Abstract
Background:
Most postsurgical scars are considered esthetically and functionally acceptable. Currently, there is no definite consensus treatment for postsurgical scarring. The purpose of this review is to shed some light on the value of scar mitigation and the efficacy of different lasers employed on postsurgical wounds.
Methods:
A systematic literature review and computational analysis were conducted to identify relevant clinical articles that pertained to the use of lasers for mitigating postsurgical scars. Articles included the National Institutes of Health–National Center for Biotechnology Information–PubMed search and sources cited from relevant studies after 1995. Trials that attributed pre- and posttreatment scores of scar severity based on a verified scar evaluation scale (eg, Patient and Observer Scar Assessment Scale, Vancouver Scar Scale, Global Assessment Scale) were chosen. Clinical assessments varied for each study. To adequately assess the efficacy of the modalities, the final scaled scar appearance scores were realigned and normalized to a standard scale for unbiased comparison.
Results:
After filtering through a total of 124 studies, 14 relevant studies were isolated and thus included in the review. Studied lasers were as follows: Pulsed dye laser (PDL), carbon dioxide, diode, potassium titanyl phosphate (KTP), and erbium glass (Er-Glass) lasers.
Conclusion:
Treatment with lasers in the postsurgical wound healing phase is safe, effective, and advised in mitigation of pathologic scar formation.
INTRODUCTION
Most postsurgical scars are considered esthetically and functionally acceptable. However, keloidal or hypertrophic scars can be symptomatic and might cause esthetic, psychological, and social distress.1 There is currently no definite, consensual measure that completely prevents postsurgical scarring. Therefore, many modalities are being explored, including laser treatment, to determine the safest and most efficacious method.2
The purpose of this review is to shed some light on the value of active scar mitigation by lasers, as compared to natural, spontaneous wound healing concerning final cosmesis of scars.
METHODS
Search Strategy
Studies were identified through the search strategy by 2 independent reviewers.
A systematic National Institutes of Health–National Center for Biotechnology Information–PubMed search was conducted to identify relevant clinical articles that pertained to the use of lasers to mitigate postsurgical scars. Message-Subject-Headings were applied. The search algorithm used was (cicatrix OR cicatrix treatment OR scar OR scars) AND (laser OR laser treatment OR laser therapy OR fractional laser OR ablative fractional laser OR nonablative fractional laser OR dye laser OR diode laser) AND (prevention OR minimizing OR early intervention OR treatment outcome). Additional studies were acquired from review articles that appeared in the search. Only human trials that attributed pre- and posttreatment scores of scar severity based on a verified scar evaluation scale [eg, Patient and Observer Scar Assessment Scale, Vancouver Scar Scale (VSS), Global Assessment Scale], published in English, after January 1, 1995, were finally included.
The final studies that fit the inclusion criteria were identified using a 2-step process. First, the titles and abstracts of acquired articles were screened. Next, the complete text was reviewed. The inclusion and exclusion criteria are shown in Figure 1.
Fig. 1.
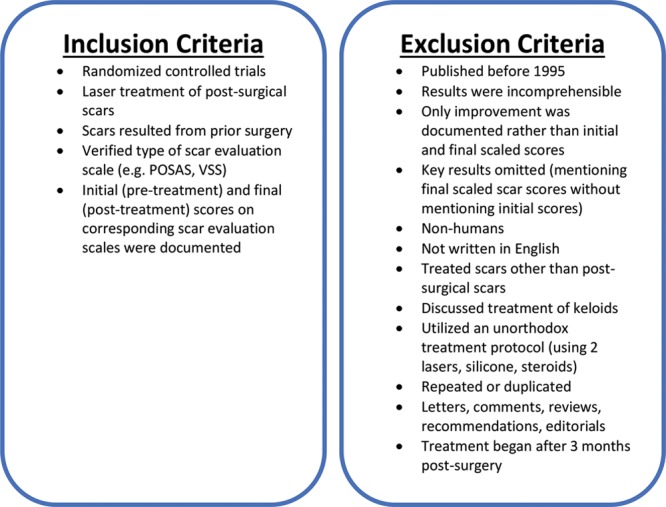
Inclusion and exclusion criteria. POSAS, Patient and Observer Scar Assessment Scale.
Data Extraction
Standardized extraction of data was compiled and consolidated in a Microsoft Excel (Microsoft, Redmond, Wash., USA) spreadsheet. Acquired data included the name of the first author, year of publication, type of surgical wound, time of first treatment relative to operation date, device(s) and medications used, number of compared treatments, number of raters, number of patients subject to each treatment arm, parameters and settings on the device, number of treatments, time interval between treatments, study design, scar evaluation scale, statistical test used, P value, results, comments, and clarifications for future considerations.
Outcome Measures, Data Normalization, and Analysis
Different studies use different clinical assessment scores. To adequately assess the efficacy of the modalities, the final scaled scar appearance scores were realigned and normalized to a standard scale for unbiased comparison. This excluded studies that only reported improvement of scars and studies that did not adequately report their final results. Scales such as the VSS, the Global Assessment Scale, the Patient and Observer Scar Assessment Scale, Visual Analog Scale, and 4- and 5-level Likert scales were normalized to a standard 0–100 scale depicting 100 as healthy skin (= best esthetic outcome) and 0 as the worst possible scar (= worst esthetic outcome). Objective scar scores were defined as those determined by physicians, and subjective scar scores were those determined by patients.
The aligned scores were then compared: treatment versus control to calculate standard score, or z score, as it pertains to a single measurement, whereas standard mean difference (SMD) compares 2 groups of measurements: treatment versus control. The SMD was calculated for both objective and subjective ratings. Studies that employed more patients were weighted heavier against studies that employed fewer patients.
RESULTS
The initial database search yielded 124 studies. Twenty-five studies were added from reference lists of reviews and filtered using the inclusion and exclusion criteria specified above.3,4 One hundred articles were excluded following title and abstract review. Thirty-five studies were excluded following full-text screening. A total of 14 studies remained and discussed in this review (Fig. 2).
Fig. 2.
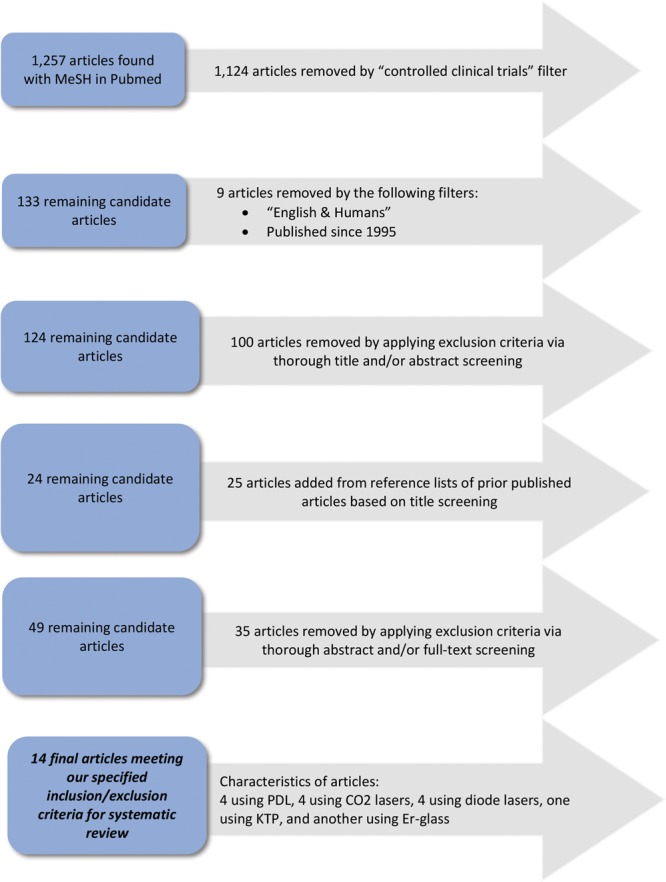
Schematic for study selection. CO2, carbon dioxide. Er, erbium.
A summary of the study characteristics is depicted in Table 1. Fourteen studies met the inclusion criteria of attempting to treat postsurgical scars with lasers and assessed the quality of scar healing with a final scar assessment score.5–18 Nine studies used split scars, 2 used controlled cohorts, 1 used a split body (breasts) comparison, and 2 were prospective pilot studies. A total of 271 scars were treated with 247 matched control scars. The number of patients per study group ranged from 5 to 40 (mean = 18.5). Pulsed dye lasers (PDL), carbon dioxide (CO2) lasers, and diode lasers were the most commonly used devices followed by erbium glass (Er-Glass) and potassium titanyl phosphate (KTP) lasers.
Table 1.
Study Characteristics
| Article | Device | Type of Control | No. Patients | Objective Scale | |
|---|---|---|---|---|---|
| Treated | Control | ||||
| Nouri et al5 | PDL | Split scar | 12 | — | VSS |
| Alam et al6 | PDL | Split scar | 17 | — | 1–4 scale |
| Conologue and Norwood7 | PDL | Split scar | 13 | — | VSS |
| Capon et al8 | Diode | Split scar | 5 | — | 1–4 scale |
| Choe et al9 | Er-glass | Separate group | 27 | 14 | VSS |
| Capon et al10 | Diode | Split scar | 30 | — | 0–3 scale |
| Carvalho et al11 | Diode | Separate group | 14 | 14 | VSS |
| Yun et al12 | KTP | Separate group | 20 | 8 | VSS |
| Lee et al13 | CO2 | Split scar | 15 | — | VSS |
| Sobanko et al14 | CO2 | Split scar | 20 | — | VSS |
| Vazquez-Martinez et al15 | PDL | Split scar | 30 | — | VSS |
| Buelens et al16 | CO2 | Split scar | 9 | — | GAS, POSAS |
| Alberti et al17 | CO2 | Separate group | 20 | 21 | VSS |
| Casanova et al18 | Diode | Split chest | 40 | — | mOSAS |
In split scar studies, the number of treated patients equals the number of controls.
GAS, Global Assessment Scale; mOSAS, modified Observer Scar Assessment Scale; POSAS, Patient Observer Scar Assessment Scale.
Various protocols were employed; laser treatments were performed 2–10 times at 2- to 10-week intervals. Most study protocols included 3–4 treatments at intervals of 2–4 weeks. For all but one study,16 the first laser treatment was scheduled after suture removal.
The VSS was used in 8 of the 15 studies. Most of the studies used multiple scales for each objective (physician) and subjective (patient) evaluations of the outcomes of scars. The objective SMD between treatment and control for all cumulative studies was calculated to be 0.777 (95% CI, 0.368–1.186). Statistically significant differences were measured between treated and untreated scars among physicians and patients.
Diode lasers treatment lead to the greatest SMD of 0.624 (95% CI, 0.322–0.925) and CO2 the lowest overall SMD with 0.43 (95% CI, 0.0794–0.780). The SMD for KTP, 2.669 (95% CI, 1.558–3.779), and Er-glass lasers, 0.876 (95% CI, 0.194–1.557), had the best results compared with controls, however, with low weights and a wide CIs. Objectively, the SMD of PDL, 0.45 (95% CI, 0.116–0.784), was similar to that of CO2. These results are shown in Figure 3.
Fig. 3.
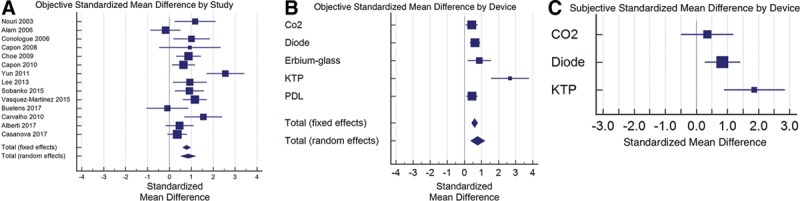
Graphical depictions of standardized mean differences between treatment and controls among (A) objective results achieved by the studies, (B) objective results achieved by specific devices, and (C) subjective results achieved by specific devices. The box size of each data point depicts the weight of the device, which was deemed dependent on the number of patients treated. The whiskers of each box depict the standard error and thus the 95% CI.
Patients’ evaluations reflected the same results, as shown in Figure 3C. The SMD for diode lasers was 0.844 (95% CI, 0.275–1.413). KTP lasers reflected an SMD of 1.868 (95% CI, 0.88–2.848) in a few patients. CO2, with an SMD of 0.353, had statistically insignificant results among patients (95% CI, −0.483 to 1.189).
DISCUSSION
Postsurgical scars are ideally flat, narrow, pale, and pliable. Abnormal scars may range from hypertrophic to keloid. Abnormal scars can be unsightly, painful, functionally, and socially limiting. Various scar mitigation options exist.19 This study provides a systematic review of trials performed over the last 2 decades that investigate mitigation of postoperative scars using a variety of single laser modalities and treatment protocols.
Four studies out of 14 did not demonstrate statistical significance with the treatment of postsurgical scars. Alam et al6 employed a single PDL treatment immediately postsurgery and concluded that more treatments are necessary to achieve a therapeutic effect. Buelens et al16 and Sobanko et al14 failed to show statistically significant improvement using fractional CO2 laser monotherapy. However, higher patient satisfaction was statistically significant for treated scars.16
Statistically, significant scar improvement was found in the remaining 10 studies. Diode, PDL, and CO2 lasers were reported to have the best results compared with controls. Although the exact mechanics are not entirely understood, diode lasers have been shown to increase heat-shock-protein 70 induction, which is known to induce collagen proliferation and modulate transforming growth factor beta (TGF-β) expression, and remodeling.8,20,21
PDL and KTP lasers best target the oxy- and deoxyhemoglobin chromophores and are best utilized to alleviate the erythema associated with highly vascular postsurgical scars.22–25 Also, PDL has been shown to upregulate p53, inhibiting cell proliferation, and reducing angiogenesis that contributes to abnormal scarring.26 Optimal results were achieved after 3 PDL treatments.5–7,15 Treatment intervals ranged from 2 to 10 weeks. All 4 studies started treatment on the day of suture removal and proved to be safe and efficacious.5,27
Both KTP and PDL lasers exhibited substantial improvement in similar categories of scar treatment; however, neither have had statistically significant results when compared with the other.28 KTP lasers have been associated with more posttreatment pain, erythema, and edema.28,29
The chromophore targeted by CO2 lasers is water, found in tissues. CO2 laser treatments are considered more aggressive and lead to considerable dermal matrix remodeling, hopefully leading to favorable remodeling of the scar.25,30 Half of the studies exploring the treatment of postsurgical scars using only CO2 lasers reported statistically insignificant results with a collectively low SMD.
Combining different modalities may augment scar mitigation. A synergistic effect can be achieved from combining PDL treatment, targeting scar vascularity and pigmentation, followed by fractional CO2, and aimed at improving the texture, pliability, and height of the scar.31–33 Also, combination of laser treatment with triamcinolone injection has shown promising results, perhaps by inhibiting fibroblasts and TGF-β.25,31,34–36 Combining Er:yttrium aluminum garnet (YAG) fractional ablation after PDL treatment improved scar pliability in addition to scar appearance.37
Our review is inherently limited by its attempt to compare different studies using various measurement scales and patient populations. This made it difficult to compare among protocols, devices, and parameters. Also, aside from 2 listed patients in one study,7 only patients less prone to hypertrophic or keloid scarring are evaluated. Future studies should explore high-risk patients such as patients with abnormal scarring history or patients undergoing midline or limb incisions. Moreover, standardized objective measuring tools such as 3D, infrared cameras, and standard scar scales should be encouraged.
CONCLUSIONS
Laser therapy is a safe and effective modality for scar mitigation. The data suggest that early intervention exhibits the best results. We recommended beginning treatment close to suture removal combining vascular and nonablative fractional resurfacing modalities for 2–4 treatments at 2- to 3-week intervals. It is imperative to monitor the wound healing process and document side effects such as erythema, discoloration, pain, and infection. Further research may help define standard treatment protocols which would benefit both evaluations of the results and clinical outcome.
Footnotes
Published online 24 April 2020.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Bock O, Schmid-Ott G, Malewski P, et al. Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res. 2006;297:433–438. [DOI] [PubMed] [Google Scholar]
- 2.Mirmanesh M, Borab Z, Gantz M, et al. Peri-procedure laser scar therapy protocol: a pilot survey of plastic surgeons’ practices. Aesthetic Plast Surg. 2017;41:689–694. [DOI] [PubMed] [Google Scholar]
- 3.Karmisholt K, Haerskjold A, Karlsmark T, et al. Early laser intervention to reduce scar formation: a systematic review. J Eur Acad Dermatol Venereol. 2018;32:1099–1110. [DOI] [PubMed] [Google Scholar]
- 4.Perez JL, Rohrich RJ. Optimizing your post-surgical scars: a systematic review on best practices in preventative scar management. Plast Reconstr Surg. 2017;140:e782, e793. [DOI] [PubMed] [Google Scholar]
- 5.Nouri K, Jimenez GP, Harrison-Balestra C, et al. 585-nm pulsed dye laser in the treatment of surgical scars starting on the suture removal day. Dermatol Surg. 2003;29:65–73. [DOI] [PubMed] [Google Scholar]
- 6.Alam M, Pon K, Van Laborde S, et al. Clinical effect of a single pulsed dye laser treatment of fresh surgical scars: randomized controlled trial. Dermatol Surg. 2006;32:21–25. [DOI] [PubMed] [Google Scholar]
- 7.Conologue TD, Norwood C. Treatment of surgical scars with the cryogen-cooled 595 nm pulsed dye laser starting on the day of suture removal. Dermatol Surg. 2006;32:13–20. [DOI] [PubMed] [Google Scholar]
- 8.Capon AC, Gossé AR, Iarmarcovai GN, et al. Scar prevention by laser-assisted scar healing (LASH): a pilot study using an 810-nm diode-laser system. Lasers Surg Med. 2008;40:443–445. [DOI] [PubMed] [Google Scholar]
- 9.Choe JH, Park YL, Kim BJ, et al. Prevention of thyroidectomy scar using a new 1,550-nm fractional erbium–glass laser. Dermatol Surg. 2009;35:1199–1205. [DOI] [PubMed] [Google Scholar]
- 10.Capon A, Iarmarcovai G, Gonnelli D, et al. Scar prevention using laser-assisted skin healing (LASH) in plastic surgery. Aesthetic Plast Surg. 2010;34:438–446. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho RL, Alcântara PS, Kamamoto F, et al. Effects of low-level laser therapy on pain and scar formation after inguinal herniation surgery: a randomized controlled single-blind study. Photomed Laser Surg. 2010;28:417–422. [DOI] [PubMed] [Google Scholar]
- 12.Yun JS, Choi YJ, Kim WS, et al. Prevention of thyroidectomy scars in Asian adults using a 532-nm potassium titanyl phosphate laser. Dermatol Surg. 2011;37:1747–1753. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Zheng Z, Roh MR. Early postoperative treatment of surgical scars using a fractional carbon dioxide laser: a split-scar, evaluator-blinded study. Dermatol Surg. 2013;39:1190–1196. [DOI] [PubMed] [Google Scholar]
- 14.Sobanko JF, Vachiramon V, Rattanaumpawan P, et al. Early postoperative single treatment ablative fractional lasing of Mohs micrographic surgery facial scars: a split-scar, evaluator-blinded study. Lasers Surg Med. 2015;47:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez-Martinez O, Eichelmann K, Garcia-Melendez M, et al. Pulsed dye laser for early treatment of scars after dermatological surgery. J Drugs Dermatol. 2015;14:1209–1212. [PubMed] [Google Scholar]
- 16.Buelens S, Van Hove AS, Ongenae K, et al. Fractional carbon dioxide laser of recent surgical scars in the head and neck region: a split-scar, evaluator-blinded study. Dermatol Surg. 2017;43(Suppl 1):S75–S84. [DOI] [PubMed] [Google Scholar]
- 17.Alberti LR, Vicari EF, De Souza Jardim Vicari R, et al. Early use of CO2 lasers and silicone gel on surgical scars: prospective study. Lasers Surg Med. 2017;49:570–576. [DOI] [PubMed] [Google Scholar]
- 18.Casanova D, Alliez A, Baptista C, et al. A 1-year follow-up of post-operative scars after the use of a 1210-nm laser-assisted skin healing (LASH) technology: a randomized controlled trial. Aesthetic Plast Surg. 2017;41:938–948. [DOI] [PubMed] [Google Scholar]
- 19.Khansa I, Harrison B, Janis JE. Evidence-based scar management: how to improve results with technique and technology. Plast Reconstr Surg. 2016;138(3 Suppl):165S–178S. [DOI] [PubMed] [Google Scholar]
- 20.Shah M, Revis D, Herrick S, et al. Role of elevated plasma transforming growth factor-beta1 levels in wound healing. Am J Pathol. 1999;154:1115–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souil E, Capon A, Mordon S, et al. Treatment with 815-nm diode laser induces long-lasting expression of 72-kDa heat shock protein in normal rat skin. Br J Dermatol. 2001;144:260–266. [DOI] [PubMed] [Google Scholar]
- 22.Alster T, Zaulyanov L, Zaulyanov-Scanlon L. Laser scar revision: a review. Dermatol Surg. 2007;33:131–140. [DOI] [PubMed] [Google Scholar]
- 23.Reiken SR, Wolfort SF, Berthiaume F, et al. Control of hypertrophic scar growth using selective photothermolysis. Lasers Surg Med. 1997;21:7–12. [DOI] [PubMed] [Google Scholar]
- 24.Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524–527. [DOI] [PubMed] [Google Scholar]
- 25.Kim DH, Ryu HJ, Choi JE, et al. A comparison of the scar prevention effect between carbon dioxide fractional laser and pulsed dye laser in surgical scars. Dermatol Surg. 2014;40:973–978. [DOI] [PubMed] [Google Scholar]
- 26.Zhibo X, Miaobo Z. Molecular mechanism of pulsed-dye laser in treatment of keloids: an in vitro study. Adv Skin Wound Care. 2010;23:29–33. [DOI] [PubMed] [Google Scholar]
- 27.Kent RA, Shupp J, Fernandez S, et al. Effectiveness of early laser treatment in surgical scar minimization: a systematic review and meta-analysis. Dermatol Surg. 2019;46:402–410. [DOI] [PubMed] [Google Scholar]
- 28.Keaney TC, Tanzi E, Alster T. Comparison of 532 nm potassium titanyl phosphate laser and 595 nm pulsed dye laser in the treatment of erythematous surgical scars: a randomized, controlled, open-label study. Dermatol Surg. 2016;42:70–76. [DOI] [PubMed] [Google Scholar]
- 29.Vas K, Gaál M, Varga E, et al. Effects of the combined PDL/nd:YAG laser on surgical scars: vascularity and collagen changes evaluated by in vivo confocal microscopy. Biomed Res Int. 2014;2014:204532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeBruler DM, Blackstone BN, Baumann ME, et al. Inflammatory responses, matrix remodeling, and re-epithelialization after fractional CO2 laser treatment of scars. Lasers Surg Med. 2017;49:675–685. [DOI] [PubMed] [Google Scholar]
- 31.Ryu HW, Cho JH, Lee KS, et al. Prevention of thyroidectomy scars in Korean patients using a new combination of intralesional injection of low-dose steroid and pulsed dye laser starting within 4 weeks of suture removal. Dermatol Surg. 2014;40:562–568. [DOI] [PubMed] [Google Scholar]
- 32.Safra T, Shehadeh W, Koren A, et al. Early intervention with pulse dye and CO2 ablative fractional lasers to improve cutaneous scarring post-lumpectomy: a randomized controlled trial on the impact of intervention on final cosmesis. Lasers Med Sci. 2019;34:1881–1887. [DOI] [PubMed] [Google Scholar]
- 33.Lee Y, Kim W. Combination laser treatment for immediate post-surgical scars: a retrospective analysis of 33 immature scars. Lasers Med Sci. 2017;32:1111–1119. [DOI] [PubMed] [Google Scholar]
- 34.Cohen JL, Geronemus R. Safety and efficacy evaluation of pulsed dye laser treatment, CO2 ablative fractional resurfacing, and combined treatment for surgical scar clearance. J Drugs Dermatol. 2016;15:1315–1319. [PubMed] [Google Scholar]
- 35.On HR, Lee SH, Lee YS, et al. Evaluating hypertrophic thyroidectomy scar outcomes after treatment with triamcinolone injections and copper bromide laser therapy. Lasers Surg Med. 2015;47:479–484. [DOI] [PubMed] [Google Scholar]
- 36.Alster T. Laser scar revision: comparison study of 585-nm pulsed dye laser with and without intralesional corticosteroids. Dermatol Surg. 2003;29:25–29. [DOI] [PubMed] [Google Scholar]
- 37.Kim HS, Kim BJ, Lee JY, et al. Effect of the 595-nm pulsed dye laser and ablative 2940-nm Er: YAG fractional laser on fresh surgical scars: an uncontrolled pilot study. J Cosmet Laser Ther. 2011;13:176–179. [DOI] [PubMed] [Google Scholar]


