Abstract
Spindles are ubiquitous oscillations during non-rapid eye movement (NREM) sleep. A growing body of evidence points to a possible link with learning and memory, and the underlying mechanisms are now starting to be unveiled. Specifically, spindles are associated with increased dendritic activity and high intracellular calcium levels, a situation favourable to plasticity, as well as with control of spiking output by feed-forward inhibition. During spindles, thalamocortical networks become unresponsive to inputs, thus potentially preventing interference between memory-related internal information processing and extrinsic signals. At the system level, spindles are co-modulated with other major NREM oscillations, including hippocampal sharp wave-ripples (SWRs) and neocortical slow waves, both previously shown to be associated with learning and memory. The sequential occurrence of reactivation at the time of SWRs followed by neuronal plasticity-promoting spindles is a possible mechanism to explain NREM sleep-dependent consolidation of memories.
This article is part of the Theo Murphy meeting issue ‘Memory reactivation: replaying events past, present and future'.
Keywords: sleep, spindles, memory, plasticity, coupling, reactivation
1. Introduction
Soon after the discovery of brain waves by Hans Berger in 1929 [1], Alfred Loomis performed electroencephalographic (EEG) recordings in resting subjects and provided the first descriptions of sleep-specific brain oscillations [2]. The EEG traces during sleep, far from being flat as expected from an ‘inactive' brain, are dominated by various patterns, including the so-called spindles, short (less than 3 s) waxing and waning oscillatory events with a typical frequency ranging from 9 to 15 Hz in humans [3–7]. The neuronal basis underlying the generation of spindles has now been clearly established and involves the interplay between the neocortex and thalamic networks spontaneously oscillating at spindle frequency [8–10].
A decade before the discovery of spindles, sleep had been shown to be beneficial for memories [11], and the relationship between sleep dynamics and memory has remained a central topic of neuroscience since then [12–16]. Specifically, a novel experience imprints a labile physiological trace in the brain that vanishes unless it is consolidated. Sleep is considered to be an ideal brain state for reprocessing memories in order to sort, consolidate and generalize recently acquired knowledge [15,17]. The exact contribution of different sleep stages for memory consolidation remains a subject of debate [13,18–20], yet it is generally accepted that non-rapid eye movement (NREM) sleep plays a key role in this process [15,20]. Oscillations during NREM sleep have been proposed to be the correlates of cellular and molecular mechanisms promoting sleep-dependent memory enhancement [12,13,19–21]. Among the different oscillations observed during NREM sleep, thalamocortical spindles have been repeatedly suggested as a mediator of cognitive enhancement and memory improvement observed during sleep [5,8,22–24].
Sleep spindles have now been the focus of a large body of literature and studied in a variety of conditions: different animal species and brain modalities, natural sleep and anaesthesia, sleep with or without associated learning paradigms, in vivo and in vitro. Here, we will first review our current understanding of the link between spindles, memory and synaptic plasticity. Then, we will review the recent data that link spindles and local regulation of the neocortical microcircuitry and how this regulation supports their role in synaptic plasticity. We will then address the question of the role of spindles at the systems level and how their co-modulation with other major oscillations during NREM sleep, in particular hippocampal sharp wave-ripples (SWRs), may constitute the physiological basis of the NREM sleep-dependent consolidation of declarative memories. Finally, we propose a model in which spindles play a central role for synaptic consolidation in the particular framework of memory systems consolidation.
2. Link between spindles, memory and plasticity
Being the most prominent sleep stage (approx. 80% of total sleep in adult humans), NREM sleep has attracted of lot of attention as the main mediator of sleep function(s). While rapid eye movement (REM) sleep has long been a focus of memory research, the discovery of experience-dependent NREM ‘replay' activity in the hippocampus provided new evidence and interest in the role of NREM sleep in memory [25–27].
(a). Thalamocortical non-rapid eye movement sleep oscillations
Cortical activity during NREM sleep differs from wakefulness in one crucial aspect: it is synchronized by slow waves (SWs). SWs are typically divided into slow oscillations (SOs less than 1 Hz) and delta (1–4 Hz), sometimes grouped into slow wave activity (SWA: 0.5–4 Hz). In thalamocortical networks, the other NREM-specific oscillations are the spindles. In humans, SWs and spindles do not occur at the same rate across sleep and their relative prevalence divides NREM sleep into three stages indicative of sleep depth [18,28]. Although spindles are found across all NREM sleep stages, spindle activity prevails in stage 2 (light sleep) while SWA increases in stage 3 (deep sleep, also called slow-wave sleep, SWS) [29]. In rodents, the architecture of NREM sleep can also be divided into three stages during which cortical dynamics share strong similarities with human electrophysiological markers defining these stages [18]. Similar to humans, SWs and spindle activity are more prominent during stages N2 and N3 in rodents but spindle intensity and number show a greater increase at the end of NREM sleep episodes [30–32], which often correspond to the transition to REM sleep.
The physiology of SWs and spindles has now been well characterized and is the focus of several comprehensive reviews [5,7–9,33–36]. These oscillations are both generated within thalamocortical networks. However, while the primary generator of SWs consists of the cortical fluctuation between UP and DOWN states of neuronal activity [9], the pacemaker of spindles resides in the thalamus. Briefly, spindles are generated by the interplay between the inhibitory cells of the thalamic reticular nucleus (TRN) of the thalamus and thalamocortical neurons in the dorsal thalamus [9,36]. Corticothalamic Inhibitory volleys from the reticular cells activate specific calcium channels (T-type current) which eventually leads to bursts of action potentials in some thalamocortical neurons. This strong volley of excitation, in turn, activates the inhibitory cells of the TRN, and this interaction repeats itself at each spindle cycle. These excitatory volleys also depolarize cortical neurons, giving rise to spindle events, as detected in EEG and local field potential (LFP) recordings (figure 1a). While the neocortex does not directly play a role in the generation of spindles, corticothalamic projections synchronize these oscillations [41].
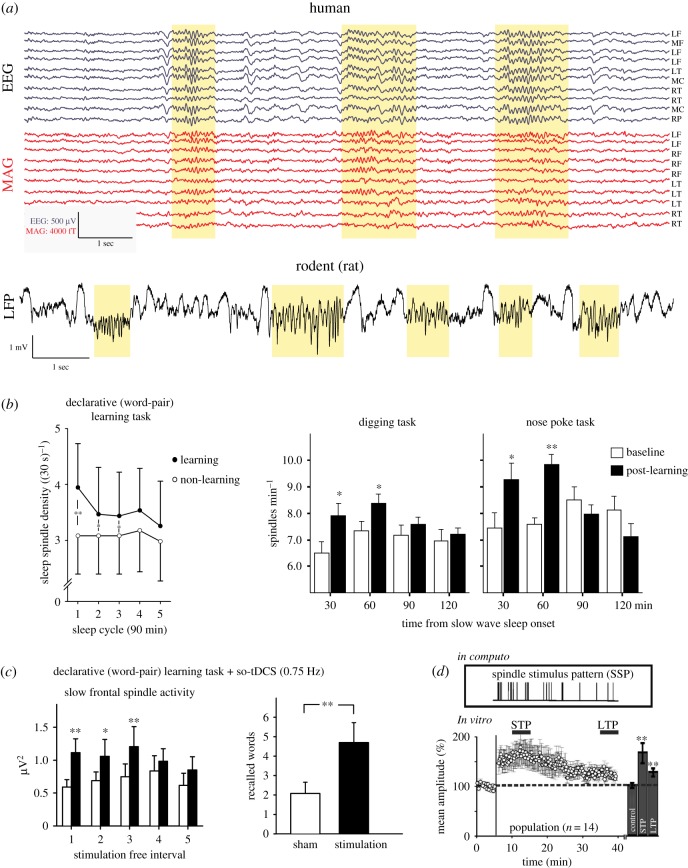
Figure 1.
Spindles, memory and plasticity. (a) Examples of spindles in human electroencephalograph (EEG) and magnetoencephalogram (MAG) (upper traces, adapted from [37]) and rat (lower trace; J. Seibt 2017, unpublished data). Spindles are highlighted in yellow. (b) Influence of learning on spindle density in the human (left graph, adapted from [38]) and rat (right graphs, adapted from [39]) frontal cortex. (c) Boosting frontal slow spindles (and SOs, not shown) with frontolateral slow oscillatory (0.75 Hz) transcranial direct current stimulation (so-tDCS) in humans improves declarative memory (adapted from [21]). (d) Naturalistic spindle stimulus pattern (SSP) promotes short- (STP) and long-term plasticity (LTP) in vitro (adapted from [40]).
Fine differences in spindle frequencies are observed across developmental stages and brain regions, yet, overall they are preserved across animal species [42]. In humans, spindles are readily visible in the EEG and can be divided into fast (12–15 Hz) and slow (9–12 Hz) spindles based on distinct peaks in the NREM power spectra [43,44]. The differentiation into fast and slow spindles is also based on topography, whereby fast spindles are preferentially expressed in the parietal/somatosensory areas and slow spindles in the frontal brain areas ([43] and see [5] for review). Although this distinction may have some functional relevance in relation to memory in humans (see below), the fast and slow components of spindles cannot be distinguished in rodent EEG/LFP power spectra [44] which limits their study in this species. However, differences in power and occurrence rate have been observed across cortical areas in rodents [45,46]. Spindles are not a unitary phenomenon, but instead, reflect the oscillatory activity of many parallel thalamocortical circuits that may or may not synchronize across circuits and modalities. In fact, intracerebral spindles recorded in epileptic patients implanted with depth electrodes are often ‘local' and more rarely ‘global' [43,47,48]. Furthermore, spindles simultaneously monitored with EEG and magnetoencephalography, believed to track the activity of different cortical dipoles, show only a weak temporal correlation [37].
While the ubiquitous nature of spindles and their characteristics across brain regions and animal species become less and less disputed, it is only in the last 20 years that their contribution to memory and brain plasticity, in general, has started to be unveiled.
(b). Link between spindles and memory
The strong activation of cortical neurons by thalamic excitatory volleys is an interesting candidate for promoting plasticity during NREM sleep [49,50]. Spindles are associated with both declarative and procedural learning (reviewed in [5]), further suggesting a fundamental link with the mechanisms responsible for reshaping network connectivity. The evidence linking SW and spindle oscillations and memory fall into three categories: correlative, causal and link with plasticity. In this section, we will review each of these categories of experimental findings.
(i). Correlative evidence
Change in NREM oscillations following learning was first evidenced in human studies. Specifically, using scalp EEG recordings, studies showed that specific spindle parameters (e.g. density, amplitude) increased in post-learning sleep for both declarative and procedural memory tasks ([22,23,38,51–55] and figure 1b). It is noteworthy that not all forms of learning upregulate spindles (e.g. [56]). In addition, modulation of spindles is also use-dependent as learning induces increase in spindles in brain regions involved in the learned tasks [55,57–60]. Those latter studies in humans also show a preferential involvement of fast spindles in memory enhancement. Comparable observations have been made for SWs, in particular SWA, that show use-dependent increase during post-learning sleep specifically over the cortical areas engaged in the learning task [61,62]. In rodents, both spindles and SWs also increase following learning and experience [39,44,63,64]. Furthermore, during sleep following perceptual visual learning in developing cats and adult mice, neurons of the visual cortex become more strongly phase-locked to spindles than in baseline condition [65,66]. Taking together, these data support a functional implication of these oscillations in offline information processing during spindles.
Beyond their role in memory, the relationship between spindles and cognition is also supported by studies showing a link between spindles and activity in the developing brain. Evidence points to a role for spindles in the early establishment of sensorimotor cortical maps [67–69]. Furthermore, changes in spindle characteristics are also closely linked to neuropathology associated with cognitive impairments such as autism spectrum disorders, schizophrenia and Alzheimer's disease [70–72]. Although the specific changes in spindles vary depending on the neuropathology (i.e. neuropsychiatric, neurodevelopmental and neurodegenerative disorders) and the spindle characteristics measured, a general trend in decreased spindles density in those pathologies have been repeatedly reported (reviewed in [5]). This suggests that variations in the number of spindles may play a particularly important role for cognitive functions. Taken together, spindles have been cast in variety of roles related to the development and maintenance of cognitive functions, including memory. These observations support a common role for spindles in neuronal plasticity—the biological substrate of memory.
(ii). Causal evidence
Do changes in NREM oscillations, especially spindles, play an active role in memory consolidation or do they only reflect physiological modifications associated with plasticity, such as increased excitability [19]? While correlative studies cannot unequivocally address this issue, it can be addressed via direct manipulations of these oscillations. The best way to establish a direct link between NREM oscillations and cognition is to perform manipulations (e.g. gain- and loss-of-function) of these network phenomena and assess the resultant effect on memory performance. Manipulations of SOs and spindles have been performed in both humans and rodents. In most human studies, non-invasive brain stimulation via transcranial electrical stimulation (tES) is used for this purpose [73]. In a seminal tES study by Marshall and colleagues, they applied transcranial direct current stimulation at the slow oscillation frequency (0.75 Hz) (so-tDCS) above frontolateral regions in young subjects. This stimulation elicits an increase in both SOs and spindles and improves performance in a declarative paired-associate memory task ([21] and figure 1c). Following this study, these results have been replicated and complemented. Using feedback-controlled transcranial alternating current stimulation (tACS) of frontal areas at spindle frequency (12 Hz) during NREM sleep, Lustenberger et al. [74] specifically enhanced spindle activity (i.e. sigma 11–16 Hz) which was accompanied with enhancement of motor memory consolidation. In addition, increasing the SOs/spindle coupling using administration of zolpidem in humans has a positive effect on performance on a verbal memory task [75]. Targeted memory reactivation (TMR) paradigm is another interesting approach [73]: presentation of a memory-related tone or odour during NREM sleep improves memory performance the following day [76,77]. In addition to enhance memory, TMR elicits a parallel increase in SOs and fast spindle incidence, suggesting a causal relationship with cognitive performance [21,53,58,78,79]. Finally, a recent study in humans used bed rocking at slow frequency (0.25 Hz) to enhance stage 3 NREM SOs and spindles. In this paradigm, memory performance improvement is positively correlated with fast spindles [80]. However, a similar approach in mice shows small effects of rocking on NREM EEG power, in particular, for the sigma band that actually displayed a strong suppression when the frequency of rocking was increased [81]. Although indicative, the influence of rocking on memory should be replicated in mice to clarify the link between NREM oscillations and cognitive performance using this behavioural approach.
In rodents, manipulations of SWs and/or spindle oscillations also point to a relationship with learning and memory. For example, optogenetic stimulation of the TRN to phase lock thalamocortical oscillations (approx. 8 Hz) with SOs and hippocampal SWRs boosts the performance of mice in a contextual fear conditioning task [82]. However, it is worth mentioning that this optogenetic stimulation increases spindle oscillations in the 7–10 Hz frequency band but also inhibits oscillations in the sigma frequency band (10–15 Hz) [83]. Thus, although thalamocortical stimulation during NREM sleep enhances memory in mice, whether the induced oscillations represent physiological spindles remains to be determined. More recently, Kim et al. [84] used optogenetic stimulation in mice to manipulate the amounts of SO/spindle or delta/spindle nesting events and show increased or decreased performance in a neuroprosthetic task (i.e. procedural memory), respectively [84]. This latter study has two implications. First, it supports growing evidence for physiological [45] and functional differences between SO and delta oscillations. Second, it further suggests that the role of spindles is not limited to strengthening of memory traces but can, depending on their coupling, also promote forgetting [84]. Altogether, manipulations of NREM sleep oscillations point at an important role of oscillatory coupling in memory, an aspect discussed in §4.
(iii). Link with plasticity
Although NREM oscillations may have a direct impact on various aspects of neuronal physiology, their influence on synaptic plasticity is a likely candidate for the mechanisms of memory consolidation. Synaptic plasticity mechanisms, such as long-term potentiation (LTP) and depression (LTD), are thought to be the cellular substrates of long-term modifications of memory-related circuits [85–89]. There are only a handful of studies that have attempted to demonstrate a link between network events during sleep and plasticity measures. In humans, the variability in LTP-like or LTD-like induction using electrical nerve stimulation paired with transcranial magnetic stimulation correlates with inter-individual variability in local changes in slow spindles and the efficacy of the paired stimulation the next day [90]. In mice, Durkin et al. used an in vivo model of cortical plasticity induced by visual experience (orientation-specific response potentiation, OSRP) and showed that optogenetic disruption of corticothalamic activity reduces both spindles and SWA. This manipulation impairs OSRP and the potentiation of neuronal responses in the visual cortex during NREM sleep post-experience [63]. Using a combination of in vivo intracellular and in vitro recordings in the cat neocortex, Chauvette et al. demonstrated that thalamocortical activity during NREM sleep can trigger cortical LTP-like responses [91]. A direct link between spindle activity and plasticity has been demonstrated in vitro by Rosanova et al. [40]. Stimulating slices of the somatosensory cortex with naturalistic spindle stimuli derived from intracellular recordings in vivo induces both short- term potentiation (STP) and LTP ([40] and figure 1d). Finally, experience-dependent increase in spindles during NREM sleep correlates with increased phosphorylation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) during REM sleep in the hippocampus [94], suggesting that plasticity initiated during NREM spindles may carry on across sleep stages.
In conclusion, spindles are crucial for the consolidation of several types of memories (i.e. declarative and procedural), as well as for the development of thalamocortical circuits and cortical functional maps, suggesting a common underlying physiological mechanism. However, how neuronal plasticity is induced and regulated during spindles in vivo remains to be determined.
3. Spindles' role in synaptic plasticity and memory: cellular, local circuits and systems
While there is now a good understanding of how spindles are generated in the thalamus, the physiology of spindles in the cortex is much less clear. Resolving this question is of prime importance to understand the role of spindles in memory and plasticity. This can only be achieved by a combination of techniques investigating intracellular and network-level properties associated with spindles, and how they relate to memory.
(a). Local: cortical activity during spindles
(i). Electrophysiological recordings
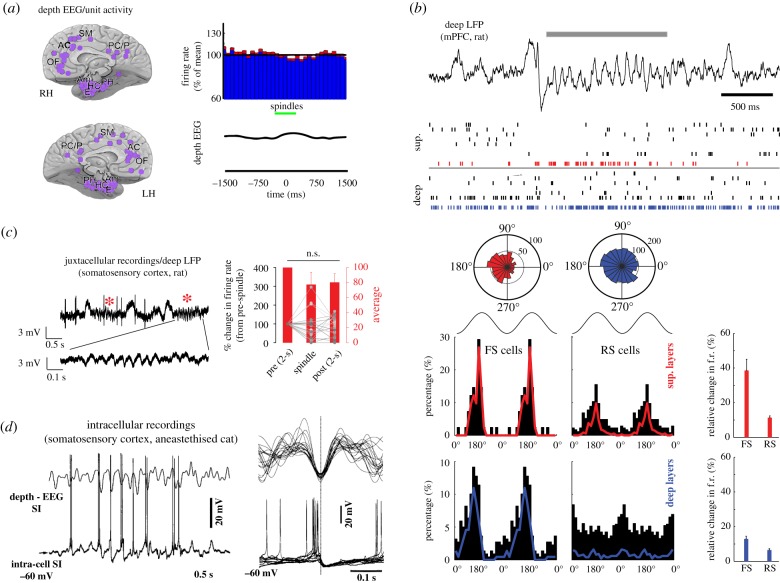
Given that thalamic neurons burst during spindles, cortical neurons must receive strong and synchronized barrage of excitatory inputs that may in turn trigger plasticity in the form of LTP [49] and augmented neuronal responses [50]. However, in vivo electrophysiological recordings in humans and rodents reveal a more complex picture. In humans, cortical neuronal spiking shows little change, if any, during spindles ([43] and figure 2a). While Andrillon et al. [43] studied more than 200 units in various brain regions, the different layers and types of neurons recorded were not specified as determining the precise location of microwires used for intracerebral human electrophysiology is generally a near-impossible task. Using extracellular recordings in naturally sleeping rats, Peyrache et al. [92] showed that spindles entrain most, but not all cortical neurons in the medial prefrontal cortex (mPFC). Specifically, inhibitory neurons across all layers show increased firing rate and phase-locking to spindles. Interestingly, while the majority of superficial excitatory neurons is modulated by spindles, spiking of excitatory neurons in deep layers (Layers 5/6) is indifferent to spindle occurrence and phase ([92] and figure 2b). The lack of modulation of deep layer excitatory neurons by spindles was confirmed in the somatosensory cortex of head-fixed rats using juxtacellular recording ([31] and figure 2c).
Figure 2.
Inhibition of cortical activity during spindles. (a) Depth EEG/unit activity recordings in various brain regions (purple circles) in humans. Average cortical firing rate (across various depths and brain regions) during spindles (green bar) (adapted from [43]). (b) Entrainment of regular spiking (RS, putative excitatory) and fast spiking (FS, putative inhibitory) neurons during spindles in the superficial and deep layers of rat medial prefrontal cortex (mPFC). Top trace and raster: example of a spindle event (grey bar) and associated firing in the superficial and deep layers of the mPFC. The red and blue dots in the raster indicate spike times of two putative FS neurons in the superficial (sup.) and deep layers, respectively. Polar histograms show the distribution of the phases of these two neurons relative to all spindles. Bottom left: distribution of preferred phases to spindles for all neurons across layers and cell types (black histogram) and only for the significantly modulated neurons (colour histograms). Bottom right: changes in firing rate (f.r.) during spindles relative to NREM sleep across cell types and layers (adapted from [92]). (c) Average change in firing rate peri-spindles (red stars) of L5 pyramidal neurons in the somatosensory cortex of head-fixed rats (adapted from [31]). (d) Intracellular recordings of pyramidal neurons activity during spindles in the primary somatosensory cortex (SI) of anaesthetized cats. Left: example of spindles sequence recorded with depth electroencephalogram (depth - EEG) together with neuronal discharge. Right: superimposition of 10 spindle cycles of the same cell. Traces were aligned to negative peaks of depth EEG. Note the excitatory post-synaptic potential followed by inhibitory post-synaptic potential followed by IPSP during spindles (adapted from [93]).
Thalamic inputs to the cortex recruit strong inhibition [95–98], especially by directly activating fast-spiking (FS), putative parvalbumin-positive (PV+) basket cells [95,98]. During spindles, FS cells increase their firing rates and are phase-locked to the oscillations [92,99]. Furthermore, the observation that deep layer pyramidal neurons are not modulated by spindles is intriguing as Layer 5 (L5) neurons receive a direct input from thalamocortical neurons [100]. One explanation is that L5 pyramidal neurons, dominated by somatic inhibition, may be indirectly silenced during spindles. This hypothesis is supported by in vivo intracellular recordings in anaesthetized cats showing inhibitory post-synaptic potentials during spontaneous spindles in the cortex ([93] and figure 2d). The reason why somatic inhibition of L5 pyramidal neurons is promoted during spindles is not clear, but computational modelling suggests that this could facilitate activation of dendrites without triggering excessive spiking during spindles ([49,93] and figure 3a). Recent Ca2+ imaging studies have provided evidence that spindles may, in fact, trigger cell- and layer-specific activation in accordance with this model (reviewed in [102,103]).
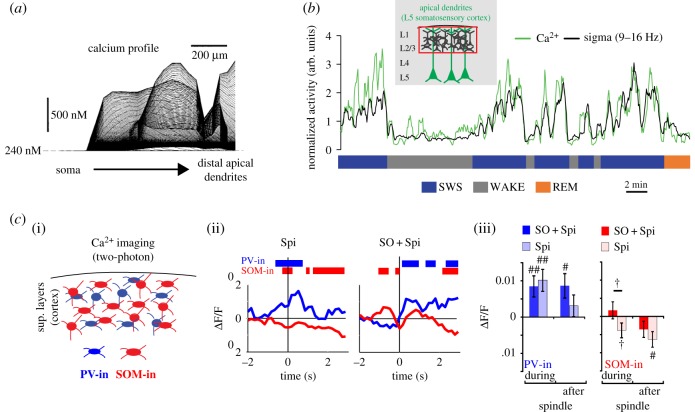
Figure 3.
Cortical microcircuit regulation during spindle activity. (a) Model of predicted deep layers (L5/6) somatic and dendritic intracellular Ca2+ activity during spindles. Instantaneous profiles taken every 0.2 ms are superimposed. Note the high Ca2+ activity only in dendrites and not in soma (adapted from [93]). (b) In vivo Ca2+ imaging (photometry) in apical dendrites of L5 neurons (somatosensory cortex) in freely behaving and sleeping rats. Superimposed activity of population of dendrites and sigma power for an approximately 30 min recording in a freely moving rat (adapted from [31]). (c) (i) In vivo Ca2+ imaging (two-photon) of cortical inhibitory neuron subpopulations (PV-in: parvalbumin inhibitory; SOM-in: somatostatin inhibitory) in superficial layers of the cortex. (ii) Mean Ca2+ activity aligned to spindles with or without SO coupling. Bars indicate significant differences compared to baseline. (iii) Ca2+ activity changes during and after spindle occurrence (adapted from [101]).
(ii). Calcium imaging
The advent of optical techniques, such as Ca2+ imaging, has provided new opportunities to monitor brain activity during natural sleep in rodents [103]. Unlike electrophysiology, Ca2+ imaging has the advantage of enabling activity monitoring in selected neuronal populations (e.g. inhibitory subpopulations) and compartments (e.g. dendrites). Imaging of pyramidal and inhibitory neurons at different cortical depths has confirmed the complex regulation of cortical activity during spindles. Using one- and two-photon imaging, Seibt et al. imaged Ca2+ activity in populations and single dendrites of L5 pyramidal neurons in rodents. They found that large populations of dendrites increase their activity and synchronization during spindle-rich oscillations ([31] and figure 3b), supporting the dendritic-centred model proposed by Contreras et al. [93]. Using two-photon imaging, Niethard et al. confirmed the electrophysiological findings, suggesting that activity in excitatory and PV+ inhibitory neurons in superficial layers (L2/3) of the cortex correlates with spindle activity ([101] and figure 3c). This latter study further showed that excitatory activity is specifically increased in spindles coupled with SOs while activity in PV+ interneurons is modulated by spindles in general. By contrast, somatostatin positive (SOM+) interneurons in the superficial layers of the cortex show a negative correlation with spindles. Because SOM+ interneurons are known to preferentially target dendrites [104], these results suggest that dendrites are disinhibited during spindles, providing a mechanism underlying increased dendritic activity at the time of spindle occurrence [49,93]. Although imaging approaches suggest that PV+ activity is increased during spindles, this has been characterized only in superficial layers. It remains to be determined if this trend is also found in the deep layers which would support the hypothesis that spindles trigger perisomatic inhibition of L5 neurons.
(b). Systems: coupling of forebrain oscillations during non-rapid eye movement sleep
One of the best examples of the contribution of spindles to learning is their postulated role in systems consolidation of declarative memories. During NREM sleep, thalamocortical spindles are co-modulated with hippocampal activity, and this coordination is believed to be essential for systems consolidation. Specifically, declarative memories are initially dependent on the integrity of the hippocampus and this dependency gradually declines as memories are consolidated in the neocortex [25,105–109]. During NREM sleep, hippocampal activity is dominated by SWRs, transient events of high neuronal synchronization, associated with a fast (200 Hz) and short-lasting (50–300 ms) oscillations in the LFP ([110] and figure 4a). SWRs during sleep are critical for memory consolidation [25,112,113] and the coupling between spindles, SOs and SWRs supports sleep-dependent memory consolidation [15,114].
Figure 4.
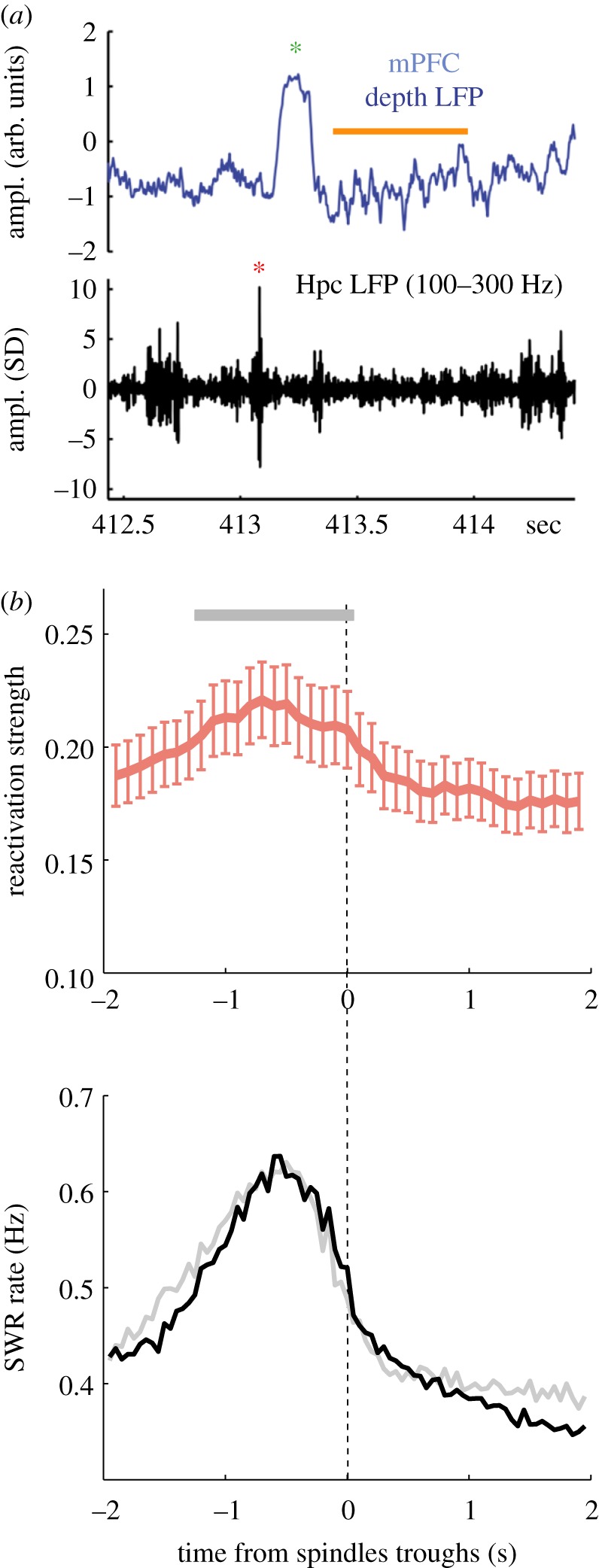
SWR and spindles comodulation and link to replay. (a) Example traces of LFP recorded simultaneously in the medial prefrontal cortex (mPFC, top, broadband) and in the CA1 pyramidal layer of the hippocampus (Hpc, bottom, filtered in the SWR-frequency range) (adapted from Peyrache et al. [92]). Red and green asterisks indicate a SWR and a SW, respectively. Spindles are highlighted with an orange bar. (b) Relationship between spindles and reactivation in the mPFC. Top: spindle-trough time average (± s.e.m.) of reactivation strength of neuronal ensembles during sleep following learning; bottom, same as top for SWR occurrence rate (adapted from [111]).
(i). Hippocampal sharp wave-ripples and the consolidation of declarative memories
During NREM sleep, patterns of neuronal population activity in the hippocampus that form with experience are spontaneously ‘replayed' (or ‘reactivated') (for review, see [115,116]), especially at times of SWRs [27,117]. This replay of population activity is critical for the consolidation of hippocampus-dependent memories [25,110] as demonstrated by direct manipulation of SWRs [112,113]. SWRs are associated with replay of activity in the neocortex, especially in the main output structures of the hippocampus such as entorhinal cortex [118,119] but see [120]), prefrontal cortex [111], striatum [121,122] and amygdala [123]. Interestingly, these brain areas are all well known for their involvement in various forms of learning. As discussed above, learning induces changes in the incidence rate of spindles and SOs (see §2b). Similarly, the occurrence rate of SWRs during NREM sleep increases after learning [124–126]. Overall, these observations suggest that learning is associated with physiological changes (e.g. cell's excitability) that directly affect NREM oscillations characteristics.
(ii). Coupling slow oscillations, spindles and sharp wave-ripples across brain regions
Declarative memories, especially episodic memories, are supposed to entail broadly distributed networks of neurons across sensory modalities [127]. Large-scale interaction between thalamocortical activity and SWRs during NREM sleep is a possible mechanism that coordinates widely distributed neuronal ensembles to promote memory consolidation [15,114].
The coordination between SWRs and spindles was first evidenced by Siapas & Wilson [128]. In this seminal study, the authors performed simultaneous recordings of the mPFC as well as CA1 region of the hippocampus and revealed that SWRs tended to occur at the onset of spindle episodes detected in mPFC ([92,128,129] and figure 4a,b). While SWRs and spindles tend to co-occur at slow timescales (approx. 10 s) irrespective of the cortical regions where spindles are detected [92,128,130], the sequential ordering of SWRs followed by spindles at faster timescale (approx. 1 s) is less pronounced in parietal cortex [130]. This could result from the relative asynchrony of frontal spindles with other brain regions, as observed in rodents [129] and humans [43].
One difficulty in assessing the coordination between SWRs and spindles is that they are both co-modulated with SOs [9,129–132]. Whether the coupling between SWRs and spindles is mediated by cortical inputs to the hippocampus [130] or by a direct communication between the thalamus and the hippocampus remains an open question. Specifically, SWRs may phase-lock to spindles by direct entorhinal projection to CA1, or indirectly via the perforant path through the dentate gyrus and CA3, both areas showing high modulation by spindles [133]. Yet, recent evidence has pointed out a complex coordination between the thalamus and SWRs [134–136], and possibly an active contribution of the thalamus to the timing of SWRs [135]. Although cortical response to SWRs during spindles is strongly diminished [92], hippocampal replay at the time of SWRs is strengthened during spindles [134]. However, the view that SWRs are ‘nested' within spindles is not necessarily correct. While SWRs are phase-locked to ongoing spindles [130], only a small proportion of SWRs do occur during spindles, at least in the prefrontal regions [92].
By using the high-channel intra-EEG recordings performed in pre-operative epileptic patients, a growing body of work focuses on the relationship between thalamocortical and hippocampal oscillations. In humans, hippocampal SWRs have recently been associated with memory functions [137,138]. Furthermore, SOs, spindles and SWRs are co-modulated, recapitulating results from the rodent literature [139,140]. As observed in rats, SWRs tend to precede frontal spindles and the delay between SWRs and spindle is less pronounced for parietal spindle [139]. Whether this reflects a ‘propagation' of SWR-triggered spindles from frontal to parietal cortex [129] remains an open question. Human studies are still in their infancy but this fast-growing field will make it possible to bridge the gap between animal research and human cognitive science.
4. The sequential hypothesis of hippocampo-cortical coupling for memory
In the previous sections, we have described (i) how spindles are associated with brain plasticity mechanisms and how they are linked with local cortical activity modulation, and (ii) how the coupling of spindles with other NREM oscillations promotes different stages of memory formation. In this section, we merge those functions and propose a model whereby spindles are mediators of local synaptic plasticity in the cortex following (and not during) network reactivation of the hippocampo-cortical network in the context of declarative memory formation.
(a). The temporal aspect of sharp wave-ripples/spindle coupling
While SWRs are intimately associated with reactivation of neocortical ensembles, simply ‘re-activating' neocortical neurons across brain regions may not be sufficient to reinforce mutual coupling between neurons and hence create a stable memory trace. Reactivation may favour a form of tagging of the synapses that are recruited during the reinstatement of the associated neuronal pattern, and the occurrence of spindles following reactivation events is perfectly suited for the synaptic consolidation of these traces. The role of NREM oscillation coupling in sleep-dependent memory consolidation was recently addressed by directly manipulating the temporal relationship between these events. By electrically stimulating the neocortex immediately after SWRs, Maingret et al. [141] showed that the SO phase is reset, leading to increased incidence of SWR-spindle sequences. Performing this stimulation during sleep following a hippocampus-dependent memory task enhances memory performance the following day [141].
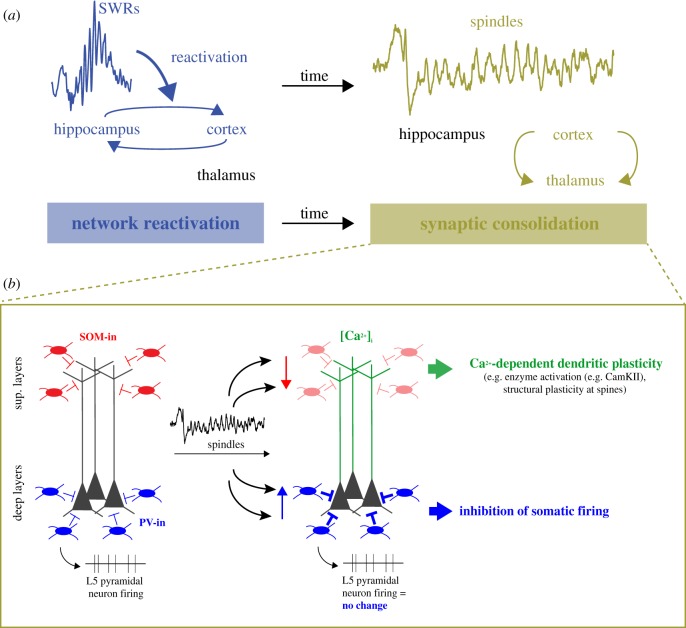
In the mPFC, neuronal reactivation is maximal prior to spindles [111] and this relationship is fully explained by the temporal coordination between SWRs and spindles (figure 4b). In this particular case, this observation rules out any specific role of spindles in reactivation, and points instead to a specific coupling between mPFC reactivation and hippocampal SWRs. Unlike other neocortical areas, the mPFC in rats receives direct input from the hippocampus [142] and plays a key role in the systems consolidation of hippocampal-dependent memories [143]. Overall, these results suggest that the sequential occurrence of SWRs and frontal spindles is important for the consolidation of hippocampus-dependent memories. According to the proposed hypothesis, synapses that undergo plasticity during spindles (‘synaptic consolidation', figure 5a) are part of the neuronal ensembles reinstated during preceding SWRs (‘network reactivation', figure 5a). Hence, the role of spindles for hippocampal-dependent memories would be similar to any other form of spindle-based learning. The only difference is that the consolidation of declarative memories in the neocortex additionally requires the hippocampus to reactivate neuronal ensembles before synapse-specific plasticity takes place.
Figure 5.
The sequential hypothesis of hippocampo-cortical coupling and plasticity role of spindles. (a) Illustration of the sequential hypothesis of hippocampo-cortical coupling. Reactivation and hippocampo-cortical network interactions during SWRs are followed by spindle events during which synaptic communication in those networks are modified via synaptic plasticity mechanisms. (b) Model of cortical circuit activity regulation of deep layers pyramidal neurons during spindles based on Ca2+ imaging, electrophysiological and computational data. During spindles, dendrites are disinhibited via decreased activity of somatostatin inhibitory (SOM-In) neurons while somatic spiking is inhibited via increase activity of parvalbumin inhibitory (PV-In) neurons. This would lead to Ca2+-dependent plasticity processes (e.g. CaMKII activation) occurring specifically in dendrites.
Several aspects of the relationship between NREM oscillations and learning remain largely unexplored. First, other studies have reported reactivation of neuronal ensembles during and not prior to spindles (e.g. [144]). These reactivations were observed in the motor cortex after training on a motor task, and similar increases in spindles have been reported in humans [22,53,55]. As discussed above, it is possible that NREM dynamics of oscillatory coupling differ between mPFC and other brain areas. Further investigations are required to address the similarities and differences between spindles and single-cell activity in different thalamocortical networks, brain regions and learning tasks. Another interesting aspect of SWR-spindle coupling is that their temporal relationship spans orders of magnitude in timescales, with SWRs preceding not only individual spindle events (at least in the mPFC) but also NREM bouts of elevated spindle event occurrence (at a timescale of approx. 10 s) [92,128]. The exact role of this slow temporal coordination remains unknown. One possibility is that successive SWRs convey related messages, as observed during awake replay events [145], and thus need orchestration at a slower timescale for coherent consolidation of extended memories.
(b). Spindles, a window for plasticity?
The occurrence of spindles following SWRs may be particularly beneficial for triggering synaptic plasticity consolidation in reactivated networks (figure 5b). However, processing inputs while the network is undergoing plasticity could interfere with the consolidation of the memory trace. Interestingly, evidence suggests that the brain becomes more unresponsive to external stimuli during spindles. Combined EEG and functional magnetic resonance imaging measures in humans have shown that acoustic stimulation during spindles fails to evoke a brain response similar to the one observed outside spindles during NREM sleep [146,147]. In mice, arousal threshold to auditory stimuli is higher during periods of high sigma power [148]. Electrophysiological recordings in the mPFC in rats also show disengagement of cortical neuronal activity from hippocampal inputs during spindles [92] suggesting that, in addition to the external inputs, the cortex is also disconnected from some intrinsic signals. However, nociceptive signals are still transmitted to the cortex [149], which may be explained for obvious survival needs. Furthermore, contrary to what human studies predicted, neurons of the primary auditory cortex of rats respond in a similar way to auditory tone during and outside spindles [150] although it is unclear if all cortical neurons are responsive. Indeed, inhibitory control of neuronal firing during spindles is maximal in L5 pyramidal cells and not necessarily so much in superficial pyramidal neurons [92,93]. Overall, the apparent disengagement of cortical networks, especially of deep cortical circuits, to incoming stimuli during spindles sets a unique time window during which cortical neurons and networks may favour consolidation, a process that would otherwise not be optimal owing to ongoing interference.
As described in §3a, convergent electrophysiological and imaging data suggest that Ca2+ concentration increases in dendrites in response to thalamocortical bursting activity during spindles without leading to increased firing, especially in L5 pyramidal cells. This decoupling between dendrites and somatic activity most likely results from shunting inhibition by PV+ neurons and is crucial for memory. An important role for the activity of cortical PV+ neurons for memory consolidation is supported by the observation that chemogenetic inhibition of PV+ neurons in the mPFC during NREM sleep impairs memory consolidation in mice [151]. This compartmentalization of increased intracellular Ca2+ in dendrites suggests that spindles may promote non-Hebbian type of plasticity [152,153] which is accompanied by localized plasticity induction within dendrites. A variety of downstream targets and processes can be activated locally in dendrites [154] such as enzymes (e.g. CaMKII), cytoskeleton remodelling (e.g. actin dynamics) and de novo protein synthesis [19] (figure 5b). It was suggested that the frequency of Ca2+ influx could favour plasticity processes [49]. Here, CaMKII might be a particularly good candidate as its activation is sensitive to rhythmic Ca2+ influx [155,156], which may be favoured during NREM oscillations, including spindles. The type and directionality of plasticity (e.g. LTP/LTD, functional/structural plasticity) promoted during spindles is unclear but spindles have been associated with mechanisms consistent with both potentiation and depression [31,40,84,157] which may depend on their coupling with SO or delta oscillations [84]. Future experiments using selective manipulation of thalamocortical oscillations during NREM sleep [45,84] combined with cellular and molecular measures of plasticity will help address this question.
5. Discussion
Brain oscillations during NREM sleep have now been studied for nearly a century but it is only in recent decades that they have been related to specific neuronal activity and memory consolidation. The three hallmarks of NREM sleep, namely SWs, spindles and SWRs, are all associated with memory enhancement and plasticity. Their coordination is now well established and is thought to be at the basis for the consolidation of memories engaging distributed neuronal assemblies.
Spindles are a particularly interesting candidate for the mechanisms supporting memory consolidation in the neocortex. Here, we have reviewed how, at the systems level, their frequency of occurrence and other intrinsic features are associated with memory and how they are coupled with hippocampal replay, another key player in memory formation. Furthermore, at the cellular level, spindles regulate Ca2+ influx in neocortical dendrites, a necessary step to trigger mechanisms associated with plasticity. While increased dendritic activity should also lead to a massive increase in neuronal spiking, studies in L5 pyramidal cells (which are the main output channel of the neocortex) show that activity in those neurons remains seemingly undisturbed by spindles, certainly controlled by shunting inhibition. This particular regulation of cortical neuron activity would enable non-Hebbian plasticity processes to occur locally in dendrites/synapses while preventing interference with ongoing activity in the circuit. The role of dendritic electrogenesis in learning has received much attention recently [158–161], and it is interesting to speculate that some of these mechanisms may be promoted during spindles as well.
In the more specific case of hippocampal-dependent memories, mPFC spindles tend to be preceded by hippocampal SWRs which are known to convey messages related to previous waking experience. This sequential ordering of SWRs and neocortical spindles may thus constitute a mechanism whereby memories are first reactivated and then consolidated.
Interestingly, the hippocampal area that is the most modulated by spindles is the dentate gyrus [133], a key structure for memory [162,163]. The relationship between activity in the dentate gyrus and spindles remains largely unexplored but may constitute a crucial component of sleep-dependent memory consolidation.
Although our knowledge of the dynamical processes at play for memory consolidation during sleep has made remarkable progress, many questions remain unanswered. In particular, a definitive demonstration of the link between plasticity processes, replay and SWs/spindles is still lacking. The development of precise imaging tools combined with advanced molecular approaches in behaving animals is very promising. Another question that will need to be addressed is the nature of the neuronal signal transmitted from the thalamus to the cortex. It has been assumed so far that dendrites are depolarized by excitatory volleys from the thalamus, yet, it is unlikely that this excitation is random and, instead, certainly convey meaningful messages. Recent evidence suggests that the pairwise coordination of thalamic neurons is preserved in NREM and REM sleep relative to the awake state [164,165] suggesting that the cortex receives similar signals independently of brain states.
Much work on spindles still needs to be carried out. This research will not only address some fundamental questions about the role of sleep but will also open new avenues for clinical research. In fact, spindles may play a protective role in the cortex in some forms of epilepsy [166]. Furthermore, spindles are diminished in schizophrenia patients [167]) and their global coordination is impaired in mouse models of schizophrenia [129]. More generally, the covariance of spindles characteristics with neurodevelopmental and neurodegenerative disorders offers a promising use of spindles as a biomarker in neuropathology. Determining a common function to spindles (and potential differences), across species, brain modalities and developmental stages [68,168] is certainly one of the great challenges in sleep research in the next decade.
Acknowledgements
The authors thank Adrian Duszkiewicz for comments on the manuscript.
Data accessibility
This article has no additional data.
Authors' contributions
A.P. and J.S. wrote the manuscript.
Competing interests
We have no competing interests.
Funding
This work was supported by a Canadian Research Chair in Systems Neuroscience grant no. (245716), a CIHR Project grant no. (155957), a NSERC Discovery grant no. (RGPIN-2018-04600), an IRDC grant no. (108877-001) to A.P., and the University of Surrey/Braithwaites foundation and the Wellcome Trust grant no. (209099/Z/17/Z) to J.S
References
- 1.Berger H. 1929. Über das Elektrenkephalogramm des Menschen. Arch. Für Psychiatr. Nervenkrankh. 87, 527–570. ( 10.1007/BF01797193) [DOI] [Google Scholar]
- 2.Loomis AL, Harvey EN, Hobart G. 1935. Potential rhythms of the cerebral cortex during sleep. Science 81, 597–598. ( 10.1126/science.81.2111.597) [DOI] [PubMed] [Google Scholar]
- 3.Coppieters ‘t Wallant D, Maquet P, Phillips C. 2016. Sleep spindles as an electrographic element: description and automatic detection methods. Neural Plast. 2016, 6783812 ( 10.1155/2016/6783812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Gennaro L, Ferrara M. 2003. Sleep spindles: an overview. Sleep Med. Rev. 7, 423–440 ( 10.1053/smrv.2002.0252). [DOI] [PubMed] [Google Scholar]
- 5.Fernandez LMJ, Luthi A. 2019. Sleep spindles: mechanisms and functions. Physiol. Rev. 100, 805–868. ( 10.1152/physrev.00042.2018) [DOI] [PubMed] [Google Scholar]
- 6.Hobson JA, Pace-Schott EF. 2002. The cognitive neuroscience of sleep: neuronal systems, consciousness and learning. Nat. Rev. Neurosci. 3, 679–693. ( 10.1038/nrn915) [DOI] [PubMed] [Google Scholar]
- 7.Lüthi A. 2014. Sleep spindles: where they come from, what they do. Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 20, 243–256. ( 10.1177/1073858413500854) [DOI] [PubMed] [Google Scholar]
- 8.Astori S, Wimmer RD, Lüthi A. 2013. Manipulating sleep spindles: expanding views on sleep, memory, and disease. Trends Neurosci. 36, 738–748. ( 10.1016/j.tins.2013.10.001) [DOI] [PubMed] [Google Scholar]
- 9.Steriade M, McCormick DA, Sejnowski TJ. 1993. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685. ( 10.1126/science.8235588) [DOI] [PubMed] [Google Scholar]
- 10.von Krosigk M, Bal T, McCormick DA. 1993. Cellular mechanisms of a synchronized oscillation in the thalamus. Science 261, 361–364. ( 10.1126/science.8392750) [DOI] [PubMed] [Google Scholar]
- 11.Jenkins JG, Dallenbach KM. 1924. Obliviscence during sleep and waking. Am. J. Psychol. 35, 605–612. ( 10.2307/1414040) [DOI] [Google Scholar]
- 12.Maquet P. 2001. The role of sleep in learning and memory. Science 294, 1048–1052. ( 10.1126/science.1062856) [DOI] [PubMed] [Google Scholar]
- 13.Navarro-Lobato I, Genzel L. 2018. The up and down of sleep: from molecules to electrophysiology. Neurobiol. Learn. Mem. 160, 3–10. ( 10.1016/j.nlm.2018.03.013) [DOI] [PubMed] [Google Scholar]
- 14.Puentes-Mestril C, Roach J, Niethard N, Zochowski M, Aton SJ. 2019. How rhythms of the sleeping brain tune memory and synaptic plasticity. Sleep 42, zsz095 ( 10.1093/sleep/zsz095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasch B, Born J. 2013. About sleep's role in memory. Physiol. Rev. 93, 681–766. ( 10.1152/physrev.00032.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stickgold R, Fosse R, Walker MP. 2002. Linking brain and behavior in sleep-dependent learning and memory consolidation. Proc. Natl Acad. Sci. USA 99, 16 519–16 521. ( 10.1073/pnas.012689199) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stickgold R, Walker MP. 2013. Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci. 16, 139–145. ( 10.1038/nn.3303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacroix MM, de Lavilléon G, Lefort J, Kanbi KE, Bagur S, Laventure S, Dauvilliers Y, Peyron C, Benchenane K.2018. Improved sleep scoring in mice reveals human-like stages. bioRxiv 489005. ( ) [DOI]
- 19.Seibt J, Frank MG. 2019. Primed to sleep: the dynamics of synaptic plasticity across brain states. Front. Syst. Neurosci. 13, 2 ( 10.3389/fnsys.2019.00002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tononi G, Cirelli C. 2014. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34. ( 10.1016/j.neuron.2013.12.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall L, Helgadóttir H, Mölle M, Born J. 2006. Boosting slow oscillations during sleep potentiates memory. Nature 444, 610–613. ( 10.1038/nature05278) [DOI] [PubMed] [Google Scholar]
- 22.Fogel SM, Smith CT. 2006. Learning-dependent changes in sleep spindles and Stage 2 sleep. J. Sleep Res. 15, 250–255. ( 10.1111/j.1365-2869.2006.00522.x) [DOI] [PubMed] [Google Scholar]
- 23.Schabus M, Gruber G, Parapatics S, Sauter C, Klösch G, Anderer P, Klimesch W, Saletu B, Zeitlhofer J. 2004. Sleep spindles and their significance for declarative memory consolidation. Sleep 27, 1479–1485. ( 10.1093/sleep/27.7.1479) [DOI] [PubMed] [Google Scholar]
- 24.Ulrich D. 2016. Sleep spindles as facilitators of memory formation and learning. Neural Plast. 2016, 1796715 ( 10.1155/2016/1796715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buzsáki G. 1989. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. ( 10.1016/0306-4522(89)90423-5) [DOI] [PubMed] [Google Scholar]
- 26.Pavlides C, Winson J. 1989. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. Off. J. Soc. Neurosci. 9, 2907–2918 ( 10.1523/JNEUROSCI.09-08-02907.1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. ( 10.1126/science.8036517) [DOI] [PubMed] [Google Scholar]
- 28.Genzel L, Kroes MCW, Dresler M, Battaglia FP. 2014. Light sleep versus slow wave sleep in memory consolidation: a question of global versus local processes? Trends Neurosci. 37, 10–19. ( 10.1016/j.tins.2013.10.002) [DOI] [PubMed] [Google Scholar]
- 29.Dijk DJ, Hayes B, Czeisler CA. 1993. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 626, 190–199 ( 10.1016/0006-8993(93)90579-C). [DOI] [PubMed] [Google Scholar]
- 30.Gottesmann C. 1996. The transition from slow-wave sleep to paradoxical sleep: evolving facts and concepts of the neurophysiological processes underlying the intermediate stage of sleep. Neurosci. Biobehav. Rev. 20, 367–387 ( 10.1016/0149-7634(95)00055-0). [DOI] [PubMed] [Google Scholar]
- 31.Seibt J, Richard CJ, Sigl-Glöckner J, Takahashi N, Kaplan DI, Doron G, de Limoges D, Bocklisch C, Larkum ME. 2017. Cortical dendritic activity correlates with spindle-rich oscillations during sleep in rodents. Nat. Commun. 8, 684 ( 10.1038/s41467-017-00735-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson BO, Levenstein D, Greene JP, Gelinas JN, Buzsáki G. 2016. Network homeostasis and state dynamics of neocortical sleep. Neuron 90, 839–852. ( 10.1016/j.neuron.2016.03.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clawson BC, Durkin J, Aton SJ. 2016. Form and function of sleep spindles across the lifespan. Neural Plast. 2016, 6936381 ( 10.1155/2016/6936381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crunelli V, Lőrincz ML, Connelly WM, David F, Hughes SW, Lambert RC, Leresche N, Errington AC. 2018. Dual function of thalamic low-vigilance state oscillations: rhythm-regulation and plasticity. Nat. Rev. Neurosci. 19, 107–118. ( 10.1038/nrn.2017.151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crunelli V, Hughes SW. 2010. The slow (<1 Hz) rhythm of non-REM sleep: a dialogue between three cardinal oscillators. Nat. Neurosci. 13, 9–17. ( 10.1038/nn.2445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCormick DA, Bal T. 1997. Sleep and arousal: thalamocortical mechanisms. Annu. Rev. Neurosci. 20, 185–215. ( 10.1146/annurev.neuro.20.1.185) [DOI] [PubMed] [Google Scholar]
- 37.Dehghani N, Cash SS, Rossetti AO, Chen CC, Halgren E. 2010. Magnetoencephalography demonstrates multiple asynchronous generators during human sleep spindles. J. Neurophysiol. 104, 179–188. ( 10.1152/jn.00198.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gais S, Mölle M, Helms K, Born J. 2002. Learning-dependent increases in sleep spindle density. J. Neurosci. Off. J. Soc. Neurosci. 22, 6830–6834. (doi:20026697). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eschenko O, Mölle M, Born J, Sara SJ. 2006. Elevated sleep spindle density after learning or after retrieval in rats. J. Neurosci. Off. J. Soc. Neurosci. 26, 12 914–12 920. ( 10.1523/JNEUROSCI.3175-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosanova M, Ulrich D. 2005. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J. Neurosci. Off. J. Soc. Neurosci. 25, 9398–9405. ( 10.1523/JNEUROSCI.2149-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. 1996. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science 274, 771–774. ( 10.1126/science.274.5288.771) [DOI] [PubMed] [Google Scholar]
- 42.Buzsáki G, Logothetis N, Singer W. 2013. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron 80, 751–764. ( 10.1016/j.neuron.2013.10.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrillon T, Nir Y, Staba RJ, Ferrarelli F, Cirelli C, Tononi G, Fried I. 2011. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J. Neurosci. Off. J. Soc. Neurosci. 31, 17 821–17 834. ( 10.1523/JNEUROSCI.2604-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mölle M, Eschenko O, Gais S, Sara SJ, Born J. 2009. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur. J. Neurosci. 29, 1071–1081. ( 10.1111/j.1460-9568.2009.06654.x) [DOI] [PubMed] [Google Scholar]
- 45.Fernandez LM, Vantomme G, Osorio-Forero A, Cardis R, Béard E, Lüthi A. 2018. Thalamic reticular control of local sleep in mouse sensory cortex. eLife 7, e39111 ( 10.7554/eLife.39111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D, Hwang E, Lee M, Sung H, Choi JH. 2015. Characterization of topographically specific sleep spindles in mice. Sleep 38, 85–96. ( 10.5665/sleep.4330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frauscher B, von Ellenrieder N, Dubeau F, Gotman J. 2015. Scalp spindles are associated with widespread intracranial activity with unexpectedly low synchrony. Neuroimage 105, 1–12. ( 10.1016/j.neuroimage.2014.10.048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nir Y, Staba RJ, Andrillon T, Vyazovskiy VV, Cirelli C, Fried I, Tononi G. 2011. Regional slow waves and spindles in human sleep. Neuron 70, 153–169. ( 10.1016/j.neuron.2011.02.043) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sejnowski TJ, Destexhe A. 2000. Why do we sleep? Brain Res. 886, 208–223 ( 10.1016/S0006-8993(00)03007-9). [DOI] [PubMed] [Google Scholar]
- 50.Steriade M, Timofeev I. 2003. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron 37, 563–576 ( 10.1016/S0896-6273(03)00065-5). [DOI] [PubMed] [Google Scholar]
- 51.Barakat M, et al. 2013. Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum. Brain Mapp. 34, 2918–2928. ( 10.1002/hbm.22116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clemens Z, Fabó D, Halász P. 2005. Overnight verbal memory retention correlates with the number of sleep spindles. Neuroscience 132, 529–535. ( 10.1016/j.neuroscience.2005.01.011) [DOI] [PubMed] [Google Scholar]
- 53.Laventure S, et al. 2016. NREM2 and sleep spindles are instrumental to the consolidation of motor sequence memories. PLoS Biol. 14, e1002429 ( 10.1371/journal.pbio.1002429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meier-Koll A, Bussmann B, Schmidt C, Neuschwander D. 1999. Walking through a maze alters the architecture of sleep. Percept. Mot. Skills 88, 1141–1159. ( 10.2466/pms.1999.88.3c.1141) [DOI] [PubMed] [Google Scholar]
- 55.Nishida M, Walker MP. 2007. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS ONE 2, e341 ( 10.1371/journal.pone.0000341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ackermann S, Hartmann F, Papassotiropoulos A, de Quervain DJ-F, Rasch B. 2015. No associations between interindividual differences in sleep parameters and episodic memory consolidation. Sleep 38, 951–959. ( 10.5665/sleep.4748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. 2012. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage 59, 2733–2742. ( 10.1016/j.neuroimage.2011.10.036) [DOI] [PubMed] [Google Scholar]
- 58.Cox R, Hofman WF, de Boer M, Talamini LM. 2014. Local sleep spindle modulations in relation to specific memory cues. Neuroimage 99, 103–110. ( 10.1016/j.neuroimage.2014.05.028) [DOI] [PubMed] [Google Scholar]
- 59.Jegou A, et al. 2019. Cortical reactivations during sleep spindles following declarative learning. Neuroimage 195, 104–112. ( 10.1016/j.neuroimage.2019.03.051) [DOI] [PubMed] [Google Scholar]
- 60.Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. 2012. Sleep spindles are locally modulated by training on a brain-computer interface. Proc. Natl Acad. Sci. USA 109, 18 583–18 588. ( 10.1073/pnas.1207532109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, Tononi G. 2006. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nat. Neurosci. 9, 1169–1176. ( 10.1038/nn1758) [DOI] [PubMed] [Google Scholar]
- 62.Huber R, Ghilardi MF, Massimini M, Tononi G. 2004. Local sleep and learning. Nature 430, 78–81. ( 10.1038/nature02663) [DOI] [PubMed] [Google Scholar]
- 63.Durkin J, Suresh AK, Colbath J, Broussard C, Wu J, Zochowski M, Aton SJ. 2017. Cortically coordinated NREM thalamocortical oscillations play an essential, instructive role in visual system plasticity. Proc. Natl Acad. Sci. USA 114, 10 485–10 490. ( 10.1073/pnas.1710613114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vyazovskiy V, Borbély AA, Tobler I. 2000. Unilateral vibrissae stimulation during waking induces interhemispheric EEG asymmetry during subsequent sleep in the rat. J. Sleep Res. 9, 367–371 ( 10.1046/j.1365-2869.2000.00230.x). [DOI] [PubMed] [Google Scholar]
- 65.Aton SJ, Suresh A, Broussard C, Frank MG. 2014. Sleep promotes cortical response potentiation following visual experience. Sleep 37, 1163–1170. ( 10.5665/sleep.3830) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aton SJ, Broussard C, Dumoulin M, Seibt J, Watson A, Coleman T, Frank MG. 2013. Visual experience and subsequent sleep induce sequential plastic changes in putative inhibitory and excitatory cortical neurons. Proc. Natl Acad. Sci. USA 110, 3101–3106. ( 10.1073/pnas.1208093110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hanganu IL, Ben-Ari Y, Khazipov R. 2006. Retinal waves trigger spindle bursts in the neonatal rat visual cortex. J. Neurosci. Off. J. Soc. Neurosci. 26, 6728–6736. ( 10.1523/JNEUROSCI.0752-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. 2004. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature 432, 758–761. ( 10.1038/nature03132) [DOI] [PubMed] [Google Scholar]
- 69.Lindemann C, Ahlbeck J, Bitzenhofer SH, Hanganu-Opatz IL. 2016. Spindle activity orchestrates plasticity during development and sleep. Neural Plast. 2016, 5787423 ( 10.1155/2016/5787423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gruber R, Wise MS. 2016. Sleep spindle characteristics in children with neurodevelopmental disorders and their relation to cognition. Neural Plast. 2016, 4724792 ( 10.1155/2016/4724792) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Manoach DS, Pan JQ, Purcell SM, Stickgold R. 2016. Reduced sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol. Psychiatry 80, 599–608. ( 10.1016/j.biopsych.2015.10.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winsky-Sommerer R, de Oliveira P, Loomis S, Wafford K, Dijk D-J, Gilmour G. 2018. Disturbances of sleep quality, timing and structure and their relationship with other neuropsychiatric symptoms in Alzheimer's disease and schizophrenia: insights from studies in patient populations and animal models. Neurosci. Biobehav. Rev. 97, 112–137. ( 10.1016/j.neubiorev.2018.09.027) [DOI] [PubMed] [Google Scholar]
- 73.Cellini N, Mednick SC. 2019. Stimulating the sleeping brain: current approaches to modulating memory-related sleep physiology. J. Neurosci. Methods 316, 125–136. ( 10.1016/j.jneumeth.2018.11.011) [DOI] [PubMed] [Google Scholar]
- 74.Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, Fröhlich F. 2016. Feedback-controlled transcranial alternating current stimulation reveals a functional role of sleep spindles in motor memory consolidation. Curr. Biol. 26, 2127–2136. ( 10.1016/j.cub.2016.06.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niknazar M, Krishnan GP, Bazhenov M, Mednick SC. 2015. Coupling of thalamocortical sleep oscillations are important for memory consolidation in humans. PLoS ONE 10, e0144720 ( 10.1371/journal.pone.0144720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rasch B, Büchel C, Gais S, Born J. 2007. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science 315, 1426–1429. ( 10.1126/science.1138581) [DOI] [PubMed] [Google Scholar]
- 77.Rudoy JD, Voss JL, Westerberg CE, Paller KA. 2009. Strengthening individual memories by reactivating them during sleep. Science 326, 1079 ( 10.1126/science.1179013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cairney SA, Guttesen AÁV, El Marj N, Staresina BP. 2018. Memory consolidation is linked to spindle-mediated information processing during sleep. Curr. Biol. 28, 948–954. e4. ( 10.1016/j.cub.2018.01.087.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rihm JS, Diekelmann S, Born J, Rasch B. 2014. Reactivating memories during sleep by odors: odor specificity and associated changes in sleep oscillations. J. Cogn. Neurosci. 26, 1806–1818. ( 10.1162/jocn_a_00579) [DOI] [PubMed] [Google Scholar]
- 80.Perrault AA, Khani A, Quairiaux C, Kompotis K, Franken P, Muhlethaler M, Schwartz S, Bayer L. 2019. Whole-night continuous rocking entrains spontaneous neural oscillations with benefits for sleep and memory. Curr. Biol. 29, 402–411.e3. ( 10.1016/j.cub.2018.12.028) [DOI] [PubMed] [Google Scholar]
- 81.Kompotis K, Hubbard J, Emmenegger Y, Perrault A, Mühlethaler M, Schwartz S, Bayer L, Franken P. 2019. Rocking promotes sleep in mice through rhythmic stimulation of the vestibular system. Curr. Biol. 29, 392–401.e4. ( 10.1016/j.cub.2018.12.007) [DOI] [PubMed] [Google Scholar]
- 82.Latchoumane C-FV, Ngo H-VV, Born J, Shin H-S. 2017. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435.e6. ( 10.1016/j.neuron.2017.06.025) [DOI] [PubMed] [Google Scholar]
- 83.Kim A, Latchoumane C, Lee S, Kim GB, Cheong E, Augustine GJ, Shin H-S. 2012. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc. Natl Acad. Sci. USA 109, 20 673–20 678. ( 10.1073/pnas.1217897109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Gulati T, Ganguly K. 2019. Competing roles of slow oscillations and delta waves in memory consolidation versus forgetting. Cell 179, 514–526.e13. ( 10.1016/j.cell.2019.08.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bear MF, Abraham WC. 1996. Long-term depression in hippocampus. Annu. Rev. Neurosci. 19, 437–462. ( 10.1146/annurev.ne.19.030196.002253) [DOI] [PubMed] [Google Scholar]
- 86.Malenka RC, Nicoll RA. 1999. Long-term potentiation: a decade of progress? Science 285, 1870–1874. ( 10.1126/science.285.5435.1870) [DOI] [PubMed] [Google Scholar]
- 87.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. 2014. Engineering a memory with LTD and LTP. Nature 511, 348–352. ( 10.1038/nature13294) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Takeuchi T, Duszkiewicz AJ, Morris RGM. 2014. The synaptic plasticity and memory hypothesis: encoding, storage and persistence. Phil. Trans. R. Soc. B 369, 20130288 ( 10.1098/rstb.2013.0288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. 2006. Learning induces long-term potentiation in the hippocampus. Science 313, 1093–1097. ( 10.1126/science.1128134) [DOI] [PubMed] [Google Scholar]
- 90.Bergmann TO, Mölle M, Marshall L, Kaya-Yildiz L, Born J, Roman Siebner H. 2008. A local signature of LTP- and LTD-like plasticity in human NREM sleep. Eur. J. Neurosci. 27, 2241–2249. ( 10.1111/j.1460-9568.2008.06178.x) [DOI] [PubMed] [Google Scholar]
- 91.Chauvette S, Seigneur J, Timofeev I. 2012. Sleep oscillations in the thalamocortical system induce long-term neuronal plasticity. Neuron 75, 1105–1113. ( 10.1016/j.neuron.2012.08.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peyrache A, Battaglia FP, Destexhe A. 2011. Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs. Proc. Natl Acad. Sci. USA 108, 17 207–17 212. ( 10.1073/pnas.1103612108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Contreras D, Destexhe A, Steriade M. 1997. Intracellular and computational characterization of the intracortical inhibitory control of synchronized thalamic inputs in vivo. J. Neurophysiol. 78, 335–350 ( 10.1152/jn.1997.78.1.335). [DOI] [PubMed] [Google Scholar]
- 94.Blanco W, et al. 2015. Synaptic homeostasis and restructuring across the sleep-wake cycle. PLoS Comput. Biol. 11, e1004241 ( 10.1371/journal.pcbi.1004241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cruikshank SJ, Lewis TJ, Connors BW. 2007. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat. Neurosci. 10, 462–468. ( 10.1038/nn1861) [DOI] [PubMed] [Google Scholar]
- 96.Haider B, Häusser M, Carandini M. 2013. Inhibition dominates sensory responses in awake cortex. Nature 493, 97–100. ( 10.1038/nature11665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Porter JT, Johnson CK, Agmon A. 2001. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J. Neurosci. 21, 2699–2710. ( 10.1523/JNEUROSCI.21-08-02699.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Swadlow HA. 2002. Thalamocortical control of feed-forward inhibition in awake somatosensory ‘barrel’ cortex. Phil. Trans. R. Soc. B 357, 1717–1727. ( 10.1098/rstb.2002.1156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hartwich K, Pollak T, Klausberger T. 2009. Distinct firing patterns of identified basket and dendrite-targeting interneurons in the prefrontal cortex during hippocampal theta and local spindle oscillations. J. Neurosci. Off. J. Soc. Neurosci. 29, 9563–9574. ( 10.1523/JNEUROSCI.1397-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Constantinople CM, Bruno RM. 2013. Deep cortical layers are activated directly by thalamus. Science 340, 1591–1594. ( 10.1126/science.1236425) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niethard N, Ngo H-VV, Ehrlich I, Born J. 2018. Cortical circuit activity underlying sleep slow oscillations and spindles. Proc. Natl Acad. Sci. USA 115, E9220–E9229. ( 10.1073/pnas.1805517115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Niethard N, Burgalossi A, Born J. 2017. Plasticity during sleep is linked to specific regulation of cortical circuit activity. Front. Neural Circuits 11, 65 ( 10.3389/fncir.2017.00065) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sigl-Glöckner J, Seibt J. 2018. Peeking into the sleeping brain: using in vivo imaging in rodents to understand the relationship between sleep and cognition. J. Neurosci. Methods 316, 71–82. ( 10.1016/j.jneumeth.2018.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tremblay R, Lee S, Rudy B. 2016. GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91, 260–292. ( 10.1016/j.neuron.2016.06.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim JJ, Fanselow MS. 1992. Modality-specific retrograde amnesia of fear. Science 256, 675–677. ( 10.1126/science.1585183) [DOI] [PubMed] [Google Scholar]
- 106.McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457. ( 10.1037/0033-295X.102.3.419) [DOI] [PubMed] [Google Scholar]
- 107.Nadel L, Moscovitch M. 1997. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr. Opin. Neurobiol. 7, 217–227 ( 10.1016/S0959-4388(97)80010-4). [DOI] [PubMed] [Google Scholar]
- 108.Scoville WB, Milner B. 1957. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry 20, 11–21. ( 10.1136/jnnp.20.1.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Squire LR, Alvarez P. 1995. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr. Opin. Neurobiol. 5, 169–177 ( 10.1016/0959-4388(95)80023-9). [DOI] [PubMed] [Google Scholar]
- 110.Buzsáki G. 2015. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. ( 10.1002/hipo.22488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. 2009. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat. Neurosci. 12, 919–926. ( 10.1038/nn.2337) [DOI] [PubMed] [Google Scholar]
- 112.Ego-Stengel V, Wilson MA. 2010. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10. ( 10.1002/hipo.20707) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. 2009. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223. ( 10.1038/nn.2384) [DOI] [PubMed] [Google Scholar]
- 114.Diekelmann S, Born J. 2010. The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126. ( 10.1038/nrn2762) [DOI] [PubMed] [Google Scholar]
- 115.O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. 2010. Play it again: reactivation of waking experience and memory. Trends Neurosci. 33, 220–229. ( 10.1016/j.tins.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 116.Tingley D, Peyrache A. 2020. On the methods for reactivation and replay analysis. Phil. Trans. R. Soc. B 375, 20190231 ( 10.1098/rstb.2019.0231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194 ( 10.1016/S0896-6273(02)01096-6). [DOI] [PubMed] [Google Scholar]
- 118.Chrobak JJ, Buzsáki G. 1994. Selective activation of deep layer (V-VI) retrohippocampal cortical neurons during hippocampal sharp waves in the behaving rat. J. Neurosci. Off. J. Soc. Neurosci. 14, 6160–6170 ( 10.1523/JNEUROSCI.14-10-06160.1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ólafsdóttir HF, Carpenter F, Barry C. 2016. Coordinated grid and place cell replay during rest. Nat. Neurosci. 19, 792–794. ( 10.1038/nn.4291) [DOI] [PubMed] [Google Scholar]
- 120.O'Neill J, Boccara CN, Stella F, Schoenenberger P, Csicsvari J. 2017. Superficial layers of the medial entorhinal cortex replay independently of the hippocampus. Science 355, 184–188. ( 10.1126/science.aag2787) [DOI] [PubMed] [Google Scholar]
- 121.Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CMA. 2009. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 7, e1000173 ( 10.1371/journal.pbio.1000173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sjulson L, Peyrache A, Cumpelik A, Cassataro D, Buzsáki G. 2018. Cocaine place conditioning strengthens location-specific hippocampal coupling to the nucleus accumbens. Neuron 98, 926–934.e5. ( 10.1016/j.neuron.2018.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Girardeau G, Inema I, Buzsáki G. 2017. Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat. Neurosci. 20, 1634–1642. ( 10.1038/nn.4637) [DOI] [PubMed] [Google Scholar]
- 124.Eschenko O, Ramadan W, Mölle M, Born J, Sara SJ. 2008. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn. Mem. 15, 222–228. ( 10.1101/lm.726008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Girardeau G, Cei A, Zugaro M. 2014. Learning-induced plasticity regulates hippocampal sharp wave-ripple drive. J. Neurosci. Off. J. Soc. Neurosci. 34, 5176–5183. ( 10.1523/JNEUROSCI.4288-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Norimoto H, et al. 2018. Hippocampal ripples down-regulate synapses. Science 359, 1524–1527. ( 10.1126/science.aao0702) [DOI] [PubMed] [Google Scholar]
- 127.Frankland PW, Bontempi B. 2005. The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130. ( 10.1038/nrn1607) [DOI] [PubMed] [Google Scholar]
- 128.Siapas AG, Wilson MA. 1998. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128 ( 10.1016/S0896-6273(00)80629-7). [DOI] [PubMed] [Google Scholar]
- 129.Phillips KG, Bartsch U, McCarthy AP, Edgar DM, Tricklebank MD, Wafford KA, Jones MW. 2012. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron 76, 526–533. ( 10.1016/j.neuron.2012.09.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sirota A, Csicsvari J, Buhl D, Buzsáki G. 2003. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl Acad. Sci. USA 100, 2065–2069. ( 10.1073/pnas.0437938100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsáki G. 2006. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron 52, 871–882. ( 10.1016/j.neuron.2006.10.023) [DOI] [PubMed] [Google Scholar]
- 132.Mölle M, Yeshenko O, Marshall L, Sara SJ, Born J. 2006. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J. Neurophysiol. 96, 62–70. ( 10.1152/jn.00014.2006) [DOI] [PubMed] [Google Scholar]
- 133.Sullivan D, Mizuseki K, Sorgi A, Buzsáki G. 2014. Comparison of sleep spindles and theta oscillations in the hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 34, 662–674. ( 10.1523/JNEUROSCI.0552-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Varela C, Wilson MA. 2019. mPFC spindle cycles organize sparse thalamic activation and recently active ca1 cells during non-REM sleep. bioRxiv 653436. ( 10.1101/653436) [DOI]
- 135.Viejo G, Peyrache A. In press. Precise coupling of the thalamic head-direction system to hippocampal ripples. Nat. Comms. ( ) [DOI] [PMC free article] [PubMed]
- 136.Yang M, Logothetis NK, Eschenko O. 2019. Occurrence of hippocampal ripples is associated with activity suppression in the mediodorsal thalamic nucleus. J. Neurosci. Off. J. Soc. Neurosci. 39, 434–444. ( 10.1523/JNEUROSCI.2107-18.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Norman Y, Yeagle EM, Khuvis S, Harel M, Mehta AD, Malach R. 2019. Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science 365, eaax1030 ( 10.1126/science.aax1030) [DOI] [PubMed] [Google Scholar]
- 138.Vaz AP, Inati SK, Brunel N, Zaghloul KA. 2019. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363, 975–978. ( 10.1126/science.aau8956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clemens Z, Mölle M, Eross L, Jakus R, Rásonyi G, Halász P, Born J. 2011. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur. J. Neurosci. 33, 511–520. ( 10.1111/j.1460-9568.2010.07505.x) [DOI] [PubMed] [Google Scholar]
- 140.Staresina BP, Bergmann TO, Bonnefond M, van der Meij R, Jensen O, Deuker L, Elger CE, Axmacher N, Fell J. 2015. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18, 1679–1686. ( 10.1038/nn.4119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. 2016. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 19, 959–964. ( 10.1038/nn.4304) [DOI] [PubMed] [Google Scholar]
- 142.Jay TM, Glowinski J, Thierry AM. 1989. Selectivity of the hippocampal projection to the prelimbic area of the prefrontal cortex in the rat. Brain Res. 505, 337–340. ( 10.1016/0006-8993(89)91464-9) [DOI] [PubMed] [Google Scholar]
- 143.Maviel T, Durkin TP, Menzaghi F, Bontempi B. 2004. Sites of neocortical reorganization critical for remote spatial memory. Science 305, 96–99. ( 10.1126/science.1098180) [DOI] [PubMed] [Google Scholar]
- 144.Ramanathan DS, Gulati T, Ganguly K. 2015. Sleep-dependent reactivation of ensembles in motor cortex promotes skill consolidation. PLoS Biol. 13, e1002263 ( 10.1371/journal.pbio.1002263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Davidson TJ, Kloosterman F, Wilson MA. 2009. Hippocampal replay of extended experience. Neuron 63, 497–507. ( 10.1016/j.neuron.2009.07.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dang-Vu TT, et al. 2011. Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proc. Natl Acad. Sci. USA 108, 15 438–15 443. ( 10.1073/pnas.1112503108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schabus M, et al. 2012. The fate of incoming stimuli during NREM sleep is determined by spindles and the phase of the slow oscillation. Front. Neurol. 3, 40 ( 10.3389/fneur.2012.00040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lecci S, Fernandez LMJ, Weber FD, Cardis R, Chatton J-Y, Born J, Lüthi A. 2017. Coordinated infraslow neural and cardiac oscillations mark fragility and offline periods in mammalian sleep. Sci. Adv. 3, e1602026 ( 10.1126/sciadv.1602026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Claude L, Chouchou F, Prados G, Castro M, De Blay B, Perchet C, García-Larrea L, Mazza S, Bastuji H. 2015. Sleep spindles and human cortical nociception: a surface and intracerebral electrophysiological study. J. Physiol. 593, 4995–5008. ( 10.1113/JP270941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sela Y, Vyazovskiy VV, Cirelli C, Tononi G, Nir Y. 2016. Responses in rat core auditory cortex are preserved during sleep spindle oscillations. Sleep 39, 1069–1082. ( 10.5665/sleep.5758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Xia F, Richards BA, Tran MM, Josselyn SA, Takehara-Nishiuchi K, Frankland PW. 2017. Parvalbumin-positive interneurons mediate neocortical-hippocampal interactions that are necessary for memory consolidation. eLife 6, e27868 ( 10.7554/eLife.27868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gambino F, Pagès S, Kehayas V, Baptista D, Tatti R, Carleton A, Holtmaat A. 2014. Sensory-evoked LTP driven by dendritic plateau potentials in vivo. Nature 515, 116–119. ( 10.1038/nature13664) [DOI] [PubMed] [Google Scholar]
- 153.Golding NL, Staff NP, Spruston N. 2002. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature 418, 326–331. ( 10.1038/nature00854) [DOI] [PubMed] [Google Scholar]
- 154.Ho VM, Lee J-A, Martin KC. 2011. The cell biology of synaptic plasticity. Science 334, 623–628. ( 10.1126/science.1209236) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dupont G, Goldbeter A. 1998. CaM kinase II as frequency decoder of Ca2+ oscillations. BioEssays News Rev. Mol. Cell. Dev. Biol. 20, 607–610. () [DOI] [PubMed] [Google Scholar]
- 156.Koninck PD, Schulman H. 1998. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science 279, 227–230. ( 10.1126/science.279.5348.227) [DOI] [PubMed] [Google Scholar]
- 157.Miyawaki H, Diba K. 2016. Regulation of hippocampal firing by network oscillations during sleep. Curr. Biol. CB 26, 893–902. ( 10.1016/j.cub.2016.02.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bittner KC, Milstein AD, Grienberger C, Romani S, Magee JC. 2017. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033–1036. ( 10.1126/science.aan3846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guerguiev J, Lillicrap TP, Richards BA. 2017. Towards deep learning with segregated dendrites. eLife 6, e22901 ( 10.7554/eLife.22901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kastellakis G, Cai DJ, Mednick SC, Silva AJ, Poirazi P. 2015. Synaptic clustering within dendrites: an emerging theory of memory formation. Prog. Neurobiol. 126, 19–35. ( 10.1016/j.pneurobio.2014.12.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mel BW, Schiller J, Poirazi P. 2017. Synaptic plasticity in dendrites: complications and coping strategies. Curr. Opin. Neurobiol. 43, 177–186. ( 10.1016/j.conb.2017.03.012) [DOI] [PubMed] [Google Scholar]
- 162.Kesner RP. 2018. An analysis of dentate gyrus function (an update). Behav. Brain Res. 354, 84–91. ( 10.1016/j.bbr.2017.07.033) [DOI] [PubMed] [Google Scholar]
- 163.Senzai Y. 2019. Function of local circuits in the hippocampal dentate gyrus-CA3 system. Neurosci. Res. 140, 43–52. ( 10.1016/j.neures.2018.11.003) [DOI] [PubMed] [Google Scholar]
- 164.Chaudhuri R, Gerçek B, Pandey B, Peyrache A, Fiete I. 2019. The intrinsic attractor manifold and population dynamics of a canonical cognitive circuit across waking and sleep. Nat. Neurosci. 22, 1512–1520. ( 10.1038/s41593-019-0460-x) [DOI] [PubMed] [Google Scholar]
- 165.Peyrache A, Lacroix MM, Petersen PC, Buzsáki G. 2015. Internally organized mechanisms of the head direction sense. Nat. Neurosci. 18, 569–575. ( 10.1038/nn.3968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gelinas JN, Khodagholy D, Thesen T, Devinsky O, Buzsáki G. 2016. Interictal epileptiform discharges induce hippocampal–cortical coupling in temporal lobe epilepsy. Nat. Med. 22, 641–648. ( 10.1038/nm.4084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferrarelli F, Huber R, Peterson MJ, Massimini M, Murphy M, Riedner BA, Watson A, Bria P, Tononi G. 2007. Reduced sleep spindle activity in schizophrenia patients. Am. J. Psychiatry 164, 483–492. ( 10.1176/appi.ajp.164.3.483) [DOI] [PubMed] [Google Scholar]
- 168.Khazipov R, Luhmann HJ. 2006. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 29, 414–418. ( 10.1016/j.tins.2006.05.007) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.