjusted hazard ratios of the individual studies. Differences for subgroups generated with the meta‐regression analyses were tested with the Wald test.
Sensitivity analysis
Additional sensitivity analyses were performed to further check the robustness of the results.
Results
Description of studies
Results of the search
A total of 5546 hits (including duplicates caused by an overlap of the three data base searches outlined above) were identified from the literature databases. Out of the 5546 references identified 447 full text publications were retrieved for assessment. Electronic searches of ongoing studies data bases retrieved 575 hits.
Baseline characteristics overall
A total of 13933 patients were evaluated in the present analysis. At randomization the median age was 60.6 years in the ESA and 59.8 years in the control group. Hb at baseline was on average 10.6 g/dL (IQR 9.6 to 12.1 g/dL) in the ES and 10.8 g/dL (IQR 9.6 to 12.5 g/dL) in the control group. 18.3% of patients in the ESA and 15.9% of patients in the control group were diagnosed with a hematological malignancy, whereas 76.6% of ESA patients and 78.5% of control patients were diagnosed with a solid tumor. 30.9% of the entire patient population was diagnosed with breast cancer and 22.1% with lung cancer, including SCLC and NSCLC. 63.1% of patients included in the current analysis were female. For details of the patient population see Figure 1, Figure 2 and Figure 3.
1.
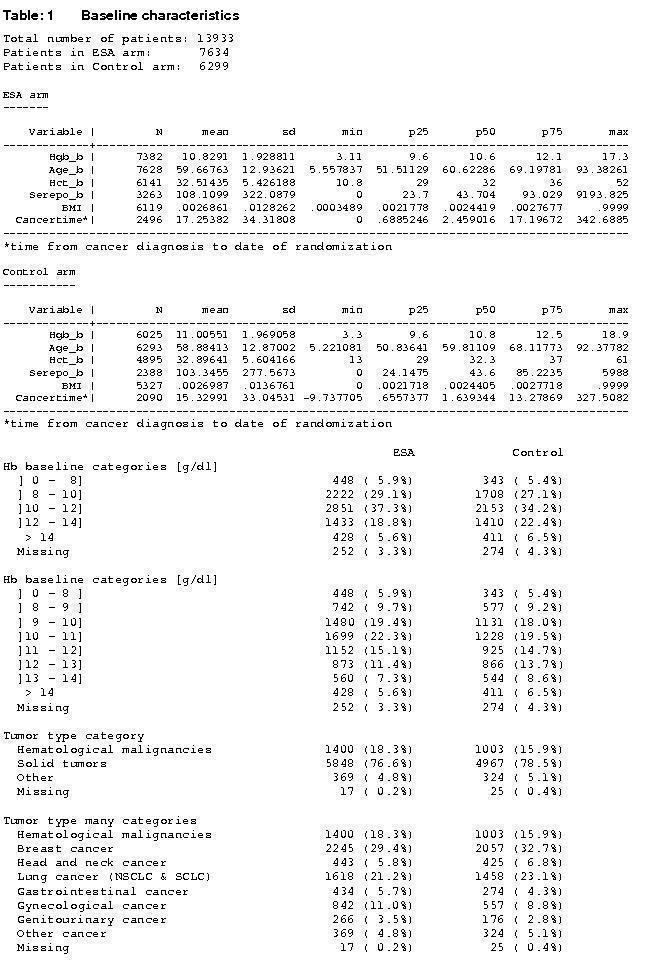
Baseline characteristics, a)
2.
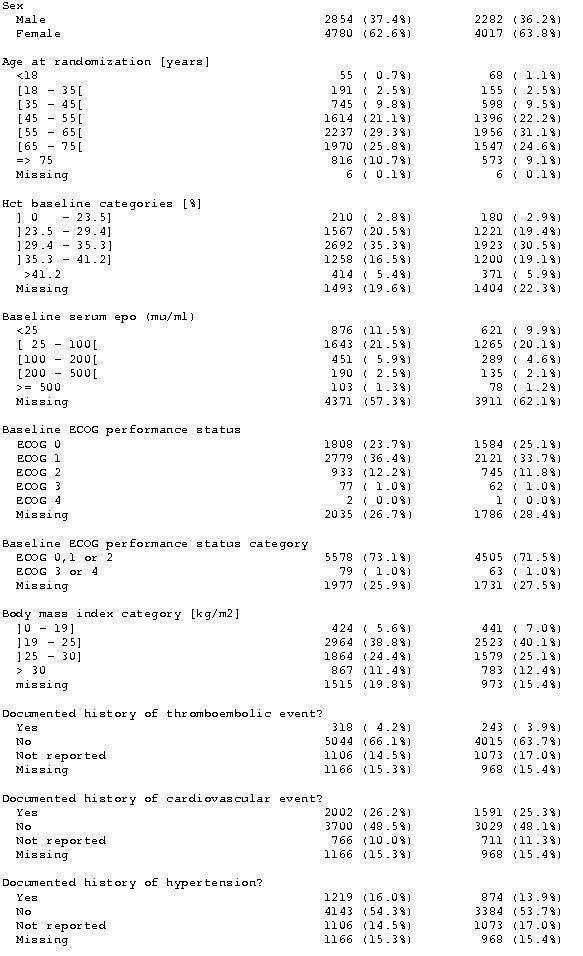
Baseline characteristics b)
3.
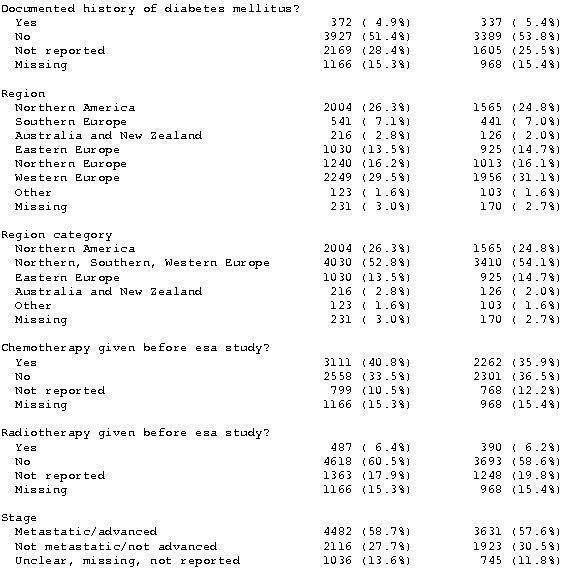
Baseline characteristics c)
Included studies
Eligible studies
A total of 63 studies were eligible for inclusion into this analysis. For 10 of the 63 studies we could not retrieve individual patient data for the present analysis (Blohmer 2003; Overgaard 2007; Bamias 2003; Watanabe 2006; Antonadou 2001; Janinis 2003; Iconomou 2003; Mystakidou 2005; Zajda 2007; Cascinu 1994). For six (Antonadou 2001; Mystakidou 2005; Cascinu 1994; Blohmer 2003; Overgaard 2007; Bamias 2003) of the ten studies aggregated survival data were reported in the literature or provided by the investigator and included in a sensitivity analysis to assess the impact of the missing studies on overall survival. In the other four studies survival data were not reported in the literature (Watanabe 2006; Janinis 2003; Iconomou 2003; Zajda 2007).
Included studies
For a total of 53 eligible studies we retrieved individual patient data, for list of included studies see 'Characteristics of studies' table. Fourty‐eight studies were provided by one of the three companies Johnson & Johnson, Roche and Amgen. Three independent investigators provided individual patient data by the means of the company (Moebus 2007; Untch 2008, Machtay 2007). Two independent investigators provided the data in the requested format directly to the collaborative group (Ray‐Coquard 2006; Thomas 2008).
Included and excluded patients
We received the data sets for 56 studies including 14393 patients. From the data set the following exclusions were made:
Total received: n=14393 patients, 56 studies
Exclusion of three studies including 187 patients, which did not meet the inclusion criteria (MF4266, MF4252 (Rau 1998), MF4253 (Kettelhack 1998).
n=14206 patients, 53 studies
Exclusion of patients without allocated study arm
MF4467 (Osterborg 2002) (n=162)
MF4250 (Osterborg 1996) (n=1)
MF4421 (Boogaerts 2003) (n=1)
n=14042 patients, 53 studies
Exclusion of ineligible study stratum: study PR99‐11‐034/044 (Razzouk 2006), children with acute lymphocytic leukemia, Non‐Hodgkin lymphoma (stratum 1, n=98), Hodgkin disease and solid tumors (stratum 2), stratum 1 was excluded.
n=13944 patients, 53 studies
For studies where the date of randomization was missing for all patients, the date of randomization was replaced with the date of first study drug as provided by the company (variable FSTTXDT from the data table DISPOSIT): study MF4421 (Boogaerts 2003).
For studies where only single patients had no date of randomization the patients were excluded from the analysis.
EPO‐INT‐3 (n=1)
DE20010033 (Untch 2008) (n=4)
MF4313 (Cazzola 1995) (n=3)
N=13936 patients, 53 studies
If both date of randomization and date of first study drug were missing in study MF4421 (Boogaerts 2003) (see above) these patients were excluded (n=3).
Total included: N=13933 patients, 53 studies
For identification of eligible trials see Figure 4.
4.
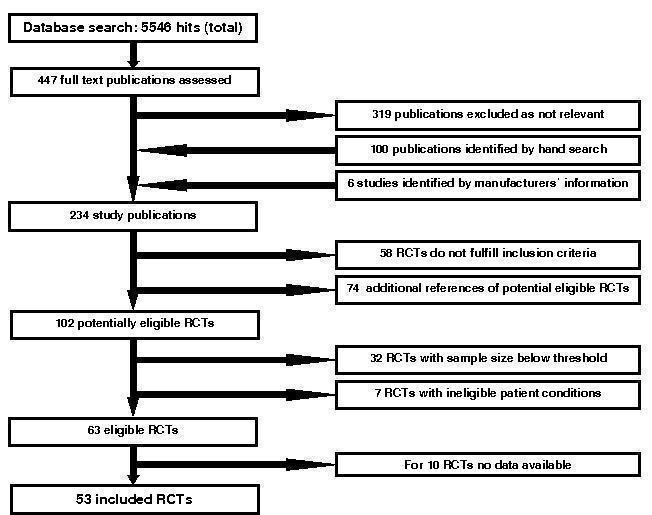
Identification of eligible trials
Characteristics of included studies
Cancer entities
Both patients with hematological malignancies and solid cancers were included in the evaluated studies. Some studies were restricted to single disease entities whereas other studies included various tumor types. Some studies were restricted to patients with identical stages of disease, whereas others included both early and advanced stages.
In detail, the following cancers were explored:
Breast cancer
Seven studies evaluated patients with breast cancer only. Of these, two studies included only patients with metastatic disease (Aapro 2008; Leyland‐Jones 2003). Two studies included only patients with non‐metastatic disease (Moebus 2007; Untch 2008). Three studies included patients with stages I to IV (Chang 2005; O'Shaugnessy 2005; Pronzato 2002).
Lung cancer
Nine studies evaluated patients with lung cancer only. Of these, five studies included patients with small cell lung cancer (SCLC) only. Goss 2005 included patients with limited disease SCLC. Pirker 2008 and EPO‐GER‐20 included patients with extensive disease SCLC. Grote 2005 included both patients with limited and extensive SCLC. Thatcher 1999 included SCLC without providing details on disease stage. Three studies included patients with non‐small cell lung cancer (NSCLC) only. Debus 2006 included NSCLC patients with inoperable stage III, Wright 2007 and Milroy 2003 included advanced stage NSCLC patients. Vansteenkiste 2002 included patients with limited and advanced stage SCLC and NSCLC.
Head and neck cancer
Three studies included patients with head and neck cancer only, including stages I‐IV (EPO‐GBR‐7) stages III and IV (Henke 2003) or non metastatic stages I‐IV only (Machtay 2007). Patients in these studies received radiotherapy.
Cervical cancer
Two studies included patients with cervical cancer only, both studies were restricted to patients in stages IIB to IVA (Thomas 2008; Strauss 2008). Patients in these studies received radiochemotherapy.
Ovarian cancer
Three studies included patients with ovarian cancer only, of these, two studies included patients with stages I‐IV (EPO‐INT‐1; Wilkinson 2006). The third study included patients in stage II‐IV (Ten Bokkel Huinink 1998).
Gastric or rectal cancer
One study was restricted to patients with gastric and rectal cancer (stages I‐III) (Vadhan‐Raj 2004). Patients received radiochemotherapy.
Multiple myeloma
Two studies were restricted to patients with multiple myeloma (Dammacco 2001; OBE/EPO‐INT‐03).
Chronic lymphocytic leukemia
Two studies included chronic lymphocytic leukemia (CLL) patients only (CC2574‐P‐174; Rose 1994). Patients received chemotherapy or corticosteroids only
Mixed cancer populations
The other 24 studies included mixed cancer populations.
Various hematological malignancies
Four studies were restricted to patients with different hematological malignancies (Hedenus 2003; Osterborg 1996; Osterborg 2002; Cazzola 1995).
Various solid tumors
Fixe studies were restricted to patients with different solid tumors (Kotasek 2003; Kotasek 2002; Oberhoff 1998; Savonije 2005; Huddart 2002)
Both solid tumors and hematological malignancies
Fifteen studies included patients with a wide range of different tumor entities, including both patients with solid cancer and hematological malignancies (Charu 2007; Ray‐Coquard 2006; Littlewood 2001; EPO‐INT‐3; Abels 1993; Henry 1995; Case 1993; Witzig 2005; Razzouk 2006; Quirt 1996; Gordon 2006; Taylor 2005; Smith 2008; Thomas 2002; Boogaerts 2003).
Cancer treatment
In thirty eight studies patients received chemotherapy during ESA treatment. In two of these studies (Moebus 2007; Untch 2008) the chemotherapy was followed by radiotherapy. However, in both studies ESA was given only during the duration of chemotherapy and the studies were therefore categorized in the chemotherapy population. In two studies (CC2574‐P‐174; Rose 1994), both studies included CLL patients only, 40% (information taken from clinical study report (CSR) (CC2574‐P‐174) and 41% (information taken from CSR (Rose 1994)) of the patients received no chemotherapy during ESA treatment. These studies were categorized as “mixed”.
Note: the investigator of these two studies (CC2574‐P‐174; Rose 1994) had recommended to evaluate the studies in the “chemotherapy” population. However, based on our predefined criteria that 70% of a study population had to receive a planned treatment to be categorized within that treatment group we decided not to include these two studies in the chemotherapy population.
In three of the included studies patients received radiotherapy only, in all of these three studies only patients with head and neck cancer were included (EPO‐GBR‐7; Henke 2003; Machtay 2007). In another five studies patients were receiving a combined chemo radiotherapy, defined as concomitant use of chemotherapy and radiotherapy. These studies included patients with cervical cancer (Strauss 2008; Thomas 2008), SCLC (Goss 2005), NSCLC (Debus 2006) and gastric and rectal cancers (Vadhan‐Raj 2004), none of these studies included patients with head and neck cancer.
In the study EPO‐GER‐22 (Debus 2006) chemotherapy was followed by radiotherapy. However, since the planned interval between chemotherapy and radiotherapy was short it was decided to classify this study as “radiochemotherapy” study. These five studies were evaluated together with the three radiotherapy studies in the radio(chemo)therapy population. In sensitivity analyses we explored whether regrouping of these studies would influence the results (see Appendix 3).
In five of the included studies patients did not receive concomitant myelosuppressive chemotherapy and/or radiotherapy (Charu 2007; Smith 2008; Gordon 2006; Wright 2007; Abels 1993).
Apart from the two studies described above (Rose 1994; CC2574‐P‐174) no other study was categorized as “mixed”, i.e. in no other study less than 70% but more than 30% of the patients were receiving either chemotherapy or radiotherapy or no anticancer treatment.
Only seven of the 48 studies, where a myelosuppressive anticancer treatment was given, had a prespecified chemotherapy or radiotherapy protocol that targeted a homogenous cancer population (Untch 2008; Witzig 2005; Debus 2006; Strauss 2008; Thomas 2008; Moebus 2007; Machtay 2007). For sensitivity analyses see Appendix 3.
ESA dosages and schedules
The frequency of ESA application ranged from seven times per week for the short lasting ESA preparations to once every four weeks for the long lasting ESA preparations. Most often ESAs were applied three times per week (26 studies) or once per week (15 studies). In the ELYPSE 4 study (Ray‐Coquard 2006) the frequency was dependent on body weight of the patients, e.g. if body weight < 45 kg patients received 2 x 10000 IU per week, if body weight 45 to 89 kg 3 x 10000 IU per week and for patients with body weight > 90 kg the dose was 4 x 10000 IU per week. In the study 20010145 (Pirker 2008) the frequency changed over time, i.e. 1 x 300 µg once per week sc weeks 1‐4 then 300 µg three times per week starting week 5 onwards.
In all but one study (Razzouk 2006) ESA was given subcutaneously. In the study by (Razzouk 2006) ESA was given intravenously.
In 19 studies ESAs were given in a fixed dose, i.e. independent from body weight. In 27 studies the individual ESA dosage was calculated based on the patient’s body weight. In six studies (Ray‐Coquard 2006; EPO‐GBR‐7; Milroy 2003; Wilkinson 2006; Pronzato 2002; Thomas 2002) the dose was adjusted, i.e. there were different fix dosages dependent on the weight or the age of the patients. For example, in the study EPO‐INT‐50 (Thomas 2002) patients with body weight < 45 kg received 3 x 5000 IU per week and patients with body weight > 45 kg received 3 x 10000 IU ESA per week. In the study MF4250 the ESA dose was titrated (Osterborg 1996).
The planned weekly Epoetin (alpha or beta) dose ranged from 21000 IU up to 63000 IU. Studies were classified based on an assumed average dose per study and not per patient. In detail: for studies where patients were receiving weight based Epoetin dosages the overall dose for the entire study was calculated based on a assumed patient weight of 70 kg. For the present analysis the doses were not calculated for the individual patient.
The planned weekly Darbepoetin dose ranged from 100 microgram up to 157.5 microgram. For patients receiving weight based Darbepoetin dosages the dose was calculated based on an assumed patient weight of 70 kg for the entire study. For the present analysis the doses were not calculated for the individual patient.
In 19 studies patients were planned to receive on average less than 40000 IU Epoetin or less than 100 micro grams Darbepoetin per week. In 12 studies patients were planned to receive 40000 IU Epoetin or 100 micro grams Darbepoetin per week. In eight studies patients were planned to receive on average more than 40000 IU Epoetin or more than 100 micrograms Darbepoetin per week. In 14 studies the planned ESA dosages depended on various factors and we could therefore not calculate a single ESA dosage per study.
The planned duration of ESA administration ranged from eight weeks up to 52 weeks. In 20 studies the duration of ESA administration was dependent on the duration of chemotherapy, i.e. ESA was given during the duration of chemotherapy. In one study Smith 2008 patients in the active study received ESA for 16 weeks and could continue ESA treatment for additional 16 weeks after the end of study period. Patients in the control group did not receive ESA. For the present analysis this study was categorized as “ESA treatment longer than 17 weeks”.
Cross‐over
In twelve studies patients in both the control arm and the active arm were allowed to receive ESAs after a defined study period (Charu 2007; Kotasek 2003; Kotasek 2002; CC2574‐P‐174; Dammacco 2001; EPO‐INT‐3; Leyland‐Jones 2003; Abels 1993; Case 1993; Henry 1995; Rose 1994; Oberhoff 1998). Our aim was to include only events and time under observation during this defined treatment period in the analysis. Therefore, these studies were evaluated for both the on study mortality and overall survival analysis restricted to the active treatment phase during which control patients did not receive ESAs.
Cross‐over studies were included in the analysis as follows:
Three studies provided by Amgen:
Charu 2007, study 53081: last actual ESA dose plus 14 days (truncated before 1. drug injection during open label phase, as provided by the investigator)
Kotasek 2003, study 35466: last actual ESA dose days plus 21 days (truncated before 1. drug injection during open label phase, as provided by the investigator)
Kotasek 2002, study 26117: last actual ESA dose days plus 28 days (truncated before 1. drug injection during open label phase, as provided by the investigator)
Eight studies provided by Johnson & Johnson (studies Dammacco 2001, Leyland‐Jones 2003, Case 1993, EPO‐INT‐3, CC2574‐P‐174 , Henry 1995, Rose 1994, Abels 1993.
All studies were truncated at termination visit plus 28 days in both arms
One study provided by Roche (Oberhoff 1998):
The study was truncated as provided by the company; i.e. for the control arm we received the data from the controlled study phase only, in the ESA arm the follow‐up was apparently longer.
For the study EPO‐INT‐76 (Leyland‐Jones 2003) it was discussed whether there was a relevant “cross‐over” after the end of the active study phase since the study was stopped prematurely. However, in the CSR it is reported that 641 patients continued in the open label phase. Of those 413 did not receive ESA and 228 (placebo 134, ESA 94) patients were treated with ESA in the open label phase. The median exposure to ESA in this population was 4.14 weeks (range 0.1; 50.1). The survival evaluation for the study EPO‐INT‐76 was therefore restricted to the active study phase. For a post hoc analysis percentages of patients receiving ESAs after the controlled phase were recorded from either the clinical study report or provided by the investigator and an exploratory survival analysis was conducted, see Appendix 4.
Hb ceiling
Hb ceiling was defined as Hb value when ESA had to be stopped. In none of the included studies the ceiling was 12 g/dL or below. In six studies the ceiling was 13 g/dL, in 20 studies 14 g/dL, in nine studies 15 g/dL and in two studies the ceiling was 16 g/dL. In nine studies the ceilings for men and women were different. In seven of these studies the ceiling was 15 g/dL for men and 14 g/dL for women, in two of the studies (EPO‐INT‐3, Machtay 2007) the ceiling was 16 g/dL for men and 14 g/dL for women. Two studies used different ceilings for different patients groups (MF4313 for Multiple myeloma (MM) Hb 13 g/dL, for NHL Hb 15 g/dL) (Cazzola 1995) or different age groups (PR99‐11‐034/044 for children aged > 12 Hb >= 15 g/dL, for children aged < 12 Hb >= 14 g/dL) (Razzouk 2006). In four studies: J89‐040 (Rose 1994), CC2574‐P‐174, I88‐036, 87‐018, 87‐019 (Henry 1995), I88‐037, 87‐016, 87‐017 (Case 1993) the ceiling was defined based on hematocrit units: ceiling hematocrit 38% in the studies I88‐036, 87‐018, 87‐019 (Henry 1995), I88‐037, 87‐016, 87‐017 (Case 1993); in the studies J89‐040 (Rose 1994) and CC2574‐P‐174 there was no explicit hematocrit ceiling reported but the Hct was to be maintained between 38% and 40%. Both studies followed similar/identical study protocols. After discussion with the investigator of these studies Hct 40% was used as ceiling for these studies. To convert the Hct based ceilings into Hb based ceilings the Hct values were multiplied with 0.34. In one study the ceiling was not reported (Abels 1993).
For two studies the ceiling was changed during the study. For EPO‐GER‐22 (Debus 2006) the initial Hb ceiling was 14 g/dL, after 17.11.2003 the ceiling was 13 g/dL. For EPO‐CAN‐15 (Goss 2005) the initial ceiling was 16 g/dL, after 1.12.2002 the ceiling was 14 g/dL. For the present analysis we computed the ceiling for each individual patient based on the ceiling that was valid on the day the patient was randomized.
Since several studies had used different ceilings for different patient populations, e.g. depending on sex, age and underlying disease, or changed the ceiling over time, ceiling categories for the analyses were constructed based on the patient level information.
Iron supplementation
In seven studies patients received a fixed iron supplementation. In 26 studies iron was given as needed following a specific protocol and in 19 studies iron was given as needed by discretion of physician or institutional policy. In none of the studies it was explicitly reported that iron should not be used. In one study (Grote 2005) iron supplementation was coded as “other”. In this study it was reported in the clinical study report how many patients received oral iron during study, but there was no statement if and how patients and physicians were advised to use iron. For the present analysis the study was evaluated in the category “iron given as needed by discretion of physician or institutional policy”.
In seven studies iron was given only in the ESA arm (Machtay 2007; Untch 2008; Moebus 2007; Debus 2006; Savonije 2005) or the policies for iron monitoring and supplementation were different in ESA and control arm (OBE/EPO‐INT‐03; EPO‐GER‐20). In the Savonije et al 2005 (Savonije 2005) study ESA patients had to receive iron mandatory by protocol, it is unclear from the clinical study report whether patients in the control arm received iron as well. In one unpublished study (OBE/EPO‐INT‐03) the iron status in the ESA arm was to be monitored and if needed supplemented. In the another unpublished study (EPO‐GER‐20) patients in the ESA arm received iron fixed and patients in the control arm received iron only if needed.
Excluded studies
see 'Characteristics of excluded studies' table
Risk of bias in included studies
Allocation
Study level parameter
Randomization and concealment of allocation
Sixteen studies were judged independently by two reviewers (JB, SK) to have reported an adequate randomization procedure, for 37 studies the method reported was judged to be unclear based on the available documents, i.e. clinical study reports, study protocols and publications if available. Thirty‐six studies were judged to have reported adequate allocation concealment, for 17 studies the method reported was judged to be unclear based on the available documents. For ten of the 53 included studies both randomization and concealment of allocation was judged to be adequate. For another eleven studies both method of randomization and concealment of allocation were judged to be unclear. For 26 studies the method of allocation was judged to be adequate but the method of randomization was unclear. For six studies the method of randomization was judged to be adequate but the method of allocation concealment was unclear.
Blinding
Placebo control
28 studies were placebo controlled and were reported to be “double‐blind”, 25 studies were open‐label studies. The assessment of the quality of the placebo control, i.e. whether patients, physicians and outcome assessors were truly masked to the treatment, is not included in the current report.
Incomplete outcome data
Drop outs
In all but four studies the numbers and reasons for withdrawal/drop out were reported in the CSRs. Details for the four studies not reporting drop outs: for three studies no clinical study report of full text publication was available and therefore information on number and reason for drop out was not available (Untch 2008; Quirt 1996; Thomas 2002). In the fourth study the number but not the reason for drop outs are reported in the statistical report, a full CSR was not available (Gordon 2006).
Selective reporting
Publication
By June 26 2008, 32 of the included studies had been published as full text, 15 had been published as abstracts only, four studies (CC2574‐P‐174; EPO‐GBR‐7; EPO‐INT‐1; EPO‐INT‐3 had been reported in the documents of the ODAC hearings in 2004, 2007 or 2008, two studies (EPO‐GER‐20 and OBE/EPO‐INT‐03) were unpublished.
For details of the study characteristics see 'Characteristics of studies' table.
Other potential sources of bias
Other design aspects
Study design (endpoint)
Five of the included studies evaluated overall survival as their primary endpoint (Pirker 2008; Aapro 2008; Leyland‐Jones 2003; Debus 2006; Untch 2008). Fifteen of the included studies evaluated overall survival as secondary endpoint. In 29 studies survival was assessed as safety or adverse event outcome. For two studies it was not reported whether survival was assessed as an endpoint or not (Dammacco 2001; O'Shaugnessy 2005). However, in both studies deaths were reported in the safety analyses chapters of the clinical study reports and the studies were therefore categorized as “mortality assessed as adverse event only”. One study was categorized as “other” (Smith 2008). In this study deaths were “reported as AEs during the study period but they were also reported during the long‐term follow‐up and these later deaths were not considered AEs since they occurred outside the AE reporting period” (communication with investigator). This study was categorized as “mortality assessed as adverse events only” in the analysis.
Long‐term follow‐up
Twenty four studies were planned for a long‐term follow‐up of at least 12 months post active study phase. Twenty‐nine studies did not fulfill this definition. For two of these studies (Ray‐Coquard 2006; Wright 2007) the investigator of the respective study had indicated that the study conducted a long‐term follow‐up, since the available study documents did not report that this follow‐up was planned, these studies were evaluated as “not designed for long‐term follow‐up”. The effect of this potential misclassification can be assessed in a sensitivity analysis.
Completed studies
Of the 53 included studies two studies (Moebus 2007; Untch 2008) were ongoing at the time of analysis. Fourteen of the included studies were terminated or halted prematurely by its own study protocol definition. Thirty‐seven studies were completed by their own study protocol definition.
Missing or not reported data
The amount of missing or not reported data for specific variables is outlined below. The distribution of missing or not reported data was generally not balanced across studies: several variables had not been provided for entire studies. For example for several studies we received no information on documented history of thromboembolic event, hypertension, diabetes mellitus or cardiovascular events, as well as no information of previous or current chemotherapy or radiotherapy. For few studies we had information of the treatment status of the patient, i.e. untreated or in complete response, partial response, stable disease etc, for 71% of the included patients this information was missing. For about 80% of patients we had no structured information on disease stage, i.e. whether the patient had limited, advanced or metastatic disease. The information on stage at diagnosis was therefore generated based on the free text entries per patient and the available study documents (Table 1).
1. Missing or not reported data per variable, in order of percentage missing.
| Missing in ESA arm | Missing in control arm | |
| Total included | 7634 | 6299 |
| Sex | 0 | 0 |
| Age | 6 (0.1%) | 6 (0.1%) |
| Tumor type* | 17 (0.2%) | 25 (0.4%) |
| Region (country) | 231 (3.0%) | 170 (2.7%) |
| Hb at baseline | 252 (3.3%) | 274 (4.3%) |
| Cancer stage at study entry (free text entry) | 761 (10.0%) | 732 (11.6%) |
| Derived variable stage (metastatic/advanced versus not) | 1036 (13.6%) | 745 (11.8%) |
| Hct at baseline | 1493 (19.6%) | 1404 (22.3%) |
| Chemotherapy given during ESA study? | 1501 (19.7%) | 1252 (19.9%) |
| BMI baseline | 1515 (19.8%) | 973 (15.4%) |
| Documented history of cardiovascular event | 1932 (25.3%) | 1679 (26.7%) |
| Chemotherapy given before ESA study? | 1965 (25.7%) | 1736 (27.6%) |
| Baseline ECOG performance status** | 2035 (26.7%) | 1786 (28.4%) |
| Radiotherapy given during ESA study? | 2097 (27.5%) | 1766 (28.0%) |
| Documented history of thromboembolic events | 2272 (29.8%) | 2041(32.4%) |
| Documented history of hypertension | 2272 (29.8%) | 2041 (32.4%) |
| Radiotherapy given before esa study? | 2529 (33.1%) | 2216 (35.2%) |
| Documented history of diabetes mellitus | 3335 (43.7%) | 2573 (40.8%) |
| Baseline serum epo (mu/ml) | 4371 (57.3%) | 3911 (62.1%) |
| Cancer treatment status at study entry | 5366 (70.3%) | 4613 (73.2%) |
| Cancer stage at study entry | 6123 (80.2%) | 5069 (80.5%) |
*For an independent study we received tumor types based on French pathology terms. To date we have not transferred these data into the uniform coding system developed and used for the present study, the data of that study are coded as “other” for the time being.
**Baseline ECOG status: If other performance score systems such as Karnofsky scores were reported these were used for the analysis but are counted as missing for the present table.
Baseline characteristics and baseline imbalances
Funnel plots were generated to investigate baseline imbalances across all included trials. For continuous variables, means for each trial arm were calculated (active and control arm) and the differences of the means for each study were plotted against the sample size of the corresponding study. For dichotomous variables, proportions for each trial arm were calculated (active and control arm) and the differences of the proportions for each study were plotted against the sample size of the corresponding study. We assessed asymmetry using random‐effects meta‐regression and derived a corresponding P value (Sterne 2001). Funnel plots include pseudo‐95% confidence interval lines, which are drawn around the summary fixed‐effect estimate (red lines).
The following variables were assessed:
Continuous: ECOG, level of serum epo, BMI, time from diagnosis of cancer to randomization, hemoglobin, hematocrit, age
Dichotomous: Sex, ECOG (low versus high), history of thromboembolic event, history of cardiovascular event, history of hypertension, history of diabetes.
Plots are shown in Appendix 5. We found no evidence of baseline imbalances across trials.
Proportional hazard assumption
For each study we plotted log‐log plots for proportional hazard assumption and conducted a Schoenfeld test for residuals. Note: on file, not provided in this review. Overall, in most studies the proportional hazard assumption was fulfilled. In one study (number 43680 (Osterborg 1996)) there was evidence that the proportional hazard assumption was not met (Schoenfeld test p=0.0309).
Censoring
Reverse Kaplan‐Meier curves to assess time to censoring for each trial are on file. In addition, we calculated the hazard ratio for being censored in the ESA arm compared to the control arm for each study and conducted a meta‐analysis based on these estimates. For this analysis patients who were censored in the original trial were considered as an event and patients who died in the original trial were censored for the purpose of this analysis. The meta‐analysis was conducted with a two‐stage random‐effects model and the Forest plot is shown in Figure 5. Overall, there was no evidence for an unbalanced censoring between the ESA and the control arm (HR for being censored when alive 0.97 (95% CI 0.91‐1.03). However, there was evidence for heterogeneity between studies: I² 65.5%, test for heterogeneity p<0.0001. In five studies (53081, 21481, 45434, 70404, 87660) the hazard for being censored was higher in the control arm compared to the ESA arm and in two studies (34917, 36158) patients in the ESA arm were more likely to be censored compared to the control arm. For these studies we compared the hazard ratio of being censored with the hazard ratio for death (Table 2).
5.
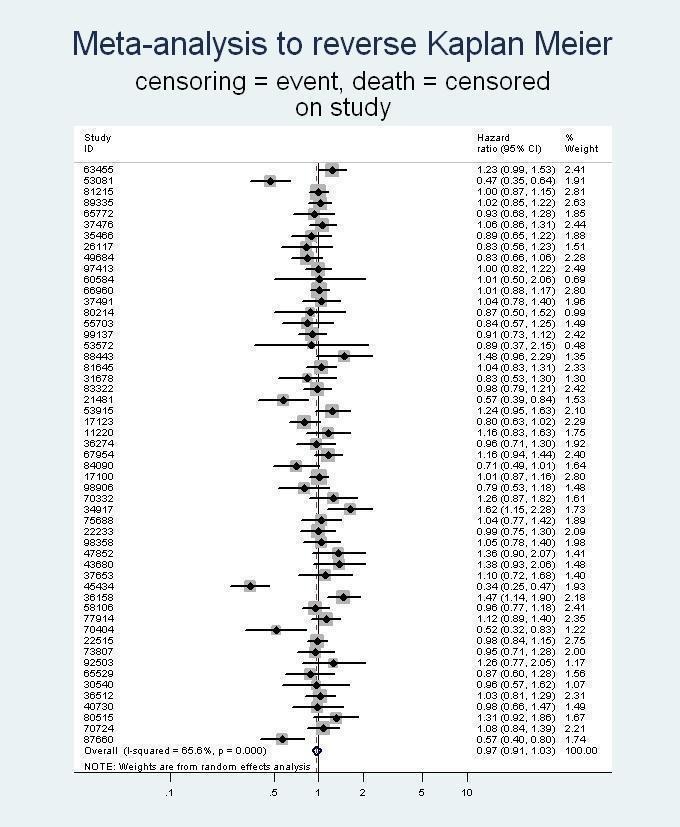
On study mortality: censoring meta‐analysis, HRs < 1,0 indicate that more patients in the control arm had the event ("censoring"), HRs > 1,0 indicate that more patients in the ESA arm had the event ("censoring") compared to controls.
2. Hazard ratios for censoring and hazard ratios for on study mortality in selected studies.
| Study number | On study censoring ESA versus control HR (95% CI)* | On study mortality ESA versus control HR (95% CI)* |
| 53081 | 0.47 (95% CI 0.35, 0.64) | 0.89 (95% CI 0.19, 4.17) |
| 21481 | 0.57 (95% CI 0.39, 0.84) | 0.94 (95% CI 0.06, 15.01) |
| 45434 | 0.34 (95% CI 0.25, 0.47) | 0.62 (95% CI 0.25, 1.58) |
| 70404 | 0.52 (95% CI 0.32, 0.83) | 0 deaths |
| 87660 | 0.57 (95% CI 0.40, 0.80) | 1.58 (95% CI 0.38, 6.61) |
| 34917 | 1.62 (95% CI 1.15, 2.28) | 1.10 (95% CI 0.45, 2.72) |
| 36158 | 1.47 (95% CI 1.14, 1.90) | 1.02 (95% CI 0.42, 2.45) |
* based on two‐stage Cox random‐effects meta‐analysis
In addition, we assessed whether in studies with a statistically significant or borderline increased or decreased hazard ratio for on study mortality, the number of censored patients was balanced between the ESA arm and the control arm, see table below. In conclusion, it seems unlikely that unbalanced censoring between the ESA and the control arm has influenced the overall estimates for ESA on mortality (Table 3).
3. Hazard ratios for censoring and hazard ratios for on study mortality in selected studies.
| Study number | On study censoring ESA versus control HR (95% CI)* | On study mortality ESA versus control HR (95% CI)* |
| 17100 | 1.01 (95% CI 0.87, 1.16) | 1.42 (95% CI 1.08, 1.86) |
| 53572 | 0.89 (95% CI 0.37, 2.15) | 1.68 (95% CI 0.95, 2.98) |
| 67954 | 1.16 (95% CI 0.94, 1.44) | 1.45 (95% CI 0.95, 2.21) |
| 81215 | 1.00 (95% CI 0.87, 1.15) | 1.37 (95% CI 1.05, 1.78) |
| 97413 | 1.00 (95% CI 0.82, 1.22) | 1.38 (95% CI 0.89, 2.13) |
* based on two‐stage Cox random‐effects meta‐analysis
Effects of interventions
On study mortality in all cancer patients
Objective 1 for on study mortality in all cancer patients
Aim: What is the effect of ESAs compared to control for on study mortality in this population and can the effect be explained by baseline imbalances of prognostic factors at patient level?
A total of 53 studies with 13933 patients were included in the analysis of on study mortality. All cancer patients regardless of anticancer treatment received were included in the present analysis. Four studies did not contribute to the present results because there were no deaths during on study period (study 22515 (Moebus 2007), 30540 (Vadhan‐Raj 2004), 66960 (Untch 2008), 70404 (Strauss 2008).
During on study phase 865 out of 7634 patients randomized to the ESA arm and 665 out of 6299 patients randomized to the control arm died. Median follow‐up was 3.71 months (IQR 2.8‐5.1 months) in the ESA arm and 3.94 months (IQR 2.9 to 5.3 months) in the control arm. The overall hazard ratio for patients receiving ESA compared to controls was 1.17 (95% CI 1.06‐1.30) during on study phase based on two‐stage log‐rank fixed‐effects meta‐analysis. Based on a Cox model stratified for study the overall result was 1.17 (95% CI 1.06‐1.30). For results of all statistical models applied, see Table 4.
4. On study mortality for all cancer patients.
| Model | ESA versus control HR (95% CI) | P value* | I² | P value** |
| Two‐stage log‐rank fixed effects model | 1.17 (95% CI 1.06‐1.30) | 0.0025 | 0% | 0.8735 |
| Two‐stage log‐rank random effects model | 1.17 (95% CI 1.06‐1.30) | 0.0025 | 0% | 0.8735 |
| Two‐stage Cox fixed effects model | 1.16 (95% CI 1.05‐1.29) | 0.0042 | 0% | 0.9303 |
| Two‐stage Cox random effects model | 1.16 (95% CI 1.05‐1.29) | 0.0042 | 0% | 0.9303 |
| Cox model stratified by study | 1.17 (95% CI 1.06‐1.30) | 0.0025 | 0.6310 |
*LR test, ** for test of heterogeneity
There was no evidence for heterogeneity between the trials (I‐square 0%, p=0.8735), for Forest plot see Figure 6, for pooled Kaplan‐Meier curve see Appendix 4. There was no evidence for small study effects: linear regression test p=0.1371, rank correlation test of funnel plot asymmetry p=0.9588. For Funnel plot see Figure 7.
6.
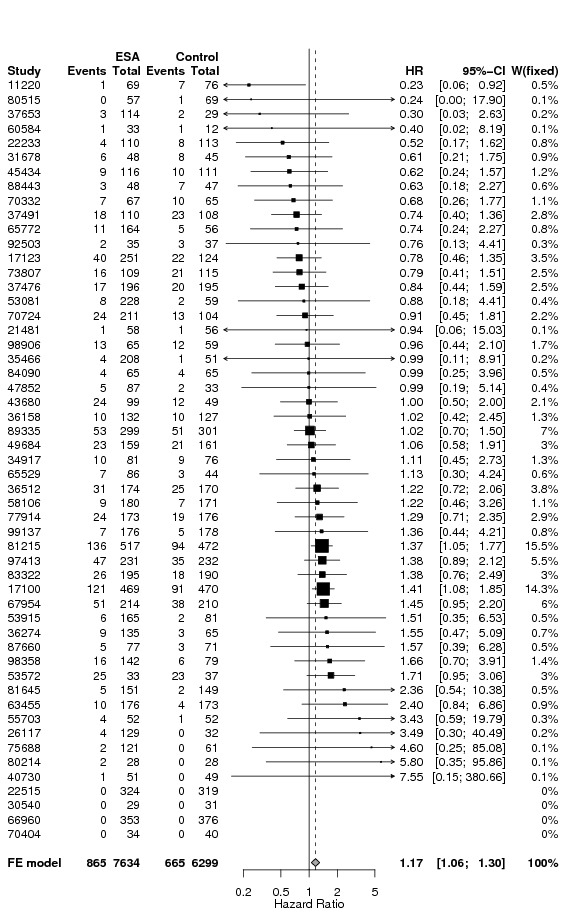
Forest plot for on study mortality in all cancer patients based on two stage log‐rank fixed‐effects meta‐analysis
7.
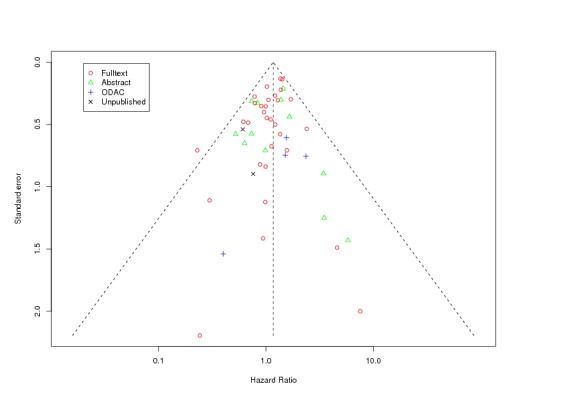
Funnel plot (based on log‐rank estimates) for on study mortality in all cancer patients
Explanation of terms used:
Full text: highest publication achieved is a full text publication
Abstract: highest publication achieved is an abstract publication
ODAC: highest publication achieved is reporting of study results in documents presented at ODAC hearings
Unpublished: to date the study was not published in any of the sources mentioned above
Date of reference: June 26th 2008
Two studies contributed more than 10% weight to the overall analysis (Leyland‐Jones 2003; Smith 2008). In the study published by Leyland‐Jones 2003 (study number 17100) 937 patients with metastatic cancer undergoing chemotherapy received ESA or placebo for 52 weeks, therefore the study has a much longer on study phase compared to other studies. In the study published by Smith et al 2008 (study number 81215) 989 patients were treated with ESA without concomitant myelosuppressive chemotherapy. The impact of single studies was assessed in an influence analysis, see Figure 8. When excluding study 17100 (Leyland‐Jones 2003), the overall HR slightly decreased and the confidence interval still excluded 1. Exclusion of any of the other studies did not markedly change the overall estimate.
8.
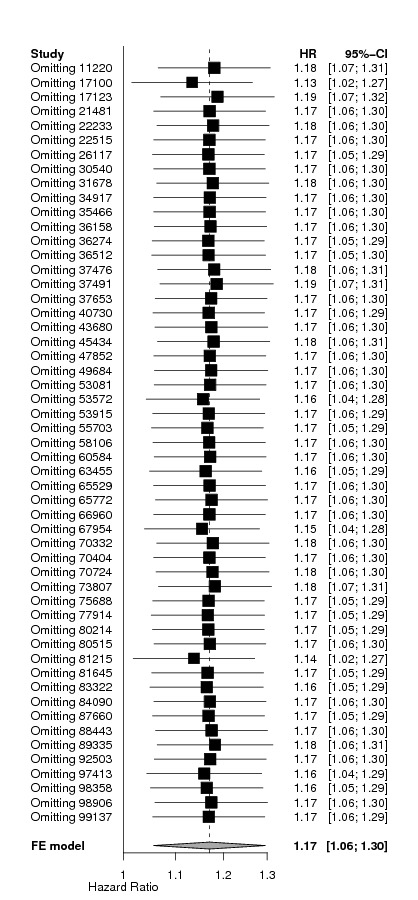
Influence analysis for on study mortality in all cancer patients
Assessment of potential confounders for objective 1
In the next step we conducted bivariate analyses: adjusting on study mortality based on the Cox model stratified by study for one variable at the time. All variables assessed relate to the individual patient data level. The results of the adjusted model were compared with the unadjusted model using LR‐Test. Results of unadjusted and adjusted models as well as P values of LR‐Test are shown in Table 5. We included only patients with full information for the respective variable; patients with missing, unknown or unreported data were excluded. Data were often missing for entire studies; therefore the overall HR might have changed because of the omission of studies. We therefore present both unadjusted and adjusted HRs based on the patient data set with available information.
5. Bivariate analyses for on study mortality in all cancer patients.
| On study mortality for all cancer patients | N included | ESA versus control Unadjusted HR (95% CI) | ESA versus control Adjusted HR (95% CI) |
P value LR‐Test |
| Total | 13933 | 1.17 (95% CI 1.06‐1.30) | ‐ | ‐ |
| Hb at baseline (continuous) | 13407 | 1.17 (95% CI 1.06‐1.30) | 1.18 (95% CI 1.07‐1.31) | 0.0000 |
| Hb at baseline (categorical 1) | 13407 | 1.17 (95% CI 1.06‐1.30) | 1.18 (95% CI 1.06‐1.31) | 0.0000 |
| Hb at baseline (categorical 2) | 13407 | 1.17 (95% CI 1.06‐1.30) | 1.18 (95% CI 1.07‐1.31) | 0.0000 |
| Tumor (categorical 1) | 13891 | 1.17 (95% CI 1.06‐1.30) | 1.17 (95% CI 1.06‐1.30) | 0.0000 |
| Tumor (categorical 2) | 13891 | 1.17 (95% CI 1.06‐1.30) | 1.16 (95% CI 1.05‐1.29) | 0.0000 |
| Sex | 13933 | 1.17 (95% CI 1.06‐1.30) | 1.16 (95% CI 1.05‐1.29) | 0.0000 |
| Age (continuous) | 13921 | 1.17 (95% CI 1.06‐1.30) | 1.17 (95% CI 1.06‐1.30) | 0.0007 |
| Age (categorical) | 13921 | 1.17 (95% CI 1.06‐1.30) | 1.18 (95% CI 1.06‐1.30) | 0.0160 |
| Hct (continuous) | 11036 | 1.18 (95% CI 1.06‐1.31) | 1.19 (95% CI 1.07‐1.32) | 0.0000 |
| Hct (categorical) | 11036 | 1.18 (95% CI 1.06‐1.31) | 1.19 (95% CI 1.07‐1.33) | 0.0000 |
| Baseline serum EPO (cont.) | 5651 | 1.11 (95% CI 0.95‐1.29) | 1.10 (95% CI 0.95‐1.28) | 0.1798 |
| Baseline serum EPO (cat.) | 5651 | 1.11 (95% CI 0.95‐1.29) | 1.10 (95% CI 0.95‐1.28) | 0.0006 |
| ECOG (0 vs 1 vs 2 vs 3 vs 4) | 10112 | 1.19 (95% CI 1.06‐1.33) | 1.17 (95% CI 1.05‐1.32) | 0.0000 |
| ECOG (0,1,2 vs 3,4) | 10225 | 1.18 (95% CI 1.06‐1.33) | 1.19 (95% CI 1.06‐1.34) | 0.0000 |
| BMI (categorical) | 11445 | 1.16 (95% CI 1.04‐1.30) | 1.17 (95% CI 1.04‐1.31) | 0.0000 |
| History of thromboembolic events | 9620 | 1.20 (95% CI 1.06‐1.34) | 1.19 (95% CI 1.06‐1.34) | 0.1105 |
| History of cardiovascular events | 10322 | 1.20 (95% CI 1.06‐1.34) | 1.19 (95% CI 1.06‐1.34) | 0.1002 |
| History of hypertension | 9620 | 1.20 (95% CI 1.06‐1.34) | 1.20 (95% CI 1.06‐1.34) | 0.8464 |
| History of diabetes mellitus | 8025 | 1.20 (95% CI 1.06‐1.35) | 1.20 (95% CI 1.06‐1.35) | 0.4497 |
| Geographical region [region_cat] | 13532 | 1.17 (95% CI 1.05‐1.29) | 1.16 (95% CI 1.05‐1.29) | 0.0001 |
| Metastatic vs non‐metastatic | 12152 | 1.21 (95% CI 1.09‐1.35) | 1.21 (95% CI 1.08‐1.35) | 0.0000 |
| Time from cancer diagnosis to randomization | 4586 | 1.17 (95% CI 0.99‐1.39) | 1.18 (95% CI 1.00‐1.40) | 0.0000 |
Based on these analyses and the number of data available for each variable, we conducted four different models, all of which are presented in Table 6. For model 1 we included the variables age, sex, and Hb at baseline and tumor type into the model. For model 2 we used the same variables as in model 1 plus stage of underlying tumor. For model 3 we used the same variables as in model 1 plus BMI and region, for model 4 we used the same variables as in model 1 and 3 plus ECOG and hematocrit. For the variables age, Hb, serum EPO and BMI the association between the exposure and the outcome was not linear (graph not shown). Therefore, these continuous variables were converted into prespecified categories. Hematocrit was converted into categories as well for the ease of interpretation. The variable “time for cancer diagnosis to randomization” was not included in the model because of too many missing data.
6. Multivariate analysis on study mortality in all cancer patients.
| On study mortality in all cancer patients | Model 1 | Model 2 | Model 3 | Model 4 |
| Patients included | n=13353 | n=11636 | n=10599 | n=6547 |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| ESA vs control unadjusted* | 1.17 (95% CI 1.06‐1.30) | 1.22 (95% CI 1.09‐1.36) | 1.16 (95% CI 1.03‐1.30) | 1.20 (95% CI 1.06‐1.37) |
| ESA vs control adjusted** | 1.17 (95% CI 1.06‐1.30) | 1.21 (95% CI 1.08‐1.35) | 1.16 (95% CI 1.03‐1.30) | 1.23 (95% CI 1.08‐1.39) |
| Hb at baseline | ||||
| Hb < 8 g/dL | 1 | 1 | 1 | 1 |
| Hb 8‐10 g/dL | 0.70 (95% CI 0.58‐0.85) | 0.66 (95% CI 0.53‐0.81) | 0.69 (95% CI 0.57‐0.85) | 0.83 (95% CI 0.62‐1.10) |
| Hb 10‐12 g/dL | 0.49 (95% CI 0.40‐0.60) | 0.46 (95% CI 0.37‐0.57) | 0.52 (95% CI 0.42‐0.65) | 0.71 (95% CI 0.51‐0.98) |
| Hb 12‐14 g/dL | 0.33 (95% CI 0.26‐0.42) | 0.31 (95% CI 0.24‐0.40) | 0.38 (95% CI 0.29‐0.49) | 0.52 (95% CI 0.35‐0.77) |
| Hb > 14 g/dL | 0.28 (95% CI 0.20‐0.39) | 0.27 (95% CI 0.20‐0.38) | 0.33 (95% CI 0.23‐0.46) | 0.45 (95% CI 0.26‐0.79) |
| Age at randomization | ||||
| 18 ‐ 35 yrs | 0.90 (95% CI 0.55‐1.46) | 1.04 (95% CI 0.61‐1.77) | 0.88 (95% CI 0.51‐1.54) | 0.79 (95% CI 0.42‐1.47) |
| 35 ‐ 45 yrs | 1 | 1 | 1 | 1 |
| 45 ‐ 55 yrs | 1.09 (95% CI 0.86‐1.39) | 1.08 (95% CI 0.84‐1.40) | 1.15 (95% CI 0.87‐1.52) | 1.03 (95% CI 0.77‐1.37) |
| 55 ‐ 65 yrs | 1.23 (95% CI 0.97‐1.54) | 1.25 (95% CI 0.98‐1.60) | 1.37 (95% CI 1.05‐1.78) | 1.19 (95% CI 0.90‐1.57) |
| 65 ‐ 75 yrs | 1.30 (95% CI 1.03‐1.64) | 1.28 (95% CI 0.99‐1.64) | 1.51 (95% CI 1.15‐1.97) | 1.33 (95% CI 1.00‐1.77) |
| > 75 ys | 1.40 (95% CI 1.07‐1.82) | 1.46 (95% CI 1.09‐1.94) | 1.52 (95% CI 1.12‐2.08) | 1.22 (95% CI 0.87‐1.71) |
| Sex | ||||
| Male | 1 | 1 | 1 | 1 |
| Female | 0.80 (95% CI 0.70‐0.91) | 0.83 (95% CI 0.72‐0.96) | 0.83 (95% CI 0.72‐0.96) | 0.84 (95% CI 0.71‐0.99) |
| Tumor category | ||||
| Hematological malignancies | 1 | 1 | 1 | 1 |
| Breast cancer | 1.55 (95% CI 1.09‐2.20) | 1.39 (95% CI 0.88‐2.19) | 1.60 (95% CI 1.08‐2.38) | 1.72 (95% CI 1.12‐2.64) |
| Head and neck cancer | 2.29 (95% CI 1.24‐4.22) | 1.84 (95% CI 0.87‐3.86) | 1.69 (95% CI 0.83‐3.44) | 1.71 (95% CI 0.71‐4.12) |
| Lung cancer | 3.15 (95% CI 2.32‐4.30) | 2.61 (95% CI 1.74‐3.91) | 2.97 (95% CI 2.06‐4.29) | 3.49 (95% CI 2.35‐5.18) |
| Gastrointestinal | 2.82 (95% CI 2.05‐3.88) | 2.54 (95% CI 1.67‐3.87) | 2.59 (95% CI 1.79‐3.77) | 2.87 (95% CI 1.92‐4.30) |
| Gynecological | 1.47 (95% CI 0.98‐2.19) | 1.22 (95% CI 0.74‐2.01) | 1.69 (95% CI 1.08‐2.64) | 2.14 (95% CI 1.31‐3.38) |
| Genitourinary | 2.16 (95% CI 1.54‐3.05) | 1.97 (95% CI 1.28‐3.03) | 2.14 (95% CI 1.44‐3.18) | 2.48 (95% CI 1.63‐3.79) |
| Other | 2.85 (95% CI 1.99‐4.07) | 2.63 (95% CI 1.67‐4.16) | 2.76 (95% CI 1.82‐4.18) | 3.01 (95% CI 1.91‐4.74) |
| Tumor stage | ||||
| Metastatic/advanced | ‐ | 1 | ‐ | ‐ |
| Not Metastatic/advanced | ‐ | 0.47 (95% CI 0.37‐0.59) | ‐ | ‐ |
| Region | ||||
| Northern America | ‐ | ‐ | 1 | 1 |
| Southern Europe | ‐ | ‐ | 1.35 (95% CI 0.90‐2.02) | 1.33 (95% CI 0.87‐2.04) |
| Australia & New Zealand | ‐ | ‐ | 1.18 (95% CI 0.75‐1.86) | 1.26 (95% CI 0.76‐2.07) |
| Eastern Europe | ‐ | ‐ | 1.66 (95% CI 1.19‐2.31) | 1.64 (95% CI 1.16‐2.31) |
| Northern Europe | ‐ | ‐ | 1.75 (95% CI 1.20‐2.55) | 1.94 (95% CI 1.31‐2.88) |
| Western Europe | ‐ | ‐ | 1.75 (95% CI 1.21‐2.51) | 1.84 (95% CI 1.25‐2.70) |
| Other | ‐ | ‐ | 1.38 (95% CI 0.74‐2.58) | 1.76 (95% CI 0.92‐3.38) |
| BMI | ||||
| < 19 kg/m² | ‐ | ‐ | 1 | 1 |
| 19‐25 kg/m² | ‐ | ‐ | 0.64 (95% CI 0.53‐0.77) | 0.65 (95% CI 0.53‐0.80) |
| 25‐30 kg/m² | ‐ | ‐ | 0.51 (95% CI 0.41‐0.62) | 0.50 (95% CI 0.40‐0.63) |
| > 30 kg/m² | ‐ | ‐ | 0.42 (95% CI 0.33‐0.54) | 0.44 (95% CI 0.34‐0.58) |
| Hct at baseline | ||||
| Hct < 23.5% | ‐ | ‐ | ‐ | 1 |
| Hct 23.5%‐29.4% | ‐ | ‐ | ‐ | 0.68 (95% CI 0.46‐1.01) |
| Hct 29.4%‐35.3% | ‐ | ‐ | ‐ | 0.52 (95% CI 0.34‐0.79) |
| Hct 35.3%‐41.2% | ‐ | ‐ | ‐ | 0.49 (95% CI 0.30‐0.79) |
| >Hct 41.2% | ‐ | ‐ | ‐ | 0.47 (95% CI 0.26‐0.84) |
| Performance score | ||||
| ECOG 0, 1 or 2 | ‐ | ‐ | ‐ | 1 |
| ECOG 3 or 4 | ‐ | ‐ | ‐ | 4.03 (95% CI 2.83‐5.74) |
*unadjusted based on the patients included in respective model, **adjusted for variables outlined in the columns
Summary points for objective 1 for on study mortality in all cancer patients
ESAs increased on study mortality in cancer patients by factor 1.17 (HR 1.17; 95% CI 1.06‐1.30, n =13933).
Available evidence does not support the hypothesis that baseline imbalances of prognostic factors analyzed influenced the overall results.
Objective 2 for on study mortality in all cancer patients
Aim: Is there a specific subgroup of patients that is at increased or decreased risk to die when receiving ESAs compared to controls? Are there design aspects at study level that influenced the effects of ESA on survival?
We tested for interaction between ESA treatment and specific variables describing patient and study characteristics, results of interaction test are outlined in Table 7, results with estimates for subgroups are outlined in Appendix 6).
7. Assessment of interaction for on study mortality in all cancer patients.
| On study mortality, all cancer patients | Patients included | P value for interaction* |
| Total included | 13933 | ‐ |
| Patient level characteristics (subgroup analysis) | ||
| Hb at baseline (continuous) | 13407 | 0.8164 |
| Hb at baseline (categorical 1) | 13407 | 0.7479 |
| Hb at baseline (categorical 2) | 13407 | 0.7917 |
| Tumor (categorical 1) | 13891 | 0.1623 |
| Tumor (categorical 2) | 13891 | 0.4697 |
| Sex | 13933 | 0.8607 |
| Age (continuous) | 13921 | 0.8677 |
| Age (categorical) | 13921 | 0.5002 |
| Hct (continuous) | 11036 | 0.5656 |
| Hct (categorical) | 11036 | 0.0110 |
| Baseline serum EPO (continuous) | 5651 | 0.2139 |
| Baseline serum EPO (categorical) | 5651 | 0.5436 |
| ECOG | 10112 | 0.6324 |
| ECOG (0,1,2 vs 3,4) | 10225 | 0.5600 |
| BMI (categorical) | 11445 | 0.7246 |
| History of thromboembolic events | 9620 | 0.0605 |
| History of cardiovascular events | 10322 | 0.6227 |
| History of hypertension | 9620 | 0.7626 |
| History of diabetes mellitus | 8025 | 0.6962 |
| Geographical region [region_cat] | 13532 | 0.1707 |
| Metastatic vs non‐metastatic | 12152 | 0.7588 |
| Planned Hb ceiling (categorical 1) | 13730 | 0.9777 |
| Planned Hb ceiling (categorical 2) | 13730 | 0.8840 |
| Study level characteristics (subset analysis) | ||
| Placebo controlled | 13933 | 0.3780 |
| Randomization (adequate vs unclear) | 13933 | 0.9848 |
| Allocation (adequate vs unclear) | 13933 | 0.2347 |
| Endpoint overall survival | 13933 | 0.4074 |
| Year of last patient randomized into study (categorical) | 13933 | 0.2351 |
| Source of data (company versus independent) | 13933 | 0.1281 |
| Patient population (chemotherapy, radiochemotherapy, radiotherapy, none, mixed) |
13933 | 0.4148 |
| Iron category | 13933 | 0.4784 |
| Planned ESA treatment duration (categorical) | 13933 | 0.3338 |
| Planned weekly ESA dosage (categorical) | 13933 | 0.1227 |
| Planned frequency of ESA administration (categorical) | 13933 | 0.0274 |
*P value for interaction based on LR test, patients with missing data are excluded from LR test
Three variables (planned frequency of ESA administration, history of thromboembolic events, hematocrit) showed a statistically significant (p<0.1) interaction term in the bivariate analyses and were included in the multivariate model (model 1). This model included the variables, age and sex, Hb at baseline and tumor category, for P values of LR tests see Table 8.
8. Assessment of selected interaction terms for on study mortality in all cancer patients, univariate and multivariate analyses.
| On study mortality all cancer patients | Patients total | ESA arm | Control arm | Bivariate analysis ESR versus control | Multivariate analysis ESR versus control adjusted for age, sex, Hb, tumor type | ||||||||
| N | n | N | % | n | N | % | HR | 95% CI | p* | HR | 95% CI | p* | |
| Hct at baseline, categorical | |||||||||||||
| < 23.5% | 390 | 55 | 210 | 26% | 24 | 180 | 13% | 2.19 | 1.35‐3.55 | 2.12 | 1.30‐3.48 | ||
| 23.5‐29.4% | 2788 | 199 | 1567 | 13% | 191 | 1221 | 16% | 0.96 | 0.78‐1.77 | 0.96 | 0.79‐1.18 | ||
| 29.4‐35.3% | 4615 | 321 | 2692 | 12% | 223 | 1923 | 12% | 1.17 | 0.99‐1.39 | 0.0110 | 1.15 | 0.97‐1.37 | 0.0191 |
| 35.3‐41.2% | 2458 | 176 | 1258 | 14% | 130 | 1200 | 11% | 1.41 | 1.12‐1.76 | 1.39 | 1.10‐1.74 | ||
| > 41.2% | 785 | 48 | 414 | 12% | 40 | 371 | 11% | 1.12 | 0.73‐1.70 | 1.15 | 0.76‐1.76 | ||
| Missing / not reported | 2897 | 66 | 1493 | 4% | 57 | 1404 | 4% | 1.09 | 0.76‐1.55 | ‐ | omitted | ‐ | |
| History of thromboembolic events | |||||||||||||
| Yes | 561 | 40 | 318 | 13% | 42 | 243 | 17% | 0.80 | 0.52‐1.23 | 0.77 | 0.50‐1.19 | 0.0440 | |
| No | 9059 | 637 | 5044 | 13% | 474 | 4015 | 12% | 1.23 | 1.09‐1.39 | 0.0605 | 1.22 | 1.08‐1.38 | |
| Missing / not reported | 4313 | 188 | 2272 | 8% | 149 | 2041 | 7% | 1.09 | 0.87‐1.35 | omitted | |||
| Planned frequency of ESA application | |||||||||||||
| Three times per week or more frequent | 6131 | 311 | 3458 | 9% | 238 | 2673 | 9% | 1.01 | 0.85‐1.20 | 1.01 | 0.85‐1.21 | ||
| Once per week | 3948 | 303 | 1972 | 15% | 231 | 1976 | 12% | 1.39 | 1.18‐1.66 | 0.0274 | 1.41 | 1.18‐1.67 | 0.0369 |
| Every second week or less frequent | 3036 | 180 | 1795 | 10% | 122 | 1241 | 10% | 1.25 | 0.99‐1.57 | 1.19 | 0.94‐1.50 | ||
| Other | 818 | 71 | 409 | 17% | 74 | 409 | 18% | 0.93 | 0.67‐1.29 | 0.96 | 0.69‐1.32 | ||
*P value from LR test for interaction. Missing data were excluded when testing for interaction.
Summary points for objective 2 for on study mortality in all cancer patients
There was no strong evidence to support the hypothesis that ESAs had different effects in sub‐populations that differed for any of the variables tested.
For three variables (ESA administration frequency, history of thromboembolic events, and hematocrit) found statistically significant (p < 0.1) in bivariate analyses multivariate analyses suggested the following:
Effect modification of Hct at baseline can only to a certain extend be explained by confounding with other patient characteristics (Hb, age, sex, tumor type). However, because of large amounts of missing data uncertainty remains.
Effect modification of history of thromboembolic events was robust in sensitivity analyses for additional patient characteristics (Hb, age, sex, tumor type); however, because of large amounts of missing data uncertainty remains.
Effect modification for planned frequency of ESA application is likely to be confounded by other study design aspects, see Appendix 4.
On study mortality in chemotherapy trials
Objective 1 for on study mortality in chemotherapy trials
Aim: What is the effect of ESAs compared to control for on study mortality in this population and can the effect be explained by baseline imbalances of prognostic factors?
A total of 38 studies with 10441 patients were included in the analysis of on study mortality analysis in patients undergoing chemotherapy. In this analysis we included only studies where at least 70% of the study population had received a myelosuppressive chemotherapy. Two studies did not contribute to the present results because there were no deaths during on study period (study 22515 (Moebus 2007), 66960 (Untch 2008)).
During on study phase 605 out of 5676 patients randomized to the ESA arm and 490 out of 4765 patients randomized to the control arm died. Median follow‐up was 4.1 months (IQR 3.0 to 5.6 months) in the ESA and 4.3 months (IQR 3.4 to 5.7 months) in the control arm. The overall hazard ratio for patients receiving ESAs compared to controls was 1.10 (95% CI 0.98‐1.24) during on study phase based on the two‐stage log‐rank fixed‐effects meta‐analysis. Based on a Cox model stratified for study the overall result was 1.10 (95% CI 0.98‐1.24). For results of all statistical models applied see Table 9. For Forest plot see Figure 9, for pooled Kaplan‐Meier curve see Appendix 4. There was no evidence for heterogeneity between the trials (I‐square 0%, p=0.7152). There was no evidence for small study effects: linear regression test p=0.1743, rank correlation test of funnel plot asymmetry p=0.7437. For Funnel plot see Figure 10.
9. On study mortality for all cancer patients.
| Model | ESA versus control HR (95% CI) | P value* | I² | P value** |
| Two‐stage log‐rank fixed effect model | 1.10 (95% CI 0.98‐1.24) | 0.1212 | 0% | 0.7152 |
| Two‐stage log‐rank random effects model | 1.10 (95% CI 0.98‐1.24) | 0.1212 | 0% | 0.7152 |
| Two‐stage Cox fixed effect model | 1.09 (95% CI 0.97‐1.23) | 0.1555 | 0% | 0.8813 |
| Two‐stage Cox random effects model | 1.09 (95% CI 0.97‐1.23) | 0.1555 | 0% | 0.8813 |
| Cox model stratified by study | 1.10 (95% CI 0.98‐1.24) | 0.121 | 0.4643 |
*LR test, ** for test of heterogeneity
9.
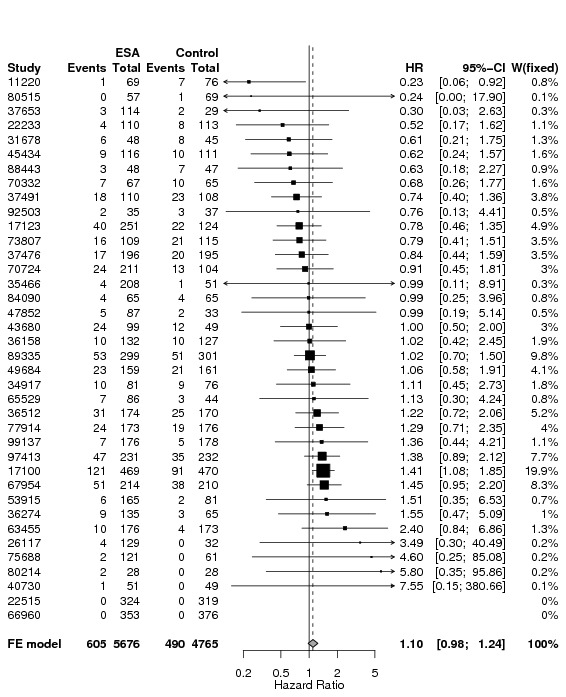
Forest plot for on study mortality in chemotherapy trials based on two‐stage log‐rank fixed‐effect meta‐analysis
10.
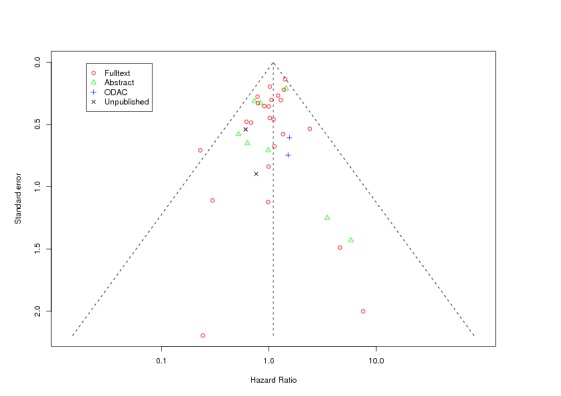
Funnel plot (based on log‐rank estimates) for on study mortality in chemotherapy trials
Explanation of terms used:
Full text: highest publication achieved is a full text publication
Abstract: highest publication achieved is an abstract publication
ODAC: highest publication achieved is reporting of study results in documents presented at ODAC hearings
Unpublished: to date the study was not published in any of the sources mentioned above
Date of reference: June 26th 2008
One study contributed 19.9% weight to the overall analysis (Leyland‐Jones 2003). As described above, in the study published by Leyland‐Jones et al 2003 (study 17100) 937 patients with metastatic cancer undergoing chemotherapy received ESA or placebo for 52 weeks, therefore the study has a much longer on study phase compared to other studies. The influence of single studies was assessed in an influence analysis, see Figure 11. Excluding study 17100 decreased the overall HR (omitting 17100: HR 1.03 (95% CI 0.90‐1.18); the margins of the confidence intervals were not influenced by exclusion of any of the other studies.
11.
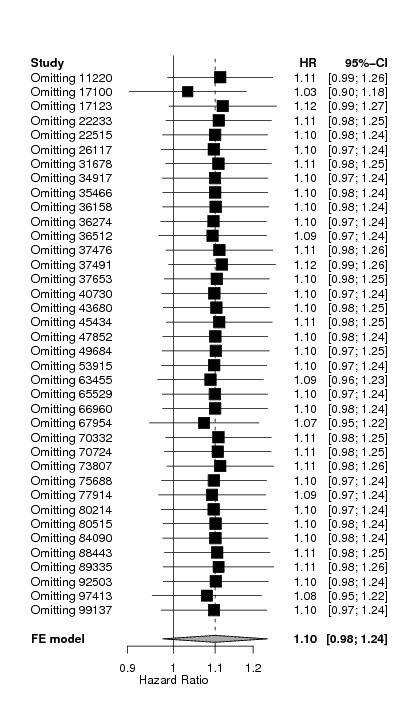
Influence analysis for on study mortality in chemotherapy trials
Assessment of potential confounders for objective 1
In the next step we conducted bivariate analyses: adjusting on study mortality based on the Cox model stratified by study for one variable at the time. All variables assessed relate to the individual patient data level. The results of the adjusted model were compared with the unadjusted model using LR‐Test. Results of unadjusted and adjusted models as well as P values of LR‐Test are shown in Table 10. We included only patients with full information for the respective variable; patients with missing, unknown or unreported data were excluded. Data were often missing for entire studies; exclusion of these studies might have affected the overall estimate. We therefore present both unadjusted and adjusted HRs for the full patient data set for each variable.
10. Bivariate analysis for on study mortality in chemotherapy trials.
| On study mortality for chemotherapy patients | N included | ESA versus control Unadjusted HR (95% confidence interval) | ESA versus control Adjusted HR (95% confidence interval) | P value LR‐Test* |
| Total | 10441 | 1.10 (95% CI 0.98‐1.24) | ‐ | ‐ |
| Hb at baseline (continuous) | 9945 | 1.10 (95% CI 0.98‐1.25) | 1.12 (95% CI 0.99‐1.26) | 0.0000 |
| Hb at baseline (categorical 1) | 9945 | 1.10 (95% CI 0.98‐1.25) | 1.12 (95% CI 0.99‐1.26) | 0.0000 |
| Hb at baseline (categorical 2) | 9945 | 1.10 (95% CI 0.98‐1.25) | 1.12 (95% CI 0.99‐1.26) | 0.0000 |
| Tumor (categorical 1) | 10399 | 1.10 (95% CI 0.97‐1.24) | 1.10 (95% CI 0.97‐1.24) | 0.0049 |
| Tumor (categorical 2) | 10399 | 1.10 (95% CI 0.97‐1.24) | 1.10 (95% CI 0.97‐1.24) | 0.0000 |
| Sex | 10441 | 1.10 (95% CI 0.98‐1.24) | 1.10 (95% CI 0.97‐1.24) | 0.0000 |
| Age (continuous) | 10430 | 1.10 (95% CI 0.98‐1.24) | 1.10 (95% CI 0.98‐1.24) | 0.0000 |
| Age (categorical) | 10430 | 1.10 (95% CI 0.98‐1.24) | 1.10 (95% CI 0.98‐1.24) | 0.0002 |
| Hct (continuous) | 7849 | 1.11 (95% CI 0.98‐1.26) | 1.12 (95% CI 0.98‐1.27) | 0.0000 |
| Hct (categorical) | 7849 | 1.11 (95% CI 0.98‐1.26) | 1.12 (95% CI 0.98‐1.27) | 0.0000 |
| Baseline serum EPO (continuous) | 3959 | 0.99 (95% CI 0.82‐1.20) | 0.99 (95% CI 0.82‐1.19) | 0.2936 |
| Baseline serum EPO (categorical) | 3959 | 0.99 (95% CI 0.82‐1.20) | 0.98 (95% CI 0.81‐1.19) | 0.0651 |
| ECOG (0 vs 1 vs 2 vs 3 vs 4) | 8057 | 1.12 (95% CI 0.98‐1.28) | 1.11 (95% CI 0.97‐1.27) | 0.0000 |
| ECOG (0,1,2 vs 3,4) | 8057 | 1.12 (95% CI 0.98‐1.28) | 1.12 (95% CI 0.98‐1.29) | 0.0000 |
| BMI (categorical) | 8882 | 1.08 (95% CI 0.94‐1.23) | 1.09 (95% CI 0.95‐1.24) | 0.0000 |
| History of thromboembolic events | 6667 | 1.11 (95% CI 0.96‐1.28) | 1.11 (95% CI 0.96‐1.28) | 0.0658 |
| History of cardiovascular events | 7369 | 1.11 (95% CI 0.96‐1.28) | 1.10 (95% CI 0.96‐1.27) | 0.0394 |
| History of hypertension | 6667 | 1.11 (95% CI 0.96‐1.28) | 1.11 (95% CI 0.96‐1.28) | 0.7143 |
| History of diabetes mellitus | 5579 | 1.09 (95% CI 0.94‐1.26) | 1.09 (95% CI 0.94‐1.27) | 0.0802 |
| Geographical region [region_cat] | 10053 | 1.09 (95% CI 0.97‐1.23) | 1.09 (95% CI 0.97‐1.24) | 0.2767 |
| Metastatic vs non‐metastatic | 8956 | 1.16 (95% CI 1.02‐1.32) | 1.15 (95% CI 1.01‐1.31) | 0.0000 |
| Time from cancer diagnosis to randomization | 3114 | 1.06 (95% CI 0.85‐1.31) | 1.06 (95% CI 0.85‐1.32) | 0.6775 |
*This test compares the adjusted with the unadjusted model. It takes into account the entire model, not only the overall hazard ratio.
Based on these analyses and the number of data available for each variable, we conducted four different models, all of which are presented in Table 11. For model 1 we included the variables age, sex, and Hb at baseline and tumor type into the model. For model 2 we used the same variables as in model 1 plus tumor stage. For model 3 we used the same variables as in model 1 plus BMI and region, for model 4 we used the same variables as in model 1 and 3 plus ECOG and hematocrit. For the continuous variables age, Hb, serum EPO and BMI the association between the exposure and the outcome was not linear (graph not shown). Therefore, these continuous variables were converted into prespecified categories. Hematocrit was converted into categories as well for the ease of interpretation. When including history of cardiovascular events into model 1, the overall effect was also not altered (data on file, not shown).
11. Multivariate models for on study mortality in chemotherapy trials.
| On study mortality chemotherapy trials | Model 1 | Model 2 | Model 3 | Model 4 |
| Patients included | n=9892 | n=8469 | n=8030 | n=5109 |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| ESA vs control, unadjusted | 1.10 (95% CI 0.98‐1.25) | 1.16 (95% CI 1.02‐1.33) | 1.07 (95% CI 0.94‐1.23) | 1.13 (95% CI 0.97‐1.31) |
| ESA vs control, adjusted | 1.12 (95% CI 0.99‐1.26) | 1.17 (95% CI 1.02‐1.33) | 1.08 (95% CI 0.95‐1.24) | 1.16 (95% CI 0.99‐1.34) |
| Hb at baseline | ||||
| Hb < 8 g/dL | 1 | 1 | 1 | 1 |
| Hb 8 ‐ 10 g/dL | 0.79 (95% CI 0.62‐1.01) | 0.73 (95% CI 0.55‐0.96) | 0.76 (95% CI 0.58‐1.00) | 0.91 (95% CI 0.61‐1.34) |
| Hb 10 ‐ 12 g/dL | 0.57 (95% CI 0.44‐0.74) | 0.53 (95% CI 0.39‐0.70) | 0.61 (95% CI 0.46‐0.82) | 0.76 (95% CI 0.50‐1.14) |
| Hb 12 ‐ 14 g/dL | 0.36 (95% CI 0.27‐0.49) | 0.33 (95% CI 0.24‐0.46) | 0.42 (95% CI 0.30‐0.57) | 0.52 (95% CI 0.33‐0.82) |
| Hb > 14 g/dL | 0.32 (95% CI 0.22‐0.47) | 0.30 (95% CI 0.20‐0.46) | 0.36 (95% CI 0.24‐0.54) | 0.45 (95% CI 0.25‐0.83) |
| Age at randomization | ||||
| 18 ‐ 35 yrs | 0.92 (95% CI 0.54‐1.57) | 1.12 (95% CI 0.62‐2.01) | 0.94 (95% CI 0.51‐1.74) | 0.77 (95% CI 0.38‐1.50) |
| 35 ‐ 45 yrs | 1 | 1 | 1 | 1 |
| 45 ‐ 55 yrs | 1.16 (95% CI 0.88‐1.51) | 1.16 (95% CI 0.86‐1.55) | 1.24 (95% CI 0.91‐1.70) | 1.08 (95% CI 0.78‐1.63) |
| 55 ‐ 65 yrs | 1.27 (95% CI 0.98‐1.64) | 1.31 (95% CI 0.99‐1.74) | 1.46 (95% CI 1.07‐1.97) | 1.19 (95% CI 0.87‐1.63) |
| 65 ‐ 75 yrs | 1.51 (95% CI 1.16‐1.97) | 1.52 (95% CI 1.14‐2.02) | 1.74 (95% CI 1.28‐2.38) | 1.52 (95% CI 1.10‐2.09) |
| > 75 yrs | 1.69 (95% CI 1.24‐2.31) | 1.93 (95% CI 1.37‐2.71) | 1.95 (95% CI 1.35‐2.81) | 1.61 (95% CI 1.08‐2.40) |
| Sex | ||||
| Male | 1 | 1 | 1 | 1 |
| Female | 0.78 (95% CI 0.66‐0.92) | 0.82 (95% CI 0.69‐0.99) | 0.84 (95% CI 0.70‐1.00) | 0.87 (95% CI 0.71‐1.07) |
| Tumor category | ||||
| Hematological malign. | 1 | 1 | 1 | 1 |
| Breast cancer | 1.36 (95% CI 0.88‐2.09) | 1.12 (95% CI 0.60‐2.11) | 1.32 (95% CI 0.81‐2.17) | 1.38 (95% CI 0.78‐2.43) |
| Head and neck cancer | 2.23 (95% CI 0.68‐7.32) | 1.59 (95% CI 0.21‐12.12) | 1.47 (95% CI 0.20‐11.07) | ‐ |
| Lung cancer | 2.78 (95% CI 1.83‐4.20) | 2.06 (95% CI 1.11‐3.80) | 2.86 (95% CI 1.70‐4.80) | 3.83 (95% CI 2.15‐6.80) |
| Gastrointestinal | 2.54 (95% CI 1.68‐3.83) | 1.90 (95% CI 1.02‐3.52) | 2.45 (95% CI 1.50‐4.01) | 2.79 (95% CI 1.60‐4.85) |
| Gynecological | 1.07 (95% CI 0.64‐1.80) | 0.61 (95% CI 0.29‐1.29) | 1.38 (95% CI 0.76‐2.50) | 2.20 (95% CI 1.10‐4.40) |
| Genitourinary | 1.34 (95% CI 0.73‐2.44) | 0.97 (95% CI 0.42‐2.26) | 1.06 (95% CI 0.47‐2.42) | 1.19 (95% CI 0.41‐3.43) |
| Other | 2.65 (95% CI 1.68‐4.17) | 2.11 (95% CI 1.10‐4.02) | 2.69 (95% CI 1.56‐4.62) | 3.17 (95% CI 1.71‐5.87) |
| Tumor stage | ||||
| Metastatic/advanced | ‐ | 1 | ‐ | ‐ |
| Not metastatic/advanced | ‐ | 0.38 (95% CI 0.28‐0.52) | ‐ | ‐ |
| Region | ||||
| Northern America | ‐ | ‐ | 1 | 1 |
| Southern Europe | ‐ | ‐ | 1.20 (95% CI 0.66‐2.17) | 1.21 (95% CI 0.64‐2.31) |
| Australia & New Zealand | ‐ | ‐ | 1.00 (95% CI 0.55‐1.81) | 1.06 (95% CI 0.52‐2.14) |
| Eastern Europe | ‐ | ‐ | 1.33 (95% CI 0.76‐2.30) | 1.32 (95% CI 0.73‐2.40) |
| Northern Europe | ‐ | ‐ | 1.25 (95% CI 0.70‐2.26) | 1.43 (95% CI 0.75‐2.74) |
| Western Europe | ‐ | ‐ | 1.50 (95% CI 0.86‐2.63) | 1.61 (95% CI 0.88‐2.95) |
| Other | ‐ | ‐ | 1.14 (95% CI 0.53‐2.43) | 1.46 (95% CI 0.66‐3.26) |
| BMI | ||||
| < 19 kg/m² | ‐ | ‐ | 1 | 1 |
| 19‐25 kg/m² | ‐ | ‐ | 0.73 (95% CI 0.57‐0.92) | 0.76 (95% CI 0.58‐1.00) |
| 25‐30 kg/m² | ‐ | ‐ | 0.61 (95% CI 0.47‐0.78) | 0.63 (95% CI 0.48‐0.85) |
| > 30 kg/m² | ‐ | ‐ | 0.50 (95% CI 0.37‐0.68) | 0.54 (95% CI 0.39‐0.76) |
| Hct at baseline | ||||
| Hct 0‐23.5% | ‐ | ‐ | ‐ | 1 |
| Hct 23.5%‐29.4% | ‐ | ‐ | ‐ | 0.71 (95% CI 0.37‐1.35) |
| Hct 29.4%‐35.3% | ‐ | ‐ | ‐ | 0.61 (95% CI 0.32‐1.16) |
| Hct 35.3%‐41.2% | ‐ | ‐ | ‐ | 0.60 (95% CI 0.31‐1.19) |
| >Hct 41.2% | ‐ | ‐ | ‐ | 0.58 (95% CI 0.27‐1.24) |
| Performance score | ||||
| ECOG 0, 1 or 2 | ‐ | ‐ | ‐ | 1 |
| ECOG 3 or 4 | ‐ | ‐ | ‐ | 3.08 (95% CI 1.99‐4.77) |
Summary points for objective 1 for on study mortality in chemotherapy trials
The hazard ratio for on study mortality in the chemotherapy population is increased by factor 1.10 for patients receiving ESAs compared to controls (HR 1.10, 95% CI 0.98‐1.24, n=10441). The evidence does not conclusively demonstrate that ESAs increase on study mortality but the evidence also does not conclusively exclude a harmful effect in this population.
Available evidence does not support the hypothesis that baseline imbalances of prognostic factors analyzed influenced the overall results.
Objective 2 for on study mortality in chemotherapy trials
Aim: Is there a specific subgroup of patients that is at increased or decreased risk to die when receiving ESAs compared to controls? Are there design aspects at study level that influenced the effects of ESA on survival?
We tested for interaction between ESA treatment and specific variables describing patient and study characteristics, results for interaction tests are shown in Table 12, results for effect estimates of subgroups are outlined in Appendix 7.
12. Assessment of interaction for on study mortality in chemotherapy trials.
| On study mortality, chemotherapy patients | N included | P value for interaction* |
| Total unadjusted (Cox model) | 10441 (100%) | ‐ |
| Patient level characteristics | ||
| Hb at baseline (continuous) | 9945 | 0.8689 |
| Hb at baseline (categorical 1) | 9945 | 0.9035 |
| Hb at baseline (categorical 2) | 9945 | 0.9881 |
| Tumor (categorical 1) | 10399 | 0.1846 |
| Tumor (categorical 2) | 10399 | 0.1509 |
| Sex | 10441 | 0.1395 |
| Age (continuous) | 10430 | 0.5684 |
| Age (categorical) | 10430 | 0.3442 |
| Hct (continuous) | 7849 | 0.5722 |
| Hct (categorical) | 7849 | 0.2189 |
| Baseline serum EPO (continuous) | 3959 | 0.9051 |
| Baseline serum EPO (categorical) | 3959 | 0.2047 |
| ECOG | 8057 | 0.5776 |
| ECOG (0,1,2 vs 3,4) | 8057 | 0.9970 |
| BMI (categorical) | 8882 | 0.6333 |
| History of thromboembolic events | 6667 | 0.1421 |
| History of cardiovascular events | 7369 | 0.9285 |
| History of hypertension | 6667 | 0.6079 |
| History of diabetes mellitus | 5579 | 0.7429 |
| Geographical region [region_cat] | 10053 | 0.3543 |
| Metastatic vs non‐metastatic | 8956 | 0.6083 |
| Planned Hb ceiling (categorical 1) | 10362 | 0.2834 |
| Planned Hb ceiling (categorical 2) | 10362 | 0.3788 |
| Study level characteristics | ||
| Placebo controlled | 10441 | 0.5349 |
| Randomization (adequate vs unclear) | 10441 | 0.8789 |
| Allocation (adequate vs unclear) | 10441 | 0.0722 |
| Endpoint overall survival | 10441 | 0.1117 |
| Year of last patient randomized into study (categorical) | 10441 | 0.1568 |
| Source of data (company versus independent) | 10441 | 0.1842 |
| Iron category | 10441 | 0.5201 |
| Planned ESA treatment duration (categorical) | 10441 | 0.2020 |
| Planned weekly ESA dosage (categorical) | 10441 | 0.2940 |
| Planned frequency ESA administration (categorical) | 10441 | 0.0544 |
*P value for interaction based on LR test, patients with missing data are excluded from LR test
Two variables (concealment of allocation, planned frequency of ESA administration) showed a statistically significant (p<0.1) interaction term in the bivariate analysis and were included in the multivariate model (model 1). This model (model 1) included the variables, age and sex, Hb at baseline and tumor category, see Table 13. Adjusting for these parameters did not markedly influence the effect estimates and the P values for interaction.
13. Interaction for on study mortality in chemotherapy trials.
| On study mortality chemotherapy patients | Bivariate ESA versus control | Multivariate ESA versus control | ||||
| Interaction term | ESA* variable | ESA* variable | ||||
| Model adjusted for | ‐ | age, sex, Hb, tumor type | ||||
| Patients included | n = 10441 | n = 9892 | ||||
| HR | 95% CI | P value LR test | HR | 95% CI | P value LR test | |
| Study level characteristics Planned frequency of ESA application | ||||||
| Three times per week or more frequent | 0.97 | 0.81‐1.17 | 0.0544 | 0.97 | 0.81‐1.18 | 0.0453 |
| Once per week | 1.35 | 1.12‐1.64 | 1.38 | 1.14‐1.68 | ||
| Every second week or less frequent | 0.92 | 0.51‐1.68 | 0.92 | 0.51‐1.68 | ||
| Other | 0.93 | 0.67‐1.29 | 0.95 | 0.67‐1.32 | ||
| Overall, unadjusted | 1.10 | 0.98‐1.24 | ‐ | 1.10 | 0.98‐1.25 | ‐ |
| Concealment of allocation | ||||||
| Adequate | 1.15 | 1.01‐1.30 | 0.0722 | 1.17 | 1.02‐1.33 | 0.0608 |
| Unclear | 0.81 | 0.57‐1.16 | 0.81 | 0.57‐1.16 | ||
| Overall, unadjusted | 1.10 | 0.98‐1.24 | ‐ | 1.10 | 0.98‐1.25 | ‐ |
Summary points for objective 2 for on study mortality in chemotherapy trials
For two variables (ESA administration frequency, concealment of allocation) found statistically significant (p < 0.1) in bivariate analyses multivariate adjustments did not markedly effect the estimates and the corresponding P values for interaction.
For both variables statistical tests for interaction had borderline significance only in both bivariate and multivariate analyses.
Overall, there is no strong evidence to support the hypothesis that ESAs had different effects in sub‐populations that differed for the variables tested in the chemotherapy population.
Overall survival in all cancer patients
Objective 1 for overall survival in all cancer patients
Aim: What is the effect of ESAs compared to control on overall survival in this population and can the effect be explained by baseline imbalances of prognostic factors at patient level?
53 studies with 13933 patients were included in the analysis of overall survival for all cancer patients. 2643 out of 7634 patients randomized to ESA and 2350 out of 6299 patients randomized to control died during longest follow‐up available. Median follow‐up was 6.2 months (IQR 3.2 to 15.4 months) in the ESA and 8.3 months (IQR 3.7 to 19.6 months) in the control arm. The overall hazard ratio for patients receiving ESA compared to controls was 1.06 (95% CI 1.00‐1.12) for longest follow‐up available based on the two‐stage log‐rank fixed‐effects model meta‐analysis. Based on a Cox model stratified for study the overall result was 1.06 (95% CI 1.00‐1.12). For results of all statistical models applied see Table 14. There was no evidence for heterogeneity between the trials (I‐square 7.1%, p=0.3288). For Forest plot see Figure 12, for pooled Kaplan‐Meier curve see Appendix 4. There was no evidence for small study effects: linear regression test p=0.7567, rank correlation test of funnel plot asymmetry p=0.602. For Funnel plot see Figure 13.
14. Overall survival for all cancer patients.
| Model | ESA versus control HR (95% CI) | P value* | I² | P value** |
| Two‐stage log‐rank fixed effect model | 1.06 (95% CI 1.00‐1.12) | 0.0464 | 7.1% | 0.3288 |
| Two‐stage log‐rank random effects model | 1.06 (95% CI 1.00‐1.13) | 0.0611 | 7.1% | 0.3288 |
| Two‐stage Cox fixed effect model | 1.06 (95% CI 1.00‐1.12) | 0.0561 | 0% | 0.6129 |
| Two‐stage Cox random effects model | 1.06 (95% CI 1.00‐1.12) | 0.0561 | 0% | 0.6129 |
| Cox model stratified by study | 1.06 (95% CI 1.00‐1.12) | 0.0462 | 0.2072 |
*LR test, ** for test of heterogeneity
12.
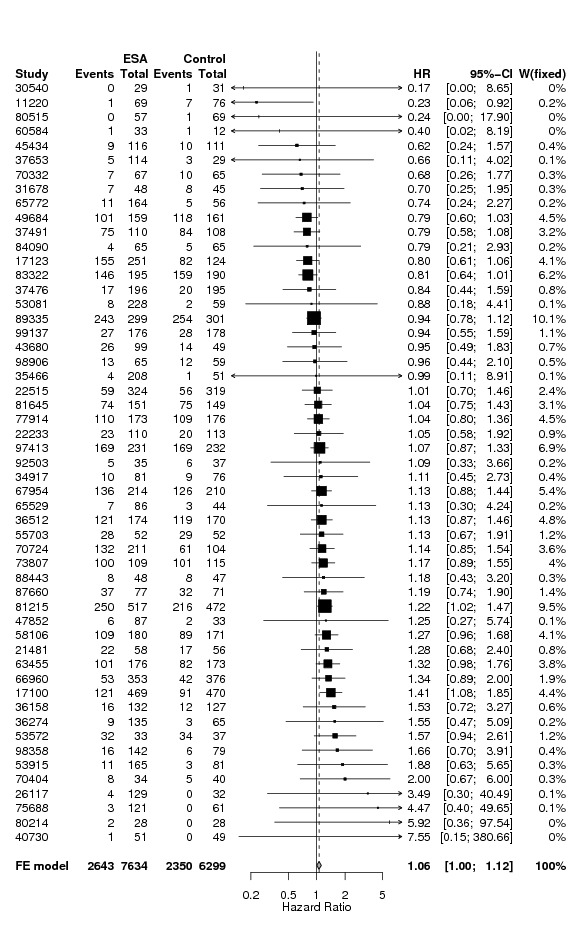
Forest plot for overall survival in all cancer patients based on two‐stage log‐rank fixed effect meta‐analysis
13.
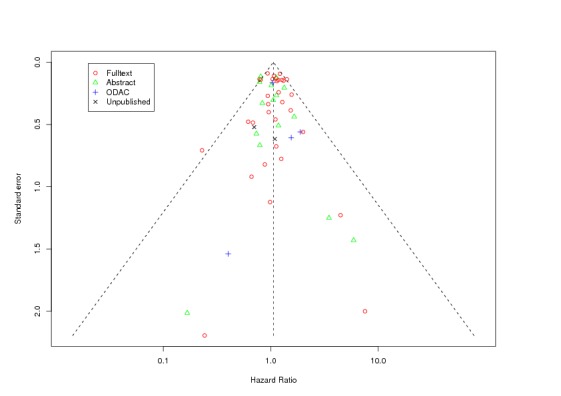
Funnel plot (based on log‐rank estimates) for overall survival in all cancer patients
Explanation of terms used:
Full text: highest publication achieved is a full text publication
Abstract: highest publication achieved is an abstract publication
ODAC: highest publication achieved is reporting of study results in documents presented at ODAC hearings
Unpublished: to date the study was not published in any of the sources mentioned above
Date of reference: June 26th 2008
Overall, 24 of the 53 included trials were designed for long‐term follow‐up, defined as planned follow‐up of at least 12 months after end of treatment phase. 14 of the 53 included studies (all of which were designed for long‐term follow‐up) had a median follow‐up of at least 12 months. Tables providing median follow‐up for both on study mortality and overall survival per study are on file. Results for studies designed for long‐term follow‐up as well as other sensitivity analyses are provided in Appendix 3.
Two studies contributed 9.5% and 10.1% weight to the overall analysis (Pirker 2008), (Smith 2008). In the study published by Smith 2008) (study number 81215) 989 patients were treated with ESA or placebo without concomitant myelosuppressive chemotherapy. In the study published by (Pirker 2008) (study number 89335) 600 patients with untreated, extensive SCLC underwent chemotherapy and were randomized to receive ESA or placebo. The influence of single studies was assessed; see Figure 14, exclusion of single studies at a time did not influence the overall result.
14.
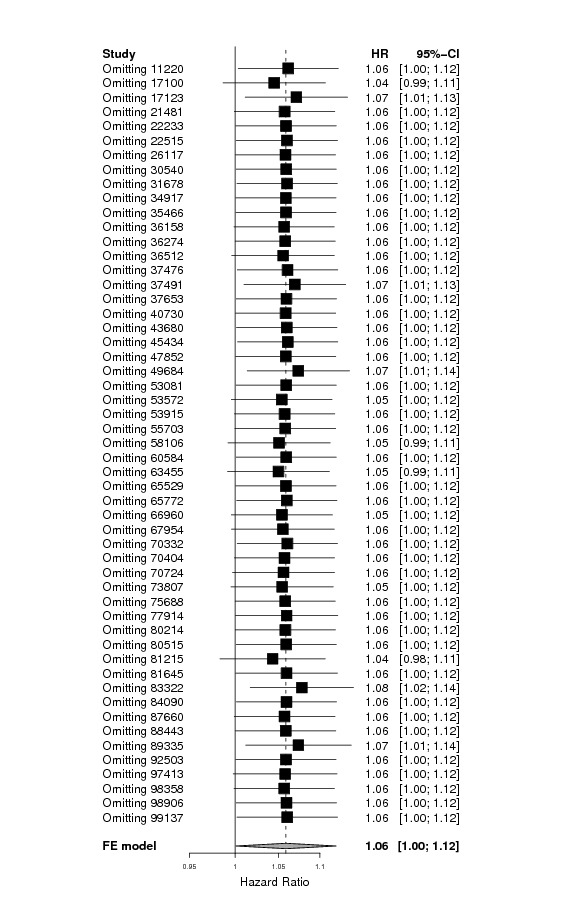
Influence analysis for overall survival in all cancer patients
Assessment of potential confounders for objective 1
In the next step we conducted bivariate analyses: adjusting overall survival based on the Cox model stratified by study for one variable at the time. All variables assessed relate to the individual patient data level. The results of the adjusted model were compared with the unadjusted model using LR‐Test. Number of patients included per variable and P values of LR‐Test are shown in Table 15. We included only patients with full information for the respective variable; patients with missing, unknown or unreported data were excluded. Data were often missing for entire studies; therefore the overall HR might have changed because of the omission of studies. We therefore present both unadjusted and adjusted HRs based on the patient data set with available information.
15. Bivariate analysis for overall survival in all cancer patients.
| Overall survival all cancer patients | Patients included | ESA versus control Unadjusted hazard ratio (95% CI) | ESA versus control Adjusted hazard ratio (95% CI) | P value LR‐Test* |
| Total | 13933 | 1.06 (95% CI 1.00‐1.12) | ‐ | ‐ |
| Hb at baseline (continuous) | 13407 | 1.06 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.12) | 0.0000 |
| Hb at baseline (categorical 1) | 13407 | 1.06 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.12) | 0.0000 |
| Hb at baseline (categorical 2) | 13407 | 1.06 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.13) | 0.0000 |
| Tumor (categorical 1) | 13891 | 1.06 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.12) | 0.0000 |
| Tumor (categorical 2) | 13891 | 1.06 (95% CI 1.00‐1.12) | 1.05 (95% CI 1.00‐1.11) | 0.0000 |
| Sex | 13933 | 1.06 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.12) | 0.0000 |
| Age (continuous) | 13921 | 1.06 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.12) | 0.0000 |
| Age (categorical) | 13921 | 1.06 (95% CI 1.01‐1.12) | 1.06 (95% CI 1.01‐1.12) | 0.0000 |
| Hct (continuous) | 11036 | 1.06 (95% CI 0.99‐1.12) | 1.06 (95% CI 1.00‐1.13) | 0.0000 |
| Hct (categorical) | 11036 | 1.06 (95% CI 0.99‐1.12) | 1.06 (95% CI 1.00‐1.13) | 0.0000 |
| Baseline serum EPO (continuous) | 5651 | 1.03 (95% CI 0.94‐1.12) | 1.03 (95% CI 0.94‐1.12) | 0.1678 |
| Baseline serum EPO (categorical) | 5651 | 1.03 (95% CI 0.94‐1.12) | 1.03 (95% CI 0.94‐1.12) | 0.0000 |
| ECOG (0 vs 1 vs 2 vs 3 vs 4) | 10112 | 1.08 (95% CI 1.01‐1.15) | 1.07 (95% CI 1.00‐1.14) | 0.0000 |
| ECOG (0,1,2 vs 3,4) | 10225 | 1.08 (95% CI 1.01‐1.15) | 1.08 (95% CI 1.01‐1.16) | 0.0000 |
| BMI (categorical) | 11445 | 1.05 (95% CI 0.99‐1.12) | 1.05 (95% CI 0.99‐1.12) | 0.0000 |
| History of thromboembolic events | 9620 | 1.05 (95% CI 0.98‐1.12) | 1.05 (95% CI 0.98‐1.12) | 0.0218 |
| History of cardiovascular events | 10322 | 1.05 (95% CI 0.99‐1.13) | 1.05 (95% CI 0.98‐1.13) | 0.0011 |
| History of hypertension | 9620 | 1.05 (95% CI 0.98‐1.12) | 1.05 (95% CI 0.98‐1.12) | 0.2436 |
| History of diabetes mellitus | 8025 | 1.06 (95% CI 0.99‐1.14) | 1.06 (95% CI 0.98‐1.14) | 0.0577 |
| Geographical region (categorical 1) | 13532 | 1.05 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.12) | 0.0000 |
| Metastatic vs non‐metastatic | 12152 | 1.06 (95% CI 1.00‐1.13) | 1.05 (95% CI 0.99‐1.12) | 0.0000 |
| Time from cancer diagnosis to randomization | 4586 | 1.06 (95% CI 0.97‐1.17) | 1.06 (95% CI 0.97‐1.16) | 0.0000 |
*The LR test compares the adjusted with the unadjusted model. It takes into account the entire model, not only the overall hazard ratio.
Based on these analyses and the number of data available for each variable, we conducted four different models, all of which are presented in Table 16. For model 1 we included the variables age, sex, Hb at baseline and tumor type into the model. For model 2 we used the same variables as in model 1 plus tumor stage. For model 3 we used the same variables as in model 1 plus BMI and region, for model 4 we used the same variables as in model 1 and 3 plus ECOG and hematocrit. For the continuous variables age, Hb, serum EPO and BMI the association between the exposure and the outcome was not linear (graph not shown). Therefore, these continuous variables were converted into prespecified categories. Hematocrit was converted into categories as well for the ease of interpretation. The variables serum EPO and time from cancer diagnosis to randomization were excluded because too many data were missing. When history of thromboembolic events and history of cardiovascular events were included in model 1 (each at a time), the overall results were also not changed (data on file).
16. Multivariate analyses for overall survival in all cancer patients.
| Overall survival all cancer patients |
Model 1 |
Model 2 |
Model 3 |
Model 4 |
| Patients included | n=13353 | n=11636 | n=10599 | n=6547 |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| ESA vs ctrl unadjusted* | 1.06 (95% CI 1.00‐1.12) | 1.06 (95% CI 1.00‐1.13) | 1.04 (95% CI 0.98‐1.11) | 1.07 (95% CI 0.99‐1.15) |
| ESA vs ctrl adjusted** | 1.06 (95% CI 1.00‐1.12) | 1.05 (95% CI 1.00‐1.12) | 1.04 (95% CI 0.98‐1.11) | 1.09 (95% CI 1.01‐1.17) |
| Hb at baseline | ||||
| Hb < 8 g/dL | 1 | 1 | 1 | 1 |
| Hb 8‐10 g/dL | 0.77 (95% CI 0.68‐0.87) | 0.72 (95% CI 0.63‐0.83) | 0.78 (95% CI 0.68‐0.90) | 0.86 (95% CI 0.70‐1.04) |
| Hb 10‐12 g/dL | 0.60 (95% CI 0.52‐0.68) | 0.56 (95% CI 0.48‐0.64) | 0.62 (95% CI 0.54‐0.71) | 0.74 (95% CI 0.60‐0.92) |
| Hb 12‐14 g/dL | 0.48 (95% CI 0.41‐0.56) | 0.45 (95% CI 0.38‐0.53) | 0.52 (95% CI 0.44‐0.61) | 0.71 (95% CI 0.55‐0.93) |
| Hb > 14 g/dL | 0.40 (95% CI 0.33‐0.48) | 0.39 (95% CI 0.32‐0.47) | 0.44 (95% CI 0.36‐0.54) | 0.69 (95% CI 0.48‐0.99) |
| Age at randomization | ||||
| 18 ‐ 35 yrs | 0.82 (95% CI 0.62‐1.07) | 0.91 (95% CI 0.68‐1.22) | 0.84 (95% CI 0.62‐1.13) | 0.65 (95% CI 0.42‐1.00) |
| 35 ‐ 45 yrs | 1 | 1 | 1 | 1 |
| 45 ‐ 55 yrs | 1.06 (95% CI 0.93‐1.21) | 1.05 (95% CI 0.91‐1.20) | 1.10 (95% CI 0.95‐1.28) | 1.16 (95% CI 0.96‐1.40) |
| 55 ‐ 65 yrs | 1.13 (95% CI 1.00‐1.28) | 1.15 (95% CI 1.01‐1.31) | 1.25 (95% CI 1.08‐1.44) | 1.32 (95% CI 1.09‐1.58) |
| 65 ‐ 75 yrs | 1.23 (95% CI 1.08‐1.39) | 1.22 (95% CI 1.07‐1.40) | 1.34 (95% CI 1.16‐1.55) | 1.34 (95% CI 1.11‐1.62) |
| > 75 ys | 1.32 (95% CI 1.14‐1.53) | 1.40 (95% CI 1.19‐1.63) | 1.39 (95% CI 1.17‐1.65) | 1.31 (95% CI 1.06‐1.63) |
| Sex | ||||
| Male | 1 | 1 | 1 | 1 |
| Female | 0.79 (95% CI 0.73‐0.84) | 0.81 (95% CI 0.75‐0.88) | 0.80 (95% CI 0.74‐0.86) | 0.77 (95% CI 0.70‐0.84) |
| Tumor category | ||||
| Hematological malign. | 1 | 1 | 1 | 1 |
| Breast cancer | 1.91 (95% CI 1.54‐2.37) | 1.57 (95% CI 1.15‐2.13) | 1.93 (95% CI 1.51‐2.46) | 2.05 (95% CI 1.59‐2.65) |
| Head and neck cancer | 2.57 (95% CI 1.87‐3.53) | 2.31 (95% CI 1.56‐3.41) | 2.56 (95% CI 1.79‐3.65) | 3.38 (95% CI 1.96‐5.83) |
| Lung cancer | 4.06 (95% CI 3.31‐4.99) | 3.06 (95% CI 2.30‐4.07) | 3.79 (95% CI 2.96‐4.86) | 3.98 (95% CI 3.07‐5.16) |
| Gastrointestinal | 3.08 (95% CI 2.49‐3.82) | 2.90 (95% CI 2.15‐3.90) | 3.11 (95% CI 2.42‐4.01) | 3.27 (95% CI 2.51‐4.26) |
| Gynecological | 2.19 (95% CI 1.70‐2.82) | 1.67 (95% CI 1.20‐2.32) | 2.33 (95% CI 1.74‐3.12) | 2.86 (95% CI 2.11‐3.88) |
| Genitourinary | 2.76 (95% CI 2.17‐3.50) | 2.36 (95% CI 1.72‐3.22) | 2.69 (95% CI 2.04‐3.55) | 2.90 (95% CI 2.18‐3.87) |
| Other | 3.21 (95% CI 2.55‐4.04) | 2.94 (95% CI 2.15‐4.01) | 3.24 (95% CI 2.48‐4.24) | 3.35 (95% CI 2.52‐4.47) |
| Tumor stage | ||||
| Metastatic or advanced | ‐ | 1 | ‐ | ‐ |
| Not metastatic/advanced | ‐ | 0.51 (95% CI 0.46‐0.57) | ‐ | ‐ |
| Region | ||||
| Northern America | ‐ | ‐ | 1 | 1 |
| Southern Europe | ‐ | ‐ | 1.33 (95% CI 1.06‐1.68) | 1.27 (95% CI 1.00‐1.61) |
| Australia & New Zealand | ‐ | ‐ | 0.97 (95% CI 0.72‐1.31) | 0.97 (95% CI 0.71‐1.32) |
| Eastern Europe | ‐ | ‐ | 1.50 (95% CI 1.23‐1.82) | 1.50 (95% CI 1.22‐1.83) |
| Northern Europe | ‐ | ‐ | 1.59 (95% CI 1.29‐1.97) | 1.61 (95% CI 1.29‐2.01) |
| Western Europe | ‐ | ‐ | 1.47 (95% CI 1.19‐1.82) | 1.47 (95% CI 1.18‐1.83) |
| Other | ‐ | ‐ | 1.23 (95% CI 0.85‐1.77) | 1.51 (95% CI 0.96‐2.37) |
| BMI | ||||
| < 19 kg/m² | ‐ | ‐ | 1 | 1 |
| 19‐25 kg/m² | ‐ | ‐ | 0.79 (95% CI 0.70‐0.88) | 0.82 (95% CI 0.71‐0.94) |
| 25‐30 kg/m² | ‐ | ‐ | 0.69 (95% CI 0.61‐0.77) | 0.70 (95% CI 0.60‐0.81) |
| > 30 kg/m² | ‐ | ‐ | 0.61 (95% CI 0.53‐0.71) | 0.61 (95% CI 0.51‐0.72) |
| Hct at baseline | ||||
| Hct <23.5% | ‐ | ‐ | ‐ | 1 |
| Hct 23.5%‐29.4% | ‐ | ‐ | ‐ | 0.84 (95% CI 0.63‐1.12) |
| Hct 29.4%‐35.3% | ‐ | ‐ | ‐ | 0.71 (95% CI 0.53‐0.96) |
| Hct 35.3%‐41.2% | ‐ | ‐ | ‐ | 0.61 (95% CI 0.44‐0.85) |
| >Hct 41.2% | ‐ | ‐ | ‐ | 0.48 (95% CI 0.32‐0.72) |
| Performance score | ||||
| ECOG 0, 1 or 2 | ‐ | ‐ | ‐ | 1 |
*unadjusted HR based on the number of patients included in the respective model **HR adjusted for the variables outlined in the respective columns
Summary points for objective 1:
Across all cancer patients analyzed, ESAs increase the risk for mortality over longest available follow‐up when compared with controls (HR 1.06, 95% CI 1.00‐1.12, n=13933).
Available evidence does not support the hypothesis that baseline imbalances of prognostic factors analyzed influenced the overall results.
Objective 2 for overall survival in all cancer patients
Aim: Is there a specific subgroup of patients that is at increased or decreased risk to die when receiving ESAs compared to controls? Are there design aspects at study level that influenced the effects of ESA on survival?
We tested for interaction between ESA treatment and specific variables describing patient and study characteristics, results are outlined in Table 17, results with subgroup effects are outlined in Appendix 8.
17. Assessment of interaction, overall survival in all cancer patients.
| Overall survival, all cancer patients | Patients included | P value for interaction |
| Total | 13933 | |
| Patient level characteristics | ||
| Hb at baseline (continuous) | 13407 | 0.7547 |
| Hb at baseline (categorical 1) | 13407 | 0.6326 |
| Hb at baseline (categorical 2) | 13407 | 0.8292 |
| Tumor (categorical 1) | 13891 | 0.2315 |
| Tumor (categorical 2) | 13891 | 0.2122 |
| Sex plus | 13933 | 0.1480 |
| Age (continuous) | 13921 | 0.3758 |
| Age (categorical) | 13921 | 0.2610 |
| Hct (continuous) | 11036 | 0.8998 |
| Hct (categorical) | 11036 | 0.0330 |
| Baseline serum EPO (continuous) | 5651 | 0.1424 |
| Baseline serum EPO (categorical) | 5651 | 0.8116 |
| ECOG | 10112 | 0.4115 |
| ECOG (0,1,2 vs 3,4) | 10225 | 0.4980 |
| BMI (categorical) | 11445 | 0.7189 |
| History of thromboembolic events | 9620 | 0.8964 |
| History of cardiovascular events | 10322 | 0.6886 |
| History of hypertension | 9620 | 0.5700 |
| History of diabetes mellitus | 8025 | 0.9435 |
| Geographical region [region_cat] | 13532 | 0.9000 |
| Metastatic vs non‐metastatic | 12152 | 0.8573 |
| Planned Hb ceiling (categorical 1) | 13730 | 0.3973 |
| Planned Hb ceiling (categorical 2) | 13730 | 0.5976 |
| Study level characteristics | ||
| Placebo controlled | 13933 | 0.2932 |
| Randomization (adequate vs unclear) | 13933 | 0.8042 |
| Allocation (adequate vs unclear) | 13933 | 0.4945 |
| Endpoint overall survival | 13933 | 0.3866 |
| Designed for long term follow up (binary) | 13933 | 0.6423 |
| Year of last patient randomized into study (categorical) | 13933 | 0.1285 |
| Source of data (company versus independent) | 13933 | 0.5736 |
| Patient population (chemotherapy, radio‐chemo‐ therapy, none, mixed) | 13933 | 0.1133 |
| Iron category | 13933 | 0.4786 |
| Planned ESA treatment duration (categorical) | 13933 | 0.7393 |
| Planned weekly ESA dosage (categorical) | 13933 | 0.8780 |
| Planned frequency of ESA administration (categorical) | 13933 | 0.0748 |
*P value for interaction based on LR test, patients with missing data are excluded from LR test
Two variables (planned frequency, Hct at baseline) showed a statistically significant (p<0.1) interaction term in the bivariate analysis and was included in the multivariate model (model 1). This model (model 1) included the variables, age and sex, Hb at baseline and tumor category; for P values of LR test see Table 18.
18. Overall survival in all cancer patient trials, test for interaction, univariate and multivariate models.
| Overall survival in all cancer patients | Bivariate ESA versus control | Multivariate ESA versus control | ||||
| Interaction term | ESA*variable | ESA*variable | ||||
| Adjusted for | ‐ | age, sex, Hb, tumor type | ||||
| HR | 95% CI | P* | HR | 95% CI | P* | |
| Patient level characteristics | ||||||
| Hct categorical | 0.0330 | 0.1343 | ||||
| Patients included | n = 11036 | n = 10972 | ||||
| < 23.5% | 1.66 | 1.18‐2.34 | 1.54 | 1.09‐2.18 | ||
| 23.5‐29.4% | 0.94 | 0.83‐1.07 | 0.96 | 0.84‐1.09 | ||
| 29.4‐35.3% | 1.10 | 0.99‐1.21 | 1.08 | 0.98‐1.19 | ||
| 35.3‐41.2% | 1.07 | 0.95‐1.21 | 1.07 | 0.95‐1.21 | ||
| > 41.2% | 1.02 | 0.82‐1.26 | 1.04 | 0.84‐1.29 | ||
| Missing | 1.08 | 0.93‐1.24 | ‐ | omitted | omitted | ‐ |
| Overall, unadjusted | 1.06 | 0.99‐1.12 | ‐ | 1.06 | 0.99‐1.12 | ‐ |
| Study level characteristics | ||||||
| Planned frequency of ESA application | 0.0748 | 0.1949 | ||||
| Patients included | n = 13933 | n = 13353 | ||||
| Three times per week or more frequent | 1.07 | 0.98‐1.18 | 1.07 | 0.97‐1.15 | ||
| Once per week | 1.06 | 0.97‐1.17 | 1.08 | 0.87‐1.18 | ||
| Every second week or less frequent | 1.20 | 1.02‐1.40 | 1.14 | 0.97‐1.34 | ||
| Other | 0.90 | 0.77‐1.05 | ‐ | 0.91 | 0.78‐1.06 | ‐ |
| Overall, unadjusted | 1.06 | 1.00‐1.39 | ‐ | 1.06 | 1.00‐1.30 | ‐ |
*P value LR test
Summary points for objective 2 for overall survival in all cancer patients
Two variables (ESA administration frequency, Hct at baseline) were found to be statistically significant (p < 0.1) in bivariate analyses. Multivariate adjustments did not markedly effect the estimates; however, corresponding P values for interaction did not reach conventional levels of significance."
Overall, available evidence does not support the hypothesis that ESAs had different effects in sub‐populations that differed for any of the variables tested for overall survival in all cancer patients.
Objective 1 for overall survival in chemotherapy trials
Aim: What is the effect of ESAs compared to control on overall survival in this population and can the effect be explained by baseline imbalances of prognostic factors?
A total of 38 studies with 10441 patients were included in the overall survival analysis of patients undergoing chemotherapy. In this analysis we included only studies where at least 70% of the study population had received a myelosuppressive chemotherapy.
1888 out of 5676 patients randomized to ESA and 1667 out of 4765 patients randomized to controls died during on study phase and subsequent follow‐up. Median follow‐up was 6.7 months (IQR 3.4 to 15.7 months) in the ESA and 8.4 months (IQR 3.7 to 19.1 months) in the control arm. The hazard ratio for overall survival in chemotherapy patients receiving ESA compared to controls was 1.04 (95% CI 0.97‐1.11) based on the two‐stage log‐rank fixed‐effects meta‐analysis. Based on a Cox model stratified for study the overall result was 1.04 (95% CI 0.97‐1.11). For results of all statistical models applied see Table 19. For Forest plot see Figure 15, for pooled Kaplan‐Meier curve see Appendix 4. There was no evidence for heterogeneity between the trials (I‐square 5.3%, p=0.3775). There was no evidence for small study effects: linear regression test p=0.7008, rank correlation test of funnel plot asymmetry p=0.6782. For Funnel plot see Figure 16. One study contributed about 14% weight to the overall analysis (Pirker 2008). In this study (Pirker 2008) (study number 89335) 600 patients with untreated, extensive SCLC underwent chemotherapy and were randomized to receive ESA or placebo. Exclusion of single studies at a time did only marginally influence the overall results, see influence analysis Figure 17.
19. Overall survival for chemotherapy trials.
| Model | ESA versus control HR (95% CI) | P value* | I² | P value** |
| Two‐stage log‐rank fixed‐effect model | 1.04 (95% CI 0.97‐1.11) | 0.2634 | 5.3% | 0.3775 |
| Two‐stage log‐rank random‐effect model | 1.04 (95% CI 0.97‐1.12) | 0.2774 | 5.3% | 0.3775 |
| Two‐stage Cox fixed‐effect model | 1.04 (95% CI 0.97‐1.11) | 0.3081 | 0% | 0.6828 |
| Two‐stage Cox random‐effects model | 1.04 (95% CI 0.97‐1.11) | 0.3081 | 0% | 0.6828 |
| Cox model stratified by study | 1.04 (95% CI 0.97‐1.11) | 0.263 | ‐ | 0.2359 |
*LR test, ** for test of heterogeneity
15.
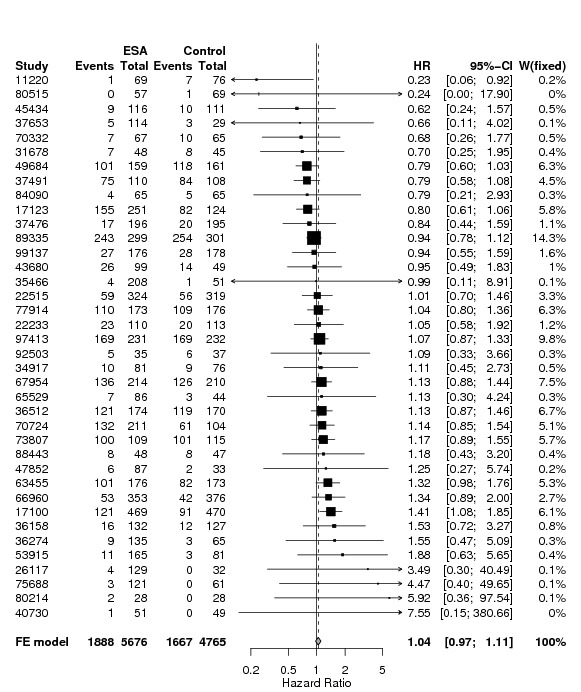
Forest plot for overall survival in chemotherapy trials based on two‐stage log‐rank fixed‐effect meta‐analysis
16.
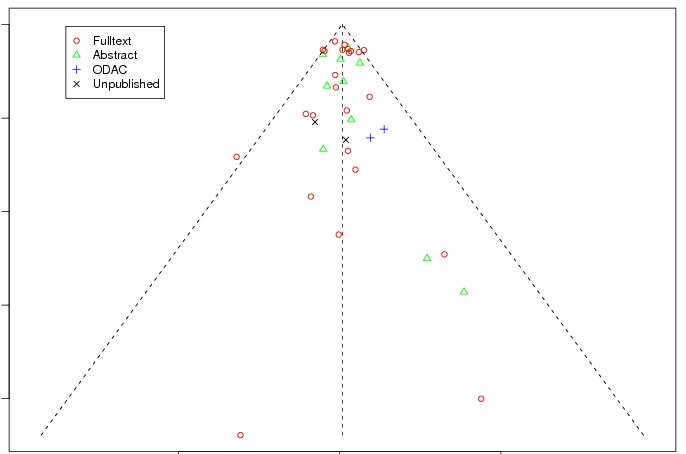
Funnel plot (based on log‐rank estimates) for overall survival in chemotherapy trials (subset analysis)
Explanation of terms used:
Full text: highest publication achieved is a full text publication
Abstract: highest publication achieved is an abstract publication
ODAC: highest publication achieved is reporting of study results in documents presented at ODAC hearings
Unpublished: to date the study was not published in any of the sources mentioned above
Date of reference: June 26th 2008
17.
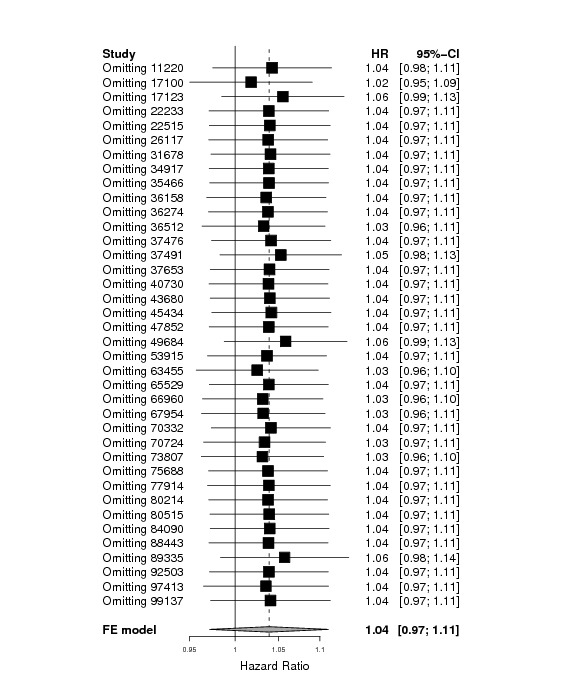
Influence analysis for overall survival in chemotherapy trials
Assessment of potential confounders for objective 1
In the next step we conducted bivariate analyses: adjusting overall survival based on the Cox model stratified by study for one variable at the time. All variables assessed relate to the individual patient data level. The results of the adjusted model were compared with the unadjusted model using LR‐Test. Results of unadjusted and adjusted models as well as P values of LR‐Test are shown in Table 20. We included only patients with full information for the respective variable; patients with missing, unknown or unreported data were excluded. Data were often missing for entire studies; therefore the overall HR might change because of the omission of specific studies. We therefore present both unadjusted and adjusted HRs based on the patient data set available for each variable.
20. Bivariate analysis for overall survival in chemotherapy trials.
| Overall survival for chemotherapy patients | Patients included | ESA versus control Unadjusted hazard ratio (95% CI) | ESA versus control Adjusted hazard ratio (95% CI) | P value LR‐Test* |
| Total | 10441 | 1.04 (95% CI 0.97‐1.11) | ‐ | ‐ |
| Hb at baseline (continuous) | 9945 | 1.04 (95% CI 0.97‐1.11) | 1.05 (95% CI 0.98‐1.12) | 0.0000 |
| Hb at baseline (categorical 1) | 9945 | 1.04 (95% CI 0.97‐1.11) | 1.05 (95% CI 0.98‐1.12) | 0.0000 |
| Hb at baseline (categorical 2) | 9945 | 1.04 (95% CI 0.97‐1.11) | 1.05 (95% CI 0.98‐1.12) | 0.0000 |
| Tumor (categorical 1) | 10399 | 1.04 (95% CI 0.97‐1.11) | 1.04 (95% CI 0.97‐1.11) | 0.0000 |
| Tumor (categorical 2) | 10399 | 1.04 (95% CI 0.97‐1.11) | 1.03 (95% CI 0.97‐1.11) | 0.0000 |
| Sex | 10441 | 1.04 (95% CI 0.97‐1.11) | 1.04 (95% CI 0.97‐1.11) | 0.0000 |
| Age (continuous) | 10430 | 1.04 (95% CI 0.97‐1.11) | 1.04 (95% CI 0.97‐1.11) | 0.0000 |
| Age (categorical) | 10430 | 1.04 (95% CI 0.97‐1.11) | 1.04 (95% CI 0.97‐1.11) | 0.0000 |
| Hct (continuous) | 7849 | 1.03 (95% CI 0.96‐1.11) | 1.04 (95% CI 0.97‐1.12) | 0.0000 |
| Hct (categorical) | 7849 | 1.03 (95% CI 0.96‐1.11) | 1.04 (95% CI 0.97‐1.12) | 0.0000 |
| Baseline serum EPO (continuous) | 3959 | 0.97 (95% CI 0.88‐1.07) | 0.97 (95% CI 0.88‐1.07) | 0.1538 |
| Baseline serum EPO (categorical) | 3959 | 0.97 (95% CI 0.88‐1.07) | 0.97 (95% CI 0.88‐1.07) | 0.0000 |
| ECOG (0 vs 1 vs 2 vs 3 vs 4) | 8057 | 1.04 (95% CI 0.97‐1.12) | 1.04 (95% CI 0.96‐1.12) | 0.0000 |
| ECOG (0,1,2 vs 3,4) | 8057 | 1.04 (95% CI 0.97‐1.12) | 1.04 (95% CI 0.97‐1.12) | 0.0000 |
| BMI (categorical) | 8882 | 1.02 (95% CI 0.95‐1.10) | 1.03 (95% CI 0.95‐1.10) | 0.0000 |
| History of thromboembolic events | 6667 | 1.04 (95% CI 0.95‐1.13) | 1.03 (95% CI 0.95‐1.12) | 0.0194 |
| History of cardiovascular events | 7369 | 1.04 (95% CI 0.96‐1.13) | 1.04 (95% CI 0.96‐1.13) | 0.0033 |
| History of hypertension | 6667 | 1.04 (95% CI 0.95‐1.13) | 1.03 (95% CI 0.95‐1.12) | 0.5565 |
| History of diabetes mellitus | 5579 | 1.04 (95% CI 0.95‐1.14) | 1.05 (95% CI 0.95‐1.15) | 0.0253 |
| Geographical region [region_cat] | 10053 | 1.03 (95% CI 0.97‐1.10) | 1.03 (95% CI 0.97‐1.11) | 0.1689 |
| Metastatic vs non‐metastatic | 8956 | 1.06 (95% CI 0.98‐1.13) | 1.04 (95% CI 0.97‐1.12) | 0.0000 |
| Time from cancer diagnosis to randomization | 3114 | 1.01 (95% CI 0.91‐1.13) | 1.01 (95% CI 0.91‐1.13) | 0.7895 |
*This test compares the adjusted with the unadjusted model. It takes into account the entire model, not only the overall hazard ratio.
Based on these analyses and the number of data available for each variable, we conducted four different models, all of which are presented in Table 21. For model 1 we included the variables age, sex, and Hb at baseline and tumor type into the model. For model 2 we used the same variables as in model 1 plus tumor stage. For model 3 we used the same variables as in model 1 plus BMI and region, for model 4 we used the same variables as in model 1 and 3 plus ECOG and hematocrit. For the continuous variables age, Hb, serum EPO and BMI the association between the exposure and the outcome was not linear (graph not shown). Therefore, these continuous variables were converted into prespecified categories. Hematocrit was converted into categories as well for the ease of interpretation. When history of thromboembolic events, history of cardiovascular events and history of diabetes mellitus were included in model 1 (each at a time) the overall results were also not altered (data on file).
21. Multivariate models for overall survival in chemotherapy trials.
| Overall survival, chemotherapy trials |
Model 1 |
Model 2 |
Model 3 |
Model 4 |
| Patients included | n=9892 | n=8469 | n=8030 | n=5109 |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| ESA vs ctrl unadjusted* | 1.04 (95% CI 0.97‐1.11) | 1.05 (95% CI 0.98‐1.13) | 1.01 (95% CI 0.94‐1.09) | 1.02 (95% CI 0.94‐1.11) |
| ESA vs ctrl adjusted** | 1.05 (95% CI 0.98‐1.12) | 1.05 (95% CI 0.98‐1.13) | 1.02 (95% CI 0.94‐1.10) | 1.04 (95% CI 0.96‐1.14) |
| Hb at baseline | ||||
| Hb < 8 g/dL | 1 | 1 | 1 | 1 |
| Hb 8‐10 g/dL | 0.85 (95% CI 0.73‐0.99) | 0.79 (95% CI 0.66‐0.94) | 0.87 (95% CI 0.74‐1.03) | 0.97 (95% CI 0.76‐1.23) |
| Hb 10‐12 g/dL | 0.67 (95% CI 0.57‐0.79) | 0.62 (95% CI 0.51‐0.74) | 0.72 (95% CI 0.60‐0.86) | 0.83 (95% CI 0.64‐1.07) |
| Hb 12‐14 g/dL | 0.53 (95% CI 0.44‐0.64) | 0.49 (95% CI 0.40‐0.60) | 0.59 (95% CI 0.48‐0.72) | 0.78 (95% CI 0.58‐1.05) |
| Hb > 14 g/dL | 0.44 (95% CI 0.35‐0.56) | 0.41 (95% CI 0.32‐0.53) | 0.48 (95% CI 0.37‐0.62) | 0.76 (95% CI 0.51‐1.13) |
| Age at randomization | ||||
| 18 ‐ 35 yrs | 0.79 (95% CI 0.59‐1.07) | 0.89 (95% CI 0.65‐1.22) | 0.83 (95% CI 0.59‐1.15) | 0.56 (95% CI 0.34‐0.91) |
| 35 ‐ 45 yrs | 1 | 1 | 1 | 1 |
| 45 ‐ 55 yrs | 1.09 (95% CI 0.94‐1.26) | 1.07 (95% CI 0.91‐1.25) | 1.15 (95% CI 0.97‐1.36) | 1.19 (95% CI 0.96‐1.46) |
| 55 ‐ 65 yrs | 1.16 (95% CI 1.01‐1.33) | 1.18 (95% CI 1.02‐1.37) | 1.32 (95% CI 1.11‐1.55) | 1.33 (95% CI 1.08‐1.63) |
| 65 ‐ 75 yrs | 1.29 (95% CI 1.11‐1.49) | 1.28 (95% CI 1.09‐1.49) | 1.42 (95% CI 1.20‐1.69) | 1.41 (95% CI 1.14‐1.73) |
| > 75 ys | 1.43 (95% CI 1.20‐1.70) | 1.54 (95% CI 1.27‐1.86) | 1.57 (95% CI 1.28‐1.93) | 1.56 (95% CI 1.22‐2.00) |
| Sex | ||||
| Male | 1 | 1 | 1 | 1 |
| Female | 0.74 (95% CI 0.68‐0.80) | 0.76 (95% CI 0.69‐0.83) | 0.76 (95% CI 0.70‐0.83) | 0.76 (95% CI 0.68‐0.84) |
| Tumor category | ||||
| Hematological malignancies | 1 | 1 | 1 | 1 |
| Breast cancer | 1.88 (95% CI 1.46‐2.42) | 1.50 (95% CI 0.98‐2.29) | 1.87 (95% CI 1.39‐2.51) | 1.98 (95% CI 1.44‐2.71) |
| Head and neck cancer | 1.84 (95% CI 0.80‐4.23) | 1.71 (95% CI 0.23‐12.7) | 2.03 (95% CI 0.28‐14.97) | 0.00 |
| Lung cancer | 4.15 (95% CI 3.19‐5.39) | 2.99 (95% CI 1.92‐4.64) | 4.37 (95% CI 3.09‐6.18) | 5.02 (95% CI 3.47‐7.26) |
| Gastrointestinal | 2.82 (95% CI 2.17‐3.67) | 2.58 (95% CI 1.66‐3.99) | 3.22 (95% CI 2.32‐4.46) | 3.58 (95% CI 2.53‐5.07) |
| Gynecological | 1.82 (95% CI 1.32‐2.51) | 1.08 (95% CI 0.66‐1.76) | 2.03 (95% CI 1.36‐3.01) | 2.89 (95% CI 1.89‐4.44) |
| Genitourinary | 2.29 (95% CI 1.54‐3.41) | 1.86 (95% CI 0.97‐3.57) | 1.91 (95% CI 1.05‐3.47) | 2.37 (95% CI 1.21‐4.63) |
| Other | 3.08 (95% CI 2.32‐4.09) | 2.57 (95% CI 1.63‐4.03) | 3.42 (95% CI 2.42‐4.83) | 4.00 (95% CI 2.77‐5.77) |
| Tumor stage | ||||
| Metastatic/advanced | ‐ | 1 | ‐ | ‐ |
| Not metastatic/advanced | ‐ | 0.48 (95% CI 0.41‐0.55) | ‐ | ‐ |
| Region | ||||
| Northern America | ‐ | ‐ | 1 | 1 |
| Southern Europe | ‐ | ‐ | 0.87 (95% CI 0.63‐1.21) | 0.82 (95% CI 0.58‐1.14) |
| Australia & New Zealand | ‐ | ‐ | 0.73 (95% CI 0.50‐1.09) | 0.69 (95% CI 0.46‐1.05) |
| Eastern Europe | ‐ | ‐ | 0.97 (95% CI 0.71‐1.31) | 0.96 (95% CI 0.70‐1.31) |
| Northern Europe | ‐ | ‐ | 1.02 (95% CI 0.75‐1.40) | 1.03 (95% CI 0.75‐1.43) |
| Western Europe | ‐ | ‐ | 1.02 (95% CI 0.75‐1.39) | 1.01 (95% CI 0.73‐1.38) |
| Other | ‐ | ‐ | 0.80 (95% CI 0.52‐1.25) | 0.97 (95% CI 0.58‐1.61) |
| BMI | ||||
| < 19 kg/m² | ‐ | ‐ | 1 | 1 |
| 19‐25 kg/m² | ‐ | ‐ | 0.83 (95% CI 0.72‐0.97) | 0.87 (95% CI 0.74‐1.03) |
| 25‐30 kg/m² | ‐ | ‐ | 0.75 (95% CI 0.64‐0.87) | 0.78 (95% CI 0.66‐0.92) |
| > 30 kg/m² | ‐ | ‐ | 0.64 (95% CI 0.54‐0.77) | 0.63 (95% CI 0.52‐0.77) |
| Hct at baseline | ||||
| Hct 0‐23.5% | ‐ | ‐ | ‐ | 1 |
| Hct 23.5%‐29.4% | ‐ | ‐ | ‐ | 0.90 (95% CI 0.60‐1.34) |
| Hct 29.4%‐35.3% | ‐ | ‐ | ‐ | 0.81 (95% CI 0.54‐1.21) |
| Hct 35.3%‐41.2% | ‐ | ‐ | ‐ | 0.70 (95% CI 0.46‐1.07) |
| >Hct 41.2% | ‐ | ‐ | ‐ | 0.55 (95% CI 0.34‐0.90) |
| Performance score | ||||
| ECOG 0, 1 or 2 | ‐ | ‐ | ‐ | 1 |
| ECOG 3 or 4 | ‐ | ‐ | ‐ | 2.24 (95% CI 1.70‐2.96) |
*unadjusted HR based on the number of patients included in the respective model ** HR adjusted for the variables outlined in the columns
Summary points for objective 1 overall survival in chemotherapy trials
Across studies with >70% of patients receiving chemotherapy, ESA treatment appeared to slightly increase the risk of mortality over longest available follow‐up (HR 1.04, 95% CI 0.97‐1.11, n=10441).
Available evidence does not support the hypothesis that baseline imbalances of prognostic factors analyzed influenced the overall results.
Objective 2 for overall survival in chemotherapy trials
Aim: Is there a specific subgroup of patients that is at increased or decreased risk to die when receiving ESAs compared to controls? Are there design aspects at study level that influenced the effects of ESA on survival?
We conducted subgroup analyses for each patient and study characteristic variable at the time and tested for interaction between ESA treatment and specific variables describing patient and study characteristics. Results of tests for interactions are outlined in Table 22, results for subgroup estimates are outlined in Appendix 9.
22. Assessment of interaction for overall survival in chemotherapy trials.
| Overall survival, chemotherapy patients | Patients included | P value for interaction |
| Total included | 10441 (100%) | |
| Patient level characteristics | ||
| Hb at baseline (continuous) | 9945 | 0.4909 |
| Hb at baseline (categorical 1) | 9945 | 0.8848 |
| Hb at baseline (categorical 2) | 9945 | 0.9844 |
| Tumor (categorical 1) | 10399 | 0.3301 |
| Tumor (categorical 2 | 10399 | 0.3287 |
| Sex | 10441 | 0.0370 |
| Age (continuous) | 10430 | 0.4055 |
| Age (categorical) | 10430 | 0.4024 |
| Hct (continuous) | 7849 | 0.2527 |
| Hct (categorical | 7849 | 0.2445 |
| Baseline serum EPO (continuous) | 3959 | 0.9996 |
| Baseline serum EPO (categorical) | 3959 | 0.4910 |
| ECOG | 8057 | 0.3408 |
| ECOG (0,1,2 vs 3,4) | 8057 | 0.9230 |
| BMI (categorical) | 8882 | 0.5227 |
| History of thromboembolic events | 6667 | 0.6838 |
| History of cardiovascular events | 7369 | 0.7809 |
| History of hypertension | 6667 | 0.9079 |
| History of diabetes mellitus | 5579 | 0.6186 |
| Geographical region [region_cat] | 10053 | 0.9283 |
| Metastatic vs non‐metastatic | 8956 | 0.6040 |
| Planned Hb ceiling (categorical 1) | 10362 | 0.5706 |
| Planned Hb ceiling (categorical 2) | 10362 | 0.7743 |
| Study level characteristics | ||
| Placebo controlled | 10441 | 0.7668 |
| Randomization (adequate vs unclear) | 10441 | 0.9035 |
| Allocation (adequate vs unclear) | 10441 | 0.2609 |
| Endpoint overall survival | 10441 | 0.5819 |
| Designed for long term follow up (binary) | 10441 | 0.4744 |
| Year of last patient randomized into study (categorical) | 10441 | 0.1793 |
| Source of data (company versus independent) | 10441 | 0.5404 |
| Iron category | 10441 | 0.4098 |
| Planned ESA treatment duration (categorical) | 10441 | 0.7156 |
| Planned weekly ESA dosage (categorical) | 10441 | 0.3738 |
| Planned frequency of ESA administration (categorical) | 10441 | 0.1562 |
*P value for interaction based on LR test, patients with missing data are excluded from LR test
Only one variable (sex) showed a statistically significant interaction term in the bivariate analysis. Women were at increased risk to die when receiving ESAs (HR 1.10, 95% CI 1.01‐1.21) compared to men (HR 0.96, 95% CI 0.87‐1.06, P value for interaction: 0.0370). When adjusting in addition for age, Hb at baseline and tumor category, the modifying effect for sex remained (P value for interaction 0.0362) (Table 23). For additional exploratory analyses see Appendix 4.
23. Overall survival in chemotherapy trials, tests for interaction, univariate and multivariate models.
| Overall survival in chemotherapy trials | Bivariate ESA versus control | Multivariate ESA versus control | ||||
| Interaction term | ESA*sex | ESA*sex | ||||
| Adjusted for | ‐ | age, sex, Hb, tumor type | ||||
| Patients excluded | ‐ | ‐ | ||||
| Patients included | n = 10441 | n = 9892 | ||||
| ESA versus control | HR | 95% CI | P* | HR | 95% CI | P* |
| Sex | ||||||
| Male | 0.96 | 0.87‐1.06 | 0.0370 | 0.97 | 0.87‐1.07 | 0.0362 |
| Female | 1.10 | 1.01‐1.21 | 1.12 | 1.02‐1.22 | ||
| Overall result, unadjusted | 1.04 | 0.97‐1.11 | ‐ | 1.04 | 0.97‐1.11 | ‐ |
*P value LR test comparing model with and without interaction term
Summary points for objective 2 for overall survival in chemotherapy patients
Within the chemotherapy population there was no convincing evidence to support the hypothesis that ESAs had different effects in sub‐populations that differed for any of the variables tested.
However, effect modification of sex cannot be explained by confounding with other patient characteristics (Hb, age, sex, tumor type), see also Appendix 4.
Survival at predefined time points
In addition to the endpoints “on study mortality” and “overall survival”, we specifically examined the following prespecified time points: survival at 4, 8, 12, 24, 36 and 60 months after randomization. We conducted these analyses in two different data sets: one analysis was based on the “on study mortality” data set. In this data set all patients were censored after the end of active treatment plus a follow‐up window of 28 days. In contrast in the overall survival analysis patients were followed up after the end of active study treatment phase (exception: studies with “cross‐over” after end of study period). When comparing the numbers of death at specific time points, the number of patients who died was higher in the overall survival data set compared to the on study mortality data set at 4, 8 and 12 months. The point estimates for HRs of overall survival appear smaller, but confidence intervals are wide, with substantial overlap. Several reasons might explain this observation: patients in both active and control arm might have received ESAs after end of study period, the underlying disease might dominate the picture after the end of ESA treatment and there might be losses to follow‐up since not all studies were designed for a long‐term active follow‐up. We conducted a sensitivity analysis for studies, which had an active follow‐up after the end of ESA treatment period at least additional 12 months, see Appendix 3.
Survival at predefined time points: including all studies
see Table 24, Table 25, Table 26, and Table 27
24. Survival at predefined time points for all cancer patients*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | ESA versus control HR (95% CI)* | P value |
| At 4 months | 1193 | 1.13 (95% CI 1.01‐1.27) | 0.036 | 1419 | 1.12 (95% CI 1.01‐1.24) | 0.038 |
| At 8 months | 1425 | 1.16 (95% CI 1.04‐1.29) | 0.006 | 2678 | 1.06 (95% CI 0.98‐1.14) | 0.140 |
| At 12 months | 1507 | 1.17 (95% CI 1.06‐1.30) | 0.002 | 3561 | 1.06 (95% CI 0.99‐1.14) | 0.071 |
| At 24 months | ‐ | ‐ | ‐ | 4537 | 1.06 (95% CI 1.00‐1.13) | 0.042 |
| At 36 months | ‐ | ‐ | ‐ | 4833 | 1.05 (95% CI 0.99‐1.12) | 0.075 |
| At 60 months | ‐ | ‐ | ‐ | 4977 | 1.06 (95% CI 1.00‐1.12) | 0.043 |
*13933 patients from all treatment populations were under observation. **based on Cox fixed‐effects model stratified by study
25. Survival at predefined time points for all chemotherapy trials*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | ESA versus control HR (95% CI)* | P value |
| At 4 months | 792 | 1.03 (95% CI 0.89‐1.18) | 0.705 | 948 | 1.06 (95% CI 0.93‐1.21) | 0.383 |
| At 8 months | 992 | 1.08 (95% CI 0.95‐1.23) | 0.225 | 1870 | 0.99 (95% CI 0.91‐1.09) | 0.886 |
| At 12 months | 1072 | 1.10 (95% CI 0.98‐1.25) | 0.117 | 2552 | 1.01 (95% CI 0.93‐1.09) | 0.797 |
| At 24 months | ‐ | ‐ | ‐ | 3246 | 1.04 (95% CI 0.97‐1.11) | 0.312 |
| At 36 months | ‐ | ‐ | ‐ | 3452 | 1.03 (95% CI 0.96‐1.10) | 0.368 |
| At 60 months | ‐ | ‐ | ‐ | 3544 | 1.04 (95% CI 0.97‐1.11) | 0.257 |
*10441 patients from the chemotherapy treatment population were under observation. **based on Cox fixed‐effects model stratified by study
26. Survival at predefined time points for radiotherapy and radiochemotherapy trials*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | Overall survival HR (95% CI)* | P value |
| At 4 months | 74 | 1.40 (95% CI 0.88‐2.23) | 0.152 | 114 | 1.16 (95% CI 0.80‐1.67) | 0.440 |
| At 8 months | 82 | 1.51 (95% CI 0.97‐2.35) | 0.067 | 300 | 1.20 (95% CI 0.95‐1.50) | 0.119 |
| At 12 months | 82 | 1.51 (95% CI 0.97‐2.35) | 0.067 | 442 | 1.12 (95% CI 0.93‐1.35) | 0.235 |
| At 24 months | ‐ | ‐ | ‐ | 686 | 1.05 (95% CI 0.91‐1.22) | 0.498 |
| At 36 months | ‐ | ‐ | ‐ | 774 | 1.02 (95% CI 0.89‐1.18) | 0.753 |
| At 60 months | ‐ | ‐ | ‐ | 826 | 1.03 (95% CI 0.90‐1.18) | 0.653 |
*1536 patients from the radiotherapy and radiochemotherapy treatment population were under observation. **based on Cox fixed‐effects model stratified by study
27. Survival at predefined time points for patients from the "mixed" treatment group*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | HR (95% CI)** ESA versus control On study mortality data set | P value | Deaths | HR (95% CI)* Overall survival data set | P value |
| At 4 months | 24 | 1.53 (95% CI 0.63‐3.69) | 0.335 | 24 | 1.53 (95% CI 0.63‐3.69) | 0.335 |
*266 patients from two studies under observation, both studies included CLL patients only, patients received either chemotherapy or corticosteroids only. Since follow up in these studies was short data are provided at 4 months only. **based on Cox fixed‐effects model stratified by study
Sensitivity analysis: survival at predefined time points including only studies with long‐term follow‐up
The outputs of Table 28, Table 29, Table 30, Table 31, Table 32, and Table 33 were restricted to studies that were designed for long‐term follow‐up. Long‐term follow‐up was defined as follow‐up of at least 12 months after end of treatment phase.
28. Survival at predefined time points in trials without concomitant radiotherapy and/or chemotherapy*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | ESA versus control HR (95% CI)* | P value |
| At 4 months | 303 | 1.35 (95% CI 1.07‐1.71) | 0.010 | 333 | 1.27 (95% CI 1.02‐1.58) | 0.035 |
| At 8 months | 327 | 1.32 (95% CI 1.06‐1.65) | 0.013 | 484 | 1.24 (95% CI 1.03‐1.48) | 0.021 |
| At 12 months | 329 | 1.33 (95% CI 1.06‐1.66) | 0.012 | 543 | 1.28 (95% CI 1.08‐1.52) | 0.005 |
| At 24 months | ‐ | ‐ | ‐ | 581 | 1.22 (95% CI 1.04‐1.44) | 0.017 |
| At 36 months | ‐ | ‐ | ‐ | 583 | 1.22 (95% CI 1.04‐1.44) | 0.017 |
*1690 patients were under observation, patients were mainly not receiving chemotherapy or radiotherapy, table truncated after end of follow up. **based on Cox fixed‐effects model stratified by study
29. Survival at predefined time points for all cancer patients, long term follow up studies only*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | ESA versus control HR (95% CI)* | P value |
| At 4 months | 790 | 1.22 (95% CI 1.06‐1.41) | 0.005 | 965 | 1.17 (95% CI 1.03‐1.33) | 0.015 |
| At 8 months | 970 | 1.25 (95% CI 1.10‐1.42) | 0.001 | 2023 | 1.08 (95% CI 0.99‐1.18) | 0.097 |
| At 12 months | 1050 | 1.26 (95% CI 1.11‐1.42) | <0.001 | 2823 | 1.08 (95% CI 1.00‐1.16) | 0.046 |
| At 24 months | ‐ | ‐ | ‐ | 3743 | 1.07 (95% CI 1.01‐1.15) | 0.032 |
| At 36 months | ‐ | ‐ | ‐ | 4028 | 1.06 (95% CI 0.99‐1.13) | 0.077 |
| At 60 months | ‐ | ‐ | ‐ | 4169 | 1.07 (95% CI 1.00‐1.13) | 0.041 |
*8974 patients from all treatment populations stemming from trials designed for long term follow up were under observation. **based on Cox fixed‐effects model stratified by study
30. Survival at predefined time points in chemotherapy trials, long term follow up studies only*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | ESA versus control HR (95% CI)* | P value |
| At 4 months | 499 | 1.14 (95% CI 0.95‐1.36) | 0.153 | 604 | 1.14 (95% CI 0.97‐1.34) | 0.119 |
| At 8 months | 658 | 1.18 (95% CI 1.01‐1.37) | 0.040 | 1346 | 1.01 (95% CI 0.91‐1.13) | 0.842 |
| At 12 months | 738 | 1.20 (95% CI 1.03‐1.39) | 0.016 | 1952 | 1.03 (95% CI 0.94‐1.13) | 0.527 |
| At 24 months | ‐ | ‐ | ‐ | 2594 | 1.05 (95% CI 0.97‐1.14) | 0.191 |
| At 36 months | ‐ | ‐ | ‐ | 2789 | 1.04 (95% CI 0.97‐1.12) | 0.290 |
| At 60 months | ‐ | ‐ | ‐ | 2878 | 1.05 (95% CI 0.98‐1.13) | 0.182 |
*6509 patients from the chemotherapy treatment population stemming from trials that were designed for long term follow up were under observation. ** Based on Cox fixed‐effects model stratified by study
31. Survival at predefined time points in radiotherapy and radiochemotherapy trials, long term follow up studies only*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | ESA versus control HR (95% CI)* | P value |
| At 4 months | 74 | 1.40 (95% CI 0.88‐2.23) | 0.152 | 114 | 1.16 (95% CI 0.80‐1.67) | 0.440 |
| At 8 months | 82 | 1.51 (95% CI 0.97‐2.35) | 0.067 | 299 | 1.21 (95% CI 0.96‐1.51) | 0.107 |
| At 12 months | 82 | 1.51 (95% CI 0.97‐2.35) | 0.067 | 441 | 1.12 (95% CI 0.93‐1.36) | 0.219 |
| At 24 months | ‐ | ‐ | ‐ | 685 | 1.06 (95% CI 0.91‐1.22) | 0.477 |
| At 36 months | ‐ | ‐ | ‐ | 773 | 1.03 (95% CI 0.89‐1.18) | 0.729 |
| At 60 months | ‐ | ‐ | ‐ | 825 | 1.03 (95% CI 0.90‐1.19) | 0.631 |
*1476 patients from the radiotherapy and radiochemotherapy treatment population stemming from trials designed for long term follow up were under observation. **based on Cox fixed‐effects model stratified by study
32. Survival at predefined time points for patients from the "mixed" treatment group, long term follow up studies only*.
| Time after date of randomization | Deaths | On study mortality data set | P value | Deaths | Overall survival data set | P value |
| At 4 months | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
*266 patients from two studies under observation, both studies included CLL patients only, patients received either chemotherapy or corticosteroids only. Both studies were not designed for long term follow‐up and are therefore not reported for this sensitivity analysis.
33. Survival at predefined time points in trials without concomitant radiotherapy and/or chemotherapy, long term follow up studies only*.
| On study mortality data set | Overall survival data set | |||||
| Time after date of randomization | Deaths | ESA versus control HR (95% CI)** | P value | Deaths | ESA versus control HR (95% CI)* | P value |
| At 4 months | 217 | 1.38 (95% CI 1.05‐1.81) | 0.018 | 247 | 1.26 (95% CI 0.98‐1.62) | 0.070 |
| At 8 months | 230 | 1.37 (95% CI 1.05‐1.78) | 0.018 | 378 | 1.23 (95% CI 1.00‐1.51) | 0.045 |
| At 12 months | 230 | 1.37 (95% CI 1.06‐1.78) | 0.018 | 430 | 1.27 (95% CI 1.05‐1.54) | 0.013 |
| At 24 months | ‐ | ‐ | ‐ | 464 | 1.22 (95% CI 1.02‐1.47) | 0.032 |
| At 36 months | ‐ | ‐ | ‐ | 466 | 1.22 (95% CI 1.02‐1.47) | 0.032 |
*989 patients were under observation, patients were mainly not receiving chemotherapy or radiotherapy, table truncated after end of follow up, only patients stemming from studies with long term follow up were included. For the no treatment population this was actually only one study. **based on Cox fixed‐effects model stratified by study
Sensitivity analyses
see Appendix 3
Exploratory analyses
see Appendix 4
Clinical relevance
To calculate the number needed to treat for an additional harmful outcome (NNTH) we applied the overall estimate for on study mortality for all cancer patients (HR 1.17; 95% CI 1.06 to 1.30) to different hypothetical cancer populations (Altman 1999). With an underlying survival probability of 95% at one year it is expected that one additional person may die for every 121 participants randomized to receive ESAs (NNTH 121, 95% CI 69 to 343). With an underlying survival probability of 80% the NNTH is 34 (95% CI 19 to 94) and 24 (95% CI 14 to 67) for a survival probability of 70%, see Table 34.
34. Clinical relevance for overall estimate of on study mortality applied to hypothetical populations.
| Underlying survival probability | ESA versus control HR (95% CI) | Number needed to treat (95% CI) |
| On study mortality, all cancer patients | ||
| 95% | 1.17 (95% CI 1.06‐1.30) | NNTH 121 (NNTH 69 to NNTH 343) |
| 80% | NNTH 34 (NNTH 19 to NNTH 94) | |
| 70% | NNTH 24 (NNTH 14 to NNTH 67) | |
| On study mortality, chemotherapy trials | ||
| 95% | 1.10 (95% CI 0.98‐1.24) | NNTH 206 (NNTH 86 to ∞ to NNTB 1026) |
| 80% | NNTH 57 (NNTH 24 to ∞ to NNTB 279) | |
| 70% | NNTH 41 (NNTH 17 to ∞ to NNTB 200) | |
We also calculated the number needed to treat for an additional harmful outcome (NNTH) for the on study mortality estimate from chemotherapy trials. Note: the confidence intervals for this estimate include 1.0 which requires special consideration when calculating confidence intervals for numbers needed to treat (Altman 1998). We applied the overall estimate for on study mortality from chemotherapy trials (HR 1.10; 95% CI 0.98 to 1.24) to different hypothetical cancer populations (Altman 1999). With an underlying survival probability of 95% at one year it is expected that one additional person may die for every 206 participants randomized to receive ESAs (95% CI NNTH 86 to ∞ to NNTB 1026). With an underlying survival probability of 80% the NNTH is 57 (95% CI NNTH 24 to ∞ to NNTB 279) and 41 (95% CI NNTH 17 to ∞ to NNTB 200) for a survival probability of 70%, see also Table 34.
Discussion
Summary of main results
This individual patient data meta‐analysis of 53 randomized clinical trials in cancer patients found that ESAs caused an estimated 17% increase in mortality relative to control during the study period and a relative increase of 6% when the longest available follow‐up was considered. The increase in mortality was less pronounced in patients receiving chemotherapy, but this difference is likely to be the product of chance.
Overall completeness and applicability of evidence
Our analysis has a number of strengths. It was based on individual patient data from 13933 patients who were enrolled in trials conducted by manufacturers and independent investigators. We had access to the study protocols and clinical study reports. All analyses were based on the intention‐to‐treat principle, i.e. all patients were evaluated in the treatment groups assigned at randomization; analyses were conducted in duplicate by two independent, experienced groups. Only factors known before the onset of treatment were considered as candidate effect modifiers. A striking finding was that although the studies included clinically diverse populations, and different ESA regimens, we detected very little, if any heterogeneity between trials. Sensitivity analyses confirmed the robustness of the overall results.
Potential biases in the review process
Data were not available for some trials, in particular RCTs with radiotherapy or radiochemotherapy (Overgaard 2007; Blohmer 2003; Antonadou 2001). However, inclusion of these studies based on the results published in the literature did not change the overall estimates. An important finding of this study is the absence of strong modifiers of the effect of ESAs on mortality. Given the large data set analyzed it seems unlikely that larger differences were missed. However, uncertainty remains since smaller differences in effects cannot be excluded with confidence.
Agreements and disagreements with other studies or reviews
While most literature‐based meta‐analyses are limited by access to aggregated data at study level only, our IPD meta‐analysis contained data on prognostic factors at patient level. Therefore, subgroup analyses based on the information for the individual patient and statistical tests for modification of results by patient and study characteristics could be analyzed across almost 14000 patients. Another advantage is the harmonized definition and analysis of different survival endpoints. I.e. we differentiated on study mortality and overall survival, which included the longest follow‐up available. While overall survival aims to detect long‐term effects, confounders occurring after the end of active study phase cannot be excluded. I.e. control patients may start ESA treatment, progression of the underlying malignancy may dominate the course of disease and follow‐up might be less rigorous leading to losses to follow‐up; all of these factors may dilute the overall effect. Indeed, the overall survival estimates in our analyses were lower compared to the on study mortality estimates. For the latter we restricted follow‐up to the study phase when patients were under close and active observation and control of both ESA medication and events. Thus, on study mortality presents the most reliable information with respect to unconfounded assessment of the effects of ESAs during treatment period. This clear definition of separate endpoints at different periods under observation distinguishes our IPD meta‐analysis from literature based meta‐analyses, which must rely on the results as reported in the literature. However, survival is often not reported or reported incompletely. For example, in the reports identified for the 51 published studies analyzed here, five studies did not report any survival data, 19 reported on study mortality, 14 overall survival and only 13 reported both endpoints; two studies were unpublished. Given the paucity of published data previous literature‐based meta‐analyses (Bohlius 2006; Bennett 2008; Seidenfeld 2006) combined on study mortality and overall survival data into one analysis, which led to an underestimation of the effect size of ESAs on mortality.
Previous analyses hypothesized that poor study designs may have produced biased results. In particular, some argued that baseline imbalances favoring the control groups might partially explain the increased mortality (Henke 2003; Leyland‐Jones 2003; Smith 2008). Our analysis found no evidence that imbalances at baseline in prognostic factors influenced the overall results. However, baseline imbalances for prognostic factors not included in the present analysis cannot be excluded. For the analysis of on study mortality in chemotherapy we observed that studies with adequate reporting of concealment of allocation reported worse effect estimates compared to studies with inadequate reporting of allocation procedures. In general, studies with adequate reporting of allocation concealment are considered to indicate studies of higher quality. Patients who were censored at a given point were often followed for only four weeks after the last drug application but not until the end of the planned treatment duration.
Epo receptors have been identified on the cell surface of numerous cancer entities. Consequently, endogenously produced or exogenously administered erythropoietins may stimulate proliferation of cancer cells expressing these receptors (Arcasoy 2003; Arcasoy 2005; Dagnon 2005; McBroom 2005; Leo 2006). However, controversy about the functionality of these receptors in tumor tissues remains (Jelkmann 2008; Sinclair 2008). Data on Epo receptor status of tumor tissues were not systematically collected in the included trials and were therefore not available for the present study.
It was also hypothesized that the increase in hemoglobin levels associated with ESAs, particularly to beyond 15 g/dL, might impair tumor control. Radiobiological data suggest that tumor hypoxia is associated with an increased resistance to radiation induced tumor cell kill due to lower production of cytotoxic free radicals (Vaupel 2001). Thus, tumor hypoxia caused either by anemia or excessively high hemoglobin levels and increased viscous resistance may result in worse treatment outcomes (Vaupel 2002). Similarly, it was argued, that high hemoglobin levels might increase the risk for fatal thromboembolic and cardiovascular events. Trials directly comparing different Hb targets in patients with renal impairment found increased mortality in patients treated to higher Hb targets (13.5 g/dL versus 11.3 g/dL) who had received higher ESA dosages (mean 11215 units per week versus 6276 IU per week) (Singh 2006; Besarab 1998). Of note, ESA dosages applied in cancer patients are on average three to four times higher than the high ESA doses reported in the study by Singh et al. We found no robust evidence for an interaction between ESA treatment hemoglobin ceilings, planned ESA dosages and mortality. However, our analysis was based on indirect comparisons only.
Other hypotheses relate to the effects of erythropoietins on the vascular system and tumor tissues. There is increasing evidence that ESAs might influence the vascular system including hematocrit‐independent hypertension, increased endothelin production and stimulation of endothelial and vascular smooth muscle cell proliferation which may contribute to an increased risk of thromboembolic and cardiovascular events independent of Hb levels (Vaziri 1999; Fisher 2003; Stohlawetz 2000; Wun 2003). Intriguingly, in our analysis patients with a history of thromboembolic events were less likely to die when receiving ESAs compared to patients without a history of thromboembolic events. One potential explanation for the observed effect is the possibility that patients with a history of thromboembolic events may have received better anticoagulation precautions during cancer therapy and this measure may have protected against the thrombogenic effects of ESAs. This is in line with a finding from a randomized trial in critically ill patients indicating that patients receiving heparin were less likely to develop thromboembolic events when receiving ESAs compared to patients not receiving heparin (Corwin 2007). However, for 31% of our entire study population history of thromboembolic events was not reported; thus, a selection bias cannot be excluded. In conclusion, the evidence reported here is too weak to establish a robust association between history of thromboembolic events and effects of ESA on mortality during study in cancer patients. There was some evidence that women were at increased risk to die when receiving ESAs compared to men. This effect modification was only observed for overall survival in chemotherapy patients, however, for all other endpoints the risk for women to die when receiving ESAs ranged between HR 1.10 and HR 1.17, although not statistically significant. The observed estimates were attenuated when excluding patients with breast cancer and other cancers that occur in women or men only. Further investigation is needed to clarify this observation.
We also observed a modifying effect of baseline Hct on mortality during active study phase and long‐term follow‐up. Patients with low hematocrit at baseline (< 23.5%) were more likely to die when receiving ESAs compared to patients with higher hematocrit values. This observed effect was robust when adjusting for other prognostic factors such as tumor stage and ECOG performance status. Similarly, patients with baseline Hb below 8 g/dL were at increased risk to die compared to others, although this effect was not statistically significant in any of the analyses. This observation may indicate that low hematocrit values are a surrogate for poor risk patients and that these patients might be more vulnerable to harm from ESAs. However, data for 21% of patients were missing leaving uncertainty to the validity of this finding.
Patients receiving ESAs three times per week or more frequently were not at increased risk to die compared to patients who received ESAs only once per week. This was observed for on study mortality analyses but not for the overall survival analyses. However, the data did not show a dose response relationship and the observed effect was confounded by other study design aspects such as planned dose of ESA, year of study conduct and primary endpoint of the study. The effect was not observed for the overall survival analysis.
Of particular interest is the possibility that ESAs have less potential harm in patients receiving chemotherapy compared to patients receiving radiochemotherapy, radiotherapy or no anticancer treatment. Mortality was increased in patients from chemotherapy trials by 10% (HR 1.10, 95% CI 0.98 to 1.24). From a statistical point of view the estimated increase in mortality from the chemotherapy trials is compatible with that obtained from other treatment group (including radiochemotherapy, radiotherapy, none and other, p=0.42 for difference). From a clinical point of view, patients not receiving myelosuppressive anticancer treatment might be more likely to experience higher hemoglobin levels leading to thromboembolic events and impaired tumor control, as discussed above. However, in the present analysis we found little evidence to support this notion.
Authors' conclusions
Implications for practice.
In conclusion, this large scale individual patient data meta‐analysis found that ESAs increase mortality in cancer patients, and such an increase is also likely in patients receiving chemotherapy. Most randomized studies and previous meta‐analyses have shown that ESAs increase hemoglobin levels, decrease the need for red blood cell transfusions and spare some patients from transfusions (Seidenfeld 2001; Bohlius 2005). A recent meta‐analysis also suggested that ESAs may effectively reduce fatigue (Minton 2008). In clinical practice the increased risks of death and thromboembolic events (Bohlius 2006; Bennett 2008) must be balanced against the possible benefits of ESAs on quality of life, taking into account the clinical circumstances and preferences of the individual patient.
Implications for research.
More data are needed on ESAs effect on quality of life and an individual patient data meta‐analysis project similar to this will be needed to address this question.
Further research is also needed to clarify mechanisms and pathways of ESAs effects at the cellular and molecular levels for both potential tumor growth stimulation and thrombogenic effects of ESAs.
What's new
| Date | Event | Description |
|---|---|---|
| 29 September 2010 | Amended | funding number added |
Acknowledgements
STEERING COMMITTEE: Julia Bohlius (project manager), Michael Clarke, Matthias Egger, Andreas Engert (principal investigator), Maryann Napoli, Margaret Piper, Dirk Rades, Martin Schumacher, Jerome Seidenfeld, Mark Somerfield, David Steensma
STATISTICAL ANALYSIS TEAM: Julia Bohlius, Corinne Brillant, Matthias Egger, Kurt Schmidlin, Martin Schumacher, Guido Schwarzer, Sven Trelle, Marcel Zwahlen
REVIEWER FOR STUDY SELECTION AND DATA EXTRACTION: Julia Bohlius, Sabine Kluge, Olaf Weingart
ADVISORY BOARD: Jesse Berlin for J&J PRD, Peter Bowers for J&J PRD, Ulrich Burger for F. Hoffmann‐La Roche Ltd, Tom Lillie for Amgen, Volker Moebus for the AGO ETC trial, Isabelle Ray‐Coquard for the ELYPSE‐4 study, Armin Scherhag for F. Hoffmann‐La Roche Ltd, Gillian Thomas for the GOG‐191 study, Dianne Tomita for Amgen, Michael Untch for the AGO PREPARE study.
HARMONIZATION OF THE ORIGINAL STUDY DATA FOR THE META‐ANALYSIS: Shamshad Ali for the GOG‐191 study, Sophie Dussart for the ELYPSE‐4 study, Alex Fleishman and the Biometrics and Data Management staff at Amgen, Viktor Nendel and the Biometrics and Data Management staff at F. Hoffmann‐La Roche Ltd, Steven Sun and the Biometrics and Data Management staff at J&J PRD.
FUNDING: This project was funded by the German Federal Ministry of Education and Research (BMBF) (grant application number: 01KG0611), "Köln Fortune" from the medical faculty of the University of Cologne and “Oncosuisse” (grant application number: OCS‐02146‐10‐2007), Switzerland
We thank Ina Monsef and Frauke Naumann for help with literature searches and Nicole Skoetz for technical assistance; John Spivak for review of the protocol, Stuart Pocock for critical review of a previous manuscript, Pia Raanani and Sue Richard for critical review of the protocol and the report of this review; Jens Blohmer for providing unpublished study results. We thank Mitchell Machtay and the Radiation Therapy Oncology Group (RTOG) for submission of study data. We would like to thank the collaborating companies for contributing study data and study documents for this project.
Appendices
Appendix 1. Search strategies
Search strategies for IPD meta‐analysis update
Database: Ovid MEDLINE(R)
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp ERYTHROPOIETIN/
2 exp ERYTHROPOIETIN, RECOMBINANT/
3 erythropoietin.mp.
4 erythropoiesis.mp.
5 exp EPOETIN ALFA/
6 epoetin.mp.
7 epo.mp.
8 epoetin alfa.mp.
9 epoetin beta.mp.
10 eprex.mp.
11 neorecormon.mp.
12 aranesp.mp.
13 procrit.mp.
14 recombinant erythropoietin.mp.
15 darbepoetin alfa.mp.
16 darbepoetin.mp.
17 RECEPTORS, ERYTHROPOIETIN/
18 CERA.mp.
19 or/1‐18
20 exp ANEMIA/dt, th [Drug Therapy, Therapy]
21 anaemia.mp.
22 anemia.mp.
23 (anemi$ adj3 cancer).mp.
24 (anaemi$ adj3 cancer).mp.
25 or/20‐24
26 exp Neoplasms/
27 malignan$.mp.
28 cancer$.mp.
29 oncolog$.tw.
30 myelodysplas$.tw.
31 chemotherapy.mp.
32 tumo?r$.mp.
33 carcinom$.mp.
34 or/26‐33
35 19 and 25
36 34 and 25
37 randomized controlled trial.pt.
38 controlled clinical trial.pt.
39 randomized controlled trials/
40 random allocation/
41 double blind method/
42 single blind method/
43 or/37‐42
44 (ANIMALS not HUMANS).sh.
45 43 not 44
46 clinical trial.pt.
47 exp clinical trials/
48 (clin$ adj25 trial$).ti,ab.
49 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
50 placebos/
51 placebo$.ti,ab.
52 random$.ti,ab.
53 research design/
54 or/46‐53
55 54 not 44
56 55 not 45
57 comparative study/
58 exp evaluation studies/
59 follow up studies/
60 prospective studies/
61 (control$ or prospectiv$ or volunteer$).ti,ab.
62 or/57‐61
63 62 not 44
64 63 not (45 or 56)
65 45 or 56 or 64
66 36 and 65
Database: Ovid (Embase)
Database: Ovid (Embase)
1 erythropoietin.mp.
2 exp ERYTHROPOIETIN/
3 exp RECOMBINANT ERYTHROPOIETIN/
4 epoetin.mp
5 epo.mp.
6 eprex.mp
7 neorecormon.mp
8 procrit.mp
9 recombinant erythropoietin.mp.
10 darbepoetin alfa.mp.
11 exp NOVEL ERYTHROPOIESIS STIMULATING PROTEIN/
12 aranesp.mp.
13 nesp.mp
14 exp darbepoetin/
15 exp darbepoietin alfa/
16 exp CONTINUOUS ERYTHROPOIESIS RECEPTOR ACTIVATOR
17 CERA.mp
18 Or/1‐17
19 exp ANEMIA/
20 anemia.mp.
21 anaemi$.tw.
22 anemi$.mp.
23 (anemi$ adj3 cancer$).mp.
24 (anaemi$ adj3 cancer$).mp.
25 Or/19‐24
26 malignan$.mp.
27 cancer$.mp.
28 exp CANCER/
29 exp NEOPLASM/
30 neoplasm$.mp.
31 oncology.mp.
32 exp ONCOLOGY/
33 exp MYELODYSPLASIA/
34 myelodysplas$.tw.
35 chemotherapy.mp.
36 exp CHEMOTHERAPY/
37 exp TUMOR/
38 tumo?r$.mp.
39 carcinom$.mp.
40 Or/26‐40
41 randomized controlled trial/
42 exp clinical trial/
43 exp controlled study/
44 double blind procedure/
45 randomization/
46 placebo/
47 single blind procedure/
48 (control$ adj (trial$ or stud$ or evaluation$ or experiment$)).mp.
49 ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).mp.
50 (placebo$ or matched communities or matched schools or matched populations).mp.
51 (comparison group$ or control group$).mp.
52 (clinical trial$ or random$).mp.
53 (quasiexperimental or quasi experimental or pseudo experimental).mp.
54 matched pairs.mp.
55 or/41‐54
56 18 and 25
57 55 and 40
58 57 and 56
CENTRAL
ID Search
#1 (erythropoietin)
#2 MeSH descriptor Erythropoietin explode all trees
#3 epoetin
#4 epo
#5 (epoetin next alfa)
#6 (epoetin next beta)
#7 (darbepoetin next alfa)
#8 eprex
#9 neorecormon
#10 aranesp
#11 procrit
#12 (recombinant near erythropoietin)
#13 "continuous erythropoietin receptor activation"
#14 "continuous erythropoietin receptor activator"
#15 CERA
#16 C.E.R.A.
#17 erythropoiesis
#18 darbepoetin
#19 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18)
#20 anemia
#21 anaemia
#22 MeSH descriptor Anemia explode all trees
#23 (anemi* near cancer)
#24 (anaemi* near cancer)
#25 (#20 OR #21 OR #22 OR #23 OR #24)
#26 (#19 AND #25)
Appendix 2. List of variables evaluated
1. Variables to assess baseline imbalances
The following list provides pre‐specified and exploratory variables that were used to assess baseline imbalances. MAIN variables, i.e. variables that were pre‐specified in advance (Langensiepen 2002) are highlighted in BOLD. All other variables are considered to be exploratory. All variables refer to patient level data, unless otherwise specified. The technical name of the variable is given in [brackets].
PATIENT
1. Hemoglobin at baseline (randomization): continuous and categorical
a. (Hb ≤ 8 g/dL versus 8 g/dL < Hb ≤ 10 g/dL versus 10 g/dL < Hb ≤ 12 g/dL versus 12 g/dL < Hb ≤ 14 g/dL versus Hb > 14 g/dL) [hgb_cat1]
b. by 1 g/dL increments, i.e. 8 g/dL < Hb ≤ 9 g/dL versus 9 g/dL < Hb ≤ 10 g/dL versus 10 g/dL < Hb ≤ 11 g/dL versus 11 g/dL < Hb ≤ 12 g/dL versus 12 g/dL < Hb ≤ 13 g/dL versus 13 g/dL < Hb ≤ 14 g/dL versus > 14 g/dL [hgb_cat2]
2. Hematocrit at baseline (randomization): continuous and categorical (Hct ≤ 23.5% versus 23.5% < Hct ≤ 29.4% versus 29.4% < Hct ≤ 35.3% versus 35.3% < Hct ≤ 41.2% versus Hct > 41.2%) [hct_cat]
Note: use hematocrit values only if measurements was made, mathematical conversions from hemoglobin to hematocrit are not allowed
3. Serum EPO level at baseline before first study drug: continuous and categorical (< 25 mU/ml versus 25 ‐< 100 mU/ml versus 100 ‐ < 200 mU/ml versus ≥ 200 mU/ml) (Littlewood 2003). Note: two categories were added: “200 ‐ < 500 mU/ml versus ≥ 500 mU/ml”) [serepo]
4. Gender: dichotomous (male versus female) [sex]
5. Age at randomization: continuous and categorical (< 18 years versus 18 to < 35 years versus 35 to < 45 years versus 45 to < 55 years versus 55 to < 65 years versus 65 to < 75 years versus ≥ 75 years) [age_cat]
6. Body mass index (BMI): continuous and categorical (BMI < 19 kg/m2 versus 19 ≤ BMI < 25 kg/m2 versus 25 ≤ BMI < 30 kg/m2 versus BMI ≥ 30 kg/m2) [bmi_cat]
7. ECOG performance score: categorical
a. each score value (0 versus 1 versus 2 versus 3 versus 4) [ecog_b]
b. 0, 1 or 2 versus 3 or 4 [ecog_cat]
8. History of thromboembolic event EXCLUDING central line associated thrombosis? Categorical (yes versus no) [hxthrom]
9. History of cardiovascular disease including coronary artery disease, myocardial infarction, atrial fibrillation or congestive heart disease? Categorical (yes versus no) [hxcardio]
10. History of hypertension? Categorical (yes versus no) [hxhyper]
11. History of diabetes mellitus? Categorical (yes versus no) [hxdiab]
12. Geographical region: categorical (Northern America versus Northern, Western, Southern Europe versus Australia/New Zealand versus Eastern Europe versus Americas versus other) [region_cat]
TUMOR
13. Tumor type with different categorizations
a. few categories (solid tumors versus hematological malignancies; note: chronic lymphocytic leukemia will be coded as lymphoma) [tumor_cat1]
b. more categories (hematological versus breast cancer versus head and neck versus lung cancer versus other cancer). Note: the categorization was changed as follows: hematological versus breast cancer versus head and neck versus lung cancer versus gastrointestinal versus gynecological versus genitourinary versus other cancer [tumor_cat2]
c. many categories (each cancer entity will be kept as separate category). Note: category c was not applied in the analysis
14. Disease stage at ESA study entry: categorical (limited disease versus locally advanced versus extensive/metastatic disease versus other). Note: data quality did only permit to dichotomize the data into metastatic or advanced versus not metastatic or not advanced. [stagem_cat1]
15. Disease status at ESA study entry: categorical (untreated versus complete response versus partial response or stable disease versus progression or progressive disease or relapsed versus not evaluable versus not evaluated). Note: data quality did not permit to use this variable.
16. Time from tumor diagnosis to randomization [cancertime]
TUMOR TREATMENT
17. Cancer treatment modality (note this replaces the analysis for chemotherapy induced anemia versus anemia of cancer):
a. Categorical at patient level (non‐platinum chemotherapy/combined modality treatment versus platinum chemotherapy/combined modality treatment versus radiotherapy versus radiochemotherapy versus none versus unclear/mixed versus other). Note: radiotherapy and radiochemotherapy were kept as separate categories [popchmg], for a sensitivity analyses both categories were collapsed into one category [popispm_cat]
2. Variables to assess study design
The following list provides pre‐specified and exploratory variables that were used to assess the study design of the included trials. MAIN variables, i.e. variables that were pre‐specified in advance (Langensiepen 2002) are highlighted in BOLD. All other variables are considered to be exploratory. All variables refer to the study level, unless otherwise specified.
1. Randomization: categorical (adequate versus unclear versus inadequate) [randomisation]
2. Concealment of allocation: categorical (adequate versus unclear versus inadequate) [allocation]
3. Placebo controlled: dichotomous (yes versus no/unclear) [placebo]
4. Blinded outcome assessment: dichotomous (yes, no/unclear; this assessment may vary between outcomes)
a. PFS: Was there independent and blinded adjudication of events and cause of deaths?
b. TEE: Was there independent and blinded adjudication of events?
5. IPD submitted by pharmaceutical company or independent investigators: categorical (pharmaceutical company versus independent investigators versus other) [source]
6. Was the outcome of interest assessed as an endpoint (primary or secondary) or as an adverse event only? dichotomous (yes (endpoint) versus no (adverse event only)) and categorical (primary versus secondary versus an adverse event only) [endpoint]. Note: this variable was only assessed categorical, not dichotomous
7. Was the study designed to assess long‐term follow‐up? dichotomous versus (yes versus no) [longfu], note: assessed in sensitivity analysis, long‐term follow‐up was defined as planned follow‐up of at least 12 months after end of active treatment period
8. Calendar year of last patient randomized per study (to be calculated based on the individual patient data): continuous [calyear] and categorical (calendar time split in 5 years period) [calyear_cat]
9. Were less than 10% of subjects within each study arm excluded from the analysis and was the ratio of exclusions between arms less than a 2:1?
10. Actual study size: continuous and dichotomous (small (n overall < 200) versus large (n overall ≥ 200)), note: not assessed
11. Prematurely terminated or halted study or completed by own study protocol: dichotomous (terminated/halted versus completed) [stop], note: assessed in sensitivity analysis
12. Median time from randomization to censoring per study, separate for each outcome (to be calculated based on the individual patient data): continuous, note: not assessed
3. Variables to assess effect modification
The following list provides pre‐specified and exploratory variables that were examined in analyses of effect modification. MAIN variables, i.e. variables that were pre‐specified in advance (Langensiepen 2002) are highlighted in BOLD. All other variables were considered to be exploratory. All variables refer to patient level data, unless otherwise specified. The technical name of the variable is given in [brackets].
PATIENT
1. Hemoglobin at baseline (randomization): continuous and categorical
a. (Hb ≤ 8 g/dL versus 8 g/dL < Hb ≤ 10 g/dL versus 10 g/dL < Hb ≤ 12 g/dL versus 12 g/dL < Hb ≤ 14 g/dL versus Hb > 14 g/dL) [hgb_cat1]
b. by 1 g/dL increments, i.e. 8 g/dL < Hb ≤ 9 g/dL versus 9 g/dL < Hb ≤ 10 g/dL versus 10 g/dL < Hb ≤ 11 g/dL versus 11 g/dL < Hb ≤ 12 g/dL versus 12 g/dL < Hb ≤ 13 g/dL versus 13 g/dL < Hb ≤ 14 g/dL versus > 14 g/dL [hgb_cat2]
2. Hematocrit at baseline (randomization): continuous and categorical (Hct ≤ 23.5% versus 23.5% < Hct ≤ 29.4% versus 29.4% < Hct ≤ 35.3% versus 35.3% < Hct ≤ 41.2% versus Hct > 41.2%) [hct_cat] Note: Use hematocrit values only if measurements was made, mathematical conversions from hemoglobin to hematocrit are not allowed.
3. Serum EPO level at baseline before first study drug: continuous and categorical (< 25 mU/ml versus 25 ‐< 100 mU/ml versus 100 ‐ < 200 mU/ml versus ≥ 200 mU/ml) (Littlewood 2003). Note: two categories were added: “200 ‐ < 500 mU/ml versus ≥ 500 mU/ml”) [serepo]
4. Gender: dichotomous (male versus female) [sex]
5. Age at randomization: continuous and categorical (< 18 years versus 18 to < 35 years versus 35 to < 45 years versus 45 to < 55 years versus 55 to < 65 years versus 65 to < 75 years versus ≥ 75 years) [age_cat]
6. Body mass index (BMI): continuous and categorical (BMI < 19 kg/m2 versus 19 ≤ BMI < 25 kg/m2 versus 25 ≤ BMI < 30 kg/m2 versus BMI ≥ 30 kg/m2) [bmi_cat]
7. ECOG performance score: categorical
a. each score value (0 versus 1 versus 2 versus 3 versus 4) [ecog_b]
b. 0, 1 or 2 versus 3 or 4 [ecog_cat]
8. History of thromboembolic event EXCLUDING central line associated thrombosis? Categorical (yes versus no) [hxthrom]
9. History of cardiovascular disease including coronary artery disease, myocardial infarction, atrial fibrillation or congestive heart disease? (yes versus no) [hxcardio]
10. History of hypertension? Categorical (yes versus no) [hxhyper]
11. History of diabetes mellitus? Categorical (yes versus no) [hxdiab]
12. Geographical region: categorical (Northern America versus Northern, Western, Southern Europe versus Australia/New Zealand versus Eastern Europe versus Americas versus other) [region_cat]
TUMOR
13. Tumor type with different categorizations
a. few categories (solid tumors versus hematological malignancies; note: chronic lymphocytic leukemia will be coded as lymphoma) [tumor_cat1]
b. more categories (hematological versus breast cancer versus head and neck versus lung cancer versus other cancer). Note: the categorization was changed as follows: hematological versus breast cancer versus head and neck versus lung cancer versus gastrointestinal versus gynecological versus genitourinary versus other cancer [tumor_cat2]
c. many categories (each cancer entity will be kept as separate category). Note: category c was not applied in the analysis
14. Disease stage at ESA study entry: categorical (limited disease versus locally advanced versus extensive/metastatic disease versus other). Note: data quality did only permit to dichotomize the data into metastatic or advanced versus not metastatic or not advanced. [stagem_cat1]
15. Disease status at ESA study entry: categorical (untreated versus complete response versus partial response or stable disease versus progression or progressive disease or relapsed versus not evaluable versus not evaluated). Note: data quality did not permit to use this variable.
16. Time from tumor diagnosis to randomization [cancertime]
TUMOR TREATMENT
17. Cancer treatment modality (note this replaces the analysis for chemotherapy induced anemia versus anemia of cancer):
a. Categorical at patient level (non‐platinum chemotherapy/combined modality treatment versus platinum chemotherapy/combined modality treatment versus radiotherapy versus radiochemotherapy versus none versus unclear/mixed versus other). Note: data quality did not allow to differentiate platinum containing versus non platinum chemotherapy.
b. Categorical at study level (mainly chemotherapy/combined modality treatment (both platinum containing and platinum free) versus mainly radiotherapy/radiochemotherapy versus none versus unclear/mixed versus other). Note: radiotherapy and radiochemotherapy were kept as separate categories [popchmg], for a sensitivity analyses both categories were collapsed into one category [popispm_cat]
ESA TREATMENT
18. Iron supplementation policy as per study protocol (study level information): categorical (fixed versus as needed by study protocol or by discretion of physician versus no iron versus no statement). [iron_cat] Note: the category “by discretion of physician” was amended to “by discretion of physician or institutional policy”.
19. Planned duration of ESA treatment as per study protocol (study level information): continuous and categorical (up to 8 weeks versus 9 to 16 weeks versus > 17 weeks versus not applicable) [plandur_cat]. Note: studies that did not indicate a specific number of weeks for ESA treatment duration were categorized as “until end of chemotherapy or radiotherapy”, if indicated.
20. Planned weekly ESA dosage as defined in the study protocol (starting dose, study level information): continuous and categorical (EPO < 40,000 IU/week or darbepoetin <100 µg/week versus EPO =40,000 IU/week or darbepo = 100 µg /week versus EPO > 40,000 IU/week or darbepoetin > 100 µg /week) [weekesa_cat]
21. Planned frequency of ESA applications as defined in the study protocol (study level information): categorical (TIW or more often versus QW versus Q2W versus Q3W versus Q4W). Note: the categorization was simplified to (TIW or more often versus QW versus Q2W or more often). [planfreq_cat] 22. Planned hemoglobin ceiling target i.e. when ESA had to be stopped according to the study protocol (study level information): continuous and categorical
a. Hb ≤ 11 versus 11 g/dL < Hb ≤ 13 g/dL versus 13 g/dL < Hb ≤ 15 g/dL versus > Hb > 15 g/dL [ceiling_cat1]
b. by 1 g/dL increments, i.e. 8 g/dL < Hb ≤ 9 g/dL versus 9 g/dL < Hb ≤ 10 g/dL versus 10 g/dL < Hb ≤ 11 g/dL versus 11 g/dL < Hb ≤ 12 g/dL versus 12 g/dL < Hb ≤ 13 g/dL versus 13 g/dL < Hb ≤ 14 g/dL versus 14 g/dL < Hb ≤ 15 g/dL versus 15 g/dL < Hb ≤ 16 g/dL versus 16 g/dL < Hb ≤ 17 g/dL versus 17 g/dL < Hb ≤ 18 g/dL versus > 18 g/dL [ceiling_cat2]
23. Maximal hemoglobin within 4 weeks before event or end of study: continuous and categorical (Hb ≤ 8 g/dL versus 8 g/dL < Hb ≤ 10 g/dL versus 10 g/dL < Hb ≤ 12 g/dL versus 12 g/dL < Hb ≤ 14 g/dL versus 14 g/dL < Hb ≤ 16 g/dL versus 16 g/dL < Hb ≤ 18 g/dL versus Hb > 18 g/dL), TIME DEPENDENT VARIABLE. Note: this variable has not been applied in the analysis.
24. Maximal hematocrit within 4 weeks before event or end of study: continuous and categorical (Hct hct ≤ 23.5% versus 23.5% < hct ≤ 29.4% versus 29.4% < hct ≤ 35.3% versus 35.3% < hct ≤ 41.2% versus 41.2% < hct ≤ 47.1% versus hct >53%), TIME DEPENDENT VARIABLE. Note: this variable has not been applied in the analysis.
4. Other protocol amendments
The variable FIX (not listed above) was amended with one category: “adjusted” for patients who received a fix dose of drug depending on their age or weight category. This category was added to differentiate between a truly weight based dosing scheme.
Appendix 3. Sensitivity analyses
Sensitivity analyses for studies with aggregated survival data
Ten studies were eligible for the IPD meta‐analysis but individual patient data could not be retrieved. For six of these studies (Antonadou 2001; Bamias 2003; Blohmer 2003; Mystakidou 2005; Overgaard 2007) results for survival were either reported in the literature or provided by the investigator. Overall, the inclusion of these results in the meta‐analyses did not lead to important changes.
Table 1: Sensitivity analyses for effect of missing studies, on study mortality
| Two‐stage log‐rank fixed‐effect meta‐analysis | Results based on IPD analysis | Including additional literature based data |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| On study mortality, all cancer patients* | 1.17 (1.06‐1.30) | 1.17 (1.06‐1.30) |
| On study mortality, chemotherapy trials | 1.10 (0.98‐1.24) | 1.11 (0.98‐1.25) |
*Not included: Overgaard 2007, no on study mortality data reported
Table 2: Sensitivity analyses for effect of missing studies, overall survival
| Two‐stage log‐rank fixed‐effect meta‐analysis | Results based on IPD analysis | Including additional literature based data |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Overall survival, all cancer patients | 1.06 (1.00‐1.12) | 1.06 (1.00‐1.11) |
| Overall survival, chemotherapy trials | 1.04 (0.97‐1.11) | 1.04 (0.97‐1.11) |
Sensitivity analyses for on study mortality in all cancer patients
Classification of studies into different treatment populations
In study 83322 (Debus 2006) patients with non‐resectable NSCLC received chemotherapy which was followed by radiotherapy. ESA was given during the treatment of chemotherapy and radiotherapy. However, only patients who achieved CR, PR or stable disease were subsequently treated with radiotherapy (39.5% of the ESA patients and 44.2% of the control patients did not receive radiotherapy, information taken from CSR). Since the chemotherapy was followed by radiotherapy after a short interval, the study was classified as “radiochemotherapy”. However, it could also be argued that the study should be classified as “combined modality treatment” because radiotherapy was given after chemotherapy or as “mixed” population, because less then 70% of the treatment population actually received radiotherapy. Both options were tested in a sensitivity analysis, results for on study mortality for the various treatment subsets and LR test for difference between subsets of studies did not change, see below.
Table 3: Sensitivity analyses for on study mortality in all cancer patients
| Two‐stage meta‐analysis based on random‐ effects Cox model | Study 83322 in radiochemotherapy treatment group | Study 83322 in mixed treatment group | Study 83322 in chemotherapy treatment group |
| ESA versus control | HR (95% CI) | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.09 ( 0.97‐1.23) | 1.10 (0.98‐1.24) |
| Radiochemotherapy | 1.47 (0.83‐2.59) | 2.34 (0.42‐13.03) | 2.34 (0.42‐13.03) |
| Radiotherapy | 1.51 (0.73‐3.12) | 1.51 (0.73‐3.12) | 1.51 (0.73‐3.12) |
| Mixed | 1.50 (0.62‐3.66) | 1.42 (0.86‐2.34) | 1.50 (0.62‐3.66) |
| None | 1.32 (1.06‐1.65) | 1.32 (1.06‐1.65) | 1.32 (1.06‐1.65) |
| Overall | 1.16 (1.05‐1.29) | 1.16 (1.05‐1.29) | 1.16 (1.05‐1.29) |
| LR test | 0.4234 | 0.3607 | 0.4290 |
Sensitivity analysis for on study mortality: mixed treatment group
In two studies with CLL patients (Rose 1994; CC2574‐P‐174 about 40% of the patients received corticosteroids and 60% of patients received chemotherapy during study. Since the definition for treatment populations was set at 70% (i.e. 70% of a trial population had to have received the planned anticancer treatment) these two studies were classified and analyzed in the “mixed” treatment population. In a sensitivity analysis we included these two studies in the “chemotherapy” population, for results see below. Overall, the results did not change.
Table 4: Sensitivity analyses for on study mortality in all cancer patients
| Two‐stage meta‐analysis based on random‐effects Cox model | Mixed treatment group separate subset | Mixed treatment group merged to chemotherapy treatment group |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.10 (0.97‐1.24) |
| Radiochemotherapy | 1.47 (0.83‐2.59) | 1.47 (0.83‐2.59) |
| Radiotherapy | 1.51 (0.73‐3.12) | 1.51 (0.73‐3.12) |
| Mixed | 1.50 (0.62‐3.66) | ‐ |
| None | 1.32 (1.06‐1.65) | 1.32 (1.06‐1.65) |
| Overall | 1.16 (1.05‐1.29) | 1.16 (1.05‐1.29) |
| LR test | 0.4234 | 0.3382 |
Sensitivity analyses for on study mortality: radiochemotherapy treatment population
In five studies patients received both radiotherapy and chemotherapy. Since patients in these studies received chemotherapy, a myelosuppressive effect of the chemotherapy cannot be excluded and it might be argued that those studies should be evaluated in the chemotherapy population. For a sensitivity analysis these patients were included in the chemotherapy treatment population, overall, the results did not change, see below.
Table 5: Sensitivity analyses for on study mortality in all cancer patients
| Two‐stage meta‐analysis based on random‐effects Cox model | Radiochemotherapy treatment group merged to radiotherapy treatment group | Radiochemotherapy treatment group merged to chemotherapy treatment group |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.11 (0.98‐1.25) |
| Radiotherapy | 1.48 (0.95‐2.32) | 1.51 (0.73‐3.12) |
| Mixed | 1.50 (0.62‐3.66) | 1.50 (0.62‐3.66) |
| None | 1.32 (1.06‐1.65) | 1.32 (1.06‐1.65) |
| Overall | 1.16 (1.05‐1.29) | 1.16 (1.05‐1.29) |
| LR test | 0.2715 | 0.4246 |
Sensitivity analysis for on study mortality: exclusion of study without date of randomization
For one study (study 36158 (Boogaerts 2003), chemotherapy population) the date of randomization was not available and was replaced with the date of “first study drug” as provided by the investigators/sponsors of the study. For a sensitivity analysis we excluded this study, for results see below. Overall, inclusion or exclusion of this study did not affect the overall results and the test for differences between treatment populations did not change.
Table 6: Sensitivity analyses for on study mortality in all cancer patients
| Two‐stage meta‐analysis based on random‐effects Cox model | Chemotherapy subset including study 36158 | Chemotherapy subset without study 36158 |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.09 (0.97‐1.24) |
| Radiochemotherapy | 1.47 (0.83‐2.59) | 1.47 (0.83‐2.59) |
| Radiotherapy | 1.51 (0.73‐3.12) | 1.51 (0.73‐3.12) |
| Mixed | 1.50 (0.62‐3.66) | 1.50 (0.62‐3.66) |
| None | 1.32 (1.06‐1.65) | 1.32 (1.06‐1.65) |
| Overall | 1.16 (1.05‐1.29) | 1.16 (1.05‐1.29) |
| LR test | 0.4234 | 0.4279 |
Sensitivity analyses for on study mortality chemotherapy patients: exclusion of studies with different concomitant treatments in active and control arm
For two studies concomitant treatments in the active and the control arm were not identical, i.e. in one study 21481 (Thomas 2008) the transfusion trigger in the ESA arm was 12 g/dL and in the control arm 10 g/dL. In another study 70404 (Strauss 2008) radiotherapy for patients in the control arm started two weeks earlier compared to patients in the ESA arm. For a sensitivity analysis these studies were excluded, for results see below. Overall, exclusion of these two studies from the radiochemotherapy population (Thomas 2008; Strauss 2008) did not change the overall result and did also not change the differences between the treatment populations.
Table 7: Sensitivity analyses for on study mortality in all cancer patients
| Two‐stage meta‐analysis based on random‐ effects Cox model | Radiochemotherapy subset including studies 21481, 70404 | Radiochemotherapy subset without studies 21481, 70404 |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.09 (0.97‐1.23) |
| Radiochemotherapy | 1.47 (0.83‐2.59) | 1.50 (0.84‐2.67) |
| Radiotherapy | 1.51 (0.73‐3.12) | 1.51 (0.73‐3.12) |
| Mixed | 1.50 (0.62‐3.66) | 1.50 (0.62‐3.66) |
| None | 1.32 (1.06‐1.65) | 1.32 (1.06‐1.65) |
| Overall | 1.16 (1.05‐1.29) | 1.16 (1.05‐1.29) |
| LR test | 0.4234 | 0.4063 |
Sensitivity analysis for on study mortality in all cancer patients: exclusion of studies with different iron policies in active and control arm
For seven studies (Machtay 2007; Untch 2008; Moebus 2007; Debus 2006; Savonije 2005; EPO‐GER‐20; OBE/EPO‐INT‐03) the iron policies in the active and the control arm were different, for a sensitivity analysis we excluded these studies from the analysis, for results see below. Overall, the results did not change.
Table 8: Sensitivity analyses for on study mortality in all cancer patients
| Two‐stage meta‐analysis based on random‐effects Cox model | Including studies with different iron policies | Excluding studies with different iron policies |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.11 (0.98‐1.26) |
| Radiochemotherapy | 1.47 (0.83‐2.59) | 4.13 (0.46‐36.94) |
| Radiotherapy | 1.51 (0.73‐3.12) | 1.48 (0.64‐3.45) |
| Mixed | 1.50 (0.62‐3.66) | 1.50 (0.62‐3.66) |
| None | 1.32 (1.06‐1.65) | 1.32 (1.06‐1.65) |
| Overall | 1.16 (1.05‐1.29) | 1.17 (1.05‐1.30) |
| LR test | 0.4234 | 0.3974 |
Sensitivity analyses for on study mortality: exclusion of studies terminated prematurely
Fourteen studies were terminated prematurely (Charu 2007; CC2574‐P‐174; Quirt 1996; Goss 2005; Wright 2007; EPO‐GBR‐7; EPO‐GER‐20; Debus 2006; Thomas 2008; Leyland‐Jones 2003; Grote 2005; OBE/EPO‐INT‐03; Vadhan‐Raj 2004; Machtay 2007), for a sensitivity analysis we excluded these studies from the analysis, for results see below. Apparently, exclusion of these studies reduced the overall effect estimate; however, the change was small.
Table 9: Sensitivity analyses for on study mortality in all cancer patients
| Two‐stage meta‐analysis based on random‐effects Cox model | Including prematurely stopped studies | Excluding prematurely stopped studies |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.05 (0.91‐1.21) |
| Radiotherapy/radiochemotherapy | 1.48 (0.95‐2.32) | 1.22 (0.46‐3.29) |
| Mixed | 1.50 (0.62‐3.66) | 1.72 (0.67‐4.41) |
| None | 1.32 (1.06‐1.65) | 1.28 (1.01‐1.63) |
| Overall | 1.16 (1.05‐1.29) | 1.11 (0.99‐1.25) |
| LR test | 0.2715 | 0.4088 |
Sensitivity analysis for on study mortality: studies designed for long‐term follow‐up.
Twenty four studies (Hedenus 2003; Smith 2008; Pirker 2008; Vansteenkiste 2002; Aapro 2008; Untch 2008; Goss 2005; Chang 2005; EPO‐GBR‐7; Debus 2006; Thomas 2008; Littlewood 2001; Milroy 2003; Thomas 2002; Leyland‐Jones 2003; Pronzato 2002; Henke 2003; Osterborg 2002; Strauss 2008; Moebus 2007; Grote 2005; OBE/EPO‐INT‐03; Savonije 2005; Machtay 2007) were designed for long‐term follow‐up, defined as follow‐up of at least 12 months after treatment period. For a sensitivity analysis we restricted the on study mortality analysis to these studies, for results see below. There is an apparent change in the chemotherapy group; however, the confidence intervals are widely overlapping.
Table 10: Sensitivity analyses for on study mortality in all cancer patients at study level
| Two‐stage meta‐analysis based on random‐effects Cox model | Including all studies | Including only studies designed for long‐term follow‐up |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.09 (0.97‐1.23) | 1.19 (1.03‐1.37) |
| Radiochemotherapy | 1.47 (0.83‐2.59) | 1.47 (0.83‐2.59) |
| Radiotherapy | 1.51 (0.73‐3.12) | 1.51 (0.72‐3.12) |
| Mixed | 1.50 (0.62‐3.66) | ‐ |
| None | 1.32 (1.06‐1.65) | 1.37 (1.05‐1.78) |
| Overall | 1.16 (1.05‐1.29) | 1.24 (1.10‐1.41) |
| LR test | 0.4234 | 0.6638 |
Sensitivity analysis for on study mortality chemotherapy population
Sensitivity analysis for on study mortality chemotherapy population patients truly receiving chemotherapy at individual patient level
We analyzed whether the mortality signal seen in the chemotherapy population can be explained by patients in these studies not receiving chemotherapy. For this analysis we included all patients from the chemotherapy trials and restricted the analysis to those patients who did receive chemotherapy as reported in the data set provided. Patients who did not receive chemotherapy and patients without reported data whether or not they received chemotherapy were excluded from the analysis. In the next step we restricted the analysis to patients who truly received chemotherapy and received at least one dose of ESA in the active arm and zero doses of ESA in the control arm, for results see table below. We then included stepwise patients from the treatment populations “mixed” and “radiochemotherapy” and restricted the analyses stepwise as outlined above, for results see below.
Table 11: Sensitivity analyses for on study mortality in chemotherapy patients
| Two‐stage meta‐analysis based on random‐effects Cox model |
ESA versus control HR (95% CI) |
P value | N included |
| Chemotherapy trials | |||
| Analysis restricted to studies reporting chemotherapy status of each patient during ESA study | 1.08 (0.95‐1.24) | 0.242 | 8732 |
| Analysis restricted to patients who actually received chemotherapy (subsets included: “chemotherapy”) | 1.10 (0.96‐1.27) | 0.172 | 8481 |
| Analysis restricted to patients who actually received chemotherapy AND ESA in active arm AND no ESA in control arm (subsets included: “chemotherapy”) | 1.09 (0.94‐1.26) | 0.257 | 8114 |
| Chemotherapy and mixed trials | |||
| Analysis restricted to studies reporting chemotherapy status of each patient during ESA study | 1.09 (0.96‐1.25) | 0.199 | 8998 |
| Analysis restricted to patients who actually received chemotherapy (subsets included: “chemotherapy” and “mixed”) | 1.12 (0.97‐1.28) | 0.112 | 8651 |
| Analysis restricted to patients who actually received chemotherapy AND ESA in active arm AND no ESA in control arm (subsets included: “chemotherapy” and “mixed”) | 1.10 (0.96‐1.27) | 0.173 | 8284 |
| Chemotherapy, mixed and radiochemotherapy trials | |||
| Analysis restricted to studies reporting chemotherapy status of each patient during ESA study | 1.11 (0.96‐1.27) | 0.153 | 9661 |
| Analysis restricted to patients who actually received chemotherapy (subsets: “chemotherapy”, “mixed” and “radiochemotherapy”) | 1.14 (1.00‐1.30) | 0.051 | 9307 |
| Analysis restricted to patients who actually received chemotherapy AND ESA in active arm AND no ESA in control arm (subsets included: “chemotherapy”, “mixed” “radiochemotherapy”) | 1.12 (0.98‐1.28) | 0.101 | 8919 |
Overall the effect of ESA on patients receiving chemotherapy did not change, i.e. the effect estimate did not decrease. Therefore it is unlikely that the observed effect of ESA in the subset chemotherapy treatment population can be explained by events in patients who did not receive chemotherapy.
Studies with prespecified chemotherapy protocols at study level
Of the 38 studies classified as chemotherapy trial, in three studies (Untch 2008; Moebus 2007; EPO‐GER‐20) a detailed protocol that specified the substance, dosage, timing and frequency of chemotherapy was part of the ESA study. We compared the results of these studies with chemotherapy studies where the chemotherapy modalities were not specified in detail, for results see below. Of note: in two (Untch 2008; Moebus 2007) of the studies with prespecified chemotherapy protocols, no patient died during on study treatment phase. Overall, there was no evidence for a difference between studies with and without prespecified study protocol.
Table 12: Sensitivity analysis for on study mortality in chemotherapy patients
| Two‐stage meta‐analysis based on random‐effects Cox model |
ESA versus control HR (95% CI) |
| Chemotherapy with prespecified chemotherapy protocol* | 0.61 (0.211.76) |
| Chemotherapy without prespecified chemotherapy protocol | 1.10 (0.97‐1.24) |
| Overall | 1.09 (0.97‐1.23) |
| LR test | 0.2702 |
*Only one study included (EPO‐GER‐20)
Table 13: Sensitivity analysis for overall survival in chemotherapy patients
| Two‐stage meta‐analysis based on random‐effects Cox model |
ESA versus control HR (95% CI) |
| Chemotherapy with prespecified chemotherapy protocol* | 1.11 (0.861.45) |
| Chemotherapy without prespecified chemotherapy protocol | 1.03 (0.96‐1.10) |
| Overall | 1.04 (0.97‐1.11) |
| LR test | 0.5937 |
*Three studies included (Untch 2008; Moebus 2007; EPO‐GER‐20)
Sensitivity analyses for radiotherapy population
Studies with prespecified radiotherapy protocols at study level
Of the eight studies classified as radiotherapy and radiochemotherapy population, in one radiotherapy study (Machtay 2007) and in three radiochemotherapy studies (Thomas 2008; Debus 2006; Strauss 2008) a detailed anti‐cancer treatment protocol was part of the ESA study. We compared the results of these studies with radiotherapy/radiochemotherapy studies where the treatment modalities were not specified in detail. There was no evidence for a difference between these two subsets of studies, for results see below.
Table 14: Sensitivity analysis for on study mortality in radiotherapy patients at study level
| Two‐stage meta‐analysis based on random‐effects Cox model |
ESA versus control HR (95% CI) |
| Radiotherapy/radiochemotherapy with prespecified treatment protocol | 1.39 (0.812.40) |
| Radiotherapy/radiochemotherapy without prespecified treatment protocol | 1.69 (0.773.73) |
| Overall | 1.48 (0.95‐2.32) |
| LR test | 0.6233 |
Table 15: Sensitivity analysis for overall survival in radiotherapy patients at study level
| Two‐stage meta‐analysis based on random‐effects Cox model |
ESA versus control HR (95% CI) |
| Radiotherapy/radiochemotherapy with prespecified treatment protocol | 1.05 (0.751.46) |
| Radiotherapy/radiochemotherapy without prespecified treatment protocol | 1.16 (0.951.41) |
| Overall | 1.06 (0.90‐1.26) |
| LR test | 0.1051 |
Sensitivity analyses for overall survival
Sensitivity analysis for overall survival: studies designed for long‐term follow‐up.
Twenty four studies (Hedenus 2003; Smith 2008; Pirker 2008; Vansteenkiste 2002; Aapro 2008; Untch 2008; Goss 2005; Chang 2005; EPO‐GBR‐7; Debus 2006; Thomas 2008; Littlewood 2001; Milroy 2003; Thomas 2002; Leyland‐Jones 2003; Pronzato 2002; Henke 2003; Osterborg 2002; Strauss 2008; Moebus 2007; Grote 2005; OBE/EPO‐INT‐03; Savonije 2005; Machtay 2007) were designed for long‐term follow‐up, defined as follow‐up of at least 12 months after treatment period. For a sensitivity analysis we restricted overall survival to these studies, for results see below. Overall, the results did not change.
Table 16: Sensitivity analysis for overall survival in all cancer patients: studies designed for long‐term follow‐up
| Two‐stage meta‐analysis based on random‐effects Cox model | Including all studies | Including only studies designed for long‐term follow‐up |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.04 (0.97‐1.11) | 1.06 (0.97‐1.15) |
| Radiochemotherapy | 0.98 (0.75‐1.27) | 1.02 (0.74‐1.41) |
| Radiotherapy | 1.17 (0.96‐1.42) | 1.17 (0.96‐1.42) |
| Mixed | 1.50 (0.62‐3.66) | ‐ |
| None | 1.22 (1.04‐1.44) | 1.22 (1.02‐1.47) |
| Overall | 1.06 (1.00‐1.12) | 1.07 (0.99‐1.15) |
| LR test | 0.11 | 0.1240 |
Sensitivity analysis for overall survival: exclusion of studies terminated prematurely
Fourteen studies were terminated prematurely (Charu 2007; CC2574‐P‐174; Quirt 1996; Goss 2005; Wright 2007; EPO‐GBR‐7 ; EPO‐GER‐20; Debus 2006; Thomas 2008; Leyland‐Jones 2003; Grote 2005; OBE/EPO‐INT‐03; Vadhan‐Raj 2004; Machtay 2007), for a sensitivity analysis we excluded these studies from the analysis, for results see below. Exclusion of these studies did not affect the overall effect estimate.
Table 17: Sensitivity analysis for overall survival in all cancer patients
| Two‐stage meta‐analysis based on random‐effects Cox model | Including prematurely stopped studies | Excluding prematurely stopped studies |
| ESA versus control | HR (95% CI) | HR (95% CI) |
| Chemotherapy | 1.04 (0.97‐1.11) | 1.01 (0.94‐1.08) |
| Radiochemotherapy | 0.98 (0.75‐1.27) | 2.00 (0.65‐6.15) |
| Radiotherapy | 1.17 (0.96‐1.42) | 1.27 (0.96‐1.69) |
| Mixed | 1.50 (0.62‐3.66) | 1.72 (0.67‐4.41) |
| None | 1.22 (1.04‐1.44) | 1.19 (1.00‐1.42) |
| Overall | 1.06 (1.00‐1.12) | 1.05 (0.98‐1.42) |
| LR test | 0.11 | 0.1128 |
Appendix 4. Exploratory analyses
Analyses that were not planned at the protocol stage are listed in this section.
Characteristics of studies included: changes over time
We evaluated changes over time of the characteristics of the included studies based on the year when the last patient was randomized into the respective study. Cut off for this binary comparison was last patient randomized before (early studies) or after 2000 (later studies). Patients in early studies were more likely to have Hb baseline < 10 g/dL (63% versus 25%) and less likely to have solid tumors (46% versus 85%). None of the early studies evaluated survival as primary endpoint and none included a stringent anticancer therapy protocol. All (100%) of the early studies applied ESA three times per week or more often compared to 31% of the more recent studies. Early studies used more likely to use chemotherapies (83% versus 66%) and no radiotherapy (0% versus 9%). Reporting of the study methods changed over time: while reporting of concealment of allocation improved over time (42% adequate in the early and 76% adequate in the late studies); reporting of randomization procedures did not improve (adequate in 42% of the early studies and 27% in the late studies). Although the study designs changed over time, the observed hazard ratios for on study mortality did not change, i.e. the percentage of studies reporting increased mortality (HR => 1.0) was identical in the early and the more recent studies (50% versus 51%), see Figure 18.
18.
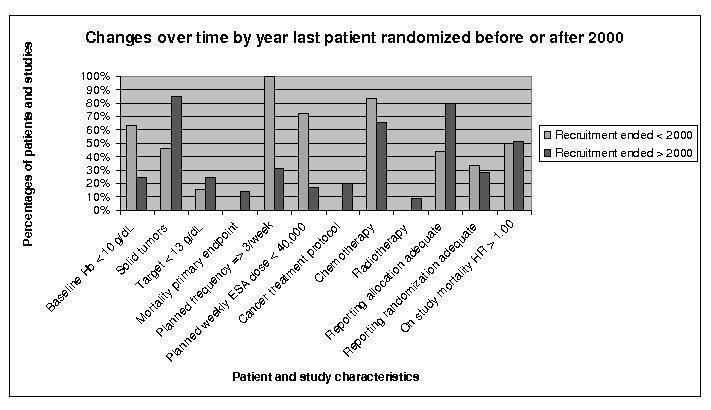
Comparing studies with last patient randomized before 2000 or after 2000
Exploratory analysis: Kaplan‐Meier curves for all endpoints
Kaplan‐Meier survival curves for all four outcomes are presented below. For these curves patient data were pooled without stratification for study, see Figure 19, Figure 20, Figure 21 and Figure 22.
19.
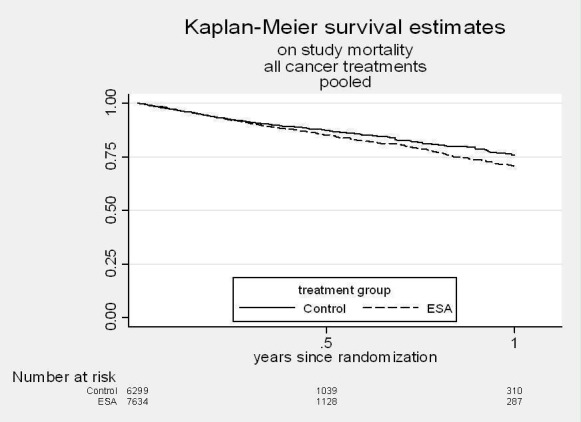
Pooled Kaplan Meier plot for on study mortality in all cancer patients
20.
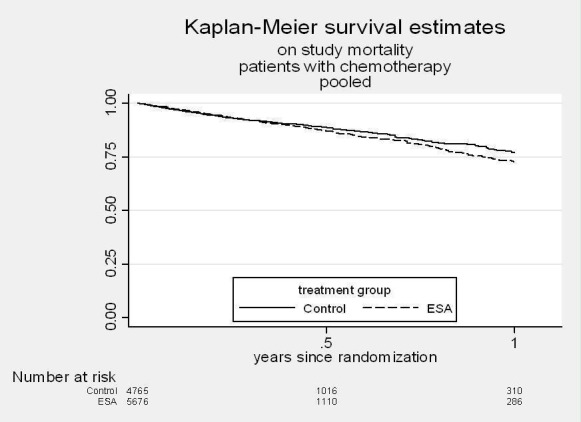
Pooled Kaplan Meier plot for on study mortality in chemotherapy patients
21.
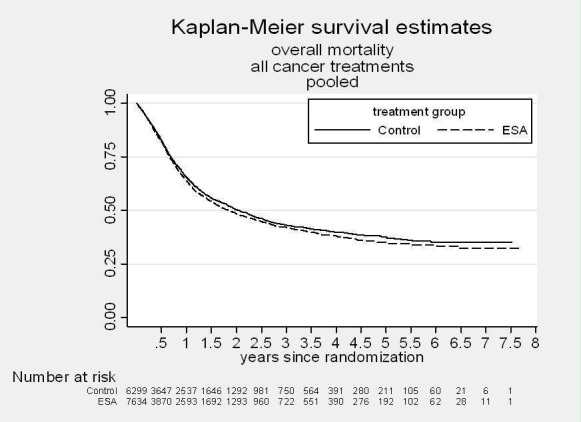
Pooled Kaplan Meier plot for overall survival in all cancer trials
22.
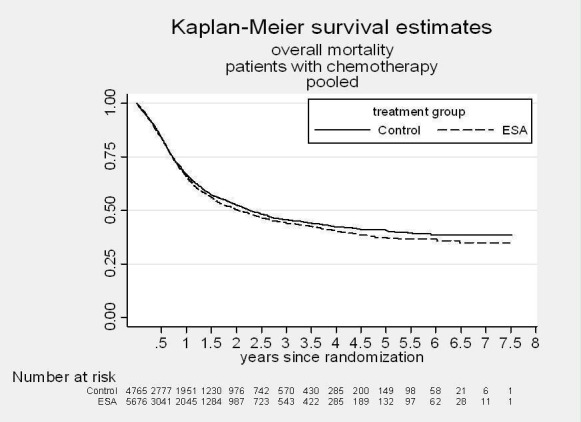
Pooled Kaplan Meier plot for overall survival in chemotherapy trials (subset analysis)
Exploratory analyses of interaction terms for on study mortality, all cancer patients
History of thromboembolic events
In the analysis of on study mortality in all cancer patients, patients with a history of thromboembolic events were less likely to die when receiving ESAs (HR 0.80, 95% CI 0.52‐1.23) compared to patients without a previous thromboembolic event and receiving ESAs (HR 1.23, 95% CI 1.09‐1.39, test for interaction: 0.0605. The effect remained after adjusting for sex, age, Hb at baseline and tumor type (P value for interaction = 0.0440), see table below. History of thromboembolic events was more often recorded in more recent studies (46% missing in studies with last patient randomized before 2000 versus 27% in the more recent studies). Patients with a history of thromboembolic events had more often a poor ECOG performance status (12% versus 6%) and high serum EPO levels (7% versus 3% serum EPO > 500) compared to patients without a positive history of thromboembolic events. There was no difference with respect to percentage of patients with metastatic disease. When adjusting for age, sex, Hb at baseline, tumor type and in addition ECOG and serum EPO level the observed effect became more pronounced, see table below. However, only 7999 out of 13933 (57%) and 4281 (31%) of patients were included in these analyses; others were excluded because of missing data. Therefore, a selection bias cannot be excluded.
Table 1: Assessment of history of thromboembolic events and effect modification, on study mortality in all cancer patients
| On study mortality all cancer patients | Bivariate ESA versus control | Multivariate ESA versus control | Multivariate ESA versus control | Multivariate ESA versus control | ||||||||
| Interaction term | ESA*HTX | ESA*HTX | ESA*HTX | ESA*HTX | ||||||||
| Model adjusted for | ‐ | age, sex, Hb, tumor type | age, sex, Hb, tumor type and ECOG | age, sex, Hb, tumor type and serum EPO | ||||||||
| Patients included | n = 9620 | n = 9467 | n = 7999 | n = 4281 | ||||||||
| HR | 95% CI | P* | HR | 95% CI | P* | HR | 95% CI | P* | HR | 95% CI | P* | |
| History of thromboembolic events (HTX) | ||||||||||||
| Yes | 0.80 | 0.52‐1.23 | 0.0605 | 0.77 | 0.50‐1.19 | 0.0440 | 0.75 | 0.48‐1.18 | 0.0338 | 0.48 | 0.25‐0.93 | 0.0129 |
| No | 1.23 | 1.09‐1.39 | 1.22 | 1.08‐1.38 | 1.25 | 1.10‐1.42 | 1.13 | 0.94‐1.34 | ||||
| Missing / not reported | 1.09 | 0.87‐1.35 | ‐ | omitted | omitted | ‐ | omitted | omitted | ‐ | omitted | omitted | ‐ |
| Overall, unadjusted | 1.20 | 1.07‐1.34 | ‐ | 1.20 | 1.07‐1.34 | ‐ | 1.21 | 1.07‐1.36 | ‐ | 1.10 | 0.93‐1.30 | ‐ |
*P value from LR test, patients with missing values were excluded from tests for interactions
Hematocrit at baseline
In the analysis of on study mortality in all cancer patients, there was some evidence that patients with a very low hematocrit at baseline (< 23.5%) had an increased risk to die compared to patients with higher hematocrit levels at baseline. Compared to patients with Hct above 23.5% at baseline, patients with low Hct had more often metastatic disease (89% versus 79%), were more often aged > 65 years (44% versus 40%) and had more often a poor ECOG performance status (4.7% versus 1.7%). Patients with low Hct values at baseline had also low Hb values and there was a correlation between Hct and Hb at baseline (correlation coefficient 0.8335). Hct data were missing for 21% of patients of the total population. In studies which recruited until 2000 (year last patient randomized) data were missing for only 8% of patients whereas for 24% of patients in the more recent studies Hct at baseline was not recorded.
After adjusting for age, sex, Hb at baseline and tumor type the effect remained, see table below. When in addition tumor stage was included in the multivariate model the effect of Hct on mortality was attenuated and the interaction test was not statistically significant. When ECOG performance status was included the effect of low Hct increased and the test for interaction was statistically significant. However, since only 9714 (70%) and 7686 (55%) of the total patient population was included in these analyses, the power for statistical tests was reduced and a selection bias cannot be excluded. For results see table below.
Table 2: Assessment of additional factors for hematocrit and interaction, on study mortality all cancer patients
| On study mortality all cancer patients | Bivariate ESA versus control HCT*ESA | Multivariate ESA versus control HCT*ESA | Multivariate ESA versus control HCT*ESA | Multivariate ESA versus control HCT*ESA | ||||||||
| Adjusted for | ‐ | age, sex, Hb, tumor type | age, sex, Hb, tumor type and tumor stage | age, sex, Hb, tumor type and ECOG | ||||||||
| Patients included | n = 11036 | n = 10972 | n = 9714 | n = 7686 | ||||||||
| HR | 95% CI | P* | HR | 95% CI | P* | HR | 95% CI | P* | HR | 95% CI | P* | |
| Hct at baseline | ||||||||||||
| < 23.5% | 2.19 | 1.35‐3.55 | 0.0110 | 2.13 | 1.30‐3.48 | 0.0191 | 1.92 | 1.13‐3.24 | 0.1220 | 2.85 | 1.47‐5.53 | 0.0254 |
| 23.5‐29.4% | 0.96 | 0.78‐1.17 | 0.96 | 0.79‐1.18 | 1.00 | 0.80‐1.24 | 1.00 | 0.80‐1.26 | ||||
| 29.4‐35.3% | 1.17 | 0.99‐1.39 | 1.15 | 0.97‐1.37 | 1.23 | 1.02‐1.48 | 1.17 | 0.96‐1.42 | ||||
| 35.3‐41.2% | 1.41 | 1.12‐1.76 | 1.39 | 1.10‐1.74 | 1.37 | 1.08‐1.72 | 1.39 | 1.07‐1.79 | ||||
| > 41.2% | 1.12 | 0.73‐1.70 | 1.15 | 0.76‐1.76 | 1.15 | 0.75‐1.75 | 1.15 | 0.71‐1.89 | ||||
| Missing | 1.09 | 0.76‐1.55 | ‐ | omitted | ‐ | omitted | ‐ | omitted | ‐ | |||
| Overall, unadjusted | 1.18 | 1.06‐1.32 | ‐ | 1.18 | 1.06‐1.32 | ‐ | 1.22 | 1.09‐1.36 | ‐ | 1.20 | 1.06‐1.35 | ‐ |
*P value LR test, missing data were excluded from LR tests
Planned frequency of ESA application
In the analysis of on study mortality in all cancer patients there was some evidence for an effect modification of planned frequency of ESA application and on study mortality in all cancer patients, i.e. patients receiving ESAs three times per week or more frequently were less likely to die compared to patients receiving ESAs only once or less often per week. This effect remained after adjusting for age, sex, Hb and tumor type. However, other aspects of study design were associated with the planned frequency of ESA application. Studies in which ESA was applied three times per week (TIW) or more often had lower average starting doses of ESAs (62% of TIW studies with ESA starting dose < 40000 per week). TIW studies were older, i.e. 63% of TIW studies randomized patients prior to calendar year 2000, whereas none of the studies that administered ESA QW or less frequently had completed randomization before 2000. In none of the TIW studies survival was assessed as primary endpoint. There were no major differences with regard to underlying chemotherapy, i.e. percentage of studies on chemotherapy, radiotherapy or no therapy was distributed equally across different application frequencies; the same applies to the planned duration of the ESA treatment. In meta‐regression analyses these factors were explored, for results see table next page. Analyses were based both on unadjusted and adjusted HRs stemming from the 53 included studies.
Table 3a: Meta‐regression analysis for planned frequency based on unadjusted hazard ratios for individual studies
| On study mortality all cancer patients | Meta‐regression ESA versus control | Meta‐regression ESA versus control | Meta‐regression ESA versus control | Meta‐regression ESA versus control | Meta‐regression ESA versus control | |||||
| Additional included variable(s) | endpoint | planned weekly ESA dose | year last patient randomized | endpoint and planned weekly dose | last patient randomized, endpoint and planned weekly dose | |||||
| HR of studies adjusted for | ‐ | ‐ | ‐ | ‐ | ‐ | |||||
| Studies included | n = 53 | n = 53 | n = 53 | n = 53 | n = 53 | |||||
| Planned frequency of ESA application | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Three times per week or more frequent | 1.09 | 0.76‐1.58 | 0.92 | 0.75‐1.14 | 0.94 | 0.68‐1.29 | 1.05 | 0.73‐1.53 | 1.00 | 0.60‐1.66 |
| Once per week | 1.44 | 1.17‐1.77 | 1.26 | 0.86‐1.84 | 1.19 | 0.76‐1.88 | 1.27 | 0.85‐1.89 | 1.19 | 0.63‐2.23 |
| Every second week or less frequent | 0.93 | 0.50‐1.73 | 0.94 | 0.59‐1.52 | 0.90 | 0.49‐1.64 | 0.80 | 0.39‐1.62 | 0.75 | 0.29‐1.93 |
| Other | 0.96 | 0.67‐1.33 | 0.71 | 0.44‐1.76 | 0.65 | 0.33‐1.31 | 0.77 | 0.47‐1.27 | 0.79 | 0.33‐1.91 |
| Test for differences between subgroups* | p = 0.0669 | p = 0.1196 | p = 0.0940 | p = 0.1560 | p = 0.4270 | |||||
*P value for test for differences between subgroups from meta‐regression (Wald test)
Table 3b: Meta‐regression analysis for planned frequency based on adjusted hazard ratios for individual studies
| On study mortality all cancer patients | Meta‐regression ESA versus control | Meta‐regression ESA versus control | Meta‐regression ESA versus control | Meta‐regression ESA versus control | Meta‐regression ESA versus control | |||||
| Additional included variable(s) | endpoint | planned weekly ESA dose | last patient randomized | endpoint and planned weekly dose | year last patient randomized, endpoint and planned weekly dose | |||||
| HR of studies adjusted for | Age, sex, Hb, tumor type | Age, sex, Hb, tumor type | Age, sex, Hb, tumor type | Age, sex, Hb, tumor type | Age, sex, Hb, tumor type | |||||
| Studies included | n = 53 | n = 53 | n = 53 | n = 53 | n = 53 | |||||
| Planned frequency of ESA application | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Three times per week or more frequent | 1.14 | 0.78‐1.67 | 0.93 | 0.74‐1.17 | 0.99 | 0.69‐1.41 | 1.08 | 0.74‐1.59 | 0.97 | 0.57‐1.68 |
| Once per week | 1.46 | 1.18‐1.80 | 1.34 | 0.91‐1.99 | 1.17 | 0.72‐1.91 | 1.39 | 0.92‐2.09 | 1.16 | 0.60‐2.26 |
| Every second week or less frequent | 0.88 | 0.46‐1.67 | 0.92 | 0.56‐1.50 | 0.87 | 0.46‐1.65 | 0.80 | 0.38‐1.66 | 0.67 | 0.25‐1.80 |
| Other | 0.91 | 0.64‐1.29 | 0.67 | 0.40‐1.10 | 0.64 | 0.31‐1.33 | 0.72 | 0.43‐1.20 | 0.72 | 0.29‐1.83 |
| Test for differences between subgroups* | p = 0.0424 | p = 0.0363 | p = 0.1668 | p = 0.0423 | p = 0.3000 | |||||
*P value for test for differences between subgroups from meta‐regression (Wald test)
Exploratory analyses of interaction terms for overall survival, chemotherapy trials
In the overall survival analysis in chemotherapy trials, sex showed a statistically significant interaction term in the bivariate analysis. Women were at increased risk to die when receiving ESAs (HR 1.10, 95% CI 1.01‐1.21) compared to men (HR 0.96, 95% cI 0.87‐1.06, P value for interaction: 0.0370). When adjusting in addition for age, Hb at baseline and tumor category, the modifying effect for sex remained (P value for interaction 0.0362). A potential explanation for this finding is the large number of female patients with breast cancer included in the analysis. I.e. of the 9892 patients included in the multivariate model testing for interaction, 4303 (43%) patients were diagnosed with breast cancer, of which 1998 (46%) had metastatic disease. When patients with breast cancer were removed from the analysis, the modifying effect of sex on overall survival in chemotherapy patients was attenuated (P value LR test model with & without interaction term for sex excluding breast cancer patients = 0.1571). In the next steps we also excluded patients with a) gynecological cancers and b) prostate and testicular cancer, restricting the analysis to cancers that can occur both in male and female patients. The effect of sex was further attenuated and the test statistic was not significant, however, 63% of the patient population was excluded from the analysis with this strategy. In none of the analyses the modifying effect of sex on survival disappeared completely, however, the differences observed were small.
Table 4: Overall survival in chemotherapy trials, tests for interaction, univariate and multivariate models
| Overall survival in chemotherapy trials | Bivariate ESA versus control | Multivariate ESA versus control | Multivariate ESA versus control | Multivariate ESA versus control | Multivariate ESA versus control | ||||||||||
| Interaction term | ESA*sex | ESA*sex | ESA*sex | ESA*sex | ESA*sex | ||||||||||
| Adjusted for | ‐ | age, sex, Hb, tumor type | age, sex, Hb, tumor type | age, sex, Hb, tumor type | age, sex, Hb, tumor type | ||||||||||
| Patients excluded | ‐ | ‐ | excluding breast cancer patients | excluding breast cancer and gynecological cancer patients | excluding breast cancer, gynecological cancer as well as prostate and testicular cancer patients | ||||||||||
| Patients included | n = 10441 | n = 9892 | n = 6257 | n = 5205 | 5128 | ||||||||||
| ESA versus control | HR | 95% CI | P* | HR | 95% CI | P* | HR | 95% CI | P* | HR | 95% CI | P* | HR | 95% CI | P* |
| Sex | |||||||||||||||
| Male | 0.96 | 0.87‐1.06 | 0.0370 | 0.97 | 0.87‐1.07 | 0.0362 | 0.97 | 0.87‐1.07 | 0.1571 | 0.97 | 0.87‐1.07 | 0.2071 | 0.97 | 0.87‐1.07 | 0.2169 |
| Female | 1.10 | 1.01‐1.21 | 1.12 | 1.02‐1.22 | 1.09 | 0.96‐1.23 | 1.07 | 0.94‐1.23 | 1.07 | 0.94‐1.23 | |||||
| Overall result, unadjusted | 1.04 | 0.97‐1.11 | ‐ | 1.04 | 0.97‐1.11 | ‐ | 1.00 | 0.93‐1.08 | ‐ | 1.00 | 0.91‐1.07 | ‐ | 0.99 | 0.92‐1.08 | ‐ |
*P value LR test comparing model with & without interaction term
Exploratory analysis for Hb change over time at study level in control arm
In this analysis we assessed the influence of myelosuppressive anticancer treatments. The only measures for myelosuppression available were Hb values in the control arm over time. Other laboratory values, such as platelets, were not requested for the present analysis. For each study we assessed whether the Hb decreased over time or not by plotting the Hb of the control arm of each study over time. Studies with Hb decrease of > 1 g/dL from baseline within 50 days were categorized as “Hb decrease”, studies with Hb within +1 g/dL to 1 g/dL margin from baseline within 50 days were categorized as “no change”. Studies with an Hb increase > 1 g/dL from baseline within 50 days were categorized as “Hb increase”. We further differentiated whether the baseline Hb of the respective study was < 10 g/dL, 10‐12 g/dL or > 12 g/dL at baseline. Please note: the classification of the studies was made at study level; the Hb curve of an individual patient was not assessed. All studies regardless of treatment population category were included in this analysis. Hb over time is only a proxy for myelosuppression and red blood cell transfusions might confound the Hb levels over time. Overall, there is no evidence for a difference between the explored groups.
Table 5: Exploratory analysis for on study mortality in all cancer patients, Hb change in control arm
| Two‐stage meta‐analysis based on random‐effects Cox model | ESA versus control HR (95% CI) |
| Hb increase | 1.18 (95% CI 0.70‐1.98) |
| Hb no change | 1.17 (95% CI 1.04‐1.32) |
| Hb decrease | 1.14 (95% CI 0.91‐1.43) |
| Unclear/not reported | 0.62 (95% CI 0.16‐2.43) |
| Overall | 1.16 (95% CI 1.05‐1.29) |
| LR test | 0.8154 |
Table 6: Exploratory analysis for on study mortality in all cancer patients, Hb change in control arm
| Two‐stage meta‐analysis based on random‐effects Cox model | ESA versus control HR (95% CI) |
| Baseline Hb < 10 g/dL & Hb no change | 1.08 (95% CI 0.90‐1.30) |
| Baseline Hb < 10 g/dL & Hb increase | 1.18 (95% CI 0.70‐1.98) |
| Baseline Hb 10‐12 g/dL & Hb decrease | 1.02 (95% CI 0.70‐1.50) |
| Baseline Hb 10‐12 g/dL & Hb no change | 1.13 (95% CI 0.91‐1.40) |
| Baseline Hb > 12 g/dL & Hb decrease | 1.21 (95% CI 0.91‐1.61) |
| Baseline Hb > 12 g/dL & Hb no change | 1.44 (95% CI 1.11‐1.88) |
| Unclear/not reported | 0.62 (95% CI 0.16‐2.43) |
| Overall | 1.16 (95% CI 1.05‐1.29) |
| LR test | 0.6180 |
Exploratory analysis for Hb change over time at study level in ESA arm
For this analysis the Hb change over time in the ESA arm for each study was plotted. Studies with an Hb increase of > 1 g/dL from baseline within 50 days were categorized as “increase”. Studies with Hb decrease of > 1 g/dL from baseline within 50 days were categorized as “decrease”, studies with Hb within +1 g/dL to 1 g/dL margin from baseline within 50 days were categorized as “no change”. We further differentiated whether the baseline Hb of the respective study was < 10 g/dL, 10‐12 g/dL or > 12 g/dL in the ESA arm. Please note: the classification of the studies was made at study level; the Hb curve of an individual patient was not assessed. All studies regardless of treatment population category were included in this analysis. Overall, there is no evidence for a difference between the explored groups.
Table 7: Exploratory analysis for on study mortality in all cancer patients, Hb change in ESA arm at study level
| Two‐stage meta‐analysis based on random‐effects Cox model | ESA versus control HR (95% CI) |
| Hb increase | 1.12 (95% CI 0.98‐1.29) |
| Hb no change | 1.23 (95% CI 1.05‐1.44) |
| Hb decrease | 1.04 (95% CI 0.48‐2.24) |
| Unclear/not reported | 0.62 (95% CI 0.16‐2.43) |
| Overall | 1.16 (95% CI 1.05‐1.29) |
| LR test | 0.7120 |
Table 8: Exploratory analysis for on study mortality in all cancer patients, Hb change in ESA arm at study level
| Two‐stage meta‐analysis based on random‐effects Cox model | ESA versus control HR (95% CI) |
| Baseline Hb < 10 g/dL & Hb no change | 1.00 (95% CI 0.50‐2.00) |
| Baseline Hb < 10 g/dL & Hb increase | 1.07 (95% CI 0.88‐1.30) |
| Baseline Hb 10‐12 g/dL & Hb no change | 1.17 (95% CI 0.83‐1.64) |
| Baseline Hb 10‐12 g/dL & Hb increase | 1.10 (95% CI 0.84‐1.46) |
| Baseline Hb > 12 g/dL & Hb decrease | 1.04 (95% CI 0.48‐2.24) |
| Baseline Hb > 12 g/dL & Hb no change | 1.25 (95% CI 1.02‐1.53) |
| Baseline Hb > 12 g/dL & Hb increase | 1.93 (95% CI 0.66‐5.67) |
| Unclear/not reported | 0.62 (95% CI 0.16‐2.43) |
| Overall | 1.16 (95% CI 1.05‐1.29) |
| LR test | 0.8420 |
Exploratory analysis for longest follow‐up available in studies with “cross‐over”
In twelve studies patients in both the control and the active treatment arm were allowed to receive ESAs after a defined treatment period. For the main analysis we included only events and time under observation during this defined treatment period in the analysis. In the overall survival, which looked at the longest follow‐up available, these studies were included only based on the events and the time period of the defined treatment period. For the purpose of a sensitivity analysis we included the longest follow‐up of these studies for the overall survival analysis as well. The percentage of patients in both the control and the ESA arm who were receiving ESAs during the “cross‐over” period, varied between studies. For details see tables below. When including cross‐over trials based on the longest follow‐up available the overall estimates were attenuated for both all cancer patients and chemotherapy trials. A cut off depending on a percentage of patients receiving ESAs was not applied in order to decide whether a specific study would be included in the analysis based on the on study or the longest follow‐up estimate. These cut‐offs were not applied because they had not been defined at the protocol stage and the percentage of patients receiving ESAs during open label phase was continuously increasing.
Table 9: Studies with “cross‐over”: percentage of total study population receiving ESA during open‐label phase
| Studies with “cross‐over”: percentage of total study population receiving ESA during open‐label phase | |||
| Study protocol | Study number | Total | Comment |
| CC2574‐P‐174 | 60584 | 93% | Data provided by company |
| J89‐040 | 98358 | 81% | Data provided by company |
| EPO‐INT‐3/ CC 2574‐P‐034 | 36274 | 76% | Data provided by company |
| H87‐032, 87‐014/OEU‐U20, 87‐015/OEU‐U21 | 98906 | 75% | Data provided by company |
| I88‐037, 87‐016, 87‐017 | 34917 | 75% | Data provided by company |
| I88‐036, 87‐018, 87‐019 | 70332 | 74% | Data provided by company |
| EPO‐INT‐2/ CC 2574‐P‐467 | 11220 | 60% | Data provided by company |
| 20000219 | 53081 | 59% | Data from clinical study report |
| 980291 | 35466 | 48% | Data from clinical study report |
| MF4321 | 45434 | 48% | Data from clinical study report |
| 980291SCH2 | 26117 | 40% | Data from clinical study report |
| EPO‐INT‐76/EPO‐CA‐489 | 17100 | 24% | Data provided by company |
Table 10: Sensitivity analyses including longest follow‐up available for studies with “cross‐over”
| Two‐stage log‐rank fixed‐effects meta‐analysis |
ESA versus control
HR (95% CI) |
P value | N included |
| Overall survival, all cancer patients | |||
| Overall survival, all cancer patients, cross‐over trials restricted to on study mortality | 1.06 (1.00‐1.12) | 0.0561 | 13933 |
| Overall survival, all cancer patients, cross‐over trials included based on longest follow‐up available | 1.04 (0.98‐1.09) | 0.1719 | 13933 |
| Overall survival, chemotherapy trials | |||
| Overall survival, chemotherapy trials, cross‐over trials restricted to on study mortality | 1.04 (0.97‐1.11) | 0.3081 | 10441 |
| Overall survival, chemotherapy trials, cross‐over trials included based on longest follow‐up available | 1.02 (0.96‐1.08) | 0.5743 | 10441 |
Exploratory analysis for current license indication
It is difficult to conduct an analysis that matches the current license indication. The main limitation is that the current indication recommends an Hb target of 12 g/dL. However, in none of the studies included in the present analysis the Hb ceiling was 12 g/dL or below. The next limitation is that the “current license indication” is an ever changing definition. Based on these considerations an analysis for the “current license indication” was not planned at the protocol for this meta‐analysis (Bohlius 2008).
Appendix 5. Funnel plots Baseline imbalances
The following figures present funnel plots of baseline imbalances.
ECOG Figure 23
23.
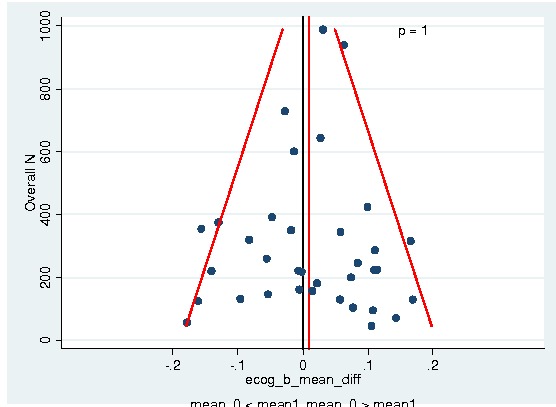
Baseline imbalances ECOG
Level of EPO serum Figure 24
24.
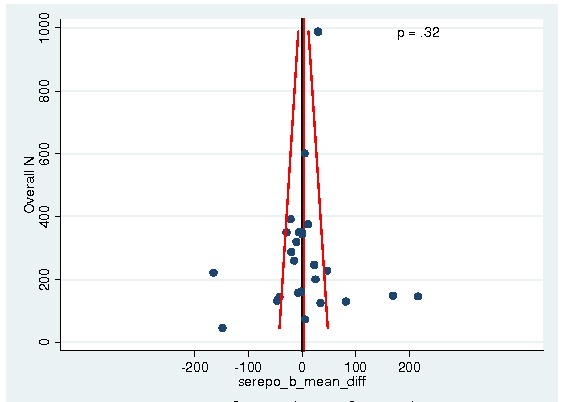
Level of EPO serum
BMI Figure 25
25.
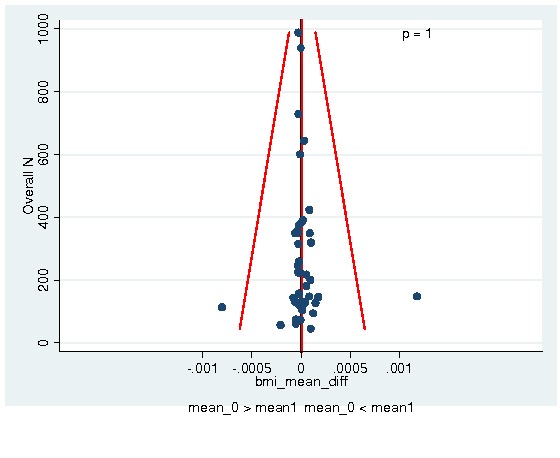
BMI
Time from cancer diagnosis to date of randomization Figure 26
26.
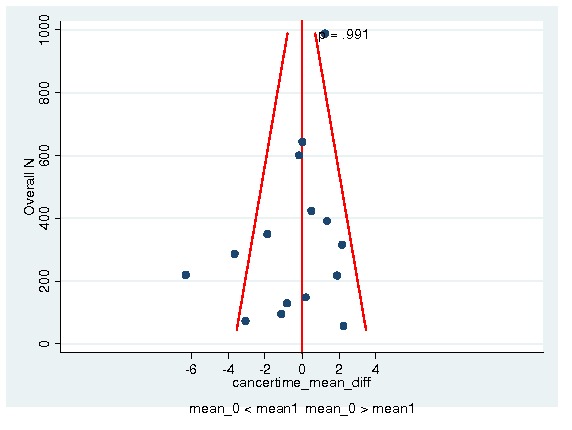
Time from cancer diagnosis to date of randomization
Hemoglobin Figure 27
27.
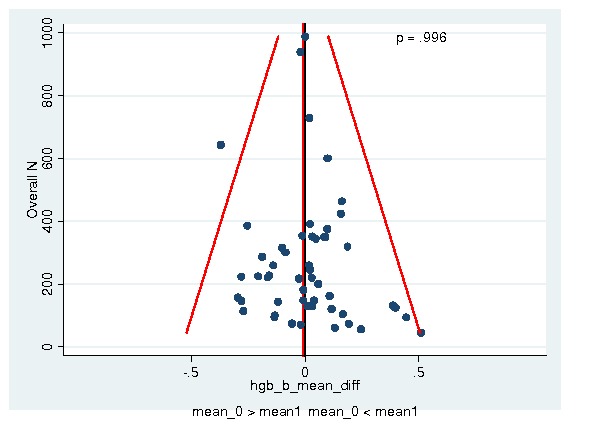
Hemoglobin
Hematocrit Figure 28
28.
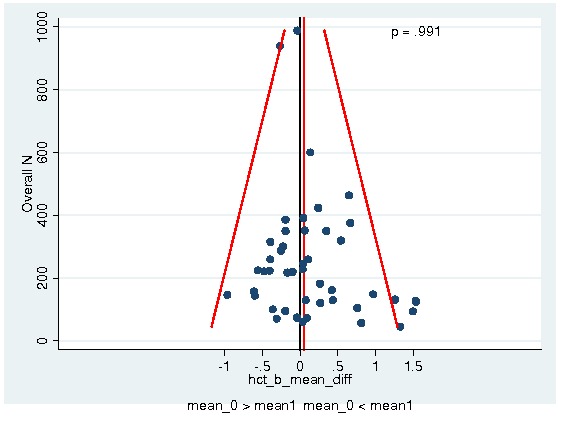
Hematocrit
Age Figure 29
29.
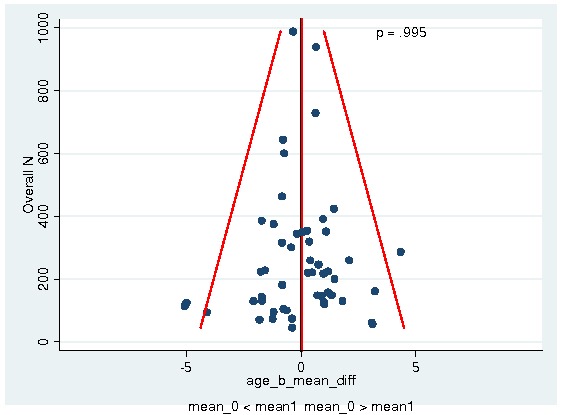
Age
Sex Figure 30
30.
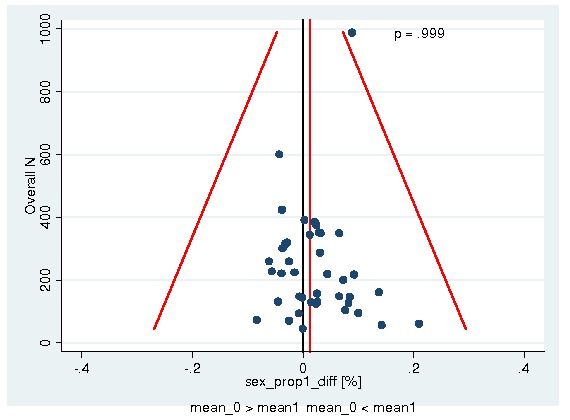
Sex
ECOG low versus high Figure 31
31.
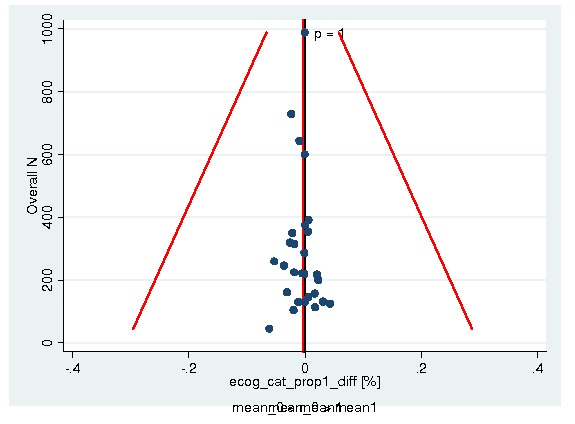
ECOG low versus high
History of thromboembolic events Figure 32
32.
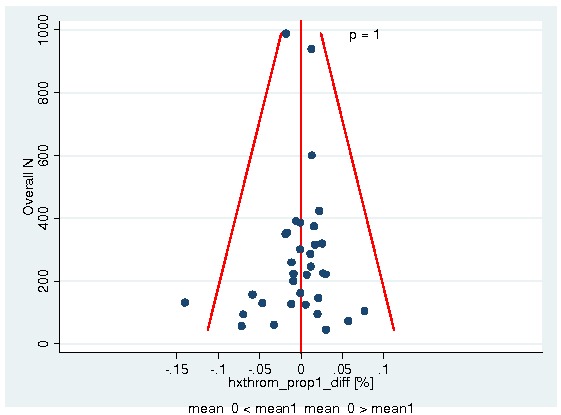
History of thromboembolic events
History of cardiovascular events Figure 33
33.
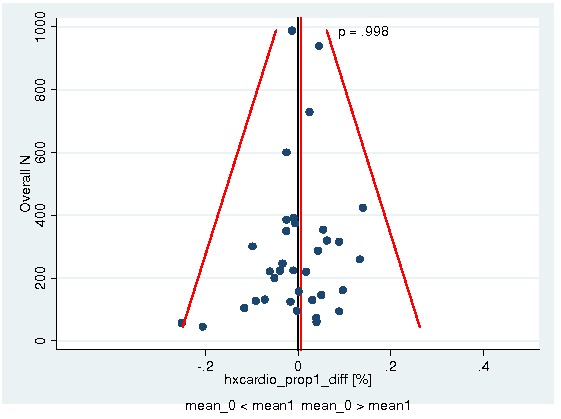
History of cardiovascular events
History of hypertension Figure 34
34.
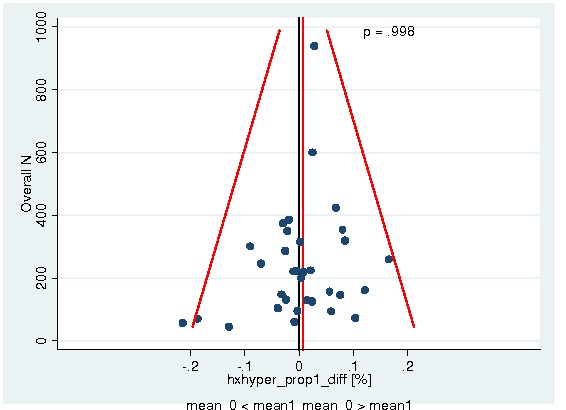
History of hypertension
History of diabetes Figure 35
35.
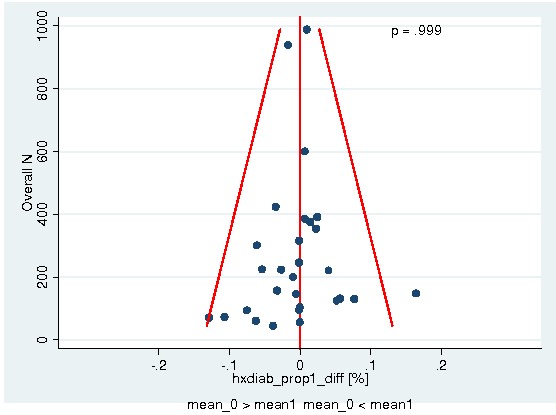
History of diabetes
Appendix 6. Assessment of interaction for mortality in all cancer patients during the active study period
| Mortality in all cancer patients during the active study period | ESA arm | Control arm | ESA versus control | |||||||
| Patients | events | sample | % | events | sample | % | HR | 95% CI | P value* | |
| Patient level characteristics | ||||||||||
| Hb at baseline (continuous) | 0.82 | |||||||||
| Hb at baseline (cat. 1) | 0.75 | |||||||||
| Hb ≤ 8 g/dL | 791 | 90 | 448 | 20% | 58 | 343 | 17% | 1.28 | 0.92‐1.78 | |
| Hb 8‐≤ 10 g/dL | 3930 | 292 | 2222 | 13% | 239 | 1708 | 14% | 1.08 | 0.91‐1.28 | |
| Hb 10‐≤ 12 g/dL | 5004 | 300 | 2851 | 11% | 220 | 2153 | 10% | 1.22 | 1.03‐1.46 | |
| Hb 12‐≤ 14 g/dL | 2843 | 141 | 1433 | 10% | 114 | 1410 | 8% | 1.28 | 1.00‐1.64 | |
| Hb > 14 g/dL | 839 | 37 | 428 | 9% | 30 | 411 | 7% | 1.06 | 0.66‐1.72 | |
| Unknown | 526 | 5 | 252 | 2% | 4 | 274 | 1% | 0.91 | 0.24‐3.40 | |
| Hb at baseline (cat. 2) | 0.79 | |||||||||
| Hb ≤ 8 g/dL | 791 | 90 | 448 | 20% | 58 | 343 | 17% | 1.28 | 0.92‐1.79 | |
| Hb 8‐≤ 9 g/dL | 1319 | 117 | 742 | 16% | 101 | 577 | 18% | 1.05 | 0.81‐1.38 | |
| Hb 9‐≤ 10 g/dL | 2611 | 175 | 1480 | 12% | 138 | 1131 | 12% | 1.11 | 0.89‐1.39 | |
| Hb 10‐≤ 11 g/dL | 2927 | 188 | 1699 | 11% | 121 | 1228 | 10% | 1.34 | 1.07‐1.69 | |
| Hb 11‐≤ 12 g/dL | 2077 | 112 | 1152 | 10% | 99 | 925 | 11% | 1.07 | 0.82‐1.41 | |
| Hb 12‐≤ 13 g/dL | 1739 | 92 | 873 | 11% | 80 | 866 | 9% | 1.22 | 0.90‐1.64 | |
| Hb 13‐≤ 14 g/dL | 1104 | 49 | 560 | 9% | 34 | 544 | 6% | 1.45 | 0.93‐2.24 | |
| Hb >14 g/dL | 839 | 37 | 428 | 9% | 30 | 411 | 7% | 1.06 | 0.65‐1.72 | |
| Unknown | 526 | 5 | 252 | 2% | 4 | 274 | 1% | 0.92 | 0.25‐3.44 | |
| Malignancy type | ||||||||||
| Tumour (cat. 1) | 0.16 | |||||||||
| Haematological malignancies | 2403 | 128 | 1400 | 9% | 79 | 1003 | 8% | 1.20 | 0.91‐1.60 | |
| Solid tumours | 10795 | 684 | 5848 | 12% | 532 | 4947 | 11% | 1.20 | 1.07‐1.35 | |
| Other | 693 | 49 | 369 | 13% | 51 | 324 | 16% | 0.81 | 0.54‐1.20 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 1.99 | 0.44‐8.94 | |
| Tumour (cat. 2) | 0.47 | |||||||||
| Haematological malignancies | 2403 | 128 | 1400 | 9% | 79 | 1003 | 8% | 1.19 | 0.90‐1.59 | |
| Breast cancer | 4302 | 224 | 2245 | 10% | 164 | 2057 | 8% | 1.34 | 1.10‐1.65 | |
| Head and neck cancer | 868 | 23 | 443 | 5% | 20 | 425 | 5% | 1.13 | 0.62‐2.07 | |
| Lung cancer | 3076 | 292 | 1618 | 18% | 243 | 1458 | 17% | 1.17 | 0.99‐1.39 | |
| Gastrointestinal cancer | 708 | 61 | 434 | 14% | 44 | 274 | 16% | 0.96 | 0.65‐1.42 | |
| Gynaecological cancer | 1399 | 40 | 842 | 5% | 27 | 557 | 5% | 1.18 | 0.72‐1.94 | |
| Genitourinary cancer | 442 | 44 | 266 | 17% | 34 | 176 | 19% | 1.02 | 0.65‐1.60 | |
| Other | 693 | 49 | 369 | 13% | 51 | 324 | 16% | 0.81 | 0.54‐1.20 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 1.96 | 0.44‐8.79 | |
| Sex | ||||||||||
| Male | 5136 | 419 | 2854 | 15% | 309 | 2282 | 14% | 1.15 | 0.99‐1.34 | 0.86 |
| Female | 8797 | 446 | 4780 | 9% | 356 | 4017 | 9% | 1.17 | 1.02‐1.35 | |
| Age | ||||||||||
| Age continuous | 0.87 | |||||||||
| Age categorical | 0.50 | |||||||||
| < 18 years | 123 | 0 | 55 | 0% | 1 | 68 | 1% | Not estimable | Not estimable | |
| ≥18‐35 years | 346 | 11 | 191 | 6% | 9 | 155 | 6% | 0.83 | 0.34‐2.01 | |
| ≥35‐45 years | 1343 | 57 | 745 | 8% | 34 | 598 | 6% | 1.36 | 0.89‐2.08 | |
| ≥45‐55 years | 3010 | 162 | 1614 | 10% | 111 | 1396 | 8% | 1.34 | 1.05‐1.71 | |
| ≥55‐65 years | 4193 | 256 | 2237 | 11% | 222 | 1956 | 11% | 1.07 | 0.89‐1.28 | |
| ≥65‐75 years | 3517 | 271 | 1970 | 14% | 210 | 1547 | 14% | 1.16 | 0.97‐1.39 | |
| ≥75 years | 1389 | 108 | 816 | 13% | 77 | 573 | 13% | 1.27 | 0.94‐1.70 | |
| Missing | 12 | 0 | 6 | 0% | 1 | 6 | 17% | Not estimable | Not estimable | |
| Hct levels at baseline | ||||||||||
| Hct continuous | 0.57 | |||||||||
| Hct categorical | 0.01 | |||||||||
| ≤23.5% | 390 | 55 | 210 | 26% | 24 | 180 | 13% | 2.19 | 1.35‐3.55 | |
| 23.5‐≤ 29.4% | 2788 | 199 | 1567 | 13% | 191 | 1221 | 16% | 0.96 | 0.78‐1.17 | |
| 29.4‐≤ 35.3% | 4615 | 321 | 2692 | 12% | 223 | 1923 | 12% | 1.17 | 0.99‐1.39 | |
| 35.3‐≤ 41.2% | 2458 | 176 | 1258 | 14% | 130 | 1200 | 11% | 1.41 | 1.12‐1.76 | |
| > 41.2% | 785 | 48 | 414 | 12% | 40 | 371 | 11% | 1.12 | 0.73‐1.70 | |
| Missing | 2897 | 66 | 1493 | 4% | 57 | 1404 | 4% | 1.09 | 0.76‐1.55 | |
| Serum Epo at baseline | ||||||||||
| Serum Epo continuous | 0.21 | |||||||||
| Serum Epo categorical | 0.54 | |||||||||
| <25 mU/ml | 1497 | 95 | 876 | 11% | 58 | 621 | 9% | 1.33 | 0.96‐1.85 | |
| 25‐<100 mU/ml | 2908 | 195 | 1643 | 12% | 171 | 1265 | 14% | 0.98 | 0.80‐1.21 | |
| 100‐<200 mU/ml | 740 | 73 | 451 | 16% | 47 | 289 | 16% | 1.08 | 0.75‐1.57 | |
| 200‐<500 mU/ml | 325 | 29 | 190 | 15% | 19 | 135 | 14% | 1.29 | 0.72‐2.31 | |
| > 500 mU/ml | 181 | 21 | 103 | 20% | 10 | 78 | 13% | 1.26 | 0.59‐2.69 | |
| Unknown | 8282 | 452 | 4371 | 10% | 360 | 3911 | 9% | 1.23 | 1.07‐1.41 | |
| Performance score | ||||||||||
| ECOG categorical | 0.63 | |||||||||
| ECOG 0 | 3392 | 86 | 1808 | 5% | 76 | 1584 | 5% | 1.15 | 0.85‐1.57 | |
| ECOG 1 | 4900 | 327 | 2779 | 12% | 250 | 2121 | 12% | 1.14 | 0.97‐1.35 | |
| ECOG 2 | 1678 | 241 | 933 | 26% | 178 | 745 | 24% | 1.21 | 1.00‐1.47 | |
| ECOG 3 | 139 | 26 | 77 | 34% | 18 | 62 | 29% | 1.30 | 0.71‐2.39 | |
| ECOG 4 | 3 | 1 | 2 | 50% | 0 | 1 | 0% | Not estimable | Not estimable | |
| ECOG missing | 3821 | 184 | 2035 | 9% | 143 | 1786 | 8% | 1.12 | 0.90‐1.39 | |
| ECOG dichotomous | 0.56 | |||||||||
| ECOG 0, 1, 2 | 10083 | 655 | 5578 | 12% | 505 | 4505 | 11% | 1.18 | 1.05‐1.33 | |
| ECOG 3, 4 | 142 | 27 | 79 | 34% | 18 | 63 | 29% | 1.42 | 0.78‐2.59 | |
| ECOG missing | 3708 | 183 | 1977 | 9% | 142 | 1731 | 8% | 1.12 | 0.89‐1.39 | |
| Body mass index | ||||||||||
| ≤ 19 kg/m² | 865 | 76 | 424 | 18% | 73 | 441 | 17% | 1.00 | 0.73‐1.39 | 0.72 |
| 19‐ ≤25 kg/m² | 5487 | 374 | 2964 | 13% | 277 | 2523 | 11% | 1.21 | 1.04‐1.42 | |
| 25‐≤ 30 kg/m² | 3443 | 193 | 1864 | 10% | 144 | 1579 | 9% | 1.14 | 0.92‐1.42 | |
| > 30 kg/m² | 1650 | 74 | 867 | 9% | 56 | 783 | 7% | 1.26 | 0.89‐1.79 | |
| Missing | 2488 | 148 | 1515 | 10% | 115 | 973 | 12% | 1.22 | 0.95‐1.57 | |
| History of thromboembolic events | ||||||||||
| Yes | 561 | 40 | 318 | 13% | 42 | 243 | 17% | 0.80 | 0.52‐1.23 | 0.06 |
| No | 9059 | 637 | 5044 | 13% | 474 | 4015 | 12% | 1.23 | 1.09‐1.39 | |
| Missing / not reported | 4313 | 188 | 2272 | 8% | 149 | 2041 | 7% | 1.09 | 0.87‐1.35 | |
| History of cardiovascular events | ||||||||||
| Yes | 3593 | 273 | 2002 | 14% | 197 | 1591 | 12% | 1.24 | 1.03‐1.49 | 0.62 |
| No | 6729 | 404 | 3700 | 11% | 319 | 3029 | 11% | 1.17 | 1.01‐1.35 | |
| Missing / not reported | 3611 | 188 | 1932 | 10% | 149 | 1679 | 9% | 1.09 | 0.87‐1.35 | |
| History of hypertension | ||||||||||
| Yes | 2093 | 140 | 1219 | 11% | 107 | 874 | 12% | 1.15 | 0.90‐1.49 | 0.76 |
| No | 7527 | 537 | 4143 | 13% | 409 | 3384 | 12% | 1.21 | 1.06‐1.37 | |
| Missing / not reported | 4313 | 188 | 2272 | 8% | 149 | 2041 | 7% | 1.09 | 0.88‐1.35 | |
| History of diabetes mellitus | ||||||||||
| Yes | 709 | 62 | 372 | 17% | 56 | 337 | 17% | 1.12 | 0.78‐1.61 | 0.70 |
| No | 7316 | 555 | 3927 | 14% | 427 | 3389 | 13% | 1.21 | 1.06‐1.37 | |
| Missing / not reported | 5908 | 248 | 3335 | 7% | 182 | 2573 | 7% | 1.11 | 0.91‐1.34 | |
| Geographical region | ||||||||||
| Northern America | 3569 | 184 | 2004 | 9% | 159 | 1565 | 10% | 1.08 | 0.87‐1.34 | 0.17 |
| Northern, Western & Southern Europe | 7440 | 403 | 4030 | 10% | 320 | 3410 | 9% | 1.08 | 0.93‐1.26 | |
| Eastern Europe | 1955 | 234 | 1030 | 23% | 151 | 925 | 16% | 1.44 | 1.17‐1.77 | |
| Australia & New Zealand | 342 | 20 | 216 | 9% | 11 | 126 | 9% | 1.42 | 0.68‐2.97 | |
| Other | 226 | 13 | 123 | 11% | 13 | 103 | 13% | 0.90 | 0.42‐1.93 | |
| Missing / not reported | 401 | 11 | 231 | 5% | 11 | 170 | 6% | 0.98 | 0.42‐2.26 | |
| Tumour stage | ||||||||||
| Metastatic / advanced | 8113 | 692 | 4482 | 15% | 527 | 3631 | 15% | 1.20 | 1.07‐1.34 | 0.76 |
| Not metastatic / not advanced | 4039 | 63 | 2116 | 3% | 45 | 1923 | 2% | 1.28 | 0.87‐1.87 | |
| Missing / not reported | 1781 | 110 | 1036 | 11% | 93 | 745 | 12% | 0.92 | 0.69‐1.22 | |
| Planned Hb ceiling | ||||||||||
| Planned Hb ceiling (cat. 1) | 0.98 | |||||||||
| ≤Hb 13.0 g/dL | 3043 | 209 | 1624 | 13% | 157 | 1419 | 11% | 1.19 | 0.97‐1.47 | |
| Hb 13.0 ‐ ≤15.0 g/dL | 10193 | 599 | 5631 | 11% | 468 | 4562 | 10% | 1.16 | 1.03‐1.32 | |
| Hb >15.0 g/dL | 494 | 29 | 259 | 11% | 23 | 235 | 10% | 1.22 | 0.70‐2.11 | |
| Other | 203 | 28 | 120 | 23% | 17 | 83 | 20% | 1.12 | 0.61‐2.06 | |
| Planned Hb ceiling (cat. 2) | 0.88 | |||||||||
| ≤Hb 13.0 g/dL | 3043 | 209 | 1624 | 13% | 157 | 1419 | 11% | 1.19 | 0.97‐1.47 | |
| Hb 13.0 – ≤14.0 g/dL | 6816 | 381 | 3733 | 10% | 322 | 3083 | 10% | 1.12 | 0.97‐1.31 | |
| Hb 14.0 – ≤15.0 g/dL | 3377 | 218 | 1898 | 11% | 146 | 1479 | 10% | 1.25 | 1.01‐1.54 | |
| >Hb 15.0 g/dL | 494 | 29 | 259 | 11% | 23 | 235 | 10% | 1.22 | 0.70‐2.11 | |
| Other | 203 | 28 | 120 | 23% | 17 | 83 | 20% | 1.12 | 0.61‐2.06 | |
| Study level characteristics | ||||||||||
| Treatment population | ||||||||||
| Treatment population (cat. 1) | ||||||||||
| Chemotherapy | 10441 | 605 | 5676 | 11% | 490 | 4765 | 10% | 1.10 | 0.98‐1.24 | 0.42 |
| Radiochemotherapy | 737 | 31 | 368 | 8% | 20 | 369 | 5% | 1.50 | 0.85‐2.63 | |
| Radiotherapy | 799 | 19 | 408 | 5% | 12 | 391 | 3% | 1.52 | 0.74‐3.14 | |
| Mixed | 266 | 17 | 175 | 10% | 7 | 91 | 8% | 1.53 | 0.63‐3.69 | |
| None | 1690 | 193 | 1007 | 19% | 136 | 683 | 20% | 1.33 | 1.06‐1.66 | |
| Treatment population (cat. 2) | ||||||||||
| Chemotherapy | 10441 | 605 | 5676 | 11% | 490 | 4765 | 10% | 1.10 | 0.98‐1.24 | 0.27 |
| Radiotherapy / radiochemotherapy | 1536 | 50 | 776 | 6% | 32 | 760 | 4% | 1.51 | 0.97‐2.35 | |
| Mixed | 266 | 17 | 175 | 10% | 7 | 91 | 8% | 1.53 | 0.63‐3.69 | |
| None | 1690 | 193 | 1007 | 19% | 136 | 683 | 20% | 1.33 | 1.06‐1.66 | |
| Iron supplementation | ||||||||||
| Fixed iron supplementation | 2589 | 71 | 1293 | 5% | 60 | 1296 | 5% | 1.17 | 0.83‐1.65 | 0.48 |
| Iron supplementation as needed | 11120 | 778 | 6232 | 12% | 584 | 4888 | 12% | 1.18 | 1.06‐1.32 | |
| Other | 224 | 16 | 109 | 15% | 21 | 115 | 18% | 0.79 | 0.41‐1.51 | |
| Planned ESA treatment duration | ||||||||||
| Up to 8 weeks | 415 | 21 | 256 | 8% | 17 | 159 | 11% | 0.96 | 0.50‐1.84 | 0.33 |
| 9‐16 weeks | 4800 | 244 | 2738 | 9% | 204 | 2062 | 10% | 1.08 | 0.89‐1.30 | |
| > 17 weeks | 3269 | 388 | 1701 | 23% | 286 | 1568 | 18% | 1.30 | 1.12‐1.52 | |
| Until end of chemo‐ or radiotherapy | 5449 | 212 | 2939 | 7% | 158 | 2510 | 6% | 1.09 | 0.88‐1.34 | |
| Planned weekly ESA dosage | ||||||||||
| < 100 µg Darbepoetin or < 40000 IU Epoetin | 4197 | 238 | 2297 | 10% | 193 | 1900 | 10% | 0.98 | 0.81‐1.19 | 0.12 |
| = 100 µg Darbepoetin or = 40000 IU Epoetin | 3081 | 240 | 1545 | 16% | 190 | 1536 | 12% | 1.36 | 1.12‐1.64 | |
| > 100 µg Darbepoetin or > 40000 IU Epoetin | 3845 | 250 | 2076 | 12% | 184 | 1769 | 10% | 1.23 | 1.01‐1.49 | |
| Other | 2810 | 137 | 1716 | 8% | 98 | 1094 | 9% | 1.11 | 0.85‐1.45 | |
| Planned frequency of ESA application | ||||||||||
| Three times per week or more frequent | 6131 | 311 | 3458 | 9% | 238 | 2673 | 9% | 1.01 | 0.85‐1.20 | 0.03 |
| Once per week | 3948 | 303 | 1972 | 15% | 231 | 1976 | 12% | 1.40 | 1.18‐1.66 | |
| Every second week or less frequent | 3036 | 180 | 1795 | 10% | 122 | 1241 | 10% | 1.25 | 0.99‐1.57 | |
| Other | 818 | 71 | 409 | 17% | 74 | 409 | 18% | 0.93 | 0.67‐1.29 | |
| Placebo controlled trial | ||||||||||
| Yes | 7657 | 594 | 4211 | 14% | 456 | 3446 | 13% | 1.21 | 1.07‐1.37 | 0.38 |
| No | 6276 | 271 | 3423 | 8% | 209 | 2853 | 7% | 1.09 | 0.91‐1.31 | |
| Randomisation | ||||||||||
| Adequate | 3882 | 303 | 2047 | 15% | 245 | 1835 | 13% | 1.17 | 0.99‐1.39 | 0.98 |
| Unclear | 10051 | 562 | 5587 | 10% | 420 | 4464 | 9% | 1.17 | 1.03‐1.33 | |
| Concealment of allocation | ||||||||||
| Adequate | 10595 | 744 | 5839 | 13% | 559 | 4756 | 12% | 1.20 | 1.08‐1.34 | 0.23 |
| Unclear | 3338 | 121 | 1795 | 7% | 106 | 1543 | 7% | 1.01 | 0.78‐1.31 | |
| Endpoint survival | ||||||||||
| Primary endpoint | 3116 | 247 | 1547 | 16% | 195 | 1569 | 12% | 1.30 | 1.08‐1.57 | 0.41 |
| Secondary endpoint | 4313 | 213 | 2282 | 9% | 161 | 2031 | 8% | 1.10 | 0.89‐1.35 | |
| Safety /adverse events | 6504 | 405 | 3805 | 11% | 309 | 2699 | 11% | 1.13 | 0.97‐1.32 | |
| Year of last patient randomized | ||||||||||
| 1990‐1994 | 1447 | 95 | 890 | 11% | 67 | 557 | 12% | 0.95 | 0.69‐1.30 | 0.24 |
| 1995‐1999 | 1725 | 95 | 1001 | 9% | 70 | 724 | 10% | 0.96 | 0.70‐1.32 | |
| 2000‐2004 | 7620 | 431 | 4105 | 10% | 337 | 3515 | 10% | 1.26 | 1.10‐1.46 | |
| 2005‐2006 | 3141 | 244 | 1638 | 15% | 191 | 1503 | 13% | 1.18 | 0.98‐1.43 | |
| Source of data | ||||||||||
| Manufacturer | 12229 | 846 | 6789 | 12% | 641 | 5440 | 12% | 1.19 | 1.07‐1.32 | 0.13 |
| Clinical study group | 1704 | 19 | 845 | 2% | 24 | 859 | 3% | 0.74 | 0.41‐1.35 | |
| *P value for likelihood‐ratio test, patients with missing data are excluded from the test, analysis based on one‐stage Cox fixed‐effects model stratified by study ESA=erythropoiesis‐stimulating agents |
||||||||||
Appendix 7. Assessment of interaction for mortality in chemotherapy trials during the active study period
| Mortality in chemotherapy trials during the active study period | ||||||||||
| ESA arm | Control arm | ESA versus control | ||||||||
| Subgroups | Patients | events | sample | % | events | sample | % | HR | 95% CI | p value* |
| Patient level characteristics | ||||||||||
| Hb at baseline (continuous) | 0.87 | |||||||||
| Hb at baseline (cat 1) | 0.90 | |||||||||
| Hb ≤ 8 g/dL | 569 | 52 | 321 | 16% | 34 | 248 | 14% | 1.20 | 0.78‐1.86 | |
| Hb 8‐≤ 10 g/dL | 2888 | 188 | 1606 | 12% | 156 | 1282 | 12% | 1.07 | 0.86‐1.33 | |
| Hb 10‐≤ 12 g/dL | 3748 | 213 | 2121 | 10% | 171 | 1627 | 11% | 1.10 | 0.90‐1.34 | |
| Hb 12‐≤ 14 g/dL | 2185 | 119 | 1108 | 11% | 100 | 1077 | 9% | 1.23 | 0.94‐1.60 | |
| Hb >14 g/dL | 555 | 29 | 286 | 10% | 25 | 269 | 9% | 0.96 | 0.56‐1.65 | |
| Unknown | 496 | 4 | 234 | 2% | 4 | 262 | 2% | 0.76 | 0.19‐3.05 | |
| Hb at baseline (cat 2) | 0.99 | |||||||||
| Hb ≤ 8 g/dL | 569 | 52 | 321 | 16% | 34 | 248 | 14% | 1.21 | 0.78‐1.86 | |
| Hb 8‐≤ 9 g/dL | 949 | 72 | 549 | 13% | 59 | 400 | 15% | 1.01 | 0.72‐1.44 | |
| Hb 9‐≤ 10 g/dL | 1939 | 116 | 1057 | 11% | 97 | 882 | 11% | 1.10 | 0.84‐1.44 | |
| Hb 10‐≤ 11 g/dL | 2074 | 113 | 1179 | 10% | 86 | 895 | 10% | 1.11 | 0.84‐1.47 | |
| Hb 11‐≤ 12 g/dL | 1674 | 100 | 942 | 11% | 85 | 732 | 12% | 1.08 | 0.81‐1.45 | |
| Hb 12‐≤ 13 g/dL | 1359 | 80 | 679 | 12% | 68 | 680 | 10% | 1.26 | 0.91‐1.74 | |
| Hb 13‐≤ 14 g/dL | 826 | 39 | 429 | 9% | 32 | 397 | 8% | 1.19 | 0.74‐1.89 | |
| Hb >14 g/dL | 555 | 29 | 286 | 10% | 25 | 269 | 9% | 0.96 | 0.56‐1.65 | |
| Unknown | 496 | 4 | 234 | 2% | 4 | 262 | 2% | 0.77 | 0.19‐3.07 | |
| Malignancy type | ||||||||||
| Tumour (cat. 1) | 0.18 | |||||||||
| Haematological malignancies | 1832 | 99 | 1034 | 10% | 65 | 798 | 8% | 1.12 | 0.81‐1.54 | |
| Solid tumours | 7967 | 464 | 4311 | 11% | 379 | 3656 | 10% | 1.14 | 0.99‐1.31 | |
| Other | 600 | 38 | 314 | 12% | 43 | 286 | 15% | 0.74 | 0.48‐1.15 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 1.96 | 0.44‐8.81 | |
| Tumour (cat. 2) | 0.15 | |||||||||
| Haematological malignancies | 1832 | 99 | 1034 | 10% | 65 | 798 | 8% | 1.11 | 0.81‐1.53 | |
| Breast cancer | 4038 | 209 | 2076 | 10% | 152 | 1962 | 8% | 1.38 | 1.12‐1.70 | |
| Head and neck cancer | 26 | 1 | 12 | 8% | 2 | 14 | 14% | 0.63 | 0.06‐6.99 | |
| Lung cancer | 2237 | 187 | 1172 | 16% | 173 | 1065 | 16% | 1.03 | 0.83‐1.26 | |
| Gastrointestinal cancer | 429 | 32 | 267 | 12% | 26 | 162 | 16% | 0.81 | 0.48‐1.37 | |
| Gynaecological cancer | 1077 | 28 | 681 | 4% | 18 | 396 | 5% | 1.06 | 0.59‐1.95 | |
| Genitourinary cancer | 160 | 7 | 103 | 7% | 8 | 57 | 14% | 0.61 | 0.22‐1.72 | |
| Other | 600 | 38 | 314 | 12% | 43 | 286 | 15% | 0.74 | 0.48‐1.15 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 1.92 | 0.43‐8.62 | |
| Sex | ||||||||||
| Male | 3125 | 241 | 1720 | 14% | 209 | 1405 | 15% | 0.99 | 0.82‐1.19 | 0.14 |
| Female | 7316 | 364 | 3956 | 9% | 281 | 3360 | 8% | 1.18 | 1.01‐1.39 | |
| Age | ||||||||||
| Age continuous | 0.57 | |||||||||
| Age categorical | 0.34 | |||||||||
| < 18 years | 123 | 0 | 55 | 0% | 1 | 68 | 1% | Not estimable | Not estimable | |
| ≥18‐35 years | 312 | 9 | 171 | 5% | 8 | 141 | 6% | 0.78 | 0.30‐2.03 | |
| ≥35‐45 years | 1135 | 45 | 620 | 7% | 28 | 515 | 5% | 1.34 | 0.83‐2.14 | |
| ≥45‐55 years | 2425 | 123 | 1311 | 9% | 93 | 1114 | 8% | 1.22 | 0.93‐1.60 | |
| ≥55‐65 years | 3233 | 175 | 1724 | 10% | 172 | 1509 | 11% | 0.93 | 0.75‐1.15 | |
| ≥65‐75 years | 2444 | 190 | 1359 | 14% | 146 | 1085 | 13% | 1.16 | 0.93‐1.44 | |
| ≥75 years | 758 | 63 | 430 | 15% | 41 | 328 | 13% | 1.28 | 0.86‐1.90 | |
| Missing / unknown | 11 | 0 | 6 | 0% | 1 | 5 | 20% | Not estimable | Not estimable | |
| Hct levels at baseline | ||||||||||
| Hct continuous | 0.57 | |||||||||
| Hct categorical | 0.22 | |||||||||
| ≤ 23.5% | 275 | 29 | 144 | 20% | 17 | 131 | 13% | 1.61 | 0.88‐2.94 | |
| 23.5‐≤ 29.4% | 2033 | 118 | 1135 | 10% | 109 | 898 | 12% | 0.96 | 0.74‐1.25 | |
| 29.4‐≤ 35.3% | 3281 | 208 | 1882 | 11% | 163 | 1399 | 12% | 1.02 | 0.83‐1.25 | |
| 35.3‐≤ 41.2% | 1801 | 152 | 931 | 16% | 115 | 870 | 13% | 1.36 | 1.07‐1.73 | |
| > 41.2% | 459 | 39 | 249 | 16% | 33 | 210 | 16% | 1.07 | 0.67‐1.71 | |
| Missing / unknown | 2592 | 59 | 1335 | 4% | 53 | 1257 | 4% | 1.04 | 0.72‐1.52 | |
| Serum Epo at baseline | ||||||||||
| Serum Epo continuous | 0.91 | |||||||||
| Serum Epo categorical | 0.20 | |||||||||
| < 25 mU/ml | 1032 | 68 | 608 | 11% | 41 | 424 | 10% | 1.34 | 0.91‐1.98 | |
| 25‐<100 mU/ml | 2083 | 110 | 1162 | 9% | 114 | 921 | 12% | 0.79 | 0.61‐1.03 | |
| 100‐<200 mU/ml | 518 | 45 | 314 | 14% | 28 | 204 | 14% | 1.14 | 0.71‐1.84 | |
| 200‐<500 mU/ml | 227 | 18 | 134 | 13% | 11 | 93 | 12% | 1.18 | 0.56‐2.51 | |
| ≥ 500 mU/ml | 99 | 8 | 57 | 14% | 4 | 42 | 10% | 1.01 | 0.30‐3.39 | |
| Missing / unknown | 6482 | 356 | 3401 | 10% | 292 | 3081 | 9% | 1.18 | 1.01‐1.38 | |
| Performance score | ||||||||||
| ECOG categorical | 0.58 | |||||||||
| ECOG 0 | 3025 | 77 | 1582 | 5% | 66 | 1443 | 5% | 1.23 | 0.89‐1.71 | |
| ECOG 1 | 3784 | 237 | 2105 | 11% | 185 | 1679 | 11% | 1.10 | 0.91‐1.34 | |
| ECOG 2 | 1140 | 137 | 623 | 22% | 114 | 517 | 22% | 1.07 | 0.84‐1.38 | |
| ECOG 3 | 105 | 15 | 57 | 26% | 13 | 48 | 27% | 0.98 | 0.46‐2.07 | |
| ECOG 4 | 3 | 1 | 2 | 50% | 0 | 1 | 0% | Not estimable | Not estimable | |
| ECOG missing / unknown | 2384 | 138 | 1307 | 11% | 112 | 1077 | 10% | 1.04 | 0.80‐1.33 | |
| ECOG dichotomous | 1.00 | |||||||||
| ECOG 0, 1, 2 | 7949 | 451 | 4310 | 10% | 365 | 3639 | 10% | 1.12 | 0.98‐1.29 | |
| ECOG 3, 4 | 108 | 16 | 59 | 27% | 13 | 49 | 27% | 1.12 | 0.54‐2.34 | |
| ECOG missing | 2384 | 138 | 1307 | 11% | 112 | 1077 | 10% | 1.03 | 0.80‐1.33 | |
| Body mass index | ||||||||||
| ≤ 19 kg/m² | 607 | 43 | 292 | 15% | 45 | 315 | 14% | 0.95 | 0.63‐1.45 | 0.63 |
| 19‐≤ 25 kg/m² | 4283 | 262 | 2318 | 11% | 208 | 1965 | 11% | 1.11 | 0.93‐1.34 | |
| 25‐≤ 30 kg/m² | 2698 | 143 | 1468 | 10% | 116 | 1230 | 9% | 1.01 | 0.79‐1.30 | |
| > 30 kg/m² | 1294 | 60 | 686 | 9% | 44 | 608 | 7% | 1.32 | 0.89‐1.94 | |
| Missing / not reported | 1559 | 97 | 912 | 11% | 77 | 647 | 12% | 1.22 | 0.90‐1.65 | |
| |
||||||||||
| History of thromboembolic events | ||||||||||
| Yes | 375 | 27 | 207 | 13% | 29 | 168 | 17% | 0.76 | 0.45‐1.28 | 0.14 |
| No | 6292 | 400 | 3469 | 12% | 320 | 2823 | 11% | 1.14 | 0.98‐1.32 | |
| Missing / not reported | 3774 | 178 | 2000 | 9% | 141 | 1774 | 8% | 1.08 | 0.86‐1.35 | |
| History of cardiovascular events | ||||||||||
| Yes | 2319 | 161 | 1295 | 12% | 126 | 1024 | 12% | 1.11 | 0.88‐1.41 | 0.93 |
| No | 5050 | 266 | 2721 | 10% | 223 | 2329 | 10% | 1.10 | 0.92‐1.31 | |
| Missing / not reported | 3072 | 178 | 1660 | 11% | 141 | 1412 | 10% | 1.08 | 0.86‐1.35 | |
| History of hypertension | ||||||||||
| Yes | 1396 | 111 | 798 | 14% | 81 | 598 | 14% | 1.18 | 0.89‐1.57 | 0.61 |
| No | 5271 | 316 | 2878 | 11% | 268 | 2393 | 11% | 1.08 | 0.92‐1.28 | |
| Missing / not reported | 3774 | 178 | 2000 | 9% | 141 | 1774 | 8% | 1.08 | 0.86‐1.35 | |
| History of diabetes mellitus | ||||||||||
| Yes | 430 | 36 | 219 | 16% | 37 | 211 | 18% | 1.01 | 0.64‐1.61 | 0.74 |
| No | 5149 | 350 | 2786 | 13% | 286 | 2363 | 12% | 1.10 | 0.94‐1.29 | |
| Missing / not reported | 4862 | 219 | 2671 | 8% | 167 | 2191 | 8% | 1.12 | 0.91‐1.37 | |
| Geographical region | ||||||||||
| Northern America | 2083 | 92 | 1088 | 8% | 95 | 995 | 10% | 0.95 | 0.71‐1.26 | 0.35 |
| Northern, Western & Southern Europe | 6082 | 341 | 3342 | 10% | 267 | 2740 | 10% | 1.05 | 0.90‐1.24 | |
| Eastern Europe | 1413 | 135 | 734 | 18% | 98 | 679 | 14% | 1.34 | 1.03‐1.73 | |
| Australia & New Zealand | 286 | 14 | 184 | 8% | 7 | 102 | 7% | 1.59 | 0.64‐3.95 | |
| Other | 189 | 13 | 106 | 12% | 13 | 83 | 16% | 0.90 | 0.42‐1.94 | |
| Missing / not reported | 388 | 10 | 222 | 5% | 10 | 166 | 6% | 1.02 | 0.42‐2.45 | |
| Tumour stage | ||||||||||
| Metastatic / advanced | 6054 | 491 | 3325 | 15% | 388 | 2729 | 14% | 1.16 | 1.01‐1.32 | 0.61 |
| Not metastatic / not advanced | 2902 | 25 | 1491 | 2% | 24 | 1411 | 2% | 1.00 | 0.57‐1.75 | |
| Missing / not reported | 1485 | 89 | 860 | 10% | 78 | 625 | 12% | 0.82 | 0.60‐1.12 | |
| Planned Hb ceiling | ||||||||||
| Planned Hb ceiling (cat 1) | 0.28 | |||||||||
| ≤Hb 13.0 g/dL | 1631 | 47 | 841 | 6% | 49 | 790 | 6% | 0.83 | 0.56‐1.25 | |
| Hb 13.0 ‐ ≤15.0 g/dL | 8451 | 523 | 4630 | 11% | 415 | 3821 | 11% | 1.14 | 1.00‐1.30 | |
| Hb >15.0 g/dL | 280 | 20 | 150 | 13% | 21 | 130 | 16% | 0.90 | 0.48‐1.67 | |
| Other | 79 | 15 | 55 | 27% | 5 | 24 | 21% | 1.43 | 0.52‐3.93 | |
| Planned Hb ceiling (cat 2) | 0.38 | |||||||||
| ≤Hb 13.0 g/dL | 1631 | 47 | 841 | 6% | 49 | 790 | 6% | 0.83 | 0.56‐1.25 | |
| Hb 13.0 – ≤14.0 g/dL | 5930 | 323 | 3200 | 10% | 277 | 2730 | 10% | 1.10 | 0.93‐1.29 | |
| Hb 14.0 – ≤15.0 g/dL | 2521 | 200 | 1430 | 14% | 138 | 1091 | 13% | 1.22 | 0.98‐1.52 | |
| >Hb 15.0 g/dL | 280 | 20 | 150 | 13% | 21 | 130 | 16% | 0.90 | 0.48‐1.67 | |
| Other | 79 | 15 | 55 | 27% | 5 | 24 | 21% | 1.43 | 0.52‐3.93 | |
| Study level characteristics | ||||||||||
| Iron supplementation | ||||||||||
| Fixed iron supplementation | 1904 | 40 | 947 | 4% | 40 | 957 | 4% | 1.00 | 0.64‐1.55 | 0.52 |
| Iron supplementation as needed | 8313 | 549 | 4620 | 12% | 429 | 3693 | 12% | 1.12 | 0.99‐1.28 | |
| Other | 224 | 16 | 109 | 15% | 21 | 115 | 18% | 0.79 | 0.41‐1.51 | |
| Planned ESA treatment duration | ||||||||||
| up to 8 weeks | 143 | 3 | 114 | 3% | 2 | 29 | 7% | 0.38 | 0.06‐2.30 | 0.20 |
| 9‐16 weeks | 3823 | 183 | 2075 | 9% | 167 | 1748 | 10% | 1.01 | 0.82‐1.25 | |
| > 17 weeks | 2280 | 252 | 1184 | 21% | 192 | 1096 | 18% | 1.27 | 1.05‐1.53 | |
| Until end of chemo‐ or radiotherapy | 4195 | 167 | 2303 | 7% | 129 | 1892 | 7% | 1.00 | 0.79‐1.26 | |
| Planned weekly ESA dosage | ||||||||||
| < 100 µg Darbepoetin or < 40000 IU Epoetin | 3733 | 208 | 2023 | 10% | 174 | 1710 | 10% | 0.96 | 0.78‐1.18 | 0.29 |
| <= 100 µg Darbepoetin or = 40000 IU Epoetin | 2200 | 179 | 1101 | 16% | 144 | 1099 | 13% | 1.29 | 1.04‐1.61 | |
| > 100 µg Darbepoetin or > 40000 IU Epoetin | 1998 | 86 | 987 | 9% | 76 | 1011 | 8% | 1.11 | 0.82‐1.51 | |
| Other | 2510 | 132 | 1565 | 8% | 96 | 945 | 10% | 1.08 | 0.83‐1.42 | |
| Planned frequency of ESA application | ||||||||||
| Three times per week or more frequent | 5016 | 267 | 2853 | 9% | 210 | 2163 | 10% | 0.97 | 0.81‐1.17 | 0.05 |
| Once per week | 3067 | 242 | 1528 | 16% | 185 | 1539 | 12% | 1.35 | 1.12‐1.64 | |
| Every second week or less frequent | 1540 | 25 | 886 | 3% | 21 | 654 | 3% | 0.92 | 0.51‐1.68 | |
| Other | 818 | 71 | 409 | 17% | 74 | 409 | 18% | 0.93 | 0.67‐1.29 | |
| Placebo controlled trial | ||||||||||
| Yes | 5473 | 379 | 2996 | 13% | 307 | 2477 | 12% | 1.13 | 0.97‐1.32 | 0.53 |
| No | 4968 | 226 | 2680 | 8% | 183 | 2288 | 8% | 1.05 | 0.86‐1.28 | |
| Randomisation | ||||||||||
| Adequate | 3258 | 244 | 1693 | 14% | 202 | 1565 | 13% | 1.11 | 0.92‐1.34 | 0.88 |
| Unclear | 7183 | 361 | 3983 | 9% | 288 | 3200 | 9% | 1.09 | 0.93‐1.28 | |
| Concealment of allocation | ||||||||||
| Adequate | 8252 | 545 | 4501 | 12% | 423 | 3751 | 11% | 1.15 | 1.01‐1.30 | 0.07 |
| Unclear | 2189 | 60 | 1175 | 5% | 67 | 1014 | 7% | 0.81 | 0.57‐1.16 | |
| Endpoint survival | ||||||||||
| Primary endpoint | 2731 | 221 | 1352 | 16% | 177 | 1379 | 13% | 1.29 | 1.06‐1.57 | 0.11 |
| Secondary endpoint | 3222 | 189 | 1730 | 11% | 147 | 1492 | 10% | 1.04 | 0.84‐1.30 | |
| Safety /adverse events | 4488 | 195 | 2594 | 8% | 166 | 1894 | 9% | 0.96 | 0.78‐1.18 | |
| Year of last patient randomized | ||||||||||
| 1990‐1994 | 1057 | 65 | 650 | 10% | 48 | 407 | 12% | 0.86 | 0.59‐1.26 | 0.16 |
| 1995‐1999 | 1725 | 95 | 1001 | 9% | 70 | 724 | 10% | 0.96 | 0.70‐1.32 | |
| 2000‐2004 | 6112 | 374 | 3263 | 11% | 298 | 2849 | 10% | 1.22 | 1.05‐1.43 | |
| 2005‐2006 | 1547 | 71 | 762 | 9% | 74 | 785 | 9% | 0.93 | 0.67‐1.29 | |
| Source of data | ||||||||||
| Manufacturer | 8851 | 587 | 4889 | 12% | 467 | 3962 | 12% | 1.12 | 0.99‐1.26 | 0.18 |
| Clinical study group | 1590 | 18 | 787 | 2% | 23 | 803 | 3% | 0.73 | 0.39‐1.36 | |
| *P value for likelihood‐ratio test, patients with missing data are excluded from the test, analysis based on one‐stage Cox fixed‐effects model stratified by study ESA= erythropoiesis‐stimulating agents | ||||||||||
Appendix 8. Assessment of interaction for overall survival in all cancer patients
| Overall survival in all cancer patients | ESA arm | Control arm | ESA versus control | |||||||
| Subgroups | Patients | events | sample | % | events | sample | % | HR | 95% CI | P value* |
| Patient level characteristics | ||||||||||
| Hb at baseline | ||||||||||
| Hb at baseline (continuous) | 0.75 | |||||||||
| Hb at baseline (cat 1) | 0.63 | |||||||||
| Hb ≤ 8 g/dL | 791 | 176 | 448 | 39% | 147 | 343 | 43% | 1.08 | 0.87‐1.35 | |
| Hb 8‐≤10 g/dL | 3930 | 725 | 2222 | 33% | 672 | 1708 | 39% | 1.02 | 0.92‐1.14 | |
| Hb 10‐≤12 g/dL | 5004 | 967 | 2851 | 34% | 777 | 2153 | 36% | 1.11 | 1.01‐1.22 | |
| Hb 12‐≤14 g/dL | 2843 | 566 | 1433 | 39% | 553 | 1410 | 39% | 1.06 | 0.95‐1.20 | |
| Hb >14 g/dL | 839 | 155 | 428 | 36% | 155 | 411 | 38% | 0.94 | 0.75‐1.18 | |
| Unknown | 526 | 54 | 252 | 21% | 46 | 274 | 17% | 1.22 | 0.82‐1.82 | |
| Hb at baseline (cat 2) | 0.83 | |||||||||
| Hb ≤ 8 g/dL | 791 | 176 | 448 | 39% | 147 | 343 | 43% | 1.08 | 0.87‐1.35 | |
| Hb 8‐≤9 g/dL | 1319 | 256 | 742 | 35% | 252 | 577 | 44% | 1.05 | 0.88‐1.25 | |
| Hb 9‐≤10 g/dL | 2611 | 469 | 1480 | 32% | 420 | 1131 | 37% | 1.02 | 0.89‐1.16 | |
| Hb 10‐≤11 g/dL | 2927 | 542 | 1699 | 32% | 414 | 1228 | 34% | 1.16 | 1.02‐1.32 | |
| Hb 11‐≤12 g/dL | 2077 | 425 | 1152 | 37% | 363 | 925 | 39% | 1.06 | 0.92‐1.22 | |
| Hb 12‐≤13 g/dL | 1739 | 377 | 873 | 43% | 371 | 866 | 43% | 1.04 | 0.90‐1.20 | |
| Hb 13‐≤14 g/dL | 1104 | 189 | 560 | 34% | 182 | 544 | 33% | 1.12 | 0.91‐1.37 | |
| Hb >14 g/dL | 839 | 155 | 428 | 36% | 155 | 411 | 38% | 0.94 | 0.75‐1.18 | |
| Unknown | 526 | 54 | 252 | 21% | 46 | 274 | 17% | 1.23 | 0.83‐1.83 | |
| Malignancy type | ||||||||||
| Tumour (cat. 1) | 0.23 | |||||||||
| Haematological malignancies | 2403 | 378 | 1400 | 27% | 286 | 1003 | 29% | 1.19 | 1.02‐1.39 | |
| Solid tumours | 10795 | 2103 | 5848 | 36% | 1916 | 4947 | 39% | 1.04 | 0.98‐1.11 | |
| Other | 693 | 158 | 369 | 43% | 145 | 324 | 45% | 0.99 | 0.82‐1.20 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 2.14 | 0.48‐9.62 | |
| Tumour (cat. 2) | 0.21 | |||||||||
| Haematological malignancies | 2403 | 378 | 1400 | 27% | 286 | 1003 | 29% | 1.18 | 1.01‐1.38 | |
| Breast cancer | 4302 | 563 | 2245 | 25% | 481 | 2057 | 23% | 1.13 | 1.00‐1.28 | |
| Head and neck cancer | 868 | 235 | 443 | 53% | 208 | 425 | 49% | 1.14 | 0.91‐1.42 | |
| Lung cancer | 3076 | 986 | 1618 | 61% | 975 | 1458 | 67% | 0.98 | 0.89‐1.07 | |
| Gastrointestinal cancer | 708 | 124 | 434 | 29% | 103 | 274 | 38% | 0.89 | 0.68‐1.16 | |
| Gynaecological cancer | 1399 | 115 | 842 | 14% | 87 | 557 | 16% | 1.13 | 0.85‐1.50 | |
| Genitourinal cancer | 442 | 80 | 266 | 30% | 62 | 176 | 35% | 1.24 | 0.89‐1.73 | |
| Other | 693 | 158 | 369 | 43% | 145 | 324 | 45% | 0.99 | 0.82‐1.20 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 2.12 | 0.47‐9.50 | |
| Sex | ||||||||||
| Male | 5136 | 1323 | 2854 | 46% | 1193 | 2282 | 52% | 1.01 | 0.94‐1.10 | 0.15 |
| Female | 8797 | 1320 | 4780 | 28% | 1157 | 4017 | 29% | 1.10 | 1.02‐1.19 | |
| Age | ||||||||||
| Age continuous | 0.38 | |||||||||
| Age categorical | 0.26 | |||||||||
| < 18 years | 123 | 0 | 55 | 0% | 1 | 68 | 1% | Not estimable | Not estimable | |
| ≥18‐35 years | 346 | 37 | 191 | 19% | 27 | 155 | 17% | 0.89 | 0.54‐1.46 | |
| ≥35‐45 years | 1343 | 196 | 745 | 26% | 147 | 598 | 25% | 1.02 | 0.82‐1.26 | |
| ≥45‐55 years | 3010 | 536 | 1614 | 33% | 439 | 1396 | 31% | 1.16 | 1.03‐1.32 | |
| ≥55‐65 years | 4193 | 818 | 2237 | 37% | 793 | 1956 | 41% | 1.01 | 0.91‐1.11 | |
| ≥65‐75 years | 3517 | 780 | 1970 | 40% | 711 | 1547 | 46% | 1.04 | 0.94‐1.15 | |
| ≥75 years | 1389 | 276 | 816 | 34% | 231 | 573 | 40% | 1.20 | 1.00‐1.43 | |
| Missing | 12 | 0 | 6 | 0% | 1 | 6 | 17% | Not estimable | Not estimable | |
| Hct levels at baseline | ||||||||||
| Hct continuous | 0.90 | |||||||||
| Hct categorical | 0.03 | |||||||||
| ≤ 23.5% | 390 | 82 | 210 | 39% | 55 | 180 | 31% | 1.66 | 1.18‐2.34 | |
| 23.5‐≤ 29.4% | 2788 | 476 | 1567 | 30% | 479 | 1221 | 39% | 0.94 | 0.83‐1.07 | |
| 29.4‐≤ 35.3% | 4615 | 945 | 2692 | 35% | 732 | 1923 | 38% | 1.10 | 0.99‐1.21 | |
| 35.3‐≤ 41.2% | 2458 | 579 | 1258 | 46% | 558 | 1200 | 47% | 1.07 | 0.95‐1.21 | |
| > 41.2% | 785 | 169 | 414 | 41% | 165 | 371 | 44% | 1.02 | 0.82‐1.26 | |
| Missing / unknown | 2897 | 392 | 1493 | 26% | 361 | 1404 | 26% | 1.08 | 0.93‐1.24 | |
| Serum Epo at baseline | ||||||||||
| Serum Epo continuous | 0.14 | |||||||||
| Serum Epo categorical | 0.81 | |||||||||
| < 25 mU/ml | 1497 | 341 | 876 | 39% | 309 | 621 | 50% | 0.97 | 0.84‐1.14 | |
| 25‐100 mU/ml | 2908 | 586 | 1643 | 36% | 548 | 1265 | 43% | 1.02 | 0.90‐1.14 | |
| 100‐200 mU/ml | 740 | 187 | 451 | 41% | 130 | 289 | 45% | 1.10 | 0.88‐1.38 | |
| 200‐500 mU/ml | 325 | 60 | 190 | 32% | 51 | 135 | 38% | 1.18 | 0.81‐1.72 | |
| > 500 mU/ml | 181 | 31 | 103 | 30% | 22 | 78 | 28% | 1.08 | 0.63‐1.88 | |
| Unknown | 8282 | 1438 | 4371 | 33% | 1290 | 3911 | 33% | 1.09 | 1.01‐1.17 | |
| Performance score | ||||||||||
| ECOG categorical | 0.41 | |||||||||
| ECOG 0 | 3392 | 351 | 1808 | 19% | 341 | 1584 | 22% | 1.06 | 0.91‐1.23 | |
| ECOG 1 | 4900 | 984 | 2779 | 35% | 814 | 2121 | 38% | 1.09 | 0.99‐1.20 | |
| ECOG 2 | 1678 | 490 | 933 | 53% | 433 | 745 | 58% | 1.01 | 0.89‐1.15 | |
| ECOG 3 | 139 | 48 | 77 | 62% | 35 | 62 | 56% | 1.18 | 0.76‐1.82 | |
| ECOG 4 | 3 | 2 | 2 | 100% | 0 | 1 | 0% | Not estimable | Not estimable | |
| ECOG missing | 3821 | 768 | 2035 | 38% | 727 | 1786 | 41% | 1.02 | 0.93‐1.14 | |
| ECOG dichotomous | 0.50 | |||||||||
| ECOG 0, 1, 2 | 10083 | 1847 | 5578 | 33% | 1604 | 4505 | 36% | 1.08 | 1.01‐1.15 | |
| ECOG 3, 4 | 142 | 50 | 79 | 63% | 35 | 63 | 56% | 1.25 | 0.81‐1.93 | |
| ECOG missing | 3708 | 746 | 1977 | 38% | 711 | 1731 | 41% | 1.02 | 0.92‐1.13 | |
| Body mass index | ||||||||||
| ≤ 19 kg/m² | 865 | 187 | 424 | 44% | 195 | 441 | 44% | 0.95 | 0.78‐1.17 | 0.72 |
| 19‐≤ 25 kg/m² | 5487 | 1098 | 2964 | 37% | 945 | 2523 | 37% | 1.06 | 0.97‐1.15 | |
| 25‐≤ 30 kg/m² | 3443 | 642 | 1864 | 34% | 543 | 1579 | 34% | 1.09 | 0.97‐1.22 | |
| > 30 kg/m² | 1650 | 250 | 867 | 29% | 224 | 783 | 29% | 1.03 | 0.86‐1.24 | |
| Missing | 2488 | 466 | 1515 | 31% | 443 | 973 | 46% | 1.10 | 0.97‐1.26 | |
| History of thromboembolic events | ||||||||||
| Yes | 561 | 128 | 318 | 40% | 107 | 243 | 44% | 1.03 | 0.80‐1.33 | 0.90 |
| No | 9059 | 1720 | 5044 | 34% | 1509 | 4015 | 38% | 1.05 | 0.98‐1.12 | |
| Missing / not reported | 4313 | 795 | 2272 | 35% | 734 | 2041 | 36% | 1.08 | 0.98‐1.20 | |
| History of cardiovascular events | ||||||||||
| Yes | 3593 | 758 | 2002 | 38% | 648 | 1591 | 41% | 1.07 | 0.96‐1.19 | 0.69 |
| No | 6729 | 1141 | 3700 | 31% | 1010 | 3029 | 33% | 1.04 | 0.96‐1.13 | |
| Missing / not reported | 3611 | 744 | 1932 | 39% | 692 | 1679 | 41% | 1.07 | 0.97‐1.19 | |
| History of hypertension | ||||||||||
| Yes | 2093 | 420 | 1219 | 34% | 373 | 874 | 43% | 1.01 | 0.88‐1.16 | 0.57 |
| No | 7527 | 1428 | 4143 | 34% | 1243 | 3384 | 37% | 1.06 | 0.98‐1.14 | |
| Missing / not reported | 4313 | 795 | 2272 | 35% | 734 | 2041 | 36% | 1.08 | 0.98‐1.20 | |
| History of diabetes mellitus | ||||||||||
| Yes | 709 | 163 | 372 | 44% | 158 | 337 | 47% | 1.05 | 0.84‐1.31 | 0.94 |
| No | 7316 | 1456 | 3927 | 37% | 1250 | 3389 | 37% | 1.06 | 0.98‐1.14 | |
| Missing / not reported | 5908 | 1024 | 3335 | 31% | 942 | 2573 | 37% | 1.06 | 0.97‐1.16 | |
| Geographical region | ||||||||||
| Northern America | 3569 | 490 | 2004 | 24% | 470 | 1565 | 30% | 1.11 | 0.98‐1.27 | 0.90 |
| Northern, Western & Southern Europe | 7440 | 1529 | 4030 | 38% | 1322 | 3410 | 39% | 1.05 | 0.98‐1.13 | |
| Eastern Europe | 1955 | 514 | 1030 | 50% | 469 | 925 | 51% | 1.03 | 0.91‐1.17 | |
| Australia & New Zealand | 342 | 40 | 216 | 19% | 28 | 126 | 22% | 1.08 | 0.66‐1.75 | |
| Other | 226 | 48 | 123 | 39% | 46 | 103 | 45% | 0.95 | 0.63‐1.43 | |
| Missing / not reported | 401 | 22 | 231 | 10% | 15 | 170 | 9% | 1.47 | 0.75‐2.89 | |
| Tumour stage | ||||||||||
| Metastatic / advanced | 8113 | 1918 | 4482 | 43% | 1698 | 3631 | 47% | 1.05 | 0.98‐1.12 | 0.86 |
| Not metastatic / not advanced | 4039 | 420 | 2116 | 20% | 408 | 1923 | 21% | 1.06 | 0.93‐1.22 | |
| Missing / not reported | 1781 | 305 | 1036 | 29% | 244 | 745 | 33% | 1.04 | 0.87‐1.23 | |
| Planned Hb ceiling | ||||||||||
| Planned Hb ceiling (cat 1) | 0.40 | |||||||||
| ≤Hb 13.0 g/dL | 3043 | 437 | 1624 | 27% | 399 | 1419 | 28% | 1.09 | 0.95‐1.25 | |
| Hb 13.0 ‐ ≤15.0 g/dL | 10193 | 2019 | 5631 | 36% | 1782 | 4562 | 39% | 1.04 | 0.97‐1.11 | |
| Hb >15.0 g/dL | 494 | 159 | 259 | 61% | 152 | 235 | 65% | 1.21 | 0.97‐1.51 | |
| Other | 203 | 28 | 120 | 23% | 17 | 83 | 20% | 1.13 | 0.61‐2.07 | |
| Planned Hb ceiling (cat 2) | 0.60 | |||||||||
| ≤Hb 13.0 g/dL | 3043 | 437 | 1624 | 27% | 399 | 1419 | 28% | 1.09 | 0.95‐1.25 | |
| Hb 13.0 – ≤14.0 g/dL | 6816 | 1142 | 3733 | 31% | 1013 | 3083 | 33% | 1.03 | 0.95‐1.13 | |
| Hb 14.0 – ≤15.0 g/dL | 3377 | 877 | 1898 | 46% | 769 | 1479 | 52% | 1.05 | 0.95‐1.15 | |
| >Hb 15.0 g/dL | 494 | 159 | 259 | 61% | 152 | 235 | 65% | 1.21 | 0.97‐1.51 | |
| Other | 203 | 28 | 120 | 23% | 17 | 83 | 20% | 1.13 | 0.61‐2.07 | |
| Study level characteristics | ||||||||||
| Treatment population | ||||||||||
| Treatment population | ||||||||||
| Chemotherapy | 10441 | 1888 | 5676 | 33% | 1667 | 4765 | 35% | 1.04 | 0.97‐1.11 | 0.11 |
| Radiochemotherapy | 737 | 204 | 368 | 55% | 211 | 369 | 57% | 0.91 | 0.75‐1.10 | |
| Radiotherapy | 799 | 220 | 408 | 54% | 196 | 391 | 50% | 1.17 | 0.96‐1.42 | |
| Mixed | 266 | 17 | 175 | 10% | 7 | 91 | 8% | 1.53 | 0.63‐3.69 | |
| None | 1690 | 314 | 1007 | 31% | 269 | 683 | 39% | 1.22 | 1.04‐1.44 | |
| Treatment population | ||||||||||
| Chemotherapy | 10441 | 1888 | 5676 | 33% | 1667 | 4765 | 35% | 1.04 | 0.97‐1.11 | 0.25 |
| Radiotherapy / radiochemotherapy | 1536 | 424 | 776 | 55% | 407 | 760 | 54% | 1.03 | 0.90‐1.18 | |
| Mixed | 266 | 17 | 175 | 10% | 7 | 91 | 8% | 1.53 | 0.63‐3.69 | |
| None | 1690 | 314 | 1007 | 31% | 269 | 683 | 39% | 1.22 | 1.04‐1.44 | |
| Iron supplementation | ||||||||||
| Fixed iron supplementation | 2589 | 468 | 1293 | 36% | 467 | 1296 | 36% | 1.00 | 0.87‐1.13 | 0.48 |
| Iron supplementation as needed | 11120 | 2075 | 6232 | 33% | 1782 | 4888 | 36% | 1.07 | 1.00‐1.14 | |
| Other | 224 | 100 | 109 | 92% | 101 | 115 | 88% | 1.17 | 0.89‐1.55 | |
| Planned ESA treatment duration | ||||||||||
| Up to 8 weeks | 415 | 55 | 256 | 21% | 47 | 159 | 30% | 1.09 | 0.74‐1.62 | 0.74 |
| 9‐16 weeks | 4800 | 667 | 2738 | 24% | 644 | 2062 | 31% | 1.02 | 0.91‐1.14 | |
| > 17 weeks | 3269 | 816 | 1701 | 48% | 747 | 1568 | 48% | 1.11 | 1.00‐1.22 | |
| Until end of chemo‐ or radiotherapy | 5449 | 1105 | 2939 | 38% | 912 | 2510 | 36% | 1.05 | 0.96‐1.14 | |
| Planned weekly ESA dosage | ||||||||||
| < 100 µg Darbepoetin or < 40000 IU Epoetin | 4197 | 832 | 2297 | 36% | 669 | 1900 | 35% | 1.04 | 0.94‐1.15 | 0.88 |
| = 100 µg Darbepoetin or = 40000 IU Epoetin | 3081 | 557 | 1545 | 36% | 536 | 1536 | 35% | 1.08 | 0.96‐1.22 | |
| > 100 µg Darbepoetin or > 40000 IU Epoetin | 3845 | 876 | 2076 | 42% | 808 | 1769 | 46% | 1.08 | 0.98‐1.19 | |
| Other | 2810 | 378 | 1716 | 22% | 337 | 1094 | 31% | 1.02 | 0.88‐1.18 | |
| Planned frequency of ESA application | ||||||||||
| Three times per week or more frequent | 6131 | 1067 | 3458 | 31% | 840 | 2673 | 31% | 1.07 | 0.98‐1.18 | 0.07 |
| Once per week | 3948 | 911 | 1972 | 46% | 886 | 1976 | 45% | 1.06 | 0.97‐1.17 | |
| Every second week or less frequent | 3036 | 347 | 1795 | 19% | 286 | 1241 | 23% | 1.20 | 1.02‐1.40 | |
| Other | 818 | 318 | 409 | 78% | 338 | 409 | 83% | 0.90 | 0.77‐1.05 | |
| Placebo controlled trial | ||||||||||
| Yes | 7657 | 1578 | 4211 | 37% | 1403 | 3446 | 41% | 1.09 | 1.01‐1.17 | 0.29 |
| No | 6276 | 1065 | 3423 | 31% | 947 | 2853 | 33% | 1.02 | 0.93‐1.12 | |
| Randomisation | ||||||||||
| Adequate | 3882 | 739 | 2047 | 36% | 636 | 1835 | 35% | 1.07 | 0.96‐1.19 | 0.80 |
| Unclear | 10051 | 1904 | 5587 | 34% | 1714 | 4464 | 38% | 1.05 | 0.99‐1.13 | |
| Concealment of allocation | ||||||||||
| Adequate | 10595 | 2176 | 5839 | 37% | 1901 | 4756 | 40% | 1.07 | 1.00‐1.14 | 0.49 |
| Unclear | 3338 | 467 | 1795 | 26% | 449 | 1543 | 29% | 1.02 | 0.89‐1.16 | |
| Endpoint survival | ||||||||||
| Primary endpoint | 3116 | 732 | 1547 | 47% | 715 | 1569 | 46% | 1.02 | 0.92‐1.13 | 0.39 |
| Secondary endpoint | 4313 | 1164 | 2282 | 51% | 985 | 2031 | 48% | 1.04 | 0.96‐1.14 | |
| Safety /adverse events | 6504 | 747 | 3805 | 20% | 650 | 2699 | 24% | 1.13 | 1.01‐1.25 | |
| Designed for long‐term follow‐up | ||||||||||
| Yes | 8974 | 2213 | 4619 | 48% | 1972 | 4355 | 45% | 1.06 | 1.00‐1.13 | 0.64 |
| No | 4959 | 430 | 3015 | 14% | 378 | 1944 | 19% | 1.03 | 0.89‐1.18 | |
| Year of last patient randomized | ||||||||||
| 1990‐1994 | 1447 | 100 | 890 | 11% | 70 | 557 | 13% | 0.96 | 0.70‐1.31 | 0.13 |
| 1995‐1999 | 1725 | 312 | 1001 | 31% | 224 | 724 | 31% | 0.97 | 0.81‐1.16 | |
| 2000‐2004 | 7620 | 1453 | 4105 | 35% | 1296 | 3515 | 37% | 1.13 | 1.04‐1.21 | |
| 2005‐2006 | 3141 | 778 | 1638 | 47% | 760 | 1503 | 51% | 0.99 | 0.89‐1.09 | |
| Source of data | ||||||||||
| Manufacturer | 12229 | 2434 | 6789 | 36% | 2151 | 5440 | 40% | 1.06 | 1.00‐1.13 | 0.57 |
| Clinical study group | 1704 | 209 | 845 | 25% | 199 | 859 | 23% | 1.00 | 0.83‐1.22 | |
| *P value for likelihood‐ratio test (test for interaction), patients with missing data are excluded from this test, analysis based on one‐stage Cox fixed‐effects model stratified by study ESA= erythropoiesis‐stimulating agents | ||||||||||
Appendix 9. Assessment of interaction for overall survival in chemotherapy trials
| Overall survival chemotherapy trials | ||||||||||
| Subgroups | Patients | ESA arm | Control arm | ESA versus control | ||||||
| events | sample | % | events | sample | % | HR | 95% CI | P value* | ||
| Patient level characteristics | ||||||||||
| Hb at baseline | ||||||||||
| Hb at baseline (continuous) | 0.49 | |||||||||
| Hb at baseline (cat. 1) | ||||||||||
| Hb ≤ 8 g/dL | 569 | 121 | 321 | 38% | 100 | 248 | 40% | 1.08 | 0.83‐1.41 | 0.88 |
| Hb 8‐≤10 g/dL | 2888 | 533 | 1606 | 33% | 504 | 1282 | 39% | 0.99 | 0.88‐1.12 | |
| Hb 10‐≤12 g/dL | 3748 | 706 | 2121 | 33% | 572 | 1627 | 35% | 1.06 | 0.95‐1.18 | |
| Hb 12‐≤14 g/dL | 2185 | 401 | 1108 | 36% | 377 | 1077 | 35% | 1.08 | 0.94‐1.24 | |
| Hb >14 g/dL | 555 | 83 | 286 | 29% | 70 | 269 | 26% | 1.13 | 0.82‐1.55 | |
| Unknown | 496 | 44 | 234 | 19% | 44 | 262 | 17% | 1.08 | 0.71‐1.65 | |
| Hb at baseline (cat. 2) | ||||||||||
| Hb ≤ 8 g/dL | 569 | 121 | 321 | 38% | 100 | 248 | 40% | 1.08 | 0.83‐1.42 | 0.98 |
| Hb 8‐≤9 g/dL | 949 | 182 | 549 | 33% | 175 | 400 | 44% | 1.00 | 0.81‐1.23 | |
| Hb 9‐≤10 g/dL | 1939 | 351 | 1057 | 33% | 329 | 882 | 37% | 0.99 | 0.85‐1.16 | |
| Hb 10‐≤11 g/dL | 2074 | 375 | 1179 | 32% | 290 | 895 | 32% | 1.08 | 0.93‐1.27 | |
| Hb 11‐≤12 g/dL | 1674 | 331 | 942 | 35% | 282 | 732 | 39% | 1.03 | 0.88‐1.20 | |
| Hb 12‐≤13 g/dL | 1359 | 287 | 679 | 42% | 275 | 680 | 40% | 1.09 | 0.92‐1.28 | |
| Hb 13‐≤14 g/dL | 826 | 114 | 429 | 27% | 102 | 397 | 26% | 1.09 | 0.83‐1.43 | |
| Hb >14 g/dL | 555 | 83 | 286 | 29% | 70 | 269 | 26% | 1.13 | 0.82‐1.55 | |
| Unknown | 496 | 44 | 234 | 19% | 44 | 262 | 17% | 1.09 | 0.71‐1.66 | |
| Malignancy type | ||||||||||
| Tumour (cat. 1) | ||||||||||
| Haematological malignancies | 1832 | 335 | 1034 | 32% | 264 | 798 | 33% | 1.13 | 0.96‐1.33 | 0.33 |
| Solid tumours | 7967 | 1410 | 4311 | 33% | 1271 | 3656 | 35% | 1.03 | 0.96‐1.12 | |
| Other | 600 | 139 | 314 | 44% | 129 | 286 | 45% | 0.90 | 0.71‐1.15 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 2.12 | 0.47‐9.54 | |
| Tumour (cat. 2) | ||||||||||
| Haematological malignancies | 1832 | 335 | 1034 | 32% | 264 | 798 | 33% | 1.12 | 0.95‐1.32 | 0.33 |
| Breast cancer | 4038 | 536 | 2076 | 26% | 454 | 1962 | 23% | 1.15 | 1.01‐1.30 | |
| Head and neck cancer | 26 | 3 | 12 | 25% | 3 | 14 | 21% | 0.49 | 0.10‐2.43 | |
| Lung cancer | 2237 | 705 | 1172 | 60% | 695 | 1065 | 65% | 0.96 | 0.87‐1.07 | |
| Gastrointestinal cancer | 429 | 84 | 267 | 31% | 65 | 162 | 40% | 0.89 | 0.64‐1.24 | |
| Gynaecological cancer | 1077 | 64 | 681 | 9% | 39 | 396 | 10% | 1.16 | 0.77‐1.73 | |
| Genitourinay cancer | 160 | 18 | 103 | 17% | 15 | 57 | 26% | 1.05 | 0.52‐2.10 | |
| Other | 600 | 139 | 314 | 44% | 129 | 286 | 45% | 0.91 | 0.72‐1.16 | |
| Missing / unknown | 42 | 4 | 17 | 24% | 3 | 25 | 12% | 2.09 | 0.47‐9.40 | |
| Sex | ||||||||||
| Male | 3125 | 806 | 1720 | 47% | 750 | 1405 | 53% | 0.96 | 0.87‐1.06 | 0.04 |
| Female | 7316 | 1082 | 3956 | 27% | 917 | 3360 | 27% | 1.10 | 1.01‐1.21 | |
| Age | ||||||||||
| Age continuous | 0.41 | |||||||||
| Age categorical | ||||||||||
| < 18 years | 123 | 0 | 55 | 0% | 1 | 68 | 1% | Not estimable | Not estimable | 0.40 |
| ≥18‐35 years | 312 | 32 | 171 | 19% | 23 | 141 | 16% | 0.95 | 0.55‐1.62 | |
| ≥35‐45 years | 1135 | 150 | 620 | 24% | 120 | 515 | 23% | 0.97 | 0.76‐1.23 | |
| ≥45‐55 years | 2425 | 392 | 1311 | 30% | 323 | 1114 | 29% | 1.15 | 0.99‐1.33 | |
| ≥55‐65 years | 3233 | 594 | 1724 | 34% | 573 | 1509 | 38% | 0.98 | 0.87‐1.10 | |
| ≥65‐75 years | 2444 | 539 | 1359 | 40% | 489 | 1085 | 45% | 1.03 | 0.91‐1.17 | |
| ≥75 years | 758 | 181 | 430 | 42% | 137 | 328 | 42% | 1.17 | 0.94‐1.47 | |
| Missing | 11 | 0 | 6 | 0% | 1 | 5 | 20% | Not estimable | Not estimable | |
| Hct levels at baseline | ||||||||||
| Hct continuous | 0.25 | |||||||||
| Hct categorical | ||||||||||
| ≤ 23.5% | 275 | 51 | 144 | 35% | 42 | 131 | 32% | 1.36 | 0.90‐2.05 | 0.24 |
| 23.5‐≤ 29.4% | 2033 | 340 | 1135 | 30% | 338 | 898 | 38% | 0.93 | 0.80‐1.08 | |
| 29.4‐≤ 35.3% | 3281 | 689 | 1882 | 37% | 531 | 1399 | 38% | 1.05 | 0.94‐1.18 | |
| 35.3‐≤ 41.2% | 1801 | 400 | 931 | 43% | 386 | 870 | 44% | 1.05 | 0.91‐1.20 | |
| > 41.2% | 459 | 84 | 249 | 34% | 66 | 210 | 31% | 1.30 | 0.94‐1.79 | |
| Missing / unknown | 2592 | 324 | 1335 | 24% | 304 | 1257 | 24% | 1.07 | 0.91‐1.25 | |
| Serum Epo at baseline | ||||||||||
| Serum Epo continuous | 1.00 | |||||||||
| Serum Epo categorical | ||||||||||
| < 25 mU/ml | 1032 | 235 | 608 | 39% | 225 | 424 | 53% | 0.91 | 0.76‐1.09 | 0.49 |
| 25‐100 mU/ml | 2083 | 434 | 1162 | 37% | 415 | 921 | 45% | 0.94 | 0.82‐1.08 | |
| 100‐200 mU/ml | 518 | 143 | 314 | 46% | 92 | 204 | 45% | 1.17 | 0.90‐1.52 | |
| 200‐500 mU/ml | 227 | 47 | 134 | 35% | 39 | 93 | 42% | 1.13 | 0.74‐1.73 | |
| > 500 mU/ml | 99 | 14 | 57 | 25% | 15 | 42 | 36% | 0.76 | 0.36‐1.58 | |
| Unknown | 6482 | 1015 | 3401 | 30% | 881 | 3081 | 29% | 1.10 | 1.01‐1.21 | |
| Performance score | ||||||||||
| ECOG categorical | ||||||||||
| ECOG 0 | 3025 | 320 | 1582 | 20% | 309 | 1443 | 21% | 1.06 | 0.90‐1.24 | 0.34 |
| ECOG 1 | 3784 | 820 | 2105 | 39% | 671 | 1679 | 40% | 1.07 | 0.96‐1.18 | |
| ECOG 2 | 1140 | 337 | 623 | 54% | 309 | 517 | 60% | 0.96 | 0.82‐1.12 | |
| ECOG 3 | 105 | 37 | 57 | 65% | 29 | 48 | 60% | 0.94 | 0.57‐1.53 | |
| ECOG 4 | 3 | 2 | 2 | 100% | 0 | 1 | 0% | Not estimable | Not estimable | |
| ECOG missing | 2384 | 372 | 1307 | 28% | 349 | 1077 | 32% | 1.03 | 0.89‐1.19 | |
| ECOG dichotomous | ||||||||||
| ECOG 0, 1, 2 | 7949 | 1477 | 4310 | 34% | 1289 | 3639 | 35% | 1.04 | 0.97‐1.13 | 0.92 |
| ECOG 3, 4 | 108 | 39 | 59 | 66% | 29 | 49 | 59% | 1.02 | 0.63‐1.65 | |
| ECOG missing | 2384 | 372 | 1307 | 28% | 349 | 1077 | 32% | 1.02 | 0.88‐1.19 | |
| Body mass index | ||||||||||
| ≤ 19 kg/m² | 607 | 107 | 292 | 37% | 116 | 315 | 37% | 0.86 | 0.66‐1.12 | 0.52 |
| 19‐≤ 25 kg/m² | 4283 | 796 | 2318 | 34% | 685 | 1965 | 35% | 1.03 | 0.93‐1.14 | |
| 25‐≤ 30 kg/m² | 2698 | 477 | 1468 | 32% | 393 | 1230 | 32% | 1.07 | 0.94‐1.23 | |
| > 30 kg/m² | 1294 | 177 | 686 | 26% | 161 | 608 | 26% | 1.00 | 0.81‐1.24 | |
| Missing | 1559 | 331 | 912 | 36% | 312 | 647 | 48% | 1.11 | 0.95‐1.30 | |
| History of thromboembolic events | ||||||||||
| Yes | 375 | 96 | 207 | 46% | 72 | 168 | 43% | 1.10 | 0.81‐1.50 | 0.68 |
| No | 6292 | 1136 | 3469 | 33% | 972 | 2823 | 34% | 1.03 | 0.94‐1.12 | |
| Missing / not reported | 3774 | 656 | 2000 | 33% | 623 | 1774 | 35% | 1.04 | 0.93‐1.17 | |
| History of cardiovascular events | ||||||||||
| Yes | 2319 | 481 | 1295 | 37% | 385 | 1024 | 38% | 1.06 | 0.92‐1.21 | 0.78 |
| No | 5050 | 802 | 2721 | 29% | 701 | 2329 | 30% | 1.03 | 0.93‐1.14 | |
| Missing / not reported | 3072 | 605 | 1660 | 36% | 581 | 1412 | 41% | 1.03 | 0.92‐1.15 | |
| History of hypertension | ||||||||||
| Yes | 1396 | 318 | 798 | 40% | 255 | 598 | 43% | 1.03 | 0.87‐1.21 | 0.91 |
| No | 5271 | 914 | 2878 | 32% | 789 | 2393 | 33% | 1.04 | 0.94‐1.14 | |
| Missing / not reported | 3774 | 656 | 2000 | 33% | 623 | 1774 | 35% | 1.04 | 0.93‐1.17 | |
| History of diabetes mellitus | ||||||||||
| Yes | 430 | 85 | 219 | 39% | 92 | 211 | 44% | 0.97 | 0.72‐1.31 | 0.62 |
| No | 5149 | 937 | 2786 | 34% | 751 | 2363 | 32% | 1.05 | 0.96‐1.16 | |
| Missing / not reported | 4862 | 866 | 2671 | 32% | 824 | 2191 | 38% | 1.03 | 0.94‐1.14 | |
| Geographical region | ||||||||||
| Northern America | 2083 | 306 | 1088 | 28% | 315 | 995 | 32% | 1.05 | 0.90‐1.23 | 0.93 |
| Northern, Western & Southern Europe | 6082 | 1131 | 3342 | 34% | 929 | 2740 | 34% | 1.05 | 0.96‐1.15 | |
| Eastern Europe | 1413 | 363 | 734 | 49% | 346 | 679 | 51% | 0.99 | 0.85‐1.14 | |
| Australia & New Zealand | 286 | 27 | 184 | 15% | 21 | 102 | 21% | 1.01 | 0.57‐1.80 | |
| Other | 189 | 45 | 106 | 42% | 44 | 83 | 53% | 0.92 | 0.61‐1.40 | |
| Missing / not reported | 388 | 16 | 222 | 7% | 12 | 166 | 7% | 1.54 | 0.71‐3.32 | |
| Tumour stage | ||||||||||
| Metastatic / advanced | 6054 | 1379 | 3325 | 41% | 1221 | 2729 | 45% | 1.03 | 0.96‐1.12 | 0.60 |
| Not metastatic / not advanced | 2902 | 248 | 1491 | 17% | 234 | 1411 | 17% | 1.09 | 0.91‐1.31 | |
| Missing / not reported | 1485 | 261 | 860 | 30% | 212 | 625 | 34% | 0.93 | 0.77‐1.12 | |
| Planned Hb ceiling | ||||||||||
| Planned Hb ceiling (cat. 1) | ||||||||||
| ≤Hb 13.0 g/dL | 1631 | 105 | 841 | 12% | 97 | 790 | 12% | 1.06 | 0.80‐1.40 | 0.57 |
| Hb 13.0 ‐ ≤15.0 g/dL | 8451 | 1664 | 4630 | 36% | 1464 | 3821 | 38% | 1.03 | 0.96‐1.10 | |
| Hb >15.0 g/dL | 280 | 104 | 150 | 69% | 101 | 130 | 78% | 1.20 | 0.91‐1.58 | |
| Other | 79 | 15 | 55 | 27% | 5 | 24 | 21% | 1.45 | 0.53‐4.00 | |
| Planned Hb ceiling (cat. 2) | ||||||||||
| ≤Hb 13.0 g/dL | 1631 | 105 | 841 | 12% | 97 | 790 | 12% | 1.06 | 0.80‐1.40 | 0.77 |
| Hb 13.0 ≤14.0 g/dL | 5930 | 969 | 3200 | 30% | 855 | 2730 | 31% | 1.03 | 0.94‐1.13 | |
| Hb 14.0 ≤15.0 g/dL | 2521 | 695 | 1430 | 49% | 609 | 1091 | 56% | 1.02 | 0.92‐1.14 | |
| >Hb 15.0 g/dL | 280 | 104 | 150 | 69% | 101 | 130 | 78% | 1.20 | 0.91‐1.58 | |
| Other | 79 | 15 | 55 | 27% | 5 | 24 | 21% | 1.45 | 0.53‐4.00 | |
| Study level characteristics | ||||||||||
| Iron supplementation | ||||||||||
| Fixed iron supplementation | 1904 | 248 | 947 | 26% | 233 | 957 | 24% | 1.12 | 0.94‐1.35 | 0.41 |
| Iron supplementation as needed | 8313 | 1540 | 4620 | 33% | 1333 | 3693 | 36% | 1.02 | 0.94‐1.09 | |
| Other | 224 | 100 | 109 | 92% | 101 | 115 | 88% | 1.17 | 0.89‐1.55 | |
| Planned ESA treatment duration | ||||||||||
| Up to 8 weeks | 143 | 5 | 114 | 4% | 3 | 29 | 10% | 0.69 | 0.13‐3.56 | 0.72 |
| 9‐16 weeks | 3823 | 591 | 2075 | 28% | 590 | 1748 | 34% | 0.99 | 0.88‐1.11 | |
| > 17 weeks | 2280 | 566 | 1184 | 48% | 531 | 1096 | 48% | 1.06 | 0.94‐1.19 | |
| Until end of chemo‐ or radiotherapy | 4195 | 726 | 2303 | 32% | 543 | 1892 | 29% | 1.07 | 0.96‐1.20 | |
| Planned weekly ESA dosage | ||||||||||
| < 100 µg Darbepoetin or < 40000 IU Epoetin | 3733 | 794 | 2023 | 39% | 645 | 1710 | 38% | 1.03 | 0.92‐1.14 | 0.37 |
| = 100 µg Darbepoetin or = 40000 IU Epoetin | 2200 | 292 | 1101 | 27% | 264 | 1099 | 24% | 1.19 | 1.00‐1.40 | |
| > 100 µg Darbepoetin or > 40000 IU Epoetin | 1998 | 498 | 987 | 50% | 496 | 1011 | 49% | 0.99 | 0.88‐1.12 | |
| Other | 2510 | 304 | 1565 | 19% | 262 | 945 | 28% | 1.01 | 0.86‐1.20 | |
| Planned frequency of ESA application | ||||||||||
| Three times per week or more frequent | 5016 | 846 | 2853 | 30% | 652 | 2163 | 30% | 1.04 | 0.94‐1.16 | 0.16 |
| Once per week | 3067 | 646 | 1528 | 42% | 614 | 1539 | 40% | 1.10 | 0.99‐1.23 | |
| Every second week or less frequent | 1540 | 78 | 886 | 9% | 63 | 654 | 10% | 1.19 | 0.85‐1.67 | |
| Other | 818 | 318 | 409 | 78% | 338 | 409 | 83% | 0.90 | 0.77‐1.05 | |
| Placebo controlled trial | ||||||||||
| Yes | 5473 | 1118 | 2996 | 37% | 1010 | 2477 | 41% | 1.03 | 0.95‐1.12 | 0.77 |
| No | 4968 | 770 | 2680 | 29% | 657 | 2288 | 29% | 1.05 | 0.95‐1.17 | |
| Randomisation | ||||||||||
| Adequate | 3258 | 649 | 1693 | 38% | 553 | 1565 | 35% | 1.04 | 0.93‐1.17 | 0.90 |
| Unclear | 7183 | 1239 | 3983 | 31% | 1114 | 3200 | 35% | 1.04 | 0.96‐1.12 | |
| Concealment of allocation | ||||||||||
| Adequate | 8252 | 1679 | 4501 | 37% | 1476 | 3751 | 39% | 1.02 | 0.95‐1.10 | 0.26 |
| Unclear | 2189 | 209 | 1175 | 18% | 191 | 1014 | 19% | 1.16 | 0.95‐1.41 | |
| Endpoint survival | ||||||||||
| Primary endpoint | 2731 | 586 | 1352 | 43% | 556 | 1379 | 40% | 1.08 | 0.96‐1.22 | 0.58 |
| Secondary endpoint | 3222 | 886 | 1730 | 51% | 738 | 1492 | 49% | 1.00 | 0.91‐1.11 | |
| Safety /adverse events | 4488 | 416 | 2594 | 16% | 373 | 1894 | 20% | 1.05 | 0.91‐1.21 | |
| Designed for long‐term follow‐up | ||||||||||
| Yes | 6509 | 1539 | 3355 | 46% | 1350 | 3154 | 43% | 1.05 | 0.98‐1.13 | 0.47 |
| No | 3932 | 349 | 2321 | 15% | 317 | 1611 | 20% | 0.99 | 0.84‐1.15 | |
| Year of last patient randomized | ||||||||||
| 1990‐1994 | 1057 | 70 | 650 | 11% | 51 | 407 | 13% | 0.88 | 0.61‐1.27 | 0.18 |
| 1995‐1999 | 1725 | 312 | 1001 | 31% | 224 | 724 | 31% | 0.97 | 0.81‐1.16 | |
| 2000‐2004 | 6112 | 1135 | 3263 | 35% | 1012 | 2849 | 36% | 1.10 | 1.01‐1.20 | |
| 2005‐2006 | 1547 | 371 | 762 | 49% | 380 | 785 | 48% | 0.94 | 0.82‐1.09 | |
| Source of data | ||||||||||
| Manufacturer | 8851 | 1701 | 4889 | 35% | 1485 | 3962 | 37% | 1.05 | 0.97‐1.12 | 0.54 |
| Clinical study group | 1590 | 187 | 787 | 24% | 182 | 803 | 23% | 0.98 | 0.80‐1.20 | |
| *P value for likelihood‐ratio test (test for interaction), patients with missing data are excluded from this test, analysis based on one‐stage Cox fixed‐effects model stratified by study ESA= erythropoiesis‐stimulating agents | ||||||||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aapro 2008.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 463, breast cancer (M1); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin beta dose = 30000 IU sc weekly hb‐target = 13‐15 d/dL planned ESA duration = 24 weeks |
|
| Outcomes | Primary: overall survival; secondary: progression free survival, tumor response rate, QoL | |
| Notes | study number = 97413 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Abels 1993.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 124, hematological malignancies, genitourinary, gastrointestinal, other cancer; no anticancer therapy | |
| Interventions | drug = Epoetin alpha dose = 100 IU/kg sc TIW hb‐target = not reported planned ESA duration = 8 weeks |
|
| Outcomes | Primary: transfusion, Hct; secondary: QoL, safety | |
| Notes | study number = 98906 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated |
| Allocation concealment? | Unclear risk | each patient was assigned a random identification number and was assigned to a treatment group by a computerized randomization schedule |
Boogaerts 2003.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 259, multiple myeloma, Non‐Hodgkin lymphoma, chronic lymphocytic leukemia, Hodgkin disease, ovarian, bone, gastrointestinal, respiratory, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin beta dose = 150 IU/kg sc TIW hb‐target = 12‐14 g/dL planned ESA duration = 12 weeks |
|
| Outcomes | Primary: QoL; secondary: direct and indirect costs | |
| Notes | study number = 36158 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Case 1993.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 157, hematological malignancies, breast, lung, gynecological, gastrointestinal, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = Hct 38%‐40% planned ESA duration = 12 weeks |
|
| Outcomes | Transfusion, Hct, QoL, safety | |
| Notes | study number = 34917 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated |
| Allocation concealment? | Unclear risk | description is unclear |
Cazzola 1995.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 143, multiple myeloma, Non‐Hodgkin lymphoma; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin beta dose = a: 1000 IU sc 7x/week, b: 2000 IU sc 7x/week; c: 5000 IU sc 7x/ week; d: 10000 IU sc 7x/week hb‐target = 11‐13 g/dL (MM), 11‐15 g/dL (NHL) planned ESA duration = 8 weeks |
|
| Outcomes | Primary: Hb response; secondary: Hb, Hct, reticulocytes, iron, ferritin, safety | |
| Notes | study number = 37653 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | randomization list |
| Allocation concealment? | Low risk | central randomization |
CC2574‐P‐174.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 45, chronic lymphocytic leukemia (any stage); concomitant therapy: other | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = Hct 38%‐40% planned ESA duration = 12 weeks |
|
| Outcomes | Primary: Hct; secondary: Hb, transfusion, QoL, safety | |
| Notes | study number = 60584 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Unclear risk | no description |
Chang 2005.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 354, breast cancer (stage I‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 12‐14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: QoL; secondary: maintain Hb above 12 g/dL, tumor response, overall survival | |
| Notes | study number = 99137 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Charu 2007.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 287, lymphoma, breast, lung, gastrointestinal, genitourinary, gynecologic, other cancer; no anticancer therapy | |
| Interventions | drug = Darbepoetin alpha dose = 3.0 µg/kg sc Q2W hb‐target = 13‐14 g/dL (women), 13‐15 g/dL (men) planned ESA duration = 12 weeks |
|
| Outcomes | Primary: hospitalization days; secondary: costs, QoL, transfusion, Hb, safety | |
| Notes | study number = 53081 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Dammacco 2001.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 145, multiple myeloma; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = 12‐14 g/dL planned ESA duration = 12 weeks |
|
| Outcomes | Primary: transfusion; secondary: Hb, Hct, reticulocytes, serum erythropoietin levels, QoL | |
| Notes | study number = 11220 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | randomization schedule prepared by RWJPRI |
| Allocation concealment? | Unclear risk | two randomization lists (patients previously transfused or not), when patient enters the study the next number was to be assigned |
Debus 2006.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 385, non‐small cell lung cancer (stage III, primarily inoperable); concomitant treatment: radiochemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 12‐14 g/dL, in 11/2003 reduced to 12‐13 g/dL planned ESA duration = during chemotherapy and radiotherapy |
|
| Outcomes | Primary: 2‐year‐survival rate; secondary: tumor response, QoL, tolerance to epoetin alpha, Hb change, transfusion, safety | |
| Notes | study number = 83322 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | randomization code provided by OrthoBiothech |
| Allocation concealment? | Unclear risk | assigned envelopes, sequentially numbered, but it is unclear whether they were sealed and opaque |
EPO‐GBR‐7.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 300, head and neck cancer (stage I‐IV); concomitant treatment: radiotherapy | |
| Interventions | drug = Epoetin alpha dose = if Hb < 12.5 10000 IU sc TIW; if Hb > 12.5 4000 IU sc TIW hb‐target = 12.5‐15 g/dL planned ESA duration = during radiotherapy |
|
| Outcomes | Primary: local disease free survival; secondary: overall survival, QoL, safety | |
| Notes | study number = 81645 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | central randomization schedule stratified by the study site was generated by the sponsor |
| Allocation concealment? | Unclear risk | no description |
EPO‐GER‐20.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 93, small cell lung cancer (extensive stage); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 10000 IU sc TIW hb‐target = 12‐14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: rate of patients with anemia; secondary: QoL, tolerability of ESA, transfusion, effectiveness of chemotherapy | |
| Notes | study number = 31678 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Patients were assigned with a randomization code provided by Janssen‐Cilag |
| Allocation concealment? | Unclear risk | assigned envelopes, sequentially numbered, but it is unclear whether they were sealed and opaque |
EPO‐INT‐1.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 246, ovarian cancer (stage I‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = a: 150 IU/kg sc TIW; b: 300 IU/kg sc TIW hb‐target = 12.5 to 14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: transfusion; secondary: Hb change, Hct, QoL | |
| Notes | study number = 53915 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | no description |
EPO‐INT‐3.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 200, breast, Non‐Hodgkin lymphoma, multiple myeloma, ovarian, small cell lung cancer, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = 12‐14 g/dL (women), 14‐16 g/dL (men) planned ESA duration = 12 weeks |
|
| Outcomes | Primary: Transfusion; secondary: Hb, QoL | |
| Notes | study number = 36274 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | according to randomization schedule prepared by RWJPRI |
| Allocation concealment? | Low risk | central randomization |
Gordon 2006.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 220, breast, non‐myeloid hematological malignancies, gastrointestinal, genitourinary, lung, gynecological, other cancer (stage I‐IV); no anticancer therapy | |
| Interventions | drug = Darbepoetin alpha dose = 6.75 µg/kg sc Q4W hb‐target = 12‐13 g/dL planned ESA duration = 16 weeks |
|
| Outcomes | Primary: Hb response; secondary: transfusion, Hb change, QoL, safety | |
| Notes | study number = 65772 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | randomization list will be centrally generated by Amgen |
| Allocation concealment? | Low risk | central randomization |
Goss 2005.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 104, small cell lung cancer (limited disease); concomitant treatment: radiochemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 14‐16 g/dL, in 10/2002 reduced to 13‐14 g/dL planned ESA duration = during chemotherapy and radiotherapy |
|
| Outcomes | Disease progression free survival, tumor response, overall survival, local disease progression, Hb, transfusion, QoL | |
| Notes | study number = 55703 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Low risk | central randomization |
Grote 2005.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 224, small cell lung cancer (limited and extensive disease); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = 14‐16 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: assess possible stimulatory effects of ESA on solid tumor growth, tumor response; secondary: overall survival, Hb, transfusion, safety | |
| Notes | study number = 73807 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated |
| Allocation concealment? | Unclear risk | description is unclear |
Hedenus 2003.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 349, Hodgkin disease, Non‐Hodgkin lymphoma, multiple myeloma, chronic lymphocytic leukemia, Waldenstrom´s disease; concomitant treatment: chemotherapy | |
| Interventions | drug = Darbepoetin alpha dose = 2.25 µg/kg sc weekly hb‐target = 13‐14 g/dL (women), 13‐15 g/dL (men) planned ESA duration = 12 weeks |
|
| Outcomes | Primary: Hb response; secondary: transfusion, Hb change, QoL, safety | |
| Notes | study number = 63455 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | based on a schedule specified by Amgen before the start of the study |
| Allocation concealment? | Low risk | central randomization |
Henke 2003.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 351, head and neck cancer (advanced, stage III, IV); concomitant treatment: radiotherapy | |
| Interventions | drug = Epoetin beta dose = 300 IU/kg sc TIW hb‐target = 12‐14 g/dL (women), 13‐15 g/dL (men) planned ESA duration = during radiotherapy |
|
| Outcomes | Primary: efficacy of radiotherapy, measured as local progression free survival; secondary: survival, progression free survival, Hb, safety, tolerability | |
| Notes | study number = 58106 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Each center had numbered packages per stratum, once randomized the lowest number had to be assigned. There was a randomization list only the statistics center had access to. In addition, there were sealed envelopes for emergencies. |
| Allocation concealment? | Low risk | coded drug packs of identical appearance |
Henry 1995.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 132, lung, gynecological, gastrointestinal, hematological malignancies, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = Hct 38%‐40% planned ESA duration = 12 weeks |
|
| Outcomes | Primary: Hct, transfusion; secondary: correction of anemia, response, QoL, safety | |
| Notes | study number = 70332 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Unclear risk | Medication boxes were used, but without identical appearance |
Huddart 2002.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 95, lung, gynecological, genitourinary, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 10000 IU sc TIW hb‐target = 12‐14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Hb response, reticulocyte, survival, QoL, safety | |
| Notes | study number = 88443 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | no description |
Kotasek 2002.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 161, lung, breast, gastrointestinal, genitourinary, gynecological, other cancer (stage I‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Darbepoetin alpha dose = a: 9 µg/kg sc Q4W, b: 12 µg/kg sc Q4W, c: 15 µg/kg sc Q4W, d: 18 µg/kg sc Q4W hb‐target = 13‐14 g/dL (women), 13‐15 g/dL (men) planned ESA duration = 12 weeks |
|
| Outcomes | Primary: safety; secondary: determine effective dose, effect of ESA, QoL feasibility | |
| Notes | study number = 26117 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Kotasek 2003.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 259, breast, gynecological, gastrointestinal, lung, genitourinary, other cancer (stage I‐IV, most patients advanced); concomitant treatment: chemotherapy | |
| Interventions | drug = Darbepoetin alpha dose = a: 4.5 µg/kg sc Q3W, b: 6.75 µg/kg sc Q3W, c: 9 µg/kg sc Q3W, d: 12 µg/kg sc Q3W, e: 13.5 µg/kg sc Q3W, f: 15 µg/kg sc Q3W hb‐target = 13‐14 g/dL (women), 13‐15 g/dL (men) planned ESA duration = 12 weeks |
|
| Outcomes | Primary: safety; secondary: determine effective dose, effect of ESA, QoL feasibility | |
| Notes | study number = 35466 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Leyland‐Jones 2003.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 939, breast cancer (stage IV, M1); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 12‐14 g/dL planned ESA duration = 52 weeks |
|
| Outcomes | Primary: overall survival; secondary: Hb, transfusion, tumor control, QoL, time to progression | |
| Notes | study number = 17100 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated |
| Allocation concealment? | Low risk | central randomization |
Littlewood 2001.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 375, breast, Non‐Hodgkin lymphoma, multiple myeloma, Hodgkin disease, chronic lymphocytic leukemia, gastrointestinal, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = 12‐15 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: transfusion; secondary: Hb, Hct, reticulocytes, predictors for response, QoL, after protocol amendment also survival | |
| Notes | study number = 17123 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated randomization schedule prepared by RWJPRI |
| Allocation concealment? | Low risk | coded drug packs of identical appearance |
Machtay 2007.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 148, head and neck cancer (stage I‐IV); concomitant treatment: radiotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 12.5‐14 g/dL (women), 13.5‐16 g/dL (men) planned ESA duration = 8 weeks |
|
| Outcomes | Primary: local regional control tumor response; secondary: overall survival, patterns of failure, local‐regional progression‐free survival, Hb, toxicity, QoL | |
| Notes | study number = 87660 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Milroy 2003.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 424, non‐small cell lung cancer (stage IIIb or IV, advanced); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = if body weight > 45 kg 10000 IU sc TIW, if body weight < 45 kg 5000 IU sc TIW hb‐target = 12.5‐14 g/dL (women), 13.5‐15 g/dL (men) planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: QoL; secondary: Hb, tumor response, survival, transfusion | |
| Notes | study number = 67954 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Moebus 2007.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 643, breast cancer (high risk, stage II/IIIA; M0); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = 13‐14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: transfusion, Hb; secondary: recurrence free survival, overall survival, relapse, QoL | |
| Notes | study number = 22515 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Low risk | central randomization |
O'Shaugnessy 2005.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 100, breast cancer (stage I, II, IIIB); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 13‐15 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: cognitive function, fatigue; secondary: QoL | |
| Notes | study number = 40730 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Low risk | coded drug packs of identical appearance |
OBE/EPO‐INT‐03.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 72, multiple myeloma; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 12‐13 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: Hb change; secondary: QoL, Hb response, transfusion, safety | |
| Notes | study number = 92503 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Oberhoff 1998.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 227, ovarian, breast, lung, genitourinary, gastrointestinal, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin beta dose = 5000 IU sc 7x per week hb‐target = 11‐14 g/dL planned ESA duration = 12 weeks |
|
| Outcomes | Primary: transfusion ; secondary: Hb response, safety | |
| Notes | study number = 45434 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Osterborg 1996.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 148, multiple myeloma, Non‐Hodgkin lymphoma, chronic lymphocytic lymphoma; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin beta dose = a: 10000 IU sc 7x/week, b: titration hb‐target = 10‐14 g/dL (women), 10‐13 g/dL (men) planned ESA duration = 24 weeks |
|
| Outcomes | Primary: transfusion; secondary: safety, Hb | |
| Notes | study number = 43680 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Osterborg 2002.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 349, multiple myeloma, Non‐Hodgkin lymphoma, chronic lymphocytic lymphoma; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin beta dose = 150 IU/kg sc TIW hb‐target = 13‐14 g/dL planned ESA duration = 16 weeks |
|
| Outcomes | Primary: transfusion free survival; secondary: Hb response, time to response, number of blood transfusions, QoL, safety | |
| Notes | study number = 77914 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | randomization program |
| Allocation concealment? | Low risk | central randomization |
Pirker 2008.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 600, small cell lung cancer (untreated, extensive stage); concomitant treatment: chemotherapy | |
| Interventions | drug = Darbepoetin alpha dose = 300 µg sc weekly for weeks 1‐4 then 300 µg Q3W starting week 5 onwards hb‐target = 13‐14 g/dL planned ESA duration = 19 weeks |
|
| Outcomes | Primary: Hb change, survival; secondary: QoL, progression‐free‐survival, tumor response, time to progression, transfusion | |
| Notes | study number = 89335 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | central randomization |
Pronzato 2002.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 223, breast cancer (stage I‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = if body weight > 45 kg 10000 IU sc TIW, if < 45 kg 5000 IU sc TIW hb‐target = 12‐14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: QoL; secondary: Hb change, tumor response | |
| Notes | study number = 22233 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Quirt 1996.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 56, lung, gynecological, hematological malignancies, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = 12.5‐14 g/dL planned ESA duration = 16 weeks |
|
| Outcomes | Primary: transfusion; secondary: QoL, costs from societal perspective, tumor response | |
| Notes | study number = 80214 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | no description |
Ray‐Coquard 2006.
| Methods | randomized controlled trial, | |
| Participants | n = 218, breast, sarcoma, lung, ovarian, other solid cancer and hematological malignancies; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = if body weight < 45 kg 10000 IU sc 2x/week, if body weight 45 kg to < 89 kg 10000 IU sc TIW, if body weight > 89 kg 10000 IU sc 4x/week hb‐target = 12‐14 g/dL planned ESA duration = 12 weeks |
|
| Outcomes | Primary: transfusion dependent anemia; secondary: QoL, Hb response predictors, Hb, toxicity, survival, costs | |
| Notes | study number = 37491 | |
Razzouk 2006.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 126, solid tumors, Hodgkin disease, Non‐Hodgkin lymphoma (patients excluded from the present meta‐analysis), acute lymphocytic leukemia (patients excluded from the present meta‐analysis); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 600 IU/kg iv weekly hb‐target = 13‐15 g/dL (age > 12 years), 13‐14 g/dL (age <12 years) planned ESA duration = 16 weeks |
|
| Outcomes | Primary: QoL; secondary: Hb, transfusion | |
| Notes | study number = 80515 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Low risk | central randomization and coded drug packs of identical appearance |
Rose 1994.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 221, chronic lymphocytic leukemia (stage III, IV); concomitant therapy: other | |
| Interventions | drug = Epoetin alpha dose = 150 IU/kg sc TIW hb‐target = Hct 38%‐40% planned ESA duration = 12 weeks |
|
| Outcomes | Primary: Hct; secondary: transfusion, QoL, safety | |
| Notes | study number = 98358 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer‐generated |
| Allocation concealment? | Unclear risk | no description |
Savonije 2005.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 315, non‐small cell lung cancer, gastrointestinal, gynecological, colorectal, small cell lung cancer, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 10000 IU sc TIW hb‐target = 13‐14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: transfusion; secondary: Hb, tumor response, QoL, survival | |
| Notes | study number = 70724 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | randomization center generates a list of subject numbers and randomly allocate numbers to the two treatment groups using a block size of six |
| Allocation concealment? | Low risk | central randomization |
Smith 2008.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 989, lung, hematological malignancies, breast, gastrointestinal, genitourinary, other cancer (stage III‐IV); no anticancer therapy | |
| Interventions | drug = Darbepoetin alpha dose = 6.75 µg/kg sc Q4W hb‐target = 12‐13 g/dL planned ESA duration = 16 weeks |
|
| Outcomes | Primary: transfusion; secondary: Hb, QoL, safety | |
| Notes | study number = 81215 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | based on a schedule specified by Amgen prior to the start of the study |
| Allocation concealment? | Low risk | central randomization |
Strauss 2008.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 74, cervical cancer (stage IIB‐IVA); concomitant treatment: radiochemotherapy | |
| Interventions | drug = Epoetin beta dose = 150 IU/kg sc TIW hb‐target = 14‐15 g/dL planned ESA duration = 12 weeks |
|
| Outcomes | Primary: tumor control failures; secondary: progression‐free survival, overall response rate, relapses/metastases, overall survival, Hb change, QoL, safety | |
| Notes | study number = 70404 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | patient randomization number will be generated by Roche |
| Allocation concealment? | Unclear risk | patient randomization numbers are to be allocated sequentially in the order in which the patients are enrolled |
Taylor 2005.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 391, non‐myeloid hematological malignancies, breast, lung, gastrointestinal, genitourinary, gynecological, other cancer (stage I‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Darbepoetin alpha dose = 300 µg sc Q3W hb‐target = 12‐13 g/dL planned ESA duration = 15 weeks |
|
| Outcomes | Primary: transfusion; secondary: Hb target achieved, number of transfusions, safety, QoL | |
| Notes | study number = 37476 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Ten Bokkel Huinink 1998.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 120, ovarian cancer (stage II‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin beta dose = a: 150 IU/kg sc TIW, b: 300 IU/kg sc TIW hb‐target = 14‐15 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: transfusion; secondary: Hb, reticulocytes, Hct, safety | |
| Notes | study number = 47852 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Thatcher 1999.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 130, small cell lung cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = a: 150 IU/kg sc TIW, b: 300 IU/kg sc TIW hb‐target = 13‐15 g/dL planned ESA duration = 26 weeks |
|
| Outcomes | Efficacy, safety, QoL | |
| Notes | study number = 65529 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | upon study entry each patient was assigned a sequential identification number which had been randomly assigned to chemotherapy with or without ESA, blocks of 6, each investigator had to treat at least 6 patients, but preferably 12 patients |
| Allocation concealment? | Unclear risk | see randomization |
Thomas 2002.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 130, breast, gastrointestinal, gynecological, other cancer; concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = if body weight > 45 kg 10000 IU sc TIW, if body weight < 45 kg 5000 IU sc TIW hb‐target = 12‐14 g/dL planned ESA duration = 12 weeks |
|
| Outcomes | Primary: Hb response; secondary: QoL, tumor response, survival, safety | |
| Notes | study number = 84090 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Thomas 2008.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 114, cervical cancer (stage IIB ‐ IV A, M0); concomitant treatment: radiochemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 13‐14 g/dL planned ESA duration = during chemotherapy and radiotherapy |
|
| Outcomes | Primary: progression‐free survival; secondary: overall survival, local control, distant recurrences, thromboembolic events | |
| Notes | study number = 21481 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Low risk | central randomization |
Untch 2008.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 729, breast cancer (M0); concomitant treatment: chemotherapy | |
| Interventions | drug = Darbepoetin alpha dose = 4.5 µg/kg sc Q2W hb‐target = 13 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: relapse free survival time, overall survival; secondary: tumor control, safety and tolerability, transfusion, Hb level, QoL | |
| Notes | study number = 66960 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | no description |
| Allocation concealment? | Unclear risk | description is unclear |
Vadhan‐Raj 2004.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 60, gastric or rectal cancer (stage I‐III); concomitant treatment: radiochemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 14‐15 g/dL planned ESA duration = 16 weeks |
|
| Outcomes | Primary: transfusions; secondary: maintain Hb levels, QoL, tumor response, safety | |
| Notes | study number = 30540 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Low risk | coded drug packs of identical appearance |
Vansteenkiste 2002.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 320, small cell lung cancer (limited and extensive), and non‐small lung cancer (stage I‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Darbepoetin alpha dose = 2.25 mg/kg sc weekly hb‐target = 13‐14 g/dL (women), 13‐15 g/dL (men) planned ESA duration = 12 weeks |
|
| Outcomes | Primary: transfusion; secondary: Hb response, Hb, transfusion timing and quantity, QoL | |
| Notes | study number = 49684 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | based on a schedule specified by Amgen before the start of the study |
| Allocation concealment? | Low risk | central randomization |
Wilkinson 2006.
| Methods | randomized controlled trial, not placebo‐controlled | |
| Participants | n = 182, ovarian cancer (stage I‐IV); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = if body weight > 45 kg 10000 IU sc TIW, if < 45 kg 5000 IU sc TIW hb‐target = 12‐14 g/dL planned ESA duration = during chemotherapy |
|
| Outcomes | Primary: Hb response; secondary: QoL, transfusion, tumor response | |
| Notes | study number = 75688 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | a prospective randomization procedure will be employed |
| Allocation concealment? | Unclear risk | assigned envelopes, sealed, but it is unclear whether they were opaque and sequentially numbered |
Witzig 2005.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 344, lung, breast, other cancer (active incurable advanced stage); concomitant treatment: chemotherapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 13‐15 g/dL planned ESA duration = 16 weeks |
|
| Outcomes | Primary: transfusion; secondary: Hb change, haemoglobin over time, predictors for response, incidence of nephrotoxicity, overall survival, tumor response, QoL | |
| Notes | study number = 36512 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Low risk | central randomization and coded drug packs of identical appearance |
Wright 2007.
| Methods | randomized controlled trial, placebo‐controlled | |
| Participants | n = 70, non‐small lung cancer (advanced stage IIIA, B and IV, recurrent disease); no anticancer therapy | |
| Interventions | drug = Epoetin alpha dose = 40000 IU sc weekly hb‐target = 12‐14 g/dL planned ESA duration = 12 weeks |
|
| Outcomes | Primary: QoL; secondary: Hb, Hct, transfusion, safety | |
| Notes | study number = 53572 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | computer generated |
| Allocation concealment? | Low risk | central randomization |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelrazik 2007 | ineligible patient characteristics (e.g. with MDS or SAA) |
| Alexopoulos 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Antonadou 2001 | no access to the individual patient data |
| Aravantinos 2003 | too small for inclusion |
| Auerbach 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Aziz 2001 | too small for inclusion |
| Bamias 2003 | no access to the individual patient data |
| Beggs 2003 | too small for inclusion |
| Bessho 1997 | ineligible patient characteristics (e.g. with MDS or SAA) |
| Bindi 2004 | too small for inclusion |
| Blayney 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Blohmer 2003 | no access to the individual patient data |
| Candelaria 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Canon 2006 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Carabantes 1999 | too small for inclusion |
| Casadevall 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Cascinu 1994 | no access to the individual patient data |
| Cazzola 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Chan 1995 | too small for inclusion |
| Charu 2007a | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Christodoulakis 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Crawford 1997 | too small for inclusion |
| Crawford 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Crawford 2007 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Daneryd 1998 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Dannemann 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Del Mastro 1997 | too small for inclusion |
| Dunphy 1999 | too small for inclusion |
| Elsaid 2001 | too small for inclusion |
| Freeman 2006 | too small for inclusion |
| Garton 1995 | too small for inclusion |
| Gebbia 1992 | too small for inclusion |
| Glaspy 2002 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Glaspy 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Glaspy 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Glaspy 2006 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Glimelius 1998 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Glossmann 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Granetto 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Hedenus 2002 | too small for inclusion |
| Hedenus 2007 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Hellström Lindberg 1998 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Henke 1999 | too small for inclusion |
| Henry 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Henry 2006 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Henry 2007 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Henze 2002 | ineligible patient characteristics (e.g. with MDS or SAA) |
| Hesketh 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Iconomou 2003 | no access to the individual patient data |
| Italian 1998 | ineligible patient characteristics (e.g. with MDS or SAA) |
| Janinis 2003 | no access to the individual patient data |
| Jitnuyanont 2001 | too small for inclusion |
| Johansson 2001 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Justice 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Kettelhack 1998 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Kosmadakis 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Kotasek 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Kotasek 2007 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Kunikane 2001 | too small for inclusion |
| Kurz 1997 | too small for inclusion |
| Mangiameli 2002 | too small for inclusion |
| Marinaccio 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Merlano 2001 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| MF4266 | ineligible patient characteristics (e.g. with MDS or SAA) |
| Miller 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Morishima 2006 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Mystakidou 2005 | no access to the individual patient data |
| Olsson 2002 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Overgaard 2007 | no access to the individual patient data |
| Pierelli 1999 | too small for inclusion |
| Policarpo 2007 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Porter 1996 | too small for inclusion |
| Rau 1998 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Rearden 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Reed 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Rosen 2003 | too small for inclusion |
| Rosenzweig 2004 | too small for inclusion |
| Rubio‐Martinez 2003 | too small for inclusion |
| Sakai 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Schwartzberg 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Schwartzberg 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Schwartzberg 2007 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Scott 2002 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Senecal 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Shi 2007 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Silvestris 1995 | too small for inclusion |
| Smith 2003 | too small for inclusion |
| Spicka 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Steensma 2006 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Stein 1991 | ineligible patient characteristics (e.g. with MDS or SAA) |
| Straus 2006 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Sweeney 1998 | too small for inclusion |
| Thompson 2000 | ineligible patient characteristics (e.g. with MDS or SAA) |
| Throuvalas 2000 | too small for inclusion |
| Tsukuda 1998 | too small for inclusion |
| Varan 1999 | too small for inclusion |
| Wagner 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Waltzman 2005 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Watanabe 2006 | no access to the individual patient data |
| Welch 1995 | too small for inclusion |
| Wurnig 1996 | too small for inclusion |
| Yilmaz 2004 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Zagari 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Zajda 2007 | no access to the individual patient data |
| Zhang 2003 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
| Zhou 2006 | ESAs were given in context with surgery, stem cell transplantation, compared different ESA dosages or ESA products (epoetin versus darbepoetin), or trials were not randomised |
Differences between protocol and review
Main difference is that the protocol covered several endpoints, i.e. survival, tumor progression, transfusion, QoL, thromboembolic events and other. In the current review only the endpoint survival is covered and the other endpoints will follow at later stages of the project. For literature based meta‐analysis see Bohlius 2004, Bohlius 2005, Bohlius 2006.
Contributions of authors
JULIA BOHLIUS: protocol development, protocol revision, literature searches and study selection, data extraction, data management, statistical analyses, first draft of review, revision of review, project management
KURT SCHMIDLIN: data management, statistical analyses, revision of review
CORINNE BRILLANT: protocol revision, data management, revision of review
GUIDO SCHWARZER: protocol development, protocol revision, statistical analyses, revision of review
SVEN TRELLE: protocol development, protocol revision, statistical analyses, revision of review
JEROME SEIDENFELD: protocol revision, extensive revision of review
MARCEL ZWAHLEN: protocol revision, statistical analyses
MICHAEL CLARKE: protocol revision, revision of review
OLAF WEINGART: protocol revision, literature searches and study selection, revision of review
SABINE KLUGE: data extraction, revision of review
MARYANN NAPOLI: revision of protocol and review, drafting plain language summary
MARGRET PIPER: protocol revision, extensive revision of review
DIRK RADES: protocol revision, revision of review
DAVID P. STEENSMA: protocol revision, revision of review
BENJAMIN DJULBEGOVIC: protocol revision
MARTIN F FEY: revision of review
ISABELLE RAY‐COQUARD: contributed the data from clinical trial, revision of review
VOLKER MOEBUS: contributed the data from clinical trial, revision of review
GILLIAN THOMAS: contributed the data from clinical trial, revision of review
MICHAEL UNTCH: contributed the data from clinical trial, revision of review
MARTIN SCHUMACHER: protocol revision, revision of review, support for statistical analyses
MATTHIAS EGGER: protocol development, protocol revision, revision of review, supervision of study ANDREAS ENGERT: protocol revision, revision of review, supervision of study
Sources of support
Internal sources
-
Köln Fortune, Germany.
Funding program “Köln Fortune”, Medical Faculty University of Cologne
External sources
-
BMBF, Germany.
The Editorial Base is funded by Federal Ministry of Education and Research (BMBF) No : 01GH0501
-
BMBF, Germany.
Project grant application NO 01KG0611, Federal Ministry of Education and Research (BMBF)
-
OncoSuisse, Switzerland.
Grant number KLS ‐021 46‐10‐2007
Declarations of interest
Julia Bohlius received honoraria and travel grants from Amgen. Andreas Engert received research funding and honoraria from Amgen, Roche and Johnson & Johnson. Gillian Thomas received research funding for the GOG‐191 study by Johnson & Johnson. Benjamin Djulbegovic received research funding from OrthoBiotech and consulted for Amgen. Volker Moebus received research funding and honoraria from Amgen, Bristol‐Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Novartis, Pfizer, and Roche. Michael Untch received research funding for the PREPARE study from Amgen and Bristol‐Myers Squibb. Margaret Piper ist employed by the Blue Cross and Blue Shield Association, the trade organization for the independent US Blue Cross Blue Shield health insurance plans, but is not involved in the determination of coverage and reimbursement policy for individual plans. All other members of the Steering Committee, the Statistical Analysis Team and Reviewers declared to have no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Aapro 2008 {published data only}
- Aapro M, Barnadas A, Leonard RC, Marangolo M, Untch M. Effects of epoetin beta treatment in patients with metastatic breast cancer receiving chemotherapy. Results of the BRAVE trial. Breast Cancer Research and Treatment 2006; Vol. 100:abstract 6095.
- Aapro M, Leonard RC, Barnadas A, Marangolo M, Untch M, Malamos N, et al. Effect of once‐weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline‐ and/or taxane‐based chemotherapy: Results of the Breast Cancer‐Anemia and the Value of Erythropoietin (BRAVE) Study. Journal of Clinical Oncology 2008;26(4):592‐8. [DOI] [PubMed] [Google Scholar]
Abels 1993 {published data only}
- Abels R. Erythropoietin for anemia in cancer patients. European Journal of Cancer 1993;29a(Suppl 2):2‐8. [DOI] [PubMed] [Google Scholar]
- Abels R. Recombinant Human Erythropoietin in the Treatment of the Anaemia of Cancer. Acta Haematologica 1992;87(Suppl 1):4‐11. [DOI] [PubMed] [Google Scholar]
- Abels RI, Larholt KM, Krantz KD, Bryant EC. Recombinant Human Erythropoietin (rHuEPO) for the Treatment of the Anemia of Cancer. Oncologist 1996;1(3):140‐50. [PubMed] [Google Scholar]
Boogaerts 2003 {published data only}
- Boogaerts M, Coiffier B, Kainz C, and the Epoetin beta QOL Working Group. Impact of epoetin beta on quality of life in patients with malignant disease. British Journal of Cancer 2003;88(7):988‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiffier B. Epoetin beta (Neorecormon©) improves quality of life in cancer‐associated anaemia to a similar degree in patients with lymphoid malignancies or solid tumours. Proceedings of the 8th Congress of the European Haematology Association 2003:abstract 0153.
- Coiffier B, Boogaerts M, Kainz C. Impact of epoetin beta versus standard care on quality of life in patients with malignant disease. Proceedings of the 6th Congress of the European Haematology Association 2001;2(Suppl 1):abstract 194. [Google Scholar]
Case 1993 {published data only}
- Abels RI, Larholt KM, Krantz KD, Bryant EC. Recombinant Human Erythropoietin (rHuEPO) for the Treatment of the Anemia of Cancer. Oncologist 1996;1(3):140‐50. [PubMed] [Google Scholar]
- Case DC, Bukowski RM, Carey RW, Fishkin EH, Henry DH, Jacobson RJ, et al. Recombinant human erythropoietin therapy for anemic cancer patients on combination chemotherapy. Journal of the National Cancer Institute 1993;85(10):801‐6. [DOI] [PubMed] [Google Scholar]
Cazzola 1995 {published data only}
- Cazzola M, Messinger D, Battistel V, Bron D, Cimino R, Enller Ziegler L, et al. Recombinant human erythropoietin in the anemia associated with multiple myeloma or non‐Hodgkin's lymphoma: dose finding and identification of predictors of response. Blood 1995;86(12):4446‐53. [PubMed] [Google Scholar]
CC2574‐P‐174 {published data only}
- Luksenburg H, Weir A, Wager R. P‐174 in: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen, Inc. and Procrit (epoetin alfa) Ortho Biotech, L.P., for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
- Pangalis GA, Poziopoulos C, Angelopoulou MK, Siakantaris MP, Panayiotidis P. Effective treatment of disease‐related anaemia in B‐chronic lymphocytic leukaemia patients with recombinant human erythropoietin. British Journal of Haematology 1995;89(3):627‐9. [DOI] [PubMed] [Google Scholar]
Chang 2005 {published data only}
- Chang J, Couture F, Young S, McWatters KL, Lau CY. Weekly epoetin alfa maintains hemoglobin, improves quality of life, and reduces transfusion in breast cancer patients receiving chemotherapy. Journal of Clinical Oncology 2005;23(12):2597‐605. [DOI] [PubMed] [Google Scholar]
Charu 2007 {published data only}
- Charu V, Belani CP, Gill AN, Bhatt M, Ben Jacob A, Tomita D, et al. A controlled, randomized, open‐label study to evaluate the effect of every‐2‐week darbepoetin alfa for anemia of cancer. Annual Meeting Proceedings of the American Society of Clinical Oncology 2004:abstract 8084.
- Charu V, Belani CP, Gill AN, Bhatt M, Tomita D, Rossi G, et al. Efficacy and safety of every‐2‐week darbepoetin alfa in patients with anemia of cancer: a controlled, randomized, open‐label phase II trial. Oncologist 2007;12(6):727‐37. [DOI] [PubMed] [Google Scholar]
- Charu V, Saidman B, Ben Jacob A, Justice GR, Maniam AS, Rearden T, et al. Improvements in fatigue are associated with early treatment with darbepoetin alfa every 3 weeks in anemic patients receiving chemotherapy. The Journal of Supportive Oncology 2005;3(2 Suppl 1):14‐5. [Google Scholar]
Dammacco 2001 {published data only}
- Dammacco F, Castoldi G, Rodjer S. Efficacy of epoetin alfa in the treatment of anaemia of multiple myeloma. British Journal of Haematology 2001;113(1):172‐9. [DOI] [PubMed] [Google Scholar]
- Dammacco F, Silvestris F, Castoldi GL, Grassi B, Bernasconi C, Nadali G, et al. The effectiveness and tolerability of epoetin alfa in patients with multiple myeloma refractory to chemotherapy. International Journal of Clinical and Laboratory Research 1998;28:127‐34. [DOI] [PubMed] [Google Scholar]
Debus 2006 {published data only}
- Debus J, Hindermann S, Morr H, Mezger J, Sebastian M, Angermund R, et al. Epoetin alfa (EPO) and survival in patients with non‐resectable NSCLC ‐ Interim results. 27th Congress of the German Cancer Society Berlin, Germany, 2006. German Medical Science 2006:abstract PO147.
- Debus J, Hindermann S, Morr H, Mezger J, Sebastian M, Angermund R, et al. Epoetin alfa (EPO) and survival in patients with non‐resectable NSCLC ‐ Interim results. Lung Cancer 2005; Vol. 49, issue Suppl 3:S57.
EPO‐GBR‐7 {published data only}
- Luksenburg H, Weir A, Wager R. EPO‐GBR‐7: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen Inc. and Procrit (epoetin alfa) Ortho Biotech L.P., for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
EPO‐GER‐20 {published data only}
- EPO‐GER‐20. Prospective, randomized, controlled, open phase‐IV study on the treatment of small cell lung cancer (SCLC) in the extensive disease (ED) stage per VALGB classification with doxorubicin, cyclophosphamide, etoposide (ACE regimen). unpublished: Angermund R, Janssen‐Cilag, personal communication.
EPO‐INT‐1 {published data only}
- Luksenburg H, Weir A, Wager R. EPO‐INT‐1: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen, Inc. and Procrit (epoetin alfa) Ortho Biotech, L.P., for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
EPO‐INT‐3 {published data only}
- Luksenburg H, Weir A, Wager R. EPO‐INT‐3: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen, Inc. and Procrit (epoetin alfa) Ortho Biotech, L.P., for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
Gordon 2006 {published data only}
- Gordon D, Nichols G, Ben Jacob A, Tomita D, Lillie T, Miller C. Treating anemia of cancer with every‐4‐week darbepoetin alfa: Final efficacy and safety results from a phase II, randomized, double‐blind, placebo‐controlled study. The Oncologist 2008;13(6):715‐24. [DOI] [PubMed] [Google Scholar]
- Gordon DH, Nichols G, Ben Jacob A, Lam H, Lillie T, Miller C. Treating anemia of cancer with darbepoetin alfa administered every 4 weeks: Final results from a phase 2, randomized, double‐Blind, placebo‐controlled study in cancer patients not receiving chemotherapy and/or radiotherapy. Blood 2006; Vol. 108, issue 11 Suppl:abstract 1304.
Goss 2005 {published data only}
- Goss G, Feld R, Bezjak A, Perry G, Melosky B, Smith C, et al. Impact of maintaining Hb with epoetin alfa on time to progression (TTP), overall survival (OS), quality of life (QOL) and transfusion reduction in limited disease SCLC patients. Lung cancer 2005; Vol. 49, issue Suppl 2:S53.
- Luksenburg H, Weir A, Wager R. EPO‐CAN‐15: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen Inc and Procrit (epoetin alfa) Ortho Biotech L.P. for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
Grote 2005 {published data only}
- Grote T, Yeilding AL, Castillo R, Butler D, Fishkin E, Henry DH, et al. Efficacy and safety analysis of epoetin alfa in patients with small‐cell lung cancer: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Oncology 2005;23(36):9377‐86. [DOI] [PubMed] [Google Scholar]
- Luksenburg H, Weir A, Wager R. N93‐004: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen Inc and Procrit (epoetin alfa) Ortho Biotech L.P. for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
Hedenus 2003 {published data only}
- 20000161. Continuing reassessment of the risks of erythropoiesis‐stimulating agents (ESAs) administered for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2007; Vol. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007‐4301b2‐02‐ FDA.pdf.
- Hedenus M, Adriansson M, San Miguel J, Kramer MH, Schipperus MR, Juvonen E, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double‐blind, placebo‐controlled study. British Journal of Haematology 2003;122(3):394‐403. [DOI] [PubMed] [Google Scholar]
- Hedenus M, Brandberg Y, Molostova V, Iosova G, Abdulkadyrov K, Messinger D, et al. Efficacy of epoetin beta in treating the anaemia of cancer in patients with haematological malignancies. Proceedings of the 6th Congress of the European Haematology Association 2001:abstract 190.
Henke 2003 {published data only}
- Henke M, Laszig R, Ruebe C, Schaefer U, Haase KD, Schilcher B, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double‐blind, placebo‐controlled trial. Lancet 2003;362:1255‐60. [DOI] [PubMed] [Google Scholar]
- Schipper J, Henke M. Erythropoietin in patients with head and neck carcinomas? [Erythropoetin bei Karzinomen im Kopf‐/Halsbereich?]. Laryngorhinootologie 2004;83(5):292‐7. [DOI] [PubMed] [Google Scholar]
Henry 1995 {published data only}
- Abels RI, Larholt KM, Krantz KD, Bryant EC. Recombinant Human Erythropoietin (rHuEPO) for the Treatment of the Anemia of Cancer. Oncologist 1996;1(3):140‐50. [PubMed] [Google Scholar]
- Henry DH, Abels RI. Recombinant human erythropoietin in the treatment of cancer and chemotherapy‐induced anemia: Results of double‐blind and open‐label follow‐up studies. Seminars in Oncology 1994;21(2 (Suppl 3)):21‐8. [PubMed] [Google Scholar]
- Henry DH, Brooks BJ, Case DC, Fishkin E, Jacobson R, Keller AM, et al. Recombinant Human Erythropoietin Therapy for Anemic Cancer Patients Receiving Cisplatin Chemotherapy. The Cancer Journal from Scientific American 1995;1:252‐60. [PubMed] [Google Scholar]
Huddart 2002 {published data only}
- Huddart RA, Welch RS, Chan S, Perren T, Atkinson R. A prospective randomised comparative‐group evaluation of epoetin alfa for the treatment of anaemia in UK cancer patients receiving platinum‐based chemotherapy. Annals of Oncology 2002; Vol. 13 (suppl 5):177.
Kotasek 2002 {published data only}
- Kotasek D, Albertsson M, Mackey J, Darbepoetin Alfa 980291 Study Group. Randomized, double‐blind, placebo‐controlled, dose‐finding study of darbepoetin alfa administered once every 3 (Q3W) or 4 (Q4W) weeks in patients with solid tumors. Proceedings of the American Society of Clinical Oncology 2002; Vol. 21:356a.
- Kotasek D, Albertsson M, Mackey J, Steger G, Rossi G, O'Byne J, et al. Once per cycle dosing of darbepoetin alfa is feasible in anemic cancer patients receiving chemotherapy. Annals of Oncology 2002; Vol. 13, issue Suppl 5:170.
Kotasek 2003 {published data only}
- Kotasek D, Berg R, Poulsen E, Colowick A. Randomized, double‐blind, placebo controlled, phase I/II dose finding study of ARANESP (TM) administered once every three weeks in solid tumor patients. Blood 2000; Vol. 96, issue 11:abstract 1268.
- Kotasek D, Steger G, Faught W, Underhill C, Poulsen E, Colowick AB, et al. Darbepoetin alfa administered every 3 weeks alleviates anaemia in patients with solid tumours receiving chemotherapy; results of a double‐blind, placebo‐controlled, randomised study. European Journal of Cancer 2003;39(14):2026‐34. [DOI] [PubMed] [Google Scholar]
Leyland‐Jones 2003 {published data only}
- Leyland‐Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncology 2003;4:459‐60. [DOI] [PubMed] [Google Scholar]
- Leyland‐Jones B, Semiglazov V, Pawlicki M, Pienkowski T, Tjulandin S, Manikhas G, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first‐line chemotherapy: A survival study. Journal of Clinical Oncology 2005;23(25):5865‐8. [DOI] [PubMed] [Google Scholar]
Littlewood 2001 {published data only}
- Aapro MS, Cella D, Zagari M. Age, anemia, and fatigue. Seminars in Oncology 2002;29(3 Suppl 8):55‐9. [DOI] [PubMed] [Google Scholar]
- Fairclough DL, Gagnon DD, Zagari MJ, Marschner N, Dicato M. Evaluation of quality of life in a clinical trial with nonrandom dropout: the effect of epoetin alfa in anemic cancer patients. Quality of Life Research 2003;12(8):1013‐27. [DOI] [PubMed] [Google Scholar]
- Fallowfield L, Gagnon D, Zagari M, Cella D, Bresnahan B, Littlewood TJ, et al. Multivariate regression analyses of data from a randomised, double‐blind, placebo‐controlled study confirm quality of life benefit of epoetin alfa in patients receiving non‐platinum chemotherapy. British Journal of Cancer 2002;87(12):1341‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallowfield L, Gagnon D, Zagari M, Cella D, Bresnahan B, Littlewood TJ, et al. Multivariate regression analyses of data from a randomised, double‐blind, placebo‐controlled study confirm quality of life benefit of epoetin alfa in patients receiving non‐platinum chemotherapy. British Journal of Cancer 2002;87(12):1341‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlewood TJ. Efficacy and quality of life outcomes of epoetin‐alpha in a double‐blind, placebo‐controlled, multicentre study of cancer patients receiving non‐platinum‐containing chemotherapy. Frontiers of radiation therapy and oncology 2002;37:34‐7. [DOI] [PubMed] [Google Scholar]
- Littlewood TJ, Bajetta E, Nortier JW, Vercammen E, Rapoport B. Effects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving non‐platinum chemotherapy: Results of a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Oncology 2001;19(11):2865‐74. [DOI] [PubMed] [Google Scholar]
- Littlewood TJ, Cella D, Nortier JW. Erythropoietin improves quality of life. Lancet Oncology 2002;3(8):459‐60. [DOI] [PubMed] [Google Scholar]
- Littlewood TJ, Kallich JD, San Miguel J, Hendricks L, Hedenus M. Efficacy of darbepoetin alfa in alleviating fatigue and the effect of fatigue on quality of life in anemic patients with lymphoproliferative malignancies. Journal of Pain & Symptom Management 2006;31(4):317‐25. [DOI] [PubMed] [Google Scholar]
- Littlewood TJ, Nortier J, Rapoport B, Pawlicki M, Wasch G, Vercammen E, et al. Epoetin alfa corrects anemia and improves quality of life in patients with hematologic malignancies receiving non‐platinum chemotherapy. Hematological Oncology 2003;21(4):169‐80. [DOI] [PubMed] [Google Scholar]
- Martin SC, Gagnon DD, Zhang L, Bokemeyer C, Marwijk KM, Hout B. Cost‐utility analysis of survival with epoetin‐alfa versus placebo in stage IV breast cancer. Pharmacoeconomics 2003;21(16):1153‐69. [DOI] [PubMed] [Google Scholar]
Machtay 2007 {published data only}
- Machtay M, Pajak T, Suntharalingam M, Hershock D, Stripp DC, Cmelak A, et al. Definitive radiotherapy +/‐ erythropoietin for squamous cell carcinoma of the head and neck: preliminary report of RTOG 99‐03. International Journal of Radiation Oncology, Biology and Physics 2004;60(Suppl 1):132. [Google Scholar]
- Machtay M, Pajak TF, Suntharalingam M, Shenouda G, Hershock D, Stripp DC, et al. Radiotherapy with or without erythropoietin for anemic patients with head and neck cancer: a randomized trial of the Radiation Therapy Oncology Group (RTOG 99‐03). International Journal of Radiation Oncology, Biology, Physics 2007;69(4):1008‐17. [DOI] [PubMed] [Google Scholar]
Milroy 2003 {published data only}
- Milroy R, Scagliotti G, Berg PM, Galanis NE, Gómez RG, Greil R, et al. Early intervention with epoetin alfa maintains hemoglobin (HB) in advanced non‐small‐cell lung cancer (NSCLC) patients. Lung Cancer 2003; Vol. 41, issue Suppl 2:S74.
Moebus 2007 {published data only}
- Moebus V, Bastert G, Kreienberg R, Eidtmann H, Cierna M, Untch M, et al. Epoetin Alpha Prevents Anemia and Transfusions of RBCS in Patients Receiving Dose‐Dense Sequential Chemotherapy. Proceedings of the American Society of Clinical Oncology 2001; Vol. 20:abstract 36.
- Moebus V, Lueck H, Thomssen C, Harbeck N, Nitz U, Kreienberg R, et al. The impact of epoetin‐alpha on anemia, red blood cell (RBC) transfusions, and survival in breast cancer patients (pts) treated with dose‐dense sequential chemotherapy: Mature results of an AGO phase III study (ETC trial). Journal of Clinical Oncology 2007; Vol. 25, issue 18 Suppl:569.
- Moebus V, Untch M, du Bois A, Lueck HJ, Thomssen C, Kuhn W, et al. Dose‐dense sequential chemotherapy with epirubicin(E), paclitaxel (T) and cyclophosphamide (C) (ETC) is superior to conventional dosed chemotherapy in high‐risk breast cancer patients (>= 4 +LN). First results of an AGO‐trial. Journal of Clinical Oncology 2004; Vol. 22, issue 14 Suppl:abstract 513.
- Untch M, Jackisch C, Lenhard MS, du Bois A, Lueck HJ, Thomssen C, et al. Epoetin‐alpha reduces red blood cell transfusions (RBC) in high‐risk breast cancer patients with adjuvant dose‐dense, sequential chemotherapy with epirubicin (E), paclitaxel (T) and cyclophosphamide (C) (ETC). Journal of Clinical Oncology 2005; Vol. 23, issue 16 Suppl:613.
O'Shaugnessy 2005 {published data only}
- O'Shaugnessy J, Vukelja S, Savin M, Holmes FA, Jones M, Royall D, et al. Effects of epoetin alfa (Procrit) on cognitive function, mood, asthenia, and quality of life in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy: a double‐blind, randomized, placebo‐controlled trial. Proceedings of the American Society of Clinical Oncology 2002:abstract 1449.
- O'Shaugnessy J, Vukelja S, Savin M, Holmes FA, Jones M, Royall D, et al. Impact of epoetin alfa on cognitive function, asthenia, and quality of life in women with breast cancer receiving adjuvant or neoadjuvant chemotherapy: analysis of 6‐month follow‐up data. Proceedings from the 25th Annual Breast Cancer Symposium 2002:abstract: 550.
- O'Shaugnessy J, Vukelja SJ, Holmes FA, Savin M, Jones M, Royall D, et al. Feasibility of quantifying the effects of epoetin alfa therapy on cognitive function in women with breast cancer undergoing adjuvant or neoadjuvant chemotherapy. Clinical Breast Cancer 2005;5(6):439‐46. [DOI] [PubMed] [Google Scholar]
OBE/EPO‐INT‐03 {published data only}
- OBE/EPO‐INT‐03. A randomized comparison of the effect of maintaining haemoglobin levels with weekly epoetin alfa or with conventional anaemia management in subjects with Multiple Myeloma undergoing chemotherapy (EMMY). unpublished: Murphy R, Janssen‐Cilag, personal communication.
Oberhoff 1998 {published data only}
- Oberhoff C, Krumeich B, Winkler UH, Hoffmann O, Schindler AE. Recombinant human erythropoietin (epoetin beta) in the treatment of chemotherapy ‐ associated anaemia: effects on blood coagulation and fibrinolysis in patients with gynecological malignancies. Annals of Hematology 2000; Vol. 79 Suppl 3:B16.
- Oberhoff C, Neri B, Amadori D, Petry KU, Gamucci T, Rebmann U, et al. Recombinant human erythropoietin in the treatment of chemotherapy‐ induced anemia and prevention of transfusion requirement associated with solid tumors: a randomized, controlled study. Annals of Oncology 1998;9(3):255‐60. [DOI] [PubMed] [Google Scholar]
- Oberhoff C, Stauch M, Wilhelm G, Musch E, Heinrich B, Neise M, et al. Prevention and therapy of anemia in tumor patients with Epoetin beta (NeoRecormon). Tumor Diagnostik und Therapie 2005;26(4):166‐71. [Google Scholar]
Osterborg 1996 {published data only}
- Osterborg A, Boogaerts MA, Cimino R, Essers U, Holowiecki J, Juliusson G, et al. Recombinant human erythropoietin in transfusion‐dependent anemic patients with multiple myeloma and non‐Hodgkin's lymphoma ‐ a randomized multicenter study. Blood 1996;87(7):2675‐82. [PubMed] [Google Scholar]
Osterborg 2002 {published data only}
- Osterborg A, Brandberg Y, Hedenus M. Impact of epoetin‐ß on survival of patients with lymphoproliferative malignancies: long‐term follow up of a large randomized study. British Journal of Haematology 2005;129:206‐9. [DOI] [PubMed] [Google Scholar]
- Osterborg A, Brandberg Y, Molostova V, Iosava G, Abdulkadyrov K, Hedenus M, et al. Randomized, double‐blind, placebo‐controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignancies. Journal of Clinical Oncology 2002;20(10):2486‐94. [DOI] [PubMed] [Google Scholar]
Pirker 2008 {published data only}
- Amgen. Aranesp(R) ``145 Study'' Shows No Difference in Survival in Patients with Small‐Cell Lung Cancer. Amgen Thousand Oaks, CA, 2007; Vol. http://wwwext.amgen.com/media/media_pr_detail.jsp?year=2007&releaseID=987476 [date of last access April 28, 2009].
- Pirker R, Ramlau RA, Schuette W, Zatloukal P, Ferreira I, Lillie T, et al. Safety and Efficacy of Darbepoetin Alfa in Previously Untreated Extensive‐Stage Small‐Cell Lung Cancer Treated With Platinum Plus Etoposide. Journal of Clinical Oncology 2008;26(14):2342‐9. [DOI] [PubMed] [Google Scholar]
Pronzato 2002 {published data only}
- Pronzato P, Cortesi E, Rijt C, Moreno‐Nogueria A, Raimundo D, Ostler P, et al. Early intervention with epoetin alfa in breast cancer (BC) patients (pts) undergoing chemotherapy (CT): results of a randomized, multicenter, phase IIIb study (EPO‐INT‐47 Study Group). Annals of Oncology 2002; Vol. 13, issue Suppl 5:168.
Quirt 1996 {published data only}
- Quirt I, Micucci S, Moran LA, Pater J, Browman G. The role of recombinant human erythropoietin (EPO) in reducing red blood cell transfusions and maintaining quality of life (QOL) in patients with lymphoma and solid tumors receiving cytotoxic chemotherapy. Results of a randomized, double‐blind, placebo‐controlled clinical trial. Blood 1996; Vol. 88, issue 10 Suppl 1:347a, abstract 1378.
Ray‐Coquard 2006 {published data only}
- Ray‐Coquard I, Dussart S, Goillot C, Mayeur D, Debourdeau P, Ghesquieres H, et al. A risk model for severe anemia to select cancer patients for primary prophylaxis with epoetin {alpha}: a prospective randomized controlled trial of the ELYPSE study group. Annals of Oncology 2009, issue doi:10.1093/annonc/mdn750. [DOI] [PubMed]
- Ray‐Coquard I, Perol D, Debourdeau P, Chabaud S, Chelghoum M, Mayeur D, et al. ELYPSE 4: A prospective randomized trial comparing Epo A in primary prophylaxis of severe anemia requiring red cells transfusion in high risk patients. Annals of Oncology 2006;17(Suppl 9):ix294. [Google Scholar]
Razzouk 2006 {published data only}
- Hinds PS, Hockenberry M, Feusner J, Hord JD, Rackoff W, Rozzouk BI. Hemoglobin response and improvements in quality of life in anemic children with cancer receiving myelosuppressive chemotherapy. Journal of Supportive Oncology 2005;3(6 Suppl 4):10‐1. [PubMed] [Google Scholar]
- Razzouk BI, Hockenberry M, Hinds PS, Feusner J, Rackoff W, Hord JD. Influence of hemoglobin response to epoetin alfa on quality‐of‐life in anemic children with cancer receiving myelosuppressive chemotherapy. Blood 2004; Vol. 104, issue 11:abstract 221.
- Razzouk BI, Hockenberry M, Hinds PS, Rackoff W, Hord JD. A double‐blind, placebo‐controlled study of once‐weekly epoetin alfa in children with cancer undergoing myelosuppressive chemotherapy. Journal of Clinical Oncology 2004; Vol. 22, issue 14S:abstract 8527. [DOI] [PubMed]
- Razzouk BI, Hord JD, Hockenberry M, Hinds PS, Feusner J, Williams D, et al. Double‐blind, placebo‐controlled study of quality of life, hematologic end points, and safety of weekly epoetin alfa in children with cancer receiving myelosuppressive chemotherapy. Journal of Clinical Oncology 2006;24(22):3583‐9. [DOI] [PubMed] [Google Scholar]
Rose 1994 {published data only}
- Rose E, Rai K, Revicki D, et al. Clinical and health status assessments in anemic chronic lymphocytic leukemia (CLL) patients treated with epoetin alfa (EPO). Blood 1994; Vol. 84, issue 10 Suppl 1:526a.
Savonije 2005 {published data only}
- Savonije J, Groeningen C, Bochove A, Pinedo H, Giaccone G. Early intervention with epoetin‐alfa during platinum‐based chemotherapy. Journal of Clinical Oncology 2004; Vol. 22, issue 14S:abstract 8111.
- Savonije JH, Groeningen CJ, Bochove A, Honkoop AH, Felius CL, Wormhoudt LW, et al. Effects of early intervention with epoetin alfa on transfusion requirement, hemoglobin level and survival during platinum‐based chemotherapy: Results of a multicenter randomised controlled trial. European Journal of Cancer 2005;41(11):1560‐9. [DOI] [PubMed] [Google Scholar]
- Savonije JH, Groeningen CJ, Wormhoudt LW, Giaccone G. Early Intervention with epoetin alfa during platinum‐based chemotherapy: an analysis of quality‐of‐life results of a multicenter, randomized, controlled trial compared with population normative data. The oncologist 2006;11(2):197‐205. [DOI] [PubMed] [Google Scholar]
Smith 2008 {published data only}
- 20010103. Continuing reassessment of the risks of erythropoiesis‐stimulating agents (ESAs) administered for the treatment of anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drugs Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2007; Vol. http://www.fda.gov/ohrms/dockets/ac/07/briefing/2007‐4301b2‐02‐ FDA.pdf.
- Glaspy J, Smith RE, Aapro M, Ludwig H, Pinter T, Smakal M, et al. Results from a phase 3 randomized, double blind, placebo controlled study of darbepoetin alfa for the treatment of anemia in cancer patients not receiving chemotherapy or radiotherapy. Haematologica 2007; Vol. 92, issue Suppl 1:136. [DOI] [PubMed]
- Smith RE, Aapro MS, Ludwig H, Pinter T, Smakal M, Ciuleanu TE, et al. Darbepoetin Alfa for the Treatment of Anemia in Patients With Active Cancer Not Receiving Chemotherapy or Radiotherapy: Results of a Phase III, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Study. Journal of Clinical Oncology 2008;26(7):1040‐50. [DOI] [PubMed] [Google Scholar]
Strauss 2008 {published data only}
- Strauss H, Haendgen G, Dunst.J, Koelbl H. Effects of anaemia correction with epoetin beta in patients with advanced cervical cancer. Proceedings of the American Society of Clinical Oncology 2003:abstract 5121.
- Strauss HG, Haendgen G, Dunst J, Haywrad CRW, Burger HU, Scherhag A, et al. Effects of anemia correction with epoetin beta in patients receiving radiochemotherapy for advanced cervical cancer. International Journal of Gynecological Cancer 2008;18(Suppl 3):515‐24. [DOI] [PubMed] [Google Scholar]
Taylor 2005 {published data only}
- Taylor K, Ganly P, Charu V, DiBenedetto J, Kracht K, Rossi G, et al. Randomized, double‐blind, placebo‐controlled study of darbepoetin alfa every 3 weeks for the treatment of chemotherapy‐induced anemia. Blood 2005; Vol. 106, issue 11:abstract 3556.
Ten Bokkel Huinink 1998 {published data only}
- Reed N, Rhan S, Hayward C, Burger H, Bokkel Huinink W. Impact of epoetin beta on the survival of anemic patients with ovarian cancer receiving platinum‐based chemotherapy. Proceedings of the American Society of Clinical Oncology 2003; Vol. 23, issue 16s:abstract 5102.
- Bokkel Huinink WW, Swart CA, Toorn DW, Morack G, Breed WP, Hillen HF, et al. Controlled multicentre study of the influence of subcutaneous recombinant human erythropoietin on anaemia and transfusion dependency in patients with ovarian carcinoma treated with platinum‐based chemotherapy. Medical Oncology 1998;15(3):174‐82. [DOI] [PubMed] [Google Scholar]
Thatcher 1999 {published data only}
- Campos E, Radford J, Steward Milroy R, Dougal M, Swindell R, et al. Clinical and in vitro effects of recombinant human erythropoietin in patients receiving intensive chemotherapy for small‐cell lung cancer. Journal of Clinical Oncology 1995;13(7):1623‐31. [DOI] [PubMed] [Google Scholar]
- Thatcher N, Campos ES, Bell DR, Steward WP, Varghese G, Morant R, et al. Epoetin alpha prevents anaemia and reduces transfusion requirements in patients undergoing primarily platinum‐based chemotherapy for small cell lung cancer. British Journal of Cancer 1999;80(3‐4):396‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2002 {published data only}
- Thomas H, McAdam KF, Thomas RJ, Joffe JK, Sugden EM, Awwad ST, et al. Early intervention with epoetin alfa for treatment of anaemia and improvement of quality of life in cancer patients undergoing myelotoxic chemotherapy. Annals of Oncology 2002; Vol. 13, issue Suppl 5:177.
Thomas 2008 {published data only}
- Luksenburg H, Weir A, Wager R. GOG‐191: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen Inc. and Procrit (epoetin alfa) Ortho Biotech L.P., for the treatment of Anemia associated with cancer chemotherapy. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
- Thomas G, Ali S, Hoebers FJP, Darcy KM, Rodgers WH, Patel M, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12.0 g/dL with erythropoietin vs above 10.0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecologic Oncology 2008;108(2):317‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Untch 2008 {published data only}
- Untch M, Fascing PA, Bauernfeind I, Conrad U, Camara O, Fett W, et al. PREPARE trial. A randomized phase III trial comparing preoperative, dose‐dense, dose‐intensified chemotherapy with epirubicin, paclitaxel and CMF with a standard dosed epirubicin/cyclophosphamide followed by paclitaxel +/‐ darbepoetin alfa in primary breast cancer: A preplanned interim analysis of efficacy at surgery. Journal of Clinical Oncology 2008; Vol. 26, issue Suppl:abstract 517.
Vadhan‐Raj 2004 {published data only}
- Vadhan‐Raj S, Skibber JM, Crane C, Buesos‐Ramos CE, Rodriguez‐Bigas MA, Feig BW, et al. Randomized, double‐blind, placebo‐controlled trial of epoetin alfa (Procrit) in patients with rectal and gastric cancer undergoing chemo‐radiotherapy (CT/RT) followed by surgery: early termination of the trial due to increased incidence of thromboembolic events (TEE). Blood 2004; Vol. 104, issue 11:797a, abstract 2915.
Vansteenkiste 2002 {published data only}
- Kallich JD, Tchekmedyian NS, Damiano AM, Shi J, Black JT, Erder MH. Psychological outcomes associated with anemia‐related fatigue in cancer patients. Oncology (Huntingt) 2002;16(9 Suppl 10):117‐24. [PubMed] [Google Scholar]
- Pirker R, Vansteenkiste J, Gateley J, Yates P, Colowick A, Musil J. A phase 3, double‐blind, placebo‐controlled, randomized study of novel erythropoiesis stimulating protein (NESP) in patients undergoing platinum treatment for lung cancer. Proceedings of the American Society of Clinical Oncology 2001; Vol. 20:abstract 1572.
- Tchekmedyian NS, Kallich J, McDermott A, Fayers P, Erder MH. The relationship between psychologic distress and cancer‐related fatigue. Cancer 2003;98(1):198‐203. [DOI] [PubMed] [Google Scholar]
- Tchekmedyian S, Glaspy J, Colowick A, Tomita D, Rossi G. Effect of darbepoetin alfa and recombinant human erythropoietin (rHuEPO) on early hemoglobin (Hb) changes in anemic cancer patients (pts). Annals of Oncology 2002; Vol. 13, issue Suppl 5:184.
- Vansteenkiste J, Pirker R, Massuti B, Barata F, Font A, Fiegl M, et al. Double‐blind, placebo‐controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapy. Journal of the National Cancer Institute 2002;94:1211‐20. [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J, Poulsen E, Rossi G, Glaspy J. Darbepoetin alfa: impact on treatment for chemotherapy‐induced anemia and considerations in special populations. Oncology (Huntingt) 2002;16(10 Suppl 11):45‐55. [PubMed] [Google Scholar]
- Vansteenkiste J, Tomita D, Rossi G, Pirker R. Darbepoetin alfa in lung cancer patients on chemotherapy: a retrospective comparison of outcomes in patients with mild versus moderate‐to‐severe anaemia at baseline. Supportive Care in Cancer 2004;12(4):253‐62. [DOI] [PubMed] [Google Scholar]
Wilkinson 2006 {published data only}
- Wilkinson PM, Antonopoulos M, Lahousen M, Lind M, Kosmidis P, Epo INT. Epoetin alfa in platinum‐treated ovarian cancer patients: results of a multinational, multicentre, randomised trial. British Journal of Cancer 2006;94(7):947‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witzig 2005 {published data only}
- Dicato M, Vercammen E, Liu KL, Xiu LX, Bowers P. Relationship of body weight to efficacy of a fixed‐dose regimen of epoetin alfa vs placebo in anemic cancer patients. Haematologica 2005; Vol. 90, issue Suppl 2:abstract 0077.
- Witzig TE, Silberstein PT, Loprinzi CL, Sloan JA, Novotny PJ, Mailliard JA, et al. Phase III, randomized, double‐blind study of epoetin alfa compared with placebo in anemic patients receiving chemotherapy. Journal of Clinical Oncology 2005;23(12):2606‐17. [DOI] [PubMed] [Google Scholar]
Wright 2007 {published data only}
- Luksenburg H, Weir A, Wager R. EPO‐CAN‐20: Safety concerns associated with Aranesp (darbepoetin alfa) Amgen Inc and Procrit (epoetin alfa) Ortho Biotech L.P. for the treatment of anemia associated with cancer chemotherapy. Oncologic Drugs Advisory Committee, Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
- Wright JR, Ung YC, Julian JA, Pritchard KI, Whelan TJ, Smith C, et al. Randomized, double‐blind, placebo‐controlled trial of erythropoietin in non‐small‐cell lung cancer with disease‐related anemia. Journal of Clinical Oncology 2007;25(9):1027‐32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelrazik 2007 {published data only}
- Abdelrazik N, Fouda M. Once weekly recombinant human erythropoietin treatment for cancer‐induced anemia in children with acute lymphoblastic leukemia receiving maintenance chemotherapy: A randomized case‐controlled study. Hematology 2007;12(6):533‐41. [DOI] [PubMed] [Google Scholar]
Alexopoulos 2004 {published data only}
- Alexopoulos C, Kotsori A. A randomized comparison of rHuEPO with darbepoetin for cancer related anemia. Annals of Oncology 2004;15 Suppl 3:abstract 832P. [Google Scholar]
Antonadou 2001 {published data only}
- Antonadou D, Cardamakis E, Puglisi M, Malamos N, Throuvalas N. Erythropoietin enhances radiation treatment efficacy in patients with pelvic malignancies. Final results of a randomized phase III study. European Journal of Cancer 2001; Vol. 37, issue Suppl 6:S144.
Aravantinos 2003 {published data only}
- Aravantinos G, Linardou H, Makridaki D, Laiou E, Zafiropoulos A, Janninis J, et al. Recombinant human erythropoietin for platinum‐based chemotherapy‐induced anaemia: A single‐centre randomised study. Journal of the Balkan Union of Oncology 2003;8(2):127‐32. [PubMed] [Google Scholar]
Auerbach 2004 {published data only}
- Auerbach M, Ballard H, Trout JR, McIlwain M, Ackerman A, Bahrain H, et al. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy‐related anemia: a multicenter, open‐label, randomized trial. Journal of Clinical Oncology 2004;22(7):1301‐7. [DOI] [PubMed] [Google Scholar]
Aziz 2001 {published data only}
- Aziz K, Hashem T, Mobarek N, Bary N, Ghoneimy I, Haddad S. Does Recombinant Human Erythropoietin Improve the Outcome of Radiation Therapy in Head and Neck Cancer Patients. Proceedings of the American Society for Therapeutic Radiology and Oncology 2001:abstract 2274.
Bamias 2003 {published data only}
- Bamias A, Aravantinos G, Kalofonos C, Timotheadou N, Siafaka V, Vlahou I, et al. Prevention of anemia in patients with solid tumors receiving platinum‐based chemotherapy by recombinant human erythropoietin (rHuEpo): A prospective, open label, randomized trial by the Hellenic Cooperative Oncology Group. Oncology 2003;64(2):102‐10. [DOI] [PubMed] [Google Scholar]
Beggs 2003 {published data only}
- Beggs VL, Disalvo WM, Meyer LP, Gragnev KH, Gibson JJ, Hoopes PJ, et al. Fatigue and plasma cytokines in a randomized double‐blind placebo‐controlled trial of epoetin alfa in patients undergoing combined modality therapy for unresectable non‐small cell lung cancer (NSCLC). Proceedings of the American Society of Clinical Oncology 2003; Vol. 22:abstract 2948.
Bessho 1997 {published data only}
- Bessho M, Hirashima K, Asano S, Ikeda Y, Ogawa N, Tomonaga M, et al. Treatment of the anemia of aplastic anemia patients with recombinant human erythropoietin in combination with granulocyte colony‐stimulating factor: a multicenter randomized controlled study. Multicenter Study Group. European Journal of Haematology 1997;58(4):265‐72. [DOI] [PubMed] [Google Scholar]
Bindi 2004 {published data only}
- Bindi M, Montemaggi P, Sabatino M, Paolelli L, Petrioli R, Morelli R, et al. Reticulocytes can represent an early indicator of the erythropoietic response to Darbepoetin alfa in the anemia by chemotherapy. Journal of Clinical Oncology 2004; Vol. 22, issue 14s:786, abstract 8245.
Blayney 2003 {published data only}
- Blayney D, Fesen M, Mirtsching BC, Katz D, Tomita D. Every‐2‐Week Darbepoetin Alfa improves hemoglobin in anemic patients with cancer undergoing chemotherapy: A stratified analysis by tumor type. Blood 2003; Vol. 102 (11):abstract 3779.
Blohmer 2003 {published data only}
- Blohmer JU, Petry K, Kolben M, Kimmig R, Boehmer D, Wuerschmidt J, et al. Adjuvant sequential chemo‐radiotherapy with vs. without erythropoietin in high‐risk patients with carcinoma of the cervix. Proceedings of the American Society of Clinical Oncology 2001; Vol. 20:abstract: 823.
- Blohmer JU, Wurschmidt F, Petry U, Weise G, Sehouli J, Kimming R, et al. Results with sequential adjuvant chemo‐radiotherapy with vs without epoetin alfa for patients with high‐risk cervical cancer: Results of a prospective, randomized, open and controlled AGO‐ and NOGGO‐intergroup study. Annals of Oncology 2004;15 (Suppl 3):abstract 447PD. [Google Scholar]
- Blohmer U, Wurschmidt U, Petry G, Weise J, Sehouli J, Kimmig R, et al. 6th interim analysis of a prospective, randomized, open and controlled AGO‐ and NOGGO‐intergroup study: Sequential adjuvant chemo‐radiotherapy with vs without epoetin alfa for pts with high‐risk cervical cancer. Proceedings of the American Society of Clinical Oncology 2003; Vol. 22:abstract 1789.
Candelaria 2005 {published data only}
- Candelaria M, Cetina L, Duenas‐Gonzalez A. Anemia in cervical cancer patients: implications for iron supplementation therapy. Medical Oncology 2005;22(2):161‐8. [DOI] [PubMed] [Google Scholar]
Canon 2006 {published data only}
- Canon JL, Vansteenkiste J, Bodoky G, Mateos MV, Bastit L, Ferreira I, et al. Randomized, double‐blind, active‐controlled trial of every‐3‐week darbepoetin alfa for the treatment of chemotherapy‐induced anemia. Journal of the National Cancer Institute 2006;98(4):273‐84. [DOI] [PubMed] [Google Scholar]
- Canon JL, Vansteenkiste J, Bodoky G, Mateos MV, Bastit L, Ferreira I, et al. Results of a randomised, double‐blind, active‐controlled trial of darbepoetin alfa administered once every 3 weeks for the treatment of anaemia in patients receiving multicycle chemotherapy. Haematologica 2005; Vol. 90, issue Suppl 2:abstract 471.
Carabantes 1999 {published data only}
- Carabantes FJ, Alonso CJ, Rius F, Benavides M, Hebrero ML, Garcia S, et al. Epoetin alfa (EPO) prevents anaemia and improves quality of life (QOL) in cancer patients (PTS) undergoing platinum‐based chemotherapy. European Journal of Cancer 1999; Vol. 35, issue S4:abstract 1482.
- Carabantes FJ, Benavides M, Trujillo R, Cobo M, Hebrero ML, Garcia S, et al. Epoetin alfa in the prevention of anemia in cancer patients undergoing platinum‐based chemotherapy (CT). A prospective randomized study. Proceedings of the American Society of Clinical Oncology 1999; Vol. 18:abstract 2303.
Casadevall 2004 {published data only}
- Casadevall N, Durieux P, Dubois S, Hemery F, Lepage E, Quarre MC, et al. Health, economic, and quality‐of‐life effects of erythropoietin and granulocyte colony‐stimulating factor for the treatment of myelodysplastic syndromes: a randomized, controlled trial. Blood 2004;104(2):321‐7. [DOI] [PubMed] [Google Scholar]
- Casadevall N, Lepage E, Durieux P, Dubois S, Dreyfus F, Quarre MC, et al. Erythropoietin (RHUEPO) plus G‐CSF in the treatment of anemia in myelodysplastic syndromes (MDS): results of a randomised trial with impact on quality of life and costs. The Hematology Journal 2001, issue Suppl 1:abstract 687.
Cascinu 1994 {published data only}
- Cascinu S, Fedeli A, Ferro E, Fedeli SL, Catalano G. Recombinant human erythropoietin treatment in cisplatin‐associated anemia: a randomized, double‐blind trial with placebo. Journal of Clinical Oncology 1994;12:1058‐62. [DOI] [PubMed] [Google Scholar]
Cazzola 2003 {published data only}
- Cazzola M, Beguin Y, Kloczko J, Spicka I, Coiffier B. Once‐weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin production. British Journal of Haematology 2003;122(3):386‐93. [DOI] [PubMed] [Google Scholar]
- Cazzola M, Coiffier B, Kloczko J, Spika I. Once weekly NeoRecormon® (epoetin beta) for the treatment of anemia associated with lymphoproliferative malignancies: results of the NOW (NeoRecormon® once weekly) study. Hematology Journal 2002, issue 3 Suppl 1:abstract 0182.
Chan 1995 {published data only}
- Chan AT, Leung WT, Lin J, Yeo W, Johnson PJ. Recombinant human erythropoietin for anaemia in Chinese cancer patients on chemotherapy. Clinical Oncology (Royal College of Radiologists) 1995;7(4):272. [DOI] [PubMed] [Google Scholar]
Charu 2007a {published data only}
- Charu V, Belani CP, Gill AN, Bhatt M, Ben Jacob A, Tomita D, et al. A controlled, randomized, open‐label study to evaluate the effect of every‐2‐week darbepoetin alfa for anemia of cancer. Annual Meeting Proceedings of the American Society of Clinical Oncology 2004:abstract 8084.
- Charu V, Saidman B, Ben Jacob A, Justice GR, Maniam AS, Tomita D, et al. A randomized, open‐label, multicenter trial of immediate versus delayed intervention with darbepoetin alfa for chemotherapy‐induced anemia. Oncologist 2007;12(10):1253‐63. [DOI] [PubMed] [Google Scholar]
Christodoulakis 2005 {published data only}
- Christodoulakis M, Tsiftsis DD, Hellenic Surgical Oncology Perioperative EPO Study Group. Preoperative epoetin alfa in colorectal surgery: a randomized, controlled study. Annals of Surgical Oncology 2005;12(9):718‐25. [DOI] [PubMed] [Google Scholar]
Crawford 1997 {published data only}
- Crawford J, Blackwell S, Shoemaker D, Pupa MR, Sparrow T, Herndon J, et al. Prevention of chemotherapy‐related anemia by recombinant human erythropoietin (EPO) in patients with small cell lung cancer receiving cyclophosphamide, doxorubicin, and etoposide (CAE) chemotherapy with G‐CSF support. Lung Cancer 1997; Vol. 18 (Suppl 1):abstract 796.
Crawford 2003 {published data only}
- Crawford J, Robert F, Perry M, Belani CP, Sarokhan B. Epoetin alfa 40,000 u once weekly maintains hemoglobin in advanced non‐small‐cell lung cancer patients receiving first‐line chemotherapy. Proceedings of the American Society of Clinical Oncology 2003;22:628, abstract 2527. [Google Scholar]
Crawford 2007 {published data only}
- Crawford J, Robert F, Perry MC, Belani C, Williams D, Anemia Prevention in NSCLC Group. A randomized trial comparing immediate versus delayed treatment of anemia with once‐weekly epoetin alfa in patients with non‐small cell lung cancer scheduled to receive first‐line chemotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer 2007;2(3):210‐20. [DOI] [PubMed] [Google Scholar]
Daneryd 1998 {published data only}
- Daneryd P, Svanberg E, Körner U, Lindholm E, Sandström R, Brevinge H, et al. Protection of metabolic and exercise capacity in unselected weight‐losing cancer patients following treatment with recombinant erythropoietin: A randomized prospective study. Cancer Research 1998;58:5374‐9. [PubMed] [Google Scholar]
- Lindholm E, Daneryd P, Korner U, Hyltander A, Fouladiun M, Lundholm K. Effects of recombinant erythropoietin in palliative treatment of unselected cancer patients. Clinical Cancer Research 2004;10(20):6855‐64. [DOI] [PubMed] [Google Scholar]
- Lonnroth C, Svensson M, Wang W, Korner U, Daneryd P, Nilsson O, et al. Survival and erythropoietin receptor protein in tumours from patients randomly treated with rhEPO for palliative care. Medical Oncology 2008;25(1):22‐9. [DOI] [PubMed] [Google Scholar]
Dannemann 2004 {published data only}
- Dannemann B, Wacholtz M, Lau H, Cheung W. Pharmacokinetics (PK) and pharmacodynamics (PD) of epoetin alfa in cancer patients with anemia receiving cyclic chemotherapy. Journal of Clinical Oncology 2004; Vol. 22, issue 14s:775, abstract 8203.
Del Mastro 1997 {published data only}
- Mastro L, Venturini M, Lionetto R, Garrone O, Melioli G, Pasquetti W, et al. Randomized phase III trial evaluating the role of erythropoietin in the prevention of chemotherapy‐induced anemia. Journal of Clinical Oncology 1997;15(7):2715‐21. [DOI] [PubMed] [Google Scholar]
Dunphy 1999 {published data only}
- Dunphy FR, Harrison BR, Dunleavy TL, Rodriguez JJ, Hilton JG, Boyd JH. Erythropoietin Reduces Anemia and Transfusions. Cancer 1999;86:1362‐7. [DOI] [PubMed] [Google Scholar]
Elsaid 2001 {published data only}
- Elsaid A, Farouk M. Significance of anemia and role of erythropoietin in radiation induced mucositis in head and neck cancer patients. International Journal of Radiation Oncology Biology Physics 2001; Vol. 51, issue 3 Suppl 1:368.
Freeman 2006 {published data only}
- Freeman III BB, Hinds P, Iacono LC, Razzouk BI, Burghen E, Stewart CF. Pharmacokinetics and pharmacodynamics of intravenous epoetin alfa in children with cancer. Pediatric Blood & Cancer 2006;47(5):572‐9. [DOI] [PubMed] [Google Scholar]
Garton 1995 {published data only}
- Garton JP, Gertz MA, Witzig TE, Greipp PR, Lust JA, Schroeder G, et al. Epoetin alfa for the treatment of the anemia of multiple myeloma. Archives of Internal Medicine 1995;155:2069‐74. [PubMed] [Google Scholar]
Gebbia 1992 {published data only}
- Gebbia V, Gebbia N, Testa A, Valenza R, Borsellino N, Rausa L, et al. Subcutaneous recombinant human erythropoietin prevents chemotherapy‐related anemia in patients with advanced cancer. International Journal of Oncology 1992;1:341‐5. [PubMed] [Google Scholar]
Glaspy 2002 {published data only}
- Glaspy JA, Jadeja JS, Justice G, Kessler J, Richards D, Schwartzberg L, et al. Darbepoetin alfa given every 1 or 2 weeks alleviates anaemia associated with cancer chemotherapy. British Journal of Cancer 2002;87(3):268‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaspy JA, Tchekmedyian NS. Darbepoetin alfa administered every 2 weeks alleviates anemia in cancer patients receiving chemotherapy. Oncology (Huntingt) 2002;16(10 Suppl 11):23‐9. [PubMed] [Google Scholar]
Glaspy 2003 {published data only}
- Glaspy JA, Jadeja JS, Justice G, Fleishman A, Rossi G, Colowick AB. A randomized, active‐control, pilot trial of front‐loaded dosing regimens of darbepoetin‐alfa for the treatment of patients with anemia during chemotherapy for malignant disease. Cancer 2003;97:1312‐20. [DOI] [PubMed] [Google Scholar]
Glaspy 2005 {published data only}
- Glaspy J, Henry D, Patel R, Tchekmedyian S, Applebaum S, Berdeaux D, et al. Effects of chemotherapy on endogenous erythropoietin levels and the pharmacokinetics and erythropoietic response of darbepoetin alfa: A randomised clinical trial of synchronous versus asynchronous dosing of darbepoetin alfa. European Journal of Cancer 2005;41(8):1140‐9. [DOI] [PubMed] [Google Scholar]
Glaspy 2006 {published data only}
- Glaspy J, Jadeja J, Justice G, Fleishman A, Rossi G, Colowick A. Optimizing the management of anemia in patients with cancer: a randomized, active‐controlled, study investigating the dosing of darbepoetin alfa. Hematology Journal 2002:abstract 0109.
- Glaspy J, Vadhan‐Raj S, Patel R, Bosserman L, Hu E, Lloyd RE, et al. Randomized comparison of every‐2‐week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy‐induced anemia: the 20030125 Study Group Trial. Journal of Clinical Oncology 2006;24(15):2290‐7. [DOI] [PubMed] [Google Scholar]
Glimelius 1998 {published data only}
- Glimelius B, Linne T, Hoffman K, Larsson L, Svensson JH, Nasman P, et al. Epoetin beta in the treatment of anemia in patients with advanced gastrointestinal cancer. Journal of Clinical Oncology 1998;16(2):434‐40. [DOI] [PubMed] [Google Scholar]
Glossmann 2003 {published data only}
- Glossmann JP, Engert A, Wassmer G, Flechtner H, Ko Y, Rudolph C, et al. Recombinant human erythropoietin, epoetin beta, in patients with relapsed lymphoma treated with aggressive sequential salvage chemotherapy ‐ results of a randomized trial. Annals of Hematology 2003;82(8):469‐75. [DOI] [PubMed] [Google Scholar]
Granetto 2003 {published data only}
- Granetto C, Ricci S, Martoni A, Pezzella G, Testore F, Mattioli R, et al. Comparing the efficacy and safety of fixed versus weight‐based dosing of epoetin alpha in anemic cancer patients receiving platinum‐based chemotherapy. Oncology Reports 2003;10(5):1289‐96. [PubMed] [Google Scholar]
Hedenus 2002 {published data only}
- Hedenus M, Hansen S, Taylor K, Arthur C, Emmerich D, Dewey C, et al. Randomized, dose‐finding study of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies. British Journal of Haematology 2002;119:79‐86. [DOI] [PubMed] [Google Scholar]
Hedenus 2007 {published data only}
- Hedenus M, Birgegard G, Nasman P, Ahlberg L, Karlsson T, Lauri B, et al. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. Leukemia 2007;21(4):627‐32. [DOI] [PubMed] [Google Scholar]
- Hedenus M, Nasman P. Epoetin beta with intravenous iron results in overall cost savings in a population of anemic patients with lymphoid malignancies not receiving chemotherapy. Blood 2007; Vol. 110, issue 11:abstract 5179.
Hellström Lindberg 1998 {published data only}
- Hellström Lindberg E, Ahlgren T, Beguin Y, Carlsson M, Carneskog J, Dahl IM, et al. Treatment of Anemia in Myelodysplastic Syndromes With Granulocyte Colony‐Stimulating Factor Plus Erythropoietin: Results From a Randomized Phase II Study and Long‐Term Follow‐Up of 71 Patients. Blood 1998;92(1):68‐75. [PubMed] [Google Scholar]
Henke 1999 {published data only}
- Henke M, Guttenberger R, Barke A, Pajonk F, Potter R, Frommhold H. Erythropoietin for patients undergoing radiotherapy: a pilot study. Radiotherapy and Oncology 1999;50(2):185‐90. [DOI] [PubMed] [Google Scholar]
Henry 2004 {published data only}
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate (FG) for increasing response to epoetin (EPO) in patients with anemia of cancer chemotherapy results of a multicenter, randomized trial. Blood 2004; Vol. 104, issue 11 Pt 2:10b, abstract 3696.
Henry 2006 {published data only}
- Henry DH, Gordan LN, Charu V, Wilhelm FE, Williams D, Xie J, et al. Randomized, open‐label comparison of epoetin alfa extended dosing (80 000 U Q2W) vs weekly dosing (40 000 U QW) in patients with chemotherapy‐induced anemia. Current Medical Research & Opinion 2006;22(7):1403‐13. [DOI] [PubMed] [Google Scholar]
- Henry DH, Williams D, Xie J, Wilhelm F. Randomized, open‐label comparison of epoetin alfa extended dosing (80,000 U every two weeks) versus weekly dosing (40,000 U weekly) in anemic patients with cancer receiving chemotherapy. Journal of Supportive Oncology 2007;5(4 Suppl 2):16‐7. [Google Scholar]
Henry 2007 {published data only}
- Henry DH, Dahl NV, Auerbach M. Is thromboembolism in cancer patients treated with erythropoietic stimulating agents related to thrombocytosis and iron restricted erythropoiesis?. Blood 2007; Vol. 110, issue 11:abstract 1625.
- Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist 2007;12(2):231‐42. [DOI] [PubMed] [Google Scholar]
Henze 2002 {published data only}
- Henze G, Michon J, Morland B, Perek D, Rizzari C, Zoubek A, et al. Phase III randomized study: efficacy of epoetin alfa in reducing blood transfusions in newly diagnosed pediatric cancer patients receiving chemotherapy. Proceedings of the American Society of Clinical Oncology 2002; Vol. 21:abstract 1547.
Hesketh 2004 {published data only}
- Hesketh PJ, Arena F, Patel D, Austin M, D'Avirro P, Rossi G, et al. A randomized controlled trial of darbepoetin alfa administered as a fixed or weight‐based dose using a front‐loading schedule in patients with anemia who have nonmyeloid malignancies. Cancer 2004;100(4):859‐68. [DOI] [PubMed] [Google Scholar]
- Hesketh PJ, Arena F, Patel D, Poulsen E, D'Avirro P, Rossi G, et al. Front‐loaded darbepoetin alfa with Q3W maintenance administered as a fixed or weight‐based dose in anemic cancer patients results in similar efficacy profiles. Proceedings of the American Society of Clinical Oncology 2003:abstract 2941.
Iconomou 2003 {published data only}
- Iconomou G, Koutras A, Rigopoulos A, Vagenakis AG, Kalofonos HP. Effect of recombinant human erythropoietin on quality of life in cancer patients receiving chemotherapy: results of a randomized, controlled trial. Journal of Pain & Symptom Management 2003;25(6):512‐8. [DOI] [PubMed] [Google Scholar]
Italian 1998 {published data only}
- Italian Cooperative Study Group. A randomized double‐blind placebo‐controlled study with subcutaneous recombinant human erythropoietin in patients with low‐risk myelodysplastic syndromes. British Journal of Haematology 1998;103:1070‐4. [DOI] [PubMed] [Google Scholar]
Janinis 2003 {published data only}
- Janinis J, Dafni U, Aravantinos G, Kalofonos HP, Papakostas P, Tsavdaridis D, et al. Quality of life (QoL) outcome of epoietin‐alfa (EPO‐A) in anemic cancer patients undergoing platinum or non‐platinum‐based chemotherapy: a randomized study conducted by the Hellenic Cooperative Oncology Group. Proceedings of the American Society of Clinical Oncology 2003; Vol. 22:789.
Jitnuyanont 2001 {published data only}
- Jitnuyanont A. Impact of therapy with recombinant human erythropoietin (r‐HuEPO) and Quality‐of‐life in anemic cancer patients. Internal Medicine Journal of Thailand 2001;17(4):283‐90. [Google Scholar]
Johansson 2001 {published data only}
- Johansson JE, Wersall P, Brandberg Y, Andersson SO, Nordstrom L, EPO‐Study Group. Efficacy of epoetin beta on hemoglobin, quality of life, and transfusion needs in patients with anemia due to hormone‐refractory prostate cancer ‐ a randomized study. Scandinavian Journal of Urology & Nephrology 2001;35(4):288‐94. [DOI] [PubMed] [Google Scholar]
Justice 2005 {published data only}
- Justice G, Kessler JF, Jadeja J, Campos L, Weick, J, et al. A randomized, multicenter study of subcutaneous and intravenous darbepoetin alfa for the treatment of chemotherapy‐induced anemia. Annals of Oncology 2005;16:1192‐8. [DOI] [PubMed] [Google Scholar]
Kettelhack 1998 {published data only}
- Kettelhack C, Hones C, Messinger D, Schlag PM. Randomized multicentre trial of the influence of recombinant human erythropoietin on intraoperative and postoperative transfusion need in anaemic patients undergoing right hemicolectomy for carcinoma. British Journal of Surgery 1998;85(1):63‐7. [DOI] [PubMed] [Google Scholar]
Kosmadakis 2003 {published data only}
- Kosmadakis N, Messaris E, Maris A, Katsaragakis S, Leandros E, Konstadoulakis MM, et al. Perioperative erythropoietin administration in patients with gastrointestinal tract cancer: prospective randomized double‐blind study. Annals of Surgery 2003;237(3):417‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotasek 2004 {published data only}
- Kotasek D, Canon JL, San Miguel J, Hedenus L, Hendricks G, Rossi K, et al. Correction/maintenance dosing (front loading) of darbepoetin alfa: final results from a randomized phase 3 active controlled trial. Blood 2004;104(11):abstract 1636. [Google Scholar]
- Kotasek D, Canon JL, San Miguel J, Hedenus M, Hendricks L, Rossi G, et al. Correction/maintenance dosing (front loading) of darbepoetin alfa: Final results from a randomized phase III active‐controlled trial. The Journal of Supportive Oncology 2005;3(2 SUPPL. 1):16‐7. [Google Scholar]
Kotasek 2007 {published data only}
- Kotasek D, Canon J‐L, Mateos MV, Hedenus M, Rossi G, Taylor K. A randomized, controlled trial comparing darbepoetin alfa correction/maintenance dosing with weekly dosing for treating chemotherapy‐induced anemia. Current Medical Research & Opinion 2007;23(6):1387‐401. [DOI] [PubMed] [Google Scholar]
Kunikane 2001 {published data only}
- Kunikane H, Watanabe K, Fukuoka M, Saijo N, Furuse K, Ikegami H, et al. Double‐blind randomized control trial of the effect of recombinant human erythropoietin on chemotherapy‐induced anemia in patients with non‐small cell lung cancer. International Journal of Clinical Oncology 2001;6:296‐301. [DOI] [PubMed] [Google Scholar]
Kurz 1997 {published data only}
- Kurz C, Marth C, Windbichler G, Lahousen M, Medl M, Vavra N, et al. Erythropoietin treatment under polychemotherapy in patients with gynecologic malignancies: a prospective, randomized, double‐blind placebo‐controlled multicenter study. Gynecologic Oncology 1997;65(3):461‐6. [DOI] [PubMed] [Google Scholar]
Mangiameli 2002 {published data only}
- Mangiameli A, Spina S, Iannetti E, Catalano D, Spadaro D, Trovato GM. Erythropoietin and cisplatin‐induced neuropathies in cancer patients. Clinica Terapeutica 2002;153(3):177‐80. [PubMed] [Google Scholar]
Marinaccio 2003 {published data only}
- Marinaccio M, Mele E, Giotta F, Cantinieri C, Cocca M. Pretreatment normalization of mild anemia with epoetin alfa: Impact on the outcome in epithelial ovarian cancer patients. Proceedings of the American Society of Clinical Oncology 2003;22:486. [Google Scholar]
Merlano 2001 {published data only}
- Merlano M, Ricci S, Martoni A. Comparing the efficacy of fixed vs. weight‐based dosing of epoetin alfa in anemic cancer patients receiving platinum‐based chemotherapy. European Journal of Cancer 2001; Vol. 37, issue Suppl 6:S346.
MF4266 {published data only}
- Luksenburg H, Weir A, Wager, R. MF4266 in: Safety Concerns Associated with Aranesp (darbepoetin alfa) Amgen, Inc. and Procrit (epoetin alfa) Ortho Biotech, L.P., for the Treatment of Anemia Associated with Cancer Chemotherapy. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research, Oncologic Drugs Advisory Committee, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
Miller 2004 {published data only}
- Miller KB, Kim HT, Greenberg P, Jagt R, Bennett JM, Tallman MS, et al. Phase III prospective randomized trial of EPO with or without G‐CSF versus supportive therapy alone in the treatment of myelodysplastic syndromes (MDS): Results of the ECOG‐CLSG trial (E1996). Blood 2004; Vol. 104, issue 11:70.
Morishima 2006 {published data only}
- Morishima Y, Ogura M, Yoneda S, Sakai H, Tobinai K, Nishiwaki Y, et al. Once‐weekly epoetin‐beta improves hemoglobin levels in cancer patients with chemotherapy‐induced anemia: A randomized, double‐blind, dose‐finding study. Japanese Journal of Clinical Oncology 2006;36(10):655‐61. [DOI] [PubMed] [Google Scholar]
Mystakidou 2005 {published data only}
- Mystakidou K, Kalaidopoulou O, Katsouda E, Parpa E, Kouskouni E, Chondros C, et al. Evaluation of epoetin supplemented with oral iron in patients with solid malignancies and chronic anemia not receiving anticancer treatment. Anticancer Research 2005;25(5):3495‐500. [PubMed] [Google Scholar]
Olsson 2002 {published data only}
- Olsson AM, Svensson JH, Sundstrom J, Bergstrom S, Edekling T, Carlsson G, et al. Erythropoietin treatment in metastatic breast cancer ‐ effects on Hb, quality of life and need for transfusion. Acta Oncologica 2002;41(6):517‐24. [DOI] [PubMed] [Google Scholar]
Overgaard 2007 {published data only}
- Overgaard J. Interim Analysis of DAHANCA 10. http://www.dahanca.dk/get_media_file.php?mediaid=125 [accessed April 1, 2009] 2006, Dec 1.
- Overgaard J, Hoff C, Sand Hansen H, Specht L, Overgaard M, Grau C, et al. Randomized study of the importance of Novel Erythropoiesis Stimulating Protein (Aranesp®) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC) ‐ the Danish Head and Neck Cancer Group DAHANCA 10 randomized trial. European Journal of Cancer Supplements 2007; Vol. 5:abstract 6LB.
Pierelli 1999 {published data only}
- Pierelli L, Perillo A, Greggi S, Salerno G, Panici PB, Menichella A, et al. Erythropoietin Addition to Granulocyte Colony‐Stimulating Factor Abrogates Life‐Threatening Neutropenia and Increases Peripheral‐Blood Progenitor‐Cell Mobilization After Epirubucin, Paclitaxel and Cisplatin Combination Chemotherapy: Results of a Randomized Comparison. Journal of Clinical Oncology 1999;17(4):1288‐95. [DOI] [PubMed] [Google Scholar]
Policarpo 2007 {published data only}
- Policarpo GD, Henry DH. Epoetin alfa for chemotherapy‐induced anemia: Assessment of two equivalent dosing regimens. Community Oncology 2007;4(3):129‐35. [Google Scholar]
Porter 1996 {published data only}
- Porter JC, Leahey A, Polise K, Bunin G, Manno CS. Recombinant human erythropoietin reduces the need for erythrocyte and platelet transfusions in pediatric patients with sarcoma: A randomized, double‐blind, placebo‐controlled trial. Journal of Pediatrics 1996;129(5):656‐60. [DOI] [PubMed] [Google Scholar]
Rau 1998 {published data only}
- Rau B, Schlag PM, Willeke F, Herfarth C, Stephan P, Franke W. Increased autologous blood donation in rectal cancer by recombinant human erythropoietin (rhEPO). European Journal of Cancer 1998;34(7):992‐8. [DOI] [PubMed] [Google Scholar]
Rearden 2004 {published data only}
- Charu V, Saidman B, Ben‐Jacob A, Justice GR, Maniam AS, Rearden T, et al. Improvements in fatigue are associated with early treatment with darbepoetin alfa every 3 weeks (Q3W) Darbepoetin alfa (DA) Treatment in Anemic Patients (pts) Receiving Chemotherapy. Blood 2004; Vol. 104:abstract 233.
- Rearden T, Charu V, Saidman B, Justice GR, Manaim AS. Results of a randomized study of every three‐week dosing (Q3W) of darbepoetin alfa for chemotherapy‐induced anemia (CIA). Blood 2003; Vol. 102, issue 11 Pt 2:abstract 3783.
- Rearden TP, Charu V, Saidman B, Ben‐Jacob A, Justice GR, Manaim AS, et al. Results of a randomized study of every three‐week dosing (Q3W) of darbepoetin alfa for chemotherapy‐induced anemia (CIA). Journal of Clinical Oncology 2004;22(14 Suppl):abstract 8064. [Google Scholar]
Reed 2005 {published data only}
- Reed SD, Radeva JI, Daniel DB, Fastenau JM, Williams D, Schulman KA. Early hemoglobin response and alternative metrics of efficacy with erythropoietic agents for chemotherapy‐related anemia. Current Medical Research & Opinion 2005;21(10):1527‐33. [DOI] [PubMed] [Google Scholar]
Rosen 2003 {published data only}
- Rosen FR, Haraf D, Brockstein B, et al. Multicenter randomized phase II study of 1 hour infusion paclitaxel, fluorouracil and hydroxyurea with concomitant hyperfractionated radiotherapy with or without erythropoietin for advanced head and neck cancer. Proceedings of the American Society of Clinical Oncology 2001:abstract 902.
- Rosen FR, Haraf DJ, Kies MS, Stenson K, Portugal L, List MA, et al. Multicenter randomized phase II study of paclitaxel (1‐hour infusion), fluorouracil, hydroxyurea, and concomitant twice daily radiation with or without erythropoietin for advanced head and neck cancer. Clinical Cancer Research 2003;9(5):1689‐97. [PubMed] [Google Scholar]
Rosenzweig 2004 {published data only}
- Rosenzweig MQ, Bender CM, Lucke JP, Yasko JM, Brufsky AM. The decision to prematurely terminate a trial of R‐HuEPO due to thrombotic events. Journal of Pain & Symptom Management 2004;27(2):185‐90. [DOI] [PubMed] [Google Scholar]
Rubio‐Martinez 2003 {published data only}
- Rubio‐Martinez A, Recaséns V, Mayayo P, Montanes A, Rubio‐Félix D, Giraldo P. Anaemia associated to multiple myeloma: response to epoetin alpha. Hematology Journal 2003; Vol. 4, issue Suppl 1:S202, abstract 258.
Sakai 2004 {published data only}
- Sakai H, Saijo N, Ohashi Y. Once‐weekly epoetin beta improves hemoglobin levels and quality of life in patients with chemotherapy‐induced anemia:A randomized, double blind, parallel group dose finding study. Annals of Oncology. 15 2004; Vol. 15, issue Suppl 3.
- Sakai H, Saijo N, Ohashi Y. Once‐weekly epoetin beta improves hemoglobin levels and quality of life in patients with chemotherapy‐induced anemia:A randomized, double blind, parallel group dose finding study. Journal of Clinical Oncology 2004;22(Suppl):abstract 8169. [DOI] [PubMed] [Google Scholar]
Schwartzberg 2004 {published data only}
- Schwartzberg LS, Yee LK, Senecal FM, Charu V, Tomita D, Wallace J, et al. A randomized comparison of every‐2‐week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy‐induced anemia in patients with breast, lung, or gynecologic cancer. Oncologist 2004;9(6):696‐707. [DOI] [PubMed] [Google Scholar]
Schwartzberg 2005 {published data only}
- Schwartzberg L, Yee L, Charu V, Tomita D, Rossi G, Senecal F. Comparable efficacy and safety of darbepoetin alfa 200 mug every 2 weeks and epoetin alfa 40,000 U weekly in patients with breast cancer: Results of a randomized comparison. The Journal of Supportive Oncology 2005;3(2 Suppl 1):30‐1. [Google Scholar]
Schwartzberg 2007 {published data only}
- Schwartzberg L, Rearden T, Yee L, Mirtsching B, Charu V, Lam H, et al. A phase II, randomized, open‐label study to assess the efficacy of extended‐dose schedule administration of darbepoetin alfa in cancer patients with chemotherapy‐induced anemia. Journal of Supportive Oncology 2007;5(4 Suppl 2):22‐3. [Google Scholar]
Scott 2002 {published data only}
- Scott SN, Boeve TJ, McCulloch TM, Fitzpatrick KA, Karnell LH. The effects of epoetin alfa on transfusion requirements in head and neck cancer patients: a prospective, randomized, placebo‐controlled study. Laryngoscope 2002;112(7 Pt 1):1221‐9. [DOI] [PubMed] [Google Scholar]
Senecal 2005 {published data only}
- Senecal FM, Yee L, Gabrail N, Charu V, Tomita D, Rossi G, et al. Treatment of chemotherapy‐induced anemia in breast cancer: results of a randomized controlled trial of darbepoetin alfa 200 microg every 2 weeks versus epoetin alfa 40,000 U weekly. Clinical Breast Cancer 2005;6(5):446‐54. [DOI] [PubMed] [Google Scholar]
Shi 2007 {published data only}
- Shi L, Hudges M, Yurgin N, Boye KS. Impact of dose frequency on compliance and health outcomes: A literature review (1966‐2006). Expert Review of Pharmacoeconomics and Outcomes Research 2007;7(2):187‐202. [DOI] [PubMed] [Google Scholar]
Silvestris 1995 {published data only}
- Silvestris F, Romito A, Fanelli P, Vacca A, Dammacco F. Long‐term therapy with recombinant human erythropoietin (rHu‐EPO) in progressing multiple myeloma. Annals of Hematology 1995;70:313‐8. [DOI] [PubMed] [Google Scholar]
Smith 2003 {published data only}
- Smith RE, Tchekmedyian NS, Chan D, Meza LA, Northfelt DW, Patel R, et al. A dose‐ and schedule‐finding study of darbepoetin alpha for the treatment of chronic anaemia of cancer. British Journal of Cancer 2003;88(12):1851‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spicka 2004 {published data only}
- Spicka I, Beguin Y. Effect of anemia correction with epoetin beta (neorecormon) once weekly on performance status and disease response. Hematology Journal 2004, issue Suppl 2:abstract 553.
Steensma 2006 {published data only}
- Steensma DP, Molina R, Sloan JA, Nikcevich DA, Schaefer PL, Rowland KM, et al. Phase III study of two different dosing schedules of erythropoietin in anemic patients with cancer. Journal of Clinical Oncology 2006;24(7):1079‐89. [DOI] [PubMed] [Google Scholar]
- Steensma DP, Molina R, Sloan JA, Nikcevich DA, Schaefer Pl, Rowland KM, et al. A Phase III Randomized Trial of Two Different Dosing Schedulesof Erythropoietin (EPO) in Patients With Cancer‐Associated Anemia: North Central Cancer Treatment Group (NCCTG) Study N02C2. Journal of Clinical Oncology Vol. 23, issue 16s:abstract 8031.
Stein 1991 {published data only}
- Stein RS, Abels RI, Krantz SB. Pharmacologic Doses of Recombinant Human Erythropoietin in the Treatment of Myelodysplastic Syndromes. Blood 1991;78(7):1658‐63. [PubMed] [Google Scholar]
Straus 2006 {published data only}
- Straus DJ, Testa M, Riggs SA, Tulpule A, Sarokhan B. Early treatment with epoetin alfa improves anemia, quality of life, and productivity in patients with hematologic malignancies and mild anemia during chemotherapy. Blood 2003; Vol. 102:abstract 1811.
- Straus DJ, Testa MA, Sarokhan BJ, Czuczman MS, Tulpule A, Turner RR, et al. Quality‐of‐life and health benefits of early treatment of mild anemia: a randomized trial of epoetin alfa in patients receiving chemotherapy for hematologic malignancies. Cancer 2006;107(8):1909‐17. [DOI] [PubMed] [Google Scholar]
Sweeney 1998 {published data only}
- Sweeney PJ, Nicolae D, Ignacio L, Chen L, Roach M, Wara W, et al. Effect of subcutaneous recombinant human erythropoietin in cancer patients receiving radiotherapy: final report of a randomized, open‐labeled, phase II trial. British Journal of Cancer 1998;77(11):1996‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 2000 {published data only}
- Thompson JA, Gilliland DG, Prchal JT, Bennett JM, Larholt K, Nelson RA, et al. Effect of recombinant human erythropoietin combined with granulocyte/macrophage colony‐stimulating factor in the treatment of patients with myelodysplastic syndrome. Blood 2000;95:1175‐9. [PubMed] [Google Scholar]
Throuvalas 2000 {published data only}
- Throuvalas NA, Antonadou D, Boufi M, Lavey R, et al. Erythropoietin decreases Transfusion Requirements during Radiochemotherapy. Proceedings of the American Society of Clinical Oncology 2000:abstract 1558.
Tsukuda 1998 {published data only}
- Tsukuda M, Yuyama S, Kohno H, Itoh K, Kokatsu T, Kawai S. Effectiveness of weekly subcutaneous recombinant human erythropoietin administration for chemotherapy‐induced anemia. Biotherapy 1998;11:21‐5. [DOI] [PubMed] [Google Scholar]
Varan 1999 {published data only}
- Buyukpamukcu M, Varan A, Kutluk T, Akyuz C. Is epoetin alfa a treatment option for chemotherapy‐related anemia in children?. Medical and pediatric oncology 2002;39(4):455‐8. [DOI] [PubMed] [Google Scholar]
- Varan A, Buyukpamukcu M, Kutluk T, Akyuz C. Recombinant human erythropoietin treatment for chemotherapy‐related anemia in children. Pediatrics 1999;103(2):E16. [DOI] [PubMed] [Google Scholar]
Wagner 2004 {published data only}
- Wagner LM, Billups CA, Furman WL, Rao BN, Santana VM. Combined use of erythropoietin and granulocyte colony‐ stimulating factor does not decrease blood transfusion requirements during induction therapy for high‐risk neuroblastoma: A randomized controlled trial. Journal of Clinical Oncology 2004;22(10):1886‐93. [DOI] [PubMed] [Google Scholar]
Waltzman 2005 {published data only}
- Waltzman R, Croot C, Justice GR, Fesen MR, Charu V, Williams D. Randomized comparison of epoetin alfa (40,000 U weekly) and darbepoetin alfa (200 microg every 2 weeks) in anemic patients with cancer receiving chemotherapy. The oncologist 2005;10(8):642‐50. [DOI] [PubMed] [Google Scholar]
- Waltzman R, Williams D. Head‐To‐Head Comparison of Epoetin Alfa (EPO) 40,000 QW vs Darbepoetin Alfa (DARB) 200 µg Q2W in Anemic Cancer Patients Receiving Chemotherapy (CT): Final Results of a Planned Interim Analysis (IA). Blood 2004; Vol. 104, issue 11:abstract 4233.
- Waltzman RJ. A randomized, active‐control, pilot trial of front‐loaded dosing regimens of darbepoetin‐alfa for the treatment of patients with anemia during chemotherapy for malignant disease. Cancer 2004;100(7):1545‐6. [DOI] [PubMed] [Google Scholar]
- Waltzman RJ, Croot C, Williams D. Final haematologic results: epoetin alfa (EPO) 40,000 U QW vs darbepoetin alfa (DARB) 200 µg Q2W in anemic cancer patients (pts) receiving chemotherapy (CT). Journal of Clinical Oncology 2005; Vol. 23, issue 16s:abstract 8030.
Watanabe 2006 {published data only}
- Watanabe M, Ezaki K, Tobinai K, Tsuboi M, Ohashi Y, Hirashima K, et al. A multicenter phase III randomized, double‐blind placebo‐controlled study of Epoetin beta administered once‐weekly for chemotherapy induced anemia (CIA) in cancer patients: Japan Erythropoietin Study Group. Annals of Oncology 2006; Vol. 17, issue Suppl 9:294.
Welch 1995 {published data only}
- Welch RS, James RD, Wilkinson PM. Recombinant human erythropoietin and platinum‐based chemotherapy in advanced ovarian cancer. Cancer Journal of the Scientific American 1995;1(4):261‐6. [PubMed] [Google Scholar]
Wurnig 1996 {published data only}
- Wurnig C, Windhager R, Schwameis E, et al. Prevention of chemotherapy‐induced anemia by the use of erythropoietin in patients with primary malignant bone tumors (a double‐blind, randomized, phase III study). Transfusion 1996;36(2):155‐9. [DOI] [PubMed] [Google Scholar]
Yilmaz 2004 {published data only}
- Yilmaz D, Cetingul N, Kantar M, Oniz H, Kansoy S, Kavakli K. A single institutional experience: is epoetin alpha effective in anemic children with cancer?. Pediatric Hematology and Oncology 2004;21(1):1‐8. [PubMed] [Google Scholar]
Zagari 2003 {published data only}
- Zagari M, Wacholz M, Xiu L. An open‐label, controlled, randomized, dose comparison study of epoetin alfa for the treatment of anemia in cancer patients receiving platinum‐containing chemotherapy. The Hematology Journal 2003;61(4 Suppl 2):abstract 0177. [Google Scholar]
Zajda 2007 {published data only}
- Zajda K, Krzakowski M. Epoetin delta in the management of anaemia in cancer patients. Journal of Clinical Oncology 2007; Vol. 25:abstract 19555.
Zhang 2003 {published data only}
- Zhang XF, Shi JG, Yang Y. Clinical observations on RHuEPO in the treatment of breast cancer chemotherapy related anemia. China Journal of Cancer Prevention and Treatment 2003;10(8):861‐2. [Google Scholar]
Zhou 2006 {published data only}
- Zhou L‐X, Wu W‐X, Li M, Yu W‐J. Clinical observation of recombinant human erythropoietin in treatment of preoperative anemia in patients with gastric cancer. Pharmaceutical Care & Research (Yaoxue Fuwu Yu Yanjiu) 2006;6(5):355‐7. [Google Scholar]
Additional references
Akaike 1974
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control 1974;19(6):716‐23. [Google Scholar]
Altman 1998
- Altman DG. Confidence intervals for the number needed to treat. BMJ 1998;317(7168):1309‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altman 1999
- Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. British Medical Journal 1999;319(7223):1492‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arcasoy 2003
- Arcasoy MO, Jiang X, Haroon ZA. Expression of erythropoietin receptor splice variants in human cancer. Biochemical Biophysical Research Communications 2003;307(4):999‐1007. [DOI] [PubMed] [Google Scholar]
Arcasoy 2005
- Arcasoy MO, Amin K, Vollmer RT, Jiang X, Mark‐Wahnefried W, Haroon ZA. Erythropoietin and erythropoietin receptor expression in human prostate cancer. Modern Pathology 2005;18(3):421‐30. [DOI] [PubMed] [Google Scholar]
Bennett 2008
- Bennett CL, Silver SM, Djulbegovic B, Samaras AT, Blau CA, Gleason KJ, et al. Venous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer‐associated anemia. Journal of the American Medical Association 2008;299(8):914‐24. [DOI] [PubMed] [Google Scholar]
Besarab 1998
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. New England Journal of Medicine 1998;339(9):584‐90. [DOI] [PubMed] [Google Scholar]
Bohlius 2004
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennet C, Engert A. Erythropoietin for patients with malignant disease. Cochrane Database of Systematic Reviews 2004, Issue 3. Art.No.: CD003407. [DOI] [PubMed] [Google Scholar]
Bohlius 2005
- Bohlius J, Langensiepen S, Schwarzer G, Seidenfeld J, Piper M, Bennett C, et al. Recombinant human erythropoietin and overall survival in cancer patients: results of a comprehensive meta‐analysis. Journal of the National Cancer Institut 2005;97(7):489‐98. [DOI] [PubMed] [Google Scholar]
Bohlius 2006
- Bohlius J, Wilson J, Seidenfeld J, Piper M, Schwarzer G, Sandercock J, et al. Recombinant human erythropoietins and cancer patients: updated meta‐analysis of 57 studies including 9353 patients. Journal of the National Cancer Institut 2006;98(10):708‐14. [DOI] [PubMed] [Google Scholar]
Bottomley 2002
- Bottomley A, Thomas R, Steen K, Flechtner H, Djulbegovic B. Human recombinant erythropoietin and quality of life: a wonder drug or something to wonder about?. Lancet Oncology 2002;3:145‐53. [DOI] [PubMed] [Google Scholar]
Boucher 1998
- Boucher KM, Slattery ML, Berry TD, Quesenberry C, Anderson K. Statistical methods in epidemiology: a comparison of statistical methods to analyze dose‐response and trend analysis in epidemiologic studies. Journal of Clinical Epidemiology 1998;51(12):1223‐33. [DOI] [PubMed] [Google Scholar]
Brines 2000
- Brines ML, Ghezzi P, Keenan S, Agnello D, Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood‐brain barrier to protect against experimental brain injury. Proceedings of National Academy of Sciences of the United States of America 2000; Vol. 97, issue 19:10526‐31. [DOI] [PMC free article] [PubMed]
Brines 2004
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta‐subunit heteroreceptor. Proceedings of Natiional Academy of Sciences of the United States of America 2004;101(41):14907‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caro 2001
- Caro JJ, Salas M, Ward A, Goss A. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer 2001;91(12):2214‐21. [PubMed] [Google Scholar]
Cella 1997
- Cella D. The functional assessment of cancer therapy‐anemia (FACT‐An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Seminars in Hematology 1997;34(Suppl 2):13‐9. [PubMed] [Google Scholar]
Clark 2002
- Clark O, Adams JR, Bennett CL, Djulbegovic B. Erythropoietin, uncertainty principle and cancer related anaemia. BMC Cancer 2002;2(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corwin 2007
- Corwin HL, Gettinger A, Fabian TC, May A, Pearl RG, Heard S, et al. Efficacy and safety of epoetin alfa in critically ill patients. New England Journal of Medicine 2007;357(10):965‐76. [DOI] [PubMed] [Google Scholar]
Cramp 2008
- Cramp F, Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2008, Issue 2, CD006145. DOI: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
D'Andrea 1989
- D'Andrea AD, Lodish HF, Wong GG. Expression cloning of the murine erythropoietin receptor. Cell 1989;57:277‐85. [DOI] [PubMed] [Google Scholar]
Dagnon 2005
- Dagnon K, Pacary E, Commo F, Antoine M, Bernaudin M, Bernaudin JF, et al. Expression of erythropoietin and erythropoietin receptor in non‐small cell lung carcinomas. Clinical Cancer Research 2005;11(3):993‐9. [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 2001
- Egger M, Smith GD, O'Rourke K. Rationale, potentials, and promise of systematic reviews. Systematic reviews in health care: meta‐analysis in context.. London: BMJ Publishing Group, 2001:3‐19. [Google Scholar]
Eisenstein 2005
- Eisenstein EL, Lemons PW, Tardiff BE, Schulman KA, Jolly MK, Califf RM. Reducing the costs of phase III cardiovascular clinical trials. American Heart Journal 2005;149(3):482‐8. [DOI] [PubMed] [Google Scholar]
Elliott 2006
- Elliott S, Busse L, Bass MB, Lu H, Sarosi I, Sinclair AM, et al. Anti‐Epo receptor antibodies do not predict Epo receptor expression. Blood 2006;107(5):1892‐5. [DOI] [PubMed] [Google Scholar]
Feldman 2006
- Feldman L, Wang Y, Rhim JS, Bhattacharya N, Loda M, Sytkowski AJ. Erythropoietin stimulates growth and STAT5 phosphorylation in human prostate epithelial and prostate cancer cells. Prostate 2006;66(2):135‐45. [DOI] [PubMed] [Google Scholar]
Fisher 2003
- Fisher JW. Erythropoietin: physiology and pharmacology update. Experimental Biology and Medicine (Maywood) 2003;228(1):1‐14. [DOI] [PubMed] [Google Scholar]
Francis 1998
- Francis JL, Biggerstaff J, Amirkhosravi A. Hemostasis and malignancy. Seminars in Thrombosis and Hemostasis 1998;24(2):93‐109. [DOI] [PubMed] [Google Scholar]
Goodnough 2003
- Goodnough LT. Risks of blood transfusion. Critical Care Medicine 2003;31(12 Suppl):678‐86. [DOI] [PubMed] [Google Scholar]
Goodnough 2005
- Goodnough LT. Risks of blood transfusion. Anesthesiology Clinics of North America 2005;23(2):241‐52. [DOI] [PubMed] [Google Scholar]
Groopman 1999
- Groopman JE, Itri LM. Chemotherapy‐induced anemia in adults: incidence and treatment. Journal of the National Cancer Institut 1999;91(19):1616‐34. [DOI] [PubMed] [Google Scholar]
Halstenson 1991
- Halstenson CE, Macres M, Katz SA, Schnieders JR, Watanabe M, Sobota JT, et al. Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clinical Pharmacology and Therapeutics 1991;50(6):702‐12. [DOI] [PubMed] [Google Scholar]
Hardee 2007
- Hardee ME, Cao Y, Fu P, Jiang X, Zhao Y, Rabbani ZN, et al. Erythropoietin blockade inhibits the induction of tumor angiogenesis and progression. PLoS ONE 2007;2(6):e549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henke 2006
- Henke M, Mattern D, Pepe M, Bezay C, Weissenberger C, Werner M, et al. Do erythropoietin receptors on cancer cells explain unexpected clinical findings?. Journal of Clinical Oncology 2006;24(29):4708‐13. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Library, Issue 4. Chichester, UK: John Wiley & Sons, Ltd., 2006. [Google Scholar]
Hockel 1993
- Hockel M, Knoop C, Schlenger K, Vorndran B, Baussmann E, Mitze M, et al. Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiotherapy Oncology 1993;26:45‐50. [DOI] [PubMed] [Google Scholar]
Holzner 2002
- Holzner B, Kemmler G, Greil R, Kopp M, Zeimet A, Raderer M, et al. The impact of hemoglobin levels on fatigue and quality of life in cancer patients. Annals of Oncology 2002;13(6):965‐73. [DOI] [PubMed] [Google Scholar]
Jelkmann 2008
- Jelkmann W, Bohlius J, Hallek M, Sytkowski AJ. The erythropoietin receptor in normal and cancer tissues. Crit Rev Oncol Hematol 2008;67(1):39‐61. [DOI] [PubMed] [Google Scholar]
Koury 1988
- Koury ST, Bondurant MC, Koury MJ. Localization of erythropoietin synthesizing cells in murine kidneys by in situ hybridization. Blood 1988;71:524‐7. [PubMed] [Google Scholar]
Koury 1990
- Koury MJ, Bondurant MC. Erythropoietin retards DNA breakdown and prevents programmed death in erythroid progenitor cells. Science 1990;248(4953):378‐81. [DOI] [PubMed] [Google Scholar]
Koury 1991
- Koury ST, Bondurant MC, Koury MJ, Semenza GL. Localization of cells producing erythropoietin in murine liver by in situ hybridization. Blood 1991;77(11):2497‐503. [PubMed] [Google Scholar]
Lai 1986
- Lai PH, Everett R, Wang FF, Arakawa T, Goldwasser E. Structural characterization of human erythropoietin. Journal of Biological Chemistry 1986 March;5(261 (7)):3116‐21. [PubMed] [Google Scholar]
Langensiepen 2002
- Langensiepen S, Bohlius J, Seidenfeld J, Piper M, Bennett CL, Schwarzer G, et al. Erythropoietin for patients with malignant disease. Cochrane Database of Systematic Reviews 2002, Issue 2. Art. No.: CD003407, DOI 10.1002/14651858.CD003407. [DOI] [PubMed] [Google Scholar]
Leo 2006
- Leo C, Horn LC, Rauscher C, Hentschel B, Liebmann A, Hildebrandt G, et al. Expression of erythropoietin and erythropoietin receptor in cervical cancer and relationship to survival, hypoxia, and apoptosis. Clinical Cancer Research 2006;12(23):6894‐900. [DOI] [PubMed] [Google Scholar]
Levine 2003
- Levine MN, Lee AY, Kakkar AK. From Trousseau to targeted therapy: new insights and innovations in thrombosis and cancer. Journal of Thrombosis and Haemostasis 2003;1(7):1456‐63. [DOI] [PubMed] [Google Scholar]
Lind 2002
- Lind M, Vernon C, Cruickshank D, Wilkinson P, Littlewood T, Stuart N, et al. The level of haemoglobin in anaemic cancer patients correlates positively with quality of life. British Journal of Cancer 2002;86(8):1243‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Littlewood 2003
- Littlewood TJ, Zagari M, Pallister C, Perkins A. Baseline and early treatment factors are not clinically useful for predicting individual response to erythropoietin in anemic cancer patients. Oncologist 2003;8(1):99‐107. [DOI] [PubMed] [Google Scholar]
Ludwig 2001
Ludwig 2004
- Ludwig H, Belle S, Barrett‐Lee P, Birgegard G, Bokemeyer C, Gascon P, et al. The European Cancer Anaemia Survey (ECAS): a large multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. European Journal of Cancer 2004;40(15):2293‐306. [DOI] [PubMed] [Google Scholar]
Luksenburg 2004
- Luksenburg H, Weir A, Wager R. Safety concerns associated with Aranesp (darbepoetin alfa) Amgen Inc and Procrit (epoetin alfa) Ortho Biotech L.P. for the treatment of anemia associated with cancer chemotherapy. Hearings before the Subcommittee on Oncologic Drugs Advisory Committee of the House Committee on Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Rockville (MD), USA, 2004; Vol. http://www.fda.gov/ohrms/dockets/ac/04/briefing/4037b2_04.pdf [date of last access March 27, 2009].
McBroom 2005
- McBroom JW, Acs G, Rose GS, Krivak TC, Mohyeldin A, Verma A. Erythropoietin receptor function and expression in epithelial ovarian carcinoma. Gynecologic Oncology 2005;99(3):571‐7. [DOI] [PubMed] [Google Scholar]
Mcentegart 1999
- Mcentegart DJ, Jadhav SP, Brown T, Channon EJ. Checks of case record forms versus the database for efficacy variables when validation programs exist. Drug Information Journal 1999;33:101‐7. [Google Scholar]
Minton 2008
- Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta‐analysis of the pharmacological treatment of cancer‐related fatigue. Journal of the National Cancer Institute 2008;100(16):1155‐66. [DOI] [PubMed] [Google Scholar]
Mohyeldin 2005
- Mohyeldin A, Lu H, Dalgard C, Lai SY, Cohen N, Acs G, et al. Erythropoietin signaling promotes invasiveness of human head and neck squamous cell carcinoma. Neoplasia 2005;7(5):537‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osterborg 2007
- Osterborg A, Aapro M, Cornes P, Haselbeck A, Hayward CR, Jelkmann W. Preclinical studies of erythropoietin receptor expression in tumour cells: impact on clinical use of erythropoietic proteins to correct cancer‐related anaemia. European Journal of Cancer 2007;43(3):510‐9. [DOI] [PubMed] [Google Scholar]
Ribatti 2007a
- Ribatti D, Marzullo A, Longo V, Poliani L. Schwann cells in neuroblastoma express erythropoietin. Journal of Neuro‐Oncology 2007;82(3):327‐8. [DOI] [PubMed] [Google Scholar]
Ribatti 2007b
- Ribatti D, Poliani PL, Longo V, Mangieri D, Nico B, Vacca A. Erythropoietin/erythropoietin receptor system is involved in angiogenesis in human neuroblastoma. Histopathology 2007;50(5):636‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rizzo 2008
- Rizzo JD, Somerfield MR, Hagerty KL, Seidenfeld J, Bohlius J, Bennett CL, et al. Use of Epoetin and Darbepoetin in Patients with Cancer. American Society of Clinical Oncology/American Society of Hematology Clinical Practice Guideline Update. Journal of Clinical Oncology 2008;26:132‐149. [DOI] [PubMed] [Google Scholar]
Royston 1999
- Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. International Journal of Epidemiology 1999;28(5):964‐74. [DOI] [PubMed] [Google Scholar]
Schrijvers 1999
- Schrijvers D, Highley M, Bruyn E, Oosterom AT, Vermorken JB. Role of red blood cells in pharmacokinetics of chemotherapeutic agents. Anticancer Drugs 1999 February; 10(2):147‐53;10(2):147‐53. [DOI] [PubMed] [Google Scholar]
Sehata 2007
- Sehata N, Walker I, Meyer R, Haynes AE, Imrie K, and Cancer Care Ontario's Program in Evidence‐Based Care's Systematic Disease Site Group. Treatment for anemia with erythropoietic agents in patients with non‐myeloid hematological malignancies: a clinical practice guideline. Report 2007:6‐12.
Seidenfeld 2001
- Seidenfeld J, Piper M, Flamm C, Hasselblad V, Armitage JO, Bennett CL, et al. Epoetin treatment of anemia associated with cancer therapy: a systematic review and meta analysis of controlled clinical trials. Journal of the National Cancer Institut 2001;93(16):1204‐14. [DOI] [PubMed] [Google Scholar]
Seidenfeld 2006
- Seidenfeld J, Piper M, Bohlius J, Weingart O, Trelle S, Engert A, et al. Comparative effectiveness of epoetin and darbepoetin for managing anemia in patients undergoing cancer treatment. Comparative Effectiveness, Review No. 3, Rockville, MD: Agency for Healthcare Research and Quality, May 2006. [PubMed]
Simmonds 2005
- Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta‐analysis of individual patient data from randomized trials: a review of methods used in practice. Clinical Trials 2005;2(3):209‐17. [DOI] [PubMed] [Google Scholar]
Sinclair 2008
- Sinclair AM, Rogers N, Busse L, Archibeque I, Brown W, Kassner PD, et al. Erythropoietin receptor transcription is neither elevated nor predictive of surface expression in human tumour cells. British Journal of Cancer 2008;98(6):1059‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2006
- Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, et al. Correction of anemia with epoetin alfa in chronic kidney disease. New England Journal of Medicine 2006;355(20):2085‐98. [DOI] [PubMed] [Google Scholar]
Smith 2005
- Smith CT, Williamson PR, Marson AG. Investigating heterogeneity in an individual patient data meta‐analysis of time to event outcomes. Statistics in Medicine 2005;24(9):1307‐19. [DOI] [PubMed] [Google Scholar]
Smith 2007
- Smith TC, Willamson PR. A comparison of methods for fixed effects meta‐analysis of individual patient data with time to event outcomes. Clinical Trials 2007;4:621‐30. [DOI] [PubMed] [Google Scholar]
Spivak 2005
- Spivak JL. The anaemia of cancer: death by a thousand cuts. Nature Reviews Cancer 2005;5(7):543‐55. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [DOI] [PubMed] [Google Scholar]
Stewart 1995
- Stewart LA, Clarke MJ. Practical methodology of meta‐analyses (overviews) using updated individual patient data. Cochrane Working Group. Statistics in Medicine 1995;14(19):2057‐79. [DOI] [PubMed] [Google Scholar]
Stohlawetz 2000
- Stohlawetz PJ, Dzirlo L, Hergovich N, Lackner E, Mensik C, Eichler HG, et al. Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood 2000;95(9):2983‐9. [PubMed] [Google Scholar]
Storring 1998
- Storring PL, Tiplady RJ, Gaines Das RE, Stenning BE, Lamikanra A, Rafferty B. Epoetin alfa and beta differ in their erythropoietin isoform compositions and biological properties. British Journal of Haematology 1998;100:79‐89. [DOI] [PubMed] [Google Scholar]
Toy 2005
- Toy P, Popovsky MA, Abraham E, Ambruso DR, Holness LG, Kopko PM, et al. Transfusion‐related acute lung injury: definition and review. Critical Care Medicine 2005 April;33(4):721‐6. [DOI] [PubMed] [Google Scholar]
Um 2007
- Um M, Gross AW, Lodish HF. A "classical" homodimeric erythropoietin receptor is essential for the anti‐apoptotic effects of erythropoietin on differentiated neuroblastoma SH‐SY5Y and pheochromocytoma PC‐12 cells. Cell Signal 2007;19(3):634‐45. [DOI] [PubMed] [Google Scholar]
Vaupel 2001
- Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Medical Oncology 2001;18(4):243‐59. [DOI] [PubMed] [Google Scholar]
Vaupel 2002
- Vaupel P, Thews O, Mayer A, Hockel S, Hockel M. Oxygenation status of gynecologic tumors: what is the optimal hemoglobin level?. Strahlentherapie und Onkologie 2002;178(12):727‐31. [DOI] [PubMed] [Google Scholar]
Vaupel 2005
- Vaupel P, Mayer A. Hypoxia and anemia: effects on tumor biology and treatment resistance. Transfusion Clinique et Biologique 2005;12(1):5‐10. [DOI] [PubMed] [Google Scholar]
Vaziri 1999
- Vaziri ND. Mechanism of erythropoietin‐induced hypertension. American Journal of Kidney Diseases 1999;33(5):821‐28. [DOI] [PubMed] [Google Scholar]
Vogelzang 1997
- Vogelzang NJ, Breitbart W, Cella D, Curt GA, Groopman JE, Horning SJ, et al. Patient, caregiver, and oncologist perceptions of cancer‐related fatigue: results of a tri part assessment survey. The Fatigue Coalition. Seminars in Hematology 1997;34(3 Suppl 2):4‐12. [PubMed] [Google Scholar]
Whitehead 2002
- Whitehead A. Meta‐analysis using individual patient data. Meta‐Analysis of Controlled Clinical Trials. Chichester: John Wiley & Sons, Ltd., 2002:90‐150. [Google Scholar]
Wilson 2007
- Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, et al. A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment. Health Technology Assessment 2007;11:1‐220. [DOI] [PubMed] [Google Scholar]
Wood 1995
- Wood PA, Hrushesky JM. Cisplatin‐associated anemia: an erythropoietin deficiency syndrome. Journal of Clinical Investigation 1995;95:1650‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wun 2003
- Wun T, Law L, Harvey D, Sieracki B, Scudder SA, Ryu JK. Increased incidence of symptomatic venous thrombosis in patients with cervical carcinoma treated with concurrent chemotherapy, radiation, and erythropoietin. Cancer 2003;98(7):1514‐20. [DOI] [PubMed] [Google Scholar]
Yasuda 2003
- Yasuda Y, Fujita Y, Matsuo T, Koinuma S, Hara S, Tazaki A, et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis 2003;24:1021‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bohlius 2008
- Bohlius J, Trelle S, Weingart O, Schwarzer G, Brillant C, Clarke M, et al. Erythropoietin or Darbepoetin for patients with cancer ‐ meta‐analysis based on individual patient data.. Cochrane Database of Systematic Reviews 2008, Issue 3. Art. No.: CD007303. DOI: 10.1002/14651858.CD007303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bohlius 2009
- Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis‐stimulating agents and mortality in patients with cancer: a meta‐analysis of randomised trials. Lancet 2009;373(9674):1532‐42. [DOI] [PubMed] [Google Scholar]


