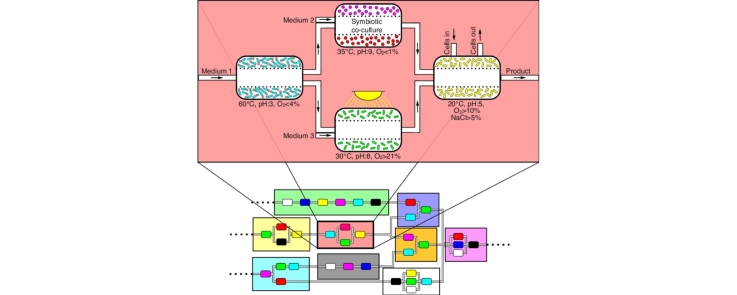
Graphical abstract
Abstract
Traditional biotechnological applications of microorganisms employ mono-cultivation or co-cultivation in well-mixed vessels disregarding the potential of spatially organized cultures. Metabolic specialization and guided species interactions facilitated through spatial isolation would enable consortia of microbes to accomplish more complex functions than currently possible, for bioproduction as well as biodegradation processes. Here, we review concepts of spatially linked microbial consortia in which spatial arrangement is optimized to increase control and facilitate new species combinations. We highlight that genome-scale metabolic network models can inform the design and tuning of synthetic microbial consortia and suggest that a standardized assembly of such systems allows the combination of ‘incompatibles’, potentially leading to countless novel applications.
Current Opinion in Biotechnology 2020, 62:137–145
This review comes from a themed issue on Environmental biotechnology
Edited by David R Johnson and Stephan Noack
For a complete overview see the Issue and the Editorial
Available online 1st November 2019
https://doi.org/10.1016/j.copbio.2019.09.015
0958-1669/© 2019 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Mankind has relied for millennia on the biochemical capabilities of microbial consortia for valuable dietary by-products—largely unaware of microbial existence or microbes’ identity. Progress in synthetic biology and biotechnology in the last century has led to the development of artificial microbial consortia composed of selected members, sometimes genetically engineered for specific tasks [1, 2, 3, 4]. Unlike traditional fermenter monocultures, consortia permit the division of labor and metabolic specialization among members, which confers certain advantages in the performance of consortia used for complex chemical pathways [1, 2, 3, 4]. Applications of such consortia include the production of chemicals, biofuels and pharmaceuticals, and services such as bioremediation and biomining (i.e. recovery of metals) [5,6]. Despite increasing numbers of industrial applications, the use of microbial consortia typically follows a simple template in which consortium members are mixed and subjected to similar culture conditions (nutrients, temperature, pH, dissolved oxygen, etc.). The spatial arrangement of consortia members observed in natural systems (as well as some industrial systems, such as microbial granules in sludge reactors), where members self-organize to exploit favorable locations (due to by-products or other local conditions), casts doubts about the optimality of the standard mixed culture practices. We hypothesize that the routine use of mixed template underutilizes the potential of directed spatial arrangement and segregation of microbial populations, which is attainable from the scale of cell assemblages to that of culture vessels. The spatial organization of microbial species shapes their interactions (and vice versa), and thus could greatly affect the function of a consortium [7, 8, 9]. Controlling the spatial organization of members of a microbial consortium and the local conditions offers a potential for overcoming limitations imposed by undifferentiated growth environments, and provides a range of tools for systematically investigating the spatio-temporal behavior of such living systems.
In the following we review current methods available for culturing spatially arranged microbial consortia, and highlight respective advantages, limitations and engineering challenges. Exerting spatial control over members of microbial consortia offers not only streamlined microbial ecological interactions, but also the possibility of linking spatial niches to address the special needs of (interacting) members that may not coexist in mixed or in natural systems. We discuss the rapidly growing field of metabolic genome-scale network models as tools for modeling complex outcomes needed for member selection and system engineering. We suggest that the untapped flexibility of assembling spatially linked microbial consortia could open new opportunities for employing a combination of species that cannot be grown in mixed cultures because of incompatible physiological requirements. We conclude that this could lead to the standardization of the construction of microbial consortia similarly to electronic circuit-boards.
Experimental consortia of spatially linked and interacting microorganisms
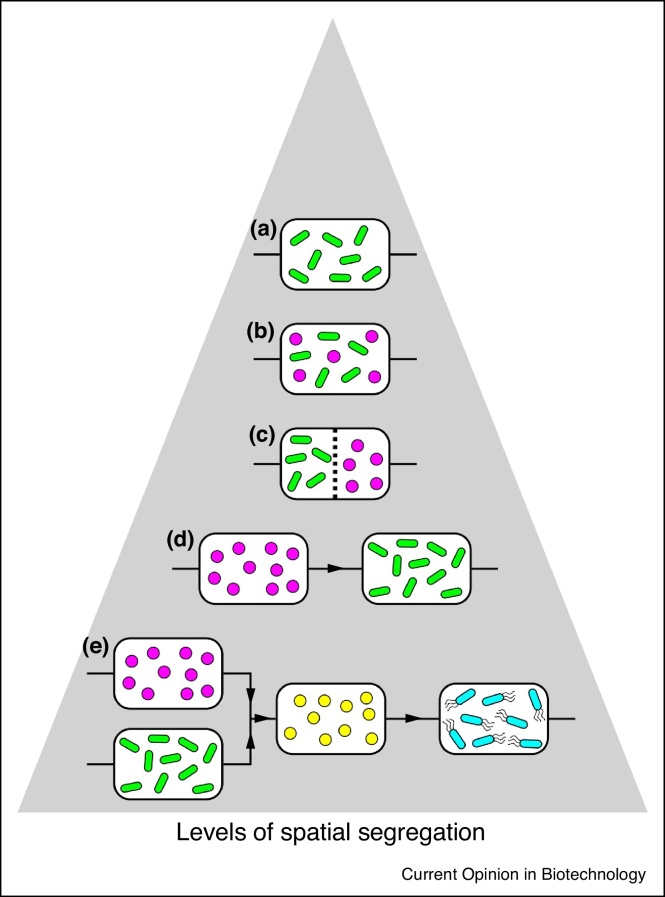
Recent studies suggested that the technical evolution from mono-cultivation to co-cultivation in microbiology represents a revolution in the biotechnological exploitation of microbes [10•]. We expand these statements by considering control over the spatial arrangement of co-cultivates with new applications of microbial consortia. The refinement of spatial organization is akin to a natural evolution from the simple co-culture design (Figure 1).
Figure 1.
From monocultures to spatially linked microbial consortia.
Increase of spatial control of microbial cultures, starting with undifferentiated environments such as monocultures (a) co-cultures (b) and co-cultures with the addition of membranes to segregate interacting members of a microbial consortium (c), followed by a full segregation (SLMC) into interconnected modules offering different environments (d) and (e).
Attempts to segregate interacting microbial members in space date back to the 1960’s. One strategy relied on culturing interacting species in the same vessel separated by permeable membranes that block macromolecules and cells [11, 12, 13]. A potential drawback of such co-cultures is that all members are exposed to the same physico-chemical conditions irrespective of their specific requirements. A different approach connects two or more bioreactors containing different microorganisms, in series or as multi-stage continuous cultures [14, 15, 16]. Surprisingly, although theoretical and experimental background have been in place for 50 years [14], such spatial-segregation solutions have not gained broader applications in current microbial biotechnology practices. Perhaps the engineering challenges of controlling all modules and fluxes individually, containing each consortium member within its module, and maintaining population sizes under distinct environmental conditions, proved to be too discouraging. The rapid changes in present-day technological landscape with new sensing devices, community member identification and advanced computational capabilities [17•,18,19••,20, 21, 22, 23, 24, 25, 26, 27, 28] (discussed below), offer new opportunities. In addition to advances in synthetic biology with powerful capabilities for genetically engineered consortium members and interspecies interactions [29, 30, 31, 32, 33, 34], optimizing the arrangement and local conditions for such members should offer a multiplier for the overall efficiency. New and promising platforms for research and applications are offered by recent advances in microfluidic devices that can host physically separated microbial populations and enable control over fluxes exchanged [10•,35, 36, 37, 38]. In terms of spatial positioning, methods such as 2D patterning using photolithography [39] or inkjet-printing [40], membranes [11, 12, 13,41], and 3D printing [42•,43] offer a range of solutions for the spatial organization of microbial populations down to the microscale. Despite these technological developments, research on microbial consortia arranged in space remains largely at the level of ‘proof of concept’, and only rarely ventures into the domain of practical applications [8].
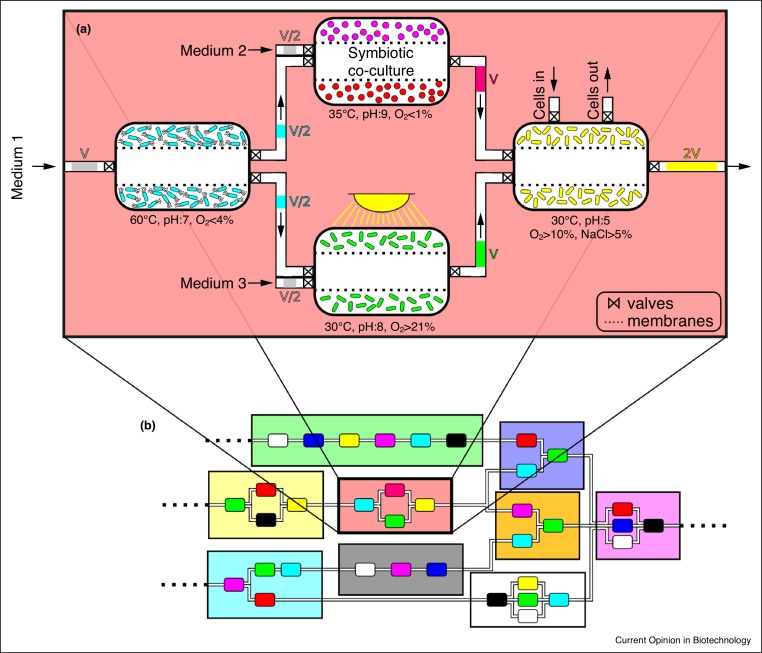
As outlined in [44•], the engineering and assembly of spatially linked microbial consortia (SLMC) involve the following key considerations: (i) the selection of microorganisms required to accomplish a specific bioprocess, (ii) the design of a spatial platform, or ‘landscape’, and (iii) operational considerations of inputs and fluxes. Different members could be placed in their respective modules, following the order of reactions of by-products of the biochemical processes, and connections between modules would be determined by the desired functions and interactions (Figure 2). Members of the consortium would be selected based on their ability to accomplish part of the overall biotransformation, without the strict requirement of being subjected to a common set of environmental conditions, as in co-cultures. This could potentially increase the range and combinations of interacting microorganisms (even allowing the assembly of consortia composed of incompatible members), with countless novel products and applications in disciplines ranging from bioremediation, pharmaceuticals and biofuels to space missions where the use of microorganisms to recycle wastes is being investigated [44•]. Despite this increased freedom of selection, considerations such as resilience to perturbations, growth characteristics, yields, nutritional requirements, secreted by-products, become important criteria when choosing members for the consortium [45].
Figure 2.
Functional elements for the culture of spatially linked microbial consortia.
(a) Represented is a hypothetical microbial consortium composed of members with incompatible requirements, as described by the different temperatures, pHs, and oxygen concentrations… Independently controlled modules containing the different members are connected to allow exchanges of metabolic by-products to achieve the desired function. Valves would control the mode (here pulses of media of volume V would be injected in the first module at regular time intervals) and rates of fluxes between modules to optimize the output of the consortium. Cells could be replaced independently as needed. Symbionts could be co-cultured if full segregation was detrimental. (b) The modularity of SLMC would allow the incorporation of microbial consortia into larger biochemical network, forming a conceptualized biological circuit board.
To activate the SLMC and initiate production, cells could be preserved by freezing, drying or lyophilization, followed by thawing or hydration to awaken the consortium [46,47]. The activation of consortium members could be synchronous or sequential, and the system may initially go through a transient phase before a steady-state is reached. Various types of membranes could be used to contain and isolate the species while allowing passage of metabolites and growth media at rates controlled by valves and pumps (or resulting from different sizes of modules) [12,13]. This scalable concept, where the type and scale of a platform would be determined by the application, could range from microfluidic devices [48] to industrial size bioreactors [14,49] and scaling could occur by parallelization or scaling up of platforms when feasible [50]. Should an adverse event (mutation, contamination) occur and be detected by the monitoring system [51], inactivation methods (physical or chemical) [47,52] could be used to sterilize the faulty module before replenishing it with fresh cells. To limit the impact of such events, redundancies, that is multiple modules fulfilling the same function in parallel, could be placed at sensitive segments, prone to failure [44•]. The monitoring of state variables (i.e. concentrations of substrates, intermediates, products, pH, oxygen concentration…), while challenging in itself, should provide the necessary information for tuning the system and assessing its performance [44•].
The review identifies the design of microbial consortia that permit the optimization of spatial organization and growth conditions for each member (Figure 2) as a promising strategy for future research and development in the cultivation of microbial consortia [44•]. A potential SLMC domain would be composed of interconnected ‘niches’ that offer differentiated conditions (temperature, pH, dissolved oxygen,…) for each consortium member, with a potential of enhancing a desired function performed by the consortium. The independent supply of nutrients to each niche or module could be designed to reduce interspecies competition for substrates, while simultaneously serving as a population control tool. In other cases, for example when mutualistic interactions are sought, co-cultivation in a single module would be applied. This could be done with or without separating the interacting populations within the module, depending on requirements for cell-to-cell contact or emergent self-organization. A SLMC would permit any combination of such single-member and mixed-members modules (Figure 2). The compartmentalization at the core of SLMC would enable mediation and control of interspecies interactions, therefore the potential for increased stability and predictability of the bioprocess. Incidentally, the potential modularity of the SLMC concept, which facilitates the design of predictable consortia in a flexible way (via ‘plug-in’ and ‘plug-out’ of modules), would provide a useful tool to the rapidly expanding field of synthetic microbial ecology [1,2,4,53,54].
The success of such systems hinges on engineering and management of the SLMC landscape, and the preselection of microbial members and prediction of their traits and metabolic interactions. New and powerful tools are offered by mathematical modeling and full genomic-based metabolic networks outlined next.
Mathematical modeling for designing and assembling microbial consortia
Mathematical modeling of interacting microbial consortia offers a means for disentangling interspecies trophic interactions and provides a framework for the design of synthetic consortia with specific metabolic functions. Although a variety of mathematical techniques and models have been used for that purpose [18,20, 21, 22, 23, 24,28], the growing availability and use of genome-scale metabolic network modeling [22,25,55] offer a breakthrough for representing and screening interactions in such complex and adaptive SLMC landscape. On the basis of whole-genome sequences, genome-scale models combine all known encoded metabolic reactions into networks amenable to mathematical analysis. Genome-scale models, whether full or reduced, have been successfully used to predict microbial functions such as growth on various substrates, and to study the effects of specific gene knockouts on cell metabolism [22,56,57]. In addition, reduced scale models, based on a subset of the metabolic reactions whilst retaining predictability for pathways of interest, are used to increase computational efficiency [58,59]. In the context of co-cultivated species, metabolic networks permit the prediction of interspecies metabolic interactions solely based on the intracellular metabolism of each member of the consortium [60]. A new landmark study strategically investigated over 2 million co-culture simulations—24 species in pairwise combinations under various environmental conditions—to identify cross-feeding interactions that provide opportunities for stable multispecies assembly [61••]. Critically, the study emphasized the determining role of the growth environment (substrate and oxygen availability) in the interspecies exchange of metabolites [61••]. Such high-throughput computational framework appears particularly well-suited as an initial step to inform the design of SLMC, namely in the choice of interacting members. However, additional computational tools may be required to specifically investigate and optimize the spatio-temporal organization of SLMC. Several recent modeling developments have used genome-scale metabolic networks in a spatially explicit context with the goal to mechanistically describe interspecies competition and trophic interactions [62,63,64•]. One such model that simulates bacterial colonies growing on agar using population-based approaches combined with flux balance analysis has demonstrated that certain interspecies processes are only possible in the context of space [62]. A current and innovative development sees the combination of genome-scale models with an individual-based representation of bacterial cells (i.e. agent-based modeling) [63,64•,65•]. Using such a model, differences in metabolic activities could be predicted within Escherichia coli colonies depending on the localization of individual cells relative to carbon and oxygen resources [63]. Further modeling extensions have permitted to include multiple species [62,63] and accommodate them in more complex environments [65•].
The computational tools and optimization strategies described above are already applied to biotechnological development: for example, genome-scale modeling of multispecies interactions was used to design more efficient bacterial consortia for the degradation of herbicide in soil [66•], and in-silico genetic engineering and spatio-temporal metabolic network models were used to improve the yield of syngas fermentation [67]. In that second study, an initial screening for beneficial gene knockouts consistently resulted in lower growth of the fermenting species Clostridium ljungdahlii. Using a spatio-temporal metabolic model of the bubble column reactor provided potential new gene targets to improve product synthesis—a discovery not possible without the context of space. Although technical and terminological hurdles still refrain the automated reconstruction of genome-scale networks [68], some methodological solutions have been delineated [69,70]. Therefore, from this point forward we expect a variety of (semi-) automated procedures and tools to rapidly increase the number of genome-scale network models available to the research community. Finally, standardization of metabolic networks would facilitate the combination of multiple network models required for consortia modeling.
The assembly of misfits
The added-value of spatially linked microbial consortia in terms of performance and function rests on alleviating the requirements for a compatible environment for the whole microbial consortium, the topological optimization of flux exchange, and the tuning of community sizes and inputs to meet the physiological capabilities of members. By segregation and optimization of each module, SLMC would allow the selection of the members based mainly on their function rather than be limited by the need to offer compatible growth conditions for every species (Figure 3). This would even allow the construction of novel artificial microbial consortia composed of members with incompatible requirements that would therefore not co-exist in nature.
Figure 3.
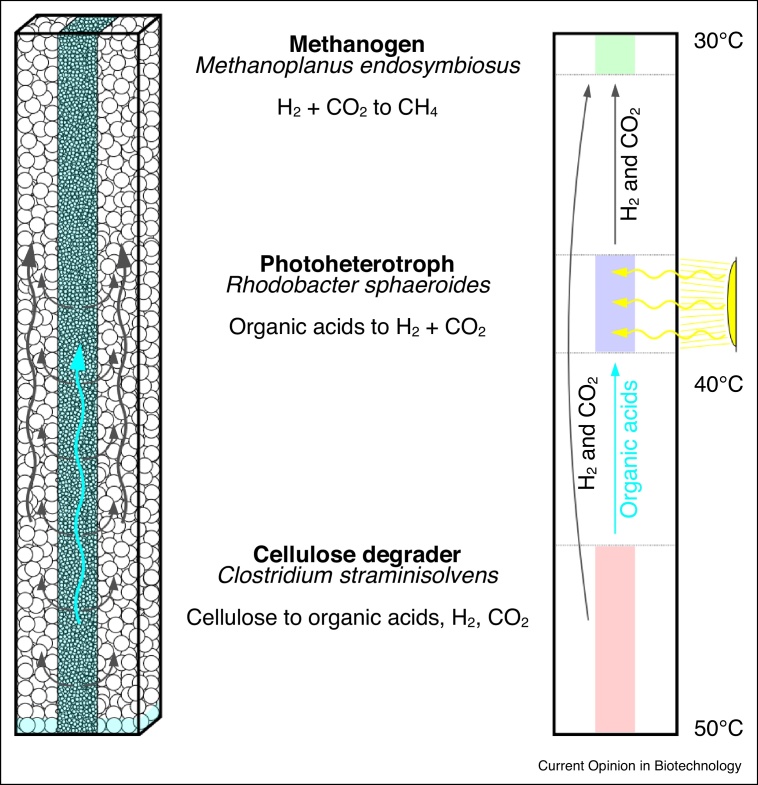
Design of a glass beads column with thermal gradient spatially segregating a microbial consortium for (anaerobic) methane production from cellulose.
In this SLMC prototype, three microbial species jointly convert cellulose to methane: a thermophilic cellulose degrader (Clostridium straminisolvens, in red), a photoheterotroph that converts organic acids to hydrogen gas (Rhodobacter sphaeroides, in purple) and a methanogen converting hydrogen gas and carbon dioxide to methane (Methanoplanus endosymbiosus, in green). Due to their incompatible thermal requirements, the different members should segregate along the thermal gradient. A central column composed of smaller glass beads would guarantee a continuous aqueous phase along the column, while the unsaturated periphery would be filled with larger beads allowing gases to be released, exchanged and consumed. Grey arrows: gas release/exchange. Blue arrow: exchange of soluble by-products.
In that context, we have designed and are currently testing a prototype of a SLMC column for the transformation of cellulose into biofuel (Figure 3). Here, the thermophilic anaerobic bacterium Clostridium straminisolvens, known for its ability to degrade filter paper [71], converts the cellulose contained in the filter at about 50–55°C and releases organic acids such as acetate and lactate, as well as hydrogen gas, carbon dioxide and traces of ethanol. The purple non-sulfur bacterium Rhodobacter sphaeroides converts photoheterotrophically at 30–35°C, acetate, lactate and ethanol to more hydrogen gas and carbon dioxide [72]. The last member, Methanoplanus endosymbiosus, a methanogenic archaeon, consumes hydrogen gas and carbon dioxide at 20–30°C to produce methane [73]. Both the photoheterotroph and the methanogen cannot grow at the minimal growth temperature required by the thermophile (on which this consortium relies to release labile carbon sources), and therefore have to occupy different niches along the thermal gradient. This experiment is intended to highlight the importance of space in the context of microbial ecology, and how this allowed the combination of 'incompatible' strains, from horizons as diverse as a composting bacterial community in Japan (C. straminisolvens), light exposed stagnant waters with low oxygen concentrations such as eutrophic ponds (R. sphaeroides), or mud from North Sea sediments (M. endosymbiosus). We hope to show that with relatively simple methods one can engineer the habitats for a community of interacting microorganisms, which should hopefully further motivate current efforts to spatially engineer microbial consortia. Ultimately, the concept of SLMC aims at harnessing and expanding on microbiological knowledge and venture in the largely underused realm of synthetic microbial ecology in an attempt to design habitats and prescribe interactions between the members of a microbial consortium, to optimize the biochemical process it catalyzes, and construct new consortia that do not exist in nature.
Conclusion and outlook
Individual members of microbial consortia may require special conditions for their optimal functioning that may not be available in a concourse within a similar environment that inherently restricts conditions for species compatibility. Present biotechnological applications often lack the structure and variety of habitats necessary to fully exploit the potential of spatial segregation when appropriate. The strategy suggested by SLMC aims at providing a diversity of environments necessary for optimizing the growth and biochemical function of each consortium member. Moreover, as illustrated in Figure 2, the intrinsic modularity of an engineered SLMC could facilitate the assembly of microbial consortia with increased control, improved stability and predictability relative to current mixed culture methods [44•]. The assembly of multiple SLMC domains could enable the construction of ‘superconsortia’ composed of interacting ‘subconsortia’ designed for a specific function, that are assembled in a ‘plug-and-play’ manner, just like letters form words from which sentences are subsequently constructed (Figure 2). Function-based libraries of consortia could be developed as the alphabet that will facilitate the construction of ‘superconsortia’, unbound by any upper complexity limit. The combination of genetic information about the microorganisms and environmental conditions (substrates, temperature, pH…), by tools like flux balance analysis (FBA), with additional thermodynamic constraints [28,57], could guide the design of such microbial consortia by providing information about substrate consumption, growth rate, production of intermediates and end product(s), towards optimizing the desired function. Should a system be diffusion limited, a combination of FBA and individual-based modeling (IBM) would offer the required and detailed spatial representation necessary for the modeling of such consortia [65•]. The practical implementation of the SLMC concept will require innovative engineering solutions to the many challenges of assembling, initiating and maintaining such ‘biological circuit boards’ (Figure 2). Recent technological developments make such an enterprise within our reach and should motivate further exploration of microbial ecosystems, hopefully resulting in novel products or applications, with benefits to mankind and the environment. Engineering new interacting communities within the framework of synthetic ecology might allow us to tap into the immense potential of the microbial world in similar ways as the modularity and scalability of the 20th century electronic revolution.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
Financial support for this work came from an Advanced Grant to D.O. by the European Research Council (ERC-3200499-‘SoilLife’), the RTD SystemsX.ch project ‘MicroscapesX’ to D.O and ETH institutional funding.
References
- 1.Hays S.G., Patrick W.G., Ziesack M., Oxman N., Silver P.A. Better together: engineering and application of microbial symbioses. Curr Opin Biotechnol. 2015;36:40–49. doi: 10.1016/j.copbio.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Jagmann N., Philipp B. Reprint of design of synthetic microbial communities for biotechnological production processes. J Biotechnol. 2014;192:293–301. doi: 10.1016/j.jbiotec.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein H.C., Carlson R.P. Microbial consortia engineering for cellular factories: in vitro to in silico systems. Comput Struct Biotechnol J. 2012;3:1–8. doi: 10.5936/csbj.201210017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindemann S.R., Bernstein H.C., Song H.S., Fredrickson J.K., Fields M.W., Shou W., Johnson D.R., Beliaev A.S. Engineering microbial consortia for controllable outputs. ISME J. 2016;10:2077–2084. doi: 10.1038/ismej.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarty N.S., Ledesma-Amaro R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019;37:181–197. doi: 10.1016/j.tibtech.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu H., Villada J.C., Lee P.K.H. Modular metabolic engineering for biobased chemical production. Trends Biotechnol. 2019;37:152–166. doi: 10.1016/j.tibtech.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim H.J., Boedicker J.Q., Choi J.W., Ismagilov R.F. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci USA. 2008;105:18188–18193. doi: 10.1073/pnas.0807935105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim H.J., Du W., Ismagilov R.F. Complex function by design using spatially pre-structured synthetic microbial communities: degradation of pentachlorophenol in the presence of Hg(ii) Integr Biol. 2011;3:126–133. doi: 10.1039/c0ib00019a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tecon R., Or D. Cooperation in carbon source degradation shapes spatial self-organization of microbial consortia on hydrated surfaces. Sci Rep. 2017;7 doi: 10.1038/srep43726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Nai C., Meyer V. From axenic to mixed cultures: technological advances accelerating a paradigm shift in microbiology. Trends Microbiol. 2018;26:538–554. doi: 10.1016/j.tim.2017.11.004. [DOI] [PubMed] [Google Scholar]; This review argues that an evolution in cultivation techniques is necessary to better understand microbial interactions and why this is relevant in many areas ranging from microbial ecology to natural product discovery. They specifically discuss current microbiological tools and recent technical advances such as microfluidics, 3D printing and single cell metabolomics, and how these could help decode microbial communication.
- 11.Ohno M., Okano I., Watsuji T., Kakinuma T., Ueda K., Beppu T. Establishing the independent culture of a strictly symbiotic bacterium Symbiobacterium thermophilum from its supporting Bacillus strain. Biosci Biotechnol Biochem. 1999;63:1083–1090. doi: 10.1271/bbb.63.1083. [DOI] [PubMed] [Google Scholar]
- 12.Manjarrez E.S., Albasi C., Riba J.P. A two-reservoir, hollow-fiber bioreactor for the study of mixed-population dynamics: design aspects and validation of the approach. Biotechnol Bioeng. 2000;69:401–408. doi: 10.1002/1097-0290(20000820)69:4<401::aid-bit6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 13.Taniguchi M., Tanaka T. Clarification of interactions among microorganisms and development of co-culture system for production of useful substances. Adv Biocheml Eng. 2004;90:35–62. doi: 10.1007/b94191. Recent Progress of Biochemical and Biomedical Engineering in Japan I, [DOI] [PubMed] [Google Scholar]
- 14.Malek I., Beran K., Hospodka J. Continuous cultivation of microorganisms. Proceedings of the Second Symposium. 1964;1:23–57. [Google Scholar]
- 15.Chang H.N., Jung K., Choi J., Lee J.C., Woo H.C. Multi-stage continuous high cell density culture systems: a review. Biotechnol Adv. 2014;32:514–525. doi: 10.1016/j.biotechadv.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Tapia F., Vazquez-Ramirez D., Genzel Y., Reichl U. Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production. Appl Microbiol Biotechnol. 2016;100:2121–2132. doi: 10.1007/s00253-015-7267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17•.Cao X., Hamilton J.J., Venturelli O.S. Understanding and engineering distributed biochemical pathways in microbial communities. Biochemistry. 2019;58:94–107. doi: 10.1021/acs.biochem.8b01006. [DOI] [PMC free article] [PubMed] [Google Scholar]; This perspective discusses the importance of interspecies, as well as microbe-habitat, interactions in shaping the function of the community, and how a mechanistic and quantitative understanding (ecological/molecular/environmental) could help us control the functions and dynamics of such systems. The predictive power of mathematical modeling, to tailor microbial communities’ behaviors, is also discussed.
- 18.DiMucci D., Kon M., Segrè D. Machine learning reveals missing edges and putative interaction mechanisms in microbial ecosystem networks. mSystems. 2018;3:1–13. doi: 10.1128/mSystems.00181-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19••.Friedman J., Higgins L.M., Gore J. Community structure follows simple assembly rules in microbial microcosms. Nat Ecol Evol. 2017;1:1–7. doi: 10.1038/s41559-017-0109. [DOI] [PubMed] [Google Scholar]; This study designed a pragmatic rule to assemble synthetic microbial communities based on the outcome of pairwise co-cultures to predict the outcomes of cultures containing three and more species. Three-species competitions (co-cultures) were predicted with ∼90% accuracy. For co-cultures containing seven or all eight members they used information from three-species competitions to reach a similar level of accuracy. This shows that a simple bottom-up approach can successfully predict community structure.
- 20.Julien-Laferriere A., Bulteau L., Parrot D., Marchetti-Spaccamela A., Stougie L., Vinga S., Mary A., Sagot M.F. A combinatorial algorithm for microbial consortia synthetic design. Sci Rep. 2016;6:1–12. doi: 10.1038/srep29182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowery N.V., Ursell T. Structured environments fundamentally alter dynamics and stability of ecological communities. Proc Natl Acad Sci USA. 2019;116:379–388. doi: 10.1073/pnas.1811887116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien E.J., Monk J.M., Palsson B.O. Using genome-scale models to predict biological capabilities. Cell. 2015;161:971–987. doi: 10.1016/j.cell.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peaudecerf F.J., Bunbury F., Bhardwaj V., Bees M.A., Smith A.G., Goldstein R.E., Croze O.A. Microbial mutualism at a distance: the role of geometry in diffusive exchanges. Phys Rev E. 2018;97 doi: 10.1103/PhysRevE.97.022411. [DOI] [PubMed] [Google Scholar]
- 24.Succurro A., Segrè D., Ebenhöh O. Emergent subpopulation behavior uncovered with a community dynamic metabolic model of Escherichia coli diauxic growth. mSystems. 2019;4:1–16. doi: 10.1128/mSystems.00230-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thommes M., Wang T., Zhao Q., Paschalidis I.C., Segrè D. Designing metabolic division of labor in microbial communities. mSystems. 2019;4 doi: 10.1128/mSystems.00263-18. e00263-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venters M., Carlson R.P., Gedeon T., Heys J.J. Effects of spatial localization on microbial consortia growth. PLoS One. 2017;12:1–20. doi: 10.1371/journal.pone.0168592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Widder S., Allen R.J., Pfeiffer T., Curtis T.P., Wiuf C., Sloan W.T., Cordero O.X., Brown S.P., Momeni B., Shou W. Challenges in microbial ecology: building predictive understanding of community function and dynamics. ISME J. 2016;10:2557–2568. doi: 10.1038/ismej.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zomorrodi A.R., Segrè D. Synthetic ecology of microbes: mathematical models and applications. J Mol Biol. 2016;428:837–861. doi: 10.1016/j.jmb.2015.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kylilis N., Tuza Z.A., Stan G.B., Polizzi K.M. Tools for engineering coordinated system behaviour in synthetic microbial consortia. Nat Commun. 2018;9 doi: 10.1038/s41467-018-05046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nguyen P.Q., Courchesne N.M.D., Duraj-Thatte A., Praveschotinunt P., Joshi N.S. Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. Adv Mater. 2018;30:1–34. doi: 10.1002/adma.201704847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volke D.C., Nikel P.I. Synthetic morphologies: getting bacteria in shape: synthetic morphology approaches for the design of efficient microbial cell factories (Adv. Biosys. 11/2018) Adv Biosyst. 2018;2 [Google Scholar]
- 32.Tanouchi Y., Smith R.P., You L. Engineering microbial systems to explore ecological and evolutionary dynamics. Curr Opin Biotechnol. 2012;23:791–797. doi: 10.1016/j.copbio.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goers L., Freemont P., Polizzi K.M. Co-culture systems and technologies: taking synthetic biology to the next level. J R Soc Interface. 2014;11 doi: 10.1098/rsif.2014.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H., Wang X. Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng. 2016;37:114–121. doi: 10.1016/j.ymben.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 35.Burmeister A., Hilgers F., Langner A., Westerwalbesloh C., Kerkhoff Y., Tenhaef N., Drepper T., Kohlheyer D., Von Lieres E., Noack S. A microfluidic co-cultivation platform to investigate microbial interactions at defined microenvironments. Lab Chip. 2019;19:98–110. doi: 10.1039/c8lc00977e. [DOI] [PubMed] [Google Scholar]
- 36.Nagy K., Ábrahám Á, Keymer J.E., Galajda P. Application of microfluidics in experimental ecology: the importance of being spatial. Front Microbiol. 2018;9:496. doi: 10.3389/fmicb.2018.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osmekhina E., Jonkergouw C., Schmidt G., Jahangiri F., Jokinen V., Franssila S., Linder M.B. Controlled communication between physically separated bacterial populations in a microfluidic device. Commun Biol. 2018;1:1–7. doi: 10.1038/s42003-018-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pham P.L.H., Rooholghodos S.A., Choy J.S., Luo X. Constructing synthetic ecosystems with biopolymer fluitrodes. Adv Biosyst. 2018;2 [Google Scholar]
- 39.Chen F., Ricken J., Xu D., Wegner S.V. Bacterial photolithography: patterning Escherichia coli biofilms with high spatial control using photocleavable adhesion molecules. Adv Biosyst. 2018 doi: 10.1002/adbi.201800269. 1800269. [DOI] [PubMed] [Google Scholar]
- 40.Choi W.S., Ha D., Park S., Kim T. Synthetic multicellular cell-to-cell communication in inkjet printed bacterial cell systems. Biomaterials. 2011;32:2500–2507. doi: 10.1016/j.biomaterials.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Hu P., Chakraborty S., Kumar A., Woolston B., Liu H., Emerson D., Stephanopoulos G. Integrated bioprocess for conversion of gaseous substrates to liquids. Proc Natl Acad Sci USA. 2016;113:3773–3778. doi: 10.1073/pnas.1516867113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42•.Schmieden D.T., Basalo Vázquez S.J., Sangüesa H., Van Der Does M., Idema T., Meyer A.S. Printing of patterned, engineered E. coli biofilms with a low-cost 3D printer. ACS Synth Biol. 2018;7:1328–1337. doi: 10.1021/acssynbio.7b00424. [DOI] [PubMed] [Google Scholar]; This study describes a new method where 3D printing is combined with genetic engineering to produce reproducible and patterned biofilm-inspired materials which survived for at least one week. Induced biofilm formation maintained its pattern and adherence to the printing substrate after removal of the matrix. This is the first time biofilm-mimicking living materials were engineered by combining spatial and genetic control.
- 43.Connell J.L., Ritschdorff E.T., Whiteley M., Shear J.B. 3D printing of microscopic bacterial communities. Proc Natl Acad Sci USA. 2013;110:18380–18385. doi: 10.1073/pnas.1309729110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Ben Said S., Or D. Synthetic microbial ecology: engineering habitats for modular consortia. Front Microbiol. 2017;8:1125. doi: 10.3389/fmicb.2017.01125. [DOI] [PMC free article] [PubMed] [Google Scholar]; This review introduces the concept of spatially linked microbial consortia (SLMC) aiming to engineering habitats to culture the members of microbial consortia. Advantages over co-cultures and opportunities and challenges are discussed, and it is suggested that the increased freedom of assembly of such synthetic consortia could even allow the combination of incompatible species.
- 45.Allison S.D., Martiny J.B.H. Colloquium paper: resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci USA. 2008;105(Suppl):11512–11519. doi: 10.1073/pnas.0801925105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan C.A., Herman N., White P.A., Vesey G. Preservation of micro-organisms by drying; a review. J Microbiol Methods. 2006;66:183–193. doi: 10.1016/j.mimet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 47.Stanbury P.F., Whitaker A., Hall S.J. Elsevier; 2017. Principles of Fermentation Technology. [Google Scholar]
- 48.Nguyen N.T., Wereley S.T., Shaegh, Mousavi Shaegh S.A. Artech House; 2019. Fundamentals and Applications of Microfluidics. [Google Scholar]
- 49.Grootjen D.R.J., Jansen M.L., van der Lans R.G.J.M., Luyben K.C.A.M. Reactors in series for the complete conversion of glucose/xylose mixtures by Pichia stipitis and Saccharomyces cerevisiae. Enzyme Microb Technol. 1991;13:828–833. [Google Scholar]
- 50.Villadsen J., Nielsen J., Lidén G. Springer US; 2011. Bioreaction Engineering Principles. [Google Scholar]
- 51.Pohlscheidt M., Charaniya S., Bork C., Jenzsch M., Noetzel T.L., Luebbert A. Encyclopedia of Industrial Biotechnology. John Wiley & Sons, Inc.; 2013. Bioprocess and fermentation monitoring; pp. 1469–1492. [Google Scholar]
- 52.Song K., Mohseni M., Taghipour F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: a review. Water Res. 2016;94:341–349. doi: 10.1016/j.watres.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 53.Dolinšek J., Goldschmidt F., Johnson D.R. Synthetic microbial ecology and the dynamic interplay between microbial genotypes. FEMS Microbiol Rev. 2016;40:961–979. doi: 10.1093/femsre/fuw024. [DOI] [PubMed] [Google Scholar]
- 54.Escalante A.E., Rebolleda-Gomez M., Benítez M., Travisano M. Ecological perspectives on synthetic biology: insights from microbial poopulation biology. Front Microbiol. 2015;6:1–10. doi: 10.3389/fmicb.2015.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terzer M., Maynard N.D., Covert M.W., Stelling J. Genome-scale metabolic networks. Wiley Interdiscip Rev Syst Biol Med. 2009;1:285–297. doi: 10.1002/wsbm.37. [DOI] [PubMed] [Google Scholar]
- 56.Oberhardt M.A., Palsson B.Ø, Papin J.A. Applications of genome-scale metabolic reconstructions. Mol Syst Biol. 2009;5:1–15. doi: 10.1038/msb.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bordbar A., Monk J.M., King Z.A., Palsson B.O. Constraint-based models predict metabolic and associated cellular functions. Nat Rev Genet. 2014;15:107–120. doi: 10.1038/nrg3643. [DOI] [PubMed] [Google Scholar]
- 58.Ataman M., Hatzimanikatis V. lumpGEM: systematic generation of subnetworks and elementally balanced lumped reactions for the biosynthesis of target metabolites. PLoS Comput Biol. 2017;13 doi: 10.1371/journal.pcbi.1005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ataman M., Gardiol D.F.H., Fengos G., Hatzimanikatis V. redGEM: systematic reduction and analysis of genome-scale metabolic reconstructions for development of consistent core metabolic models. PLoS Comput Biol. 2017;13 doi: 10.1371/journal.pcbi.1005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zomorrodi A.R., Islam M.M., Maranas C.D. d-OptCom: dynamic multi-level and multi-objective metabolic modeling of microbial communities. ACS Synth Biol. 2014;3:247–257. doi: 10.1021/sb4001307. [DOI] [PubMed] [Google Scholar]
- 61••.Pacheco A.R., Moel M., Segrè D. Costless metabolic secretions as drivers of interspecies interactions in microbial ecosystems. Nat Commun. 2019;10 doi: 10.1038/s41467-018-07946-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study investigates the potential for intermediate metabolites to promote interspecies trophic interactions using pairwise growth simulations based on genome scale metabolic networks. The screening of 24 species indicates that there is a large number of exchanged metabolites and that anoxic conditions can promote mutualistic interactions.
- 62.Harcombe W.R., Riehl W.J., Dukovski I., Granger B.R., Betts A., Lang A.H., Bonilla G., Kar A., Leiby N., Mehta P. Metabolic resource allocation in individual microbes determines ecosystem interactions and spatial dynamics. Cell Rep. 2014;7:1104–1115. doi: 10.1016/j.celrep.2014.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cole J.A., Kohler L., Hedhli J., Luthey-Schulten Z. Spatially-resolved metabolic cooperativity within dense bacterial colonies. BMC Syst Biol. 2015;9:1–17. doi: 10.1186/s12918-015-0155-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64•.Bauer E., Zimmermann J., Baldini F., Thiele I., Kaleta C. BacArena: individual-based metabolic modeling of heterogeneous microbes in complex communities. PLoS Comput Biol. 2017;13:1–22. doi: 10.1371/journal.pcbi.1005544. [DOI] [PMC free article] [PubMed] [Google Scholar]; This research article describes the combination of constraint-based modeling, with flux balance analysis and individual-based modeling (BacArena) to study microbial metabolism and biofilm formation by Pseudomonas aeruginosa and model community of the human gut composed of seven microorganisms. The P. aeruginosa biofilm model showed that cross-feeding resulted in spatial phenotypical differentiation over time. The human gut model highlighted the importance of nutrient gradients for niche formations and community structure and prompted novel hypotheses about interspecies metabolic interactions. This study illustrates the significance of spatial and temporal multi-scale modeling approaches.
- 65•.Borer B., Ataman M., Hatzimanikatis V., Or D. Modeling metabolic networks of individual bacterial agents in heterogeneous and dynamic soil habitats (IndiMeSH) PLoS Comput Biol. 2019;15 doi: 10.1371/journal.pcbi.1007127. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article introduces a mathematical model of bacterial life in soil (IndiMeSH), combining an individual-based representation of bacterial cells where growth is calculated using metabolic networks with a detailed representation of the aqueous phase in a porous matrix. Various case studies highlight the versatility and predictive capability of the model by comparing to well established spatiotemporal metabolic network models and experimental data.
- 66•.Xu X., Zarecki R., Medina S., Ofaim S., Liu X., Chen C., Hu S., Brom D., Gat D., Porob S. Modeling microbial communities from atrazine contaminated soils promotes the development of biostimulation solutions. ISME J. 2019;13:494–508. doi: 10.1038/s41396-018-0288-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; The investigators constructed genome-scale metabolic models for Arthrobacter (a degrader of the herbicide atrazine) and four non-degrading soil microorganisms sensitive to atrazine. Their model predicted which consortia would enhance atrazine degradation, and confirmed this experimentally.
- 67.Chen J., Henson M.A. In silico metabolic engineering of Clostridium ljungdahlii for synthesis gas fermentation. Metab Eng. 2016;38:389–400. doi: 10.1016/j.ymben.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 68.Karp P.D., Weaver D., Latendresse M. How accurate is automated gap filling of metabolic models? BMC Syst Biol. 2018;12:1–11. doi: 10.1186/s12918-018-0593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Monk J., Nogales J., Palsson B.Ø. Optimizing genome-scale network reconstructions. Nat Biotechnol. 2014;32:447–452. doi: 10.1038/nbt.2870. [DOI] [PubMed] [Google Scholar]
- 70.Machado D., Andrejev S., Tramontano M., Patil K.R. Fast automated reconstruction of genome-scale metabolic models for microbial species and communities. Nucleic Acids Res. 2018;46:7542–7553. doi: 10.1093/nar/gky537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kato S., Haruta S., Cui Z.J., Ishii M., Yokota A., Igarashi Y. Clostridium straminisolvens sp. nov., a moderately thermophilic, aerotolerant and cellulolytic bacterium isolated from a cellulose-degrading bacterial community. Int J Syst Evol Microbiol. 2004;54:2043–2047. doi: 10.1099/ijs.0.63148-0. [DOI] [PubMed] [Google Scholar]
- 72.Imhoff J.F., Trüper H.G., Pfennig N. Rearrangement of the species and genera of the phototrophic “Purple Nonsulfur Bacteria.”. Int J Syst Bacteriol. 1984;34:340–343. [Google Scholar]
- 73.Van Bruggen J.J.A., Zwart K.B., Hermans J.G.F., van Hove E.M., Stumm C.K., Vogels G.D. Isolation and characterization of Methanoplanus endosymbiosus sp. nov., an endosymbiont of the marine sapropelic ciliate Metopus contortus Quennerstedt. Arch Microbiol. 1986;144:367–374. [Google Scholar]