Abstract
Background:
The World Health Organization ranked migraine as the 19th worldwide disease causing disability. Recent insights into the pathogenesis of migraine headache substantiate a neuronal hyperexcitability and inflammation involving compressed peripheral craniofacial nerves, and these trigger points can be eliminated by surgery. In this study, we report our experience with minimally invasive surgical procedures for frontal migraine headache treatment.
Methods:
From June 2011 to May 2019, we performed 70 frontal migraine decompression surgeries of both supratrochlear and supraorbital nerves (65 bilateral and 5 unilateral) by an endoscopic or transpalpebral approach. In 24 patients (34.2%), frontal migraine emerges as a secondary trigger point following primary occipital and/or temporal migraine surgery.
Results:
After a mean follow-up of 24 months (range, 3–97 months), patients with frontal trigger site migraine reported a 94% positive response to surgery (32% complete relief and 62% significant improvement); 6% had no change in their symptoms.
Conclusions:
Based on our experience, the operation has not caused any serious complication or side effects, and surgical decompression of supraorbital and supratrochlear nerves might be recommended to patients who suffer from a moderate to severe chronic frontal migraine not responding to conventional therapy.
INTRODUCTION
Migraine headache (MH) is a common disabling disorder with high prevalence (it affects 1.7%–4% of the world’s adult population) and high socioeconomic and personal impacts.1 Indeed, it is ranked as the third most prevalent disorder and the seventh-highest specific cause of disability worldwide.2 The first approach is usually a combination of pharmacologic treatments (both abortive and preventive drugs) and nonpharmacologic interventions like behavioral and lifestyle changes. Despite all the available conservative options, a quite relevant group of MH patients remains refractory and does not achieve a satisfactory relief. In 1999, Guyuron et al3 first described elimination or improvement of MH in a group of cosmetic patients who underwent corrugator supercilii muscle resection for forehead rejuvenation surgery. In more recent years, further evidences and anatomic studies were determinant to validate the surgical approach. Although the pathophysiology of MHs remains a matter of debate, it is a common belief that chronic compression to the terminal branches of trigeminal nerve caused by surrounding structures (eg, muscles, vessels, or fascial bands) is responsible for its origin.4 Four main migraine trigger zones have been described to be addressed by surgical procedures: frontal (site I: supraorbital and supratrochlear nerves), temporal (site II: zygomatic–temporal branch of the trigeminal nerve and/or auriculotemporal nerve), endonasal (site III: trigeminal end branches), and occipital (site IV: great occipital nerve).4 The aim of this article was to describe the currently available surgical options to treat frontal migraine (site I) with specific regard for the author’s technique.
MATERIALS AND METHODS
Surgical Treatment
The supraorbital nerve is a sensory nerve originating from the frontal branch of the ophthalmic division of the trigeminal nerve. In the majority of the cases, it passes through a supraorbital notch, which can be occasionally completed by a fibrous band. It can also exit through a foramen situated 1.5 mm above the supraorbital rim.5 At this point, the nerve divides into a superficial and a deep branch, although it can split before exiting the supraorbital rim in a minority of the cases.5 Here the nerve displays an intimate relationship with the corrugator supercilii muscle. The reason why some patients do not respond to the surgical decompression of the only supraorbital nerve and need a more medial muscular resection is that the supratrochlear nerve may be involved.5 The supratrochlear nerve is the smallest terminal branch of the frontal nerve, which itself originates from the ophthalmic division of the trigeminal nerve. It emerges between the trochlea and the supraorbital foramen. The exit point of the supraorbital nerve can be either a foramen or a notch, with findings of a notch present on both sides being more frequent. The floor of the notch is in fact a fibrous band which surrounds the nerve. The nerve then ascends through the forehead and passes through the fat pad behind the orbicularis oculi, and it pierces the corrugator muscle. The point where it enters the muscles has a mean distance of 16.4 mm from the midsagittal line and of 2.3 mm from the supraorbital rim.5 Another source of compression can be the interaction of nerves with the vascular structures. The main vessels that may be involved are the supratrochlear and the supraorbital arteries. The supratrochlear artery passes through the frontal notch and runs medial to the nerve and can be found around the medial canthal vertical line. The artery often crosses underneath the nerve deep to the corrugator from medial to lateral. It then pierces the corrugator supercilii and reaches the subcutaneous layer from 15 to 25 mm above the supraorbital rim.5 The supraorbital artery can be found in a vertical line corresponding to the medial limbus of the cornea, sharing its course with the supraorbital nerve.
Patients who suffered from frontal MH can be treated with an endoscopic approach or a transpalpebral approach. In our experience, we performed both procedures to decompress supraorbital and supratrochlear nerves,4–13 although 97% (68) of the patients underwent an endoscopic approach. Endoscopic nerve decompression, however, was not performed in patients with long foreheads (8 cm measured from the anterior hairline to the supraorbital ridge) or in patients with significant curvature to the forehead because endoscopic access would have been difficult or impossible. Transpalpebral approach for frontal trigger site deactivation was performed by means of a supratarsal crease incision involving up to two-thirds of the medial limit of the caudal portion of the conventional upper blepharoplasty incision. The upper eyelid, glabellar area, and the lower forehead were infiltrated with local anesthesia composed by 40-ml carbocaine 1% + 40-cc sodium chloride 0.9%, and 20-ml sodium bicarbonate 8.4%. After raising a skin–orbicularis oculi muscle flap above the level of the septum, the orbicularis muscle was dissected in a cephalic direction. The dissection was continued to the supraorbital rim. The corrugator supercilii muscle protecting the supraorbital and supratrochlear nerves was elevated, and by dissection, the exposure of depressor supercilii muscle was performed. After selective myotomy of depressor and corrugator supercilii muscles, the lateral fibers of the procerus muscle encasing the supratrochlear nerve were dissected. Once the supraorbital (Fig. 1) and supratrochlear nerves were isolated, they were decompressed by the cauterization of the concomitant (usually ectatic) arteries. The cutaneous access was closed with absorbable sutures, and strips were positioned at the level of superior eyelids bilaterally. The endoscopic selective myotomies technique was performed with a single access by means of a specifically modified (Fig. 2) endoscope (Karl Storz, Tuttlingen, Germany). With the patient supine and the head in a neutral position, frontal trigger nerves were located. Skin markings were drawn above the eyebrow bilaterally, at the mid-pupillary line (supraorbital nerve) and about 1.5 cm medially (supratrochlear nerve) (Fig. 3). Local anesthesia with diluted 40-ml carbocaine 1% + 40-ml sodium chloride 0.9% and 20-ml sodium bicarbonate 8.4% was injected in the forehead, between the glabellar region and about 2 cm behind the anterior hairline. A single, 1.5-cm incision was then performed on the midline, 1 cm behind the frontal hairline. The surrounding frontal tissues were dissected above the periosteum layer. The lateral anatomic limit of the undermined area was the temporal region, bilaterally. To lift the frontal skin during the endoscopic procedure (and better visualize the anatomic structures), nylon 1-0 sutures were placed in the superciliary region at each side of both supratrochlear and supraorbital nerves bilaterally (Fig. 4). Our modified endoscope consists of a 9-mm trocar with an air/insufflator/suction triple valve, a straight Hopkins telescope with fiber-light transmission, a Wittmöser operating sheath with a connection for high-frequency diathermy, and a specifically designed elliptical-tipped wire loop electrode for electrocautery. The modified endoscope was inserted through the incision in the subgaleal/subfrontal plane and was used to perform endoscopically assisted section of the corrugator supercilii, depressor supercilii, and procerus muscles bilaterally, with the purpose of decompressing the supraorbital and supratrochlear nerves bilaterally (Fig. 5). At the end of the procedure, after an accurate hemostasis, the cutaneous access was closed with absorbable suture, without any drainage; a compressive bandage was positioned all around the undermined region.
Fig. 1.
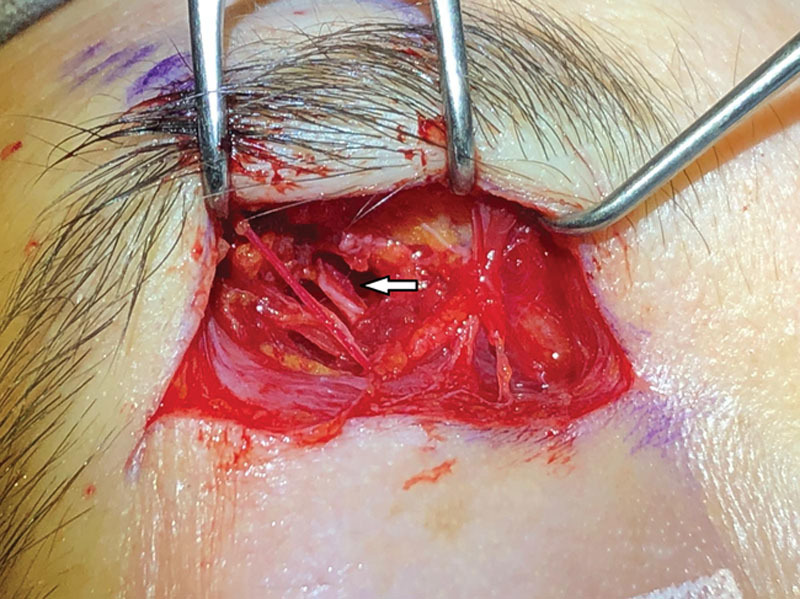
Supratarsal crease approach. White arrow: left supraorbital nerve.
Fig. 2.
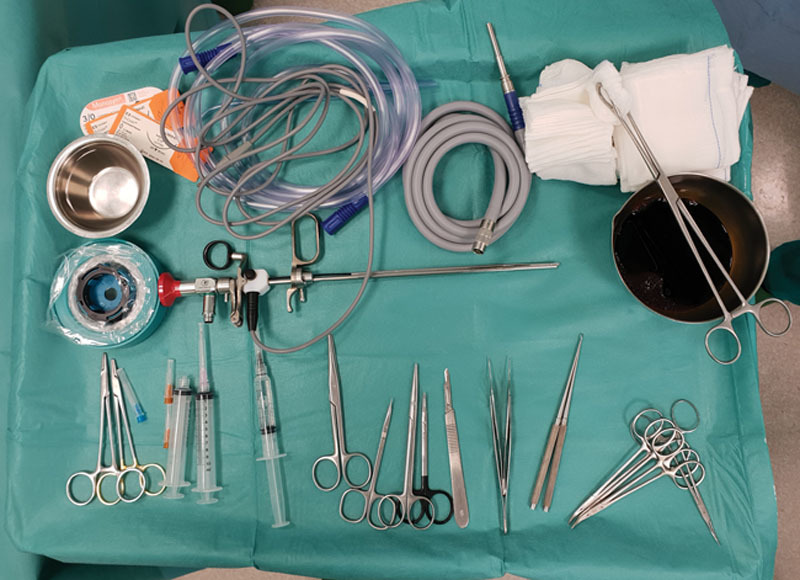
Modified endoscope with 9-mm trocar with an air/insufflator/suction triple valve, a straight Hopkins telescope with fiber-light transmission, a Wittmöser operating sheath with a connection for high-frequency diathermy, and a specifically designed elliptical-tipped wire loop electrode for electrocautery.
Fig. 3.

Cutaneous markings of the right supratrochlear (blue) and supraorbital (red) nerves.
Fig. 4.
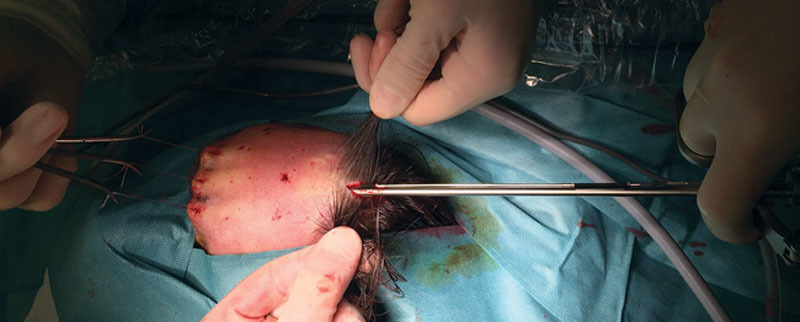
To lift the frontal skin during the endoscopic procedure (and better visualize the anatomic structures), nylon 1-0 sutures were placed in the superciliary region at each side of both supratrochlear and supraorbital nerves bilaterally.
Fig. 5.
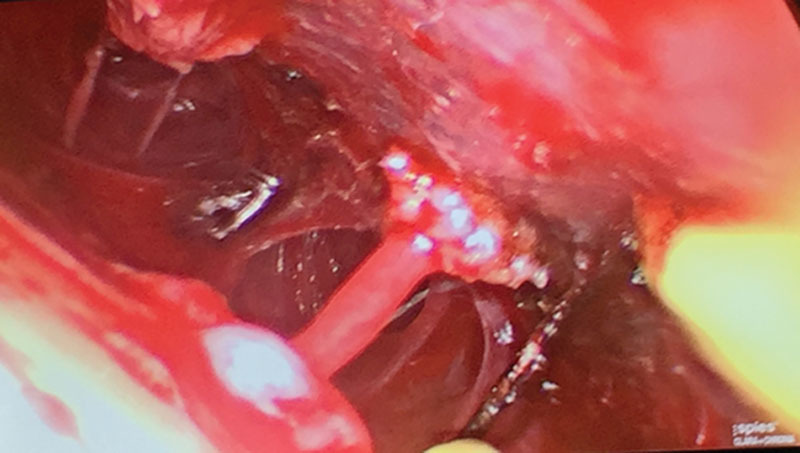
Endoscopically assisted section of the corrugator supercilii and depressor supercilii muscles lateral to the left supraorbital nerve.
Complications
Migraine surgery is regarded as a minimally invasive procedure, but all patients undergoing frontal decompression surgery experience frontal and/or upper eyelid edema of various degrees. Usually, the edema resolves by the fifth postoperative day. Ecchymosis of both upper and lower eyelids follows surgery. No treatment needs to be given because these collateral events resolve by themselves; boric water applications 3 times a day may help the process of reabsorption of the edema. As previously stated, the only hypothetical serious complication that may occur within the 12 hours following the surgery is the compression of the optical nerve due to the drop of the edema into the posterior orbicular space. In these cases, prompt recognition of patient’s sight modification is mandatory to urgently decompress the optic nerve. Patients with particularly thin skin of the frontal region may develop postoperative burn-like scar because of the endoscopic electrocautery. Temporarily, anesthesia occurs in almost all patients, which lasts 163 days on average.14–21 Other minor and transient complications reported are lasting intense itching after surgery (5.7%); hypertrophic scar (2.7%); incisional cellulitis (1%), that resolve with oral antibiotics; and transient mild incisional alopecia or hair thinning after endoscopic approach (5%).14–21
RESULTS
From June 2011 to May 2019, we have performed 259 MH decompression surgeries to treat patients with frontal, occipital, or temporal migraine trigger sites. Among them, 70 frontal migraine decompression surgeries were performed (65 bilateral and 5 unilateral; 56 women and 14 men; age range, 27–72 years; mean, 49.5 years). A comprehensive headache questionnaire was submitted to each patient before surgery, and data regarding age, sex, age at onset, migraines per month (in days), associated symptoms, severity (on a scale from 1 to 10), inability to work per month (in days), health status, history of neck trauma, and familiar history were collected. Migraine Disability Assessment Scale was also used to evaluate the degree of disability for each patient.22 After a mean follow-up of 24 months (range, 3–97 months), patients with frontal trigger site migraine reported a 94% positive response to surgery (32% complete relief and 62% significant improvement, intended as over 50% reduction in duration, frequency, and intensity of headache), whereas 6% had no change in their symptoms. In the case of multiple trigger points, an essential step was detecting the precise site of pain onset (the trigger point). Although patients might report diffuse headache, once they were asked to locate where the pain begins, they could precisely pinpoint it with one fingertip and that was where the surgical treatment had to be performed to release the putative nerve branch. Among the total patients underwent MH frontal decompression surgeries, 24 patients (34.2%) experienced secondary trigger point emergence following primary occipital and/or temporal migraine surgery. Among these, 20 patients had 2 trigger points (18 frontal and occipital, 2 frontal and temporal), whereas 4 patients had all three trigger points; all of them were treated accordingly, by decompression of the corresponding trigger point(s). All patients continue to experience a quality of life better than before surgery, and all would have the surgery again.
DISCUSSION
In 2000, Guyuron et al3 was the first to show in a retrospective study the relation between MH and corrugator supercilii muscle resection, as he reported that 80% of patients described elimination or improvement in their headaches following corrugator supercilii muscle avulsion for forehead rejuvenation surgery. The striking results reported by surgeons performing decompression surgeries of trigger points in MH sufferers strongly support peripheral etiology of MH. Indeed, various authors independently reported success rate higher than 80% in resolving or at least improving MH by decompressing irritated nerve from surrounding structures.4–13 Because surgical deactivation of peripheral sensory nerves has been demonstrated to be effective for the treatment of MH, positive surgical outcome also has significant economic value because it leads to cost savings by cutting expenses associated with medications, doctor visits, and other financial burdens relating to MH.23 Global positive response rate of frontal trigger point deactivation is 94%.4–13 However, when frontal MH is approached first, other trigger points might be unmasked in the postoperative periods. In our experience, patients usually complained of migraine, starting from the occipital or temporal site, which they did not experience in the past. Elimination rate of frontal migraine has the highest variability, performed either by endoscopic or transpalpebral approach.4–13 Poggi et al24 reported a 16.7% complete elimination rate of frontal MH, and Guyuron et al16 described a 57.1% resolution rate, whereas Janis et al15 achieved complete relief of frontal MH only in 8.7% of patients. This discrepancy may partially be explained by variation in the technique: Guyuron et al16 and Poggi et al24 performed the frontal glabellar muscle avulsion, whereas Janis et al15 resected only the corrugator. We have reported a 32% resolution of frontal MH by means of endoscopic myotomies of glabellar muscles or transpalpebral nerve decompression. Special relevance should be given to the close nerve/artery relationship that may intersect or intertwine each other, perhaps promoting irritation and therefore triggering MH attacks.25,26 Transpalpebral approach allowed treating the vascular compression of supraorbital and supratrochlear nerves by corresponding ectatic arteries. The learning curve and the experience of the operator also play an important role when evaluating clinical outcomes.
CONCLUSIONS
In this study, we confirmed previous data in the literature, strengthening the role of a peripheral nerve compression (trigger points) in MHs. Based on our experience, because the operation has not caused any serious complication or side effects and has provided excellent results, surgical decompression of supraorbital and supratrochlear nerves could be recommended to patients who suffer from a moderate to severe chronic frontal migraine not responding to medications. With the same results, at present, we prefer to adopt a transpalpebral approach that allows a better anatomic exposure of the nerves and related structures.
Table 1.
Results of Frontal Migraine Decompression Surgeries from June 2011 to May 2019
| Surgical outcomes | Rates | Results |
|---|---|---|
| Treatment response | ||
| Positive response | 94% | Complete relief: 62% |
| No response | 6% | Partial relief: 32% |
| Trigger points | ||
| Multiple trigger points | 34% | Two trigger points (occipital or temporal trigger point): 83% |
| No multiple trigger points | 66% | Three trigger points (occipital and temporal trigger points): 17% |
PATIENT CONSENT STATEMENT
The patient provided written consent for the use of her image.
Footnotes
Published online 29 April 2020.
Presented, in part, at the 62nd National Congress of the Japan Society of Plastic and Reconstructive Surgery, May 15–17, 2019, Sapporo, Japan; at the 30th European Association of Plastic Surgeons (EURAPS) Annual Meeting, May 23–25, 2019, Helsinki, Finland; at the 10th Congress of World Society for Reconstructive Microsurgery, June 12–15, 2019, Bologna, Italy; at the British Association of Plastic, Reconstructive and Aesthetic Surgery (BAPRAS) Summer Scientific Meeting and Trainee Day, June 26–29, 2019, Bournemouth, United Kingdom; at the Eighth Edition of International Conference and Exhibition on Surgery and Transplantation, July 1–2, 2019, Valencia, Spain; at the 88th Annual Meeting of the American Society of Plastic Surgeons, September 19–23, 2019, San Diego, CA; and at the 68th National Congress of the Italian Society of Plastic, Reconstructive and Esthetic Surgery (SICPRE), September 26–28, 2019, Palermo, Italy.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Mosser SW, Guyuron B, Janis JE, et al. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004;113:693–684; 700.discussion 684 [DOI] [PubMed] [Google Scholar]
- 2.Dash KS, Janis JE, Guyuron B. The lesser and third occipital nerves and migraine headaches. Plast Reconstr Surg. 2005;115:1752–684; discussion 684. [DOI] [PubMed] [Google Scholar]
- 3.Guyuron B, Varghai A, Michelow BJ, et al. Corrugator supercilii muscle resection and migraine headaches. Plast Reconstr Surg. 2000;106:429–684; discussion 684. [DOI] [PubMed] [Google Scholar]
- 4.Bertozzi N, Simonacci F, Lago G, et al. Surgical therapy of temporal triggered migraine headache. Plast Reconstr Surg Glob Open. 2018;6:e1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raposio E, Bertozzi N, Bordin C, et al. Turker H. Surgical therapy of migraine and tension-type headaches. In: Current Perspectives on Less-known Aspects of Headache. 2017:Zagreb, Croatia: InTech; 93–114. [Google Scholar]
- 6.Raposio E, Caruana G. Raposio E. Minimally invasive endoscopic surgical treatment of headache. In: Atlas of Endoscopic Plastic Surgery. 2016:New York, N.Y.: Springer; 17–21. [Google Scholar]
- 7.Caruana G, Bertozzi N, Boschi E, et al. Endoscopic forehead surgery for migraine therapy personal technique. Ann Ital Chir. 2014;85:583–586. [PubMed] [Google Scholar]
- 8.Raposio E, Caruana G. Frontal endoscopic myotomies for chronic headache. J Craniofac Surg. 2015;26:e201–e203. [DOI] [PubMed] [Google Scholar]
- 9.Caruana G, Grignaffini E, Raposio E. Endoscopic forehead muscle resection for nerve decompression: a modified procedure. Plast Reconstr Surg Glob Open. 2015;3:e342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polotto S, Simonacci F, Grignaffini E, et al. Surgical treatment of frontal and occipital migraines: a comparison of results. Plast Reconstr Surg Glob Open. 2016;4:e653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raposio E, Caruana G. Tips for the surgical treatment of occipital nerve-triggered headaches. Eur J Plast Surg. 2017;40:177–182. [Google Scholar]
- 12.Raposio E, Caruana G. Frontal endoscopic myotomies for chronic headache. J Craniofac Surg. 2015;26:e201–e203. [DOI] [PubMed] [Google Scholar]
- 13.Raposio E, Bertozzi N, Bordin C, et al. Kozubski W. Surgical therapy of headaches: minimally invasive approaches. In: Clinical Advances in Head & Neck Surgery. 2018:Las Vegas, Nev: Intech Open; 1–23. [Google Scholar]
- 14.Chmielewski L, Liu MT, Guyuron B. The role of occipital artery resection in the surgical treatment of occipital migraine headaches. Plast Reconstr Surg. 2013;131:351e–356e. [DOI] [PubMed] [Google Scholar]
- 15.Janis JE, Barker JC, Javadi C, et al. A review of current evidence in the surgical treatment of migraine headaches. Plast Reconstr Surg. 2014;134:131–141. [DOI] [PubMed] [Google Scholar]
- 16.Guyuron B, Kriegler JS, Davis J, et al. Comprehensive surgical treatment of migraine headaches. Plast Reconstr Surg. 2005;115:1–9. [PubMed] [Google Scholar]
- 17.Guyuron B, Reed D, Kriegler JS, et al. A placebo-controlled surgical trial of the treatment of migraine headaches. Plast Reconstr Surg. 2009;124:461–468. [DOI] [PubMed] [Google Scholar]
- 18.Guyuron B, Kriegler JS, Davis J, et al. Five-year outcome of surgical treatment of migraine headaches. Plast Reconstr Surg. 2011;127:603–608. [DOI] [PubMed] [Google Scholar]
- 19.Punjabi A, Brown M, Guyuron B. Emergence of secondary trigger sites after primary migraine surgery. Plast Reconstr Surg. 2016;137:712e–716e. [DOI] [PubMed] [Google Scholar]
- 20.Guyuron B, Nahabet E, Khansa I, et al. The current means for detection of migraine headache trigger sites. Plast Reconstr Surg. 2015;136:860–867. [DOI] [PubMed] [Google Scholar]
- 21.Tolhurst DE, Carstens MH, Greco RJ, et al. The surgical anatomy of the scalp. Plast Reconstr Surg. 1998;102:478. [PubMed] [Google Scholar]
- 22.Stewart WF, Lipton RB, Dowson AJ, et al. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56(6 suppl 1):S20–S28. [DOI] [PubMed] [Google Scholar]
- 23.Faber C, Garcia RM, Davis J, et al. A socioeconomic analysis of surgical treatment of migraine headaches. Plast Reconstr Surg. 2012;129:871–877. [DOI] [PubMed] [Google Scholar]
- 24.Poggi JT, Grizzell BE, Helmer SD. Confirmation of surgical decompression to relieve migraine headaches. Plast Reconstr Surg. 2008;122:115–684; discussion 684. [DOI] [PubMed] [Google Scholar]
- 25.Gfrerer L, Raposio E, Ortiz R, et al. Surgical treatment of migraine headache: back to the future. Plast Reconstr Surg. 2018;142:1036–1045. [DOI] [PubMed] [Google Scholar]
- 26.Raposio E. Atlas of Surgical Therapy for Migraine and Tension-Type Headache. 2019New York, N.Y.: Springer. [Google Scholar]


