Abstract
STUDY QUESTION
Is pre-conception 25(OH)D associated with the per cycle probability of conception, i.e fecundability, in a prospective cohort study?
SUMMARY ANSWER
There are suggestive associations of high 25(OH)D (at least 50 ng/ml) with increased fecundability and low 25(OH)D (<20 ng/ml) with reduced fecundability, but the estimates were imprecise.
WHAT IS KNOWN ALREADY
Vitamin D has been associated with reproductive function and fertility in animal studies, but few human studies exist.
STUDY DESIGN, SIZE, DURATION
This community-based prospective cohort study included 522 women attempting to become pregnant between 2010 and 2016. The women completed online daily and monthly diaries until a positive home pregnancy test was observed or 12 months had elapsed.
PARTICIPANTS/MATERIALS, SETTING, METHODS
The study included women from central North Carolina who were aged 30–44 with no history of infertility, with no more than 3 months of attempt time at recruitment. Women recorded vaginal bleeding so that the ongoing number of attempt cycles could be counted and used to quantify a woman’s pregnancy attempt time. Blood collected at the study entry was analysed for 25(OH)D using liquid chromatography tandem mass spectrometry. Associations with fecundability were estimated with a log-binomial discrete time-to-event model.
MAIN RESULTS AND THE ROLE OF CHANCE
Among 522 women, 257 conceived during the study. The mean age was 33 years and the mean 25(OH)D was 36 ng/ml. There was an estimated 10% higher fecundability with each 10 ng/ml increase in 25(OH)D (fecundability ratio (FR) 1.10, 95% CI: 0.96, 1.25). The suggestive dose-response association with the continuous measure of 25(OH)D was driven by women in the lowest and the highest categories of 25(OH)D. Compared to women with 25(OH)D of 30–40 ng/ml, women below 20 ng/ml had an estimated 45% reduction in fecundability (FR (CI): 0.55 (0.23, 1.32)), and women with at least 50 ng/ml had an estimated 35% increase in fecundability (FR (CI): 1.35 (0.95, 1.91)). Across these three categories (25(OH)D of <20 ng/ml, 30–40 ng/ml and > 50 ng/ml), the probability of taking longer than 6 months to conceive was, respectively, 51% (17%, 74%), 28% (17%, 39%) and 15% (10%, 37%).
LIMITATIONS, REASONS FOR CAUTION
While the distribution of 25(OH)D was wide, the number of observed cycles with high 25(OH)D (N = 107) or low 25(OH)D (N = 56) was small.
WIDER IMPLICATIONS OF THE FINDINGS
Our findings are consistent with prior reports of reduced fertility in women with 25(OH)D concentrations below the clinically defined deficiency level (20 ng/ml). Further studies are needed to evaluate the possible reproductive benefits of considerably higher 25(OH)D concentration (>50 ng/ml).
STUDY FUNDING/COMPETING INTEREST(S)
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under award numbers R00HD079659 and R01HD067683 and supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, under projects ES103086, ES049003 and ES044003. ClearBlue ovulation predictor kits were generously donated to AMZJ and AJW by Swiss Precision Diagnostics. Drs Wilcox and Jukic report non-financial support from Swiss Precision Diagnostics during the conduct of the study; Dr Jukic reports non-financial support from Theralogix, LLC, outside the submitted work. Otherwise there are no competing interests.
Trial registration number
N/A
Keywords: fertility, conception, vitamin D, time to pregnancy, pregnant
Introduction
Vitamin D is known for its role in calcium absorption and bone health, but interest in its role in reproduction has been growing (Lerchbaum and Obermayer-Pietsch, 2012). Vitamin D3 is synthesized in the skin in response to ultraviolet-B radiation. Vitamin D3 and D2 can also be obtained from the diet through several sources including fortified dairy products and fatty fish. Vitamin D is converted in the liver to 25-hydroxyvitamin D (25(OH)D) that is the clinical biomarker of vitamin D status (Dietary Reference Intakes for Calcium and Vitamin D, 2011; Holick et al., 2011). 25(OH)D is then converted to its active form, 1,25-dihydroxyvitamin D, by the enzyme 1α-hydroxylase, a product of the CYP27B1 gene. Mice that lack either CYP27B1 or the vitamin D receptor exhibit arrested follicular development, uterine hypoplasia, prolonged estrous cycles and subfertility (Yoshizawa et al., 1997; Kinuta et al., 2000; Panda et al., 2001; Sun et al., 2010; Dicken et al., 2012). Rats fed a vitamin D deficient diet also exhibit subfertility and reduced litter size (Halloran and DeLuca, 1980; Hickie et al., 1983; Kwiecinksi et al., 1989; Johnson and DeLuca, 2002).
In women undergoing IVF, higher vitamin D status has been associated with improved live birth rates (Lv et al., 2016). Among women with polycystic ovarian syndrome (PCOS), vitamin D has been associated with improvements in spontaneous ovulation (Thys-Jacobs et al., 1999; Rashidi et al., 2009) and IVF success (Pal et al., 2016). In community-based samples of women, three recent studies have reported associations between low 25(OH)D and long or irregular menstrual cycles (Jukic et al., 2015, 2016, 2018). Three studies have examined vitamin D and fertility in women who were not undergoing fertility treatment. One small study of 145 women reported an increased odds of conception with higher 25(OH)D levels, but CIs were wide and time to pregnancy was not examined as a continuum (Moller et al., 2012). Another small study of 132 women reported increased fertility among women with a 25(OH)D level of at least 20 ng/ml compared to women with <20 ng/ml (Fung et al., 2017). The final study reported increased conception rates for women with 25(OH)D levels of at least 30 ng/ml (compared with <30 ng/ml), but only included women with a history of pregnancy loss (Mumford et al., 2018).
The objective of this analysis was to examine the association between circulating 25(OH)D and time to pregnancy in the Time to Conceive study, a prospective community-based cohort of over 500 women who were enrolled early in their attempt to become pregnant and were followed for conception.
Methods
Study design
Time to Conceive was a prospective, time-to-pregnancy cohort study (2008–2016) (Steiner et al., 2011). Women, from the Raleigh-Durham-Chapel Hill area of North Carolina, who were discontinuing contraception were recruited through mass emails, introductory letters and web and radio advertising. Eligible women were ages 30 to 44 and reported having been trying to get pregnant for 3 months or less. Women were excluded if they reported a history of infertility, PCOS, endometriosis, a partner with infertility or current breastfeeding. Participants were asked to keep daily diaries (described below) and to self-report demographic data, reproductive history, contraceptive history and other behaviors through an online questionnaire. Women were also asked to schedule a study visit on Day 2, 3 or 4 of their next menses. If they missed this window, they were asked to come in at the beginning of the subsequent menses.
At the study visit, after giving written informed consent, women provided a blood sample and received pregnancy test kits. In 2010, the study protocol was amended to add the collection of whole blood spots, which were dried and stored frozen. The present analysis is limited to the cohort of women who enrolled in or after 2010 and provided a blood spot. Of the 788 women who gave a blood sample and had some follow-up data, 556 women also had a measure of 25(OH)D (Fig. 1).
Figure 1.
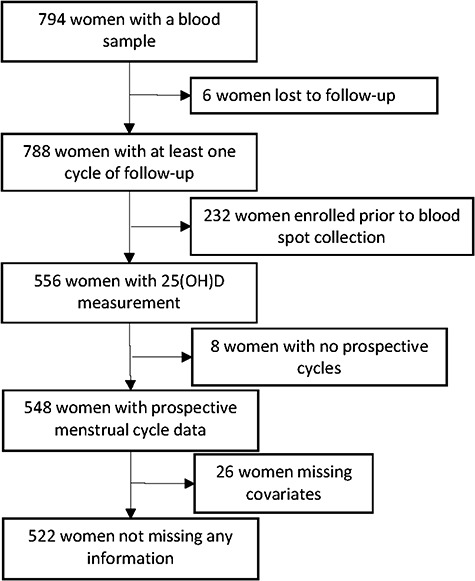
Women included in this analysis from the Time to Conceive cohort, 2010–2016.
Women were asked to complete an online daily diary and a monthly diary for up to 4 months, but only monthly diaries afterwards until a positive pregnancy test was observed or 12 months had elapsed, whichever came first. In the daily diary, women recorded vaginal bleeding, results of their pregnancy tests and vitamin supplement intake. For the latter, participants searched a database based on active ingredient or brand name. They chose each appropriate vitamin from a drop-down menu and entered it into their daily diary. Data from daily diaries was used to identify the start of each menses (Crawford et al., 2016). The first of two consecutive days with menstrual bleeding (not spotting) was considered the day of menses onset. The monthly diary asked about tobacco use, alcohol intake, caffeine consumption and exercise frequency in the preceding month.
Participants were asked to begin testing for pregnancy on Day 28 of the menstrual cycle, and to then test every third day thereafter (i.e. Day 31, 34, etc.) until the test was positive or bleeding began. Women were withdrawn from follow-up if they began fertility treatment or stopped trying to conceive. Women in this analysis were enrolled between February 2010 and July 2015. All study activities were approved by the University of North Carolina IRB.
25(OH)D
25(OH)D was extracted from 6 mm punches from stored blood spots, collected at enrollment, using previously described methods at ZRT laboratories (Beaverton, OR) (Newman et al., 2009; Larkin et al., 2011). 25(OH)D3 and 25(OH)D2 were quantified through liquid chromatography-tandem mass spectrometry, using standardization with the NIST vitamin D standard reference materials. ZRT was certified by the Vitamin D External Quality Assessment Scheme (DEQAS) at the time of the assays (2013–2016) and maintains that certification today. 25(OH)D measured in dried blood spots shows good agreement with plasma measures (Heath et al., 2014). Blinded replicate samples indistinguishable from test samples were also sent to the laboratory. Based on these samples, the intra-assay coefficient of variation was 6.3% and the inter-assay coefficient of variation was 7.7% and there were no differences among batches. Blood spots from 556 women were measured for 25(OH)D (Fig. 1).
Observed attempt time and conception
‘Observed attempt time’ was defined as the number of menstrual cycles from blood draw until the occurrence of either a positive pregnancy test or a censoring event (12 cycles of attempt, withdrawal from the study or beginning fertility treatment). Conception was presumed when there was a positive home pregnancy test.
The number of cycles a woman had been trying to conceive at the time of the blood draw was determined using several participant-reported dates: (i) the date she stopped using contraception, (ii) the date she started having regular intercourse without doing anything to prevent pregnancy and (iii) the date her last pregnancy ended. Using these dates and her average menstrual cycle length, we calculated the number of menstrual cycles the participant had been attempting to conceive prior to the study blood draw. In some cases, this assessment of the cycle number of attempt was inconsistent with the participants’ self-report of being within three cycles of attempt when screened by telephone. This inconsistency was addressed with a sensitivity analysis described below. Of the 556 women with a blood spot, 8 women did not provide any menstrual cycle information after their blood draw, leaving 548 women (Fig. 1).
Covariates
Covariates of interest included age at the beginning of each menstrual cycle and self-reported demographics at baseline: race (Caucasian, African-American, other), gravidity, BMI and education. Each month, participants also reported their number of alcoholic drinks per month, number of caffeinated drinks per day and the number of cigarettes smoked per day. Participants were asked about use of other nicotine products, but none were reported. Since cigarette smoking was rarely reported in the prospective diaries, smoking status at baseline (current, former, never) was used in all analyses. If women did not have at least one prospective monthly report of caffeine or alcohol, her baseline information was used.
Monthly reports were matched with each menstrual cycle of attempt. Missing monthly reports were imputed by taking the previous month’s values, and if this month was missing, the next previous month was used, and so on. If a previous month was not available, a subsequent month’s values were used. Of the 548 women available, 26 women were missing information on covariates, leaving 522 women (Fig. 1). Each month, participants also reported, in categories, their average amount of vigorous exercise (0 h, <1, 1–3, 4–7, >7 h per week). Exercise was not captured on the baseline questionnaire and was therefore more often missing (N = 514 women had exercise information).
Ovulation was identified using ovulation predictor kits (Jukic et al., 2018), basal body temperature or cervical mucus monitoring. For basal body temperature, ovulation day was identified by replacing each day of a cycle with a three-day average (the day prior, the day of interest and the subsequent day) and then scanning for the steepest five-day slope of those averages. For cervical mucus, ovulation day was identified as the last day of fertile cervical mucus during the cycle. If ovulation day could not be identified using these methods, we assigned Day 15 (the mode of the population based on the ovulation predictor kit) as the day of ovulation. Results did not differ when the imputed ovulation days were used versus when they were excluded (data not shown). Using the identified ovulation day, the pattern of sexual intercourse during the fertile window was described based on frequency and timing of sexual intercourse over the six day window ending with the day of ovulation (Weinberg et al., 1994). This pattern can be used to estimate the probability of conception in that cycle based on intercourse alone (Weinberg et al., 1994). This probability was divided into quintiles for analysis and is referred to as ‘fertile window intercourse’.
Statistical analysis
We estimated adjusted fecundability ratios (FR) and 95% CI using log-binomial regression. In this model, conception (yes/no) was the outcome and an intercept was estimated for each cycle of attempt (rather than one overall intercept) by including attempt cycle as a categorical predictor. Including the attempt cycle number allows for the natural decrease in fecundability across cycles and also accommodates delayed entry, which allows for left truncation. For example, a woman whose blood draw occurred early in her third cycle, initiating her follow up as a participant in the study, would only contribute information to the model beginning at cycle 3. Women who were lost to follow-up before conceiving were censored in their last fully observed cycle. Five women contributed two pregnancies to the analysis and their attempts were inverse-weighted by cluster size.
We examined 25(OH)D both as a continuous linear variable and in categories (<20, 20–<30, 30–<40, 40–<50 and ≥50 ng/ml). The lowest category (<20 ng/ml) corresponds to ‘at risk of vitamin D deficiency’, based on the Institute of Medicine and Endocrine Society guidelines and the 20–<30 category is the definition of ‘insufficiency’ based on the Endocrine Society guidelines (Dietary Reference Intakes for Calcium and Vitamin D, 2011; Holick et al., 2011).
Covariates that were assessed as potential confounders were age, race, time since contraceptive estrogen use, fertile window intercourse, education, obesity, alcohol, caffeine and exercise. We evaluated several parameterisations of age with fecundability using a univariable discrete time model described above. We used Akaike’s Information Criterion (AIC) to choose the best-fitting parameterisation of age in each model. All other continuous variables (BMI, caffeine, alcohol and exercise) were evaluated in a similar way: several parameterisations were compared using the AIC from the fecundability model, after adjustment for age and race. Similarly, we examined the univariate associations between each covariate and 25(OH)D. Variables that were not independently associated with both vitamin D and fecundability were not included in the final models. The final model included age, race, education and fertile window intercourse. (Additional adjustment for exercise is presented as a sensitivity analysis in Supplementary Table SI.)
To make the findings more clinically applicable, we used the adjusted log-binomial model to estimate the proportion of women at each specific 25(OH)D category who would not conceive within six cycles of trying (with all other covariates fixed at their referent level). First, the estimated probability of conception at each level of 25(OH)D and at each cycle number was calculated, among couples in that category who are still trying at that cycle number, i.e. conditional on having not conceived earlier. Let that estimated probability of conception at cycle k, given that they are still trying at cycle k, be denoted pk, and let 1 minus pk be denoted qk. Then the estimated proportion of couples who would require more than six cycles to conceive is the product of the qk over k = 1 to k = 6. Ninety-five percent CIs were calculated by generating 1000 bootstrapped samples.
Sensitivity analyses
We performed several sensitivity analyses that are presented in Supplementary Table SI.
First, a single measured value of 25(OH)D may become less relevant over time, with possible changes in vitamin D levels due to season of the year or alterations in supplement use. To account for this, we estimated an extrapolated time-varying cycle-specific 25(OH)D level using the measured 25(OH)D and the values of the predictors at the time of the blood draw (see below and (Jukic et al., 2018)). The measured 25(OH)D was used in all analyses, but the imputed cycle-specific value is presented in Supplementary Table SI.
Second, women did not always attend the clinic visit at the first menses after enrolment and could have been beyond three cycles of attempt at the blood draw. To examine the influence of left truncation, we performed a sensitivity analysis by restricting to women who had been within six cycles of the start of their attempt when they had their blood drawn.
Third, related to the previous analysis, we examined the association between 25(OH)D and fecundability when limiting the cycles to those that occurred within three cycles or 90 days of the blood draw.
Fourth, to examine the influence of women who may have been peri-menopausal or who potentially had undiagnosed PCOS, we completed a sensitivity analysis excluding women with AMH values that were low (<0.7 ng/ml) or high (>7.5 ng/ml).
Fifth, adjustment for gravidity is not advisable when the exposure may have affected previous pregnancies (Howards et al., 2012), thus adjustment for gravidity was also presented as a sensitivity analysis.
Finally, we adjusted the results for level of exercise for those women who provided this information.
Estimating cycle-specific 25(OH)D
We estimated a time-varying cycle-specific 25(OH)D level for cycles after the blood draw.
First, we identified variables for which we had longitudinal data that could change with each menstrual cycle (season, supplement use and time since estrogen use (Mikkelsen et al. 2013; Harmon et al., 2016)). For the menstrual cycle in which the blood draw occurred, season was defined by assigning the date of the blood draw to a radian measure based on its distance from January 1. Season for the menstrual cycles that occurred after the blood draw were defined by the date of the first day of menses. Supplement use was calculated from the daily diary as the average international units (IUs) of vitamin D intake for each menstrual cycle. The average IUs of vitamin D intake was calculated by summing the total IUs consumed during a cycle, divided by the number of days a woman recorded in the daily diary for that cycle. On the baseline questionnaire, women reported their contraceptive use for each of the months immediately before beginning their pregnancy attempt (since hormonal contraception affects fecundability for ~3 months post-cessation (Mikkelsen et al., 2013)). This information was used to determine how much time had elapsed since she had last taken an estrogen-containing contraceptive, which may also affect 25(OH)D levels (Harmon et al., 2016).
Second, using only the cycles in which the blood draw occurred, we modeled the measured 25(OH)D values as a function of season (including the cosine and sine for the radian measure described above (Galbraith, 1988)), supplement use and time since estrogen use. We also included interactions between race and obesity with season and/or supplement use where they were important (P < 0.2).
Third, the difference between the predicted 25(OH)D and the observed 25(OH)D was calculated. We then used the predictive model to estimate 25(OH)D in other observed menstrual cycles using the date of the cycle, the time since estrogen use and the supplement use reported for that menstrual cycle. The residual that had been calculated in step three was added to the modeled 25(OH)D to obtain the final estimated 25(OH)D level for the cycle. We refer to this measure as ‘cycle-specific 25(OH)D’.
Ethical approval
The Institutional Review Board at the University of North Carolina at Chapel Hill reviewed and approved this protocol.
Results
Univariable description
Of the 522 women, 257 conceived and the median number of cycles in the study was 3. The mean age of the women was 33 years and the mean 25(OH)D was 36 ng/ml and the SD was 11 ng/ml (median (interquartile range): 35 ng/ml (29–41)). Women who entered the study in their first attempt cycle tended to have slightly higher 25(OH)D than those entering later, mean 25(OH)D was 38 ng/ml in cycle 1, 35 in cycle 2, 36 in cycle 3, 32 in cycle 4 and 36 for cycle 5 or higher. African-American women, women with less education and overweight or obese women had lower 25(OH)D (Table I). Women with higher levels of exercise or who had recently used an estrogen-containing contraceptive had higher 25(OH)D, as did women with higher alcohol consumption. The average age among women who did not conceive was 34.6 compared with 32.9 among women who conceived. Women who did not conceive had a mean 25(OH)D of 34.4 ng/ml compared with 36.5 ng/ml among women who conceived.
Table I.
Distribution of 25(OH)D among eligible participants from their first cycle in Time to Conceive (N = 522).
| 25(OH) D (ng/ml) | ||||||||||||
| Overall | <20 | 20—<30 | 30—<40 | 40—<50 | > = 50 | |||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Age | ||||||||||||
| 29–30 | 103 | (20) | 2 | (10) | 20 | (15) | 52 | (25) | 18 | (17) | 11 | (24) |
| 31–32 | 148 | (28) | 4 | (20) | 40 | (29) | 63 | (30) | 28 | (26) | 13 | (29) |
| 33–35 | 153 | (29) | 6 | (30) | 50 | (36) | 53 | (25) | 32 | (30) | 12 | (27) |
| 36–40 | 98 | (19) | 7 | (35) | 24 | (18) | 37 | (17) | 24 | (22) | 6 | (13) |
| >40 | 20 | (4) | 1 | (5) | 3 | (2) | 7 | (3) | 6 | (6) | 3 | (7) |
| Race | ||||||||||||
| African-American | 40 | (8) | 9 | (45) | 16 | (12) | 11 | (5) | 2 | (2) | 2 | (4) |
| Caucasian | 413 | (79) | 5 | (25) | 94 | (69) | 175 | (83) | 97 | (90) | 42 | (93) |
| Other | 69 | (13) | 6 | (30) | 27 | (20) | 26 | (12) | 9 | (8) | 1 | (2) |
| Education | ||||||||||||
| Some college or less | 37 | (7) | 8 | (40) | 13 | (9) | 10 | (5) | 5 | (5) | 1 | (2) |
| College graduate | 104 | (20) | 3 | (15) | 26 | (19) | 48 | (23) | 18 | (17) | 9 | (20) |
| Some graduate school or master’s degree | 241 | (46) | 2 | (10) | 56 | (41) | 102 | (48) | 57 | (53) | 24 | (53) |
| Terminal degree (MD, PhD) | 140 | (27) | 7 | (35) | 42 | (31) | 52 | (25) | 28 | (26) | 11 | (24) |
| Body mass index | ||||||||||||
| <20 | 68 | (13) | 1 | (5) | 10 | (7) | 36 | (17) | 17 | (16) | 4 | (9) |
| 20–25 | 275 | (53) | 8 | (40) | 67 | (49) | 111 | (52) | 58 | (54) | 31 | (69) |
| >25–30 | 102 | (20) | 2 | (10) | 35 | (26) | 39 | (18) | 20 | (19) | 6 | (13) |
| >30 | 77 | (15) | 9 | (45) | 25 | (18) | 26 | (12) | 13 | (12) | 4 | (9) |
| Gravid | ||||||||||||
| Yes | 242 | (46) | 11 | (55) | 64 | (47) | 105 | (50) | 44 | (41) | 18 | (40) |
| No | 280 | (54) | 9 | (45) | 73 | (53) | 107 | (50) | 64 | (59) | 27 | (60) |
| Time since estrogen use | ||||||||||||
| More than 3 months | 432 | (83) | 20 | (100) | 123 | (90) | 174 | (82) | 84 | (78) | 31 | (69) |
| One month or less | 28 | (5) | 0 | 0 | 5 | (4) | 8 | (4) | 11 | (10) | 4 | (9) |
| Two months | 19 | (4) | 0 | 0 | 3 | (2) | 8 | (4) | 4 | (4) | 4 | (9) |
| Three months | 43 | (8) | 0 | 0 | 6 | (4) | 22 | (10) | 9 | (8) | 6 | (13) |
| 25(OH) D (ng/ml) | ||||||||||||
| Overall | <20 | 20—<30 | 30—<40 | 40—<50 | > = 50 | |||||||
| N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | N | (%) | |
| Poorly timed intercourse in the fertile window | ||||||||||||
| No | 238 | (46) | 11 | (55) | 63 | (46) | 91 | (43) | 54 | (50) | 19 | (42) |
| Yes | 284 | (54) | 9 | (45) | 74 | (54) | 121 | (57) | 54 | (50) | 26 | (58) |
| Attempt cycle at blood draw | ||||||||||||
| 0a | 13 | (2) | 0 | 0 | 3 | (2) | 5 | (2) | 3 | (3) | 2 | (4) |
| 1 | 103 | (20) | 1 | (5) | 31 | (23) | 28 | (13) | 28 | (26) | 15 | (33) |
| 2 | 213 | (41) | 6 | (30) | 51 | (37) | 103 | (49) | 36 | (33) | 17 | (38) |
| 3 | 103 | (20) | 6 | (30) | 23 | (17) | 44 | (21) | 22 | (20) | 8 | (18) |
| 4 | 59 | (11) | 6 | (30) | 19 | (14) | 23 | (11) | 11 | (10) | 0 | 0 |
| > = 5 | 31 | (6) | 1 | (5) | 10 | (7) | 9 | (4) | 8 | (7) | 3 | (7) |
| Average number of alcoholic drinks per month during prospective diary collection | ||||||||||||
| 0—<1.5 | 164 | (31) | 11 | (55) | 57 | (42) | 61 | (29) | 25 | (23) | 10 | (22) |
| 1.5—<8.25 | 177 | (34) | 5 | (25) | 43 | (31) | 80 | (38) | 32 | (30) | 17 | (38) |
| 8.25–80 | 181 | (35) | 4 | (20) | 37 | (27) | 71 | (33) | 51 | (47) | 18 | (40) |
| Average number of caffeinated drinks per day during prospective diary collection | ||||||||||||
| 0–0.80 | 123 | (24) | 4 | (20) | 36 | (26) | 51 | (24) | 18 | (17) | 14 | (31) |
| >0.80–1.0 | 241 | (46) | 10 | (50) | 61 | (45) | 102 | (48) | 48 | (44) | 20 | (44) |
| >1–1.95 | 158 | (30) | 6 | (30) | 40 | (29) | 59 | (28) | 42 | (39) | 11 | (24) |
| How many hours exercised vigorously in the past month | ||||||||||||
| Missing | 21 | (4) | 0 | 0 | 7 | (5) | 6 | (3) | 6 | (6) | 2 | (4) |
| 0 | 58 | (11) | 5 | (25) | 18 | (13) | 20 | (9) | 13 | (12) | 2 | (4) |
| <1 | 98 | (19) | 2 | (10) | 25 | (18) | 46 | (22) | 19 | (18) | 6 | (13) |
| 1–3 | 194 | (37) | 10 | (50) | 52 | (38) | 85 | (40) | 30 | (28) | 17 | (38) |
| 4–7 | 122 | (23) | 2 | (10) | 30 | (22) | 44 | (21) | 34 | (31) | 12 | (27) |
| >7 | 29 | (6) | 1 | (5) | 5 | (4) | 11 | (5) | 6 | (6) | 6 | (13) |
aThe 13 women had their blood drawn prior to starting their attempt at pregnancy, for example, while using a barrier method for contraception.
25(OH)D and fecundability
Unadjusted, there was a 6% increase in fecundability for each 10 ng/ml increase in 25(OH)D (FR (95% CI): 1.06 (0.98, 1.14)). With full adjustment, there was a 10% increase in fecundability for each 10 ng/ml increase in 25(OH)D (Table II).
Table II.
Associations between 25(OH)D and fecundability among participants from the Time to Conceive cohort (N = 522 women).
| Number of cycles | FR (95% CI)a | |
|---|---|---|
| 25(OH)D, per 10 ng/ml increase | 1282 | 1.10 (0.96, 1.25) |
| <20 ng/ml | 56 | 0.55 (0.23, 1.32) |
| 20–30 | 318 | 1.17 (0.89, 1.53) |
| 30–40 | 523 | 1 |
| 40–50 | 278 | 0.97 (0.73, 1.31) |
| ≥50 | 107 | 1.35 (0.95, 1.91) |
aAdjusted for age, race, education and fertile window intercourse.
In the unadjusted models, when compared with a 25(OH)D level between 30 and 40 ng/ml, 25(OH)D <20 ng/ml was associated with lower fecundability (FR(95% CI): 0.50 (0.21, 1.18)) while a 25(OH)D of at least 50 ng/ml was associated with higher fecundability (FR (95% CI): 1.26 (0.87, 1.81)). In the fully adjusted model, low 25(OH)D was associated with lower fecundability (FR (95% CI): 0.55 (0.23, 1.32)), although the magnitude of effect was smaller, and the CI was wide (Table II). The point estimate was unchanged when examined as a dichotomous variable, i.e. when comparing women with clinical deficiency (<20 ng/ml) to all other women (> = 20 ng/ml), FR (95% CI): 0.52 (0.22, 1.23). On the other hand, in the fully adjusted model, women with a 25(OH)D level of at least 50 ng/ml had 35% higher fecundability (CI: 0.95, 1.91) (Table II). Additional adjustment for season did not materially alter these results (FR (CI): 1.32 (0.93, 1.87)). These results were also not changed when excluding the seven current smokers (FR (CI):1.35 (0.95, 1.91)) or when adjusting for paternal age and obesity (FR (CI): 1.33 (0.93, 1.91)).
The estimated probability of taking longer than six menstrual cycles to conceive was 51% in women with 25(OH)D <20 ng/ml (95% CI: 0.17, 0.74), 28% in women with 25(OH)D of 30–40 ng/ml (95% CI: 0.17, 0.39) and 15% in women with 25(OH)D >50 ng/ml (95% CI: 0.10, 0.37).
The results were slightly weaker using the predicted cycle-specific value of 25(OH)D, although the sample size of cycles was also smaller (Supplementary Table SI). The results in Table II were robust to the other sensitivity analyses, which resulted in FRs of 1.27 to 1.50 (Supplementary Table SI).
Discussion
In women of late reproductive age with no known fertility problems, we found suggestive associations between low levels of 25(OH)D and lower fecundability and between high levels of 25(OH)D and increased fecundability. The level of 25(OH)D with the highest fecundability (50 ng/ml or greater) and estimated cumulative probability of pregnancy is higher than the levels recommended by either the Institute of Medicine or the Endocrine Society (Dietary Reference Intakes for Calcium and Vitamin D, 2011; Holick et al., 2011). The relevance of higher levels of 25(OH)D for fertility has not been previously explored in a community-based sample. However, a previous study reported that among women with PCOS undergoing IVF, a 25(OH)D level of at least 40 ng/ml was associated with improved outcomes (Pal et al., 2016). A recent meta-analysis of five studies also found that higher 25(OH)D improved IVF success (Lv et al., 2016), although the authors noted heterogeneity across those studies. Our results are consistent with this literature and suggest that higher levels of 25(OH)D may also be relevant for improved reproductive function more generally. Furthermore, low vitamin D levels have previously been associated with long or irregular menstrual cycles (Jukic et al., 2015, 2016, 2018). Thus, lower levels of vitamin D may lead to both long cycles and a decreased probability of conception in each cycle. These effects would generally combine to increase the calendar time required to achieve conception.
Three other studies have examined 25(OH)D and fecundability. A study of 132 healthy women in the Northeast US found three times the odds of clinical pregnancy within six menstrual cycles among women with a 25(OH)D of at least 30 ng/ml (compared with <30 ng/ml) (Fung et al., 2017). The estimated fecundability ratio in that study was 1.19 (0.74, 1.92). Another study followed 145 Danish women attempting pregnancy and found no association of 25(OH)D with conceiving within about 6 months (Moller et al., 2012); however, this analysis did not examine time to pregnancy as a continuum. Finally, a recent study of women with a history of pregnancy loss reported an increased conception rate with higher 25(OH)D (Mumford et al., 2018). The adjusted fecundability odds ratio comparing women with vitamin D sufficiency (≥30 ng/ml) to women with insufficiency (<30 ng/ml) was 1.13 (CI: 0.95, 1.34). This is weaker than the result we have reported here for women with high 25(OH)D levels. However, in the previous studies, results were not presented for even higher levels of 25(OH)D. It is possible that higher levels of 25(OH)D drove the associations with conception or fecundability in all the previous studies.
Our results are also consistent with numerous animal studies that have demonstrated an effect of lower vitamin D on fertility (Halloran and DeLuca, 1980; Hickie et al., 1983; Kwiecinksi et al., 1989; Yoshizawa et al., 1997; Kinuta et al., 2000; Panda et al., 2001; Johnson and DeLuca, 2002; Sun et al., 2010; Dicken et al., 2012). However, the underlying mechanism of this association is unclear. The further associations of vitamin D with menstrual cycles and ovulation timing (Jukic et al., 2015, 2016, 2018) suggest that vitamin D may play a role in ovulation or the hypothalamic–pituitary–ovarian axis. One study in mice suggests that vitamin D may influence ovarian angiogenic factors that in turn influence the development of ovarian follicles (Sun et al., 2010). Other animal studies suggest that low vitamin D is associated with hypergonadotropic hypogonadism, including decreased ovarian aromatase gene expression and activity, decreased estrogen synthesis and increased pituitary LH and FSH (Kinuta et al., 2000; Sun et al., 2010). Finally, one study of mice reported that the vitamin D receptor is expressed in gonadotropin-releasing hormone (GnRH) neurons, which might suggest a role for vitamin D in hypothalamic signaling (Dicken et al., 2012).
In humans, little is known regarding vitamin D and hormone levels, and the existing studies differ in their underlying study populations and the timing of biospecimen collection. Higher circulating 25(OH)D has been associated with both higher (Zhao et al., 2017) and lower estradiol (Knight et al., 2010), but an absence of association has also been reported (Chang et al., 2014). One study reported a decrease in progesterone with higher 25(OH)D (Knight et al., 2010); however, progesterone was measured on Day 21 of the menstrual cycle without accounting for ovulation timing, which is important given the reported association of low 25(OH)D with delayed ovulation (Jukic et al., 2018). Interestingly, 25(OH)D has been positively correlated with testosterone (Chang et al., 2014; Zhao et al., 2017).
Vitamin D has also been associated with uterine receptivity and embryonic implantation, which might also be relevant mechanisms for the observed associations with fecundability. An in vitro study of human endometrium reported that active vitamin D upregulated HOXA10 expression, which is known to influence uterine receptivity and embryonic implantation (Du et al., 2005). Extravillous trophoblast invasion is increased in vitro when treated with either active vitamin D or 25(OH)D (Chan et al., 2015). Pregnancy rates may be lower in vitamin D-deficient recipients of donor oocytes, suggesting that uterine receptivity is influenced by vitamin D (Rudick et al., 2014), although another study found no difference (Fabris et al., 2017). A randomized trial of vitamin D reported improved endometrial thickness in women who received vitamin D supplementation (Abedi et al., 2019). Fertilisation is another possible mechanism underlying the observed associations as some IVF studies have reported improved fertilisation rates in vitamin D sufficient women (Firouzabadi et al., 2014; Abadia et al., 2016), although another study showed no association (Fabris et al., 2017).
In the IVF literature more broadly, studies are conflicting. Several studies report increased IVF success for women with higher serum 25(OH)D (Ozkan et al., 2010; Rudick et al., 2012; Garbedian et al., 2013; Polyzos et al., 2014) or follicular fluid 25(OH)D levels (Ozkan et al., 2010). However, other studies of women using artificial reproductive technologies report no association of vitamin D with various measures of IVF success (Fabris et al., 2014; Franasiak et al., 2015; Abadia et al., 2016; Butts et al., 2019). The reason for these differences is unclear but may be due to small sample sizes or the underlying distribution of race or ethnicity or of causes of infertility in each study sample. For example, women with PCOS specifically, may have a higher probability of artificial reproductive technology success if they are not vitamin D deficient (Ott et al., 2012; Butts et al., 2019; Cunningham et al., 2019). It is also possible that women undergoing IVF differ systematically across studies.
This study was larger than all but one prior study, by including over 500 women, with a wide distribution of 25(OH)D which enabled detection of associations at the high end of 25(OH)D. This study is further strengthened by the use of prospective daily diary information as well as monthly reports, which were used to define menstrual cycles during the attempt time. However, dietary information was not collected in these diaries which could lead to residual confounding if foods that provide vitamin D influence fecundability independently of vitamin D. We did have information regarding alcohol and caffeine use. A limitation is that this analysis relies on a single blood sample drawn early in the attempt to become pregnant. To address this, we built an imputation model to estimate the cycle-specific 25(OH)D level in post-blood sampling cycles as the seasons changed. This refined estimate did not substantially change the results. Women in this study were generally well-educated, which may limit generalizability to other populations.
Conclusions
Although imprecise, there were suggestive associations of high 25(OH)D (at least 50 ng/ml) with increased fecundability and low 25(OH)D (<20 ng/ml) with reduced fecundability. Reproductive function may be improved at 25(OH)D levels that are higher than current clinical guidelines, which recommend at least 20 ng/ml (Dietary Reference Intakes for Calcium and Vitamin D, 2011) or at least 30 ng/ml (Holick et al., 2011) of 25(OH)D. Our results are consistent with animal and human literature. Future studies should confirm the association and investigate the mechanism underlying it. Possible mechanisms include the influence of vitamin D on reproductive endocrinology, follicle development and uterine receptivity in humans.
Supplementary data
Supplementary data are available at Human Reproduction online.
Supplementary Material
Acknowledgements
We thank Dr Katie O’Brien and Dr Kelly Ferguson for their comments on a draft of this manuscript.
Authors’ roles
A.M.Z.J. conceived of the study, performed the data analysis and drafted the manuscript. D.D.B., C.R.W., A.J.W. and D.R.M. provided input on the data analysis strategy and design and edited the manuscript. A.Z.S. designed the Time to Conceive study, provided input on the data analysis strategy and design and edited the manuscript. All authors approved the final submitted draft.
Funding
ClearBlue ovulation predictor kits were generously donated to A.M.Z.J. and A.J.W. by Swiss Precision Diagnostics. This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NIH) under award numbers R00HD079659 and R01HD067683 and supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, under projects ES103086, ES049003, ES044003 and ES103333. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest
Drs Wilcox and Jukic report non-financial support from Swiss Precision Diagnostics during the conduct of the study. Dr Jukic reports non-financial support from Theralogix, LLC outside the submitted work.
References
- Abadia L, Gaskins AJ, Chiu YH, Williams PL, Keller M, Wright DL, Souter I, Hauser R, Chavarro JE, Environment, and Team Reproductive Health Study . Serum 25-hydroxyvitamin D concentrations and treatment outcomes of women undergoing assisted reproduction. Am J Clin Nutr 2016;104:729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi S, Taebi M, Nasr Esfahani MH. Effect of vitamin D supplementation on intracytoplasmic sperm injection outcomes: a randomized double-blind placebo-controlled trial. Int J Fertil Steril 2019;13:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts SF, Seifer DB, Koelper N, Senapati S, Sammel MD, Hoofnagle AN, Kelly A, Krawetz SA, Santoro N, Zhang H et al. Vitamin D deficiency is associated with poor ovarian stimulation outcome in PCOS but not unexplained infertility. J Clin Endocrinol Metab 2019;104:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SY, Susarla R, Canovas D, Vasilopoulou E, Ohizua O, McCabe CJ, Hewison M, Kilby MD. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta 2015;36:403–409. [DOI] [PubMed] [Google Scholar]
- Chang EM, Kim YS, Won HJ, Yoon TK, Lee WS. Association between sex steroids, ovarian reserve, and vitamin D levels in healthy nonobese women. J Clin Endocrinol Metab 2014;99:2526–2532. [DOI] [PubMed] [Google Scholar]
- Crawford NM, Pritchard DA, Herring AH, Steiner AZ. Prospective evaluation of the impact of intermenstrual bleeding on natural fertility. Fertil Steril 2016;105:1294–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TK, Allgar V, Dargham SR, Kilpatrick E, Sathyapalan T, Maguiness S, Mokhtar Rudin HR, Abdul Ghani NM, Latiff A, Atkin SL. Association of vitamin D metabolites with embryo development and fertilization in women with and without PCOS undergoing subfertility treatment. Front Endocrinol (Lausanne) 2019;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicken CL, Israel DD, Davis JB, Sun Y, Shu J, Hardin J, Neal-Perry G. Peripubertal vitamin D(3) deficiency delays puberty and disrupts the estrous cycle in adult female mice. Biol Reprod 2012;87:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press, 2011. [PubMed] [Google Scholar]
- Du H, Daftary GS, Lalwani SI, Taylor HS. Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol 2005;19:2222–2233. [DOI] [PubMed] [Google Scholar]
- Fabris AM, Cruz M, Iglesias C, Pacheco A, Patel A, Patel J, Fatemi H, Garcia-Velasco JA. Impact of vitamin D levels on ovarian reserve and ovarian response to ovarian stimulation in oocyte donors. Reprod Biomed Online 2017;35:139–144. [DOI] [PubMed] [Google Scholar]
- Fabris A, Pacheco A, Cruz M, Puente JM, Fatemi H, Garcia-Velasco JA. Impact of circulating levels of total and bioavailable serum vitamin D on pregnancy rate in egg donation recipients. Fertil Steril 2014;102:1608–1612. [DOI] [PubMed] [Google Scholar]
- Firouzabadi RD, Rahmani E, Rahsepar M, Firouzabadi MM. Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet 2014;289:201–206. [DOI] [PubMed] [Google Scholar]
- Franasiak JM, Molinaro TA, Dubell EK, Scott KL, Ruiz AR, Forman EJ, Werner MD, Hong KH, Scott RT Jr. Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol 2015;212:e1–e6. [DOI] [PubMed] [Google Scholar]
- Fung JL, Hartman TJ, Schleicher RL, Goldman MB. Association of vitamin D intake and serum levels with fertility: results from the lifestyle and fertility study. Fertil Steril 2017;108:302–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith RF. Trigonometric Regression In: Armitage P, Colton T (eds). Encyclopedia of Biostatistics. Chichester: Wiley, 1988. [Google Scholar]
- Garbedian K, Boggild M, Moody J, Liu KE. Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open 2013;1:E77–E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran BP, DeLuca HF. Effect of vitamin D deficiency on fertility and reproductive capacity in the female rat. J Nutr 1980;110:1573–1580. [DOI] [PubMed] [Google Scholar]
- Harmon QE, Umbach DM, Baird DD. Use of estrogen-containing contraception is associated with increased concentrations of 25-hydroxy vitamin D. J Clin Endocrinol Metab 2016;101:3370–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AK, Williamson EJ, Ebeling PR, Kvaskoff D, Eyles DW, English DR. Measurements of 25-hydroxyvitamin D concentrations in archived dried blood spots are reliable and accurately reflect those in plasma. J Clin Endocrinol Metab 2014;99:3319–3324. [DOI] [PubMed] [Google Scholar]
- Hickie JP, Lavigne DM, Woodward WD. Reduced fecundity of vitamin D deficient rats. Comp Biochem Physiol A Comp Physiol 1983;74:923–925. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine S. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF, Poole C, Kaufman JS, Weinberg CR. ‘Toward a clearer definition of confounding’ revisited with directed acyclic graphs. Am J Epidemiol 2012;176:506–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LE, DeLuca HF. Reproductive defects are corrected in vitamin d-deficient female rats fed a high calcium, phosphorus and lactose diet. J Nutr 2002;132:2270–2273. [DOI] [PubMed] [Google Scholar]
- Jukic AM, Steiner AZ, Baird DD. Lower plasma 25-hydroxyvitamin D is associated with irregular menstrual cycles in a cross-sectional study. Reprod Biol Endocrinol 2015;13:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AMZ, Upson K, Harmon QE, Baird DD. Increasing serum 25-hydroxyvitamin D is associated with reduced odds of long menstrual cycles in a cross-sectional study of African American women. Fertil Steril 2016;106:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukic AMZ, Wilcox AJ, McConnaughey DR, Weinberg CR, Steiner AZ. 25-hydroxyvitamin D and long menstrual cycles in a prospective cohort study. Epidemiology 2018;29:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology 2000;141:1317–1324. [DOI] [PubMed] [Google Scholar]
- Knight JA, Wong J, Blackmore KM, Raboud JM, Vieth R. Vitamin D association with estradiol and progesterone in young women. Cancer Causes Control 2010;21:479–483. [DOI] [PubMed] [Google Scholar]
- Kwiecinksi GG, Petrie GI, DeLuca HF. 1,25-dihydroxyvitamin D3 restores fertility of vitamin D-deficient female rats. Am J Physiol 1989;256:E483–E487. [DOI] [PubMed] [Google Scholar]
- Larkin EK, Gebretsadik T, Koestner N, Newman MS, Liu Z, Carroll KN, Minton P, Woodward K, Hartert TV. Agreement of blood spot card measurements of vitamin D levels with serum, whole blood specimen types and a dietary recall instrument. PLoS One 2011;6:e16602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol 2012;166:765–778. [DOI] [PubMed] [Google Scholar]
- Lv SS, Wang JY, Wang XQ, Wang Y, Xu Y. Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 2016;293:1339–1345. [DOI] [PubMed] [Google Scholar]
- Mikkelsen EM, Riis AH, Wise LA, Hatch EE, Rothman KJ, Sorensen HT. Pre-gravid oral contraceptive use and time to pregnancy: a Danish prospective cohort study. Hum Reprod 2013;28:1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller UK, Streym S, Heickendorff L, Mosekilde L, Rejnmark L. Effects of 25OHD concentrations on chances of pregnancy and pregnancy outcomes: a cohort study in healthy Danish women. Eur J Clin Nutr 2012;66:862–868. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Garbose RA, Kim K, Kissell K, Kuhr DL, Omosigho UR, Perkins NJ, Galai N, Silver RM, Sjaarda LA et al. Association of preconception serum 25-hydroxyvitamin D concentrations with livebirth and pregnancy loss: a prospective cohort study. Lancet Diabetes Endocrinol 2018;6:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MS, Brandon TR, Groves MN, Gregory WL, Kapur S, Zava DT. A liquid chromatography/tandem mass spectrometry method for determination of 25-hydroxy vitamin D2 and 25-hydroxy vitamin D3 in dried blood spots: a potential adjunct to diabetes and cardiometabolic risk screening. J Diabetes Sci Technol 2009;3:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J, Wattar L, Kurz C, Seemann R, Huber JC, Mayerhofer K, Vytiska-Binstorfer E. Parameters for calcium metabolism in women with polycystic ovary syndrome who undergo clomiphene citrate stimulation: a prospective cohort study. Eur J Endocrinol 2012;166:897–902. [DOI] [PubMed] [Google Scholar]
- Ozkan S, Jindal S, Greenseid K, Shu J, Zeitlian G, Hickmon C, Pal L. Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 2010;94:1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal L, Zhang H, Williams J, Santoro NF, Diamond MP, Schlaff WD, Coutifaris C, Carson SA, Steinkampf MP, Carr BR et al. Vitamin D status relates to reproductive outcome in women with polycystic ovary syndrome: secondary analysis of a multicenter randomized controlled trial. J Clin Endocrinol Metab 2016;101:3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A 2001;98:7498–7503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos NP, Anckaert E, Guzman L, Schiettecatte J, Van Landuyt L, Camus M, Smitz J, Tournaye H. Vitamin D deficiency and pregnancy rates in women undergoing single embryo, blastocyst stage, transfer (SET) for IVF/ICSI. Hum Reprod 2014;29:2032–2040. [DOI] [PubMed] [Google Scholar]
- Rashidi B, Haghollahi F, Shariat M, Zayerii F. The effects of calcium-vitamin D and metformin on polycystic ovary syndrome: a pilot study. Taiwan J Obstet Gynecol 2009;48:142–147. [DOI] [PubMed] [Google Scholar]
- Rudick B, Ingles S, Chung K, Stanczyk F, Paulson R, Bendikson K. Characterizing the influence of vitamin D levels on IVF outcomes. Hum Reprod 2012;27:3321–3327. [DOI] [PubMed] [Google Scholar]
- Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA. Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril 2014;101:447–452. [DOI] [PubMed] [Google Scholar]
- Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, Baird DD. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol 2011;117:798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Xie H, Ji J, Zhou X, Goltzman D, Miao D. Defective female reproductive function in 1,25(OH)2D-deficient mice results from indirect effect mediated by extracellular calcium and/or phosphorus. Am J Physiol Endocrinol Metab 2010;299:E928–E935. [DOI] [PubMed] [Google Scholar]
- Thys-Jacobs S, Donovan D, Papadopoulos A, Sarrel P, Bilezikian JP. Vitamin D and calcium dysregulation in the polycystic ovarian syndrome. Steroids 1999;64:430–435. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Gladen BC, Wilcox AJ. Models relating the timing of intercourse to the probability of conception and the sex of the baby. Biometrics 1994;50:358–367. [PubMed] [Google Scholar]
- Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y et al. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet 1997;16:391–396. [DOI] [PubMed] [Google Scholar]
- Zhao D, Ouyang P, Boer IH, Lutsey PL, Farag YM, Guallar E, Siscovick DS, Post WS, Kalyani RR, Billups KL et al. Serum vitamin D and sex hormones levels in men and women: the multi-ethnic study of atherosclerosis (MESA). Maturitas 2017;96:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


