Abstract
RNA technology is transforming life science research and medicine, but many applications are limited by the accessibility, cost, efficacy, and tolerability of delivery systems. Here we report the first members of a new class of dynamic RNA delivery vectors, oligo(serine ester)-based charge-altering releasable transporters (Ser-CARTs). Composed of lipid-containing oligocarbonates and cationic oligo(serine esters), Ser-CARTs are readily prepared (one flask) by a mild ring-opening polymerization using thiourea anions and, upon simple mixing with mRNA, readily form complexes that degrade to neutral serine-based products, efficiently releasing their mRNA cargo. mRNA/Ser-CART transfection efficiencies of >95% are achieved in vitro. Intramuscular or intravenous (iv) injections of mRNA/Ser-CARTs into living mice result in in vivo expression of a luciferase reporter protein, with spleen localization observed after iv injection.
Messenger RNA (mRNA) is advancing fundamental research and medicine through its ability to induce the transient catalytic expression of target proteins in vitro, in vivo, and ex vivo. Applications of mRNA include protein replacement therapy, gene editing, vaccination, and cancer immunotherapy.1 However, the challenge of developing synthetically accessible, affordable, safe, and effective delivery vectors that extracellularly protect and intracellularly release mRNA have hampered applications, driving demand for improved delivery systems.2,3 Current delivery strategies focus on mechanical methods and viral and nonviral vectors.4–6 Mechanical methods that temporarily render the cellular membrane permeable are limited to accessible tissues and ex vivo techniques, often suffer from poor cell viability, and encounter scalability challenges.7 Viral vectors offer broader administration options but are coupled with cost and immunogenicity concerns and cargo size limitations.8,9 These restrictions have stimulated interest in nonviral vectors, typically lipid nanoparticles and cationic polymers that form electrostatic complexes with polyanionic nucleic acids.7,10–16 Despite advances in nonviral vectors, challenges with accessibility, formulation, efficacy, tolerability, and targetability have prompted the search for improved delivery systems, with a particular emphasis on degradable vectors.17–22
We recently reported a new class of synthetic biodegradable gene delivery materials, dubbed charge-altering releasable transporters (CARTs) (e.g., 1).23 These first-generation CARTs 1 are amphipathic diblock co-oligomers consisting of a lipophilic oligocarbonate sequence followed by a cationic morpholinone-derived α-amino ester backbone. These transporters operate through an unprecedented mechanism in which the cationic oligo(α-amino ester) block electrostatically complexes the anionic nucleic acid cargo and subsequently undergoes an irreversible rearrangement to neutral small molecules (e.g., 2), resulting in cargo release (Scheme 1A). While morpholinone-based CARTs 1 are effective for mRNA and plasmid delivery in many cell lines, including Tlymphocytes, these first-generation transporters represent only one subclass of a potentially broad and unexplored platform of charge-altering vectors for gene delivery.23–27
Scheme 1. mRNA Release Mechanisms of (A) Morpholinone-Derived CARTs 123 and (B) Ser-CARTs 3a.
aBoth systems utilize activated α-amino esters, with oligo(serine esters) rearranging to neutral serine-based products via O−N acyl shifts.
Here we report the synthesis and evaluation of a new class of charge-altering vectors based on oligo(serine esters), denoted as Ser-CARTs (3). Differing from CARTs with oligocationic backbones, Ser-CARTs incorporate a charge-altering side-chain amine to complex the mRNA cargo and produce neutral serine-based byproducts upon degradation, resulting in mRNA release (Scheme 1B). In addition to their biocompatibility and expected rearrangement into peptides 4,28–30 oligo(serine esters) were selected for study over other degradable amine-functionalized polyesters18,31 because of their activating α-amino ester motif. Studies suggest that the rapid rearrangement of morpholinone-derived oligo(α-amino esters) is partially due to the activation of a backbone ammonium group positioned α to the ester repeating unit.23 In this study, we synthesized and characterized the degradation of side-chain ammonium-containing oligo(serine esters), which are structural isomers of oligo(serine amides) 4.32 We demonstrate that Ser-CARTs are readily formed (one flask) and efficiently deliver mRNA in cultured cells and live mice.
To study Ser-CARTs, we developed a polymerization method that avoids the control issues and harsh conditions previously reported for oligo(serine ester) synthesis.30,33–37 Our procedure benefits from the commercial availability of the N-trityl-L-serine lactone monomer (serine lactone) and an organocatalytic ring-opening polymerization (OROP)38–43 strategy, utilizing a thiourea anion catalyst recently developed for the OROP of lactones and cyclic carbonates (Figure 1).44
Figure 1.
Poly(serine ester) synthesis.
Specifically, we found that the OROP of serine lactone with 1(3,5-bis(trifluoromethyl)phenyl)-3-cyclohexylthiourea (TU) and potassium hydride (KH) in the presence of an alcohol initiator proceeds at room temperature in hours to generate trityl-protected poly(serine esters) 5a−9a with predictable molecular weights (Mn = 7−17 kDa) and narrow dispersities (Đ = 1.11−1.24), avoiding the previously reported multimodal distributions (Tables 1 and S1).35,36 After polymer isolation, trityl groups are removed using 1% TFA to yield cationic poly(serine esters) 5b−9b with no significant decrease in molecular weight, as determined by end-group analysis (Figure S1). This facile controlled polymerization of serine lactone is noteworthy, as prior reports suggested that β-lactone OROP is inefficient.45–47
Table 1.
Poly(serine ester) Characterizationa
| ROH | [M]b | [M]/[ROH] | conv.(%)c | DPd | Mn(kDa)e | Đ | |
|---|---|---|---|---|---|---|---|
| 5a | BnOH | 1.0 | 50 | >95 | 62 | 16.7 | 1.17 |
| 6a | BnOH | 0.5 | 50 | 89 | 47 | 12.6 | 1.24 |
| 7a | BnOH | 1.5 | 50 | 93 | 61 | 15.6 | 1.20 |
| 8a | BnOH | 1.0 | 20 | >95 | 20 | 7.7 | 1.21 |
| 9a | PyOH | 1.0 | 50 | >95 | 49 | 15.8 | 1.21 |
Polymerizations were run for 4 h at room temperature in toluene.
Serine lactone molar concentration.
Determined by NMR spectroscopy.
Degree of polymerization, determined by NMR end-group analysis after dialysis.
Determined by gel-permeation chromatography.
As studies indicate that the ring opening of β-lactones can occur by two mechanisms,48 we performed the stoichiometric ring opening of serine lactone with 1 equiv of benzyl alcohol using the KH/TU catalyst. Analysis of the resulting product by HMBC NMR indicated that ring opening proceeded through acylation of benzyl alcohol by the lactone to generate the alkoxy-terminated benzyl serine ester rather than by nucleophilic attack at the β-carbon48 to generate the carboxylate (Figure S2).
Having developed an effective poly(serine ester) synthesis, we next investigated whether poly(serine esters) would rearrange in biologically relevant pH regimes in the absence of an mRNA cargo. While uncomplexed poly(serine ester) 9b (degree of polymerization (DP) = 47) is stable under its generation conditions, at pH 7.4 it begins to degrade in minutes, producing in hours the known rearrangement product, oligo(serine amide) 4,28,29 and also a previously unreported product, dimerized serine diketopiperazine (DKP, 10), as confirmed by NMR and LC−MS analyses (Figure 2A; also see the Supporting Information). Analysis of the degradation 9b at pH 7.4 revealed that the DKP yield increased over 24 h, resulting in final yields of 55% DKP and 45% oligo(serine amides) of various lengths (Figure 2B). While prior reports indicated that the aqueous degradation of poly(serine esters) generates poly(serine amides) by a series of O-to-N acyl shifts,28,29 our studies uncovered DKP as a significant serine-based byproduct.
Figure 2.
(A) Proposed rearrangement of poly(serine esters). (B) Time-dependent yield of DKP 10 when 9b was subjected to pH 7.4-buffered D2O at room temperature (trial 1 ■, trial 2 ●, trial 3 ▲).
We propose that the degradation of poly(serine esters) to generate DKP 10 follows a charge-altering mechanism (Figure 2A) related to that proposed for the degradation of morpholinone-based poly(α-amino esters) (Figure S3) but now involving a primary side-chain amine.23,28,29 For pH values at which some of the pendant ammonium groups are deprotonated, nucleophilic attack of the resultant primary amine on an adjacent H-bond-activated ester carbonyl (five-membered O−N acyl shift) would generate an amide and contract the polymer backbone, positioning the proximal amine for a six-membered O−N acyl shift to liberate DKP. Importantly, the formation of the expected oligo(serine amide) 4 and the newly observed DKP 10 provide charge-altering transformations from cationic α-amino esters to neutral β-hydroxyamides that are critical for CART-mediated mRNA complexation, delivery, and release.23
Having shown that uncomplexed poly(serine esters) degrade to neutral products, we explored the use of Ser-CARTs for polyanion complexation and delivery, focusing on mRNA. As lipid domains are vital in polyanion delivery vehicles,10,24,49–53 we generated a series of amphiphilic diblock co-oligomers composed of a dodecyl (C12)- or oleyl (C18)-modified oligocarbonate sequence and a cationic oligo(serine ester) sequence from carbonate monomer 11 or 12 and serine lactone, respectively, using our thiourea anion catalyst system. Co-oligomers 13a−16a (R′ = dodecyl: n = 10, m = 15; n = 10, m = 17; n = 19, m = 17; R′ = oleyl: n = 15, m = 38) were synthesized by a straightforward three-component, step-economical (one-flask) procedure using alcohol initiators (Figure 3 and Table S2). Deprotection of 13a−16a with 1% TFA afforded cationic Ser-CARTs 13b−16b.
Figure 3.
Synthesis of Ser-CARTs 13b−16b.
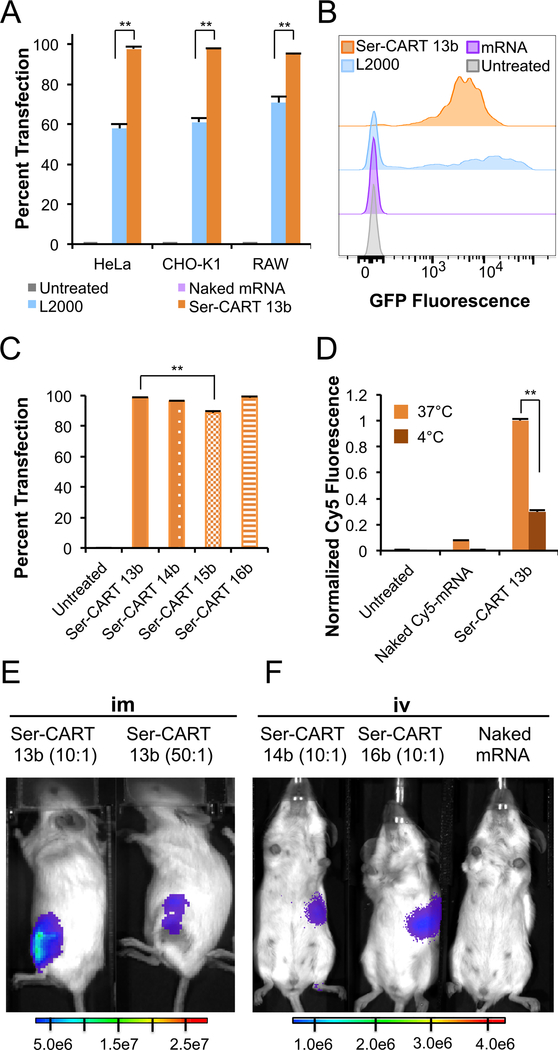
To assess the efficacy of Ser-CARTs for mRNA delivery and expression, we investigated the transfection of cultured cells using Ser-CARTs complexed with mRNA encoding green fluorescent protein (EGFP) and analyzed by flow cytometry the total fluorescence and percentage of cells transfected. Notably, mRNA/Ser-CART polyplexes are produced by simple mixing of mRNA with Ser-CARTs. To optimize the in vitro formulation, we screened charge ratios of 5:1 to 100:1 (cation:anion (+/−)) using dodecyl-Ser-CART 13b formulated with EGFP mRNA for delivery into HeLa cells. The highest fluorescence was observed at a charge ratio of 50:1 (+/−) under serum-free conditions, and the intracellular EGFP expression was confirmed by confocal and fluorescence microscopy (Figures S4–S6). Using this charge ratio, we compared EGFP mRNA delivery using Ser-CART 13b to that using the commercial transfection reagent Lipofectamine 2000 (L2000) as a positive control and to that using naked EGFP mRNA. Significantly, 13b-mediated EGFP mRNA delivery resulted in highly efficient (>95%) transfection in multiple cell lines (HeLa, CHO-K1, Raw-Blue), markedly outperforming L2000 (55−71% transfection) (Figure 4A). The transfection efficiency of 13b is consistent with that of morpholinone-based CARTs 1, highlighting the importance of the charge-altering block for mRNA delivery (Figure S7). Additionally, greater fluorescence was observed with 13b-mediated EGFP mRNA delivery than with L2000-mediated delivery in HeLa cells (Figure 4B). Ser-CART 14b also exhibited >95% transfection for EGFP mRNA delivery in HeLa cells, suggesting that the initiator does not significantly influence transfection, as pyrene butanol was used for 13b and benzyl alcohol for 14b (Figure 4C). In contrast to dodecyl-Ser-CARTs 13b and 14b, lower transfection levels were observed with more lipid-rich 15b. Oleyl-Ser-CART 16b also resulted in >95% transfection. Ser-CARTs retain these high transfection efficiencies when stored at 0 °C under nitrogen (Figure S8). Notably, formulation of EGFP mRNA with poly(serine ester)47 9b or DKP 10 resulted in negligible fluorescence (Figure S9). Importantly, DKP 10 was found to be nontoxic at concentrations up to 500 μM in HeLa cells (Figure S10).
Figure 4.
Delivery of mRNA/Ser-CARTs in vitro and in vivo. mRNA/ Ser-CARTs were formulated at a 50:1 (+/−) ratio unless otherwise specified. (A) Percent transfection of HeLa, CHO-K1, or Raw-Blue (RAW) cells using L2000 or EGFP mRNA/13b. (B) Histogram of HeLa cells treated with naked EGFP mRNA, L2000, or mRNA/13b. (C) Percent transfection of HeLa cells treated with EGFP mRNA/ 13b−16b. (D) Cy5-labeled mRNA/13b uptake into HeLa cells after 1 h incubation at 4 or 37 °C. (E, F) Representative bioluminescence images of mice 7 h after (E) im injection with fLuc mRNA/13b at 10:1 (+/−) (left, n = 4) or 50:1 (+/−) (right, n = 2) and (F) iv injection with fLuc mRNA/14b (left, n = 4), fLuc mRNA/16b (middle, n = 3), both at 10:1 (+/−), or naked fLuc mRNA (right, n = 1). Results in (A), (C), and (D) are averages of three or more experiments. Error bars represent ±SD. **, p < 0.0004.
We next explored the temperature-dependent uptake of Ser-CARTs. HeLa cells treated with Cy5-labeled mRNA/13b at 4 °C resulted in a 70% reduction in Cy5 fluorescence relative to cells incubated at 37 °C, indicating mainly endocytic uptake of the polyplexes, as incubation at 4 °C inhibits endocytosis (Figure 4D).54
Analysis of the EGFP mRNA/Ser-CART polyplexes by dynamic light scattering indicated hydrodynamic diameters of <190 nm (dodecyl-based 13b, ∼154 nm; oleyl-based 16b, ∼174 nm), which are significantly smaller than those of morpholinone-based CARTs (∼250 nm) (Table S3).23 Zeta potential measurements were used to study the time-dependent surface charge. When added to RNase-free water, mRNA/Ser-CARTs were initially positive (13b, 37 ± 6 mV; 16b, 52 ± 10 mV) but over 1 h became negative (13b, −20 ± 7 mV; 16b, −15 ± 5 mV), consistent with rearrangement of the cationic oligo(serine ester) block to neutral products (Figure S11).
Encouraged by these in vitro studies, we explored the in vivo utility of Ser-CARTs for mRNA delivery using two different modes of administration in female BALB/c mice. Luciferase (fLuc)-coding mRNA was chosen as a model reporter gene since luciferase expression can be quantitatively monitored in real time in living mice.55,56 After fLuc mRNA delivery in vitro was confirmed (Figure S12), fLuc mRNA/13b polyplexes were administered via intramuscular (im) injection into mice at a 10:1 or 50:1 (+/−) ratio, and expression was visualized after 7 h by bioluminescence imaging (Figure 4E).25 Both conditions resulted in protein expression, but mice treated at the lower 10:1 (+/−) ratio resulted in enhanced luciferase expression (Figure S13). At the 10:1 (+/−) ratio, fLuc mRNA/14b or 16b polyplexes administered to mice via intravenous (iv) tail vein injection resulted in luciferase expression localized in the spleen, a target organ for several therapeutic indications (Figures 4F and S14). Importantly, mRNA/Ser-CARTs formulated at the 10:1 (+/−) ratio resulted in improved cell viability (78−87% relative to untreated HeLa cells) compared with 50:1 (+/−) in vitro (Figure S15).
In conclusion, mRNA delivery with the readily synthesized (one flask) Ser-CARTs results in efficient transfection and high protein expression in vitro and in vivo. Further benefits of Ser-CARTs over first-generation CARTs include the degradation of the oligo(serine ester) block at biological pH into serine peptides (oligo(serine amides) and DKP), the commercial availability of the monomer, and the smaller size of the polyplexes. The accessibility, tunability, effectiveness, and organ selectivity of mRNA/Ser-CART polyplexes bode well for their use in biomedical research and therapeutic applications. Furthermore, this study establishes the generality of charge-altering architectures for polyanion delivery. We are currently exploring Ser-CARTs for targeting, cotransfections, and clinical indications with an initial emphasis on vaccination and immunotherapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the NSF (CHE-1607092 to R.M.W. and CHE848280 to P.A.W.), the NIH (CA031845 to P.A.W.), and the Child Health Research Institute at Stanford University and the Stanford SPARK Translational Research Program (R.M.W and P.A.W). R.L.M. was supported by an Abbott Laboratories Stanford Graduate Fellowship. C.R.T. acknowledges support from the National Institute of Biomedical Imaging and Bioengineering of the NIH under Award F32EB021161. Flow cytometry data were collected in the Stanford Shared FACS Facility using NIH S10 Shared Instrument Grant S10RR027431-01. We gratefully acknowledge Dr. Timothy Blake, Dr. Colin McKinlay, and Prof. Ronald Levy for helpful discussions, B. K. Marshall for fluorescence microscopy, Prof. Lynette Cegelski for use of tissue-culture equipment, and Prof. Richard Zare for use of the Malvern Zetasizer.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.9b03154.
Experimental details, including representative spectra and biological assays (PDF)
REFERENCES
- (1).Sahin U; Kariko K; Tú ¨reci, Ö. mRNA-based therapeutics∿ developing a new class of drugs. Nat. Rev. Drug Discovery 2014, 13, 759–780. [DOI] [PubMed] [Google Scholar]
- (2).Stanton MG Current Status of Messenger RNA Delivery Systems. Nucleic Acid Ther. 2018, 28, 158–165. [DOI] [PubMed] [Google Scholar]
- (3).Dowdy SF Overcoming cellular barriers for RNA therapeutics. Nat. Biotechnol 2017, 35, 222–229. [DOI] [PubMed] [Google Scholar]
- (4).Gresham TL; Jansen JE; Shaver FW; Gregory JT βPropiolactone. II. Reactions with Salts of Inorganic Acids. J. Am. Chem. Soc 1948, 70 (3), 999–1001. [Google Scholar]
- (5).Hajj KA; Whitehead KA Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater 2017, 2, 17056. [Google Scholar]
- (6).Kaczmarek JC; Kowalski PS; Anderson DG Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med. 2017, 9 (1), 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Guan S; Rosenecker J Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017, 24, 133–143. [DOI] [PubMed] [Google Scholar]
- (8).Cotrim AP; Baum BJ Gene Therapy: Some History, Applications, Problems, and Prospects. Toxicol. Pathol 2008, 36, 97–103. [DOI] [PubMed] [Google Scholar]
- (9).Yacoub N. a.; Romanowska M; Haritonova N; Foerster J Optimized production and concentration of lentiviral vectors containing large inserts. J. Gene Med 2007, 9, 579–584. [DOI] [PubMed] [Google Scholar]
- (10).Fenton OS; Kauffman KJ; McClellan RL; Appel EA; Dorkin JR; Tibbitt MW; Heartlein MW; DeRosa F; Langer R; Anderson DG Bioinspired Alkenyl Amino Alcohol Ionizable Lipid Materials for Highly Potent In Vivo mRNA Delivery. Adv. Mater 2016, 28, 2939–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wender PA; Huttner MA; Staveness D; Vargas JR; Xu AF Guanidinium-Rich, Glycerol-Derived Oligocarbonates: A New Class of Cell-Penetrating Molecular Transporters That Complex, Deliver, and Release siRNA. Mol. Pharmaceutics 2015, 12, 742–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Miyata K; Oba M; Nakanishi M; Fukushima S; Yamasaki Y; Koyama H; Nishiyama N; Kataoka K Polyplexes from Poly(aspartamide) Bearing 1,2-Diaminoethane Side Chains Induce pH-Selective, Endosomal Membrane Destabilization with Amplified Transfection and Negligible Cytotoxicity. J. Am. Chem. Soc 2008, 130, 16287–16294. [DOI] [PubMed] [Google Scholar]
- (13).Yan Y; Xiong H; Zhang X; Cheng Q; Siegwart DJ Systemic mRNA Delivery to the Lungs by Functional Polyester-based Carriers. Biomacromolecules 2017, 18 (12), 4307–4315. [DOI] [PubMed] [Google Scholar]
- (14).Wu Y; Smith AE; Reineke TM Lipophilic Polycation Vehicles Display High Plasmid DNA Delivery to Multiple Cell Types. Bioconjugate Chem. 2017, 28 (8), 2035–2040. [DOI] [PubMed] [Google Scholar]
- (15).Shrestha R; Elsabahy M; Luehmann H; Samarajeewa S; Florez-Malaver S; Lee NS; Welch MJ; Liu Y; Wooley KL Hierarchically Assembled Theranostic Nanostructures for siRNA Delivery and Imaging Applications. J. Am. Chem. Soc 2012, 134 (42), 17362–17365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Dhande YK; Wagh BS; Hall BC; Sprouse D; Hackett PB; Reineke TM N-Acetylgalactosamine Block-co-Polycations Form Stable Polyplexes with Plasmids and Promote Liver-Targeted Delivery. Biomacromolecules 2016, 17 (3), 830–840. [DOI] [PubMed] [Google Scholar]
- (17).Zhao N; Qi J; Zeng Z; Parekh P; Chang C-C; Tung CH; Zu Y Transfecting the hard-to-transfect lymphoma/leukemia cells using a simple cationic polymer nanocomplex. J. Controlled Release 2012, 159, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lim Y.-b.; Kim C.-h.; Kim K; Kim SW; Park J.-s. Development of a Safe Gene Delivery System Using Biodegradable Polymer, Poly[α-(4-aminobutyl)-L-glycolic acid]. J. Am. Chem. Soc 2000, 122 (27), 6524–6525. [Google Scholar]
- (19).Putnam D; Langer R Poly(4-hydroxy-L-proline ester): Low Temperature Polycondensation and Plasmid DNA Complexation. Macromolecules 1999, 32 (11), 3658–3662. [Google Scholar]
- (20).Luten J; van Nostrum CF; De Smedt SC; Hennink WE Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J. Controlled Release 2008, 126, 97–110. [DOI] [PubMed] [Google Scholar]
- (21).Borguet YP; Khan S; Noel A; Gunsten SP; Brody SL; Elsabahy M; Wooley KL Development of Fully Degradable Phosphonium-Functionalized Amphiphilic Diblock Copolymers for Nucleic Acids Delivery. Biomacromolecules 2018, 19 (4), 1212–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Hao J; Kos P; Zhou K; Miller JB; Xue L; Yan Y; Xiong H; Elkassih S; Siegwart DJ Rapid Synthesis of a Lipocationic Polyester Library via Ring-Opening Polymerization of Functional Valerolactones for Efficacious siRNA Delivery. J. Am. Chem. Soc 2015, 137 (29), 9206–9209. [DOI] [PubMed] [Google Scholar]
- (23).McKinlay CJ; Vargas JR; Blake TR; Hardy JW; Kanada M; Contag CH; Wender PA; Waymouth RM Chargealtering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl. Acad. Sci. U. S. A 2017, 114, E448–E456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Benner NL; Near KE; Bachmann MH; Contag CH; Waymouth RM; Wender PA Functional DNA Delivery Enabled by Lipid-Modified Charge-Altering Releasable Transporters (CARTs). Biomacromolecules 2018, 19, 2812–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).McKinlay CJ; Benner NL; Haabeth OA; Waymouth RM; Wender PA Enhanced mRNA delivery into lymphocytes enabled by lipid-varied libraries of charge-altering releasable transporters. Proc. Natl. Acad. Sci. U. S. A 2018, 115, E5859–E5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Haabeth OAW; Blake TR; McKinlay CJ; Tveita AA; Sallets A; Waymouth RM; Wender PA; Levy R Local delivery of Ox40l, Cd80, and Cd86 mRNA kindles global anti-cancer immunity. Cancer Res. 2019, 79, 1624–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Haabeth OAW; Blake TR; McKinlay CJ; Waymouth RM; Wender PA; Levy R mRNA vaccination with charge-altering releasable transporters elicits human T cell responses and cures established tumors in mice. Proc. Natl. Acad. Sci. U. S. A 2018, 115 (39), E9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Jebors S; Enjalbal C; Amblard M; Subra G; Mehdi A; Martinez J Switchable polymer-grafted mesoporous silica’s: from polyesters to polyamides biosilica hybrid materials. Tetrahedron 2013, 69, 7670–7674. [Google Scholar]
- (29).Tailhades J; Blanquer S; Nottelet B; Coudane J; Subra G; Verdie P; Schacht E; Martinez J; Amblard M From Polyesters to Polyamides Via O-N Acyl Migration: An Original Multi-Transfer Reaction. Macromol. Rapid Commun 2011, 32, 876–880. [DOI] [PubMed] [Google Scholar]
- (30).Wei Y; Li X.-y.; Jing X.-b.; Chen X.-s.; Huang Y.-b. Preparation of poly(serine ester)s by ring-opening polymerization of N-trityl serine lactone under catalysis of ZnEt2. Chem. Res. Chin. Univ 2013, 29, 177–182. [Google Scholar]
- (31).de Gracia Lux C; Olejniczak J; Fomina N; Viger ML; Almutairi A Intramolecular cyclization assistance for fast degradation of ornithine-based poly(ester amide)s. J. Polym. Sci., Part A: Polym. Chem 2013, 51 (18), 3783–3790. [Google Scholar]
- (32).Kohn J; Langer R Polymerization reactions involving the side chains of α-L-amino acids. J. Am. Chem. Soc 1987, 109 (3), 817–820. [Google Scholar]
- (33).Fietier I; Le Borgne A; Spassky N Synthesis of functionaĺ polyesters derived from serine. Polym. Bull 1990, 24, 349–353. [Google Scholar]
- (34).Zhou Q-X; Kohn J Preparation of Poly(L-serine ester): A Structural Analogue of Conventional Poly(L-serine). Macromolecules 1990, 23, 3399–3406. [Google Scholar]
- (35).Wei Y; Li X; Jing X; Chen X; Huang Y Synthesis and characterization of α-amino acid-containing polyester: poly[(εcaprolactone)- co -(serine lactone)]. Polym. Int 2013, 62, 454–462. [Google Scholar]
- (36).Rossignol H; Boustta M; Vert M Synthetic poly(β-hydroxyalkanoates) with carboxylic acid or primary amine pendent groups and their complexes. Int. J. Biol. Macromol 1999, 25, 255–264. [DOI] [PubMed] [Google Scholar]
- (37).Aluri R; Jayakannan M One-pot two polymers: ABB′ melt polycondensation for linear polyesters and hyperbranched poly(esterurethane)s based on natural L-amino acids. Polym. Chem 2015, 6 (25), 4641–4649. [Google Scholar]
- (38).Kamber NE; Jeong W; Waymouth RM; Pratt RC; Lohmeijer BGG; Hedrick JL Organocatalytic Ring-Opening Polymerization. Chem. Rev 2007, 107, 5813–5840. [DOI] [PubMed] [Google Scholar]
- (39).Kiesewetter MK; Shin EJ; Hedrick JL; Waymouth RM Organocatalysis: Opportunities and Challenges for Polymer Synthesis. Macromolecules 2010, 43, 2093–2107. [Google Scholar]
- (40).Tang J; Chen EY-X Increasing complexity in organopolymerization of multifunctional γ-butyrolactones. Eur. Polym. J 2017, 95, 678–692. [Google Scholar]
- (41).Kowalski PS; Capasso Palmiero U; Huang Y; Rudra A; Langer R; Anderson DG Ionizable Amino-Polyesters Synthesized via Ring Opening Polymerization of Tertiary Amino-Alcohols for Tissue Selective mRNA Delivery. Adv. Mater 2018, 30 (34), 1801151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Jones CH; Chen C-K; Jiang M; Fang L; Cheng C; Pfeifer BA Synthesis of Cationic Polylactides with Tunable Charge Densities as Nanocarriers for Effective Gene Delivery. Mol. Pharmaceutics 2013, 10 (3), 1138–1145. [DOI] [PubMed] [Google Scholar]
- (43).McKinlay CJ; Waymouth RM; Wender PA Cell Penetrating, Guanidinium-Rich Oligophosphoesters: Effective and Versatile Molecular Transporters for Drug and Probe Delivery. J. Am. Chem. Soc 2016, 138 (10), 3510–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang X; Jones GO; Hedrick JL; Waymouth RM Fast and selective ring-opening polymerizations by alkoxides and thioureas. Nat. Chem 2016, 8, 1047–1053. [DOI] [PubMed] [Google Scholar]
- (45).Fastnacht KV; Spink SS; Dharmaratne NU; Pothupitiya JU; Datta PP; Kiesewetter ET; Kiesewetter MK Bis- and TrisUrea H-Bond Donors for Ring-Opening Polymerization: Unprecedented Activity and Control from an Organocatalyst. ACS Macro Lett. 2016, 5 (8), 982–986. [DOI] [PubMed] [Google Scholar]
- (46).Kazakov OI; Datta PP; Isajani M; Kiesewetter ET; Kiesewetter MK Cooperative Hydrogen-Bond Pairing in Organocatalytic Ring-Opening Polymerization. Macromolecules 2014, 47 (21), 7463–7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Lohmeijer BGG; Pratt RC; Leibfarth F; Logan JW; Long DA; Dove AP; Nederberg F; Choi J; Wade C; Waymouth RM; Hedrick JL Guanidine and Amidine Organocatalysts for Ring-Opening Polymerization of Cyclic Esters. Macromolecules 2006, 39 (25), 8574–8583. [Google Scholar]
- (48).Sosnowski S; Slomkowski S; Penczek S On the ambident reactivity of beta-lactones in their reactions with alcoholates initiating polymerization. Macromolecules 1993, 26 (20), 5526–5527. [Google Scholar]
- (49).deRonde BM; Posey ND; Otter R; Caffrey LM; Minter LM; Tew GN Optimal Hydrophobicity in Ring-Opening Metathesis Polymerization-Based Protein Mimics Required for siRNA Internalization. Biomacromolecules 2016, 17, 1969–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Priegue JM; Crisan DN; Martínez-Costas J; Granja JR; Fernandez-Trillo F; Montenegro J In Situ Functionalized Polymers for siRNA Delivery. Angew. Chem., Int. Ed 2016, 55, 7492–7495. [DOI] [PubMed] [Google Scholar]
- (51).deRonde BM; Torres JA; Minter LM; Tew GN Development of Guanidinium-Rich Protein Mimics for Efficient siRNA Delivery into Human T Cells. Biomacromolecules 2015, 16 (10), 3172–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Gehin C; Montenegro J; Bang E-K; Cajaraville A; Takayama S; Hirose H; Futaki S; Matile S; Riezman H Dynamic Amphiphile Libraries To Screen for the “Fragrant” Delivery of siRNA into HeLa Cells and Human Primary Fibroblasts. J. Am. Chem. Soc 2013, 135 (25), 9295–9298. [DOI] [PubMed] [Google Scholar]
- (53).Louzao I; García-Fandiño R; Montenegro J Hydrazone modulated peptides for efficient gene transfection. J. Mater. Chem. B 2017, 5 (23), 4426–4434. [DOI] [PubMed] [Google Scholar]
- (54).Wender PA; Galliher WC; Goun EA; Jones LR; Pillow TH The design of guanidinium-rich transporters and their internalization mechanisms. Adv. Drug Delivery Rev 2008, 60 (4), 452–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Contag CH; Bachmann MH Advances in In Vivo Bioluminescence Imaging of Gene Expression. Annu. Rev. Biomed. Eng 2002, 4, 235–260. [DOI] [PubMed] [Google Scholar]
- (56).Wender PA; Goun EA; Jones LR; Pillow TH; Rothbard JB; Shinde R; Contag CH Real-time analysis of uptake and bioactivatable cleavage of luciferin-transporter conjugates in transgenic reporter mice. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (25), 10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.