Abstract
Anthropogenic noise levels are globally rising with profound impacts on ecosystems and the species that live in them. Masking or distraction by noise can interfere with relevant sounds and thereby impact ecological interactions between individuals of the same or different species. Predator–prey dynamics are particularly likely to be influenced by rising noise levels, with important population- and community-level consequences, as species may differentially adapt to noise disturbance. Acoustic noise can, however, also impair the use of visual information by animals through the process of cross-sensory interference, possibly impacting species interactions that have so far been largely ignored by noise impact studies. Here, we assessed how noise affected the performance of great tit (Parus major) foraging on cryptic prey. Birds trained individually to search for paper moths differing in the level of camouflage with the test background were tested in the presence and absence of noise. We found that noise significantly increased approach and attack latencies, but that the effect depended on the level of crypsis. Noise increased latencies for cryptic prey targets, but not for conspicuous and colour-matched prey targets. Our results show that noise can interfere with the processing of visual information, particularly in difficult tasks such as separating objects from a similar looking background. These results have important ecological and evolutionary implications as they demonstrate how globally rising anthropogenic noise levels can influence the arms race between predators and prey across sensory domains.
Keywords: anthropogenic noise, cross-sensory interference, visual predator, species interactions, cryptic prey
1. Introduction
Human activities such as industry and urbanization are rapidly transforming terrestrial and aquatic ecosystems on Earth [1,2]. A recently recognized global disturbance concerns the effect of anthropogenic noise impacts on animals [3–5]. Our current knowledge on noise is mainly restricted to reduced detection of relevant sounds and how this, in turn, can alter intra- and interspecific relations of a wide range of taxa [6–8]. For example, acoustic communication in many species of birds, frogs, mammals and insects is impaired by overlapping levels of traffic noise [9–12]. Similarly, noise can reduce the detection and use of acoustic cues generated by predators or their prey [13–15]. The majority of animal species, however, rely on more than one sense and often combine new information with previously obtained knowledge to make appropriate decisions [16–18]. Therefore, acoustic noise has the potential to interfere with processing of information from other sensory modalities or cognitive domains [19–22].
Such cross-modal impact of anthropogenic acoustic noise can be mediated via direct and indirect pathways. Noise can directly impair multisensory perception via masking within the acoustic channel and thereby affect behaviours such as multimodal communication [23,24]. Noise may also require increased processing capacity by auditory nuclei and thereby interfere with the detection and use of information derived from other sensory inputs [20,25], a process referred to as cross-sensory interference [17]. Finally, noise can indirectly affect higher level cognitive processing through feedback from behavioural or physiological responses [14,26,27]. Noise could, for example, increase perceived predation risk, leading to physiological changes such as circulating stress hormones [28–30]. These physiological responses may in turn affect cognitive processing, alter decision-making and ultimately lead to a notable difference in an animal's behaviour [17]. Importantly, all of the three different processes listed above can translate into reduced performance for a given task and thus force animals to adapt or suffer long-term consequences.
Foraging birds provide an excellent system for an in-depth study on the impact of noise on non-acoustic information processing. Many bird species are specialized in finding visually cryptic prey, such as nocturnal moths that mimic the visual backgrounds on which they rest during the day [31,32]. Presenting foraging birds tasks that differ in the amount and type of visual information processing can thus be used to quantify a cross-sensory noise impact with a high level of detail. A wide range of studies has experimentally documented noise impacts on the behaviour and physiology of birds [33–36], including in a foraging context [14,30]. However, we often lack knowledge on the underlying mechanism of these impacts, which hampers our understanding of their long-term consequences.
Great tits (Parus major) are a very suitable model system to assess whether anthropogenic noise can impact avian insectivores that are foraging for cryptic prey. Great tits are considered visual specialists and are often tested on visual foraging tasks [37,38]. Furthermore, the impact of anthropogenic noise on the behaviour and reproduction of great tits is well documented [36,39]. On the short term (minutes or hours), noise has been shown to affect the behaviour and perception of both male and female birds [40–42]. On the long term (days to weeks), birds have been shown to adapt behaviourally [43], though the link to long-term fitness consequences is less clear [44]. Long-term nest-box exposure found no effect of noise on breeding performance, suggesting that any long-term effects of noise could be owing to an impact on foraging efficiency away from the nest-box [44].
Here, we present data on the effect of anthropogenic noise on a captive population of great tits consisting of males and females originating from two lines selected for divergent levels of exploratory behaviour (fast or slow) and that consequently differ in their average personality traits [45,46]. Animals that differ in these behavioural traits are known to differ in how they assess and respond to environmental conditions, including anthropogenic noise pollution [42,47]. Furthermore, birds from our selection lines differ in their genetic make-up, and demonstrating a difference in performance under noisy conditions between these lines would be a first indication of adaptive phenotypic variation that could be linked to genomic variation.
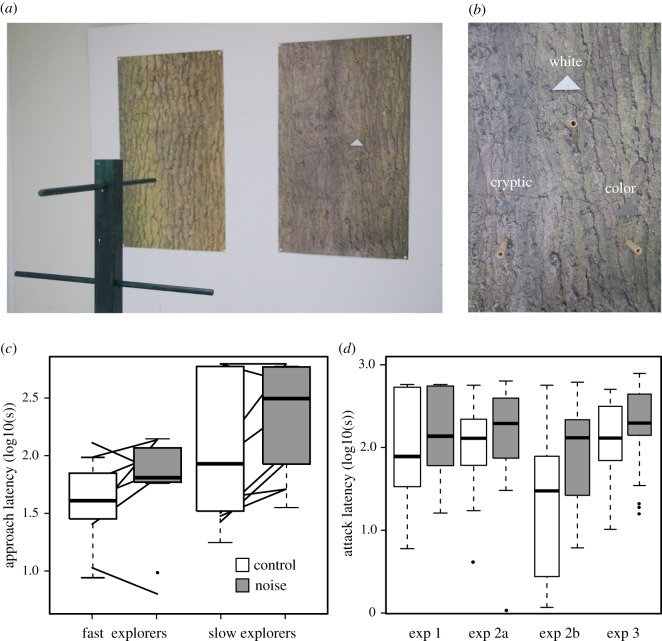
We exposed individual birds to quiet and noisy conditions in an experimental room where they could freely search for prey items, namely moths made out of colour-printed triangular piece of paper with a mealworm attached to it. Birds were trained to approach and attack one or two prey targets attached to an experimental board with two large oak tree backgrounds printed on it (figure 1a). In a first experiment, we tested birds on their latency to approach and attack conspicuously coloured prey items (either black or white print) in order to assess a general impact of noise. In a second experiment, we tested birds on their latency to approach and attack one of three prey targets differing in their level of camouflage with the background (i.e. crypsis, figure 1b) in the presence and absence of anthropogenic noise exposure to assess cross-sensory interference. In a final experiment, we presented two prey types differing in camouflage and tested birds on their attack latency in the presence and absence of noise.
Figure 1.
Noise consistently affects foraging for artificial prey in great tits. (a) Photo of the experimental room showing the perch from which birds started to search for prey targets on one of two life-size printed oak trees. Targets were either presented on one of the trees or on both (in which case, their position was mirrored). (b) Close-up of the three different prey types used during the experiments. A triangular piece of paper was pinned together with a food reward (mealworm or wax moth) to one of the background trees. We provided eight wooden landing perches per oak tree from which birds could attack the target. We either used a conspicuous pattern (black or white), a pattern matching the average colour of the background (colour), or a pattern that was copy-pasted from another background tree (cryptic). (c) Results of experiment 1 demonstrating that noise increases latency to approach conspicuous prey, irrespective of the selection line from which birds were taken (see text for details on selection line birds). Boxplots depict model estimates. Lines depict raw data per individual. Note that latency on the y-axis is log10 transformed. (d) Results across experiments demonstrate that noise consistently and repeatedly increases attack latencies. Note that test conditions differed across experimental days. Between the first and second run of experiment 2 (indicated with a and b), only the number of targets per trial was reduced from two to one, which may explain the strong drop in attack latency as birds got presumably better at the task between these two consecutive testing days. See text for full statistics. (Online version in colour.)
2. Material and methods
(a). Study location and species
We relied on captive great tits (P. major) that originated from a bidirectional selection experiment and were housed at the Netherlands Institute for Ecology (NIOO-KNAW). Experiments as described here did not involve animal experiments as described in the law, which was confirmed by the Institute for Animal Experiments of NIOO-KNAW. Lines were selected for three generations for divergent levels of exploratory behaviour, with a high line for fast exploratory behaviour and a low line for slow exploratory behaviour. Selection was conducted on a combination of the reactions to a novel environment and a novel object (either fast or slow) in a novel environment (see [45] for details on their background and selection regime). We started off with 24 great tits (12 males, 12 females and 12 of each line evenly divided over the sexes) and managed to train 17 birds to carry out the experimental tasks repeatedly and within a set amount of time during the training phase (less than 20 min).
(b). Experimental set-up and training scheme
The birds were trained and tested in a room (4.0 × 2.4 × 2.5 m) that was situated adjacent to their housing cages. Birds were first trained to get a food reward by approaching a board (1.5 × 2.0 m) placed at the far side of the room (opposite the entrance door). The board contained several small wooden perches (4 cm length) with visual targets and food reward pinned to the board (see below). The room was lit by two full spectrum daylight high-frequency fluorescent lights (Activa 172, Philips, Eindhoven, The Netherlands), with the one closest to the board being blocked with white paper to avoid the casting of a shadow that could provide the birds with an alternative target cue. In the morning, prior to training, birds were exposed to their visual targets (all types) in their housing cages (50 × 90 × 90 cm) to familiarize them with the prey items. Birds would enter the experimental room individually and voluntarily from their cage when we opened a small slide and turned on the lights inside the test room.
During the first training round, birds were forced to land on the small wooden perches (no other landing perch provided) with the target directly above it. During subsequent training rounds, we provided a single, central landing perch (large pole with two orthogonal 0.45 m long horizontal perches attached at 1.20 and 1.45 m height) situated at different distances from the board with the targets. Birds were trained on conspicuous targets (half of them on black targets on a white background, the other half on white targets on a coloured background, see below for stimuli descriptions). To avoid familiarization with these conspicuous targets, we switched colours between training and testing rounds. Birds varied greatly in the number of training trials they needed to land on the perches with the target stimuli. The training phase ended when the bird would approach and attack the targets on the board from the central landing perch at least once. The testing phase always started 1 or more days after the end of the training phase.
(c). Visual target and background stimuli
We used moths as our visual targets that consisted of triangular-shaped printed pieces of paper (2.5 cm width, 2.0 cm height) with a mealworm or waxmoth larvae attached to the back and sticking out underneath the paper by roughly one-third of their body. These moth targets were pinned on either a white background (black targets during the training phase only) or a life-sized printed picture of the base of an oak tree (83.4 cm height, 50–55 cm wide, printed using a Canon Colourwave on HVO 130 gram paper). Tree bark pictures were taken from eight large oaks (greater than 1 m diameter at the base) from two different locations in the vicinity of Leiden in January/February in 2017. Pictures were taken with a Canon EOS 7D mark II camera (Canon Inc.) and a Canon EF 100 mm F/2.8 L USM iS macro, with ISO-values of 400–800 and diaphragm of 5.6–7.1. Pictures were colour and white-balance calibrated with a colourchecker (x-rite, colourchecker passport) and digitally adjusted in Photoshop CS6 (Adobe Inc.). We created three different visual stimuli: (i) a conspicuous stimulus, which consisted of a paper moth target (either white and without print, or black-printed); (ii) a colour stimulus, consisting of a paper moth target with a homogeneous print matching the averaged coloration of the printed background tree; and (iii) a cryptic stimulus, consisting of a paper moth target with printed bark pattern (thus matching with the background in both pattern as well as coloration). For the cryptic stimuli, we copy-pasted parts of the life-sized tree images from the centre of the stem. We used the colour average tool in Photoshop CS6 to create the colour stimuli from the cryptic stimuli (thus creating matching stimuli that only differed in patterning, not in average coloration).
(d). Noise exposure treatment
We tested birds on their foraging behaviour for different prey targets during traffic-like noise exposure and control sound conditions. The exposure treatment consisted of 1 h of pink noise (artificial filtered white noise with a spectral bias towards the low frequencies, with the same amount of energy in each octave band) created in Audacity. The spectral and temporal properties of the noise files matched recordings made approximately 100 m from a busy highway (see Halfwerk et al. [39,40] for power spectra). Sound was broadcast through a full-range speaker (Peerless TG9FD10-08, 3.5 inch, 8 ohm), placed 1 m from the landing perch and 2 m from the board on the ground. The speaker was attached to a stereo integrated amplifier (renkforce SAP-702) connected to a laptop outside the experimental room. An additional speaker was placed in the room with the housing cages and also played noise during the experiment. We did this with the aim to avoid the birds associating the noise only with the experimental room. Sound levels were set to 65 dB (using a RadioShack SPL-meter at 0.5 m distance, set to fast response, C-weighing, max). Control sound conditions consisted of playback of silence (ambient noise levels in the experimental room ranged from 43 to 48 dB[A]). On each experimental day, we started with a 1 h playback of noise to let the bird acclimatize to the noise treatment while still in their cages.
(e). Experimental procedures
We tested birds on foraging trials with different noise as well as visual target treatments. The order of the trials was randomized and balanced across individuals and birds received a maximum of six trials per experimental day. Each trial started with a 30 s period of either control playback or playback of noise with gradually increasing noise levels. After this phase, we opened the slide to an individual's cage from inside the room and turned on the lights after having left the room. We noted the time from the start of the experiment (lights on) to landing on the central perch. When a bird landed on the nearest perch to attack the target, or when it hovered in front of the target, we stopped the trial. When a bird would immediately fly to the board from its cage, we would abandon the trial. In all these cases, birds would land on the top of the board and move from perch to perch to find the target. When a bird would fly to the ground and search for food for more than several seconds, we also abandoned the trial. Abandoned trials were repeated only once at the end of the day.
We used eight different oak trees as a source for target stimuli and test background. Targets were never tested on the background image they were made from (natural cryptic prey would also never perfectly match its background) and each stimulus was only used once. To ensure that birds were paying attention to the target and not the background tree when searching for food, we started training them on two background trees separated by 30 cm with the training targets placed either on the left or right tree (figure 1a). During the experiments, we kept using this set-up, although we randomized the eight background trees between experimental days.
We attached wooden perches in four rows and two columns on each of these trees (thus 16 possible target locations in total) with rows and columns being 20 cm apart from each other. All target locations were placed at least 8 cm from the edge of the printed tree. For experiments 1 and 3, we placed two targets on each of the background trees, keeping row number (1–4) and column number (1–2) the same between the left and right tree. Row number and column were randomized between trials. For experiment 2, we ran two sets of six trials (three targets × two noise conditions). On the first day of experiment 2, we tested birds on two targets placed on both trees, whereas on the second day of experiment 2, we tested birds on one of the trees (with the side randomly chosen). All birds moved directly to the tree with the target during this second round of trials, demonstrating that they were paying attention to the visual target cues.
For our first experiment, birds were tested for their foraging with conspicuous targets that were either white (paper only) or black (black ink printed on white paper). Half of the birds were trained to approach and attack a black target and tested on a white target during noise and quiet playback treatments, whereas the other half was trained on white and tested on black targets. For this experiment, we managed to test 17 birds (10 from the slow and seven from the fast selection line, nine females and eight males) on their foraging efficiency in noise and in relation to the selection line they came from. The central starting perch was placed 50 cm away from the board with the targets.
After the first experiment, we included another training round in which birds had to initiate their attack from the central perch at a distance of 1 m. Furthermore, we trained birds on the two other stimuli (colour and cryptic) to ensure that individuals had been equally exposed to the different visual targets. We continued with a subset of birds (n = 12) that had attacked the targets and obtained the food reward during this additional training round at least once. One bird was removed a posteriori from our dataset as it turned out that this bird always flew to the same spot of the experimental board and started searching for the target from there.
For our second experiment, we tested 12 birds (six from the slow and six from the fast selection line) on conspicuous (only white), coloured and cryptic targets in the presence and absence of noise. Birds were tested using a balanced full-factorial design. This experiment spanned two experimental rounds, carried out on different days. On the first day, birds were tested on two of the same targets, their location on the two background trees matched within a trial. On the second day, birds were tested on only one target, with target side (left or right tree) randomized.
For the third experiment, we presented 12 birds three different combinations of our target stimuli (white–colour/white–cryptic/colour–cryptic) and scored their attack latency and choice of first attack in the presence and absence of noise. This final experiment also acted as a probe test to ensure birds had been trained to use the visual cues and not some non-visual cue to find and attack the target.
(f). Behavioural measurements
Birds were observed and video-recorded with a Panasonic WV-CP500 Super Dynamic 5 camera connected to a desktop pc running Ethovision XT (v. 11, Noldus) fixed onto the door of the experimental room. Individual behaviour was scored directly on screen by one of the experimenters as well as from videos by someone blind to the noise treatment. We scored the start time of the experiment (defined as the moment the lights were switched on inside the test chamber) and the time taken to land on the central perch, the time taken (in seconds) to approach the experimental board (from start of the experiment). Furthermore, we scored the time taken (from the perch) to attack the prey target, defined as an individual landing on, taking the food reward, or hovering in front of one of the 16 small perches with the target. In some cases, birds would approach the board, land on a small perch without the target and hop from perch to perch until the target was reached. In these cases, attack time refers to the time it took until they reached the target perch. The scores taken by the experimenter and blind observer matched in all but a few cases (less than 2%), in which case the values from the blind observer were taken as correct. In the few cases (less than 5%) where the video was lost owing to a program crash, the scores from the experimenter were taken.
(g). Statistical analyses
We analysed the data by constructing generalized linear mixed models (GLMM) using the package lme4 (v. 1.1–19) in the program R (v. 3.5.2; [48,49]). For latency data, we used models with a Poisson error distribution and logit-link function. For choice data, we created models with a binomial error structure and log-link function. We first explored various null models (excluding fixed effects of interest) to find the random structure that best fitted our data (following [50]) based on their Akaike information criterion (AICc, corrected for small sample size). Random intercept terms included individual or order of trials. Additionally, we modelled individual as random slope effect, regressing latencies against trial order. This latter approach was based on our observations that individual birds differed greatly in their behaviour in relation to the trial order. Finally, we explored models with individuals nested in one of the two selection lines. We selected the null model that best fitted our data and added various fixed terms, depending on the experiment (see the electronic supplementary material for information for the results of this model selection procedure and comparison of the best fitted model, the random slope model, with the random intercept model).
For experiment 1, we created full models with latency to approach and attack as dependent variable and noise treatment (control/noise) and selection line (fast/slow) as fixed effects. For experiment 2, the full models on latency data had noise treatment (control/noise) and prey target (conspicuous/colour/cryptic) as fixed effects. During experiment 3, birds were presented one of three possible target combinations (white–colour/white–cryptic/colour–cryptic). Models on latency data from experiment 3 had only noise treatment (control/noise) as fixed effects, as models including the interaction between attack choice and noise treatment failed to run.
We compared models using likelihood ratio tests and Wald statistics to assess the significance of single effects and their interactions [50]. Effect sizes and standard errors of final models are reported in the electronic supplementary material, information. For experiment 2, two birds had only approached the targets during one or two treatment conditions. Adding or removing these birds did not change the test results.
3. Results
(a). Anthropogenic noise reduces foraging efficiency
In a first experiment, we tested whether noise can affect great tit foraging for conspicuous prey (white paper or black-printed paper). Individual birds (n = 17) varied substantially in their latency to approach and attack prey targets during the experiment. Although this variation was to some extent explained by selection lines (figure 1c), latencies between the two groups were not significantly different (GLMM; approach: χ21 = 1.72, p = 0.19, figure 1c; attack: χ21 = 0.89, p = 0.35). Noise exposure significantly increased, however, both approach (χ21 = 8.52, p = 0.003; figure 1c) and attack latency (χ21 = 4.13, p = 0.042, exp 1 in figure 1d), but the impact did not differ between the selected lines for exploration (no significant interaction of noise × selection line: all p > 0.44).
(b). Anthropogenic noise impairs visual perception of cryptic prey
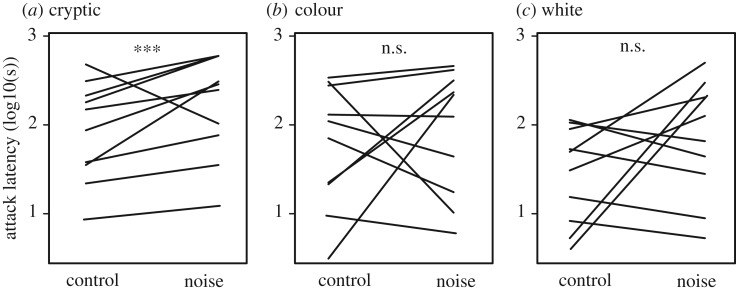
In a second experiment, we continued with the subset of birds (n = 12) that repeatedly attacked prey items across multiple training days. These birds were additionally trained to attack conspicuous (black or white), colour-matched (colour) and colour- and pattern-matched (cryptic) prey targets (figure 1b) in the absence of the oak tree test background. Birds were tested in two rounds, first on two targets of the same level of crypsis (exp 2a in figure 1d), followed by one target (exp 2b in figure 1d), randomly placed on the left or right tree in our set-up. During this second round, all birds approached the tree containing the target first, demonstrating they were paying attention to the target. When we averaged the responses over the 2 days of experiment 2, we found the latency to approach and to attack different prey targets to be dependent on the level of crypsis (GLMM; approach: χ22 = 686.0, p < 0.001; attack: χ22 = 656.6, p < 0.001; figure 2). Great tits were fastest in attacking the conspicuous prey, intermediate in the colour-matched prey and slowest in attacking the prey that matched both colour and pattern of the oak tree background (figure 2). As in experiment 1, noise exposure increased approach (χ21 = 13.80, p < 0.001) and attack latencies (χ21 = 595.0, p < 0.001), but importantly, the noise impact depended on the type of prey target (interaction noise×target stimuli; approach: χ22 = 545.9, p < 0.001; attack: χ22 = 457.6, p < 0.0001; figure 2). A post hoc comparison revealed that noise exposure significantly increased attack latencies for cryptic prey targets (z-value = 2.83, p = 0.014; figure 2a), but not for the colour-matched (z-value = −0.25, p = 0.99; figure 2b) and conspicuous prey targets (z-value = −1.58, p = 0.25; figure 2c).
Figure 2.
Effect of noise on foraging for cryptic prey. Attack latencies were highest for cryptic (a), intermediate for coloured (b), and lowest for conspicuous (c) prey targets. Noise exposure increased attack latencies, but its impact depended on the type of prey target. Noise only consistently increased attack latencies for cryptic prey (a) and not for colour-matched (b), or conspicuous prey (c). Lines depict raw data averaged per individual over treatment conditions. Data are shown for the 10 individuals that responded during all treatment conditions. ***p < 0.01. See text for full statistics.
(c). Noise repeatedly impacts foraging behaviour
In a third experiment, we presented birds (n = 12) with two different prey targets simultaneously and found attack latencies to increase during noise exposure (χ21 = 386.2, p < 0.001; figure 1d).
4. Discussion
Our results demonstrate that anthropogenic noise affects the foraging behaviour of great tits that were trained to visually hunt for prey. Birds took longer to approach and to attack artificial prey targets during trials with increased noise levels. Importantly, noise had the strongest impact when birds were tested on the most difficult visual task, namely searching for our cryptic prey stimuli. Interestingly, noise did not significantly reduce foraging performance during trials where the prey was only camouflaged in one stimulus dimension, namely when our stimulus matched the overall background coloration. Furthermore, noise reduced foraging for conspicuous prey during experiment 1, but not during experiment 2. These data suggest that great tits can habituate to repeated noise exposure for relatively simple visual tasks, but not for more complex tasks, such as detecting prey that are camouflaged in more than one dimension (at least combined colour and pattern). We also found that birds took longer to attack during noise exposure when presenting them two targets that differed in their level of crypsis. The latter finding may suggest that noise can also influence the decision-making process, though careful experimental design is needed to discriminate between effects of visual detection and the actual choice for a given target. For example, some birds may not have seen the less conspicuous target during experiment 3, and therefore did not actually chose which target to attack.
Studies on a range of taxa have shown that anthropogenic noise can impact animals on the short term by masking important acoustic signals and cues, by distraction, or by misleading their risk-assessment systems [20,40]. Whether noise has within-individual long-term effects or can lead to adaptive evolutionary responses remains less clear [3]. One of the few long-term field studies also carried out on great tits revealed that noise can mask male–female communication on the first day of exposure, but that males can adapt by moving closer to their mate over the course of several days [43]. Contrarily, long-term exposure to greater sage-grouse during the mating season revealed chronic levels of corticosterone, suggesting that individuals were continuously stressed by the increase in perceived predation risk caused by noise [29]. Although we exposed birds to four experimental days of noise and although test conditions differed across day, our data suggest at least that birds do not quickly habituate to the presence of noise during a foraging task. A long-term impact on performance can lead to reduced survival and reproduction, especially in situations where animals may not be able to cope behaviourally, for instance, by avoiding noisy areas, or by changing their communicative or perceptual mechanisms [23,36]. Importantly, populations found in noise-polluted areas are forced to adapt or go extinct when animals cannot adjust individually. Interestingly, recent common garden experiments with frogs collected near roads or from urban areas suggested that populations can adapt to sensory pollution, although breeding experiments are required to reveal causal (onto)genetic mechanisms [51,52].
In our first experiment, we demonstrated an impact of noise on the attack of conspicuous prey, which disappeared during the second experiment. These observations suggest that birds can habituate to noise exposure, at least for relatively simple visual perception tasks. We tested birds on a similar number of trials to cryptic prey and found no habituation over the subsequent testing days. Noise exposure also increased attack latencies in our third experiment, although the complex experimental design makes interpretation of these results difficult. Our breeding lines that had been selected for fast and slow exploratory behaviour varied largely in approach and attack latency to conspicuous prey, but did not differ in their response to noise.
In conclusion, we have shown that noise can interfere with visual processing during foraging tasks, making it harder for birds to find cryptic compared to less-cryptic prey in noise-polluted areas. These results have important ecological and evolutionary consequences. On the one hand, noise could alter the arms race between visual predators and visually camouflaged prey. Areas that mainly differ in background noise, either natural or anthropogenic, may selectively favour one prey species over the other, depending on their coloration and patterning. On the other hand, noise may force animals to adapt by changing the way they process environmental information. In noisy areas, such as cities, we might expect local predators to be less distracted by noise. Changes to any perceptual or cognitive process will probably come at additional costs and any adaptation to noise as well as their associated consequences for species interactions, such as predator–prey dynamics, will ultimately depend on a whole suite of environmental factors.
Supplementary Material
Acknowledgements
We thank the support staff at NIOO that helped out with animal care and construction of the set-up. Judith Smit is thanked for blindly scoring of the videos and Andrew Cronin for proofreading our manuscript. We are grateful to Dr Hans Slabbekoorn and Prof. Innes Cuthill for their advice on experimental design and stimulus preparation.
Ethics
Experiments were approved by the animal experiment committee (DEC) of the NIOO.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.sf7m0cg24 [53].
Authors' contributions
W.H. conceived the idea, designed and carried out experiments, analysed the data and wrote the paper. K.v.O. designed the experiments, discussed the results and wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
The research was funded through an NWO Veni grant (no. 863.15.006).
References
- 1.Steffen W, et al. 2015. Planetary boundaries: guiding human development on a changing planet. Science 347, 1259855 ( 10.1126/science.1259855) [DOI] [PubMed] [Google Scholar]
- 2.Vitousek PM, Mooney HA, Lubchenco J, Melillo JM. 1997. Human domination of Earth's ecosystems. Science 277, 494–499. ( 10.1126/science.277.5325.494) [DOI] [Google Scholar]
- 3.Swaddle JP, et al. 2015. A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol. Evol. 30, 550–560. ( 10.1016/j.tree.2015.06.009) [DOI] [PubMed] [Google Scholar]
- 4.Shannon G, et al. 2016. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 91, 982–1005. ( 10.1111/brv.12207) [DOI] [PubMed] [Google Scholar]
- 5.Francis CD, Barber JR. 2013. A framework for understanding noise impacts on wildlife: an urgent conservation priority. Front. Ecol. Environ. 11, 305–313. ( 10.1890/120183) [DOI] [Google Scholar]
- 6.Slabbekoorn H. 2013. Songs of the city: noise-dependent spectral plasticity in the acoustic phenotype of urban birds. Anim. Behav. 85, 1089–1099. ( 10.1016/j.anbehav.2013.01.021) [DOI] [Google Scholar]
- 7.Bunkley JP, McClure CJ, Kawahara AY, Francis CD, Barber JR. 2017. Anthropogenic noise changes arthropod abundances. Ecol. Evol. 7, 2977–2985. ( 10.1002/ece3.2698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis CD, Ortega CP, Cruz A. 2011. Vocal frequency change reflects different responses to anthropogenic noise in two suboscine tyrant flycatchers. Proc. R. Soc. B 278, 2025–2031. ( 10.1098/rspb.2010.1847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JWC, Narins PA. 2005. Anthropogenic sounds differentially affect amphibian call rate. Biol. Conserv. 121, 419–427. ( 10.1016/j.biocon.2004.05.017) [DOI] [Google Scholar]
- 10.Schmidt R, Morrison A, Kunc HP. 2014. Sexy voices—no choices: male song in noise fails to attract females. Anim. Behav. 94, 55–59. ( 10.1016/j.anbehav.2014.05.018) [DOI] [Google Scholar]
- 11.Lampe U, Reinhold K, Schmoll T. 2014. How grasshoppers respond to road noise: developmental plasticity and population differentiation in acoustic signalling. Funct. Ecol. 28, 660–668. ( 10.1111/1365-2435.12215) [DOI] [Google Scholar]
- 12.Parks SE, Johnson M, Nowacek D, Tyack PL. 2011. Individual right whales call louder in increased environmental noise. Biol. Lett. 7, 33–35. ( 10.1098/rsbl.2010.0451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunkley JP, Barber JR. 2015. Noise reduces foraging efficiency in pallid bats (Antrozous pallidus). Ethology 121, 1116–1121. ( 10.1111/eth.12428) [DOI] [Google Scholar]
- 14.Quinn JL, Whittingham MJ, Butler SJ, Cresswell W. 2006. Noise, predation risk compensation and vigilance in the chaffinch Fringilla coelebs. J. Avian Biol. 37, 601–608. ( 10.1111/j.2006.0908-8857.03781.x) [DOI] [Google Scholar]
- 15.Siemers BM, Schaub A. 2011. Hunting at the highway: traffic noise reduces foraging efficiency in acoustic predators. Proc. R. Soc. B 278, 1646–1652. ( 10.1098/rspb.2010.2262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munoz NE, Blumstein DT. 2012. Multisensory perception in uncertain environments. Behav. Ecol. 23, 457–462. ( 10.1093/beheco/arr220) [DOI] [Google Scholar]
- 17.Halfwerk W, Slabbekoorn H. 2015. Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biol. Lett. 11, e20141051 ( 10.1098/rsbl.2014.1051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens M. 2013. Sensory ecology, behaviour, and evolution, 1st edn Oxford, UK: Oxford University Press. [Google Scholar]
- 19.Simpson SD, Purser J, Radford AN. 2014. Anthropogenic noise compromises antipredator behaviour in European eels. Glob. Change Biol. 21, 586–593. ( 10.1111/gcb.12685) [DOI] [PubMed] [Google Scholar]
- 20.Chan AAY-H, Giraldo-Perez P, Smith S, Blumstein DT. 2011. Anthropogenic noise affects risk assessment and attention: the distracted prey hypothesis. Biol. Lett. 6, 458–461. ( 10.1098/rsbl.2009.1081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miedema HME. 2007. Annoyance caused by environmental noise: elements for evidence-based noise policies. J. Social Issues 63, 41–57. ( 10.1111/j.1540-4560.2007.00495.x) [DOI] [Google Scholar]
- 22.Morris-Drake A, Kern JM, Radford AN. 2016. Cross-modal impacts of anthropogenic noise on information use. Curr. Biol. 26, R911–R912. ( 10.1016/j.cub.2016.08.064) [DOI] [PubMed] [Google Scholar]
- 23.Gomes DGE, Page RA, Geipel I, Taylor RC, Ryan MJ, Halfwerk W. 2016. Bats perceptually weight prey cues across sensory systems when hunting in noise. Science 353, 1277–1280. ( 10.1126/science.aaf7934) [DOI] [PubMed] [Google Scholar]
- 24.Preininger D, Boeckle M, Freudmann A, Starnberger I, Sztatecsny M, Hödl W. 2013. Multimodal signaling in the small sorrent frog (Micrixalus saxicola) in a complex acoustic environment. Behav. Ecol. Sociobiol. 67, 1449–1456. ( 10.1007/s00265-013-1489-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Purser J, Radford AN. 2011. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS ONE 6, 8 ( 10.1371/journal.pone.0017478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wale MA, Simpson SD, Radford AN. 2013. Noise negatively affects foraging and antipredator behaviour in shore crabs. Anim. Behav. 86, 111–118. ( 10.1016/j.anbehav.2013.05.001) [DOI] [Google Scholar]
- 27.McLaughlin KE, Kunc HP. 2013. Experimentally increased noise levels change spatial and singing behaviour. Biol. Lett. 9, 20120771 ( 10.1098/rsbl.2012.0771) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crino OL, Johnson EE, Blickley JL, Patricelli GL, Breuner CW. 2013. Effects of experimentally elevated traffic noise on nestling white-crowned sparrow stress physiology, immune function and life history. J. Exp. Biol. 216, 2055–2062. ( 10.1242/jeb.081109) [DOI] [PubMed] [Google Scholar]
- 29.Blickley JL, Blackwood D, Patricelli GL. 2012. Experimental evidence for the effects of chronic anthropogenic noise on abundance of greater sage-grouse at leks. Conserv. Biol. 26, 461–471. ( 10.1111/j.1523-1739.2012.01840.x) [DOI] [PubMed] [Google Scholar]
- 30.Ware HE, McClure CJ, Carlisle JD, Barber JR. 2015. A phantom road experiment reveals traffic noise is an invisible source of habitat degradation. Proc. Natl Acad. Sci. USA 112, 12 105–12 109. ( 10.1073/pnas.1504710112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cuthill IC, Hiby E, Lloyd E. 2006. The predation costs of symmetrical cryptic coloration. Proc. R. Soc. B 273, 1267–1271. ( 10.1098/rspb.2005.3438) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cuthill IC, Stevens M, Sheppard J, Maddocks T, Párraga CA, Troscianko TS. 2005. Disruptive coloration and background pattern matching. Nature 434, 72–74. ( 10.1038/nature03312) [DOI] [PubMed] [Google Scholar]
- 33.Blickley JL, Word KR, Krakauer AH, Phillips JL, Sells SN, Taff CC, Wingfield JC, Patricelli GL. 2012. Experimental chronic noise is related to elevated fecal corticosteroid metabolites in lekking male greater sage-grouse (Centrocercus urophasianus). PLoS ONE 7, e50462 ( 10.1371/journal.pone.0050462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meillère A, Brischoux F, Angelier F. 2015. Impact of chronic noise exposure on antipredator behavior: an experiment in breeding house sparrows. Behav. Ecol. 26, 569–577. ( 10.1093/beheco/aru232) [DOI] [Google Scholar]
- 35.Potvin DA, MacDougall-Shackleton SA. 2015. Traffic noise affects embryo mortality and nestling growth rates in captive zebra finches. J. Exp. Zool. A Ecol. Genet. Physiol. 323, 722–730. ( 10.1002/jez.1965) [DOI] [PubMed] [Google Scholar]
- 36.Halfwerk W, Slabbekoorn H. 2009. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim. Behav. 78, 1301–1307. ( 10.1016/j.anbehav.2009.09.015) [DOI] [Google Scholar]
- 37.Thorogood R, Kokko H, Mappes J. 2018. Social transmission of avoidance among predators facilitates the spread of novel prey. Nat. Ecol. Evol. 2, 254–261. ( 10.1038/s41559-017-0418-x) [DOI] [PubMed] [Google Scholar]
- 38.Erichsen JT, Krebs J, Houston A. 1980. Optimal foraging and cryptic prey. J. Anim. Ecol. 49, 271–276. ( 10.2307/4288) [DOI] [Google Scholar]
- 39.Halfwerk W, Holleman LJM, Lessells CM, Slabbekoorn H. 2011. Negative impact of traffic noise on avian reproductive success. J. Appl. Ecol. 48, 210–219. ( 10.1111/j.1365-2664.2010.01914.x) [DOI] [Google Scholar]
- 40.Halfwerk W, Bot S, Buikx J, Van Der Velde M, Komdeur J, Ten Cate C, Slabbekoorn H. 2011. Low songs lose potency in urban noise conditions. Proc. Natl Acad. Sci. USA 108, 14 549–14 554. ( 10.1073/pnas.1109091108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pohl NU, Leadbeater E, Slabbekoorn H, Klump GM, Langemann U. 2012. Great tits in urban noise benefit from high frequencies in song detection and discrimination. Anim. Behav. 83, 711–721. ( 10.1016/j.anbehav.2011.12.019) [DOI] [Google Scholar]
- 42.Naguib M, Van Oers K, Braakhuis A, Griffioen M, De Goede P, Waas JR. 2013. Noise annoys: effects of noise on breeding great tits depend on personality but not on noise characteristics. Anim. Behav. 85, 949–956. ( 10.1016/j.anbehav.2013.02.015) [DOI] [Google Scholar]
- 43.Halfwerk W, Bot S, Slabbekoorn H. 2012. Male great tit song perch selection in response to noise-dependent female feedback. Funct. Ecol. 26, 1339–1347. ( 10.1111/j.1365-2435.2012.02018.x) [DOI] [Google Scholar]
- 44.Halfwerk W, Both C, Slabbekoorn H. 2016. Long-term nestbox noise experiments reveal an impact on nest-site selection but not on reproduction. Behav. Ecol. 27, 1592–1600. ( 10.1093/beheco/arv204) [DOI] [Google Scholar]
- 45.Drent PJ, Oers KV, Noordwijk AJV. 2003. Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51. ( 10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smit JA, van Oers K. 2019. Personality types vary in their personal and social information use. Anim. Behav. 151, 185–193. ( 10.1016/j.anbehav.2019.02.002) [DOI] [Google Scholar]
- 47.Read J, Jones G, Radford AN. 2014. Fitness costs as well as benefits are important when considering responses to anthropogenic noise. Behav. Ecol. 25, 4–7. ( 10.1093/beheco/art102) [DOI] [Google Scholar]
- 48.Bates D, Mächler M, Bolker B, Walker S. 2014. Fitting linear mixed-effects models using lme4. arXiv, 1406.5823.
- 49.Team RC. 2012. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 50.Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York, NY: Springer Science & Business Media. [Google Scholar]
- 51.Tennessen JB, Parks SE, Swierk L, Reinert LK, Holden WM, Rollins-Smith LA, Walsh KA, Langkilde T. 2018. Frogs adapt to physiologically costly anthropogenic noise. Proc. R. Soc. B 285, 20182194 ( 10.1098/rspb.2018.2194) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Halfwerk W, et al. 2019. Adaptive changes in sexual signalling in response to urbanization. Nat. Ecol. Evol. 3, 374 ( 10.1038/s41559-018-0751-8) [DOI] [PubMed] [Google Scholar]
- 53.Halfwerk W, van Oers K. 2020. Data from: Anthropogenic noise impairs foraging for cryptic prey via cross-sensory interference Dryad Digital Repository. ( 10.5061/dryad.sf7m0cg24) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Halfwerk W, van Oers K. 2020. Data from: Anthropogenic noise impairs foraging for cryptic prey via cross-sensory interference Dryad Digital Repository. ( 10.5061/dryad.sf7m0cg24) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.sf7m0cg24 [53].