Summary:
The reversed glove sleeve technique is a simple, available, reproducible, and cost-effective method of achieving “no touch” breast implant insertion. It allows a new glove to be used for each side, thus reducing the risk of contamination by reusing a sleeve/funnel for the subsequent implant insertion. The link between bacterial contamination of breast implants and capsular contracture is established. Further prospective evaluation of this technique is underway to show if there is benefit in reducing the risk of capsular contracture.
INTRODUCTION
Bacterial contamination of breast implants by biofilm has been shown to significantly potentiate capsular contracture.1 Surgical strategies to minimize handling of prostheses and reduce potential bacterial contamination have been shown to reduce the incidence of capsular contracture.2,3 Minimizing implant contact through “no touch” is one of the strategies that has been investigated.4 First described in both orthopedic and urological literature, the “no touch” technique was adapted for breast augmentation in 1993 by Mladick using a submuscular technique with a saline prosthesis implant.5 The rationale behind this was to reduce bacterial contamination of the implant as it is passed through the skin and subcutaneous tissues. Both skin and breast tissues have been shown to harbor significant numbers of pathogenic bacteria, especially coagulase-negative Gram-positive cocci, which have been shown to be contributory to progressive capsular contracture in both in vivo and in vitro studies.6–8
The concept of the sleeve was first described by Dolsky9 due to the difficulty introducing polyurethane implants. The sleeve technique for implantation of polyurethane prostheses was highly effective; therefore, the distributed polyurethane prosthesis included a sleeve for implantation.10 Commercially available products such as the “Keller Funnel” provide a sleeve for introduction of prostheses to minimize skin contact. The product is most suited for introduction of smooth-textured, round prostheses. The effectiveness of the product is shown by Moyer et al11 in a cadaveric model which showed significant reduction in skin contact using the funnel. Clinical studies have also supported its utility in potentially reducing the risk of capsular contracture.12,13
The reversed glove sleeve presented in this article creates a “no touch” funnel with the ability to use the technique at any time on any prosthesis if the commercially available product is not available. Other simple readily available techniques are described for saline prostheses,14 but to our knowledge this is the first description for a silicone textured and shaped breast prosthesis.
SURGICAL TECHNIQUE
The reversed glove sleeve technique requires the use of a single large sterile latex-free glove. We trialed varying types/brands of surgical gloves and found that the latex-free glove (size 8 or 8.5) was best at minimizing friction and allowing the implant to slide. The IMF incision required to accommodate the sleeve and prosthesis averaged 6cm. After preparation of the product and appropriate lavage of the pocket/implant with betadine and/or antibiotic solution (that also serves as a lubricant between prosthesis and glove), the technique is performed as demonstrated in video (see Video [online], which demonstrates the author’s technique) and in Figs. 1–4 as follows:
Fig. 1.
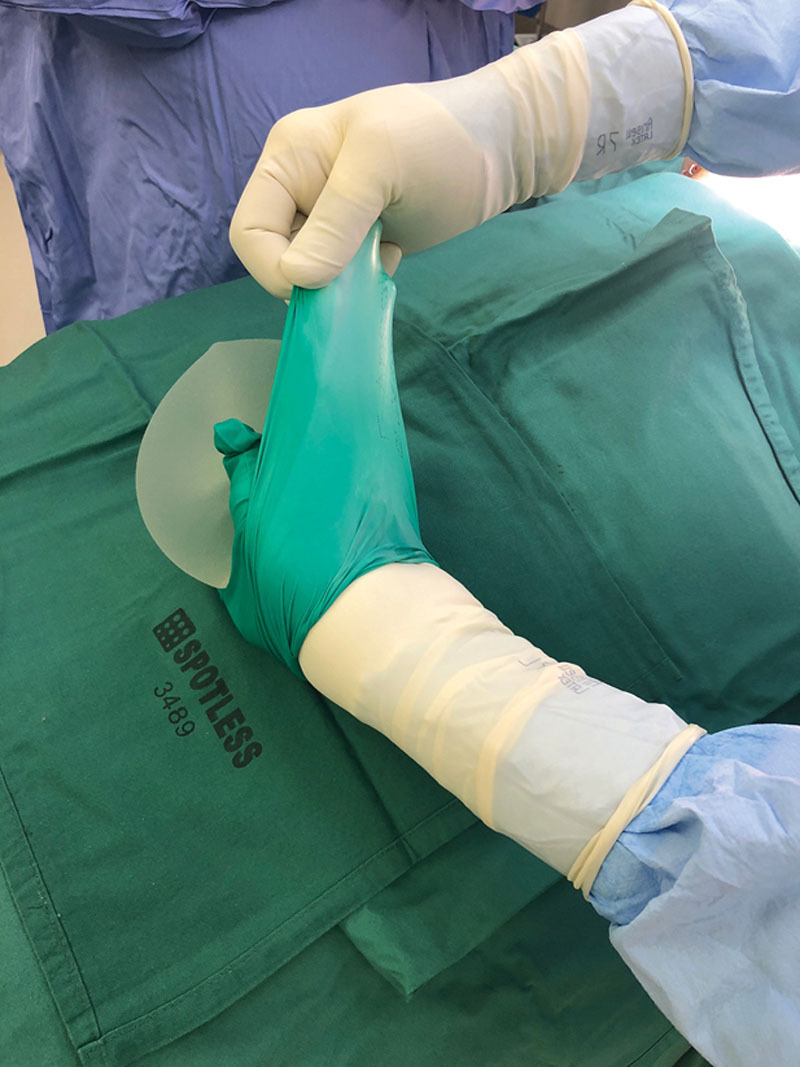
Placement of prosthesis (textured in this depiction) into sterile glove.
Fig. 4.

Successful implantation with minimal skin and parenchymal contact.
Video 1. Reversed Glove Sleeve. Video 1 from “The Reversed Glove Sleeve: A readily available and cost-effective way to achieve “no touch” breast implant insertion”.
Fig. 2.
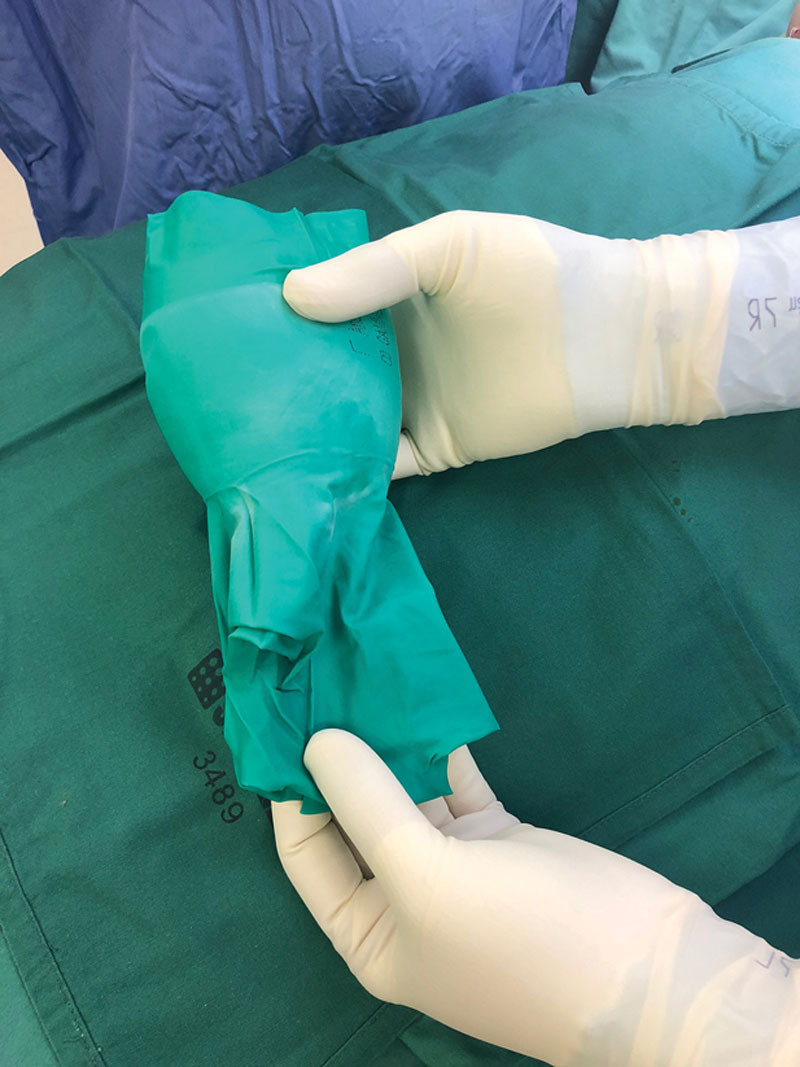
Adjustment of glove so that it overhangs the prosthesis, creating a funnel.
Fig. 3.
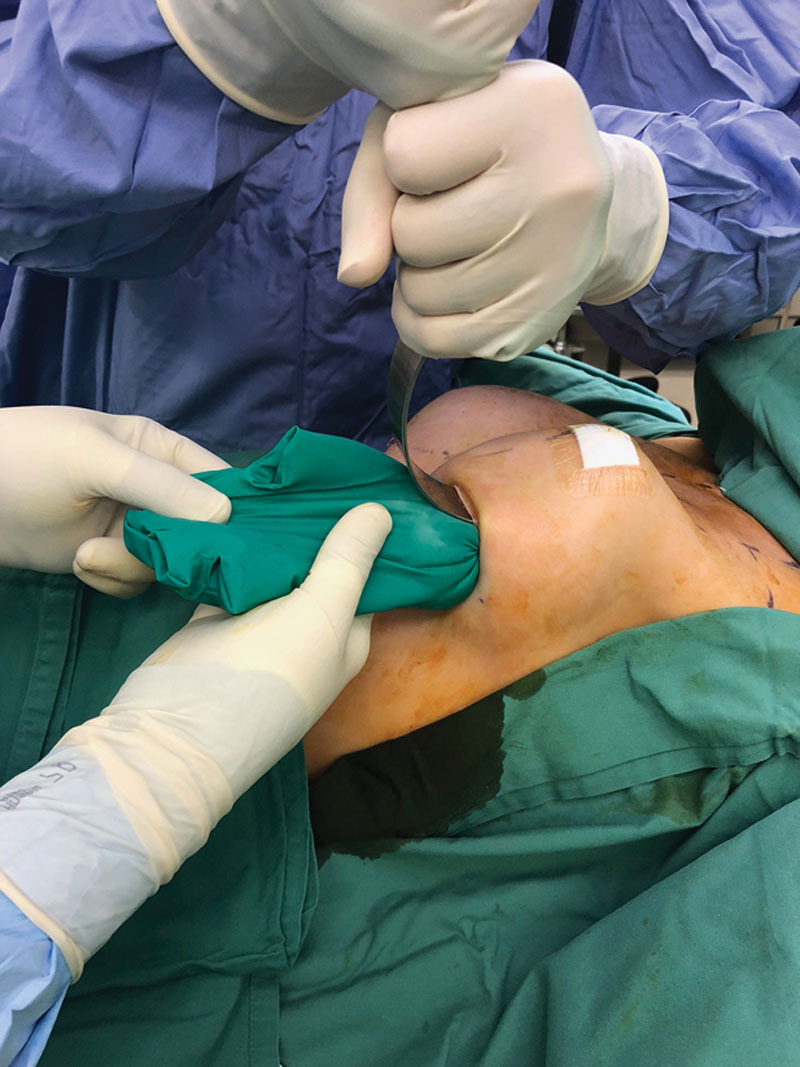
Implantation using the funnel and the proximal tail of the glove to squeeze the prothesis out.
The surgeon puts on an extra sterile glove (which is to act as the sleeve) and picks up the implant with this additionally gloved hand.
While the surgeon holds firmly onto the implant, the surgeon uses his/her other hand to roll the glove inside-out and over the top of the implant so that the implant is completely enveloped by the glove. The glove cuff should overhang the end of the implant to ensure that it does not come into contact with skin or breast parenchyma until it is fully inserted.
Meanwhile, the assistant retracts the skin to open the breast pocket to accommodate the cuff of the glove and then the prosthesis.
The overhang of the glove acts as the funnel and it is placed into the incision made at the inframammary fold.
The surgeon then squeezes the implant into the breast pocket while avoiding unnecessary contact with skin or parenchyma.
The glove is inspected by the surgeon and theater team to ensure it is intact.
UTILIZATION
To date, this technique has been used by the senior author in 83 cases without failure. Breakdown of cases is 73 anatomic textured (size ranges 225–445 cm3) and 10 smooth (size range 225–300 cm3). We used a 8.0 glove in about 50% and a 8.5 glove in 50% with a recent preference to using the large glove size. The largest inserted prosthesis is 445 cc. There have been no reported incidences of postoperative breast infection. The long-term benefits of the technique with respect to capsular contracture can not be evaluated by this technique alone and further prospective study is required.
CONCLUSIONS
The reversed glove sleeve technique is a simple, available, reproducible, and cost-effective method of achieving “no touch” breast implant insertion. It allows a new glove to be used for each side, thus reducing the risk of contamination by reusing a sleeve/funnel for the subsequent implant insertion. Further prospective evaluation of this technique is underway to show if there is benefit in reducing the risk of capsular contracture.
Footnotes
Published online 27 April 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. This study was supported by departmental resources.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Chong SJ, Deva AK. Understanding the etiology and prevention of capsular contracture: translating science into practice. Clin Plast Surg. 2015;42:427–436. [DOI] [PubMed] [Google Scholar]
- 2.Giordano S, Peltoniemi H, Lilius P, et al. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative study. Aesthet Surg J. 2013;33:675–680. [DOI] [PubMed] [Google Scholar]
- 3.Adams WP, Jr, Conner WC, Barton FE, Jr, et al. Optimizing breast-pocket irrigation: the post-betadine era. Plast Reconstr Surg. 2001;107:1596–1601. [DOI] [PubMed] [Google Scholar]
- 4.Deva AK, Adams WP, Jr, Vickery K. The role of bacterial biofilms in device-associated infection. Plast Reconstr Surg. 2013;132:1319–1328. [DOI] [PubMed] [Google Scholar]
- 5.Mladick RA. “No-touch” submuscular saline breast augmentation technique. Aesthetic Plast Surg. 1993;17:183–192. [DOI] [PubMed] [Google Scholar]
- 6.Pajkos A, Deva AK, Vickery K, et al. Detection of subclinical infection in significant breast implant capsules. Plast Reconstr Surg. 2003;111:1605–1611. [DOI] [PubMed] [Google Scholar]
- 7.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plast Reconstr Surg. 2010;126:835–842. [DOI] [PubMed] [Google Scholar]
- 8.McGuire P, Reisman NR, Murphy DK. Risk factor analysis for capsular contracture, malposition, and late seroma in subjects receiving natrelle 410 form-stable silicone breast implants. Plast Reconstr Surg. 2017;139:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolsky RL. Inserting the Meme prosthesis. Plast Reconstr Surg. 1984;73:466–468. [DOI] [PubMed] [Google Scholar]
- 10.Castello MF, Han S, Silvestri A, et al. A simple method to inset and position polyurethane-covered breast implants. Aesthetic Plast Surg. 2014;38:365–368. [DOI] [PubMed] [Google Scholar]
- 11.Moyer HR, Ghazi B, Saunders N, et al. Contamination in smooth gel breast implant placement: testing a funnel versus digital insertion technique in a cadaver model. Aesthet Surg J. 2012;32:194–199. [DOI] [PubMed] [Google Scholar]
- 12.Newman AN, Davison SP. Effect of keller funnel on the rate of capsular contracture in periareolar breast augmentation. Plast Reconstr Surg Glob Open. 2018;6:e1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flugstad NA, Pozner JN, Baxter RA, et al. Does implant insertion with a funnel decrease capsular contracture? A preliminary report. Aesthet Surg J. 2016;36:550–556. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Blanchet NP. An easy and cost-effective method to perform the “No-Touch” technique in saline breast augmentation. Aesthet Surg J. 2015;35:NP176–NP178. [DOI] [PubMed] [Google Scholar]


