Abstract
Objective:
This study investigated the audiological and tinnitus outcomes of cochlear implantation (CI) in adults with single-sided deafness (SSD) and tinnitus.
Study Design:
Multicentered prospective, non-randomized intervention study.
Setting:
Six French CI centers.
Patients:
Twenty-six patients with SSD and incapacitating tinnitus (Tinnitus Handicap Inventory [THI] >58) underwent cochlear implantation.
Interventions:
First, CIs delivered only masking white noise stimulation for 1 month and then standard CI stimulation.
Main Outcome Measures:
Before and after CI surgery, patients completed the THI, Tinnitus Reaction Questionnaire (TRQ), Subjective Tinnitus Severity Scale (STSS), and two visual analogue scales quantifying tinnitus loudness and annoyance. Speech perception in spatialized noise was tested at 13 months.
Results:
The first month of white noise stimulation triggered a significant improvement in THI scores (72 ± 9 to 55 ± 20, p < 0.05). No change was observed for the other measures. After 1 year of standard CI stimulation, 23 patients (92%) reported a significant improvement in tinnitus. This improvement started 1 to 2 months after CI and exceeded 40% improvement for 14 patients (54%). Average speech-in-noise perception after 1 year significantly improved for the 23 patients who completed these measures.
Conclusions:
CI is efficacious to reduce the handicap of patient with SSD and incapacitating tinnitus, leading to a decrease in reported tinnitus and partial restoration of binaural hearing abilities.
Keywords: Binaural hearing, Cochlear implant, Tinnitus, Unilateral hearing loss, White noise masking
Single-sided deafness (SSD) describes hearing impairment in one ear and normal hearing in the contralateral ear. It is defined as pure-tone average (PTA) hearing thresholds at 0.5, 1, 2, and 4 kHz more than or equal to 70 dB HL in the poorer ear and less than or equal to 30 dB HL in the better ear (1). SSD affects sound localization (2) and leads to effortful listening (3,4). Patients with SSD often experience associated tinnitus, which can have a negative impact on quality of life and lead to anxiety and depression. Tinnitus treatment includes psychoactive drugs (e.g., antidepressants or anxiolytics) and psychological therapy (5). For example, cognitive behavioral therapy can be offered together with a hearing aid and/or masking sound generator (e.g., (6)). Current SSD treatment involves the provision of hearing devices that transfer sounds from the deaf side to the contralateral ear with contralateral routing of offside signal (CROS) or bone conduction. They do not stimulate the deprived auditory pathways of the impaired ear and therefore do not relieve tinnitus (1).
Cochlear implantation (CI) partially restores auditory input with direct electrical stimulation of the auditory nerve. In cases of bilateral severe-to-profound hearing loss and tinnitus, it reduces tinnitus in 29 to 100% of cases and eliminates tinnitus in 20 to 60% of cases (7–13). Auditory pathway stimulation could trigger a decrease in perceived tinnitus, without the need for further stimulation with for example masking noise (14–16). In the first published clinical study of CI for SSD patients with ipsilateral incapacitating tinnitus, tinnitus loudness was reduced in 20 of the 21 subjects and full effect was reported as early as 1 month after CI activation (17). Many studies have since reproduced tinnitus reduction from CI in SSD patients (18–29). However, the samples included in these studies were heterogeneous. Few studies had strict inclusion criteria regarding hearing loss severity on the un-implanted side (19,23) or reported their inclusion criteria for tinnitus severity (22,23). Some patients had hearing loss in their good ear and were within or close to the criteria for CI; some patients had mild tinnitus. The results support how CI stimulation improves tinnitus, but more evidence is required to support CI as an efficacious tinnitus treatment in patients with SSD. Furthermore, various scales and questionnaires were used in these studies to evaluate tinnitus and its impact. Thus, comparisons between studies remain difficult. Several studies only used a visual analogue scale (VAS) to investigate tinnitus intensity. Also, the psychosocial impact of tinnitus pre- and post-CI is important to measure. CIs could improve binaural hearing abilities for SSD patients, including sound localization (30–32) and speech-in-noise perception (19,29,32,33).
The present study evaluated the benefit of CI on incapacitating tinnitus 1 year after surgery with two types of stimulation: 1) white noise stimulation and 2) standard stimulation. A homogenous group of patients with SSD and incapacitating tinnitus with normal or near normal contralateral hearing was included. Disability and annoyance related to tinnitus were investigated as well as speech-in-noise perception and binaural effects.
MATERIALS AND METHODS
Study Design
This multicentered prospective study was open and non-randomized. Patients acted as their own controls. The study was registered in the U.S. National Library of Medicine clinical trial database (ID: NCT02966366). Data were collected in six French cochlear implantation centers between August 2013 and May 2017. The study was approved by the French health authorities (ANSM: 2012-A01453-40) and the Ile-de-France VI (Paris, France) ethics committee (IRB: 2012-A01453-40).
Population Description
The inclusion criteria were:
-
-
Adult (≥18 years of age)
-
-
SSD (PTA hearing thresholds at 0.5, 1, 2, and 4 kHz ≥70 dB HL in the poorer ear and <35 dB HL at 2 kHz in the better ear)
-
-
Native French speaker
-
-
Ipsilateral incapacitating tinnitus at enrolment:
-
∘
Tinnitus Handicap Inventory score (THI) more than 58 (scores could range from 0 to 100).
-
∘
VAS assessment of tinnitus annoyance more than or equal to 8 (scores could range from 0 to 10).
-
∘
Duration more than or equal to 1 year.
-
∘
Attempted more than or equal to one form of tinnitus treatment for more than or equal to 1 year without satisfactory treatment effect.
-
∘
-
-
Normal vestibular function contralateral to the SSD.
Based on preoperative CT scans and additional evaluation, patients were excluded if they presented middle ear pathology, cochlear ossification, retro-cochlear pathology, documented severe central auditory processing disorder, significant depression (Beck's Depression Inventory score >16), or if a psychologist or psychiatrist recommended against CI.
Study Procedure
The patients were seen twice over the 2 months preceding implantation surgery. All patients underwent standard CI surgery with a 20-channel Digisonic SP system (Oticon Medical, Vallauris, France) and received standard audiology and speech therapy care.
For the first month after CI activation patients received white noise stimulation: they did not receive any standard sound stimulation. Thereafter, patients received standard stimulation. Patients were instructed to use their CI more than or equal to 5 hours per day and to log their daily CI usage in a diary. All patients who completed the 7- and 13-month visits complied. Average daily usage ranged from 1 to 8 hours during the first month following CI activation (mean: 5 ± 1.5, median: 5), and from 5 to 15 hours during the follow-up period (mean: 9 ± 3, median: 9). Data logging of CI usage was not available.
Tinnitus severity was assessed before implantation (pre-CI), at activation, and at 1, 2, 4, 7, and 13 months post-activation using two VAS (34) evaluating loudness and annoyance and three questionnaires translated and validated in French (Fig. 1).
FIG. 1.
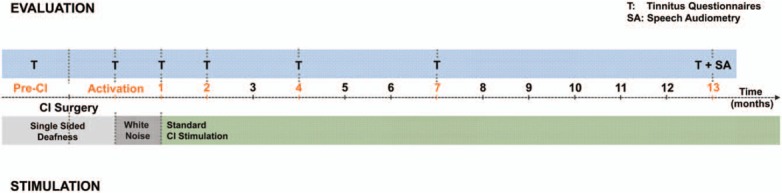
Study design. Top bar (blue) represents the different visit for tinnitus (T) evaluation and speech audiometry (SA) test. The bottom bar indicates the type of stimulation provided through CI (white noise or standard stimulation). CI indicates cochlear implantation.
-
1)
The THI (35,36) measures disability associated with tinnitus. Its 25 items have three response options: no (scored 0), sometimes (scored 2), and yes (scored 4). Total scores range 0 to 100 and describe severity: slight (0–16); mild (18–36); moderate (38–56); severe (58–76), and; catastrophic (78–100).
-
2)
The Tinnitus Reaction Questionnaire (TRQ; (37,38)) measures psychological distress associated with tinnitus. Its 26 items target general distress, interference with daily activities, severity of tinnitus, and avoidance, with five response options: not at all (scored 0), a little of the time (scored 1), some of the time (scored 2), a good deal of the time (scored 3), and almost all of the time (scored 4). Total scores range 0 to 104. Scores more than 60 indicate significant psychological distress.
-
3)
The Subjective Tinnitus Severity Scale (STSS; (39,40)) measures tinnitus severity. Its 16 items correspond to three sub-domains: intrusion, dominance, and distress with two response options: no (scored 0, except for six reverse-scoring items) and yes (scored 1, except for six reverse-scoring items). Total scores range 0 to 16.
Speech perception and binaural hearing were evaluated at 13 months post-CI activation using a spatialized speech-in-noise perception test. Stimuli included French monosyllabic words (Fournier lists) with free-field stationary white noise in best aided condition (i.e., with contralateral hearing aid for patients wearing one). The percentage of words correctly identified was measured at –3 and +5 dB signal-to-noise ratio (SNR) in three different spatial configurations: signal and noise presented from the front (S0N0), signal presented from the front and noise presented on the CI side (S0NCI), and noise presented from the front and signal presented on the CI side (SCIN0). All hearing tests were performed with CI turned off and then with CI on.
Binaural effects were evaluated through three effects: the summation effect (difference CI on versus CI off in the S0N0 spatial configuration), the squelch effect (difference CI on versus CI off in the S0NCI spatial configuration), and the head shadow effect (difference S0NCI spatial configuration and SCIN0 spatial configuration; (33,41)).
Statistical Analyses
Statistica 10 (StatSoft, Maisons-Alfort, France) was used. Shapiro-Wilk normality tests, with an alpha value at or above 0.0014, indicated a normal distribution of data: only STSS scores on activation day was significant (W = 0.83, p = 0.00071), suggesting non-normal distribution for this subset of the data. All residuals however followed a normal distribution (all ps > 0.0014). Tinnitus questionnaires were analyzed with repeated-measures analyses of variance (ANOVAs). Speech perception scores were transformed into rationalized arcsine units (RAU) (42) for ANOVAs where they were the dependent variable. Although RAU values were used for statistical analyses, raw scores are reported. A level of p < 0.05 was used to determine statistical significance.
RESULTS
Twenty-six patients were included. Of these, one patient had a severe accident 1 week after the CI activation and was excluded from measures post-CI. Two patients withdrew from the study after the 4-month visit: one patient experienced an aggravation of the tinnitus and one experienced no benefit from CI stimulation. All data collected on these two patients up to and including the 4-month visit were kept for analysis. Twenty-three patients completed the 7-month visit and the final 13-month visit when speech audiometry and binaural effects were measured. Missing data were kept as missing and no data imputation took place.
The sample included 17 men and nine women, with a mean age of 54.2 ± 10 years [27–69]. Table 1 summarizes tinnitus characteristics before CI surgery. Tinnitus duration ranged 1 to 21 years, with a mean of 7.2 ± 5 years. The contralateral ear had normal or near-normal hearing with mean pure-tone thresholds of 18 ± 11, 18 ± 10, 23 ± 11, and 39 ± 19 dB HL, at 500, 1000, 2000, and 4000 Hz, respectively.
TABLE 1.
Tinnitus characteristics before cochlear implant surgery (n = 26)
| n (%) | |
| Tinnitus and hearing loss | |
| Side | |
| Right | 8 (31%) |
| Left | 18 (69%) |
| Etiology | |
| Sensorineural hearing loss | 9 (35%) |
| Trauma (noise, pressure, or cranial fracture) | 5 (19%) |
| Middle ear surgery | 4 (15%) |
| Unknown | 8 (31%) |
| Tinnitus | |
| Occurrence | |
| Progressive | 7 (27%) |
| Immediately with hearing loss | 19 (73%) |
| Type | |
| Constant | 17 (85%) |
| Fluctuating (non pulsatile) | 3 (15%) |
| THI | |
| Severe handicap (58–76) | 18 (69%) |
| Catastrophic handicap (78–100) | 8 (31%) |
| Tinnitus treatment trialed before CI surgery | |
| Medical treatment (antidepressants, anxiolytics, etc.) | 15 (58%) |
| Sound therapy (masking sound, hearing aids, CROS devices) | 9 (35%) |
| Cognitive behavioral therapy | 4 (15%) |
| Alternative therapy (osteopathy, sophrology, acupuncture, magnetism) | 5 (31%) |
| >1 inefficacious therapy tested | 15 (58%) |
CI indicates cochlear implantation; CROS, contralateral routing of offside signal; THI, Tinnitus Handicap Inventory.
Tinnitus appeared with hearing loss for 19 patients (73%) and after hearing loss for the remaining seven patients (27%). Seventeen patients (85%) had a constant tinnitus and three patients described a tinnitus fluctuating in intensity, but not pulsatile. All patients had trialed tinnitus treatment without significant relief.
Tinnitus Handicap Inventory Scores
Eighteen patients (69%) had a severe handicap and eight patients (31%) had a catastrophic handicap (Fig. 2A). The mean score before surgery was 72 ± 9 and decreased progressively to 26 ± 20 at 13 months post-CI (F[6, 132] = 27, p < 0.05). At study completion, nine patients had a slight or no handicap, seven had mild handicap, six had moderate handicap, one had severe handicap, and none had catastrophic handicap (Fig. 2B). THI scores were unchanged at CI activation (62 ± 18; post hoc Bonferroni corrected test, p = 0.34) but started to significantly improve 1 month later to 55 ± 20, i.e., after 1 month of white noise stimulation (all subsequently corrected ps < 0.05).
FIG. 2.
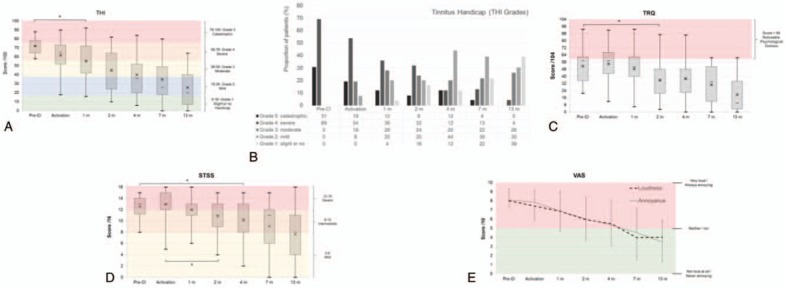
Results on questionnaires and VAS. A, Evolution of the THI score during follow-up. Box and whisker plots are minimum values, 1st quartile, median (horizontal bar), mean (cross), 3rd quartile, and maximal values. The different color-shaded areas indicate the different grades of THI: grade 1 (0–16, green), grade 2 (18–36, blue), grade 3 (38–56, yellow), grade 4 (58–76, orange), and grade 5 (78–100, red). B, Evolution of the THI grades during follow-up. Proportion of tested patients in different tinnitus handicap grades from THI. C, Evolution of the TRQ scores during follow-up. Noticeable psychological distress is in the red shaded area (scores >60). Box and whisker plots show minimum values, the 1st quartile, median (horizontal bar), mean (cross), 3rd quartile, and maximal value. D, Evolution of the STSS scores during follow-up. The grades are indicated by color-shaded areas: scores <8 = mild—yellow, 8 < scores < 12 = intermediate—orange, and scores >12 = severe—red. Box and whiskers plots show the minimum value, 1st quartile, median (horizontal bar), mean (cross), 3rd quartile, and maximal value. E, VAS loudness and annoyance outcomes during follow-up. Results are expressed as mean with standard deviation. Positive judgments are in the green area (not intense/not annoying) and negative ratings are in the red area (intense/annoying). STSS indicates Subjective Tinnitus Severity Scale; THI, Tinnitus Handicap Inventory; TRQ, Tinnitus Reaction Questionnaire; VAS, visual analogue scale.
Tinnitus Reaction Questionnaire Scores
The mean TRQ score was 51.6 ± 18 before CI surgery. Psychological distress was noticeable with a TRQ score more than or equal to 60 for 12 patients (48%). TRQ scores significantly decreased at 2 months post-activation compared with pre-CI scores and further decreased to 19.5 ± 19 at 13 months post-activation (F(6,132) = 18.5, p < 0.05). After 1 year of conventional CI stimulation, only one patient continued to experience tinnitus with a score more than 60. Eight patients (34.8%) had no further psychological distress (scores ≤10) (Fig. 2C).
Subjective Tinnitus Severity Scale Scores
Before CI surgery, mean STSS scores were 12.5 ± 2, with scores more than 12 (severe tinnitus) for 69% of patients. All other patients scored between 8 and 12 (intermediate tinnitus) (Fig. 2D). STSS scores were significantly reduced at 4 months compared with pre-CI scores and reached an average of 7.6 ± 4 at 13 months, corresponding to mild tinnitus (F (6, 132) = 15, p < 0.05). Individual scores indicated that at 13 months post-activation, two patients continued having STSS scores more than 12 (8.7%). Eight had scores between 8 and 12, and 13 were less than 8 (56.5%).
Tinnitus Loudness and Annoyance on VAS
The VAS means of tinnitus loudness and annoyance were 8.0 ± 1 and 8.1 ± 1, respectively, before surgery (Fig. 2E). Both VAS triggered similar ratings: Pearson's correlation coefficient between both VAS scores was significant at each session, with r(173) = 0.89, p < 0.05, corresponding to a coefficient of determination R2 = 0.79. Therefore, scores were averaged on one scale for each patient. A one-way ANOVA with factor Sessions showed a significant main effect (F(6, 132) = 27.3, p < 0.05), and a Bonferroni corrected post hoc evaluation indicated that scores improved compared with pre-CI (8.1 ± 0.9) at 2 months post-activation (6.0 ± 2.3) and further to 3.8 ± 2.5 at 13 months (ps < 0.05).
Tinnitus Evaluations: Individual Observations and Individual Variability
To summarize scores at 7 and13 months post-activation, they were normalized into a percent scale relative to the pre-CI score for each patient. At 13 months, scores from the different tests correlated significantly with each other (r(23) = 0.72, ps < 0.05). Therefore, an aggregate score averaged the scores of the four tinnitus questionnaires at 7 and 13 months post-activation (Fig. 3). Tinnitus significantly improved between 7 and 13 months post-activation (F(1, 22) = 10.9, p < 0.05). The mean tinnitus improvement was 40.5% ± 27 at 7 months post-CI (range, 0.12–99.0%) and increased further to 54.0% ± 27 at 13 months (range, 13.6–100.0%). Scores were stable for six patients and improved for 17 patients. Four patients had a slow improvement of the tinnitus outcomes less than 10% at 7 months but reached more than 10% at 13 months. Lastly, one patient exhibited a decreased in benefit between the 7 and the 13 months visit (from 57 to 30% improvement). All 23 patients attending the 13-month visit reported improvement of their tinnitus on more than or equal to two of the four questionnaires.
FIG. 3.
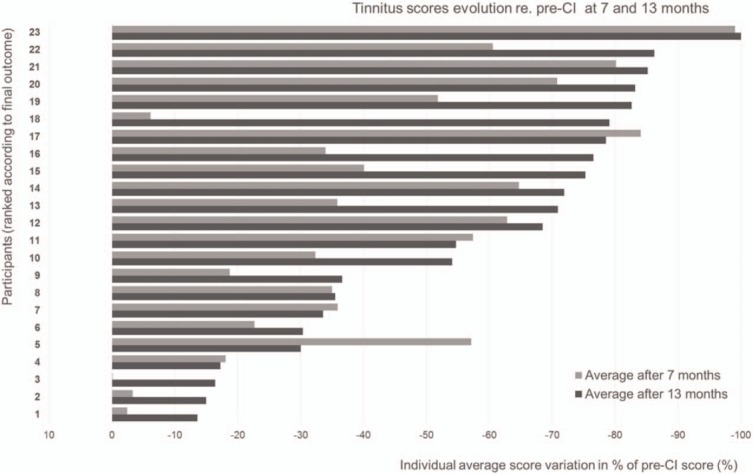
Inter-individual evolution of tinnitus questionnaires and VAS improvement between 7 months (light gray) and 13 months (dark gray). Aggregate mean normalized scores, compared with the Pre-CI score, considered as 100 (a 50% decrease corresponds to scores being divided by two, compared with Pre-CI scores, a 100% decrease corresponds to scores going from any pre-CI score to 0). CI indicates cochlear implantation; VAS, visual analogue scale.
Speech Perception and Binaural Effects
CI stimulation improved speech perception at +5 dB SNR in all configurations tested (23 patients, Figure 4, F(1, 22) = 6.5, p < 0.05; SNR: F(1, 22) = 262.3, p < 0.05; Configuration F(2, 44) = 12.8, p < 0.05). Moreover, in binaural conditions, the combined head-shadow and squelch effect in the SCIN0 configuration was improved (p < 0.05). However, the summation effect and the squelch effect did not reach significance.
FIG. 4.
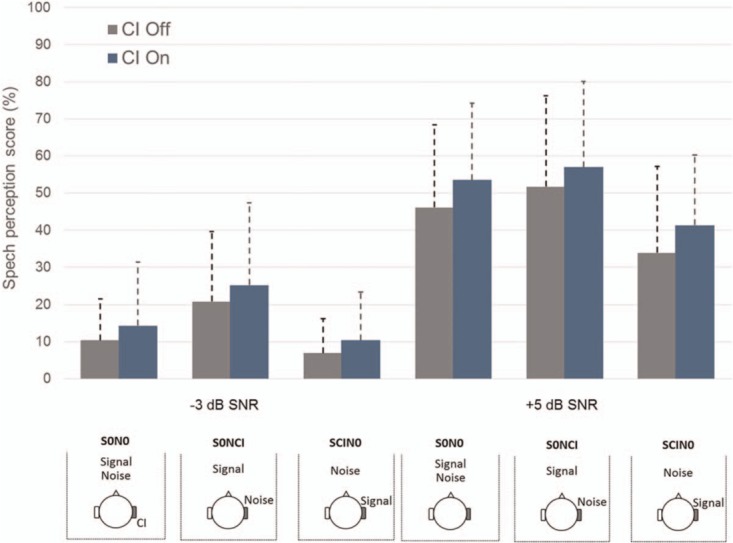
Speech spatialized noise outcomes at 13 months. Mean scores are expressed in percent correct word identification, CI off (gray) and CI on (blue). Patients were tested at –3 and +5 dB SNR in spatialized white noise. CI indicates cochlear implantation; SNR, signal-to-noise ratio.
DISCUSSION
The present study assessed prospectively the benefit of CI stimulation on incapacitating tinnitus associated with SSD. Tinnitus was monitored using three validated questionnaires (THI, TRQ, and STSS) and two VAS.
Treatment Effect
Restoring the sound input to the deaf ear of SSD patients reduced tinnitus, with 23 of the 25 patients (92%) responding to CI treatment. Two patients withdrew because of limited benefits. This is in line with a review showing that among 30 patients included in five studies, 87% exhibited improvement, 13% showed no improvement, and no patient experienced worsening symptoms (24).
Time Course of Treatment Effect
Significant improvement was observed on the tinnitus measures in the months following implantation: 1 month for THI, 2 months for VAS and TRQ, and 4 months for STSS. Progressive tinnitus reduction was observed until last follow-up without any plateau. Another study observed a maximum decrease of tinnitus severity 1 month after CI activation (17). Such decrease was not observed in the present study after 1 month of white noise stimulation, suggesting that unmeaningful stimulation was partially efficient to reduce severe tinnitus, either through a masking effect or re-activation of the peripheral pathways. In a previous study, various unmeaningful stimuli such as noise, sine waves, music, and environmental sounds delivered through a CI efficiently suppressed tinnitus (43). The characteristics of the white noise stimulation used could explain its limited effect. Indeed, studies showed a positive effect of unmeaningful stimulation when the stimulation electrodes, frequency, and levels were personalized for every CI user (14,15). Results of white noise stimulation could have been additionally affected by the specific inclusion criteria. The Van de Heyning et al. (17) participants were not selected according to their tinnitus severity: the rapid relief noticed could be due to less disabling tinnitus at the time of inclusion.
Tinnitus Measures
To date, the THI has seldom been used to monitor tinnitus handicap after CI in SSD patients. One exception is the report of a decrease in THI scores in 12 SSD patients already after 1 month of standard CI stimulation (44). The homogeneous population in terms of tinnitus severity could explain this benefit shortly after activation. Nevertheless, other studies with patients experiencing various severities of tinnitus reported stable ratings after a few months of CI use (17,27).
CI treatment had a positive impact on the psychological distress associated with tinnitus (TRQ), with the effect observed 2 months post-activation. Only one patient still noted a score more than 60, indicating significant psychological distress, despite consistent CI usage. Similar results of reduction on psychological distress have been shown previously (29).
The STSS, reporting tinnitus severity, showed the highest variability in answers and a slower improvement in scores, reaching significance 4 months post-activation. At 13 months, over half of the patients had STSS scores less than 8, indicating mild tinnitus. The slow improvement in scores may result from the questionnaire format. Indeed, the STSS was the only questionnaire where response options were binary (yes/no). Patients who experienced tinnitus improvement but still perceived some tinnitus when removing their CI processor could have rated their tinnitus as unchanged. In its current form, the STSS seems to be of limited value to assess CI treatment effect. The STSS could be more valid if respondents were asked to answer according to when wearing their CI processor.
Tinnitus loudness and annoyance were significantly improved 2 months after activation, corresponding to 1 month of standard CI stimulation. Similarly, a significant tinnitus reduction 1 month after CI surgery which remained stable after 3 months has been observed previously (27). However, tinnitus relief may disappear once the CI processor is turned off (17,27): in one study, 65% of subjects reported that tinnitus relief had disappeared 1 minute after CI processor switch off (27).
All tinnitus measures were correlated and when comparing their responsiveness, THI showed the largest range of both individual responses and changes over time. THI offers a wide continuous scoring of tinnitus disability from 0 to 100 and the categories describing scores from slight to catastrophic are useful. Present results suggest THI as a valid and responsive measure of tinnitus treatment effect.
Binaural Hearing
Speech-in-noise perception at –3 and +5 dB SNR improved 13 months post-CI. Perception scores were better in all tested spatial configurations when the CI processor was turned on versus off. A significant combined squelch and head-shadow effect was observed (p = 0.02). This observation is coherent with former work showing at least partial restoration of binaural hearing abilities in SSD patients, with an improvement of speech-in-noise perception (19,29,33). The mechanisms, however, are still unknown. No significant “pure” squelch effect was observed. This is in line with previous work reporting a significant squelch effect only for patients using a hearing aid on the contralateral ear (19,26,33). Because CIs translate sounds into electrical pulse trains delivered at a fixed stimulation rate, they poorly restore temporal fine structure information. CIs cannot transfer low-frequency information associated with fine temporal structure coding. Conversely, they reproduce level differences occurring between the two ears, which are more relevant for high-frequency sounds.
CI is currently the only hearing rehabilitation that both improves tinnitus severity and binaural hearing in SSD patients. There seems to be an interaction between tinnitus severity and audiological outcomes in SSD patients. It has already been shown that tinnitus in one ear could interfere with speech-in-noise perception in the non-tinnitus ear (25). Long-term outcomes over 10 years of CI with patients with SSD or asymmetric hearing loss have showed that SSD patients rate tinnitus reduction as the primary benefit of CI while the latter group of patients rate hearing improvement as the primary benefit of CI (27). This highlights the need for clinical guidelines for CI patients with SSD, focusing on tinnitus over the first months and progressively moving to binaural hearing once tinnitus is reduced.
Neural Plasticity
The fact that SSD patients have normal or near-normal contralateral hearing could slow or limit both tinnitus reduction and the acquisition of binaural hearing abilities. The sudden loss of auditory input in one side triggers central auditory plasticity, including a progressive reorganization of auditory pathways and auditory brain areas potentially associated with phantom sensations in the ipsilateral ear (45–48). Restoration of auditory input via CI elicits reversed plasticity likely to affect tinnitus perception (49). Brain plasticity that started when restoring hearing input would continue over years. It is likely that SSD patients experience difficulties to adapt to CI stimulation because of sound comparison with their normal-hearing ear. Patients may observe a benefit only when they accept a stimulation level than can mask, at least partly, the tinnitus (15,50).
While masking could fail to reduce tinnitus in some patients, electrical stimulation with precise location, time, and intensity could suppress the neurophysiological responses associated with tinnitus (14). In the present study, white noise stimulation during the first month after surgery somewhat reduced tinnitus, suggesting that energetic masking or re-activation of the auditory pathways through electrical stimulation may alleviate tinnitus without eliciting an immediate maximal reduction. The difficulty to adapt to CI stimulation may also explain the relatively low audiological benefit that SSD patients experience (51). Even if improvements in speech-in-noise and spatial localization abilities can be observed long-term, tinnitus relief started early (significant between 1 and 3 months post-activation) and reached levels of improvement that makes CI an interesting treatment option (52,53).
It could be that duration of hearing loss and tinnitus influences the time course of CI outcomes in adults with SSD. Interestingly, several previous studies report positive hearing and tinnitus outcomes in patient samples that include people with longstanding hearing loss of over 10 years (17,28,33,49).
Strengths and Limitations
The present study benefited from clear inclusion criteria and from a thorough longitudinal evaluation of outcomes. However, it is well known that placebo effects can occur in tinnitus treatment trials (54), and such effects could have partly influenced the results observed in the present study. During the informed consent procedure, careful care was taken to inform patients of expected CI outcomes, and not to speculate on expected CI outcomes for the two types of stimulation tested in the study. This was done to minimize potential bias in patient report of tinnitus treatment outcomes. While four different patient reported outcome measures of tinnitus were used and were found to be correlated, the Tinnitus Functional Index is another measure of tinnitus, and it was especially designed to be responsive to treatment and would be relevant to use in future similar studies (55). Future research should shed light on the effect of duration of hearing loss and tinnitus on CI outcomes in SSD patients and therefore inform candidacy indicators for CI in this population. Similarly, it would be interesting to investigate optimal CI settings, including thresholds and comfortable loudness levels, to best alleviate tinnitus in SSD patients.
CONCLUSION
After 1 year of follow-up, 92% of SSD patients with CI displayed reduced handicap linked to their incapacitating tinnitus starting at 13 months post-activation. Hearing abilities progressively developed after tinnitus relief, which lead to significant improvement in speech-in-noise perception at 1 year after activation, suggesting partial restoration of binaural hearing abilities. Further research should investigate long term socio-economic benefit such as workplace participation and health care utilization to quantify the cost-utility of CI in adults with SSD and tinnitus.
Acknowledgments
The authors thank the medical teams, audiologists, and speech-pathologists of the participating centers for their excellent patient care during the study and beyond. Ariane Laplante-Lévesque and Edward Overstreet provided helpful comments on the manuscript.
Footnotes
Statement and Ethics: Subjects have given their written informed consent. The study protocol was approved by the research institute's committee on human research. The study was registered in the U.S. National Library of Medicine clinical trial database (ID: NCT02966366). The study was carried out in accordance with the Helsinki Declaration and was approved by the French health authorities (ANSM: 2012-A01453-40) and the Ile-de-France VI (Paris, France) ethics committee (IRB: 2012-A01453-40).
Conflicts of Interest and Source of Funding: The authors acknowledge the support of Oticon Medical (Vallauris, France) who provided the cochlear implant systems and their accessories. Four of the authors, M.A., D.G., M.H., and S.S., are employees of Oticon Medical. These authors were involved in the study design and provided support during data analysis and manuscript preparation. The remaining authors have no conflict of interest to report.
REFERENCES
- 1.Van de Heyning P, Távora-Vieira D, Mertens G, et al. Towards a unified testing framework for single-sided deafness studies: a consensus paper. Audiol Neurotol 2016; 21:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douglas SA, Yeung P, Daudia A, Gatehouse S, O’Donoghue GM. Spatial hearing disability after acoustic neuroma removal. Laryngoscope 2007; 117:1648–1651. [DOI] [PubMed] [Google Scholar]
- 3.Feuerstein JF. Monaural versus binaural hearing: ease of listening, word recognition, and attentional effort. Ear Hear 1992; 13:80–86. [PubMed] [Google Scholar]
- 4.Alhanbali S, Dawes P, Lloyd S, Munro KJ. Self-reported listening-related effort and fatigue in hearing-impaired adults. Ear Hear 2017; 38:39–48. [DOI] [PubMed] [Google Scholar]
- 5.Thompson DM, Hall DA, Walker D-M, Hoare DJ. Psychological therapy for people with tinnitus: a scoping review of treatment components. Ear Hear 2017; 38:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sereda M, Davies J, Hall DA. Pre-market version of a commercially available hearing instrument with a tinnitus sound generator: feasibility of evaluation in a clinical trial. Int J Audiol 2017; 56:286–294. [DOI] [PubMed] [Google Scholar]
- 7.Ito J. Tinnitus suppression in cochlear implant patients. Otolaryngol Head Neck Surg 1997; 117:701–703. [DOI] [PubMed] [Google Scholar]
- 8.Aschendorff A, Pabst G, Klenzner T, Laszig R. Tinnitus in cochlear implant users: the Freiburg experience. Int Tinnitus J 1998; 4:162–164. [PubMed] [Google Scholar]
- 9.Quaranta N, Wagstaff S, Baguley DM. Tinnitus and cochlear implantation. Int J Audiol 2004; 43:245–251. [DOI] [PubMed] [Google Scholar]
- 10.Pan T, Tyler RS, Ji H, Coelho C, Gehringer AK, Gogel SA. Changes in the tinnitus handicap questionnaire after cochlear implantation. Am J Audiol 2009; 18:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amoodi HA, Mick PT, Shipp DB, et al. The effects of unilateral cochlear implantation on the tinnitus handicap inventory and the influence on quality of life. Laryngoscope 2011; 121:1536–1540. [DOI] [PubMed] [Google Scholar]
- 12.Kompis M, Pelizzone M, Dillier N, Allum J, DeMin N, Senn P. Tinnitus before and 6 months after cochlear implantation. Audiol Neurotol 2012; 17:161–168. [DOI] [PubMed] [Google Scholar]
- 13.Kim D-K, Bae S-C, Park K-H, et al. Tinnitus in patients with profound hearing loss and the effect of cochlear implantation. Eur Arch Otorhinolaryngol 2013; 270:1803–1808. [DOI] [PubMed] [Google Scholar]
- 14.Zeng FG, Tang Q, Dimitrijevic A, Starr A, Larky J, Blevins NH. Tinnitus suppression by low-rate electric stimulation and its electrophysiological mechanisms. Hear Res 2011; 277:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JE, Zeng FG. Tinnitus suppression by electric stimulation of the auditory nerve. Front Syst Neurosci 2012; 29:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arts RA, George EL, Chenault MN, Stokroos RJ. Optimizing intracochlear electrical stimulation to suppress tinnitus. Ear Hear 2015; 36:125–135. [DOI] [PubMed] [Google Scholar]
- 17.Van de Heyning P, Vermeire K, Diebl M, Nopp P, Anderson I, De Ridder D. Incapacitating unilateral tinnitus in single-sided deafness treated by cochlear implantation. Ann Otol Rhinol Laryngol 2008; 117:645–652. [DOI] [PubMed] [Google Scholar]
- 18.Buechner A, Brendel M, Lesinski-Schiedat A, et al. Cochlear implantation in unilateral deaf subjects associated with ipsilateral tinnitus. Otol Neurotol 2010; 31:1381–1385. [DOI] [PubMed] [Google Scholar]
- 19.Arndt S, Aschendorff A, Laszig R, et al. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol 2011; 32:39–47. [DOI] [PubMed] [Google Scholar]
- 20.Arts RAGJ, George ELJ, Janssen M, Griessner A, Zierhofer C, Stokroos RJ. Tinnitus suppression by intracochlear electrical stimulation in single sided deafness – A prospective clinical trial: follow-up. PLoS One 2016; 11:e0153131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arts RAGJ, George ELJ, Stokroos RJ, Vermeire K. Review: Cochlear implants as a treatment of tinnitus in single-sided deafness. Curr Opin Otolaryngol Head Neck Surg 2012; 20:398–403. [DOI] [PubMed] [Google Scholar]
- 22.Punte AK, Vermeire K, Hofkens A, De Bodt M, De Ridder D, Van de Heyning P. Cochlear implantation as a durable tinnitus treatment in single-sided deafness. Cochlear Implants Int 2011; 12:26–29. [DOI] [PubMed] [Google Scholar]
- 23.Ramos Á, Polo R, Masgoret E, et al. Cochlear implant in patients with sudden unilateral sensorineural hearing loss and associated tinnitus. Acta Otorrinolaringol Esp 2012; 63:15–20. [DOI] [PubMed] [Google Scholar]
- 24.Blasco MA, Redleaf MI. Cochlear implantation in unilateral sudden deafness improves tinnitus and speech comprehension: meta-analysis and systematic review. Otol Neurotol 2014; 35:1426–1432. [DOI] [PubMed] [Google Scholar]
- 25.Mertens G, Punte AK, De Ridder D, de Heyning PV. Tinnitus in a single-sided deaf ear reduces speech reception in the nontinnitus ear. Otol Neurotol 2013; 34:662–666. [DOI] [PubMed] [Google Scholar]
- 26.Mertens G, Punte AK, De Bodt M, Van de Heyning P. Binaural auditory outcomes in patients with postlingual profound unilateral hearing loss: 3 years after cochlear implantation. Audiol Neurotol 2015; 20:67–72. [DOI] [PubMed] [Google Scholar]
- 27.Mertens G, De Bodt M, Van de Heyning P. Cochlear implantation as a long-term treatment for ipsilateral incapacitating tinnitus in subjects with unilateral hearing loss up to 10 years. Hear Res 2016; 331:1–6. [DOI] [PubMed] [Google Scholar]
- 28.Távora-Vieira D, Marino R, Krishnaswamy J, Kuthbutheen J, Rajan GP. Cochlear implantation for unilateral deafness with and without tinnitus: a case series. Laryngoscope 2013; 123:1251–1255. [DOI] [PubMed] [Google Scholar]
- 29.Távora-Vieira D, Marino R, Acharya A, Rajan GP. The impact of cochlear implantation on speech understanding, subjective hearing performance, and tinnitus perception in patients with unilateral severe to profound hearing loss. Otol Neurotol 2015; 36:430–436. [DOI] [PubMed] [Google Scholar]
- 30.Gartrell BC, Jones HG, Kan A, Buhr-Lawler M, Gubbels SP, Litovsky RY. Investigating long-term effects of cochlear implantation in single-sided deafness: a best practice model for longitudinal assessment of spatial hearing abilities and tinnitus handicap. Otol Neurotol 2014; 35:1525–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeitler DM, Dorman MF, Natale SJ, Loiselle L, Yost WA, Gifford RH. Sound source localization and speech understanding in complex listening environments by single-sided deaf listeners after cochlear implantation. Otol Neurotol 2015; 36:1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buss E, Dillon MT, Rooth MA, et al. Effects of cochlear implantation on binaural hearing in adults with unilateral hearing loss. Trends Hear 2018; 22:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeire K, Van de Heyning P. Binaural hearing after cochlear implantation in subjects with unilateral sensorineural deafness and tinnitus. Audiol Neurotol 2009; 14:163–171. [DOI] [PubMed] [Google Scholar]
- 34.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 1990; 13:27–36. [DOI] [PubMed] [Google Scholar]
- 35.Newman CW, Jacobson GP, Spitzer JB. Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 1996; 122:143–148. [DOI] [PubMed] [Google Scholar]
- 36.Ghulyan-Bédikian V, Paolino M, Giorgetti-D’Esclercs F, Paolino F. Psychometric properties of a French adaptation of the Tinnitus Handicap Inventory. Encéphale 2010; 36:390–396. [DOI] [PubMed] [Google Scholar]
- 37.Wilson PH, Henry J, Bowen M, Haralambous G. Tinnitus reaction questionnaire: psychometric properties of a measure of distress associated with tinnitus. J Speech Lang Hear Res 1991; 34:197–201. [PubMed] [Google Scholar]
- 38.Meric C, Pham E, Chéry-Croze S. Translation and validation of the subjective scale of Tinnitus distress measure (Tinnitus Reaction Questionnaire, Wilson et al., 1991). Encéphale 1997; 23:442–446. [PubMed] [Google Scholar]
- 39.Halford JBS, Anderson SD. Tinnitus severity measured by a subjective scale, audiometry and clinical judgement. J Laryngol Otol 1991; 105:89–93. [DOI] [PubMed] [Google Scholar]
- 40.Meric C, Pham E, Chéry-Croze S. Translation and validation of the subjetive scale of Tinnitus severity. J Fr ORL 1996; 45:409–412. [Google Scholar]
- 41.Schleich P, Nopp P, D’Haese P. Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant. Ear Hear 2004; 25:197–204. [DOI] [PubMed] [Google Scholar]
- 42.Studebaker GA. A “rationalized” arcsine transform. J Speech Lang Hear Res 1985; 28:455–462. [DOI] [PubMed] [Google Scholar]
- 43.Tyler RS, Keiner AJ, Walker K, et al. A series of case studies of tinnitus suppression with mixed background stimuli in a cochlear implant. Am J Audiol 2015; 24:398–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holder JT, O’Connell B, Hedley-Williams A, Wanna G. Cochlear implantation for single-sided deafness and tinnitus suppression. Am J Otolaryngol 2017; 38:226–229. [DOI] [PubMed] [Google Scholar]
- 45.Ponton CW, Vasama J-P, Tremblay K, Khosla D, Kwong B, Don M. Plasticity in the adult human central auditory system: evidence from late-onset profound unilateral deafness. Hear Res 2010; 154:32–44. [DOI] [PubMed] [Google Scholar]
- 46.Kral A, Hubka P, Tillein J. Strengthening of hearing ear representation reduces binaural sensitivity in early single-sided deafness. Audiol Neurotol 2015; 20:7–12. [DOI] [PubMed] [Google Scholar]
- 47.Pross SE, Chang JL, Mizuiri D, Findlay AM, Nagarajan SS, Cheung S. Temporal cortical plasticity in single-sided deafness: a functional imaging study. Otol Neurotol 2015; 36:1443–1449. [DOI] [PubMed] [Google Scholar]
- 48.Eggermont JJ. Acquired hearing loss and brain plasticity. Hear Res 2017; 343:176–190. [DOI] [PubMed] [Google Scholar]
- 49.Firszt JB, Reeder RM, Holden TA, Burton H, Chole RA. Changes in auditory perceptions and cortex resulting from hearing recovery after extended congenital unilateral hearing loss. Front Syst Neurosci 2013; 7:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vernon JA. Masking of tinnitus through a cochlear implant. J Am Acad Audiol 2000; 11:293–294. [PubMed] [Google Scholar]
- 51.Finke M, Strauß-Schier A, Kludt E, Büchner A, Illg A. Speech intelligibility and subjective benefit in single-sided deaf adults after cochlear implantation. Hear Res 2017; 348:112–119. [DOI] [PubMed] [Google Scholar]
- 52.Hoare DJ, Kowalkowski VL, Kang S, Hall DA. Systematic review and meta-analyses of randomized controlled trials examining tinnitus management. Laryngoscope 2011; 121:1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SH, Byun JY, Yeo SG, Park MS. Tinnitus retraining therapy in unilateral tinnitus patients with single side deafness. J Int Adv Otol 2016; 12:72–76. [DOI] [PubMed] [Google Scholar]
- 54.Duckert LG, Rees TS. Placebo effect in tinnitus management. Otolaryngol Head Neck Surg 1984; 92:697–699. [DOI] [PubMed] [Google Scholar]
- 55.Meikle MB, Henry JA, Griest SE, et al. The tinnitus functional index: Development of a new clinical measure for chronic, intrusive tinnitus. Ear Hear 2012; 33:153–176. [DOI] [PubMed] [Google Scholar]


