Abstract
Background:
Tear trough (TT) treatment with hyaluronic acid soft tissue fillers is an increasingly popular aesthetic procedure. The traditional needle technique is cited many times in the literature with no studies looking at the results, complications and satisfaction rate with the use of the cannula device instead. The aims of this study are to describe the experience of 4 aesthetic doctors in the treatment of TT deformity and assess complications and side effects, overall satisfaction and improvement.
Methods:
Twenty-four patients were included (48 TTs) that fulfilled the inclusion and exclusion criteria and they were assessed over a 4-week period, looking at the complications, side effects, satisfaction rate, and others with the cannula technique for the medial TT.
Results:
Twenty-two women and 2 men each had the medial TT filler supra-periosteally using a cannula device. They were all reviewed at the 2-week stage +/− the 4-week stage. 100% of patients noted an overall improvement to the TTs and 75% were satisfied with their results with the other 25% requiring further filler to be satisfied. There were no major complications and only a small number of minor side effects like mild bruising and swelling that lasted up to 4 weeks.
Conclusions:
TT treatment, if performed using a cannula with a maximum of 1ml used in one sitting between both eyes, according to this study, is a safe treatment with a very low pain rating and with no major complications and high patient satisfaction.
INTRODUCTION
Over the past few years, tear trough (TT) treatment with hyaluronic acid (HA) dermal fillers has become an increasingly popular option for “under eye” rejuvenation across all ages. The TT extends from the medial canthus inferiorly and laterally roughly 2 cm in most patients1 and becomes more prominent as we age due to loss of bone and fat.2 The TT deformity itself was first described by Flowers in 19931 where he proposed the placement of a small silicone-based implant to improve the hollowness. Following Flowers, many forms of grading scales and assessment techniques have been developed to accurately describe/measure TT deformities. For example, Lambros,3 Hirmand,4 and Sadick et al.5 With rejuvenating treatments in this area more increasingly requested, HA dermal fillers in this region provide an excellent and safe option.
In this article, whilst it was open-label, we report our results using Teosyal Puresense Redensity 2 (TEOXANE SA, Geneva, Switzerland) which contains 15 mg/ml of HA (a mix of crosslinked and non-crosslinked high molecular weight HA). Its overall degree of modification with BDDE is 5.5% plus a dermo-restructuring complex which contains 14 essential nutrients; vitamin B6, zinc, copper, 3 antioxidants (glutathione, N-acetyl-L-cysteine, and alpha-lipoic acid) and 8 amino acids (glycine, lysine, threonine, proline, isoleucine, leucine, valine, and arginine). The product is a licensed product for use within the area in 80 different countries worldwide. For the sake of consistency, each practitioner involved in the study used a cannula device for delivery of product into the TT region, what we are proposing to be a preferred method, and not using the traditional needle method.
A number of articles have discussed the results, patient satisfaction, and complications with TT treatment, however, they have all been using the needle technique or a combination. This study is focused on the cannula technique to improve TT deformity.
The aims of this study are to describe the experience of 4 aesthetic doctors and complete a prospective study on male and female patients undergoing TT deformity treatment over a 3 month period, between the ages of 18–65 to assess:
(1) Most common complications within the 4-week period after TT treatment with a cannula device in the supra-periosteal plane;
(2) Age-specific complications;
(3) Best time to review a TT treatment and what is the average time it takes to fully heal;
(4) Best time to do second session/second milliliter in patients that require it;
(5) Is 1 ml maximum satisfactory for most patients overall? What percentage need more? Is this affected by certain ethnicities and ages?;
(6) Are patients satisfied by 1 ml maximum in one sitting?
Overall, the aim is to provide more treatment specific, evidence-based information and after care for patients as well as for practitioners. Furthermore, to create the first UK-based study on TTs describing the experience of patients and doctors using the cannula technique.
METHODS
All patients considered to be included in the study signed an informed consent form. The criteria for being entered into the study were divided into patient-specific and treatment-specific criteria. We also classified inclusion and exclusion criteria. The patient-specific inclusion and exclusion criteria are listed in Table 1. Exclusion criteria included points that could reduce the longevity and efficacy of the results, for example, anyone over the age of 65 as it may skew results due to extensive soft tissue and bony loss over this age.6
Table 1.
Patient-specific Inclusion and Exclusion Criteria for the Study
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patient consented to be part of the study | Smoker |
| Clear indication for TT filler using Lambros and Hirmand criteria | Performs heavy exercise >4 times per week |
| Under the age of 18 or over the age of 65 | |
| Regular sunbed, sauna, and steam room user (>1 per week) | |
| Allergy to HA filler, lidocaine or local anesthetic cream | |
| Have had TT filler in the past | |
| Patient has semi-permanent or permanent fillers in situ in TT region or have had previous surgery in the area | |
| Current infection in the area |
The treatment-specific criteria, including and detailing the inclusion and exclusion criteria are listed in Table 2. This was a multi-practitioner and multi-centre study, therefore to get consistency and reliability of the data, all practitioners needed to follow these criteria. These included the use of a cannula, the medial TT must be filled in the treatment plan for the patient +/− palpebromalar and nasojugal grooves (Fig. 1). The filler was placed in the supra-periosteal plane for all patients. Whilst more superficial placement is reported in the literature, practitioner experience and other evidence states that this has a very high risk of causing lumpiness, Tyndall effect and unsatisfactory outcomes.
Table 2.
Treatment-specific Inclusion and Exclusion Criteria for the Study
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Cannula used for treatment for medial TT | Unable to carry out review |
| Medial TT must be filled at supra-periosteal plane, at or inferior to orbital rim | |
| Only 1 ml used as maximum total between both eyes | |
| Patient reviewed at 2 weeks and 4 weeks (this can be face-to-face, video call or telephone consultation with supporting photography) |
Fig. 1.
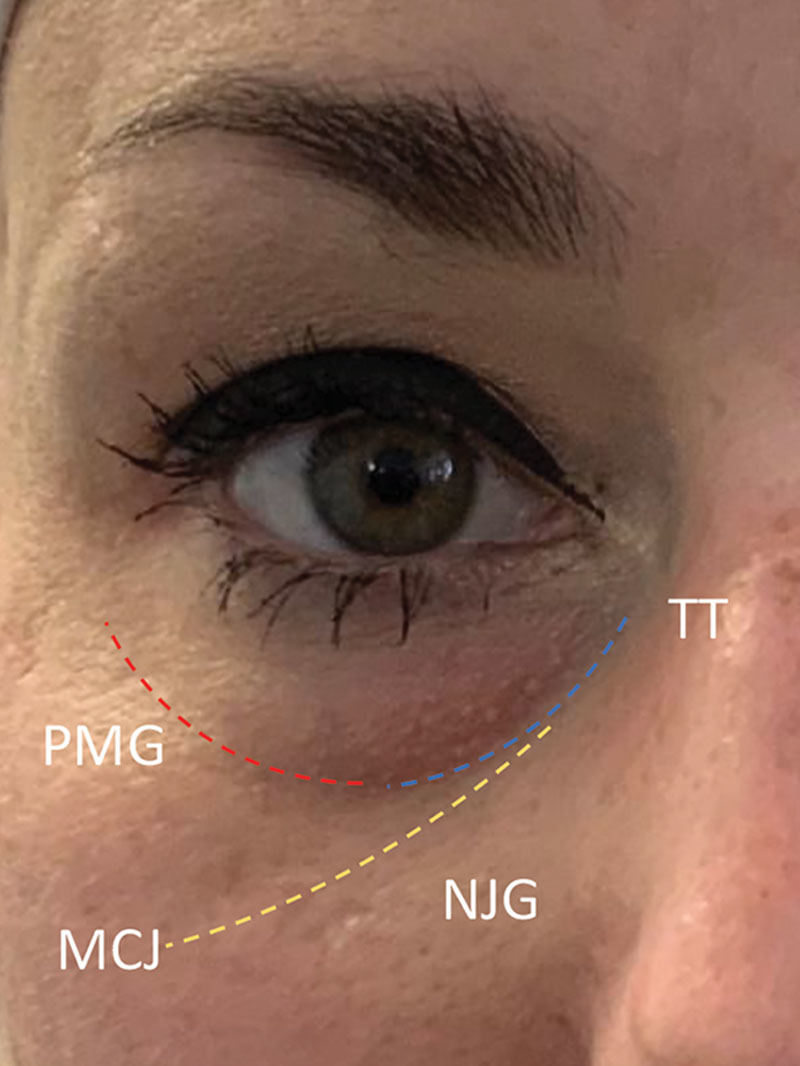
Illustrating the “true” TT deformity, the palpebromalar groove (PMG) the nasojugal groove (NJG) and the mid-cheek junction (MCJ).10
A 14-day and 4-week review either face-to-face, video call, or telephone consultation with supporting photography was accepted as review. If a review failed to be conducted, this fell under the exclusion criteria and the patient was excluded from the study. There were no touch-ups planned until the 4-week mark.
For each consented patient fulfilling all criteria, a pro-forma was completed by the treating practitioner which included a quantitative grading of each TT deformity using Lambros3 and Hirmand4 criteria. Lambros (Table 3) emphasized the importance of assessing 4 key components when considering TT rejuvenation (including skin quality, defined hollowing, minimal orbital fat presence, and no skin pigmentation changes). Lambros suggested that with all 4 of these present, the chances of getting a good patient outcome were increased, therefore we have used this as an assessment scale. Hirmand (Fig. 2), on the other hand, used clinical evaluation of the depth and extent of the groove itself—grade 1 meaning the groove is confined to the medial trough, grade 2 included part of the lateral trough, and grade 3 the full circumferential depression along the inferior orbital rim.
Table 3.
Lambros Criteria3
| Lambros Criteria Score (Total Out of 4): |
|---|
| Good skin quality (0 = poor, 1 = good) |
| Groove definition (0 = poor, 1 = good) |
| Orbital Fat presence (0 = present, 1 = not present) |
| Pigmentation (0 = present, 1 = not present) |
Fig. 2.
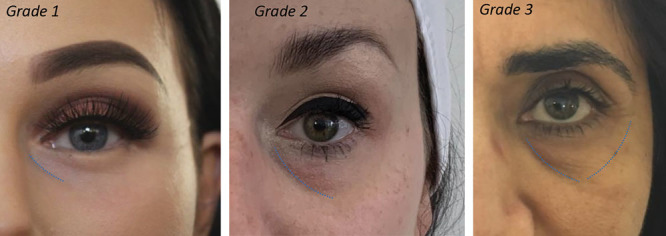
Hirmand grading.
All treating practitioners in this study were medical doctors. For each patient, the filler, lot number, and expiry date were noted on the pro-forma as well as cannula size and length, total amount in each side, technique used (micro-droplet, bolus, linear thread, or other) and whether the lateral trough was filled. If the patients had other treatments carried out on that day, this was also noted. Next, immediate side effects were noted Including the severity of immediate swelling or if any bruising was present (nil, mild, moderate, or severe), grading of how painful the treatment was out of 10 (10 being the worst pain) and any others. At the 14-day review, the same was assessed in terms of bruising, swelling, tenderness and any others (eg, heamosiderin deposition, puffiness, asymmetry, filler migration, post-inflammatory hyperpigmentation, infection, Tyndall effect, blindness, nodules, others). Patients were asked to express whether they were satisfied/happy with the treatment and whether they saw an improvement. If everything was normal at this stage then the 4-week review was not required. At the 4-week review, the same assessment was made as at day 14.
RESULTS
A total of 50 eyes were treated of 25 patients in the study by 4 medical doctors. All patients gave written consent to be in the study. One patient was excluded due to being a smoker. Therefore 24 patients were included.
There were 22 females and 2 males with an average age of 34.8 years overall with the female mean average at 34.8 years and the male mean average at 34.0 years. Breakdown of ethnicity was as follows: Caucasian (n = 14), South Asian (n = 7), Afro Caribbean (n = 1), Middle Eastern (n = 1), and Southeast Asian (n = 1).
TT grading for Lambros criteria was equal in all patients under both eyes (right and left under eyes, grade 1 n = 0, grade 2 n =1, grade 3 n = 10, and grade 4 n = 13). Twenty-one out of 24 patients had equal Hirmand grading under both eyes (right and left under eyes, grade 1 n = 14, grade 2 n = 11, and grade 3 n = 6). Three out of 24 patients had different Hirmand grading between each under eyes (2/3 were right eye grade 1 and left eye grade 2, 1/3 had grade 3 for the right under eye and grade 2 for left under eye).
The medial trough was filled for 100% of patients at the supraperiosteal plane at or inferior to orbital rim using a cannula (27G n = 2 and 25G n = 22, 50 mm length n = 5/24 and 38 mm length n = 19/24). For all patients, injectors used the microdroplet +/− linear threading technique.
The minimum amount of filler placed under a single TT in one session was 0.2 ml and the maximum placed under a single TT in one session was 0.6 ml. Two out of 24 patients had their lateral TT filled. The mean average amount of filler placed under a TT in this study was 0.43 ml (n = 48 TTs).
Nine out of 24 patients had other treatments performed on the same day (6/9 included orbicularis oculi “crows feet” toxin injections, 3/9 were upper face toxin—frontalis, glabella, and orbicularis oculi crows feet lines, 2/9 were lip fillers, 1/9 was nose filler, 1/9 was medial cheek filler).
Immediate injection site reactions of affected 1/24 who had moderate swelling immediately after on the right side only, 22/24 had mild swelling on at least one side. Immediate bruising was rarely seen with 1/24 who had mild right-sided and moderate left-sided bruising at the entry point of the cannula immediately after treatment. The average score for pain was 1.7/10. No other complications reported immediately after treatment except for one patient who reported pre-syncopal symptoms that resolved after lying the patient flat with legs raised for 3 minutes.
At day 14, 6/24 patients had mild swelling and 1/24 with moderate swelling. In terms of bruising, only 2 patients had bruising at this stage with 1 patient having mild unilateral bruising and 1 patient having moderate bilateral bruising at the entry point of the cannula. Four out of 24 patients experienced puffiness at 2 weeks, 1/24 had mild asymmetry, 1/24 experienced a unilateral watery eye, 1/24 had an overall minimal difference.
Patient satisfaction at 2 weeks was high with 17/24 (71%) patients satisfied with treatment and 7/24 patients not satisfied, with majority of these needing a touch-up or further session of treatment.
Two out of 24 patients had swelling at 4 weeks posttreatment and with both of these, the swelling was bilateral and mild. No patients had any bruising at 4 weeks posttreatment. One patient had puffiness in the mornings only at the 4 -week mark.
Patient satisfaction at 4 weeks went up to 18/24 (75%) and 6/24 patients were not satisfied by 4 weeks. Of these, 1/24 wanted to continue working on pigmentation issues, 4/6 required further treatment with further filler but were unable to afford it at the time and 1/6 felt she needed more but the doctor felt objectively the groove was filled to its maximum. 100% of patients by this point felt an overall improvement in the appearance of their TTs.
We assessed the parallel between the Lambros and Hirmand grading systems and the quantity of filler used. The average used for Lambros grade 2 was 0.38 ml, grade 3 0.43 ml, and grade 4 was 0.42 ml. Therefore, no real parallel was seen here (Table 4).
Table 4.
Average Amount Placed in TT Relative to Lambros Grading
| Lambros | |||||
|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | |||
| Right | Left | Right | Left | Right | Left |
| 0.35 ml | 0.4 ml | 0.43 ml | 0.43 ml | 0.45 ml | 0.39 ml |
| Average = 0.38 ml (n = 2) | Average = 0.43 ml (n = 20) | Average = 0.42 ml (n = 26) | |||
Each figure is an average of all figures within the grade. Each TT assessed separately, therefore, total = 48 TTs and 24 patients.
The average used for Hirmand grade 1 was 0.32 ml, grade 2 was 0.45 ml, and grade 3 was 0.46 ml. This was slightly more apparent from grade 1 to grade 2, but grades 2 and 3 were relatively similar (Table 5).
Table 5.
Average Amount Placed in TT Relative to Hirmand Grading
| Hirmand | |||||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | |||
| Right | Left | Right | Left | Right | Left |
| 0.35 ml | 0.28 ml | 0.46 ml | 0.44 ml | 0.48 ml | 0.44 ml |
| Average = 0.32 (n = 10) | Average = 0.45 ml (n = 25) | Average = 0.46 ml (n = 13) | |||
Each figure is an average of all figures within the grade. Each TT assessed separately, therefore, total = 48 TTs and 24 patients, as many patients had differing right and left TT Hirmand grade.
We also assessed these grading systems and whether they had any correlation with patient satisfaction (Figs. 3–4). All patients that were Hirmand grade 1 (n = 4) were satisfied with treatment by week 2, 100% satisfaction rate. For Hirmand grade 2 patients (n = 13) (patients categorized into highest grade if 2 grades present between each eye), 8/13 satisfied at 2 weeks and 11/13 satisfied at 4 weeks, so an 85% satisfaction rate by week 4. And for Hirmand grade 3 (n = 7), 5/7 satisfied at 2 weeks and dropped to 3/7 patients that were satisfied at 4 weeks, giving a 43% satisfaction rate by week 4. Four out of 4 that were dissatisfied at week 4 required further treatment with more filler, but could not afford it at the time.
Fig. 3.
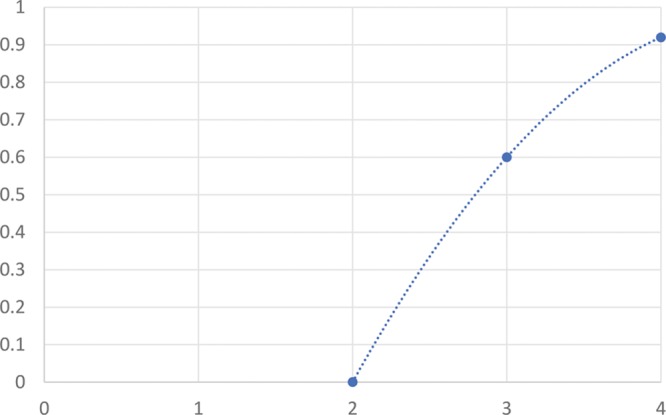
Patient satisfaction rate (by week 4) vs Hirmand grade.
Fig. 4.
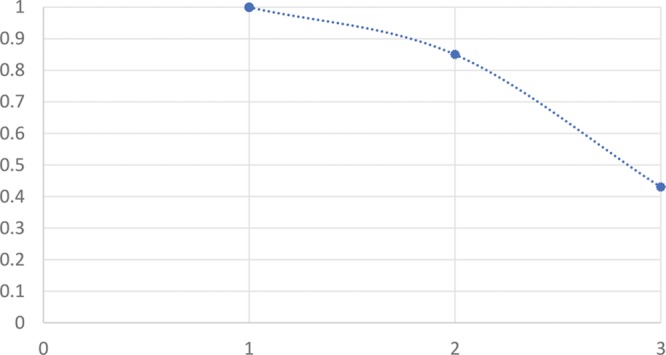
Patient satisfaction rate (by week 4) vs Lambros grade.
Lambros grading showed a similar pattern, with grade 2 patients have 0% satisfaction rate by week 4 (n = 1), grade 3 60% satisfaction rate by week 4 (n = 10), and grade 4 with 92% satisfaction rate at week 4 (n = 13); the higher the Lambros grade, the more “amenable” to TT filler the patient is3, meaning a better satisfaction rate.
Six patients had crows feet toxin, 5/6 were satisfied with overall treatment by 4 weeks despite a range of Hirmand and Lambros grades between them all. One out of 6 was not; she required more filler and was the oldest in this group of patients at 38 years old.
There were no major complications reported in this study. Four out of 24 patients reported puffiness with the first 2 weeks and no correlation was found with the quantity of filler used.
There were 8/24 patients that were over the ages of 35, 3/8 were dissatisfied at 4 weeks, giving this group of patients a 38% dissatisfaction rate with 1 ml. There were 16/24 patients that were 35 years or under, 3/16 were dissatisfied at 4 weeks, giving the group of patients a 19% dissatisfaction rate. Figures 5–8 show examples of results and satisfied and non-satisfied patients.
Fig. 5.
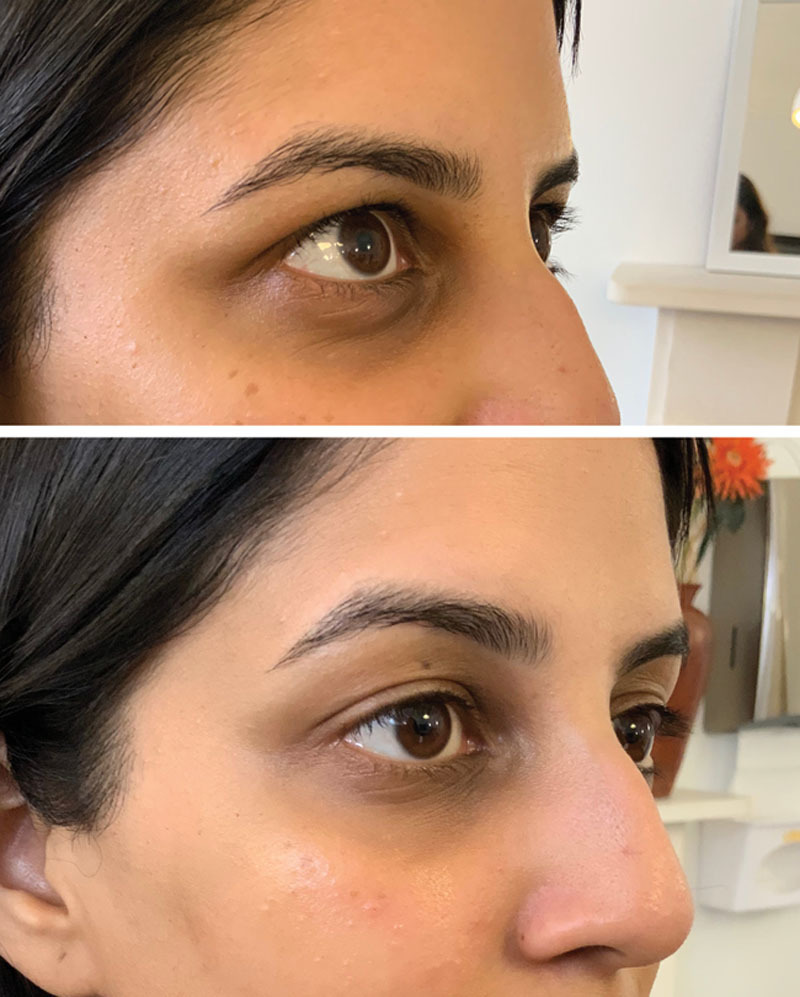
This patient was classed as Hirmand grade 2 and Lambros grade 3 (pigmentation present) bilaterally. She had 0.5 ml per TT and no other treatments. This patient was not satisfied fully at the 4-week mark as she wanted to work more on pigmentation. She did note an overall improvement to her TT.
Fig. 8.

This patient was classed as Hirmand grade 1 and Lambros grade 4 bilaterally. She had 0.2 ml L TT and 0.25 ml R TT. This patient was happy with the results.
Fig. 6.
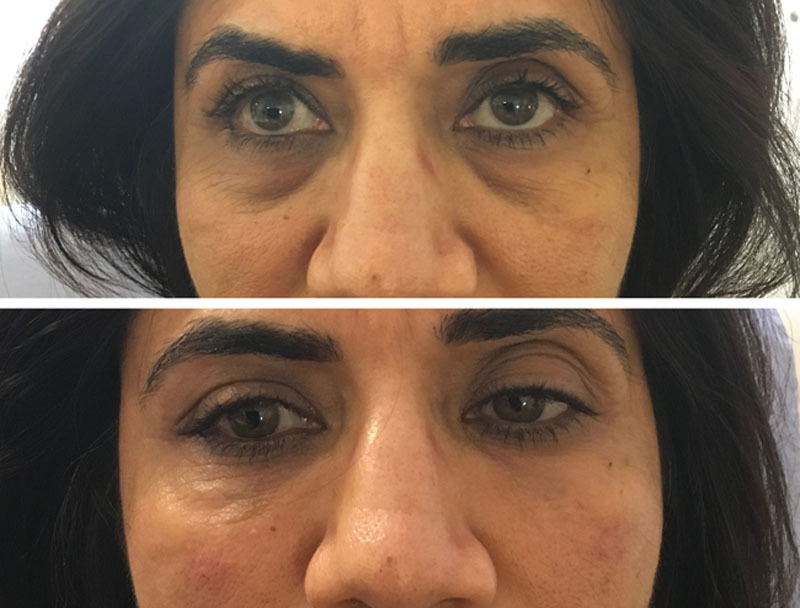
This was a slight older patient with deep TT. She was classified as Hirmand grade 3 bilaterally and Lambros grade 3 bilaterally. Here right TT was deeper than the left. She had 0.55 ml in the right TT and 0.45 ml in the left TT. This patient was dissatisfied at the 4-week stage as she felt she needed more as did the practitioner, she was financially unable to get a second session for the time being. She noted an overall improvement in her TT.
Fig. 7.
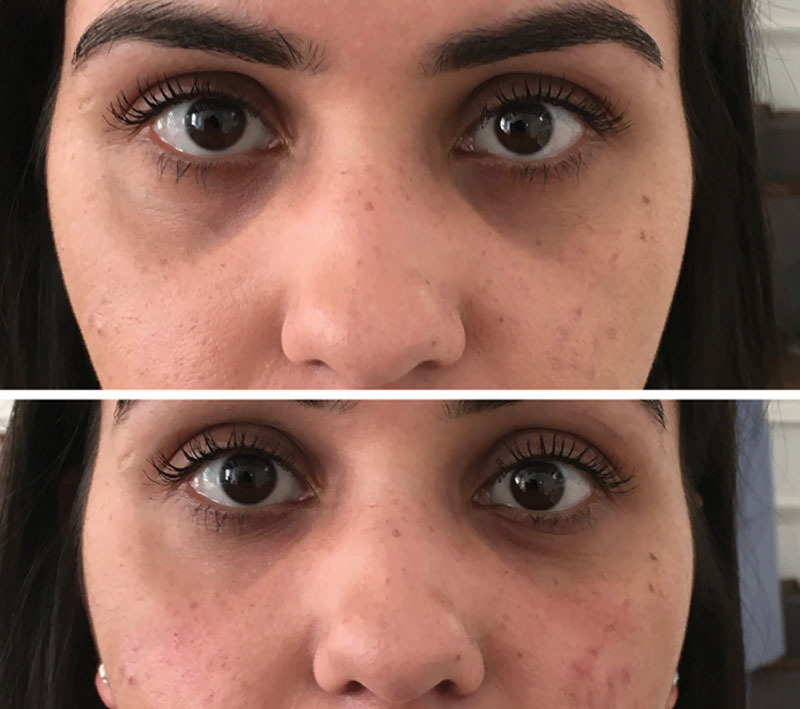
This patient was classed as Hirmand grade 2 and Lambros grade 3 (pigmentation present) bilaterally. She had 0.5 ml per TT and no other treatments. This patient was satisfied with the results.
DISCUSSION
Previous similar studies have assessed the treatment of the TT, but none have assessed it using purely the cannula method in the supra-periosteal plane. Overall, the results showed no major complications like vascular occlusion or large hematomas, and no complications that were considered serious using this method. Whilst there were mild complications like swelling, these fully resolved. Moreover, patients also reported a very low average pain score during and after treatment.
Interestingly, there was a good correlation seen between under and over 35-year-olds with regards to satisfaction rate and predictability. The older age group were more likely to be dissatisfied with 1 ml of filler between both TTs, at a rate of 38%, and required more filler generally. The younger age group were less likely to be dissatisfied with 1 ml of filler between both TTs, at a rate of 19%. The main hindrance to patient satisfaction seems to be financial as these patients require further treatment, hence, it may be worth explaining to all patients over 35 that almost 4/10 patients over this age require a second session, preparing them and their treatment plan.
Berguiga and Galatoire7 article on the same filler in the TT region on 151 patients with the needle technique reported 11.3% bruising (17/151) including 2 with moderate and 1 with severe bruising immediately after treatment. Moreover, this bruising lasted in 12/17 patients at the one month mark. In comparison, in this study, the use of the cannula technique may have led to only one patient suffering mild/moderate bruising at the cannula entry point, which resolved by week 4. Four patients in the Berguiga and Galatoire7 study suffered from blue discoloration, zero patients experienced this in this current study. This could be due to the use of the cannula and deep supra-periosteal placement. Interestingly, they found swelling in 22/151 patients immediately that continued at the 4-week mark in 13/151 patients with some patients suffering moderate and severe swelling even at this stage. Conversely, in this study, no patients had any swelling at the 4-week stage. This coveys that the best time to actually review patients to consider further treatment in the same area, for example, to add more filler to a very deep hollow, should be 4 weeks.
In addition, compared to more articles that discuss complications after treating this region, we again found a much lower rate of bruising. Fifty-two percent of cases in the Viana et al (2011) article8 suffered bruising; a prospective study where 25 patients underwent TT rejuvenation with soft tissue filler using a 30 gauge needle and serial puncture technique. In Goldberg and Fiaschetti’s 2006 article9 on filler in the periorbital region using a different filler (Restylane) and using the needle with bolus and threading techniques in 121 patients, they found that 11% of these patients needed hyaluronidase injections to dissolve some of the filler and to improve the irregularities. There were no such cases with this filler or technique.
It is important to assess both Lambros and Hirmand classes, as one does not dictate amount of filler to be used necessarily. There is a high satisfaction rate of 75% overall, with the main reason for the dissatisfaction being “required more filler” and not being able to afford the treatment financially at the time of the study. Another interesting finding was that 83% of patients that had adjuvant treatments on the same day as their TT treatment, for example, crows feet toxin, were satisfied at the 4-week mark. This may be something to consider mentioning to all relevant patients. Moreover, for the older patients especially, mid-facial fillers will help improve and support the TT region and is something that should be discussed with relevant patients also.
In the future, the aim will be to improve the study with specific focus on certain age groups, larger sample size and review patients at the 6-month and 1-year mark to assess longevity. Patient satisfaction after all treatment sessions for the TT should be assessed in future studies as financial restraint seemed to be the main reason satisfaction rate was not 100%. All patients did note an overall improvement.
CONCLUSIONS
TT treatment, if performed using a cannula with a maximum of 1 ml used in one setting between both eyes, according to this study, is a safe treatment with a very low pain rating and with no major complications. Compared to other studies looking at the TT treatment with needles, this study has shown significantly less risk of and rate of bruising and major complications like hematoma or need for hyaluronidase. Whilst there are common side effects like immediate swelling, these are temporary and fully resolved in all patients with this technique.
There is a high satisfaction rate with this technique and Teosyal Redensity 2 filler and all patients experienced an overall improvement in the appearance of their TT s after a 1 ml of filler as a maximum in one sitting. Patients over the age of 35 should be advised at the consultation phase that there is a higher chance of them requiring more than one session/1 ml divided between both eyes. The best time to review patients using this technique is at the 4-week mark where if more filler is required, this should be the minimum time frame to wait.
Overall, this technique and filler type are safe with a high satisfaction rate and low complication rate.
Footnotes
Published online 29 April 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Flowers RS. Tear trough implants for correction of tear trough deformity. ClinPlast Surg. 1993;20:403–15. [PubMed] [Google Scholar]
- 2.Lambros VS. Observations on periorbital and midface aging. Plast Reconstr Surg. 2007;120:1367–1376; discussion 1377. doi: [DOI] [PubMed] [Google Scholar]
- 3.Lambros VS. Hyaluronic acid injections for correction of the tear trough deformity. Plast Reconstr Surg. 2007;120(6 Suppl):74S–80S. [DOI] [PubMed] [Google Scholar]
- 4.Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125:699–708. [DOI] [PubMed] [Google Scholar]
- 5.Sadick NS, Bosniak SL, Cantisano-Zilkha M, et al. Definition of the tear trough and the tear trough rating scale. J Cosmet Dermatol. 2007;6:218–222. [DOI] [PubMed] [Google Scholar]
- 6.Mendleson B, Wong CH. Changes in the facial skeleton with aging: implications and clinical applications in facial rejuvenation. Aesthetic Plast Surg. 2012;36:753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berguiga M, Galatoire O. Tear trough rejuvenation: a safety evaluation of the treatment by a semi-cross-linked hyaluronic acid filler. Orbit. 2017;36:22–26. [DOI] [PubMed] [Google Scholar]
- 8.Viana GA, Osaki MH, Cariello AJ, et al. Treatment of the tear trough deformity with hyaluronic acid. Aesthet Surg J. 2011;31:225–231. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg RA, Fiaschetti D. Filling the periorbital hollows with hyaluronic acid gel: initial experience with 244 injections. Ophthalmic Plast Reconstr Surg. 2006;22:335–684; discussion 684. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Hong G. Definitions of groove and hollowness of the infraorbital region and clinical treatment using soft-tissue filler. Arch Plast Surg. 2018;45:214–221. [DOI] [PMC free article] [PubMed] [Google Scholar]


