Abstract
Background:
Delayed-onset adverse reactions to hyaluronic acid (HA) fillers are uncommon but have received increased attention, particularly with regard to late-onset nodules. Globally, there is a need for comprehensive prevention and management strategies.
Methods:
Experts with clinical practices in diverse regions of the world and extensive experience in managing complications related to HA fillers convened to propose and evaluate approaches to prevent delayed-onset adverse reactions after HA filler administration and manage late-onset nodules.
Results:
The expert panel agreed to define delayed-onset adverse reactions as those presenting more than 4 weeks posttreatment, with swelling, induration, and nodulation being the most common clinical signs. The panel recommended 5 general key approaches for the prevention of delayed-onset reactions (patient selection, anatomic location of injection/product selection, aseptic technique, injection procedure/filler, and posttreatment care). Strategies recommended for managing late-onset nodules included oral antibiotics, oral steroids, nonsteroidal anti-inflammatory drugs if needed, hyaluronidase for noninflammatory nodules (recognizing the limitations and regional availability of this treatment), intralesional antibiotics, intralesional immunosuppressive drugs such as steroids and fluorouracil, and surgical excision as a last resort. The panel noted that late-onset nodules may vary in both clinical presentation and etiology, making them challenging to address or prevent, and stressed individualized treatment based on clinical presentation. Regional differences in aseptic protocols, antibiotic selection, and steroid formulations were described.
Conclusion:
Insights from global experts on approaches to prevent and manage delayed-onset adverse reactions following HA filler administration, including late-onset nodules, support clinicians worldwide in optimizing patient outcomes and safety.
INTRODUCTION
The number of facial esthetic injectable procedures performed worldwide in 2017 was nearly 8.6 million, representing an increase of about 50% since 2011.1,2 There is an especially high demand for injections of dermal filler products, used to smooth facial lines and replace volume lost through aging.3–6 Injections of hyaluronic acid (HA)-based fillers accounted for more than 3 million procedures internationally in 2017.2 The appeal of HA filler injections includes minimal postprocedural downtime and immediately visible results.3,4,6
The overall safety profile of HA fillers is favorable, and adverse immune reactions are rare.3,4,6 Common, minor adverse events may include localized transient reactions, such as erythema, bruising, and pain, whereas less common, severe complications may include nodules, vascular occlusion, and visual disturbances or ocular alterations.7 Reactions may range from mild to serious and occur soon after injection or with a delayed onset.8–13Delayed-onset adverse reactions, though uncommon, are being recognized more frequently as an important area of concern with HA fillers.4,13–21 These reactions include cyclic or persistent edema and erythema and late-onset inflammation and/or nodules.9 Late-onset nodules had an incidence of 1.0% per patient in a recent retrospective study19 and may arise from a number of factors, including hypersensitivity, foreign body reaction, injection placement, infection, sterile abscess, or biofilm development.6,9
Many consensus recommendations and practice guidelines have been published to advise clinicians about the diagnosis, prevention, and treatment of complications associated with HA fillers.3,6,22–26 However, few guidelines account for regional practice differences11,27 or focus exclusively on delayed-onset reactions.3,6,22–26 This article will present a global perspective on approaches to the prevention of delayed-onset adverse reactions after HA filler injections in general and the management of late-onset nodules in particular.
OBJECTIVES AND METHODOLOGY
In October 2018, the sponsor invited 22 experts, selected based on the high volume of HA filler procedures performed, extensive experience in managing complications related to HA fillers, and clinical practice locations in diverse regions of the world, to participate in the Global HA Filler Complications Management Advisory Board. The objective was to generate streamlined guidance on the diagnosis, prevention, and management of delayed-onset adverse reactions associated with HA fillers. An initial literature search was performed to explore the current body of work on delayed-onset adverse reactions, including nodules. Topics extracted from the literature search provided the foundation for survey questions developed by the sponsor. An independent agency, Metaplan (Princeton, NJ), administered the survey to the expert panel and then performed semistructured qualitative interviews of the experts in preparation for the subsequent live meeting (Fig. 1).
Fig. 1.
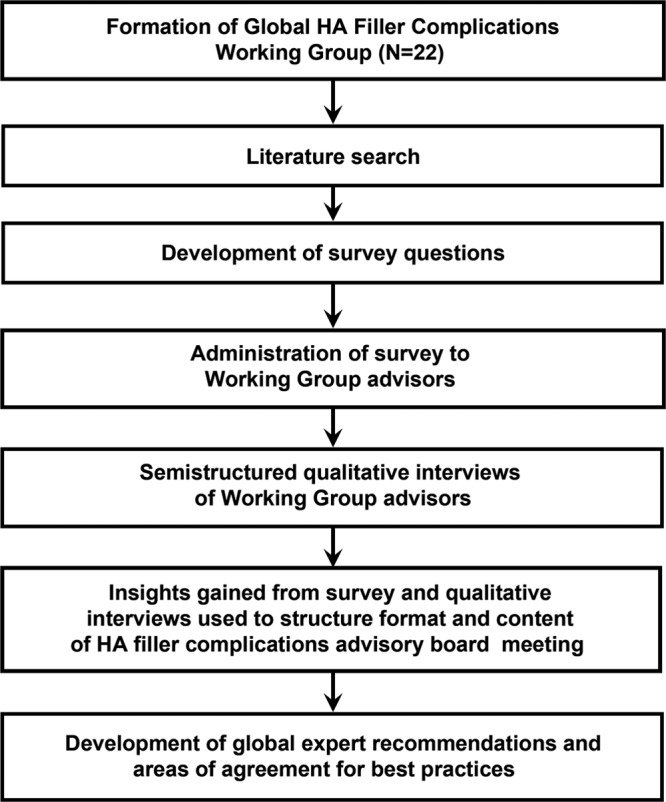
Global Hyaluronic Acid Filler Complications Working Group process.
In January 2019, the experts convened in Windsor, England, with a goal of achieving best-practice recommendations on how to prevent delayed-onset adverse reactions and manage late-onset nodules, giving consideration to regional differences. Insights from the premeeting survey and qualitative interviews guided the advisory board discussions, the goal of which was to establish global strategies and algorithms based on the clinical experience of the attendees. The expert panel proposed and evaluated the prevention and management recommendations for publication. The cause and differential diagnosis of late-onset nodules will be covered in a separate publication.
RECOMMENDATIONS AND AREAS OF AGREEMENT
Key Characteristics Associated with Delayed-onset Adverse Reactions
Clinical opinions vary on the postinjection time period that defines “delayed onset” (ie, 1–3 weeks,6 4 weeks,11,26 6 weeks,23 or >14 days after injection8). The panel agreed to categorize adverse reactions presenting 4 or more weeks after HA filler treatment as delayed onset. It was also agreed that although clinical presentations of delayed-onset adverse reactions may vary, the most common signs include swelling, induration, and nodulation. Other delayed-onset adverse reactions after HA filler administration may include erythema, discoloration, persistent intermittent-delayed edema (edema or swelling in or next to the filler site), scars, severe edema, telangiectasia, foreign body granuloma, filler migration, and neovascularization.6,11,20,27–29 Clinicians should consider that not all delayed-onset reactions need treatment to resolve, which is supported by reports in the literature.4,14,19,20
The panel agreed that pathological diagnosis is generally not required to determine treatment for delayed-onset adverse reactions, but may be helpful in formulating a customized treatment strategy. Regardless of the clinical presentation, a rational treatment protocol can be developed. The panel concluded that the time to complete resolution of similar clinical presentations is often difficult to predict and varies from weeks to months, as supported by the literature.20,29,30
Among the possible delayed-onset adverse reactions following HA filler procedures, late-onset nodules were the main focus of the panel’s review and discussion. The panel agreed that all HA fillers have the potential to cause late-onset nodules. Nodules were clinically differentiated as “inflammatory,” also referred to as “hot” (ie, red, painful, swollen), and “noninflammatory,” or “cold.” The panel noted that in the literature, inflammatory and noninflammatory nodules have been associated with different causative factors, and their prevention or management strategies can vary.3,8,22,29–31 Inflammatory nodules, which usually present initially with erythema and other signs of inflammation such as injection-site swelling, as seen in persistent intermittent-delayed edema, may result from infection (possibly biofilms) or foreign body reactions. Superficial injections, low-grade filler reactions, or improper filler placement are factors that may be associated with noninflammatory nodules.6,13 Noninflammatory nodules that result from improper filler placement are generally seen immediately after injection, whereas inflammatory nodules may emerge from days to years after injection.18
Approaches to Preventing Delayed-onset Adverse Reactions
The panel agreed on 5 areas of focus aimed at the general prevention of delayed-onset adverse reactions after HA filler administration: patient selection, anatomic location and product selection, aseptic technique, injection procedure and filler, and posttreatment care (Table 1). These areas have been widely accepted as important considerations in optimizing outcomes in facial esthetic procedures with HA fillers.8,20,26,30 Approaches to the prevention of delayed-onset adverse reactions are shown in Table 2. These included patient-related factors, preventive aseptic/clean procedures, and injection procedure and volume considerations.
Table 1.
Areas of Focus in the Prevention of Delayed-onset Adverse Reactions after Hyaluronic Acid Filler Administration
| Patient Selection | Anatomic Location and Product Selection | Aseptic Technique | Injection Procedure and Filler | Posttreatment Care |
|---|---|---|---|---|
| High importance in preventing delayed-onset adverse reactions | Risk factors for delayed-onset adverse reactions | One of the most important preventive factors | Contributes to the level of risk | Patient education can minimize the risk of delayed-onset adverse reactions |
| Utilize patient selection criteria | Select product based on anatomic location | Alcohol alone is not sufficient | Should be selected based on both product and anatomic location | Posttreatment care is critical to discuss with patients |
| Assess the presence of previous filler | Anatomic location affects the level of risk | Patients must have a clean face for the procedure | Delay the following until healed:Dental workApplication of makeup, creams, or lotions | |
| Prevent or delay injection in the following cases: | ||||
| Recent dental workAny procedure compromising the skin barrierActive facial infection or inflammationActive systemic infection or inflammationActive autoimmunedisease | Clean technique is critical; do not touch the needle or the hair of the patient | |||
Table 2.
Clean Injection Procedures and Other Recommended Approaches to the Prevention of Delayed-onset Adverse Reactions
| Factors Related to Prevention | Underlying Issues | Best Practices |
|---|---|---|
| Patient-related factors | Periprocedural factorsLocal infection | Exclude periprocedural factorsAssess in a patient consultation before the procedure |
| Systemic infection | ||
| Systemic inflammation | Create patient awareness of potential adverse reactions | |
| Dental work | Set expectations | |
| Skin barrier | Explain that reactions can be managed | |
| Topical inflammation | ||
| Immunization | Time procedure appropriately | |
| Medication | Consider any planned surgery or dental work | |
| History of reaction to previous fillers; consider a sensitivity test | Delay if needed | |
| Predisposing host factorsProcedures that have the potential to introduce bacteria | Ensure posttreatment education | |
| Minor surgical procedures | Emphasize aseptic practices and other posttreatment precautions for the patient | |
| Aseptic/clean procedures | Introduction of bacteria at the time of injection Physician or staff not following aseptic techniques Only cleaning part of the face Not using a strong-enough skin disinfectant Lack of patient education on posttreatment care Not knowing when the needle entry point is closed Touching the site after injection, introducing bacteria Resistance to change (continued makeup application, face touching, etc.) |
Clean and reclean Use “continuous prep” technique—ie, cleansing the entire face, removing makeup, and then applying antiseptic to the full face; initiating treatment in areas away from the bacteria-prone nose and mouth; cleaning and recleaning each injection site immediately before treatment; and using a disposable dressing tray Clean the whole face with CHG, an alcohol-based antiseptic solution (70%–75% alcohol), or alcohol-based CHGSpray gauze with chlorhexidine and/or isopropanol (isopropyl alcohol), or with povidone iodineHave patient clean hands during the procedure Use and change gloves Wear gloves for surgical aseptic procedures Change gloves after prepBe hypervigilant with perioral injections: change gloves if finger touched inside the mouth Ensure the right tools Use a sterile, disposable dressing tray for each treatment Use the smallest recommended needle size for the filler Educate the patientCommunicate pre- and posttreatment Dos and Don’ts, possibly using a messaging appEducate the patient about aftercare, especially the need to avoid manipulation of the treated area and to keep hands clean Explain to patients that makeup can lead to infection (recommend initially avoiding makeup or applying only new, previously unused makeup) Other Teach continuous prep technique Have extra disposable dressing trays with small quantities of sanitary supplies on hand for all procedures Do not be too harsh with prep/injection Use antiseptic cream postinjection |
| Injection procedure and volume | Needle dulls from hitting boneFanning technique may introduce more bacteria and create more trauma, leading to an inflammatory cascade The bigger the needle, the more bacteria get introducedLarger volumes and greater numbers of sites treated allow a larger area for introducing foreign bodies and/or may lead to mechanical irritation |
Inject the right productUse the appropriate product appropriately Use appropriate techniqueUse a gentle, nontraumatic techniqueEmploy a slow injection speed Inject at the right sites Limit entrance sites Do not inject using an intraoral approach Pay extra attention in mucosal areas Select injection volume wisely Exceeding 0.2 cc is not advisable Practice asepsis Treat existing internal infections appropriately and early Maintain as much asepsis as possible Do not touch the cannula to the skin Change needles frequently |
CHG, chlorhexidine gluconate.
Aseptic/clean practices should address recontamination during the injection procedure. The introduction of a foreign body (eg, filler) during a procedure can increase the opportunity for microorganisms to survive skin entry and cause infection.32 Laboratory and clinical studies confirm the risk of bacterial contamination with every needle pass through the skin; however, rapid degradation and phagocytosis may address the invading bacteria.33 The panel advocated implementing a “continuous prep” technique (Table 2). The panel recommended changing needles frequently, but also noted that regional practice differences may affect the practicality of this approach; for example, recapping needles is not permitted in hospital environments in some countries. Gauze used to clean the area of injection or applied to reduce bleeding after the injection should be dampened with antiseptic. However, avoid over soaking the gauze with the antiseptic to prevent the solution dripping on the patient’s face or splashing into the patient’s eyes. Based on panel experience, the choice of antiseptic solution [eg, chlorhexidine gluconate (CHG) and isopropanol (isopropyl alcohol)] may be geographically dependent. Further, CHG may be preferable to isopropyl alcohol because it disrupts microbial cell membranes and binds to keratin proteins in the skin, providing persistent antimicrobial activity when dry, whereas fresh swabs of alcohol must be reapplied to maintain antiseptic effect during the procedure.32,34–36 Although there are reports of splash risk and subsequent corneal and otologic toxicity with CHG during the head, neck, and ophthalmic surgeries,35,37 the panel considered the risk of ocular injury with CHG less relevant to facial filler procedures performed with a local anesthetic. Nonetheless, CHG should be applied conservatively to the face. Other acceptable antiseptics are povidone iodine, chloroxylenol, polihexanide, or hypochlorous acid. Using CHG during initial skin preparation and alcohol before each injection may also be effective.
Aseptic practices that patients also must observe were described as critical to minimizing adverse reactions. Experts agreed that patients should delay applying makeup after the procedure, but the recommended duration for this delay was based on clinical judgment. Recommendations varied between 24 hours (recognizing that may be impractical for some patients), at least 4 hours, and 5 minutes (based on evidence that blood may clot rapidly at the site of injection and form a barrier against infection).38 Use of brand-new makeup after the procedure is preferable, but asking patients to purchase makeup may be unrealistic in most cases. In some geographical areas, providing patients with unopened makeup samples may be a viable option. Patients should wash hands immediately before treatment, avoid touching the area to be treated, keep hands clean after treatment, and avoid touching or manipulating the treated area. Aggressive handling of the area may cause the filler to migrate. It is important to continuously train and educate nurses and other assisting medical staff to emphasize the advice and guidance being provided to the patient. The panel agreed that clinicians should provide patient consent forms that explain aseptic guidelines and potential adverse reactions and require the patient to review and acknowledge them.
Other preventative approaches recommended by the panel include patient-related factors such as the periprocedural presence of infection, inflammation, or predisposing hypersensitivity and external risks of bacterial infection, such as planned or recent dental or surgical procedures. As suggested by other clinicians, aseptic preventative measures may be expected to optimize the likelihood of prevention if the adverse reaction is a nodule with a bacterial etiology.15,24
The panel also addressed the injection procedure and volume of HA filler used as factors in preventing complications. Larger boluses may also cause mechanical irritation, eventually triggering an inflammatory cascade, as supported by several studies.26,31,33 The panel recommended not exceeding an injection volume of 0.2 cc per bolus for most facial areas. Some authors recommended using no more than 0.1 cc per bolus. Larger bolus volumes (0.5 cc) occasionally may be appropriate for regions of the face where product rheology and anatomy result in smooth dispersion of the product (temple) or confined projection (chin), depending on the patient’s individual needs. The panel recommended taking extra precaution when injecting the perioral areas or to inject these areas last because of the intrinsically high presence of bacteria. For the same reason, direct injection through or into oral mucosa is discouraged; contamination with local bacteria can lead to a potentially higher number of adverse reactions.18 Consensus recommendations and practice guidelines concur that a larger bolus volume of HA may increase the risk of foreign body reactions and other complications22,23,25 and that larger volumes may be a risk factor for granulomatous reactions or infectious processes.22,39 Ultimately, the panel suggested being reasonably conservative about the total volume used and the number of sites injected.
The panel noted that late-onset nodules, in particular, may have an etiology that is challenging to address or prevent. For example, a 2019 report described the emergence of the red, firm, and painful swelling at the location of HA filler injections in 14 patients, days after the onset of a flu-like illness.40 The patients had received prior HA filler injections, with the last injection occurring 2–10 months before the reaction.40 According to the report, a type IV hypersensitivity reaction initiated by T lymphocytes following the HA injection and an influenza infection may have played a role in these late-onset nodules.40 Additionally, multiple reports of late-onset nodules have demonstrated that they peak in the fall and winter months, possibly owing to the cold and flu season; in fact, cold and flu symptoms have occurred immediately before nodule onset in several documented cases.4,20 The panel agreed that evidence suggests viral or bacterial infections as an immunological trigger. A foreign body granulomatous reaction may be the cause in cases where nodules form several months to years after treatment.18 The panel cautioned, however, that a granuloma is primarily a histopathological description.
Managing Late-onset Nodules
The panel noted that not all late-onset nodules require medical treatment to resolve, a recommendation that is supported by the literature.4,14,19,20 Watchful waiting is acceptable for noninflammatory nodules. Nodule size does not determine the need for treatment; rather, the presence of factors such as inflammation, systemic signs and symptoms, coexisting infections, and functional or cosmetic impairments direct treatment. Where the treatment of late-onset nodules is required, the panel agreed on several strategies to guide their management, including the limitations of hyaluronidase; the effectiveness of oral antibiotics, intralesional antibiotics, oral steroids, intralesional steroids, and anti-inflammatory medication; and the consideration of surgical excision as a last resort (Table 3).
Table 3.
Guidance for Managing Late-onset Nodules after Hyaluronic Acid Filler Administration
| Hyaluronidase may not provide resolution as the sole treatment |
| Less likely to resolve nodule if treatment is delayed |
| Should not be the first course of action for all late-onset nodules |
| Should only be used with concomitant antibiotics in cases of suspected active infection |
| May require repeat injections for full resolution of late-onset nodules |
| H2 antagonists/antihistamines and topical steroids may improve the symptoms of late-onset nodules, but they do not effectively treat the cause |
| Oral antibiotics, intralesional antibiotics, oral steroids, and nonsteroidal anti-inflammatory drugs are effective against late-onset nodules |
| 5-FU and intralesional steroids should be used when other treatment modalities have been exhausted |
| Surgical excision should be considered only as a last resort |
| Combination therapy is the most effective way of treating late-onset nodules |
5-FU, 5-fluorouracil.
There are regional differences and personal preferences in the timing and placement of steroids in the recommended treatment plan. The panel has also had mixed results with injecting hyaluronidase into the affected area to dissolve any remaining HA, with some authors reporting the failure of hyaluronidase when not combined with other treatments. The failure of hyaluronidase may be more common for nodules that develop after injection of more cohesive and tightly crosslinked HA filler products. Degradation of HA filler using hyaluronidase is dose dependent, with 30 units of hyaluronidase per 0.1 mL of highly cohesive filler demonstrating effectiveness, in vivo.41 Clinical practice supports injecting high doses of hyaluronidase directly into the center of the nodule every 48 hours until resolution. Ultrasound, when available, may be used to detect the amount, location, and depth of injected filler at the site of the nodule and guide the delivery of hyaluronidase.42–44
A management algorithm for inflammatory and noninflammatory nodules is shown in Figure 2; it includes an example of an antibiotic treatment regimen for inflammatory nodules. A complete history and physical examination are important when obtaining a clinical profile to guide the management of delayed-onset adverse reactions. What other facial conditions are present, where, and for how long? Are there skin barrier function alterations, concurrent or systemic skin infections, or a history of systemic or local infections? Is the nodule inflammatory or noninflammatory? The panel’s algorithm (Fig. 2) recommends hyaluronidase for managing noninflammatory nodules and antibiotics for managing inflammatory nodules, and the panel agreed that the early treatment paradigm may also include steroids, hyaluronidase, and massage for inflammatory nodules. Some authors have reported success with oral prednisone once daily for 1 week or less, administered early in the treatment paradigm, with or without an antibiotic.
Fig. 2.
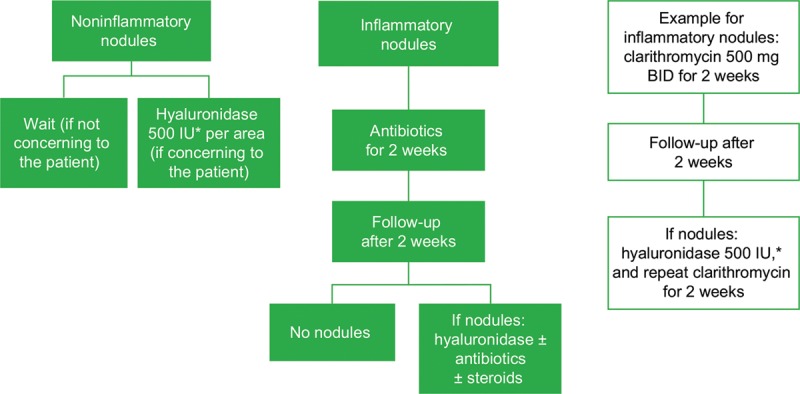
Management of late-onset nodules after hyaluronic acid filler administration. *Hyaluronidase dose may vary based on the area treated; lower doses may be required for areas such as the tear trough, whereas higher doses may be necessary for the midface. BID, twice daily.
An overview of the panel’s recommended algorithm for single-start antibiotics in the treatment of late-onset nodules, based on the assumption of bacterial causation, is shown in Table 4. The panel cautioned that fluoroquinolone antibiotics, which include ciprofloxacin and moxifloxacin, should no longer be used as first-line antibiotic therapy to address late-onset nodules because of significant side effects, which include cardiovascular toxicity; potentially fatal aortic ruptures and tears; disabling, possibly permanent tendon, muscle, joint, and central nervous system damage; seizures; altered mental status; hepatotoxicity; and dysglycemia.45–47 The panel recommended that clinicians refer to the antimicrobial guidelines in their geographical region before prescribing a specific antibiotic. The panel agreed that the response to single-antibiotic treatment may be observed within days but warned that the time course of antibiotics may be dependent on the amount of filler initially injected. A poor response to the treatments recommended in the algorithm may necessitate administration of clarithromycin or doxycycline and, if these are not effective, a second antibiotic, a steroid, or colchicine/nonsteroidal anti-inflammatory drugs. Additionally, the degree of inflammation dictates the duration of treatment needed.
Table 4.
Single-start Antibiotic Treatment Approach
| Types and rationale for single-start antibiotic |
| Bacterial spread, sinusitis: doxycycline, augment with cephalexin |
| Dental: amoxicillin and clindamycin/cephalosporins/amoxicillin and clavulanate potassium |
| UTI: cephalexin, metronidazole, amoxicillin and clavulanate potassium |
| Gastroenteritis: metronidazole, clindamycin |
| Low-grade bacteria commensal, low-grade inflammation and infection: doxycycline |
| If very fluctuant and large, do incision and drainage |
| Antibiotics should be administered concurrently with this procedure |
UTI, urinary tract infection.
Algorithms for the use of broad-spectrum antibiotics and oral steroids in the treatment of late-onset nodules are shown in Figures 3 and 4, respectively. Regional differences are important to consider, as a high incidence of infectious diseases in some countries makes the use of oral steroids less desirable; steroid options also vary between countries.
Fig. 3.
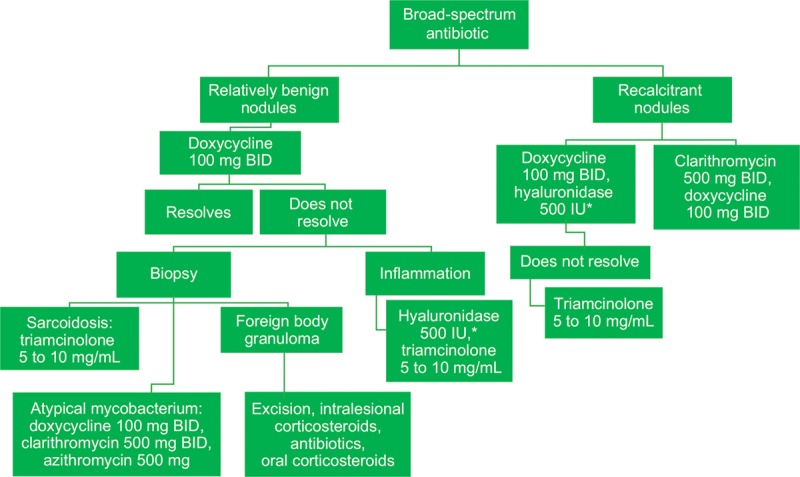
Broad-spectrum antibiotic treatment for late-onset nodules after hyaluronic acid filler administration. *Hyaluronidase dose may vary based on the area treated; lower doses may be required for areas such as the tear trough, whereas higher doses may be necessary for the midface. BID, twice daily.
Fig. 4.
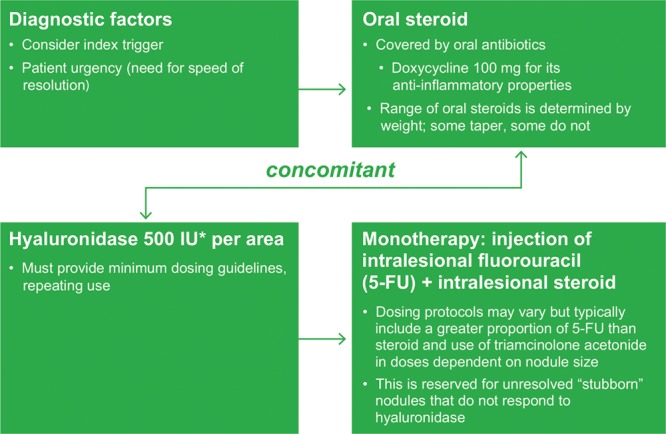
Oral steroid treatment algorithm for late-onset nodules after hyaluronic acid filler administration. *Hyaluronidase dose may vary based on the area treated; lower doses may be required for areas such as the tear trough, whereas higher doses may be necessary for the midface.
A summary of global treatment strategies for late-onset nodules as a complication of HA filler injections is presented in Figure 5. The panel advised that nonsteroidal anti-inflammatory drugs, antihistamines, or oral steroids may be used at any stage in the algorithm to reduce the signs and symptoms of inflammation, but clinicians should be aware that stopping steroid use may lead to rebound effects, and the use of steroids may have immunosuppressive effects in cases of existing bacterial infection. The panel cautioned that clinicians should rule out an abscess and examine the nodule for the presence of fluctuance. The panel also agreed that abscess management requires incision and drainage. Sterile abscesses are characterized by negative cultures and may represent a biofilm reaction. They can be difficult to treat.23 The most serious error is to diagnose an active infection as a “late inflammatory reaction,” which may lead to permanent tissue injury, because the infection may lead to permanent deformity (indentation). Proper incision and drainage, leaving a small silicone, gauze mesh, or rubber drain segment in place overnight, is critical to successful outcomes when managing an active infection; incision with aspiration using a syringe was considered less effective by the panel.
Fig. 5.
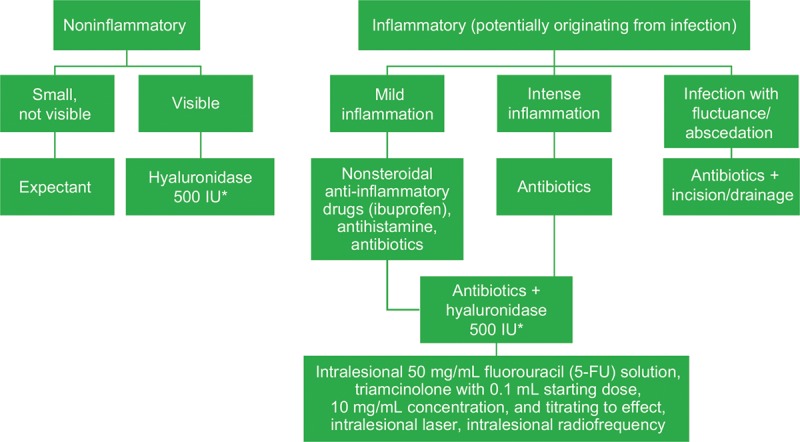
Summary of global treatment strategies for late-onset nodules after hyaluronic acid filler administration. *Hyaluronidase dose may vary based on the area treated; lower doses may be required for areas such as the tear trough, whereas higher doses may be necessary for the midface.
Inflammation may be an immune response triggered by infection, and inflammatory nodules should be treated with antibiotics; the panel suggested that it would be safe to assume that an infectious process underlies an inflammatory nodule and to rule out infection as a first step. For refractory nodules, intralesional fluorouracil (5-FU; 50 mg/mL) combined with an intralesional corticosteroid may be used when other treatment modalities (hyaluronidase, oral steroids) have been exhausted. Dosing protocols recommended for this combination included 7 to 9 parts 5-FU to 1 to 3 parts triamcinolone acetonide or an injectable betamethasone dipropionate solution (not available in all regions), in aliquots of 0.1 mL to 0.15 mL per injection; an 80:20 ratio of 5-FU and triamcinolone acetonide (10 or 40 mg depending on nodule size and duration); and small aliquots of a mixture of 1 cc 5-FU and 0.1 cc triamcinolone acetonide 40 mg injected at 1-month intervals until resolution, with volume dependent on nodule size. The latter strategy was recommended not only for treatment-resistant nodules but also for nodules from permanent fillers. The panel stressed injecting 5-FU/steroid combinations intralesionally, instead of intradermally, to avoid tissue atrophy. Failure to improve after corticosteroid injections should prompt the clinician to consider the possibility of an infectious etiology.8 It must be emphasized that proper diagnosis leads to proper treatment.
CONCLUSIONS
This is the first expert guidance that focuses solely on approaches in the prevention and management of delayed-onset adverse reactions, including late-onset nodules, after administration of HA fillers. Perspectives related to geographical differences in medical management strategies have been provided, which may impact the ability to reach full agreement on the management of late-onset nodules from a global perspective. Further investigations focusing on differences such as variations in aseptic protocols, choice of antibiotic therapies, and variations in steroid formulations will further help with regional management of late-onset nodules with HA fillers. More evidence-based data on the prevention and treatment of late-onset nodules may still be necessary; however, the recommendations provided here can raise awareness of this HA-associated complication in clinical practice.
ACKNOWLEDGMENTS
The authors would like to acknowledge the contributions of the other participants in the Global Hyaluronic Acid Filler Complications Management Advisory Board: Seyfi Akbay, MD; Sue Ellen Cox, MD; David Eccleston, MB, ChB, BCAM; Steve Fagien, MD; Becky Fitzgerald, MD; David Funt, MD; Michael Kane, MD; Yi-Hua Liao, MD, PhD; Isabelle Rousseaux, MD; Gloria Trocchi, MD; Mohammed Al Turkmani, MD; Fernando Urdiales, MD; and Wu Yan, MD, PhD.
Footnotes
Published online 29 April 2020.
Disclosure: Writing and editorial assistance with manuscript preparation was provided to the authors by Regina Kelly, MA, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, N.J., and was sponsored by Allergan plc, Dublin, Ireland. No honoraria or other forms of payment were made for authorship. Dr Batniji is a consultant, speaker, and advisory board member for Allergan plc. Dr Goodman is an advisory board member, clinical investigator, and consultant for Allergan plc; and is a speaker, advisory board member, and consultant for Galderma. Dr Jones is an investigator, consultant, and advisory board member for Allergan plc; and an investigator for Galderma. Dr Philipp-Dormston is an investigator, consultant, speaker, and advisory board member for Allergan plc and Galderma. Dr Heydenrych is an advisory board member and consultant for Allergan plc. Dr Delorenzi is an advisory board member and consultant for Allergan plc; and is a medical director for Allergan Canada and Merz Canada (complications management). Dr Trindade De Almeida is a speaker, advisory board member, and investigator for Allergan plc and Merz. Dr De Boulle is an advisory board member and consultant for Allergan plc and Laboratoires Genevrier. A. Swift is an advisory board member, speaker, and investigator for Allergan plc, Merz, and Galderma.
REFERENCES
- 1.International Society of Aesthetic Plastic Surgery. ISAPS international survey on aesthetic/cosmetic procedures performed in 2011. Available at: http://www.isaps.org/Media/Default/global-statistics/ISAPS-Results-Procedures-2011.pdf. Accessed January 9, 2020
- 2.International Society of Aesthetic Plastic Surgery. ISAPS international survey on aesthetic/cosmetic procedures performed in 2017. Available at: https://www.isaps.org/wp-content/uploads/2018/10/ISAPS_2017_International_Study_Cosmetic_Procedures.pdf. Accessed January 9, 2020
- 3.Snozzi P, van Loghem JAJ. Complication management following rejuvenation procedures with hyaluronic acid fillers-an algorithm-based approach. Plast Reconstr Surg Glob Open. 2018;6:e2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humphrey S, Carruthers J, Carruthers A. Clinical experience with 11,460 ml of a 20-mg/ml, smooth, highly cohesive, viscous hyaluronic acid filler. Dermatol Surg. 2015;41:1060–1067. [DOI] [PubMed] [Google Scholar]
- 5.Jones D, Flynn T. Jones D. Hyaluronic acids: clinical applications. In: Injectable Fillers: Principles and Practice. 2010:Oxford, United Kingdom: Wiley-Blackwell; 158–174. [Google Scholar]
- 6.Philipp-Dormston WG, Bergfeld D, Sommer BM, et al. Consensus statement on prevention and management of adverse effects following rejuvenation procedures with hyaluronic acid-based fillers. J Eur Acad Dermatol Venereol. 2017;31:1088–1095. [DOI] [PubMed] [Google Scholar]
- 7.Haneke E. Managing complications of fillers: rare and not-so-rare. J Cutan Aesthet Surg. 2015;8:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg. 2009;35(suppl 2):1672–1680. [DOI] [PubMed] [Google Scholar]
- 9.Funt D, Pavicic T. Dermal fillers in aesthetics: an overview of adverse events and treatment approaches. Clin Cosmet Investig Dermatol. 2013;6:295–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alijotas-Reig J, Fernández-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45:97–108. [DOI] [PubMed] [Google Scholar]
- 11.Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42:498–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artzi O, Loizides C, Verner I, et al. Resistant and recurrent late reaction to hyaluronic acid-based gel. Dermatol Surg. 2016;42:31–37. [DOI] [PubMed] [Google Scholar]
- 13.Correa-Selm LM, Rogachefsky A, Lee K. Mangement of nodules post-filler injection. Available at: https://www.the-dermatologist.com/content/management-nodules-post-filler-injection. Accessed January 9, 2020.
- 14.Goodman GJ. An interesting reaction to a high- and low-molecular weight combination hyaluronic acid. Dermatol Surg. 2015;41(suppl 1):S164–S166. [DOI] [PubMed] [Google Scholar]
- 15.DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561–575. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Aceñero MJ, Zamora E, Borbujo J. Granulomatous foreign body reaction against hyaluronic acid: report of a case after lip augmentation. Dermatol Surg. 2003;29:1225–1226. [DOI] [PubMed] [Google Scholar]
- 17.Park TH, Seo SW, Kim JK, et al. Clinical experience with hyaluronic acid-filler complications. J Plast Reconstr Aesthet Surg. 2011;64:892–896. [DOI] [PubMed] [Google Scholar]
- 18.Ledon JA, Savas JA, Yang S, et al. Inflammatory nodules following soft tissue filler use: a review of causative agents, pathology and treatment options. Am J Clin Dermatol. 2013;14:401–411. [DOI] [PubMed] [Google Scholar]
- 19.Sadeghpour M, Quatrano NA, Bonati LM, et al. Delayed-onset nodules to differentially crosslinked hyaluronic acids: comparative incidence and risk assessment. Dermatol Surg. 2019;45:1085–1094. [DOI] [PubMed] [Google Scholar]
- 20.Beleznay K, Carruthers JD, Carruthers A, et al. Delayed-onset nodules secondary to a smooth cohesive 20 mg/ml hyaluronic acid filler: cause and management. Dermatol Surg. 2015;41:929–939. [DOI] [PubMed] [Google Scholar]
- 21.Shahrabi Farahani S, Sexton J, Stone JD, et al. Lip nodules caused by hyaluronic acid filler injection: report of three cases. Head Neck Pathol. 2012;6:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Signorini M, Liew S, Sundaram H, et al. ; Global Aesthetics Consensus Group. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers-evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137:961e–971e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31:110–121. [DOI] [PubMed] [Google Scholar]
- 24.Cassuto D, Sundaram H. A problem-oriented approach to nodular complications from hyaluronic acid and calcium hydroxylapatite fillers: classification and recommendations for treatment. Plast Reconstr Surg. 2013;132(4 suppl 2):48S–58S. [DOI] [PubMed] [Google Scholar]
- 25.De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heydenrych I, Kapoor KM, De Boulle K, et al. A 10-point plan for avoiding hyaluronic acid dermal filler-related complications during facial aesthetic procedures and algorithms for management. Clin Cosmet Investig Dermatol. 2018;11:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trindade de Almeida A, Banegas R, Boggio R, et al. Diagnosis and treatment of hyaluronic acid adverse events: Latin American expert panel consensus recommendations. Surg Cosmet Dermatol. 2017;9:204–213. [Google Scholar]
- 28.Urdiales-Gálvez F, Delgado NE, Figueiredo V, et al. Preventing the complications associated with the use of dermal fillers in facial aesthetic procedures: an expert group consensus report. Aesthetic Plast Surg. 2017;41:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narins RS, Coleman WP, III, Glogau RG. Recommendations and treatment options for nodules and other filler complications. Dermatol Surg. 2009;35(suppl 2):1667–1671. [DOI] [PubMed] [Google Scholar]
- 30.King M, Bassett S, Davies E, et al. Management of delayed onset nodules. J Clin Aesthet Dermatol. 2016;9:E1–E5. [PMC free article] [PubMed] [Google Scholar]
- 31.Winslow CP. The management of dermal filler complications. Facial Plast Surg. 2009;25:124–128. [DOI] [PubMed] [Google Scholar]
- 32.National Collaborating Centre for Women’s and Children’s Health (UK). Intraoperative phase. Surgical Site Infection: Prevention and Treatment of Surgical Site Infection. 2008:London, United Kingdom: RCOG Press; 50–90. [PubMed] [Google Scholar]
- 33.Saththianathan M, Johani K, Taylor A, et al. The role of bacterial biofilm in adverse soft-tissue filler reactions: a combined laboratory and clinical study. Plast Reconstr Surg. 2017;139:613–621. [DOI] [PubMed] [Google Scholar]
- 34.Kyllo RL, Alam M. Risk, prevention, diagnosis, and management of post-operative cutaneous infection. Curr Dermatol Rep. 2019;8:80–84. [Google Scholar]
- 35.Alam M, Cohen JL, Petersen B, et al. Association of different surgical sterile prep solutions with infection risk after cutaneous surgery of the head and neck. JAMA Dermatol. 2017;153:830–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dumville JC, McFarlane E, Edwards P, et al. Preoperative skin antiseptics for preventing surgical wound infections after clean surgery. Cochrane Database Syst Rev. 2015:CD003949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinsapir KD, Woodward JA. Chlorhexidine keratitis: safety of chlorhexidine as a facial antiseptic. Dermatol Surg. 2017;43:1–6. [DOI] [PubMed] [Google Scholar]
- 38.Macrae FL, Duval C, Papareddy P, et al. A fibrin biofilm covers blood clots and protects from microbial invasion. J Clin Invest. 2018;128:3356–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemperle G, Gauthier-Hazan N, Wolters M, et al. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009;123:1842–1863. [DOI] [PubMed] [Google Scholar]
- 40.Turkmani MG, De Boulle K, Philipp-Dormston WG. Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin Cosmet Investig Dermatol. 2019;12:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shumate GT, Chopra R, Jones D, et al. In vivo degradation of crosslinked hyaluronic acid fillers by exogenous hyaluronidases. Dermatol Surg. 2018;44:1075–1083. [DOI] [PubMed] [Google Scholar]
- 42.Schelke LW, Decates TS, Velthuis PJ. Ultrasound to improve the safety of hyaluronic acid filler treatments. J Cosmet Dermatol. 2018;17:1019–1024. [DOI] [PubMed] [Google Scholar]
- 43.Aquino Cavallieri F, de Almeida Balassiano LK, de Bastos JT, et al. Persistent, intermitent delayed swelling PIDS: late adverse reaction to hyaluronic acid fillers. Surg Cosmet Dermatol. 2017;9:218–222. [Google Scholar]
- 44.Skrzypek E, Gornicka B, Skrzypek DM, et al. Granuloma as a complication of polycaprolactone-based dermal filler injection: ultrasound and histopathology studies. J Cosmet Laser Ther. 2019;21:65–68. [DOI] [PubMed] [Google Scholar]
- 45.Kuula LSM, Viljemaa KM, Backman JT, et al. Fluoroquinolone-related adverse events resulting in health service use and costs: a systematic review. PLoS One. 2019;14:e0216029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. Food and Drug Administration. FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. Available at: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse. Accessed January 9, 2020
- 47.U.S. Food and Drug Administration. FDA warns about increased risk of ruptures or tears in the aorta blood vessel with fluoroquinolone antibiotics in certain patients. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-increased-risk-ruptures-or-tears-aorta-blood-vessel-fluoroquinolone-antibiotics. Accessed January 9, 2020.


