Abstract
Synchronous displays are hallmarks of many animal societies, ranging from the pulsing flashes of fireflies, to military marching in humans. Such displays are known to facilitate mate attraction or signal relationship quality. Across many taxa, synchronous male displays appear to be driven by competition, while synchronous displays in humans are thought to be unique in that they serve a cooperative function. Indeed, it is well established that human synchrony promotes cooperative endeavours and increases success in joint action tasks. We examine another system in which synchrony is tightly linked to cooperative behaviour. Male bottlenose dolphins form long-lasting, multi-level, cooperative alliances in which they engage in coordinated efforts to coerce single oestrus females. Previous work has revealed the importance of motor synchrony in dolphin alliance behaviour. Here, we demonstrate that allied dolphins also engage in acoustic coordination whereby males will actively match the tempo and, in some cases, synchronize the production of their threat vocalization when coercing females. This finding demonstrates that male dolphins are capable of acoustic coordination in a cooperative context and, moreover, suggests that both motor and acoustic coordination are features of coalitionary behaviour that are not limited to humans.
Keywords: communication, cooperation, synchrony, alliance formation, bottlenose dolphin, coordinated behaviour
1. Introduction
Behaviours that involve ritualized movement or the coordination or synchronization of signals can be found in a diverse array of taxa. Synchronous movements among individuals are ubiquitous in human societies, with examples including dancing and marching in unison [1]. In non-human animals, well-known examples of synchronous visual signals include claw waving in male fiddler crabs [2] and the flashing of male fireflies [3,4], both of which function in mate attraction. The coordination of acoustic signals also plays a key role in mate attraction in both insects and anurans [5]. For example, male katydids precisely synchronize their acoustic signals in the presence of females [6]. In all these cases, synchrony is defined as the precise coincidence of events in time [7], with such precise synchrony shown to be competitive rather than cooperative, as signallers are vying to be the leading male in order to attract the female [2,6].
Interestingly, while competition appears to drive many synchronous animal displays [8,9], the proximate and ultimate causes of human synchrony are strongly linked to cooperation. Over the years, extensive experimental work has revealed the important role that human synchrony plays in promoting in-group bonding [10–12], fostering cooperation [13–15], and diminishing the perceived formidability of competitors [10]. Furthermore, humans that engage in synchronous behaviour may have increased success in subsequent joint action tasks, whereby coordinated action sharpens the perceptual and motor skills required to participate in collaborative endeavours [14]. As such, the relationship between social bonding and coordinated behaviour in humans is well established, with both physical and verbal synchrony promoting affiliation and enhancing cooperative effort [16].
There are, however, many other forms of temporal coordination with regards to signal production [17]. These include duetting, defined as coupled, simultaneous, and/or alternating chorusing, which does not necessarily involve synchrony [17]. Duetting is found in anurans [5], gibbons [18], lemurs [19], and numerous birds [20], in fact, some species are capable of such precise temporal alternation during duets that it sounds as if only one individual is singing [20,21]. In most cases, duets occur between mated pairs and appear to facilitate the cooperative defence of shared resources [18,22], promote pair bonding [18,19,23], or advertise relationship quality [24]. Turn-taking is a similar form of acoustic coordination that has received much interest in recent years, with individuals timing their vocal output to avoid overlap during exchanges. Humans [25], meerkats [26], and marmosets [27] all engage in turn-taking, for example. Coordinated behaviours can, therefore, take a number of forms with varying temporal characteristics.
Here, we examine another system in which motor synchrony is tightly linked to cooperative behaviour. In Shark Bay, Western Australia, male Indo-Pacific bottlenose dolphins [28] form long-lasting, cooperative alliances [29], which engage in coordinated efforts to compete with rival alliances over access to females [29,30]. The core unit of male social organization is the ‘second-order alliance', typically comprised of 4–14 males. Within these second-order alliances, pairs or trios of allied males, known as ‘first-order alliances', work together to herd single oestrus females during events termed ‘consortships’ [29]. Multiple first-order alliances from the same second-order alliance may participate in attempts to steal females from competing alliances, or defend against such attempts [29]. These strong alliance relationships can last for decades and are critical to each male's reproductive success [29]. This is because males cannot monopolise and defend females on their own due to the intense competition for receptive females, minimal sexual size dimorphism, and because the three-dimensional habitat impedes coerced mating by single males [29].
Motor synchrony has been shown to play an important role in promoting cooperation between these allied dolphins, purportedly acting as a signal of alliance unity [31–33]. First-order allies will surface side-by-side synchronously, usually less than a metre apart, and break the surface within 80–120 ms of each other [32]. The males frequently perform elaborate and synchronous physical displays in the female's presence [32,34]. These displays include a variety of synchronous underwater and aerial leaps and turns. Furthermore, synchrony between second-order allies is most common during bouts of social behaviour with female consorts [32].
During consortships, males use female-directed aggression to constrain her movement and keep her away from competing males [29]. To facilitate this, males produce threat vocalizations called ‘pops’, which are narrow-band, low frequency (peak energy 1–2 kHz), pulsed calls that are produced in repetitive trains [35]. Pop trains are produced almost exclusively during consortships, and function as an agonistic ‘come-hither' demand that induces the female to remain close to the popping male [35,36] and may facilitate guard-switching between males [37]. Pops are, therefore, a largely coercive signal with a strong association with physical threats [35]. While pop trains were thought to be produced primarily by individual males, recent observations of multiple males apparently coordinating their pop production required investigation.
In this study, we investigated whether allied male dolphins engaged in acoustic coordination when cooperating in the herding of single females, as a means of furthering our understanding of the evolutionary importance of cooperative, coordinated behaviour in promoting collective action across non-human taxa. Given the prevalence of physical synchrony in this cooperative context, we hypothesized that males might also engage in acoustic synchrony. With our long-term (greater than 30 years) dataset on the association histories of well-known individuals as a basis, we used a combination of contemporary behavioural observations, acoustic recordings, and individual animal localization to (i) assess whether males coordinated pop production in cooperative contexts and (ii) determine the extent to which acoustic coordination occurred among allied males across the population.
2. Methods
Our long-term dolphin research has been run on a seasonal basis (typically austral winter-spring) off Monkey Mia in the eastern gulf of Shark Bay since 1982, and off Useless Loop in the western gulf of Shark Bay since 2007. Detailed association, behavioural, and ranging data have been collected since the mid-1980s in the eastern gulf, and as part of systematic sampling in the western gulf study area since its inception. We used survey data to estimate the proportion of time spent together by different individuals. A ‘survey’ is defined by a minimum 5-min observation of dolphin group composition and behaviour, where ‘group' is defined using the 10 m ‘chain rule' (where all individuals were considered part of the same group if they were within 10 m of any other individual [38]). These data are used to calculate association indices using the simple ratio index, which is an estimate of the proportion of time two animals spend together (0 for pairs of animals that never associate; 1 for pairs always seen together). Male alliances are defined both by their association indices (equal or greater than 0.2 for second-order alliance partners) and their functional behaviour, e.g. cooperating in the herding and defence of females [38–40].
(a). Data collection
Data for this study on male alliance behaviour were collected in the months of June–November from 2016 to 2018 in Shark Bay's eastern gulf, and May–September in 2016 and 2018 in the western gulf. Behavioural and acoustic data were collected from a small (less than 7 m) research vessel, from which we towed an array consisting of four HTI-96 Min series hydrophones (flat frequency response: 0.002–30 kHz ±1 dB) as per King et al. [39]. Recordings were made onto a TASCAM DR-680 MKII multi-track recorder at a sampling rate of 96 kHz. A spoken track that was synchronized with the acoustic recording was used to note the bearing (compass bearing, where the vessel's bow was 0°), distance (m), and identification of the focal animals at each surfacing. Voice notes were also used to describe notable behaviours (i.e. physical synchrony, displays, aggression) performed by alliance members.
During observations of focal groups of adult males (i.e. first-order alliances), the engine was switched off whenever possible to maximize the signal-to-noise ratio of the recordings. Acoustic data were collected during both focal follows and opportunistic recordings. During each focal follow, the following variables were verified every 5 min: group composition, predominant group behavioural state, and predominant group spread. All occurrences of changes to group composition or important behavioural events were also recorded during the focal follows. During opportunistic recordings, continuous sampling was used to record any behavioural changes. Focal follows and opportunistic recordings lasted between 60 and 300 min. Behavioural state definitions and consortship criteria from the Shark Bay Dolphin Research Ethogram are provided in the electronic supplementary material.
(b). Acoustic analysis
Acoustic recordings were analysed by inspecting spectrograms (FFT length 1024, Hamming window) in Adobe Audition CC (v. 10.0.2). Pops were visually identified and graded as either individual male pop trains or instances of possible coordinated pop production (referred to as ‘multi-male’ hereafter). The former represented the predominant type of pop train recorded, where pops are produced in a stereotyped sequence, and the latter represented cases of rapid popping with irregular timing of pop intervals. To characterize variation in the temporal properties of individual and multi-male pop trains, we manually measured the time between consecutive pops within a train, termed the ‘inter-pop interval' (IPI); and the time between consecutive pop trains, termed the ‘inter-train interval' (ITI). ITIs were at least twice the length of the preceding IPI, and typically featured a short tonal component at approximately 5 kHz. Consecutive pop trains were grouped into sequences. Sequence boundaries were defined by acoustic assessment, where the assessor could hear the vocalizing animal cease pop production for a period of time notably longer than the preceding ITIs for that sequence of trains, and where there was no terminal tonal component.
(c). Acoustic localization
Localization was used to determine the spatial and temporal arrangement of consecutive pops in both individual and multi-male pop trains. Acoustic localization was performed using the Matlab-based TOADY program [41]. Localization error of the array was calculated using custom-written Matlab routines to calculate two-dimensional averaged MINNA (minimum number of receiver array) localizations using the methods described in Wahlberg et al. [42] and Schulz et al. [43]. The array was calibrated using two different pop trains previously recorded from this population. Acoustic localization errors for pop directions (n = 50) were calculated as 100% within ±15° of the true location, 94% within ±10°, and 68% within ±5°. However, variation in estimated direction within a train was low, with less than 2° difference between sequential pops in a train produced by an individual male. Only vocalizations with a high signal-to-noise ratio were used for localization. If pops in multi-male trains were partly overlapped, only the non-overlapping portions were localized, and the bearing compared to the preceding and subsequent pops in the train.
(d). Statistical analysis
All statistical procedures were conducted in R 3.4.4 (R project for statistical computing; GNU project). To determine whether the distribution of IPIs differed between individual and multi-male pop trains, we built a linear mixed-effects model (lme using nlme package in R) with IPI as our response variable. The model predictor was pop train type as a nominal variable (individual or multi-male) with second-order alliance membership included as a random effect. To account for the violation of the homoscedasticity assumption, we explicitly modelled the differences in variance between pop train type using the ‘varIdent' function (nlme package in R). The full model was compared to a null model containing only the random effect. Model selection was performed by ranking them using log-likelihood (logLik) and Akaike's information criterion (AIC). Visual assessment of the residuals confirmed that they were normally distributed. To check for model stability, we used the ‘influence' function (car package in R) to assess the influence of each grouping level of the random effect (second-order alliance). The model selection table is provided in the electronic supplementary material.
To assess whether males modified temporal characteristics of pops during multi-male trains, we first characterized individual pop trains across our entire dataset. We calculated the slope of the regression line for each individual pop train to determine whether there was a tendency for IPIs to increase or decrease over the duration of the pop train. To explore within and between individual variation in more detail we assessed smooth linear change in IPI over the duration of the pop train for six individual males from four different second-order alliances. We then used Pearson product-moment correlation to test whether males (i.e. male A and male B) synchronized their pop tempo (IPI) when producing pops in multi-male trains. To test whether this correlation was higher than expected by chance, a null model was constructed by randomly pairing each of male A's pop trains with randomly selected pop trains produced by another male, matched for the number of pops. This procedure was repeated 1000 times to generate 1000 null correlation coefficients from randomly permuted datasets. The true correlation coefficient was then compared to this null distribution to determine whether it was higher than expected by chance (alpha level = 0.05), which would indicate that the males attempt to match their partner's tempo when producing pops in multi-male trains.
Next, to test whether the males coordinated their pop train production by starting and stopping their pop trains at the same time, we calculated the total overlap of male A and male B's pop trains in a multi-male sequence. This was calculated by using acoustic localization to determine the start and end of each male's pop train, and calculating the percentage of time over a multi-male pop train sequence where the pop trains overlapped. For each multi-male pop train sequence, we took 1 s either side of the sequence as our start and endpoint for the time shift analysis. However, if another vocalization type occurred shortly before or after the pop train sequence, we then took the time between the pop train sequence starting/ending and the vocalization. Thus, the minimum time between the multi-male pop train sequence and the start or endpoint for the time shift analysis was 250 ms and the maximum was 1 s. This meant the time shift analysis was concentrated over the multi-pop train sequence. As such, a high percentage overlap is expected to occur by chance levels. The null model was constructed by performing the time shift analysis where the pop trains of one male were shifted by a given interval relative to the pop trains of the other male. We linked the start and end of the entire sequence and shifted the sequences relative to one another by a randomly selected time shift (a distribution ranging from 100 ms to the maximum length of the sequence in 100 ms increments), resulting in a different set of overlaps, and a different computed overlap percentage (similar to [26]). An overlap probability distribution from 1000 time shift randomizations was generated and compared to the observed value.
3. Results
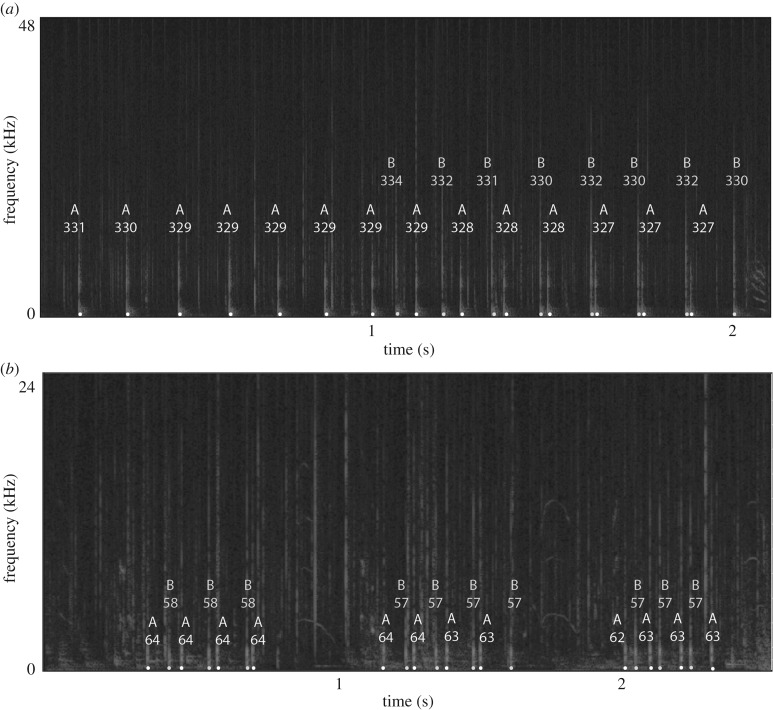
Acoustic data were analysed from 13 focal group follows of seven different second-order alliances (comprising a total of 59 males), with a total recording time of 22 h and 5 min from the austral springs of 2016–2018. All recordings were made during consortships and featured both pop trains produced by individual males (figure 1a), and instances of possible coordinated pop production (referred to as ‘multi-male' hereafter, figure 1b).
Figure 1.
Spectrograms of individual and multi-male pop trains from one second-order alliance (down-sampled to 48 kHz, FFT length: 1024, Hamming window function). (a) Individual male pop trains. (b) Multi-male pop trains. (Online version in colour.)
A total of 6082 IPIs from 453 pop trains were measured: 2415 intervals from trains produced by individual males (281 trains) and 3667 from multi-male trains (172 trains). A summary of individual male and multi-male pop train characteristics are provided in table 1. Multi-male pop trains were, on average, longer with more pops per train and shorter IPIs. This is because in multi-male trains, two males rapidly alternate pop production, so we were measuring the IPIs between consecutive pops i.e. between two different males. Comparison of the interval distributions between individual male and multi-male pop trains confirmed that they clearly differed (linear mixed-effects model: estimate = −0.048, CI: −0.051 to −0.046; P < 0.0001; electronic supplementary material, figure S1).
Table 1.
Summary of individual male and multi-male pop train characteristics. Mean and range of number of pops per train, inter-pop interval, and pop train length.
| individual male pop trains | multi-male pop trains | |
|---|---|---|
| mean number of pops per train | 9.6 (range: 2–49) | 22.3 (range: 3–194) |
| mean inter-pop interval between consecutive pops (s) | 0.115 (range: 0.016–0.377) | 0.064 (range: 0.0006–0.372) |
| mean pop train length (s) | 0.96 (range: 0.21–5.84) | 1.70 (range: 0.19–25.5) |
(a). Individual identification
Localization of a subset of pops returned consistent bearings for consecutive pops in trains produced by individual males (95 pops, 16 trains across five second-order alliances). Consecutive pops within the same train differed on average by 0.2° (s.d. = 1.93°, min = 0.00°, max 6.55°). Electronic supplementary material, figure S2 illustrates two examples of individual male pop trains produced by males from different second-order alliances with associated bearing information. While the bearings change over the course of the pop trains, they do so in a predictable way, either steadily increasing to infer the individuals' movement in a constant direction (electronic supplementary material, figure S2a), or with small variation around one bearing, inferring the animal remained stationary while vocalizing (electronic supplementary material, figure S2b). By contrast, multi-male pop trains returned two dominant bearings, which alternated with consecutive pops (244 pops, 20 trains across five second-order alliances). Bearings between consecutive pops varied by 4.43° on average (s.d. = 3.94°, min = 0.08°, max 17.46°), however, bearing differences between every second pop were comparable to those measured for individual male trains, varying by 0.23° on average (s.d. = 1.96°, min = 0.00°, max 16.84°). Despite not always being able to identify which two of the three allied males were engaging in this behaviour (due to their proximity), the consistent difference in bearings between consecutive pops in multi-male trains confirmed that the pops were being produced by two different males. Figure 2 illustrates two examples of multi-male trains from different second-order alliances, with bearing information demonstrating the alternation of pops achieved by two different males. Additional examples of localized multi-male pop trains are provided in electronic supplementary material, figure S2c,d.
Figure 2.
Localized multi-male pop trains from two different second-order alliances. Spectrogram of multi-male pop trains; (a) sampled at 96 kHz, FFT length: 1024, Hamming window function and (b) down-sampled to 48 kHz, FFT length: 1024, Hamming window function. Both panels show pops produced by two different males (A in white and B in yellow) from two different second-order alliances, and the localized bearing of each pop in relation to the research vessel (0° is the research vessel's bow). Each localized pop is also identified with a coloured dot (corresponding to male A or B) at the base of the pop. (Online version in colour.)
(b). Tempo adjustment
There was no consistent systematic change in IPI over time in individual male pop trains (figure 3). Individuals may increase or decrease the IPI in any given train as shown by the distribution of positive and negative slopes (figure 3a). This is further supported by figure 3b, where the variability both within and between individuals is apparent. The pop trains presented were produced in a 5-min time period for each male (different recordings for all males except individuals SMO + COO), with males producing trains that both increase and decrease in IPI.
Figure 3.
Variation in individual male pop trains. (a) Density plot of the slopes from the linear regression of 279 individual male pop trains (total of 2692 pops) recorded from seven different second-order alliances (one value not visible in density plot, x = 0.028); (b) smoothed linear change in IPI over time in a subset of localized pop trains produced by six individual males from four different second-order alliances. (Online version in colour.)
We used acoustic localization to explore the temporal patterning of each male's pops, when they called as part of a multi-male pop train, in more detail. Interestingly, IPIs were highly correlated between males when they coordinated pop production in these multi-male trains (Pearson correlation: r96 = 0.91, P < 0.0001). Figure 4 shows the distribution of localized pop intervals for two males in multi-male pop trains (i.e. the IPI for pops produced by male A and the IPI for pops produced by male B) with corresponding Pearson correlation coefficients. Four of five male dyads from different second-order alliances significantly correlated their tempo, even as the tempo changed over the duration of the pop trains (figure 4a). Permutations revealed that the correlation coefficients for all four pairs were significantly stronger than expected if the males were modulating their tempo independently of one another (figure 4b). We should note that, for the fifth pair (AC alliance; figure 4), only one extended pop train was used for this analysis (3–10 pop trains were included for the other four alliances; figure 4). However, even though they did not show a correlation in tempo, they did appear to maintain a constant tempo with little variation between individuals (i.e. male A range = ±0.01 s, male B range = ±0.03 s, difference between male A and male B range = ±0.01 s).
Figure 4.
Analysis of individual pop tempo when produced in multi-male pop trains. (a) IPI of male A (n = 96) against the IPI of male B immediately following male A (n = 96), across multiple pop trains in the same pop sequence, with corresponding Pearson correlation coefficient; colour-coded by second-order alliance membership (green, RR alliance; grey, KS alliance; red, AC alliance; blue, SB alliance; yellow, PB alliance). Note, this figure does not represent the change in IPI over time but the correlation of consecutive IPIs between male dyads. (b) Null model of the expected correlation coefficients based on 1000 permutations of pop trains produced in multi-male pop trains with pop trains produced by an individual male (matched for number of pops). The dotted line indicates the observed correlation coefficient, with plots colour-coded by second-order alliance membership. Note, given the lack of correlation for the AC alliance, no null model was created. (Online version in colour.)
(c). Pop train co-occurrence
We present three cases of male dyads from three different second-order alliances coordinating pop train production, i.e. they started and stopped their pop trains in unison (figure 2b; electronic supplementary material, figure S3). While multi-male pop production is frequent, we only used examples where we were able to localize the start and end of each pop train for each participating male. Electronic supplementary material, figure S3 shows the multi-male pop trains where the percentage overlap of pop trains averaged 83% (range: 76–86%). A randomized time shift analysis revealed that the percentage overlap for all three dyads was significantly higher (P < 0.05) than expected if the males were vocalizing independently of one another (percentage overlap at chance levels averaged 51%; electronic supplementary material, figure S3). As the time shift analysis was concentrated over the multi-pop train sequence, a high percentage overlap was expected to occur by chance levels. Notably, the observed percentage overlaps significantly exceeded this value. These sequences contained 4–7 pop trains and averaged 6.3 s in length (range: 5.5–7.3 s).
Finally, acoustic coordination between allied males was not restricted to particular alliances or areas. A list of all the second-order alliances occurring in both the eastern and western gulfs of Shark Bay where multi-male popping has been recorded is presented in electronic supplementary material, table S1.
4. Discussion
We show that multi-level dolphin alliances perform acoustic coordination in a cooperative context. While allied males frequently engage in motor synchrony when working together to coerce single oestrus females [31–33], we illustrate here that these males also engage in vocal coordination, whereby males will actively match the production and tempo of their pop vocalizations. Individual male pop trains can vary in a number of attributes, including the number of pops per train, the tempo of popping, and the length of inter-train intervals, even within the same pop sequence. Yet multi-male pop trains were generally highly coordinated, with males matching each other's tempo of pop production, even as the tempo changed within the train. Males also coordinated the length and timing of their trains, so that their pop trains overlapped significantly above chance levels. Multi-male pop trains were recorded in second-order alliances across our entire study area in both the eastern and western gulfs of Shark Bay. Given the distance between the gulfs relative to dolphin home range sizes [44] and that both sexes are philopatric [45], acoustic coordination may thus be customary in this population and not a behavioural strategy limited to a select number of alliances.
Intervals between pops produced by each individual within localized multi-male pop exchanges averaged 100 ms (figure 4), revealing that tempo matching between males can be strikingly precise, given the rate at which these pops are produced (x̄ = 10 pops per s, approx. 600 beats per min (BPM)). Humans can quickly and precisely synchronize to an external beat at 67–200 BPM [46,47], and a sulphur-crested cockatoo was capable of synchronization at around 100 BPM [48], both notably slower than the 600 BPM we report here. However, studies on both these species tested their capacity for rhythmic entrainment with a metronome, where motor synchrony, such as tapping a finger or bobbing a head, occurred at a phase offset of 0° (i.e. behaviour occurs at the same time as the beat). In our study, males did not always overlap individual pops, nor did we quantify phase offset, thus we did not find evidence of acoustic synchrony i.e. the precise coincidence of pops in time. In fact, if the signal to the female is based on presenting a unified front of two cooperating males, it is possible that males avoid more precise phase locking of individual pops to prevent masking. However, males did match pop train production where trains significantly overlapped. Given that single pops are rarely, if ever, produced and the vocal unit appears to be the pop train, we suggest that synchrony may be occurring at the level of the pop train.
It is possible that the males are being entrained by some unknown factor rather than actively coordinating with each another. However, poor underwater visibility in Shark Bay would make vocal entrainment on a physical signal extremely challenging, and no other acoustic signal was found to be associated with multi-male pop production, so we deem this highly unlikely. The bottlenose dolphin's vocal flexibility and propensity for synchronizing their movement with social partners [31,32] suggest that they should be able to synchronize auditory output. Given that the range of individual IPIs over which males can make tempo adjustments in localized multi-male trains (43–173 ms) is similar to the range of values found for dolphin motor synchrony (77–150 ms [32]), this species seems capable of coordination across modalities. To address this with greater certainty, playback experiments could be used to determine how readily individuals adjust their pop production to coordinate with a simulated popping male, and whether they are capable of precise acoustic synchrony.
It has previously been hypothesized that human pulse perception and entrainment evolved as a result of sexual selection, i.e. multi-male vocal displays helped attract migrating females [17,49,50]. Recently, however, it was shown that males and females are comparable in their vocal entrainment abilities [17,50], and thus a more favourable hypothesis is that the human capacity to perceive and synchronize with rhythms evolved to facilitate cooperative social interactions [17,50]. Indeed, cooperation is wide-spread in human societies and synchrony is an effective mechanism for promoting cooperation by strengthening social attachment among group members [15]. We show that both motor synchrony and acoustic coordination play an important role in the cooperative interactions between male dolphins in Shark Bay [31,32,39]. In the context of consortships, allied male dolphins work together to herd a female and defend her from rival alliances, yet they are also competing for a resource that is indivisible (fertilization).
It has been suggested that alliance synchrony during consortships might reduce tension between males in a context that requires them to cooperate successfully [32]. The mechanism underlying this relationship may be hormonal, as studies have linked synchronous behaviour to the release of the neuropeptide oxytocin in humans, which promotes trust and cooperation [51,52] and improves social communication [53]. While a link between synchrony and oxytocin release is yet to be demonstrated in dolphins, a link between oxytocin and prosocial behaviour has been demonstrated in other non-human animals, such as meerkats [54], chimpanzees [55], and grey seals [56] (reviewed in [57]). A positive feedback system may, therefore, exist between oxytocin release and coordinated behaviour, which would not only promote in-group trust and cooperation but also help regulate stress between allied males in competitive contexts (e.g. consortships). Instances of synchronous behaviour in other taxa and the selective pressures driving their evolution are important to consider when interpreting the motivation behind coordinated displays. Coordinated displays appear to play an important role in promoting cooperative partnerships in human societies [1,15,17]. Our work suggests that acoustic coordination in dolphins also promotes cooperative behaviour, providing further evidence that coordination, in both motor and acoustic forms, can be a collective feature of cooperation that enhances inclusive fitness by facilitating joint action tasks.
Supplementary Material
Acknowledgements
We thank RAC Monkey Mia Dolphin Resort, Shark Bay Resources, Monkey Mia Wildsights, and the DBCA's Shark Bay Rangers for their continued support and assistance. We thank all field assistants for their help during this study. We thank three anonymous reviewers and the handling editor for their constructive comments.
Ethics
Permits for the scientific use of animals were obtained from the Department of Biodiversity, Conservation and Attractions (DBCA), Western Australia. The University of Western Australia, University of Zürich and University of Bristol granted animal ethics approvals.
Data accessibility
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.r2280gb9h [58].
Authors' contributions
B.L.M. and S.L.K. conceived study; S.L.K., R.C.C., and M.K. acquired funding; S.L.K., B.L.M., S.J.A., R.C.C., and M.K. collected data; B.L.M. and S.L.K. conducted analysis and drafted the manuscript; all authors edited the manuscript and approved submission.
Competing interests
We declare we have no competing interests.
Funding
S.L.K. was supported by the Branco Weiss Fellowship—Society in Science. S.L.K. and R.C.C. were supported by a grant from the National Geographic Society (050R-17). M.K. was supported by the Swiss National Science Foundation (31003A_149956).
References
- 1.McNeill WM. 1995. Keeping together in time: dance and drill in human history. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Backwell PRY. 2018. Synchronous waving in fiddler crabs: a review. Curr. Zool. 65, 83–88. ( 10.1093/cz/zoy053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buck J. 1988. Synchronous rhythmic flashing of fireflies II. Q. Rev. Biol. 63, 265–289. ( 10.1086/415929) [DOI] [PubMed] [Google Scholar]
- 4.Buck J. 1938. Synchronous rhythmic flashing of fireflies. Q. Rev. Biol. 13, 301–314. ( 10.1086/516403) [DOI] [PubMed] [Google Scholar]
- 5.Greenfield M. 1994. Synchronous and alternating choruses in insects and anurans: common mechanisms and diverse functions. Am. Zool. 34, 605–615. ( 10.1093/icb/34.6.605) [DOI] [Google Scholar]
- 6.Greenfield MD, Roizen I. 1993. Katydid synchronous chorusing is an evolutionarily stable outcome of female choice. Nature 364, 618–620. ( 10.1038/364618a0) [DOI] [Google Scholar]
- 7.Ravignani A. 2017. Agree on definitions of synchrony. Nature 545, 158 ( 10.1038/545158c) [DOI] [PubMed] [Google Scholar]
- 8.Ravignani A, Verga L, Greenfield MD. 2019. Interactive rhythms across species: the evolutionary biology of animal chorusing and turn-taking. Ann. N.Y. Acad. Sci. 1453, 12–21. ( 10.1111/nyas.14230) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartbauer M, Römer H. 2016. Rhythm generation and rhythm perception in insects: the evolution of synchronous choruses. Front. Neurosci. 10, 1–15. ( 10.3389/fnins.2016.00223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fessler DMT, Holbrook C. 2014. Marching into battle: synchronized walking diminishes the conceptualized formidability of an antagonist in men. Biol. Lett. 10, 20140592 ( 10.1098/rsbl.2014.0592) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Launay J, Tarr B, Dunbar RIM. 2016. Synchrony as an adaptive mechanism for large-scale human social bonding. Ethology 122, 779–789. ( 10.1111/eth.12528) [DOI] [Google Scholar]
- 12.Tarr B, Launay J, Cohen E, Dunbar R. 2015. Synchrony and exertion during dance independently raise pain threshold and encourage social bonding. Biol. Lett. 11, 1–4. ( 10.1098/rsbl.2015.0767) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reddish P, Fischer R, Bulbulia J. 2013. Let's dance together: synchrony, shared intentionality and cooperation. PLoS ONE 8, e71182 ( 10.1371/journal.pone.0071182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valdesolo P, Ouyang J, DeSteno D. 2010. The rhythm of joint action: synchrony promotes cooperative ability. J. Exp. Soc. Psychol. 46, 693–695. ( 10.1016/j.jesp.2010.03.004) [DOI] [Google Scholar]
- 15.Wiltermuth SS, Heath C. 2008. Synchrony and cooperation. Psychol. Sci. 20, 1–5. ( 10.1111/j.1467-9280.2008.02253.x) [DOI] [PubMed] [Google Scholar]
- 16.von Zimmermann J, Richardson DC. 2016. Verbal synchrony and action dynamics in large groups. Front. Psychol. 7, 1–10. ( 10.3389/fpsyg.2016.02034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravignani A, Bowling DL, Fitch WT. 2014. Chorusing, synchrony, and the evolutionary functions of rhythm. Front. Psychol. 5, 1–15. ( 10.3389/fpsyg.2014.01118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geissmann T. 2002. Duet-splitting and the evolution of gibbon songs. Biol. Rev. Camb. Phil. Soc. 77, 57–76. ( 10.1017/S1464793101005826) [DOI] [PubMed] [Google Scholar]
- 19.Méndez-Cárdenas MG, Zimmermann E. 2009. Duetting: a mechanism to strengthen pair bonds in a dispersed pair-living primate (Lepilemur edwardsi)? Am. J. Phys. Anthropol. 139, 523–532. ( 10.1002/ajpa.21017) [DOI] [PubMed] [Google Scholar]
- 20.Brumm H, Slater P. 2007. Animal communication: timing counts. Curr. Biol. 17, 521–523. ( 10.1016/j.cub.2007.04.053) [DOI] [PubMed] [Google Scholar]
- 21.Mann NI, Dingess KA, Slater PJB. 2006. Antiphonal four-part synchronized chorusing in a Neotropical wren. Biol. Lett. 2, 1–4. ( 10.1098/rsbl.2005.0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall ML, Peters A. 2008. Coordination between the sexes for territorial defence in a duetting fairy-wren. Anim. Behav. 76, 65–73. ( 10.1016/j.anbehav.2008.01.010) [DOI] [Google Scholar]
- 23.Hall ML. 2004. A review of hypotheses for the functions of avian duetting. Behav. Ecol. Sociobiol. 55, 415–430. ( 10.1007/s00265-003-0741-x) [DOI] [Google Scholar]
- 24.Hall ML, Magrath RD. 2007. Temporal coordination signals coalition quality. Curr. Biol. 17, 406–407. ( 10.1016/j.cub.2007.04.022) [DOI] [PubMed] [Google Scholar]
- 25.Levinson SC. 2016. Turn-taking in human communication - origins and implications for language processing. Trends Cogn. Sci. 20, 6–14. ( 10.1016/j.tics.2015.10.010) [DOI] [PubMed] [Google Scholar]
- 26.Demartsev V, Strandburg-Peshkin A, Ruffner M, Manser M. 2018. Vocal turn-taking in meerkat group calling sessions. Curr. Biol. 28, 3661–3666. ( 10.1016/j.cub.2018.09.065) [DOI] [PubMed] [Google Scholar]
- 27.Takahashi DY, Narayanan DZ, Ghazanfar AA. 2013. Coupled oscillator dynamics of vocal turn-taking in monkeys. Curr. Biol. 23, 2162–2168. ( 10.1016/j.cub.2013.09.005) [DOI] [PubMed] [Google Scholar]
- 28.Allen SJ, Bryant KA, Kraus RHS, Loneragan NR, Kopps AM, Brown AM, Gerber L, Krützen M. 2016. Genetic isolation between coastal and fishery-impacted, offshore bottlenose dolphin (Tursiops spp.) populations. Mol. Ecol. 25, 2735–2753. ( 10.1111/mec.13622) [DOI] [PubMed] [Google Scholar]
- 29.Connor RC, Krützen M. 2015. Male dolphin alliances in Shark Bay: changing perspectives in a 30-year study. Anim. Behav. 103, 223–235. ( 10.1016/j.anbehav.2015.02.019) [DOI] [Google Scholar]
- 30.Connor RC, Smolker RA, Richards AF. 1992. Two levels of alliance formation among male bottlenose dolphins (Tursiops sp.). Proc. Natl Acad. Sci. USA 89, 987–990. ( 10.1073/pnas.89.3.987) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connor RC. 2007. Dolphin social intelligence: complex alliance relationships in bottlenose dolphins and a consideration of selective environments for extreme brain size evolution in mammals. Phil. Trans. R. Soc. B 362, 587–602. ( 10.1098/rstb.2006.1997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connor RC, Smolker R, Bejder L. 2006. Synchrony, social behaviour and alliance affiliation in Indian Ocean bottlenose dolphins, Tursiops truncatus. Anim. Behav. 72, 1371–1378. ( 10.1016/j.anbehav.2006.03.014) [DOI] [Google Scholar]
- 33.McCue LM, Cioffi WR, Heithaus MR, Barrè L, Connor RC. 2020. Synchrony, leadership, and association in male Indo-pacific bottlenose dolphins (Tursiops aduncus). Ethology, 1–10. ( 10.1111/eth.13025) [DOI] [Google Scholar]
- 34.Connor RC, Smolker RA, Richards AF. 1992. Dolphin alliances and coalitions. In Coalitions and alliances in humans and other animals, pp. 415–443. Oxford, UK: Oxford University Press. [Google Scholar]
- 35.Connor RC, Smolker RA. 1996. ‘Pop’ goes the dolphin: a vocalization male bottlenose dolphins produce during consortships. Behaviour 133, 643–662. ( 10.1163/156853996X00404) [DOI] [Google Scholar]
- 36.Vollmer NL, Hayek LC, Heithaus MR, Connor RC. 2015. Further evidence of a context-specific agonistic signal in bottlenose dolphins: the influence of consortships and group size on the pop vocalization. Behaviour 152, 1979–2000. ( 10.1163/1568539X-00003311) [DOI] [Google Scholar]
- 37.King S, Allen S, Krützen M, Connor R. 2019. Vocal behaviour of allied male dolphins during cooperative mate guarding. Anim. Cogn. 22, 991–1000. ( 10.1007/s10071-019-01290-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smolker RA, Richards AF, Connor RC, Pepper JW. 1992. Sex differences in patterns of association among Indian Ocean bottlenose dolphins. Behaviour 123, 38–69. ( 10.1163/156853992X00101) [DOI] [Google Scholar]
- 39.King SL, Friedman WF, Allen SJ, Gerber L, Jensen FH, Wittwer S, Connor RC, Krutzen M. 2018. Bottlenose dolphins retain individual vocal labels in multi-level alliances. Curr. Biol. 28, 1993–1999. ( 10.1016/j.cub.2018.05.013) [DOI] [PubMed] [Google Scholar]
- 40.Gerber L, et al. 2019. Affiliation history and age similarity predict alliance formation in adult male bottlenose dolphins. Behav. Ecol., 1–10. ( 10.1093/beheco/arz195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quick NJ, Rendell LE, Janik VM. 2008. A mobile acoustic localisation system for the study of free-ranging dolphins during focal follows. Mar. Mammal Sci. 24, 979–989. ( 10.1111/j.1748-7692.2008.00231.x) [DOI] [Google Scholar]
- 42.Wahlberg M, Møhl B, Madsen PT. 2001. Estimating source position accuracy of a large-aperture hydrophone array for bioacoustics. J. Acoust. Soc. Am. 109, 397–406. ( 10.1121/1.1329619) [DOI] [Google Scholar]
- 43.Schulz TM, Rendell LE, Whitehead H. 2006. A remotely-piloted acoustic array for studying sperm whale vocal behavior. Can. Acoust. 34, 54–55. [Google Scholar]
- 44.Randić S, Connor RC, Sherwin WB, Krützen M. 2012. A novel mammalian social structure in Indo-Pacific bottlenose dolphins (Tursiops sp.): complex male alliances in an open social network. Proc. R. Soc. B 279, 3083–3090. ( 10.1098/rspb.2012.0264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krutzen M, Sherwin WB, Berggren P, Gales N. 2004. Population structure in an inshore cetacean revealed by microsatellite and mtDNA analysis: bottlenose dolphins in Shark Bay, Western Australia. Mar. Mammal Sci. 20, 28–47. ( 10.1111/j.1748-7692.2004.tb01139.x) [DOI] [Google Scholar]
- 46.Repp B. 2013. Sensorimotor synchronization: a review of the tapping literature. Psychon. Bull. Rev. 12, 969–992. ( 10.3758/BF03206433) [DOI] [PubMed] [Google Scholar]
- 47.Patel AD. 2014. The evolutionary biology of musical rhythm: was Darwin wrong? PLoS Biol. 12, 1–6. ( 10.1371/journal.pbio.1001821) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel AS, Iversen JR, Bregman MR. 2009. Experimental evidence for synchronization to a musical beat in a nonhuman animal. Curr. Biol. 19, 827–830. ( 10.1016/j.cub.2009.03.038) [DOI] [PubMed] [Google Scholar]
- 49.Merker B. 2000. Synchronous chorusing and the origins of music. Music. Sci. 3, 59–73. ( 10.1177/10298649000030S105) [DOI] [Google Scholar]
- 50.Bowling DL, Herbst CT, Fitch WT. 2013. Social origins of rhythm? Synchrony and temporal regularity in human vocalization. PLoS ONE 8, e80402 ( 10.1371/journal.pone.0080402) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Dreu CKW, Greer LL, Van Kleef GA, Shalvi S, Handgraaf MJJ. 2011. Oxytocin promotes human ethnocentrism. Proc. Natl Acad. Sci. USA 108, 1262–1266. ( 10.1073/pnas.1015316108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Dreu CKW, Greer LL, Handgraaf MJJ, Shalvi S, Van Kleef GA, Baas M, Ten Velden FS, Van Dijk E, Feith SWW. 2010. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science 328, 1408–1411. ( 10.1126/science.1189047) [DOI] [PubMed] [Google Scholar]
- 53.Spengler FB, Scheele D, Marsh N, Kofferath C, Flach A, Schwarz S, Stoffel-Wagner B, Maier W, Hurlemann R. 2017. Oxytocin facilitates reciprocity in social communication. Soc. Cogn. Affect. Neurosci. 12, 1325–1333. ( 10.1093/scan/nsx061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madden JR, Clutton-Brock TH. 2011. Experimental peripheral administration of oxytocin elevates a suite of cooperative behaviours in a wild social mammal. Proc. R. Soc. B 278, 1189–1194. ( 10.1098/rspb.2010.1675) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbuhler K, Deschner T. 2013. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc. R. Soc. B 280, 20122765 ( 10.1098/rspb.2012.2765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robinson KJ, Twiss SD, Hazon N, Moss S, Pomeroy PP. 2017. Positive social behaviours are induced and retained after oxytocin manipulations mimicking endogenous concentrations in a wild mammal. Proc. R. Soc. B 284, 20170554 ( 10.1098/rspb.2017.0554) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crockford C, Deschner T, Ziegler TE, Wittig RM. 2014. Endogenous peripheral oxytocin measures can give insight into the dynamics of social relationships: a review. Front. Behav. Neurosci. 8, 1–14. ( 10.3389/fnbeh.2014.00068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore BL, Connor RC, Allen SJ, Krützen M, King SL. 2020. Data from: Acoustic coordination by allied male dolphins in a cooperative context. Dryad Digital Repository ( 10.5061/dryad.r2280gb9h). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Moore BL, Connor RC, Allen SJ, Krützen M, King SL. 2020. Data from: Acoustic coordination by allied male dolphins in a cooperative context. Dryad Digital Repository ( 10.5061/dryad.r2280gb9h). [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.r2280gb9h [58].