Abstract
Background:
Prepectoral breast reconstruction has reemerged as a popular option for prosthetic-based breast reconstruction. Recent published literature highlights good outcomes; however, techniques are evolving and options exist for different technologies. The aim of this study is to evaluate short-term complication rates of prepectoral reconstructions using Cortiva acellular dermal matrix.
Methods:
A multicenter retrospective study was conducted of all patients who underwent mastectomy with immediate direct-to-implant or 2-stage prepectoral breast reconstruction with Cortiva (RTI Surgical, Alachua, Fla.) between January 2016 and September 2018. The incidence of surgical complications was determined and studied against patient demographics and procedural details.
Results:
One-hundred eighteen patients met the inclusion criteria for a total of 183 individual breasts reconstructed with prepectoral implant. Average length of follow-up was 9.26 months (range, 1.0 month to 2.5 years). Thirty-two breasts (17.49%) experienced 1 or more complications. Prepectoral reconstruction was successful 89.07% of the time. Infection was the most common cause of both reoperation and implant failure, with 7.65% of all breasts requiring washout and 5.46% failing prosthetic reconstruction secondary to infection.
Conclusions:
Surgical outcomes for prepectoral breast reconstruction using 2-stage and direct-to-implant are similar and comparable to the literature for dual-plane reconstruction, with infection being the main cause of failure.
INTRODUCTION
Prepectoral breast reconstruction continues to gain acceptance as a safe and effective technique for prosthetic-based breast reconstruction. Leaving the pectoralis muscle in place is inherently less invasive and has been demonstrated to result in quicker postoperative recovery, decreased pain scores, and improved esthetics compared with the current gold standard dual-plane technique.1,2 Early studies have shown prepectoral reconstruction with the use of acellular dermal matrix (ADM) produces a more natural projection, decreased rates of capsular contracture, and elimination of animation deformity.3–6 Although results remain favorable, the recent resurgence of this technique suffers from limited short- and long-term data on prepectoral reconstruction outcomes.
Historically, more aggressive mastectomy procedures and their resultant inadequate soft tissue support led to high incidence of infection, capsular contracture, and implant loss when placed within the subcutaneous plane.7 The subsequent shift from subcutaneous to subpectoral reconstruction provided more substantial implant coverage and ultimately led to improved outcomes, although not without both functional and esthetic consequences.8,9 Disruption of the pectoral muscle has been shown to result in decreased strength of the ipsilateral arm, whereas muscle contraction and spasm around a submuscular implant have been shown to cause pain in as many as 50% of women at least 1 year after surgery.10–12 In addition, both complete and partial muscular coverage approaches have been associated with unnatural breast animation on contraction.13 Although analysis of ADM use in partial muscular coverage procedures (ie, dual-plane technique) has revealed improved breast contour, projection, and inframammary fold definition, the other aforementioned functional complications and animation deformity remain.14,15
Several fundamental differences exist today which have primed both breast and plastic surgeons for the revival of prepectoral reconstruction. Changes in mastectomy techniques to include a more generous layer of subcutaneous tissue between dermis and breast epithelium have helped to address the principle concern of insufficient soft tissue coverage.16–19 This, paired with the popularization of ADM and its role to safely bolster the breast pocket, is credited in part for early evidence of improved outcomes with prepectoral reconstruction in the modern era.20–25 A review of recent literature describes overall complication rates with immediate ADM-assisted prepectoral reconstruction ranging from 10%23 to 17.9%24 and rates of implant loss from 1.2%24 to 10.2%.22 Importantly, several studies have demonstrated prepectoral and subpectoral prosthetic reconstructions to have comparable overall complication rates.24,26,27
Various ADM products known to incorporate favorably exist, and it is important that surgeons know the options available for use in prepectoral reconstruction. The aim of this study is to evaluate short-term complication rates of direct-to-implant (DTI) and 2-stage prepectoral breast reconstruction using Cortiva human ADM.
PATIENTS AND METHODS
A multicenter retrospective study was conducted of all patients who underwent mastectomy with immediate implant-based prepectoral breast reconstruction with Cortiva (RTI Surgical, Alachua, Fl.) between January 2016 and September 2018. Data were collected from a prospectively maintained database at Emory University Hospital (A.L.) and Cancer Treatment Centers of America in Chicago (D.Z.L.). Both DTI and 2-stage procedures were included. Surgical technique included only those approaches which achieved either full (360 degrees) or partial (180 degrees) wrap coverage of the prosthesis with Cortiva.28
The incidence of major surgical complications was determined and studied against patient demographics and procedural details. The data collected included patient age, body mass index, comorbidities, oncologic history, radiation exposure, and mastectomy procedure details. A complication was defined as major if it required readmission or return to the operating room within 60 days from reconstruction. Incidence of major infection, mastectomy skin or nipple necrosis, spontaneous implant exposure, hematoma, seroma, and delayed wound healing complications were documented. Reconstruction was deemed successful if the patient had an implant in the prepectoral space at the time of most recent follow-up, whereas reconstruction was considered a failure if the implant or expander was removed without subsequent replacement or exchange. Capsular contracture was documented if a patient presented with Baker grade III or IV contracture or required operative capsulectomy. Data were analyzed, and significance between variables was determined using a type I error of 5% (α = 0.05).
RESULTS
One-hundred eighteen patients met the inclusion criteria for a total of 183 individual breasts reconstructed with prepectoral implant. The average age was 52 years with an average body mass index of 26.52 (Table 1). Forty-four patients had preoperative (n = 9) or postoperative (n = 35) radiation treatment (Table 2). The types of mastectomy included nipple-sparing (n = 103, 56.28%), skin-sparing (n = 72, 39.34%), or skin-reducing (n = 8, 4.37%) (Table 3). Sixty-five patients underwent immediate bilateral mastectomy and reconstruction, whereas 53 patients had only unilateral procedures. There were 136 DTI reconstructions and 47 tissue expander (TE) reconstructions (Table 4). The average DTI implant volume was 407.90 mL (range, 130–700 mL) (Table 5). Average length of follow-up was 9.26 months (range, 1.0 month to 2.5 years).
Table 1.
Patient Demographics
| All Patients (n* = 118) (%) | Patients Who Experienced Major Complication (n* = 25) (%) | |
|---|---|---|
| Mean age at surgery (yr) | 52 (30–86) | 53 (32–67) |
| Mean BMI | 26.52 (18–41.1) | 27.81 (19.7–41.1) |
| Current smoker | 2 (1.69) | 1 (4.00) |
| Diabetic | 11 (9.32) | 2 (8.00) |
| Hypertensive | 38 (32.20) | 13 (52.00) |
*n expressed as number of patients.
BMI, body mass index.
Table 2.
Major Complication Rates and Outcomes by Radiation Exposure
| Major Complication | Pre- or Postmastectomy Radiation Exposure (n* = 44) | No Radiation Exposure (n* = 139) | P |
|---|---|---|---|
| All causes | 7 (15.90) | 25 (17.99) | 0.057 |
| Infection | 1 (2.27) | 13 (9.35) | 0.106 |
| Mastectomy skin/nipple necrosis | 2 (4.55) | 5 (3.60) | 0.534 |
| Implant exposure | 1 (2.27) | 2 (1.44) | 0.564 |
| Hematoma | 0 (0) | 3 (2.16) | 0.436 |
| Seroma | 1 (2.27) | 2 (1.44) | 0.564 |
| Delayed wound healing | 2 (4.55) | 0 (0) | |
| Implant/expander failure | 4 (9.10) | 15 (10.79) | 0.501 |
*n expressed as number of breasts.
Table 3.
Mastectomy Procedure Details
| All Breasts (n* = 183) | Major Complication Group (n* = 32) | Implant/Expander Failure Group (n = 19) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 183) (%) | DTI (n = 136) (%) | TE (n = 47) (%) | All (n = 32) (%) | DTI (n = 23) (%) | TE (n = 9) (%) | All (n = 19) (%) | DTI (n = 14) (%) | TE (n = 5) (%) | |
| NSM | 103 (56.28) | 94 (69.10) | 9 (19.10) | 12 (37.5) | 11 (47.83) | 1 (11.11) | 8 (42.11) | 8 (57.14) | 0 (0) |
| SSM | 72 (39.34) | 42 (30.90) | 30 (63.80) | 20 (62.5) | 12 (52.17) | 8 (88.89) | 11 (57.87) | 6 (42.86) | 5 (100.00) |
| SRM | 8 (4.37) | 0 (0) | 8 (17.00) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
*n expressed as number of breasts.
NSM, nipple-sparing mastectomy; SRM, skin-reducing mastectomy; SSM, skin-sparing mastectomy.
Table 4.
Major Complication Rates and Outcomes by Direct-to-Implant versus 2-stage Reconstruction
| Major Complication* | DTI (n† = 136) (%) | TE (n† = 47) (%) | P |
|---|---|---|---|
| All causes | 23 (16.91) | 9 (19.15) | 0.82 |
| Infection | 10 (7.35) | 4 (8.51) | >0.05 |
| Mastectomy skin/nipple necrosis | 5 (3.68) | 2 (4.26) | >0.05 |
| Implant exposure | 1 (0.74) | 2 (4.26) | >0.05 |
| Hematoma | 3 (2.21) | 0 (0) | >0.05 |
| Seroma | 3 (2.21) | 0 (0) | >0.05 |
| Delayed wound healing | 1 (0.74) | 1 (2.13) | >0.05 |
| Implant/expander failure‡ | 14 (10.29) | 5 (10.64) | 1.00 |
*Defined as any complication which required OR or readmission <60 d from primary reconstruction.
†n expressed as number of breasts.
‡Defined as removal of implant/expander without subsequent replacement or exchange.
OR, operating room.
Table 5.
Major Complication Rates and Outcomes for DTI Reconstructions by Implant Volume
| DTI ≤450 mL (n* = 93) (%) | DTI >450 mL (n* = 43) (%) | P | |
|---|---|---|---|
| Major complication† | 13 (14.00) | 10 (23.30) | 0.220 |
| Implant failure‡ | 6 (6.50) | 8 (18.60) | 0.018 |
| Capsular contracture§ | 7 (7.50) | 2 (4.20) | 0.719 |
*n expressed as number of breasts.
†Defined as any complication which required OR or readmission <60 d from primary reconstruction.
‡Defined as removal of implant/expander without subsequent replacement or exchange.
§Included only patients who presented with Baker grade III/IV or those requiring operative capsulectomy.
OR, operating room.
Twenty-five patients (21.19%) and 32 breasts (17.49%) had 1 or more major complications, with 7 patients experiencing bilateral complications (Table 6). There was no statistical difference in the major complication rate when comparing DTI and TE reconstructions (P = 0.824) (Table 4). Infection was the most common reason for reoperation, occurring in 7.65% of all breasts. Prepectoral reconstruction was successful 89.62% of the time with infection being the inciting complication leading to failure in 52.63% of cases (Figs. 1, 2). Baker III/IV capsular contracture was found in 5.4% of breasts (n = 10).
Table 6.
Complication Rates and Outcomes among All Breasts in Series (n* = 183)
| n (%) | |
|---|---|
| Major complications† | |
| All causes | 32 (17.49) |
| Infection | 14 (7.65) |
| Mastectomy skin/nipple necrosis | 7 (3.83) |
| Implant exposure | 3 (1.64) |
| Hematoma | 3 (1.64) |
| Seroma | 3 (1.64) |
| Delayed wound healing | 2 (1.09) |
| Implant/expander failure‡ | |
| All causes | 19 (10.38) |
| Infection | 10 (5.46) |
| Implant exposure | 8 (4.37) |
| Seroma | 1 (0.55) |
| Capsular contracture§ | 10 (5.46) |
*n expressed as number of breasts.
†Defined as any complication which required OR or readmission <60 d from primary reconstruction.
‡Defined as removal of implant/expander without subsequent replacement or exchange.
§Included only patients who presented with Baker grade III/IV or those requiring operative capsulectomy.
OR, operating room.
Fig. 1.

This is a 47-year-old woman with breast cancer who underwent a bilateral areolar and skin-sparing mastectomy and direct-to-implant reconstruction. Her 500-mL gel implants were both placed in the prepectoral space with anterior ADM. She is shown at 1-year follow-up and is deferring additional nipple reconstruction.
Fig. 2.
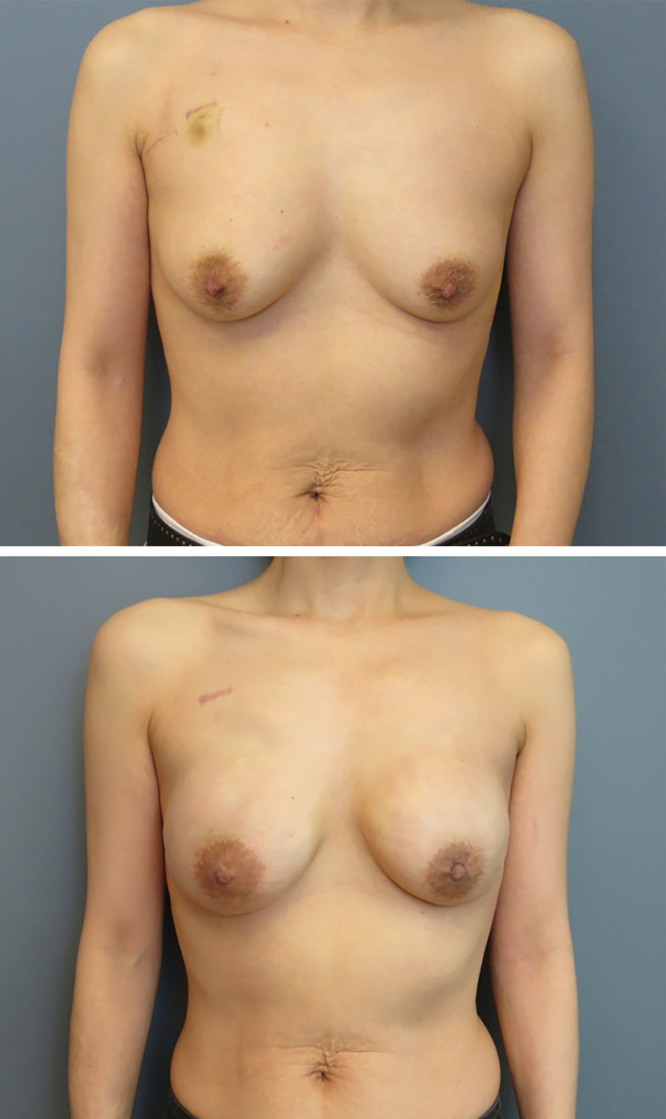
This is a 39-year-old woman with left breast cancer who underwent a bilateral nipple-sparing mastectomy and prepectoral direct-to-implant reconstruction with anterior coverage ADM. The implant size was 250-mL moderate plus gel. She is shown 1.5 years following completion of left breast irradiation with a soft symmetric breast. She has some rippling in the upper pole on the left that could be addressed with autologous fat grafting.
There was no statistical difference between major complication rates of DTI reconstructions using implant volumes ≤450 mL versus those that used >450 mL (P = 0.220) (Table 5). However, patients with implants >450 mL were statistically more likely to experience implant failure (P = 0.018).
A full wrap technique was used in 115 breasts, whereas another 68 breasts were reconstructed with partial, anterior-only coverage. Of those breasts reconstructed using the full wrap technique, 16.52% (n = 19) experienced a major complication compared with 19.12% (n = 13) for partial coverage only.
Of note, 27.78% of all skin-sparing mastectomies performed experienced a major complication compared with 11.65% of all nipple-sparing mastectomies. The failure rate was 15.28% for skin-sparing mastectomies compared with 7.77% for nipple-sparing mastectomies (Table 3).
DISCUSSION
Advancements in both breast oncology and the tools available for reconstruction have changed the landscape of breast reconstruction, making pursuit for improved esthetic and functional outcomes a reality for plastic surgeons. Recent literature seems to corroborate a role for prepectoral prosthesis placement in achieving these ideals, specifically as it relates to decreased animation deformity, capsular contracture formation, and postoperative pain scores. Correspondingly, the popularity of prosthetic-based reconstruction in the prepectoral position has increased.
In our cohort of 183 breasts, prepectoral reconstruction was successful in 89.62% of cases. Over half of cases of implant failure were attributable to infection. Our overall rate of infection requiring operative washout was high at 7.65% of all breasts. In comparison, 2 large retrospective reviews of 353 and 135 prepectoral reconstructions with AlloDerm (LifeCell Corp., Branchburg, N.J.) demonstrated infection rates of 4.5% (Sigalove et al29) and 2.0% (Woo et al23), respectively. Differences in patient exclusion criteria cannot be ignored, however, because both of these studies excluded patients with history of radiation exposure. The inclusion of these patients in our report may explain our rates of infection and failure, given the known association between radiation exposure and increased risk of implant failure.30–32 Overall, our incidence of infection was within acceptable limits of published ADM-assisted breast reconstruction outcomes, where one meta-analysis of 16 retrospective cohort studies reported a pooled infection complication rate of 5.7% (95% CI, 4.3%–7.3%).33
Many different types of human ADM are available and have been described.34–38 This report describes our experience with Cortiva. The use of ADM in prepectoral breast reconstruction provides support for the implant and improved coverage. More importantly, it works to create a more favorable interface between the prosthesis and skin flaps. Previous clinical and histologic reports have shown similar clinical outcomes when comparing Alloderm to Cortiva,37,39 with histologic evidence demonstrating lower levels of TGF-β in the Cortiva group.38 Evidence that prepectoral prosthesis with the use of ADM may limit capsule formation is growing. Our experience supports this with a low incidence of capsular contracture formation (5.46%), albeit guarded given the short-term follow-up of this study. In addition, there was no significant difference in complications when comparing the full wrap versus partial coverage cohort.
Based on our data, there did not seem to be a difference in complications when comparing 2-staged TE reconstruction versus DTI. The prepectoral DTI approach did seem to be safer when used with implants <450 mL, likely due to the initial smaller breast size being protective against complications.
The purpose of this study was to evaluate short-term complications of prepectoral reconstructions using Cortiva. Our study was multicentered and included patients with history of radiation exposure. The authors recognize this as a strength of this study, given the known association with radiation exposure as a cause of implant infection and failure.30–32 A limitation of this report was the retrospective nature of data abstraction. Because this was not a randomized controlled trial, patients were selected to be good candidates for prepectoral reconstruction at the surgeon’s discretion. Thus, our results may not be applicable to the general population. For future studies, longer follow-up was planned and will incorporate patient satisfaction scores and more adequate follow-up of long-term outcomes including capsular contracture.
CONCLUSIONS
Preliminary outcomes from this report suggest that prepectoral reconstruction with ADM is once again safe and feasible. The short-term major complication outcomes in this study of 183 breasts compare favorably with other immediate prepectoral reconstruction results in the literature.21 However, the heterogeneous prepectoral candidate exclusion criteria among patient cohorts in published reports, and the spectrum of technical considerations related to the use of ADM, make direct comparisons challenging. A call for a more standardized approach to prepectoral outcomes reporting may be needed, whereby there may be utility to data capture and comparison by means of a validated method like the American College of Surgeons National Surgical Quality Improvement Program. Large prospective studies comparing the use of different acellular dermal matrices are also needed before prepectoral implant placement may be considered the new standard of care for breast reconstruction.
Footnotes
Published online 23 April 2020.
Disclosure: Drs. Liu and Losken are speakers for RTI surgical. Neither of the other authors has any financial disclosures.
REFERENCES
- 1.Cattelani L, Polotto S, Arcuri MF, et al. One-step prepectoral breast reconstruction with dermal matrix-covered implant compared to submuscular implantation: functional and cost evaluation. Clin Breast Cancer. 2018;18:e703–e711. [DOI] [PubMed] [Google Scholar]
- 2.Schaeffer CV, Dassoulas KR, Thuman J, et al. Early functional outcomes after prepectoral breast reconstruction: a case-matched cohort study. Ann Plast Surg. 2019;82(6S Suppl 5):S399–S403. [DOI] [PubMed] [Google Scholar]
- 3.Gabriel A, Sigalove S, Sigalove NM, et al. Prepectoral revision breast reconstruction for treatment of implant-associated animation deformity: a review of 102 reconstructions. Aesthet Surg J. 2018;38:519–526. [DOI] [PubMed] [Google Scholar]
- 4.Salzberg CA, Ashikari AY, Koch RM, et al. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (Alloderm). Plast Reconstr Surg. 2011;127:514–524. [DOI] [PubMed] [Google Scholar]
- 5.Onesti MG, Maruccia M, Di Taranto G, et al. Clinical, histological, and ultrasound follow-up of breast reconstruction with one-stage muscle-sparing “wrap” technique: a single-center experience. J Plast Reconstr Aesthet Surg. 2017;70:1527–1536. [DOI] [PubMed] [Google Scholar]
- 6.Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plast Reconstr Surg. 2010;126:1842–1847. [DOI] [PubMed] [Google Scholar]
- 7.Schlenker JD, Bueno RA, Ricketson G, et al. Loss of silicone implants after subcutaneous mastectomy and reconstruction. Plast Reconstr Surg. 1978;62:853–861. [DOI] [PubMed] [Google Scholar]
- 8.Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. 1981;67:312–317. [DOI] [PubMed] [Google Scholar]
- 9.Woo KJ, Park JW, Mun GH, et al. Does the use of acellular dermal matrix increase postoperative complications of the first-stage reconstruction of immediate expander-implant breast reconstruction: a matched cohort study. Ann Plast Surg. 2017;79:341–345. [DOI] [PubMed] [Google Scholar]
- 10.de Haan A, Toor A, Hage JJ, et al. Function of the pectoralis major muscle after combined skin-sparing mastectomy and immediate reconstruction by subpectoral implantation of a prosthesis. Ann Plast Surg. 2007;59:605–610. [DOI] [PubMed] [Google Scholar]
- 11.Hage JJ, van der Heeden JF, Lankhorst KM, et al. Impact of combined skin sparing mastectomy and immediate subpectoral prosthetic reconstruction on the pectoralis major muscle function: a preoperative and postoperative comparative study. Ann Plast Surg. 2014;72:631–637. [DOI] [PubMed] [Google Scholar]
- 12.Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: a survey of 282 women. Pain. 1996;66:195–205. [DOI] [PubMed] [Google Scholar]
- 13.Spear SL, Schwartz J, Dayan JH, et al. Outcome assessment of breast distortion following submuscular breast augmentation. Aesthetic Plast Surg. 2009;33:44–48. [DOI] [PubMed] [Google Scholar]
- 14.Nahabedian MY. Acellular dermal matrices in primary breast reconstruction: principles, concepts, and indications. Plast Reconstr Surg. 2012;130(5 Suppl 2):44S–53S. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim AM, Koolen PG, Ganor O, et al. Does acellular dermal matrix really improve aesthetic outcome in tissue expander/implant-based breast reconstruction? Aesthetic Plast Surg. 2015;39:359–368. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell GP, Storm-Dickerson T, Whitworth P, et al. Advances in nipple-sparing mastectomy: oncological safety and incision selection. Aesthet Surg J. 2011;31:310–319. [DOI] [PubMed] [Google Scholar]
- 17.Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg. 2014;101:899–911. [DOI] [PubMed] [Google Scholar]
- 18.Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg. 2011;127:27–33. [DOI] [PubMed] [Google Scholar]
- 19.Headon HL, Kasem A, Mokbel K. The oncological safety of nipple-sparing mastectomy: a systematic review of the literature with a pooled analysis of 12,358 procedures. Arch Plast Surg. 2016;43:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorkin M, Qi J, Kim HM, et al. Acellular dermal matrix in immediate expander/implant breast reconstruction: a multicenter assessment of risks and benefits. Plast Reconstr Surg. 2017;140:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee A, Nahabedian MY, Gabriel A, et al. Early assessment of post-surgical outcomes with pre-pectoral breast reconstruction: a literature review and meta-analysis. J Surg Oncol. 2018;117:1119–1130. [DOI] [PubMed] [Google Scholar]
- 22.Jafferbhoy S, Chandarana M, Houlihan M, et al. Early multicentre experience of pre-pectoral implant based immediate breast reconstruction using Braxon. Gland Surg. 2017;6:682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo A, Harless C, Jacobson SR. Revisiting an old place: single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J. 2017;23:545–553. [DOI] [PubMed] [Google Scholar]
- 24.Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;140:432–443. [DOI] [PubMed] [Google Scholar]
- 25.Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: first multicenter European report on 100 cases. Breast J. 2017;23:670–676. [DOI] [PubMed] [Google Scholar]
- 26.Bettinger LN, Waters LM, Reese SW, et al. Comparative study of prepectoral and subpectoral expander-based breast reconstruction and Clavien IIIB score outcomes. Plast Reconstr Surg Glob Open. 2017;5:e1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77–e86. [DOI] [PubMed] [Google Scholar]
- 28.Sigalove S. Options in acellular dermal matrix-device assembly. Plast Reconstr Surg. 2017;140(6S Prepectoral Breast Reconstruction):39S–42S. [DOI] [PubMed] [Google Scholar]
- 29.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg. 2017;139:287–294. [DOI] [PubMed] [Google Scholar]
- 30.Lee KT, Mun GH. Prosthetic breast reconstruction in previously irradiated breasts: a meta-analysis. J Surg Oncol. 2015;112:468–475. [DOI] [PubMed] [Google Scholar]
- 31.Chetta MD, Aliu O, Zhong L, et al. Reconstruction of the irradiated breast: a national claims-based assessment of postoperative morbidity. Plast Reconstr Surg. 2017;139:783–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearney AM, Brown MS, Soltanian HT. Timing of radiation and outcomes in implant-based breast reconstruction. J Plast Reconstr Aesthet Surg. 2015;68:1719–1726. [DOI] [PubMed] [Google Scholar]
- 33.Ho G, Nguyen TJ, Shahabi A, et al. A systematic review and meta-analysis of complications associated with acellular dermal matrix-assisted breast reconstruction. Ann Plast Surg. 2012;68:346–356. [DOI] [PubMed] [Google Scholar]
- 34.Lee KT, Mun GH. A meta-analysis of studies comparing outcomes of diverse acellular dermal matrices for implant-based breast reconstruction. Ann Plast Surg. 2017;79:115–123. [DOI] [PubMed] [Google Scholar]
- 35.Liu DZ, Mathes DW, Neligan PC, et al. Comparison of outcomes using Alloderm versus FlexHD for implant-based breast reconstruction. Ann Plast Surg. 2014;72:503–507. [DOI] [PubMed] [Google Scholar]
- 36.Hinchcliff KM, Orbay H, Busse BK, et al. Comparison of two cadaveric acellular dermal matrices for immediate breast reconstruction: a prospective randomized trial. J Plast Reconstr Aesthet Surg. 2017;70:568–576. [DOI] [PubMed] [Google Scholar]
- 37.Keifer OP, Jr, Page EK, Hart A, et al. A complication analysis of 2 acellular dermal matrices in prosthetic-based breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4:e800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moyer HR, Hart AM, Yeager J, et al. A histological comparison of two human acellular dermal matrix products in prosthetic-based breast reconstruction. Plast Reconstr Surg Glob Open. 2017;5:e1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh RP, Tenenbaum MM, Yan Y, et al. Cortiva versus Alloderm ready-to-use in prepectoral and submuscular breast reconstruction: prospective randomized clinical trial study design and early findings. Plast Reconstr Surg Glob Open. 2018;6:e2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


