Abstract
Neural activity during ripples has attracted great theoretical and experimental attention over the last three decades. Perhaps one reason for such interest is that ripples occur during quiet waking moments and during sleep, times when we reflect and dream about what has just occurred and what we expect to happen next. The hope is that understanding such ‘offline’ activity may yield insights into reflection, planning, and the purposes of sleep. This review focuses on the mechanisms by which neurons organize during these high-frequency events. In studying ripples, broader principles have emerged that relate intrinsic neural properties, network topology and synaptic plasticity in controlling neural activity. Ripples, therefore, serve as an excellent model for studying how properties of a neural network relate to neural dynamics.
This article is part of the Theo Murphy meeting issue ‘Memory reactivation: replaying events past, present and future’.
Keywords: ripple, sequence, plasticity
1. Introduction
According to the standard model of memory consolidation, hippocampal synapses change quickly to afford fast learning, thereby bearing the brunt of interference-dependent forgetting [1]. Long-term memory storage occurs in the cortex, which changes more slowly, to update existing semantic structure via a hippocampal teacher signal. A body of work has been motivated by the hypothesis that the teaching signal is provided by the reactivation of waking patterns during sharp-wave ripples [1–3]. Despite the appeal of this framework and the empirical evidence that ripples are important for memory expression [4,5], it is not known what aspects of ripple activity are important for neural computation. Several options exist, such as the synchronous burst of hippocampal activity, the coordination of this burst with receptive downstream regions, the identity of which hippocampal neurons fire together during the ripple and the sequence of those neurons relative to one another. From the perspective of the standard consolidation model, the identity and order of the hippocampal neurons matter, as the set of neurons firing during the ripple defines a pointer to a particular cortical module, and the hippocampal sequence sets the order in which those cortical modules unfold [6,7].
Upon this theoretical backdrop, we will review the current state of knowledge concerning why certain neurons fire as they do during sharp-wave ripples. First, we overview and discuss findings related to the physiology that produces the approximately 140–250 Hz ripple (see Mechanisms of ripple rhythmogenesis). We then consider studies that shed light on how neurons become selectively recruited during these population bursts (see Mechanisms driving neural organization during ripples). It has recently become clear that replay and ripple-band activity are not unique to the CA1 region of the hippocampus. Therefore, the last section is dedicated to summarizing the evidence for extra-hippocampal ripples (see Ripple oscillations beyond the hippocampus), with the goal of identifying more universal organizing principles that allow sequences of neurons to unfold when networks are pushed with a strong excitatory drive.
2. Mechanisms of ripple rhythmogenesis
(a). What controls ripple frequency in the hippocampus?
Synchronous neural depolarization is needed for CA1 to generate a ripple. The ‘sharp-wave’ in ‘sharp-wave ripple (SPW-R)’ refers to the fact that the pyramidal layer ripple oscillation is usually accompanied by a negative deflection in the local field potential (LFP) in the CA1 stratum radiatum [8], generated by the current sink associated with glutamatergic inputs from the CA3 via the Schaffer collaterals. However, it has been suggested that any sufficiently strong input could trigger a ripple response, and subsets of ripples are observed without a sharp wave [9], which may be driven by inputs from CA2 [10], the entorhinal cortex [11] or after hyperpolarization-induced rebound spiking [12]. Notably, ripple-like events, termed ‘induced high-frequency oscillations (iHFOs)’, can be elicited by non-rhythmic optogenetic depolarization of CA1 pyramidal neurons, suggesting that the circuitry intrinsic to CA1 is sufficient for ripple generation [13]. Acute optogenetic silencing of CA3 pyramidal neurons strongly reduces the incidence of CA1 ripples [11,14], showing that, in the intact brain, CA3 is the main ripple driver for CA1.
Several hypotheses have been put forth for how excitatory barrages result in the organized ripple. The main candidates have been: excitatory to inhibitory feedback loops, reciprocal inhibition between GABAergic interneurons (through synapses or gap junctions [15]), pacemaking by excitation [16] or via an axonal plexus formed by pyramidal cells axons connected by gap junctions [17]. The role of rhythmic inhibition was recognized by early work [8], published shortly after the initial report describing neuronal participation in ripples [18] and has largely stood the test of time.
A seemingly parsimonious explanation for the CA1 ripple would be an inheritance of the rhythm from area CA3, as high-frequency oscillations are found in CA3, particularly in reduced preparations such as acute slices [19–21]. In vivo, CA3 neurons are indeed active prior to SPW-Rs [22–25], but the population frequency is slower than that of CA1 ripples, and the regions lack strong oscillatory phase coherence [23,25]. Despite the lack of rhythmic coupling, it was recently suggested that a low frequency, CA3-derived gamma rhythm paces discrete transitions in CA1 ripple sequencing [26]. Subsequent analysis, however, showed that the apparent gamma oscillation reflected the spectral decomposition of the interval between bursts of ripples [27]. That CA1 ripples persist after genetic disruption of CA3 synaptic vesicle binding [28] shows that CA1 can produce ripples without CA3 input (albeit at a lower frequency).
Observations of high-frequency oscillations in hippocampal slices persisting in the absence of calcium in the bath (inhibiting synaptic transmission) and which are blocked by halothane (a gap-junction blocker) [29] prompted the axon plexus hypothesis, which posits that gap junctions between pyramidal cell axons allow electrical activity to spread between pyramidal cells to support ripples [17,29,30]. A key tenet of this theory is that the action potentials during ripples are necessarily antidromic, initiating in the axon (depolarized by an electrically coupled partner pyramidal cell) and travelling to the soma, a phenomenon which was observed in acute slices [17,29,31]. However, in freely behaving/sleeping mice, intracellular pyramidal cell action potential waveforms lack the signatures of antidromic spikes, reflecting an exclusively orthodromic origin [32]. Furthermore, ripples persist in connexin knock-out mice that lack normal gap junctions [33,34]. Therefore, the data strongly imply that such a gap-junction-mediated mechanism is not likely to drive the in vivo rhythm.
The roles for excitation and inhibition in pacing the ripple rhythm are also a matter of debate [13,35]. Though the spiking output of both CA1 pyramidal cells and GABAergic interneurons show phase-locked activity during ripples [13,25,32,36,37], the phasic modulation of the driving currents is less clear, and the timing of excitatory and inhibitory post-synaptic potential/post-synaptic currents has been controversial [35,38–40].
Early work under urethane anaesthesia suggested that the primary driver of the ripple frequency is inhibition, as experimental hyperpolarization of Vm below the Cl− reversal potential or loading with supraphysiological chloride produced a near phase reversal in the Vm rhythm in relation to that of the LFP, as expected if fluctuations in the intracellular membrane were dictated by GABAA receptors [8]. This evidence has recently been challenged by Hulse et al. [38], who instead found that the intracellular ripple phase continuously changes with the membrane potentials between the GABAA and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) reversal potentials, indicative of additional involvement of AMPA receptor-mediated currents and rhythmic excitation. However, these experiments were done under current-clamp conditions, which offer only limited temporal resolution. These issues were addressed by Gan et al. [39], who voltage-clamped pyramidal neurons in awake mice to measure synaptic currents, and used intracellular loading of cells with QX-314, to clamp at depolarized potentials without spike induction, and caesium to decrease the electrotonic length and amplify signal-to-noise of distal synaptic currents. Using this technique, the authors found that inhibitory post-synaptic currents (IPSCs), and not excitatory post-synaptic currents (EPSCs), were phase-locked to the LFP ripples, demonstrating that inhibition is at the least the major driver of the ripple frequency and arguing against models in which either CA3 or the sparse excitatory recurrent connections in CA1 sustain the oscillation [41].
CA1 parvalbumin-positive (PV+) GABAergic interneurons are the best candidate for the source of this inhibition. Fast-spiking, PV+ cells show strong phase locking to ripples [13,25,42–45] and can pace the CA1 pyramidal cell spiking [13]. Optogenetic inhibition of PV+ interneurons reduces inhibitory conductances and ripple duration and disrupts the IPSC phase locking to the ripple [39]. Local picrotoxin administration in urethane-anaesthetized mice was shown to inhibit ripple oscillations, suggesting a requirement for GABAA receptors [13]. The abundance of the evidence thus supports the hypothesis that GABAA-receptor-mediated currents, activated by inputs from PV+ interneurons (and possibly others [36]), control the frequency of the ripple oscillation in CA1.
If ripples can be generated locally, how does CA1 show such tight phase synchrony during large-amplitude ripple events [46]? Artificial, optogenetic generation of iHFOs does not increase the firing rate of neighbouring neurons (200 µm) away, but does drive the firing phase of distant interneurons, and not pyramidal cells, to be synchronized with the local oscillation [13]. This influence on phasic interneuron activity probably synchronizes anatomically separated rhythm generators, as ripple-band coherence between nearby electrodes is eliminated with picrotoxin, thus showing a role for GABAergic signalling in such coordination [13]. To complete the loop, the optogenetic drive of fast-spiking interneurons synchronizes pyramidal spikes at ripple frequency [13]. Together, this work shows an important role of feedback inhibition in both coordinating and pacing CA1 ripples.
Assuming that ripples are the result of local CA1 pyramidal-interneuron (PYR-INT) and interneuron-interneuron (INT-INT) loops, what dictates the network frequency, and can the observed spectral properties of ripples allow us to understand anything about the underlying network computations? This is an important issue because learning is often accompanied by changes in ripple frequency, duration, rate of occurrence and amplitude [5,47–50].
There have been several modelling approaches to elucidate the mechanisms that control neural synchrony during ripples. The conclusions of this modelling work highly depend on the parameters of the model, in particular, the degree to which the system is driven by stochastic inputs, often referred to as ‘noise’, and the nature of the recurrent connections. On one extreme, the classic approach is to study the network as weakly coupled oscillators [51–53]. These types of models (e.g. pyramidal-interneuron gamma/interneuronal gamma) are typically employed in a low noise regime in which each neuron fires on every cycle of the network oscillation. This does not appear to be the case in the hippocampus, as pyramidal cells do not fire on every ripple cycle, or regularly, although certain classes of inhibitory interneurons do show regular periodic firing during ripples [36].
Instead, pyramidal cell firing is sparse and the firing of any individual neuron cannot indicate whether a ripple is present or not. Brunel & Wang [54] modelled ripples as a recurrent network with PYR-INT and INT-INT loops where the activity of neurons is dominated by stochastic synaptic noise. In these types of networks, recurrent inhibitory loops oscillate faster, with the period set by the time constant of the rise time of the inhibitory conductances. PYR-INT networks naturally resonate slower, as the feedback signal must pass through two types of synapses whose time constants summate to set the overall network frequency. The frequency of a network composed of both types of recurrence is therefore a weighted compromise between INT-INT networks and PYR-INT networks that tends to higher frequencies when INT-INT networks dominate and lower when PYR-INT networks are most active [54].
In contrast with the modelling predictions from Brunel & Wang [54], increasing the drive to CA1 (either with large Schaffer collateral input or optogenetic stimulation) causes an increase in ripple frequency [13], without affecting the degree of intracellular depolarization [38]. Large current sinks in the stratum radiatum recruit more pyramidal cells while the degree of interneuron recruitment is roughly stable [25]. That the recruitment of more pyramidal cells is associated with faster network frequency suggests that the rhythm is being set by factors beyond a balance between excitatory and inhibitory loops. In fact, genetically reducing the excitatory drive to PV+ interneurons does not change ripple frequency [55], but does increase ripple power, results that can be theoretically realized in a network of inhibitory loops receiving rhythmic input [56]. These results, and others [57], suggest the purpose of feedback inhibition onto pyramidal cells is to sculpt ripple membership through lateral inhibition, rather than set ripple pace.
Even the models that place a central role for recurrent inhibition in setting ripple frequency differ fundamentally in the mechanism. For example, one model emphasized that, throughout the ripple, the CA1 circuit synchronizes and, in doing so, leaves the noise-driven regime. The shift from external stochastic inputs to recurrent synchronized drive causes the network frequency to become more yoked to the slower rate of the individual members and for the rhythm to persist longer than would be expected, given equivalent drive in the initial noise-dominated regime [58]. An opposing conclusion was drawn by Malerba et al. [59], who suggested instead that feedback loops are hardly needed in CA1 to generate a rhythm as strong input can reset the phase of relatively independent inhibitory neurons thus generating strong coherence [59,60]. This reset, in turn, phase-locks pyramidal cells to produce the network ripple [59]. A key difference between the models is the degree to which neural activity is dominated by external stochastic inputs, where in Marlerba et al. [59], the noise level is so high that the system lives in the non-periodic, asynchronous regime even during ripples [61], such that feedback connections terminate the ripple event by amplifying noise—thus explaining why ripples can show a narrow distribution of duration and a wide distribution of frequency [59]. Clearly, more work is needed—in particular recordings of EPSCs/IPSCs in inhibitory neurons—before the field can settle on a satisfactory model for ripple rhythmogenesis.
3. Mechanisms driving neural organization during ripples
(a). What controls which neurons fire during any particular ripple?
Neurons fire action potentials according to the temporal pattern of synaptic excitation and inhibition they receive and the interaction between these currents and the intrinsic properties of the neuron. Although the learning and memory field often focuses on the pattern of synaptic drive creating heterogeneity in the postsynaptic response, intrinsic differences between neurons probably play a large role in dictating the identity of neuron firing at any given moment.
What is the evidence for such a role of intrinsic variability in determining ripple recruitment and, therefore, replay content? It has been noted that there is a strong correlation in neural firing rate across a variety of different conditions including different environments, different stages of sleep and during ripples [62]. Statistical models of place field allotment in large, featureless linear tracks show that field distribution can be well explained by a model in which any given cell lays down a place field in a random location at a fixed probability, which varies across neurons [63]. More recent studies show that this probability is fixed across environments and over weeks of recordings, suggesting that this heterogeneity is driven by differences in intrinsic neural excitability (potentially including number/strength of synaptic inputs) [64]. In support of this hypothesis, neurons that had a place field in a novel environment showed a lower threshold to spiking with intracellular current injection prior to that first-time exposure [65]. Although ripple recruitment was not addressed in that study, this observation dovetails with the preplay literature (see below) in suggesting that a neuron's recruitment is owing in large part to the intrinsic properties of that cell. This point was emphasized by Grosmark & Buzsaki [66] who found that high firing rate cells were most likely to have place fields and showed the largest degree of ripple recruitment before and after a first-time experience.
To study ripple recruitment in the absence of synaptic drive from CA3, two studies used pyramidal-specific optogenetic activation to induce artificial high-frequency oscillations and cell spiking. Both studies [5,67] found a strong correlation between the recruitment of neurons in the natural ripples and those that fired with optogenetic stimulation. Because the excitatory drive occurred randomly, these results suggest that intrinsic excitability strongly biases ripple membership and that lateral inhibition prevents the firing of those less excitable neurons, which presumably received the same strong optogenetic drive.
The origin of the firing rate heterogeneity is unknown, and part of this dispersion probably derives from asymmetries in the synaptic inputs. This hypothesis was tested in an ex vivo experiment in which the neurons that fired during a first-time experience were tagged with a fluorescent protein under control of a modified enhancer sequence for the immediate early gene Arc [68]. Then, mice were sacrificed, and acute slices, including CA1 and CA3, were prepared to compare the Arc+ and Arc− neurons (fluorescent and non-fluorescent, respectively). The authors found that the tagged (Arc+, fluorescent) neurons were more likely to be recruited into the in vitro ripple. Critically, the tagged neurons showed a higher excitatory : inhibitory (E : I) ratio, which was driven exclusively by higher amplitude EPSPs, showing that differences in excitation and not inhibition drive ripple recruitment, at least in vitro [68]. These findings complement those of Pouille et al. [69] who systematically varied the stimulation strength of Schaffer collaterals while monitoring evoked EPSPs in a pair of simultaneously recorded pyramidal cells in vitro. They found that the spiking threshold was dynamic and varied with the strength of excitatory inputs, with the propensity for one neuron to fire over another largely explained by heterogeneities in the distribution of excitatory, and not inhibitory, currents [69].
In vivo estimations of E : I balance have also found that ripple recruitment can be predicted through synaptic mechanisms. Using intracellular recordings in urethane-anaesthetized rats, Valero et al. clustered ripples by LFP similarity and held neurons at varying membrane potentials to estimate the driving force at each moment. Those subsets of ripples for which a neuron participated were associated with higher driving forces, thus showing a synaptic mechanism for selective recruitment into one ripple type over another [57].
Whatever the mechanism that causes one neuron to fire more often than another, it is clear that this drive cannot sustain high rates throughout the duration of the ripple. A recent report found that the high firing rate cells fire early in the ripple and then become silent, unable to be driven again even with the application of strong optogenetic stimulation time-locked to the ripple [5]. This finding suggests that inhibition, that affects all neurons during ripples [32], interacts with excitability and synaptic drive to establish sequential firing order. Accordingly, low-firing rate cells tended to fire towards the end of the ripple, probably after being released from the lateral inhibition imposed by their high-firing rate neighbours [5].
Though in vitro results have suggested a privileged role for feed-forward excitation, and not inhibition, in dictating ripple recruitment, an in vivo study found that an anatomical bias for feed-forward inhibition dictates ripple recruitment according to the laminar location of the pyramidal cell ([10,70], but see [71]). Deep neurons (those closer to stratum oriens) receive stronger feed-forward inhibition during ripples and are therefore less likely to be recruited, as shown through a more negative driving force to both natural ripples and CA3 electrical stimulation [70]. These differences were probably owing to the higher density of boutons of PV+ interneurons in deeper neurons, versus the higher degree of pyramidal to interneuron connectivity for the superficially positioned pyramids [70,72,73]. Whether learning-related changes in inhibitory connectivity also play a role in ripple recruitment remains unknown [74].
(b). How does learning affect which neurons are recruited into ripples?
Because replay is quantified as a change from baseline, the enhancement of waking-related neural correlations in subsequent sleep suggests that something must have changed the circuit. Taking the simplistic assumption that specific sensory constellations drive hippocampal activity during learning [75], one possibility is that, by virtue of being active at time t, there is a higher likelihood of being active at time t + 1 [76–78]. On a molecular level, this excitability tail could be owing to a number of factors, including phosphorylated cAMP-response element binding protein [79] or potentially other mechanisms affecting K+ channels [80]. In support of the idea that experience-related changes in excitability explain recruitment into ripples, the strength and rate of replay are highest during waking ripples and are biased towards the present location, with the rate of replay decaying rapidly over the sleep following a training session [81].
Though appealing in its simplicity, observations in the replay literature demonstrate that the selection process is more complex. For example, in one experiment, rats were exposed to a radial arm maze with a subset of the arms cordoned off. Once part of the maze was familiar to the subjects, the barriers to the remaining arms were lifted and the rats were exposed to both highly familiar and novel locations in rapid succession. The pattern of neural reactivation showed that pairs of neurons with place fields on a novel arm of a radial arm maze were more likely to be co-active at short latencies than pairs with overlapping place fields on familiar arms, even when the rat was standing in the familiar space [82]. Subsequent experiments that focused more specifically on sequential activity during ripples found that replay was more likely for events that happened more remotely (minutes ago) in a maze where rats ran blocks of trials following one of two paths [83]. These findings show that recent experience alone is insufficient to explain the statistics of neural recruitment into ripples.
Perhaps a more dominant view is that synaptic plasticity among the neurons that are co-active during learning, and in particular those in CA3 [75,84,85], store sequences of neural activity that drives the replay seen in CA1 [86]. To investigate how synaptic plasticity contributes to replay, several studies have targeted the classic pathways known to be involved in long-term potentiation. In one such study, knocking out the NR1 N-methyl-D-aspartate receptor (NMDAR) in CA3 pyramidal cells had a dual effect of decreasing the strength of the replay of CA1 neurons in a novel environment and increasing the degree to which pre-experience patterns dominated post-experience ripples [87]. These findings suggest that the normal function of CA3 NMDARs is to allow novel experiences to alter the pre-learning baseline structure to reinforce the synchronies that occurred during learning. Pharmacological antagonism of the NMDAR with systemic 3-(2-carboxypiperazin-4-yl)propyl-1-Phosphonic acid delivery prevents the preferential expression of reward-related replay [88] and diminishes the replay of a novel track, while preserving the reactivation of neurons representing familiar spaces [89]. Consistent with this ‘fire together wire together’ framework, the number of coincident firings during learning is a strong predictor of subsequent co-activity during ripples [90].
The preponderance of replay for certain types of goal locations and experiences [88,91–94] suggests that coincident activity may be necessary but not sufficient for future replay. As neuromodulators boost synaptic plasticity [95,96], and replay is thought to depend upon those physiological changes, the same neuromodulators should affect replay dynamics as well. Using the dopamine transporter promoter to target ChR2 expression to dopaminergic neurons in the ventral tegmental area, McNamara et al. [97] found that optogenetic stimulation of midbrain dopamine somata, or their axons in CA1, during learning increased the reactivation rate of ensembles coding novel environments and goal locations, a boost that depended upon D1/D5 receptor signalling [97].
Replay is often described as being temporally compressed, because the time it takes to run between the centres of place fields is approximately 10–15 times longer than the duration of a ripple, and it is this sequence of place field locations that are typically correlated with SPW-R activity [98]. However, during movement through a series of place fields, the firing of rodent hippocampal neurons is organized by theta, and a temporal compression is observed in the so-called theta sequences which can be between 10 and 20 times the rate in which subjects pass through the centre of the place field [83,99,100]. Therefore, any mechanistic account of replay may not need to deal with how to compress neural activity, if we assume a link between the compression that occurs during theta and replay sequences observed during ripples.
To test whether theta sequences drive replay, an elegant set of studies was conducted that preserved the rate code of place fields while disrupting the fine-time scale organization during theta. In one such experiment, rats were moved through their environment on a motorized model train with a built-in treadmill. When the treadmill was turned off, place fields were present, but phase precession and theta sequences were disrupted. Turning on the treadmill, forced the rats to run, which reinstated theta and the associated theta sequencing of place fields [101,102]. Replay was strongly enhanced when the treadmill was turned on, and the dominance of forward over reverse replay was only present in this condition, as expected if the hippocampal system encoded the forward-going theta sequences [103].
This interpretation of the ripple function assumes that learned experiences are replayed during the ripple. However, there is a large correlation in the neural activity before and after learning. More remarkably, there is also a large correlation in that temporal structure before learning and during, even if the learning is taking place in a bit of space that is novel to the subject [104]. This is the phenomenon of ‘preplay’ that challenges not only our notion that sensory inputs define neural states during learning but also the theory that this sensory state is then learned by the hippocampus to be played back during ripples—the existence of preplay suggests that those ripple sequences existed all along and define the order of upcoming place-cell sequences.
One idea is that ripple activity is an exploration of the existing topological structure. When decoding replayed positions during sleep, the statistics of these events looked as if the subject were moving through the environment along random trajectories—as though the virtual travel could move along any vector at every position. The cumulative experience of the subject could not predict this kind of Brownian drift, suggesting that replay must be more than broadcasting recent theta sequences [105]. Indeed, one critical difference between theta sequences and replay sequences is that, unlike theta sequences which strictly move in the forward direction, replay events playout can go in the forward or reverse direction [106], challenging the notion that these replayed sequences are baked in during earlier sequences [83].
Two recent studies [107,108] have most explicitly challenged this link between learning-related plasticity and replay by using a dissociable developmental timeline of the emergence of theta sequences versus ripple sequences. In Farooq & Dragoi [108], young rats (p15–p22) had place fields, and theta, but lacked theta sequences, which did not emerge until p23. On the other hand, ripple sequences emerged by p17. However, at this young age, preplay and replay were equivalent and the increase in replay sequences above the pre-exploration baseline was not observed until p23. Clearly, replay before p23 could not have arisen owing to learned theta sequences, as these did not exist in young animals.
A preponderance of evidence has accumulated to demonstrate that learning-dependent plasticity alters the structure of replay events. However, the existence and strength of preplay and the fact that preplay/replay emerge prior to theta sequences shows that a great deal of structure is present a priori, and it remains a possibility that no new sequences are formed during learning, but rather plasticity acts to bias an existing reservoir to dwell longer in certain states above others.
4. Ripple oscillations beyond the hippocampus
The comparative study of similar phenomena across brain regions can offer meaningful insights about their mechanism and function and has been a useful tool in studying other brain rhythms, such as gamma oscillations [109]. Accumulating evidence from recent years points to the existence of high-frequency oscillations in the ripple band outside of the hippocampal formation, in various cortical and subcortical areas. These new findings pose an opportunity to study the analogies in the anatomical substrate and network properties shared by such regions where ripple oscillations are observed in order to draw more general conclusions about their origin.
Cortical ripples have been described in a variety of areas including somatosensory [110], motor areas [111] and olfactory cortices [112], as well as parahippocampal [113–115] and associational areas [111,116,117]. There is some controversy regarding the expression of cortical ripples in primary cortical areas: while some studies observed fast oscillations in the ripple band in S1 of ketamine-anaesthetized and drug-free cats [110], or in secondary motor area of freely behaving rats [111], a recent study using large-scale spatial coverage recordings of cortical areas in rats reported the sparsity of ripple events in these areas [116]. In humans, the incidence of fast oscillations (80–120 Hz) was similar between primary cortices and associational areas, although only the latter showed coupling to ripples in the medial temporal lobe (MTL) [117]. These differences could, in part, arise from different brain states (i.e. anesthetized versus drug-free), differences between species and different definitions of the ripple band. Extra-hippocampal ripples were also reported in subcortical structures, but these reports are scarcer and include the amygdala and neighbouring endopiriform nucleus [118] as well as faster rhythms in the lateral septum [119].
(a). Cortical ripples are embedded within lower frequency rhythms
A common hallmark of cortical ripples is their coupling to slower, large-amplitude LFP fluctuations. The identity of these rhythms, however, seems to be less crucial for ripple generation. While some reports, particularly from parahippocampal regions, linked cortical ripples to the trough of a cortical ‘sharp wave’ [113,114,120], other studies, mostly in neocortical regions, found that cortical ripples were phase locked to the trough of local spindles [111] or delta waves [110,116]. Thus, it appears that unlike the clear separation of the ripple and sharp-wave components in the hippocampus, in the cortex, the two are anatomically superimposed (figure 1a). Remarkably, optogenetic stimulation of CaMKII-positive cells in L5 of S1 gave rise to fast oscillations in the ripple band [13], suggesting that any strong, tonic, anatomically concentrated stimulus of excitatory cells is sufficient to set in motion the network interactions that underlie ripple oscillations.
Figure 1.
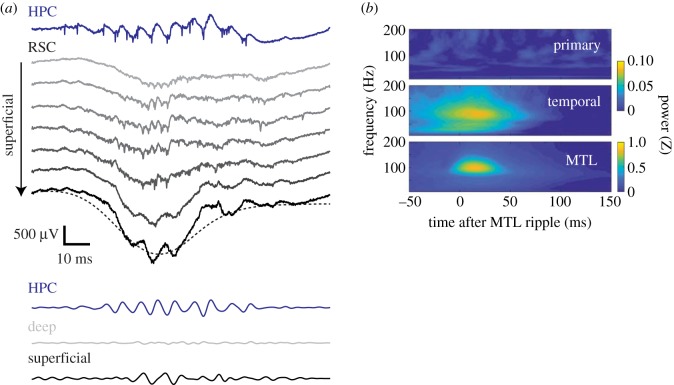
(a) Cortical ripples in the retrosplenial cortex (RSC). Exemplary trace showing a ripple recorded in the CA1 and eight simultaneously recorded channels in the retrosplenial cortex ranging from deep (top, light grey) to superficial (bottom, black). Note that retrosplenial ripple is localized to superficial layers. Bottom channel is overlaid with a low-passed filtered version (dashed line) to illustrate the cortical sharp wave, in which the ripple is embedded. (b) Ripple oscillations in the MTL are coupled to cortical ripples in temporal association area, but not in the primary cortex. HPC, hippocampal cortex. From Vaz et al. [117], with permission. (Online version in colour.)
Another similarity between hippocampal and cortical ripple oscillations is the involvement of interneurons. Using juxtacellular recordings in naturally sleeping rats, Averkin et al. [111] demonstrated that the firing of identified PV+ interneurons recruited during spindle-nested cortical ripples follows the firing of L3 pyramidal neurons by approximately 1–3 ms, suggesting similar PYR-INT ripple dynamics to those observed in the CA1 [44]. These findings were later corroborated by extracellular recordings from different associational areas [116,120]. The involvement of interneurons in extra-hippocampal ripples was recently demonstrated in the lateral septum, where direct optogenetic activation of GABAergic neurons resulted in ripple-like iHFOs [119]. These observations are in-line with similar in vitro results from the hippocampus [40]; yet in contrast with the hippocampus, the lateral septum is composed only of GABAergic cells, suggesting that the underlying mechanism of ripple generation in those areas could be quite distinct.
(b). Potential mechanisms of extra-hippocampal ripple induction
As the mechanisms underlying the generation of ripples in the hippocampus are still heavily debated and given the scarcity of information about extra-hippocampal ripples, in particular with respect to sub-threshold inputs, the conclusions that can be drawn about the mechanisms must be rather speculative. Therefore, we will try to outline some general commonalities shared by extra-hippocampal areas where ripple oscillations were reported with the hope of identifying the physical properties of networks that can sustain these high-frequency oscillations.
The majority of studies reporting cortical ripple oscillations observed a laminar profile where the majority of identified events were strongly localized to superficial layers [111,113,116,120]. A possible explanation could be that these layers are most strongly aligned with the current sink driven by the slower, large-amplitude oscillation that provides the strong synchronous excitation necessary for ripple generation and in which ripples are typically embedded. This view is supported by the strong projections from the CA1 subfield and the subiculum to superficial aspects of L5 medial entorhinal cortex (MEC) and by the subicular projections to the L2/3 retrosplenial cortex (RSC), which both contribute to the cortical ‘sharp wave’ in those layers [113,120]. According to this view, the neural networks underlying ripple oscillations may be present in both deep and superficial layers [13], yet under physiological conditions, only superficial layers are exposed to sufficiently strong depolarizing currents needed to fully engage this network in the ripple band.
Another possibility is that superficial and deep cortical layers comprise different E : I conductance ratios that favour the emergence of ripples in superficial layers when compared with deep layers. This scenario can result from a different PYR-INT connectivity scheme and may be related to the higher cell body density in superficial layers in cortical areas where ripple oscillations are readily observed, such as the piriform cortex [112] and the RSC, which are reminiscent of that in the CA of the hippocampus. Consistent with the idea of different recruitment of local interneurons across layers, a recent study reported marked differences in the E : I ratio of superficial and deep RSC pyramidal cells [121].
Moreover, cell-intrinsic properties of different cortical neurons were suggested to contribute to sub-threshold resonance and pacemaker properties in the gamma band [122–124]. Consistently, superficial pyramids in the RSC were shown to fundamentally differ from deep pyramidal cells and from superficial cells in primary cortical areas in a variety of intrinsic properties including their compact dendritic morphology, the lack of spike frequency accommodation and the high input resistance, rendering those cells highly excitable [120,125]. Further work is needed in examining whether those unique properties contribute to ripple generation and whether they generalize across areas where cortical ripples have been observed.
(c). Coupling between hippocampal and extra-hippocampal ripples
A central question pertains to the relationship between extra-hippocampal and hippocampal ripples. Extra-hippocampal ripples could arise from, or be influenced by, hippocampal ripples, they could occur independently of the hippocampus or, alternatively, extra-hippocampal and hippocampal ripples could be triggered by a common third party. The coincidence of CA1 ripples with cortical spindles and delta oscillations [126,127] suggests that these rhythms are primary candidates for such outside coordination.
The answer to this question seems to vary across studies and the brain area under investigation. While Averkin et al. [111] observed no preferential timing of hippocampal SPW-R with respect to spindle-nested neocortical ripples in secondary motor and parietal cortices, Khodagholy et al. [116] reported the temporal coupling of SPW-R to cortical ripples, but not to gamma oscillations, in parietal and midline cortices. These results were later expanded to human subjects by Vaz et al. [117], who reported the coupling of MTL ripples to ripples in the lateral temporal cortex but not to ripples in primary cortices (figure 1b). Ripples in the parietal cortex were strongly modulated by slow oscillations [116], which are capable of temporally biasing the timing of hippocampal SPW-Rs within a window of 30–200 ms [126–130], suggesting that both cortical and hippocampal ripples may be coordinated by cortical slow oscillations. Such a mechanism could explain why cortical ripples are coupled to hippocampal ripples, even in areas such as the posterior parietal cortex which are not directly interconnected with the hippocampus.
Ripple oscillations were also observed in areas that receive direct hippocampal output such as the subicular complex and MEC [113] and the granular RSC [120]. Ripple oscillations along the subicular–entorhinal axes were shown to decrease in amplitude and to possess fewer cycles as a function of increasing distance from CA1. Moreover, cells in the subicular complex and MEC were phase-locked to individual ripple cycles in CA1, suggesting a wave-by-wave propagation of ripples along those hippocampal output axes. Likewise, retrosplenial ripples showed a moderate phase coherence with dorsal hippocampal ripples and superficial RSC units, which receive direct hippocampal inputs, but not deep RSC cells which do not, were phase locked to the remote hippocampal ripple waves [120]. Similar to MEC ripples, those RSC ripples were also of lower amplitude compared with CA1 ripples, and a fraction of RSC ripples occurred in the absence of a substantial increase in CA1 ripple power. While these RSC ripples could be driven by unrecorded ripples in other positions along the septo-temporal axis of the hippocampus [46], they most likely were triggered by inputs other than those from the hippocampus. This view is supported by the high proportion of cortical sharp waves in superficial layers of the RSC which are not correlated with hippocampal ripples and far exceed the rate of CA1 ripples [120]. Finally, a causal relationship between hippocampal and cortical ripples was recently suggested by Nitzan et al. [120], who showed that optogenetic stimulation of subicular bursting cells, which give rise to hippocampal–RSC projections, resulted in ripple-band activity in superficial, but not deep layers, of the RSC. Conversely, optogenetic inhibition of subicular fibres in the RSC resulted in a reduction in RSC ripple incidence, suggesting that hippocampal inputs are necessary, at least in part, for the induction of ripples in cortical areas directly interconnected with the hippocampus.
In summary, ripple oscillations can be observed in both palaeocortical areas immediately interconnected with the hippocampus, as well as neocortical networks. However, we suggest that a distinction between the two should be made, as ripples in palaeocortical areas show stronger coupling with hippocampal ripples, as assessed by phase locking of field potential and spikes. We propose that the selective spread of ripples to these regions is owing to an anatomical continuum of the same mechanisms responsible for the propagation of ripple activity within the hippocampus [46], namely networks dominated by lateral inhibition reacting to strong phasic excitatory drive. Interregional phase coupling may play an important role in maintaining coherent sequential structure across areas as the replay was shown to be coherent between CA1 and deep layers of the MEC [131], where ripples are also coherent [113]. By contrast, the relationship between neocortical ripples and hippocampal ripples is more variable, and while they do appear to be temporally coupled, an active propagation from the hippocampus is less likely and coupling to a more global rhythm must be considered.
(d). Cortical ripples in memory consolidation and retrieval
The effect of hippocampal–cortical ripple coupling on memory performance was recently addressed by two studies. The first showed that the coupling of ripples in the hippocampus and posterior parietal cortex increased during sleep following the learning of a spatial task [116]. However, as cortical ripples were also shown to be strongly coupled to slow waves [116], which are themselves up-modulated following learning [132], thus raising the possibility that this increased coupling may reflect an indirect effect. Similarly, the coupling between MTL ripples and ripples in the temporal association cortex, but not the primary or prefrontal cortex, of awake humans was specifically increased before successful memory retrieval in verbal memory tasks [117]. Together, these studies suggest that the coupling between hippocampal and cortical ripples provides a brief temporal window for enhanced communication between the hippocampus and other cortical areas, although this has not been conclusively demonstrated. Because the coupling of hippocampal ripples to cortical oscillations such as delta-waves or thalamo-cortical spindles is known to be important for memory [133–135], it remains to be investigated whether, and how, those cortical rhythms orchestrate this cross-structural ripple coupling.
While the role of hippocampal ripples in memory consolidation has been linked to replay of behaviourally relevant sequences from previous experience, it is not clear whether such sequences also exist during extra-hippocampal ripples. Studies from various cortical areas, even those not receiving direct CA1 output, show coordinated hippocampal–cortical replay [136–141]. Yet, no study to date has reported the involvement of cortical fast oscillation in cortical replay, and, although cortical replay is associated with spindles and delta waves [142], the overall relationship between the coupling of hippocampal–cortical ripples and coordinated replay is unclear. The fact that different subsets of ripples (as clustered by LFP profile) are associated with distinct activity patterns across most of the brain [9] presents a challenge for determining the contribution of specific hippocampal replay events in driving divergent responses in efferent regions.
5. Conclusion
More than four decades have passed since ripple oscillations were first described in the rodent hippocampus [143], yet the function and mechanism of this phenomenon are far from fully understood. Throughout this review, we emphasize the physiological mechanisms that pace rhythmic activity and pattern neural activity during ripples, thus defining replay ‘content’.
Strong input can drive ripple-band activity in hippocampal, cortical and even some subcortical structures. Numerous models seek to explain ripple rhythmogenesis. We emphasize that many models are not mutually exclusive and operate under different physiological circumstances; however, a preponderance of evidence points towards a crucial role of interneurons in pacing neural activity at the ripple band. Phase resetting of independent oscillators with common resonant properties can induce strong coherence. This fact presents a challenge in identifying the role of synaptic inputs in driving ripples within the hippocampus and coupling of activity between the hippocampus and the cortex. Ultimately, better perturbation experiments and models will be required to untangle these recurrent knots.
We point out three interacting mechanisms that can set the sequence of neural recruitment during ripples: differences in the balance of feed-forward excitation and inhibition, differences in intrinsic excitability and the pattern of lateral inhibition. Crucially, these mechanisms are very likely to interact, especially under a scenario where neighbouring cells are exposed to the same external drive. Learning-related plasticity in any of these loci could drive changes in replay content after the experience. Of course, these features of a neural network do not uniquely come into play during recruitment into ripples, and we suggest that the ripple recruitment may serve as a model for how learning-related changes affect subsequent network dynamics.
6. Open questions
| (1) Do interneurons receive phasic excitatory inputs during ripples? |
| (2) To what degree does replay reflect the increase in strength of existing patterns versus the encoding of new patterns enforced by sensory inputs? |
| (3) How does learning affect INT-PYR plasticity? |
| (4) How do theta and ripple sequences interact and to what extent do ripple sequences depend on theta? |
| (5) Are hippocampal and cortical ripples generated by the same mechanisms? |
| (6) Why are cortical ripples observed only in certain cortical areas (namely associational cortices)? |
| (7) How are hippocampal and cortical ripples synchronized? |
| (8) Are cortical ripples in different areas coupled with each other? |
| (9) Are cortical ripples necessary for memory consolidation? |
| (10) What is the role of cell-intrinsic properties in promoting ripple expression and recruitment? |
| (11) How does intrinsic excitability dictate sequential firing patterns, and does plasticity in neural excitability affect replay statistics? |
Acknowledgements
We would like to thank Richard Kempter, Nikolaus Maier, Manuel Valero, Roman Huszar and Kaiser Arndt for providing helpful feedback on earlier drafts of this manuscript.
Data accessibility
Data available upon publication at https://buzsakilab.com/wp/resources/.
Authors' contributions
S.M.K., N.N. and D.F.E. researched and wrote the review.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the National Institute of Mental Health (grant no. K99 MH118423).
References
- 1.McClelland JL, McNaughton BL, O'Reilly RC. 1995. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol. Rev. 102, 419–457. ( 10.1037/0033-295X.102.3.419) [DOI] [PubMed] [Google Scholar]
- 2.Buzsaki G. 1989. Two-stage model of memory trace formation: a role for ‘noisy’ brain states. Neuroscience 31, 551–570. ( 10.1016/0306-4522(89)90423-5) [DOI] [PubMed] [Google Scholar]
- 3.Kumaran D, Hassabis D, McClelland JL. 2016. What learning systems do intelligent agents need? Complementary learning systems theory updated. Trends Cogn. Sci. 20, 512–534. ( 10.1016/j.tics.2016.05.004) [DOI] [PubMed] [Google Scholar]
- 4.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. 2009. Selective suppression of hippocampal ripples impairs spatial memory. Nat. Neurosci. 12, 1222–1223. ( 10.1038/nn.2384) [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Ruiz A, Oliva A, Fermino De Oliveira E, Rocha-Almeida F, Tingley D. 2019. Long-duration hippocampal sharp wave ripples improve memory. Science 364, 1082–1086. ( 10.1126/science.aax0758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buzsaki G, Tingley D. 2018. Space and time: the hippocampus as a sequence generator. Trends Cogn. Sci. 22, 853–869. ( 10.1016/j.tics.2018.07.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teyler TJ, DiScenna P. 1986. The hippocampal memory indexing theory. Behav. Neurosci. 100, 147–154. ( 10.1037/0735-7044.100.2.147) [DOI] [PubMed] [Google Scholar]
- 8.Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, Buzsaki G. 1995. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J. Neurosci. 15, 30–46. ( 10.1523/JNEUROSCI.15-01-00030.1995) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez-Villegas JF, Logothetis NK, Besserve M. 2015. Diversity of sharp-wave-ripple LFP signatures reveals differentiated brain-wide dynamical events. Proc. Natl Acad. Sci. USA 112, E6379–E6387. ( 10.1073/pnas.1518257112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliva A, Fernandez-Ruiz A, Buzsaki G, Berenyi A. 2016. Role of hippocampal CA2 region in triggering sharp-wave ripples. Neuron 91, 1342–1355. ( 10.1016/j.neuron.2016.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto J, Tonegawa S. 2017. Direct medial entorhinal cortex input to hippocampal CA1 is crucial for extended quiet awake replay. Neuron 96, 217–227. ( 10.1016/j.neuron.2017.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. 1995. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature 378, 75–78. ( 10.1038/378075a0) [DOI] [PubMed] [Google Scholar]
- 13.Stark E, Roux L, Eichler R, Senzai Y, Royer S, Buzsáki G. 2014. Pyramidal cell-interneuron interactions underlie hippocampal ripple oscillations. Neuron 83, 467–480. ( 10.1016/j.neuron.2014.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davoudi H, Foster DJ. 2019. Acute silencing of hippocampal CA3 reveals a dominant role in place field responses. Nat. Neurosci. 22, 337–342. ( 10.1038/s41593-018-0321-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pais I, Hormuzdi SG, Monyer H, Traub RD, Wood IC, Buhl EH, Whittington MA, Lebeau FEN. 2003. Sharp wave-like activity in the hippocampus in vitro in mice lacking the gap junction protein connexin 36. J. Neurophysiol. 89, 2046–2054. ( 10.1152/jn.00549.2002) [DOI] [PubMed] [Google Scholar]
- 16.Maier N, et al. 2011. Coherent phasic excitation during hippocampal ripples. Neuron 72, 137–152. ( 10.1016/j.neuron.2011.08.016) [DOI] [PubMed] [Google Scholar]
- 17.Traub RD, Bibbig A. 2000. A model of high-frequency ripples in the hippocampus based on synaptic coupling plus axon-axon gap junctions between pyramidal neurons. J. Neurosci. 20, 2086–2093. ( 10.1523/JNEUROSCI.20-06-02086.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. 1992. High-frequency network oscillation in the hippocampus. Science 256, 1025–1027. ( 10.1126/science.1589772) [DOI] [PubMed] [Google Scholar]
- 19.Kubota D, Colgin LL, Casale M, Brucher FA, Lynch G. 2003. Endogenous waves in hippocampal slices. J. Neurophysiol. 89, 81–89. ( 10.1152/jn.00542.2002) [DOI] [PubMed] [Google Scholar]
- 20.Maier N, Güldenagel M, Söhl G, Siegmund H, Willecke K, Draguhn A. 2002. Reduction of high-frequency network oscillations (ripples) and pathological network discharges in hippocampal slices from connexin 36-deficient mice. J. Physiol. 541, 521–528. ( 10.1113/jphysiol.2002.017624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papatheodoropoulos C, Kostopoulos G. 2002. Spontaneous, low frequency (approximately 2–3 Hz) field activity generated in rat ventral hippocampal slices perfused with normal medium. Brain Res. Bull. 57, 187–193. ( 10.1016/S0361-9230(01)00738-9) [DOI] [PubMed] [Google Scholar]
- 22.Hunt DL, Linaro D, Si BL, Romani S, Spruston N. 2018. A novel pyramidal cell type promotes sharp-wave synchronization in the hippocampus. Nat. Neurosci. 21, 985 ( 10.1038/s41593-018-0172-7) [DOI] [PubMed] [Google Scholar]
- 23.Sullivan D, Csicsvari J, Mizuseki K, Montgomery S, Diba K, Buzsaki G. 2011. Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: influence of dentate and entorhinal cortical activity. J. Neurosci. 31, 8605–8616. ( 10.1523/JNEUROSCI.0294-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csicsvari J, Hirase H, Mamiya A, Buzsaki G. 2000. Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron 28, 585–594. ( 10.1016/S0896-6273(00)00135-5) [DOI] [PubMed] [Google Scholar]
- 25.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. 1999. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J. Neurosci. 19, RC20 ( 10.1523/JNEUROSCI.19-16-j0001.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfeiffer BE, Foster DJ, CELLS PLACE. 2015. Autoassociative dynamics in the generation of sequences of hippocampal place cells. Science 349, 180–183. ( 10.1126/science.aaa9633) [DOI] [PubMed] [Google Scholar]
- 27.Oliva A, Fernandez-Ruiz A, Fermino de Oliveira E, Buzsaki G. 2018. Origin of gamma frequency power during hippocampal sharp-wave ripples. Cell Rep. 25, 1693–1700. ( 10.1016/j.celrep.2018.10.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakashiba T, Buhl DL, McHugh TJ, Tonegawa S. 2009. Hippocampal CA3 output is crucial for ripple-associated reactivation and consolidation of memory. Neuron 62, 781–787. ( 10.1016/j.neuron.2009.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Draguhn A, Traub RD, Schmitz D, Jefferys JGR. 1998. Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature 394, 189–192. ( 10.1038/28184) [DOI] [PubMed] [Google Scholar]
- 30.Bahner F, et al. 2011. Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proc. Natl Acad. Sci. USA 108, E607–E616. ( 10.1073/pnas.1103546108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheffzük C, Kukushka VI, Vyssotski AL, Draguhn A, Tort ABL, Brankaä KJ. 2011. selective coupling between theta phase and neocortical fast gamma oscillations during REM-sleep in mice. PLoS ONE 6, e28489 ( 10.1371/journal.pone.0028489) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.English DF, Peyrache A, Stark E, Roux L, Vallentin D, Long MA, Buzsáki GA. 2014. Excitation and inhibition compete to control spiking during hippocampal ripples: intracellular study in behaving mice. J. Neurosci. 34, 16 509–16 517. ( 10.1523/JNEUROSCI.2600-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buhl DL, Harris KD, Hormuzdi SG, Monyer H, Buzsaki G. 2003. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J. Neurosci. 23, 1013–1018. ( 10.1523/JNEUROSCI.23-03-01013.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzsaki G. 2015. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188. ( 10.1002/hipo.22488) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nimmrich V, Maier N, Schmitz D, Draguhn A. 2005. Induced sharp wave-ripple complexes in the absence of synaptic inhibition in mouse hippocampal slices. J. Physiol. (Lond.) 563, 663–670. ( 10.1113/jphysiol.2004.079558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klausberger T, Márton LF, Baude A, Roberts JDB, Magill PJ, Somogyi P. 2004. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat. Neurosci. 7, 41–47. ( 10.1038/nn1159) [DOI] [PubMed] [Google Scholar]
- 37.Klausberger T, Magill PJ, Márton LF, Roberts JDB, Cobden PM, Buzsáki GA, Somogyi P. 2003. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature 421, 844–848. ( 10.1038/nature01374) [DOI] [PubMed] [Google Scholar]
- 38.Hulse BK, Moreaux LC, Lubenov EV, Siapas AG. 2016. Membrane potential dynamics of ca1 pyramidal neurons during hippocampal ripples in awake mice. Neuron 89, 800–813. ( 10.1016/j.neuron.2016.01.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gan J, Weng SM, Pernia-Andrade AJ, Csicsvari J, Jonas P. 2017. Phase-locked inhibition, but not excitation, underlies hippocampal ripple oscillations in awake mice in vivo. Neuron 93, 308–314. ( 10.1016/j.neuron.2016.12.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlingloff D, Kali S, Freund TF, Hajos N, Gulyas AI. 2014. Mechanisms of sharp wave initiation and ripple generation. J. Neurosci. 34, 11 385–11 398. ( 10.1523/JNEUROSCI.0867-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Memmesheimer RM. 2010. Quantitative prediction of intermittent high-frequency oscillations in neural networks with supralinear dendritic interactions. Proc. Natl Acad. Sci. USA 107, 11 092–11 097. ( 10.1073/pnas.0909615107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varga C, Golshani P, Soltesz I. 2012. Frequency-invariant temporal ordering of interneuronal discharges during hippocampal oscillations in awake mice. Proc. Natl Acad. Sci. USA 109, E2726–E2734. ( 10.1073/pnas.1210929109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klausberger T, et al. 2005. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J. Neurosci. 25, 9782–9793. ( 10.1523/JNEUROSCI.3269-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G. 1999. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J. Neurosci. 19, 274–287. ( 10.1523/JNEUROSCI.19-01-00274.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Quyen ML, Bragin A, Staba R, Crepon B, Wilson CL, Engel J. 2008. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J. Neurosci. 28, 6104–6110. ( 10.1523/JNEUROSCI.0437-08.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel J, Schomburg EW, Berenyi A, Fujisawa S, Buzsaki G. 2013. Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J. Neurosci. 33, 17 029–17 041. ( 10.1523/JNEUROSCI.2036-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ponomarenko AA, Li JS, Korotkova TM, Huston JP, Haas HL. 2008. Frequency of network synchronization in the hippocampus marks learning. Eur. J. Neurosci. 27, 3035–3042. ( 10.1111/j.1460-9568.2008.06232.x) [DOI] [PubMed] [Google Scholar]
- 48.Nguyen DP, Kloosterman F, Barbieri R, Brown EN, Wilson MA. 2009. Characterizing the dynamic frequency structure of fast oscillations in the rodent hippocampus. Front. Integr. Neurosci. 3, 11 ( 10.3389/neuro.07.011.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramadan W, Eschenko O, Sara SJ. 2009. Hippocampal sharp wave/ripples during sleep for consolidation of associative memory. PLoS ONE 4, e6697 ( 10.1371/journal.pone.0006697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eschenko O, Ramadan W, Molle M, Born J, Sara SJ. 2008. Sustained increase in hippocampal sharp-wave ripple activity during slow-wave sleep after learning. Learn. Mem. 15, 222–228. ( 10.1101/lm.726008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han SK, Kurrer C, Kuramoto Y. 1995. Dephasing and bursting in coupled neural oscillators. Phys. Rev. Lett. 75, 3190–3193. ( 10.1103/PhysRevLett.75.3190) [DOI] [PubMed] [Google Scholar]
- 52.Wang XJ, Rinzel J. 1992. Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comput. 4, 84–97. ( 10.1162/neco.1992.4.1.84) [DOI] [Google Scholar]
- 53.Kopell N, Ermentrout GB. 1986. Symmetry and phaselocking in chains of weakly coupled oscillators. Commun. Pure Appl. Math. 39, 623–660. ( 10.1002/cpa.3160390504) [DOI] [Google Scholar]
- 54.Brunel N, Wang XJ. 2003. What determines the frequency of fast network oscillations with irregular neural discharges? I. Synaptic dynamics and excitation-inhibition balance. J. Neurophysiol. 90, 415–430. ( 10.1152/jn.01095.2002) [DOI] [PubMed] [Google Scholar]
- 55.Racz A, Ponomarenko AA, Fuchs EC, Monyer H. 2009. Augmented hippocampal ripple oscillations in mice with reduced fast excitation onto parvalbumin-positive cells. J. Neurosci. 29, 2563–2568. ( 10.1523/JNEUROSCI.5036-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramirez-Villegas JF, Willeke KF, Logothetis NK, Besserve M. 2018. Dissecting the synapse and frequency-dependent network mechanisms of in vivo hippocampal sharp wave-ripples. Neuron 100, 1224 ( 10.1016/j.neuron.2018.09.041) [DOI] [PubMed] [Google Scholar]
- 57.Valero M, Averkin RG, Fernandez-Lamo I, Aguilar J, Lopez-Pigozzi D, Brotons-Mas JR, Cid E, Tamas G, Menendez De La Prida L. 2017. Mechanisms for selective single-cell reactivation during offline sharp-wave ripples and their distortion by fast ripples. Neuron 94, 1234 ( 10.1016/j.neuron.2017.05.032) [DOI] [PubMed] [Google Scholar]
- 58.Donoso JR, Schmitz D, Maier N, Kempter R. 2018. Hippocampal ripple oscillations and inhibition-first network models: frequency dynamics and response to GABA modulators. J. Neurosci. 38, 3124–3146. ( 10.1523/JNEUROSCI.0188-17.2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malerba P, Krishnan GP, Fellous JM, Bazhenov M. 2016. Hippocampal CA1 ripples as inhibitory transients. PLoS Comput. Biol. 12, e1004880 ( 10.1371/journal.pcbi.1004880) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakao H, Arai KS, Nagai K, Tsubo Y, Kuramoto Y. 2005. Synchrony of limit-cycle oscillators induced by random external impulses. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 72, 026220 ( 10.1103/PhysRevE.72.026220) [DOI] [PubMed] [Google Scholar]
- 61.Brunel N, Hakim V. 2008. Sparsely synchronized neuronal oscillations. Chaos 18, 015113 ( 10.1063/1.2779858) [DOI] [PubMed] [Google Scholar]
- 62.Mizuseki K, Buzsaki G. 2013. Preconfigured, skewed distribution of firing rates in the hippocampus and entorhinal cortex. Cell Rep. 4, 1010–1021. ( 10.1016/j.celrep.2013.07.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rich PD, Liaw HP, Lee AK. 2014. Place cells. Large environments reveal the statistical structure governing hippocampal representations. Science 345, 814–817. ( 10.1126/science.1255635) [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Briguglio J, Romani S, Lee A. 2019. The statistical structure of the hippocampal code for space as a function of time, context, and value. bioRxiv 615203 ( 10.1101/615203) [DOI] [PubMed]
- 65.Epsztein J, Brecht M, Lee AK. 2011. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron 70, 109–120. ( 10.1016/j.neuron.2011.03.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grosmark AD, Buzsaki G. 2016. Diversity in neural firing dynamics supports both rigid and learned hippocampal sequences. Science 351, 1440–1443. ( 10.1126/science.aad1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stark E, Roux L, Eichler R, Buzsáki G. 2015. Local generation of multineuronal spike sequences in the hippocampal CA1 region. Proc. Natl Acad. Sci. USA 112, 10 521–10 526. ( 10.1073/pnas.1508785112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mizunuma M, et al. 2014. Unbalanced excitability underlies offline reactivation of behaviorally activated neurons. Nat. Neurosci. 17, 503–505. ( 10.1038/nn.3674) [DOI] [PubMed] [Google Scholar]
- 69.Pouille F, Marin-Burgin A, Adesnik H, Atallah BV, Scanziani M. 2009. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat. Neurosci. 12, 1577–1585. ( 10.1038/nn.2441) [DOI] [PubMed] [Google Scholar]
- 70.Valero M, et al. 2015. Determinants of different deep and superficial CA1 pyramidal cell dynamics during sharp-wave ripples. Nat. Neurosci. 18, 1281–1290. ( 10.1038/nn.4074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mizuseki K, Diba K, Pastalkova E, Buzsaki G. 2011. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat. Neurosci. 14, 1174–1181. ( 10.1038/nn.2894) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee S-H, Marchionni I, Bezaire M, Varga C, Danielson N, Lovett-Barron M, Losonczy A, Soltesz I. 2014. Parvalbumin-positive basket cells differentiate among hippocampal pyramidal cells. Neuron 82, 1129–1144. ( 10.1016/j.neuron.2014.03.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.English DF, Mckenzie S, Evans T, Kim K, Yoon E, Buzsáki G. 2017. Pyramidal cell-interneuron circuit architecture and dynamics in hippocampal networks. Neuron 96, 505–520. ( 10.1016/j.neuron.2017.09.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.McKenzie S. 2017. Inhibition shapes the organization of hippocampal representations. Hippocampus. 28, 659–671. ( 10.1002/hipo.22803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cutsuridis V, Hasselmo M. 2011. Spatial memory sequence encoding and replay during modeled theta and ripple oscillations. Cogn. Comput. 3, 554–574. ( 10.1007/s12559-011-9114-3) [DOI] [Google Scholar]
- 76.Howard MW. 2018. Memory as perception of the past: compressed time in mind and brain. Trends Cogn. Sci. 22, 124–136. ( 10.1016/j.tics.2017.11.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cai DJ, et al. 2016. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118. ( 10.1038/nature17955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lisman J, Cooper K, Sehgal M, Silva AJ. 2018. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat. Neurosci. 21, 309–314. ( 10.1038/s41593-018-0076-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. 2009. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat. Neurosci. 12, 1438–1443. ( 10.1038/nn.2405) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pignatelli M, Ryan TJ, Roy DS, Lovett C, Smith LM, Muralidhar S, Tonegawa S. 2019. Engram cell excitability state determines the efficacy of memory retrieval. Neuron 101, 274–284. ( 10.1016/j.neuron.2018.11.029) [DOI] [PubMed] [Google Scholar]
- 81.Kudrimoti HS, Barnes CA, McNaughton BL. 1999. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J. Neurosci. 19, 4090–4101. ( 10.1523/JNEUROSCI.19-10-04090.1999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cheng S, Frank LM. 2008. New experiences enhance coordinated neural activity in the hippocampus. Neuron 57, 303–313. ( 10.1016/j.neuron.2007.11.035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. 2010. Hippocampal replay is not a simple function of experience. Neuron 65, 695–705. ( 10.1016/j.neuron.2010.01.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jackson JC, Johnson A, Redish AD. 2006. Hippocampal sharp waves and reactivation during awake states depend on repeated sequential experience. J. Neurosci. 26, 12 415–12 426. ( 10.1523/JNEUROSCI.4118-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zarnadze S, Bäuerle P, Santos-Torres J, Böhm C, Schmitz D, Geiger JRP, Dugladze T, Gloveli T. 2016. Cell-specific synaptic plasticity induced by network oscillations. eLife 5, e14912 ( 10.7554/eLife.14912) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wallenstein GV, Hasselmo ME. 1997. Functional transitions between epileptiform-like activity and associative memory in hippocampal region CA3. Brain Res. Bull. 43, 485–493. ( 10.1016/S0361-9230(97)00003-8) [DOI] [PubMed] [Google Scholar]
- 87.Dragoi G, Tonegawa S. 2013. Development of schemas revealed by prior experience and NMDA receptor knock-out. eLife 2, e01326 ( 10.7554/eLife.01326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dupret D, O'Neill J, Pleydell-Bouverie B, Csicsvari J. 2010. The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat. Neurosci. 13, 995–1002. ( 10.1038/nn.2599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Silva D, Feng T, Foster DJ. 2015. Trajectory events across hippocampal place cells require previous experience. Nat. Neurosci. 18, 1772–1779. ( 10.1038/nn.4151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O'Neill J, Senior TJ, Allen K, Huxter JR, Csicsvari J. 2008. Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat. Neurosci. 11, 209–215. ( 10.1038/nn2037) [DOI] [PubMed] [Google Scholar]
- 91.Michon F, Sun JJ, Kim CY, Ciliberti D, Kloosterman F. 2019. Post-learning hippocampal replay selectively reinforces spatial memory for highly rewarded locations. Curr. Biol. 29, 1436 ( 10.1016/j.cub.2019.03.048) [DOI] [PubMed] [Google Scholar]
- 92.Ambrose RE, Pfeiffer BE, Foster DJ. 2016. Reverse replay of hippocampal place cells is uniquely modulated by changing reward. Neuron 91, 1124–1136. ( 10.1016/j.neuron.2016.07.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singer AC, Karlsson MP, Nathe AR, Carr MF, Frank LM. 2010. Experience-dependent development of coordinated hippocampal spatial activity representing the similarity of related locations. J. Neurosci. 30, 11 586–11 604. ( 10.1523/JNEUROSCI.0926-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pfeiffer BE, Foster DJ. 2013. Hippocampal place-cell sequences depict future paths to remembered goals. Nature 497, 74–79. ( 10.1038/nature12112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosen ZB, Cheung S, Siegelbaum SA. 2015. Midbrain dopamine neurons bidirectionally regulate CA3-CA1 synaptic drive. Nat. Neurosci. 18, 1763–1771. ( 10.1038/nn.4152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lisman JE, Grace AA. 2005. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron 46, 703–713. ( 10.1016/j.neuron.2005.05.002) [DOI] [PubMed] [Google Scholar]
- 97.McNamara CG, Tejero-Cantero Á, Trouche S, Campo-Urriza N, Dupret D. 2014. Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nat. Neurosci. 17, 1658–1660. ( 10.1038/nn.3843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee AK, Wilson MA. 2002. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194. ( 10.1016/S0896-6273(02)01096-6) [DOI] [PubMed] [Google Scholar]
- 99.Dragoi G, Buzsáki G. 2006. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron 50, 145–157. ( 10.1016/j.neuron.2006.02.023) [DOI] [PubMed] [Google Scholar]
- 100.Foster DJ, Wilson MA. 2007. Hippocampal theta sequences. Hippocampus 17, 1093–1099. ( 10.1002/hipo.20345) [DOI] [PubMed] [Google Scholar]
- 101.Cei A, Girardeau G, Drieu C, Kanbi KE, Zugaro M. 2014. Reversed theta sequences of hippocampal cell assemblies during backward travel. Nat. Neurosci. 17, 719–724. ( 10.1038/nn.3698) [DOI] [PubMed] [Google Scholar]
- 102.Terrazas A, et al. 2005. Self-motion and the hippocampal spatial metric. J. Neurosci. 25, 8085–8096. ( 10.1523/JNEUROSCI.0693-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Drieu C, Todorova R, Zugaro M. 2018. Nested sequences of hippocampal assemblies during behavior support subsequent sleep replay. Science 362, 675–679. ( 10.1126/science.aat2952) [DOI] [PubMed] [Google Scholar]
- 104.Dragoi G, Tonegawa S. 2011. Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469, 397–401. ( 10.1038/nature09633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stella F, Baracskay P, O'Neill J, Csicsvari J. 2019. Hippocampal reactivation of random trajectories resembling Brownian diffusion. Neuron 102, 450–461. ( 10.1016/j.neuron.2019.01.052) [DOI] [PubMed] [Google Scholar]
- 106.Diba K, Buzsáki G. 2007. Forward and reverse hippocampal place-cell sequences during ripples. Nat. Neurosci. 10, 1241–1242. ( 10.1038/nn1961) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muessig L, Lasek M, Varsavsky I, Cacucci F, Wills TJ. 2019. Coordinated emergence of hippocampal replay and theta sequences during post-natal development. Curr. Biol. 29, 834–840. ( 10.1016/j.cub.2019.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farooq U, Dragoi G. 2019. Emergence of preconfigured and plastic time-compressed sequences in early postnatal development. Science 363, 168–173. ( 10.1126/science.aav0502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buzsaki G, Wang XJ. 2012. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 35, 203–225. ( 10.1146/annurev-neuro-062111-150444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grenier FO, Timofeev I, Steriade M. 2001. Focal synchronization of ripples (80–200 Hz) in neocortex and their neuronal correlates. J. Neurophysiol. 86, 1884–1898. ( 10.1152/jn.2001.86.4.1884) [DOI] [PubMed] [Google Scholar]
- 111.Averkin RG, Szemenyei V, Borde S, Tamas G. 2016. Identified cellular correlates of neocortical ripple and high-gamma oscillations during spindles of natural sleep. Neuron 92, 916–928. ( 10.1016/j.neuron.2016.09.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manabe H, Kusumoto-Yoshida I, Ota M, Mori K. 2011. Olfactory cortex generates synchronized top-down inputs to the olfactory bulb during slow-wave sleep. J. Neurosci. 31, 8123–8133. ( 10.1523/JNEUROSCI.6578-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chrobak JJ, Buzsaki G. 1996. High-frequency oscillations in the output networks of the hippocampal-entorhinal axis of the freely behaving rat. J. Neurosci. 16, 3056–3066. ( 10.1523/JNEUROSCI.16-09-03056.1996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Collins DR, Lang EJ, Pare D. 1999. Spontaneous activity of the perirhinal cortex in behaving cats. Neuroscience 89, 1025–1039. ( 10.1016/S0306-4522(98)00396-0) [DOI] [PubMed] [Google Scholar]
- 115.Axmacher N, Elger CE, Fell J. 2008. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain 131, 1806–1817. ( 10.1093/brain/awn103) [DOI] [PubMed] [Google Scholar]
- 116.Khodagholy D, Gelinas JN, Buzsaki G. 2017. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358, 369–372. ( 10.1126/science.aan6203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vaz AP, Inati SK, Brunel N, Zaghloul KA. 2019. Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363, 975–978. ( 10.1126/science.aau8956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ponomarenko AA, Korotkova TM, Haas HL. 2003. High frequency (200 Hz) oscillations and firing patterns in the basolateral amygdala and dorsal endopiriform nucleus of the behaving rat. Behav. Brain Res. 141, 123–129. ( 10.1016/S0166-4328(02)00327-3) [DOI] [PubMed] [Google Scholar]
- 119.Tingley D, Buzsáki G. 2020. Routing of hippocampal ripples to subcortical structures via the lateral septum. Neuron 105, 138–149e5. ( 10.1016/j.neuron.2019.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nitzan N, McKenzie S, Beed P, English DF, Oldani S, Tukker JJ, Buzáki G, Schmitz D. 2020. Propagation of hippocampal ripples to the neocortex by way of a subiculum-retrosplenial pathway. bioRxiv 966770. ( 10.1101/2020.02.27.966770) [DOI]
- 121.Sempere-Ferrandez A, Martinez S, Geijo-Barrientos E. 2019. Synaptic mechanisms underlying the intense firing of neocortical layer 5B pyramidal neurons in response to cortico-cortical inputs. Brain Struct. Funct. 224, 1403–1416. ( 10.1007/s00429-019-01842-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Llinas RR, Grace AA, Yarom Y. 1991, In vitro neurons in mammalian cortical layer 4 exhibit intrinsic oscillatory activity in the 10- to 50-Hz frequency range. Proc. Natl Acad. Sci. USA 88, 897–901. ( 10.1073/pnas.88.3.897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nunez A, Amzica F, Steriade M. 1992. Voltage-dependent fast (20–40 Hz) oscillations in long-axoned neocortical neurons. Neuroscience 51, 7–10. ( 10.1016/0306-4522(92)90464-D) [DOI] [PubMed] [Google Scholar]
- 124.Gray CM, McCormick DA. 1996. Chattering cells: superficial pyramidal neurons contributing to the generation of synchronous oscillations in the visual cortex. Science 274, 109–113. ( 10.1126/science.274.5284.109) [DOI] [PubMed] [Google Scholar]
- 125.Kurotani T, Miyashita T, Wintzer M, Konishi T, Sakai K, Ichinohe N, Rockland KS. 2013. Pyramidal neurons in the superficial layers of rat retrosplenial cortex exhibit a late-spiking firing property. Brain Struct. Funct. 218, 239–254. ( 10.1007/s00429-012-0398-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Siapas AG, Wilson MA. 1998. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 21, 1123–1128. ( 10.1016/S0896-6273(00)80629-7) [DOI] [PubMed] [Google Scholar]
- 127.Sirota A, Csicsvari J, Buhl D, Buzsaki G. 2003. Communication between neocortex and hippocampus during sleep in rodents. Proc. Natl Acad. Sci. USA 100, 2065–2069. ( 10.1073/pnas.0437938100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Molle M, Yeshenko O, Marshall L, Sara SJ, Born J. 2006. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J. Neurophysiol. 96, 62–70. ( 10.1152/jn.00014.2006) [DOI] [PubMed] [Google Scholar]
- 129.Isomura Y, Sirota A, Ozen S, Montgomery S, Mizuseki K, Henze DA, Buzsäki G. 2006. Integration and segregation of activity in entorhinal-hippocampal subregions by neocortical slow oscillations. Neuron 52, 871–882. ( 10.1016/j.neuron.2006.10.023) [DOI] [PubMed] [Google Scholar]
- 130.Battaglia FP, Sutherland GR, McNaughton BL. 2004. Hippocampal sharp wave bursts coincide with neocortical ‘up-state’ transitions. Learn. Mem. 11, 697–704. ( 10.1101/lm.73504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ólafsdóttir HF, Carpenter F, Barry C. 2016. Coordinated grid and place cell replay during rest. Nat. Neurosci. 19, 792–794. ( 10.1038/nn.4291) [DOI] [PubMed] [Google Scholar]
- 132.Huber R, Ghilardi MF, Massimini M, Tononi G. 2004. Local sleep and learning. Nature 430, 78–81. ( 10.1038/nature02663) [DOI] [PubMed] [Google Scholar]
- 133.Latchoumane CV, Ngo HV, Born J, Shin HS. 2017. Thalamic spindles promote memory formation during sleep through triple phase-locking of cortical, thalamic, and hippocampal rhythms. Neuron 95, 424–435. ( 10.1016/j.neuron.2017.06.025) [DOI] [PubMed] [Google Scholar]
- 134.Novitskaya Y, Sara SJ, Logothetis NK, Eschenko O. 2016. Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn. Mem. 23, 238–248. ( 10.1101/lm.040923.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. 2016. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 19, 959–964. ( 10.1038/nn.4304) [DOI] [PubMed] [Google Scholar]
- 136.Gomperts SN, Kloosterman F, Wilson MA. 2015. VTA neurons coordinate with the hippocampal reactivation of spatial experience. eLife 4, e5360 ( 10.7554/eLife.05360) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yu JY, Liu DF, Loback A, Grossrubatscher I, Frank LM. 2018. Specific hippocampal representations are linked to generalized cortical representations in memory. Nat. Commun. 9, 2209 ( 10.1038/s41467-018-04498-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang W, Shin JD, Frank LM, Jadhav SP. 2017. Hippocampal-prefrontal reactivation during learning is stronger in awake compared with sleep states. J. Neurosci. 37, 11 789–11 805. ( 10.1523/JNEUROSCI.2291-17.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jadhav SP, Rothschild G, Roumis DK, Frank LM. 2016. Coordinated excitation and inhibition of prefrontal ensembles during awake hippocampal sharp-wave ripple events. Neuron 90, 113–127. ( 10.1016/j.neuron.2016.02.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Girardeau G, Inema I, Buzsaki G. 2017. Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat. Neurosci. 20, 1634–1642. ( 10.1038/nn.4637) [DOI] [PubMed] [Google Scholar]
- 141.Ji D, Wilson MA. 2007. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 10, 100–107. ( 10.1038/nn1825) [DOI] [PubMed] [Google Scholar]
- 142.Peyrache A, Khamassi M, Benchenane K, Wiener SI, Battaglia FP. 2009. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat. Neurosci. 12, 919–926. ( 10.1038/nn.2337) [DOI] [PubMed] [Google Scholar]
- 143.O'Keefe J. 1976. Place units in the hippocampus of the freely moving rat. Exp. Neurol. 51, 78–109. ( 10.1016/0014-4886(76)90055-8) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available upon publication at https://buzsakilab.com/wp/resources/.