Abstract
Effective sampling of marine communities is essential to provide robust estimates of species richness and abundance. Baited Remote Underwater Video Stations (BRUVS) are a useful tool in assessment of fish assemblages, but research on the optimal sampling period required to record common and rare elasmobranch species is limited. An appropriate ‘soak time’ (time elapsed between settlement of the BRUVS on the seabed and when it is hauled off the seabed) requires consideration, since longer soak times may be required to record species rare in occurrence, or sightings in areas of generally low elasmobranch abundance. We analysed 5352 BRUVS deployments with a range of soak times across 21 countries in the Coral Triangle and Pacific Ocean, to determine the optimal soak time required for sampling reef-associated elasmobranchs, considering species rarity, and community abundance at each site. Species were categorised into 4 ‘rarity’ groups (very rare to common), by their relative occurrence in the dataset, defined simply by the proportion of BRUVS on which they occurred. Individual BRUVS were categorised into 3 ‘abundance’ groups (low to high) by overall relative elasmobranch abundance, defined as total number of all elasmobranchs sighted per unit of sampling effort. The effects of BRUVS soak times, and levels of rarity and abundance groupings, on the time to first sighting (TFS) and time to maximum number of elasmobranchs observed (tMaxN) were examined. We found that TFS occurred earlier for species groups with high occurrence, and on BRUVS with high elasmobranch abundance, yet longer soak times were not essential to observe rarer species. Our models indicated an optimum of 95% of both sighting event types (TFS, tMaxN) was recorded within 63–77 minutes, and a soak time of 60 minutes recorded 78–94% of the elasmobranch sighting events recorded (78–94% of TFS events and 82–90% of tMaxN events), when species rarity and abundance on BRUVS was accounted for. Our study shows that deployments of ~ 77 minutes are optimal for recording all species we observed, although 60 minutes soak time effectively samples the majority of elasmobranch species in shallow coral reef habitats using BRUVS.
Introduction
The abundance of elasmobranchs can be highly variable [1, 2] and will be dependent on species distributions, environmental conditions, and local fishing pressure [3, 4]. Their distributions can cross political borders and concerns about conservation status of some species can clash with the interests of commercial and recreational fisheries where they are seen as targets for exploitation [5, 6] or competitors for teleost resources (e.g. [7]). It is therefore important to have a reliable, defensible methodology to make confident comparisons of elasmobranch status within and among locations and jurisdictions.
There are a variety of fishery-independent methods available for estimating the abundance of elasmobranchs [8]. Two of the most common for tropical sharks are underwater visual census (UVC) and baited remote underwater video stations (BRUVS). Both methods have been used in coral reef habitats with UVC most commonly applied to enumerate reef sharks [9–12]. However, BRUVS have been increasingly applied to assessment of shark assemblages over the last two decades [1, 13–16].
One of the main benefits of the baited video technique is that it removes the need for skilled observers in the field, yet is easily standardised for application to a variety of locations and depths. UVC requires a scuba diver highly skilled in fish identification to conduct each survey and there is no visual record of the sample. The presence of video footage permits visualisation of the seascapes in the field of view and species identifications in an archive that can be interrogated repeatedly for a variety of research questions [17]. Due to the use of bait as an attractant, easy replication, and capacity to sample a variety of depths and habitats, BRUVS are an ideal method for studying broad-scale presence and abundance of elasmobranch species without capturing and handling animals (e.g. [2, 14, 18–21]).
The status of reef-associated elasmobranchs has been the subject of numerous studies which have produced varying perspectives about their conservation status and the efficacy of their management [2, 5, 22]. However, the currently available data are heavily biased to carcharhinid sharks with relatively little known about the status of other elasmobranchs that use coral reefs [23]. Reports of declines in the abundance of reef-associated sharks due to overfishing (e.g. [9, 11, 22]) have focussed the use of BRUVS surveys to detect effects of marine protected areas (MPA). Several studies have revealed increased numbers of elasmobranchs in areas closed to fishing [16, 21, 24, 25] indicating the efficacy of BRUVS to detect the effects of fishing. However, there may also be sources of variability in the size of reef shark populations beyond those related to fishing. For example, highly variable baseline estimates of sharks were recorded at a number of Pacific Ocean reefs which were largely attributed to differences in ocean productivity [26]. Further study over broad spatial scales is required to help refine our understanding of variability in reef elasmobranch populations and the factors driving these patterns. This requires capacity to assess relative abundance and compare communities among a variety of sites in a standardised way.
The application of BRUVS provides an opportunity to sample a wide array of depths and habitat types using multiple units simultaneously (e.g. lagoon, slope, inter-reef habitats) and to survey large areas more efficiently than UVC surveys. A large number of BRUVS can be deployed in quick succession to sample a broader area than is surveyed in a UVC transect. The amount of time a BRUVS is deployed on the seabed (‘soak time’) is constrained by the travelling time between sampling sites, the handling time in setting and retrieving the units, camera storage capacity and battery life. A common presumption is that longer soak times would be needed in areas of low abundance (e.g. [27]), and that differing soak times could produce differing results depending on how readily elasmobranchs are attracted to the BRUVS.
Although sharks and rays are easily attracted to bait, it is unclear how they interact with BRUVS units. It could be assumed that in areas of high abundance BRUVS are likely to be placed in proximity to local sharks. In these instances individuals could be expected to appear in the BRUVS field of view early in the deployment, and accumulate to high numbers. In contrast, it could also be assumed that in areas of low overall elasmobranch abundance where individuals are sparsely distributed, or for particular species that are rarely encountered in the region, that likelihood of deploying a BRUVS in their proximity is low and hence arrival times would be longer (e.g. [28]). For example, a soak time of 5–6 hours was suggested as appropriate for detecting the presence of nearshore white sharks Carcharodon carcharias, since their mean time to first sighting was 148 minutes for a maximum of one individual at any time [20]. While 1 hour soak times have been suggested for sharks [8], these assumptions have not been tested across multiple locations, and fundamental questions about how BRUVS accumulate sightings of elasmobranchs warrant exploration.
The Global FinPrint project (https://globalfinprint.org/) has conducted BRUVS deployments in coral reef ecosystems around the world to estimate the relative abundance of reef-associated elasmobranchs. Countries within the Coral Triangle and Pacific Ocean comprise a wide diversity and abundance of elasmobranchs, with species varying in rarity (i.e. occurrence on BRUVS), and sites ranging from low to high overall abundance (i.e. overall elasmobranch sightings per unit effort for each BRUVS). We examined this data to identify the optimal soak time required to capture the events of the time to first sighting (TFS) and time to maximum number of elasmobranchs observed (tMaxN), compared with the commonly used soak time of 60 minutes. The soak time required to effectively capture these sighting events was assessed under varying levels (categories) of: (1) species rarity: the occurrence of each species on the individual BRUVS in the dataset, grouped into categories of species rarity; and (2) overall elasmobranch abundance on individual BRUVS. These findings provide a basis and recommendations for future research using BRUVS to help define the occurrence and relative abundance of tropical, reef-associated elasmobranchs.
Materials and methods
Our study included data from 5352 baited remote underwater video station (BRUVS) deployments of the Global FinPrint project in 21 countries. Deployments were conducted in coral reef habitats in depths from 1–70 m. Each BRUVS consisted of a lightweight aluminium frame fitted with a video camera (GoPro Hero4 Silver, GoPro Inc. USA; https://www.gopro.com) within a housing overlooking a bait bag positioned 1.5 m from the camera (Fig 1). Each BRUVS was baited with approximately 1 kg of oily fish (primarily from families Clupeidae and Scombridae). BRUVS were separated by at least 500 m at any time to reduce the likelihood of individuals occurring on multiple cameras [29].
Fig 1. BRUVS deployed on seabed in reef habitat.
BRUVS showing bait arm, camera within housing and rope attached to surface float. Credit: S Lindfield, Coral Reef Research Foundation Palau.
Video and data analysis
Data comprised standardised legacy data and records from videos analysed using the FinPrint Annotator (v.1.1.44.0) which were examined by two readers, with reader discrepancies, outliers and a subset of images reviewed by a master annotator to ensure accuracy in species identification. Elasmobranchs were identified to species, and the maximum number of individuals of each species seen together in one frame (‘MaxN’, a conservative measure of relative abundance [29]), was recorded for each BRUVS video. Videos were analysed from the time the BRUVS settled BRUVS on the seabed (start time), with the ‘time to first sighting’ (TFS) recorded for each species, and MaxN updated if the number increased at a later point in the video.
Four measurements obtained from BRUVS were used in this study: the ‘soak time’; ‘time to first sighting’ (TFS); MaxN; and ‘time to MaxN’ (tMaxN). The ‘soak time’ was measured as the minutes elapsed between the start time and the instant at which the BRUVS was hauled off the seabed (the end time). For instances where bait was entirely removed from a BRUVS, the time at which this occurred was classified as the end time and marked completion of the soak, for standardisation among BRUVS. Elasmobranch observations beyond the end time were not included in analyses. The ‘time to first sighting’ (TFS) was the time elapsed between the start of the sampling period and the first record of a particular species in the field of view. The ‘time to MaxN’ (tMaxN) for each species was the time elapsed between the start of the sampling period and the MaxN event, representing the time at which maximum relative abundance was observed. These metrics have been reviewed [17, 29] and this study focuses on comparisons between event timing and soak time from the two datasets: TFS data; and tMaxN data, which includes an additional 1337 BRUVS, for which only MaxN for each species was recorded (S1 Table). All times were measured in decimal minutes, rounded upward to the nearest whole minute for all analyses.
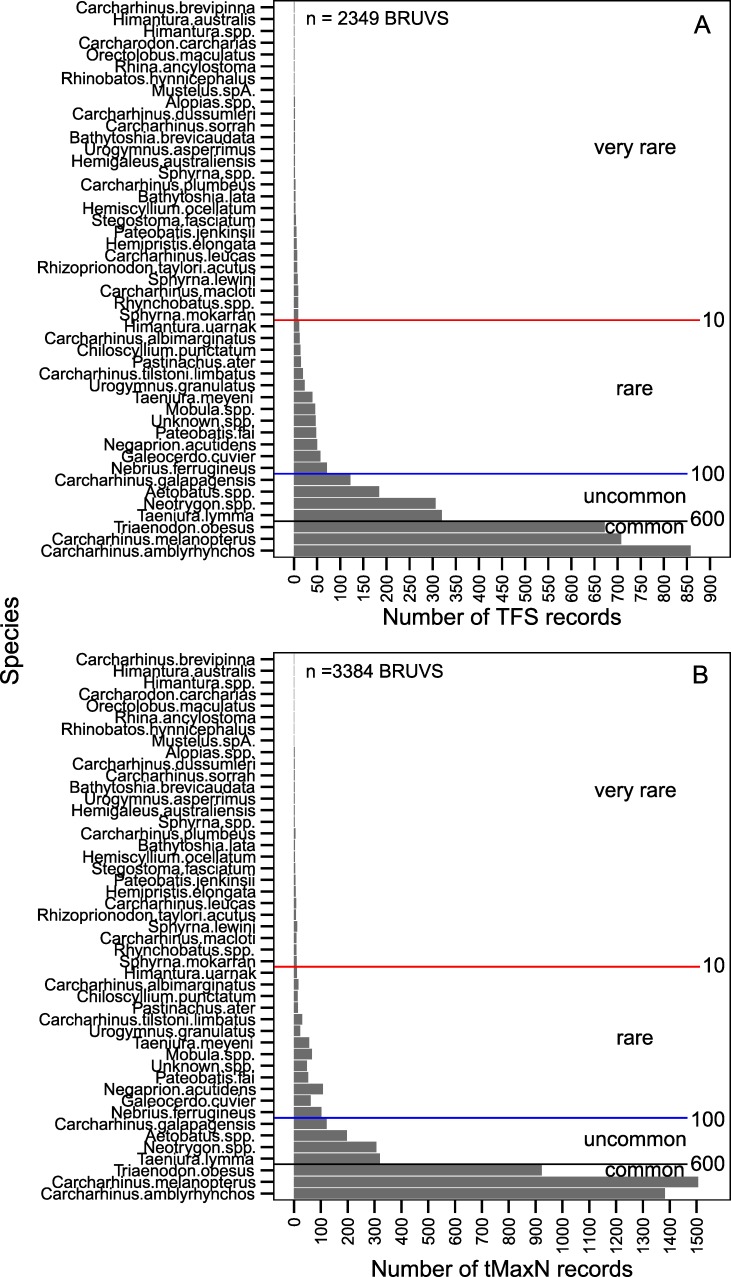
Relative elasmobranch abundance for each BRUVS sample was calculated as sightings per unit effort (SPUE), being MaxN values summed across species on individual BRUVS divided by the soak time, multiplied by 60 to provide an hourly rate. SPUE was used to assign three categories of overall elasmobranch abundance on individual BRUVS: ‘low’ (SPUE ≤ 1); ‘medium’ (SPUE > 1 and SPUE ≤ 3, which incorporated the global, median SPUE) and ‘high’ (SPUE > 3). Records for each species were assigned to four levels of ‘occurrence’ (‘rarity’) based on the number of BRUVS samples on which they were recorded in the TFS dataset (2349 BRUVS, Fig 2). These species categories were: ‘very rare’ (occurrence on ≤ 10 BRUVS), ‘rare’ (11–100), ‘uncommon’ (101–600), and ‘common’ (> 600). Rarity and abundance groups were used as predictors with soak time in the analyses, and all analyses were conducted in the R environment [30].
Fig 2.
Relative occurrence of 47 species of elasmobranchs in the TFS dataset (A; n = 2349) and tMaxN dataset (B; n = 3384). Species are ranked from top to bottom by the number of records in the TFS dataset. Species were grouped for some analyses by the cut points shown for levels of rarity: ‘very rare’ (occurrence on ≤ 10 BRUVS); ‘rare’ (11–100), ‘uncommon’ (101–600); and ‘common’ (≥ 600 BRUVS).
Summaries of proportions of events by time
Where cumulative frequency shows the frequency of an event in an interval, the empirical cumulative density function (ECDF, a step function) was used to estimate the fraction of observations of TFS and tMaxN that were less than or equal to a specified value (using the ecdf function in R). The ECDF plots were used to find the 75th and 95th percentiles of TFS and tMaxN by species occurrence (4 levels of rarity), and by abundance on individual BRUVS (3 levels based on SPUE).
Modelling the effect of soak time, rarity and abundance on timing of events
To identify patterns in TFS and tMaxN with soak time, species rarity, and overall abundance, Aggregated Boosted Regression Trees (ABT, using abt package in R: [31, 32]) were used. ABTs modelled the relationship between the TFS and tMaxN events with the predictors: species rarity (4 levels based on occurrence); overall elasmobranch abundance (3 levels based on SPUE per BRUVS); and soak time. We identified the optimal soak times, and considered the commonly used soak time of 60 minutes as sufficient if ABTs indicated the majority of events occurred within 60 minutes, i.e. for all categories of abundance and species rarity. ABTs use the benefits of both regression techniques and classification by machine learning to derive a single, optimal tree by cross-validation (see [33, 34]). The final ABT object is a collection of five trees and results are presented as partial effects plots for the main effects of soak time, abundance and species rarity groups, and partial interaction plots of the effect of soak time by abundance group and soak time by rarity group. The ABT approach is ideal for large datasets with missing values, large outliers, correlated predictors and unequal sample sizes. While regression models summarise how much variation is explained by model fits, ABTs summarise how much variation is predicted, and an equivalent of the pseudo-R-squared value from regression is produced [33].
BRUVS were excluded from the ABT analyses if soak time > 126 (i.e. 2.1% of total BRUVS with > 2 hours and 6 minutes), due to scarcity of cases with very long soaks beyond this time. Responses were transformed by 4th root to reduce the skew in the data, and the models were event0.25 ~ soak time + species rarity group + abundance group. Five-fold cross-validation was used for all models, and a range of learning rates from 0.01 (fast) to 0.001 (very slow) were tested with tree sizes of 1000 and 5000.
Results
There were 47 elasmobranch species recorded from 21 countries in 5352 BRUVS samples, with sightings on 63.2% of the deployments (S1 Table). Information on TFS was available for 2349 BRUVS and data were obtained for tMaxN from 3384 BRUVS (S1 Table).
The MaxN recorded for each species was highly skewed, with an overall median of MaxN = 1, a maximum of 21, and a mean of 1.6. The very high values were caused by passage of schools of rays or sharks through the field of view. Total relative abundance per BRUVS ranged from 1 to 32, with a median of 2 and a mean of 2.5 for tMaxN data. The predominance of MaxN = 1 implied that tMaxN was equivalent to TFS for most species and most BRUVS. The overall SPUE per BRUVS ranged from 0.2 to 62.4, with a median of 1.5 sighting hr-1 and mean of 2.4 sighting hr-1. Soak times for seven of the eight BRUVS with SPUE >25 were of 30 minutes soak time or less, caused by removal of the bait by predators.
Timing of events based on species rarity
Three species comprised the ‘common’ category of species rarity based on occurrence: grey reef shark (Carcharhinus amblyrhynchos), blacktip reef shark (C. melanopterus), and whitetip reef shark (Triaenodon obesus) (Fig 2). The ‘uncommon’ category included three rays and one shark species: spotted fantail ray (Taenura lymma), maskrays (Neotrygon spp.), eagle rays (Aetobatus spp.), and the Galapagos shark (Carcharhinus galapagensis) (Fig 2). Most other species were classified as ‘very rare’ (27) or ‘rare’ (13) and occurred on 86 (3.7%) and 418 (17.8%) of the 2349 BRUVS in the TFS dataset, respectively. The most common species, C. amblyrhynchos, was recorded on 858 (36.5%) of the 2349 BRUVS where TFS information was available, 1382 of the 3384 BRUVS with TMaxN data (40.8%; Fig 2A and 2B), and had an overall recorded abundance (total summed MaxN) of 2989 individuals. The larger tMaxN dataset (Fig 2B) showed similar rankings due to the prevalence of MaxN = 1 for most species and most BRUVS (e.g. S1 Fig).
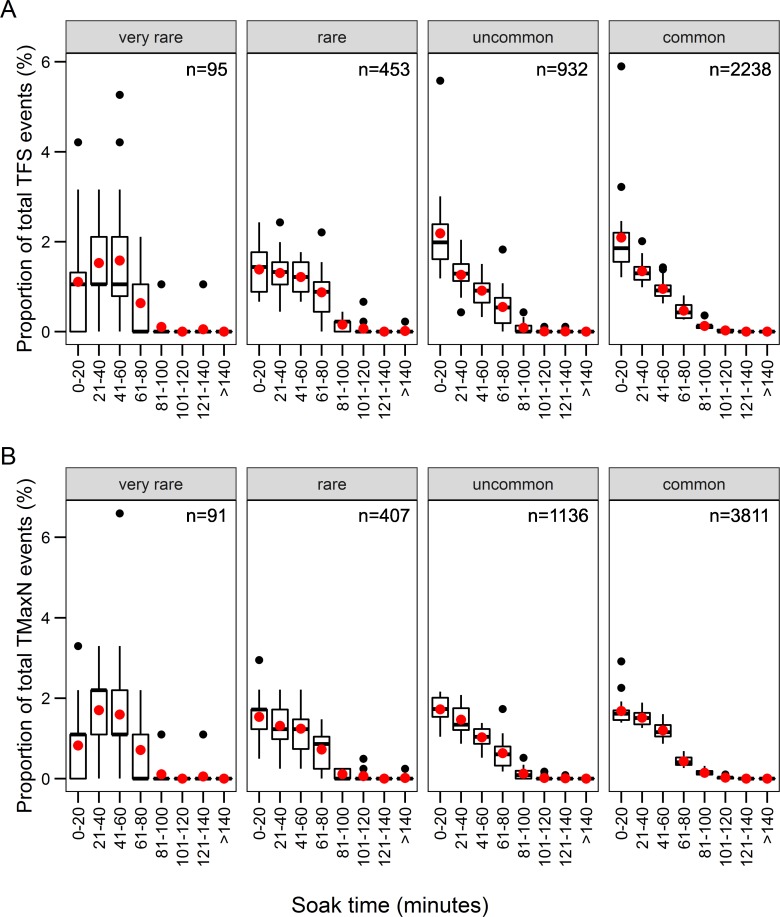
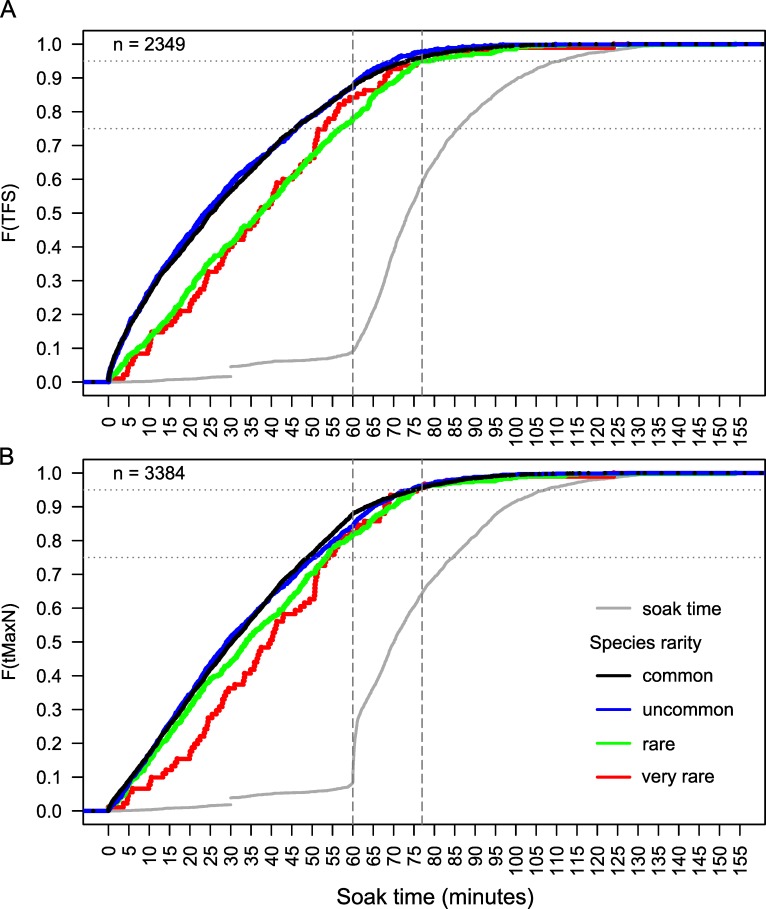
For each level of species rarity, counts of TFS and TMaxN event observations for each minute were calculated and represented as a proportion of the total observations per level of rarity. Boxplots of the event proportions observed per minute for 20 minute time bins showed no evidence that rarer species were being sighted later in videos, or that the highest proportions peaked later than those of the more common categories (Fig 3). While the proportion of events was lower in the first 0–20 minutes for the ‘very rare’ species compared to other three rarity groups, the greatest proportion of all species groups based on rarity were observed within the first 60 minutes of soak time, with < 60 minutes comprising the majority of TFS and TMaxN events. However, empirical cumulative density functions for the four categories of species rarity gave a clearer picture separating the two most common species groups from the other two groups (Fig 4). About 78–88% of all TFS events and 82–88% of all tMaxN events occurred for all species groups by 60 minutes soak time, and 95% of recorded TFS and tMaxN events for all species groups had occurred by ~ 77 minutes. ‘Common’ and ‘uncommon’ species had almost identical cumulative density functions for both TFS and tMaxN, which occurred earlier than for ‘rare’ and ‘very rare’ species. The timing of tMaxN was delayed by about 10 minutes for ‘very rare’ species between about 10 and 50 minutes soak time, after which the curves converged (Fig 4B).
Fig 3. Proportions of species in rarity groups by soak time (minutes).
Species groups were categorised as ‘very rare’ (occurrence on ≤ 10 BRUVS), ‘rare’ (11–100), ‘uncommon’ (101–600), and ‘common’ (≥ 600 BRUVS) species in the TFS dataset (A; n = 2349) and tMaxN (B; n = 3384) dataset (see Fig 2A). The boxes show the medians (black lines) and means (red points) within the first and third quartiles of the data. The ‘whiskers’ represent 1.5 times the interquartile ranges, and outliers in events proportions are shown.
Fig 4. Time to first sighting (TFS: A) and time to maximum MaxN (tMaxN: B) by species rarity group.
Empirical cumulative density functions (ECDF) of the TFS and tMaxN by 4 species groups based on their rarity (see Fig 2A). The ECDF for soak times are also shown for each dataset. Horizontal lines on the y-axis show 75th and 95th percentiles in the ECDF. Vertical lines on the x-axis represents a soak time of 60 and 77 minutes.
Timing of events based on relative abundance
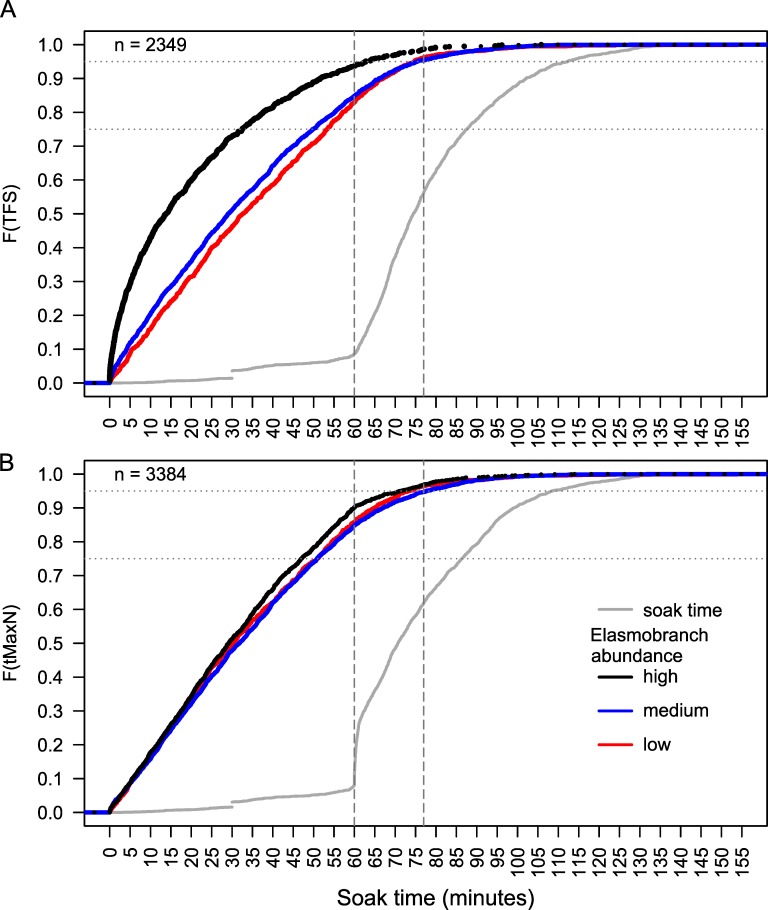
Approximately 83–94% of all TFS events and 85–90% of all tMaxN events occurred by 60 minutes of soak time for BRUVS of all elasmobranch abundance (SPUE) groups (Fig 5). At least 95% of all TFS and tMaxN events for all groups had occurred by ~ 77 minutes soak time. Elasmobranchs were sighted faster (10–15 minutes earlier) on BRUVS where higher overall abundance was recorded, compared to BRUVS with medium and low SPUE, particularly within the first 60 minutes (Fig 5A). Thus, a shorter optimal soak time of 63 minutes captured 95% of TFS events from BRUVS categorised by high elasmobranch abundance (Fig 5A), linked also to a number of sightings recorded immediately on BRUVS settlement to the seafloor. Little difference among abundance groups was observed for tMaxN events, with marginal difference between the high versus medium and low curves (Fig 5B).
Fig 5. Time to first sighting (TFS: A) and time to maximum MaxN (tMaxN: B) by abundance group.
Empirical cumulative density functions (ECDF) of the TFS and tMaxN by 3 groups based on relative abundance of all elasmobranchs (SPUE) per BRUVS. The ECDF for soak times are also shown for each dataset. Horizontal lines on the y-axis show 75th and 95th percentiles in the ECDF. Vertical lines on the x-axis represents a soak time of 60 and 77 minutes.
Correlations between soak time and events
The transformed events of TFS (n = 3652 on 2314 BRUVS) and tMaxN (n = 5393 on 3347 BRUVS) for 47 species were modelled as responses to the predictors soak time, species rarity and abundance groups for ABT analyses. Less than ~ 10% of soak times ended before ~ 60 minutes, and less than 10% of soaks were longer than ~ 100 minutes (Figs 6B and 7B). Mean soak times in the datasets (< 126 minutes) for TFS and tMaxN were 76 and 73.5 minutes respectively. The global, mean TFS and tMaxN occurred at less than half of these values at 30.6 and 33.5 minutes, indicating mean values had been reached within the first half of most deployments. There were positive correlations of 0.30 and 0.27 between soak time and TFS and soak time and tMaxN.
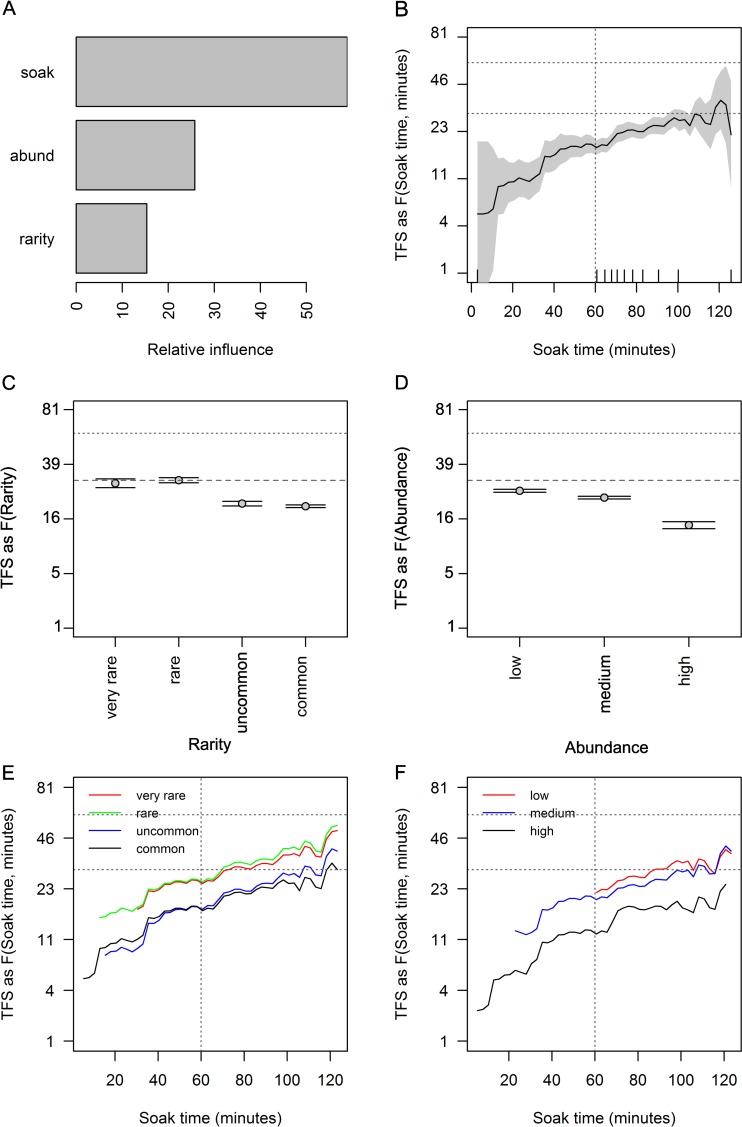
Fig 6. Partial effects plots of soak time, species rarity and abundance groups on TFS.
Relative influence of each predictor (A), the effects of each predictor: soak time (B); species rarity groups (based on occurrence, C); and abundance groups (based on SPUE per BRUVS, D); are shown when the other predictors were held to their average value. The partial effect of soak time given the 4 species rarity groups (E) and 3 abundance groups (F) are indicated. Shading and errors bars are 2 standard errors. The y axes are back-transformed responses from the 4th root scale, expressed in minutes. The rugs on the x-axis of panel (B) are ten percentiles in the distribution of the soak times. Horizontal lines on panels show mean TFS (30.6 minutes) and 60 minutes and vertical lines indicate a soak time of 60 minutes.
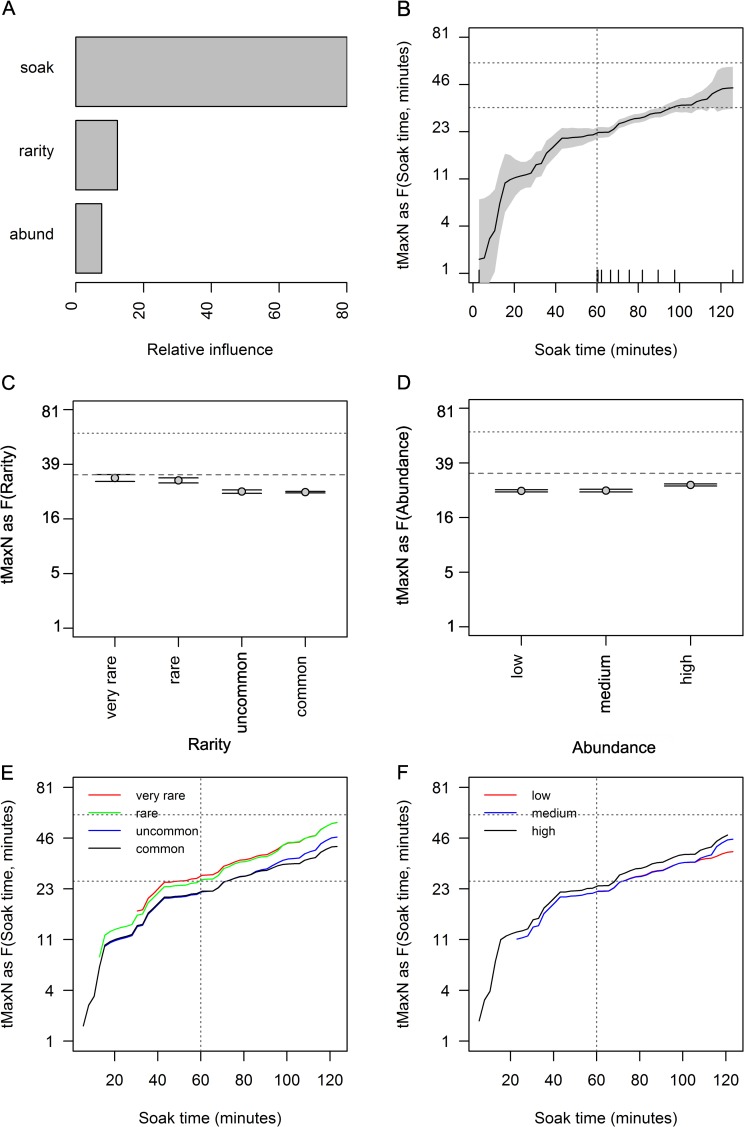
Fig 7. Partial effects plots of soak time, species rarity and abundance groups on tMaxN.
Relative influence of each predictor (A), the effects of each predictor: soak time (B); species rarity groups (based on occurrence, C); and abundance groups (based on SPUE per BRUVS, D); are shown when the other predictors were held to their average value. The partial effect of soak time given the 4 species rarity groups (E) and 3 abundance groups (F) are indicated. Horizontal lines show mean tMaxN (33.5 minutes) and 60 minutes. All other conventions follow Fig 6.
The effect of soak time on time to first sighting (TFS)
The best model for TFS incorporated all three predictors with second-order interactions (depth = 3). The relative prediction error of 0.85, equated to an R-squared of only 15.3%, and the relative influence of soak was 58.9%, with a generally increasing relationship between soak time and mean predicted TFS (species and BRUVS pooled) (Fig 6A and 6B). The relative influence of abundance group was 25.8% and 15.3% for rarity. The slope in the response of TFS0.25 to soak time was generally positive, but relatively small over the range of soak times where 80% of the data occurred, i.e. ~ 9 minutes between 60 and 100 minutes (Fig 6B).
For BRUVS where elasmobranchs were classified as high in relative abundance, the TFS for a given soak time was observed faster than for BRUVS where SPUE of elasmobranchs were low to medium (Fig 6D and 6F). Likewise, predicted TFS for the common and uncommon rarity groups were earlier than for the rare and very rare groups (Fig 6C and 6E). The curves for each group ended below TFS = 60 minutes and majority below ~ 46 minutes, for the entire set of soak times up to 126 minutes (Fig 6F), although the uncertainty was high in the top 10 percent of soak times (Fig 6B).
The effect of soak time on time to MaxN (tMaxN)
The best model for tMaxN incorporated all three predictors (soak time, rarity and abundance groups with interaction depth = 2), with a high relative prediction error (0.94), equating to a very small R-squared of 6.3%. The model for tMaxN with soak time alone had a marginally lower R-squared of 6.1%. The relative influence soak time was 80.1%, with relative influence of 12.3% and 7.6% for rarity and abundance groups respectively (Fig 7A). There was a positive increase in tMaxN of about 8 minutes between soak times of 60 and 90 minutes, with greater uncertainty and fewer samples for soak times beyond 100 minutes (Fig 7B). Little difference in tMaxN was observed by rarity or abundance groups (Fig 7C–7F). Although soak time explained a small proportion of the overall model deviance, this analysis indicates tMaxN events were observed within 60 minutes of soak time, and the majority of the curves predicted tMaxN events occurred prior to a tMaxN ~ 46 minutes (Fig 7B, 7E and 7F).
Discussion
Optimal soak times for BRUVS are a trade-off between logistical constraints and statistical performance to detect differences in selected metrics between samples. Logistical challenges include balancing handling time of the hardware in the field, the need to maximise spatial replication whilst maintaining independence of samples, and the desire to minimise video interrogation times in the laboratory. Statistical constraints include the desire to reach asymptotes in species accumulation curves or species counts [35, 36], minimise the ratio of the standard error to the mean of the metrics of interest (‘precision’, sensu [37]), maximise the ‘power’ to detect differences amongst treatments [38, 39, 40], or minimise mis-classification rates of models on a validation data set [41]. All four of these statistical constraints have been examined for fish assemblages recorded by different BRUVS soak times (see [27, 42–46]). Yet, for elasmobranchs in coral reef environments, an optimal soak time has been lacking. Based on our results, first sightings occurred earlier for species groups with high occurrence, and on BRUVS with high elasmobranch SPUE, yet sightings of rarer species were not restricted to longer soak times. A soak time of 60–77 minutes recorded 78%-95% of all sighting records for reef elasmobranchs regardless of species rarity, and BRUVS-level abundance. These results confirm that a BRUVS soak time of ~ 77 minutes is optimal, while 60 minutes sufficiently captures the majority of sightings relevant for reef-associated elasmobranch community surveys.
Our ability to test the effect of varying soak times on these metrics for elasmobranchs extends research on the dynamics of visits to baited cameras by teleosts. Soak times for teleost community studies typically range from 30 to 90 minutes [47] and are most commonly 60 minutes by Australian standard [48, 49]. Thus most evaluations of optimal soak times have been confined to these limits, but results vary by habitat type. For example, A soak time of 15 minutes was the shortest duration able to capture abundance and length metrics for Hawaiian ‘bottom fish’, whilst 30 and 40 minute soak times generated data that did not significantly differ [45]. Another comparison of soak times (20, 40 and 60 minutes) in terms of species richness, MaxN, times to first arrival and times to MaxN values for Hawaiian bottom fish assemblages, found soak times of 60 minutes were considerably more powerful for detecting statistical differences in sessile ‘macropiscivores’ and ‘generalist macropiscivores’ [42]. Yet either 30 or 60 minute soak times were found to provide a reasonable estimate of rocky reef fish diversity (< 25 species) and relative abundance for comparative purposes [44]. Mean species richness, mean total MaxN and mean species-specific MaxN did not differ among soak times of 30, 60 and 90 minutes in estuarine habitats, but ‘precision’ rose with increasing sampling time for small fish on seagrass and sandy substrata [43]. Precision of species richness and abundance of a semi-pelagic fish assemblage improved when soak time was extended from 60 to 120 minutes on pelagic stereo-BRUVS, but not when soak times were increased to 180 minutes [27]. Therefore testing of effects of soak time in different habitats and for different species groups is crucial to interpretation of relative abundance data.
Studies using BRUVS to specifically identify and count elasmobranchs have used soak times of 60 minutes [14], 90 minutes [1, 13], 300 minutes [20], and up to 615 minutes [28]. Some of these studies offer inference about the timing of arrival of ‘rare’ species. For example, the time to first appearance of juvenile white sharks (Carcharodon carcharias) ranged from 15 to 299 minutes with a mean of 148 ± 15 minutes [20], and mean arrival times of the Greenland shark (Somniosus microcephalus) was found between 118 to 280 minutes [28]. These studies focus on species occurring in low numbers, which may require longer soak times. Our tests of the effects of soak time for reef-associated elasmobranchs with boosted regression trees indicated that soak time was not a major driver of events concerning times of arrival and attainment of MaxN, accounting for only 9% and 8% of the total predicted variation in TFS and tMaxN, respectively. This result likely reflects the high potential for sighting reef-associated elasmobranchs on BRUVS deployed in clear water reef habitats. There were obviously other major factors not analysed here that governed elasmobranch dynamics, such as micro-habitat type in the field of view, reef type and fishing pressure. These factors are important in other studies of reef-associated elasmobranch abundance [1, 16].
Our analyses showed a slight trend for delay in events for a given soak time at sites where overall elasmobranch abundance was classified as ‘low’ or ‘medium’. The times MaxN was observed differed little between abundance groups, but for sites characterised by high abundance (SPUE > 3 elasmobranchs), TFS occurred earlier. This was obvious within the first 60 minutes (94% of events observed), particularly for deployments where elasmobranchs were viewed immediately on settlement of the BRUVS on the seabed. The trend in TFS is in accordance with a presumption that arrival times to BRUVS will be faster at sites inhabited by more elasmobranchs. For example, mean arrival times of S. microcephalus was found to occur within the first half of long (up to 10 hour) soak times with a significant negative exponential relationship between first arrival times and total individuals sighted [28]. In contrast, a four-fold increase in relative abundance of the grey reef shark (Carcharhinus amblyrhynchos) on BRUVS was not accompanied by any significant change in TFS or tMaxN in the field of view [16]. Our comparison of soak times across a broad geographic range and groupings based on abundance groups, adds valuable information in this space.
Events (TFS and tMaxN) in the Global FinPrint datasets occurred, on average, within ~ 46 minutes in ABT models for all abundance groups, and the mean, global TFS and tMaxN occurred at 30.6 and 33.5 minutes across the variety of soak times recorded. The ECDFs indicated that approximately 83–94% of all TFS and 85–90% of all tMaxN events occurred by 60 minutes, and 95% by 77 minutes of soak time, for all abundance groups. Abundance groups also indicated that while first sightings of species occurred faster on BRUVS where elasmobranch SPUE was highest, ABT models predicted that all observations were observed within the first 60 minutes. These analyses imply that regardless of BRUVS-specific relative abundance, soak times of 60–77 minutes effectively survey elasmobranch assemblages of associated with shallow, coral reefs in the Pacific and Coral Triangle in daylight hours. These results may also apply to other coral reef systems, as the rates of elasmobranch sightings (SPUE) in our abundance groups are comparable to other studies, including the Tubbataha no-take Marine Reserve in the Philippines which was identified as a “hotspot” for elasmobranchs [50].
We found no evidence that rarer species were sighted only on the longest video records or that counts of their maximum number peaked later than those of more abundant species. Species groups classified as ‘very rare’ showed a delay of only ~10 minutes in the timing of tMaxN, but 78–88% of all TFS and 82–88% of all tMaxN events had occurred by 60 minutes, and 95% by 77 minutes soak time, for all species groups. This trend supports an expectation of slightly later arrival of rarer species in the field of view, as BRUVS deployments are more likely to coincidentally overlap with the presence of a common species than a rare one. A soak time of 60 minutes appeared effective in recording the majority of species and individuals, and ~ 77 minutes would be optimal to survey patterns of elasmobranch diversity for all species groups, regardless of rarity. The short delay in arrival of rarest species matches our understanding of the use of habitat by reef-associated elasmobranchs. Tropical ichthyofaunas, are composed of a core group of common species closely associated with coral reef habitat types, and a larger pool of rare, transient species that move through the reefal habitat or use it facultatively (see [51]). Most research has indicated the more common reef sharks (e.g. C. amblyrhynchos, C. melanopterus, T. obesus) rarely move over large distances and tend to have fairly consistent activity spaces on the scale of several kilometres [24, 52–55], and would be obvious members of the core, reef-associated elasmobranch fauna. In contrast, rarer species in our dataset (e.g. tiger sharks, Galeocerdo cuvier) may have species-specific habitat preferences, broader movements, or nocturnal foraging patterns that reduce their incidental interaction with the BRUVS field of view (e.g. [56, 57]). The single records of the spinner shark (C. brevipinna), white shark (Carcharodon carcharias), thresher shark (Alopias spp.) and the reticulate whipray (Himantura australis), amongst many other singletons, could be considered transients. While a ~ 77 minute soak time may be more optimal than 60 minutes for research solely targeting one of these rarer transients in this region, increased replication of 60 minute BRUVS or complementary sampling via other approaches such as 360 degree cameras [58, 59] would likely be beneficial.
While species richness varied among BRUVS, the majority of MaxN values were only 1 individual for most species on most BRUVS. This may be because separate visits of single, different, elasmobranchs in the field of view are not recognised in the conservatism of the MaxN metric [60]. Low MaxN suggests that reef-associated elasmobranchs are not typically found in large groups and/or very high local abundances. Most reef elasmobranchs are not known to aggregate as readily as some pelagic or inshore species (e.g. [61, 62]). Local abundances might also reflect fishing pressure, and most of the sites sampled here have at least some level of fishing pressure, and populations are thought to be in decline in some where targeted fishing of elasmobranchs occurs [26, 63, 64]. The corollary of MaxN = 1 is TFS = tMaxN, which limited the contrasts and ranges available in our separate analysis of these metrics. It also prevented species-specific modelling of accumulation of MaxN with soak time, which is known to be an indicator of relative abundance when combined with TFS [65]. We used MaxN in levels of SPUE for each BRUVS, and species rarity groupings, as predictors to explain the effects of soak time on the timing of events, and regardless of the reasons behind differences in relative abundance, 95% of TFS and tMaxN events were observed within 63–77 minutes soak time.
Conclusions and recommendations
We have concluded that a sampling time of 63–77 minutes is optimal for sighting individuals for defining relative abundance of rare to common species, at sites with varying levels of elasmobranch abundance, on shallow tropical reefs across a broad geographic area. Extending deployments and video analysis beyond 60 minutes could have undesirable logistical consequences and questionable benefits, and may not be necessary since 60 minutes appears sufficient to capture the majority of sighting events. Understanding the effectiveness of the commonly used 60 minute soak time is important for robust comparisons among tropical locations, especially for rare or uncommon species. We recommend that researchers routinely record all events relating to timing of first arrival and subsequent timing of increase in MaxN to gain the most insight into patterns of relative abundance and the dynamics of elasmobranchs (and teleosts) in the field of view of BRUVS. Unless specifically investigating a single very rare transient species in areas with few elasmobranchs, greater benefit is likely gained through increased replication of 60 minute BRUVS than extending soak times. These metrics are fundamental to addressing questions about the status of populations when using the BRUVS technique, and we provide a baseline for the areas within the regions sampled. We recommend pilot studies compare a range of sampling durations using these methods to gain an understanding of completeness of the biodiversity inventories assessed.
Supporting information
Percentage of BRUVS where elasmobranchs were recorded is shown.
(PDF)
(PNG)
Acknowledgments
We would like to thank all contributors to the Global FinPrint Project sampling in the Pacific and Coral Triangle, particularly CS Sherman, A Schlaff, S Bierwagen, S Lindfield, M Heithaus, E Harvey, J Goetze, M Rees, J Asher, S Moore, J Cramp, LM Sjamsul Qamar, E Clua, A Wirsing and L Vigliola. We would like to extend our gratitude to E Lédée, B D’Alberto and the Global FinPrint volunteer BRUVS analysts at James Cook University.
Data Availability
The data underlying this study are owned by a third party, the Global FinPrint Project (https://globalfinprint.org/). Data can be accessed by contacting Demian Chapman (FinPrint Principal Investigator, dchapman@fiu.edu) and Florida International University will facilitate access to data for the purpose of re-running analyses. Regional and global results from this data are yet to be published. The lead author (l.currey@aims.gov.au) can provide scripts to re-run all analyses if requested.
Funding Statement
This work is a contribution of the Global FinPrint Project, supported by Paul G Allen Philanthropies under grant number 11861, Pew Foundation, and Florida International University's Tropical Conservation Institute. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Acuña-Marrero D, Smith ANH, Salinas-de-León P, Harvey ES, Pawley MDM, Anderson MJ. Spatial patterns of distribution and relative abundance of coastal shark species in the Galapagos Marine Reserve. Mar Ecol Prog Ser. 2018;593:73–95. [Google Scholar]
- 2.Espinoza M, Cappo M, Heupel MR, Tobin AJ, Simpfendorfer CA. Quantifying shark distribution patterns and species-habitat associations: Implications of marine park zoning. PLoS ONE. 2014;9(9):e106885 10.1371/journal.pone.0106885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spaet JLY, Nanninga GB, Berumen ML. Ongoing decline of shark populations in the Eastern Red Sea. Biol Conserv. 2016;201:20–8. 10.1016/j.biocon.2016.06.018 WOS:000384782800003. [DOI] [Google Scholar]
- 4.Dulvy NK, Simpfendorfer CA, Davidson LNK, Fordham SV, Brautigam A, Sant G, et al. Challenges and priorities in shark and ray conservation. Curr Biol. 2017;27(11):R565–R72. 10.1016/j.cub.2017.04.038 WOS:000402814600040. [DOI] [PubMed] [Google Scholar]
- 5.Heupel MR, Williams AJ, Welch DJ, Ballagh A, Mapstone BD, Carlos G, et al. Effects of fishing on tropical reef associated shark populations on the Great Barrier Reef. Fish Res. 2009;95(2–3):350–61. 10.1016/j.fishres.2008.10.005 WOS:000262350300025. [DOI] [Google Scholar]
- 6.Vianna GMS, Meekan MG, Ruppert JLW, Bornovski TH, Meeuwig JJ. Indicators of fishing mortality on reef-shark populations in the world's first shark sanctuary: the need for surveillance and enforcement. Coral Reefs. 2016;35(3):973–7. 10.1007/s00338-016-1437-9 WOS:000382019400025. [DOI] [Google Scholar]
- 7.Mitchell JD, McLean DL, Collin SP, Langlois TJ. Shark depredation in commercial and recreational fisheries. Rev Fish Biol Fish. 2018;28(4):715–48. 10.1007/s11160-018-9528-z WOS:000451724000003. [DOI] [Google Scholar]
- 8.Harvey ES, Santana-Garcon J, Goetze JS, Saunders BJ, Cappo M. The use of stationary underwater video for sampling sharks In: Carrier J, Heithaus MS, CA, editors. Shark Research: emerging technologies and applications for the field and laboratory. Boca Raton: CRC Press Taylor and Francis; 2019. [Google Scholar]
- 9.Graham NAJ, Spalding MD, Sheppard CRC. Reef shark declines in remote atolls highlight the need for multi-faceted conservation action. Aquat Conserv: Mar Freshwat Ecosyst. 2010;20(5):543–8. 10.1002/aqc.1116 WOS:000280636900007. [DOI] [Google Scholar]
- 10.McCauley DJ, McLean KA, Bauer J, Young HS, Micheli F. Evaluating the performance of methods for estimating the abundance of rapidly declining coastal shark populations. Ecol Appl. 2012;22(2):385–92. 10.1890/11-1059.1 [DOI] [PubMed] [Google Scholar]
- 11.Ward-Paige CA, Mora C, Lotze HK, Pattengill-Semmens C, McClenachan L, Arias-Castro E, et al. Large-scale absence of sharks on reefs in the greater-Caribbean: A footprint of human pressures. PLoS ONE. 2010;5(8):e11968 10.1371/journal.pone.0011968 WOS:000280605400010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rizzari JR, Frisch AJ, Magnenat KA. Diversity, abundance, and distribution of reef sharks on outer-shelf reefs of the Great Barrier Reef, Australia. Mar Biol. 2014;161(12):2847–55. 10.1007/s00227-014-2550-3 WOS:000345050800011. [DOI] [Google Scholar]
- 13.Brooks EJ, Sloman KA, Sims DW, Danylchuk AJ. Validating the use of baited remote underwater video surveys for assessing the diversity, distribution and abundance of sharks in the Bahamas. Endanger Species Res. 2011;13(3):231–43. [Google Scholar]
- 14.De Vos L, Watson RGA, Gotz A, Attwood CG. Baited remote underwater video system (BRUVs) survey of chondrichthyan diversity in False Bay, South Africa. Afr J Mar Sci. 2015;37(2):209–18. 10.2989/1814232x.2015.1036119 WOS:000358666800009. [DOI] [Google Scholar]
- 15.Jabado RW, Kyne PM, Pollom RA, Ebert DA, Simpfendorfer CA, Ralph GM, et al. Troubled waters: Threats and extinction risk of the sharks, rays and chimaeras of the Arabian Sea and adjacent waters. Fish and Fisheries. 2018;19(6):1043–62. 10.1111/faf.12311 [DOI] [Google Scholar]
- 16.Speed CW, Cappo M, Meekan MG. Evidence for rapid recovery of shark populations within a coral reef marine protected area. Biol Conserv. 2018;220:308–19. 10.1016/j.biocon.2018.01.010 WOS:000429765000034. [DOI] [Google Scholar]
- 17.Cappo M, Harvey E, Shortis M, editors. Counting and measuring fish with baited video techniques—an overview. Cutting-edge technologies in fish and fisheries science Australian Society for Fish Biology Workshop Proceedings; 2006 28–29 August; Hobart, Tasmania: Australian Society for Fish Biology; 2007.
- 18.Asher J, Williams ID, Harvey ES. An assessment of mobile predator populations along shallow and mesophotic depth gradients in the Hawaiian Archipelago. Sci Rep. 2017;7:18 10.1038/s41598-017-00050-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetze JS, Langlois TJ, McCarter J, Simpfendorfer CA, Hughes A, Leve JT, et al. Drivers of reef shark abundance and biomass in the Solomon Islands. PLoS ONE. 2018;13(7):e0200960 10.1371/journal.pone.0200960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harasti D, Lee KA, Laird R, Bradford R, Bruce B. Use of stereo baited remote underwater video systems to estimate the presence and size of white sharks (Carcharodon carcharias). Mar Freshw Res. 2017;68(7):1391–6. 10.1071/mf16184 WOS:000404481900019. [DOI] [Google Scholar]
- 21.White J, Simpfendorfer CA, Tobin AJ, Heupel MR. Application of baited remote underwater video surveys to quantify spatial distribution of elasmobranchs at an ecosystem scale. J Exp Mar Biol Ecol. 2013;448:281–8. 10.1016/j.jembe.2013.08.004 [DOI] [Google Scholar]
- 22.Robbins WD, Hisano M, Connolly SR, Choat JH. Ongoing collapse of coral-reef shark populations. Curr Biol. 2006;16(23):2314–9. 10.1016/j.cub.2006.09.044 WOS:000242642300022. [DOI] [PubMed] [Google Scholar]
- 23.Heupel MR, Papastamatiou YP, Espinoza M, Green ME, Simpfendorfer CA. Reef shark science—Key questions and future directions. Front Mar Sci. 2019;6 10.3389/fmars.2019.00012 WOS:000457827300001. [DOI] [Google Scholar]
- 24.Espinoza M, Heupel MR, Tobin AJ, Simpfendorfer CA. Residency patterns and movements of grey reef sharks (Carcharhinus amblyrhynchos) in semi-isolated coral reef habitats. Mar Biol. 2014;162(2):343–58. 10.1007/s00227-014-2572-x WOS:000348564300010. [DOI] [Google Scholar]
- 25.Goetze JS, Fullwood LAF. Fiji’s largest marine reserve benefits reef sharks. Coral Reefs. 2013;32(1):121–5. 10.1007/s00338-012-0970-4 [DOI] [Google Scholar]
- 26.Nadon MO, Baum JK, Williams ID, McPherson JM, Zgliczynski BJ, Richards BL, et al. Re-creating missing population baselines for Pacific reef sharks. Conserv Biol. 2012;26(3):493–503. 10.1111/j.1523-1739.2012.01835.x WOS:000304135300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santana-Garcon J, Newman SJ, Langlois TJ, Harvey ES. Effects of a spatial closure on highly mobile fish species: an assessment using pelagic stereo-BRUVs. J Exp Mar Biol Ecol. 2014;460:153–61. 10.1016/j.jembe.2014.07.003 [DOI] [Google Scholar]
- 28.Devine BM, Wheeland LJ, Fisher JAD. First estimates of Greenland shark (Somniosus microcephalus) local abundances in Arctic waters. Sci Rep. 2018;8(1):974 10.1038/s41598-017-19115-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cappo M, Speare P, De'ath G. Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. J Exp Mar Biol Ecol. 2004;302(2):123–52. 10.1016/j.jembe.2003.10.006 ISI:000221071500001. [DOI] [Google Scholar]
- 30.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. Available: https://www.R-project.org/ [Google Scholar]
- 31.De'ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88(1):243–51. 10.1890/0012-9658(2007)88[243:btfema]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 32.De'ath G. Modified version of Greg Ridgeway's abt package. abt: Aggregated Boosted Trees, R package version 4.0. 2017.
- 33.Schonlau M. Boosted regression (Boosting): An introductory tutorial and a stata plugin. The Stata Journal. 2005;5(3):330–54. 10.1177/1536867X0500500304 [DOI] [Google Scholar]
- 34.Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77(4):802–13. 10.1111/j.1365-2656.2008.01390.x WOS:000256539800020. [DOI] [PubMed] [Google Scholar]
- 35.De Vos L, Götz A, Winker H, Attwood CG. Optimal BRUVs (baited remote underwater video system) survey design for reef fish monitoring in the Stilbaai Marine Protected Area. Afr J Mar Sci. 2014;36(1):1–10. 10.2989/1814232X.2013.873739 [DOI] [Google Scholar]
- 36.Gotelli NJ, Colwell RK. Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett. 2001;4(4):379–91. 10.1046/j.1461-0248.2001.00230.x WOS:000170417800015. [DOI] [Google Scholar]
- 37.Cochran WG, Cox GM. Experimental Designs. 2nd ed ed. New York: John Wiley and Sons; 1957. [Google Scholar]
- 38.Faul F, Erdfelder E, Buchner A, Lang A-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 39.Bicknell AWJ, Sheehan EV, Godley BJ, Doherty PD, Witt MJ. Assessing the impact of introduced infrastructure at sea with cameras: A case study for spatial scale, time and statistical power. Mar Environ Res. 2019;147:126–137. 10.1016/j.marenvres.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 40.Schramm KD, Harvey ES, Goetze JS, Travers MJ, Warnock B, Saunders BJ. A comparison of stereo-BRUV, diver operated and remote stereo-video transects for assessing reef fish assemblages. J Exp Mar Biol Ecol. 2020;524:151273. [Google Scholar]
- 41.Picard RR, Cook R. Cross-validation of regression models. J Am Stat Assoc. 1984;79(387):575–83. 10.1080/01621459.1984.10478083 [DOI] [Google Scholar]
- 42.Asher J. A deeper look at Hawaiian coral reef fish assemblages: A comparison of survey approaches and assessments of shallow to mesophotic communities [PhD Thesis]. Western Australia: Curtin University; 2017.
- 43.Gladstone W, Lindfield S, Coleman M, Kelaher B. Optimisation of baited remote underwater video sampling designs for estuarine fish assemblages. J Exp Mar Biol Ecol. 2012;429:28–35. 10.1016/j.jembe.2012.06.013 WOS:000308282900005. [DOI] [Google Scholar]
- 44.Harasti D, Malcolm H, Gallen C, Coleman MA, Jordan A, Knott NA. Appropriate set times to represent patterns of rocky reef fishes using baited video. J Exp Mar Biol Ecol. 2015;463:173–80. 10.1016/j.jembe.2014.12.003 WOS:000348628400022. [DOI] [Google Scholar]
- 45.Misa W, Richards BL, DiNardo GT, Kelley CD, Moriwake VN, Drazen JC. Evaluating the effect of soak time on bottomfish abundance and length data from stereo-video surveys. J Exp Mar Biol Ecol. 2016;479:20–34. 10.1016/j.jembe.2016.03.001 WOS:000375365900003. [DOI] [Google Scholar]
- 46.Unsworth RKF, Peters JR, McCloskey RM, Hinder SL. Optimising stereo baited underwater video for sampling fish and invertebrates in temperate coastal habitats. Estuar Coast Shelf Sci. 2014;150:281–7. 10.1016/j.ecss.2014.03.020 WOS:000346217800009. [DOI] [Google Scholar]
- 47.Whitmarsh SK, Fairweather PG, Huveneers C. What is Big BRUVver up to? Methods and uses of baited underwater video. Rev Fish Biol Fish. 2016;27:53–73. [Google Scholar]
- 48.Harvey E, McLean D, Frusher S, Haywood M, Newman S, Williams A. The use of BRUVs as a tool for assessing marine fisheries and ecosystems: a review of the hurdles and potential. University of Western Australia, 2013. [Google Scholar]
- 49.Langlois T, Williams J, Monk J, Bouchet P, Currey L, Goetze J, et al. Marine sampling field manual for benthic stereo BRUVS (Baited Remote Underwater Videos) In: Przeslawski R, Foster S, editors. Field manuals for marine sampling to monitor Australian waters: National Environmental Science Programme (NESP); 2018. p. 82–104. [Google Scholar]
- 50.Murray R, Conales S, Araujo G, Labaja J, Snow SJ, Pierce SJ, et al. Tubbataha Reefs Natural Park: the first comprehensive elasmobranch assessment reveals global hotspot for reef sharks. J Asia Pac Biodivers. 2019;12(1):49–56. 10.1016/j.japb.2018.09.009 [DOI] [Google Scholar]
- 51.Magurran AE, Henderson PA. Explaining the excess of rare species in natural species abundance distributions. Nature. 2003;422(6933):714–6. 10.1038/nature01547 WOS:000182272300039. [DOI] [PubMed] [Google Scholar]
- 52.Barnett A, Abrantes KG, Seymour J, Fitzpatrick R. Residency and spatial use by reef sharks of an isolated seamount and its implications for conservation. PLoS ONE. 2012;7(5):e36574 10.1371/journal.pone.0036574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bond ME, Babcock EA, Pikitch EK, Abercrombie DL, Lamb NF, Chapman DD. Reef sharks exhibit site-fidelity and higher relative abundance in marine reserves on the Mesoamerican Barrier Reef. PLoS ONE. 2012;7(3):e32983 10.1371/journal.pone.0032983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chin A, Tobin AJ, Heupel MR, Simpfendorfer CA. Population structure and residency patterns of the blacktip reef shark Carcharhinus melanopterus in turbid coastal environments. J Fish Biol. 2013;82(4):1192–210. 10.1111/jfb.12057 WOS:000317291700006. [DOI] [PubMed] [Google Scholar]
- 55.Papastamatiou Y, Itano D, J. Dale J, Meyer C, N. Holland K. Site fidelity and movements of sharks associated with ocean-farming cages in Hawaii. Mar Freshw Res. 2011;61(12):1366–75. 10.1071/MF10056 [DOI] [Google Scholar]
- 56.Heupel MR, Lédée EJI, Simpfendorfer CA. Telemetry reveals spatial separation of co-occurring reef sharks. Mar Ecol Prog Ser. 2018;589:179–92. [Google Scholar]
- 57.Meekan MM, Cappo MM, Carleton JJ, Marriott R. Surveys of shark and fin-fish abundance on reefs within the MOU74 Box and Rowleys Shoals using baited remote underwater video systems. Australian Institute of Marine Science Townsville, Australian Government Department of the Environment and Heritage; 2006. [Google Scholar]
- 58.Kilfoil JP, Wirsing AJ, Campbell MD, Kiszka JJ, Gastrich KR, Heithaus MR, et al. Baited Remote Underwater Video surveys undercount sharks at high densities: insights from full-spherical camera technologies. Mar Ecol Prog Ser. 2017;585:113–21. [Google Scholar]
- 59.Whitmarsh SK, Huveneers C, Fairweather P, G. What are we missing? Advantages of more than one viewpoint to estimate fish assemblages using baited video. Royal Society Open Science. 2018;5(5):171993 10.1098/rsos.171993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sherman CA, Chin A, Heupel MR, Simpfendorfer CA. Are we underestimating elasmobranch abundances on baited remote underwater video systems (BRUVS) using traditional metrics? J Exp Mar Biol Ecol. 2018;503:80–5. [Google Scholar]
- 61.Blaylock RA. A massive school of cownose rays, Rhinoptera bonasus (Rhinopteridae), in lower Chesapeake Bay, Virginia. Copeia. 1989;1989(3):744–8. 10.2307/1445506 [DOI] [Google Scholar]
- 62.Litvinov F. Fish visitors to seamounts: Aggregations of large pelagic sharks above seamounts In: Pitcher TJ, Morato T, Hart PJB, Clark MR, Haggan N, Santos RS, editors. Seamounts: ecology, fisheries & conservation. Oxford: Blackwell Publishing; 2007. p. 202–206. [Google Scholar]
- 63.Blaber SJM, Dichmont CM, White W, Buckworth R, Sadiyah L, Iskandar B, et al. Elasmobranchs in southern Indonesian fisheries: the fisheries, the status of the stocks and management options. Rev Fish Biol Fish. 2009;19(3):367–91. 10.1007/s11160-009-9110-9 [DOI] [Google Scholar]
- 64.Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, et al. Extinction risk and conservation of the world’s sharks and rays. eLife. 2014;3:e00590 10.7554/eLife.00590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cappo M, Harvey E, Shortis M. Counting and measuring fish with baited video techniques—an overview. Cutting-edge technologies in fish and fisheries science Australian Society for Fish Biology Workshop Proceedings; 2006 28–29 August; Hobart, Tasmania: Australian Society for Fish Biology; 2007.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Percentage of BRUVS where elasmobranchs were recorded is shown.
(PDF)
(PNG)
Data Availability Statement
The data underlying this study are owned by a third party, the Global FinPrint Project (https://globalfinprint.org/). Data can be accessed by contacting Demian Chapman (FinPrint Principal Investigator, dchapman@fiu.edu) and Florida International University will facilitate access to data for the purpose of re-running analyses. Regional and global results from this data are yet to be published. The lead author (l.currey@aims.gov.au) can provide scripts to re-run all analyses if requested.