Supplemental Digital Content is Available in the Text.
Keywords: Chronic pain, Memory bias, Pain-related information, Meta-analysis, Systematic review
Abstract
Pain-related memory biases have been frequently explored in individuals with chronic pain, and along with attentional and interpretation biases are hypothesised to contribute to the onset and/or maintenance of chronic pain. The aim of this review is to provide a systematic review and synthesis of studies exploring memory recall biases for pain-related information in individuals with chronic pain relative to healthy controls and the recall of neutral information. Studies were identified through a search of Medline, PsychINFO, Web of Science, CINAHL, Cochrane Library, and Open Grey databases. Search terms were memory, recall, recognition, and bias*, intersected with pain. Eighteen studies meeting the inclusion criteria were included. Subset meta-analyses are also reported from 12 studies with relevant between-groups data (comparing recall in chronic pain vs healthy control groups) and 12 studies with relevant within-groups data (eg, comparing recall of pain-related/emotional vs neutral words). Between-groups analysis revealed significantly weaker recall bias for affective-pain words in individuals with chronic pain relative to healthy controls, but only when nondepressed chronic pain individuals were included. No significant differences were found between groups in the recall of sensory-pain, illness-related, or depression-related words. Within-groups analysis revealed individuals with chronic pain show a significant recall bias favouring sensory-pain words relative to neutral and affective-pain words, and a bias for illness-related words relative to depression-related words. A recall bias favouring neutral words was found in healthy individuals. Evidence for the presence of pain-related memory biases in patients with chronic pain is inconclusive. Further methodologically rigorous research is required.
1. Introduction
Individuals with chronic pain show pain-related attentional17,86,101 and interpretation88 biases compared with healthy individuals. Theoretical accounts of emotional processing and chronic pain also predict memory biases favouring the recall of pain-related information in people with chronic pain,4,5,72 and it has been argued different forms of cognitive bias interact and influence one another.28,40 The Threat Interpretation Model100 proposes an interpretation bias favouring the pain-related meaning of ambiguous information is necessary, but not sufficient, for an attentional bias to be observed. Biases in memory have not been incorporated into this model, although memory, attention, and interpretation processes interact with each other.14,41 More recently, van Ryckeghem et al.102 have argued the relationship between pain-related attentional, interpretation, and memory biases is bidirectional, which is likely due to shared underlying mechanisms (ie, motivational and contextual variables), and that the co-occurrence of multiple forms of bias may have cumulative effects on pain-related outcomes. Further to testing these predictions and contributing to a more comprehensive theoretical model of pain-related cognitive biases, an understanding of memory biases in chronic pain is important because it has been speculated they may exacerbate or maintain the experience of pain.46,58 Across the broader literature, evidence of memory bias has been reported in meta-analyses of anxious37,59 and depressed56 populations, and the causal role of such biases in the development of anxiety and depression debated. A review of existing evidence for memory biases in chronic pain will make an important contribution to the chronic pain cognitive bias field and help guide future research into their clinical implications.
Pincus and Morley72 provided an excellent narrative review of the cognitive bias literature up to 2001. In their review of the memory bias literature, which included 5 studies with adults and 2 studies with children, they explored recall biases for pain-/illness-related words. A meta-analysis was not conducted nor were specific between-groups effect sizes comparing chronic pain vs pain-free controls computed. Only within-groups effect sizes were calculated where possible comparing recall for pain-related words vs control words, although insufficient information is provided on the exact procedures undertaken. For adults with chronic pain, effect sizes pertaining to the recall of sensory-pain vs neutral words were small to large across 2 studies (0.33–0.78). Nevertheless, and in the absence of between-groups meta-analyses, Pincus and Morley concluded there to be robust evidence for memory biases in patients with chronic pain. The specificity of bias and comorbidity of depression were also considered by the authors in their narrative review, concluding that biases exist towards sensory-pain words in patients with chronic pain, along with biases towards broader health- and illness-related words in patients who were concurrently depressed or distressed. Considering the overall cognitive bias literature (ie, attention, interpretation, and memory biases), they concluded patients with chronic pain demonstrated preferential processing of sensory-pain stimuli in particular. However, numerous studies have been published since this review. A more recent review from Rusu et al.84 also highlighted evidence of memory biases in individuals with chronic pain, in particular towards sensory-pain words. It was noted, however, that more recent studies have not replicated this finding, and that future research is needed exploring the influence of moderating variables such as patient depression on memory biases. While informative and timely, neither a systematic search nor a meta-analysis was conducted as part of this review.
The aim of this systematic review is to provide an updated synthesis of studies exploring memory biases for pain-related (sensory-pain, affective-pain, pain images), illness-related, and emotional (depression-related, negative) information in adults with chronic pain, and a subset meta-analysis comparing memory biases in patients with chronic pain relative to healthy, pain-free individuals, and also relative to the recall of neutral words. Although there is a growing body of research exploring the influence of memory on pain and pain outcomes in children and adolescents,66,67 a review of the paediatric cognitive bias literature specifically has recently been published,49 with a further systematic review and meta-analysis of this literature currently underway.52 The present review will therefore focus on the adult memory bias literature only, addressing the following questions: (1) Are adults with chronic pain characterised by a memory bias specifically favouring the recall of pain-related information compared with healthy controls? and (2) Are adults with chronic pain characterised by a memory bias favouring the recall of pain-related information relative to neutral information?
2. Methods
2.1. Literature search
PRISMA guidelines were followed,60 and although the protocol was not registered with PROSPERO,78 it is available on request. Studies were identified through a search of Web of Science (title), MEDLINE, PsychINFO and CINAHL (title, subject terms), Cochrane Library (title, abstract, key words), and Open Grey (main search field) databases (the full search strategy is provided in supplementary material 1, available at http://links.lww.com/PR9/A62). Search terms were memory, recall, recognition, and bias*, intersected with pain. The names of known researchers in the chronic pain cognitive bias field were also used as search terms in the databases. Finally, reference lists of all obtained articles were inspected. All searches were made from database inception. The initial literature search was conducted by DS, and all potentially eligible records were independently reviewed by D.E.S. and C.L. with any disagreements resolved by discussion.
2.2. Inclusion and exclusion criteria
For inclusion in the review, each study was required to meet the following criteria:
Available in the English language until November 12, 2019.
Explored memory recall biases for presented pain-related or illness-related information and provided relevant data.
Included a sample of adults (≥18 years old) with chronic pain lasting 3 months or longer.
Cognitive biases may differ between children and adults, and therefore, studies recruiting a paediatric sample43,46 were not included. Patterns of cognitive bias in adult populations may be confounded by recurrent episodes of pain and pain management attempts,49 and developmental factors may also affect patterns of cognitive bias. Indeed, adolescence is a sensitive period of brain development31 and is associated with improvements in attentional shift, response inhibition, processing speed, and emotional capacity.107 A separate systematic review of the paediatric pain-related cognitive bias literature is therefore currently underway from our research group,52 in the same way separate systematic reviews of anxious youth have been published.24,54 The present review also only includes studies assessing memory biases for symbolic representations of pain in the form of visually presented pain-related words or images or words presented aurally, in line with recent attentional17,86,101 and interpretation bias88 reviews. Several reviews of the relationship between working memory and long-term memory and chronic pain have been published. Berryman et al.7 concluded individuals with chronic pain perform worse on working memory tests than healthy controls, with moderate effects found consistently across studies and paradigms. Mazza et al.57 found evidence of moderate declines in both working memory and long-term memory performances in patients with chronic pain. Although it could not be concluded there were long-term storage impairments, patients with chronic pain exhibited more specifically encoding or retrieving difficulties compared with controls.
2.3. Data extraction
Data from eligible studies were extracted into standardized, prepiloted forms (developed by C.L. and D.E.S.) by D.E.S. which were subsequently checked for accuracy by C.L. Where data were unavailable or insufficient for analysis, study authors were contacted through email requesting missing data.
2.4. Study quality assessment
Various bespoke tools have been used in former chronic pain cognitive bias reviews to assess study quality, featuring items relevant to the particular form of cognitive bias under review.17,81,86,88 We therefore developed a tool to assess quality of memory bias studies specifically, which was based partly on these previously used tools and partly on a tool recently developed for cross-sectional studies.23 A preliminary version of this tool was piloted, and further feedback obtained from an independent expert in the chronic pain cognitive bias field (see Acknowledgements). The tool includes 15 items covering a range of issues relevant to empirical research in general (eg, clear description of samples recruited, reporting of a priori power calculation) and memory bias research specifically (eg, appropriate paradigm and stimuli used to assess memory bias, stimuli rated on valence and arousal). Assessment was based on information in the report. Two authors (D.E.S. and K.R.) independently performed the risk of bias assessment (Kappa = 0.784), with disagreements resolved by discussion where necessary with the third author (C.L.) (Table S1, http://links.lww.com/PR9/A62). A discussion of the results is provided, although a summary score was not computed as these have been found to be unreliable, are not always transparent, and pose difficulties in assigning weightings to different items.38,39
According to the GRADE working group if the total number of participants in a systematic review is less than that required for a single adequately powered intervention (a threshold known as the optimal information size [OIS]), the quality of evidence may be downgraded.34 Although proposed in relation to clinical interventions, the OIS is nevertheless a useful criterion to evaluate quality of evidence. Power calculations were therefore conducted in GPower27 for both small (d = 0.30) and medium (d = 0.50) effect sizes, using commonly accepted conventions (2-tailed,30 power level of 0.80,16 alpha of 0.0562,98).
2.5. Meta-analytic procedures
For inclusion in meta-analysis, each study had to provide independent data pertaining to, or enabling the calculation of, effect sizes and SDs of appropriate memory bias measures. The magnitude of memory bias was explored through between-groups and within-groups analyses.59 Between-groups analyses examined differences between chronic pain and healthy control groups in the recall of pain-related/emotional information. Within-groups analyses examined differences between the recall of pain-related/emotional and neutral information in chronic pain and healthy control groups separately. These 2 types of analysis address different forms of bias, and it may be argued both are required to infer the presence of memory biases in a specific population.59,83 To explore the bias specificity in more depth,53 within-groups analyses were also conduced where possible comparing recall of sensory-pain vs affective-pain words and recall of pain-related/illness-related vs negative/depression-related words.
2.5.1. Between-groups procedures
Hedges' adjusted g effect sizes (standardized mean difference) for between-group comparisons (chronic pain group vs healthy control group) were computed using group mean values and SDs in Review Manager 5.3.80 A random-effects model was used, which assumes the average effect size varies between studies, and therefore, heterogeneity is to be expected.10 Although random-effects models have less statistical power than fixed-effects models, results may be generalised to similar studies not included in the actual analysis.10,82 Cochrane's Q and the I2 statistic were used to assess study heterogeneity. With Cochrane's Q, a significant result is indicative of heterogeneity. The I2 statistic describes the percentage of variability in effect estimates due to heterogeneity as opposed to sampling error.39 Where evidence of significant heterogeneity was found, sensitivity analyses were conducted to explore the robustness of findings and with all decisions fully documented.39
2.5.2. Within-groups procedures
Cohen's d effect sizes were computed for within-group analyses (eg, recall for pain-related vs neutral words) based on study mean values and SDs. Based on recent recommendations,18,48 the average SD was used in these computations, as correlations between measures were not available. A random-effects analysis was used to compute average effect sizes using ESCI (Exploratory Software for Confidence Intervals).18 Based on the recommendation of Cumming,18 an unbiased estimate of the population effect size, referred to as dunb, was computed in ESCI and used in within-groups analyses. This adjustment is advocated as d overestimates the population effect size, especially for smaller sample sizes.18,19,36 A positive effect indicates greater recall for pain-related words than neutral words, whereas a negative effect indicates the opposite recall pattern. Cochrane's Q and the I2 statistic were used to assess study heterogeneity, and where significant, sensitivity analyses were conducted to explore the robustness of findings and with all decisions fully documented.39
2.5.3. Meta-regression
Meta-regression was planned to explore whether memory bias scores were significantly predicted by individual difference variables such as current pain intensity, anxiety, or depression. It was not possible to perform any meta-regression, however, because none of the analyses included data from 10 or more studies as recommended by the Cochrane Collaboration.39
2.6. Meta-analytic methodological decisions
A number of methodological decisions were made further to the stated inclusion and exclusion criteria, and which are provided in Supplementary Material 2, available at http://links.lww.com/PR9/A62.
3. Results
3.1. Search results
The literature search and study selection process is shown in the PRISMA flow diagram in Figure 1. From an initial identification of 6357 records, 18 studies meeting the inclusion criteria were retained for the review, of which 12 provided data for inclusion in the subset meta-analyses (see Table 1 for study characteristics). Data from 12 studies were available for use in the between-groups meta-analysis and 12 studies the within-groups meta-analyses.
Figure 1.
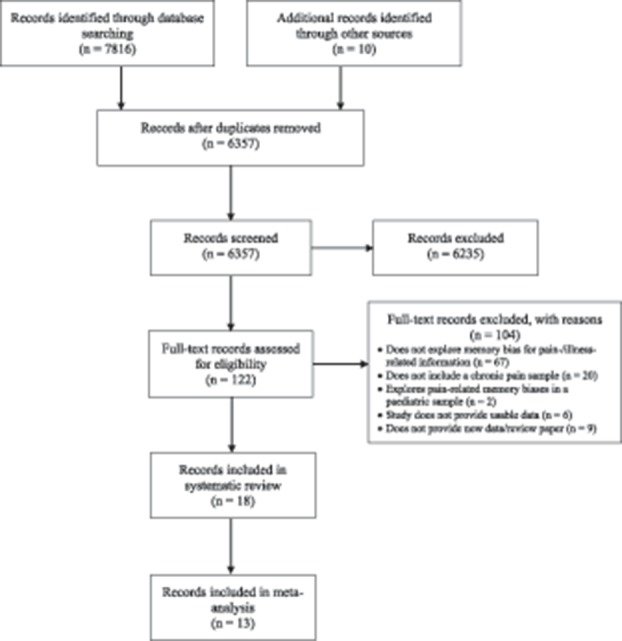
Flow of records for inclusion in the narrative review and meta-analysis of memory biases in chronic pain.
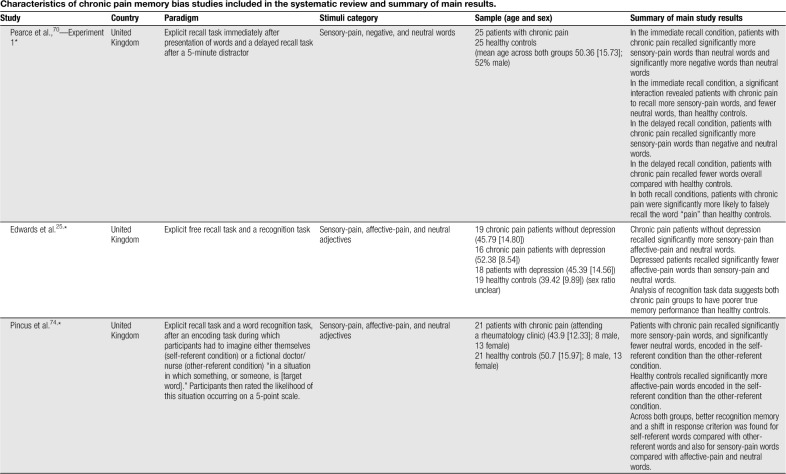
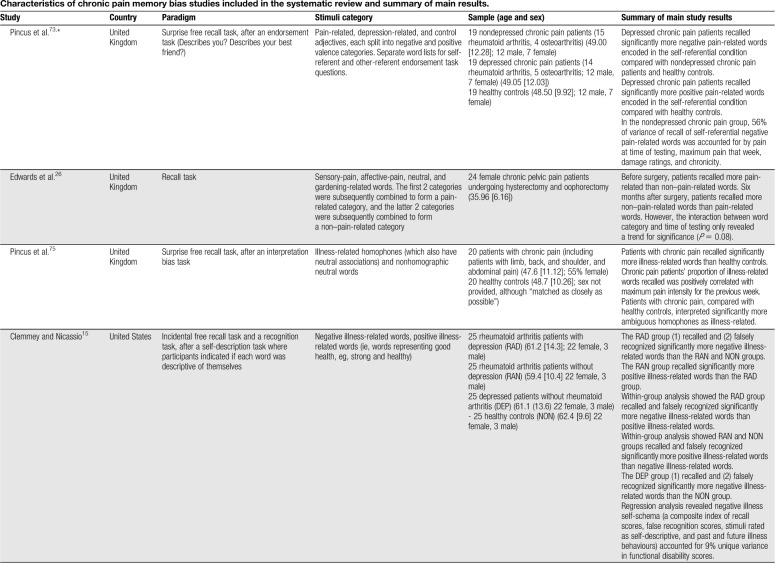
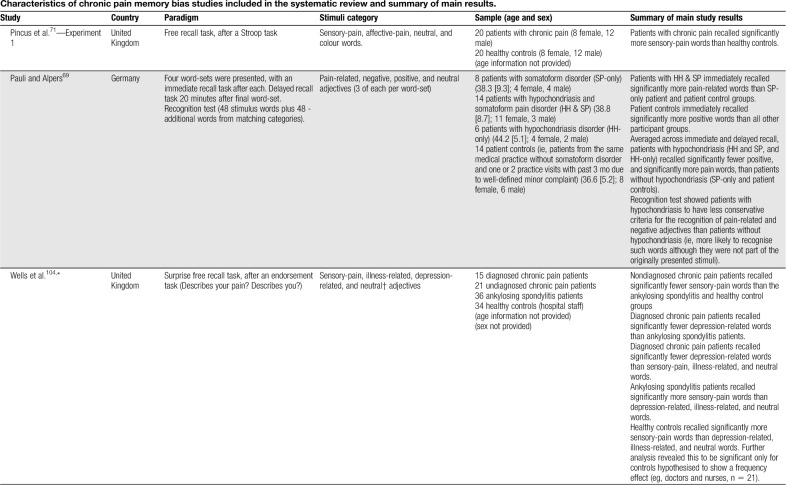
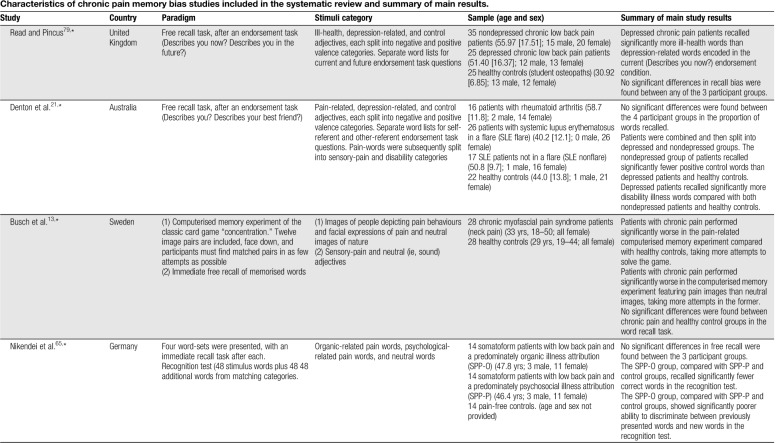
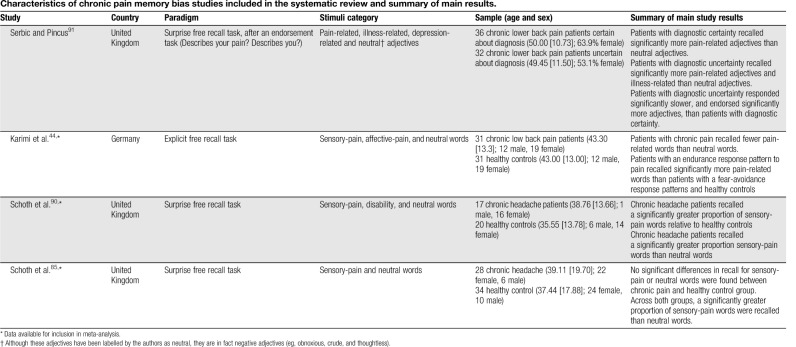
Table 1.
Characteristics of chronic pain memory bias studies included in the systematic review and summary of main results.
3.2. Methodological quality
All studies clearly stated their aims or objectives, recruited samples representative of the intended population, and provided sufficient details on methods and statistical analyses that would allow for replication. All but one study specified in the methods section the analyses to be conducted which were subsequently reported in the results (Ref. 75: reported all details in the results section). The chronic pain group was clearly defined in 9 studies,13,25,44,65,73,79,85,90,91 and the control group in 7 studies.13,44,73,79,85,90,104 In 17 studies, all participants completed the experiment as intended, or the protocol for handling missing data was provided and followed as intended. In the remaining study,26 the protocol for handling missing data at follow-up was not provided. Authors' conclusions were justified by the results in all studies, although authors did not discuss study limitations in 2 studies.25,73 Seven studies assessed and reported anxiety and depression and considered potential or actual influences on patterns of memory bias.21,69,71,75,85,90,91
A number of particularly notable limitations were identified. Only 2 controlled studies matched their chronic pain and control groups on age, sex, and education.44,70 No study rated their stimuli on valence and arousal and reported the data, and only 3 studies performed a power calculation and reported the results.44,79,91 An appropriate and identical testing environment was only clearly specified in 2 studies.69,75 No study was deemed to have used an appropriate paradigm and stimuli which were described clearly. This item had 2 subsections, which required both to be answered “yes” for the overall item to be rated as “yes.” Twelve studies matched their stimuli on relevant dimensions,21,25,26,44,65,71,73–75,85,90,91 although importantly none used a paradigm that has been trialed, piloted, or published previously and reported psychometric properties. Although there were broad similarities between the free recall tasks adopted in the studies, the majority differed on important features such as mode of stimuli presentation (computer, audio, written), completion of previous tasks featuring the stimuli (Stroop, homophone, sentence generation, visual-probe, spatial cueing tasks), task instructions (explicit recall, surprise recall after an endorsement task [type of endorsement also varied]), presence of absence of a distractor task, and time allocated for recall. In many instances, aspects of the methodology were altered from previous studies without any reported piloting. This, coupled with the lack of reporting of paradigm psychometric properties, resulted in all studies being rated as “no” for this item.
3.3. Systematic review
To address the 2 research questions in turn, the narrative synthesis first discusses between-groups results followed by within-groups results.
3.3.1. Between-groups biases
Sensory-pain words were the most common type of stimuli used. Three without an explicit self-endorsement task reported significantly greater recall of sensory-pain words in patients with chronic pain relative to healthy controls,70,71,90 whereas 4 found no significant differences between groups.13,25,44,85 Karimi et al.44 combined sensory and affective words and found patients with an endurance response pattern recalled significantly more pain-related words than patients with a fear-avoidance response pattern and healthy controls.
Some studies have explored recall after a previous endorsement task, 2 of which presented participants with a cue question to facilitate encoding of words in relation to the self (Describes your pain? Describes you?). Wells et al.104 found patients with chronic pain who had not received a medical diagnosis recalled significantly fewer sensory-pain words than a healthy control group of medical professionals and patients with ankylosing spondylitis. Another study presented participants with negative illness words which were encoded in self-referent (Describes you?) and other-referent (Describes your best friend?) conditions and later divided into sensory-pain and disability categories. No evidence was found for enhanced recall of sensory-pain words in those with chronic pain relative to healthy controls.21 Pincus et al.74 presented participants with word lists and for each word required the participant to imagine either themselves (self-referent condition) or another person (other-referent condition) in a situation involving that word. No differences in recall were found between chronic pain and healthy control groups. Overall across the reviewed studies, there is no evidence of a significant between-groups effect favouring enhanced sensory-pain recall in patients with chronic pain after a previous endorsement task.
No evidence of an enhanced recall bias for affective-pain words (eg, cruel, punishing, and horrible) specifically has been reported in patients with chronic pain relative to healthy controls25,26,44,71,74 (no study included an endorsement task). Broader categories of pain-, illness-, and health-related words have also been used (for simplicity, this category is referred to as “illness-related”). Considering studies without a previous endorsement task, one showed significantly greater recall of illness-related (eg, die, pain, and heal) words in patients with chronic pain than healthy controls.75 Another found no evidence of recall bias in patients with chronic headache relative to healthy controls for ambiguous words with disability and neutral meanings (eg, disorder, invalid, and handicap).90 Two studies have explored memory biases in patients diagnosed with somatoform pain disorders. Pauli and Alpers69 found patients with somatoform pain disorders and hypochondriasis recalled significantly more pain-related words (eg, stinging, unpleasant, and miserable) than patients with somatoform pain disorders only and a patient control group (ie, patients without somatoform disorder and one or 2 practice visits within the past 3 months). Nikendei et al.65 recruited somatoform patients with low back pain and a predominately organic illness attribution (SPP-O), somatoform patients with low back pain and a predominately psychosocial illness attribution (SPP-P), and pain-free controls. Memory for words related to organic causes (eg, weak bones, strain, and rheumatism) and psychosocial causes (eg, emotional stress, depression, and divorce) was explored. The results showed no significant differences in recall between the 3 participant groups.
Two studies using a previous endorsement task reported patients with chronic pain to recall significantly more negative illness-related words than healthy controls. Pincus et al.73 found depressed chronic pain patients to recall significantly more negative pain-related words (eg, hurting, vulnerable, and uncomfortable) encoded in the self-referential condition compared with nondepressed chronic pain patients and healthy controls. Clemmey and Nicassio15 found patients with rheumatoid arthritis with depression to recall significantly more negative illness-related words (eg, sick, diseased, and painful) than healthy controls, but no difference was found between patients with rheumatoid arthritis without depression and healthy controls. Denton et al.21 reported no evidence of bias for negative illness words (eg, hurting, aching, and vulnerable) in patients with rheumatoid arthritis or systemic lupus erythematosus, although post hoc analysis found depressed patients to recall a significantly greater proportion of disability-related words than healthy controls and nondepressed patients. Two studies reported no significant differences between participant groups.79,104
For depression/negative words, 2 studies without an explicit self-endorsement task have not reported any significant biases in patients with chronic pain relative to healthy controls.69,70 Of those studies featuring an endorsement task, Wells et al.104 found diagnosed chronic pain patients recalled significantly fewer depression-related words than ankylosing spondylitis patients, although no differences were found to healthy controls. The remaining 3 studies found no evidence of bias towards depression/negative words relative to healthy controls.21,73,79
One study explored memory bias for pain-related images. Busch et al.13 used a computerised memory game involving 12 image pairs randomised and presented “face down” in a 6 × 4 grid. Participants revealed each image by selecting it with the mouse. The task of the participant was to match identical images in as few moves (ie, mouse clicks) as possible. Participants completed one game featuring pain-related images (images of models displaying pain behaviours, including holding their head/neck/back in pain) and one game featuring neutral images (nature scenes). Patients with chronic pain performed significantly worse in the pain-related game than healthy controls, taking more moves to solve the game.
3.3.2. Within-groups biases
For sensory-pain words, 2 studies without an explicit self-endorsement task reported significantly greater recall of sensory-pain words relative to neutral words in those with chronic pain.70,90 Another found patients with chronic pain without depression recalled significantly more sensory-pain words than neutral and affective-pain.25 An uncontrolled study revealed chronic pelvic pain patients before surgery recalled significantly more pain-related words than non–pain-related words, although sensory- and affective-pain word categories were combined. Six months after surgery, half of the patients were completely pain-free, and the results showed a greater number of non–pain-related words recalled than pain-related words.26 Also combining sensory- and affective-pain words, one study found patients with chronic pain recalled fewer pain-related words than neutral words.44
Considering studies using a previous endorsement task, one reported patients with ankylosing spondylitis and healthy controls to recall significantly more sensory-pain words than neutral, illness-related, and depression-related words.104 One uncontrolled study found chronic pain patients with diagnostic certainty, and patients with diagnostic uncertainty, to recall significantly more sensory-pain words than neutral words.91 Pincus et al.74 found patients with chronic pain recalled significantly more sensory-pain words encoded in a self-referent condition than those encoded in an other-referent condition, but reported no difference in recall compared with neutral words. Three studies reported no significant within-groups biases for sensory-pain words relative to neutral words,13,21,71 although one reported that a significantly greater proportion of sensory-pain words were recalled than neutral words across both chronic pain and healthy control groups.85
Regarding affective-pain words, as noted, one study showed chronic pelvic pain patients to recall significantly more pain-related words than non–pain-related words, with the pain-related words comprising sensory- and affective-pain adjectives.26 Another reported patients with chronic pain recalled fewer pain-related words (sensory and affective-pain words combined) than neutral words.44 Three studies reported no significant biases for affective-pain words relative to neutral words in patients with chronic pain.25,71,74 Of those studies using illness-related words without a previous endorsement task, one uncontrolled study found chronic pain patients with diagnostic uncertainty recalled significantly more illness-related adjectives (eg, suffering, disabled, and dependent) than neutral words.91 No other study without65,69,75,90 or with15,21,73,79,104 an endorsement task found evidence of bias for illness-related words relative to neutral words.
Considering studies using depression/negative words without an explicit self-endorsement task, one study found patients with chronic pain recalled significantly more negative words than neutral words during immediate recall, but not during delayed (5-minute) recall.70 Another reported no significant recall biases for depression/negative words relative to neutral words.69 Considering studies featuring an endorsement task, Wells et al.104 found diagnosed chronic pain patients recalled significantly fewer depression-related words than neutral words. The remaining 3 studies reported no significant recall biases for depression/negative words relative to neutral words.21,73,79,91 In the only study to use pain-related images, Busch et al.13 found that, contrary to their hypothesis, patients with chronic pain took significantly more moves to solve the pain-related game than the neutral game.
4. Meta-analysis results
4.1. Between-groups analyses
Meta-analyses are presented comparing patients with chronic pain to healthy controls on recall memory biases for pain-related (sensory-pain and affective-pain combined), sensory-pain, affective-pain, illness-related, depression-related, and negative words. Full details for each analysis are provided in Table 2, and data are presented in a series of forest plots in Figure 2. Additional analyses exploring biases with depressed and nondepressed chronic pain groups separately are provided in supplementary material 3 and Table S2, available at http://links.lww.com/PR9/A62. A power calculation revealed the OIS for between-groups analyses to be 128 participants for a medium effect size and 352 participants for a small effect size.
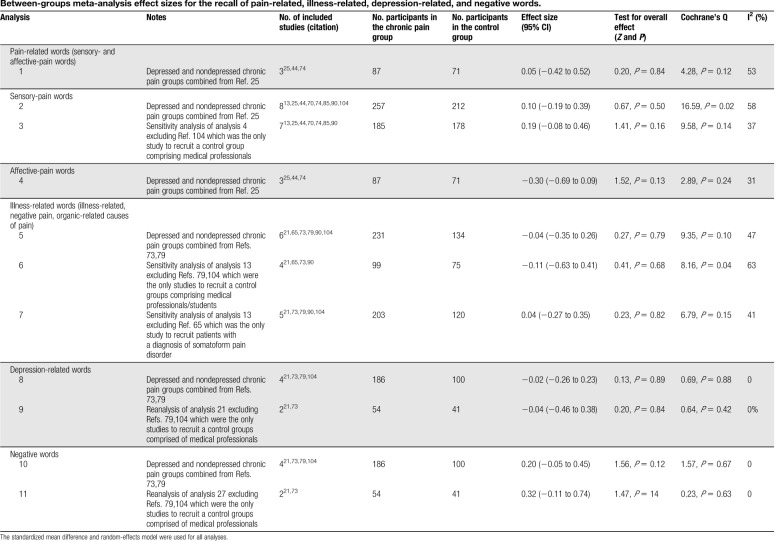
Table 2.
Between-groups meta-analysis effect sizes for the recall of pain-related, illness-related, depression-related, and negative words.
Figure 2.
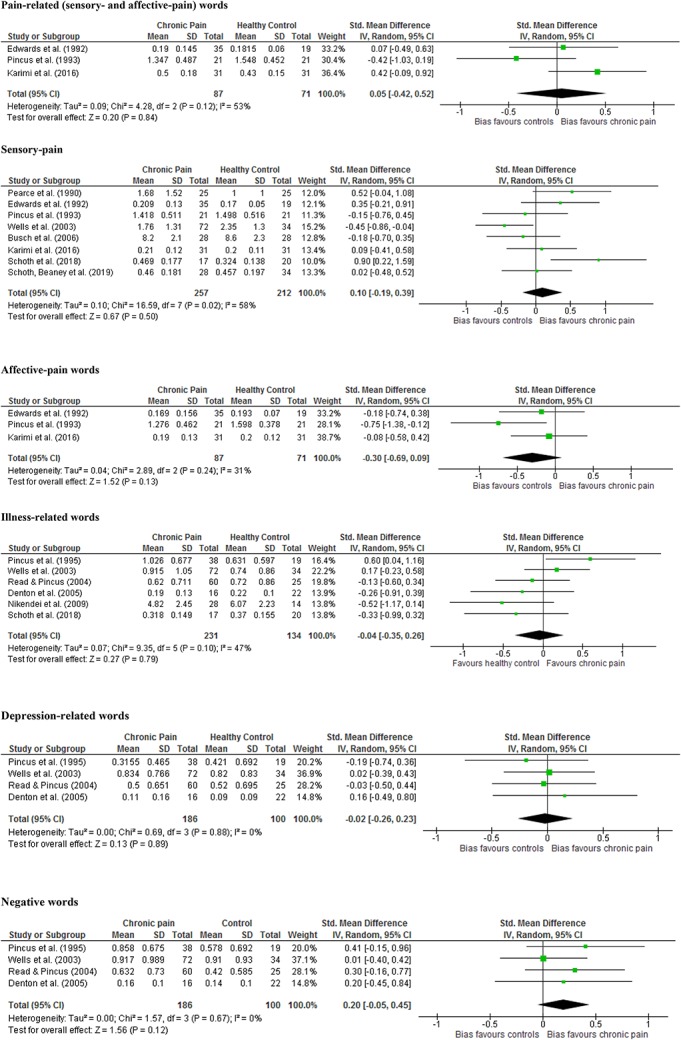
Between-groups forest plots created in Review Manager showing overall effect sizes for individual studies for pain-related, sensory-pain, affective-pain, illness-related, depression-related, and negative words ordered by publication date.
4.1.1. Pain-related words
Three studies included data from both sensory- and affective-pain word categories.25,44,74 No significant differences in recall were found between chronic pain and healthy control groups (analysis 1: chronic pain n = 87, healthy control n = 71; Hedges' g = 0.05, P = 0.84).
4.1.2. Sensory-pain words
Eight studies included data from sensory-pain words.13,25,44,70,74,85,90,104 No significant differences in recall were found between chronic pain and healthy control groups (analysis 2: chronic pain n = 257, healthy control n = 212; Hedges' g = 0.10, P = 0.50). Significant heterogeneity was found, and therefore, sensitivity analyses was conducted excluding the only study to recruit a control group comprising medical professionals.104 Between-group differences remained nonsignificant (analysis 3: chronic pain n = 185, healthy control n = 178; Hedges' g = 0.19, P = 0.16).
4.1.3. Affective-pain words
Three studies included data from affective-pain words.25,44,74 No significant differences in recall were found between chronic pain and healthy control groups (analysis 4: chronic pain n = 87, healthy control n = 71; Hedges' g = −0.30, P = 0.13).
4.1.4. Illness-related words
Six studies included data from illness-related (illness-related, negative pain, organic-related causes of pain) words.21,65,73,79,90,104 No significant differences in recall were found between chronic pain and healthy control groups (analysis 5: chronic pain n = 231, healthy control n = 134 Hedges' g = −0.04, P = 0.79). An additional analysis was conducted excluding studies recruiting control groups comprising medical professionals/students79,104 and which was nonsignificant (analysis 6: chronic pain n = 99, healthy control n = 75; Hedges' g = −0.11, P = 0.68). Another analysis was conducted excluding the only study to recruit patients with a diagnosis of somatoform pain disorder65 and which again was nonsignificant (analysis 7: chronic pain n = 203, healthy control n = 120; Hedges' g = 0.04, P = 0.82).
4.1.5. Depression-related words
Four studies included data from depression-related words.21,73,79,104 No significant differences in recall were found between chronic pain and healthy control groups (analysis 8: chronic pain n = 186, healthy control n = 100; Hedges' g = −0.02, P = 0.89). An additional analysis were conducted excluding studies recruiting control groups comprising medical professionals/students79,104 and which was nonsignificant (analysis 9: chronic pain n = 54, healthy control n = 41; Hedges' g = −0.04, P = 0.84).
4.1.6. Negative words
Four studies included data from negative words.21,73,79,104 No significant differences in recall were found between chronic pain and healthy control groups (analysis 10: chronic pain n = 186, healthy control n = 100; Hedges' g = 0.20, P = 0.12). An additional analysis was conducted excluding studies recruiting control groups comprising medical professionals/students79,104 and which was nonsignificant (analysis 11: chronic pain n = 54, healthy control n = 41; Hedges' g = 0.32, P = 0.14).
4.2. Within-groups analyses
Meta-analyses are presented comparing memory recall biases for pain-related (sensory- and affective-pain combined), sensory-pain and affective-pain words relative to neutral words in patients with chronic pain and healthy individuals separately. Analysis was not conducted with illness-related words as only one study included neutral words.65 Analyses were not conducted for depression and negative words as studies using this word categories included negative and/or positive adjectives as control words rather than neutral words specifically.21,73,79,104 Full details for each analysis are provided in Table 3, and data for patients with chronic pain (where applicable including depressed and nondepressed patients combined) are presented in a series of forest plots in Figure 3. Sufficient data were also available to perform analyses comparing recall biases for sensory-pain relative to affective-pain words, illness-related relative to negative words, and illness-related to depression-related words. Additional analyses exploring biases with depressed and nondepressed chronic pain groups separately are provided in supplementary material 3 and Table S3, available at http://links.lww.com/PR9/A62.
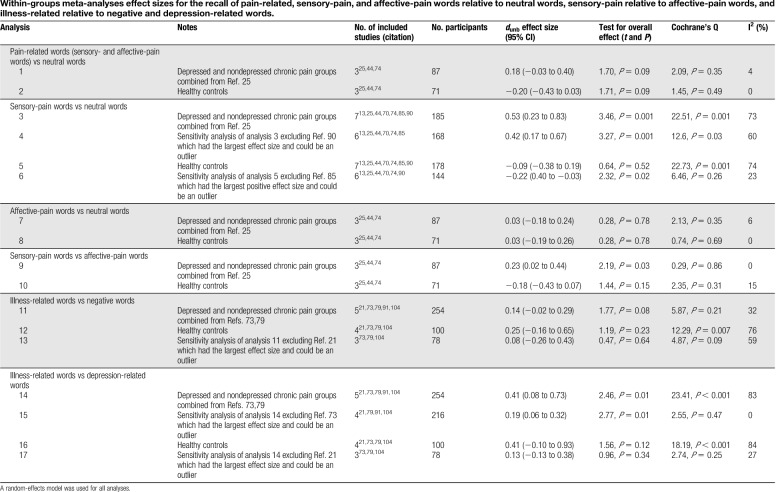
Table 3.
Within-groups meta-analyses effect sizes for the recall of pain-related, sensory-pain, and affective-pain words relative to neutral words, sensory-pain relative to affective-pain words, and illness-related relative to negative and depression-related words.
Figure 3.
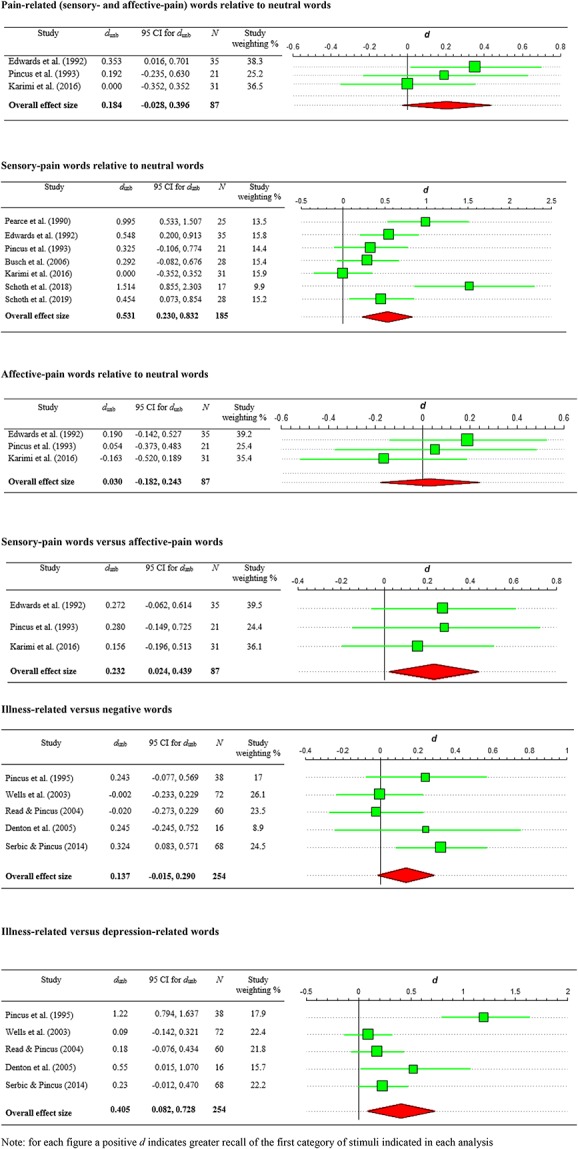
Within-groups forest plots created using ESCI for chronic pain patients' recall bias effect sizes.
4.2.1. Pain-related words vs neutral words
Three studies included data from both sensory- and affective-pain word categories along with neutral words.25,44,74 No significant recall bias was found for patients with chronic pain (analysis 1: n = 87; dunb = 0.18, P = 0.09) or healthy controls (analysis 2: n = 71; dunb = −0.20, P = 0.09).
4.2.2. Sensory-pain words vs neutral words
Seven studies included data from sensory-pain and neutral words.13,25,44,70,74,85,90 Significant recall bias was found for patients with chronic pain favouring the recall of sensory-pain words (analysis 3: n = 185; dunb = 0.53, P = 0.001), but no significant bias was observed in healthy controls (analysis 5: n = 178; dunb = −0.09, P = 0.52). Significant heterogeneity was found in each analysis and therefore sensitivity analyses excluding the study with the largest effect size that could potentially be an outlier (Ref. 90 for chronic pain patient analysis, and Ref. 85 for healthy control analysis). Recall bias remained significant for patients with chronic pain (analysis 4: n = 168; dunb = 0.42, P = 0.001). Bias was also significant for healthy controls favouring recall of neutral words over sensory-pain words (analysis 6: n = 144; dunb = −0.22, P = 0.02).
4.2.3. Affective-pain words vs neutral words
Three studies included data from affective-pain and neutral words.25,44,74 No significant recall bias was found for patients with chronic pain (analysis 7: n = 87; dunb = 0.03, P = 0.78) or healthy controls (analysis 8: n = 71; dunb = 0.03, P = 0.78).
4.2.4. Sensory-pain words vs affective-pain words
Three studies included data from sensory- and affective-pain words.25,44,74 Significant recall bias was found favouring sensory-pain words for patients with chronic pain (analysis 9: n = 87; dunb = 0.23, P = 0.03). No significant bias was found for healthy controls (analysis 10: n = 71; dunb = −0.18, P = 0.15).
4.2.5. Illness-related vs negative words
Five studies included data from illness-related and negative words.21,73,79,91,104 No significant recall bias was found for patients with chronic pain (analysis 11: n = 254; dunb = 0.14, P = 0.08). No significant difference was found for healthy controls (analysis 12: n = 100; dunb = 0.25, P = 0.23) nor after removal of the study with the largest effect size which could potentially be an outlier (analysis 13: n = 78; dunb = 0.08, P = 0.64).
4.2.6. Illness-related vs depression-related words
Five studies included data from illness-related and negative words.21,73,79,91,104 Significant recall bias was found favouring illness-related words for patients with chronic pain (analysis 14: n = 254; dunb = 0.41, P = 0.01). This effect remained significant in a sensitivity analysis removing the study with the largest effect size which could potentially be an outlier (analysis 15: n = 216; dunb = 0.19, P = 0.01). No significant recall bias was found for healthy controls (analysis 16: n = 100; dunb = 0.41, P = 0.12) nor after removal of the study with the largest effect size which could potentially be an outlier (analysis 17: n = 78; dunb = 0.13, P = 0.34).
4.3. Publication bias
The inspection of funnel plots is not recommended when fewer than 10 studies are included in the meta-analysis.39 Therefore, no funnel plots were inspected in the present meta-analyses, which included at most 8 studies.
5. Discussion
The aim of this systematic review was to determine whether adults with chronic pain are characterised by a memory bias specifically favouring the recall of pain-related information. Between-groups analysis revealed patients with chronic pain, relative to healthy controls, show significantly weaker memory recall bias for affective-pain words. However, this result was only significant with the inclusion of the nondepressed chronic pain group from Edwards et al.25. Within-groups analysis showed patients with chronic pain had a significant recall bias for sensory-pain words relative to neutral words and affective-pain words, and a significant recall bias for illness-related words relative to depression-related words. Healthy individuals showed significantly greater recall bias for neutral words relative to sensory-pain words. No significant evidence of memory recall bias was found when sensory-pain– and affective-pain–related words were combined.
Inconsistent evidence for the presence of pain-related memory biases has been found when comparing the results of the between- and within-groups meta-analyses, and there is also variation between the results of individual studies. These differences are likely due in part to a number of methodological limitations identified in the individual studies included in this review. For example, none of the studies rated their stimuli on valence and arousal, although emotion has a complex relationship with memory6 and research has shown arousing and highly valanced words are better recalled than neutral words.45 It is important researchers therefore include detailed information on stimuli characteristics such as these in their reports. Furthermore, few studies reported using an appropriate and identical testing environment for all participants, although different testing environments could potentially influence recall due to the presence or absence of environmental cues (eg, hospital or clinical environments may contain more pain-related cues than university laboratories). Few studies also reported matching chronic pain and control groups on age, sex, and education level, although individual difference variables such as these may also influence memory either individually or through their interaction.20,33,94
Overall, the present results provide only partial support for the predictions of relevant theoretical models,5,72 and are not in line with the overall conclusion from Pincus and Morley72 that robust evidence for memory biases exist in chronic pain. It is not uncommon for systematic reviews to reach different conclusions, however,42,61 especially if they are not directly compatible, as is the case in this instance. More specifically: (1) Pincus and Morley included studies from both adult and paediatric samples, including 2 paediatric studies which reported evidence of significant sensory-pain memory biases43,46; (2) only the present review included a meta-analysis of study effect sizes (although Pincus and Morley did report within-groups effect sizes where possible); (3) the present review included 10 additional studies published since Pincus and Morley's review that have reported mixed results; and (4) the present review clearly separated within- and between-groups effects. The results of the present review are more akin with the review from Rusu and et al.,84 who note evidence of memory biases has been found in individuals with chronic pain, yet results are not consistent and more recent studies have not replicated this finding.
The only conclusion shared by all 3 reviews is the existence of an enhanced recall bias favouring sensory-pain words relative to neutral words in adults with chronic pain, which itself is supportive of the view that such words are particularly relevant to patients and favour enhanced processing.17,35,72,100 Affective-pain words may be less threatening than sensory-pain words, and threat is argued as an important component in the salience of pain-related information and whether cognitive biases are shown.100 Patients may therefore find it easier to avoid affective-pain words than sensory-pain words, although unfortunately no study included in this review provided ratings on arousal or threat. Although sensory and affective dimensions of pain are intimately related they are nevertheless distinguishable,47,77 and the existence of different patterns of cognitive bias is not surprising (as shown in the attentional bias literature17).
The present review also found individuals with chronic pain showed significantly greater recall bias for sensory-pain words than affective-pain words. It is therefore unsurprising no bias was found when sensory- and affective-pain words were combined. A significant between-groups effect revealing weaker biases for affective-pain words in patients with chronic pain was only found when the nondepressed chronic pain group from Edwards et al.25 was included; no difference was found with the inclusion of the depressed chronic pain group or when both depressed and nondepressed groups were combined. This result is difficult to interpret and should be considered with caution as the analysis included only 3 studies with evidence of moderate heterogeneity. Nevertheless, emerging research suggests avoidance of affective-pain information may have negative outcomes. A prospective study of acute and subacute low back pain patients showed attentional avoidance of affective-pain information at baseline predicted chronicity at 3 and 6 months.93 Another study with healthy individuals found training attention towards affective-pain words, compared with training attention away, resulted in significantly greater experimental pain threshold but also greater distress at tolerance.99 It is feasible attentional avoidance of affective-pain words would lead to a poorer recall of such stimuli.8,28 Further research exploring the potential clinical implications of avoiding affective-pain information, as measured through different forms of cognitive bias, is warranted.
Between-groups analysis found no evidence of significant bias for sensory-pain words in patients with chronic pain relative to healthy controls. Research shows poorer memory performance in patients with chronic pain relative to healthy controls,22,68 and such differences may at least partly explain the lack of between-group effects. Considering evidence shows declines in working memory with increasing age,29 chronic pain and healthy control groups should be matched for age. However, this was only reported for 2 studies included in the sensory-pain meta-analysis,44,70 and for one study, relatively large differences were apparent.25 Healthy samples were also recruited from a variety of locations, and included psychology students, hospital staff, and individuals from evening classes and a community centre, yet only 2 studies in this analysis reported matching control and chronic pain groups on education.44,70 Healthy individuals were found to show significantly greater recall of neutral words than sensory-pain words, although considering the heterogeneity within these samples, we recommend caution in the interpretation of this result. Overall, and similar to the attentional17,86 and interpretation bias88 literature, patterns of within- and between-group biases can vary within the same study, and we encourage researchers to be explicit when describing their results.
A number of studies used broader categories of words reflecting illness and organic-related causes of pain (referred to as “illness-related”; eg, 4 studies included in the meta-analysis used the words vulnerable, ill, suffering, and uncomfortable). The meta-analysis showed no significant between-group effects. The narrative review suggests additional individual difference variables may be important, however, as a number of studies reported recall biases in depressed chronic pain patients relative to controls, but not in patients without depression relative to controls.15,21,73 Within-groups analysis showed individuals with chronic pain to recall significantly more illness-related words than depression-related words (an effect remaining significant with the inclusion of patients with and without depression). Interpretation biases for broader illness-related information have been observed in patients with chronic pain relative to controls.88 By contrast, between-group differences have not been observed in the attentional bias literature for words and images reflecting antecedents or consequences of pain.17,87 We agree with other researchers that it is important for future studies to continue exploring the specificity of cognitive biases in chronic pain and their clinical implications,100 including differences between sensory-pain and broader illness-related stimuli.
Potential clinical implications of pain-related attentional and interpretation biases have been raised.51,88,92 Facilitated recall of pain-related information may enhance emotional distress, which in turn may encourage pain behaviours.74 Of the 5 studies reporting correlational analyses between pain-related recall specifically and patient functioning, 3 reported no significant associations.13,21,71 Pincus et al.73 found, in nondepressed chronic pain patients, 56% of the variance of recall of self-referential negative pain-related words was accounted for by pain at time of testing, maximum pain that week, physical damage ratings (provided by a physician for each patient on a 5-cm visual analogue scale), and chronicity. For depressed chronic pain patients, however, significant negative correlations were found between recall of self-referential negative pain-related words and pain at time of testing, maximum pain that week, damage ratings, and activity. In a subsequent study, the proportion of illness-related homophones recalled was significantly and positively correlated with maximum pain intensity from the previous week.75 Longitudinal research is particularly needed exploring causal relationships between recall biases with pain characteristics and pain-related distress.
Evidence of attentional17,86 and interpretation88 biases has been found in patients with chronic pain, and it has been argued that normal cognitive processes are cyclical in nature64 and that different forms of cognitive bias influence and interact with one another.40,100 Despite this, conclusive evidence for pain-related memory biases does not currently exist in the chronic pain literature. Between-groups analyses found no evidence of biases for sensory-pain words, although significant within-group effects were found relative to neutral words. It should be noted, however, that not all studies in the present review reported matching pain-related and neutral words on length and frequency of use. Considerable research has explored how word length and frequency influence recall, with evidence that shorter words are better recalled than longer words,2 and high frequency words better recalled than low frequency words.76 However, these effects are not always consistently reported,12,63 and important variations in study design and sample characteristics can influence the pattern of results found. Although it is beyond the scope of this review to discuss this literature in detail, it is important to emphasise that researchers should consider stimulus properties such as these when developing their stimuli lists and carefully report such details.
Furthermore, “neutral” is a rather broad term which can be misleading, as in 2 studies which labelled negative adjectives (eg, obnoxious, crude, and thoughtless) as neutral.91,104 As noted, it is important to carefully match emotional and neutral information in memory bias studies, and future research should always assess stimuli on valence and arousal. Significant within-groups bias was also shown for sensory-pain words relative to affective-pain words, although again limitations are apparent as the word categories were not matched on length or frequency. It is also important to acknowledge that the source of bias is not clear when comparing 2 emotional/threatening categories of information,3 although comparisons with neutral stimuli in the same study can help explain these effects.
Much like attentional17 and interpretation89 biases, differing methods may be used to explore memory recall biases, including surprise and explicit tasks. These 2 different approaches have not been directly compared in the chronic pain field nor have the reliability of such paradigms been assessed. The latter is important, however, because between-groups effects may not be detected should the paradigms used be unreliable.59 Furthermore, the self-reference effect has been extensively documented,96,97 although only 3 studies included in the meta-analysis explored whether self-referent encoding facilitates greater recall for pain-related information than other-referent encoding.21,73,74 Although only one found evidence supporting the self-reference effect,74 this nevertheless remains an avenue for future investigation. Finally, one study in this review used a novel computerised memory game with pictures that recorded manual responses (ie, number of mouse clicks), in addition to immediate recall of a list of memorised words.13 Although some evidence was shown for differences in performance between the 2 paradigms (ie, patients with chronic pain performed significantly worse than healthy controls in the computerised memory experiment but not the free recall task), the inclusion of different stimuli makes such comparisons difficult. We encourage researchers to further develop and explore alternatives to the use of simple word lists when researching memory biases, although once again, it is important that reliability and psychometric properties are fully assessed.
Experimental32 and clinical research66 has shown memory of previous pain significantly contributes to the subsequent experience of pain, while clinical assessment of chronic pain is largely based on the patient's ability to recall their pain experience.50 Memory for pain has been extensively studied for many decades, although there is still debate regarding the accuracy of patients' memories of pain.1 Nevertheless, some research has shown patients with chronic pain overestimate their pain during later recall.11,95 One possibility is that patients who overestimate their previous pain episodes may also demonstrate significantly greater recall biases for pain-related information (ie, representations of pain). However, the relationship between biased recall of pain and memory recall biases for pain-related information has yet to be explored.
Further to recall of symbolic representations of pain, research has also explored the relationship between autobiographical memory and pain. Liu et al.55 administered the Autobiographical Memory Test105 which presents a series of negative and positive cue words to participants, who for each word were asked to describe what it reminded them of. Patients with chronic pain retrieved significantly more overgeneral memories, significantly slower, than healthy controls. Vucurovic et al.103 recruited participants with fibromyalgia and healthy controls, who were instructed to describe 5 self-defining memories of events from at least 1 year earlier. Participants with fibromyalgia retrieved less-specific self-defining memories (similar to the results of Liu and et al.55) with a more negative emotional valence than healthy controls, although the number of pain memories retrieved did not differ between the 2 groups. However, divergent findings have been reported. Wright and Morley106 presented participants with pain-related and neutral cue words, who then subsequently retrieved a personal event from their past associated with the cue. Patients with chronic pain retrieved significantly more memories incorporating elements of physical pain, which was attributable to memories of themselves in chronic pain. However, this between-group effect was not due to patients with chronic pain showing differential sensitivity to pain-related cues specifically. Although contrasting to the results of Vucurovic et al.,103 these 2 studies used different methodologies with different patient groups, and therefore, direct comparison should be avoided. Although it is beyond the scope of the present review to discuss this literature in depth, this body of research nevertheless highlights differences in retrieval of autobiographical memories between individuals with chronic pain and healthy controls. One possibility for future research is to investigate whether recall biases for symbolic representations of pain are associated with, or are predicted by, biases in retrieval of autobiographical memories.
Unfortunately, data were not available from all studies for inclusion in the subset meta-analyses, and in some instances, meta-analysis was conducted with as few as 3 studies. The OIS for between-groups analyses was 128 participants for a medium effect size which was met in all but 6 analyses. The OIS was 352 participants for a small effect size, however, which was not met in 28 of the between-group analyses. The limited number of studies also prevented the use of meta-regression to explore the potential influence of covariates such as pain intensity at the time of testing.9,39 It should also be noted that although all studies included in this review met our inclusion and exclusion criteria, specific pain diagnosis varied between studies and within the analyses conducted. Although we are unable to ascertain any consistent evidence that certain pain diagnoses are more likely to be associated with memory recall biases than other pain diagnoses, this is a difficult assessment to make given that studies included in this review differed not only on pain diagnosis but also the precise stimuli and methods used. A limitation of the present review is that it was not registered on PROSPERO.78 Although PROSPERO is mainly used for registering systematic reviews of interventions, it good practice to register all systematic reviews in some capacity online. In summary, inconclusive evidence is presented for pain-related memory biases in chronic pain. However, numerous methodological limitations have been raised pertaining to the studies included in the present review, and it is apparent that further, rigorous research is needed.
Disclosures
The authors have no conflicts of interest to declare.
This review was supported by the ESRC (ES/I904026/1).
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A62.
Supplementary Material
Acknowledgements
The authors thank Professor Tamar Pincus (Royal Holloway, University of London) for her helpful comments on a previous version of the study quality assessment tool developed for the present review.
Author contributions: All authors significantly contributed to this article and have discussed the results and commented on the submitted version.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Adamczyk WM, Farley D, Wiercioch-Kuzianik K, Bajcar EA, Buglewicz E, Nastaj J, Gruszka A, Bąbel P. Memory of pain in adults: a protocol for systematic review and meta-analysis. Syst Rev 2019;8:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Baddeley AD, Thomson N, Buchanan M. Word length and the structure of short-term memory. J Verbal Learn Verbal Behav 1975;14:575–89. [Google Scholar]
- [3].Bar-Haim Y, Lamy D, Lee P, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull 2007;133:1–24. [DOI] [PubMed] [Google Scholar]
- [4].Beck AT. Cognitive therapy and the emotional disorders. New York: American Library, 1976. [Google Scholar]
- [5].Beck AT, Emery G, Greenberg RL. Anxiety disorders and phobias; a cognitive perspective. New York: Basic Books, 1985. [Google Scholar]
- [6].Bergmann HC, Rijpkema M, Fernandez G, Kessels RP. The effects of valence and arousal on associative working memory and long-term memory. PLoS One 2012;7:e52616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Berryman C, Stanton TR, Jane Bowering K, Tabor A, McFarlane A, Lorimer Moseley G. Evidence for working memory deficits in chronic pain: a systematic review and meta-analysis. PAIN 2013;154:1181–96. [DOI] [PubMed] [Google Scholar]
- [8].Blaut A, Paulewicz B, Szastok M, Prochwicz K, Koster E. Are attentional bias and memory bias for negative words causally related? J Behav Ther Exp Psychiatry 2013;44:293–9. [DOI] [PubMed] [Google Scholar]
- [9].Borenstein M, Hedges LV, Higgins J, Rothstein HR. Introduction to meta-analysis. Cornwell: Wiley Online Library, 2009. [Google Scholar]
- [10].Borenstein M, Hedges LV, Higgins J, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- [11].Broderick JE, Schwartz JE, Vikingstad G, Pribbernow M, Grossman S, Stone AA. The accuracy of pain and fatigue items across different reporting periods. PAIN 2008;139:146–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brysbaert M, Mandera P, Keuleers E. The word frequency effect in word processing: an updated review. Curr Dir Psychol Sci 2018;27:45–50. [Google Scholar]
- [13].Busch H, Montgomery W, Melin B, Lundberg U. Visuospatial and verbal memory in chronic pain patients: an explorative study. Pain Pract 2006;6:179–85. [DOI] [PubMed] [Google Scholar]
- [14].Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr Opin Neurobiol 2007;17:177–84. [DOI] [PubMed] [Google Scholar]
- [15].Clemmey PA, Nicassio PM. Illness self-schemas in depressed and nondepressed rheumatoid arthritis patients. J Behav Med 1997;20:273–90. [DOI] [PubMed] [Google Scholar]
- [16].Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Hillsdale: Erlbaum, 1988. [Google Scholar]
- [17].Crombez G, Van Ryckeghem D, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. PAIN 2013;154:497–510. [DOI] [PubMed] [Google Scholar]
- [18].Cumming G. Understanding the new statistics: effect sizes, confidence intervals, and meta-analysis. New York: Routledge, 2012. [Google Scholar]
- [19].Cumming G. The new statistics: why and how. Psychol Sci 2014;25:7–29. [DOI] [PubMed] [Google Scholar]
- [20].Danckert SL, Craik FI. Does aging affect recall more than recognition memory? Psychol Aging 2013;28:902. [DOI] [PubMed] [Google Scholar]
- [21].Denton FJ, Sharpe L, Schrieber L. Cognitive bias in systemic lupus erythematosus. Eur J Pain 2005;9:5–14. [DOI] [PubMed] [Google Scholar]
- [22].Dick BD, Rashiq S. Disruption of attention and working memory traces in individuals with chronic pain. Anesth Analg 2007;104:1223–9. [DOI] [PubMed] [Google Scholar]
- [23].Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6:e011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dudeney J, Sharpe L, Hunt C. Attentional bias towards threatening stimuli in children with anxiety: a meta-analysis. Clin Psychol Rev 2015;40:66–75. [DOI] [PubMed] [Google Scholar]
- [25].Edwards LC, Pearce S, Collett BJ, Pugh R. Selective memory for sensory and affective information in chronic pain and depression. Br J Clin Psychol 1992;31:239–48. [DOI] [PubMed] [Google Scholar]
- [26].Edwards LC, Pearce SA, Beard RW. Remediation of pain-related memory bias as a result of recovery from chronic pain. J Psychosom Res 1995;39:175–81. [DOI] [PubMed] [Google Scholar]
- [27].Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instr Comput 1996;28:1–11. [Google Scholar]
- [28].Everaert J, Koster EHW, Derakshan N. The combined cognitive bias hypothesis in depression. Clin Psychol Rev 2012;32:413–24. [DOI] [PubMed] [Google Scholar]
- [29].Fabiani M, Zimmerman B, Gratton G. Chapter 11–working memory and aging: a review. In: Jolicoeur P, Lefebvre C, Martinez-Trujillo J, editors. Mechanisms of sensory working memory: Attention and performance XXV. San Diego: Academic Press, 2015. p. 131–48. [Google Scholar]
- [30].Field A. Discovering statistics using IBM SPSS statistics. London: Sage, 2013. [Google Scholar]
- [31].Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends Cogn Sci 2015;19:558–66. [DOI] [PubMed] [Google Scholar]
- [32].Gedney JJ, Logan H. Pain related recall predicts future pain report. PAIN 2006;121:69–76. [DOI] [PubMed] [Google Scholar]
- [33].Graves LV, Moreno CC, Seewald M, Holden HM, Van Etten EJ, Uttarwar V, McDonald CR, Delano-Wood L, Bondi MW, Woods SP. Effects of age and gender on recall and recognition discriminability. Arch Clin Neuropsychol 2017;32:972–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux P, Montori VM, Freyschuss B, Vist G. GRADE guidelines 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 2011;64:1283–93. [DOI] [PubMed] [Google Scholar]
- [35].Haggman SP, Sharpe LA, Nicholas MK, Refshauge KM. Attentional biases toward sensory pain words in acute and chronic pain patients. J Pain 2010;11:1136–45. [DOI] [PubMed] [Google Scholar]
- [36].Hedges LV. Distribution theory for Glass's estimator of effect size and related estimators. J Educ Stat 1981;6:107–28. [Google Scholar]
- [37].Herrera S, Montorio I, Cabrera I, Botella J. Memory bias for threatening information related to anxiety: an updated meta-analytic review. J Cogn Psychol 2017;29:832–54. [Google Scholar]
- [38].Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ Case Rep 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. West Sussex, United Kingdom: The Cochrane Collaboration, 2008. [Google Scholar]
- [40].Hirsch CR, Clark DM, Mathews A. Imagery and interpretations in social phobia: support for the combined cognitive biases hypothesis. Behav Ther 2006;37:223–36. [DOI] [PubMed] [Google Scholar]
- [41].Intaitė M, Duarte JV, Castelo-Branco M. Working memory load influences perceptual ambiguity by competing for fronto-parietal attentional resources. Brain Res 2016;1650:142–51. [DOI] [PubMed] [Google Scholar]
- [42].Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. Can Med Assoc J 1997;156:1411–16. [PMC free article] [PubMed] [Google Scholar]
- [43].Johnson R, Spence SH. Pain, affect, and cognition in children: 2. Recall bias associated with pain. In: Gebhart GF, Hammond DL, Jensen TS, editors. Proceedings of the 7th World Congress on Pain. Seattle: IASP Press, 1994. [Google Scholar]
- [44].Karimi Z, Pilenko A, Held SM, Hasenbring MI. Recall bias in patients with chronic low back pain: individual pain response patterns are more important than pain itself!. Int J Behav Med 2016;23:12–20. [DOI] [PubMed] [Google Scholar]
- [45].Kensinger EA, Corkin S. Memory enhancement for emotional words: are emotional words more vividly remembered than neutral words? Mem Cognit 2003;31:1169–80. [DOI] [PubMed] [Google Scholar]
- [46].Koutantji M, Pearce SA, Oakley DA, Feinmann C. Children in pain: an investigation of selective memory for pain and psychological adjustment. PAIN 1999;81:237–44. [DOI] [PubMed] [Google Scholar]
- [47].Kunz M, Lautenbacher S, LeBlanc N, Rainville P. Are both the sensory and the affective dimensions of pain encoded in the face? PAIN 2012;153:350–8. [DOI] [PubMed] [Google Scholar]
- [48].Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol 2013;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lau JY, Heathcote LC, Beale S, Gray S, Jacobs K, Wilkinson N, Crombez G. Cognitive biases in children and adolescents with chronic pain: a review of findings and a call for developmental research. J Pain 2018;19:589–98. [DOI] [PubMed] [Google Scholar]
- [50].Lefebvre JC, Keefe FJ. Memory for pain: the relationship of pain catastrophizing to the recall of daily rheumatoid arthritis pain. Clin J Pain 2002;18:56–63. [DOI] [PubMed] [Google Scholar]
- [51].Liossi C. Attentional biases in chronic pain: do they exist and does it really matter? PAIN 2012;153:9–10. [DOI] [PubMed] [Google Scholar]
- [52].Liossi C, Broadbent P, Schoth DE, Williams G. A systematic review and meta-analysis of cognitive biases for pain-related information in children and adolescents and their parents. PROSPERO: International prospective register of systematic reviews, 2018. [Google Scholar]
- [53].Liossi C, Schoth DE. Attention toward interpersonal stimuli in individuals with and without chronic daily headache. J Neurol Neurosurg 2016;3:1–7. [Google Scholar]
- [54].Lisk S, Vaswani A, Linetzky M, Bar-Haim Y, Lau JY. Systematic review and meta-analysis: eye-tracking of attention to threat in child and adolescent anxiety. J Am Acad Child Adolesc Psychiatry 2019;59:88–99.e1. [DOI] [PubMed] [Google Scholar]
- [55].Liu X, Liu Y, Li L, Hu Y, Wu S, Yao S. Overgeneral autobiographical memory in patients with chronic pain. Pain Med 2014;15:432–9. [DOI] [PubMed] [Google Scholar]
- [56].Marchetti I, Everaert J, Dainer-Best J, Loeys T, Beevers CG, Koster EH. Specificity and overlap of attention and memory biases in depression. J Affect Disord 2018;225:404–12. [DOI] [PubMed] [Google Scholar]
- [57].Mazza S, Frot M, Rey AE. A comprehensive literature review of chronic pain and memory. Prog Neuropsychopharmacol Biol Psychiatry 2018;87:183–92. [DOI] [PubMed] [Google Scholar]
- [58].Meyer P, Karl A, Flor H. Pain can produce systematic distortions of autobiographical memory. Pain Med 2015;16:905–10. [DOI] [PubMed] [Google Scholar]
- [59].Mitte K. Memory bias for threatening information in anxiety and anxiety disorders: a meta-analytic review. Psychol Bull 2008;134:886. [DOI] [PubMed] [Google Scholar]
- [60].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Plos Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Moja L, Del Rio MPF, Banzi R, Cusi C, D'Amico R, Liberati A, Lodi G, Lucenteforte E, Minozzi S, Pecoraro V. Multiple systematic reviews: methods for assessing discordances of results. Intern Emerg Med 2012;7:563–8. [DOI] [PubMed] [Google Scholar]
- [62].Nakagawa S, Foster TM. The case against retrospective statistical power analyses with an introduction to power analysis. Acta Ethologica 2004;7:103–8. [Google Scholar]
- [63].Neath I, Bireta TJ, Surprenant AM. The time-based word length effect and stimulus set specificity. Psychon Bull Rev 2003;10:430–4. [DOI] [PubMed] [Google Scholar]
- [64].Neisser U. Cognitive psychology. East Norwalk: Appleton-Century-Crofts, 1967. [Google Scholar]
- [65].Nikendei C, Waldherr S, Schiltenwolf M, Herzog W, Röhrig M, Walther S, Weisbrod M, Henningsen P, Hanel G. Memory performance related to organic and psychosocial illness attributions in somatoform pain disorder patients. J Psychosom Res 2009;67:199–206. [DOI] [PubMed] [Google Scholar]
- [66].Noel M, Chambers CT, McGrath PJ, Klein RM, Stewart SH. The influence of children's pain memories on subsequent pain experience. PAIN 2012;153:1563–72. [DOI] [PubMed] [Google Scholar]
- [67].Noel M, Chambers CT, Petter M, McGrath PJ, Klein RM, Stewart SH. Pain is not over when the needle ends: a review and preliminary model of acute pain memory development in childhood. Pain Manag 2012;2:487–97. [DOI] [PubMed] [Google Scholar]
- [68].Oosterman JM, Derksen LC, van Wijck AJ, Veldhuijzen DS, Kessels RP. Memory functions in chronic pain: examining contributions of attention and age to test performance. Clin J Pain 2011;27:70–5. [DOI] [PubMed] [Google Scholar]
- [69].Pauli P, Alpers GW. Memory bias in patients with hypochondriasis and somatoform pain disorder. J Psychosom Res 2002;52:45–53. [DOI] [PubMed] [Google Scholar]
- [70].Pearce SA, Isherwood S, Hrouda D, Richardson PH, Erskine A, Skinner J. Memory and pain: tests of mood congruity and state dependent learning in experimentally induced and clinical pain. PAIN 1990;43:187–93. [DOI] [PubMed] [Google Scholar]
- [71].Pincus T, Fraser L, Pearce S. Do chronic pain patients “Stroop” on pain stimuli? Br J Clin Psychol 1998;37:49–58. [DOI] [PubMed] [Google Scholar]
- [72].Pincus T, Morley S. Cognitive-processing bias in chronic pain: a review and integration. Psychol Bull 2001;127:599–617. [DOI] [PubMed] [Google Scholar]
- [73].Pincus T, Pearce S, McClelland A, Isenberg D. Endorsement and memory bias of self‐referential pain stimuli in depressed pain patients. Br J Clin Psychol 1995;34:267–77. [DOI] [PubMed] [Google Scholar]
- [74].Pincus T, Pearce S, McClelland A, Turner-Stokes L. Self-referential selective memory in pain patients. Br J Clin Psychol 1993;32:365–74. [DOI] [PubMed] [Google Scholar]
- [75].Pincus T, Pearce S, Perrott A. Pain patients' bias in the interpretation of ambiguous homophones. Br J Med Psychol 1996;69:259–66. [DOI] [PubMed] [Google Scholar]
- [76].Poirier M, Saint-Aubin J. Word frequency effects in immediate serial recall: item familiarity and item co-occurrence have the same effect. Memory 2005;13:325–32. [DOI] [PubMed] [Google Scholar]
- [77].Price DD, Harkins SW, Baker C. Sensory-affective relationships among different types of clinical and experimental pain. PAIN 1987;28:297–307. [DOI] [PubMed] [Google Scholar]
- [78].PROSPERO. PROSPERO: International prospective register of systematic reviews. National Institute for Health Research. [Google Scholar]
- [79].Read J, Pincus T. Cognitive bias in back pain patients attending osteopathy: testing the enmeshment model in reference to future thinking. Eur J Pain 2004;8:525–31. [DOI] [PubMed] [Google Scholar]
- [80].RevMan. Review manager 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. [Google Scholar]
- [81].Roelofs J, Peters ML, Zeegers MPA, Vlaeyen JWS. The modified stroop paradigm as a measure of selective attention towards pain-related stimuli among chronic pain patients: a meta analysis. Eur J Pain 2002;6:273–81. [DOI] [PubMed] [Google Scholar]
- [82].Rosenthal R. Writing meta-analytic reviews. Psychol Bull 1995;188:183–92. [Google Scholar]
- [83].Russo R, Fox E, Bowles RJ. On the status of implicit memory bias in anxiety. Cogn Emot 1999;13:435–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Rusu AC, Gajsar H, Schlüter MC, Bremer YI. Cognitive biases toward pain: implications for a neurocognitive processing perspective in chronic pain and its interaction with depression. Clin J Pain 2019;35:252–60. [DOI] [PubMed] [Google Scholar]
- [85].Schoth DE, Beaney R, Broadbent P, Zhang J, Liossi C. Attentional, interpretation and memory biases for sensory-pain words in individuals with chronic headache. Br J Pain 2019;13:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Schoth DE, Delgado Nunes V, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clin Psychol Rev 2012;32:13–25. [DOI] [PubMed] [Google Scholar]
- [87].Schoth DE, Liossi C. Specificity and time-course of attentional bias in chronic headache: a visual-probe investigation. Clin J Pain 2013;29:583–90. [DOI] [PubMed] [Google Scholar]
- [88].Schoth DE, Liossi C. Biased interpretation of ambiguous information in patients with chronic pain: a systematic review and meta-analysis of current studies. Health Psychol 2016;35:944–56. [DOI] [PubMed] [Google Scholar]
- [89].Schoth DE, Liossi C. A systematic review of experimental paradigms for exploring biased interpretation of ambiguous information with emotional and neutral associations. Front Psychol 2017;8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schoth DE, Parry L, Liossi C. Combined cognitive biases for sensory-pain and disability information in individuals with chronic headache. J Health Psychol 2018;23:1610–21. [DOI] [PubMed] [Google Scholar]
- [91].Serbic D, Pincus T. Diagnostic uncertainty and recall bias in chronic low back pain. PAIN 2014;155:1540–6. [DOI] [PubMed] [Google Scholar]
- [92].Sharpe L. Attentional biases in pain: more complex than originally thought? PAIN 2014;155:439–40. [DOI] [PubMed] [Google Scholar]
- [93].Sharpe L, Haggman S, Nicholas M, Blake DF, Refshauge K. Avoidance of affective pain stimuli predicts chronicity in patients with acute low back pain. Pain Headache 2014;155:45–52. [DOI] [PubMed] [Google Scholar]
- [94].Souza-Talarico JNd, Caramelli P, Nitrini R, Chaves EC. The influence of schooling on working memory performance in elderly individuals without cognitive decline. Dement Neuropsychol 2007;1:276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Stone AA, Schwartz JE, Broderick JE, Shiffman SS. Variability of momentary pain predicts recall of weekly pain: a consequence of the peak (or salience) memory heuristic. Pers Soc Psychol Bull 2005;31:1340–6. [DOI] [PubMed] [Google Scholar]
- [96].Sui J, Humphreys GW. The integrative self: how self-reference integrates perception and memory. Trends Cogn Sci 2015;19:719–28. [DOI] [PubMed] [Google Scholar]
- [97].Symons CS, Johnson BT. The self-reference effect in memory: a meta-analysis. Psychol Bull 1997;121:371–94. [DOI] [PubMed] [Google Scholar]
- [98].Tabachnick BG, Fidell LS. Using multivariate statistics. 6th ed Boston: Pearson, 2012. [Google Scholar]
- [99].Todd J, Sharpe L, Colagiuri B. Attentional bias modification and pain: the role of sensory and affective stimuli. Behav Res Ther 2016;83:53–61. [DOI] [PubMed] [Google Scholar]
- [100].Todd J, Sharpe L, Johnson A, Nicholson Perry K, Colagiuri B, Dear BF. Towards a new model of attentional biases in the development, maintenance, and management of pain. PAIN 2015;156:5189–1600. [DOI] [PubMed] [Google Scholar]
- [101].Todd J, van Ryckeghem DM, Sharpe L, Crombez G. Attentional bias to pain-related information: a meta-analysis of dot-probe studies. Health Psychol Rev 2018;12:419–36. [DOI] [PubMed] [Google Scholar]
- [102].Van Ryckeghem DM, Noel M, Sharpe L, Pincus T, Van Damme S. Cognitive biases in pain: an integrated functional–contextual framework. PAIN 2019;160:1489–93. [DOI] [PubMed] [Google Scholar]
- [103].Vucurovic K, Dupont-Gaudin C, Raucher-Chéné D, Kaladjian A, Cuervo-Lombard CV. Fibromyalgia patients make scarce reference to pain in self-defining memories. Compr Psychiatry 2019;90:30–6. [DOI] [PubMed] [Google Scholar]
- [104].Wells HJ, Pincus T, McWilliams E. Information processing biases among chronic pain patients and ankylosing spondylitis patients: the impact of diagnosis. Eur J Pain 2003;7:105–11. [DOI] [PubMed] [Google Scholar]
- [105].Williams JM, Broadbent K. Autobiographical memory in suicide attempters. J Abnorm Psychol 1986;95:144. [DOI] [PubMed] [Google Scholar]
- [106].Wright J, Morley S. Autobiographical memory and chronic pain. Br J Clin Psychol 1995;34:255–65. [DOI] [PubMed] [Google Scholar]
- [107].Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Curr Opin Neurobiol 2007;17:251–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A62.