Abstract
Objective
Recent results from the Cardiovascular Trial of the Testosterone Trials showed that testosterone treatment of older men with low testosterone was associated with greater progression of noncalcified plaque (NCP). We evaluated the effect of anthropometric measures and cardiovascular biomarkers on plaque progression in individuals in the Testosterone Trial.
Methods
The Cardiovascular part of the trial included 170 men aged 65 years or older with low testosterone. Participants received testosterone gel or placebo gel for 12 months. The primary outcome was change in NCP volume from baseline to 12 months, as determined by coronary computed tomography angiography (CCTA). We assayed several markers of cardiovascular risk and analyzed each marker individually in a model as predictive variables and change in NCP as the dependent variable.
Results
Of 170 enrollees, 138 (73 testosterone, 65 placebo) completed the study and were available for the primary analysis. Of 10 markers evaluated, none showed a significant association with the change in NCP volume, but a significant interaction between treatment assignment and waist-hip ratio (WHR) (P = 0.0014) indicated that this variable impacted the testosterone effect on NCP volume. The statistical model indicated that for every 0.1 change in the WHR, the testosterone-induced 12-month change in NCP volume increased by 26.96 mm3 (95% confidence interval, 7.72-46.20).
Conclusion
Among older men with low testosterone treated for 1 year, greater WHR was associated with greater NCP progression, as measured by CCTA. Other biomarkers and anthropometric measures did not show statistically significant association with plaque progression.
Keywords: noncalcified coronary artery plaque, coronary artery disease, testosterone replacement
Lower serum testosterone concentration has been associated with adverse cardiovascular disease (CVD) outcomes (1, 2). There are conflicting reports regarding the effect of testosterone treatment on CVD risk. Some retrospective studies reported more CVD events in men taking testosterone, while others did not (3–7). The Testosterone Trials (TTrials) comprised 7 coordinated placebo-controlled clinical trials designed to assess the effects of testosterone treatment in older men who had low testosterone concentrations for no apparent reason other than age (8). In the Cardiovascular Trial, testosterone treatment for 1 year compared with placebo was associated with significantly greater progression of coronary artery noncalcified plaque (NCP) volume measured by serial coronary computed tomography angiography (CCTA) (9).
Serum markers such as total cholesterol, high-density lipoprotein (HDL), low density lipoprotein (LDL), and hemoglobin A1C (HbA1c), have been recognized as significant risk factors for developing coronary artery plaque and future CVD events (10, 11). There are contradictory reports about the association of biomarkers and the extent, progression of atherosclerosis, and coronary events (12–14).Inflammatory markers such as C-reactive protein (CRP) have been reported to be associated with plaque progression in some studies (15, 16), while other reports found no association (17, 18). Anthropometric measures such as waist-hip ratio (WHR) and waist circumference are predictors of myocardial infarction risk (19, 20). Abdominal obesity can lead to increases in insulin and glucose levels and is a central feature of metabolic syndrome. Several observational studies have shown a link between low endogenous sex hormones and metabolic syndrome (21–23). One large cross-sectional study reported that higher testosterone and sex hormone–binding globulin (SHBG) levels in older men were independently associated with reduced risk of metabolic syndrome and higher insulin sensitivity (24).
The aim of the current study is to evaluate the impact of baseline anthropometric measures and cardiovascular biomarkers on the progression of coronary artery plaque volume in the 138 men who participated in the Cardiovascular Trial of the TTrials. We also assessed the interaction of anthropometric measures and cardiovascular biomarkers with testosterone treatment for atherosclerotic plaque progression.
Methods
Study design
The TTrials comprised 7 double-blind, placebo-controlled randomized controlled trials. The overall study design of the TTrials, as well as that of the Cardiovascular Trial, has been published (8, 25). To qualify for the TTrials overall, a participant had to qualify for at least 1 of 3 main trials (Sexual Function Trial, Physical Function Trial, and Vitality Trial). Qualified men could also participate in any of other trials, if respective eligibility criteria were met. The participants were allocated to receive testosterone or placebo gel for 1 year (8, 9). Institutional review boards of all participating sites approved the TTrials and Cardiovascular Trial protocols. All participants provided written consent. Trial conduct and participant safety were supervised by an independent safety and data monitoring board.
Participants
The TTrials included men ≥ 65 years of age who had symptoms and objective evidence of low libido, physical dysfunction and/or low vitality, and serum testosterone levels that averaged < 275 ng/dL on 2 morning samples. Men who were at moderate or high risk for prostate cancer, who had had a myocardial infarction within the previous 3 months, or who had systolic blood pressure >160 mm Hg or diastolic blood pressure >100 mm Hg, were excluded (8).
Exclusion criteria specifically for the Cardiovascular Trial included circumstances that either made coronary computed tomography angiography (CCTA) technically unfeasible (inability to hold breath for 10 seconds, a prior diagnosis of tachycardia or irregular heart rhythm [eg, atrial fibrillation], weight > 136 kg, or a history of coronary artery bypass graft surgery) or that increased the risk of performing the CCTA (estimated glomerular filtration rate < 60 mL/min/1.73 m2 or known allergy to iodinated contrast) (9, 25).
Testosterone treatment
Participants were assigned to receive either testosterone as a 1% gel in a pump bottle (AndroGel) or a placebo gel, by a double-blinded method for 1 year. The initial testosterone dose was 5 g/day and was adjusted to maintain the serum concentrations within normal range for young men (280–873 ng/dL) measured at a central laboratory (Quest Clinical Trials) at months 1, 2, 3, 6, and 9. To maintain blinding, whenever dose adjustments were made in a man receiving testosterone treatment, the dose was also changed in a man receiving placebo (8).
Assessments
The concentrations of cardiovascular biomarkers were measured on serum samples drawn at baseline and months 3 and 12 and stored at −80 C. These assays were performed at the Laboratory for Clinical Biochemistry Research, University of Vermont, and University of Minnesota, as described previously (7, 9). At months 3, 6, 9, and 12, clinical variables were measured.
Details of coronary artery plaque volume by CCTA assessment have been published (25). In brief, coronary artery plaque volume was assessed by CCTA at 9 of the 12 TTrials clinical sites. Precontrast scans for evaluation of coronary artery calcium density and postcontrast scans for evaluation of coronary artery plaque volume were performed at baseline and 12 months. Scans were assessed at a central reading center (Harbor-UCLA Medical Center) by readers who were blinded both to treatment group and date of scan. Quantitative plaque assessment was conducted according to a previously defined protocol (26) using semi-automated plaque analysis software (QAngioCT Research Edition Version 2.0.5; Medis Medical Imaging Systems). Based on the guidelines of the Society of Cardiovascular Computed Tomography, 17-segment coronary artery model vessels greater than 1.5 mm were evaluated (27). The volumes of 4 types of coronary artery plaque (low attenuation, fibrous-fatty, fibrous, and dense calcified) were calculated by Hounsfield unit threshold. The primary outcome was change in NCP volume from baseline to month 12. NCP was defined as the sum of the fibrous, fibrous fatty, and low attenuation plaque. Secondary outcomes were change in calcified plaque volume and change in coronary artery score. Details of intra- and inter-observer variability have been published. The intra-class correlations (ICCs) and coefficient of variation (CVs) were 0.99 and 7.8 %, respectively, for intra-observer variability. The ICC and CV were 0.95 and 19.9 %, respectively, for inter-observer variability (9).
Statistical analyses
The following markers were available for study: total cholesterol; non-HDL cholesterol; HDL; LDL; total cholesterol/HDL ratio; triglycerides; HbA1c; glucose, insulin; homeostasis model assessment (HOMA); d-dimer; troponin; CRP; interleukin-6 (IL-6); SHBG; weight; body mass index (BMI); waist circumference; and WHR. We evaluated the inter-correlation of the baseline values of these markers, separately within groups where substantial inter-correlation was expected: lipid markers, metabolic markers, markers of inflammation, and clinical markers. We then excluded from further study the marker showing correlation > 0.5 with the most other markers, and then eliminated any marker with correlation > 0.5 with the selected marker from further consideration. We retained any other markers with correlation < 0.5 with the selected marker. If 2 markers showed high correlation with the same number of other markers, we selected the 1 with the lowest correlation with the remaining markers. We also included SHBG, d-dimer, and troponin without testing for correlation with other markers, since they did not fit into the any of the 4 categories noted above.
We tested each selected marker separately in a regression model, including treatment as a covariate as well as age (over or under 75), baseline testosterone (over or under 200 ng/mL) and an interaction term of the marker with treatment. Any variable showing a significant association with the change in plaque volume after adjusting for multiple comparisons using the Holm procedure (28) was to be included in a multivariable model, assessing all potentially predictive variables simultaneously.
Secondary analyses included testing association of the selected markers with change in calcified plaque volume and with coronary artery calcium score, using the same approach as above.
Results
Of 138 men who were enrolled, 73 received testosterone treatment and 65 received placebo. The baseline characteristics of the participants in the Cardiovascular Trial were previously reported (9). At baseline, the mean (standard deviation [SD]) age was 71.2 (5.7) years. The majority of participants were white (81%) and had relatively high rates of cardiovascular risk factors, including hypertension, hyperlipidemia, obesity, and diabetes. At baseline, the mean (SD) BMI was 30.6 (3.8) in the testosterone group and 30 (3.5) in the placebo group; the mean weight was 94 kg and the mean WHR was 1.0 in each treatment group. The calculated 10-year risk of cardiovascular events was relatively high: a mean risk of 27% (95% confidence interval [CI], 6.4%-47.6%) in the placebo group and 24% (95% CI, 2.6%-45.4%) in the testosterone group.
Of the 19 markers initially evaluated, 10 remained for further study after removing those that were highly correlated with other markers, as described above. These 10 remaining markers were HDL cholesterol, non-HDL cholesterol, D-dimer, IL-6, CRP, insulin, HgbA1C, SHBG, weight, and WHR (Table 1). Among these 10 measures, only the baseline WHR interaction with treatment showed a significant association with the progression of NCP volume at 12 months (Table 2, Figure 1). The baseline values of WHR ranged from 0.9 to 1.2. Because it was the interaction term that met the threshold based on the multiple comparisons adjustment (P = 0.0014 compared to threshold value from the Holm multiple comparisons procedure of 0.0056), we evaluated WHR separately for the 2 treatment groups. The association was seen only in the testosterone group (P = 0.007). The model indicates that for every 0.1 change in the WHR, the effect of testosterone on the 12-month change in NCP volume would increase by 26.96 mm3 (95% CI, 7.72-46.20).
Table 1.
Baseline Values of Anthropometric Measures and Biomarkers
| Assay | Statistic | Testosterone | Placebo |
|---|---|---|---|
| HDL, mg/dL | Mean (SD) | 44.2 (11.7) | 49.1 (16.0) |
| Median (IQR) | 43 (36, 51) | 46 (39, 56) | |
| Non-HDL, mg/dL | Mean (SD) | 116.8 (36.6) | 126 (38) |
| Median (IQR) | 113 (91, 140) | 120 (109, 137) | |
| D-dimer, mg/L | Mean (SD) | 0.70 (0.52) | 0.71 (0.62) |
| Median (IQR) | 0.58 (0.39, 0.81) | 0.51 (0.40, 0.76) | |
| IL-6, pg/mL | Mean (SD) | 1.7 (3.0) | 1.6 (2.2) |
| Median (IQR) | 1.2 (0.80, 1.6) | 1.1 (0.83, 1.5) | |
| CRP, mg/L | Mean (SD) | 5.3 (17.7) | 2.8 (4.2) |
| Median (IQR)) | 1.8 (0.78, 2.7) | 1.4 (0.82, 2.4) | |
| Insulin, µIU/mL | Mean (SD) | 19.4 (13.8) | 17.7 (17.1) |
| Median (IQR) | 15.6 (10.1, 24.1) | 14.3 (10.6, 18.5) | |
| HbA1c, % | Mean (SD) | 6.3 (0.79) | 6.2 (0.75) |
| Median (IQR) | 6.0 (5.7, 6.8) | 6.0 (5.8, 6.4) | |
| SHBG, nmol/L | Mean (SD) | 30.4 (15.1) | 29.6 (13.2) |
| Median (IQR) | 27.2 (20.2, 35.6) | 28.5 (20.4, 35.5 | |
| Weight, kg | Mean (SD) | 94.8 (14.0) | 93.8 (14.6) |
| Median (IQR) | 91.5 (84, 103) | 93 (84, 104) | |
| Waist-hip ratio | Mean (SD) | 1.0 (0.06) | 1.0 (0.07) |
| Median (IQR) | 1.0 (0.96, 1.0) | 1.0 (0.97, 1.0) |
Abbreviations: CRP, C-reactive protein; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; IL-6, interleukin-6; IQR, interquartile range; SD, standard deviation; SHBG, sex hormone–binding globulin.
Table 2.
Association of Baseline Anthropometric Measures and Biomarkers with 12-Month Change in NCP Volume. Based on Linear Regression Models Including Terms for Site, Age (over/under 75), Baseline Testosterone Level (over/under 200 ng/Dl), Baseline NCP volume, Predictor Variable, and Interaction of Predictor Variable with Treatment. P Values Not Adjusted for Multiple Comparisons
| Predictor variable | Effect Estimate (Standard error) | 95% CI | Nominal P value |
|---|---|---|---|
| HDL | 0.31 (0.38) | −0.43, 1.06 | 0.40 |
| HDL × treatment | −0.75 (0.65) | −2.03, 0.53 | 0.25 |
| Non-HDL | 0.034 (0.16) | −0.29, 0.35 | 0.84 |
| Non-HDL × treatment | −0.83 (0.23) | −05.4, 0.37 | 0.71 |
| D-dimer | −19.4 (9.93) | −39.1, 0.25 | 0.053 |
| D-dimer × treatment | 15.92 (14.98) | −13.74, 45.58 | 0.29 |
| IL-6 | −2.81 (2.78) | −8.32, 2.69 | 0.31 |
| IL-6 × treatment | 2.50 (3.32) | −4.07, 9.08 | 0.45 |
| CRP | −1.48 (1.45) | −4.36, 1.39 | 0.31 |
| CRP × treatment | 1.30 (1.49) | −1.64, 4.25 | 0.38 |
| Insulin | 0.53 (0.36) | −0.66, 0.76 | 0.88 |
| Insulin × treatment | −0.26 (0.57) | −1.40, 0.87 | 0.65 |
| HbA1c | 0.34 (9.00) | −17.5, 18.2 | 0.97 |
| HbA1c × treatment | 5.15 (11.88) | −18.82, 28.70 | 0.67 |
| SHBG | 0.24 (0.49) | −0.73, 1.21 | 0.63 |
| SHBG × treatment | −0.83 (0.60) | −2.02, 0.36) | 0.17 |
| Weight | −0.44 (0.42) | −1.27, 0.40 | 0.30 |
| Weight × treatment | 0.80 (0.59) | −0.37, 1.97 | 0.18 |
| Waist-hip ratio | −87.7 (93.2) | −272.2, 96.7 | 0.35 |
| Waist-hip ratio × treatment | 429.4 (131.4) | 169.4, 689.5 | 0.0014 |
Abbreviations: CRP, C-reactive protein; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; IL-6, interleukin-6; IQR, interquartile range; SD, standard deviation; SHBG, sex hormone–binding globulin.
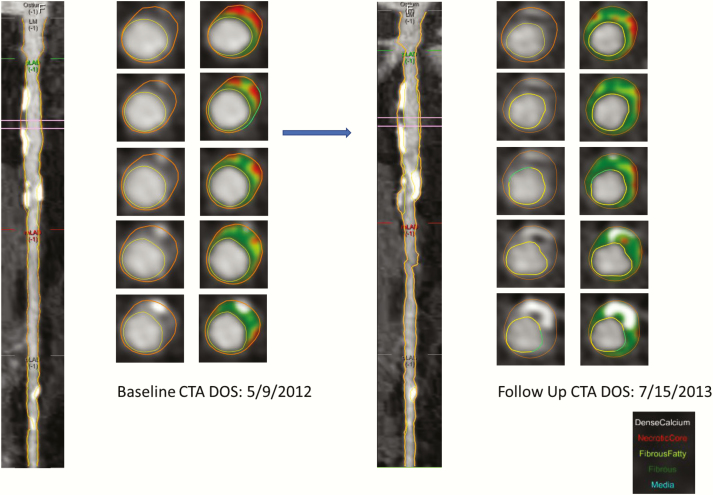
Figure 1.
An example of plaque progression in proximal left anterior descending coronary artery over 1 year in participants enrolled in testosterone trial.
None of the cardiovascular risk markers were statistically significantly associated with change in calcified plaque or coronary artery calcium score when applying the multiple comparisons correction.
Discussion
We report that in older hypogonadal men participating in the Cardiovascular Trial of the TTrials there was a significant association between baseline WHR and progression of NCP volume measured by CCTA after 1 year of testosterone treatment. Among men taking testosterone, larger WHRs were associated with greater progression of NCP.
There are strong associations among presence of visceral adipose tissue, insulin sensitivity, dyslipidemia, and increase in inflammation and hypertension (29, 30). Visceral adipose tissue stores can be measured by computed tomography, dual-energy X-ray absorptiometry, or magnetic resonance imaging, but these modalities are too expensive and time consuming for day-to-day use (31, 32). WHR is closely related to visceral fat and commonly measured in clinical practice (33). Meta-analyses of 28 114 patients from 15 prospective studies showed that for every 0.01 increase in WHR, there was a 5% increase in risk of future CVD events (33). Our data indicate that for every 0.1 increase in WHR, there was 26 mm3 greater increase in progression of NCP volume in patients treated with testosterone replacement therapy.
NCP volume, as assessed by CCTA, has been associated with CVD events. In a large single-center trial conducted by Zu et al (34), the cumulative probability of 3-year major adverse cardiovascular events (including cardiac death, nonfatal myocardial infarction, or coronary revascularization) increased across the strata for cardiac computed tomography plaque characteristics (5.5% for calcified plaque, 22.7% for NCP, and 37.7% for mixed plaque; P < 0.001)
WHR and waist circumference, measures of central obesity or abdominal obesity, have been associated with reduced total testosterone levels (35, 36). A potential mechanism for this inverse relationship may involve increased leptin levels, which are hypothesized to interfere with luteinizing hormone stimulation of androgen production and decreased SHBG in central obesity (37). Another plausible mechanism of decreased testosterone in obese individuals is increased aromatase activity in visceral adipose tissue, which leads to higher conversion of testosterone to estradiol (38). Androgen deprivation therapy, as given to patients with prostate cancer, has shown to significantly increase BMI, total weight, and body fat mass and to decrease lean body mass (39, 40). Hence, several studies have investigated the hypothesis that testosterone replacement therapy may decrease visceral fat stores and improve the metabolic profile in men. However, there are conflicting reports on the effects of testosterone replacement on visceral fat. Some studies reported testosterone replacement therapy decreases visceral fat, while others showed no association (41, 42). In a study of 261 patients in a prospective longitudinal registry, testosterone replacement was associated with a significant reduction in obesity parameters (eg, waist circumference, BMI) and cholesterol values over the 5-year study period (43). However, randomized controlled clinical trials reported no impact of testosterone replacement on weight, BMI, and metabolic syndrome (41, 44). A previous paper from the TTrials also did not show any changes in WHR, waist circumference, and BMI in men treated with testosterone for 12 months compared with placebo-treated men (7).
These results are hypothesis-generating and warrant further investigation of the interaction of visceral adipose tissue stores and testosterone treatment. To our knowledge, no other studies have examined the interaction of testosterone replacement therapy and central obesity on CVD outcomes. The strengths of our trial included requiring all men to have unequivocally low testosterone at baseline, a placebo-controlled design, and blinded central review of baseline and 12 month scans. An important limitation of our study is the use of a surrogate marker of heart disease, NCP, rather than a clinical outcome. Another limitation is that the results apply only to men ≥ 65 years of age with low testosterone (9).
Our results are exploratory and do not conclusively demonstrate that the markers we assessed have no associations with NCP progression; however, the lack of statistically significant findings suggest that if such associations do exist, they are not likely to be strong. We conclude that among older men receiving testosterone treatment, those with higher vs lower WHR may experience greater increases in NCP volume. Future trials should evaluate the interaction of testosterone treatment and surrogate markers of abdominal obesity and visceral fat stores.
Acknowledgments
We acknowledge with great appreciation and fondness the critical and sustained contributions of Dr. Elizabeth Barrett-Connor to the Testosterone Trials. As a member of the TTrials Steering Committee, she was instrumental in their development, conduct, and interpretation. As the principal investigator of the University of California, San Diego site, she set a high standard in the conduct of a clinical trial site.
Financial Support: The Testosterone Trials were supported by a grant from the National Institute on Aging, National Institutes of Health (U01 AG030644), supplemented by funds from the National Heart, Lung and Blood Institute, National Institute of Neurological Diseases and Stroke, and National Institute of Child Health and Human Development. AbbVie (formerly Solvay and Abbott Laboratories) generously provided funding, AndroGel, and placebo gel.
Clinical Trial Information: Clinicaltrials.gov identifier: NCT00799617.
Glossary
Abbreviations
- BMI
body mass index
- CCTA
coronary computed tomography angiography
- CI
confidence interval
- CRP
C-reactive protein
- CVD
cardiovascular disease
- HbA1c
hemoglobin A1C
- HDL
high-density lipoprotein
- IL-6
interleukin-6
- LDL
low-density lipoprotein
- NCP
coronary artery noncalcified plaque
- SD
standard deviation
- SHBG
sex hormone–binding globulin
- TTrials
Testosterone Trials
- WHR
waist-hip ratio
Additional Information
Disclosure Summary: Dr. Ellenberg reports grants from the National Institutes of Health (NIH) and grants from AbbVie, Inc, during the conduct of the study and grants from AbbVie, Inc., outside the submitted work; Dr. Lewis reports grants from NIH and grants from AbbVie during the conduct of the study; Dr. Wenger reports grants from Alnylam Pharmaceuticals, grants and personal fees from Gilead Sciences, grants from NHLBI, grants from Pfizer, grants from the Society for Women’s Health Research, personal fees from Amgen, personal fees from AstraZeneca, and personal fees from Merck outside the submitted work; Dr. Budoff reports grants from NIH during the conduct of the study and grants from General Electric outside the submitted work; Dr. Barrett-Connor has nothing to disclose; Dr. Swerdloff reports grants from The Bone Trial of the Testosterone Trial during the conduct of the study; grants and other support from Clarus, grants from Lipesene, grants and other support from Antares, outside the submitted work; Dr. Stephens-Shields reports grants from the National Institute on Aging (NIA) and from AbbVie during the conduct of the study; Dr. Bhasin reports grants from NIA during the conduct of the study; grants and personal fees from Abbvie, grants and personal fees from Lilly, grants from Transition Therapeutics, grants and personal fees from Regeneron, outside the submitted work; in addition, Dr. Bhasin has a patent pending for a free testosterone calculator and has equity interest in FPT, LLC; Dr. Cauley has nothing to disclose; Dr. Crandall has nothing to disclose; Dr. Cunningham reports personal fees from AbbVie, personal fees from Apricus, personal fees from Besins, personal fees from Clarus Therapeutics, personal fees from Endo Pharma, personal fees from Ferring, personal fees from Lilly, personal fees from Pfizer, personal fees from Repros Therapeutics, outside the submitted work; Dr. Ensrud reports grants from NIA, during the conduct of the study; Dr. Gill has nothing to disclose; Dr. Matsumoto reports personal fees from AbbVie outside the submitted work; Dr. Molitch reports grants from NIH, grants from Abbott Laboratories, during the conduct of the study; personal fees from Abbvie (Abbott Laboratories), personal fees from Eli Lilly & Co., personal fees from Pfizer, outside the submitted work; X. Hou has nothing to disclose; Dr. Snyder reports grants from NIA, NIH, grants and nonfinancial support from AbbVie (formerly Solvay and Abbott Laboratories), during the conduct of the study.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
References
- 1. Khaw KT, Dowsett M, Folkerd E, et al. . Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694–2701. [DOI] [PubMed] [Google Scholar]
- 2. Smith GD, Ben-Shlomo Y, Beswick A, Yarnell J, Lightman S, Elwood P. Cortisol, testosterone, and coronary heart disease: prospective evidence from the Caerphilly study. Circulation. 2005;112(3):332–340. [DOI] [PubMed] [Google Scholar]
- 3. Baillargeon J, Urban RJ, Kuo YF, et al. . Risk of Myocardial Infarction in Older Men Receiving Testosterone Therapy. Ann Pharmacother. 2014;48(9):1138–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finkle WD, Greenland S, Ridgeway GK, et al. . Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. Plos One. 2014;9(1):e85805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050–2058. [DOI] [PubMed] [Google Scholar]
- 6. Vigen R, O’Donnell CI, Barón AE, et al. . Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013;310(17):1829–1836. [DOI] [PubMed] [Google Scholar]
- 7. Mohler ER 3rd, Ellenberg SS, Lewis CE, et al. . The effect of testosterone on cardiovascular biomarkers in the testosterone trials. J Clin Endocrinol Metab. 2018;103(2):681–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snyder PJ, Ellenberg SS, Cunningham GR, et al. . The testosterone trials: seven coordinated trials of testosterone treatment in elderly men. Clin Trials. 2014;11(3):362–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Budoff MJ, Ellenberg SS, Lewis CE, et al. . Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317(7):708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao Q, Yu S, Xiong W, et al. . Waist-hip ratio as a predictor of myocardial infarction risk: a systematic review and meta-analysis. Medicine. 2018;97(30):e11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diederichsen SZ, Grønhøj MH, Mickley H, et al. . CT-Detected growth of coronary artery calcification in asymptomatic middle-aged subjects and association with 15 biomarkers. JACC Cardiovasc Imaging. 2017;10(8):858–866. [DOI] [PubMed] [Google Scholar]
- 12. Anroedh SS, Akkerhuis KM, Oemrawsingh RM, et al. . Associations of 26 circulating inflammatory and renal biomarkers with near-infrared spectroscopy and long-term cardiovascular outcome in patients undergoing coronary angiography (ATHEROREMO-NIRS Substudy). Curr Atheroscler Rep. 2018;20(10):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Battes LC, Cheng JM, Oemrawsingh RM, et al. . Circulating cytokines in relation to the extent and composition of coronary atherosclerosis: results from the ATHEROREMO-IVUS study. Atherosclerosis. 2014;236(1):18–24. [DOI] [PubMed] [Google Scholar]
- 14. Cheng JM, Oemrawsingh RM, Garcia-Garcia HM, et al. . Relation of C-reactive protein to coronary plaque characteristics on grayscale, radiofrequency intravascular ultrasound, and cardiovascular outcome in patients with acute coronary syndrome or stable angina pectoris (from the ATHEROREMO-IVUS study). Am J Cardiol. 2014;114(10):1497–1503. [DOI] [PubMed] [Google Scholar]
- 15. Alman AC, Kinney GL, Tracy RP, et al. . Prospective association between inflammatory markers and progression of coronary artery calcification in adults with and without type 1 diabetes. Diabetes Care. 2013;36(7):1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wadwa RP, Kinney GL, Ogden L, et al. . Soluble interleukin-2 receptor as a marker for progression of coronary artery calcification in type 1 diabetes. Int J Biochem Cell Biol. 2006;38(5-6):996–1003. [DOI] [PubMed] [Google Scholar]
- 17. Gauss S, Klinghammer L, Steinhoff A, et al. . Association of systemic inflammation with epicardial fat and coronary artery calcification. Inflamm Res. 2015;64(5):313–319. [DOI] [PubMed] [Google Scholar]
- 18. Kronmal RA, McClelland RL, Detrano R, et al. . Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115(21):2722–2730. [DOI] [PubMed] [Google Scholar]
- 19. Cao Q, Yu S, Xiong W, et al. . Waist-hip ratio as a predictor of myocardial infarction risk: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(30):e11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tigbe WW, Granat MH, Sattar N, Lean MEJ. Time spent in sedentary posture is associated with waist circumference and cardiovascular risk. Int J Obes (Lond). 2017;41(5):689–696. [DOI] [PubMed] [Google Scholar]
- 21. Barrett-Connor E, Khaw KT. Endogenous sex hormones and cardiovascular disease in men. A prospective population-based study. Circulation. 1988;78(3):539–545. [DOI] [PubMed] [Google Scholar]
- 22. Gyllenborg J, Rasmussen SL, Borch-Johnsen K, Heitmann BL, Skakkebaek NE, Juul A. Cardiovascular risk factors in men: The role of gonadal steroids and sex hormone-binding globulin. Metabolism. 2001;50(8):882–888. [DOI] [PubMed] [Google Scholar]
- 23. Simon D, Charles MA, Nahoul K, et al. . Association between plasma total testosterone and cardiovascular risk factors in healthy adult men: The Telecom Study. J Clin Endocrinol Metab. 1997;82(2):682–685. [DOI] [PubMed] [Google Scholar]
- 24. Muller M, Grobbee DE, den Tonkelaar I, Lamberts SW, van der Schouw YT. Endogenous sex hormones and metabolic syndrome in aging men. J Clin Endocrinol Metab. 2005;90(5):2618–2623. [DOI] [PubMed] [Google Scholar]
- 25. Abd Alamir M, Ellenberg SS, Swerdloff RS, et al. . The Cardiovascular Trial of the Testosterone Trials: rationale, design, and baseline data of a clinical trial using computed tomographic imaging to assess the progression of coronary atherosclerosis. Coron Artery Dis. 2016;27(2):95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Papadopoulou SL, Neefjes LA, Garcia-Garcia HM, et al. . Natural history of coronary atherosclerosis by multislice computed tomography. JACC Cardiovasc Imaging. 2012;5(3 Suppl):S28–S37. [DOI] [PubMed] [Google Scholar]
- 27. Leipsic J, Abbara S, Achenbach S, et al. . SCCT guidelines for the interpretation and reporting of coronary CT angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2014;8(5):342–358. [DOI] [PubMed] [Google Scholar]
- 28. Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979:6(2)65–70. [Google Scholar]
- 29. Pouliot MC, Després JP, Nadeau A, et al. . Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41(7):826–834. [DOI] [PubMed] [Google Scholar]
- 30. Tchernof A, Lamarche B, Prud’Homme D, et al. . The dense LDL phenotype. Association with plasma lipoprotein levels, visceral obesity, and hyperinsulinemia in men. Diabetes Care. 1996;19(6):629–637. [DOI] [PubMed] [Google Scholar]
- 31. Kamel EG, McNeill G, Han TS, et al. . Measurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and women. Int J Obes Relat Metab Disord. 1999;23(7):686–692. [DOI] [PubMed] [Google Scholar]
- 32. Onat A, Avci GS, Barlan MM, Uyarel H, Uzunlar B, Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int J Obes Relat Metab Disord. 2004;28(8):1018–1025. [DOI] [PubMed] [Google Scholar]
- 33. de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–856. [DOI] [PubMed] [Google Scholar]
- 34. Hou ZH, Lu B, Gao Y, et al. . Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc Imaging. 2012;5(10):990–999. [DOI] [PubMed] [Google Scholar]
- 35. Pasquali R, Casimirri F, Cantobelli S, et al. . Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism. 1991;40(1):101–104. [DOI] [PubMed] [Google Scholar]
- 36. Svartberg J. Epidemiology: testosterone and the metabolic syndrome. Int J Impot Res. 2007;19(2):124–128. [DOI] [PubMed] [Google Scholar]
- 37. Rosmond R, Wallerius S, Wanger P, Martin L, Holm G, Björntorp P. A 5-year follow-up study of disease incidence in men with an abnormal hormone pattern. J Intern Med. 2003;254(4):386–390. [DOI] [PubMed] [Google Scholar]
- 38. Kalyani RR, Dobs AS. Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr Opin Endocrinol Diabetes Obes. 2007;14(3):226–234. [DOI] [PubMed] [Google Scholar]
- 39. Chen Z, Maricic M, Nguyen P, Ahmann FR, Bruhn R, Dalkin BL. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer. 2002;95(10):2136–2144. [DOI] [PubMed] [Google Scholar]
- 40. Smith MR, Finkelstein JS, McGovern FJ, et al. . Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87(2):599–603. [DOI] [PubMed] [Google Scholar]
- 41. Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnoea: a randomised placebo-controlled trial. Eur J Endocrinol. 2012;167(4):531–541. [DOI] [PubMed] [Google Scholar]
- 42. Lunenfeld B. The relationship between sex hormones and the metabolic syndrome. Acta Biomed. 2010;81 Suppl 1:79–84. [PubMed] [Google Scholar]
- 43. Yassin DJ, Doros G, Hammerer PG, Yassin AA. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J Sex Med. 2014;11(6):1567–1576. [DOI] [PubMed] [Google Scholar]
- 44. Boyanov MA, Boneva Z, Christov VG. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male. 2003;6(1):1–7. [PubMed] [Google Scholar]