Abstract
Supergenes, or linked groups of alleles that are inherited together, present excellent opportunities to understand gene–behaviour relationships. In white-throated sparrows (Zonotrichia albicollis), a supergene on the second chromosome associates with a more aggressive and less parental phenotype. This supergene includes the gene for vasoactive intestinal peptide (VIP), a neuropeptide known to play a causal role in both aggression and parental behaviour. Here, using a free-living population, we compared the levels of VIP mRNA between birds with and without the supergene. We focused on the anterior hypothalamus and infundibular region, two brain regions containing VIP neurons known to play a causal role in aggression and parental behaviour, respectively. First, we show that the supergene enhances VIP expression in the anterior hypothalamus and that expression positively predicts vocal aggression independently of genotype in both sexes. Next, we show that the supergene reduces VIP expression in the infundibular region, which suggests reduced secretion of prolactin, a pro-parental hormone. Thus, the patterns of VIP expression in these two regions are consistent with the enhanced aggression and reduced parental behaviour of birds with the supergene allele. Our results illustrate mechanisms by which elements of genomic architecture, such as supergenes, can contribute to the evolution of alternative behavioural phenotypes.
Keywords: aggression, behavioural polymorphism, parental behaviour, prolactin, territoriality, white-throated sparrow
1. Introduction
To understand how behaviour evolves, we must understand how it is genetically inherited. The relationships between genes and behaviours are complicated, however, because behaviour is multiply determined. White-throated sparrows (Zonotrichia albicollis) exhibit genetically based alternative behavioural phenotypes, making this species a good model for studying gene–behaviour associations. Two plumage morphs (figure 1), white-striped and tan-striped, occur in both sexes and are equally prevalent. Within each sex, white-striped birds express higher levels of vocal and physical aggression in response to territorial intrusions than do tan-striped birds [1,3]. By contrast, tan-striped birds provision nestlings at higher rates than do white-striped birds; this morph difference in parental effort is most evident in males [1,4].
Figure 1.
White-throated sparrows occur in two plumage morphs, tan-striped and white-striped. The morphs differ with respect to endocrine profiles and social behaviour [1]. Photo credit B. Horton. Reprinted with permission of the Society for Integrative and Comparative Biology (see [2]). (Online version in colour.)
The plumage morphs in this species are determined by a series of inversions on the second chromosome,1 called ZAL2m [7]. White-striped birds are heterozygous for this rearrangement, which constitutes a ‘supergene’ in that it is inherited together as a unit [6]. Tan-striped birds are homozygous for the standard arrangement, called ZAL2. Because mating pairs are almost exclusively made up of one white-striped and one tan-striped bird [8], ZAL2m exists in a near-constant state of heterozygosity; ZAL2m/ZAL2m homozygotes are extremely rare [9,10]. This situation results in suppression of recombination between ZAL2 and ZAL2m, causing genetic differences such as single-nucleotide polymorphisms to accumulate inside the supergene [6].
Because the behavioural differences between the morphs are ultimately attributable to this genetic differentiation, each gene inside the supergene is a potential candidate for mediating the behavioural phenotypes. Included among those genes captured by the ZAL2m rearrangement is the one encoding vasoactive intestinal peptide (VIP), a neuropeptide that has been associated with a variety of social behaviours in birds, including parenting and aggression [11,12]. There are no nonsynonymous changes between the ZAL2 and ZAL2m alleles of VIP [13]; in other words, the protein sequences do not differ. It is thus unlikely that the function of the protein itself, for example, its activity or affinity for receptors, differs between the morphs. Cis-regulatory regions, on the other hand, contain polymorphisms [13] that could affect the level of gene expression and thus the abundance and/or distribution of VIP mRNA in tissues. We therefore hypothesized that VIP mRNA is differentially expressed between the morphs. Because of the clear causal role of VIP in both territorial aggression and parental care in other avian species (reviewed by [11,14]), such a finding would connect differentiation of genetic sequence and differentiation of the behavioural phenotypes.
We quantified VIP mRNA expression in two populations of VIP cells, the anterior hypothalamus and the infundibular region of the hypothalamus, which are thought to play key roles in territorial aggression and parental provisioning, respectively, in other avian species. VIP immunoreactivity in the anterior hypothalamus is positively correlated with aggression in song sparrows (Melospiza melodia) and field sparrows (Spizella pusilla) [15]; VIP knockdown in this cell population inhibited aggression in violet-eared waxbills (Uraeginthus granatinus) [16]. The infundibular region contains a population of VIP neurons that project to the median eminence and stimulate the hypophyseal secretion of prolactin [17–19], which has been shown to play a causal role in parental behaviour in birds (reviewed by [14]).
Here, we tested the hypothesis that white-striped and tan-striped white-throated sparrows, which differ with respect to their VIP allelic genotype, also differ with respect to VIP expression in the anterior hypothalamus and infundibular region. We tested this hypothesis by measuring gene expression and behaviour in free-living birds during two breeding stages. The first group of birds was studied early in the breeding season when behavioural responses to territorial intrusions are high [1]. The second group was studied during the second half of the nestling stage, when parental demands are high. Morph differences in VIP expression during these stages in the anterior hypothalamus and infundibular region, respectively, would suggest that differential regulation of the VIP gene may contribute to morph differences in social behaviour in this species.
2. Material and methods
(a). Behavioural observations and tissue collection
Our methods of behavioural observation and tissue collection have been published elsewhere [1,20–22] and are described in detail in the electronic supplementary material. Briefly, we studied free-living white-throated sparrows in the Hemlock Stream Forest near Argyle, Maine, USA, in two consecutive breeding seasons. We characterized behaviour and collected tissues from birds during two phases of breeding, hereafter referred to as the ‘early breeding’ and ‘nestling’ stages. The early breeding stage coincides with the highest levels of territorial aggression in white-throated sparrows [1,23]. During early breeding, we quantified song rate in response to simulated territorial intrusions according to the methods described by Horton et al. [1]. Singing in this context is a component of territorial aggression, and in this study population, song rates in response to simulated territorial intrusions are higher in white-striped birds than tan-striped birds in both sexes [1]. During the nestling stage, and on different territories than those studied during early breeding, we quantified nestling provisioning rates (the number of feeding trips per hour) by parental adults when nestlings were five and six days old according to the methods described by Horton et al. [1]. In this study population, parental white-striped males provision nestlings at lower rates than do parental tan-striped males; white-striped and tan-striped females provision young at similar rates [1]. Our sample during both stages consisted entirely of opposite morph pairs, the typical pair type for this species [8].
We captured focal birds on the day following their last behavioural observation. Blood samples were collected and analysed for gonadal steroid concentrations according to the methods described by Horton et al. [1]; time to capture and to acquire the blood sample did not vary according to morph in either sex or stage [22]. Immediately after capture and blood sampling, whole brains were extracted and rapidly frozen on powdered dry ice at the site.
(b). Labelling and quantification of vasoactive intestinal peptide mRNA
We labelled VIP mRNA expression in sets of 20 µm sections using in situ hybridization with an 35S-labelled riboprobe (see the electronic supplementary material and [24]). Tissue from each sex and breeding stage was run separately; that is, we performed separate runs of in situ hybridization on tissue from early breeding males, early breeding females, nestling stage males and nestling stage females. With the exception of the early breeding females, each group was further divided into two separate runs of in situ hybridization with year and morph balanced across them. Thus, the tissue was processed in a total of seven runs of in situ hybridization.
To quantify VIP mRNA, we used ImageJ to measure the average grey value of VIP mRNA signal in high-resolution scans of the autoradiographic films (see the electronic supplementary material Methods). We quantified signal in the anterior hypothalamus and infundibular region bilaterally (see the electronic supplementary material, figure S1). Grey values for VIP signal in each of two to four sections containing the region were corrected for background by subtracting the grey value of a nearby region with no discernable VIP signal. For each region of interest, these corrected grey values were then averaged across hemispheres and sections for each individual.
(c). Data analysis
VIP mRNA expression was quantified for 38 birds (n = 12 tan-striped males, 13 white-striped males; seven tan-striped females, six white-striped females) collected during early breeding, and 41 birds (n = 11 tan-striped males, 10 white-striped males; nine tan-striped females, 11 white-striped females) collected during the nestling stage. VIP expression in the anterior hypothalamus was quantifiable for all birds, but damage to the infundibular region prohibited reliable quantification of VIP expression in this region for two early breeding tan-striped females and one nestling stage white-striped female. We recorded reliable behavioural data for all birds in which VIP expression was measured except for one early breeding tan-striped male, one nestling stage tan-striped male and one nestling stage white-striped male; spontaneous song rates were recorded during the nestling stage for n = 15 males (eight tan-striped, seven white-striped).
We tested for morph differences in VIP mRNA expression using a two-step approach. We first conducted one-way MANCOVAs to examine the main effect of morph on VIP expression in both brain regions (anterior hypothalamus and infundibular region). Then, if a significant main effect of morph or region × morph interaction was found, we proceeded with one-way ANCOVAs to examine the main effect of morph on VIP expression within regions. MANCOVAs and ANCOVAs included year, Julian day, run of in situ hybridization, plasma testosterone level and plasma oestradiol level as covariates. Separate analyses were conducted for each sex and breeding stage. Gene expression and hormone data were square root transformed to address heteroscedasticity and non-normality.
When morph differences in gene expression and behaviour were found within a sex and breeding stage, we looked for relationships between VIP expression in the anterior hypothalamus and song rate, or between VIP expression in the infundibular region and nestling provisioning rate, as appropriate. We first examined zero-order correlations between gene expression and behaviour. Then, we conducted multivariate analyses to examine the extent to which gene expression, morph or hormone level (testosterone in males, oestradiol in females) predicted individual variation in behaviour when controlling for the effects of all other variables. These multivariate models also included year, Julian day, and run of in situ hybridization as predictor variables. For zero-order correlations, we present Pearson correlation coefficients (r) and associated p-values. For multivariate analyses, we present partial correlation coefficients (r) and associated p-values. Data for gene expression, hormone level and behaviour were square root transformed for correlation analyses to address heteroscedasticity and non-normality. Analyses were performed using JMP® v. 14 (SAS Institute).
3. Results
When VIP mRNA expression in both the anterior hypothalamus and infundibular region was examined using omnibus MANCOVAs, there was clear evidence of morph-dependent variation in expression in both sexes during both breeding stages (table 1). In males, there was a significant main effect of morph on VIP expression during early breeding (F1,18 = 9.1, p = 0.008) and a significant brain region × morph interaction during the nestling stage (F1,18 = 42.9, p < 0.001). In females, there was a significant interaction between region and morph during early breeding (F1,5 = 6.9, p = 0.047), a significant main effect of morph (F1,12 = 8.0, p = 0.015) and a significant region × morph interaction (F1,12 = 5.3, p = 0.041) during the nestling stage.
Table 1.
Results (F and p-values) for analyses of the effects of colour morph on VIP mRNA expression in the anterior hypothalamus (AH) and infundibular region (INF) of breeding white-throated sparrows. (For the MANCOVAs (left), which consider gene expression in both brain regions, values are shown for the overall effect of morph and the region × morph interaction. For the ANCOVAs (right), values for the main effects of morph within regions are shown. Separate analyses were conducted for each sex and study (breeding stage). Significant effects are shown in italics; sample sizes are shown in figure 2.)
| sex | MANCOVAs (both regions) |
ANCOVAs (effect of morph within region) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| study | morph |
region × morph |
region | early breeding |
nestling stage |
|||||
| F | p | F | p | F | p | F | p | |||
| males | early breeding | 9.05 | 0.008 | 1.00 | 0.330 | AH | 6.42 | 0.021 | 20.35 | <0.001 |
| nestling stage | 3.87 | 0.069 | 42.92 | <0.001 | INF | 1.92 | 0.182 | 13.13 | 0.003 | |
| females | early breeding | 1.84 | 0.233 | 6.86 | 0.047 | AH | 13.52 | 0.010 | 6.25 | 0.027 |
| nestling stage | 8.04 | 0.015 | 5.26 | 0.041 | INF | 0.65 | 0.458 | 0.51 | 0.490 | |
(a). Vasoactive intestinal peptide mRNA expression in the anterior hypothalamus
Region-specific ANCOVAs showed morph differences in VIP expression in the anterior hypothalamus in both sexes during both breeding stages (table 1 and figure 2). In males, there was a significant main effect of morph in the anterior hypothalamus during early breeding (F1,18 = 6.4, p = 0.021) and during the nestling stage (F1,14 = 20.4, p < 0.001). During both breeding stages, VIP expression in the anterior hypothalamus was significantly higher in white-striped males than in tan-striped males (figure 2a).
Figure 2.
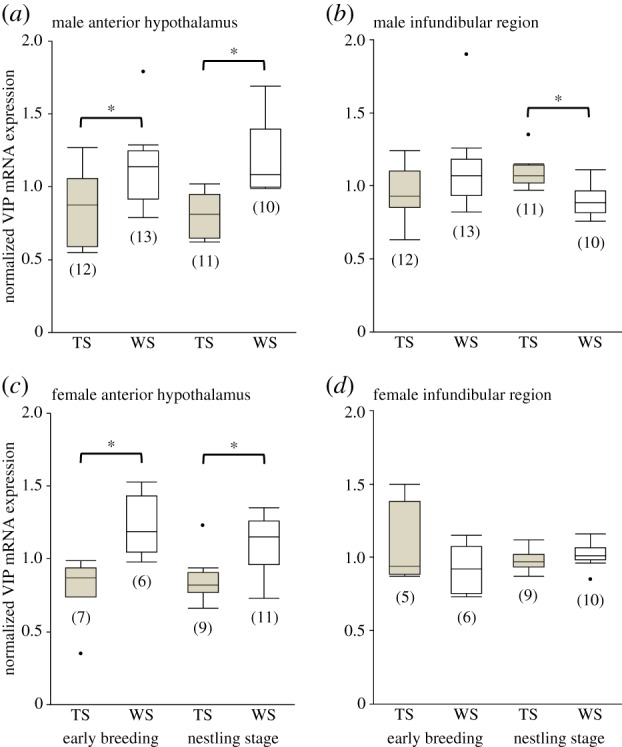
Variation in VIP mRNA expression in the anterior hypothalamus (a,c) and infundibular region (b,d) in the brains of male (a,b) and female (c,d) white-throated sparrows according to colour morph and breeding stage. Please refer to panels (c) and (d) for the full x-axis labels. Within each region, values were normalized to the series mean, such that 1.0 on the y-axis represents the mean corrected grey value within sex and breeding stage; mean normalized values ± s.e. are shown. Asterisks denote significant differences between morphs (table 1). TS, tan-striped morph; WS, white-striped morph; numbers in parentheses denote sample sizes.
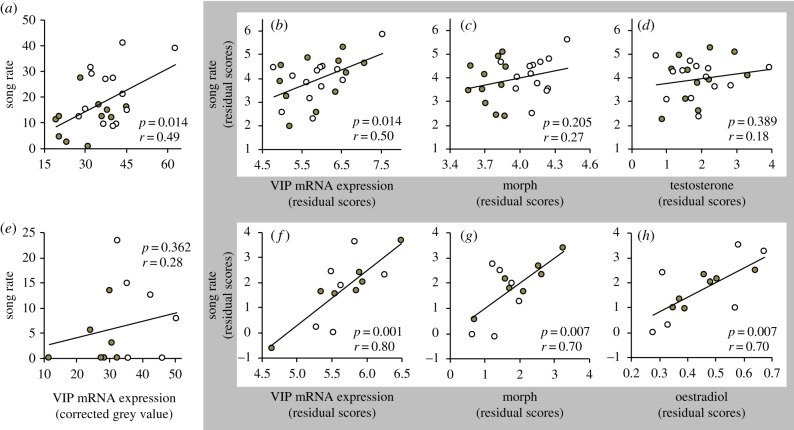
In early breeding males, there was a significant and positive zero-order correlation between VIP expression in the anterior hypothalamus and song rate during simulated territorial intrusions (r = 0.49, p = 0.014; figure 3a). Indeed, a multivariate analysis revealed that when VIP expression in the anterior hypothalamus, morph, and testosterone were included in the same model, VIP expression significantly predicted individual variation in male song rate (r = 0.50, p = 0.014; figure 3b), but morph (r = 0.27, p = 0.205; figure 3c) and testosterone (r = 0.18, p = 0.389; figure 3d) did not. In parental males, however, VIP expression in the anterior hypothalamus was not directly correlated with spontaneous song rate during the nestling stage (zero-order correlation r = 0.14, p = 0.178), and a multivariate analysis revealed that spontaneous song rate during the nestling stage was predicted by testosterone (r = 0.81, p = 0.019), but not VIP expression (r = 0.03, p = 0.933) or morph (r = 0.38, p = 0.323).
Figure 3.
VIP mRNA expression in the anterior hypothalamus predicts individual variation in song rate (number of territorial songs exhibited during 10 min simulated territorial intrusions) of male (a–d; n = 24) and female (e–h; n = 13) white-throated sparrows. (a,e) Zero-order correlations showing the relationship between song rate and VIP expression in the anterior hypothalamus without correcting for other potential sources of variation in behaviour. VIP expression is plotted as the grey value of the region, corrected for background and averaged across measurements within individual. Partial correlations, shown in the shaded area, depict the relationship between song number and (b,f) VIP mRNA expression, (c,g) morph, and plasma (d) testosterone or (h) oestradiol levels when controlling for the effects of all other variables on behaviour. Multivariate models also included year, Julian day and in situ hybridization run as covariates. Filled circles denote tan-striped birds; open circles denote white-striped birds.
In females, there was a significant main effect of morph on VIP expression in the anterior hypothalamus during early breeding (F1,6 = 13.52, p = 0.010) and during the nestling stage (F1,13 = 6.3, p = 0.027). As was seen in males, VIP expression was significantly higher in white-striped than in tan-striped females during both breeding stages (figure 2c). There was no apparent zero-order correlation between VIP expression in the anterior hypothalamus and song rate during simulated territorial intrusions for females (r = 0.28, p = 0.362; figure 3e). A multivariate analysis revealed that VIP expression in the anterior hypothalamus (r = 0.80, p = 0.001), morph (r = 0.70, p = 0.007) and oestradiol (r = 0.70, p = 0.007) were each significantly and positively related to song rate in females when all three variables were included in the same model (figure 3f–h).
(b). Vasoactive intestinal peptide mRNA expression in the infundibular region
In males, morph differences in VIP expression in the infundibular region depended on breeding stage (table 1 and figure 2b). There was no effect of morph on VIP expression in the infundibular region during early breeding (F1,18 = 1.9, p = 0.182), but during the nestling stage, VIP expression in this region was significantly higher in parental tan-striped males than in parental white-striped males (F1,14 = 13.1, p = 0.003). VIP expression in the infundibular region was not, however, directly correlated with male nestling provisioning rate (zero-order correlation r = 0.22, p = 0.377). The multivariate analysis showed that male nestling provisioning rate was predicted by morph (r = 0.58, p = 0.031), but not VIP expression in the infundibular region (r = 0.42, p = 0.135) or testosterone (r = 0.01, p = 0.828).
In females, VIP expression in the infundibular region did not vary according to morph during early breeding (F1,5 = 0.7, p = 0.458) or during the nestling stage (F1,12 = 0.5, p = 0.490; table 1 and figure 2d).
4. Discussion
In this study, we showed that the expression of VIP in the white-throated sparrow brain is a potential mediator of alternative behavioural phenotypes. In both breeding stages, expression in the anterior hypothalamus of the more aggressive phenotype (white-striped) exceeded that of tan-striped birds (figure 2a,c). This expression predicted aggressive behaviour, independently of morph and plasma sex steroids, in both sexes (figure 3). In addition, we showed that the expression of VIP in the infundibular region, which regulates prolactin secretion, is higher in the more parental (tan-striped) morph. The latter effect was noted only in parental males, which fits with previous reports that the morph difference in nestling provisioning is most pronounced in males [1,4].
The strongest evidence that VIP expression in the anterior hypothalamus is causal for aggression in songbirds comes from a series of studies in the violet-eared waxbill, a highly territorial estrildid finch. During resident-intruder tests, FOS immunoreactivity was induced in an area of the anterior hypothalamus that precisely overlaps the VIP cell population [15]. When VIP expression in the anterior hypothalamus was experimentally knocked down via antisense oligonucleotides, aggressive behaviour was profoundly inhibited in both sexes [15]. The latter result strongly suggests that VIP expression, specifically in the anterior hypothalamus, is required for the expression of territorial aggression. Although VIP expression in the anterior hypothalamus has not been experimentally manipulated in sparrows, VIP immunoreactivity in this region is positively correlated with aggression in song sparrows and field sparrows [16]; this result suggests that the behavioural function of VIP cells in the anterior hypothalamus may be conserved in white-throated sparrows.
Ventral to the anterior hypothalamus, in the tuberal hypothalamus, lies another population of VIP neurons. This cell group, which we refer to as the infundibular region, projects to the median eminence and releases VIP into the portal vasculature [18,19]. From there, VIP enters the anterior pituitary and stimulates the production of the peptide hormone prolactin [17,25], which has been strongly and positively associated with parental behaviour across vertebrates. Although much of the research on this association in birds has been correlational in design, there is clear experimental evidence of a causal effect of prolactin on parental behaviour (reviewed by [14]), including nestling provisioning. In zebra finches (Taeniopygia guttata), for example, the pharmacological inhibition of prolactin synthesis dramatically reduced the amount of time parents spent feeding nestlings [26]. VIP manipulations in poultry have shown effects on parental behaviour; immunization against VIP reduced nesting activity in turkeys [27] and increased nest abandonment in incubating bantam hens [28]. In the current study, although VIP expression was higher in the more parental morph, it did not correlate directly with nestling provisioning rate. This result may be owing to the fact that the effects of infundibular VIP expression on parental behaviour are most likely indirect and mediated by prolactin, which could introduce variation and obscure relationships between gene expression and behaviour. In future work, it will be important to measure prolactin levels in the plasma when considering the potential relationship between VIP and parental behaviour.
Our results are consistent with experimental evidence of a role for VIP in parental provisioning specifically. In zebra finches, exogenous treatment with VIP did not increase feeding behaviour in all animals; rather, it altered the division of labour within pairs. In saline-treated pairs, females did more provisioning than did males. By contrast, VIP-treated pairs shared provisioning responsibilities more equally [29]. This finding is interesting in the context of white-throated sparrows because in pairs with a white-striped male and a tan-striped female, females do the majority of provisioning. By contrast in pairs with a tan-striped male and a white-striped female, provisioning is shared equally between the sexes [1]. Perhaps morph differences in the division of labour can be attributed, at least in part, to altered VIP secretion, whereby higher infundibular VIP expression promotes greater provisioning effort by tan-striped males compared with white-striped males.
The morph differences in VIP expression in both the anterior hypothalamus and infundibular region are probably caused by differentiation of cis-regulatory regions of the VIP gene. The 2 kb region upstream of the transcription start site, for example, contains dozens of polymorphisms [30]. Cis-regulatory variation can impact transcription via several mechanisms (see [31]), such as when polymorphisms occur in transcription factor bindings sites, enhancers or DNA sequences that could be methylated. Given the robust morph differences in VIP expression observed in this study, we hypothesize that the ZAL2 and ZAL2m alleles are differentially regulated and probably show allelic imbalance in both the anterior hypothalamus and infundibular region. The fact that the morph differences in VIP expression go in different directions in these two regions suggests further that cis-regulation could depend on epigenetic modifications or local availability of transcription factors that bind differentially to the two alleles. These possibilities represent directions for future research.
The behaviours that we hypothesize are affected by VIP, aggression and parental behaviour, are also affected by plasma sex steroids in this and related species [32,33]. In the current study, VIP mRNA in the anterior hypothalamus predicted territorial singing even when controlling for plasma testosterone and oestradiol in males and females, respectively (figure 3). These results suggest that morph differences in these sex steroids [1] cannot, alone, explain morph differences in territorial singing. Our findings from an earlier study also support this claim; when levels of sex steroids were experimentally equalized, morph differences in vocal aggression persisted [34]. We note here, however, that there was a positive correlation between plasma oestradiol and territorial singing in females even when the VIP signal in the anterior hypothalamus was held constant (figure 3), so at least part of the effect of sex steroids on aggression cannot be completely explained by VIP expression.
5. Conclusion
The white-throated sparrow is an important model with which we can understand how genome architecture contributes to social behaviour and vice versa [35]. The ZAL2m rearrangement in this species has captured a number of genes that contribute to social behaviour [6,13,20,36] and therefore represents a classic example of a supergene with the potential for important coadaptation. Here, we have shown that the neuropeptide VIP is a potential mediator of morph differences in both territorial singing and provisioning, which are the two most well-known morph differences in behaviour. Because the neuroendocrine and hormonal control of these behaviours is well-studied and excellent genomic resources are available [13,21,30], this species is likely to provide much additional insight into how differentiation of specific genetic sequence can have profound effects on behaviour.
Supplementary Material
Acknowledgments
The authors wish to thank J. Davis, C. Horoszko, Y. Hu, H. Jeong, J. Liang, C. MacDowell, J. Thomas and W. Zinzow-Kramer for technical assistance in Atlanta; A. Annis, E. Burns, J. Cava, A. Cornell, C. Gurguis, C. Henry, J. Michaud and C. McKee for field assistance in Maine. We thank the Forest Society of Maine and J. Metzler for permission to conduct our field study at the Hemlock Stream Forest.
Endnote
Ethics
All research was carried out with the approval of the Emory University Institutional Animal Care and Use Committee and with the appropriate state and federal permits for banding and scientific collection (Maine Inland Fisheries and Wildlife, permit nos 2010–295 and 2011–295; US Geological Survey, USGS permit no. 23369; US Fish and Wildlife Service permit no. MB009702).
Data accessibility
The data supporting this article can be obtained from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.w0vt4b8n1 [37].
Authors' contributions
B.M.H. and D.L.M. designed the research. B.M.H. and C.M.M. conducted the research. B.M.H. analysed the data. B.M.H., M.R.P. and D.L.M. wrote the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by NIH 1R01MH082833 to D.L.M.
References
- 1.Horton BM, Moore IT, Maney DL. 2014. New insights into the hormonal and behavioural correlates of polymorphism in white-throated sparrows, Zonotrichia albicollis. Anim. Behav. 93, 207–219. ( 10.1016/j.anbehav.2014.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maney DL, Horton BM, Zinzaw-Kramer WM. 2015. Estrogen receptor alpha as a mediator of life-history trade-offs. Int. Comp. Biol. 55, 323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kopachena JG, Falls JB. 1993. Aggressive performance as a behavioral correlate of plumage polymorphism in the white-throated sparrow (Zonotrichia albicollis). Behaviour 124(3/4), 249–266. ( 10.1163/156853993X00605) [DOI] [Google Scholar]
- 4.Kopachena JG, Falls JB. 1993. Re-evaluation of morph-specific variations in parental behavior of the white-throated sparrow. Wilson Bulletin 105, 48–59. [Google Scholar]
- 5.Ladjali-Mohammedi K, Bitgood JJ, Tixier-Boichard M, Ponce de Leon FA. 1999. International System for Standardized Avian Karyotypes (ISSAK): standardized banded karyotypes of the domestic fowl (Gallus domesticus). Cytogenet. Cell Genet. 86, 271–276. ( 10.1159/000015318) [DOI] [PubMed] [Google Scholar]
- 6.Thomas JW, et al. 2008. The chromosomal polymorphism linked to variation in social behavior in the white-throated sparrow (Zonotrichia albicollis) is a complex rearrangement and suppressor of recombination. Genetics 179, 1455–1468. ( 10.1534/genetics.108.088229) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thorneycroft HB. 1975. A cytogenetic study of the white-throated sparrow, Zonotrichia albicollis. Evolution 29, 611–621. ( 10.1111/j.1558-5646.1975.tb00855.x) [DOI] [PubMed] [Google Scholar]
- 8.Falls JB, Kopachena JG. 2010. White-throated sparrow (Zonotrichia albicollis). In The birds of North America (ed. Poole A.). Ithaca, NY: Cornell Laboratory of Ornithology. [Google Scholar]
- 9.Davis JK, Mittel LB, Lowman JJ, Thomas PJ, Maney DL, Martin CL, Thomas JW. 2011. Haplotype-based genomic sequencing of a chromosomal polymorphism in the white-throated sparrow (Zonotrichia albicollis). J. Hered. 102, 380–390. ( 10.1093/jhered/esr043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horton BM, et al. 2013. Behavioral characterization of a white-throated sparrow homozygous for the ZAL2m chromosomal rearrangement. Behav. Genet. 43, 60–70. ( 10.1007/s10519-012-9574-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kingsbury MA. 2015. New perspectives on vasoactive intestinal polypeptide as a widespread modulator of social behavior. Curr. Opinion Behav. Sci. 6, 139–147. ( 10.1016/j.cobeha.2015.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kingsbury MA, Wilson LC. 2016. The role of VIP in social behavior: neural hotspots for the modulation of affiliation, aggression, and parental care. Integr. Comp. Biol. 56, 1238–1249. ( 10.1093/icb/icw122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuttle EM, et al. 2016. Divergence and functional degradation of a sex chromosome-like supergene. Curr. Biol. 26, 344–350. ( 10.1016/j.cub.2015.11.069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smiley KO. 2019. Prolactin and avian parental care: new insights and unanswered questions. Horm. Behav. 111, 114–130. ( 10.1016/j.yhbeh.2019.02.012) [DOI] [PubMed] [Google Scholar]
- 15.Goodson JL, Kelly AM, Kingsbury MA, Thompson RR. 2012. An aggression-specific cell type in the anterior hypothalamus of finches. Proc. Natl Acad. Sci. USA 109, 13 847–13 852. ( 10.1073/pnas.1207995109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodson JL, Wilson LC, Schrock SE. 2012. To flock or fight: neurochemical signatures of divergent life histories in sparrows. Proc. Natl Acad. Sci. USA 109, 10 685–10 692. ( 10.1073/pnas.1203394109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maney DL, Schoech SJ, Sharp PJ, Wingfield JC. 1999. Effects of vasoactive intestinal peptide on plasma prolactin in passerines. Gen. Comp. Endocrinol. 113, 323–330. ( 10.1006/gcen.1998.7220) [DOI] [PubMed] [Google Scholar]
- 18.Péczely P, Kiss JZ. 1988. Immunoreactivity to vasoactive intestinal polypeptide (VIP) and thyreotropin-releasing hormone (TRH) in hypothalamic neurons of the domesticated pigeon (Columba livia). Alterations following lactation and exposure to cold. Cell Tissue Res. 251, 485–494. ( 10.1007/BF00215858) [DOI] [PubMed] [Google Scholar]
- 19.Macnamee MC, Sharp PJ, Lea RW, Sterling RJ, Harvey S. 1986. Evidence that vasoactive intestinal polypeptide is a physiological prolactin-releasing factor in the bantam hen. Gen. Comp. Endocrinol. 62, 470–478. ( 10.1016/0016-6480(86)90057-2) [DOI] [PubMed] [Google Scholar]
- 20.Horton BM, et al. 2014. Estrogen receptor α polymorphism in a species with alternative behavioral phenotypes. Proc. Natl Acad. Sci. USA 111, 1443–1448. ( 10.1073/pnas.1317165111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zinzow-Kramer WM, et al. 2015. Genes located in a chromosomal inversion are correlated with territorial song in white-throated sparrows. Genes Brain Behav. 14, 641–654. ( 10.1111/gbb.12252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grogan KE, Horton BM, Hu Y, Maney DL. 2019. A chromosomal inversion predicts the expression of sex steroid-related genes in a species with alternative behavioral phenotypes. Mol. Cell. Endocrinol. 495, 110517 ( 10.1016/j.mce.2019.110517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horton BM, Hauber ME, Maney DL. 2012. Morph matters: aggression bias in a polymorphic sparrow. PLoS ONE 7, e48705 ( 10.1371/journal.pone.0048705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leung CH, Abebe DF, Earp SE, Goode CT, Grozhik AV, Mididoddi P, Maney DL. 2011. Neural distribution of vasotocin receptor mRNA in two species of songbird. Endocrinology 152, 4865–4881. ( 10.1210/en.2011-1394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Halawani ME, Silsby JL, Mauro LJ. 1990. Vasoactive intestinal peptide is a hypothalamic prolactin-releasing neuropeptide in the turkey (Meleagris gallopavo). Gen. Comp. Endocrinol. 78, 66–73. ( 10.1016/0016-6480(90)90048-Q) [DOI] [PubMed] [Google Scholar]
- 26.Smiley KO, Adkins-Regan E. 2018. Lowering prolactin reduces post-hatch parental care in male and female zebra finches (Taeniopygia guttata). Horm. Behav. 98, 103–114. ( 10.1016/j.yhbeh.2017.12.011) [DOI] [PubMed] [Google Scholar]
- 27.Sharp PJ, Sterling RJ, Talbot RT, Huskisson NS. 1989. The role of hypothalamic vasoactive intestinal polypeptide in the maintenance of prolactin secretion in incubating bantam hens: observations using passive immunization, radioimmunoassay and immunohistochemistry. J. Endocrinol. 122, 5–13. [DOI] [PubMed] [Google Scholar]
- 28.El Halawani ME, Pitts GR, Sun S, Silsby JL, Sivanandan V. 1996. Active immunization against vasoactive intestinal peptide prevents photo-induced prolactin secretion in turkeys. Gen. Comp. Endocrinol. 104, 76–83. [DOI] [PubMed] [Google Scholar]
- 29.Smiley KO, Adkins-Regan E. 2018. Factors that influence the onset of parental care in zebra finches: roles for egg stimuli and prolactin. Behav. Proc. 153, 47–54. ( 10.1016/j.beproc.2018.05.002) [DOI] [PubMed] [Google Scholar]
- 30.Sun D, Huh I, Zinzow-Kramer WM, Maney DL, Yi SV. 2018. Rapid regulatory evolution of a non-recombining autosome linked to divergent behavioral phenotypes. Proc. Natl Acad. Sci. USA 115, 2794–2799. ( 10.1073/pnas.1717721115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maney DL. 2017. Polymorphisms in sex steroid receptors: from gene sequence to behavior. Front. Neuroendocrinol. 47, 47–65. ( 10.1016/j.yfrne.2017.07.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoech SJ, Ketterson ED, Nolan V Jr, Sharp PJ, Buntin JD. 1998. The effect of exogenous testosterone on parental behavior, plasma prolactin, and prolactin binding sites in dark-eyed juncos. Horm. Behav. 34, 1–10. ( 10.1006/hbeh.1998.1455) [DOI] [PubMed] [Google Scholar]
- 33.Wingfield JC. 1984. Androgens and mating systems: testosterone-induced polygyny in normally monogamous birds. Auk 101, 665–671. ( 10.2307/4086893) [DOI] [Google Scholar]
- 34.Maney DL, Lange HS, Raees MQ, Reid AE, Sanford SE. 2009. Behavioral phenotypes persist after gonadal steroid manipulation in white-throated sparrows. Horm. Behav. 55, 113–120. ( 10.1016/j.yhbeh.2008.09.002) [DOI] [PubMed] [Google Scholar]
- 35.Rubenstein DR, et al. 2019. Coevolution of gene architecture and social behavior. Trends Ecol. Evol. 34, 844–855. [DOI] [PubMed] [Google Scholar]
- 36.Merritt JR, Grogan KE, Zinzow-Kramer WM, Sun D, Ortlund EA, Yi SV, Maney DL. 2020. A behavioral polymorphism caused by a single gene inside a supergene. bioRxiv 897637 ( 10.1101/2020.01.13.897637) [DOI] [PMC free article] [PubMed]
- 37.Horton BM, Michael CM, Prichard MR, Maney DL. 2020. Data from: Vasoactive intestinal peptide as a mediator of the effects of a supergene on social behavior Dryad Digital Repository. ( 10.5061/dryad.w0vt4b8n1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Horton BM, Michael CM, Prichard MR, Maney DL. 2020. Data from: Vasoactive intestinal peptide as a mediator of the effects of a supergene on social behavior Dryad Digital Repository. ( 10.5061/dryad.w0vt4b8n1) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The data supporting this article can be obtained from the Dryad Digital Repository: https://dx.doi.org/10.5061/dryad.w0vt4b8n1 [37].