Abstract
Sharp-wave ripples are complex neurophysiological events recorded along the trisynaptic hippocampal circuit (i.e. from CA3 to CA1 and the subiculum) during slow-wave sleep and awake states. They arise locally but scale brain-wide to the hippocampal target regions at cortical and subcortical structures. During these events, neuronal firing sequences are replayed retrospectively or prospectively and in the forward or reverse order as defined by experience. They could reflect either pre-configured firing sequences, learned sequences or an option space to inform subsequent decisions. How can different sequences arise during sharp-wave ripples? Emerging data suggest the hippocampal circuit is organized in different loops across the proximal (close to dentate gyrus) and distal (close to entorhinal cortex) axis. These data also disclose a so-far neglected laminar organization of the hippocampal output during sharp-wave events. Here, I discuss whether by incorporating cell-type-specific mechanisms converging on deep and superficial CA1 sublayers along the proximodistal axis, some novel factors influencing the organization of hippocampal sequences could be unveiled.
This article is part of the Theo Murphy meeting issue ‘Memory reactivation: replaying events past, present and future’.
Keywords: ripples, replay, preplay, deep–superficial
1. Introduction
The hippocampus is crucial for episodic memory, an ability that relies on the formation of a cognitive map [1]. According to this theory, a set of hippocampal pyramidal cells fire selectively to map relationships between locations where events happen. During encoding, firing sequences of hippocampal cells are coordinated by theta (4–12 Hz) and gamma oscillations (40–80 Hz) and activated in a manner ordered by experience [2]. In a given theta cycle, spikes from place cells organize along the past, present and future events [3–7]. This mechanism allows behavioural sequences to be chunked into theta sequences [4,8,9]. Presumably, theta sequences facilitate encoding of information by plasticity mechanisms relying on the relative timing between spikes [10,11].
Offline reinstating of this activity has been traditionally viewed as a form of replaying sequences for their subsequent consolidation via high-frequency induced plasticity [12–14]. Replay was initially described during sleep in association with fast oscillations or ripples, but it was also observed during exploratory pauses [15–17]. Moreover, the content of replay (i.e. the organization and identity of neuronal sequences) was shown to vary substantially across states and behaviour [18]. In the light of this new evidence, it was proposed that replay may serve a wider range of cognitive functions than originally thought [19,20].
Here, I first review recent data on hippocampal replay studied in rodents to illustrate how our conceptions have evolved to accommodate a more complex perspective. In spite of this conceptual shift, we still lack a physiological understanding on how different forms of replay are generated. A major difficulty in updating this view is that our current models have not yet considered the cell-type and region specificity of hippocampal microcircuits. However, emerging data suggest a specific organization of the hippocampus along the dorso-ventral (or septo-temporal), proximodistal (transverse) and deep–superficial (radial) axes [21–26]. I discuss whether, by considering physiological, microcircuit and neuromodulatory factors in the transverse and radial axes of the dorsal hippocampus, different mechanisms may help to explain biases of hippocampal replay.
2. Complexities of retrospective hippocampal replay
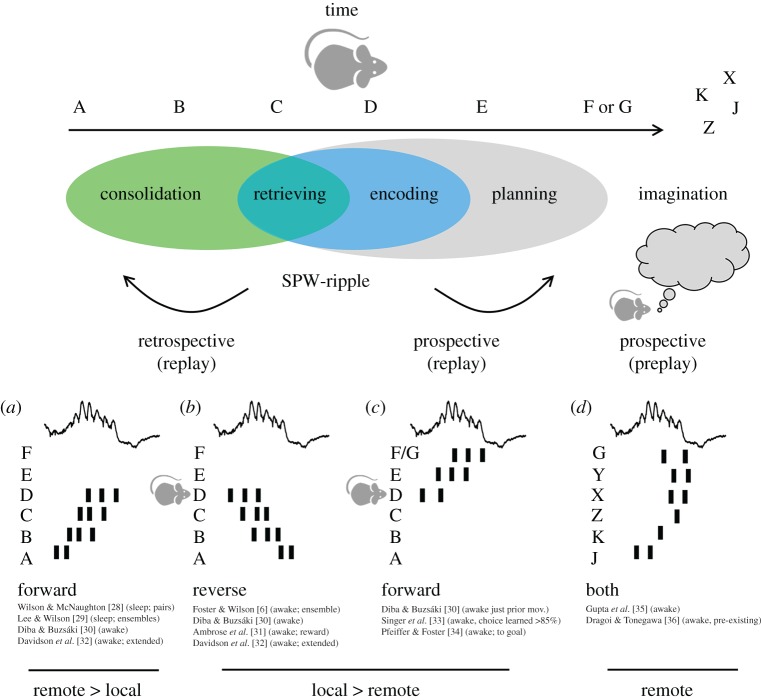
Hippocampal replay of neuronal sequences is a rather complex phenomenon. Replay is typically associated with a particular physiological event called the sharp-wave ripple [27]. It consists of short multi-neuronal sequences (approx. 40–100 ms) carrying information about the temporal organization of experience (figure 1). Originally viewed as a basic mechanism underlying memory consolidation during sleep [12], retrospective replay reflects ordered firing from a set of neuronal ensembles that were previously activated by experience [28,29].
Figure 1.
Miscellaneous patterns of replay potentially underlying different processes of memory consolidation, retrieval, planning and imagination. As the animal experiences a succession of events (represented by letters), some hippocampal neurons fire selectivity to build an abstract representation or cognitive map. During periods associated with sleep and immobility, sequences of these ‘place cells’ are co-activated in an orderly manner coordinated by sharp-wave ripples (SPW-ripples). The order of replay reveals the multifactorial influence of brain state and microcircuit physiology, as well as other procedural and cognitive factors. According to use, replay can be retrospective (engaging sequences already experienced) or prospective (engaging sequences ahead in time). According to the order, replay can be forward (in the same order as experienced) or reverse (opposite to experienced). Some sequences are not linked to experience and reflect a sort of preplay of events never seen before. Finally, according to the subject ‘location’ in the event space, replay can be local or remote. (a) Forward retrospective replay occurring remotely during sleep was the first form of replay reported in the literature [28,29]. Later reports showed it is also present locally during awake immobility and exploratory pauses [30] (b) Reverse retrospective replay is more typically present in awake conditions [6,30] and strongly influenced by novelty, reward values or goal-oriented tasks [31]. Forward and reverse replay can be concatenated along several sharp-wave ripples to accommodate extended experience [32]. (c) Forward prospective replay of already experienced neuronal sequences is typically seen before running for a goal or during choice learning [30,33,34]. (d) Preplay depicts sequences never experienced before and is correlated to or predictive of the activity during the future experience [35,36]. Preplay, which can be forward or reverse, is detectable in awake rest and in slow-wave sleep, and it has been proposed to play a major role in rapid encoding of novel information. (Online version in colour.)
The organization of replay was early challenged by the discovery that sequence order is influenced by the brain state [6,30]. During sleep, sequential firing is typically organized forward (i.e. in a similar order to that experienced; figure 1a), but when replayed during awake immobility the order can reverse (figure 1b). Physiologically, different neuromodulatory influences during sleep and awake states help to explain part of this bias [37]. The role of place fields and place field tails suggested a contribution of the animal's current location in the emergence of awake replay [30,38], but remote places were also seen to trigger sequences [39]. The terms centripetal (away from the animal) and centrifugal (towards the animal) were introduced to identify replay from a more egocentric perspective (see [40] for an early reference to the terms).
Additional complexities were noted in these studies. When rodents were trained to run back and forth along a linear track, the forward and reverse orders were dissociated [30]. At the end of the track, when the animal was drinking and immobile, sequences replayed forwardly, while after running and preceding drinking they replayed in reverse order. This quasi-directional effect suggests a higher level of organization emerging from complex associations among different ensembles [41]. In subsequent studies, it was noted that forward and reverse sequences intermixed in long tracks to incorporate the spatial intricacies of the maze [32,42]. Sequence integrity was not uniform, suggesting that extended replay (up to 600 ms) during concatenated sharp-wave ripples may depend specifically on direct inputs from layer III of the entorhinal cortex [43,44].
In general, forward replay appears more frequent than reverse replay [30,32]. However, additional variables can influence the organization of retrospective sequences. Sensory interference (sounds) can bias the content of replay during sleep to reactivate memory traces associated with auditory cues [45,46]. Reverse replay can be modulated by changing rewards [31], a manipulation that also affects the rate of sharp-wave ripples [47]. This brings reverse replay to a unique position to integrate reward-predictive information into specific neuronal sequences. Consistently, during spatial learning, sequences reorganize to represent new goal locations [48] under the influence of dopaminergic signals from the ventral tegmental area (VTA), locus coeruleus and the dorsal raphe nucleus [40,49–51]. Thus, replay and sharp-wave ripples can actually scale to a system level to serve different cognitive abilities in interaction with other brain regions (e.g. the striatum [52,53], visual cortex [54], cingulate cortex [55], prefrontal cortex [56], auditory cortex [46], amygdala [57], mediodorsal thalamus [58]). Accordingly, forward and reverse retrospective replay can contribute to retrieving information more dynamically during awake states [59,60].
3. Prospective hippocampal replay defies the rule
Research on the intriguing nature of replay gained a new impetus with the discovery of prospective coding during sharp-wave ripples (figure 1c). Prospective coding is a more general phenomenon [61,62], but identification of similar processes during ripples challenged the dominant view. Using spatial alternation tasks requiring short-term memory, it was found that forward replay occurring just before making a decision depicted future place cell sequences ahead of the animal [33]. Moreover, increased activation of these ripple-associated neuronal sequences predicted correct choices, whereas during incorrect trials synchrony remained at chance levels. A role of the animal's location in this form of replay was proposed based on the observation that sequences started in place and ended at the goal sites [34]. This form of replay is dependent on the memory for recent experiences and therefore it may reflect an interaction between neuronal processes underlying encoding, retrieving and planning [20].
However, using a complex maze with several bifurcations and detours in a choice task with daily changing contingencies, it was noted that the replay content may not be determined solely by the recency of events [35]. Up to that moment, it was believed that replay was intimately associated with sequences built up by experience (theta sequences) [4,11]. Surprisingly, the new experiments revealed sequences of trajectories never experienced before by the animal [35]. This more complex organization was related to subsequent observations of preplay (figure 1d), a phenomenon by which future place cell sequences can be recorded during sharp-wave ripples preceding exploration [36]. Pre-existing ensembles were proposed to underlie the formation of sequences that can be repurposed for acquiring new experiences while others are generated de novo [63–67]. The existence of these rigid and reconfigurable hippocampal ensembles may be supported by the skewed distribution of relevant physiological parameters such as synaptic weights and firing rate [64,68,69]. Thus, replay may also contribute constructive cognitive roles such as imagination, a hippocampal-dependent ability that can be conceptualized as a mental travel through the cognitive map [20,70].
In the view of all the evidence reviewed so far, replay can be considered a multifactorial event and hippocampal sharp-wave ripples the underlying physiological mechanism. However, our understanding is still fragmented. How can a palette of sequences ordered back and forth in time and space emerge from an apparently unidirectional event (i.e. from CA3 to CA1) such as the sharp-wave ripple?
4. Can replay organize differently along CA1?
Sharp-waves reflect a population synaptic event presumably emerging from the recurrent CA3 microcircuit, whereas ripples can be generated locally in CA1 [27]. During states dominated by a lower cholinergic tone (i.e. immobility and slow-wave sleep), CA3 neurons are released from sustained GABAergic inhibition and population bursts emerge from transient increases in synchrony [71,72]. These synchronous discharges from CA3 pyramidal cells concurrently activate synaptic currents in the apical dendrites of CA1 neurons, resulting in a transient sharp potential at the stratum pyramidale [73]. Together with CA1 pyramidal cells, some GABAergic interneurons discharge during sharp-wave ripples [74]. Thus, dedicated pyramidal–interneuron interactions shape the local expression of ripples [75]. The relative ‘independency’ between the sharp-wave and the ripple presumably allows for the expression of several but coherent events along the proximodistal and septo-temporal axes [76,77]. The term independency refers to different local ripple mechanisms associated with a given sharp-wave, as well as their independent occurrence at different loci. Thus, multiple neuronal sequences can be activated during sharp-waves, reflecting different ensemble patterns of CA3–CA1 neurons [78,79].
Consistently with this view, intracellular recordings of CA1 pyramidal cells typically show depolarizing responses shaped by phasic excitation/inhibition during sharp-wave ripples both in awake and anaesthetized conditions [73,80–82]. However, this picture was challenged by reports of intermixed hyperpolarized responses able to silence some CA1 neurons [83,84]. Morphological reconstruction revealed a specific organization across the deep (closer to oriens) and superficial (closer to the stratum radiatum) sublayers [83]. Part of this selection can be explained by a radial gradient of perisomatic inhibition interacting with behavioural and state-dependent effects to bias neuronal firing during sleep and awake ripples [83]. Other microcircuit factors may equally contribute to sublayer segregation of hippocampal activities [26,83,85].
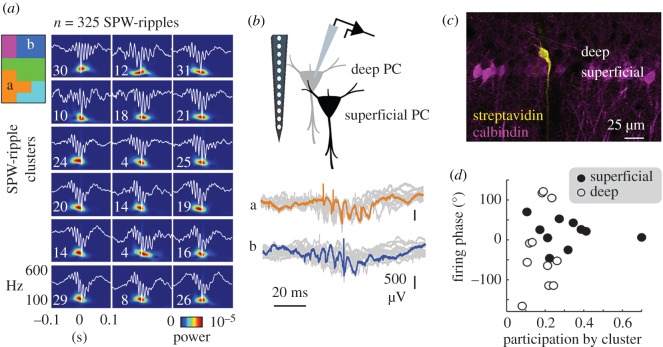
While investigating the mechanisms for selective reactivation of morphologically identified CA1 cells, some additional differences were noted [86]. Unsupervised clustering of sharp-wave ripple events allows us to evaluate how variability of single-cell firing could be explained by the participation of different ensembles, as captured by extracellular signatures [86,87] (figure 2a). When applied to recordings of morphologically identified cells, it was noted that deep cells participated less than superficial cells [83,86] and that their spike timing along sharp-waves was more variable (figure 2b,c). For instance, the cell shown in figure 2b fired earlier during sharp-wave ripples in the orange cluster and later for ripples in the blue cluster. This wider range of firing variability may suggest more flexibility for forward and reverse replay in deep than superficial cells in a sequence (figure 2d).
Figure 2.
Flexibility of neuronal firing from CA1 pyramidal cells during sharp-wave ripples (SPW-ripples). (a) Unsupervised classification of individual sharp-wave ripple events clusters of similar local field potential signatures to be identified. The number of events in each group is indicated. Clusters of groups with topological similarities in the high-dimensional space are identified by colours in the inset scheme. (b) Single CA1 pyramidal cells were recorded juxtacellularly during sharp-wave ripples and their firing was grouped per cluster. Note different timing of the same cell in the orange and blue cluster indicated before. (c) Juxtacellular recorded cells are labelled for morphological identification with streptavidin, and classified as deep or superficial depending on their location within the calbindin-positive sublayer. (d) Distribution of the preferred firing phase during the sharp-wave from different clusters in deep and superficial cells. Note wider distribution in deep cells indicating more flexibility. Data from [86]. (Online version in colour.)
These data suggest that ripple-associated replay may proceed differently across CA1 sublayers. A radial organization of CA1 neuronal firing was early noted [88]. Deep CA1 cells fire at higher rates and have broader place fields as compared with superficial cells [88,89]. The nature of these fields is also qualitatively different: deep cells are more tightly linked to somatosensory landmarks and influenced by rewards, while superficial cell firing is more contextual [89,90]. Superficial place fields apparently provide a more stable representation of a given context while deep cells are more flexible. Their ability to form theta sequences is strongly determined by different phase precession dynamics: deep cells exhibit a wider phase range than superficial cells and can even shift phases during rapid eye movement (REM) sleep [88,91]. Therefore, physiological mechanisms may be in place to influence neuronal activity across CA1; whether they could explain the variety of hippocampal replay is unknown.
5. Potential biases of hippocampal replay across deep–superficial sublayers
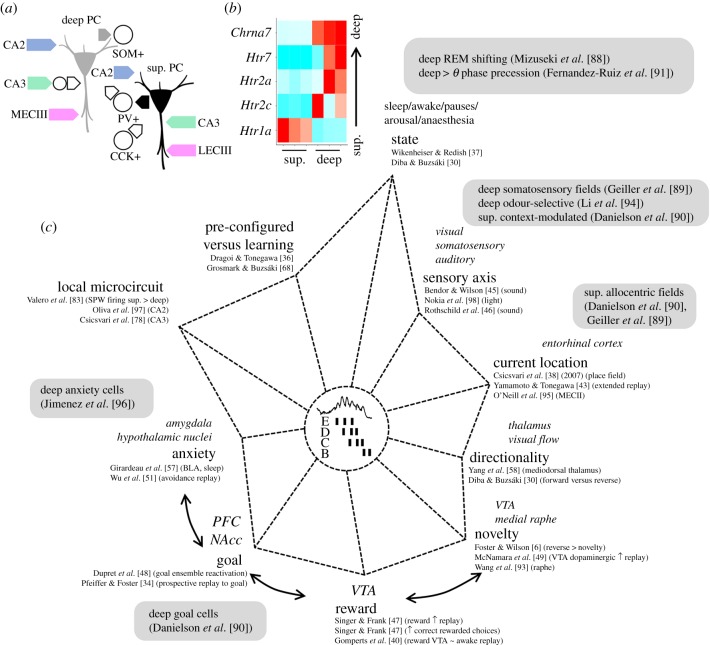
By considering genetic, microcircuit and behavioural factors, different mechanisms could be proposed to bias replay. First, cell-type and region-specific microcircuits wire differentially along sublayers (figure 3a). Deep cells at CA1 regions closest to CA2 (proximal CA1) receive more inputs from the medial entorhinal cortex, whereas superficial cells located closer to subiculum (distal CA1) are better connected to the lateral entorhinal cortex [99]. Therefore, the way sensory modalities integrate into the CA1 region should be determined by this dedicated wiring. Consistently, non-spatial and spatial information segregates radially [89,90,94] and proximodistally in CA1 [100–102]. Intrahippocampal circuits also wire differently across sublayers. Superficial cells are more responsive to CA3 inputs than deep cells owing to differences of feed-forward inhibition [83], which together with the proximodistal organization of Schaffer collaterals will determine how contextual information from dentate gyrus (DG) and CA3 enters into CA1 [103–105]. Deep cells, in contrast, are more strongly activated by CA2 [106,107], which may help to accommodate other cognitive representations such as social memory and delay signals during immobility [108–111].
Figure 3.
Biases of hippocampal replay can affect deep and superficial (sup.) CA1 pyramidal cells differently. (a) Known deep superficial local microcircuit motifs. Deep cells are more strongly activated by inputs from CA2 (at basal dendrites) and the medial entorhinal cortex. Inputs from CA3 cells onto deep cells are strongly interfaced by feed-forward inhibition. Superficial cells receive more innervation from the lateral entorhinal cortex and direct CA3 inputs. Importantly, lateral and medial entorhinal inputs to deep and superficial cells organize proximodistally along the traverse CA1 axis. Superficial cells mainly recruit PV+ basket cells whereas deep cells are biased for SOM+ interneurons. In return, innervation by PV+ basket cells is larger over deep cells while CCK+ basket cells preferentially target superficial cells. (b) Differential transcriptomic expression of serotoninergic (Htr) and cholinergic receptor genes (Chrn) along the deep and superficial layers. Normalized gene expression values from three different replicates are shown. Data from https://hipposeq.janelia.org/ [92]. (c) Multifactorial axes biasing the content and organization of replays during sharp-wave ripples. Different influences on deep and superficial CA1 pyramidal cells have been reported along these axes (grey boxes). The relative axis length is not necessarily informative [6,30,34,36–38,40,43,45–51,57,58,68,78,88–91,93–98]. PC, pyramidal cell, SOM+, somatostatin-positive interneuron; PV+, parvalbumin-positive basket cell; CCK+, cholecystokinin-positive basket cell; MECIII, medial entorhinal cortex layer III; LECIII, lateral entorhinal cortex layer III. (Online version in colour.)
Different innervation by local GABAergic interneurons also shapes the dynamics of CA1 cells [112], especially across behavioural states [113–115]. Parvalbumin (PV) and cholecystokinin (CCK) basket cells target deep and superficial cells differently [83,116] so that their feed-forward and feedback activation can gate information differently across sublayers [91,116–118]. In addition, transcriptomic differences regulate the laminar expression of neuromodulatory receptors such as G-protein coupled receptor 5-HT1a for serotonin (enriched at superficial cells) or the nicotine receptor for acetylcholine (enriched in the deep sublayer) [92] (figure 3b).
Therefore, data support integration of distinct physiological influences across CA1. Under this scenario, it is tempting to consider whether these mechanisms could provide explanatory axes for a variety of replays (figure 3c). For instance, state (including brief exploratory pauses) has a major influence [30,119], and this is supported by the physiology of sharp-wave ripples in response to different neuromodulators [120]. Sensory inputs [45,46] as well as the animal's location can bias the replay content, a property that presumably depends on entorhinal inputs segregated along CA1 [99]. Accordingly, co-activations of neuronal sequences in entorhinal layers can independently control the input/output flow of the hippocampal replay [95,121]. Novelty and reward can reverse replay in time [6,47] owing to the influence of the VTA, nucleus accumbens and medial prefrontal cortex [40,49,122,123]. Aversive inputs from the amygdala can also affect hippocampal replay [51,57].
Along many of these axes, a deep–superficial organization of neuronal firing has been described and can therefore influence sequence dynamics (figure 3c; grey boxes). State-dependent shifts of the preferred theta phase and wider precession dynamics are reported for deep CA1 cells [88,91]. Place field of deep cells is more typically linked with somatosensory and olfactory inputs [89,94] while superficial maps are more contextual and allocentric [90]. The activity of deep cells is more affected by goals, rewards and anxiety [90,96]. Given that sharp-wave ripple physiology is influenced by all these factors [27,124,125], I hypothesize that the deep–superficial hippocampal axis may contribute to shape replay. Are there microcircuit substrates for such an effect?
6. A proximodistal and deep–superficial perspective of hippocampal replay
According to the classical view, sharp-wave ripples represent a complex event built from two different processes [27]. Sharp-waves result from convergent depolarization of the dendrites of CA1 pyramidal cells in response to CA3 firing distributed through Schaffer collaterals [78]. By contrast, ripples emerge locally in CA1 from feedback interactions between pyramidal cells and interneurons [75]. It is believed that the recruitment process starts more typically at the distal CA3a region (close to CA2) and runs as a neuronal avalanche towards CA1.
However, sharp-wave activity can also backpropagate to the proximal CA3c area (close to DG) before invading CA1 [71,78]. Driven by CA3c firing, mossy cells from the hilus are recruited and integrated into the flow [126]. Moreover, recent evidence suggests that some CA2 cells may independently support the generation of sharp-wave ripples markedly during awake states [97], possibly owing to recurrent connectivity [127] and in interaction with a specific subset of CA3a cells [128]. Therefore, different initiating regions distribute along the hilus–CA3–CA2 axis to trigger local ripples in CA1 [105,124,97,129]. If diversity of replay can be explained by any physiological mechanism, the multiple sharp-wave initiating loci must be one key. In support of this view, individual ripple events vary along CA1 [77]. Such high-dimensional dynamics can be disentangled with appropriate methods ([86,87,130,131]; see also figure 2).
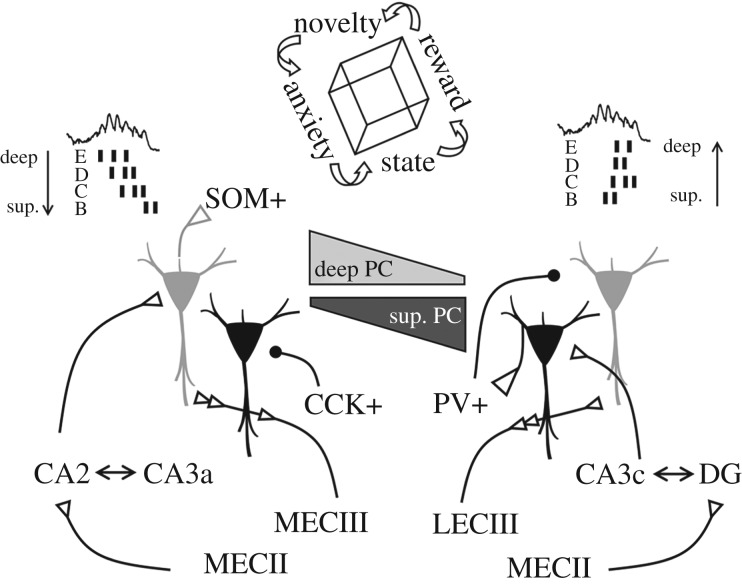
I propose bringing the focus to models of sharp-wave ripples consisting of multiple initiating loci instead of considering a single triggering area. By considering different spots along upstream pre-synaptic regions, the activity can flow differently in CA1 to control the replay order more precisely by discharging cells at different timing (figure 4). Under this perspective, the proximodistal hilus–CA3–CA2–CA1 axis represents a primary source of modulation, but entorhinal inputs as well [43,132]. Adherence of individual cells to particular ripple clusters (i.e. replay content) reflects that the underlying orthogonalization may also segregate across CA1 sublayers [75,83,86]. Therefore, the proximodistal organization of pre-synaptic ensembles interacts radially in CA1. For instance, sharp-wave events initiated by CA3c cells would more likely result in the feed-forward inhibition of CA2 cells [83,133] and consequently would discharge mostly superficial CA1 cells. By contrast, sharp-wave ripples initiated by CA3a–CA2 pyramidal cells could rather flow through deep CA1 sublayers first (figure 4). Connectivity between CA3 cells, hilar mossy cells and dentate granule cells should further contribute to shaping firing content during these events by incorporating additional sequences and influencing reactivation [105,126,129]. Selection of different sequences can be triggered by single cells [71,134], which together with plasticity can help to reconfigure and to establish new ensembles [135]. Recent evidence supports the idea that such a process may indeed occur randomly [66].
Figure 4.
Potential mechanisms for sequence orthogonalization across deep and superficial CA1 sublayers. Different responsiveness of deep and superficial (sup.) cells to a collection of pre-synaptic inputs from CA2 to CA3 regions may support different timing for activation in a sequence, which together with dedicated inhibitory control will determine firing selection across CA1 sublayers. Entorhinal inputs converging differently in deep and superficial cells across the proximodistal axis could additionally bias firing depending on the animal location and salient sensory information. State and emotional factors represent additional orthogonalizing factors.
Given the ‘independency’ of ripples from the sharp-wave event, local CA1 circuits could also contribute to the way sequences can be replayed. While recurrent connectivity between CA1 pyramidal cells is very low, di-synaptic inhibitory circuits interface between sublayers [25,26]. Thus, driven by specific spatio-temporal patterns of GABAergic inhibition the content and order of replay could further unfold [74,136,137]. Interneuronal interactions, but possibly GABAergic projecting cells as well [115,118,138], could organize spatially separated ensembles into coherent sequences [75]. For example, superficial CA1 cells preferentially activate PV+ basket cells while deep cells apparently do so with SOM+ interneurons [91,116]. Therefore, cell-type-specific local ripple generators can provide additional mechanisms for orthogonalization (figure 4). Indeed, optogenetic manipulations able to induce artificial CA1 ripples suggest that deep and superficial sublayers may actually form distinct ensembles [139].
Computational models of replay support the existence of multiple attractors resulting from different buffers of activity [5,140]. In some of these models, direct inputs bypassing the intrahippocampal circuit are required to favour encoding of reversed association [5,141]. In most cases, the experience is critical in establishing the organization of auto-associative ensembles and thus the order and content of replay. While retrospective and prospective firing can be simulated with these premises, evidence of preplay has questioned whether sequence reactivation during sharp-wave ripples requires encoding or rather it may result from existing ensembles [67,142]. Pre-determined cell-type-specific connectivity can favour pre-configured attractors. For instance, a single CA3 pyramidal cell typically gives rise to a number of collaterals projecting in different directions onto its neighbouring cells [143–145], with the pattern of connectivity depending on the location of the pre-synaptic soma [146,147]. Novel synapse labelling methods have revealed Schaffer collateral connections with CA1 are enriched between neurons sharing similar developmental periods [148]. Strikingly, CA3 connectivity with early developed deep CA1 pyramidal cells appears more highly structured and non-random than with late developed superficial cells [149]. This suggests there is a level of determinism that provides additional functional constraints. The way in which pre-configured and learned sequences coexist and interact remains to be understood.
Overrepresentation of reward, goal and aversive contexts bias replay for specific events [48,51,150–153]. The hippocampal sharp-wave ripple generator interacts brain-wide to hierarchically orchestrate these representations. New evidence supports that hippocampal–amygdala and hippocampal–hypothalamic interactions are more likely to involve subpopulations of deep cells [96,116] and that deep CA1 cells are more influenced by goals and rewards [90]. Similarly, state-dependent changes may affect differently deep and superficial layers given their different innervation by some GABAergic interneurons [113] and gradient expression of receptors for some neuromodulatory transmitters [154]. Consistently, deep cells phase shift during theta oscillations associated with REM sleep [88]. Therefore, different behavioural and emotional states could also contribute to orthogonalize replay (figure 4).
7. Conclusion
Understanding replay is challenging. Most of the work done so far has adopted conceptually separated views. On the one hand, sequence analysis has enabled identifying the organization of neuronal ensembles during replay and using them to decode neuronal representations. On the other hand, a physiological perspective has helped to pinpoint microcircuit mechanisms responsible for the basic field potential signatures of sharp-wave ripples. We need to fill the gap between the mechanisms and function of these events.
To better understand the complexities of replay, an updated view of sharp-wave ripple physiology has to incorporate the critical influence of cell-type-specific subcircuits that wire brain-wide the proximodistal and radial axes of CA1. We then need to exploit this specificity to evaluate how replay dynamics can be precisely controlled. Here, I have reviewed the evidence and discussed recent data supporting that firing of deep and superficial CA1 cells can be influenced differently during sharp-wave ripples. I suggest that cell-type- and input-specific connectivity together with radial expression of receptors and intrinsic properties may provide substrates for biasing hippocampal replay back and forth. How these variables can specifically affect the content and order of replay remains to be examined.
I propose relying on these mechanisms to force conceptual shifts regarding our understanding of the way replay is established and used to guide behaviour. Based on this review and emerging cell-type and region-specific data the following research questions and hypotheses can be addressed:
-
—
Are sharp-wave ripples multifocal? I hypothesize events may arise from different loci and engage different sets of neuronal sequences along the septo-temporal, proximodistal and deep–superficial axes of the hippocampus.
-
—
What is the role of cortical and subcortical inputs? Based on the different connectivity with deep and superficial pyramidal cells, contrasting and dynamic effects across CA1 sublayers can be predicted.
-
—
What determines the direction of propagation of sharp-wave ripples and how does it relate with replay order and content? I hypothesize that the generating loci, as well as participating GABAergic interneurons and dynamically fluctuating inputs, will bias replay in a predictable way.
-
—
To what extent can experience modify replay of deep and superficial subcircuits? Given their different developmental origin I predict different connectivity and plasticity rules determining preplay ability across sublayers.
-
—
Can manipulations of the replay content differently affect cognition? Given different brain-wide connectivity of deep and superficial cells, specific behavioural effects can be predicted.
The advent of super-resolution optoelectrodes and multicellular voltage imaging [139,155–158], in combination with single-cell transcriptomic public atlases and gene editing techniques [92,154,159–161], will certainly many of these questions to be addressed at unprecedented levels of detail in forthcoming years [162–164].
Acknowledgements
Thanks to Attila Gulyás, the two reviewers and participants of the Memory Reactivation workshop for inspiring discussions.
Data accessibility
This article has no additional data.
Competing interests
I declare no competing interests.
Funding
My research on sharp-wave ripples is currently supported by the Spanish Ministry of Science, Innovation and Universities (grant no. RTI2018-098581-B-I00).
References
- 1.O'Keefe J. 1978. The hippocampus as a cognitive map. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Buzsáki G, Moser EI. 2013. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 16, 130–138. ( 10.1038/nn.3304) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. 1996. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172. () [DOI] [PubMed] [Google Scholar]
- 4.Dragoi G, Buzsáki G. 2006. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50, 145–157. ( 10.1016/j.neuron.2006.02.023) [DOI] [PubMed] [Google Scholar]
- 5.Koene RA, Hasselmo ME. 2008. Reversed and forward buffering of behavioral spike sequences enables retrospective and prospective retrieval in hippocampal regions CA3 and CA1. Neural Netw. 21, 276–288. ( 10.1016/j.neunet.2007.12.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster DJ, Wilson MA. 2006. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683. ( 10.1038/nature04587) [DOI] [PubMed] [Google Scholar]
- 7.Huxter JR, Senior TJ, Allen K, Csicsvari J. 2008. Theta phase-specific codes for two-dimensional position, trajectory and heading in the hippocampus. Nat. Neurosci. 11, 587–594. ( 10.1038/nn.2106) [DOI] [PubMed] [Google Scholar]
- 8.Lisman J, Redish AD. 2009. Prediction, sequences and the hippocampus. Phil. Trans. R. Soc. B 364, 1193–1201. ( 10.1098/rstb.2008.0316) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Keefe J, Recce ML. 1993. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330. ( 10.1002/hipo.450030307) [DOI] [PubMed] [Google Scholar]
- 10.Lisman JE, Jensen O. 2013. The θ-γ neural code. Neuron 77, 1002–1016. ( 10.1016/j.neuron.2013.03.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta MR. 2015. From synaptic plasticity to spatial maps and sequence learning. Hippocampus 25, 756–762. ( 10.1002/hipo.22472) [DOI] [PubMed] [Google Scholar]
- 12.Buzsáki G. 1989. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. ( 10.1016/0306-4522(89)90423-5) [DOI] [PubMed] [Google Scholar]
- 13.O'Neill J, Pleydell-Bouverie B, Dupret D, Csicsvari J. 2010. Play it again: reactivation of waking experience and memory. Trends Neurosci. 33, 220–229. ( 10.1016/j.tins.2010.01.006) [DOI] [PubMed] [Google Scholar]
- 14.Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. 1992. High-frequency network oscillation in the hippocampus. Science 256, 1025–1027. ( 10.1126/science.1589772) [DOI] [PubMed] [Google Scholar]
- 15.Nádasdy Z, Hirase H, Czurkó A, Csicsvari J, Buzsáki G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507. ( 10.1523/JNEUROSCI.19-21-09497.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster DJ. 2017. Replay comes of age. Annu. Rev. Neurosci. 40, 581–602. ( 10.1146/annurev-neuro-072116-031538) [DOI] [PubMed] [Google Scholar]
- 17.Skaggs WE, McNaughton BL. 1996. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science 271, 1870–1873. ( 10.1126/science.271.5257.1870) [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer BE. 2017. The content of hippocampal ‘replay’. Hippocampus 30, 6–18. ( 10.1002/hipo.22824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ólafsdóttir HF, Bush D, Barry C. 2018. The role of hippocampal replay in memory and planning. Curr. Biol. 28, R37–R50. ( 10.1016/j.cub.2017.10.073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joo HR, Frank LM. 2018. The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci. 19, 744–757. ( 10.1038/s41583-018-0077-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones MW, McHugh TJ. 2011. Updating hippocampal representations: CA2 joins the circuit. Trends Neurosci. 34, 526–535. ( 10.1016/j.tins.2011.07.007) [DOI] [PubMed] [Google Scholar]
- 22.Witter MP, Moser EI. 2006. Spatial representation and the architecture of the entorhinal cortex. Trends Neurosci. 29, 671–678. ( 10.1016/j.tins.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 23.Strange BA, Witter MP, Lein ES, Moser EI. 2014. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 15, 655–669. ( 10.1038/nrn3785) [DOI] [PubMed] [Google Scholar]
- 24.Igarashi KM, Ito HT, Moser EI, Moser M-B. 2014. Functional diversity along the transverse axis of hippocampal area CA1. FEBS Lett. 588, 2470–2476. ( 10.1016/j.febslet.2014.06.004) [DOI] [PubMed] [Google Scholar]
- 25.Soltesz I, Losonczy A. 2018. CA1 pyramidal cell diversity enabling parallel information processing in the hippocampus. Nat. Neurosci. 21, 484–493. ( 10.1038/s41593-018-0118-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valero M, de la Prida LM. 2018. The hippocampus in depth: a sublayer-specific perspective of entorhinal–hippocampal function. Curr. Opin. Neurobiol. 52, 107–114. ( 10.1016/j.conb.2018.04.013) [DOI] [PubMed] [Google Scholar]
- 27.Buzsáki G. 2015. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. ( 10.1002/hipo.22488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson MA, McNaughton BL. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679. ( 10.1126/science.8036517) [DOI] [PubMed] [Google Scholar]
- 29.Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. ( 10.1016/S0896-6273(02)01096-6) [DOI] [PubMed] [Google Scholar]
- 30.Diba K, Buzsáki G. 2007. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 10, 1241–1242. ( 10.1038/nn1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambrose RE, Pfeiffer BE, Foster DJ. 2016. Reverse replay of hippocampal place cells is uniquely modulated by changing reward. Neuron 91, 1124–1136. ( 10.1016/j.neuron.2016.07.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davidson TJ, Kloosterman F, Wilson MA. 2009. Hippocampal replay of extended experience. Neuron 63, 497–507. ( 10.1016/j.neuron.2009.07.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singer AC, Carr MF, Karlsson MP, Frank LM. 2013. Hippocampal SWR activity predicts correct decisions during the initial learning of an alternation task. Neuron 77, 1163–1173. ( 10.1016/j.neuron.2013.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeiffer BE, Foster DJ. 2013. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79. ( 10.1038/nature12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. 2010. Hippocampal replay is not a simple function of experience. Neuron 65, 695–705. ( 10.1016/j.neuron.2010.01.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dragoi G, Tonegawa S. 2011. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469, 397–401. ( 10.1038/nature09633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wikenheiser AM, Redish AD. 2013. The balance of forward and backward hippocampal sequences shifts across behavioral states. Hippocampus 23, 22–29. ( 10.1002/hipo.22049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Csicsvari J, O'Neill J, Allen K, Senior T. 2007. Place-selective firing contributes to the reverse-order reactivation of CA1 pyramidal cells during sharp waves in open-field exploration. Eur. J. Neurosci. 26, 704–716. ( 10.1111/j.1460-9568.2007.05684.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson MP, Frank LM. 2009. Awake replay of remote experiences in the hippocampus. Nat. Neurosci. 12, 913–918. ( 10.1038/nn.2344) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomperts SN, Kloosterman F, Wilson MA. 2015. VTA neurons coordinate with the hippocampal reactivation of spatial experience. eLife 4, e5360 ( 10.7554/eLife.05360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pfeiffer BE, Foster DJ. 2015. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science 349, 180–183. ( 10.1126/science.aaa9633) [DOI] [PubMed] [Google Scholar]
- 42.Wu X, Foster DJ. 2014. Hippocampal replay captures the unique topological structure of a novel environment. J. Neurosci. 34, 6459–6469. ( 10.1523/JNEUROSCI.3414-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamamoto J, Tonegawa S. 2017. Direct medial entorhinal cortex input to hippocampal CA1 is crucial for extended quiet awake replay. Neuron 96, 217–227. ( 10.1016/j.neuron.2017.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliva A, Fernández-Ruiz A, Fermino de Oliveira E, Buzsáki G.. 2018. Origin of gamma frequency power during hippocampal sharp-wave ripples. Cell Rep. 25, 1693–1700. ( 10.1016/j.celrep.2018.10.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bendor D, Wilson MA. 2012. Biasing the content of hippocampal replay during sleep. Nat. Neurosci. 15, 1439–1444. ( 10.1038/nn.3203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rothschild G, Eban E, Frank LM. 2017. A cortical–hippocampal–cortical loop of information processing during memory consolidation. Nat. Neurosci. 20, 251–259. ( 10.1038/nn.4457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer AC, Frank LM. 2009. Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64, 910–921. ( 10.1016/j.neuron.2009.11.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. 2010. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 13, 995–1002. ( 10.1038/nn.2599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. 2014. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci. 17, 1658–1660. ( 10.1038/nn.3843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y, Romani S, Lustig B, Leonardo A, Pastalkova E. 2015. Theta sequences are essential for internally generated hippocampal firing fields. Nat. Neurosci. 18, 282–288. ( 10.1038/nn.3904) [DOI] [PubMed] [Google Scholar]
- 51.Wu C-T, Haggerty D, Kemere C, Ji D. 2017. Hippocampal awake replay in fear memory retrieval. Nat. Neurosci. 20, 571–580. ( 10.1038/nn.4507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pennartz CMA, Lee E, Verheul J, Lipa P, Barnes CA, McNaughton BL. 2004. The ventral striatum in off-line processing: ensemble reactivation during sleep and modulation by hippocampal ripples. J. Neurosci. 24, 6446–6456. ( 10.1523/JNEUROSCI.0575-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CMA. 2009. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 7, e1000173 ( 10.1371/journal.pbio.1000173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji D, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107. ( 10.1038/nn1825) [DOI] [PubMed] [Google Scholar]
- 55.Remondes M, Wilson MA. 2015. Slow-γ rhythms coordinate cingulate cortical responses to hippocampal sharp-wave ripples during wakefulness. Cell Rep. 13, 1327–1335. ( 10.1016/j.celrep.2015.10.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jadhav SP, Rothschild G, Roumis DK, Frank LM. 2016. Coordinated excitation and inhibition of prefrontal ensembles during awake hippocampal sharp-wave ripple events. Neuron 90, 113–127. ( 10.1016/j.neuron.2016.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Girardeau G, Inema I, Buzsáki G. 2017. Reactivations of emotional memory in the hippocampus–amygdala system during sleep. Nat. Neurosci. 20, 1634–1642. ( 10.1038/nn.4637) [DOI] [PubMed] [Google Scholar]
- 58.Yang M, Logothetis NK, Eschenko O. 2019. Occurrence of hippocampal ripples is associated with activity suppression in the mediodorsal thalamic nucleus. J. Neurosci. 39, 434–444. ( 10.1523/JNEUROSCI.2107-18.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ólafsdóttir HF, Carpenter F, Barry C. 2017. Task demands predict a dynamic switch in the content of awake hippocampal replay. Neuron 96, 925–935. ( 10.1016/j.neuron.2017.09.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey AA, Tanaka Y, van der Meer MAA. 2019. Reward revaluation biases hippocampal replay content away from the preferred outcome. Nat. Neurosci. 22, 1450–1459. ( 10.1038/s41593-019-0464-6) [DOI] [PubMed] [Google Scholar]
- 61.Ferbinteanu J, Shapiro ML. 2003. Prospective and retrospective memory coding in the hippocampus. Neuron 40, 1227–1239. ( 10.1016/s0896-6273(03)00752-9) [DOI] [PubMed] [Google Scholar]
- 62.Frank LM, Brown EN, Wilson M. 2000. Trajectory encoding in the hippocampus and entorhinal cortex. Neuron 27, 169–178. ( 10.1016/s0896-6273(00)00018-0) [DOI] [PubMed] [Google Scholar]
- 63.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. 2008. Internally generated cell assembly sequences in the rat hippocampus. Science 321, 1322–1327. ( 10.1126/science.1159775) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Roth Z, Pastalkova E. 2016. Synchronized excitability in a network enables generation of internal neuronal sequences. eLife 5, e20697 ( 10.7554/eLife.20697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu K, Sibille J, Dragoi G. 2018. Generative predictive codes by multiplexed hippocampal neuronal tuplets. Neuron 99, 1329–1341. ( 10.1016/j.neuron.2018.07.047) [DOI] [PubMed] [Google Scholar]
- 66.Stella F, Baracskay P, O'Neill J, Csicsvari J. 2019. Hippocampal reactivation of random trajectories resembling Brownian diffusion. Neuron 102, 450–461. ( 10.1016/j.neuron.2019.01.052) [DOI] [PubMed] [Google Scholar]
- 67.Dragoi G, Tonegawa S. 2013. Distinct preplay of multiple novel spatial experiences in the rat. Proc. Natl Acad. Sci. USA 110, 9100–9105. ( 10.1073/pnas.1306031110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grosmark AD, Buzsáki G. 2016. Diversity in neural firing dynamics supports both rigid and learned hippocampal sequences. Science 351, 1440–1443. ( 10.1126/science.aad1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buzsáki G, Mizuseki K. 2014. The log-dynamic brain: how skewed distributions affect network operations. Nat. Rev. Neurosci. 15, 264–278. ( 10.1038/nrn3687) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Penny WD, Zeidman P, Burgess N. 2013. Forward and backward inference in spatial cognition. PLoS Comput. Biol. 9, e1003383 ( 10.1371/journal.pcbi.1003383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de la Prida LM, Huberfeld G, Cohen I, Miles R. 2006. Threshold behavior in the initiation of hippocampal population bursts. Neuron 49, 131–142. ( 10.1016/j.neuron.2005.10.034) [DOI] [PubMed] [Google Scholar]
- 72.Schlingloff D, Káli S, Freund TF, Hájos N, Gulyás AI. 2014. Mechanisms of sharp wave initiation and ripple generation. J. Neurosci. 34, 11 385–11 398. ( 10.1523/JNEUROSCI.0867-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. 1995. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J. Neurosci. 15, 30–46. ( 10.1523/JNEUROSCI.15-01-00030.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Klausberger T, Somogyi P. 2008. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57. ( 10.1126/science.1149381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G. 2014. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480. ( 10.1016/j.neuron.2014.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chrobak JJ, Buzsáki G. 1996. High-frequency oscillations in the output networks of the hippocampal–entorhinal axis of the freely behaving rat. J. Neurosci. 16, 3056–3066. ( 10.1523/JNEUROSCI.16-09-03056.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel J, Schomburg EW, Berényi A, Fujisawa S, Buzsáki G. 2013. Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J. Neurosci. 33, 17 029–17 041. ( 10.1523/JNEUROSCI.2036-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Csicsvari J, Hirase H, Mamiya A, Buzsáki G. 2000. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28, 585–594. ( 10.1016/S0896-6273(00)00135-5) [DOI] [PubMed] [Google Scholar]
- 79.Stark E, Roux L, Eichler R, Buzsáki G. 2015. Local generation of multineuronal spike sequences in the hippocampal CA1 region. Proc. Natl Acad. Sci. USA 112, 10 521–10 526. ( 10.1073/pnas.1508785112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.English DF, Peyrache A, Stark E, Roux L, Vallentin D, Long MA, Buzsáki G. 2014. Excitation and inhibition compete to control spiking during hippocampal ripples: intracellular study in behaving mice. J. Neurosci. 34, 16 509–16 517. ( 10.1523/JNEUROSCI.2600-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Maier N, et al. 2011. Coherent phasic excitation during hippocampal ripples. Neuron 72, 137–152. ( 10.1016/j.neuron.2011.08.016) [DOI] [PubMed] [Google Scholar]
- 82.Bahner F, et al. 2011. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc. Natl Acad. Sci. USA 108, E607–E616. ( 10.1073/pnas.1103546108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Valero M, Cid E, Averkin RG, Aguilar J, Sanchez-Aguilera A, Viney TJ, Gomez-Dominguez D, Bellistri E, de la Prida LM. 2015. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat. Neurosci. 18, 1281–1290. ( 10.1038/nn.4074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hulse BK, Moreaux LC, Lubenov EV, Siapas AG. 2016. Membrane potential dynamics of CA1 pyramidal neurons during hippocampal ripples in awake mice. Neuron 89, 800–813. ( 10.1016/j.neuron.2016.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Navas-Olive A, Valero M, de Salas A, Jurado-Parras T, Averkin RG, Gambino G, Cid E, de la Prida LM. In preparation. Multimodal determinants of phase-locked dynamics across deep-superficial hippocampal sublayers during theta oscillations. https://biorxiv.org/cgi/content/short/2020.03.15.991935v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valero M, Averkin RG, Fernandez-Lamo I, Aguilar J, Lopez-Pigozzi D, Brotons-Mas JR, Cid E, Tamas G, Menendez de la Prida L. 2017. Mechanisms for selective single-cell reactivation during offline sharp-wave ripples and their distortion by fast ripples. Neuron 94, 1234–1247. ( 10.1016/j.neuron.2017.05.032) [DOI] [PubMed] [Google Scholar]
- 87.Reichinnek S, Künsting T, Draguhn A, Both M. 2010. Field potential signature of distinct multicellular activity patterns in the mouse hippocampus. J. Neurosci. 30, 15 441–15 449. ( 10.1523/JNEUROSCI.2535-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mizuseki K, Diba K, Pastalkova E, Buzsáki G. 2011. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat. Neurosci. 14, 1174–1181. ( 10.1038/nn.2894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geiller T, Fattahi M, Choi J-S, Royer S. 2017. Place cells are more strongly tied to landmarks in deep than in superficial CA1. Nat. Commun. 8, 14531 ( 10.1038/ncomms14531) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Danielson NB, Zaremba JD, Kaifosh P, Bowler J, Ladow M, Losonczy A. 2016. Sublayer-specific coding dynamics during spatial navigation and learning in hippocampal area CA1. Neuron 91, 652–665. ( 10.1016/j.neuron.2016.06.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fernández-Ruiz A, Oliva A, Nagy GA, Maurer AP, Berényi A, Buzsáki G. 2017. Entorhinal-CA3 dual-input control of spike timing in the hippocampus by theta-gamma coupling. Neuron 93, 1213–1226. ( 10.1016/j.neuron.2017.02.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cembrowski MS, Wang L, Sugino K, Shields BC, Spruston N. 2016. Hipposeq: a comprehensive RNA-seq database of gene expression in hippocampal principal neurons. eLife 5, e14997 ( 10.7554/eLife.14997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang DV, Yau HJ, Broker CJ, Tsou JH, Bonci A, Ikemoto S. . 2015. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat Neurosci. 18, 728–735. ( 10.1038/nn.3998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Y, et al. 2017. A distinct entorhinal cortex to hippocampal CA1 direct circuit for olfactory associative learning. Nat. Neurosci. 20, 559–570. ( 10.1038/nn.4517) [DOI] [PubMed] [Google Scholar]
- 95.O'Neill J, Boccara CN, Stella F, Schoenenberger P, Csicsvari J. 2017. Superficial layers of the medial entorhinal cortex replay independently of the hippocampus. Science 355, 184–188. ( 10.1126/science.aag2787) [DOI] [PubMed] [Google Scholar]
- 96.Jimenez JC, et al. 2018. Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97, 670–683. ( 10.1016/j.neuron.2018.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oliva A, Fernández-Ruiz A, Buzsáki G, Berényi A. 2016. Role of hippocampal CA2 region in triggering sharp-wave ripples. Neuron 91, 1342–1355. ( 10.1016/j.neuron.2016.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nokia MS, Mikkonen JE, Penttonen M, Wikgren J. 2020. Disrupting neural activity related to awake-state sharp wave-ripple complexes prevents hippocampal learning. Front. Behav. Neurosci. 6, 84 ( 10.3389/fnbeh.2012.00084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Masurkar AV, Srinivas KV, Brann DH, Warren R, Lowes DC, Siegelbaum SA. 2017. Medial and lateral entorhinal cortex differentially excite deep versus superficial CA1 pyramidal neurons. Cell Rep. 18, 148–160. ( 10.1016/j.celrep.2016.12.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bellistri E, Aguilar J, Brotons-Mas JR, Foffani G, de la Prida LM. 2013. Basic properties of somatosensory-evoked responses in the dorsal hippocampus of the rat. J. Physiol. 591, 2667–2686. ( 10.1113/jphysiol.2013.251892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Henriksen EJ, Colgin LL, Barnes CA, Witter MP, Moser M-B, Moser EI. 2010. Spatial representation along the proximodistal axis of CA1. Neuron 68, 127–137. ( 10.1016/j.neuron.2010.08.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Beer Z, Vavra P, Atucha E, Rentzing K, Heinze H-J, Sauvage MM. 2018. The memory for time and space differentially engages the proximal and distal parts of the hippocampal subfields CA1 and CA3. PLoS Biol. 16, e2006100 ( 10.1371/journal.pbio.2006100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee H, Wang C, Deshmukh SS, Knierim JJ. 2015. Neural population evidence of functional heterogeneity along the CA3 transverse axis: pattern completion versus pattern separation. Neuron 87, 1093–1105. ( 10.1016/j.neuron.2015.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Neunuebel JP, Knierim JJ. 2014. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427. ( 10.1016/j.neuron.2013.11.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sasaki T, Piatti VC, Hwaun E, Ahmadi S, Lisman JE, Leutgeb S, Leutgeb JK. 2018. Dentate network activity is necessary for spatial working memory by supporting CA3 sharp-wave ripple generation and prospective firing of CA3 neurons. Nat. Neurosci. 21, 258–269. ( 10.1038/s41593-017-0061-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kohara K, et al. 2014. Cell type-specific genetic and optogenetic tools reveal hippocampal CA2 circuits. Nat. Neurosci. 17, 269–279. ( 10.1038/nn.3614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nasrallah K, Therreau L, Robert V, Huang AJY, McHugh TJ, Piskorowski RA, Chevaleyre V. 2019. Routing hippocampal information flow through parvalbumin interneuron plasticity in area CA2. Cell Rep. 27, 86–98. ( 10.1016/j.celrep.2019.03.014) [DOI] [PubMed] [Google Scholar]
- 108.Mankin EA, Sparks FT, Slayyeh B, Sutherland RJ, Leutgeb S, Leutgeb JK. 2012. Neuronal code for extended time in the hippocampus. Proc. Natl Acad. Sci. USA 109, 19 462–19 467. ( 10.1073/pnas.1214107109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Okuyama T, Kitamura T, Roy DS, Itohara S, Tonegawa S. 2016. Ventral CA1 neurons store social memory. Science 353, 1536–1541. ( 10.1126/science.aaf7003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernandez-Lamo I, Gomez-Dominguez D, Sanchez-Aguilera A, Oliva A, Morales AV, Valero M, Cid E, Berenyi A, Menendez de la Prida L. 2019. Proximodistal organization of the CA2 hippocampal area. Cell Rep. 26, 1734–1746. ( 10.1016/j.celrep.2019.01.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kay K, Sosa M, Chung JE, Karlsson MP, Larkin MC, Frank LM. 2016. A hippocampal network for spatial coding during immobility and sleep. Nature 531, 185–190. ( 10.1038/nature17144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Freund TF, Buzsáki G. 1998. Interneurons of the hippocampus. Hippocampus 6, 347–470. () [DOI] [PubMed] [Google Scholar]
- 113.Lapray D, et al. 2012. Behavior-dependent specialization of identified hippocampal interneurons. Nat. Neurosci. 15, 1265–1271. ( 10.1038/nn.3176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Katona L, Lapray D, Viney TJ, Oulhaj A, Borhegyi Z, Micklem BR, Klausberger T, Somogyi P. 2014. Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron 82, 872–886. ( 10.1016/j.neuron.2014.04.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Katona L, Micklem B, Borhegyi Z, Swiejkowski DA, Valenti O, Viney TJ, Kotzadimitriou D, Klausberger T, Somogyi P. 2017. Behavior-dependent activity patterns of GABAergic long-range projecting neurons in the rat hippocampus. Hippocampus 27, 359–377. ( 10.1002/hipo.22696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee S-H, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I. 2014. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82, 1129–1144. ( 10.1016/j.neuron.2014.03.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leão RN, et al. 2012. OLM interneurons differentially modulate CA3 and entorhinal inputs to hippocampal CA1 neurons. Nat. Neurosci. 15, 1524–1530. ( 10.1038/nn.3235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kitamura T, Pignatelli M, Suh J, Kohara K, Yoshiki A, Abe K, Tonegawa S. 2014. Island cells control temporal association memory. Science 343, 896–901. ( 10.1126/science.1244634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wikenheiser AM, Redish AD. 2011. Changes in reward contingency modulate the trial-to-trial variability of hippocampal place cells. J. Neurophysiol. 106, 589–598. ( 10.1152/jn.00091.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Buzsáki G, Czopf J, Kondákor I, Kellényi L. 1986. Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res. 365, 125–137. ( 10.1016/0006-8993(86)90729-8) [DOI] [PubMed] [Google Scholar]
- 121.Ólafsdóttir HF, Carpenter F, Barry C. 2016. Coordinated grid and place cell replay during rest. Nat. Neurosci. 19, 792–794. ( 10.1038/nn.4291) [DOI] [PubMed] [Google Scholar]
- 122.Peyrache A, Battaglia FP, Destexhe A. 2011. Inhibition recruitment in prefrontal cortex during sleep spindles and gating of hippocampal inputs. Proc. Natl Acad. Sci. USA 108, 17 207–17 212. ( 10.1073/pnas.1103612108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rajasethupathy P, et al. 2015. Projections from neocortex mediate top-down control of memory retrieval. Nature 526, 653–659. ( 10.1038/nature15389) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsáki G. 2011. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci. 31, 8605–8616. ( 10.1523/JNEUROSCI.0294-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheng S, Frank LM. 2008. New experiences enhance coordinated neural activity in the hippocampus. Neuron 57, 303–313. ( 10.1016/j.neuron.2007.11.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Swaminathan A, Wichert I, Schmitz D, Maier N. 2018. Involvement of mossy cells in sharp wave-ripple activity in vitro. Cell Rep. 23, 2541–2549. ( 10.1016/j.celrep.2018.04.095) [DOI] [PubMed] [Google Scholar]
- 127.Okamoto K, Ikegaya Y. 2019. Recurrent connections between CA2 pyramidal cells. Hippocampus 29, 305–312. ( 10.1002/hipo.23064) [DOI] [PubMed] [Google Scholar]
- 128.Hunt DL, Linaro D, Si B, Romani S, Spruston N. 2018. A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus. Nat. Neurosci. 21, 985–995. ( 10.1038/s41593-018-0172-7) [DOI] [PubMed] [Google Scholar]
- 129.Sun Q, Sotayo A, Cazzulino AS, Snyder AM, Denny CA, Siegelbaum SA. 2017. Proximodistal heterogeneity of hippocampal CA3 pyramidal neuron intrinsic properties, connectivity, and reactivation during memory recall. Neuron 95, 656–672. ( 10.1016/j.neuron.2017.07.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramirez-Villegas JF, Logothetis NK, Besserve M. 2015. Diversity of sharp-wave–ripple LFP signatures reveals differentiated brain-wide dynamical events. Proc. Natl Acad. Sci. USA 112, E6379–E6387. ( 10.1073/pnas.1518257112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Malvache A, Reichinnek S, Villette V, Haimerl C, Cossart R. 2016. Awake hippocampal reactivations project onto orthogonal neuronal assemblies. Science 353, 1280–1283. ( 10.1126/science.aaf3319) [DOI] [PubMed] [Google Scholar]
- 132.Chenani A, Sabariego M, Schlesiger MI, Leutgeb JK, Leutgeb S, Leibold C. 2019. Hippocampal CA1 replay becomes less prominent but more rigid without inputs from medial entorhinal cortex. Nat. Commun. 10, 1341 ( 10.1038/s41467-019-09280-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chevaleyre V, Siegelbaum SA. 2010. Strong CA2 pyramidal neuron synapses define a powerful disynaptic cortico-hippocampal loop. Neuron 66, 560–572. ( 10.1016/j.neuron.2010.04.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miles R, Wong RK. 1983. Single neurones can initiate synchronized population discharge in the hippocampus. Nature 306, 371–373. ( 10.1038/306371a0) [DOI] [PubMed] [Google Scholar]
- 135.Sadowski JHLP, Jones MW, Mellor JR. 2016. Sharp-wave ripples orchestrate the induction of synaptic plasticity during reactivation of place cell firing patterns in the hippocampus. Cell Rep. 14, 1916–1929. ( 10.1016/j.celrep.2016.01.061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Taxidis J, Anastassiou CA, Diba K, Koch C. 2015. Local field potentials encode place cell ensemble activation during hippocampal sharp wave ripples. Neuron 87, 590–604. ( 10.1016/j.neuron.2015.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cannon J, Kopell N, Gardner T, Markowitz J. 2015. Neural sequence generation using spatiotemporal patterns of inhibition. PLoS Comput. Biol. 11, e1004581 ( 10.1371/journal.pcbi.1004581) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Basu J, Zaremba JD, Cheung SK, Hitti FL, Zemelman BV, Losonczy A, Siegelbaum SA. 2016. Gating of hippocampal activity, plasticity, and memory by entorhinal cortex long-range inhibition. Science 351, aaa5694 ( 10.1126/science.aaa5694) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu F, Stark E, Ku P-C, Wise KD, Buzsáki G, Yoon E. 2015. Monolithically integrated μLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Neuron 88, 1136–1148. ( 10.1016/j.neuron.2015.10.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jensen O, Lisman JE. 2005. Hippocampal sequence-encoding driven by a cortical multi-item working memory buffer. Trends Neurosci. 28, 67–72. ( 10.1016/j.tins.2004.12.001) [DOI] [PubMed] [Google Scholar]
- 141.Treves A, Rolls ET. 1992. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus 2, 189–199. ( 10.1002/hipo.450020209) [DOI] [PubMed] [Google Scholar]
- 142.Farooq U, Dragoi G. 2019. Emergence of preconfigured and plastic time-compressed sequences in early postnatal development. Science 363, 168–173. ( 10.1126/science.aav0502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ishizuka N, Weber J, Amaral DG. 1990. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J. Comp. Neurol. 295, 580–623. ( 10.1002/cne.902950407) [DOI] [PubMed] [Google Scholar]
- 144.Sik A, Tamamaki N, Freund TF. 1993. Complete axon arborization of a single CA3 pyramidal cell in the rat hippocampus, and its relationship with postsynaptic parvalbumin-containing interneurons. Eur. J. Neurosci. 5, 1719–1728. ( 10.1111/j.1460-9568.1993.tb00239.x) [DOI] [PubMed] [Google Scholar]
- 145.Li X-G, Somogyi P, Ylinen A, Buzsáki G. 1994. The hippocampal CA3 network: an in vivo intracellular labeling study. J. Comp. Neurol. 339, 181–208. ( 10.1002/cne.903390204) [DOI] [PubMed] [Google Scholar]
- 146.Wittner L, Henze DA, Záborszky L, Buzsáki G. 2006. Hippocampal CA3 pyramidal cells selectively innervate aspiny interneurons. Eur. J. Neurosci. 24, 1286–1298. ( 10.1111/j.1460-9568.2006.04992.x) [DOI] [PubMed] [Google Scholar]
- 147.Wittner L, Miles R. 2007. Factors defining a pacemaker region for synchrony in the hippocampus. J. Physiol. 584, 867–883. ( 10.1113/jphysiol.2007.138131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Druckmann S, Feng L, Lee B, Yook C, Zhao T, Magee JC, Kim J. 2014. Structured synaptic connectivity between hippocampal regions. Neuron 81, 629–640. ( 10.1016/j.neuron.2013.11.026) [DOI] [PubMed] [Google Scholar]
- 149.Kwon O, Feng L, Druckmann S, Kim J. 2018. Schaffer collateral inputs to CA1 excitatory and inhibitory neurons follow different connectivity rules. J. Neurosci. 38, 5140–5152. ( 10.1523/JNEUROSCI.0155-18.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI. 2001. Accumulation of hippocampal place fields at the goal location in an annular watermaze task. J. Neurosci. 21, 1635–1644. ( 10.1523/JNEUROSCI.21-05-01635.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hok V, Lenck-Santini P-P, Roux S, Save E, Muller RU, Poucet B. 2007. Goal-related activity in hippocampal place cells. J. Neurosci. 27, 472–482. ( 10.1523/JNEUROSCI.2864-06.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gauthier JL, Tank DW. 2018. A dedicated population for reward coding in the hippocampus. Neuron 99, 179–193. ( 10.1016/j.neuron.2018.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Michon F, Sun J-J, Kim CY, Ciliberti D, Kloosterman F. 2019. Post-learning hippocampal replay selectively reinforces spatial memory for highly rewarded locations. Curr. Biol. 29, 1436–1444. ( 10.1016/j.cub.2019.03.048) [DOI] [PubMed] [Google Scholar]
- 154.Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N. 2016. Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron 89, 351–368. ( 10.1016/j.neuron.2015.12.013) [DOI] [PubMed] [Google Scholar]
- 155.Stringer C, Pachitariu M, Steinmetz N, Reddy CB, Carandini M, Harris KD. 2019. Spontaneous behaviors drive multidimensional, brainwide activity. Science 364, eaav7893 ( 10.1126/science.aav7893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jun JJ, et al. 2017. Fully integrated silicon probes for high-density recording of neural activity. Nature 551, 232–236. ( 10.1038/nature24636) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Adam Y, et al. 2019. Voltage imaging and optogenetics reveal behaviour-dependent changes in hippocampal dynamics. Nature 569, 413–417. ( 10.1038/s41586-019-1166-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Piatkevich KD, et al. 2019. Population imaging of neural activity in awake behaving mice. Nature 574, 413–417. ( 10.1038/s41586-019-1641-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lein E, Borm LE, Linnarsson S. 2017. The promise of spatial transcriptomics for neuroscience in the era of molecular cell typing. Science 358, 64–69. ( 10.1126/science.aan6827) [DOI] [PubMed] [Google Scholar]
- 160.Fenno LE, et al. 2014. Targeting cells with single vectors using multiple-feature Boolean logic. Nat. Methods 11, 763–772. ( 10.1038/nmeth.2996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Madisen L, et al. 2015. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958. ( 10.1016/j.neuron.2015.02.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gridchyn I, Schoenenberger P, O'Neill J, Csicsvari J. . In press. Assembly-specific disruption of hippocampal replay leads to selective memory deficit. Neuron. ( 10.1016/j.neuron.2020.01.021) [DOI] [PubMed] [Google Scholar]
- 163.Shin JD, Tang W, Jadhav SP. 2019. Dynamics of awake hippocampal-prefrontal replay for spatial learning and memory-guided decision making. Neuron 104, 1110–1125.e7. ( 10.1016/j.neuron.2019.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tingley D, Buzsáki G. 2020. Routing of hippocampal ripples to subcortical structures via the lateral septum. Neuron 105, 138–149.e5. ( 10.1016/j.neuron.2019.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.