Abstract
Background:
The objective of this study was to evaluate ON-state resting state functional connectivity (FC) from the mesencephalic locomotor regions (MLR) to distributed sensorimotor cortical regions in patients with Freezing of Gait (FOG) and its association with gait performance.
Methods:
54 individuals with PD were recruited for this study (50% of whom had FOG). All individuals received a resting state functional MRI in the ON state, and underwent a series of gait assessments during single and dual task conditions. FC with the MLR was calculated using a whole brain seed to voxel approach wherein the left and right MLR seeds were extracted from a published atlas. General linear regression was used to determine differences in connectivity between the individuals with (‘freezers’) and without (‘non-freezers’) FOG as well as the correlation between MLR connectivity and gait performance in the freezers.
Results:
Freezers had significantly higher MLR connectivity to a network of sensorimotor regions compared to non-freezers. Additionally, among the freezers, higher FC with these regions was related to longer single-task and dual-task performance. There were no regions in which non-freezers had higher connectivity than freezers (p<0.05, FWE corrected clusters for all analyses).
Conclusion:
These data support the hypothesis that freezers have significantly higher ON-state FC between the MLR and a network of cortical structures than non-freezers. Additionally, this elevated connectivity is directly related to worsening FOG severity. These data add to a theoretical foundation which suggests that cortical hyperconnectivity to the MLR is central to the underlying pathophysiology of FOG.
Keywords: Parkinson, Freezing of Gait, Connectivity, resting state, fMRI, Gait
Introduction
Freezing of gait (FOG) is a debilitating condition which occurs in the majority of patients with Parkinson’s Disease (PD) and for which there are no effective therapies. Freezing in these patients increases the frequency of falls and negatively impacts overall quality of life[1]. FOG is defined as a reduction in the ability to initiate gait and maintain forward movement which can be triggered or alleviated by external cues[2]. FOG may respond to dopaminergic therapy, however, response is often incomplete[3, 4]. The pathophysiology of FOG is poorly understood although multiple conceptual models have been proposed[5]. One emerging hypothesis is that FOG is due to a disruption in neural networks that contribute to automatic gait. In this framework, automaticity of gait occurs in the absence of executive control. When this automaticity is lost however, additional cognitive resources are required to select appropriate motor responses[6]. Therefore, increased reliance on cognitive resources can be seen as a compensatory strategy for a loss of gait automaticity[6]. This compensatory response may be represented by an increased reliance on cortical structures by subcortical locomotor regions associated with automatic gait.
One locomotor region of particular interest is the pedunculopontine nucleus (PPN). This brainstem structure is part of the mesencephalic locomotor region (MLR) which has been implicated in gait control including initiation, turning, stopping, and avoiding obstacles[7]. To investigate cortical governance of locomotion we investigated group differences in functional connectivity (FC) between the MLR and other areas of the brain. We postulated that this approach would shed light on how the brain adapts to MLR degeneration and a loss of automaticity of gait. Overall, defining the locomotor network, including cortical and subcortical structures is a critical step in elucidating the pathophysiological changes underlying FOG. Given our knowledge of the MLR as an area of automatic gait control, which is known to degenerate in PD[8], we aimed to determine whether changes in cortical synchrony between the MLR and cortical regions was associated with FOG. To determine changes that were specific to FOG we designed our study to compare FC changes (in the ON state) between participants with PD and FOG, and those with PD without FOG. In a similar design, Fling et al. reported increased OFF state FC between the MLR and SMA in 8 freezers compared to controls and FC between these regions correlated positively with worsening FOG severity[9]. Other FC studies of FOG have uncovered other important aspects of the pathophysiology of FOG including a reduction in FC in the executive network [10, 11], functional decoupling of the basal ganglia and the cognitive control network[12] and the relationship of visual and attention networks to freezing behavior[13, 14]. However, there have been no further investigations of top-down control of gait in FOG since the initial report by Fling et al. Given the clinical implications of these findings, we set out to conduct the largest study of this type in freezers, and to determine if increased cortical control of FOG persists in the ON state.
In order to investigate the relevance of FC differences between freezers and non-freezers we studied the relationship to objective measures of gait behavior. Metrics involving walking while performing a concurrent cognitive task (dual tasks) are of particular interest for questions of automaticity and cortical governance of gait, and can be seen as surrogate markers of gait automaticity or lack thereof[15]. Associations between these markers and connectivity between disease and control groups are informative, not only in terms of identifying clinically relevant connections and therapeutic targets, but also in determining the direction of the dysfunction. Thus, we additionally hypothesized that if increased cortical control of gait is not an effective compensatory response it would be positively correlated with markers of FOG severity.
Methods
Participants:
All patients included in this study were recruited from the Movement Disorders clinic at the Medical University of South Carolina and the Ralph H Johnson VA hospital and gave informed written consent. All participants exhibited UK Brain Bank diagnostic criteria for Parkinson’s disease. Subjects were determined to be freezers based on their response to item 1 from the new freezing of gait questionnaire (nFOG-Q) [16], item 14 of the Unified Parkinson’s Disease Rating Scale (UPDRS) and confirmed by assessment by a movement disorders neurologist. There was no discordance between the final determination of FOG status between the subject and the neurologist. Exclusion criteria included deep brain stimulation implantation, MRI contraindications, dementia, (<26 on the mini-mental status examination) and gait dysfunction other than from PD. Fifty-four individuals completed the study (27 PD patients with FOG and 27 age and gender matched PD patients without FOG, Table 1). All individuals received a resting state functional MRI (rs-fMRI) in the ON state, clinical evaluation, and gait evaluation. Foam padding and earplugs were used to reduce head motion and noise. Levodopa equivalent daily dose (LEDD) was significantly greater (p<0.0001) in the freezers (1199.9±618.3mg) than non-freezers (651.0±319.3mg). Disease duration was greater by approximately 2 years in freezers when compared with non-freezers (Table 1). The vast majority had all evaluations including rs-fMRI performed on consecutive days, and all occurred within 6 months. The presence of hallucinations were evaluated using Part I, Question 2 of the UPDRS. Both groups had a subset of participants which experienced “benign hallucinations with insight retained” or episodes of “vivid dreaming”, however none experienced “occasional” or “frequent” hallucinations which interfered in daily activities (see Table 1).
Table 1.
Demographics and Clinical Assessments
| Demographics and Clinical Assessments |
PD Freezers | PD Non-Freezers |
|---|---|---|
| Sample size | n=27 | n=27 |
| Male/ Female | 20/7 | 22/5 |
| Age (years old ± SD) | 67.2 ± 7.1 years | 67.9 ± 5.7 years |
| UPDRS-III ON (±SD) | 17.8 ± 7.0 | 18.2 ± 7.3 |
| UPDRS-III OFF (±SD) | 24.7 ± 8.0 | n/a |
| Thought Disorder | VD = 3, BH = 3 | VD = 10, BH = 2 |
| Clinical Fluctuations (±SD) | 1.93±2.02 | 0.33±0.83 |
| Freezing of Gait Questionnaire (±SD) | 20.25 ± 4.7 | n/a |
| LEDD (±SD) | 1199.9±618.3 mg | 651.0±319.3 mg |
| Duration of Disease(±SD) | 8.7 ± 5.3 years | 6.8 ± 4.0 years |
| Walking Evaluation: Single task (seconds) | ||
| Time Up and Go: OFF | 43.84 ± 32.85 sec | n/a |
| Time Up and Go: ON** | 27.88 ± 9.65 sec | 21.06 ± 4.3 sec |
| Time to Turn: OFFΛ | 10.66 ± 9.76 sec | n/a |
| Time to Turn: ON** | 6.93 ± 4.17 sec | 4.33 ± 1.02 sec |
| Walking Evaluation: Dual task (seconds) | ||
| Time Up and Go: OFF# | 49.34 ± 29.92 sec | n/a |
| Time Up and Go: ONΛ ** | 37.11 ± 21.00 sec | 24.14 ± 4.8 sec |
| Time to Turn: OFFΛ | 24.56 ± 37.47 sec | n/a |
| Time to Turn: ON* | 11.80 ± 13.88 sec | 5.04 ± 1.15 sec |
For PD Freezers: Several individuals had abnormally long values when OFF. These data points were excluded if they were >2xSD of the exclusive mean.
#23 individuals (4 excluded),
Λ 26 individuals (1 excluded). For thought disorders the number of participants in each group with VD (“vivid dreaming”) and BH (“benign hallucinations with insight retained”) is displayed as responses to Part I, Question 2 of the UPDRS. None reported occasional or frequent hallucinations which interfered in daily activities.
Significant differences in group gait time denoted by
p<0.05
p<0.01.
Behavioral Assessments:
Behavioral assessments were collected and included the new Freezing of Gait Questionnaire (nFOG-Q), and the UPDRS parts 1-4 in the ON and OFF state as a measure of PD motor severity. OFF assessments were made at least 12 hours OFF all dopaminergic medication, ON assessments were made at least 30 minutes after taking the first dose of medications on the same day. Since subjects in the PD-control group experienced no (or minimal) motor fluctuations clinically (score of 0.33 +/− 0.83 on clinical fluctuations questions of the UPDRS, part 4 compared to 1.93 +/− 2.02 in the FOG group), evaluations were only performed in the ON state for this group. Subjects completed two timed up and go (TUG) trials on the GaitRite digital walkway in the OFF state, which were averaged to yield the single task TUG time variable. This was followed by two more trials in the OFF state with a dual task (serial 7s and every other letter of the alphabet), which were averaged to yield the TUG time dual task variable. Turn duration was calculated as the time when subjects stepped off of the GaitRite mat onto the M2 mat, where they turned around a cone placed at a predetermined distance at the center of the mat to standardize the distance of the turn[17]. Demographic and gait data (ON/OFF with and without dual task) were compared between freezers and PD-controls. Comparisons were performed using a 2-paired t-test were considered significant if p<0.05 (See Table 1).
Functional and structural MRI assessment:
T1 structural MRI scans were acquired for each participant prior to the functional scan (TR=2300ms, TE=2.26ms, TI = 900ms, slice thickness= 1mm, field of view = 256 mm, flip angle= 8 degrees) on a 3T Siemens Trio MRI scanner with a 32-channel head coil. Following the structural scan, participants were asked to fixate on a white cross for 4 minutes and 30 seconds during which T2* functional MRI scans were acquired (TR=2200ms, 36 transverse slices, thickness=3.0 mm, interleaved or ascending slice order, 119 volumes).
Functional connectivity analysis (Supplementary Figure 1):
The fMRI data were preprocessed using a default pipeline specified in the CONN toolbox (Version 17.f) as implemented in Matlab (Mathworks R2017b). Preprocessing steps included realignment, slice-timing correction, detection of volumes exceeding 0.9 mm of motion (ART toolbox), segmentation, normalization (MNI space), and spatial smoothing (8mm kernel). In recognition of the impact head motion has on resting-state FC, we compared quality assurance metrics of movement between groups. The number of volumes scrubbed was not significantly different between the freezers (3.7±6.7 volumes) and non-freezers (4.7±7.8 volumes). Mean movement did not differ between the freezer (0.20±0.15mm) and non-freezer (0.20 ±0.11mm) groups. Data were de-noised using CompCor (CSF/White matter) and by regression of confounding effects including realignment (head motion) parameters, and scrubbing. Band-pass filtering was performed using a frequency window of 0.008-0.09 Hz. Seed to voxel FC was performed using bivariate correlations. Seed regions included the left and right PPN as defined by the Harvard Ascending Arousal Network (AAN) Atlas in MNI152 space (Edlow 2012 et al.)(Supplementary Figure 2). This PPN ROI is an elongated oval shape with a center of gravity at x=±8, y=−26, z=−18 and contains a total of 94 1mm voxels. The BOLD time-course from these regions were extracted from unsmoothed data within the PPN seed.
The differences in FC between the groups was assessed using a general linear regression framework directly comparing the groups. Voxelwise differences (representing whole brain connectivity with the PPN) between freezer and non-freezer groups were carried out using a 2-sample t-test (voxel threshold p<0.01, clusters FWE corrected p<0.05). Secondary analyses were performed to evaluate if LEDD or disease duration would affect between group differences in PPN connectivity when included as covariates. The relationship between behavioral performance metrics and connectivity was evaluated in the freezers using a linear regression (voxel threshold p>0.005, FWE corrected clusters p<0.05).
Results
Functional connectivity with the left PPN:
In the non-freezers, there was significant FC between the left PPN and 9 clusters in the brain. These clusters included a total of 2592 voxels and were located in the left and right SMA, occipital pole, left middle and inferior frontal gyri, and the left cerebellum. Local connectivity within the PPN included 1184 voxels. There were no areas negatively correlated with the left PPN (voxel threshold p<0.01, FWE corrected clusters p<0.05).
In the freezers, there was significant FC between the left PPN and 6 clusters in the brain. These clusters included a total of 3831 voxels. Like the non-freezers, the clusters were located in the left and right SMA, occipital pole, and the inferior and middle gyri. Unlike the non-freezers however, there was no significant FC with the cerebellum. There were no areas negatively correlated with the left PPN (voxel threshold p<0.01, FWE corrected clusters p<0.05).
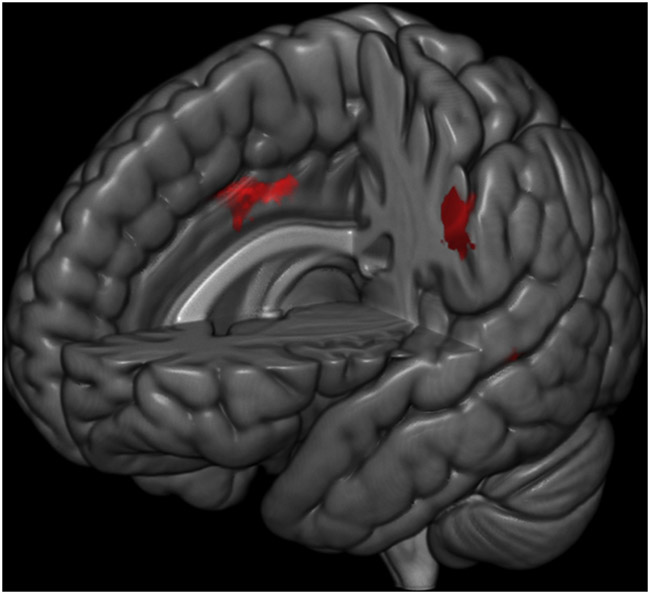
The freezers had significantly greater FC than the non-freezers in a network of neural regions (Table 2, Figure 1). The three significant clusters included: 1) left and right midcingulate cortex (an area that projects to the spinal cord and is involved in multisensory orientation of the head and body in space)[18], 2) the supramarginal gyrus, and 3) the left temporal pole, extending into the entorhinal cortex, a key area for spatial integration (voxel threshold p<0.01, family-wise error corrected clusters p<0.05)[19]. When correcting for disease duration all three of these clusters remained significant (see Supplementary Table 1). After including LEDD as a covariate, the temporal and entorhinal cortex clusters remained significant (FWE p<0.05) however the midcingulate cluster was only significant at p<0.05, uncorrected.
Table 2.
Areas of differences in Functional Connectivity between the Freezers and Non-freezers
| P value (FWE) |
Cluste r Size |
Max t | MNI Coordinates |
Cortical Brodmann Area |
Left or Right |
Description | |||
|---|---|---|---|---|---|---|---|---|---|
| Freezers greater than NonFreezers | 0.002 | 445 | 3.89 | −8 | 0 | 38 | 24 & 32 | L&R | Middle Cingulate |
| 0.025 | 305 | 4.13 | −54 | −26 | 40 | 40 | L | Supramarginal Gyrus | |
| 0.030 | 296 | 4.14 | −22 | 4 | −38 | 38 | L | Temporal Pole/Entorhinal | |
| Nonfreezers greater than Freezers | |||||||||
Areas wherein connectivity to the left PPN is different in Freezers versus Non-Freezers (Family Wise Error Correction, p<.05 at cluster level; p>0.005 voxel level)
Figure 1.
Areas wherein Freezers had significantly higher functional connectivity than the Non-Freezers
There were no areas in which the non-freezers had greater connectivity with the left PPN than the freezers. This result was consistent when including LEDD or disease duration as covariates.
Functional connectivity with the right PPN:
In the non-freezers, there was significant FC between the right PPN and 5 clusters in the brain. These clusters included a total of 4336 voxels and were located in the entorhinal cortex, cerebellum, and temporal pole. Local connectivity within the PPN included 2291 voxels. There were no areas negatively correlated with the right PPN (voxel threshold p<0.01, FWE corrected clusters p<0.05).
In the freezers, there was significant FC between the right PPN and 6 clusters in the brain. These clusters included a total of 3342 voxels. The clusters were located in the middle temporal gyrus and the anterior cingulate cortex bilaterally. There were no areas negatively correlated with the right PPN (voxel threshold p<0.01, FWE corrected clusters p<0.05).
There was no significant difference in right PPN connectivity between the freezers and non-freezers. This lack of difference in PPN connectivity between groups was consistent when correcting for disease duration or LEDD.
Relationship between Gait and PPN connectivity.
To determine whether left PPN connectivity was related to gait performance in freezers, a general linear regression was applied to compare FC with scores on 1) nFOG-Q, 2) Dual task performance in the OFF state (including Total TUG and turn duration in the OFF state), and 3) Dual task performance in the ON state (including Total TUG and turn duration in the ON state) (voxel threshold p<0.005, FWE corrected clusters p<0.05). Before the behavioral variables were entered into the regression analysis, they were evaluated for outliers (2 standard deviations from the mean). Three participants were removed from the turn duration analysis in the Dual Task (n=24 data points). One participant was removed from the turn duration analysis in the Single Task (n=26 data points). There were no outliers for total time up and go for either the Single Task or Dual Task conditions (n=27) in the OFF state. No one was excluded during the ON state.
Freezing of Gait questionnaire.
There was no significant correlation between elevated total nFOG-Q score and elevated connectivity with the PPN.
Single Task Walking Assessment.
(Table 3, Figure 2) There was a significant correlation between OFF state TUG and PPN connectivity to 3 clusters including the left inferior and middle temporal cortices as well as the Pre-SMA bilaterally. There was also a correlation between OFF state turn duration and PPN connectivity to 3 clusters including the left inferior and middle temporal cortices as well as the left premotor cortex (lateral to the SMA).
Table 3.
Areas that significantly correlated with Clinical Assessments in the Freezers.
| Behavioral Assesment |
P value (FWE) |
Cluste r Size |
Max t | MNI Coordinates |
Cortical Brodmann Area |
Left or Right |
Description | ||
|---|---|---|---|---|---|---|---|---|---|
| Single Task Performance | |||||||||
| Time Up & Go | 0.008 | 239 | 4.09 | −52 | −10 | −40 | 20 | L | Inferior temporal |
| 0.013 | 224 | 4.40 | 6 | 22 | 60 | 6 | L & R | Pre-SMA (Rostral SMA) | |
| 0.032 | 191 | 5.31 | −68 | −38 | −6 | 21 | L | Middle temporal | |
| Time to Turn | <0.000 | 474 | 4.80 | −50 | −6 | −40 | 20 | L | Inferior temporal |
| 0.002 | 293 | 4.98 | −68 | −38 | −6 | 21 | L | Middle temporal | |
| 0.019 | 211 | 3.59 | −50 | −2 | 52 | 6 | L | Lateral premotor cortex | |
| Dual Task Performance | |||||||||
| Time Up & Go | <0.001 | 639 | 5.67 | −18 | 28 | 60 | 6 | L&R | Pre-SMA (Rostral SMA) |
| <0.001 | 359 | 4.66 | −60 | −10 | −34 | 20 & 21 | L | Inferior temporal | |
| 0.025 | 196 | 4.13 | 14 | −46 | 18 | 23 | R | Posterior cingulate | |
| 0.035 | 184 | 4.10 | −66 | −56 | 14 | 39 & 40 | L | Supramarginal gyrus | |
| 0.049 | 173 | 6.07 | 10 | −24 | 76 | 6 | R | SMA (Caudal SMA) | |
| Time to Turn | <0.001 | 964 | 4.77 | −10 | −6 | −10 | N/A | L | Thalamus |
| <0.001 | 842 | 7.58 | −48 | −2 | −40 | 20 | L | Inferior temporal | |
| 0.001 | 460 | 5.94 | 54 | 8 | −22 | 38 | R | Temporal pole | |
| 0.001 | 449 | 4.08 | 4 | −22 | 74 | 6 | L&R | SMA (Caudal SMA) | |
Areas wherein slower behavioral performance was correlated with higher functional connectivity from the PPN in Freezers. with greater functional connectivity to the PPN in Freezers relative to Non-Freezers (Family Wise Error Correction, p<.05 at cluster level; p<0.005 voxel level).
Figure 2.

Areas wherein elevated functional connectivity with the PPN was significantly correlated with total time up and go (left column) and time to turn (right column) during Single Task performance (top row) and Dual Task performance (bottom row).
Dual Task Walking Assessment.
There was a significant correlation between OFF state TUG and PPN connectivity to 5 regions including the bilateral Pre-SMA, left inferior temporal cortex and supramarginal gyrus, as well as the right posterior cingulate and SMA (caudal to the Pre-SMA cluster). There was also a correlation between OFF state turn duration and PPN connectivity to 4 regions including a cluster with local maxima in the thalamus and substantia nigra/MLR as well as the left inferior temporal cortex, right temporal pole (extending into the entorhinal cortex) and the bilateral SMA.
Discussion
Here we report the results of the largest resting state functional connectivity study comparing PD patients with FOG to those without FOG in the ON state. Our findings support the notion that cortical control of gait is increased in FOG. Specifically this study not only confirms but extends previous work[9] in FOG by demonstrating that increased FC between the MLR and the SMA (as well as multiple other cortical structures involved in sensorimotor integration) persists in the ON state. Furthermore, our findings support the concept that increased cortical control of gait is either not effective or detrimental to FOG since we consistently see a positive correlation between MLR-cortical connectivity and markers of FOG severity. In FOG, increased cortical control may arise as a compensatory mechanism in response to a loss of automaticity of gait. In fact, increased FC between sensorimotor and attentional networks has been reported in PD and interpreted as a compensatory response to loss of automaticity [20]. Despite this effort to compensate for this deficit, FOG severity worsens with increased cortical control. Thus, an increase in cortical control is likely to be either ineffective, or even detrimental to FOG.
FC between the MLR and the SMA correlated positively with walking and turning in the dual task condition, off dopaminergic medications. In addition, FC from the MLR to the posterior cingulate and other areas involved in sensorimotor integration were also positively correlated with these tasks. The PPN has been demonstrated to play an important role in a network of regions involved in postural sensory integration [21, 22]. This may be mediated by thalamic efferents, which were also shown to have higher connectivity in freezers than non-freezers in the present study.
It is important to note that increased FC between these cortical structures and brainstem locomotor centers was associated with worsening performance on these tasks in freezers, not in PD controls. If increased cortical control were an effective adaptive response, we would expect an improvement in these measures when cortical control increases. Since we, and other groups, have consistently seen worsening of markers of FOG severity with increased FC between MLR and cortical structures, only two explanations remain: 1) increased cortical control is a maladaptive response, or 2) it is an ineffective adaptation.
Attention to the MLR as a biomarker for gait automaticity comes from early work showing that stimulation of the MLR in decerebrate cats could elicit locomotion. Recent work in rats shows worsening gait with lesioning, and improvement with pharmacogenetic stimulation[23]. Although inconsistent, preliminary studies in humans show low frequency deep brain stimulation (DBS) of 10-25Hz within the PPN area can lead to gait improvement [24] [25]. Aside from its key role in locomotion, the PPN has a number of structural and functional connections to motor and prefrontal cortical regions which may explain why activity in these regions is altered in the context of PPN degeneration [26].
When interpreting our findings it is important to note that there is a lack of consensus over the precise location of the PPN in humans[24] and which structures within the MLR contain true markers of locomotion[23]. Furthermore, the accuracy of the normalization and differences in the precise anatomical location of PPN for individuals has made creating a standardized ROI for the PPN difficult. Previous studies have used a variety of coordinates in the form of spherical ROIs (x = ±6, y =−30, z = −19) [9] or as an elongated oval shape (x = ±7.1, y = −32, z = −22)[27]. We chose to use the Ascending Arousal Network (AAN) atlas-based ROI for the PPN in order to increase the chances of reproducibility in future studies. This atlas was created specifically for connectivity-based analyses and used histological data from an ex vivo brain specimen, in addition to diffusion MRI and Paxinos human atlas as a reference[28]. Although individualized ROIs for participants were considered, the atlas provided consistency in the ROI size and shape. It is likely that the PPN ROI includes cuneiform and reticulospinal structures and therefore the PPN and MLR nomenclature is used interchangeably.
We did see prominent laterality in our results, finding differences between groups only with the left PPN-MLR. Asymmetric PPN structural connectivity has been reported, with reductions in structural connectivity in PD on the right PPN[29]. Interestingly, this reduction was predominantly observed in the right hemisphere of freezers indicating a lateralization to white matter deficits[27]. The behavioral importance of these structural changes is supported by studies which have associated PPN connectivity and white matter asymmetry with performance during dual task walking. This may explain increased FC on the left but not right PPN.
Although performing imaging during the ON state is a significant contribution to our understanding of so called ON-FOG or dopa-unresponsive FOG, it is also a limitation since we cannot rule out that differences in PPN connectivity were not affected by L-DOPA dose. Specifically, freezers had consistently higher L-DOPA doses than non-freezers. Despite this confound, when including L-DOPA dose as a covariate, our results remained largely consistent. Additionally, interpreting the association between PPN connectivity and freezing behavior is complicated by the notion that scanning was performed during the ON state while freezing behavioral correlations were observed in participants during the OFF state. This limitation does not call into question the finding of persistent increases in FC between the MLR and SMA, but the behavioral correlation. This is likely explained by the fact that OFF state gait evaluations are superior to ON state evaluations at capturing actual FOG severity. Another important consideration are variables with a colinear relationship with freezing (i.e. disease duration and cognitive deficits) which may contribute to differences in brain connectivity within freezers. While we were able to correct for disease duration differences between the groups by including it as a covariate in our analysis, we did not have a reliable way of correcting for cognitive deficits. Additionally, PPN dysfunction has previously been implicated in REM behavior disorder which was not directly assessed in this study[30]. Finally, unlike objective gait assessments, connectivity changes did not correlate with the nFOGQ, a subjective questionnaire with inherent biases and limitations.
Supplementary Material
Supplementary Table 1. Areas of differences in Functional Connectivity between the Freezers and Non-freezers corrected for disease duration
Supplementary Figure 3. Scatter plots of the correlation between PPN connectivity strength and gait performance (TUG and time to turn).
Supplementary Figure 2. Depiction of MLR/PPN ROI
Supplementary Figure 1. Functional connectivity analysis pipeline.
Cortico-mesencephalic functional connectivity is increased in Freezing of Gait
Increased cortico-mesencephalic connectivity correlates with worsening gait in freezers
Increased cortico-mesencephalic connectivity persists in the ON state in freezers
Acknowledgments:
We would like to thank the MRI technicians at MUSC’s Center for Biomedical Imaging and Shonna Jenkins for her help with participant visits and data collection.
Financial Disclosures: Grants: Dr. Revuelta has received grant support from Boston Scientific. Advisory boards: Dr. Revuelta serves on the advisory board for Lundbeck and Boston Scientific. Stock Ownership in medically related fields, intellectual property rights, consultancies, expert testimony, employment, contracts, honoraria, royalties, other: none.
Funding Sources: Barmore Fund for Parkinson’s Research, the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, supported by NIH/NCATS Grant Number UL1TR000062, NIH NINDS Grant Number 1K23NS091391-01A1, American Heart Association Predoctroral Fellowship (17PRE33660857).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures/ Conflict of Interest relating to manuscript: none
References:
- 1.Perez-Lloret S, et al. , Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA Neurol, 2014. 71(7): p. 884–90. [DOI] [PubMed] [Google Scholar]
- 2.Nutt JG, et al. , Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol, 2011. 10(8): p. 734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suppa A, et al. , l-DOPA and Freezing of Gait in Parkinson's Disease: Objective Assessment through a Wearable Wireless System. Front Neurol, 2017. 8: p. 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fasano A and Lang AE, Unfreezing of gait in patients with Parkinson's disease. Lancet Neurol, 2015. 14(7): p. 675–7. [DOI] [PubMed] [Google Scholar]
- 5.Nieuwboer A and Giladi N, Characterizing freezing of gait in Parkinson's disease: Models of an episodic phenomenon. Mov Disord, 2013. 28(11): p. 1509–19. [DOI] [PubMed] [Google Scholar]
- 6.Vandenbossche J, et al. , Freezing of gait in Parkinson's disease: disturbances in automaticity and control. Frontiers in Human Neuroscience, 2012. 6: p. 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mena-Segovia J, Bolam JP, and Magill PJ, Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? Trends Neurosci, 2004. 27(10): p. 585–8. [DOI] [PubMed] [Google Scholar]
- 8.French IT and Muthusamy KA, A Review of the Pedunculopontine Nucleus in Parkinson's Disease. Front Aging Neurosci, 2018. 10: p. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fling BW, et al. , Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One, 2014. 9(6): p. e100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tessitore A, et al. , Resting-state brain connectivity in patients with Parkinson's disease and freezing of gait. Parkinsonism Relat Disord, 2012. 18(6): p. 781–7. [DOI] [PubMed] [Google Scholar]
- 11.Lenka A, et al. , Freezing of gait in Parkinson's disease is associated with altered functional brain connectivity. Parkinsonism Relat Disord, 2016. 24: p. 100–6. [DOI] [PubMed] [Google Scholar]
- 12.Gilat M, et al. , Dysfunctional Limbic Circuitry Underlying Freezing of Gait in Parkinson's Disease. Neuroscience, 2018. 374: p. 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velu PD, et al. , Effect of visual feedback on the occipital-parietal-motor network in Parkinson's disease with freezing of gait. Front Neurol, 2014. 4: p. 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maidan I, et al. , Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson's disease. Parkinsonism Relat Disord, 2019. 63: p. 77–82. [DOI] [PubMed] [Google Scholar]
- 15.Clark DJ, Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci, 2015. 9: p. 246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nieuwboer A, et al. , Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture, 2009. 30(4): p. 459–63. [DOI] [PubMed] [Google Scholar]
- 17.Revuelta GJ, et al. , Pilot study of atomoxetine in patients with Parkinson's disease and dopa-unresponsive Freezing of Gait. Transl Neurodegener, 2015. 4: p. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt BA, Midcingulate cortex: Structure, connections, homologies, functions and diseases. J Chem Neuroanat, 2016. 74: p. 28–46. [DOI] [PubMed] [Google Scholar]
- 19.Fyhn M, et al. , Spatial representation in the entorhinal cortex. Science, 2004. 305(5688): p. 1258–64. [DOI] [PubMed] [Google Scholar]
- 20.Onu M, et al. , Increased connectivity between sensorimotor and attentional areas in Parkinson's disease. Neuroradiology, 2015. 57(9): p. 957–68. [DOI] [PubMed] [Google Scholar]
- 21.Kim K, et al. , Thalamic cholinergic innervation makes a specific bottom-up contribution to signal detection: Evidence from Parkinson's disease patients with defined cholinergic losses. Neuroimage, 2017. 149: p. 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller ML, et al. , Thalamic cholinergic innervation and postural sensory integration function in Parkinson's disease. Brain, 2013. 136(Pt 11): p. 3282–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albin RL, et al. , Targeting the pedunculopontine nucleus in Parkinson's disease: Time to go back to the drawing board. Mov Disord, 2018. 33(12): p. 1871–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snijders AH, et al. , Physiology of freezing of gait. Annals of Neurology, 2016. 80(5): p. 644–659. [DOI] [PubMed] [Google Scholar]
- 25.Thevathasan W, et al. , Pedunculopontine nucleus deep brain stimulation in Parkinson's disease: A clinical review. Mov Disord, 2018. 33(1): p. 10–20. [DOI] [PubMed] [Google Scholar]
- 26.Muthusamy KA, et al. , Connectivity of the human pedunculopontine nucleus region and diffusion tensor imaging in surgical targeting. J Neurosurg, 2007. 107(4): p. 814–20. [DOI] [PubMed] [Google Scholar]
- 27.Fling BW, et al. , Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain, 2013. 136(8): p. 2405–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edlow BL, et al. , Neuroanatomic connectivity of the human ascending arousal system critical to consciousness and its disorders. J Neuropathol Exp Neurol, 2012. 71(6): p. 531–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fling BW, et al. , Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain, 2013. 136(Pt 8): p. 2405–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallea C, et al. , Pedunculopontine network dysfunction in Parkinson's disease with postural control and sleep disorders. Mov Disord, 2017. 32(5): p. 693–704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Areas of differences in Functional Connectivity between the Freezers and Non-freezers corrected for disease duration
Supplementary Figure 3. Scatter plots of the correlation between PPN connectivity strength and gait performance (TUG and time to turn).
Supplementary Figure 2. Depiction of MLR/PPN ROI
Supplementary Figure 1. Functional connectivity analysis pipeline.